Abstract
Savoring is an emotion regulation technique that aims to increase, sustain and deepen positive emotion. It has been incorporated into several novel, “positive affect” interventions for anxiety, depression and chronic pain, but has not been studied in a laboratory setting. As such, it is unknown whether savoring can modulate subjective and neural correlates of emotion-processing and whether savoring might exert a persistent effect on stimulus processing (i.e., modulating response at subsequent encounter). Here, 49 participants savored or viewed positive and neutral pictures, before seeing the same pictures again approximately 20 minutes later without instructions to savor (or view) pictures. Subjective valence and arousal ratings and the picture-elicited late positive potential (LPP) were assessed during both tasks. Results showed that savoring increased participant ratings of picture pleasantness and arousal as well as a picture-elicited LPP. Moreover, pictures that had previously been savored continued to elicit higher ratings during the subsequent picture viewing task. A larger LPP was observed for previously savored positive and neutral pictures during an early portion of picture viewing; later on during picture viewing, this effect was limited to positive pictures only (i.e., it was not evident for neutral pictures). Results validate savoring as an effective and durable means of increasing positive emotion and are discussed in the context of a broader emotion regulation literature, which has primarily examined the downregulation of negative picture processing.
Keywords: ERP, late positive potential, LPP, savoring, emotion regulation
1. INTRODUCTION
Savoring is an emotion regulation strategy aimed at increasing, sustaining and deepening positive emotion (Bryant, 1989). More specifically, savoring is a present-moment focused technique used to amplify pleasurable aspects of one’s experience (Bryant et al., 2011a), rather than trying to re-interpret stimuli. In recent years, savoring has been incorporated into novel, positive-affect-based interventions that aim to reduce anxiety or depression by increasing positive emotion. In some studies, these interventions have been found to be just as effective as or even more effective than more traditional therapeutic approaches that aim to regulate negative emotion (Craske et al., 2019; see also, Smith & Hanni, 2019). Moreover, savoring has been found to be helpful in treating chronic pain and substance abuse, by teaching patients to increase pleasant emotional response to everyday events (Garland et al., 2015; Garland & Howard, 2018). Yet despite the promise of savoring as an effective emotion regulation technique in these clinical contexts, savoring has not yet been investigated in a controlled lab context. For example, it is unknown whether savoring can modulate neural and subjective correlates of emotion processing, and whether savoring can exert a lasting change on the processing of stimuli.
Savoring is the process of generating, enhancing and maintaining positive emotion via experiential absorption (Bryant et al., 2011b). Savoring can be accomplished by sensory-perceptual sharpening/luxuriating, which can increase physical and psychological pleasure; marveling, which elicits feelings of awe; and gratitude – i.e., giving thanks for what is good (Jose et al., 2012). Importantly, savoring is a present-moment focused technique, which involves willfully seeking out and attending to pleasurable aspects of the here-and-now (Bryant & Veroff, 2007), rather than trying to change the meaning of stimuli. In its present-moment focus, savoring is similar to mindfulness, a form of mediation (Kabat-Zinn, 1994) that has also been incorporated into recent therapeutic approaches (Chiesa & Serretti, 2011). However, mindfulness differs from savoring because mindfulness aims at acknowledgment and acceptance of all present-moment experiences and thoughts and their transient nature, whereas savoring emphasizes experiential absorption in pleasurable experiences, in particular. Moreover, mindfulness encourages its practitioners to observe their own experiences and thoughts, thereby creating some distance from them (Bishop et al., 2004). Therefore, savoring is similar to mindfulness in promoting increased attention to ongoing events and stimuli, but it is different than mindfulness because it promotes preferential attention to the positive and willful deepening of engagement with what is pleasurable.
To examine the effect of savoring in a lab context, we used the late positive potential (LPP), an event-related potential that has often been used to assess the downregulation of negative emotion. The LPP is typically measured at parietal electrodes, begins approximately 300 ms after stimulus onset and is larger for emotional compared to neutral stimuli (Cuthbert et al., 2000; Hajcak et al., 2010). The LPP persists throughout the duration of stimulus presentation – e.g., 6000 ms or longer (Cuthbert et al., 2000), and is believed to measure motivated attention toward stimuli (Bradley, 2009). Not only is the LPP larger for negative and positive compared to neutral stimuli, but it is also sensitive to more fine-grained distinctions in stimulus salience. For example, the LPP is larger to personally relevant stimuli, such as pictures of relatives or one’s own face or name (Grasso & Simons, 2011; Tacikowski & Nowicka, 2010) and to pictures that have been denoted as targets (Schupp et al., 2007). In addition, the LPP is smaller when participants are asked to reduce their emotional response to a picture (e.g., Hajcak & Nieuwenhuis, 2006). This has been shown using a variety of techniques, including cognitive reappraisal, which involves changing the meaning of stimuli (Hajcak & Nieuwenhuis, 2006; Moser et al., 2009; Parvaz et al., 2012), distraction, which involves thinking of something else (Thiruchselvam et al., 2011; Uusberg et al., 2014) and suppression, which involves inhibiting outward expression of emotion (Cai et al., 2016; Moser et al., 2006).
Yet, the immediate success of an emotion regulation technique may not be the only outcome that matters. For example, in a therapeutic context, there may be benefits to an emotion regulation technique that persists across time to affect the way an individual responds to the same stimulus at a subsequent encounter. Not only would a persistent effect of emotion regulation save the individual from having to enact the same technique again, but potentially, persistently altered emotional responses might generalize to other, similar situations and stimuli. Notably, the LPP has been used to show that the downregulation of negative emotion regulation appears to persist across time. That is, in a passive picture viewing task, negative and neutral pictures that were previously reappraised elicited a smaller LPP when encountered approximately 30 minutes later (MacNamara, Ochsner, et al., 2011). However, the durability of positive emotion upregulation has not been examined.
Though the LPP is typically measured at parietal electrodes (MacNamara, 2018; Suess & Abdel Rahman, 2015), preliminary evidence has suggested that emotion regulation strategies such as reappraisal might also lead to an increase in the frontal LPP (Moser et al., 2014; Shafir et al., 2015). For instance, positive reappraisal of negative pictures has been found to increase the early frontal LPP (750–1050 ms post stimulus onset) and reduce a later, parietal LPP (1000–6000 ms post-stimulus onset; Moser et al., 2014). Similarly, a larger frontal LPP has been found early on (800–1100 ms post-stimulus onset) during reappraisal (i.e., downregulation) of highly arousing negative pictures (compared to distraction and watch trials; Shafir et al., 2015). Therefore, it has been suggested that the frontal LPP might reflect cognitive effort associated with willful modulation of stimulus salience (Moser et al., 2014; Shafir et al., 2015). However, a larger late frontal LPP (e.g., 1000 ms onwards) has also been observed for passive viewing of emotional compared to neutral stimuli (Hajcak et al., 2010, 2011), and for negatively described compared to neutrally described pictures (MacNamara et al., 2009) – i.e., experimental conditions that should not involve increased effort. And indeed, the distribution of the LPP is known to become more frontal over time (Foti et al., 2009; Hajcak et al., 2010). Therefore, one possibility is that early frontal positivities might be associated with cognitive effort, whereas at least in some tasks, the larger late frontal LPP might simply reflect increased stimulus salience.
In comparison to the multitude of studies that have examined the downregulation of negative emotion, there have been relatively few studies that have investigated the upregulation of positive emotion. Two studies found that when participants were permitted to use any technique they wanted to increase their emotional response to positive pictures, both the (parietal) LPP and picture ratings were increased (Baur et al., 2015; Bernat et al., 2011). However, when participants were instructed to use reappraisal to increase their response to positive pictures, the LPP was not increased (Krompinger et al., 2008). Therefore, different emotion regulation strategies might be required when the goal is to increase rather than decrease emotional responding. Identification of additional strategies for increasing neural and subjective response to positive stimuli could also provide alternatives that could be compared to existing strategies (e.g., reappraisal) in terms of their suitability to different situations or individual preference/ability.
In the current study, we sought to determine whether savoring could effectively modulate picture processing when enacted in a lab context. We were also interested in knowing whether savoring would exert a lasting change in emotional response; that is, whether pictures that had previously been savored would be processed differently at a subsequent encounter – a question of relevance to positive affect therapies that aim to exert lasting improvements in patient well-being (e.g., Craske et al., 2019). We hypothesized that savoring would increase the LPP and subjective ratings of picture pleasantness and arousal elicited by positive and neutral pictures1. Furthermore, we thought that these effects would persist across time and would be evident when pictures were presented without instructions to savor, approximately 20 minutes later.
2. METHOD
2.1. Participants
Fifty-two undergraduate students (36 female; M = 18.98, SD = 0.87) participated in the experiment for course credit. Three participants failed to complete one of the tasks; therefore 49 participants (34 female; M = 19, SD = 0.89) were included in the final sample. The sample size was determined by our a priori decision to run the study for a single semester, with the total N resulting from the number of undergraduates who signed up to complete the study during this time. Study procedures were in compliance with the Helsinki Declaration of 1975 (as revised in 1983), and were approved by the Texas A&M University institutional review board.
2.2. Materials
One hundred pictures (50 positive; 50 neutral) were selected from the International Affective Picture System (IAPS; Lang et al., 2005) and Emotional Picture Set (Wessa et al., 2010)2. Pictures were chosen from two databases in order to provide more options for picture selection. These positive and neutral pictures were assigned to savor or view trials (25 positive view, 25 neutral view, 25 positive savor, 25 neutral savor). In order to counterbalance the pictures savored or viewed across participants, two lists of pictures were made. The positive pictures assigned to “positive view” on one list were assigned to “positive savor” on the other list and neutral pictures that were assigned to “neutral view” on one list were assigned to “neutral savor” on the other list. Pictures were displayed in color and filled the monitor screen (which measured 48.26 cm, diagonally). Participants were seated approximately 60 cm from the screen, and the pictures occupied about 40° of visual angle horizontally and vertically. Each participant saw all pictures exactly one time.
The Self-Assessment Manikin 9-point scales (SAM; Bradley & Lang, 1994) were used by participants in both tasks to rate the valence and arousal of each picture. Response options ranged from 1–9, with higher numbers indicating more pleasant valence and more arousing pictures.
2.3. Procedure
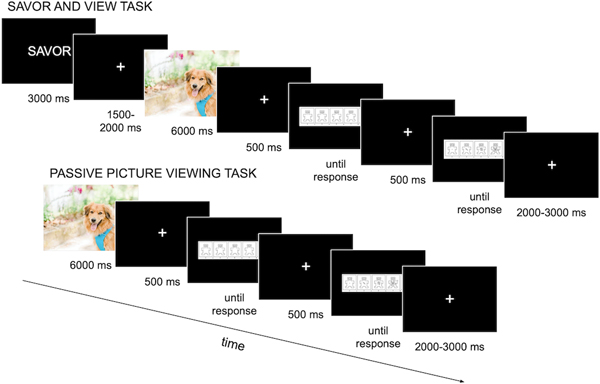
When participants arrived, they were directed to a laboratory room where they were consented and were asked to complete a demographic questionnaire. Next, participants performed a savor and view task, which lasted approximately 25–30 minutes; this was followed by a passive picture viewing task, which lasted approximately 20 minutes. We used two tasks so that participants would receive a break between tasks, to reduce potential carryover effects (e.g., so participants were clear that they were simply being asked to view pictures in the second task and not to change their response in any way), and to allow more time to pass between savoring and picture viewing, thereby increasing confidence in the durability of effects. There were no tasks completed between the savor and view task and the passive picture viewing task. Figure 1 depicts example trials from the savor and view task (top) and the passive picture viewing task (bottom).
Figure 1.
A depiction of sample trials from the savor and passive picture viewing tasks.
2.3.1. Savor and View Task
Participants were instructed that they would be viewing positive and neutral pictures. They were told that before each picture was presented, they would see the word “VIEW” or “SAVOR”. Participants were told that when they saw the word “VIEW”, they should view the upcoming picture as they normally would, without trying to change their emotional response. They were told that if they saw the word “SAVOR”, they should try to savor the positive emotions they felt in response to the picture. Prior to beginning the task, participants were instructed in savoring using the following script:
“In this task, you will be shown neutral and positive pictures. On some trials, you will see the word “SAVOR” before the picture comes onscreen. On these trials, we would like you to try to increase your positive feelings towards the picture, by focusing your attention on and really allowing yourself to feel the positive aspects of the picture. For example, you might focus on the sensory aspects of the picture, like how beautiful the colors or shapes are, how delicious a picture of food looks or how healthy and strong a person looks. You might really draw your attention to these pleasurable aspects of the picture, blocking out other distractions and trying to make the pleasurable feelings last as long as possible. Depending on the picture, you might feel gratitude or awe or you might just take a moment to enjoy life’s simple pleasures (like a beautiful sunset or a smiling face). No matter which of these techniques you use, we’d like you to try to increase and sustain your positive feelings about the picture. This might be more difficult with neutral pictures, but even if it’s a picture of something that’s not particularly pleasant to you, we’d like you to try to find something in it that you can appreciate.”
Subsequently, participants completed four practice trials in which they attempted to savor two positive pictures and two neutral pictures (in random order). Following each practice trial, the experimenter asked the participant how s/he had savored the picture and provided additional examples of how this might be done. For example, for a picture of a diver: “For this picture, you might think about how thrilling or wonderful it would feel to be the diver. Or, you might just admire the grace and beauty of this diver – his health and strength, the blue color of the water, the excitement of being at a sporting event.” And for a picture of a clock: “So this picture is a neutral picture – it’s probably going to be a little bit harder to savor this picture than some of the others. However, the point of savoring is to find anything you can enjoy or experience as pleasurable in the picture, and to really emphasize that. So for the clock, we could think about how it’s so symmetrical, so organized. It’s such a useful machine. It keeps a rhythm; it could be soothing to watch the hands go around – sort of meditative. The black border is a perfect circle. It might also remind us of elementary school, or some other time when we saw clocks like this.” At the end of each practice trial, participants rated the picture using the SAM valence and arousal scales.
After the practice, participants began the real experiment. At the beginning of each trial, the word “SAVOR” or “VIEW” was displayed in white text on a black background for 3000 ms. Following this, a fixation cross was presented for 1500–2000 ms, followed by a positive or neutral picture for 6000 ms. After picture offset, a fixation cross was shown for 500 ms followed by the valence rating (until response), a fixation cross for 500 ms and the arousal rating (until response). The intertrial interval consisted of a white fixation cross presented on a black background, and varied randomly from 2000–3000 ms.
There were two versions of each task, counterbalanced across participants. In both versions, there were five blocks of 20 trials each, resulting in a total of 100 trials across the task. Each block consisted of five positive pictures that were presented on view trials (“positive view”), five positive pictures that were presented on savor trials (“positive savor”), five neutral pictures that were presented on view trials (“neutral view”) and five neutral pictures that were presented on savor trials (“neutral savor”). Within these constraints, trial order was random. Following every block of 20 trials, participants received a self-timed break. Participants were asked to keep their eyes on the screen the entire time.
2.3.2. Passive Picture Viewing Task
After completing the savor and view task, the experimenter instructed the participant in the passive picture viewing task. In this task, participants were asked to simply view positive and neutral pictures and at the end of each trial, to rate each picture using the valence and arousal scales they had used previously. Before beginning the task, participants completed four practice trials (two positive, two neutral), that were shown in random order. During the task, participants viewed all of the pictures that they had seen previously. Therefore, they saw 25 positive pictures that had previously been presented on view trials (“positive view”), 25 pictures that had previously been presented on savor trials (“positive savor”), 25 neutral pictures that had previously been presented on view trials (“neutral view”) and 25 neutral pictures that had previously been presented on savor trials (“neutral savor”).
Each trial began with the 6000 ms presentation of a positive or neutral picture. After picture offset, a fixation cross was shown for 500 ms followed by the valence rating (until response), a fixation cross for 500 ms and the arousal rating (until response). The intertrial interval consisted of a white fixation cross presented on a black background, and varied randomly from 2000–3000 ms.
There were five blocks of 20 trials each, resulting in a total of 100 trials across the task. Each block consisted of five positive view, five neutral view, five positive savor and five neutral savor trials; within these constraints, trial order was random. Following every block of 20 trials, participants received a self-timed break. Participants were asked to keep their eyes on the screen the entire time.
2.4. Electroencephalographic Recording and Data Reduction
Continuous EEG recordings were collected using an ActiCap and the ActiCHamp amplifier system (Brain Products GmbH, Gilching Germany). Thirty-two electrode sites were used based on the 10/20 system. The electrooculogram (EOG) was recorded from four facial electrodes: two that were placed approximately 1 cm above and below the right eye, forming a bipolar channel to measure vertical eye movement and blinks and two that were placed approximately 1 cm beyond the outer edges of each eye, forming a bipolar channel to measure horizontal eye movements. The EEG data were digitized at 24-bit resolution and a sampling rate of 1000 Hz.
EEG data were processed offline using BrainVision Analyzer 2 software (Brain Products GmbH). Data was processed separately for the savor and view task and the passive picture viewing task. For each task, data were segmented for each trial beginning 200 ms prior to stimulus onset and lasting throughout the entire duration of picture presentation (6200 ms in total); baseline correction for each trial was performed using the 200 ms pre-stimulus period. The signal from each electrode was re-referenced to the average of the left and right mastoids (TP9/10) and band-pass filtered with high-pass and low-pass filters of 0.01 and 30 Hz, respectively. Eyeblink and ocular corrections used the method developed by Miller, Gratton, and Yee (1988). Artifact analysis was used to identify a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100-ms intervals. Trials were also inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-to-trial basis.
The LPP during both tasks was scored at 1) a pooling of Fz, Cz, Fc1, and Fc2 (Langeslag & van Strien, 2013; Moser et al., 2014) and 2) a pooling of Pz, Oz, O1, and O2 (MacNamara, 2018; Suess & Abdel Rahman, 2015), from 400–1000 ms and 1000–6000 ms3. Electrode poolings were determined from examining condition headmaps, in which amplitudes were larger at parietal sites in the earlier time window and at fronto-central sites in the later time window. Electrode pools were also selected based on research indicating that the LPP, which is a broadly distributed midline component consisting of multiple, overlapping positivities (Foti et al., 2009), is typically maximal at parietal sites early on during stimulus presentation, and becomes more frontally maximal over time (Foti et al., 2009; Hajcak et al., 2010). Therefore, poolings were chosen so that they would not overlap with each other, but would still capture the full spatial distribution of the LPP as it changed over time.
2.5. Data Analyses
Valence and arousal ratings were analyzed using 2 (picture type: neutral, positive) X 2 (condition: view, savor) repeated measures analyses of variance (ANOVA), performed separately for each task. The LPP was analyzed using 2 (picture type: neutral, positive) X 2 (condition: view, savor) repeated measures ANOVAs, performed separately for each task and for each time window (400–1000 ms and 1000–6000 ms) and electrode pooling (fronto-central and parieto-occipital). The number of rejected trials were also analyzed using a 2 (picture type: neutral, positive) X 2 (condition: view, savor) repeated measures ANOVA. Significant effects were followed up using paired t-tests, as appropriate. All analyses were performed using SPSS statistical software version 25.0 (IBM, Armonk, NY).
3. RESULTS
All data are open and available on the Open Science Framework (https://osf.io/tuc6x/) and we report all conditions, measures, manipulations, and data exclusions. Table 1 presents mean valence and arousal ratings and LPP amplitudes, shown separately for the four conditions in each task. The average percent of trials rejected per participant for the savor and view task ranged from 3% to 8% of trials (Positive Savor: M = 5.68%, SD = 0.09; Positive View: M = 4.99%, SD = 0.08; Neutral Savor: M = 5.08%, SD = 0.08; Neutral View: M = 5.63%, SD = 0.08). The percentage of trials rejected did not vary by picture type, condition or the interaction between picture type X condition, ps > .083. The average percent of trials rejected per participant for the passive picture viewing task ranged from 3% to 5% of trials (Positive Savor: M = 3.53%, SD = 0.05; Positive View: M = 3.72%, SD = 0.06; Neutral Savor: M = 3.87%, SD = 0.06; Neutral View: M = 4.33%, SD = 0.06). The percentage of trials rejected did not vary by picture type, condition or the interaction between picture type X condition, ps > .171.
Table 1.
Mean (SD) LPP and Picture Ratings
| Savor and View Task | Passive Picture Viewing | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Neutral View | Neutral Savor | Positive View | Positive Savor | Neutral View | Neutral Savor | Positive View | Positive Savor | |
| Fronto-central (400–1000 ms; μV) | −0.52 (4.02) | 0.58 (4.10) | 2.58 (4.01) | 4.69 (4.53) | −2.16 (3.73) | −0.88 (4.15) | 2.09 (4.64) | 2.74 (4.56) |
| Parieto-occipital (400–1000 ms; μV) | 2.74 (3.48) | 3.12 (3.85) | 3.66 (3.56) | 4.71 (3.72) | 3.58 (3.59) | 3.73 (3.69) | 4.61 (4.35) | 5.93 (4.39) |
| Fronto-central (1000–6000 ms; μV) | 1.66 (5.62) | 3.96 (5.68) | 3.66 (5.06) | 6.75 (6.02) | −0.40 (5.13) | 0.77 (5.91) | 3.40 (6.52) | 4.09 (6.65) |
| Parieto-occipital (1000–6000 ms; μV) | −2.21 (4.17) | −2.16 (5.07) | −1.46 (4.29) | −0.22 (5.35) | −1.10 (4.01) | −1.03 (3.87) | −0.46 (4.28) | 1.68 (5.25) |
| Valence | 4.42 (1.03) | 5.66 (1.00) | 6.38 (0.94) | 7.14 (0.60) | 4.36 (1.09) | 4.54 (0.93) | 6.47 (0.81) | 6.67 (0.69) |
| Arousal | 2.47 (1.09) | 3.08 (1.34) | 4.22 (1.50) | 4.76 (1.44) | 2.41 (1.14) | 2.46 (1.09) | 4.19 (1.43) | 4.30 (1.44) |
Note: Valence and arousal were rated on the 1 to 9 Likert SAM scales with higher numbers indicating more pleasant and arousing affect.
3.1. Subjective Ratings
3.1.1. Savor and View Task
There was a main effect of picture type for valence ratings, F(1, 48) = 310.24, p < .001, ηp2 = .87, such that positive pictures were rated as more pleasant than neutral pictures. There was also a main effect of condition, F(1, 48) = 81.28, p < .001, ηp2 = .63, indicating that the pictures presented on savor trials were rated as more pleasant than those presented on view trials. These effects were qualified by an interaction between picture type X condition, F(1, 48) = 18.34, p < .001, ηp2 = .28, such that the effect of savoring was larger for neutral pictures than for positive pictures. For arousal ratings, there was also a main effect of picture type, F(1, 48) = 202.87, p < .001, ηp2 = .81, indicating that positive pictures were rated as more arousing than neutral pictures. Additionally, there was a main effect of condition, F(1, 48) = 51.11, p < .001, ηp2 = .52, such that pictures presented in the savor condition were rated as more arousing than pictures presented in the view condition. The interaction between picture type X condition did not reach significance for arousal ratings, p = .509.
3.1.2. Passive Picture Viewing Task
There was a main effect of picture type for valence ratings, F(1, 48) = 306.78, p < .001, ηp2 = .87, such that positive pictures were rated as more pleasant than neutral pictures. There was also a main effect of condition, F(1, 48) = 18.89, p < .001, ηp2 = .28, indicating that participants rated pictures that had been presented in the savor condition as more pleasant than those that had been presented in the view condition. The interaction between picture type X condition did not reach significance for valence ratings, p = .838. For arousal ratings, there was a main effect of picture type, F(1, 48) = 154.52, p < .001, ηp2 = .76, such that positive pictures were rated as more arousing than neutral pictures. There was also a main effect of condition for arousal ratings, F(1, 48) = 3.98, p = .052, ηp2 = .08, indicating that pictures that had been presented in the savor condition were rated as more arousing than pictures that had been presented in the view condition. The interaction between picture type X condition did not reach significance for arousal ratings, p = .5254.
3.2. LPP
3.2.1. Savor and View Task
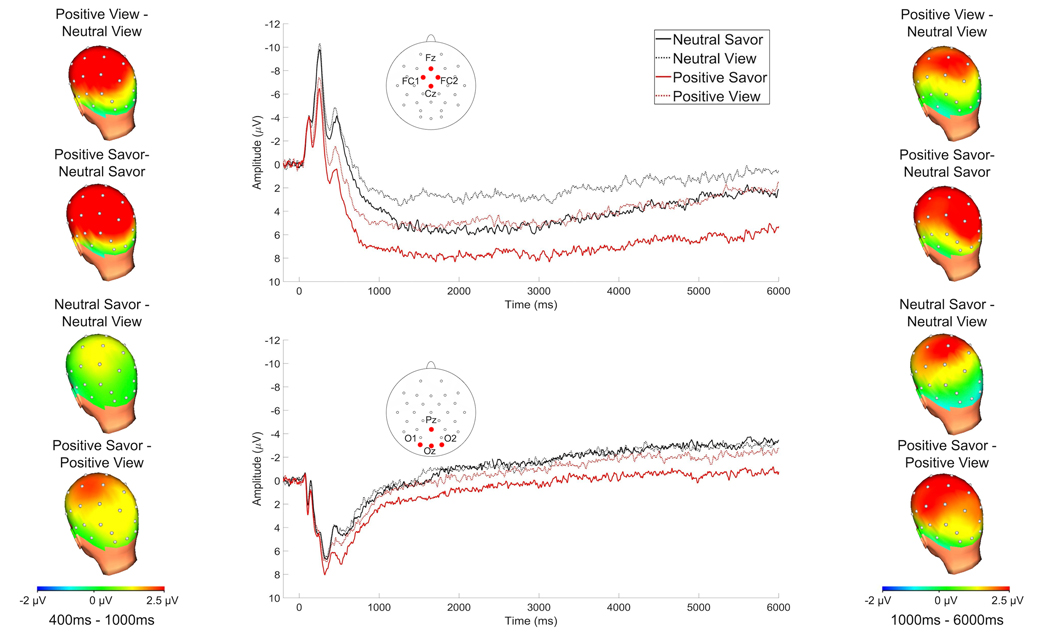
Figure 2 depicts the grand-averaged waveforms for the savor and view task at the fronto-central and parieto-occipital sites at which the LPP was scored, as well as headmaps depicting voltage differences between conditions, from 400 – 1000 ms (left) and 1000 – 6000 ms (right) following picture onset.
Figure 2.
Grand-averaged waveforms elicited by pictures in each of the four conditions in the savor and view task at fronto-central (top center) and parieto-occipital (bottom center) poolings where the LPP was scored and headmaps depicting voltage differences, from 400–1000 ms (left) and 1000–6000 ms (right) after picture onset.
3.2.1.1. 400–1000 ms
At the fronto-central pooling, a main effect of picture type, F(1, 48) = 100.30, p < .001, ηp2 = .68 indicated that positive pictures elicited larger amplitudes than neutral pictures. Additionally, at the fronto-central pooling, a main effect of condition, F(1, 48) = 22.46, p < .001, ηp2 = .32 indicated that pictures presented on savor trials elicited larger a LPP than those presented on view trials. The two -way interaction between picture type X condition did not reach significance at the fronto-central pooling, p = .14. For the parieto-occipital pooling, a main effect of picture type, F(1, 48) = 16.06, p < .001, ηp2 = .25 indicated that positive pictures elicited larger amplitudes than neutral pictures. Moreover, a main effect of condition, F(1, 48) = 8.67, p = .005, ηp2 = .15 indicated that pictures presented on savor trials elicited a larger LPP than those presented on view trials. The two-way interaction between picture type X condition did not reach significance at the parieto-occipital pooling, p = .220.
3.2.1.2. 1000–6000 ms
At the fronto-central pooling, a main effect of picture type, F(1, 48) = 18.45, p < .001, ηp2 = .28 indicated that positive pictures elicited larger amplitudes than neutral pictures. Additionally, at the fronto-central pooling, a main effect of condition, F(1, 48) = 19.41, p < .001, ηp2 = .29 indicated that savored pictures elicited a larger LPP than those presented on view trials. The two-way interaction between picture type X condition did not reach significance at the fronto-central pooling, p = .500. At the parieto-occipital pooling, a main effect of picture type, F(1, 48) = 5.93, p = .019, ηp2 = .11 indicated that positive pictures elicited larger amplitudes than neutral pictures. However, at the parieto-occipital pooling, the main effect of condition did not reach significance, p = .114. The two-way interaction between picture type X condition did not reach significance at the parieto-occipital pooling, p = .222.
3.2.2. Passive Picture Viewing Task
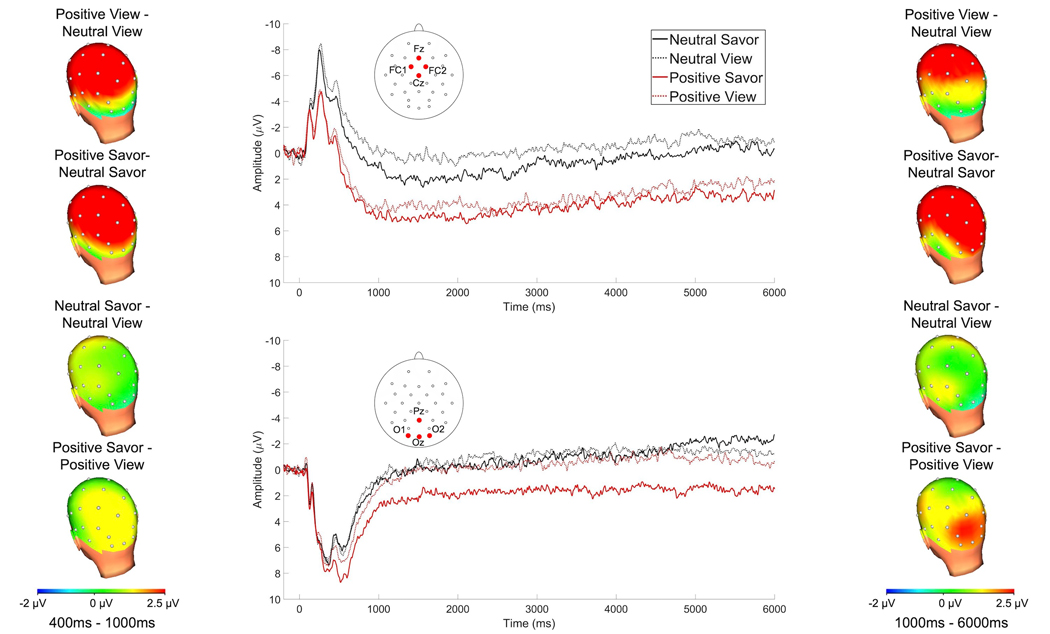
Figure 3 depicts the grand-averaged waveforms for the passive picture viewing task at the fronto-central and parieto-occipital sites at which the LPP was scored, as well as headmaps depicting voltage differences between conditions from 400 – 1000 ms (left) and 1000 – 6000 ms (right) following picture onset.
Figure 3.
Grand-averaged waveforms elicited by pictures in each of the four conditions in the passive picture viewing task at fronto-central (top center) and parieto-occipital (bottom center) poolings where the LPP was scored and headmaps depicting voltage differences, from 400–1000 ms (left) and 1000–6000 ms (right) after picture onset.
3.2.2.1. 400–1000 ms
At the fronto-central pooling, a main effect of picture type, F(1, 48) = 71.31, p < .001, ηp2 = .60 indicated that positive pictures elicited larger amplitudes than neutral pictures. Additionally, at the fronto-central pooling, a main effect of condition, F(1, 48) = 5.85, p = .019, ηp2 = .11 indicated that pictures that had been presented on savor trials elicited a larger LPP than those that had been presented on view trials. The two-way interaction between picture type X condition did not reach significance at the fronto-central pooling, p = .485. For the parieto-occipital pooling, a main effect of picture type, F(1, 48) = 20.95, p < .001, ηp2 = .30 indicated that positive pictures elicited larger amplitudes than neutral pictures. Moreover, a main effect of condition, F(1, 48) = 8.42, p = .005, ηp2 = .15 indicated that pictures that had been presented on savor trials elicited a larger LPP than those that had been presented on view trials. The two-way interaction between picture type X condition did not reach significance at the parieto-occipital pooling, p = .062.
3.2.2.2. 1000–6000 ms
At the fronto-central pooling, a main effect of picture type, F(1, 48) = 42.64, p < .001, ηp2 = .47 indicated that positive pictures elicited larger amplitudes than neutral pictures. However, at the fronto-central pooling, the main effect of condition did not reach significance, p = .107. The two-way interaction between picture type X condition did not reach significance at the fronto-central pooling, p = .722. At the parieto-occipital pooling, a significant main effect of picture type, F(1, 48) = 14.84, p < .001, ηp2 = .24 indicated that positive pictures elicited larger amplitudes than neutral pictures. Moreover, a main effect of condition, F(1, 48) = 7.31, p = .009, ηp2 = .13 indicated that pictures that had been presented on savor trials elicited a larger LPP than those presented on view trials. This was qualified by an interaction between picture type X condition, F(1, 48) = 4.33, p = .040, ηp2 = .08. Follow-up tests indicated that positive pictures that had been presented on savor trials elicited a larger LPP than viewed pictures, t(48) = 3.11, p = .003, but there was no effect of condition for neutral pictures, p = .905.
4. DISCUSSION
To-date, the field of emotion regulation has focused nearly exclusively on the downregulation of negative emotion. Here, we performed the first experimental investigation of savoring, a technique for increasing positive emotion that is based on experiential absorption, and we examined the persistence of savoring’s effects over time (i.e., 20 minutes later). Results showed that savoring increased both subjective and neural response to positive and neutral pictures. Moreover, during a subsequent encounter with the same stimuli (in the absence of instructions to savor), pictures that had previously been savored were rated as more pleasant and more arousing and continued to elicit a larger LPP, compared to pictures that had been not been savored. Therefore, savoring appears to be an effective and lasting means of increasing both positive emotion and the electrocortical processing of pictures.
Prior work that has attempted to increase positive emotion using other emotion regulation techniques has yielded mixed results (Baur et al., 2015; Bernat et al., 2011; Krompinger et al., 2008). Some of these techniques, such as cognitive reappraisal, likely increase cognitive load, because participants are required to generate an alternative interpretation of a picture, and then hold that interpretation in mind for the remainder of picture presentation. Working memory load has been shown to reduce the parietal LPP (MacNamara, Ferri, et al., 2011). Therefore, one possibility is that cognitively demanding techniques such as reappraisal may be contraindicated when the goal is to increase and sustain emotional picture processing. Indeed, in one study in which positive pictures were described in positive compared to neutral terms (i.e., when participants were given stimulus reappraisals instead of generating them themselves), positively described positive pictures did elicit a larger parietal LPP (Liu et al., 2019), suggesting that when reappraisal of positive pictures is not effortful, it may successfully increase picture salience.
In contrast to reappraisal, savoring may be less effortful, because it does not rely on meaning change (Strauss et al., 2016). Therefore, savoring might be effective in increasing positive emotion in part because it does not incur undue cognitive demands. Nonetheless, during the early time window of the savor and view task, we found that savoring increased both the parieto-occipital and fronto-central LPP, the latter of which has been hypothesized to reflect cognitive effort during reappraisal (Moser et al., 2014; Shafir et al., 2015). Moreover, during the later time window, savoring only increased the fronto-central LPP. Therefore, we cannot rule out the possibility that savoring is an effortful emotion regulation technique. Indeed, in the absence of a direct comparison with reappraisal, it is impossible to determine whether savoring is less cognitively demanding, or whether it is more (or less) effective than cognitive reappraisal at increasing positive emotion under certain circumstances. Instead, the current results provide the first evidence that savoring is an effective means of increasing subjective and neural response to pictures and that it provides a viable alternative to other emotion regulation strategies such as reappraisal, whose effectiveness with positive stimuli is questionable.
In future work, it may also be important to compare savoring and mindfulness. Both savoring and mindfulness involve increased attention to the present moment, however only savoring involves an exclusive focus on the positive aspects of experience (Lindsay & Creswell, 2017). Depending on the way in which mindfulness has been operationalized, it has been found to decrease (Zhang et al., 2019) or increase (Egan et al., 2018) the LPP to positive, negative and neutral stimuli. In thinking about how savoring might exert a different effect than mindfulness, we suspect that if participants were asked to savor a negative picture (i.e., seek out and attend to any pleasurable/least negative aspects of the picture, while attempting to increase positive emotion to the picture), a) that the LPP would be reduced, not increased and b) that participants would rate these pictures as less negative. This would be in keeping with our prior work, in which attention to the positive aspects of a negative picture reduced the LPP (Ferri et al., 2013; Hajcak et al., 2013). Therefore, we suspect that savoring would exert a different effect on negative compared to positive pictures (unlike mindfulness). In sum, we believe that while there is some overlap in mechanism between mindfulness and savoring, that these approaches are not equivalent and that savoring is not reducible to increased attention.
For an emotion regulation technique to be effective at improving well-being (e.g., in a clinical context), it should be capable of inducing a lasting change in stimulus processing. Cognitive reappraisal has been found to reduce the processing of negative pictures 30 minutes later (MacNamara, Ochsner, et al., 2011). By contrast, distraction, which may effectively reduce the processing of stimuli in the short-term, appears to increase neural and subjective response upon subsequent encounter with stimuli, perhaps because stimuli seem more salient when they are not processed as deeply/not fully attended at initial encounter (Paul et al., 2016). Therefore, not all emotion regulation techniques that are effective in the short-term may be desirable in the longer-term. Here, pictures that had previously been savored continued to elicit increased ratings of pleasantness and arousal as well as a larger LPP approximately 20 minutes later, demonstrating that savoring exerts a lasting effect on the LPP. Not every task manipulation leaves a lasting effect on the LPP, even with much shorter lapses in time. For example, pictures that elicit a larger LPP in one block of a task because they are denoted as targets do not continue to elicit a larger LPP during subsequent task blocks (e.g., Schupp et al., 2007). Therefore, it is significant that savoring persisted to increase the LPP elicited by pictures presented 20 minutes later (i.e., this is not something that happens in every task). Nonetheless, given recent evidence that other emotion regulation techniques, such as reappraisal, can persist up to 24 hours later (Denny et al., 2015; Hermann et al., 2017), future research may wish to explore the extent of this lasting effect over longer periods of time.
In regards to the topography of the effects observed here, prior work has found that in the absence of a pictorial stimulus, imagining emotional scenarios (MacNamara, 2018; Suess & Abdel Rahman, 2015) also increased the parieto-occipital LPP. Therefore, an increased parieto-occipital LPP might reflect processes that are shared between imagining and savoring, such as the representation of embellished stimulus features that are not evident in the visual percept/cannot be seen. For example, if a participant initially savored a picture of a flower by focusing on the color of the flower, when the participant saw the flower again, s/he might imagine the color of the flower as brighter or deeper than a participant who had initially seen the picture in the view condition. In regards to the specificity of effects in the late time window to positive pictures only during the passive view task, one possibility is that savoring-based enhancements in picture-processing are more memorable because positive stimuli are better suited to savoring than neutral stimuli. Nonetheless, we did observe a main effect of condition during the early time window in the passive view task, indicating that even neutral pictures that had been savored continued to elicit a larger LPP early on during subsequent encounter, though this effect did not persist throughout the duration of stimulus presentation.
When participants were actively engaged in savoring pictures (i.e., during the savor and view task), the LPP was increased for both neutral and positive pictures in both time windows. These results are in line with prior work, which found that the LPP to both emotional and neutral pictures was sensitive to extrinsic manipulations of stimulus salience (i.e., picture descriptions; MacNamara et al., 2009), indicating that effects were not restricted to emotional stimuli. Moreover, during the savor and view task, the effect of savoring on valence ratings was larger for neutral compared to positive pictures. This may be because there was more “room” for savoring to increase valence ratings for neutral pictures; by contrast, ratings for positive pictures may have been constrained by a ceiling effect. Similar results have been observed previously; that is, negative compared to neutral descriptions have been shown to more strongly modulate valence ratings for neutral compared to negative pictures (MacNamara et al., 2009).
While the current results provide the first experimental validation of savoring as a technique for increasing positive emotion, it is worth noting that the current study measured emotional responses to positive and neutral pictures (e.g., self-reported valence and arousal ratings), rather than participants’ ongoing mood or affect. As such, direct connections to positive-affect-based interventions that incorporate savoring are limited. Additionally, a limitation of this study is that we did not ask participants if they used savoring or an alternative technique to increase positive emotion; therefore, we cannot attribute the results to savoring with complete certainty.
In sum, the current results show that savoring is an effective and lasting means of increasing subjective and neural response to positive pictures. Results validate this untested emotion regulation technique, increase confidence in the notion that it is possible to willfully increase positive emotion and deliver a paradigm for investigating a potential mechanism of change in novel psychiatric interventions.
Acknowledgements
A. MacNamara was supported by National Institute of Mental Health grant, K23 MH105553 during preparation of this manuscript. A. MacNamara is a consultant for Aptinyx Inc.
Footnotes
We expected that savoring would modulate the LPP and subjective emotional response to positive and neutral pictures because our prior work using negative pictures had suggested that extrinsic manipulations can increase the salience of neutral pictures. For example, negatively described neutral pictures have been found to elicit larger a LPP and more negative ratings compared to neutrally described neutral pictures (Foti & Hajcak, 2008), and these effects have been found to persist to affect picture viewing 30 minutes later (MacNamara, Ochsner, et al., 2011).
The pictures were positive (008, 013, 020, 027, 047, 056, 076, 1602,2050, 2530, 2005, 331, 4130, 4490, 4599, 4668, 4692, 4697, 5628, 7200, 7280, 7350, 7480, 8186, 8497, 004, 011, 016, 023, 040, 055, 066, 1500, 1722, 2151, 2655, 330, 2311, 4460, 4575, 4604, 4681, 4693, 5202, 071, 7279, 7281, 7472, 8021, 8465) and neutral (081, 7045, 7059, 7037, 123, 7060, 7234, 140, 162, 7233, 176, 2026, 7061, 275, 283, 335, 7313, 363, 5500, 7009, 7950, 7019, 7030, 7023, 7235, 093, 196, 106, 121, 129, 133, 139, 141, 166, 174, 188, 2039, 2270, 280, 299, 338, 5020, 5520, 7016, 7018, 7026, 7032, 7170, 7496). Picture numbers < 1000 are from the EmoPics image set and image numbers > 1000 are from the IAPS image set.
When the late LPP was analyzed during shorter time windows (1000–3500 ms and 3500–6000 ms), results were in line with those presented in the main text.
Unstandardized residuals were used to examine correlations between the valence and arousal ratings and the LPP for both time windows and poolings for each task. Residual scores were used because they allow for better isolation of the variance unique to each condition and effective for analyses involving individual differences (Meyer et al., 2017). The residual scores for each task were created by entering responses to the view condition as a predictor of responses to the savor condition (averaged across picture type), computed separately for each variable (LPP, valence and arousal ratings). Unstandardized residuals from these regressions were saved for each participant and were used in subsequent correlational analyses. For the savor and view task, there were no significant associations between valence or arousal ratings and LPPs (all ps > .08, rs < .26). Similarly, for the picture viewing task, there were no significant associations between valence or arousal ratings and the LPP (all ps > .14, rs < .22).
References
- Baur R, Conzelmann A, Wieser MJ, & Pauli P. (2015). Spontaneous emotion regulation: Differential effects on evoked brain potentials and facial muscle activity. International Journal of Psychophysiology, 96(1), 38–48. 10.1016/j.ijpsycho.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Bernat EM, Cadwallader M, Seo D, Vizueta N, & Patrick CJ (2011). Effects of Instructed Emotion Regulation on Valence, Arousal, and Attentional Measures of Affective Processing. Developmental Neuropsychology, 36(4), 493–518. 10.1080/87565641.2010.549881 [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, & Devins G. (2004). Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice, 11(3), 230–241. 10.1093/clipsy.bph077 [DOI] [Google Scholar]
- Bradley MM (2009). Natural selective attention: Orienting and emotion. Psychophysiology, 46(1), 1–11. 10.1111/j.1469-8986.2008.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Bryant FB (1989). A Four-Factor Model of Perceived Control: Avoiding, Coping, Obtaining, and Savoring. Journal of Personality, 57(4), 773–797. 10.1111/j.1467-6494.1989.tb00494.x [DOI] [Google Scholar]
- Bryant FB, Chadwick ED, & Kluwe K (2011a). Understanding the Processes that Regulate Positive Emotional Experience: Unsolved Problems and Future Directions for Theory and Research on Savoring. International Journal of Wellbeing, 1(1). 10.5502/ijw.v1i1.18 [DOI] [Google Scholar]
- Bryant FB, Chadwick ED, & Kluwe K. (2011b). Understanding the Processes that Regulate Positive Emotional Experience: Unsolved Problems and Future Directions for Theory and Research on Savoring. International Journal of Wellbeing, 1(1), Article 1. 10.5502/ijw.v1i1.18 [DOI] [Google Scholar]
- Bryant FB, & Veroff J. (2007). Savoring: A new model of positive experience (pp. xv, 278). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Cai A, Lou Y, Long Q, & Yuan J. (2016). The Sex Differences in Regulating Unpleasant Emotion by Expressive Suppression: Extraversion Matters. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.01011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, & Serretti A. (2011). Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Research, 187(3), 441–453. 10.1016/j.psychres.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D. (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457–471. 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Denny BT, Inhoff MC, Zerubavel N, Davachi L, & Ochsner KN (2015). Getting Over It: Long-Lasting Effects of Emotion Regulation on Amygdala Response. Psychological Science, 26(9), 1377–1388. 10.1177/0956797615578863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Schmidt J, Hajcak G, & Canli T. (2013). Neural correlates of attentional deployment within unpleasant pictures. NeuroImage, 70, 268–277. 10.1016/j.neuroimage.2012.12.030 [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G. (2008). Deconstructing Reappraisal: Descriptions Preceding Arousing Pictures Modulate the Subsequent Neural Response. Journal of Cognitive Neuroscience, 20(6), 977–988. 10.1162/jocn.2008.20066 [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J. (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, & Howard MO (2015). Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: Exploratory ERP findings from a pilot RCT. Journal of Behavioral Medicine, 38(2), 327–336. 10.1007/s10865-014-9607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, & Howard MO (2018). Enhancing Natural Reward Responsiveness Among Opioid Users Predicts Chronic Pain Relief: EEG Analyses From a Trial of Mindfulness-Oriented Recovery Enhancement. Journal of the Society for Social Work and Research, 9(2), 285–303. 10.1086/697685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, & Simons RF (2011). Perceived parental support predicts enhanced late positive event-related brain potentials to parent faces. Biological Psychology, 86(1), 26–30. 10.1016/j.biopsycho.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Foti D, Ferri J, & Keil A. (2013). The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biological Psychology, 92(3), 447–455. 10.1016/j.biopsycho.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Nieuwenhuis S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6(4), 291–297. 10.3758/CABN.6.4.291 [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-Related Potentials, Emotion, and Emotion Regulation: An Integrative Review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D. (2011). ERPs and the Study of Emotion. The Oxford Handbook of Event-Related Potential Components. 10.1093/oxfordhb/9780195374148.013.0222 [DOI] [Google Scholar]
- Hermann A, Kress L, & Stark R. (2017). Neural correlates of immediate and prolonged effects of cognitive reappraisal and distraction on emotional experience. Brain Imaging and Behavior, 11(5), 1227–1237. 10.1007/s11682-016-9603-9 [DOI] [PubMed] [Google Scholar]
- Jose PE, Lim BT, & Bryant FB (2012). Does savoring increase happiness? A daily diary study. The Journal of Positive Psychology, 7(3), 176–187. 10.1080/17439760.2012.671345 [DOI] [Google Scholar]
- Kabat-Zinn J. (1994). Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. Hachette Books. [Google Scholar]
- Krompinger JW, Moser JS, & Simons RF (2008). Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion, 8(1), 132–137. 10.1037/1528-3542.8.1.132 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual (Technical Report A-8). University of Florida. https://www2.unifesp.br/dpsicobio/adap/instructions.pdf [Google Scholar]
- Langeslag SJE, & van Strien JW (2013). Up-regulation of emotional responses to reward-predicting stimuli: An ERP study. Biological Psychology, 94(1), 228–233. 10.1016/j.biopsycho.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Liu W, Liu F, Chen L, Jiang Z, & Shang J. (2019). Cognitive Reappraisal in Children: Neuropsychological Evidence of Up-Regulating Positive Emotion From an ERP Study. Frontiers in Psychology, 10. 10.3389/fpsyg.2019.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A. (2018). In the mind’s eye: The late positive potential to negative and neutral mental imagery and intolerance of uncertainty. Psychophysiology, 55(5), e13024. 10.1111/psyp.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Ferri J, & Hajcak G. (2011). Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective, & Behavioral Neuroscience, 11(3), 321–331. 10.3758/s13415-011-0036-z [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, & Hajcak G. (2009). Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion, 9(4), 531–543. 10.1037/a0016251 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Ochsner KN, & Hajcak G. (2011). Previously reappraised: The lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience, 6(3), 348–358. 10.1093/scan/nsq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G. (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches: Difference scores and ERPs. Psychophysiology, 54(1), 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, & Simons RF (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43(3), 292–296. 10.1111/j.1469-8986.2006.00402.x [DOI] [PubMed] [Google Scholar]
- Moser JS, Hartwig R, Moran TP, Jendrusina AA, & Kross E. (2014). Neural markers of positive reappraisal and their associations with trait reappraisal and worry. Journal of Abnormal Psychology, 123(1), 91–105. 10.1037/a0035817 [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, & Simons RF (2009). Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology, 46(1), 17–27. 10.1111/j.1469-8986.2008.00721.x [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, & Hajcak G. (2012). Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective & Behavioral Neuroscience, 12(4), 730–740. 10.3758/s13415-012-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Kathmann N, & Riesel A. (2016). The costs of distraction: The effect of distraction during repeated picture processing on the LPP. Biological Psychology, 117, 225–234. 10.1016/j.biopsycho.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, & Hamm AO (2007). Selective Visual Attention to Emotion. Journal of Neuroscience, 27(5), 1082–1089. 10.1523/JNEUROSCI.3223-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir R, Schwartz N, Blechert J, & Sheppes G. (2015). Emotional intensity influences pre-implementation and implementation of distraction and reappraisal. Social Cognitive and Affective Neuroscience, 10(10), 1329–1337. 10.1093/scan/nsv022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, & Hanni AA (2019). Effects of a Savoring Intervention on Resilience and Well-Being of Older Adults. Journal of Applied Gerontology, 38(1), 137–152. 10.1177/0733464817693375 [DOI] [PubMed] [Google Scholar]
- Strauss GP, Ossenfort KL, & Whearty KM (2016). Reappraisal and Distraction Emotion Regulation Strategies Are Associated with Distinct Patterns of Visual Attention and Differing Levels of Cognitive Demand. PloS One, 11(11), e0162290. 10.1371/journal.pone.0162290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suess F, & Abdel Rahman R. (2015). Mental imagery of emotions: Electrophysiological evidence. NeuroImage, 114, 147–157. 10.1016/j.neuroimage.2015.03.063 [DOI] [PubMed] [Google Scholar]
- Tacikowski P, & Nowicka A. (2010). Allocation of attention to self-name and self-face: An ERP study. Biological Psychology, 84(2), 318–324. 10.1016/j.biopsycho.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, & Gross JJ (2011). The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biological Psychology, 87(1), 84–92. 10.1016/j.biopsycho.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Uusberg A, Thiruchselvam R, & Gross JJ (2014). Using distraction to regulate emotion: Insights from EEG theta dynamics. International Journal of Psychophysiology, 91(3), 254–260. 10.1016/j.ijpsycho.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, & Schönfelder S. (2010). EmoPics: Subjektive und psychophysiologische Evaluationen neuen Bildmaterials für die klinisch-bio-psychologische Forschung. Z. Klin. Psychol. Psychother, 1, S11–S77. [Google Scholar]