Abstract
Objective
To assess the efficacy and safety of bevacizumab (BEV) in patients with glioma.
Design
Systematic review and meta-analysis.
Participants
Adults aged 18 years and above, whose histology was confirmed to be malignant glioma.
Primary and secondary outcome measures
The main indicators included progression-free survival (PFS) rate and overall survival (OS) rate, and the secondary indicators were adverse reactions.
Results
A total of 11 clinical centre trials were included in this study for meta-analysis, including 2392 patients. The results of the meta-analysis showed that the median PFS rate of the BEV group was significantly higher than that of the non-BEV group (p<0.00001). When comparing PFS between two groups, we found that the PFS in the BEV group was higher than that in the non-BEV group at 6 months (OR 3.31, 95% CI 2.74 to 4.00, p<0.00001), 12 months (OR 2.05, 95% CI 1.70 to 2.49, p<0.00001) and 18 months (OR 1.31, 95% CI 1.02 to 1.69, p=0.03). But at 24 months (OR 0.83, 95% CI 0.50 to 1.37, p=0.47), there was no significant difference between the two groups. At 30 months (OR 0.62, 95% CI 0.39 to 0.97, p=0.04), the PFS of the BEV group was lower than that of the non-BEV group. Moreover, The results showed that BEV had no significant effect on improving OS, but the adverse reaction in BEV group was significantly higher than that in non-BEV group.
Conclusion
The evidence suggests that BEV can significantly prolong the PFS of patients with glioma within 18 months and shorten the PFS of patients after 30 months. This limitation may be related to the subgroup of patients, the change of recurrence mode, the optimal dose of drug, the increase of hypoxia, the enhancement of invasiveness and so on. Therefore, it is necessary to carry out more samples and higher quality large-scale research in the future.
Keywords: neurosurgery, oncology, head & neck tumours
Strengths and limitations of this study.
We used the Cochrane criteria to assess the risk of bias.
The heterogeneity was explored by sensitivity, subgroup.
The quality of included studies was largely moderate to high.
The preoperative symptoms and the scope and degree of surgical resection are not taken into account.
Introduction
Brain glioma is the most common primary intracranial tumour, accounting for about 27% of central nervous system tumours and 80% of intracranial malignant tumours.1 The median survival time reported with brain glioma is 14–16 months.2 The surgical intervention combined with radiotherapy and chemotherapy are often followed for treatment of such cases, but because of its high invasive nature, it often relapses in a short time with poor prognosis. The emergence of temozolomide has considerably delayed the development of glioma to some extent, but the survival rate and quality of life of patients are still very low. Therefore, looking for better drugs to prevent and delaying the postoperative recurrence of glioma have become the focus of current research. In recent years, more and more studies have shown that malignant glioma is the tumour with the highest degree of vascularisation.3 The nature of proliferation is characterised by obvious proliferative vascular lumen and with abnormal proliferation of neovascularisation which participates in the construction of tumour microenvironment.4 It is closely related to the growth, invasion, and metastasis of the tumour, and positively correlated with the extent of malignancy and prognosis of the tumour. Recently, the unique biological characteristics of gliomas indicated that angiogenic factors may play an important role in its treatment and have become the focus of research.
Humanised antivascular endothelial growth factor monoclonal antibody-bevacizumab (BEV),5 as a representative drug of antiangiogenic therapy, was approved for recurrent glioblastoma by Food and Drug Administration (FDA) in 20096 and was listed in China in 2010 by China Food and Drug Administration (CFDA). According to the radiological response rate, BEV has been approved for recurrent glioblastoma in the USA and many other countries.7 8 Although BEV has become an important part of high-grade glioma (HGG) therapy, the safety and long-term efficacy of BEV are not clear. Therefore, we conducted a clinical meta-analysis to evaluate the safety and adverse reactions of BEV in patients with HGG, in order to provide a reference for clinical application.
Methods
This study was mainly based on the literature research, hence there is no need for ethical identification.
Patient and public involvement
No patients or members of the public were involved in the design or conduct of this study.
Search strategy
We collected all the clinical experimental studies of antiangiogenic therapy in the treatment of gliomas, retrieved through a database search including PubMed, Embase, The Cochrane Library, WanFang, Chinese Periodical Full-Text Database and Chinese Biomedical Literature Service System, the time span is from the establishment of the database to April 2020. The search strategy followed included a combination of subject words and free words, and the retrieval strategy was determined after several pre-searches. The main search words included: “glioma”, “angiogenesis inhibitors”, “vascular endothelial growth factors”, “VEGF”, and “clinical study”. Additionally, we also manually searched the reference list of all articles on this topic to check and enhance the retrieval of other related publications. All search results are evaluated according to the statement of ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’.
Selection criteria
Studies were included if they fulfilled the following criteria: (1) study subjects: the participants were adults aged 18 years and above, whose histology was confirmed to be malignant glioma. They may have undergone some form of surgery to achieve histological diagnosis (biopsy or resection); (2) study type: the clinical control study; (3) intervention: BEV group must include BEV, which can be used alone or in combination with multiple drugs. The control group (non-BEV) refers to treatment that did not include antiangiogenesis agents, which can be placebo or supportive therapy, or active intervention (such as chemotherapy). (4) Outcome indicators: included in accordance with the following arbitrary outcome indicators: (1) main indicators: progression-free survival (PFS) rate, defined as the time from randomisation to death or disease progression of any cause, and overall survival (OS) rate, defined as the time from randomisation to death; (2) key indicators: adverse events classified according to the WHO or the General terminology Standard of the National Cancer Institute (NCI-CTCAE (CTCAE2017)), including the percentage of treatment-related deaths.
Studies were excluded if they fulfilled the following conditions: non-clinical control studies, incomplete abstract information, conference papers, reviews and case reports. In addition, the literature of repeated publication and incomplete data that cannot extract valid data were excluded.
Data extraction
Literature screening, data extraction and cross-checking were carried out by two independent researchers according to the initial inclusion and exclusion criteria, if there were any differences, they were discussed or judged with the assistance of a third person. For missing data, we contacted the author if possible. During the literature screening, the title and the abstract were read initially, after excluding obviously irrelevant literature, the full text was read to determine whether to include it or not. On matching the inclusion criteria of requirements, the following contents were extracted: (1) the basic information, including title, author, published country, publication date, research type; (2) study subjects, including the number of cases in each group, average age; (3) interventional factors, including the specific details of exposure factors, follow-up time and so on and (4) the outcome indicators.
Quality assessment
Using the Cochrane collaboration tool, the risk of bias in individual studies was assessed from seven aspects (sequence generation, allocation hiding, uninformed participants and people, incomplete outcome data, selective reports, and other biases and risks).9 Finally, each project was evaluated at three levels: low risk, unclear and high risk. The two authors conducted independent quality assessments and any differences among them were resolved through discussions with a third research expert.
Statistical analysis
Analysis of outcome index
PFS, OS and adverse reactions were analysed by Meta with RevMan5.1 software. The dichotomy data is expressed as the combined risk ratio (RR) or RR (HR). The measurement data is expressed as the mean difference. The interval estimation was expressed by 95% CI, and the test level of the effect quantity was α=0.05. The test for heterogeneity used I2 statistics. If there is no significant heterogeneity among studies (I2 ≤50%), we used the fixed effects model for data consolidation. While there is significant heterogeneity (I2 >50%) between the results of the study, the random effects model for data analysis would be used.
Sensitivity analysis
Simultaneously, STATA V.15.1 was used for sensitivity analysis, adopt the method of examining the impact of individual studies and eliminate them one by one, if the value obtained is within the CI on both sides, the result is stable. Otherwise, they were regarded as unstable. If the results are unstable, it is proved that the elimination research has a great impact on the overall research results. We will conduct a professional analysis of the elimination research to find out the reasons for its impact on the results and study it. Studies included in literature >10 were used to detect publication bias by funnel chart.
Result
Literature screening
A total of 1108 related literature were obtained in the initial examination. After screening the literature one by one, a total of 1123 patients were included in 11 clinical studies.10–20 The flow chart and the results of literature retrieval are shown in figure 1.
Figure 1.

Document screening process and results. PubMed (n=259), The Cochrane Library (n=153), EMbase (n=155), Chinese Periodical Full-Text Database (n=118), Chinese Biomedical Literature Service System (n=358), WanFang (n=65).
Basic characteristics of the inclusion study
For the inclusion study, the basic information for inclusion is completed using pre-developed forms (tables 1 and 2).
Table 1.
Basic information for inclusion in the study
| Study | State | Research type | Cases (experimental/control) | Ages (experimental/control) | Follow-up time | Outcome |
| Olivier et al10 | France | RCT | 458/463 | 20–84/18–79 | The last patient was hospitalised for 17 months | 1-year and 2-year survival rates, safety and quality of life, PFS, OS |
| Qianru11 | China | RCT | 25/24 | 24–71/27–74 | The median follow-up time was 7.9 months | Disease control rate, median survival time, OS, PFS |
| Herrlinger et al12 | Germany | RCT | 116/54 | 25–78/26–78 | Long-term follow-up until death | PFS-6, PFS, OS |
| Gilbert et al13 | Germany | RCT | 320/317 | >18 | 6 cycles | OS, PFS |
| Chen et al14 | USA | Non-RCT | 57/79/23 | 30–77/24–82/19–78 | >1 year | OS, PFS, adverse reactione |
| Hualong et al15 | China | RCT | 31/31 | 18–70/19–69 | 4 months | PFS6, DCR, adverse reaction |
| Zhang et al16 | China | RCT | 20/20 | 24–74 | 5.2–18 months | PFS6, OS12 |
| Jiaqi et al17 | China | RCT | 27/27 | 53.6±9.7/54.7±8.8 | 6 months–2 years | RR, DCR, adverse reaction |
| Lai et al18 | USA | RCT | 70/110 | 31.3–75.8/20.5–90 | >42 months | OS, PFS, adverse reaction |
| Chauffert et al19 | Britain | RCT | 60/60 | 43–69/43–71 | 6 months |
OS, PFS, adverse reaction |
| Balana et al20 | Spain | RCT | 48/45 | 36–75/43–75 | OS, PFS, adverse reaction |
DCR, Dynamic Contrast Ratio; OS, overall survival; PFS, progression-free survival; RCT, randomised controlled trial; RR, risk ratio.
Table 2.
Basic characteristics of the inclusion study
| Study | Male | Female | Open biopsy | Partial resection | Complete resection | Experimental/control |
| Chinot et al10 | 282 (61.6)/ 298 (64.4) |
176 (38.4)/ 165 (35.6) |
60 (13.1)/ 44 (9.5) |
210 (45.9)/ 223 (48.2) |
188 (41.0)/ 196 (42.3) |
BEV+RT–TMZ/ Placebo+RT–TMZ |
| Qianru11 | 14/12 | 11/12 | / | 15/16 | 10/8 | BEV+TMZ/TMZ |
| Herrlinger et al12 | 80 (69.0)/ 34 (63.0) |
36 (31.0)/ 20 (37.0) |
0/2 (;3.7) | 58 (;50.0)/ 27 (;50.0) |
58 (50.0)/ 25 (46.3) |
BEV+IRI/TMZ |
| Gilbert et al13 | / | / | / | / | / | Bevacizumab/placebo |
| Chen et al14 | 30 (;53)/ 45 (;57)/ 15 (;65) |
57/79/23 27 (;47)/ 34 (;43)/ 8 (;35) |
34 (;60)/ 44 (;56)/ 14 (;61) |
20 (;35)/ 33 (;42)/ 9 (;39) |
3 (;5)/ 2 (;2)/ 0 (;0) |
Bevacizumab monotherapy /bevacizumab combination /non-bevacizumab |
| Hualong et al15 | 19/18 | 12/13 | / | / | / | TMZ+BEV/TMZ |
| Zhixian et al16 | 22 | 18 | / | 18 | 22 | BEV+TMZ/Gamma knife+TMZ |
| Jiaqi et al17 | 16/14 | 11/13 | / | / | / | TMZ+BEV/TMZ |
| Albert et al18 | 31/40 | 39/70 | 2/23 | 40/40 | 28/47 | RT+TMZ+BV/RT/TMZ |
| Chauffert et al19 | 26/23 | 34/37 | / | / | / | BEV+IRI/TMZ+RT |
| Balana et al20 | 31/25 | 17/20 | 42/35 | / | / | TMZ+BEV/TMZ |
BEV, bevacizumab; BV, Bevacizumab; IRI, Irinotecan; RT, radiotherapy; TMZ, temozolomide.
Risk of bias assessment
The results of the bias risk assessment included in the study are shown in figure 2.
Figure 2.
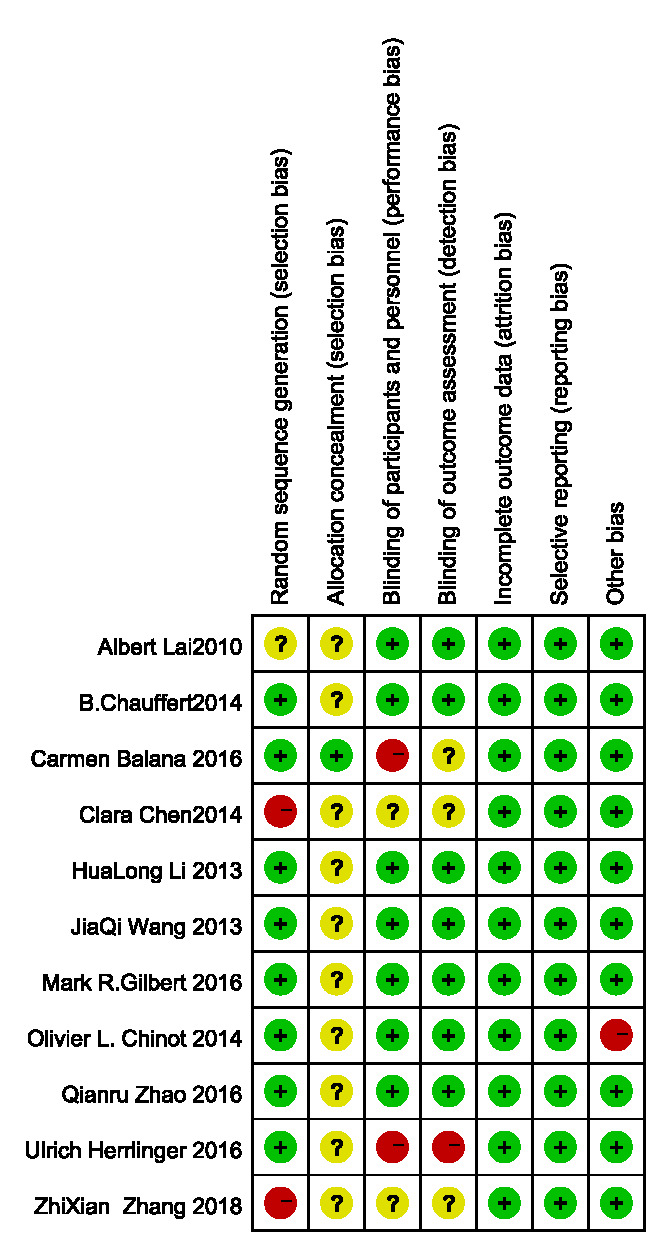
Bias risk assessment form.
Meta-analysis results
Progression-free survival
Seven studies10 12–14 18–20 reported median PFS (BEV group, n=1160) and non-BEV group (n=1027). There was no significant difference in the heterogeneity test (I2=34%<50%), so the fixed effect model was used for data analysis. Results suggested that the median PFS of gliomas treated with BEV was significantly longer than that of malignant gliomas treated with non-BEV (HR 0.71, 95% CI 0.65 to 0.78, p<0.00001), as shown in figure 3.
Figure 3.

HR of median progression-free survival in bevacizumab (BEV) group and non-BEV group in the treatment of glioma.
Ten studies10–19 compared PFS ratios at different follow-up between the BEV group and the non-BEV group. There was a significant difference in the total heterogeneity test (I2=71%>50%), so the random effect model was used. Through the results found it was found that the PFS in the BEV group was higher than that in the non-BEV group at 6 months (OR 3.31, 95% CI 2.74 to 4.00, p<0.00001), 12 months (OR 2.05, 95% CI 1.70 to 2.49, p<0.00001) and 18 months (OR 1.31, 95% CI 1.02 to 1.69, p=0.03). But at 24 months (OR 0.83, 95% CI 0.50 to 1.37, p=0.47), p>0.05, so there was no significant statistical difference between the two groups. At 30 months (OR 0.62, 95% CI 0.39 to 0.97, p=0.04), 0.61<1, the diamond pattern falls on the group that supports non-BEV group, so the PFS of the BEV group was lower than that of the non-BEV group as shown in figure 4.
Figure 4.
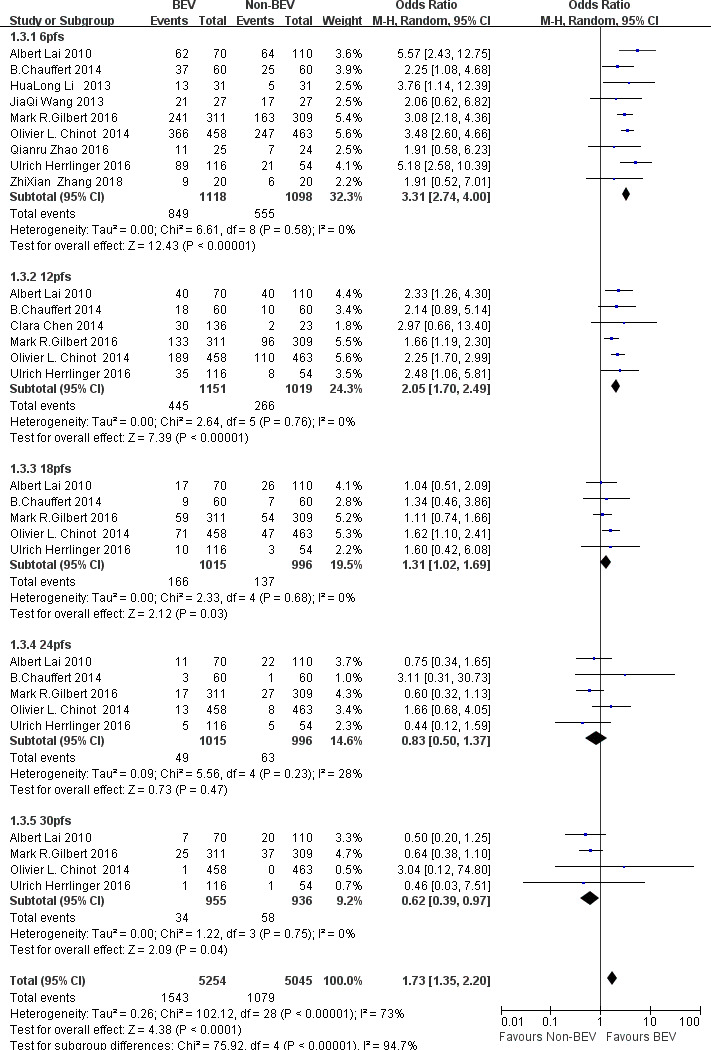
OR of progression-free survival (PFS) at each follow-up time in bevacizumab (BEV) group and non-BEV group in the treatment of glioma.
OS time
Seven studies10 12–14 18–20 reported the median OS time, and there was a significant difference in the total heterogeneity test (I2=71%>50%), so the random effect model was used. Results suggesting that there was no significant difference in median OS time between the BEV group and non-BEV group (HR 0.90, 95% CI 0.73 to 1.10, p=0.30), as shown in figure 5.
Figure 5.
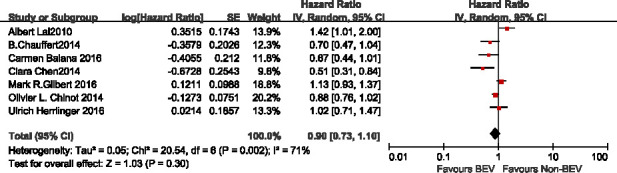
HR of median overall survival in bevacizumab (BEV) group and non-BEV group in the treatment of glioma.
Six studies10 12–14 18 19 compared OS ratios at different follow-up between the BEV group and the non-BEV group. there was no significant difference in the heterogeneity test (I2=38%<50%), so the fixed effect model was used for data analysis. Through the results found it was found that the OS in the BEV group was higher than that in the non-BEV group at 6 months (OR 1.41; 95% CI 1.07 to 1.84; p=0.01), 12 months(OR 1.31; 95% CI 1.09 to 1.58; p=0.005). But at 18 months (OR 0.95; 95% CI 0.79 to 1.14; p=0.58), 24 months (OR 1.10; 95% CI 0.89 to 1.35; p=0.39) and 30 months (OR 0.90; 95% CI 0.69 to 1.18; p=0.44), p>0.05, so there was no significant statistical difference between the two groups, as shown in figure 6.
Figure 6.
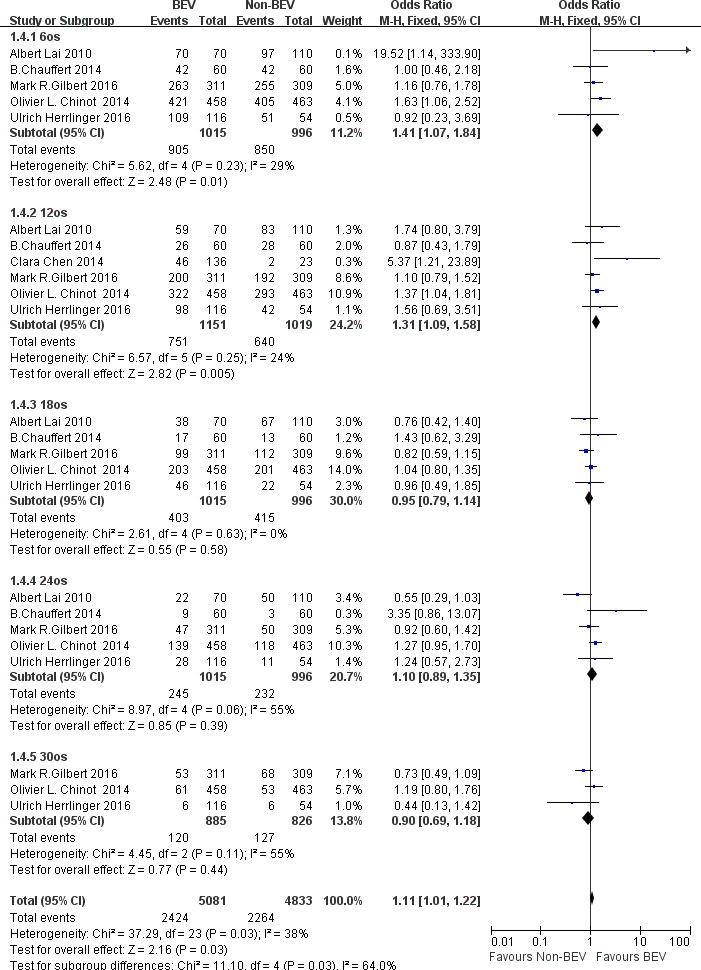
OR of overall survival (OS) at each follow-up time in the treatment of glioma in the bevacizumab (BEV) group and non-BEV group.
Adverse reaction
As shown in figure 7, there were six studies10 11 13–15 that compared adverse reactions between the BEV group and the non-BEV group. There was a significant difference in the total heterogeneity test (I2=54%>50%), and the random effect model was used. The results showed the combined OR values of hypertension, haemorrhage, hematencephalon, albuminuria and thromboembolism as follows: hypertension (OR 5.14, 95% CI 3.79 to 6.96, p<0.00001), haemorrhage (OR 2.62, 95% CI 1.96 to 3.49, p<0.00001), hematencephalon (OR 2.26, 95% CI 1.08 to 4.72, p=0.03), albuminuria (OR 4.04, 95% CI 2.56 to 6.37, p<0.00001) and thromboembolism (OR 1.57, 95% CI 0.88 to 2.77, p=0.13). Through the results found it was found that the adverse reactions in the BEV group was higher than that in the non-BEV group.
Figure 7.
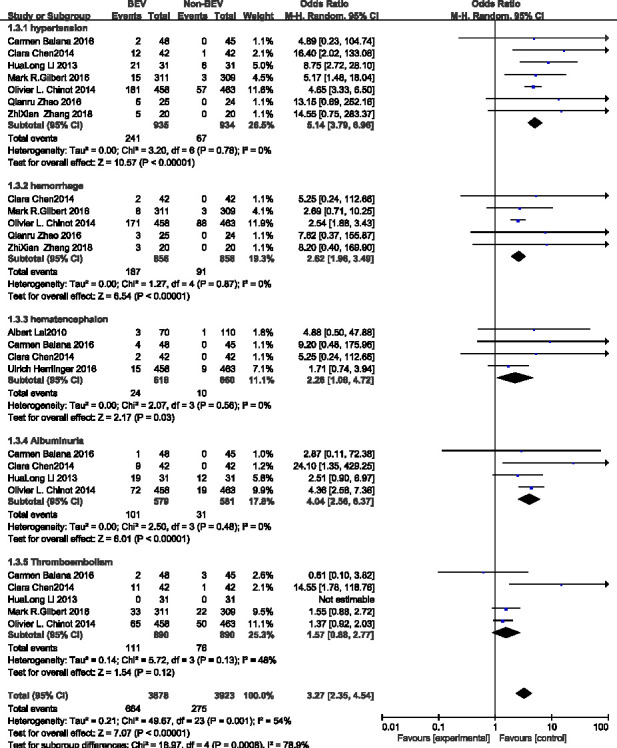
OR of adverse reactions in the treatment of glioma in the bevacizumab (BEV) group and non-BEV group.
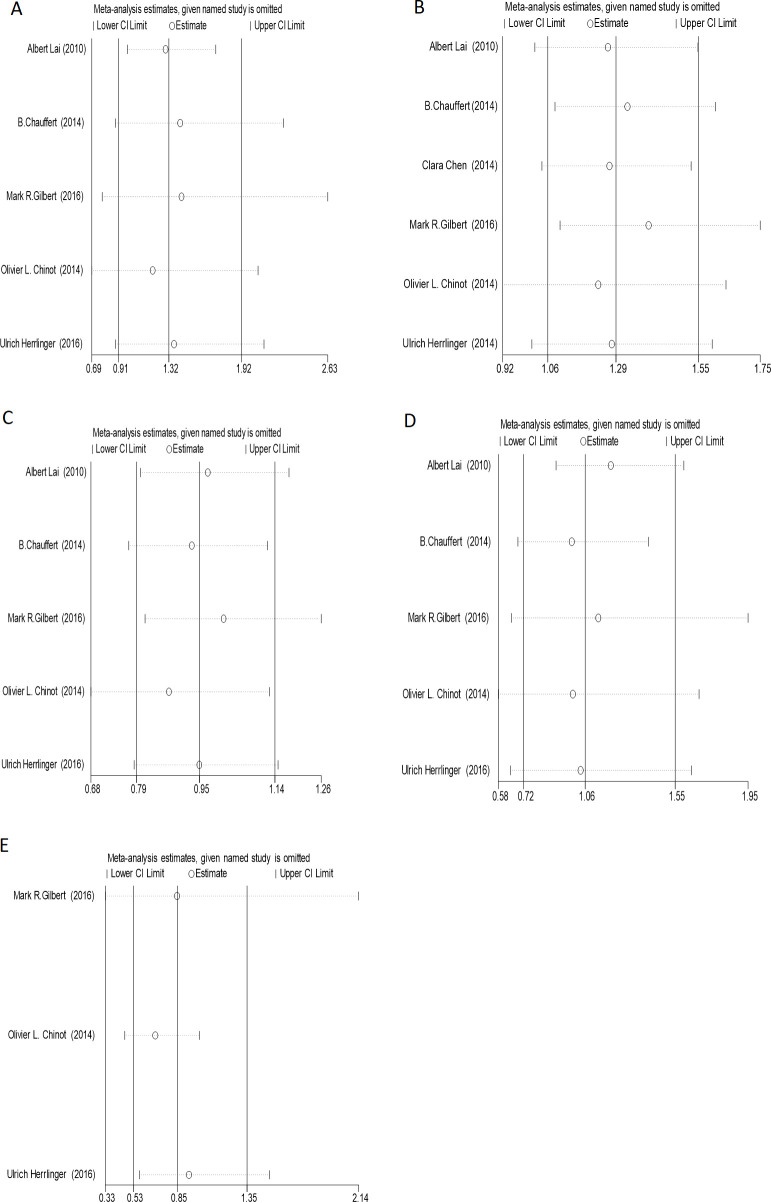
Sensitivity analysis
The sensitivity test was used to evaluate the stability of OS, PFS and adverse reactions in the included literature, which showed that all values remained in the CI on both sides after one by one elimination. Hence, it can be concluded that all the included literature is stable, as shown in figures 8 and 9.
Figure 8.
(A) The sensitivity analysis of PFS6; (B) the sensitivity analysis of PFS12; (C) the sensitivity analysis of PFS18; (D) the sensitivity analysis of PFS24; (E) the sensitivity analysis of PFS30. PFS, progression-free survival.
Figure 9.
(A) The sensitivity analysis of OS6; (B) the sensitivity analysis of OS12; (C) the sensitivity analysis of OS18; (D) the sensitivity analysis of OS24; (E) the sensitivity analysis of OS30. OS, overall survival.
Publication bias
As shown in figure 10, the funnel chart was mainly concentrated at the top. Moreover, the symmetry was also proper, so it was concluded that the possibility of publication bias was small.
Figure 10.
(A) Funnel chart of progression-free survival (PFS) at each follow-up time; (B) funnel chart of overall survival (OS) at each follow-up time.
Discussion
According to histopathological and clinical features, gliomas are divided into astrocytoma, oligodendroglioma, oligodendroglioma and ependymoma, which are the most common malignant tumours derived from neuroepithelium. Although the technical level of surgery, radiotherapy and chemotherapy21 in the treatment of glioma has been greatly improved, but the recurrence rate and mortality rate are still high, so there is an urgent need for a new treatment. Glioma affects the body through a variety of pathophysiological processes, in which angiogenesis plays an important role in the occurrence and development of glioma, so blocking angiogenesis has become a new direction of treatment. BEV is an anti-(VEGF) antibody against vascular endothelial growth factor,22 which acts mainly by competing against VEGF, and binding to VEGFR on the target cell membrane. Pope et al23 and other studies have shown that the high surface of VEGF affects blood vessel density and tumour grade. Some studies have shown that Ang2/Tie224 25 and STAT326 are two important signal pathways in antiangiogenic therapy, which play a good role in inhibiting peritumoural oedema and the increase of neurological symptoms. In order to better understand the advantages and disadvantages of BEV on glioma, this study has a better understanding of the efficacy and safety of BEV through systematic review.
The results of our study showed that the PFS of BEV group was higher than that of non-BEV group during the follow-up period of <18 months, but when the follow-up time was 30 months, the PFS of BEV group was lower than that of non-BEV; meanwhile, it was found that the OS in the BEV group was higher than that in the non-BEV group at 6 months, 12 months, but after 12 months, there was no statistically significant difference between the BEV group and the non-BEV group. The study of Li et al27 showed that the PFS time at 24 months and 36 months in the BEV group was lower than that in the non-BEV group. The results of Liao et al28 showed that a higher incidence of PFS could be obtained by adding BEV to newly diagnosed Glioblastoma (GB), and this combined treatment did not improve OS. The AVA glio29 trial showed that patients treated with BEV had significant advantages in PFS (6.2 months vs 10.6 months) and maintenance of life quality, but showed no advantages in OS (16.8 months vs 16.7 months). 2.2% of patients treated with BEV confirmed false progression, compared with 9.3% of patients treated with non-BEV. Vredenburgh et al30 found in a single-group clinical phase II experimental study that the median PFS of BEV combined with temozolomide and radiotherapy reached nearly two times the standard of 3–14 months, however, the OS was not significant improvement. Chinot et al10 and Gilbert et al31 conducted phase III clinical trials with a placebo control group, the results showed that PFS increased by 40%–71% compared with the control group. Special related research on OS, Brandes et al32 and Wick et al33 also found that BEV failed to improve OS of patients with glioma in a randomised study analysing BEV. From the above research, BEV can improve the PFS of glioma patients within 18 months, but the PFS of patients may be reduced after 30 months. It has no obvious significance to improve OS.
This study showed that after the application of BEV, there were five common adverse reactions: hypertension, haemorrhage, hematencephalon, albuminuria and thromboembolism. A phase II trial of Japanese34 showed that the most common side effects were albuminuria, hypertension, haemorrhage, fever and epilepsy. Studies35 showed that the incidence of adverse reactions above grade 3 was 27.1%–46.4%, the most common events were thromboembolism, hypertension, epilepsy, fatigue and intestinal perforation. Zhang36 searched 20 articles about adverse reactions caused by BEV, and found that the main adverse reactions were cardiovascular and haematological diseases. Norden et al37 evaluated 64 glioma patients who received BEV anticoagulant therapy and 64 glioma patients who did not receive anticoagulant therapy. The results showed that the incidence of intracranial haemorrhage and other bleeding in patients treated with anticoagulants was significantly higher than that in patients with BEV alone, but the incidence of severe intracranial haemorrhage was within an acceptable range. Therefore, when using BEV clinically, it is necessary to closely observe drug adverse reactions, monitor blood pressure, coagulation function and other indicators, and deal with symptoms in time.
From the above research results, it can be concluded that long-term use of BEV does not increase the patient’s PFS, BEV can improve the PFS of glioma patients within 18 months, but the PFS of patients may be reduced after 30 months. Kaka et al found38 that BEV could have a role in the treatment of particular subgroups of patients with newly diagnosed Glioblastoma multiforme (GBM). Several studies10 39 have found that the median PFS of patients with methylation is longer than that of methylguanine DNA methyl transferase (MGMT) unmethylated tumours treated with radiotherapy (RT) and temozolomide (TMZ) combined with BEV. Phillips and colleagues40 found that BEV combined with standard TMZ and RT can improve the survival rate of neurotumours, while poorly differentiated mesenchymal tumours may make tumours resistant to BEV over time. Adilijiang and colleagues41 found that treatment with BEV and TMZ results in the upregulation of certain microenvironment related genes in IDH1 mutant tumours in vitro, specifically those involving immune response and extracellular matrix organisation. Therefore, the question of whether the limitation of BEV in the treatment of gliomas is due to fixed subsets deserves constant attention.
Studies have shown42 43 that antiangiogenic therapy can lead to a transition of glioma to a more aggressive phenotype. In retrospective analysis44 45 a trend toward enhanced infiltrative disease was seen in BEV-treated glioma patients suggesting that enhanced tumour inhibition may be a consequence of VEGF signalling blockade. Weathers et al46 show that determining the best biological dose and the subgroup of patients most likely to obtain long-lasting benefits can improve the durability of BEV. Levin et al47 found treatment for recurrent GBM with BEV appears to improve survival at a dose lower than that in the FDA drug insert. Study48 suggests that the higher dosage of BEV used may have impacted survival benefits. Animal models49 also suggest that higher dose of anti-VEGF treatment, resulting in more hypoxia, may increase tumour aggressiveness. Tamura et al50 found that high doses and long-term use of anti-VEGF/VEGFR may lead to hypoxia. Weathers et al46 proposed in tumours where excessive vascular pruning takes place, hypoxia exacerbated by antiangiogenic therapy is likely responsible for initiating a cascade of events. As mentioned above, there are many possible reasons for the limited efficacy of antiangiogenic therapy. But the lack of a long-lasting response to current antiangiogenic treatment underscores the need for a better understanding of how to use antiangiogenic therapy to optimise radiation and chemotherapy treatments.
Conclusions
The evidence suggests that BEV can significantly prolong the PFS of patients with glioma within 18 months and shorten the PFS of patients after 30 months. This limitation may be related to the subgroup of patients, the change of recurrence mode, the optimal dose of drug, the increase of hypoxia, the enhancement of invasiveness and so on. BEV treatment has no obvious meaning in improving OS, and it has some side effects, which are acceptable, but we still need to pay close attention to it and take active measures to reduce the side effects. Therefore, it is necessary to carry out more samples and higher quality large-scale research in the future.
Supplementary Material
Footnotes
Contributors: HW and JG contributed to conception and design. HW, TW and KW contributed to data acquisition or analysis and interpretation of data. HW, JG, TW, ZW and TS were involved in drafting the manuscript or revising it critically for important intellectual content. All authors have given final approval of the version to be published. HW is the guarantor of this study.
Funding: This study was funded by The First Affiliated Hospital of Xi'an Jiaotong University.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article. No additional data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Brem S, Tsanaclis AM, Gately S, et al. Immunolocalization of basic fibroblast growth factor to the microvasculature of human brain tumors. Cancer 1992;70:2673–80. [DOI] [PubMed] [Google Scholar]
- 2.Xia S, Lal B, Tung B, et al. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro Oncol 2016;18:507–17. 10.1093/neuonc/nov171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Pavlidis N, Jelic S, et al. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol 2005;16 Suppl 1:i64–5. 10.1093/annonc/mdi834 [DOI] [PubMed] [Google Scholar]
- 4.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweet JA, Feinberg ML, Sherman JH. The role of Avastin in the management of recurrent glioblastoma. Neurosurg Clin N Am 2012;23:331–41. 10.1016/j.nec.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009;14:1131–8. 10.1634/theoncologist.2009-0121 [DOI] [PubMed] [Google Scholar]
- 7.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740–5. 10.1200/JCO.2008.16.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. JCO 2009;27:4733–40. 10.1200/JCO.2008.19.8721 [DOI] [PubMed] [Google Scholar]
- 9.HJP G. CochraneHandbook for systematic reviews of interventions version 5.1.0 (updated March 2011). Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 2014;5:S38. [Google Scholar]
- 10.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. 10.1056/NEJMoa1308345 [DOI] [PubMed] [Google Scholar]
- 11.Qianru Z. Efficacy and safety of bevacizumab combined with temozolomide in the treatment of recurrent malignant gliomas. Zhengzhou University, 2016. [PMC free article] [PubMed] [Google Scholar]
- 12.Herrlinger U, Schäfer N, Steinbach JP, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol 2016;34:1611–9. 10.1200/JCO.2015.63.4691 [DOI] [PubMed] [Google Scholar]
- 13.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med Overseas Ed 2014;370:699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Ravelo A, Yu E, et al. Clinical outcomes with bevacizumab-containing and non-bevacizumab-containing regimens in patients with recurrent glioblastoma from US community practices. J Neurooncol 2015;122:595–605. 10.1007/s11060-015-1752-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hualong L, Ming Q, Xiaoqin H. Efficacy of bevacizumab combined with temozolomide in the treatment of malignant recurrent gliomas. Anhui Medicine 2020;41:66–8. [Google Scholar]
- 16.Zhixian Z, Hou G, Jian L. Clinical efficacy of gamma knife combined with bevacizumab in the treatment of postoperative malignant gliomas. Journal of Kunming Medical University 2018;39:33–7. [Google Scholar]
- 17.Jiaqi W, Yong L, Zheng O. Clinical analysis of bevacizumab combined with temozolomide in the treatment of glioblastoma. Oncology Pharmacy 2013;3:274–7. [Google Scholar]
- 18.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011;29:142–8. 10.1200/JCO.2010.30.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauffert B, Feuvret L, Bonnetain F, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: final results of the TEMAVIR study from ANOCEF†. Ann Oncol 2014;25:1442–7. 10.1093/annonc/mdu148 [DOI] [PubMed] [Google Scholar]
- 20.Balana C, De Las Penas R, Sepúlveda JM, et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: the GENOM 009 randomized phase II trial. J Neurooncol 2016;127:569–79. 10.1007/s11060-016-2065-5 [DOI] [PubMed] [Google Scholar]
- 21.Yuntao L, Qi Songtao O, et al. Surgical and therapeutic strategies for recurrent malignant gliomas in refractory sites. Chinese Journal of Modern Neurological Diseases 2012;12:682–90. [Google Scholar]
- 22.Haixia C. Hair cell astrocytoma with vascular central arrangement: case report and literature review. Chinese Journal of Modern Neurology 2013;13:342–8. [Google Scholar]
- 23.Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology 2011;76:432–7. 10.1212/WNL.0b013e31820a0a8a [DOI] [PubMed] [Google Scholar]
- 24.Park J-S, Kim I-K, Han S, et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 2016;30:953–67. 10.1016/j.ccell.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 25.Labussière M, Cheneau C, Prahst C, et al. Angiopoietin-2 may be involved in the resistance to bevacizumab in recurrent glioblastoma. Cancer Invest 2016;34:39–44. 10.3109/07357907.2015.1088948 [DOI] [PubMed] [Google Scholar]
- 26.de Groot J, Liang J, Kong L-Y, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget 2012;3:1036–48. 10.18632/oncotarget.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Hou M, Lu G, et al. The prognosis of anti-angiogenesis treatments combined with standard therapy for newly diagnosed glioblastoma: a meta-analysis of randomized controlled trials. PLoS One 2016;11:e0168264. 10.1371/journal.pone.0168264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao K-L, Huang S, Wu Y-P. The prognosis for patients with newly diagnosed glioblastoma receiving bevacizumab combination therapy: a meta-analysis. Onco Targets Ther 2018;11:3513–20. 10.2147/OTT.S156723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinot O, Cloughesy T, Henriksson R. Efficacy and safety of bevacizumab (bv) plus standard combination temozolomide (T) and radiotherapy (RT) in newly diagnosed glioblastoma: final results from a AVA glio. Eur J Cancer 2013;49:S774–5. [Google Scholar]
- 30.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2012;82:58–66. 10.1016/j.ijrobp.2010.08.058 [DOI] [PubMed] [Google Scholar]
- 31.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandes AA, Finocchiaro G, Zagonel V, et al. At-11 * final results from the randomized phase II trial AVAREG (ML25739) with bevacizumab (BEV) or fotemustine (FTM) in recurrent GBM. Neuro Oncol 2014;16:v10–22. 10.1093/neuonc/nou237.11 [DOI] [Google Scholar]
- 33.Wick W, Brandes AA, Gorlia T, et al. LB-05PHASE III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol 2015;17:v1.5–v1. 10.1093/neuonc/nov306 [DOI] [Google Scholar]
- 34.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–40. 10.1200/JCO.2008.19.8721 [DOI] [PubMed] [Google Scholar]
- 35.Nagane M, Nishikawa R, Narita Y, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 2012;42:887–95. 10.1093/jjco/hys121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z. Statistical analysis of adverse reactions caused by bevacizumab. Clinical Medical Engineering 2012;19:129–30. [Google Scholar]
- 37.Norden AD, Bartolomeo J, Tanaka S, et al. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol 2012;106:121–5. 10.1007/s11060-011-0642-1 [DOI] [PubMed] [Google Scholar]
- 38.Kaka N, Hafazalla K, Samawi H, et al. Progression-Free but no overall survival benefit for adult patients with bevacizumab therapy for the treatment of newly diagnosed glioblastoma: a systematic review and meta-analysis. Cancers 2019;11:1723. 10.3390/cancers11111723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157–73. 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 41.Adilijiang A, Hirano M, Okuno Y, et al. Next generation sequencing-based transcriptome predicts bevacizumab efficacy in combination with temozolomide in glioblastoma. Molecules 2019;24:3046. 10.3390/molecules24173046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piao Y, Liang J, Holmes L, et al. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res 2013;19:4392–403. 10.1158/1078-0432.CCR-12-1557 [DOI] [PubMed] [Google Scholar]
- 43.Lu KV, Chang JP, Parachoniak CA, et al. Vegf inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012;22:21–35. 10.1016/j.ccr.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779–87. 10.1212/01.wnl.0000304121.57857.38 [DOI] [PubMed] [Google Scholar]
- 45.Soda Y, Myskiw C, Rommel A, et al. Mechanisms of neovascularization and resistance to anti-angiogenic therapies in glioblastoma multiforme. J Mol Med 2013;91:439–48. 10.1007/s00109-013-1019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weathers S-P, de Groot J. Resistance to antiangiogenic therapy. Curr Neurol Neurosci Rep 2014;14:443. 10.1007/s11910-014-0443-y [DOI] [PubMed] [Google Scholar]
- 47.Levin VA, Mendelssohn ND, Chan J, et al. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol 2015;122:145–50. 10.1007/s11060-014-1693-x [DOI] [PubMed] [Google Scholar]
- 48.Lorgis V, Maura G, Coppa G, et al. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol 2012;107:351–8. 10.1007/s11060-011-0748-5 [DOI] [PubMed] [Google Scholar]
- 49.Keunen O, Johansson M, Oudin A, et al. Anti-Vegf treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A 2011;108:3749–54. 10.1073/pnas.1014480108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura R, Tanaka T, Akasaki Y, et al. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol 2019;37:2. 10.1007/s12032-019-1329-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article. No additional data are available.