Abstract
OBJECTIVES AND METHODS:
In September 2018, the government of India banned 328 fixed dose combinations (FDCs), 24 of which are combinations containing topical steroids. To assess what impact can be expected from this regulatory action, we analyzed reports of adverse drug events due to topical corticosteroids at a hospital-based pharmacovigilance center between January 2017 and August 2018.
RESULTS:
Among 34 different steroid-containing FDCs responsible for 485 reports of ADEs with topical steroids, only three preparations, accounting for 50.10% of ADEs, come under the umbrella of the recent ban. Clobetasone propionate (68.87%) and betamethasone (28.45%) were the corticosteroids most frequently associated with adverse events. Most of the steroid preparations (87.84%) had been bought without a prescription for the treatment of dermatophytoses (76.70%). Males (77.73%) were predominantly affected, and nearly half (47.43%) of the patients were between 21 and 30 years of age. Skin atrophy (50.10%), striae (25.54%), and hypopigmentation (19.79%) were the major ADEs.
CONCLUSION:
Nearly half of the cutaneous adverse effects were due to topical steroid combinations which are still widely available over the counter.
Keywords: Adverse effects, fixed dose combinations, self-administration, topical steroids
Introduction
The Central Drugs and Standards Control Organization has banned many fixed dose combinations (FDCs) of topical corticosteroids (TCs). The Indian Association of Dermatologists, Venereologists, and Leprologists have also advocated against topical steroid abuse.[1] Yet, a large number of irrational FDCs of topical steroids with other drugs, especially antibacterials and antifungals have been spared by the ban and are still available without a prescription in India. This encourages their inappropriate use for treatment of cutaneous fungal infections, acne, undiagnosed rashes, and for lightening of skin by prescribers, chemists as well as the general public.[2,3] Topical steroids are known to cause a number of adverse effects including erythema, skin atrophy, striae, acne, photosensitivity, hypertrichosis, rosacea, and talengectiasis.[4,5] A constellation of these features on the face has been termed topical steroid dependent/damaged face (TSDF).[6] Inappropriate use of topical steroid containing creams may also contribute to the upsurge in drug-resistant superficial fungal infections in the country in recent years.[7,8]
We therefore undertook this study to investigate the topical steroid-related adverse drug events (ADEs) at a hospital-based pharmacovigilance center under the Pharmacovigilance Programme of India (PvPI).
Methods
All ADEs associated with topical corticosteroids, reported to a hospital-based pharmacovigilance center in a tertiary care hospital in Central India between January 2017 and August 2018 were included. The cutoff date of August 31, 2018 was chosen for data collection as the Government of India notified a ban on 328 FDCs, including 24 topical preparations containing corticosteroids with effect from September 14, 2018. The ADEs reported were analyzed for nature of adverse effect, topical steroid used, whether prescribed or bought without prescription, and the clinical indication for use of topical steroid. All adverse effects were reported to National Coordinating Centre, PvPI through Vigiflow.
Compliance with ethical standards
The study site is a designated Regional Training Centre for Pharmacovigilance cum Adverse Drug Monitoring Centre under the PvPI. All Adverse Drug Reaction reports received at the center are sent through Vigiflow to the Indian Pharmacopeia Commission, Ghaziabad, which is the National Coordinating Centre for the PvPI. Data used in the study have been collected as a part of the routine functioning of the centre. Patient and reporter identities were kept strictly confidential. Patient consent was obtained before using patient photographs for the study, and measures were taken to mask the face of the patient where applicable. None of the authors have any conflict of interest in the study. No funding was received from any source for the conduct of the study.
Results
A total of 485 ADEs with topical corticosteroids were reported during the study period, which made up 44.21% of total ADEs and 54.62% of cutaneous reactions reported to the ADR monitoring center during the study period. Males were predominantly affected (77.73%). The mean age was 25.44 years, with 47.43% of the patients aged between 21 and 30 years [Table 1]. Skin atrophy (50.10%), striae [25.54%, Figure 1], and hypopigmentation [19.79%, Figure 2] were the major adverse effects reported [Table 2]. TSDF was reported in 46 (9.48%) cases and comprised features such as erythema, acne, hypertrichosis, telangiectasia, hypopigmentation, rosacea, photosensitivity, skin atrophy, or striae [Figure 3]. A number of patients also presented with flaring up of tinea infection, leading to extensive tinea (32 patients) or modification and masking of its features, that is, tinea incognito [34 patients, Figure 4]. Thighs (40.0%) and face (34.43%) were the major sites of these reactions [Table 3]. Clobetasone propionate (68.87%) and betamethasone (28.45%) alone or in various combinations with other drugs were the most frequent suspected agents [Table 4]. The most frequently used single TC containing FDC was a preparation comprising clobetasol propionate 0.05% + ofloxacin 0.75% + ornidazole 2% + terbinafine hydrochloride 1%, which was responsible for adverse effects in 29.69% of the cases. The most common indication for the TCs [Table 5] was dermatophytoses (76.70%). Antifungals were present in 71.18% preparations, with terbinafine (27.85%), miconazole (20.50%), and tolnaftate (19.15%) being the most common agents [Table 6]. There was one FDC, being used by 63 patients, which contained two antifungal agents, that is ketoconazole and tolnaftate. Antibacterials were present in 79.88% of FDCs. Gentamicin (42.55%) and ofloxacin (27.85%) were the most common antibacterials combined with TCs. Ornidazole (27.85%) and clioquinol (iodochlorhydroxyquinoline) were also a part of a number of preparations (23.71%).
Table 1.
Demographic profile of patients with cutaneous adverse effects with topical steroids
| Number of patients (n=485), n (%) | |
|---|---|
| Male | 377 (77.73) |
| Female | 108 (22.27) |
| Total | 485 (100) |
| Age | |
| <21 | 159 (32.78) |
| 21-30 | 230 (47.43) |
| >30 | 96 (19.79) |
| Total | 485 (100) |
Mean age=25.44 years
Figure 1.
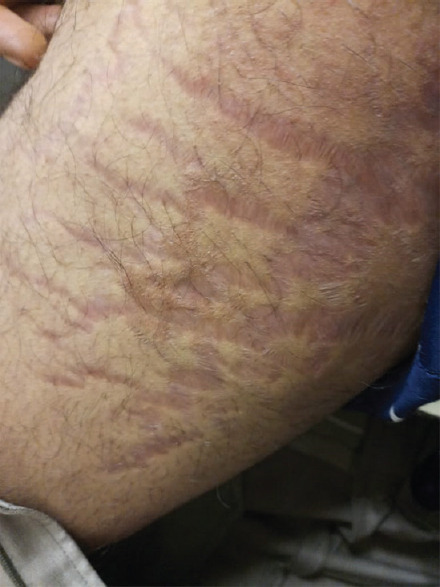
Striae on thighs due to topical steroid use
Figure 2.
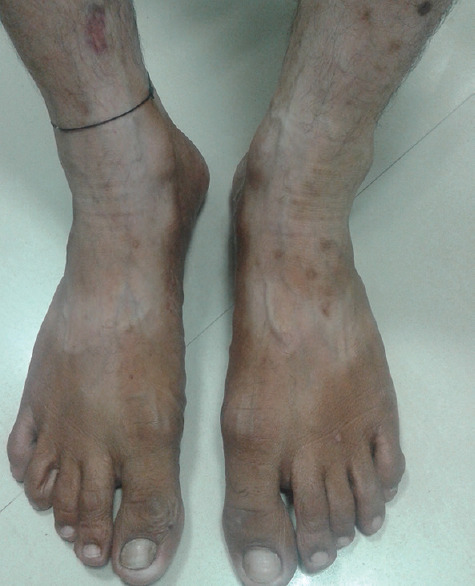
Hypopigmentation of legs and feet due to topical steroid use
Table 2.
Types of adverse drug reactions due to topical steroid - containing preparations
| ADR | Number of patients (n=485), n (%)* |
|---|---|
| Skin atrophy | 243 (50.10) |
| Hypopigmentation | 96 (19.79) |
| Striae | 119 (25.54) |
| Tinea incognito/extensive tinea | 66 (13.61) |
| TSDF | 46 (9.48) |
| Acne | 47 (9.69) |
| Erythema | 43 (8.87) |
| Photosensitivity | 35 (7.22) |
| Other | 102 (21.03) |
*One patient may have more than one ADR. ADR=Adverse drug reaction, TSDF=Topical steroid damaged face
Figure 3.
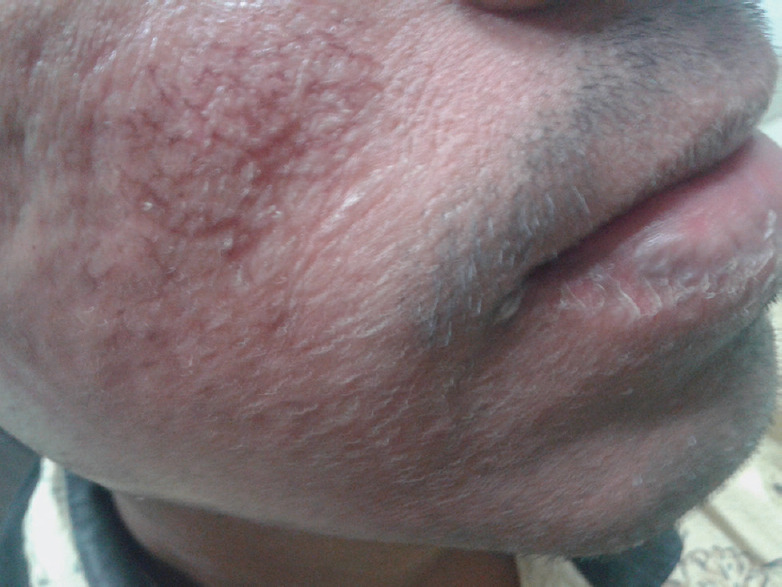
Topical steroid damaged face with skin atrophy, hypopigmentation and telangiectasia
Figure 4.
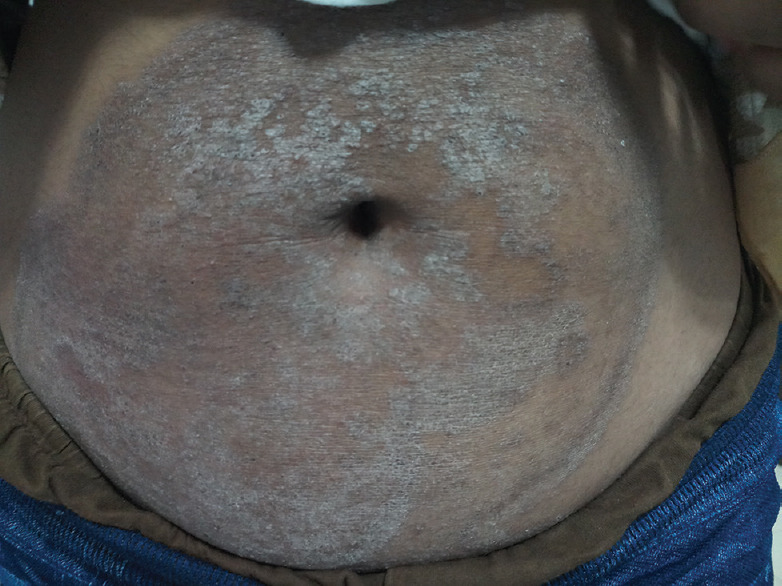
Tinea incognito
Table 3.
Sites of involvement, lag period and outcome of adverse effects due to topical steroid-containing preparations
| Number of patients (n=485), n (%) | |
|---|---|
| Site* | |
| Thighs | 194 (40.00) |
| Face | 167 (34.43) |
| Genital region | 70 (14.43) |
| Rest of lower limbs | 36 (7.42) |
| Trunk or neck | 40 (8.25) |
| Uper limbs | 14 (2.89) |
| Extensive | 68 (14.02) |
| Lag period | |
| <3 months | 220 (45.36) |
| 3 months-1 year | 237 (48.87) |
| >1 year | 28 (5.77) |
| Total | 485 (100) |
| Outcome | |
| Abated fully | 204 (42.06) |
| Abated partially | 57 (11.75) |
| Not abated | 221 (45.57) |
| Unknown | 3 (0.61) |
| Total | 485 (100) |
*Many patient had lesions on more than one site. Mean lag period: 152.03 days (~5 months)
Table 4.
Topical steroid preparations implicated in reported adverse effects
| Number of patients (n=485), n (%)* | |
|---|---|
| Clobetasol propionate 0.05% | 334 (68.87) |
| Alone | 7 (1.44) |
| Combinations | 327 (67.42) |
| Clobetasol propionate with- | |
| Ofloxacin + ornidazole + terbinafine** | 144 (29.69) |
| Gentamicin/neomycin + antifungal (miconazole/clotrimazole) | 64 (13.20) |
| Ketoconazole + tolnaftate gentamicin + clioquinol** | 63 (12.99) |
| Gentamicin/neomycin | 38 (7.84) |
| Salicylic acid | 11 (2.27) |
| Other combinations | 7 (1.44) |
| Betamethasone | 138 (28.45) |
| Alone | 20 (4.12) |
| Combinations | 118 (24.33) |
| Betamethasone with- | |
| Gentamicin/neomycin + antifungal (miconazole/clotrimazole) | 46 (9.48) |
| Gentamicin + tolnaftate + clioquinol** | 36 (7.42) |
| Gentamicin/neomycin | 12 (2.47) |
| Clioquinol | 16 (3.30) |
| Other combinations | 8 (1.65) |
| Mometasone in combinations | 33 (6.80) |
| Other steroids | 12 (2.47) |
| Total | 517# |
*28 patients were using more than one steroid preparation, **Banned by Government of India with effect from September 13, 2018
Table 5.
Indications for use of topical steroids
| Indication | Number of patients (n=485), n (%) |
|---|---|
| Tinea | 372 (76.70) |
| Acne | 45 (9.28) |
| Skin lightening | 28 (5.77) |
| Melasma | 19 (3.92) |
| Other | 22 (4.54) |
| Total | 485 |
Table 6.
Antimicrobials and antifungal agents in topical steroid preparations
| Drug | Number of preparations (n=517), n (%) |
|---|---|
| Types of combinations | |
| Steroids + antifungals + antibiotics | 361 (69.83) |
| Steroids + antibiotics | 413 (79.88) |
| Steroids + antifungals | 368 (71.18) |
| Antibacterials | 413 |
| Gentamicin | 220 (42.55) |
| Ofloxacin | 144 (27.85) |
| Neomycin | 48 (9.28) |
| Nadifloxacin | 1 (0.19) |
| Antifungals | 431 (83.37)* |
| Terbinafine | 144 (27.85) |
| Miconazole | 106 (20.50) |
| Tolnaftate | 99 (19.15) |
| Ketoconazle | 64 (12.38) |
| Clotrimazole | 16 (3.10) |
| Other | 2 (0.39) |
| Antiprotozoals | 249 (48.16) |
| Ornidazole | 144 (27.85) |
| Clioquinol | 115 (23.71) |
*63 preparations contained 2 antifungals (Ketoconazole and Tolnaftate)
Most of the topical steroids had been bought from a chemist without a prescription (87.84%). In 45.36% patients, adverse effects appeared within 3 months, while it took 3 months to 1 year for adverse effects to manifest in 48.87% of cases [Table 3]. In 45.57% of patients, the reactions had not abated, while they abated only partially in 11.75% till last follow-up [Table 3].
Four patients had also received injection triamcinolone in addition to topical steroids. One of these developed Cushingoid facies in addition to skin atrophy and flare of dermatophytosis. There was no case of systemic adverse effect with the use of topical steroids alone.
Discussion
After its March 2016 ban of 344 FDC drugs was stayed by the Delhi High Court later the same year, the Government of India has once again succeeded in prohibiting the use of 328 FDCs on September 14, 2018,[9] citing lack of therapeutic justification. Out of the 328 FDCs banned, 24 are combinations containing topical steroids. While this is a step in the right direction, there are over a thousand brands of topical steroids, most of them combinations products, for sale in India,[2] which are not covered by the ban. These are widely available, that too without a prescription, owing to an ambiguity regarding the exclusion of topical preparations from the list of prescription only or Schedule H drugs in the Drugs and Cosmetics Rules 1945.[10] In our study, we came across 34 different preparations of topical steroids, 29 of which were FDCs, with a large majority of the patients (87.84%) having procured them over the counter. Only three of these FDCs are now banned. These include a preparation containing clobetasol propionate 0.05% + terbinafine 1% + ofloxacin 0.75% + ornidazole 2%, which was the most common TC containing FDC suspected to be responsible for an ADR in 144 (29.69%) cases in our study and has been a widely used TC combination in the country in recent years.[10,11] Two other FDCs which are now banned were an FDC containing clobetasol propionate 0.05% + ketoconazole 2% + tolnaftate 1% + gentamicin 0.1% + clioquinol 1% (63 cases) and another containing betamethasone 0.05% + gentamicin 0.1% + tolnaftate 1% + clioquinol 1% (36 cases). In all, these three preparations made up 50.10% of all cases, which means that half of the adverse effects were due to TC containing preparations which the manufacturers are still free to market.
Another cause of concern is that FDC preparations containing particular topical corticosteroids, antifungals, and antimicrobials have been banned. This can be overcome simply by changing from one TC or antifungal to another, or coming up with another permutation and combination which is just as irrational as the banned FDC and defeats the whole purpose of the ban. An example is Panderm plus (clobetasol propionate 0.05% + terbinafine hydrochloride 1% + olfoxacin 0.75% + ornidazole 2%) which was among the most widely misused TC combination and is now banned. It has already been replaced by Panderm plus plus, which contains clobetasol propionate 0.05% + miconazole nitrate 2% + neomycin sulfate 0.5%, another irrational FDC, but profiting on the existing market niche for Panderm plus. This could be avoided by a blanket ban on preparations containing topical steroids and antifungals and/or antimicrobials.
Clobetasone propionate 0.05%, classified as a super potent steroid,[12] was the most frequent steroid implicated in adverse reactions in our study population, followed by the high potency steroid betamethasone dipropionate 0.05% and its medium potency salt betamethasone valerate 0.1%. The widespread use of potent corticosteroids is reflected in other studies as well.[13]
The use of topical antibacterial agents along with topical corticosteroids is difficult to rationalize. While majority of the steroid preparations were being used for dermatophytoses (76.70%), antibacterials were present in 79.88% of them. Similarly, many of the preparations included antiprotozoal agents, that is, ornidazole (27.85%) or clioquinol (23.71%) even though there is no rationale of their combination.
Skin atrophy (50.10%), striae (25.54%), and hypopigmentation (19.79%) were the common adverse reactions seen in our study, more than reported from other parts of the country.[3,13,14] Resistant and extensive dermatophytoses is another problem, at least partly due to the indiscriminate use of topical steroid and antifungal combinations.[7,8] A related problem is exaggeration of tinea accompanied by modification or masking of its features, which has been termed tinea incognito.[15,16] In our study, 32 patients had a flaring up of tinea infection, leading to extensive tinea, while 34 patients were diagnosed as tinea incognito after TC use.
The term “Topical steroid dependent/damaged face (TSDF)” was introduced by Lahiri in 2008.[6] While a constellation of features has been described as a part of this entity, the common features in our study in patients who were diagnosed with TSDF were erythema, acne, hypertrichosis, telangiectasia, hypopigmentation, rosacea, photosensitivity, skin atrophy, and striae.
More males than females presented with ADRs due to TCs, a finding similar to some studies,[13] but unlike other reports.[3,14] While majority of the men were using steroids for dermatophytoses (68.04% of all patients), a number of males were also using these creams for skin lightening (12 males out of 28 patients who used TC for skin lightening) or acne (24 males out of 45 patients) on the face. Abuse of TC creams for fairness is fairly common not only in India[2,3,17] but also in Africa.[18] Although the original Kligman's formula for melasma or its modifications contained mild steroids such as hydrocortisone, many newer combinations claiming to be modified Kligman's formula contain mometasone, a medium potency steroid.[19] Triple combinations consisting of mometasone furoate 0.1% + hydroquinone 0.2% + tretinoin 0.025% were being used as fairness creams or to help reduce pigmentation in 31 patients in our study.
Although it is advised that super potent topical corticosteroids such as clobetasol propionate not be used for a duration longer than 14 days,[12] the average duration of use was about 5 months (152.03 days) in our study, with maximum duration of use being 2 years. Application of medium or high potency steroids on the face for prolonged, unsupervised periods is an important contributor to the surge in adverse effects with TCs.[20]
On the other hand, while it usually takes 6 months for adverse effects with TCs to appear,[7] a large proportion (45.36%) of the adverse effects reported to our AMC appeared after <6 months of steroid use. This may be due to the fact that most of the cases in our study had been using super potent, high, or medium potency steroids.
Conclusion
The indiscriminate use of topical corticosteroid-containing FDCs continues to be a problem in India. Although many of these FDCs have now been banned by the government, optimism over the impact of the ban on the burden of these adverse effect is tempered by the fact that many irrational preparations have escaped the prohibition, and those banned are already being replaced with new but still irrational combinations. Efforts to reduce inappropriate and prolonged use of topical steroids in irrational combinations are still important and required to decrease the morbidity of adverse effects caused. This can be achieved through increasing the awareness among both, the prescriber and the end user, since much of the use is through self-administration of over the counter products.
Financial support and sponsorship
This study was financially supported by Pharmacovigilance Programme of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to acknowledge Dr. Sanjiv Chaudhary, Additional Professor and the residents of Department of Dermatology, All India Institute of Medical Sciences, Bhopal, for reporting cases of steroid induced adverse effects to the ADR Monitoring Centre. We also take this opportunity to thank the Indian Pharmacopeia Commission, Ghaziabad, National Coordinating Centre, PvPI, for their logistics support of adverse drug reaction monitoring activities at All India Institute of Medical Sciences Bhopal, which has made this study possible.
References
- 1.Nabar K. News of activity report of IADVL's taskforce against topical steroid abuse: Tireless efforts bringing fruits. Indian J Drugs Dermatol. 2015;1:56–7. [Google Scholar]
- 2.Kumar S, Goyal A, Gupta YK. Abuse of topical corticosteroids in India: Concerns and the way forward. J Pharmacol Pharmacother. 2016;7:1–5. doi: 10.4103/0976-500X.179364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraswat A, Lahiri K, Chatterjee M, Barua S, Coondoo A, Mittal A, et al. Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients. Indian J Dermatol Venereol Leprol. 2011;77:160–6. doi: 10.4103/0378-6323.77455. [DOI] [PubMed] [Google Scholar]
- 4.Fisher DA. Adverse effects of topical corticosteroid use. West J Med. 1995;162:123–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Lahiri K, Coondoo A. Topical Steroid Damaged/Dependent Face (TSDF): An entity of cutaneous pharmacodependence. Indian J Dermatol. 2016;61:265–72. doi: 10.4103/0019-5154.182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is antifungal resistance a cause for treatment failure in dermatophytosis: A study focused on tinea corporis and cruris from a tertiary centre? Indian Dermatol Online J. 2018;9:90–5. doi: 10.4103/idoj.IDOJ_137_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Government of India. Directorate of General Health Services. Prohibition of 328 Fixed Dose Combinations by the Central Government Vide Gazette Notification nos. S.O. 4379 (E) to S.O. 4706 (E)-reg. 2018 September 14; [Google Scholar]
- 10.Pande S. Steroid containing fixed drug combinations banned by government of India: A big step towards dermatologic drug safety. Indian J Drugs Dermatol. 2016;2:1–2. [Google Scholar]
- 11.Verma SB. Topical corticosteroid misuse in India is harmful and out of control. BMJ. 2015;351:h6079. doi: 10.1136/bmj.h6079. [DOI] [PubMed] [Google Scholar]
- 12.Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371–8. doi: 10.4103/0378-6323.178903. [DOI] [PubMed] [Google Scholar]
- 13.Meena S, Gupta LK, Khare AK, Balai M, Mittal A, Mehta S, et al. Topical corticosteroids abuse: A clinical study of cutaneous adverse effects. Indian J Dermatol. 2017;62:675. doi: 10.4103/ijd.IJD_110_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bains P. Topical corticosteroid abuse on face: A clinical study of 100 patients. Int J Res Dermatol. 2016;2:40–5. [Google Scholar]
- 15.Dutta B, Rasul ES, Boro B. Clinico-epidemiological study of tinea incognito with microbiological correlation. Indian J Dermatol Venereol Leprol. 2017;83:326–31. doi: 10.4103/ijdvl.IJDVL_297_16. [DOI] [PubMed] [Google Scholar]
- 16.Arenas R, Moreno-Coutiño G, Vera L, Welsh O. Tinea incognito. Clin Dermatol. 2010;28:137–9. doi: 10.1016/j.clindermatol.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Rathi S. Abuse of topical steroid as cosmetic cream: A social background of steroid dermatitis. Indian J Dermatol. 2006;51:154. [Google Scholar]
- 18.Nnoruka E, Okoye O. Topical steroid abuse: Its use as a depigmenting agent. J Natl Med Assoc. 2006;98:934–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Majid I. Mometasone-based triple combination therapy in melasma: Is it really safe? Indian J Dermatol. 2010;55:359–62. doi: 10.4103/0019-5154.74545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coondoo A. Topical corticosteroid misuse: The Indian scenario. Indian J Dermatol. 2014;59:451–5. doi: 10.4103/0019-5154.139870. [DOI] [PMC free article] [PubMed] [Google Scholar]


