Abstract
Objective
The objective of this scoping review was to map the current situation and available evidence and gaps on rabies morbidity, mortality, integrated rabies surveillance programmes, and existing prevention and control strategies in Africa.
Methods
We conducted a systematic scoping review following the Joanna Briggs methodology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews checklist. Medline, Embase, CINAHL (EBSCOHost), Scopus, Web of Science and rabies web conferences were used to search for peer-reviewed publications between January 1946 and May 2020. Two researchers reviewed the studies and extracted data based on author (year) and region, study design and data collection duration, participants/comparators, interventions, control conditions/exposures and outcomes (rabies mortality and morbidity) and key findings/gaps/challenges. The results were reported narratively using Arksey and O’Malley’s methodological framework.
Results
Electronic search yielded 2775 records, of which 43 studies were included. A total of 543 714 bite victims were censored through the included studies. Most of the victims were less than 15 years of age. The studies included rabies morbidity (21) and mortality (15) fluctuating in space and time across Africa depending on countries’ rabies prevention and control practices (16). Others were surveillance (nine studies); surveillance and prevention (five studies); management and control (seven studies); and surveillance, prevention and control (six studies). We found challenges in rabies reporting, existing dog vaccination programmes and post-exposure prophylaxis availability or compliance.
Conclusion
This study found challenges for dog rabies control and elimination in Africa and the need for a policy to drive the goal of zero dog-transmitted rabies to humans by 2030.
This is an open-access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build on this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated and the use is non-commercial (see http://creativecommons.org/licenses/by-nc/4.0/).
Keywords: epidemiology, infectious diseases, public health
Strengths and limitations of this study.
We conducted an extensive search of published and grey literature to identify studies to include in the scoping review.
Pulling together data from both published and grey literature from the Ministries of Health gave us an opportunity to understand the breadth of rabies epidemiology and how surveillance, prevention and control would be a critical tool in implementing effective control of rabies across Africa.
We conducted screening of identified articles and extraction of data in duplicate.
We reported the results narratively as it was not possible to combine data from different studies conducted using different study designs and different population groups.
Background
The natural history of rabies disease in Africa is not well known, but it is well accepted that the disease must have been present in northern Africa for hundreds of years, particularly as an urban dog disease and was also associated with cycles in the Middle East.1 European colonisation influenced the spread of dog rabies in Western and Central Africa.2 In many sub-Saharan African countries, rabies has become epizootic only in the 19th and 20th centuries involving domestic dogs and free-ranging wildlife species.1–3
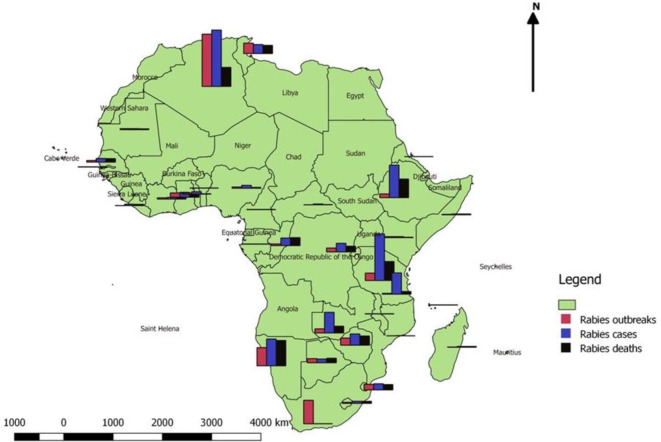
More than 59 000 people die of rabies worldwide every year,4 5 99% of them in African and Asian countries where dog rabies is endemic.4 6–10 Due to the lack of laboratory confirmation, sporadic epidemiological surveillance and unreported clinical cases in developing countries, current mortality estimates almost certainly under-represent the true incidence of human rabies deaths.4 8–10 Rabies is responsible for an estimated 21 000–25 000 deaths annually in Africa.4 11 12 Figure 1 shows a map illustrating rabies distribution in 32 African countries considering rabies outbreaks in animals, cases and deaths in humans.13 In 2011, a total of 33 African countries reported 1607 outbreaks of rabies, 2779 cases and 1524 deaths.13 Data show that rabies accounts for 7.2% of all animal disease outbreaks reported, making it the disease with the highest number of outbreak reports in Africa in 2011.13 Algeria, Namibia, Eswatini (former Swaziland), Tunisia, Uganda, Zambia and Zimbabwe reported high morbidity and mortality with 563 cases (33.9% deaths), 269 cases (94% deaths), 62 cases (88.7% deaths), 91 cases (90% deaths), 466 cases (40.9%), 207 cases (32.8% deaths) and 114 cases (80.7% deaths), respectively.13
Figure 1.
Human rabies distribution in 32 African countries (2011).
Dog rabies predominates throughout most of Africa; the domestic dog is the principal reservoir host as well as the most important source of infection for people.14 In addition, there are many other lyssaviruses (also referred to as rabies-related viruses) reported from African countries. Most of these rabies-related viruses have been associated with obscure hosts including specific bat species and shrews, partly attributable to the difficulty in biosurveillance of these viruses. RABV, however, spreads in terrestrial mammalian hosts in Africa and has not been associated with bat infections as it is the case in the Americas. While all mammals (domestic and wild) are susceptible to RABV infection, some are able to retain those virus variants adapted to their species while others are only reported as dead-end hosts.15 Rabies has been reported in both domesticated species and wildlife. These are sometimes diagnosed with rabies virus infection in Zambia, South Africa, Ethiopia, Kenya, Tanzania, Zimbabwe and Egypt.15–21
Most reported cases of rabies in wild carnivorous species included yellow mongoose (Cynictis penicillata) and bat-eared fox (Otocyon megalotis)22 as well as critically endangered wild dogs (Lycaon pictus).19 23–25 In Ethiopia, rabies outbreaks were described in the endangered Ethiopian wolf (Canis simensis) population.26 27 In 2014 and 2015, RABV infection was also observed in two wild dogs and a spotted hyaena (Crocuta crocuta) in the Madikwe Game Reserve, North West Province of South Africa, in Ethiopia and in Nigeria,21 25 28 monkeys and jackals (C. adustus and C. mesomelas).16 17 20 21 The review of 20 studies across Africa has revealed that bite victims account for 91.9% (48 092 dog bites), cat bite for 2.9%, jackal bite for 0.8% and 4.41% for others (monkey, donkey, horse, rat, pig, rabbit, Honey badger, kudu, goat, cattle, eland and hyaena).29–48
Mass vaccination of dogs as a key component of national rabies elimination programmes has been successful in eliminating dog-transmitted rabies in Europe, North and Latin America, and Japan.49–51 By far, the most significant public health threat comes from RABV, and over 99% of all globally reported human cases are caused by exposure to unvaccinated dogs infected with canine RABV variant, mostly in Asia and Africa.52
In most of Africa, and specifically Western and Central African countries, notification of rabies disease is not mandatory, so epidemiological data are scarce.53 Human rabies could be prevented by the immediate administration of post-exposure prophylaxis (PEP) following exposure to rabid animals.5 45 However, people in low-income countries often do not receive these life-saving treatments because either PEP treatment is costly and not readily available, or because of lack of rabies awareness, people might not go to hospital for treatment.5 9 54 The lack of effective educational outreach at community level had led to gaps in knowledge as to the best way to avoid animal bites and administer first aid following bites or other potential rabies exposures.55 A recent study has shown considerable in-country variability in the availability of rabies vaccines and immunoglobulin vaccine supply system, administration route (intramuscular (IM) vs subcutaneous (SC), cost of vaccine and rabies immunoglobulin (RIG). In a global survey conducted in rabies-endemic countries, 49 of the 54 African countries were rated as moderate to high risk for human rabies, while 16 of the 23 countries that responded to the survey had inadequate surveillance systems.12 56
One major barrier is the difficulty of consistently achieving the required coverage of 70% of the dog population across the hard-to-reach landscapes that characterise much of sub-Saharan Africa.57–59 Reviewing dog vaccination coverage in African countries, only South Africa, Tanzania, Algeria, Morocco and Egypt had the dog vaccination coverage of 63%, 37.24%, 23.7%, 25% and 23.7%, respectively.60 61 In all other African countries, dog vaccination coverage was below 18% with further below 5% in some cases.60 The analysis of the above data and the consideration of the framework for the elimination of dog rabies suggested by Wallace et al and others stipulated the existing coverage of dog rabies vaccination, that was directly associated with the number of years it would take to achieve rabies elimination.62 Theoretically, Global Dog Rabies Elimination Route consisting of a 13-year time frame would be an ample time for even the least developed rabies prevention systems to achieve elimination by 2030 if completely committed to this achievement.62 This system divided countries into three categories: (1) phase I—preparation (dog vaccination >18%), (2) phase II—vaccination of dogs (dog vaccination: <18% and >70%) and (3) phase III—70% continued vaccination of dogs. African countries have been categorised into phase I, II and III but with no data on dog vaccination.63 The available data indicate that most African countries were still at the preparation phase since ‘zero rabies by 2020’ was initiated 5 years ago. Although the feasibility of reaching 70% dog vaccination coverage has been shown through pilot projects in a wide range of settings, African countries still struggle to achieve a 70% yearly dog vaccination rate.10 14 In Africa, dog mass vaccination systems have demonstrated some effectiveness as a proof of principle in countries such as South Africa,49–64 Tanzania,49 65–67 Malawi49 68 and Chad.54 69–71
Inadequate education for veterinarians and physicians, insufficient resources for proper confirmatory diagnosis and risk assessment, and the lack of effective communication channels between Ministries of Health and Agriculture frequently have led to failures of prophylactic intervention, even in regions where vaccines and immunoglobulins were available.55 A recent study conducted in Africa and Asia revealed that RIGs were found to be less available than the vaccine, with access restricted in almost two-thirds of the countries surveyed.72 Eleven (11) African countries had comprehensive access to RIG. Of the seven countries with broad access to vaccines, six of them had a national rabies prevention programme or policy. Two of the countries had only a monitoring programme/strategy in place.72 This is worrisome as it exposes a huge absence of surveillance and prevention policies in most African countries. The absence of a robust monitoring process is mostly attributed to the lack of rabies in national communicable disease plans and reporting systems at national level in Africa.73 Therefore, widespread under-reporting is likely to occur in many affected countries due to lack of health information, civil registration and vital statistical systems, and inaccessibility of clinical care and diagnostic confirmation73 as symptoms of the disease may be non-specific and similar to other encephalitic infections. Even where data exist in Africa, the lack of communication and exchange of data between the animal and human health sectors also hinders the collection, storage and reporting of coherent data to international databases.73 The WHO meeting in Geneva in 2018 on ‘Moving Progress towards Rabies Elimination’ pointed out that political engagement is a key factor with governments providing leadership role in the coordination of elimination strategies.12 The global collection of data on deaths from any neglected disease is a huge challenge, and early attempts to collate data for human deaths from canine rabies were no exception.74 Due to the lack of regular reporting of rabies cases to the WHO from many member states, the RabNet Database was closed down in 2011.75 Therefore, rabies is not reportable in many African countries, which restricts data collection by structured surveillance systems.73 One Health evolved from the recognition that an interdisciplinary approach is required to understand complex health problems, and that the health of humans and animals is inextricably linked.76 Rabies requires a comprehensive, strategic, and targeted control and prevention approach with collaboration from human, animal, and environmental health disciplines at local, national, and global levels to achieve more effective control.77 In fact, most of African countries lack a One Health approach to prevent human rabies deaths.78
The WHO, the World Organization for Animal Health (OIE), the Food and Agriculture Organization and the Global Alliance for Rabies Control (GARC) have developed a strategic global plan to end canine-mediated rabies by 2030.7 12 79 This initiative provides a concerted approach to the prevention of rabies, combined with the strengthening of human and veterinary health systems. These would enable reaching out to the most underserved communities in the world by engaging, encouraging and supporting all countries to lead and improve elimination efforts.73 This scoping review was therefore designed to map the evidence on rabies morbidity, mortality, integrated rabies surveillance, prevention and control in African countries. Its objectives were: (1) to assess the extent of available research on the morbidity and mortality of rabies due to animal bites conducted in Africa; (2) to identify research gaps in the literature on the impact of rabies in Africa so as to effectively plan public health intervention; (3) to ascertain the current level of rabies disease surveillance, prevention and control that exists in African countries; (4) to assess the published adverse events and complications associated with human rabies vaccination in African countries; (5) to assess the different types of vaccines used and the effectiveness of locally produced and imported vaccines in treating rabies in different parts of Africa; and (6) to assess rabies morbidity and mortality associated with dogs and contact with a suspect animal in humans.
Methodology
Study design
This paper used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews checklist80 and the Joanna Briggs Institute guidelines81 as a norm for reporting scoping reviews. The analysis was conducted in accordance with the structure suggested by Arksey and O'Malley,82 further developed by Tricco et al.83
Eligibility criteria
The search was conducted from 1 January 1946 until 30 May 2020. A PICO (Population/Interventions/Comparisons/Outcomes) search framework was set, where P (humans infected with rabies in African countries), I (integrated rabies disease surveillance, prevention and control), C (little or no integrated rabies disease surveillance, prevention and control) and O (reduced human morbidity and mortality of rabies associated with animal bites) were chosen. The included studies are described in online supplemental table 1.
bmjopen-2020-048551supp001.pdf (247.2KB, pdf)
Electronic search
We conducted a systematic search of the electronic databases Medline (OVID), Embase (OVID), CINAHL (EBSCOHost), Scopus (Elsevier), Web of Science and web conferences (rabiesalliance.org, www.who-rabies-bulletin.org and https://www.oie.int/). The search techniques were limited to English. The main search strategy was listed in online supplemental material 1. EndNote V.X9 reference manager was used to remove duplicates. JLT reviewed all the papers identified by title in order to pick those that were potentially appropriate, with a clear bias towards retention. The abstracts of all the studies chosen on the basis of their titles were independently reviewed by two reviewers (PN and JLT) and any variations were preserved in the study.
bmjopen-2020-048551supp002.pdf (76.6KB, pdf)
Data charting process
For all studies selected at the abstract level, data were extracted and plotted in the table, covering author (year) and region, study design and data collection duration, participants/comparators, interventions and control conditions/exposures, outcomes (rabies mortality and morbidity), and key findings/gaps/challenges. The final decision to include studies was taken on the basis of this data extraction and whether it met the inclusion/exclusion requirements, based on an independent review by two authors (PN and JLT) and a discussion of any differences; the third author (RT) was available for consultation if consensus could not be reached. Our inclusion criteria were the following: rabies occurrence or mortality rates, all ages included, only studies performed in Africa, studies in which at least one intervention included monitoring, prevention and control of rabies, and only quantitative studies.
A data extraction sheet has been developed and used to extract data from included papers. The data collection sheet included: author, region, year, study design, level of evidence, sample size description, interventions or exposures, results, and key findings/gaps/challenges. Two reviewers (PN and JLT) worked separately at all levels of the study. The results were then compared and any variations were addressed and resolved by PN and JLT. The third author (RT), who also summarised the findings, was consulted when a discrepancy could not be resolved. The evaluation of the probability of bias, the methodological standard of the included studies, was not assessed due to the scoping review of the study.82
Data items
Seven items were listed in the data collection chart table. We included the first author, the year of publication of the study and the country (item 1), study design and period of data collection (item 2), the sample size, mean or median age, and gender (item 3). The intervention and control conditions/exposures included surveillance, prevention and control of rabies and any other form of intervention used in human rabies (item 4). The number or rate of human rabies morbidity included annually or during the study period (item 5). Human rabies mortality recorded the death or mortality rate annually or during the study period (item 6). Main findings/gaps/challenges included other outcomes such as data gaps found, available research evidence as well as PEP or vaccine adverse events (item 7).
Critical appraisal of individual sources of evidence
The methodological quality of included studies was not evaluated due to the scoping nature of the review.83
Synthesis of results
Studies were summarised based on author (year) and country, study designs, participants and comparator, interventions and control conditions/exposures, key outcomes, gaps, findings and challenges. Interventions were subdivided into rabies prevention, surveillance, control and management. Where data were not clearly provided to compute morbidity and mortality rates, we reported the results narratively, as recommended in scoping reviews methodological framework.82
Results
Study selection
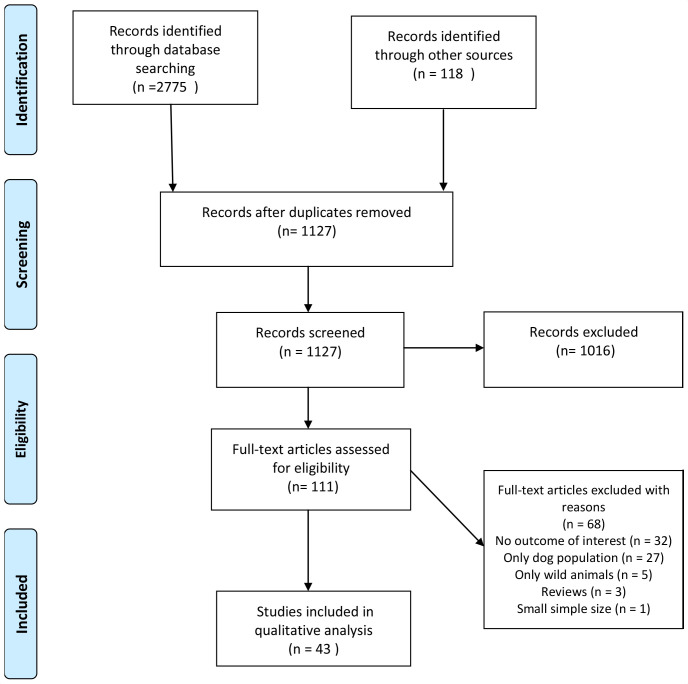
The searches from the five electronic databases hit a total of 2775 records (Medline: 696, Embase: 952, CINAHL: 289, Scopus: 431 and Web of Science: 407) that led to a total of 1127 titles and abstracts that were screened after the removal of duplicates. We retained 111 of these based on their title and abstract screening. The full-text screening’s stage led to 43 potential articles relevant to our scoping review. The scoping review flow chart was described in figure 2.
Figure 2.
Flow diagram of human rabies mortality and morbidity associated with animal bites in Africa.
Study characteristics
The review reported only quantitative studies on rabies surveillance, prevention and control. Thirty-two quantitative studies were retrospective cohort with 8 months to 14 years of study duration,21 29–31 33 34 36 38–46 48 84–98 three studies were mixed designs (retrospective and cross-sectional study),47 99 100 three were prospective cohort studies,32 35 101 two were cross-sectional studies,102 103 one was case–control,37 one was clinical trial104 and a randomised control trial.105
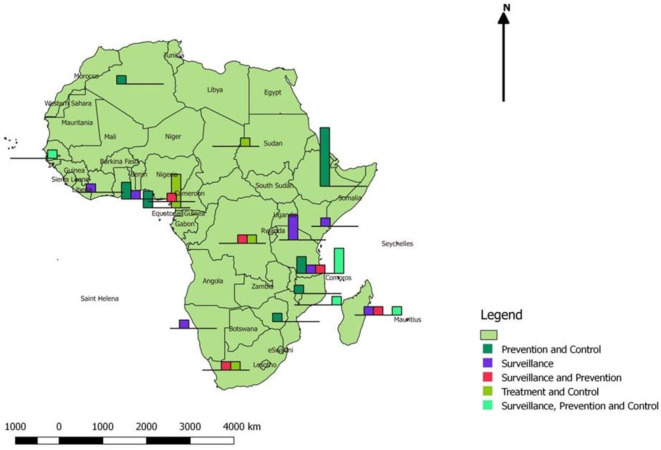
We grouped the included studies into five categories based on rabies interventions, namely (1) prevention and control, (2) surveillance, (3) surveillance and prevention, (4) treatment and control, and (5) surveillance, prevention and control. Figure 3 shows the distribution of studies according to the intervention in 19 African countries. We also summarised all studies included in the final analyses in online supplemental table 1 which included seven parts in line with the specified framework for data synthesis: (1) rabies morbidity, (2) rabies mortality, (3) interventions for rabies control, (4) rabies disease surveillance, prevention and control, (5) available research evidence, (6) research gaps identified, and (7) adverse events and complications associated with rabies vaccination.
Figure 3.
Distribution of 43 studies in African countries.
Patient characteristics
A total of 543 714 bite victims were recorded in included studies. Age-specific and sex-specific distribution revealed that the most fatal cases belonged to age groups 0–14 years.29 33 35 38 43 45 46 48 84 91 92 94 96 100 102 Other studies identified rabies victims of 15 years and above.30 38 39 42 46 84 95 96 100 103 The median age was 18 years in most of the studies and ranged from 1 to 95 years. Most of the children were males.45 46 94 96 However, another study has indicated that females have more animal-related bites than males.29 Young children are at higher risk of contracting rabies in the absence of PEP and wound care due to the location of the bites they incur.102 Based on the extent and depth of injury, 1567 victims were recorded. Extent and depth of injury was classified as broken skin (11.1%), scratch (9.25%), superficial (loss of epidermis only) (36.38%), deep (27.31%) and simple (affect only one tissue) (12.38%).29 31 101 103 Further, 10 006 bites were described in regard to the site of exposure among which included the head/neck/face (5.08%), leg/feet (61.06%), arm/hand (23.23%), buttocks/trunk (10.32 %) or multiple (0.31%).29–33 42 47 93 95 99 102
Individuals suspected to have rabies were clinically managed by the chief doctor paediatricians and nurses.32 36 37 40 84 94 99 103 The main treatments were wound management, antibiotics, and prophylaxis against tetanus and rabies. Nine studies reported wound management as part of PEP.32 37 40 47 93–96 Reports were collected in hospitals, treatment and health centres, health clinics and pharmacies.103
Study outcomes
Rabies morbidity
Twenty-one studies reported human rabies morbidity in Africa (table 1).30 34–39 41 43 46 48 84 86 88–92 97 99–101 103 Among them, five studies were undertaken in Tanzania, the first study estimated an average annual incidence per 100 000 bites of 37.1, 11.3 and 33.5 in the human population districts of Ulanga (193 280 inhabitants), Kilombero (321 611 inhabitants) and Serengeti (176 057 inhabitants), respectively.89 The second study found that the incidence of bite patients seeking PEP declined substantially (>50%) from 2011 to 2015.90 The third study estimated an annual incidence of ~58 cases per 100 000,41 the fourth study reported a mean incidence of 74 bites considered at risk of rabies transmission per 100 000 persons per year,97 and the last study conducted in Tanzania revealed an average of 75.6 and 19.3 probable rabies exposures per 100 000 persons per year.99 Three studies conducted in Ghana reported 54 dog bite victims bitten by rabies-positive dogs within 3 years,84 13 cases of human rabies in a 6-year retrospective records review38 and an annual incidence of rabies cases of 172 per 100 000 people.48 Four studies reported rabies morbidity in Ethiopia. Yizengaw et al reported a high incidence rate of rabies exposure during spring (360, 39%) and summer (244, 26.4%) seasons and a total of 924 human rabies exposure cases received the anti-rabies PEP from September 2015 to August 2017.100 The incidence of human rabies exposure was reported to be 40 per 100 000 persons in Ethiopia,33 annual estimated rabies incidence of 2.33 cases per 100 000 in humans,101 and the incidence of human rabies exposure cases calculated per 100 000 persons was 35.8, 63.0, 89.8 and 73.1 in 2012, 2013, 2014 and 2015, respectively.93 A study conducted in Zimbabwe found among rabies suspect, 42 (73.7%) were positive.39 In Madagascar, 9 of the 11 suspected human cases tested from 2005 to 2010 with a laboratory-confirmed rabies.86 In Uganda, a total of 208 720 patients with animal bite injuries were treated at health facilities across the country.88 Ivory Coast reported 50 cases of human rabies with annual incidence of 0.06–0.08 per 100 000.35 The incidence of human injuries caused by animal bites was 289 per 100 000 persons with the highest incidence reported at 302 per 100 000 and lowest at 121 per 100 000 persons in Kenya.38 Another study undertaken in Malawi reported 14 paediatric rabies cases during the study period.92 A 6-year retrospective study revealed 31 positive cases of human rabies in Madagascar.46 In Democratic Republic of Congo (DRC), a 5-year retrospective study found 29 positive to rabies in a total of 5053 dog bites recorded in the veterinary clinics,34 and Frey et al estimated an annual incidence of bites from suspected rabid animals of 12.9/100 000 and an incidence of 0.7 human rabies deaths/100 000 in Chad.103 Namibia reported above 16 cases per year from 2011 until 2015 with a maximum of 23 cases observed in 2015 with an incidence of 1.0 and 2.4 per 100 000 inhabitants and per year on average.43
Table 1.
Mapping human rabies morbidity rate
| Country (region, district and town) | Human rabies morbidity rate | Study duration |
| Chad (N’Djamena) | An annual incidence of bites from suspected rabid animals of 12.9/100 000103 | 7 months, September 2008–April 2009103 |
| Democratic Republic of Congo (Kinshasa) | 29 positive to rabies in 5053 dog bites recorded in the veterinary clinics34 | 5 years, 2009–201334 |
| Ethiopia (national level; Ababa and outside of Addis Ababa; North Gondar administrative zone) | A total of 924 human rabies reported.101 The incidence of human rabies exposure was reported to be 40 per 100 000 people in Ethiopia33; annual estimated rabies incidence of 2.33 cases per 100 000 in humans101; the incidence of human rabies exposure cases calculated per 100 000 people was 35.8, 63.0, 89.8 and 73.1 every year91 | 1 year, January 2016–31 December 2016; 2 years, 2015–2017; 3 years, 2012–2015; 11 months, April 2009–March 201033 91 100 101 |
| Ghana (Techiman municipality; eastern region of Ghana) | 54 dog bite victims bitten by rabies-positive dogs84; 13 cases of human38; an annual incidence of rabies cases of 172 per a population of 100 00048 | 4 years, 2009–2012; 6 years, 2011–2016; 2 years, 2013–201538 48 84 |
| Ivory Coast (national level) | Annual incidence of 0.06–0.08 per 100 00035 | 3 years, 2014–201635 |
| Kenya (Machakos and Kitui counties in lower eastern region; Kisumu county in Lake Victoria basin; Nandi county in Central Rift Valley and Kilifi coastal region) | Human injuries caused by animal bites, incidence of 289 per 100 000 persons30 | 6 years, 2011–201630 |
| Madagascar (national level) | 9 of the 11 suspected human cases tested with a laboratory-confirmed rabies86 31 positive cases of human rabies reported46 |
6 years, 2006–2011; 6 years, 2005–201046 86 |
| Malawi (Blantyre) | 14 paediatric rabies cases reported92 | 6 years, 2012–201792 |
| Namibia (Kavango) | An incidence of 1.0 and 2.4 per 100 000 inhabitants and per year on average43 | 7 years, 2011–201743 |
| Tanzania (Mwanza region; Tabora; Shinyanga; Mara; Ulanga; Kilombero; Serengeti; Dodoma region; Ngorongoro districts in northern Tanzania and in the 11 districts in southern Tanzania) | Average annual incidence per 100 000 bites of 37.1, 11.3 and 33.5 in human population89 An incidence of bite patients seeking PEP declined substantially (>50%)95 An annual incidence of 58 cases per 100 00041 Mean incidence of 74 bites considered at risk of rabies transmission per 100 000 persons per year97 An average of 75.6 and 19.3 probable rabies exposures per 100 000 persons per year99 |
5 years, 2002–2006; 4 years, 2002–2006; January 2011–January 2013; 7 years, 2008–2014; 2002–2017; 2011–2017; 2011–201641 89 90 97 99 |
| Uganda (national level) | 208 720 patients with animal bite injuries treated across the country88 | 14 years, 2001–201588 |
| Zimbabwe (national level) | Among rabies suspect, 42 (73.7%) were positive39 | 11 years, 1992–200339 |
PEP, post-exposure prophylaxis.
Rabies mortality
Sixteen studies reported rabies-related mortality in Africa (table 2).21 31 37 40 42 44 45 85 87–89 98 99 101–103 Ethiopia reported three studies among which 320 people, diagnosed clinically, died of rabies in a 5-year retrospective study conducted in the national level,21 386 human rabies fatalities were reported in an 8-year retrospective study with an annual range of 35–58 deaths in Addis Ababa and outside of Addis Ababa.45 There were also 32 cases of human rabies recorded from which 3 people ended with fatality in North Gondar administrative zone.101 Four studies assessed rabies mortality in Tanzania from which Ulanga, Kilombero and Serengeti districts reported human rabies mortality rates of 2.4, 0.8 and 1.4/100 000 per year, respectively.89 Sixteen human deaths (1291 bite victims) due to rabies were reported within the Integrated Bite Case Management Study across 20 districts in 4 regions in Southern, Central and Northern Tanzania.98 Other studies reported 28 deaths from suspected rabies cases during the 5-year period in the two districts, an average of 1.5/100 000 per year in Serengeti and 2.3/100 000 in Ngorongoro.44 Fourteen (14) among 1005 bite victims died showing clinical signs of rabies within 5 years.99 Three (3) studies identified rabies mortality in Uganda. Among them, there were 592 (95% CI 345 to 920) deaths,102 where one dose of PEP was sufficient for protection following a rabid animal bite. Another research estimated a total of 371 deaths of rabies with a cumulative total of 117 085 rabies cases in 9 years87 and a 14-year retrospective study revealed a total of 486 suspected human rabies deaths among 208 720 patients with animal bite injuries treated at health facilities across the country.88 A study undertaken in Moramanga district (Madagascar) recorded an annual incidence of 42–110 rabies exposures and 1–3 deaths per 100 000 persons.31 An estimated seven rabies deaths (95% CI 4 to 10 deaths) per year was recorded in N’Djamena (Chad).101 A study conducted in Algeria excluding Sahara region found an annual average of 20.6 human rabies deaths.90 A total of 14 cases of fatal rabies with 12 deaths were reported in Maputo city, which is the capital of Mozambique.37 An average annualised rabies attack rate of 136 rabies cases per 100 000 dog bite injuries (7 of 5139) with 6 of 7 deaths were reported in South Africa.42 There were patients with serious rabies manifestations and the case fatality rate of 100% in a study conducted in Kinshasa (DRC).40
Table 2.
Mapping human rabies mortality rate
| Country (region, district and town) | Morbidity rate | Study duration |
| Algeria (national level excluding Sahara region) | An annual average of 20.6 human rabies deaths85 | 13 years, 2006–201885 |
| Chad (N’Djamena) | An estimated 7 rabies deaths (95% CI 4 to 10 deaths) per year103 | 8 months, September 2008–April 2009103 |
| Democratic Republic of Congo (Kinshasa) | Case fatality rate of 100%40 | 8 months, December 2008–July 200940 |
| Ethiopia (national level, Ababa and outside of Addis Ababa, North Gondar administrative zone) | 320 people died of rabies in 5 years21 386 human rabies fatalities were reported with annual range of 35–58 deaths45 32 cases in human rabies recorded101 |
5 years, 1997–2001, 8 years, 2001–2009; 11 months, April 2009–March 2010; 1 year, January 2016–31 December 2016; 2 years, 2015–2017; 3 years, 2012–2015; 11 months, April 2009–March 201021 33 45 91 100 101 |
| Madagascar (Moramanga district) | An annual incidence of 42–110 rabies exposures and 1–3 deaths per 100 000 persons31 | 1 year, 2016–201731 |
| Mozambique (Maputo city) | A total of 14 cases of fatal rabies with 12 deaths34 | 3 months, April–July 201434 |
| South Africa (Uthungulu District of Kwazulu-Natal province) |
An average annualised rabies attack rate of 136 rabies cases per 100 000 dog bite injuries (7 of 5139) with 6 of 742 | 3 years, 2008–201042 |
| Tanzania (Ulanga, Kilombero and Serengeti districts; 20 districts in 4 regions in Southern, Central and Northern Tanzania; Serengeti and Ngorongoro) | Human rabies mortality rates of 2.4; 0.8 and 1.4/100 000 per year, respectively89 16 human deaths (1291 bite victims) due to rabies were reported99 28 deaths from suspected rabies cases during the 5-year period in the two districts, an average of 1.5/100 000 per year and 2.3/100 00044 14 among 1005 bite victims died showing clinical signs of rabies within 5 years99 |
5 years, 2002–2006; 3 years, 2006–2008; 2002–2017; 2011–2017; 2011–201644 89 99 |
| Uganda (national level; 10 districts) | 592 (95% CI 345 to 920) deaths102 An estimated total of 371 deaths of rabies with a cumulative total of 117 085 rabies cases in 9 years87 A total of 486 suspected human rabies deaths among 208 720 patients in 14 years88 |
8 years, 2001–2009; 14 years, 2001–2015; 3 months87 88 102 |
Rabies disease surveillance, prevention and control
Rabies prevention and control
The review summarised rabies prevention and control based on rabies exposure status, PEP, dog vaccination and seasonality. Among 36 741 bite victims recorded in studies reporting PEP29 41 45 84 91 92 97 the PEP was initiated based on WHO grade of exposure.106 We found 505 bites in grade 1 (8.78%), 2050 in grade 2 (35.63%) and 3199 in grade 3 (55.59%).32 33 42 95 96 The overall PEP course among bite victims varied between 24% and 99%.29 84 We reported 2652 bites victims in studies reporting PEP and mass dog vaccination.33 38 39 85 100 Dog vaccination coverage varied from 14.1% to 68.78%.33 85 In Ethiopia and Zimbabwe, the dog vaccination decreased significantly across the study and also the health status of most dogs involved in biting was unknown.39 100 Rabies prevention and control also depended on the seasonality. In Ethiopia, two studies reported season-wise rabies exposure. The first study reported rabies exposure during spring (360 of 924, 39%) and summer (244 of 924, 26.4%) seasons.100 The second study that found the highest human rabies exposures were reported in spring (April–June) followed by winter (January–March), while the lowest distribution of human rabies exposure was recorded in autumn (October–December).33 In Nigeria, Osaghae reported the prevalence of dog bite was highest, 41 of 81 (50.6%), during the hot season (April–June) and low, 14 of 81 (17.3%), during the wet season (July–October).93 Another study conducted in Nigeria recorded the highest number of dog bites with two peaks in April and October 2008.47 However, the number of dog bite cases was lowest. For all years, the numbers of dog bite cases recorded were lowest at the beginning of the year and dog bites increased during the last 3 months (October–December) of the year 2006.47 Animal-to-human rabies transmission was highest during the dry months of July–November in Zimbabwe.39 In DRC, a study found there was no seasonal difference observed for rabies occurrence either for clinical cases or confirmed cases throughout the study period.34 In Tanzania, each year, the majority of rabies cases were recorded during the period June–October.41 In the dry season, significantly fewer rabies-positive cases were reported than in the rainy season in Namibia.43 In Chad, more rabies records per month were collected during the hot, dry season months (March and April), than during the dry season months (September–February).103 In Senegal, dog bite victims were higher in dry season (November–May) than rainy season (June–October).32 Besides, bite victims in rural areas took longer, on average, to receive PEP than those in urban areas.89 97 100 Probable human rabies cases were higher in rural than urban areas.33 35
Rabies surveillance, prevention and control
The findings revealed that 39 802 bite victims were recorded in rabies surveillance and prevention interventions. The studies included laboratory, database and network surveillance.34 36 42 46 90 The rabies prevention included PEP and dog vaccination. Laboratory surveillance has improved rabies diagnosis,34 36 42 46 90 and database and network surveillance improved mortality and morbidity records35 42 90 and allowed better estimates of the true rabies burden.36 Furthermore, compliance with PEP regimens was significantly higher, rating from 83.7% to 93% in two studies.42 90 In contrast, dog vaccination remained low (10%–12.6%).36 46
We found five studies including rabies disease surveillance, prevention and control.31 32 37 44 98 99 Among them, three studies32 37 44 have reported significantly improved rabies morbidity and mortality and also PEP uptake. The PEP uptake was 71% and it dramatically reduced the risk of developing rabies.39 Another study did not report any death with high number of patients receiving PEP.32
A combined strategy of mass dog vaccination, enhanced surveillance, and expanded access to PEP reduced the annual incidence of rabies exposures and deaths annually in Madagascar.31 Strict measures such as vaccination of dogs in the neighbourhoods where human rabies cases had occurred, mass vaccination campaign of dogs, participation of private veterinary clinics in animal vaccination, collection of stray dogs in selected neighbourhoods, and community education regarding prevention and control measures had drastically reduced rabies cases in humans to 14 during the study period in Mozambique.37
Post-exposure management
We found seven studies on rabies management and control. Among them, six studies addressed wound management and PEP.40 47 93–95 103 Wound severity was graded as follows: 0=no apparent injury seen, 1=skin scratch with no bleeding, 2=minor wound with some bleeding, 3=deep or multiple injuries.95 The reported severity of the wound was classified as deep wound, lacerated wound, superficial wound or scratch.103 The severity of the injury was determined using the WHO dog bite injury grading system.106 Soap, water, wound dressing, tetanus prophylaxis, anti-rabies vaccination, intravenous fluids, diazepam and antibiotics were also part of the management. The overall review reported 5754 bites managed according to the WHO dog bite injury grading system, 3199 (55.59%) were grade 3; 2050 (35.63%) were grade 2 and 505 (8.78%) were grade 1. In 52 cases (7%), the grade of the bite was not recorded.30 31 40 93 97
Available research evidence on human rabies
Rabies cases in various committees emphasise the need for active surveillance by following up of people bitten by animals and mass dog vaccinations to alleviate the zoonotic threat of the virus.87 Strengthening rabies surveillance, controlling rabies in dogs, proper post-exposure management, increasing the awareness of the community and ensuring availability of PEP at lower health facilities are the best approaches of eliminating rabies.88 92 101 Other studies have demonstrated that reinforcement of rabies surveillance system can improve rabies reporting, which ultimately allows for better estimates of the true rabies burden in the countries.34 36 42 90 Compliance with PEP regimens was significantly higher for patients following the implementation of automated reminders in comparison with patients attending normal clinics.42 90 Other studies concluded that preventing dog bites would most effectively reduce bite injuries by improving public health education among children below 15 years.42 Public health education is also enhanced by encouraging early PEP initiation and completion, development and implementation of responsible dog ownership, animal behaviour educational programmes as well as improving human and veterinary health linkages.30 42 Evidence also showed that no rabies victim in Mozambique received full post-exposure vaccination and the factors significantly associated with human rabies were: age <15 years (p=0.05), bite by stray dog (p=0.002), deep wound (p=0.02), bite in the head (p=0.001), bite by unimmunised dog (p=0.01), no use of soap and water (p=0.001), and no PEP (p=0.01).39 Studies have shown that all the rabies vaccines including suckling mouse brain virus (SMBV), fetal bovine kidney virus (FBKV), purified chicken embryo cell rabies vaccine, purified vero cell rabies vaccine, sheep brain anti-rabies vaccine, human diploid-cell vaccine and purified equine RIG were efficacious. However, the WHO and OIE contraindicated SMBV and FBKV in both animals and humans.29 31 32 36 45 46 102 104 105 Furthermore, a clinical trial with a purified chicken embryo cell rabies vaccine dose used intramuscularly every 2 years generated ineffective immune response to rabies virus104 as the Zagreb protocol (two intradermal injections of 0.1 mL at two sites, deltoids and/or thighs, on days 0, 3, 7 and 28) was not applied. Even though a randomised control trial showed antibody response 26.7% of SMBV recipients and 28.6% of FBKV recipients within a week, both SMBV and FBKV were equally efficacious and well tolerated,105 however those vaccines are contraindicated by the WHO because of its association with neurological adverse reactions (severe allergic encephalomyelitis). Furthermore, these vaccines are inferior to modern vaccine in terms of potency and immunogenicity.107 Table 3 describes the available research evidence on human rabies in Africa.
Table 3.
Mapping human rabies evidence identified in Africa
| Evidence map | Studies | Impacts/outcomes |
| Strengthening rabies surveillance | 35 36 42 87 88 90 92 101 | Reinforcement of rabies surveillance system that can improve rabies reporting and increase the awareness of the community, and ensuring availability of PEP at lower health facilities are the best approaches of eliminating rabies. |
| Automated short message service (SMS) reminders and telephone contacts | 42 44 90 98 99 | Compliance with PEP regimens was significantly higher for patients following the implementation of automated SMS reminders and telephone contacts. |
| Public health education (PEP initiation and completion, responsible dog ownership, behaviour educational programmes and veterinary health linkages) | 30 42 47 88 92 101 | Lack of enforcement of regulations for licensing of dogs and rabies vaccination increased human rabies morbidity and mortality. |
| Accurate rabies diagnostic | 31 35 36 38 39 46 86 99 | The diagnosis of dog bite and rabies was clinical and laboratory based. This improved accurate rabies cases reporting. |
| Mass dog vaccination | 39 85 92 | Even though the 70% coverage was not achieved, there was an inverse relationship between dog vaccination coverage and dog rabies cases during the study period. |
| SMBV, FBKV, purified chicken embryo cell rabies vaccine, purified vero cell rabies vaccine, sheep brain anti-rabies vaccine, human diploid-cell vaccine and purified equine rabies immunoglobulin (RIG) (Zagreb protocol) | 29 31 32 35 46 102 104 105 | Studies have shown that all the rabies vaccines and RIG were efficacious and well tolerated. However, the WHO contraindicated SMBV and FBKV. |
| Effective rabies control and management | 36 42 85 92 | PEP, mass dog vaccination and WHO dog bite injury grading system |
| Integrated bites case management/rabies disease surveillance, prevention and control | 31 32 37 44 98 99 | Studies have shown the importance of coordinated surveillance, prevention and control in the eradication of rabies. |
FBKV, fetal bovine kidney virus; PEP, post-exposure prophylaxis; SMBV, suckling mouse brain virus.
Adverse events and complications associated with rabies vaccination
Adverse events and complications associated with rabies vaccination were reported based on SMBV, FBKV, purified chicken embryo cell rabies vaccine, purified vero cell rabies vaccine, sheep brain anti-rabies vaccine, human diploid-cell vaccine and purified equine RIG. All the four-dose or Zagreb regimen was reported in all the RIGs.108 Among the studies reporting rabies vaccination, only one study using a purified vero cell rabies vaccine at day 0 (two doses), day 7 (one dose) and day 21 (one dose) study found that adverse events occurred in 6% of the patients with two doses and after the third dose, 3% developed adverse events. However, most of the adverse events were minor and associated with headache, fever and pain at the injection site that occurred simultaneously on the same day of the vaccine injection.32 Other studies did not report any adverse events and complications associated with rabies vaccination.29 31 36 45 46 102 104 105
Research gaps identified
In this review, we identified 66.67% African countries reporting poor rabies diagnostic capacity, 50% reported the lack of coordinated surveillance, 50% showed the lack of PEP course completion, 22.22% had insufficient rabies control and 77.78% had low dog vaccination coverage. Insufficient knowledge and practice on rabies prevention was also identified as a gap. However, we did not find enough studies to evaluate this gap in Africa (table 4).
Table 4.
Mapping research gaps and strengths in Africa
| African countries | Mapping research gaps and strengths in Africa | |||||
| Diagnostic capacity | Coordinated surveillance | Lack of PEP course completion/PEP unavailable | Inefficient control | Insufficient knowledge and practice on rabies prevention | Low dog vaccination coverage (<70%) | |
| Algeria | N/A | N/A | N/A | √90 | N/A | X90 |
| Cameroon | √36 | √36 | X36 | √36 | N/A | X36 |
| Chad | X108 | X108 | X108 | X108 | N/A | X108 |
| Democratic Republic of Congo | X34 40 | X34 | X34 40 | X34 42 | N/A | X42 |
| Ethiopia | X21 33 45 | N/A | X91 | X33 91 101 | X101 | X33 91 101 |
| Ghana | X48 | X48 | X48 84 | X38 48 84 | N/A | X38 84 |
| Ivory Coast | √35 | √35 | X35 | X35 | N/A | X35 |
| Kenya | X30 | X30 | X30 | X30 | N/A | N/A |
| Madagascar | √31 46 86 | X46 86 | X31 46 | X31 46 86 | N/A | X31 46 |
| Malawi | X92 | √92 | √92 | √92 | N/A | N/A |
| Mozambique | X37 | X37 | X37 | X37 | N/A | X37 |
| Namibia | X43 | X43 | N/A | X43 | N/A | N/A |
| Nigeria | X93 94 | N/A | X47 93–95 | X47 93–95 | N/A | X47 |
| Senegal | X32 | √32 | X32 | X32 | √32 | X32 |
| Tanzania | X90 97 | √89 97 | X41 44 89 97 99 | X41 44 89 97 99 | X44 | N/A |
| Uganda | X87 88 102 | X87 88 102 | X102 | X87 88 102 | N/A | X87 102 |
| South Africa | √42 | √42 | X42 96 | √42 | N/A | N/A |
| Zimbabwe | √39 | √39 | N/A | X39 | N/A | X39 |
PEP, post-exposure prophylaxis.
The recorded data available so far have shown the underestimation of rabies diagnosis, PEP and fatal human cases, and could be attributed to poor diagnostic capacity and the absence of national rabies surveillance system. In African countries, rabies diagnostic is mostly clinical.21 30 32–34 37 40 42 43 45 48 87–89 92–94 97 102 103 Among 11 studies, including human rabies surveillance, only 4 reported adequate and successful surveillance,35 36 42 90 12 studies reported lack of accurate data or non-existing surveillance data30 34 37 42 43 46 48 86–88 102 103 (table 4). Other studies reported that dog bite victims did not complete the post-exposure anti-human rabies vaccine course and were not likely to receive PEP31 32 35 41 42 44 84 89 94 97 99 (table 4). The exposure victims considered to be at risk of rabies either did not receive any PEP or did not receive all PEP vaccinations due to unavailability, shortage, cost barriers, insufficient knowledge about prompt PEP, category 1 exposure injury or misadvice.36 40 42 44 47 48 95 98 99 A study has reported that the lack of PEP was the cause of 100% fatality rate in DRC.40 There was significant difference between rural and urban exposure cases in respect of the time of arrival to the hospital, and living in rural areas was statistically associated with loss to follow-up after the first dose.34 47 97 100 There was also high human rabies exposure rate in children and in the rural community.31 34 97 100 Insufficient knowledge about rabies’ dangers and prevention, particularly prompt PEP, as well as wound management, was the main cause of rabies deaths.44 A higher proportion of human rabies exposures was caused by unprovoked dogs and of these, the majority were unvaccinated.33 47 Dog vaccination remains an urgent intervention gap. Among 18 studies conducted in 9 countries, none of them reported the target of 70% of dog vaccination (table 4). The highest dog vaccination rate was reported in Algeria (67.3%)85 and the lowest in Madagascar (10%).46
Discussion
This is to our knowledge the first scoping review synthesising publicly available data on rabies in Africa and weighing such data in support of the global goal of ‘zero human rabies deaths by 2030’. The purpose of this scoping analysis was to provide a summary of evidence on rabies morbidity, mortality, integrated rabies surveillance, prevention and control in Africa. Overall, studies have shown that African countries face a range of problems based on rabies surveillance, prevention and control that have negative effects on rabies mortality and morbidity. Reviewing rabies morbidity and mortality rates across Africa, data obtained fluctuated largely over time and space in various countries, as well as in different regions or districts across the same area. While some countries may have shown significant improvement in rabies morbidity and mortality data, the morbidity and mortality rates in Africa generally remain high. Included studies showed no standardisation in reporting human rabies outcomes, human rabies morbidity and mortality rates were reported in terms of annual incidence and number of infected human rabies and deaths related to rabies. Moreover, small-scale studies may not reflect the national or regional human rabies morbidity and mortality rates. Therefore, it was difficult to have an accurate picture per country and assess human rabies situation between African countries.
These are the consequences of a lack of laboratory rabies confirmation, epidemiological surveillance, inadequate mass dog vaccination and PEP policy, and unreported clinical cases in African countries. Lack of monitoring data on rabies or low data quality is problematic, resulting in rabies being poorly addressed in most African countries. Results have also shown that of the 11 countries in which rabies surveillance has been applied, only 4 studies reported that surveillance decreased rabies morbidity and mortality.35 36 42 90 Comparing old and new data (before and after the ‘zero rabies by 2030’ target), rabies diagnosis and surveillance have not improved in most of the African countries. As a result, well-structured rabies surveillance enhanced the reporting of morbidity and mortality and also has a visible impact on rabies elimination strategy in Africa. While strategies have been subdivided into surveillance, prevention, control and management of rabies (see table of included studies), only three studies have shown the efficacy of the combination of surveillance, prevention and control of rabies.32 37 44 However, passive surveillance has shown its limitations in rabies elimination because cases are reported clinically with or without laboratory-based strategies, inducing inaccurate diagnostic tools, scarcity of laboratory confirmation and poor reporting system.30 43 46 87 88 102 This is why both passive and active surveillance are preferable to strengthen rabies monitoring and reporting in African countries.109 Strengthening rabies surveillance is also the foundation of the provision of actionable data for efficient management of wildlife diseases.110 Besides, the review has shown that strengthening surveillance, prevention and management of rabies has shown good evidence in three separate studies.32 37 44 Coordination of surveillance, prevention and control of rabies can play an important role in the eradication of rabies in Africa. It is worth noting that specific awareness of when and where the disease occurs is essential to the formulation of prevention, control and elimination strategies.110
As seen above, the implementation of different rabies interventions at national level has never reached African countries. It is vital that African countries achieve the 2030 target of eliminating human rabies by providing readily accessible and affordable PEP in all countries in the continent where rabies infection is endemic. The exposed victims considered to be at risk of rabies either did not receive any PEP or did not receive complete PEP vaccinations due to unavailability, shortage, cost barriers, insufficient knowledge about prompt PEP or misadvice.36 40 44 48 95 98 99 This could be emulated from Thailand, which has significantly reduced human deaths from rabies to fewer than 10 cases per year by educating the public and health workers and delivering PEP free of charge across the country before mass dog vaccination achieved the minimum 70% coverage.111 112 When provided correctly and in a timely manner, rabies PEP is almost 100% effective in the prevention of the disease.72 113 The findings of the review revealed that the dog bite victims found to be at risk of rabies either did not receive PEP or did not receive all the PEP vaccines due to unavailability, shortages, cost barriers, long distance travel to the hospital or misadvice.31 32 35 36 40 41 44 48 84 89 94 95 97–99 It is important to remember that PEP, combined with other treatments such as soap, water, wound dressing, antibiotics, tetanus prophylaxis and anti-rabies vaccination, has been shown to be beneficial for dog bite victims. Two studies have shown that compliance with PEP regimens was substantially higher for patients who did not receive PEP after automated reminders.42 90 Taken together, our results point to a suboptimal system requiring specific improvements to achieve prompt provision of rabies PEP for persons exposed to rabies.112
Statistical modelling studies show that the annual vaccination of 70% of the canine population would induce adequate herd immunity to effectively eradicate canine rabies and subsequent human exposure.10 44 The lowest and highest reviewed dog vaccination rates were 10% and 67.3%, respectively,10 46 and no reliable data on dog vaccination were reported in most of the studies. This is because many campaigns, if conducted, struggle to achieve a 70% vaccination rate.10 14 This is due to husbandry practices, rabies knowledge, geographical area/location and the ages of dogs.114 Evidence has shown the dog mass vaccination systems have demonstrated some effectiveness in reducing human rabies morbidity and mortality in countries such as KwaZulu-Natal, South Africa,49 65 Serengeti, Tanzania,49 66 68 Malawi44 69 and Chad.70 71 However, the Tanzanian study has shown that, if vaccine coverage was not sustained, rabies infection would resurface extremely quickly.34 Despite effective monitoring of rabies at the Tanzania study site from 1998 to 2001, vaccine coverage decreased from 2001 to 2003 resulting in a new rabies outbreak, with human exposures increasing by six times in 2003 relative to previous years.44 Further, free mass dog vaccination intervention has proven to increase dog vaccination coverage. Government and stakeholders should work actively to provide a free sustainable dog vaccination.
Besides, the evidence has also illustrated the effectiveness of other strategies, such as mobile phone touch tracing strategies, new rabies vaccines, integrated bite case management and wound management, which were correlated with PEP and/or mass dog vaccination. Africa is yet to recognise rabies as an immediate public health problem; this may be due to a lack of awareness of the burden of disease and inadequate surveillance. Policies should be put in place to raise awareness of rabies at grassroots level and coordination between the appropriate agencies for improvements of the policies.47 54
The World Animal Health Information System is a well-established global animal disease reporting system that reproduces the data submitted by countries to the OIE, but is also constrained by the under-reporting problems inherent in national reporting systems.115 116 The need for regional One Health-oriented reporting network has therefore become apparent.117 118 The development of rabies-specific regional bulletins has been extremely effective in the Pan-American Health Organization field.117 The database such as the New Latin American Rabies Surveillance System should be applicable in Africa.
Indeed, the elimination of rabies is not feasible without African cooperation. No single country will retain rabies-free status unless it is brought under control in neighbouring countries.113 Regionally organised efforts are required to eradicate human rabies, taking into account country-specific needs and sociocultural acceptability.113 Canine rabies-endemic regions have formed international rabies networks based on the successful Meeting of Rabies Program Directors of the Americas (REDIPRA) model which enables them to create a unified and directed approach towards elimination within their regions.119 REDIPRA meetings can be considered a model for coordination and governance in the world.7 118 119 In Africa, the Pan-African Rabies Control Network (PARACON) was formed under the secretariat of GARC, as an Africa-focused advisory and networking initiative.119 It was established in order to unify all sub-Saharan African countries and any related rabies networks in a One Health approach towards rabies control and elimination.119 The PARACON facilitates the development and implementation of national rabies elimination strategies, with a focus on sustainability through governmental support.119 However, the probability of meeting the 2030 goal without African and international solidarity is low, as more than two-thirds of countries are in the low-level human development community.120 Leading countries should serve as role models, sharing their knowledge and skills so that no nation is left behind. African unification with international support will enable the common goal of zero human rabies deaths to be achieved by 2030.120 Therefore, regional network support, channel and pool efforts and support-monitoring platforms will help make much progress.121 Partnerships are important to the achievement of an objective and the last mile is going to be the most demanding.121 In addition, contact and collaboration between human health and veterinary systems is also critical for the follow-up of both human and animal cases.122 Data collected on alleged cases of human rabies, human exposures and rabid animals must be constantly reviewed and effectively disseminated.122 Communication between the various national levels of healthcare administration is a crucial means of disseminating outcomes.122 Finally, stakeholders need to be engaged in the long term to ensure that surveillance is effective.122 Knowing that the Global Strategic Plan is catalytic and not intended to replace the strategies and commitments of individual countries,121 African countries should emphasise gaps, challenges, barriers and evidence applicable to the various districts, countries and regions as indicated in this present study. One Health interventions are provided by approaches to the prevention of human rabies deaths.123 In African countries, the Ministries of Health, Animal Resources, Natural Resources, Environment and Tourism are in charge of implementing the canine and human rabies programmes in accordance with local, national and international bodies.
Human rabies is 100% preventable through two complementary measures: first, PEP, which involves administration of RIG and a multidose course of rabies vaccination to people bitten by suspected rabid animals; second, mass vaccination of animal reservoirs (primarily domestic dogs, the reservoir in the vast majority of human cases), which reduces the risk of human exposure and can ultimately result in rabies virus elimination.123
New rabies control tests and technologies that have been developed, such as oral rabies vaccine (ORV), may be considered as an additional tool for the canine rabies control and elimination. ORV is effective, for instance, in skunks, red foxes and raccoons.124 ORV has been demonstrated to be effective for the oral immunisation of foxes, some of them being competitors for long baits year consumption. Switzerland eradicated wild rabies since 1985.122 However, strategies to eliminate human rabies in Africa should adapt the REDIPRA model in African context, which emphasises that people exposed to rabies have timely access to quality immunobiologicals, that appropriate levels of vaccination coverage in dogs in highly enzootic areas are maintained, that national rabies plans are strengthened and that systematic implementation is ensured, that the surveillance system for human rabies transmitted by dogs is strengthened and that systematic implementation is ensured, and that training and the development of a laboratory quality control system, particularly in highly enzootic areas, strengthen education, communication and advocacy in enzootic areas, to ensure the continuous political support that is necessary, develop and adopt a guide that delineates the requirements for declaring countries or areas free of human rabies transmitted by dogs.119
Conclusion
This comprehensive scoping review is of crucial importance in assessing various pieces of evidence of human rabies morbidity, mortality, monitoring, prevention and management. Rabies control strategies and case effects and available studies established to address various gaps are also important in the management of rabies in Africa. The analysis included past, existing and future viewpoints that are important for African countries to achieve zero rabies transmitted by dogs to humans by 2030. The findings of 43 studies included 32 quantitative retrospective studies, 3 mixed designs (retrospective and cross-sectional studies), 3 prospective cohort studies, 2 cross-sectional studies, 1 case–control, 1 clinical trial and 1 randomised control study. Mapping the outcomes, the review included rabies morbidity (21 studies), mortality (15 studies), rabies prevention and control (16 studies), surveillance (9 studies), surveillance and prevention (5 studies), management and control (7 studies), surveillance, prevention and control (6 studies), strong research evidence (14 studies), rabies vaccination or PEP adverse events (4 studies), and research gaps (41 studies).
Evidence has shown that human rabies morbidity and mortality remain high compared with rabies globally, and human rabies morbidity and mortality fluctuate in time and space across different African countries. In order to better understand this, the review has shown that monitoring, prevention and control of rabies are inadequate and insufficient in most African countries. This is attributable to a variety of gaps and challenges across African countries. In addition, this study found insufficient and ineffective surveillance of rabies, unavailability of PEP, high cost, lack of information on prevention of rabies, and poor or non-existent data on dog vaccination. However, few studies have shown a thorough design of rabies measures such as enhanced surveillance of rabies, regulation of rabies in dogs, proper post-exposure treatment, improved community awareness and availability of PEP in all rural areas, use of cell phone intervention to enhance surveillance of rabies, prevention and control of enhanced rabies morbidity, and more. In addition, African countries can learn about different community-based obstacles that can interfere with surveillance, prevention and control of rabies disease. This is important to point out that no single country will preserve rabies-free status unless it is brought under control in neighbouring countries.119 That is why African countries should build a forum for rabies that may be significant to exchange data and experience on rabies. Finally, African countries can also look at futuristic rabies innovations such as ORV.
Supplementary Material
Footnotes
Twitter: @pnyasulu
Contributors: PN initiated the study and drafted the project concept. PN and JLT conducted the data extraction. Both authors wrote the review in consultation with JW, RT, AM, SVN, JLT, LN, NEN, GKH, MTG, AA, SD, RB and CD. All the authors reviewed and approved the final version of the manuscript. PN is the guarantor of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Since this is a scoping review, there is no institutional requirement to obtain ethical clearance from the Health Research Ethics Committee of the Faculty of Medicine and Health Sciences, Stellenbosch University. We used data from open source and publicly available accessed on different databases as described in the methods.
References
- 1.Nel LH. Discrepancies in data reporting for rabies, Africa. Emerg Infect Dis 2013;19:529–33. 10.3201/eid1904.120185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talbi C, Holmes EC, de Benedictis P, et al. Evolutionary history and dynamics of dog rabies virus in Western and central Africa. J Gen Virol 2009;90:783–91. 10.1099/vir.0.007765-0 [DOI] [PubMed] [Google Scholar]
- 3.Nel LH, Rupprecht CE. Emergence of lyssaviruses in the Old World: the case of Africa. In: Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission. 315. Springer, 2007: 161–93. [DOI] [PubMed] [Google Scholar]
- 4.Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 2015;9:e0003709. 10.1371/journal.pntd.0003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desa G, Birasa D, Deneke Y, et al. Assessment of knowledge, attitude and practice (KAP) of community toward rabies in Medawelabu district, bale zone, Ethiopia. Int J Res Granthaalayah 2020;8:29–42. 10.29121/granthaalayah.v8.i3.2020.124 [DOI] [Google Scholar]
- 6.Cleaveland S, Lankester F, Townsend S, et al. Rabies control and elimination: a test case for one health. Vet Rec 2014;175:188–93. 10.1136/vr.g4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Rabies: rationale for investing in the global elimination of dog-mediated human rabies, 2015. Available: https://apps.who.int/iris/handle/10665/185195
- 8.Fooks AR, Banyard AC, Horton DL, et al. Current status of rabies and prospects for elimination. Lancet 2014;384:1389–99. 10.1016/S0140-6736(13)62707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 2005;83:360–8. doi:/S0042-96862005000500012 [PMC free article] [PubMed] [Google Scholar]
- 10.Lavan RP, King AIM, Sutton DJ, et al. Rationale and support for a one health program for canine vaccination as the most cost-effective means of controlling zoonotic rabies in endemic settings. Vaccine 2017;35:1668–74. 10.1016/j.vaccine.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 11.Dodet B, Tejiokem MC, Aguemon A-R, et al. Human rabies deaths in Africa: breaking the cycle of indifference. Int Health 2015;7:4–6. 10.1093/inthealth/ihu071 [DOI] [PubMed] [Google Scholar]
- 12.Adesina MA, Olufadewa II, OgaH YI, et al. Incidence and mortality from a neglected tropical disease (rabies) in 28 African countries. Folia Vet 2020;64:46–51. 10.2478/fv-2020-0016 [DOI] [Google Scholar]
- 13.Interafrican Bureau for Animal Resources . Rabies distribution, 2013. Available: https://www.au-ibar.org/rabies#Distribution_Map_for_Africa
- 14.Cleaveland S. Epidemiology and control of rabies: the growing problem of rabies in Africa. Trans R Soc Trop Med Hyg 1998;92:131–4. [DOI] [PubMed] [Google Scholar]
- 15.Knobel D, Conan A. Dog ecology and rabies control in Africa world small animal veterinary association world Congress proceedings. Age 2014;4:23. [Google Scholar]
- 16.Röttcher D, Sawchuk AM. Wildlife rabies in Zambia. J Wildl Dis 1978;14:513–7. 10.7589/0090-3558-14.4.513 [DOI] [PubMed] [Google Scholar]
- 17.Botros BA, Salib AW, Mellick PW, et al. Antigenic variation of wild and vaccine rabies strains of Egypt. J Med Virol 1988;24:153–9. 10.1002/jmv.1890240204 [DOI] [PubMed] [Google Scholar]
- 18.Burrows R. Rabies in African wild dogs of Tanzania. J Wildl Dis 1994;30:297–9. 10.7589/0090-3558-30.2.297 [DOI] [PubMed] [Google Scholar]
- 19.Kat PW, Alexander KA, Smith JS, et al. Rabies and African wild dogs in Kenya. Proc Biol Sci 1995;262:229–33. 10.1098/rspb.1995.0200 [DOI] [PubMed] [Google Scholar]
- 20.Foggin CM. The epidemiological significance of jackal rabies in Zimbabwe. In: Rabies in the tropics. Springer, 1985: 399–405. [Google Scholar]
- 21.Tefera G, Yimer E, Geyid A. Endemic existence of rabies in Ethiopia. Ethiop Med J 2002;40:163–10. [PubMed] [Google Scholar]
- 22.Nel LH, Sabeta CT, von Teichman B, et al. Mongoose rabies in southern Africa: a re-evaluation based on molecular epidemiology. Virus Res 2005;109:165–73. 10.1016/j.virusres.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 23.Woodroffe R, Prager KC, Munson L, et al. Contact with domestic dogs increases pathogen exposure in endangered African wild dogs (Lycaon pictus). PLoS One 2012;7:e30099. 10.1371/journal.pone.0030099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheepers JL, Venzke KAE. Attempts to reintroduce African wild dogs Lycaon pictus into Etosha National Park, Namibia. S Afr J Wildlife Res 1995;25:138–40. [Google Scholar]
- 25.Sabeta C, Ngoepe EC. Controlling dog rabies in Africa: successes, failures and prospects for the future. Rev Sci Tech 2018;37:439–49. 10.20506/rst.37.2.2813 [DOI] [PubMed] [Google Scholar]
- 26.Johnson N, Mansfield KL, Marston DA, et al. A new outbreak of rabies in rare Ethiopian wolves (canis simensis). Arch Virol 2010;155:1175–7. 10.1007/s00705-010-0689-x [DOI] [PubMed] [Google Scholar]
- 27.Marino J, Sillero-Zubiri C, Deressa A, et al. Rabies and distemper outbreaks in smallest Ethiopian wolf population. Emerg Infect Dis 2017;23:2102–4. 10.3201/eid2312.170893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomori O. Wild life rabies in Nigeria: experimental infection and transmission studies with the shrew (Crocidura sp.). Ann Trop Med Parasitol 1980;74:151–6. 10.1080/00034983.1980.11687325 [DOI] [PubMed] [Google Scholar]
- 29.Ramos JM, Melendez N, Reyes F, et al. Epidemiology of animal bites and other potential rabies exposures and anti-rabies vaccine utilization in a rural area in southern Ethiopia. Ann Agric Environ Med 2015;22:76–9. 10.5604/12321966.1141372 [DOI] [PubMed] [Google Scholar]
- 30.Ngugi JN, Maza AK, Omolo OJ, et al. Epidemiology and surveillance of human animal-bite injuries and rabies post-exposure prophylaxis, in selected counties in Kenya, 2011-2016. BMC Public Health 2018;18:996. 10.1186/s12889-018-5888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajeev M, Edosoa G, Hanitriniaina C, et al. Healthcare utilization, provisioning of post-exposure prophylaxis, and estimation of human rabies burden in Madagascar. Vaccine 2019;37 Suppl 1:A35–44. 10.1016/j.vaccine.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diallo MK, Diallo AO, Dicko A, et al. Human rabies post exposure prophylaxis at the Pasteur Institute of Dakar, Senegal: trends and risk factors. BMC Infect Dis 2019;19:321. 10.1186/s12879-019-3928-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebru G, Romha G, Asefa A, et al. Risk factors and spatio-temporal patterns of human rabies exposure in northwestern Tigray, Ethiopia. Ann Glob Health 2019;85:119. 10.5334/aogh.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twabela AT, Mweene AS, Masumu JM, et al. Overview of animal rabies in Kinshasa Province in the Democratic Republic of Congo. PLoS One 2016;11:e0150403. 10.1371/journal.pone.0150403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiembré I, Broban A, Bénié J, et al. Human rabies in Côte d'Ivoire 2014-2016: results following reinforcements to rabies surveillance. PLoS Negl Trop Dis 2018;12:e0006649. 10.1371/journal.pntd.0006649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofeu CL, Broban A, Njifou Njimah A, et al. Improving systematic rabies surveillance in Cameroon: a pilot initiative and results for 2014-2016. PLoS Negl Trop Dis 2018;12:e0006597. 10.1371/journal.pntd.0006597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomão C, Nacima A, Cuamba L, et al. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: implications for public health interventions for rabies control. PLoS Negl Trop Dis 2017;11:e0005787. 10.1371/journal.pntd.0005787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Punguyire DT, Osei-Tutu A, Aleser EV, et al. Level and pattern of human rabies and dog bites in Techiman Municipality in the middle belt of Ghana: a six year retrospective records review. Pan Afr Med J 2017;28:281. 10.11604/pamj.2017.28.281.14218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfukenyi DM, Pawandiwa D, Makaya PV, et al. A retrospective study of wildlife rabies in Zimbabwe, between 1992 and 2003. Trop Anim Health Prod 2009;41:565–72. 10.1007/s11250-008-9224-4 [DOI] [PubMed] [Google Scholar]
- 40.Muyila DI, Aloni MN, Lose-Ekanga MJ, et al. Human rabies: a descriptive observation of 21 children in Kinshasa, the Democratic Republic of Congo. Pathog Glob Health 2014;108:317–22. 10.1179/2047773214Y.0000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazigo HD, Okumu FO, Kweka EJ, et al. Retrospective analysis of suspected rabies cases reported at bugando referral Hospital, mwanza, Tanzania. J Glob Infect Dis 2010;2:216. 10.4103/0974-777X.68530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubheka V, Govender P, Margot B, et al. Dog bites and human rabies in the Uthungulu district of KwaZulu-Natal Province, 2008–2010: a review of surveillance data. South African J Epidemiol Infect 2013;28:33–40. 10.1080/10158782.2013.11441517 [DOI] [Google Scholar]
- 43.Hikufe EH, Freuling CM, Athingo R, et al. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011-2017. PLoS Negl Trop Dis 2019;13:e0007355. 10.1371/journal.pntd.0007355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampson K, Dobson A, Kaare M, et al. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis 2008;2:e339. 10.1371/journal.pntd.0000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deressa A, Ali A, Bayene M, et al. The status of rabies in Ethiopia: a retrospective record review. Ethiop J Health Dev 2010;24:127–32. 10.4314/ejhd.v24i2.62961 [DOI] [Google Scholar]
- 46.Andriamandimby S, Héraud J-M, Ramiandrasoa R, et al. Surveillance and control of rabies in La reunion, Mayotte, and Madagascar. Vet Res 2013;44:77–9. 10.1186/1297-9716-44-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alabi O, Nguku P, Chukwukere S, et al. Profile of dog bite victims in Jos plateau state, Nigeria: a review of dog bite records (2006-2008). Pan Afr Med J 2014;18 Suppl 1:12. 10.11604/pamj.supp.2014.18.1.4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adomako B-Y, Baiden F, Sackey S, et al. Dog bites and rabies in the eastern region of Ghana in 2013–2015: a call for a One-Health approach. J Trop Med 2018;2018:1–5. 10.1155/2018/6139013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Athingo R, Tenzin T, Shilongo A, et al. Fighting Dog-Mediated rabies in Namibia-Implementation of a rabies elimination program in the Northern communal areas. Trop Med Infect Dis 2020;5. 10.3390/tropicalmed5010012. [Epub ahead of print: 17 Jan 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller T, Demetriou P, Moynagh J, et al. Rabies elimination in Europe: a success story. in: in rabies control: towards sustainable prevention at the source, compendium of the OIE, global conf. on rabies control, Incheon-Seoul, 7-9 September. 2012, 2011: 31–44. [Google Scholar]
- 51.Velasco-Villa A, Escobar LE, Sanchez A, et al. Successful strategies implemented towards the elimination of canine rabies in the Western hemisphere. Antiviral Res 2017;143:1–12. 10.1016/j.antiviral.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization . Who expert consultation on rabies: second report, 2013. Available: https://apps.who.int/iris/handle/10665/85346 [PubMed]
- 53.Dodet B, Africa Rabies Bureau (AfroREB) . The fight against rabies in Africa: from recognition to action. Vaccine 2009;27:5027–32. 10.1016/j.vaccine.2009.06.030 [DOI] [PubMed] [Google Scholar]
- 54.Kayali U, Mindekem R, Yémadji N, et al. Coverage of pilot parenteral vaccination campaign against canine rabies in N'Djaména, Chad. Bull World Health Organ 2003;81:739–44. [PMC free article] [PubMed] [Google Scholar]
- 55.Franka R, Smith TG, Dyer JL, et al. Current and future tools for global canine rabies elimination. Antiviral Res 2013;100:220–5. 10.1016/j.antiviral.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 56.Zemanová S, Korytár Ľ., Benkő Z, et al. Ecological factors of transmission, persistence and circulation of pathogens in bat populations. Folia Vet 2019;63:32–40. 10.2478/fv-2019-0005 [DOI] [Google Scholar]
- 57.Hampson K, Dushoff J, Cleaveland S, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol 2009;7:e1000053. 10.1371/journal.pbio.1000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minyoo AB, Steinmetz M, Czupryna A, et al. Incentives increase participation in mass dog rabies vaccination clinics and methods of coverage estimation are assessed to be accurate. PLoS Negl Trop Dis 2015;9:e0004221. 10.1371/journal.pntd.0004221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lankester F, Davis A, Kinung'hi S, et al. An integrated health delivery platform, targeting soil-transmitted helminths (STh) and canine mediated human rabies, results in cost savings and increased breadth of treatment for STh in remote communities in Tanzania. BMC Public Health 2019;19:1398. 10.1186/s12889-019-7737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Global Alliance for Rabies Control . Recent research November 2017, 2017. Available: https://rabiesalliance.org/resource/recent-research-november-2017
- 61.MEEREB . Rabies diagnostic and epidemiological data,, 2018. Available: https://www.fondation-merieux.org/wp-content/uploads/2017/11/4th-meereb-2018-florence-cliquet.pdf
- 62.Wallace RM, Mehal J, Nakazawa Y, et al. The impact of poverty on dog ownership and access to canine rabies vaccination: results from a knowledge, attitudes and practices survey, Uganda 2013. Infect Dis Poverty 2017;6:97. 10.1186/s40249-017-0306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jibat T, Hogeveen H, Mourits MCM. Review on dog rabies vaccination coverage in Africa: a question of dog accessibility or cost recovery? PLoS Negl Trop Dis 2015;9:e0003447. 10.1371/journal.pntd.0003447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shwiff SA, Hatch B, Anderson A, et al. Towards canine rabies elimination in KwaZulu-Natal, South Africa: assessment of health economic data. Transbound Emerg Dis 2016;63:408–15. 10.1111/tbed.12283 [DOI] [PubMed] [Google Scholar]
- 65.Cleaveland S, Kaare M, Tiringa P, et al. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 2003;21:1965–73. 10.1016/S0264-410X(02)00778-8 [DOI] [PubMed] [Google Scholar]
- 66.Kaare M, Lembo T, Hampson K, et al. Rabies control in rural Africa: evaluating strategies for effective domestic dog vaccination. Vaccine 2009;27:152–60. 10.1016/j.vaccine.2008.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hatch B, Anderson A, Sambo M, et al. Towards canine rabies elimination in south-eastern Tanzania: assessment of health economic data. Transbound Emerg Dis 2017;64:951–8. 10.1111/tbed.12463 [DOI] [PubMed] [Google Scholar]
- 68.Gibson AD, Handel IG, Shervell K, et al. The vaccination of 35,000 dogs in 20 working days using combined static point and door-to-door methods in Blantyre, Malawi. PLoS Negl Trop Dis 2016;10:e0004824. 10.1371/journal.pntd.0004824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Léchenne M, Oussiguere A, Naissengar K, et al. Operational performance and analysis of two rabies vaccination campaigns in N’Djamena, Chad. Vaccine 2016;34:571–7. 10.1016/j.vaccine.2015.11.033 [DOI] [PubMed] [Google Scholar]
- 70.Anyiam F, Léchenne M, Mindekem R. Cost estimate of canine rabies elimination by parenteral mass vaccination in Chad, 2017. Available: https://edoc.unibas.ch/57353/1/Lechenne_thesis_edoc%20version.pdf [DOI] [PubMed]
- 71.Zinsstag J, Lechenne M, Laager M, et al. Vaccination of dogs in an African City interrupts rabies transmission and reduces human exposure. Sci Transl Med 2017;9:eaaf6984. 10.1126/scitranslmed.aaf6984 [DOI] [PubMed] [Google Scholar]
- 72.Sreenivasan N, Li A, Shiferaw M, et al. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa, 2017-2018. Vaccine 2019;37 Suppl 1:A6–13. 10.1016/j.vaccine.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization . 2016: the beginning of the end of rabies? 2016. Available: https://www.who.int/rabies/resources/tlgh_rabies_oct_2016/en/
- 74.Bögel K, Motschwiller E. Incidence of rabies and post-exposure treatment in developing countries. Bull World Health Organ 1986;64:883. [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor LH, Knopf L. Partners for rabies prevention. surveillance of human rabies by National authorities-a global survey. Zoonoses Public Health 2015;62:543–52. [DOI] [PubMed] [Google Scholar]
- 76.Kamani TM, Kazwala R, Mfinanga S, et al. One health: a concept led by Africa, with global benefits. Vet Rec 2015;176:496–7. 10.1136/vr.h2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acharya KP, Acharya N, Phuyal S, et al. One-health approach: a best possible way to control rabies. One Health 2020;10:100161. 10.1016/j.onehlt.2020.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Otu A, Effa E, Meseko C, et al. Africa needs to prioritize one health approaches that focus on the environment, animal health and human health. Nat Med 2021;27:943–6. 10.1038/s41591-021-01375-w [DOI] [PubMed] [Google Scholar]
- 79.Jarvis S. Aiming for elimination of dog-mediated human rabies cases by 2030. Vet Rec 2016;178:86–7. 10.1136/vr.i51 [DOI] [PubMed] [Google Scholar]
- 80.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 81.Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–6. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 82.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 83.Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol 2016;16:15. 10.1186/s12874-016-0116-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edward FD, Osei-Tutu A, Gbeddy K, et al. A retrospective study of rabies cases at Techiman municipal, Ghana, 2009 – 2012. Int J Infect Dis 2014;21:179. 10.1016/j.ijid.2014.03.795 [DOI] [Google Scholar]
- 85.Kardjadj M, Yahiaoui F, Ben-Mahdi M-H. Incidence of human dog-mediated zoonoses and demographic characteristics/vaccination coverage of the domestic dog population in Algeria. Rev Sci Tech 2019;38:809-821. 10.20506/rst.38.3.3028 [DOI] [PubMed] [Google Scholar]
- 86.Reynes J-M, Andriamandimby SF, Razafitrimo GM, et al. Laboratory surveillance of rabies in humans, domestic animals, and bats in Madagascar from 2005 to 2010. Adv Prev Med 2011;2011:6 10.4061/2011/727821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nyakarahuka L, Tweheyo R. Occurrence and mortality rates from rabies in Uganda, 2001-2009. Int J Infect Dis 2012;16:e458. 10.1016/j.ijid.2012.05.659 [DOI] [Google Scholar]
- 88.Masiira B, Makumbi I, Matovu JKB, et al. Long term trends and spatial distribution of animal bite injuries and deaths due to human rabies infection in Uganda, 2001-2015. PLoS One 2018;13:e0198568. 10.1371/journal.pone.0198568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sambo M, Cleaveland S, Ferguson H, et al. The burden of rabies in Tanzania and its impact on local communities. PLoS Negl Trop Dis 2013;7:e2510. 10.1371/journal.pntd.0002510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mtema Z, Changalucha J, Cleaveland S, et al. Mobile phones as surveillance tools: implementing and evaluating a large-scale intersectoral surveillance system for rabies in Tanzania. PLoS Med 2016;13:e1002002. 10.1371/journal.pmed.1002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teklu GG, Hailu TG, Eshetu GR. High incidence of human rabies exposure in northwestern tigray, Ethiopia: a four-year retrospective study. PLoS Negl Trop Dis 2017;11:e0005271. 10.1371/journal.pntd.0005271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmer BL, Gamble L, Foster R, et al. Assessment of the impact on paediatric rabies at Queen Elizabeth central Hospital, Blantyre, Malawi, following a mass canine rabies vaccination programme. Int J Infect Dis 2019;79:64. 10.1016/j.ijid.2018.11.165 [DOI] [Google Scholar]
- 93.Osaghae DO. Animal and human bites in children. West Afr J Med 2011;30:421–4. [PubMed] [Google Scholar]
- 94.Ogundare EO, Olatunya OS, Oluwayemi IO, et al. Pattern and outcome of dog bite injuries among children in Ado-Ekiti, Southwest Nigeria. Pan Afr Med J 2017;27:81. 10.11604/pamj.2017.27.81.7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abubakar SA, Bakari AG. Incidence of dog bite injuries and clinical rabies in a tertiary health care institution: a 10-year retrospective study. Ann Afr Med 2012;11:108–11. 10.4103/1596-3519.93534 [DOI] [PubMed] [Google Scholar]
- 96.Kent SJW, Naicker B, Wood DR. Demographics and management of dog bite victims at a level two hospital in KwaZulu-Natal. S Afr Med J 2012;102:845–7. 10.7196/SAMJ.5934 [DOI] [PubMed] [Google Scholar]
- 97.De Nardo P, Gentilotti E, Vairo F, et al. A retrospective evaluation of bites at risk of rabies transmission across 7 years: the need to improve surveillance and reporting systems for rabies elimination. PLoS One 2018;13:e0197996. 10.1371/journal.pone.0197996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lushasi K, Steenson R, Bernard J, et al. One health in practice: using integrated bite case management to increase detection of rabid animals in Tanzania. Frontiers in Public health 2020;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Changalucha J, Steenson R, Grieve E, et al. The need to improve access to rabies post-exposure vaccines: lessons from Tanzania. Vaccine 2019;37:A45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yizengaw E, Getahun T, Mulu W, et al. Incidence of human rabies virus exposure in northwestern Amhara, Ethiopia. BMC Infect Dis 2018;18:1–7. 10.1186/s12879-018-3500-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jemberu WT, Molla W, Almaw G, et al. Incidence of rabies in humans and domestic animals and people's awareness in North Gondar zone, Ethiopia. PLoS Negl Trop Dis 2013;7:e2216. 10.1371/journal.pntd.0002216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fèvre EM, Kaboyo RW, Persson V, et al. The epidemiology of animal bite injuries in Uganda and projections of the burden of rabies. Trop Med Int Health 2005;10:790–8. 10.1111/j.1365-3156.2005.01447.x [DOI] [PubMed] [Google Scholar]
- 103.Frey J, Mindekem R, Kessely H, et al. Survey of animal bite injuries and their management for an estimate of human rabies deaths in N'Djaména, Chad. Trop Med Int Health 2013;18:1555–62. 10.1111/tmi.12202 [DOI] [PubMed] [Google Scholar]
- 104.Olugasa BO, Odeniyi AO, Adeogun AO, et al. Antibody levels against rabies among occupationally exposed individuals in a Nigerian university. Vet Ital 2010;46:21–8. [PubMed] [Google Scholar]
- 105.Harry TO, Adeiga A, Anyiwo CE, et al. Anti-rabies treatment of dog-bite victims in Lagos, Nigeria: trial of suckling mouse brain and fetal bovine kidney cell rabies vaccines. Vaccine 1984;2:257–60. 10.1016/0264-410X(84)90040-9 [DOI] [PubMed] [Google Scholar]
- 106.World Health Organization Technical Report Series . Who expert consultation on rabies, 2005. Available: https://apps.who.int/iris/bitstream/handle/10665/85346/9789240690943_eng.pdf?sequence=1 [PubMed]
- 107.World Health Organization . Who expert consultation on Rabies-World health. Available: https://apps.who.int/iris/bitstream/handle/10665/85346/9789241209823_eng.pdf?sequence=1
- 108.Ren J, Yao L, Sun J, et al. Zagreb regimen, an abbreviated intramuscular schedule for rabies vaccination. Clin Vaccine Immunol 2015;22:1–5. 10.1128/CVI.00531-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banyard AC, Horton DL, Freuling C, et al. Control and prevention of canine rabies: the need for building laboratory-based surveillance capacity. Antiviral Res 2013;98:357–64. 10.1016/j.antiviral.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 110.Kirby JD, Chipman RB, Nelson KM, et al. Enhanced rabies surveillance to support effective oral rabies vaccination of raccoons in the eastern United States. Trop Med Infect Dis 2017;2:34. 10.3390/tropicalmed2030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilde H, Ghai S, Hemachudha T. Rabies: still a silent killer targeting the poor. Vaccine 2017;35:2293–4. 10.1016/j.vaccine.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 112.Wambura G, Mwatondo A, Muturi M, et al. Rabies vaccine and immunoglobulin supply and logistics: challenges and opportunities for rabies elimination in Kenya. Vaccine 2019;37 Suppl 1:A28–34. 10.1016/j.vaccine.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.World Health Organization . WHO expert consultation on rabies: third report. 1012 edition. World Health Organization, 2018. [Google Scholar]
- 114.Hergert M, le Roux K, Nel LH. Risk factors associated with nonvaccination rabies status of dogs in KwaZulu-Natal, South Africa. Vet Med 2016;7:75–83. 10.2147/VMRR.S103859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor LH, Hampson K, Fahrion A, et al. Difficulties in estimating the human burden of canine rabies. Acta Trop 2017;165:133–40. 10.1016/j.actatropica.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scott TP, Coetzer A, Fahrion AS, et al. Addressing the disconnect between the estimated, reported, and true rabies data: the development of a regional African rabies Bulletin. Front Vet Sci 2017;4:18. 10.3389/fvets.2017.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scott TP, Coetzer A, de Balogh K, et al. The Pan-African rabies control network (PARACON): a unified approach to eliminating canine rabies in Africa. Antiviral Res 2015;124:93–100. 10.1016/j.antiviral.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 118.Gongal G, Wright AE. Human rabies in the who Southeast Asia region: forward steps for elimination. Adv Prev Med 2011;2011:1–5. 10.4061/2011/383870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vigilato MAN, Molina-Flores B, Del Rio Vilas VJ, et al. Canine rabies elimination: governance principles. Rev Sci Tech 2018;37:707–9. 10.20506/rst.37.2.2859 [DOI] [PubMed] [Google Scholar]
- 120.Mbilo C, Coetzer A, Bonfoh B, et al. Dog rabies control in West and central Africa: a review. Acta Trop 2021;224:105459. 10.1016/j.actatropica.2020.105459 [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization . Driving progress towards rabies elimination, 2019. Available: file:///C:/Users/Staff/Downloads/WHO-CDS-NTD-NZD-2019.04-eng.pdf
- 122.Broban A, Tejiokem MC, Tiembré I, et al. Bolstering human rabies surveillance in Africa is crucial to eliminating canine-mediated rabies. PLoS Negl Trop Dis 2018;12:e0006367. 10.1371/journal.pntd.0006367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cleaveland S, Sharp J, Abela-Ridder B, et al. One health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos Trans R Soc Lond B Biol Sci 2017;372:20160168. 10.1098/rstb.2016.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tataryn J, Buck PA. The Canadian rabies management plan: an integrated approach to the coordination of rabies activities in Canada. Can Commun Dis Rep 2016;42:135–6. 10.14745/ccdr.v42i06a05 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048551supp001.pdf (247.2KB, pdf)
bmjopen-2020-048551supp002.pdf (76.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information.