Abstract
The purpose of this state-of-the-art review is to update the American College of Chest Physicians 2006 guideline on global physiology and pathophysiology of cough. A review of the literature was conducted using PubMed and MEDLINE databases from 1951 to 2019 and using prespecified search terms. We describe the basic phenomenology of cough patterns, behaviors, and morphological features. We update the understanding of mechanical and physiological characteristics of cough, adding a contemporary view of the types of cough and their associated behaviors and sensations. New information about acoustic characteristics is presented, and recent insights into cough triggers and the patient cough hypersensitivity phenotype are explored. Lastly, because the clinical assessment of patients largely focuses on the duration rather than morphological features of cough, we review the morphological features of cough that can be measured in the clinic. This is the first of a two-part update to the American College of Chest Physicians 2006 cough guideline; it provides a more global consideration of cough phenomenology, beyond simply the mechanical aspects of a cough. A greater understanding of the typical features of cough, and their variations, may allow a more informed interpretation of cough measurements and the clinical relevance for patients.
Key Words: cough, pathophysiology, phenomenology, physiology
Abbreviations: CPD, compression phase duration; EMG, electromyography; ER, expiration reflex
The purpose of this review is to update in two parts the section on global physiology and pathophysiology of cough in the 2006 American College of Chest Physicians cough guidelines.1 The authors performed a review of the literature by using PubMed and MEDLINE from 1951 to 2019 and using the search terms shown in Table 1. The terms used to describe cough types in the literature are variable and inconsistent.2 Physicians categorize cough by its duration and cause.2, 3, 4 Mechanistic researchers classify cough as induced cough, voluntary cough, and spontaneous cough when referring to study methodology. Others describe cough as sensitized (hypertussia and allotussia), typically triggered by heterogeneous stimuli, or desensitized (hypotussia). Finally, coughs can be defined by physiological characteristics, sound properties, and patterns. There is no universally accepted way to classify cough types, but an understanding of the phenomenology (a term used here to describe the patterns, behaviors, and morphological features of cough) may provide insight into underlying pathophysiological and neurobiological mechanisms.
Table 1.
MeSH Search Terms
|
MeSH = Medical Subject Headings.
Part 1 of this update will summarize the motor and sensory traits of cough, presenting typical descriptive characteristics; physiological mechanics of cough; how cough is assessed; and, where available, how cough characteristics can differ between health and disease. Part 2 of the update will describe more applied topics of the demographic characteristics of patients with cough, the clinical conditions affecting cough mechanics, and the relationship between cough and the role of airway secretions in cough clearance.
Cough and Related Sensorimotor Processes in the Clinical Setting
Classical Cough, Expiratory Reflexes, and the Urge to Cough
Cough can occur reflexively or voluntarily. Cough is commonly induced in the experimental or clinical setting by way of inhaled challenges involving the use of tussive agents, such as capsaicin from hot chili peppers. Cough challenges are often described as cough reflex testing, although the true involvement of reflexes vs volitional responses has not been assessed. Induced cough is often distinguished from spontaneous cough occurring in disease because, although both are often induced by irritant stimuli, the latter reflects naturally occurring cough in which the tussive triggers may be endogenous (eg, mucus, refluxate, or inflammation), exogenous (eg, cold air, perfume, or smoke), or perhaps cognitive (voluntary cough) but are unlikely to be homogeneous for all people. Reflex coughing, like many reflexes, involves neural processes that are somewhat simpler in organization, integrated at the level of the brainstem. Voluntary control of coughing, however, requires more complex neural processing at higher cortical brain levels and has been described as behavioral regulation of coughing. People can voluntarily produce a cough, with or without accompanying airway stimuli, as well as regulate cough intensity and even voluntarily suppress cough entirely for periods of time.
These broad types of cough are important to distinguish conceptually. For example, the study of induced and voluntary cough allows precisely controlled experimental conditions to be used for comparisons between cough in patients with disease and that in healthy volunteers and provides insights into disease mechanisms and drug target engagement, which is often difficult when assessing spontaneous cough. However, one must be cognizant that studies of voluntary and induced cough do not necessarily predict therapeutic effects on spontaneous coughing or cough severity in disease. For example, drugs that antagonize the capsaicin receptor (transient receptor potential vanilloid 1) result in effective attenuation of capsaicin-induced cough responses in healthy volunteers and patients with chronic cough, yet they fail to reduce spontaneous coughing in patients with chronic refractory cough, suggesting this mechanism is not universally relevant to the disease.5
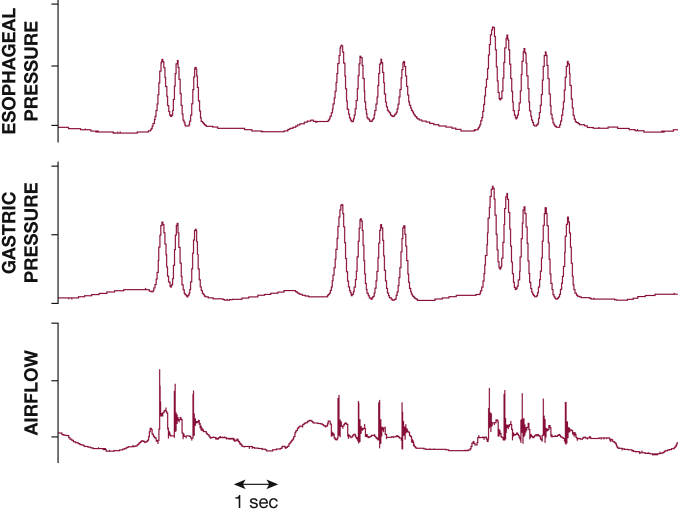
Coughs also can occur as isolated events or within bouts or epochs (Fig 1). Patients with spontaneous cough often complain of these as coughing fits, which are perceived as contributing to the severity of cough.4,6 Although these bouts are accepted as a series of expulsive efforts, whether each bout must originate from separate breaths is uncertain, and a variety of definitions have been used in the literature.7 Studies involving acoustic cough counting have defined bouts as continuous periods of coughing with less than 2-s pauses.8,9 However, most cough frequency data still report the number of coughs as a total number of events regardless of whether in a bout or not, and, in fact, the two are well correlated.10
Figure 1.
Bouts of coughs, characterized by multiple expulsive efforts occurring close together.
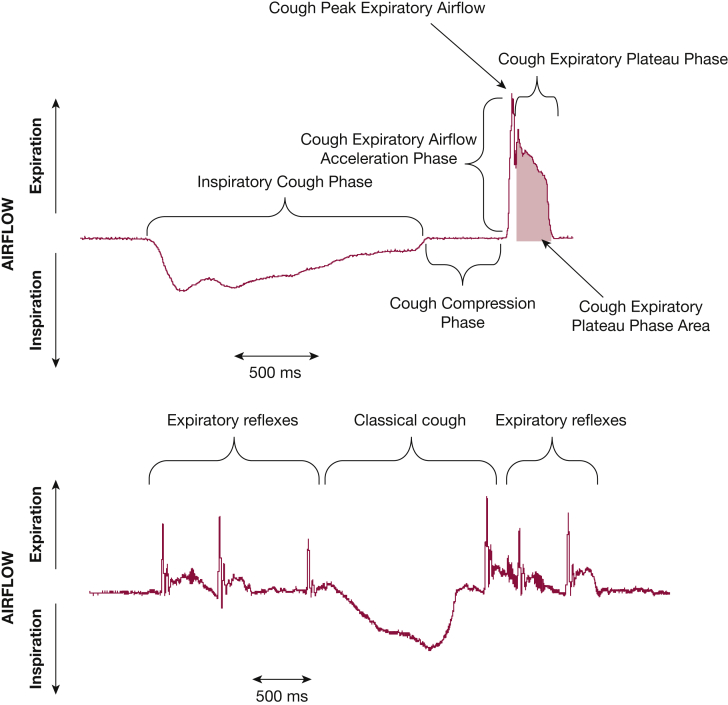
Where there is glottic closure and expiratory effort but without the preceding inspiration, the event is termed an expiration reflex (ER), which differs from a classical cough (Fig 2).7 It is not uncommon for cough reaccelerations during coughing bouts to be considered ERs,11,12 but it is equally important that ERs can be evoked in isolation by using mechanical stimuli around the glottal folds or trachea.13,14 The rationale for recognizing classical cough and ER as separate entities is suggested by the possible role for ERs in preventing aspiration and pneumonia.7,14, 15, 16 Another consideration is that ERs are likely to occur from lower lung volumes than are coughs after an inspiration and therefore will generate lower flows.17 The counterarguments are that it is at present difficult to differentiate them in clinical practice because they sound similar and current monitoring methods (as well as most patients and physicians) consider all such events as coughs.18
Figure 2.
A, The three-phase flow pattern of a classical cough is characterized by an initial inspiratory phase followed by cessation of flow during the compression phase (glottis closure) and then rapid expulsion of air during the expiratory phase. B, Expiration reflexes and classical cough. Flow trace depicting a series of three expiration reflexes (characterized by lack of preceding inspiration) followed by a single classical cough and two expiration reflexes.
Although the subject is not well studied, it is possible that in patients with chronic cough, spontaneous coughing is made up of a mixture of classical coughs, ERs, and cough reaccelerations. Some coughs could be reflexive, whereas others could be under various levels of volitional control. This subject, again, has not been well studied.
Cough Triggers and the Concept of Cough Hypersensitivity
Traditionally, physicians have viewed cough solely as a symptom of an underlying lung disease or arising as a consequence of an acute inflammatory or infective insult. But the cough may persist long after the initiating insult has resolved, and this chronicity is a source of considerable morbidity. Physicians experienced in the management of chronic cough are readily aware of how troubled their patients are by spasms of cough provoked by everyday activities including talking or laughing and changes in ambient air temperature or exposure to aerosols or perfumes.19, 20, 21 Many patients also describe abnormal sensations such as a persisting itch or tickle in the throat or the feeling of a “lump” in the back of the throat.22 These clinical observations have given rise to the unifying clinical concept of cough hypersensitivity syndrome, defined by the European Respiratory Society Task Force as a “disorder characterized by troublesome coughing often triggered by low levels of thermal, mechanical or chemical exposure.”23
The triggering of cough by relatively innocuous stimuli suggests heightened sensitivity of the sensory nerve pathways alluded to earlier that normally serve to detect and respond to harmful airway irritants. In these circumstances, the cough should not be considered as a symptom but rather as a disease entity caused by a disordered nervous system.24 The pathological mechanisms responsible for how such nerves become pathologically excitable is unknown, but inflammation-induced injury causing functional changes of the neural pathways seems possible.25 The notion that cough triggered by relatively inoffensive stimuli (allotussia) might be similar to allodynia (pain response from stimuli that do not normally provoke pain) and excessive coughing in response to a noxious exposure (hypertussia) could be considered equivalent to hyperalgesia (abnormally increased sensitivity to pain) supports the view that mechanistic parallels may exist between cough hypersensitivity syndrome and neuropathic pain.26 The logical extension of this concept has prompted clinical trials designed to evaluate the efficacy of neuromodulatory drugs, such as morphine, gabapentin, pregabalin, and amitriptyline, more traditionally used to treat pain.27 The European Respiratory Society guidelines on the diagnosis and treatment of chronic cough have made a conditional recommendation (albeit on low-quality evidence) that a trial of such agents be offered to adult patients with chronic refractory cough.28
This clinical phenomenon whereby innocuous sensory stimuli evoke a strong urge to cough or trigger bouts of coughing is in part due to a disorder in the communication of sensory information from the airway to the brain. Equally as important is the cognitive awareness of this information and the processing responsible for the generation of a cough. The complex role of cognition in cough regulation is discussed later.
Behavioral Considerations in the Regulation of Cough
Cough is cognitively controlled by discriminative and affective cortical neural mechanisms. The discriminative element provides the patient with an assessment of the cough stimulus (eg, what the intensity is, some capacity to localize) and can precede the motor cough response. Affective neural systems superimpose reward-aversion value judgments onto the cough response (eg, how does it make me feel?). Affective mechanisms may therefore promote suppression or potentiation of the motor cough behavior. Cognitive suppression and/or modulation of cough is of importance in regulating the cough motor pattern. The cognitive awareness of a cough stimulus can promote an urge to cough, much like thirst promotes an urge to drink. It has been suggested that the urge to cough reflects activation of a motivational neural system in the brain that promotes voluntary cough or other behaviors to help alleviate the sensations accompanying airway irritation.29 Although these sensory experiences could be considered the premotor phase of cough, they do not precede all coughs, an urge to cough being reported in 69% of patients with chronic cough, and not all throat irritation or urge to cough will evolve into actual coughing, as they can be suppressed or satisfied by other maneuvers.20,29 Functional brain imaging has shown activation of cortical and subcortical regions during capsaicin-induced urge to cough that differs between patients with chronic cough and healthy volunteers.30
The threshold of the urge to cough can be critical for initiating cough; a weak urge to cough (high threshold) means patients will not voluntarily cough and will clear their airways with weak stimuli. The implications of an increased urge-to-cough threshold are increased risk of aspiration as weak cough and delayed airway clearance. The relationship between a high urge-to-cough threshold and aspiration-related lung infection needs to be investigated. Cough is additionally subject to strong placebo or suggestive suppression, mediated by cognitive processes in the higher brain.31, 32, 33
Physiological Mechanics of Cough
The major function of cough is to engage high-velocity airflow to clear the airways. Cough airflow is generated by contracting expiratory muscles while the glottis is closed, thus producing high positive subglottic pressures.34 When a cough is initiated, the normal cough motor pattern is characterized9 by a stereotypic inspiration (inspiratory phase) followed by complete closure of the glottis, allowing compression of the thorax and increasing subglottic pressure (compression phase). These phases are followed by rapid opening of the glottis, resulting in a high-velocity airflow (peak expiratory airflow phase), a high expiratory airflow rate (plateau phase) that is sustained for a variable duration, with the cough ending by expiratory airflow returning to baseline (Fig 2A).
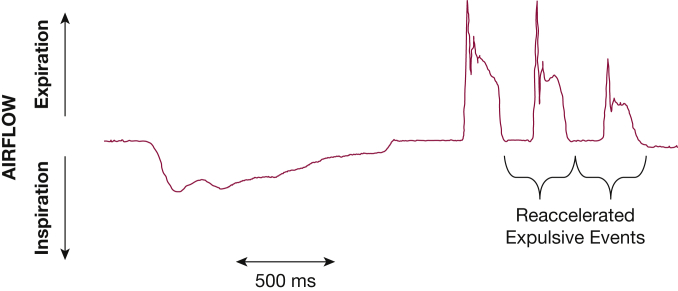
This single expulsive effort is the classical definition of a cough9 that must be amended to include bouts of expulsive events after a single inspiration.35 Initiation of a cough most commonly results in a large inspiration followed by multiple expulsive events during the decrease in expired volume (Fig 3). An expulsive event after the initial cough expiratory sequence is characterized by reclosure of the glottis, a compression phase of equal duration followed by rapid glottis opening, and a peak expiratory airflow phase that is usually less than the initial event but reaccelerates expiratory airflow (Fig 3).35 This pattern of multiple expulsive events for a single inspiration has been termed cough reacceleration.12,35 The most common coughing pattern is two expulsive events for a single inspiration.12 Increasing the cough stimulus intensity causes an increased number of expulsive events—cough reaccelerations—for a single inspiration. Multiple cough reaccelerations recorded in a flow-volume loop have been reported as cough spiking.36,37
Figure 3.
Example of diminishing cough strength during cough reaccelerations within a bout. An initial inspiratory effort is followed by multiple expiratory events efforts that have sequentially less flow as the bout progresses.
The advantage of cough reacceleration expulsive events after an inspiration is the repeated shear forces applied to the airway. The initial cough expiratory airflow acceleration phase produces the shear forces dislodging material from the walls of the airway. The sustained cough expiratory plateau phase moves the material through the center of the airway airflow stream. When a cough bout occurs, the second expulsive event occurs while the material moves toward the exit of the airways. Transient reclosure of the glottis allows the subglottic pressure to again increase, thus generating renewed shear forces when the glottis is reopened. This mechanism keeps the material moving toward the opening of the airways without an interrupting inspiratory phase, avoiding material reattachment to the airway wall and sustaining clearance. With strong cough stimuli, the number of cough reaccelerations increases for each inspiratory volume.12,35 The critical importance of high airflow velocity on shearing forces dislodging material from the wall of the airway results in the documented relevance of cough expiratory airflow volume acceleration and cough peak expiratory airflow velocity measurements for cough efficacy and strength.37, 38, 39, 40
Functional Relevance of the Cough Motor Pattern
The magnitude of the airflow rate during the expiratory peak airflow and plateau phases of cough is directly related to the initial inspired lung volume.12,17 Thus, the inspired volume primes the thoracic system volume for generating expiratory pressures and airflows. The greater the cough priming inspiratory volume, the greater the subglottic pressure that is a combination of volume-dependent elastic recoil and expiratory muscle pump force. The magnitude of the inspiratory volume is also proportional to the number of cough reaccelerations.12 Active expiratory muscle contraction and elastic recoil of the thoracic system against a closed glottis results in a rapidly increasing subglottic pressure.34 During this compression phase, the end-inspiratory total respiratory compliance, the magnitude of the expiratory muscle contraction, the tightness of glottic closure, and the duration of the compression phase determine the peak subglottic pressure. There appears to be a subglottic pressure threshold for glottis opening, although it has received little investigation. The subglottic pressure determines the magnitude of the cough expiratory airflow acceleration on opening of the glottis and is correlated with the clearance of the airways.
Cough peak expiratory airflow (Fig 2A) has also been correlated with the successful clearance of the airway.39, 40, 41, 42 The initial expiratory cough airflow rapidly accelerates, reaches a peak, and then rapidly declines to a sustained cough expiratory airflow plateau. The peak airflow spike (Fig 2A) is of short duration. The subsequent cough expiratory plateau phase (Fig 2A) is the result of sustained active expiratory muscle contraction and respiratory system elastic recoil. The cough plateau phase is often extended to sustain expulsive forces, especially when patients feel that they have not cleared their airway. The respiratory system elastic recoil is dependent on the total thoracic volume and decreases as air is exhaled. The airflow rate during the plateau phase sustains the airway shear and proximal propulsion of material from the airways.11,43 As lung volume decreases during the plateau phase, sustaining the airflow rate requires increasing expiratory muscle contraction.11,35 The plateau phase is usually abruptly terminated, and expiratory airflow rate returns to baseline.
When a cough bout (multiple expulsive events) occurs with a single inspiration, this return to baseline becomes a new compression phase with glottis closure (Fig 3). Similar to the initial pattern, the glottic closure results in respiratory system elastic recoil increasing subglottic pressure in combination with a resurgence of expiratory muscle contraction.35 The second compression phase generally has a duration similar to that of the initial compression phase that is again terminated by rapid opening of the glottis, initiating a second cough peak airflow spike (Fig 3). The second expulsive event often has a diminished cough expiratory airflow acceleration rate and decreased cough peak expiratory airflow rate primarily because of the lower lung volume. Each subsequent expulsive event for multiple cough reaccelerations has a lower initial airflow acceleration, peak expiratory airflow, and plateau airflow rate (Fig 3).11 Reaccelerated cough expulsive events can occur throughout the expired volume from the end-inspiratory lung volume to lung volumes below functional residual capacity.17,44 The expiratory muscle activity required to produce each expulsive event may increase as lung volume decreases.35
Physiological Measures of Cough
Airflow
Flow is easily measured in voluntary cough performed in the laboratory but challenging to measure during induced cough or in the ambulatory setting. The classical cough flow pattern is of an inspiration followed by cessation of flow during glottis closure and then rapid expiration followed plateau and termination. The peak flow during the expiratory phase is the most extensively measured physiological characteristic. Peak cough flow has been used to assess respiratory muscle function, airway clearance function, and suitability for extubation from invasive ventilation.45, 46, 47, 48 Peak flows during maximum voluntary cough can reach > 800 L/min and can be greater than flows observed during peak expiratory flow rate maneuvers.49, 50, 51 Measurement of flow during induced cough is more technically challenging but is significantly lower than maximum voluntary cough flow.48,52, 53, 54, 55 Flow during spontaneous cough has seldom been studied, and may be altered by the measuring equipment, but has been reported as less than that of maximum voluntary cough, although higher than that of induced cough.53
Several other flow dynamic characteristics have been described. Compression phase duration (CPD), the period between cessation of flow after inspiration and onset of the expiratory flow in the expulsive phase, varies in the literature, but estimates in the region of 0.30 s are reported in healthy people.34,53 CPD cannot be measured via flow for ERs because of the lack of preceding inspiration but could be studied using other modalities such as chest wall motion and volume via impedance bands, optoelectronic plethysmography, or electromyography (EMG). CPD (and cough duration) is shorter for coughs occurring within bouts than in single coughs.17 Conditions after laryngectomy predictably result in loss of the compression phase, but reasons for prolonged CPD are more complex and may include reduced motor drive and impaired laryngeal function.55,56 The expiratory rise time, the time between onset and peak expiratory flow, has been reported in a small study of healthy adults and ranged from 51 to 73 ms, varying with sex and height,51 but can be significantly longer in patients with amyotrophic lateral sclerosis, in patients with diseases with unsafe swallowing, and in patients with chronic airways disease.57,58
Pressure
Cough gastric pressure is a reflection of intraabdominal pressure and can be measured using a balloon catheter and pressure transducer system.59 Often used to assess expiratory muscle function, cough gastric pressures can exceed 300 mm Hg during maximum voluntary cough, although values of 214 and 165 mm Hg are reported for healthy male and female patients, respectively.60 The pattern is typically of a spike in pressure with rapid increase and decrease coinciding with expiratory flow (Fig 1). Esophageal pressure is a measure of intrathoracic pressure and can also be measured with the same balloon catheter system and has a similar pattern.59 Esophageal pressure increases during the compression and expulsion phases of cough and can also reach pressures as high as 300 mm Hg.61 Both pressure measurement methods are limited by their invasive nature; requirement for assessment in the laboratory setting; and being subject to effects from body position, state of rest of the abdominal muscles, and external compression.62,63
Electromyography
Abdominal muscle EMG has been explored as a measure of cough for more than 30 years, but accessory muscle EMG has also been studied.64,65 Abdominal EMG correlates well with flow during voluntary cough and is repeatable during induced cough challenges.49,66, 67, 68 However, when voluntary coughs are studied from a range of different lung volumes and with different efforts applied, then, unlike flow, the EMG activity is largely independent of lung volume and is mainly determined by cough effort. The exceptions are coughs performed from below functional residual capacity, such as those occurring at the end of cough bouts; these are associated with the highest EMG signals.69 The study of EMG has allowed the observation that voluntary cough is associated with coordinated sequential activation of the main expiratory and accessory muscles, whereas induced cough is associated with simultaneous activation of both muscle groups with greater EMG activity but shorter duration.64
A limitation of EMG is the inability to compare values between subjects or between experimental sessions, and normalization methods are required for data analysis.64,66,69,70 Factors such as electrode position, contact between electrode and skin, resting muscle state, and inherent skin resistance can affect the EMG measures, and signal contamination from ECG, and limited potential for automation has also made EMG challenging to develop as a clinical measure.71
Sound
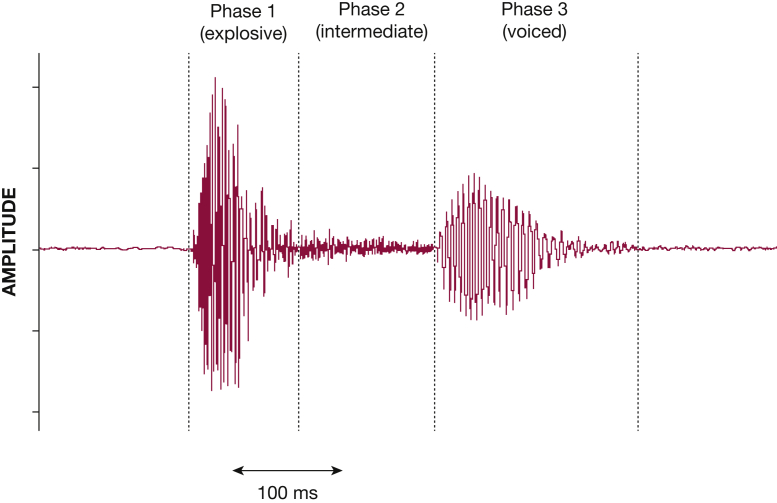
The characteristic cough sound waveform is composed of three distinct phases: explosive, intermediate, and voiced (Fig 4). Although all coughs contain the first phase, the voiced phase may be absent in approximately one-third of subjects.72, 73, 74, 75 In health, the duration of a typical cough sound is approximately 410 ms, but in disease it can be longer—up to 600 ms in asthma or bronchitis and up to 1 s in acute viral cough.76, 77, 78, 79 The sound signal amplitude and power (calculated after transforming the sound signal into its frequency domain) correlate with flow, pressure, and cough EMG during voluntary cough, although the strength of the relationships has varied among studies.69,75,80,81
Figure 4.
A typical three-phase cough sound shown in the time domain. The first phase, explosive, relates to the expulsion of air through the glottis and is followed in most cases by a voiced phase, with a gradually diminishing sound signal; a quiet intermediate phase occurs between the two.
An area of interest is the ability to discern the underlying cause from the cough sound. Physicians are able to discriminate wet from dry cough sounds by ear, but they are less able to differentiate between causes such as fibrosis, asthma, COPD, or bronchiectasis.82 Analysis of sound properties by using signal-processing techniques can also help differentiate between wet and dry cough but have additionally been reported to enable identification of asthma, COPD, pertussis, and pneumonia; however, there is currently no validated diagnostic system available for the clinical setting.75,83, 84, 85, 86, 87 Although spontaneous and voluntary coughs were assessed in these studies, there is a lack of data comparing cough sound properties of spontaneous, induced, and voluntary cough directly within subjects. It is not known whether ERs have sound qualities different from those of classical coughs.
Cough Assessment
Assessment of cough requires differentiation between the strength of the individual cough and the total cough response. The strength of a cough is a function of the individual cough motor pattern. A high-magnitude cough response is characterized by a large number of expulsive events.
Cough assessment requires the occurrence of a cough when the patient has instruments placed for recording cough sounds, airflow, and/or motor patterns. This assessment usually requires that cough is induced under controlled conditions. Cough without an external tussive stimulus can be induced by asking the individual to perform a voluntary cough. Reflex cough can be induced by stimulation of airway cough receptors by using capsaicin, citric acid, distilled water fog, or similar airway irritants. Voluntary and reflex cough have similar motor patterns, with the inspiratory phase, compression phase, and cough expiratory phase.88 Induced cough is useful for evaluating the cough motor pattern. Induced reflex cough is useful for assessing cough motor pattern, cough stimulus threshold, urge to cough, and cough sensitivity to a specific stimulus. Voluntary cough is useful in assessing cough motor pattern and voluntary cough strength. Both voluntary and reflex-induced cough reliably generate a cough but do not allow for the assessment of spontaneous cough. Reflex and voluntary induced cough may be insensitive to antitussive treatment.89
Assessment of spontaneous cough has been investigated with ambulatory cough monitors.90,91 Spontaneous cough monitors are effective in assessing cough frequency and cough sound intensity as a measure of cough strength. Two systems are commonly used. The first is a semiautomated cough monitoring system (VitaloJAK; Vitalograph Ltd) that records sound and requires a manual analysis to discriminate cough sounds during the cough counting process. The second is a semiautomated system (Leicester Cough Monitor; Birring and Matos) with user input used to train the detection and analysis algorithm; validation results have shown it to be able to differentiate cough sounds from noncough sounds such as throat clearing.92 Parameters derived from ambulatory cough monitors allow for the assessment of antitussive treatments in more natural environments and may better reflect treatment efficacy. Although cough frequency monitoring is widely practiced, the uptake of cough sound intensity monitoring in clinical practice has been limited by the lack of validation data against physiological measures of cough intensity in the ambulatory setting.80 Ambulatory cough monitors usually do not allow for the recording of cough airflow and cough motor pattern.
Dysfunction and/or disruption of the cough motor pattern that results in reduced cough effectiveness is defined as dystussia. One dystussic complication of cough motor pattern is inadequate closure of the glottis (leak) during the compression phase.70,93 This inadequate closure results in reduced subglottic pressure, decreased initial expiratory airflow acceleration, reduced peak cough expiratory airflow, and reduced expulsive forces in the airways; hence, it results in inadequate clearance of material from the airways. Another complication resulting in dystussia is decreased expiratory muscle force-generating capacity.66 Reduced expiratory muscle strength results in decreased subglottic pressure during the compression phase, reduced initial cough expiratory airflow rate, decreased cough peak expiratory airflow, reduced plateau phase expiratory airflow, and inadequate clearance of material from the airway.
Qualitative patient perspectives highlight both the frequency and intensity of coughs as determinants of cough severity, in addition to degree of effect or disruption.4 Cough monitoring has focused primarily on recording cough frequency, but there is recognition that the addition of cough intensity monitoring may be valuable.
Cough Frequency
Current cough frequency monitoring systems generally work by recording ambient sound continuously over a 24-hour period followed by off-line analysis, either manually or via semiautomated analysis, to determine cough counts.92,94 The monitoring systems count all recorded events as coughs regardless of whether they are classical coughs or ERs because the current methodology for cough detection is not able to discriminate between them. Cough frequency is most commonly quantified as the total number of events per hour or day, but the merits of quantifying in bouts or time spent coughing have also been shown.10 Cough frequency data exist for healthy adults (8-30 coughs per day), as well as for various pulmonary diseases, and cough frequency monitoring has changed the standards by which novel cough therapies are being evaluated.91,95
Cough Intensity
Cough intensity is often considered as the harshness or violence of coughing perceived by patients. However, there is lack of consensus about whether the mechanical properties of cough events can reflect perceived cough intensity to provide an objective intensity measure, so there is generally little agreement on the best measures of cough intensity. Direct measures of cough strength assessments are typically made using cough airflow patterns, including the initial cough expiratory airflow acceleration, the cough peak expiratory airflow rate, and the area under the cough expiratory airflow plateau phase. When surface EMGs are recorded, the integrated EMG from abdominal and intercostal areas are correlated with cough expiratory airflow rate only as a difference within a single subject recording session.49
The impracticalities of measuring EMG or pressure or flow in the ambulatory setting have prompted the study of sound as a potential cough intensity monitoring measure, but further studies are needed to determine whether this is a clinically useful outcome measure.69,80,81,96 Other auditory assessments of cough have been used to assess cough strength indirectly, and they are often performed by counting the number of expulsive events elicited by spontaneous, induced, or voluntary cough. There is a direct correlation between the number of expulsive events, cough frequency recorded from cough sounds, and the reported cough strength.97 Auditory sound intensity and duration for a single expulsive event are also reported as a measure of cough strength.80,81 Cough strength can be assessed by behavioral magnitude production tasks.53,69 The subject is asked to produce a weak, moderate, or strong cough during simultaneous measurement of auditory, airflow, and/or EMG outputs.53,69,80 Cognitive cough strength magnitude production tasks are correlated with peak airflow, cough sound, and integrated EMG area.53,69,80
In the future, studies are needed to assess the clinical relevance of these cough patterns. Studies should focus on the relationships among cough phenomenology, the cause of cough, and the effect on patients (patient-reported cough severity and quality of life). The changes of these morphological features in response to antitussive treatments in clinical trials and the potential to monitor patients by using recent advances in technology should also be assessed.
Conclusions
This update to the American College of Chest Physicians 2006 cough guideline1 reviews the advances in the knowledge of cough physiology and pathophysiology, specifically describing the features and patterns of different cough types, the triggers, and the regulatory processes, with relevance to patients with chronic cough. The terminology used to describe cough types is varied; consequently, it is important to define the type of cough under assessment and recognize the usual characteristics to support interpretation of findings and, more importantly, clinical relevance. With the development of improved less invasive and advanced portable technologies, there is major potential for more detailed assessments of cough to become widespread for remote diagnostics and monitoring. A better knowledge and understanding of cough phenomenology will surely support this.
Acknowledgments
Author contributions: All authors participated in conceiving of the content to be covered in this article, writing specific sections of the first draft, and reading and editing and approving of the final draft that was prepared by K. K. L., L. M., and S. B. M.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. A. S. reports personal fees from Algernon Pharmaceuticals; AstraZeneca; Attenua, Inc; Bayer AG; BELLUS Health, Inc; Boehringer Ingelheim; Menlo Therapeutics, Inc; Merck & Co, Inc; NeRRe Therapeutics; and Shionogi, Inc. L. M. reports personal fees from Chiesi Farmaceutici, SpA; GlaxoSmithKline plc; Merck & Co, Inc; NeRRe Therapeutics; and Shionogi, Inc; grant support from Merck & Co, Inc; and other support from AstraZeneca; Boehringer Ingelheim; and Chiesi Farmaceutici, SpA. S. B. M. declares personal fees from Merck & Co, Inc, and NeRRe Therapeutics and grant support from Merck & Co, Inc. S. S. B. reports personal fees from Bayer AG; BELLUS Health, Inc; Boehringer Ingelheim; Menlo Therapeutics, Inc; Merck & Co, Inc; NeRRe Therapeutics; Shionogi, Inc; and LCM cough analysis service (to King's College Hospital). None declared (K. K. L., P. W. D., R. S. I.).
∗CHEST Expert Cough Panel Collaborators: Abd Moain Abu Dabrh, MBBCh, MS (Mayo Clinic, Jacksonville, FL), Kenneth W. Altman, MD, PhD (Geisinger Commonwealth School of Medicine, Danville, PA), Alan F. Barker, MD (Oregon Health & Science University, Portland, OR), Surinder S. Birring, MBChB, MD (Division of Asthma, Allergy and Lung Biology, King's College London, Denmark Hill, London, United Kingdom), Fiona Blackhall, MD, PhD (University of Manchester, Department of Medical Oncology, Manchester, England), Donald C. Bolser, PhD, (College of Veterinary Medicine, University of Florida, Gainesville, FL), Christopher Brightling, MBBS, PhD, FCCP (University of Leicester, Glenfield Hospital, Leicester, United Kingdom), Anne B. Chang, MBBS, PhD, MPH, (Royal Children's Hospital, QLD, Australia), Paul Davenport, PhD (Department of Physiological Sciences, University of Florida, Gainesville, FL), Ali A. El Solh, MD, MPH (University at Buffalo, State University of New York, Buffalo, NY), Patricio Escalante, MD, MSc, FCCP (Mayo Clinic, Rochester, MN), Stephen K. Field, MD (University of Calgary, Calgary, AB, Canada), Dina Fisher, MD, MSc (University of Calgary, Respiratory Medicine, Calgary, AB, Canada), Cynthia T. French, PhD, FCCP (UMass Memorial Medical Center, Worcester, MA), Cameron Grant, MB ChB, PhD (University of Auckland, New Zealand), Susan M. Harding, MD, FCCP, (Division of Pulmonary, Allergy and Critical Care Medicine, University of Alabama at Birmingham, Birmingham, AL), Anthony Harnden, MB ChB, MSc (University of Oxford, Oxford, England), Adam T. Hill, MB ChB, MD (Royal Infirmary and University of Edinburgh, Edinburgh, Scotland), Richard S. Irwin, MD, Master FCCP (UMass Memorial Medical Center, Worcester, MA), Vivek Iyer, MD, MPH Mayo Clinic, Rochester MN), Peter J. Kahrilas, MD (Feinberg School of Medicine, Northwestern University, Chicago, IL), Joanne Kavanagh, MBChB, (Division of Asthma, Allergy and Lung Biology, King's College London, Denmark Hill, London, United Kingdom), Karina A. Keogh, MD (Mayo Clinic, Rochester, MN), Kefang Lai, MD, PhD (First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China), Andrew P. Lane, MD (Johns Hopkins University School of Medicine, Baltimore, MD), Kaiser Lim, MD (Mayo Clinic, Rochester, MN), J. Mark Madison, MD, FCCP (UMass Memorial Medical Center, Worcester, MA), Mark A. Malesker, PharmD, FCCP (Creighton University School of Pharmacy and Health Professions, Omaha, NE), Lorcan McGarvey, MD (The Queens University Belfast, Belfast, United Kingdom), M. Hassan Murad, MD, MPH (Mayo Clinic, Rochester, MN), Mangala Narasimhan, DO, FCCP (Hofstra-Northwell Health, Manhasset, NY), Peter Newcombe, PhD (School of Psychology University of Queensland, QLD, Australia), John Oppenheimer, MD (UMDNJ-Rutgers University), Bruce Rubin, MEngr, MD, MBA (Virginia Commonwealth University, Richmond, VA), Richard J. Russell, MBBS (University of Leicester, Glenfield Hospital, Leicester, United Kingdom), Jay H. Ryu, MD, FCCP (Mayo Clinic, Rochester, MN), Sonal Singh, MD, MPH (UMass Memorial Medical Center, Worcester, MA), Maeve P. Smith, MB ChB, MD (University of Alberta, Edmonton, AB, Canada), Susan M. Tarlo, MBBS, FCCP (Toronto Western Hospital, Toronto, ON, Canada), Anne E. Vertigan, PhD, MBA, BAppSc (SpPath) (John Hunter Hospital, NSW, Australia).
Other contributions: We thank Nancy Harger, MLS, an education and clinical services librarian working in the University of Massachusetts Medical School Library in Worcester, MA, who undertook all the searches for each section in this article. Information presented was obtained from both animal and human experimental work.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/CHEST-Guidelines.
Contributor Information
Lorcan McGarvey, Email: l.mcgarvey@qub.ac.uk.
Stuart B. Mazzone, Email: stuart.mazzone@unimelb.edu.au.
CHEST Expert Cough Panel:
AbdMoain Abu Dabrh, Kenneth W. Altman, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald C. Bolser, Christopher Brightling, Anne B. Chang, Paul Davenport, Ali A. El Solh, Patricio Escalante, Stephen K. Field, Dina Fisher, Cynthia T. French, Cameron Grant, Susan M. Harding, Anthony Harnden, AdamT. Hill, Richard S. Irwin, Vivek Iyer, Peter J. Kahrilas, Joanne Kavanagh, Karina A. Keogh, Kefang Lai, AndrewP. Lane, Kaiser Lim, J. Mark Madison, MarkA. Malesker, Lorcan McGarvey, M. Hassan Murad, Mangala Narasimhan, Peter Newcombe, John Oppenheimer, Bruce Rubin, Richard J. Russell, Jay H. Ryu, Sonal Singh, Maeve P. Smith, Susan M. Tarlo, and Anne E. Vertigan
References
- 1.McCool F.D. Global physiology and pathophysiology of cough. Chest. 2006;129(1 suppl):48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 2.Chung K.F., Bolser D., Davenport P., Fontana G., Morice A., Widdicombe J. Semantics and types of cough. Pulm Pharmacol Ther. 2009;22(2):139–142. doi: 10.1016/j.pupt.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin R.S., Baumann M.H., Bolser D.C., et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernon M., Leidy N.K., Nacson A., Nelsen L. Measuring cough severity: perspectives from the literature and from patients with chronic cough. Cough. 2009;5:5. doi: 10.1186/1745-9974-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalid S., Murdoch R., Newlands A., et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol. 2014;134(1):56–62. doi: 10.1016/j.jaci.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Everett C.F., Kastelik J.A., Thompson R.H., Morice A.H. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007;3:5. doi: 10.1186/1745-9974-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana G.A., Widdicombe J. What is cough and what should be measured? Pulm Pharmacol Ther. 2007;20(4):307–312. doi: 10.1016/j.pupt.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.K., Matos S., Evans D.H., White P., Pavord I.D., Birring S.S. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 2013;187(9):991–997. doi: 10.1164/rccm.201209-1686OC. [DOI] [PubMed] [Google Scholar]
- 9.Morice A.H., Fontana G.A., Belvisi M.G., et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29(6):1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 10.Kelsall A., Decalmer S., Webster D., et al. How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J. 2008;32(1):175–179. doi: 10.1183/09031936.00101307. [DOI] [PubMed] [Google Scholar]
- 11.Davenport P.W., Bolser D.C., Vickroy T., et al. The effect of codeine on the urge-to-cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20(4):338–346. doi: 10.1016/j.pupt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegland K.W., Troche M.S., Davenport P.W. Cough expired volume and airflow rates during sequential induced cough. Front Physiol. 2013;4:167. doi: 10.3389/fphys.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishino T., Tagaito Y., Isono S. Cough and other reflexes on irritation of airway mucosa in man. Pulm Pharmacol. 1996;9(5-6):285–292. doi: 10.1006/pulp.1996.0037. [DOI] [PubMed] [Google Scholar]
- 14.Tatar M., Hanacek J., Widdicombe J. The expiration reflex from the trachea and bronchi. Eur Respir J. 2008;31(2):385–390. doi: 10.1183/09031936.00063507. [DOI] [PubMed] [Google Scholar]
- 15.Stephens R.E., Addington W.R., Miller S.P., Anderson J.W. Videofluoroscopy of the diaphragm during voluntary and reflex cough in humans. Am J Phys Med Rehabil. 2003;82(5):384. doi: 10.1097/01.PHM.0000064731.36291.61. [DOI] [PubMed] [Google Scholar]
- 16.Widdicombe J., Fontana G. Cough: what's in a name? Eur Respir J. 2006;28(1):10–15. doi: 10.1183/09031936.06.00096905. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.A., Aliverti A., Quaranta M., et al. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol. 2012;590(3):563–574. doi: 10.1113/jphysiol.2011.213157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morice A.H. Rebuttal: cough is an expiratory sound. Lung. 2008;186(suppl 1):S7–S9. doi: 10.1007/s00408-007-9039-5. [DOI] [PubMed] [Google Scholar]
- 19.McGarvey L., McKeagney P., Polley L., MacMahon J., Costello R.W. Are there clinical features of a sensitized cough reflex? Pulm Pharmacol Ther. 2009;22(2):59–64. doi: 10.1016/j.pupt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Hilton E., Marsden P., Thurston A., Kennedy S., Decalmer S., Smith J.A. Clinical features of the urge-to-cough in patients with chronic cough. Respir Med. 2015;109(6):701–707. doi: 10.1016/j.rmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Won H.K., Kang S.Y., Kang Y., et al. Cough-related laryngeal sensations and triggers in adults with chronic cough: symptom profile and impact. Allergy Asthma Immunol Res. 2019;11(5):622–631. doi: 10.4168/aair.2019.11.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vertigan A.E., Bone S.L., Gibson P.G. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology. 2013;18(6):948–956. doi: 10.1111/resp.12103. [DOI] [PubMed] [Google Scholar]
- 23.Morice A.H., Millqvist E., Belvisi M.G., et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014;44(5):1132. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 24.McGarvey L., Gibson P.G. What is chronic cough? Terminology. J Allergy Clin Immunol Pract. 2019;7(6):1711–1714. doi: 10.1016/j.jaip.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 25.McGovern A.E., Short K.R., Kywe Moe A.A., Mazzone S.B. Translational review: neuroimmune mechanisms in cough and emerging therapeutic targets. J Allergy Clin Immunol. 2018;142(5):1392–1402. doi: 10.1016/j.jaci.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Chung K.F., McGarvey L., Mazzone S.B. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1(5):414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 27.Gibson P., Wang G., McGarvey L., Vertigan A.E., Altman K.W., Birring S.S. Treatment of unexplained chronic cough: CHEST Guideline and Expert Panel Report. Chest. 2016;149(1):27–44. doi: 10.1378/chest.15-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morice A.H., Millqvist E., Bieksiene K., et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport P.W. Urge-to-cough: what can it teach us about cough? Lung. 2008;186(suppl 1):S107–S111. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 30.Mazzone S.B., McLennan L., McGovern A.E., Egan G.F., Farrell M.J. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176(4):327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- 31.Hutchings H.A., Morris S., Eccles R., Jawad M.S. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87(5):379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee P.C., Cotterill-Jones C., Eccles R. Voluntary control of cough. Pulm Pharmacol Ther. 2002;15(3):317–320. doi: 10.1006/pupt.2002.0365. [DOI] [PubMed] [Google Scholar]
- 33.Eccles R. The powerful placebo in cough studies? Pulm Pharmacol Ther. 2002;15(3):303–308. doi: 10.1006/pupt.2002.0364. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara N., von Leden H., Werner-Kukuk E. The physical parameters of cough: the larynx in a normal single cough. Acta Otolaryngol. 1966;61(6):495–510. doi: 10.3109/00016486609127088. [DOI] [PubMed] [Google Scholar]
- 35.Vovk A., Bolser D.C., Hey J.A., et al. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther. 2007;20(4):423–432. doi: 10.1016/j.pupt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beardsmore C.S., Wimpress S.P., Thomson A.H., Patel H.R., Goodenough P., Simpson H. Maximum voluntary cough: an indication of airway function. Bull Eur Physiopathol Respir. 1987;23(5):465–472. [PubMed] [Google Scholar]
- 37.Chaudri M.B., Liu C., Hubbard R., Jefferson D., Kinnear W.J. Relationship between supramaximal flow during cough and mortality in motor neurone disease. Eur Respir J. 2002;19(3):434–438. doi: 10.1183/09031936.02.00082702. [DOI] [PubMed] [Google Scholar]
- 38.Kim J., Davenport P., Sapienza C. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr. 2009;48(3):361–366. doi: 10.1016/j.archger.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Smith Hammond C.A., Goldstein L.B., Zajac D.J., Gray L., Davenport P.W., Bolser D.C. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 40.Ward K., Rao P., Reilly C.C., et al. Poor cough flow in acute stroke patients is associated with reduced functional residual capacity and low cough inspired volume. BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickey B.F. What it takes for a cough to expel mucus from the airway. Proc Natl Acad Sci U S A. 2018;115(49):12340–12342. doi: 10.1073/pnas.1817484115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren S., Li W., Wang L., et al. Numerical analysis of airway mucus clearance effectiveness using assisted coughing techniques. Sci Rep. 2020;10(1):2030. doi: 10.1038/s41598-020-58922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J., Li Y. Human cough as a two-stage jet and its role in particle transport. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilozni D., Lavie M., Ofek M., Sarouk I., Efrati O. Cough characteristics and FVC maneuver in cystic fibrosis. Respir Care. 2014;59(12):1912–1917. doi: 10.4187/respcare.03290. [DOI] [PubMed] [Google Scholar]
- 45.Szeinberg A., Tabachnik E., Rashed N., et al. Cough capacity in patients with muscular dystrophy. Chest. 1988;94(6):1232–1235. doi: 10.1378/chest.94.6.1232. [DOI] [PubMed] [Google Scholar]
- 46.Polkey M.I., Lyall R.A., Green M., Nigel Leigh P., Moxham J. Expiratory muscle function in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 1998;158(3):734–741. doi: 10.1164/ajrccm.158.3.9710072. [DOI] [PubMed] [Google Scholar]
- 47.Smina M., Salam A., Khamiees M., Gada P., Amoateng-Adjepong Y., Manthous C.A. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 48.Kulnik S.T., Birring S.S., Hodsoll J., Moxham J., Rafferty G.F., Kalra L. Higher cough flow is associated with lower risk of pneumonia in acute stroke. Thorax. 2016;71(5):474–475. doi: 10.1136/thoraxjnl-2015-207810. [DOI] [PubMed] [Google Scholar]
- 49.Fontana G.A., Pantaleo T., Lavorini F., Boddi V., Panuccio P. A noninvasive electromyographic study on threshold and intensity of cough in humans. Eur Respir J. 1997;10(5):983–989. doi: 10.1183/09031936.97.10050983. [DOI] [PubMed] [Google Scholar]
- 50.Ross B.B., Gramiak R., Rahn H. Physical dynamics of the cough mechanism. J Appl Physiol. 1955;8(3):264–268. doi: 10.1152/jappl.1955.8.3.264. [DOI] [PubMed] [Google Scholar]
- 51.Feinstein A.J., Zhang Z., Chhetri D.K., Long J. Measurement of cough aerodynamics in healthy adults. Ann Otol Rhinol Laryngol. 2017;126(5):396–400. doi: 10.1177/0003489417694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward K., Seymour J., Steier J., et al. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. Eur Respir J. 2010;36(6):1383–1390. doi: 10.1183/09031936.00010510. [DOI] [PubMed] [Google Scholar]
- 53.Lee K.K., Ward K., Rafferty G.F., Moxham J., Birring S.S. The intensity of voluntary, induced, and spontaneous cough. Chest. 2015;148(5):1259–1267. doi: 10.1378/chest.15-0138. [DOI] [PubMed] [Google Scholar]
- 54.Wheeler Hegland K., Troche M.S., Brandimore A.E., Davenport P.W., Okun M.S. Comparison of voluntary and reflex cough effectiveness in Parkinson's disease. Parkinsonism Relat Disord. 2014;20(11):1226–1230. doi: 10.1016/j.parkreldis.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavorini F., Fontana G.A., Pantaleo T., et al. Fog-induced cough with impaired respiratory sensation in congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 2007;176(8):825–832. doi: 10.1164/rccm.200612-1870OC. [DOI] [PubMed] [Google Scholar]
- 56.Pitts T., Bolser D., Rosenbek J., Troche M., Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia. 2008;23(3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plowman E.K., Watts S.A., Robison R., et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia. 2016;31(3):383–390. doi: 10.1007/s00455-015-9687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piirila P., Sovijarvi A.R. Differences in acoustic and dynamic characteristics of spontaneous cough in pulmonary diseases. Chest. 1989;96(1):46–53. doi: 10.1378/chest.96.1.46. [DOI] [PubMed] [Google Scholar]
- 59.American Thoracic Society/European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 60.Man W.D.C., Kyroussis D., Fleming T.A., et al. Cough gastric pressure and maximum expiratory mouth pressure in humans. Am J Respir Crit Care Med. 2003;168(6):714–717. doi: 10.1164/rccm.200303-334BC. [DOI] [PubMed] [Google Scholar]
- 61.Sharpey-Schafer E.P. Effects of coughing on intra-thoracic pressure, arterial pressure and peripheral blood flow. J Physiol. 1953;122(2):351–357. doi: 10.1113/jphysiol.1953.sp005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hebbard G.S., Reid K., Sun W.M., Horowitz M., Dent J. Postural changes in proximal gastric volume and pressure measured using a gastric barostat. Neurogastroenterol Motil. 1995;7(3):169–174. doi: 10.1111/j.1365-2982.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 63.Vanderstappen G., Texter E.C., Jr. Response of the physiologic gastroesophageal sphincter to increased intra-abdominal pressure. J Clin Invest. 1964;43:1856–1868. doi: 10.1172/JCI105059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lasserson D., Mills K., Arunachalam R., Polkey M., Moxham J., Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax. 2006;61(8):699–705. doi: 10.1136/thx.2005.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox I.D., Wallis P.J., Apps M.C., et al. An electromyographic method of objectively assessing cough intensity and use of the method to assess effects of codeine on the dose-response curve to citric acid. Br J Clin Pharmacol. 1984;18(3):377–382. doi: 10.1111/j.1365-2125.1984.tb02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontana G.A., Pantaleo T., Lavorini F., Benvenuti F., Gangemi S. Defective motor control of coughing in Parkinson's disease. Am J Respir Crit Care Med. 1998;158(2):458–464. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 67.Fontana G.A., Pantaleo T., Lavorini F., Mutolo D., Polli G., Pistolesi M. Coughing in laryngectomized patients. Am J Respir Crit Care Med. 1999;160(5 pt 1):1578–1584. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]
- 68.Strohl K.P., Mead J., Banzett R.B., Loring S.H., Kosch P.C. Regional differences in abdominal muscle activity during various maneuvers in humans. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(6):1471–1476. doi: 10.1152/jappl.1981.51.6.1471. [DOI] [PubMed] [Google Scholar]
- 69.McGuinness K., Ward K., Reilly C.C., Morris J., Smith J.A. Muscle activation and sound during voluntary single coughs and cough peals in healthy volunteers: insights into cough intensity. Respir Physiol Neurobiol. 2018;257:42–50. doi: 10.1016/j.resp.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Chellini E., Magni C., Lavorini F., Rucci L., Fontana G.A. Motor features of voluntary cough following partial laryngectomy for glottal carcinoma. Bratisl Lek Listy. 2011;112(3):115–119. [PubMed] [Google Scholar]
- 71.Lavorini F., Fontana G.A., Chellini E., Magni C., Pistolesi M., Widdicombe J. Respiratory expulsive efforts evoked by maximal lung emptying. Chest. 2011;140(3):690–696. doi: 10.1378/chest.10-1084. [DOI] [PubMed] [Google Scholar]
- 72.Doherty M.J., Wang L.J., Donague S., et al. The acoustic properties of capsaicin-induced cough in healthy subjects. Eur Respir J. 1997;10(1):202–207. doi: 10.1183/09031936.97.10010202. [DOI] [PubMed] [Google Scholar]
- 73.Korpas J., Sadlonova J., Vrabec M. Analysis of the cough sound: an overview. Pulm Pharmacol. 1996;9(5-6):261–268. doi: 10.1006/pulp.1996.0034. [DOI] [PubMed] [Google Scholar]
- 74.Murata A., Taniguchi Y., Hashimoto Y., Kaneko Y., Takasaki Y., Kudoh S. Discrimination of productive and non-productive cough by sound analysis. Intern Med. 1998;37(9):732–735. doi: 10.2169/internalmedicine.37.732. [DOI] [PubMed] [Google Scholar]
- 75.Thorpe C.W., Toop L.J., Dawson K.P. Towards a quantitative description of asthmatic cough sounds. Eur Respir J. 1992;5(6):685–692. [PubMed] [Google Scholar]
- 76.Debreczeni L.A., Korpas J., Salat D. Spectral analysis of cough sounds recorded with and without a nose clip. Bull Eur Physiopathol Respir. 1987;23(suppl 10):57S–61S. [PubMed] [Google Scholar]
- 77.Kelemen S.A., Cseri T., Marozsan I. Information obtained from tussigrams and the possibilities of their application in medical practice. Bull Eur Physiopathol Respir. 1987;23(suppl 10):51S–56S. [PubMed] [Google Scholar]
- 78.Korpas J., Sadlonova J., Salat D., Masarova E. The origin of cough sounds. Bull Eur Physiopathol Respir. 1987;23(suppl 10):47S–50S. [PubMed] [Google Scholar]
- 79.Van Hirtum A., Berckmans D. Assessing the sound of cough towards vocality. Med Eng Phys. 2002;24(7-8):535–540. doi: 10.1016/s1350-4533(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 80.Lee K.K., Matos S., Ward K., et al. Sound: a non-invasive measure of cough intensity. BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pavesi L., Subburaj S., Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest. 2001;120(4):1121–1128. doi: 10.1378/chest.120.4.1121. [DOI] [PubMed] [Google Scholar]
- 82.Smith J.A., Ashurst H.L., Jack S., Woodcock A.A., Earis J.E. The description of cough sounds by healthcare professionals. Cough. 2006;2:1. doi: 10.1186/1745-9974-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abeyratne U.R., Swarnkar V., Triasih R., Setyati A. Cough sound analysis: a new tool for diagnosing pneumonia. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5216–5219. doi: 10.1109/EMBC.2013.6610724. [DOI] [PubMed] [Google Scholar]
- 84.Al-Khassaweneh M., Bani Abdelrahman R. A signal processing approach for the diagnosis of asthma from cough sounds. J Med Eng Technol. 2013;37(3):165–171. doi: 10.3109/03091902.2012.758322. [DOI] [PubMed] [Google Scholar]
- 85.Knocikova J., Korpas J., Vrabec M., Javorka M. Wavelet analysis of voluntary cough sound in patients with respiratory diseases. J Physiol Pharmacol. 2008;59(suppl 6):331–340. [PubMed] [Google Scholar]
- 86.Pramono R.X., Imtiaz S.A., Rodriguez-Villegas E. A cough-based algorithm for automatic diagnosis of pertussis. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swarnkar V., Abeyratne U.R., Amrulloh Y.A., Chang A. Automated algorithm for wet/dry cough sounds classification. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:3147–3150. doi: 10.1109/EMBC.2012.6346632. [DOI] [PubMed] [Google Scholar]
- 88.Magni C., Chellini E., Lavorini F., Fontana G.A., Widdicombe J. Voluntary and reflex cough: similarities and differences. Pulm Pharmacol Ther. 2011;24(3):308–311. doi: 10.1016/j.pupt.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 89.Dicpinigaitis P.V., Morice A.H., Birring S.S., et al. Antitussive drugs: past, present, and future. Pharmacol Rev. 2014;66(2):468–512. doi: 10.1124/pr.111.005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryan N.M., Birring S.S., Gibson P.G. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 91.Abdulqawi R., Dockry R., Holt K., et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 92.Birring S.S., Fleming T., Matos S., Raj A.A., Evans D.H., Pavord I.D. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J. 2008;31(5):1013–1018. doi: 10.1183/09031936.00057407. [DOI] [PubMed] [Google Scholar]
- 93.Vertigan A.E., Kapela S.M., Kearney E.K., Gibson P.G. Laryngeal dysfunction in cough hypersensitivity syndrome: a cross-sectional observational study. J Allergy Clin Immunol Pract. 2018;6(6):2087–2095. doi: 10.1016/j.jaip.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 94.Smith J.A., McGarvey L.P.A., Badri H., et al. Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough. Int J Clin Pharmacol Ther. 2017;55(9):712–719. doi: 10.5414/CP202804. [DOI] [PubMed] [Google Scholar]
- 95.Yousaf N., Monteiro W., Matos S., Birring S.S., Pavord I.D. Cough frequency in health and disease. Eur Respir J. 2013;41(1):241–243. doi: 10.1183/09031936.00089312. [DOI] [PubMed] [Google Scholar]
- 96.Kerem E., Wilschanski M., Miller N.L., et al. Ambulatory quantitative waking and sleeping cough assessment in patients with cystic fibrosis. J Cyst Fibros. 2011;10(3):193–200. doi: 10.1016/j.jcf.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 97.Martin Nguyen A., Bacci E., Dicpinigaitis P., Vernon M. Quantitative measurement properties and score interpretation of the Cough Severity Diary in patients with chronic cough. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620915155. 1753466620915155. [DOI] [PMC free article] [PubMed] [Google Scholar]