Abstract
Anterior gradient 2, AGR2, is a small, 20 kDa protein that plays a vital role in oxidative protein folding in the endoplasmic reticulum. AGR2 is involved in several signal transduction pathways that are essential for cell survival. It was initially discovered in the African clawed frog, Xenopus laevis, where it plays an important function in embryonic development. Akin to several other developmental genes, it is also frequently deregulated in cancer, where it plays a decisive role in tumor initiation, progression and metastasis. In this review, we have summarized currently known AGR2 functions, its expression and function in embryonic and cancer development, as well as its potential as a candidate tumor biomarker and promising new target for cancer immunotherapy.
Keywords: AGR2, anterior gradient 2, embryonic development, cancer, tumor biomarker, therapeutic target
Introduction
Anterior gradient 2 (AGR2) is a 154 amino acid protein disulfide isomerase (PDI) with a single central cysteine residue [1]. It is a resident endoplasmic reticulum (ER) protein highly expressed in mucus-secreting cells and human endocrine tissues [1,2]. The AGR2 gene belongs to a small family of AGR genes (AGR1, 2 and 3), and is localized on chromosome 7p21.3 in the human genome [3]. It plays an important role in embryonic development [4,5] and tissue regeneration [6] and has been linked with the initiation and progression of several cancer types [7-12].
In this review, we illustrate the expression of AGR2 in the embryonic development of several species, including human, summarize its role across normal and diseased states, and describe its promising function in the detection and treatment of cancer.
AGR2 as a developmental gene
AGR2 was initially identified in the African clawed frog, Xenopus laevis, where it is expressed in the anterior region of the ectoderm and plays an important role in the ectodermal patterning and formation of anterior ectodermal structures such as cement gland and early forebrain [4,5]. The cement gland is the mucus-secreting organ required for embryo attachment to a solid support before the development of swimming and feeding abilities [13]. AGR2 has also been shown to be involved in the regeneration of the limbs and the tail in frog tadpoles [6].
AGR2 was detected in mucus-secreting organs of other species as well. In embryos of zebrafish Danio rerio, it is required for the terminal differentiation of mucin-producing goblet cells [14,15]. In the adult fish, it is expressed in organs containing mucus-secreting cells including gill, esophagus, swim bladder, and intestine [15].
In mice, Agr2 gene plays an important function in intestinal mucus production and gut protection, as mice lacking AGR2 have an increased risk of the development of colitis [2]. Moreover, the loss of AGR2 expression causes hyperplasia and defective lineage maturation in AGR2 null mouse model, indicating that AGR2 plays a role in maintaining the proliferation of glandular stomach cells [16]. Similarly, the loss of AGR2 expression leads to loss of tissue regeneration ability in the mouse pancreatitis model [17]. The AGR2 has been shown to regulate cellular proliferation and lobuloalveolar development in the mammary gland in mice, with the highest AGR2 expression observed during late pregnancy and lactation [18].
In this study, we demonstrate for the first time that AGR2 is also expressed during human embryonic development (Figure 1). The human embryonic period is divided into 23 Carnegie stages, based on the external and internal morphological development of the embryo, covering the first 8 weeks post-ovulation. AGR2 expression was first observed in embryos in Carnegie stage 19 (CS19), week 7 of embryo development onwards, initially in the esophagus, trachea, liver, and stomach (Figure 1A, 1B). Its expression persisted in later stages in the gastrointestinal tract, where it was observed in the esophagus and stomach in CS20 (Figure 1C, 1D) and duodenum, stomach, pylorus and herniated intestines in CS23 (Figure 1E-H). In the post-conception stage, AGR2 expression was also observed in the rectum in post-conception weeks (PCW) 9, 10, 12 and 14, and anus in PCW10 (Figure 1I-K).
Figure 1.
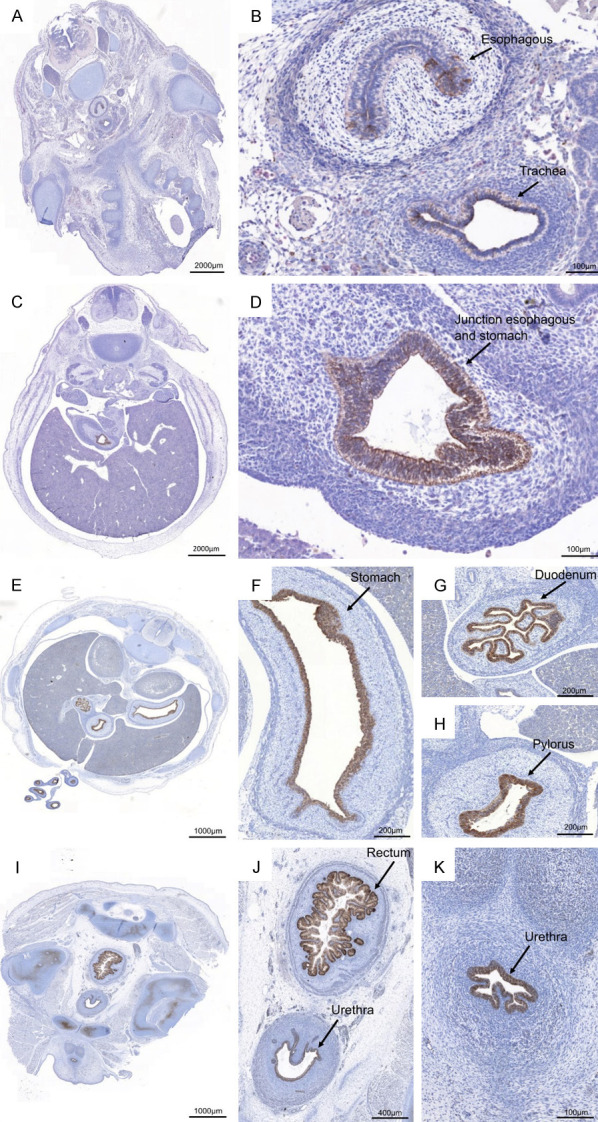
AGR2 expression in the gastrointestinal tract of the human embryo. The human embryonic and fetal material from 3 to 20 weeks of development was obtained from the MRC-Welcome Trust Development Human Developmental Biology Resource (HDBR) tissue bank. Two sections of the human embryo and fetal tissues were used for each stage from Carnegie Stage 19 (CS) of embryonic development up to post-conception week 14 (PCW14). The AGR2 antibody was optimized for Formalin-Fixed Paraffin-Embedded (FFPE) tissues. The immunostaining was performed, and the pictures were scanned using Panoramic 250 3D Histech. AGR2 was found to be expressed across the gastrointestinal tract in several stages of embryonic development: in esophagus and trachea in CS19 (A, B); in the junction of esophagus and stomach; in CS20 (C, D), herniated intestines, stomach, duodenum, and pylorus in CS23 (E-H); and rectum and urethra in PCW10 (I-K).
In addition to the gastrointestinal tract, AGR2 expression was detected in the pigmented layer of the retina in CS22 (Supplementary Figure 1A, 1B) and a skeletal system in ilium (PCW9 and PCW12), ischium (PCW12), pubis (CS23 and PCW10), head of the femur (CS23 and PCW10) and vertebral body (PCW10) (Supplementary Figure 1C-H) as well as in the urogenital system, including ureter (PCW9 and PCW14), urethra (PCW10) and bladder (PCW9 and PCW14), (Supplementary Figure 1I-K).
In adult human tissues, the highest level of AGR2 expression was observed in the gastrointestinal tract (from the stomach to rectum) and genitourinary tract (urinary bladder and female and male genitalia), as well as in respiratory epithelia of nasopharynx and bronchus [19].
Since Virchow’s notion in 1840 that new formations (neoplasms) arise “in accordance with the same law, which regulates embryonic development” [20], abundant evidence was amassed that a number of pathways involved in embryonic and fetal development are later on re-utilized in both the control of postnatal cell growth and differentiation as well as in oncogenesis [21-23]. AGR2 is one of the genes that corroborate this association between embryonic development and cancer.
AGR2 in health and disease
AGR2 is a member of the protein disulfide isomerase (PDI) superfamily [24], which is an expanding family of enzymes that are predominantly expressed in the ER. Structural characteristics of PDI proteins include a carboxyl-terminal KTEL motif as well as at least one thioredoxin-like structural domain with a CXXS motif [11,24-28]. The main function of the PDI family, including AGR2, is the regulation of protein folding. Under stress conditions, AGR2 activates the unfolded protein response (UPR) pathway in the ER, which is one of the key cellular defense mechanisms. AGR2 expression is commonly elevated during ER stress and directly controlled by ER signaling [29]. Accordingly, the siRNA-mediated knockdown of AGR2 can inhibit the ER-associated protein degradation process and disable cellular response to the ER stress [30].
The unstructured N-terminal region of AGR2 is responsible for the cellular adhesion, while the folded domain is essential for dimerization via intermolecular salt bridges [31]. The AGR2 dimerization process in ER can be inhibited by the ER stress, resulting in secretion of AGR2 monomers and activation of pro-inflammatory responses. This can lead to the development of Crohn’s and inflammatory bowel disease (IBD). Interestingly, the level of dysregulation of the AGR2 dimerization process is positively correlated with the severity of both diseases [10]. Additionally, AGR2 dimerization is also required for the interaction of AGR2 with other ER-resident proteins, such as heat-shock protein A5 (HSPA5), which in turn induces the activation of the UPR pathway [32].
AGR2 is essential in the production of gel-forming mucins: MUC1 [33], MUC2 [2], MUC5AC and MUC5B [34] in the intestinal epithelial layer, which are essential for the protection of the gastrointestinal tract (GI) against several pathogens, including the majority of viruses and bacteria that cause various human pathologies [35]. In lung tissues, AGR2 has been described to activate the allergen-induced overproduction of mucins during asthma [34]. Furthermore, AGR2 is involved in the stimulation of the wound healing process in the skin by increasing the migration of keratinocytes and the recruitment of fibroblasts [36]. AGR2 has been also shown to regulate angiogenesis by enhancing the activity of vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2) via direct binding and stimulation of their homodimerization processes [37]. Finally, AGR2 can also play a significant role in metabolism, inducing the expression of lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK1), kallikrein 2 (HK2), enolase 1-α (ENO1), as well as glucose uptake and lactate production [38].
Several nuclear, cytosolic, and plasma membrane proteins have been shown to bind AGR2 [39]. For example, under normal conditions, AGR2 has been shown to interact with isoform 1 of uracil-DNA glycosylase (UNG1) protein, which plays a crucial role in activating base-excision repair in mitochondria [40]. Moreover, AGR2 has been also reported to interact with transmembrane emp24 domain-containing protein 2 (TMED2), which can be found in ER and Golgi and functions in vesicular protein trafficking [41].
Widespread interest in AGR2 is however, raised due to its increasingly recognized role in tumor initiation, development, progression, and its resistance to therapy [11,28]. AGR2 is overexpressed in different types of solid tumors, including prostate [12], breast [9], lung [10], pancreatic [11], ovarian (8), and oral cancers [7]. A comprehensive summary of the AGR2 expression pattern in various tumor tissues is provided in Table 1.
Table 1.
AGR2 expression in cancer
| Ovarian cancer | The elevated levels of AGR2 in plasma are positively correlated to survival in ovarian cancer [8]. |
| Breast cancer | AGR2 expression correlates with poor outcome of patients with ER-positive breast cancer [83]. |
| Cervical cancer | Bioinformatics analysis has revealed that AGR2 is highly expressed in cervical cancer tissue [67], and AGR2 is a potential prognostic factor for this cancer [85]. |
| Endometrial cancer | AGR2 is overexpressed in endometrial cancers and positively associated with high expression of estrogen alpha, progesterone, and androgen receptors [100]. |
| Prostate cancer | AGR2 expression is elevated in prostate cancer [12] and initiates the invasion of prostate cancer cells [76]. |
| Lung cancer | AGR2 is overexpressed in NSCLC [10] and lung adenocarcinoma [101] and is associated with poor survival, especially in younger patients [87]. Immunostaining analysis of 95 NSCLC samples showed elevated AGR2 expression in 66% of the cases [10]. AGR2 expression at the mRNA level is related to lymph nodes metastasis in NSCLC [102]. |
| Nasopharyngeal carcinoma | AGR2 concentration in serum of nasopharyngeal carcinoma (NPC) patients is significantly elevated in comparison to healthy controls. AGR2 serum levels can be used as a marker for the clinical prognosis of NPC [103]. |
| Pancreatic adenocarcinoma | AGR2 was found to be induced in sporadic and familial PanIN lesions, PDAC cells, circulating tumor cells, and metastases. The invasiveness of pancreatic cancer cells correlates with the level of AGR2 expression [54]. |
| Oral cancer | High expression of AGR2 is associated with oral tumor metastasis [7]. |
| Gastric cancer | Elevated expression of AGR2 is related to the progression of gastric cancer and poor survival [104]. |
| Esophageal adenocarcinoma | AGR2 promotes tumor growth of esophageal adenocarcinoma [105]. |
| Cholangiocarcinoma | Upregulation of AGR2 in cholangiocarcinoma promotes cancer cell proliferation, migration, and invasion [106]. |
| Ampullary cancer | AGR2 upregulates proliferation and invasion of ampullary cancer cells [107]. |
| Biliary tract cancer | The AGR2 expression was found to be decreasing with biliary tract cancer progression [108]. |
| Fibrolamellar carcinoma | AGR2 is overexpressed in the majority of fibrolamellar carcinomas [109]. |
| Colorectal cancer | Loss of AGR2 activity is a prognostic factor for colorectal cancer. AGR2 is responsible for the sensitivity of colorectal cancer cells to chemotherapy [110]. |
| Bladder cancer | During bladder cancer, cells secrete AGR2 into urine at higher levels than healthy cells [111]. |
| Papillary thyroid carcinoma | AGR2 is a marker for survival, invasion, and migration of papillary thyroid carcinomas [112]. |
| Head and neck squamous cell carcinoma | AGR2 expression is associated with cancer stem cell and epithelial-mesenchymal transition in high-grade head and neck squamous cell carcinoma [57]. |
| Glioblastoma | The stromal cell-derived factor 1 activates AGR2 to promote EMT progression and the development of glioblastoma [113]. Hypoxia-inducible factor 1 has been shown to regulate AGR2 inducing growth and angiogenesis in glioblastoma [114]. |
| Pituitary adenoma | The serum AGR2 protein levels are significantly elevated in the serum of pituitary adenoma (PA) patients in comparison to healthy controls [70]. |
| Chronic myelogenous leukemia | Upregulation of AGR2 was observed in TKI-resistant chronic myelogenous leukemia (CML) cells [95]. |
Since the first description of AGR2 expression in human breast cancer in 1998 [42], the extent of AGR2 roles that it plays as an active component of a number of signal transduction pathways has emerged (Figure 2).
Figure 2.
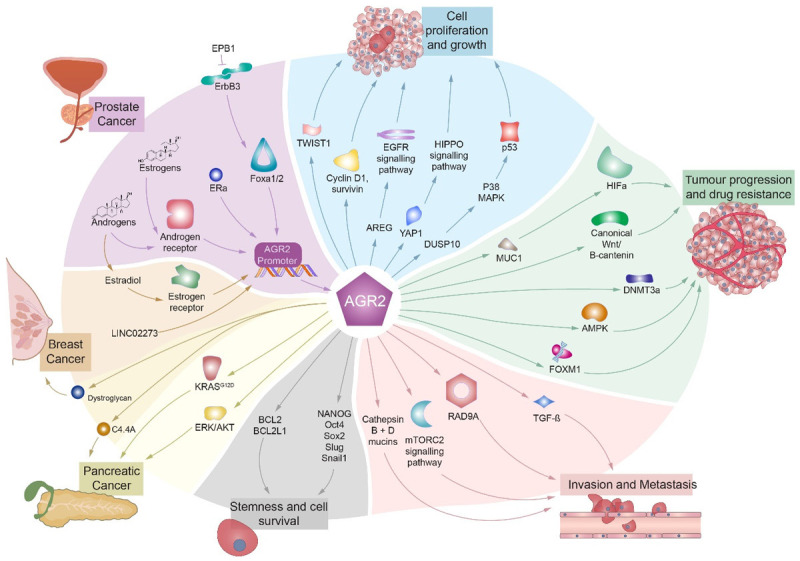
Summary of main AGR2 pathways. Figure shows regulation of cell proliferation and growth (in blue), tumor progression and drug resistance (in green), invasion and metastasis (in red), stemness and cell survival (in grey), pancreatic cancer (in yellow), breast cancer (in orange), prostate cancer (in purple).
AGR2 is involved in cellular proliferation and growth through several pathways (Figure 2. Cell proliferation and growth; blue color). It targets and regulates Hippo signaling pathway co-activator, yes-associated protein 1 (YAP-1), as well as amphiregulin (AREG), which interacts with epidermal growth factor receptor (EGFR) promoting cellular growth in adenocarcinoma cells [43]. Additionally, AGR2 was identified as an inhibitor of p53 transcriptional response after DNA damage, proving that AGR2 plays a vital role in tumor initiation and progression [44]. AGR2 up-regulates Dual Specificity Phosphatase 10 (DUSP10), which in consequence inhibits p38 mitogen-activated protein kinase (p38 MAPK), preventing the activation of tumor suppressor p53 [45]. Twist Family BHLH Transcription Factor 1 (Twist1) protein increases AGR2 promoter activity via direct binding to the E-box motif, inducing proliferation and growth of breast cancer cells [46]. Finally, a recent study has shown that AGR2 can trigger lung tumor cell proliferation through repressing the tumor suppressor p21CIP1 [47].
AGR2 was revealed to be a target of canonical Wnt/β-catenin pathway and is a cancer stem cell marker responsible for enhancing cell proliferation and progression of colorectal cancer [48]. Moreover, the epigenetic regulation of AGR2 by DNA (cytosine-5)-methyltransferase 3A (DNMT3a) was shown to facilitate resistance to 5-azacytidine in colorectal cancer [49]. Additionally, AGR2 was reported to control metformin-dependent activation of AMP-activated protein kinase (AMPK) and silencing of AGR2 induced colorectal cancer sensitivity to chemotherapy [50]. Forkhead box M1 transcription factor (FOXM1) is directly bound to and transcriptionally activates human AGR2 gene promoter stimulating an invasive phenotype in lung cancer cells in vivo [51]. Finally, the glucose metabolism induced by AGR2 can trigger activation of the MUC1/HIF-1α pathway causing progression of endometrial carcinoma [38] (Figure 2. Tumor progression and drug resistance; green color).
The cell cycle checkpoint control protein RAD9A was shown to activate the AGR2 expression to promote tumor metastasis and invasion [52]. Recently, AGR2 was established to induce tumor metastasis via regulation of the mTOR Complex 2 (mTORC2) pathway [53]. AGR2 can also promote tumor cell dissemination via posttranscriptional activation of cathepsins B (CTSB) and D (CTSD) [54]. Moreover, AGR2 has been shown to maintain epithelial phenotype by preventing the activation of transforming growth factor-beta (TGF-β) involved in epithelial-mesenchymal transition (EMT) during tumor invasion and metastasis [55]. The knockdown of AGR2 in the metastatic xenograft mouse model reduced tumor growth and metastasis of head and neck squamous cell carcinoma (HNSCC) [56] (Figure 2. Invasion and Metastasis; red color).
Knockdown of AGR2 in HNSCC cells caused downregulation of pro-survival markers (Survivin, Bcl2, Bcl2l1, and Cyclin D1), EMT markers (Slug and Snail) and stem cell markers (Nanog, Sox2 and OCT4), suggesting that AGR2 is involved in EMT and self-renewal of cancer stem cells [57] (Figure 2. Stemness and cell survival; grey color).
Interestingly, activation of oncogenic Kirsten rat sarcoma (KRAS) mutation KRASG12D triggered upregulation of the Agr2 gene, indicating that AGR2 protein is activated downstream of KrasG12D in pancreatic cancer cells [58]. Additionally, mothers against decapentaplegic homolog 4 (SMAD4) was shown to induce AGR2 expression, that in turn up-regulated MUC1 contributing to the development of pancreatic intraepithelial neoplasia (PanIN) and its progression to PDAC [33]. The silencing of AGR2 in vitro inhibited ERK/AKT pathway, reduced migration and invasion, and increased apoptosis in pancreatic cancer cells [59]. AGR2 has been also shown to bind and activate Ly6/PLAUR domain-containing protein 3 to stimulate the growth of pancreatic tumors in mice, and blocking of the AGR2-C4.4A pathway reduced pancreatic tumor growth, metastasis, and increased survival of mice in the orthotopic mouse model [60] (Figure 2. Pancreatic cancer; yellow color).
AGR2 can be detected in different cellular localizations: intracellular (iAGR2) and extracellular (eAGR2). Interestingly, while healthy cells mainly express iAGR2 in the ER, cancer cells display increased expression of eAGR2 on the cellular surface [54]. The iAGR2 was shown to interact with estrogen receptor alpha (ERα), while eAGR2 induces insulin-like growth factor 1 (IGF-1) signaling via activation of ER-α in breast cancer [61]. Aside from ERα in breast cancer, cyclin D1, pSrc, c-Myc, and survivin were also demonstrated to be expressed downstream of AGR2 [62]. Moreover, AGR2 was shown to interact with C4.4A and dystroglycan (DAG-1) proteins involved in breast cancer metastasis formation through the regulation of estrogen receptor adhesion and functioning [63]. Additionally, AGR2 has been shown to interact with nuclear transcription factor hypoxia-induced factor (HIF)-1α, which has been associated with human breast cancer chemoresistance [64] (Figure 2. Breast cancer; orange color).
In prostate cancers, androgens and estrogens have been shown to stimulate the androgen receptor binding to the AGR2 promoter [65]. AGR2 promoter can be also activated by ERα or erb-b2 receptor tyrosine kinase 3 (ErbB3) which enhances Foxa1 and Foxa2. Moreover, ErbB3-binding protein 1 (EBP1) can suppress ErbB3 inhibiting the AGR2 promoter activity in prostate cancer cells [66] (Figure 2. Prostate cancer highlighted; purple color).
AGR2 activity is regulated via multiple ways, in addition to already mentioned, several microRNA molecules have been proposed as a potential regulator of AGR2 activity. For example, p53 has been shown to activate miR-3647-5p that in turn suppresses AGR2 expression, inhibiting proliferation and promoting apoptosis of cervical cancer [67], and miR-1291 have been revealed to significantly inhibit AGR2 expression in a pancreatic adenocarcinoma cell line, PANC-1 [68].
Apart from its functional roles in cancer, AGR2 was also established as a promising biomarker for cancer detection as well as a compelling therapeutic target.
AGR2 as a biomarker for cancer detection
AGR2 is attracting considerable interest as a promising biomarker for the detection of the most common cancers due to its elevated expression patterns in premalignant lesions, primary tumors, and metastases. Being a secreted molecule, it can be detected in several biological fluids, including serum, plasma, and urine, being therefore a promising non-invasive biomarker. Importantly, AGR2 was also found to be expressed in circuiting tumor cells (CTCs) in blood specimens of patients with advanced and metastatic cancers and was associated with poor clinical outcome in such patients [69].
The serum AGR2 protein levels were found to be significantly elevated in pituitary adenoma (PA) patients [70], as well as in plasma [8] and serum [71] of ovarian cancer patients. Combined in a panel with cancer antigen 125 (CA125) and midkine (MDK), AGR2 has been evaluated as a potential diagnostic biomarker for symptomatic ovarian cancer patients [72].
AGR2 is also considered a potential biomarker for pancreatic adenocarcinoma (PDAC) detection as it was found at elevated levels in the pancreatic juice of patients with early-stage cancer [73]. Enzyme-linked immunosorbent assay (ELISA) analysis of plasma samples has demonstrated a significant increase of biomarker profile comprising of AGR2, syncollin, olfactomedin-4 (OLFM4), polymeric immunoglobulin receptor (plgR), and collagen alpha-1(VI) chain in pancreatic cancer patients when assessed against healthy controls [74]. In a patient-derived xenograft model of PDAC, AGR2 is overexpressed in pancreatic cancer stem cells (CSCs), indicating that AGR2 plays a role in PDAC initiation and may be a potential marker of CSCs [75].
Additionally, AGR2 expression has been detected at increased levels in urine sediment of prostate cancer patients [76], suggesting that the development of AGR2 urine ELISA-based test could be useful for the detection of this cancer [77]. Interestingly, AGR2 expression was significantly raised in blood at both messenger RNA (mRNA) and protein level in patients with metastatic prostate cancer [78]. Likewise, immunostaining of prostate cancer has revealed a high expression of AGR2 in bone metastasis [79]. A targeted mass spectrometric assay has been recently developed for quantification of AGR2 in urine and serum specimens [80], which now requires further validation for the early detection of clinically significant prostate cancer in a large cohort study.
The meta-analysis of 20 studies, including 3285 patients, has revealed that increased AGR2 expression is correlated with poor overall survival (OS) of patients with solid tumors, especially with breast cancer [81]. In fact, AGR2 is a well-characterized candidate biomarker for early breast cancer, where it correlates with poor outcome of patients with ER-positive breast cancer [82,83]. A recent in vivo study has shown that the expression of novel long noncoding RNA called LINC02273 is significantly increased in metastatic lesions compared to primary breast tumors. When combined, LINC02273 and AGR2 act as an independent prognostic factor that can be used to predict the OS of breast cancer patients [84]. Moreover, AGR2 has been described as a potential prognostic factor for cervical cancer [85], and the serum AGR2 was shown to be a useful biomarker for early detection, prediction of recurrence, and prognosis of lung adenocarcinomas [86,87].
Finally, when tested in pre-diagnostic samples, AGR2 combined with CA125, HE4, CHI3L1 and PEBP4 achieved 85.7% sensitivity at 95.4% specificity for detection of ovarian cancer up to 1 year before diagnosis, providing a significant improvement in comparison to CA125 alone [88].
The above studies thus highlight the promising role of AGR2 as both diagnostic and prognostic biomarker, but also suggest that combining AGR2 with other biomarkers and biomarker panels may be a promising strategy for increasing the accuracy of earlier cancer detection. The existence of a number of commercially available anti-AGR2 antibodies provides a first step in the development of ELISAs, lateral flow or other biosensor-type assays that would enable, now necessary, larger scale clinical validation of promising role of AGR2 as a cancer biomarker.
AGR2 as a therapeutic target
The first antibody developed against AGR2 was the mouse monoclonal antibody, called 184A, which was shown to inhibit the growth of breast cancer cells in vitro [89]. Subsequent studies have produced the humanized version of this antibody, 18A4Hu I, and reported that it suppresses the growth of AGR2+ ovarian cancer xenograft [90]. The combinational approach using 18A4 with bevacizumab (targeting VEGF-A) was demonstrated to inhibit tumor growth in an ovarian cancer xenograft mouse model [37]. Recently, the 18A4 antibody was shown to improve survival and prevent AGR2-induced tumor progression by regulating p53 and MAPK pathways, without any toxic effects on major organs in a preclinical lung cancer mice model [91].
We have previously reported for the first time that AGR2 is localized on the surface of PDAC cells. Combined with its expression in both pre-malignant and malignant cells, as well as in CSC and CTCs in pancreatic cancer, indicated that AGR2 could thus present a promising pan-target for immunotherapy in this malignancy [54]. Recently, we have also demonstrated the successful production of two human-mouse chimeric anti-AGR2 antibodies, P3A5 (IgG2a) and P1G4 (IgG1), that when incubated with human blood, initiated the cellular lysis of eAGR2+ PC3 prostate cancer cells without any damage to iAGR2+ healthy cells, indicating that these antibodies could potentially be implemented for the treatment of several eAGR2 positive solid tumors [92].
The mouse monoclonal antibodies against AGR2 and C4.4A were developed and tested in vivo on orthotopic tumors in nude mice models (AsPc-1-Aggressive Cell Model, CaPan-2-Stromal Model and CaPan-2-Regression Study), resulting in significantly reduced tumor growth, metastasis, and increased mouse survival [60]. Moreover, the synthetic single-chain variable fragment monoclonal antibody, scFv4, that can be expressed in mammalian cells has been designed and demonstrated to bind the N-terminal of AGR2 with high affinity [93].
Interestingly, the transduction of dendritic cells with a recombinant adenovirus encoding the AGR2 gene allowed for AGR2 expression without any significant changes in dendritic cell viability and cytokine secretion. Moreover, it induced the production of AGR2-specific cytotoxic T lymphocytes that were capable of lysing AGR2-expressing colorectal cancer cell lines [94].
While AGR2 is known for its role in solid tumors, due to its interaction with miR-217, AGR2 was also proposed as a potential therapeutic target for the treatment of hematological malignancies, including chronic myelogenous leukemia (CML) [95].
An additional reason for AGR2 targeting is provided by an increasing number of studies that demonstrate the influence of AGR2 on chemotherapy resistance in tumor tissues. For example, AGR2 was shown to enhance the resistance of cells to gemcitabine treatment in pancreatic cancer [96], and it stimulates the development of tamoxifen resistance in breast cancers, proving further that AGR2 is a relevant target for the development of breast cancer therapy [83,97,98]. Recently, a study by Cocce et al identified a new targetable pathway for endocrine therapy-resistant breast cancers, based on the transcription factors FOXA1, the membrane receptor LYPD3, and its ligand AGR2. They show that the inhibition of the activity of this pathway using blocking antibodies directed against either LYPD3 or AGR2 inhibits the growth of challenging endocrine therapy-resistant breast cancers in the preclinical model, again providing the rationale for development of humanized antibodies against AGR2 [99].
As the above studies indicate, it is encouraging to see that based on the exceptionally promising properties of AGR2, its favorable localization on cancer cell surface, and the ‘holistic’ expression in all cancer stages, from stem cells to CTCs and metastases, the conditions are now ripe for its translational development. Two published patents: a US Patent for blocking monoclonal antibodies to AGR2 and its receptor C4.4A (https://patents.justia.com/patent/10428159) and another one for AGR2 blocking antibody (https://patents.google.com/patent/EP2749573A1/en) by Sanofi China, offer a tangible hope of testing of anti-AGR2 antibodies in clinical trials in the near future.
Conclusion
In this review, we have summarized the current knowledge on AGR2 and presented some novel data on its expression in normal human development. Although initially discovered as a developmental gene, AGR2 is generating a considerable amount of interest due to its overexpression in most common cancers, and its important role in tumor initiation, progression, and metastasis. Taken together, this highlights an important role of this protein in both early embryonic development and tumorigenesis. A greater understanding of this relationship will likely continue to contribute to the generation of novel, more effective anti-cancer approaches in humans, both in the field of early detection and in developing novel treatments based on AGR2 targeting.
Acknowledgements
We would like to thank Dr. Steven Lisgo from the Institute of Genetic Medicine, Newcastle University, and Dr. Dianne Gerelli from the Institute of Child Health, University College London for their assistance with the human embryonic and fetal material which was provided by the Joint MRC/Wellcome Trust grant #099175/Z/12/Z Human Developmental Biology Resource (http://hdbr.org).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bergstrom JH, Berg KA, Rodriguez-Pineiro AM, Stecher B, Johansson ME, Hansson GC. AGR2, an endoplasmic reticulum protein, is secreted into the gastrointestinal mucus. PLoS One. 2014;9:e104186. doi: 10.1371/journal.pone.0104186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N, Erle DJ. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petek E, Windpassinger C, Egger H, Kroisel PM, Wagner K. Localization of the human anterior gradient-2 gene (AGR2) to chromosome band 7p21.3 by radiation hybrid mapping and fluorescencein situ hybridisation. Cytogenet Cell Genet. 2000;89:141–142. doi: 10.1159/000015594. [DOI] [PubMed] [Google Scholar]
- 4.Tereshina MB, Ivanova AS, Eroshkin FM, Korotkova DD, Nesterenko AM, Bayramov AV, Solovieva EA, Parshina EA, Orlov EE, Martynova NY, Zaraisky AG. Agr2-interacting Prod1-like protein Tfp4 from Xenopus laevis is necessary for early forebrain and eye development as well as for the tadpole appendage regeneration. Genesis. 2019;57:e23293. doi: 10.1002/dvg.23293. [DOI] [PubMed] [Google Scholar]
- 5.Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–130. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova AS, Tereshina MB, Ermakova GV, Belousov VV, Zaraisky AG. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci Rep. 2013;3:1279. doi: 10.1038/srep01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YT, Ho CL, Chen PK, Chen YL, Chang CF. Anterior gradient 2: a novel sensitive tumor marker for metastatic oral cancer. Cancer Lett. 2013;339:270–278. doi: 10.1016/j.canlet.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Edgell TA, Barraclough DL, Rajic A, Dhulia J, Lewis KJ, Armes JE, Barraclough R, Rudland PS, Rice GE, Autelitano DJ. Increased plasma concentrations of anterior gradient 2 protein are positively associated with ovarian cancer. Clin Sci (Lond) 2010;118:717–725. doi: 10.1042/CS20090537. [DOI] [PubMed] [Google Scholar]
- 9.Fritzsche FR, Dahl E, Pahl S, Burkhardt M, Luo J, Mayordomo E, Gansukh T, Dankof A, Knuechel R, Denkert C, Winzer KJ, Dietel M, Kristiansen G. Prognostic relevance of AGR2 expression in breast cancer. Clin Cancer Res. 2006;12:1728–1734. doi: 10.1158/1078-0432.CCR-05-2057. [DOI] [PubMed] [Google Scholar]
- 10.Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I, Dietel M, Kristiansen G. Expression of AGR2 in non small cell lung cancer. Histol Histopathol. 2007;22:703–708. doi: 10.14670/HH-22.703. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008;68:7811–7818. doi: 10.1158/0008-5472.CAN-08-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–259. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 13.Sive H, Bradley L. A sticky problem: the Xenopus cement gland as a paradigm for anteroposterior patterning. Dev Dyn. 1996;205:265–280. doi: 10.1002/(SICI)1097-0177(199603)205:3<265::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Lu YF, Li IC, Hwang SP. Zebrafish Agr2 is required for terminal differentiation of intestinal goblet cells. PLoS One. 2012;7:e34408. doi: 10.1371/journal.pone.0034408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih LJ, Lu YF, Chen YH, Lin CC, Chen JA, Hwang SP. Characterization of the agr2 gene, a homologue of X. laevis anterior gradient 2, from the zebrafish, Danio rerio. Gene Expr Patterns. 2007;7:452–460. doi: 10.1016/j.modgep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Wodziak D, Tun M, Bouley DM, Lowe AW. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J Biol Chem. 2013;288:4321–4333. doi: 10.1074/jbc.M112.433086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wodziak D, Dong A, Basin MF, Lowe AW. Anterior gradient 2 (AGR2) induced epidermal growth factor receptor (EGFR) signaling is essential for murine pancreatitis-associated tissue regeneration. PLoS One. 2016;11:e0164968. doi: 10.1371/journal.pone.0164968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Salmans ML, Geyfman M, Wang H, Yu Z, Lu Z, Zhao F, Lipkin SM, Andersen B. The estrogen-responsive Agr2 gene regulates mammary epithelial proliferation and facilitates lobuloalveolar development. Dev Biol. 2012;369:249–260. doi: 10.1016/j.ydbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjostedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlen M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virchow RLK. Berlin: August Hirschwald; 1858. Cellular pathology. [Google Scholar]
- 21.Naxerova K, Bult CJ, Peaston A, Fancher K, Knowles BB, Kasif S, Kohane IS. Analysis of gene expression in a developmental context emphasizes distinct biological leitmotifs in human cancers. Genome Biol. 2008;9:R108. doi: 10.1186/gb-2008-9-7-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aiello NM, Stanger BZ. Echoes of the embryo: using the developmental biology toolkit to study cancer. Dis Model Mech. 2016;9:105–114. doi: 10.1242/dmm.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Huang Q, Wei GH. The role of hox transcription factors in cancer predisposition and progression. Cancers (Basel) 2019;11:528. doi: 10.3390/cancers11040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Galligan JJ, Petersen DR. The human protein disulfide isomerase gene family. Hum Genomics. 2012;6:6. doi: 10.1186/1479-7364-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlov G, Maattanen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–3936. doi: 10.1111/j.1742-4658.2010.07793.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Dong A, Lowe AW. AGR2 gene function requires a unique endoplasmic reticulum localization motif. J Biol Chem. 2012;287:4773–4782. doi: 10.1074/jbc.M111.301531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hrstka R, Murray E, Brychtova V, Fabian P, Hupp TR, Vojtesek B. Identification of an AKT-dependent signalling pathway that mediates tamoxifen-dependent induction of the pro-metastatic protein anterior gradient-2. Cancer Lett. 2013;333:187–193. doi: 10.1016/j.canlet.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Salmans ML, Zhao F, Andersen B. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker. Breast Cancer Res. 2013;15:204. doi: 10.1186/bcr3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higa A, Mulot A, Delom F, Bouchecareilh M, Nguyen DT, Boismenu D, Wise MJ, Chevet E. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J Biol Chem. 2011;286:44855–44868. doi: 10.1074/jbc.M111.275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P, Clarke C, Barraclough DL, Jowitt TA, Rudland PS, Barraclough R, Lian LY. Metastasis-promoting anterior gradient 2 protein has a dimeric thioredoxin fold structure and a role in cell adhesion. J Mol Biol. 2013;425:929–943. doi: 10.1016/j.jmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Ryu J, Park SG, Lee PY, Cho S, Lee DH, Kim GH, Kim JH, Park BC. Dimerization of pro-oncogenic protein Anterior Gradient 2 is required for the interaction with BiP/GRP78. Biochem Biophys Res Commun. 2013;430:610–615. doi: 10.1016/j.bbrc.2012.11.105. [DOI] [PubMed] [Google Scholar]
- 33.Norris AM, Gore A, Balboni A, Young A, Longnecker DS, Korc M. AGR2 is a SMAD4-suppressible gene that modulates MUC1 levels and promotes the initiation and progression of pancreatic intraepithelial neoplasia. Oncogene. 2013;32:3867–3876. doi: 10.1038/onc.2012.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–185. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Mangukiya HB, Mashausi DS, Guo H, Negi H, Merugu SB, Wu Z, Li D. Anterior gradient 2 is induced in cutaneous wound and promotes wound healing through its adhesion domain. FEBS J. 2017;284:2856–2869. doi: 10.1111/febs.14155. [DOI] [PubMed] [Google Scholar]
- 37.Guo H, Zhu Q, Yu X, Merugu SB, Mangukiya HB, Smith N, Li Z, Zhang B, Negi H, Rong R, Cheng K, Wu Z, Li D. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene. 2017;36:5098–5109. doi: 10.1038/onc.2017.132. [DOI] [PubMed] [Google Scholar]
- 38.Gong W, Ekmu B, Wang X, Lu Y, Wan L. AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF-1α pathway. Hum Cell. 2020;33:790–800. doi: 10.1007/s13577-020-00356-4. [DOI] [PubMed] [Google Scholar]
- 39.Delom F, Mohtar MA, Hupp T, Fessart D. The anterior gradient-2 interactome. Am J Physiol Cell Physiol. 2019;318:C40–C47. doi: 10.1152/ajpcell.00532.2018. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Hu Y, Gong Y, Zhang W, Liu C, Wang Q, Deng H. Hydrogen peroxide mediated mitochondrial UNG1-PRDX3 interaction and UNG1 degradation. Free Radic Biol Med. 2016;99:54–62. doi: 10.1016/j.freeradbiomed.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Maurel M, Obacz J, Avril T, Ding YP, Papadodima O, Treton X, Daniel F, Pilalis E, Hörberg J, Hou W, Beauchamp MC, Tourneur-Marsille J, Cazals-Hatem D, Sommerova L, Samali A, Tavernier J, Hrstka R, Dupont A, Fessart D, Delom F, Fernandez-Zapico ME, Jansen G, Eriksson LA, Thomas DY, Jerome-Majewska L, Hupp T, Chatziioannou A, Chevet E, Ogier-Denis E. Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med. 2019;11:e10120. doi: 10.15252/emmm.201810120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 43.Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–18310. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, Kernohan N, Hupp TR. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–547. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Hrstka R, Bouchalova P, Michalova E, Matoulkova E, Muller P, Coates PJ, Vojtesek B. AGR2 oncoprotein inhibits p38 MAPK and p53 activation through a DUSP10-mediated regulatory pathway. Mol Oncol. 2016;10:652–662. doi: 10.1016/j.molonc.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SY, Yun J, Kim SJ, Kang S, Kim DY, Kim YJ, Park JH, Jang WB, Ji ST, Ha JS, Hong Van LT, Truong Giang LT, Rethineswaran VK, Kim DH, Song P, Kwon SM. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem Biophys Res Commun. 2019;516:149–156. doi: 10.1016/j.bbrc.2019.05.191. [DOI] [PubMed] [Google Scholar]
- 47.Fessart D, de Barbeyrac C, Boutin I, Grenier T, Richard E, Begueret H, Bernard D, Chevet E, Robert J, Delom F. Extracellular AGR2 triggers lung tumour cell proliferation through repression of p21(CIP1) Biochim Biophys Acta Mol Cell Res. 2021;1868:118920. doi: 10.1016/j.bbamcr.2020.118920. [DOI] [PubMed] [Google Scholar]
- 48.Dahal Lamichane B, Jung SY, Yun J, Kang S, Kim DY, Lamichane S, Kim YJ, Park JH, Jang WB, Ji ST, Dehua L, Ha JS, Kim YH, Kwon SM. AGR2 is a target of canonical Wnt/beta-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem Biophys Res Commun. 2019;515:600–606. doi: 10.1016/j.bbrc.2019.05.154. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Hu J, Luo Z, Zhou C, Huang L, Zhang H, Chi J, Chen Z, Li Q, Deng M, Chen J, Tao K, Wang G, Wang L, Wang Z. AGR2 is controlled by DNMT3a-centered signaling module and mediates tumor resistance to 5-Aza in colorectal cancer. Exp Cell Res. 2019;385:111644. doi: 10.1016/j.yexcr.2019.111644. [DOI] [PubMed] [Google Scholar]
- 50.Martisova A, Sommerova L, Kuricova K, Podhorec J, Vojtesek B, Kankova K, Hrstka R. AGR2 silencing contributes to metformin-dependent sensitization of colorectal cancer cells to chemotherapy. Oncol Lett. 2019;18:4964–4973. doi: 10.3892/ol.2019.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, Breuhahn K, Whitsett JA, Kalinichenko VV, Kalin TV. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13:e1007097. doi: 10.1371/journal.pgen.1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broustas CG, Hopkins KM, Panigrahi SK, Wang L, Virk RK, Lieberman HB. RAD9A promotes metastatic phenotypes through transcriptional regulation of anterior gradient 2 (AGR2) Carcinogenesis. 2019;40:164–172. doi: 10.1093/carcin/bgy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiemann K, Garri C, Lee SB, Malihi PD, Park M, Alvarez RM, Yap LP, Mallick P, Katz JE, Gross ME, Kani K. Loss of ER retention motif of AGR2 can impact mTORC signaling and promote cancer metastasis. Oncogene. 2019;38:3003–3018. doi: 10.1038/s41388-018-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, Brentnall TA, Bronner MP, Feakins RM, Timms JF, Brennan C, Lemoine NR, Crnogorac-Jurcevic T. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sommerova L, Ondrouskova E, Vojtesek B, Hrstka R. Suppression of AGR2 in a TGF-β-induced Smad regulatory pathway mediates epithelial-mesenchymal transition. BMC Cancer. 2017;17:546. doi: 10.1186/s12885-017-3537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp Cell Res. 2012;318:1788–1798. doi: 10.1016/j.yexcr.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6:8807–8821. doi: 10.18632/oncotarget.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu QG, Li YJ, Yao L. Knockdown of AGR2 induces cell apoptosis and reduces chemotherapy resistance of pancreatic cancer cells with the involvement of ERK/AKT axis. Pancreatology. 2018;18:678–688. doi: 10.1016/j.pan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Arumugam T, Deng D, Bover L, Wang H, Logsdon CD, Ramachandran V. New blocking antibodies against novel AGR2-C4.4A pathway reduce growth and metastasis of pancreatic tumors and increase survival in mice. Mol Cancer Ther. 2015;14:941–951. doi: 10.1158/1535-7163.MCT-14-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Zhu Q, Chen H, Hu L, Negi H, Zheng Y, Ahmed Y, Wu Z, Li D. Binding of anterior gradient 2 and estrogen receptor-alpha: dual critical roles in enhancing fulvestrant resistance and IGF-1-induced tumorigenesis of breast cancer. Cancer Lett. 2016;377:32–43. doi: 10.1016/j.canlet.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Vanderlaag KE, Hudak S, Bald L, Fayadat-Dilman L, Sathe M, Grein J, Janatpour MJ. Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin. Breast Cancer Res. 2010;12:R32. doi: 10.1186/bcr2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fletcher GC, Patel S, Tyson K, Adam PJ, Schenker M, Loader JA, Daviet L, Legrain P, Parekh R, Harris AL, Terrett JA. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–585. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Zhu Q, Hu L, Chen H, Wu Z, Li D. Anterior gradient 2 is a binding stabilizer of hypoxia inducible factor-1α that enhances CoCl2-induced doxorubicin resistance in breast cancer cells. Cancer Sci. 2015;106:1041–1049. doi: 10.1111/cas.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bu H, Schweiger MR, Manke T, Wunderlich A, Timmermann B, Kerick M, Pasqualini L, Shehu E, Fuchsberger C, Cato AC, Klocker H. Anterior gradient 2 and 3--two prototype androgen-responsive genes transcriptionally upregulated by androgens and by oestrogens in prostate cancer cells. FEBS J. 2013;280:1249–1266. doi: 10.1111/febs.12118. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Ali TZ, Zhou H, D’Souza DR, Lu Y, Jaffe J, Liu Z, Passaniti A, Hamburger AW. ErbB3 binding protein 1 represses metastasis-promoting gene anterior gradient protein 2 in prostate cancer. Cancer Res. 2010;70:240–248. doi: 10.1158/0008-5472.CAN-09-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu R, Qian M, Zhou T, Cui P. TP53 mediated miR-3647-5p prevents progression of cervical carcinoma by targeting AGR2. Cancer Med. 2019;8:6095–6105. doi: 10.1002/cam4.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tu MJ, Pan YZ, Qiu JX, Kim EJ, Yu AM. MicroRNA-1291 targets the FOXA2-AGR2 pathway to suppress pancreatic cancer cell proliferation and tumorigenesis. Oncotarget. 2016;7:45547–45561. doi: 10.18632/oncotarget.9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LW, O’Hara SM. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 70.Tohti M, Li J, Tang C, Wen G, Abdujilil A, Yizim P, Ma C. Serum AGR2 as a useful biomarker for pituitary adenomas. Clin Neurol Neurosurg. 2017;154:19–22. doi: 10.1016/j.clineuro.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Park K, Chung YJ, So H, Kim K, Park J, Oh M, Jo M, Choi K, Lee EJ, Choi YL, Song SY, Bae DS, Kim BG, Lee JH. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011;43:91–100. doi: 10.3858/emm.2011.43.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rice GE, Edgell TA, Autelitano DJ. Evaluation of midkine and anterior gradient 2 in a multimarker panel for the detection of ovarian cancer. J Exp Clin Cancer Res. 2010;29:62. doi: 10.1186/1756-9966-29-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen R, Pan S, Duan X, Nelson BH, Sahota RA, de Rham S, Kozarek RA, McIntosh M, Brentnall TA. Elevated level of anterior gradient-2 in pancreatic juice from patients with pre-malignant pancreatic neoplasia. Mol Cancer. 2010;9:149. doi: 10.1186/1476-4598-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makawita S, Smith C, Batruch I, Zheng Y, Rückert F, Grützmann R, Pilarsky C, Gallinger S, Diamandis EP. Integrated proteomic profiling of cell line conditioned media and pancreatic juice for the identification of pancreatic cancer biomarkers. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.008599. M111.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dumartin L, Alrawashdeh W, Trabulo SM, Radon TP, Steiger K, Feakins RM, di Magliano MP, Heeschen C, Esposito I, Lemoine NR, Crnogorac-Jurcevic T. ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene. 2017;36:3094–3103. doi: 10.1038/onc.2016.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bu H, Bormann S, Schafer G, Horninger W, Massoner P, Neeb A, Lakshmanan VK, Maddalo D, Nestl A, Sultmann H, Cato AC, Klocker H. The anterior gradient 2 (AGR2) gene is overexpressed in prostate cancer and may be useful as a urine sediment marker for prostate cancer detection. Prostate. 2011;71:575–587. doi: 10.1002/pros.21273. [DOI] [PubMed] [Google Scholar]
- 77.Wayner EA, Quek SI, Ahmad R, Ho ME, Loprieno MA, Zhou Y, Ellis WJ, True LD, Liu AY. Development of an ELISA to detect the secreted prostate cancer biomarker AGR2 in voided urine. Prostate. 2012;72:1023–1034. doi: 10.1002/pros.21508. [DOI] [PubMed] [Google Scholar]
- 78.Kani K, Malihi PD, Jiang Y, Wang H, Wang Y, Ruderman DL, Agus DB, Mallick P, Gross ME. Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate. 2013;73:306–315. doi: 10.1002/pros.22569. [DOI] [PubMed] [Google Scholar]
- 79.Vitello EA, Quek SI, Kincaid H, Fuchs T, Crichton DJ, Troisch P, Liu AY. Cancer-secreted AGR2 induces programmed cell death in normal cells. Oncotarget. 2016;7:49425–49434. doi: 10.18632/oncotarget.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi T, Gao Y, Quek SI, Fillmore TL, Nicora CD, Su D, Zhao R, Kagan J, Srivastava S, Rodland KD, Liu T, Smith RD, Chan DW, Camp DG 2nd, Liu AY, Qian WJ. A highly sensitive targeted mass spectrometric assay for quantification of AGR2 protein in human urine and serum. J Proteome Res. 2014;13:875–882. doi: 10.1021/pr400912c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian SB, Tao KX, Hu J, Liu ZB, Ding XL, Chu YN, Cui JY, Shuai XM, Gao JB, Cai KL, Wang JL, Wang GB, Wang L, Wang Z. The prognostic value of AGR2 expression in solid tumours: a systematic review and meta-analysis. Sci Rep. 2017;7:15500. doi: 10.1038/s41598-017-15757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Innes HE, Liu D, Barraclough R, Davies MP, O’Neill PA, Platt-Higgins A, de Silva Rudland S, Sibson DR, Rudland PS. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hrstka R, Nenutil R, Fourtouna A, Maslon MM, Naughton C, Langdon S, Murray E, Larionov A, Petrakova K, Muller P, Dixon MJ, Hupp TR, Vojtesek B. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010;29:4838–4847. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- 84.Xiu B, Chi Y, Liu L, Chi W, Zhang Q, Chen J, Guo R, Si J, Li L, Xue J, Shao ZM, Wu ZH, Huang S, Wu J. LINC02273 drives breast cancer metastasis by epigenetically increasing AGR2 transcription. Mol Cancer. 2019;18:187. doi: 10.1186/s12943-019-1115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, Chung JY, Kim TJ, Lee JW, Kim BG, Bae DS, Choi CH, Hewitt SM. Genomic network-based analysis reveals pancreatic adenocarcinoma up-regulating factor-related prognostic markers in cervical carcinoma. Front Oncol. 2018;8:465. doi: 10.3389/fonc.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung K, Nishiyama N, Yamano S, Komatsu H, Hanada S, Wei M, Wanibuchi H, Suehiro S, Kakehashi A. Serum AGR2 as an early diagnostic and postoperative prognostic biomarker of human lung adenocarcinoma. Cancer Biomark. 2011-2012;10:101–107. doi: 10.3233/CBM-2012-0234. [DOI] [PubMed] [Google Scholar]
- 87.Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, Chia D, Goodglick L, Liu AY. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer. 2015;15:655. doi: 10.1186/s12885-015-1658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitwell HJ, Worthington J, Blyuss O, Gentry-Maharaj A, Ryan A, Gunu R, Kalsi J, Menon U, Jacobs I, Zaikin A, Timms JF. Improved early detection of ovarian cancer using longitudinal multimarker models. Br J Cancer. 2020;122:847–856. doi: 10.1038/s41416-019-0718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu ZH, Zhu Q, Gao GW, Zhou CC, Li DW. Preparation, characterization and potential application of monoclonal antibody 18A4 against AGR2. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:49–51. [PubMed] [Google Scholar]
- 90.Guo H, Chen H, Zhu Q, Yu X, Rong R, Merugu SB, Mangukiya HB, Li D. A humanized monoclonal antibody targeting secreted anterior gradient 2 effectively inhibits the xenograft tumor growth. Biochem Biophys Res Commun. 2016;475:57–63. doi: 10.1016/j.bbrc.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 91.Negi H, Merugu SB, Mangukiya HB, Li Z, Zhou B, Sehar Q, Kamle S, Yunus FU, Mashausi DS, Wu Z, Li D. Anterior Gradient-2 monoclonal antibody inhibits lung cancer growth and metastasis by upregulating p53 pathway and without exerting any toxicological effects: a preclinical study. Cancer Lett. 2019;449:125–134. doi: 10.1016/j.canlet.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 92.Liu AY, Kanan AD, Radon TP, Shah S, Weeks ME, Foster JM, Sosabowski JK, Dumartin L, Crnogorac-Jurcevic T. AGR2, a unique tumor-associated antigen, is a promising candidate for antibody targeting. Oncotarget. 2019;10:4276–4289. doi: 10.18632/oncotarget.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohtar MA, Low T, Vojtesek B, Jamal R, Hupp T. The development of a synthetic scFv monoclonal antibody targeting pro-oncogenic AGR2. Front Pharmacol. 2018;9 [Google Scholar]
- 94.Lee HJ, Hong CY, Kim MH, Lee YK, Nguyen-Pham TN, Park BC, Yang DH, Chung IJ, Kim HJ, Lee JJ. In vitro induction of anterior gradient-2-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses as a potential therapy for colorectal cancer. Exp Mol Med. 2012;44:60–67. doi: 10.3858/emm.2012.44.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan B, Yang J, Wang X, Xu K, Ikezoe T. miR-217 sensitizes chronic myelogenous leukemia cells to tyrosine kinase inhibitors by targeting pro-oncogenic anterior gradient 2. Exp Hematol. 2018;68:80–88. e82. doi: 10.1016/j.exphem.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Hong X, Li ZX, Hou J, Zhang HY, Zhang CY, Zhang J, Sun H, Pang LH, Wang T, Deng ZH. Effects of ER-resident and secreted AGR2 on cell proliferation, migration, invasion, and survival in PANC-1 pancreatic cancer cells. BMC Cancer. 2021;21:33. doi: 10.1186/s12885-020-07743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hengel SM, Murray E, Langdon S, Hayward L, O’Donoghue J, Panchaud A, Hupp T, Goodlett DR. Data-independent proteomic screen identifies novel tamoxifen agonist that mediates drug resistance. J Proteome Res. 2011;10:4567–4578. doi: 10.1021/pr2004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wright TM, Wardell SE, Jasper JS, Stice JP, Safi R, Nelson ER, McDonnell DP. Delineation of a FOXA1/ERalpha/AGR2 regulatory loop that is dysregulated in endocrine therapy-resistant breast cancer. Mol Cancer Res. 2014;12:1829–1839. doi: 10.1158/1541-7786.MCR-14-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cocce KJ, Jasper JS, Desautels TK, Everett L, Wardell S, Westerling T, Baldi R, Wright TM, Tavares K, Yllanes A, Bae Y, Blitzer JT, Logsdon C, Rakiec DP, Ruddy DA, Jiang T, Broadwater G, Hyslop T, Hall A, Laine M, Phung L, Greene GL, Martin LA, Pancholi S, Dowsett M, Detre S, Marks JR, Crawford GE, Brown M, Norris JD, Chang CY, McDonnell DP. The lineage determining factor GRHL2 collaborates with FOXA1 to establish a targetable pathway in endocrine therapy-resistant breast cancer. Cell Rep. 2019;29:889–903. e810. doi: 10.1016/j.celrep.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamal A, Valentijn A, Barraclough R, Rudland P, Rahmatalla N, Martin-Hirsch P, Stringfellow H, Decruze SB, Hapangama DK. High AGR2 protein is a feature of low grade endometrial cancer cells. Oncotarget. 2018;9:31459–31472. doi: 10.18632/oncotarget.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pizzi M, Fassan M, Balistreri M, Galligioni A, Rea F, Rugge M. Anterior gradient 2 overexpression in lung adenocarcinoma. Appl Immunohistochem Mol Morphol. 2012;20:31–36. doi: 10.1097/PAI.0b013e3182233f9f. [DOI] [PubMed] [Google Scholar]
- 102.Inoue M, Hiyama K, Nakabayashi K, Morii E, Minami M, Sawabata N, Shintani Y, Nakagiri T, Susaki Y, Maeda J, Higashiyama M, Okami J, Yoshida Y, Ding J, Otomo Y, Okumura M. An accurate and rapid detection of lymph node metastasis in non-small cell lung cancer patients based on one-step nucleic acid amplification assay. Lung Cancer. 2012;78:212–218. doi: 10.1016/j.lungcan.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, Wang W, Liu Z, Jiang Y, Lu J, Xie H, Tang F. AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin Chim Acta. 2018;484:323–327. doi: 10.1016/j.cca.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, Wang Y, Chen J, Huang Y, He X, Zhao Z. AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett. 2016;11:2075–2083. doi: 10.3892/ol.2016.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 106.Yosudjai J, Inpad C, Chomwong S, Dana P, Sawanyawisuth K, Phimsen S, Wongkham S, Jirawatnotai S, Kaewkong W. An aberrantly spliced isoform of anterior gradient-2, AGR2vH promotes migration and invasion of cholangiocarcinoma cell. Biomed Pharmacother. 2018;107:109–116. doi: 10.1016/j.biopha.2018.07.154. [DOI] [PubMed] [Google Scholar]
- 107.Kim SJ, Jun S, Cho HY, Lee DC, Yeom YI, Kim JH, Kang D. Knockdown of anterior gradient 2 expression extenuates tumor-associated phenotypes of SNU-478 ampulla of Vater cancer cells. BMC Cancer. 2014;14:804. doi: 10.1186/1471-2407-14-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SJ, Kim DH, Kang D, Kim JH. Expression of anterior gradient 2 is decreased with the progression of human biliary tract cancer. Tohoku J Exp Med. 2014;234:83–88. doi: 10.1620/tjem.234.83. [DOI] [PubMed] [Google Scholar]
- 109.Vivekanandan P, Micchelli ST, Torbenson M. Anterior gradient-2 is overexpressed by fibrolamellar carcinomas. Hum Pathol. 2009;40:293–299. doi: 10.1016/j.humpath.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riener MO, Thiesler T, Hellerbrand C, Amann T, Cathomas G, Fritzsche FR, Dahl E, Bahra M, Weichert W, Terracciano L, Kristiansen G. Loss of anterior gradient-2 expression is an independent prognostic factor in colorectal carcinomas. Eur J Cancer. 2014;50:1722–1730. doi: 10.1016/j.ejca.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 111.Ho ME, Quek SI, True LD, Seiler R, Fleischmann A, Bagryanova L, Kim SR, Chia D, Goodglick L, Shimizu Y, Rosser CJ, Gao Y, Liu AY. Bladder cancer cells secrete while normal bladder cells express but do not secrete AGR2. Oncotarget. 2016;7:15747–15756. doi: 10.18632/oncotarget.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Di Maro G, Salerno P, Unger K, Orlandella FM, Monaco M, Chiappetta G, Thomas G, Oczko-Wojciechowska M, Masullo M, Jarzab B, Santoro M, Salvatore G. Anterior gradient protein 2 promotes survival, migration and invasion of papillary thyroid carcinoma cells. Mol Cancer. 2014;13:160. doi: 10.1186/1476-4598-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu C, Liu Y, Xiao L, Guo C, Deng S, Zheng S, Zeng E. The involvement of anterior gradient 2 in the stromal cell-derived factor 1-induced epithelial-mesenchymal transition of glioblastoma. Tumour Biol. 2016;37:6091–6097. doi: 10.1007/s13277-015-4481-0. [DOI] [PubMed] [Google Scholar]
- 114.Hong XY, Wang J, Li Z. AGR2 expression is regulated by HIF-1 and contributes to growth and angiogenesis of glioblastoma. Cell Biochem Biophys. 2013;67:1487–1495. doi: 10.1007/s12013-013-9650-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


