Abstract
Transcranial magnetic stimulation (TMS) is a popular modulatory technique for the noninvasive diagnosis and therapy of neurological and psychiatric diseases. Unfortunately, current modulation strategies are only modestly effective. The literature provides strong evidence that the modulatory effects of TMS vary depending on device components and stimulation protocols. These differential effects are important when designing precise modulatory strategies for clinical or research applications. Developments in TMS have been accompanied by advances in combining TMS with neuroimaging techniques, including electroencephalography, functional near-infrared spectroscopy, functional magnetic resonance imaging, and positron emission tomography. Such studies appear particularly promising as they may not only allow us to probe affected brain areas during TMS but also seem to predict underlying research directions that may enable us to precisely target and remodel impaired cortices or circuits. However, few precise modulation strategies are available, and the long-term safety and efficacy of these strategies need to be confirmed. Here, we review the literature on possible technologies for precise modulation to highlight progress along with limitations with the goal of suggesting future directions for this field.
Keywords: Transcranial magnetic stimulation, Modulation strategies, Precise stimulation target, Coil location, Individual treatment paradigm
Introduction
In the last three decades, transcranial magnetic stimulation (TMS) has developed as a popular modulatory technique that allows for the noninvasive diagnosis and therapy of neurological and psychiatric diseases [1–3]. Although current modulation strategies are effective for some patients, many others with similar clinical profiles receive little benefit [4].
TMS is able to cause the rapid depolarization of neurons and alter cortical excitability during (online) and after (offline) the stimulation period [5]. However, the magnitude of current density falls sharply with distance from the cortical surface under general stimulation intensities, inducing cortical fields to a depth of ~ 2 cm and an area of 100–200 mm2 [6]. Although the stimulation target is commonly a single brain region, TMS effects can be mediated via distributed networks [7, 8]. Local effects are produced by action potentials induced in the targeted regions, while remote effects occur when the action potentials of the targeted region propagate to distant regions through polysynaptic connections. Since neurological and psychiatric disorders can be conceptualized as disorders of neural networks, not brain regions, the ability of local effects to propagate to distant regions is important for using TMS in diagnosis and therapy.
The potential value of connectivity-based targeting methods for the TMS treatment of depression has been demonstrated [9, 10], but it is difficult to identify the optimal stimulation target and coil position to obtain the best outcome, since the process is so complex. In addition, there is little consensus about the therapeutic effects in neurological and neuropsychiatric conditions other than depression. One reason for this failure is that precise modulation strategies of TMS have not been sufficiently discussed. Thus, we need to consider possible precise strategies to improve the therapeutic effects of targeted brain modulation.
There are a few review articles on precise stimulation with TMS with a variety of foci, including designing geometric models of the TMS coil [11, 12], improving navigation systems [13–15], optimizing the coil location and orientation [15, 16], and providing new stimulation targets [9, 10, 17–19]. However, investigation of the network mechanisms of TMS and the precise localization of targets, the two most important issues, have not been thoroughly discussed. Moreover, considering all of these in one review might lead to a better understanding than considering any of them alone.
The present review considers the precise modulation for TMS from several different perspectives. First, we focus on the basic description of TMS, including devices, stimulation protocols, and applications in diagnosis, therapy, and exploration, all of which may be useful for optimizing treatment and identifying precise stimulation targets. Then, we review the neuroimaging methodologies that can be integrated with TMS. These techniques may provide a network perspective that could improve the therapeutic effects of TMS and lead to individualized treatments. Finally, we consider several questions for future studies into precise modulation strategies for TMS.
Basic Description of TMS Devices, Stimulation Protocols, and Applications
Components and Principles of TMS Devices
The first TMS device was developed by Barker and colleagues in 1985 [20]. It was based on principles derived from Faraday’s experiment showing that alternating current in a primary circuit is capable of inducing the same current in an isolated secondary circuit if the two circuits are in close proximity. TMS devices consist of a main unit, a group of capacitors, and a stimulation coil. The main unit is used to set the amount of current and releases the current pulse at a given time via specialized circuitry. The capacitors accumulate high loads of electric charge, which are drawn from power supply lines. During TMS, the operator selects a monophasic or diphasic current pulse. Then, the main unit activates an electrical switch and the capacitors send the given amount of charge to the coil. The coil placed above the scalp generates a time-varying magnetic field in the air and head. In the cortex, this magnetic field produces transitory currents to evoke cortical activity.
According to the Biot–Savart–Laplace law, the area of the evoked cortical activity depends on the magnetic field strength and the coil geometry. The magnetic field strength is reduced proportionate to the distance between the coil and the targeted area. Thus, in most clinical TMS treatments, the peak magnetic field strength underneath the coil center is very strong (> 1 Tesla).
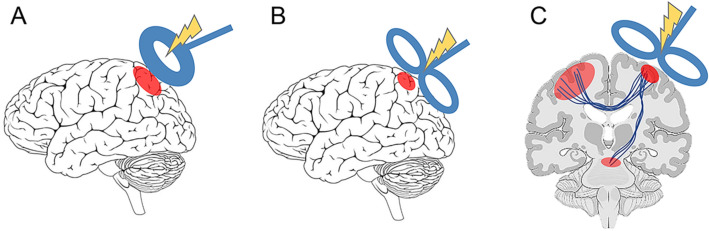
Currently, different coils are designed for different desired stimulation effects, including both the depth of penetration and the distribution across the brain. For the first TMS device, Barker and colleagues designed a circular coil with high-density circular wire windings. The circular coil, which is the simplest design, has a high penetration power, which is useful for peripheral stimulation. However, the stimulation effect is not very focal, and the spatial selectivity is > 4 cm2 (Fig. 1A). Then, a figure-of-eight coil made of two side-by-side round coils, each 25–70 mm in diameter, was designed by Ueno and colleagues [21]. The figure-of-eight coil induces a more localized electrical field at the junction where the two round coils sum, allowing more selective stimulation (1.5–2 cm2) than a circular coil [22] (Fig. 1B).
Fig. 1.
The modulatory effects of circular and figure-of-eight coils in TMS. A The modulation effect of a circular coil has high penetration power, but the activated brain area is not focal. B The modulation effect of a figure-of-eight coil is more focal but has limited penetration. C TMS effects may result from active initiation of action potentials in stimulated neurons or alterations in brain networks.
The strength of the initial current passing through the coil contributes to the strength of the currents induced in the cortical layers. In general, the penetration of the coils is limited since the induced currents are negligible less than a few centimeters from the cortical surface [6]. To penetrate deeper into the cortex, a number of coil models, such as the H-shaped coil, have been designed [23, 24]. A recent study evaluated the clinical outcome of two TMS protocols delivered by an H-shaped coil and a figure-of-eight coil in major depressive disorder [25] and found that the H-shaped coil group had a better response rate and greater reduction in depression severity than the figure-of-eight coil group. However, the superficial cortical layers under an H-shaped coil are exposed to the strongest field [12, 23, 26–28]. Thus, the figure-of-eight coil is still most common for clinical and academic uses.
Modulation Effects of Stimulation Protocols
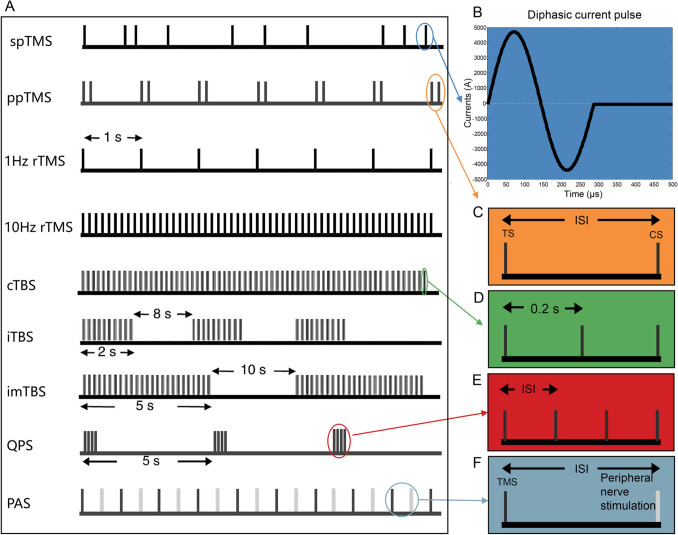
The initial current through TMS coils can be delivered as a single pulse, paired pulses, or a train of pulses (Fig. 2A). Single-pulse TMS (spTMS) produces transitory currents in the cortex and transiently depolarizes neurons. By using the stimulus intensity needed to elicit a change in a patient when the coil is focused on the motor [29] or visual [30, 31] systems as the standard, spTMS has become a standard protocol for selecting the appropriate stimulation intensity required to elicit a change in other brain areas [32].
Fig. 2.
Stimulation protocols for TMS. A The initial current through TMS coils can be delivered as a single pulse, paired pulses, or a train of pulses (modified from ref. [164]). B A diphasic current pulse. C In ppTMS, the interstimulus intervals (ISIs) between the test stimulus (TS) and the conditioning stimulus (CS) can be changed to allow different stimulation effects. D The TBS pattern consists of a burst of three 50-Hz pulses in trains repeated every 200 ms. E QPS consists of repeated trains of four monophasic TMS pulses, and the ISIs can be changed. F PAS consists of repetitive low-frequency pairings of electrical stimulation of a peripheral nerve with TMS over the contralateral M1.
Paired pulse TMS (ppTMS) is commonly applied to assess the excitability of intracortical circuits by stimulating the primary motor cortex (M1) [33]. This approach can result in a decrease or increase in the amplitude of the conditioned motor evoked potentials (MEPs) by changing the interstimulus intervals between the conditioning stimulus and the test stimulus (Fig. 2C) [34–36]. The increase or decrease in the amplitude of the MEP is termed intracortical facilitation or intracortical inhibition, respectively.
Repetitive TMS (rTMS) delivers a train of pulses at equal interstimulus intervals. In addition, trains of rTMS pulses can be applied at different frequencies. rTMS with specific frequencies can induce a persistent modulation of cortical excitability [37–40]. The induced online (during stimulation) and offline (after stimulation) neuromodulatory effects of rTMS have provided insight into the role of specific brain regions in terms of their plasticity and behavior. The conventional finding has been that low-frequency (≤ 1 Hz) rTMS inhibits cortical excitability whereas high-frequency (5–20 Hz) rTMS facilitates cortical excitability [37, 39–43].
rTMS is a family of widely-used neuromodulation techniques. However, the effects induced during or after the period of stimulation are still limited. Several alternative patterned modulation protocols have been used to try to induce plastic changes in the human cortex and have shown differences in their relevance to modulatory effects. Emerging patterned modulation protocols include theta-burst stimulation (TBS), quadripulse TMS (QPS), and paired associative stimulation (PAS) (Fig. 2A). Generally, these protocols have more complex stimulation patterns and induced effects (Fig. 3). TBS and QPS protocols have complex timings and produce longer-lasting effects on specific brain regions. PAS combines TMS with other modulation techniques such as peripheral nerve stimulation and produces facilitatory or inhibitory effects on specific excitatory pathways between two connected brain regions.
Fig. 3.
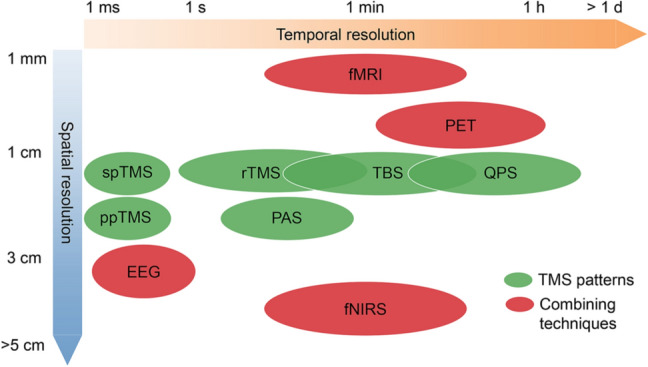
Temporal and spatial resolution for modulatory effects of TMS patterns and neuroimaging techniques, which are helpful for evaluating the online and offline responses to TMS modulation.
The TBS pattern, which consists of a burst of three 50-Hz pulses in trains repeated every 200 ms (Fig. 2D), is designed to mimic theta rhythms [44]. TBS can be delivered in a continuous, intermediate, or intermittent manner. TBS protocols are able to induce longer-lasting effects than conventional high- or low-frequency rTMS paradigms [45–50]. In addition, continuous TBS takes only 20–40 s to apply and decreases cortical excitability, whereas intermittent TBS takes only 3 min total and facilitates cortical excitability.
QPS consists of repeated trains of four monophasic TMS pulses, which are separated by interstimulus intervals of 1.5–1250 ms (Fig. 2E) [51]. Studies have shown that QPS protocols are able to induce longer-lasting effects than conventional rTMS protocols [52]. For example, QPS at short intervals (e.g., 5 ms) facilitate MEPs for > 75 min, whereas QPS at long intervals (e.g., 50 ms) inhibit MEPs for > 75 min.
PAS consists of repetitive low-frequency pairings of electrical stimulation of a peripheral nerve with TMS over the contralateral M1 (Fig. 2F). PAS can be applied to increase or decrease corticospinal excitability by spike-timing-dependent, plasticity-like mechanisms [53]. The change in corticospinal excitability depends on the relative timing of the interaction between the pair of stimuli [54]. PAS increases excitability when the intervals between the stimuli are equal or when an interval is a few milliseconds longer than the N20 latency, which refers to the 20-ms negative response after a median nerve somatosensory-evoked potential. In contrast, PAS suppresses excitability when the stimulus interval is shorter than the N20 latency.
In conclusion, modulatory effects are characterized by important differences in terms of stimulation protocols (Fig. 3), including the frequency indicated by the TMS stimuli per second, intensity determined as a percentage of maximum stimulator output, and the time course of events when TMS occurs with respect to a trigger signal. However, studies evaluating modulatory effects on brain functions and their refractoriness to treatment are few in number. These findings of modulatory effects bring into question whether the therapeutic effect of TMS may be enhanced by changing the stimulation protocols.
Current TMS Applications
Because of its ability to facilitate or inhibit cortical excitation at local or distant sites, TMS has become a diagnostic and therapeutic technique in neurological and psychiatric disorders. In addition, TMS is an exploratory tool in the field of brain functioning.
Use of TMS as a Diagnostic Tool
TMS is particularly useful for studying movement disorders because it is able to probe motor cortical physiology by eliciting MEPs [55]. Thus, it has been used to estimate the degree of intra- and inter-hemispheric and corticospinal connectivity following brain lesions. Several parameters, such as central motor conduction time (CMCT), resting motor threshold, active motor threshold, and the amplitude of the MEPs obtained using high stimulator intensities, have been used to increase the diagnostic sensitivity [56–58]. These parameters seem to have the highest reliability compared with other parameters, such as silent period, short‐interval intracortical inhibition, intracortical facilitation, short‐latency afferent inhibition or facilitation, and MEP recruitment, which are less reliable [56, 57, 59–62].
CMCT is used to estimate the conduction time of corticospinal fibers from M1. It is generally measured when a target muscle is activated in a healthy individual, in this way obtaining a measure of the shortest latency from the cortex to the muscle. Since the spinal motor neuron pool is near the firing threshold, the earliest descending corticospinal volley is the most likely to cause a discharge. In disease states, the CMCT is likely to be prolonged because of the loss of large descending fibers or the demyelination of central motor pathways, leading to impaired summation of the descending volleys at the motor neuron [63].
Studies have concluded that a prolonged CMCT is strong evidence for a clinical diagnosis of Parkinson's disease (PD), or atypical parkinsonism, such as multiple system atrophy or progressive supranuclear palsy [55, 64, 65]. The corticospinal tract is damaged in these diseases, so the CMCT is prolonged. In addition, resting motor threshold, active motor threshold, and MEPs are frequently used to measure motor cortex excitability in PD [66].
A short silent period [67–69] and reduced short‐interval intracortical inhibition [70, 71] are frequently reported in dystonia. However, some studies found that discrepancies in the silent period [72, 73] and short‐interval intracortical inhibition [74, 75] between patients with dystonia and healthy individuals are within the normal range due to great inter-individual variability. In addition, the MEP amplitudes after PAS in dystonia can also be either normal or reduced [76, 77].
TMS has been widely used to diagnose disorders in the motor cortex because it supplies valid and reliable metrics, but it has rarely been used in sensory areas because the metrics are variable and qualitatively different. However, recent studies have shown that ppTMS-induced phosphenes may be used to identify disease severity or prognosis based on cortical excitability and inhibition at either the local or network level in the visual system [35]. In addition to its use for movement disorders, TMS has been reported to be useful along with routine assessment in patients with Alzheimer’s disease and holds great promise in diagnosis and in increasing diagnostic confidence [78] to a degree that is comparable to the well-established amyloidosis biomarkers [79].
Consequently, neurophysiological measures with high reliability can be used as biomarkers for early diagnosis and disease monitoring. However, at least for now, TMS cannot be used to determine the nature or cause of a lesion.
Use of TMS as a Therapeutic Tool
The greatest area of application for TMS currently is in therapy for neuropsychiatric disorders. An evidence-based guideline on the therapeutic use of TMS for conditions such as depression, pain, stroke, movement disorders, and schizophrenia has been established by a group of European experts [3]. The antidepressant effect of high-frequency (HF) rTMS of the left dorsolateral pre-frontal cortex (DLPFC) and its analgesic effect on the M1 contralateral to the painful side are recommended as level A, which means definite efficacy. The antidepressant effect of three TMS protocols, low-frequency (LF) rTMS of the right DLPFC, LF-rTMS of the contralesional M1 in chronic motor stroke, and HF-rTMS of the left DLPFC for the negative symptoms of schizophrenia, are recommended as level B (probable efficacy).
Treatments for several additional conditions have been recommended as level C (possible efficacy): HF rTMS of the bilateral M1 areas on the motor symptoms of PD, continuous TBS of the contralesional left posterior parietal cortex in hemispatial neglect, LF rTMS of the epileptic focus in epilepsy, HF rTMS of the right DLPFC in post-traumatic stress disorder, and HF rTMS of the left DLPFC in cigarette smoking.
A repair model and an interactive model have been proposed to explain the therapeutic effect of TMS [80, 81]. The repair model posits that TMS reshapes the dysfunction caused by disease, but there is no evidence. The interactive model proposes that TMS helps the brain to restore itself and thus promotes natural adaptations to injury or chronic disease. Indeed, the long-term potentiation-like and long-term depression-like effects of offline TMS protocols strengthen and weaken plasticity phenomena respectively by interacting with the brain network [82–84]. Unfortunately, these studies were conducted on a small scale or performed at a single center, so the results are difficult to evaluate.
The therapeutic efficacy of TMS has been tested against a wide range of psychiatric conditions, predominantly depression. Specifically, the efficacy and safety of rTMS in depression has been confirmed by massive-scale multicenter trials and meta-analyses over the last 20 years [45, 85–87]. Neuroimaging evidence has shown that activity in the left DLPFC is reduced in patients with depression [4]. Thus, HF-rTMS is applied in several daily sessions to enhance the activity of the left DLPFC and prolong the offline effects [45]. Recent studies have reported that changes in functional connectivity may predict the clinical outcome of treatment for major depressive disorder and may help to define precise strategies for stimulation [17, 88]. TMS has also been applied to other psychiatric conditions, such as post-traumatic stress disorder [89], addiction disorders [90], obsessive-compulsive disorder [91], and the negative symptoms of schizophrenia [92].
The therapeutic efficacy of TMS has also been tested against neurological conditions, such as migraine headache and other forms of neuropathic pain as well as post-stroke deficits. Studies have shown that spTMS is effective and safe as a treatment for migraine [93]. Thus, the US FDA approved an spTMS device to relieve the pain caused by migraine with aura in 2017. To reduce neuropathic pain, HF-rTMS is applied to the M1 contralateral to the painful side [94]. In addition, prolonged continuous TBS has been showed to have more analgesic effects and a shorter treatment duration than classical HF-rTMS [46], a finding that has considerable clinical potential. Another study has shown that two rTMS protocols, HF-rTMS of the ipsilesional M1 to increase excitability and LF-rTMS of the contralesional M1 to decrease excitability, may be used to improve motor abilities in stroke patients [95]. However, these modalities of stimulation must be considered with caution because the real impact of rTMS in daily practice remains unknown.
A robust series of studies has demonstrated that TMS has significant therapeutic benefit in the therapy of neuropsychiatric disorders. Furthermore, TMS can be applied in other neurological situations [10], such as spasticity, PD, epilepsy, and attention-deficit/hyperactivity disorder. TMS has also been applied to monitor intraoperative neurophysiology during tumor resections [96]. While the breadth of indications for TMS therapy expands, stimulation parameters in routine clinical practice remain to be established and optimized.
Use of TMS as an Exploratory Tool
As an exploratory tool, TMS can been used to map and elucidate cortical function in a variety of neuropathological states because it can directly manipulate cortical sites to establish causal relationships. When TMS is applied to stimulate some brain areas, the output shows a clear signature. For example, TMS over the motor cortex [29, 97] and the visual cortex [35] causes a twitch and induces phosphenes, respectively. The stimulation effects on the motor cortex can be assessed by the resulting muscle responses, which can be recorded by electromyography. In addition, TMS effects in the visual cortex have been assessed by average phosphene sizes in a recent study [35]; this may provide a valid and reliable method for measuring cortical excitability and inhibition in the visual cortex.
However, many regions, such as the prefrontal cortex, the occipital face area, and the inferior frontal gyrus, are behaviorally silent after TMS and do not produce an immediately observable response. Electrophysiological and neuroimaging techniques [98–101] have been used to evaluate the online and offline responses to TMS modulation. Thus, TMS can be coupled with electrophysiological and neuroimaging techniques to probe the anatomical and functional interactions causally. A recent study explored the feedback from the secondary visual cortex (V2) to V1 during contextual modulation by testing participants and using offline TMS-fMRI and showed that the feedback was mostly inhibitory [102], a finding that corroborates recent reports on monkey electrophysiology [103].
Although the above TMS applications have made great progress, the TMS technique still has low clinical treatment efficacy [104] and undesirable covariates in experimental situations due to the failure to provide consistent stimulation, which can reduce the statistical effectiveness of scientific findings. However, there is potential to improve its efficacy further if more precise TMS strategies can be designed and studied. Moreover, combining neuroimaging techniques is the way to reveal the modulatory effects of TMS, and this is important for designing precise modulatory strategies for clinical or research applications.
Combining Neuroimaging Techniques to Reveal the Modulatory Effects of Precise TMS
Suprathreshold stimulation of M1 with spTMS elicits MEPs. While studies have benefited from knowing about cortical excitability quantified by the amplitude of MEPs, measuring the physiological response of many regions of therapeutic and psychiatric interest other than M1 has not been easy. In these regions, neuroimaging techniques are helpful for evaluating the online and offline responses to TMS stimulation [105]. Thus, these techniques not only provide a practical solution for closing the loop between locating the appropriate brain regions and choosing the optimal modulation systems but also potentially enable the identification of optimal treatment paradigms by examining individual responses [106].
Integrating TMS with neuroimaging methodologies has revealed different spatial or temporal effects of TMS (Fig. 3). These methodologies include the EEG, positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (fNIRS).
Combining TMS with EEG
Combining TMS with EEG can track the temporal dynamics of brain activity and thus be used to evaluate the effective time-resolved connectivity. This technique has a high temporal resolution of a few milliseconds. In addition, a large number of scalp sites can be sampled by using EEG.
EEG signals may be applied to record a linear projection of the postsynaptic currents that are indirectly induced by TMS with high temporal resolution and can thus be used to evaluate local cortical excitability and effective connectivity in the nervous system during TMS [98]. TMS–EEG is able to convey precise temporal information about the order in which connected cortical areas are activated, thereby enabling the defining of the causal interactions between two brain areas within functional networks and identifying whether the interactions are excitatory or inhibitory [107]. In addition, combining TMS–EEG with structural neuroimaging provides an opportunity to activate cortical regions at a more precise spatial resolution.
However, the strong magnetic field produced by TMS induces an electric field in the EEG electrodes, nearby skin, muscles, or other conductors and thereby activates muscle and nerve [108]. These factors can generate large-amplitude artifacts in the EEG signals. A tapping sensation or a loud click during TMS can generate sensory-evoked potentials or auditory-evoked potentials, which can also generate artifacts.
Recently, some technologies, such as dedicated TMS-compatible EEG equipment, online or offline noise removal techniques, and a number of control conditions, have been provided to minimize such noise [109]. Taking the above points into account, TMS–EEG allows the evaluation of the spatio-temporal pattern of neural activity from the cortical neurons (but not from deep-brain structures), although it mainly provides measures of effective connectivity in the time domain.
Combining TMS with fMRI
fMRI and TMS are increasingly popular techniques that can be used to non-invasively measure brain connectivity in human subjects. Collecting fMRI data during TMS experiments provides a high spatial resolution and a reduced temporal resolution of a few seconds or minutes.
fMRI is an important method for the in vivo investigation of cognitive processes in the human brain by detecting changes associated with blood flow and oxygenation and relies on the fact that blood flow and neural activity are coupled. fMRI uses the blood-oxygen-level dependent (BOLD) contrast to map neural activity in the brain. The basis for BOLD contrast is that paramagnetic deoxyhemoglobin possesses a strong magnetic moment, which can be compared with diamagnetic oxyhemoglobin. The presence of deoxyhemoglobin in a capillary causes a susceptibility difference in and around blood vessels and surrounding tissue. This difference can be measured by appropriate MR imaging sequences.
In addition to detecting BOLD responses in response to specific stimuli, there are two kinds of fMRI: task-related and resting-state fMRI. Task-related fMRI is applied to detect BOLD changes when subjects are performing a task if the brain is experiencing a perturbation, such as that caused by TMS. This method can identify the cortical areas that are activated by the event, thus revealing the functional anatomy [98]. Resting-state fMRI is applied to detect low-frequency fluctuations in the BOLD signal (usually <0.1 Hz) and is generally applied while the subject is relaxing. This method is able to identify the synchronization between different brain regions, thus revealing the functional connectivity and networks.
TMS can be delivered during the process of acquiring fMRI data (online TMS–fMRI) or before/after fMRI (offline TMS–fMRI). Earlier research demonstrated that the online TMS–fMRI method can greatly advance our understanding of the immediate and rapid TMS-induced changes in cortical networks [110]. Although the feasibility of this method has also been demonstrated [100, 111], there are challenges. The most crucial challenge in online TMS–fMRI experiments is that they are significantly affected by the signal-to-noise ratio due to adverse interactions between the strong magnetic field of the TMS and that of fMRI. fMRI and MRI use the same scanner, which generates a strong magnetic field and radio waves to produce an image of the blood flow in the brain. The large magnets and gradients of an MRI scanner can induce a reverse current in the TMS coils, which makes the entire process quite uncontrolled. Therefore, to achieve a proper combination process, several methods have been proposed, such as using special TMS coils constructed from non-magnetic materials [100] or using specialized TMS coils with a thin dispersion of weakly-ferromagnetic stainless-steel foil attached to the back [112]. In contrast, the offline TMS–fMRI method is easily applied because the TMS and fMRI are separated in time and space. Moreover, offline fMRI is generally used to guide the target cortical areas. For example, a virtual lesion is often used to navigate the fMRI-identified brain regions [113].
Combining TMS with fNIRS
Combining TMS with fNIRS enables the measurement of changes in cortical hemoglobin concentrations based on the optical properties of the investigated medium. Its temporal resolution is lower than EEG and higher than PET and fMRI. However, TMS–fNIRS enables researchers to easily study both the online and offline effects of TMS because optically-measured fNIRS signals are not intrinsically subject to electromagnetic interference.
The fNIRS technique takes advantage of the optical window to provide a non-invasive measurement. The term optical window refers to the natural transparency of tissue to near-infrared light (650–900 nm). Because of the ability of cortical hemoglobin to absorb light, the chromophore concentration can be estimated by changes in light intensity. Thus, neural activity during or after TMS can be measured by fNIRS through hemodynamic changes due to their relatively tight coupling with neural activation [106].
Although the fNIRS technique is not susceptible to electromagnetic interference, care should be taken to ensure that individual fNIRS systems are noise-free during the TMS operation and properly shielded to prevent hardware damage from electromagnetic interference [101].
Combining TMS with PET
PET is a nuclear imaging technique that uses radioactive tracers and gamma ray detection to visualize and measure the tissue concentrations of molecules of interest. This technique has allowed researchers to measure changes in neurotransmitters and synaptic activity in the stimulated region or remote areas (anatomically and functionally connected areas) [98]. In particular, PET radiotracers that bond to dopamine receptors have been studied and widely used to study DA release [114].
Based on the concurrent use of PET, TMS can be used to directly assess the connectivity of cortical-subcortical neural networks [115]. Combining TMS with PET enables the localization of TMS-related changes in cerebral blood flow with high sensitivity and to detect such changes equally well in cortical and subcortical structures, which reveals the connectivity of cortical-subcortical neural networks. Thus, PET imaging can enhance our understanding of the underlying neural mechanisms of TMS and brain connectivity [99].
PET has been used to investigate the exact regions of TMS activation [116–118], a process that is helpful for gaining a better understanding of the factors that result in TMS effects. These studies have provided evidence that the orientation of the cortical columns in the sulcal banks in relationship to the direction of the TMS-induced electric field vector is a significant factor. By using this evidence, we may design more precise coil placement methods that can be used for most TMS applications.
Summary and Future Directions for Improving Precise TMS Strategies
In the previous section, we analyzed possible strategies for improving the precision of TMS, all of which have made distinct contributions. Technological and methodological advances in TMS allow for a greater stimulation effect. Furthermore, the integration of TMS with neuroimaging methodologies can reveal the spatial and temporal effects of TMS.
However, the existing TMS procedures are still limited in the precision of their spatial and temporal targeting. Reproducibility in identifying brain stimulation targets, identifying coil locations, and locating the TMS coil is also a major issue. These unaddressed issues for precise stimulation require future study. The factors that most need to be further investigated can be divided into identifying brain stimulation targets, identifying coil locations, locating the TMS coil, individualizing the TMS treatment paradigm, and recording intra-individual responses (Fig. 4).
Fig. 4.
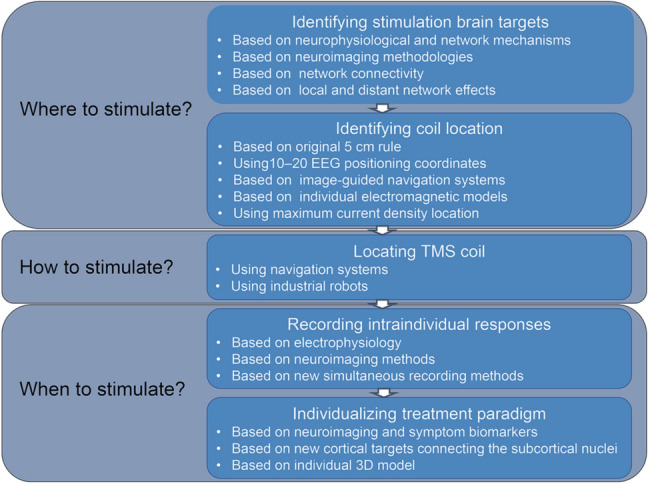
Flow diagram for identifying potential precise TMS strategies.
Identifying Currently Unknown Optimal Stimulation Sites May Facilitate More Precise Targets for Brain Stimulation
The therapeutic efficacy of rTMS against psychiatric and neurological conditions, such as depression [85] and migraine [93], has been tested in many studies. Studies have reported an association between lesions in the left DLPFC and depression [119, 120]. Thus, protocols for applying rTMS to the left DLPFC have received USFDA approval for therapeutic clinical use in depression. However, the views about treatment targets based on focal brain lesions differ [17, 19]. The network connectivity and neurophysiological and network-related mechanisms of TMS effects may help to decide new and more precise brain stimulation targets. Identifying currently unknown optimal stimulation sites may facilitate more precise stimulation.
Network Connectivity May Help to Identify New Brain Stimulation Targets
Many studies have investigated whether network connectivity helps in identifying accurate stimulation targets [10, 121–124]. Whether rTMS produces both local and distant network effects has been discussed. To evoke changes at a distance, the effects must be conveyed through anatomical and functional network connectivity that links different regions of the same cerebral circuit [99, 125–128]. The location of the distant activated areas may be predicted by information about connectivity between the initial stimulation target and distant brain regions [129]. Thus, information about network connectivity may improve our knowledge of the regions that will be activated under rTMS and thus suggest potential targets for stimulation. For example, rTMS may relieve depression by modulating functional connectivity in cortical networks, so new targets may be recognized based on knowledge of functional connectivity. More precise atlases of network connectivity, such as the human Brainnetome atlas [130], should provide more accurate targets. Recent studies have demonstrated that lesion locations associated with depression can be mapped to a specific circuit [131–133]. Moreover, stimulation sites aligned with brain lesions mapped to the depression circuit are effective for improving post-stroke depression. This depression circuit may help identify stimulation targets that map to the depression circuit rather than to a single region.
Neurophysiological Mechanisms of TMS Effects May Help to Decide More Precise Targets for Brain Stimulation
TMS techniques are used to activate neuronal firing temporarily and reversibly in a targeted brain region or in distant regions connected with the targeted region [134, 135] (Fig. 1C). TMS generally interferes with brain function, creating a virtual lesion, but it may augment cortical activity in some situations. Animal studies have provided several mechanisms to explain the modulation effects, including that TMS actively initiates action potentials, modifies membrane resting potentials and thresholds, changes synaptic connectivity, affects timing dynamics of cellular gating components, and influences channel properties leading to subsequent alterations in spontaneous activity [136, 137]. While each of these perspectives has provided a unique contribution, they converge in several important aspects.
In general, online TMS effects are thought to result from an active initiation of action potentials in the stimulated neurons [138] and networks [135, 139]. However, offline TMS effects may result from either cortical facilitation or cortical inhibition. TMS protocols are thought to alter the long-term excitability of stimulated cells and networks following stimulation [6, 140, 141]. The long-term effects are often described as long-term potentiation-like or long-term depression-like, with increased or decreased synaptic strength lasting minutes or more [82–84]. However, there is still significant debate as to the mechanisms by which offline TMS alters activity [27, 142–145]. An appealing hypothesis is that these long-term effects originate from changes in synaptic plasticity [146]. Synaptic plasticity is the ability of a synapse to modulate its synaptic strength in response to changes in neuronal activity, an important neurochemical foundation of learning and memory [147, 148]. The expression of long-term potentiation and long-term depression either respectively strengthens or weakens the strength of synaptic tranmisssion [9, 27, 149]. Indeed, many studies have shown that rTMS induces long-term changes in glutamatergic neurotransmission to principal neurons [145, 150]. The pivotal role of post-synaptic Ca2+ in determining whether a glutamatergic synapse is potentiated or depressed has also been investigated [146, 151–153]. Although TMS-induced plasticity shares certain properties reminiscent of N-methyl-D-aspartate glutamatergic plasticity, this association must be taken with caution since direct demonstrations of the physiological mechanisms in humans have not been made.
It is increasingly recognized that alterations in brain networks may play the same role in neural and psychiatric disease as alterations in brain region and may be the reason for many of the behavioral manifestations of such diseases [154]. In general, human brain connectivity can be divided into anatomical connectivity and functional network connectivity. Characterizing anatomical network connectivity predominantly relies on diffusion tensor imaging (DTI), a neuroimaging technique that enables the measurement of the restricted diffusion of water molecules along white matter fiber tracks. Functional network connectivity is predominantly obtained using EEG, PET, fMRI, or fNIRS. TMS can be used to perturb human brain connectivity, and these neuroimaging techniques can, then, be used to reveal the spatial and temporal effects of TMS. These types of human neuroimaging studies have revealed that TMS effects may change the plasticity of networks in the brain, both their synaptic plasticity and their network plasticity. Synaptic plasticity may improve the functioning of the brain at the synaptic level. Similar to synaptic plasticity, network plasticity could result from remodeling other levels of brain organization. Such plastic changes may improve the functioning of the brain by modifying its structure and altering the connection between functional networks [155, 156].
Structural modifications are similar to the macroscopic changes that have been induced in training experiments [157, 158], in that they reflect the rapid adjustment of neuronal systems at the cellular level. Several neuroimaging studies have suggested that TMS relieve neurological or psychiatric disorders by modulating abnormal structural and functional connectivity in cortical networks [39, 128, 159–162]. It is thought that TMS modulates synaptic strength in both the local region and functionally connected regions [38, 163–165]. Thus, these studies suggest that structural and functional networks can be alternative targets that can activate the targeted brain region.
In summary, human neuroimaging studies have provided strong evidence that structural and functional networks can be modulated by TMS and may provide new stimulation targets. However, the neurophysiological mechanisms of the modulatory effects have not yet been demonstrated, and more direct evidence from human studies is lacking.
The Optimal Position for the Coil Still Needs to Be Determined by Future Studies
Various strategies have been implemented to determine the coil location. These methods include following: the original 5–cm rule [166], using 10–20 EEG positioning coordinates [167], and applying image-guided navigation systems [168]. The premise of these strategies is that the region directly underneath the center of the TMS coil is the most likely to be activated and induce action potentials. Hence, the TMS coil is typically positioned on the area of the scalp nearest to the brain target. However, the activated area is not necessarily underneath the center of the TMS coil [169]. A method for projecting the center of gravity has also been used to predict the activated areas. Critical principles related to tissue-specific conductance and boundary effects should also be considered [170].
Many studies have estimated the distribution of the TMS‐induced electrical fields using individual electromagnetic models to guide coil positioning [97, 171, 172]. However, these methods cannot define the exact stimulation coil location and orientation, so researchers can only choose a coarse coil position in which the induced electric field at the stimulation target seems to be maximal. A recent study investigated the electrical field strength and optimal TMS coil orientation and used them to generate an atlas [173] that is useful for obtaining effective stimulation. This computational study confirmed that the electromagnetic model is useful for identifying an accurate coil position. However, the optimal positioning of the coil still needs to be developed in future studies.
Studies have proposed that the brain region with the maximum induced current is most likely to be maximally activated [174, 175]. Specifically, currents have their maximum impact at the axonal boundaries or bends in the fibers of individual neurons, such as the axon–soma and axon–bouton boundaries, which result in geometric discontinuities. The location of the maximum current density may be predicted via mathematical modeling, depth electrode recordings, phantom studies, imaging studies, and electromagnetic modeling [9, 137, 176–180]. To date, electromagnetic modeling has been the primary method for predicting cortical locations with current density maxima obtained by solving Maxwell's equations. Using this approach, investigators may be able to find a relationship between the cortical locations and the optimal coil location and orientation in future work. If treatment targets of rTMS do prove their worth, it will be important to develop an optimization algorithm based on the coil location and orientation. Therefore, optimizing the coil location and orientation may be a new strategy for improving the therapeutic effectiveness of rTMS.
However, the technical limitations of precise stimulation include the quality of the MRI and fMRI, the way the MRI/fMRI are combined with TMS, and the accuracy of the electrical field modeling [181–183]. With the development of imaging technologies, image-processing algorithms, and numerical methods [170, 184, 185], several groups have calculated the induced electrical field in an anatomically realistic head model obtained from MRI and DTI data [13, 186–188]. MRI data are used to rebuild a tailored finite element head model and DTI data are used to map anisotropic conductivity information [189]. This technique offers the opportunity to track axonal fibers to further improve neuro-navigational procedures. It should be noted, however, that although complex models offer the possibility of increasing coil targeting, it comes at a significant computational cost. Recently, deep neural network models [190] and a realistic five-compartment head model [191] have been applied to estimate the induced electrical field; these may be useful for real-time, high-precision navigation.
The Precision of Coil Location Positioning by Navigation Systems Still Needs to Be Improved
Navigation systems have been developed to facilitate the use of rTMS, in order to ensure that the stimulating coil targets the correct anatomical or functional landmarks [181]. An infrared camera in the navigation system is used to detect trackers placed on the stimulating coil and on a headband worn by the subject, ensuring the consistency of coil placement [192, 193]. In addition, navigation systems guide the positioning of the TMS coil by working in cooperation with a computational model and neuroimaging data to identify the brain stimulation target and coil location [194]. Although precision has been improved substantially by using navigated rTMS, the coil targeting accuracy is still limited to ~6 mm.
Currently, few industrial robots are applied in navigation systems to guarantee precise coil placement and to achieve repeatable stimulation results. Robotized positioning of the coil offers some advantages over conventional navigation systems (function-guided, stereotactic-guided, or image-guided) in that it is tightly controlled based on the surface reconstruction of the individual brain and shows high intra-individual reproducibility. Because of its high degree of automatization, it is also investigator-independent.
Individual TMS Treatment Paradigms Should Be Determined
In recent years, there has been increased emphasis on individualized treatment because intrinsic factors, including genetics, gender, age, brain morphology, and brain connectivity, may contribute to individual variability. As a result, individual outcomes of rTMS treatment may be variable. If new rTMS protocols (stimulation target, coil location, and locating coil) do prove their worth, it will become important to individualize the rTMS treatment paradigm.
However, designing patient-specific treatment paradigms is a monumental task. Approaches to accomplishing this task vary mainly in the information sources used for brain target and coil localization and the methods for integrating the information. Broadly speaking, the information sources fall into three categories. First, recent studies have suggested that neuroimaging [195, 196], a model of neurocircuit dysfunction [168], and clinical symptom scales [197] predict the outcomes of rTMS treatment. Therefore, individual brain targets may be refined in the future based on neuroimaging and symptom biomarkers. The effective stimulation target, such as cortical regions connecting to subcortical nuclei, may be determined by performing tractography on an individual’s DTI data. Furthermore, it is important to use an individual 3-D model to simulate the induced electrical field, using patients’ MRI or DTI data to build it. Using such techniques can improve individual coil target localization.
Real-time Intra-individual Responses Should Be Recorded to Provide More Accurate Stimulation Procedures
There is a small but certain degree of intra-individual variability that is reflected in within-person changes during repeated measurements. Thus, a neuroscientist must be aware of real-time individual responses to accurately plan the stimulation procedures. Electrophysiology, neuroimaging methods, and new simultaneous recording methods may help neuroscientists to solve this problem.
Electrophysiological and neuroimaging methods have been used to study the interactions between intracortical, cortico-cortical, and cortico-subcortical regions during TMS modulation. However, these methods may generate artifacts, so they usually cannot be used to simultaneously record individual responses, as discussed in the above section. A recent in vivo study recorded the single-neuron activity of alert non-human primates within 1 ms after the TMS pulse [198]. A TMS coil was designed that enabled direct acquisition of neuronal signals in awake monkeys. Application of these tools may facilitate the refinement of experimental and treatment protocols. Furthermore, techniques that can simultaneously record individual responses provide a practical solution for closing the loop between locating the appropriate brain regions and choosing the optimal modulation systems and can thus potentially enable clinicians to identify optimal treatment paradigms using intra-individual responses.
Conclusions
Precise TMS modulation is an intensive area of ongoing neuromodulation application aimed at identifying precise brain targets and identifying TMS coil locations, locating the TMS coil precisely, and providing individual and intra-individual TMS treatment paradigms. The authors hope that the studies discussed above will provide insights that merit consideration in designing and carrying out precise TMS modulation. Further research is required to determine the effects of different modulation strategies on the safety and efficacy of precise stimulation. However, although the characteristics of the human brain pose challenges for the design and execution of precise TMS strategies, the unique opportunity these modalities offer for achieving exact special and temporal effects is unmatched.
Acknowledgements
This review was supported by the Chinese Academy of Sciences, Science and Technology Service Network Initiative (KFJ-STS-ZDTP-078), the National Natural Science Foundation of China (31620103905), the Science Frontier Program of the Chinese Academy of Sciences (QYZDJ-SSW-SMC019), and the National Key R&D Program of China (2017YFA0105203). We would like to thank Ming Song, Lingzhong Fan, Nianming Zuo, Zihui Qi, and Hao Liu for their help with manuscript preparation. And we appreciate Drs. Edmund F. and Rhoda E. Perozzi for editing assistance.
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Gangliang Zhong, Email: gangliang.zhong@ia.ac.cn.
Zhengyi Yang, Email: zhengyi.yang@nlpr.ia.ac.cn.
Tianzi Jiang, Email: jiangtz@nlpr.ia.ac.cn.
References
- 1.Chalah MA, Ayache SS. Noninvasive brain stimulation and psychotherapy in anxiety and depressive disorders: A viewpoint. Brain Sci. 2019;9:E82. doi: 10.3390/brainsci9040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Agata F, Peila E, Cicerale A, Caglio MM, Caroppo P, Vighetti S, et al. Cognitive and neurophysiological effects of non-invasive brain stimulation in stroke patients after motor rehabilitation. Front Behav Neurosci. 2016;10:135. doi: 10.3389/fnbeh.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ. A study of the pattern of response to rTMS treatment in depression. Depress Anxiety. 2016;33:746–753. doi: 10.1002/da.22503. [DOI] [PubMed] [Google Scholar]
- 5.Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- 6.Wagner T, Rushmore J, Eden U, Valero-Cabre A. Biophysical foundations underlying TMS: Setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex. 2009;45:1025–1034. doi: 10.1016/j.cortex.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrillon G, Sollmann N, Kurcyus K, Razi A, Krieg SM, Riedl V. The physiological effects of noninvasive brain stimulation fundamentally differ across the human cortex. Sci Adv. 2020;6:eaay2739. doi: 10.1126/sciadv.aay2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent Theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014;34:12049–12056. doi: 10.1523/JNEUROSCI.1776-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson MT, Fulcher BD, Fung PK, Robinson PA, Fornito A, Rogasch NC. Biophysical modeling of neural plasticity induced by transcranial magnetic stimulation. Clin Neurophysiol. 2018;129:1230–1241. doi: 10.1016/j.clinph.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Buckner RL, Liu HS, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez LJ, Goetz SM, Peterchev AV. Design of transcranial magnetic stimulation coils with optimal trade-off between depth, focality, and energy. J Neural Eng. 2018;15:046033. doi: 10.1088/1741-2552/aac967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WeissLucas C, Tursunova I, Neuschmelting V, Nettekoven C, Oros-Peusquens AM, Stoffels G, et al. Functional MRI versus navigated TMS to optimize M1 seed volume delineation for DTI tractography. A prospective study in patients with brain tumours adjacent to the corticospinal tract. Neuroimage Clin. 2017;13:297–309. doi: 10.1016/j.nicl.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pommier B, Vassal F, Boutet C, Jeannin S, Peyron R, Faillenot I. Easy methods to make the neuronavigated targeting of DLPFC accurate and routinely accessible for rTMS. Clin Neurophysiol. 2017;47:35–46. doi: 10.1016/j.neucli.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: A comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koponen LM, Nieminen JO, Mutanen TP, Stenroos M, Ilmoniemi RJ. Coil optimisation for transcranial magnetic stimulation in realistic head geometry. Brain Stimul. 2017;10:795–805. doi: 10.1016/j.brs.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ning LP, Makris N, Camprodon JA, Rathi Y. Limits and reproducibility of resting-state functional MRI definition of DLPFC targets for neuromodulation. Brain Stimul. 2019;12:129–138. doi: 10.1016/j.brs.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MD, Liu HS, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downar J, Daskalakis ZJ. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 2013;6:231–240. doi: 10.1016/j.brs.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 21.Ueno S, Tashiro T, Harada K. Localized stimulation of neural tissues in the brain by means of a paired configuration of time-varying magnetic fields. J Appl Phys. 1988;64:5862–5864. [Google Scholar]
- 22.Jalinous R. Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:10–25. doi: 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24:31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- 24.Levkovitz Y, Roth Y, Harel EV, Braw Y, Sheer A, Zangen A. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2730–2744. doi: 10.1016/j.clinph.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 25.Filipčić I, Šimunović Filipčić I, Milovac Ž, Sučić S, Gajšak T, Ivezić E, et al. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res. 2019;114:113–119. doi: 10.1016/j.jpsychires.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–370. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ren C, Tarjan PP, Popović DB. A novel electric design for electromagnetic stimulation—the Slinky coil. IEEE Trans Biomed Eng. 1995;42:918–925. doi: 10.1109/10.412658. [DOI] [PubMed] [Google Scholar]
- 29.Franza M, Sorrentino G, Vissani M, Serino A, Blanke O, Bassolino M. Hand perceptions induced by single pulse transcranial magnetic stimulation over the primary motor cortex. Brain Stimul. 2019;12:693–701. doi: 10.1016/j.brs.2018.12.972. [DOI] [PubMed] [Google Scholar]
- 30.Konen CS, Haggard P. Multisensory parietal cortex contributes to visual enhancement of touch in humans: A single-pulse TMS study. Cereb Cortex. 2014;24:501–507. doi: 10.1093/cercor/bhs331. [DOI] [PubMed] [Google Scholar]
- 31.Silvanto J. Is primary visual cortex necessary for visual awareness? Trends Neurosci. 2014;37:618–619. doi: 10.1016/j.tins.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khammash D, Simmonite M, Polk TA, Taylor SF, Meehan SK. Probing short-latency cortical inhibition in the visual cortex with transcranial magnetic stimulation: A reliability study. Brain Stimul. 2019;12:702–704. doi: 10.1016/j.brs.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirota Y, Sommer M, Paulus W. Strength-duration relationship in paired-pulse transcranial magnetic stimulation (TMS) and its implications for repetitive TMS. Brain Stimul. 2016;9:755–761. doi: 10.1016/j.brs.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Battaglia F, et al. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161:114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- 38.Ma QY, Geng Y, Wang HL, Han B, Wang YY, Li XL, et al. High frequency repetitive transcranial magnetic stimulation alleviates cognitive impairment and modulates hippocampal synaptic structural plasticity in aged mice. Front Aging Neurosci. 2019;11:235. doi: 10.3389/fnagi.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards G, Agosta S, Herpich F, Contò F, Parrott D, Tyler S, et al. Prolonged neuromodulation of cortical networks following low-frequency rTMS and its potential for clinical interventions. Front Psychol. 2019;10:529. doi: 10.3389/fpsyg.2019.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 41.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 42.Beynel L, Appelbaum LG, Luber B, Crowell CA, Hilbig SA, Lim W, et al. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies. Neurosci Biobehav Rev. 2019;107:47–58. doi: 10.1016/j.neubiorev.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 44.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 45.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of Theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 46.Moisset X, Goudeau S, Poindessous-Jazat F, Baudic S, Clavelou P, Bouhassira D. Prolonged continuous Theta-burst stimulation is more analgesic than ‘classical’ high frequency repetitive transcranial magnetic stimulation. Brain Stimul. 2015;8:135–141. doi: 10.1016/j.brs.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 47.de Martino E, Fernandes AM, Galhardoni R, De Oliveira Souza C, De Andrade DC, Graven-Nielsen T. Sessions of prolonged continuous Theta burst stimulation or high-frequency 10 hz stimulation to left dorsolateral prefrontal cortex for 3 days decreased pain sensitivity by modulation of the efficacy of conditioned pain modulation. J Pain. 2019;20:1459–1469. doi: 10.1016/j.jpain.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Lowe CJ, Manocchio F, Safati AB, Hall PA. The effects of Theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: A systematic review and meta-analysis. Neuropsychologia. 2018;111:344–359. doi: 10.1016/j.neuropsychologia.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten years of Theta burst stimulation in humans: Established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Chung SW, Hill AT, Rogasch NC, Hoy KE, Fitzgerald PB. Use of Theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;63:43–64. doi: 10.1016/j.neubiorev.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Hamada M, Terao HR, Shirota Y, Nakatani-Enomoto S, Furubayashi T, et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. 2008;586:3927–3947. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura K, Groiss SJ, Hamada M, Enomoto H, Kadowaki S, Abe M, et al. Variability in response to quadripulse stimulation of the motor cortex. Brain Stimul. 2016;9:859–866. doi: 10.1016/j.brs.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 54.Ziemann U, Ilić TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Brown KE, Lohse KR, Mayer IMS, Strigaro G, Desikan M, Casula EP, et al. The reliability of commonly used electrophysiology measures. Brain Stimul. 2017;10:1102–1111. doi: 10.1016/j.brs.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Hermsen AM, Haag A, Duddek C, Balkenhol K, Bugiel H, Bauer S, et al. Test-retest reliability of single and paired pulse transcranial magnetic stimulation parameters in healthy subjects. J Neurol Sci. 2016;362:209–216. doi: 10.1016/j.jns.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 58.Pellegrini M, Zoghi M, Jaberzadeh S. The effect of transcranial magnetic stimulation test intensity on the amplitude, variability and reliability of motor evoked potentials. Brain Res. 2018;1700:190–198. doi: 10.1016/j.brainres.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Cacchio A, Cimini N, Alosi P, Santilli V, Marrelli A. Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clin Neurophysiol. 2009;120:414–419. doi: 10.1016/j.clinph.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- 61.Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Gonzalez Rothi LJ. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin Neurophysiol. 2006;117:1037–1046. doi: 10.1016/j.clinph.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Beaulieu LD, Flamand VH, Massé-Alarie H, Schneider C. Reliability and minimal detectable change of transcranial magnetic stimulation outcomes in healthy adults: A systematic review. Brain Stimul. 2017;10:196–213. doi: 10.1016/j.brs.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider SA, Talelli P, Cheeran BJ, Khan NL, Wood NW, Rothwell JC, et al. Motor cortical physiology in patients and asymptomatic carriers of parkin gene mutations. Mov Disord. 2008;23:1812–1819. doi: 10.1002/mds.22025. [DOI] [PubMed] [Google Scholar]
- 65.Benussi A, Dell'Era V, Cantoni V, Ferrari C, Caratozzolo S, Rozzini L, et al. Discrimination of atypical parkinsonisms with transcranial magnetic stimulation. Brain Stimul. 2018;11:366–373. doi: 10.1016/j.brs.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Latorre A, Rocchi L, Berardelli A, Bhatia KP, Rothwell JC. The interindividual variability of transcranial magnetic stimulation effects: Implications for diagnostic use in movement disorders. Mov Disord. 2019;34:936–949. doi: 10.1002/mds.27736. [DOI] [PubMed] [Google Scholar]
- 67.Filipović SR, Ljubisavljević M, Svetel M, Milanović S, Kacar A, Kostić VS. Impairment of cortical inhibition in writer's cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–170. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- 68.Chen R, Wassermann EM, Caños M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- 69.Edwards MJ, Huang YZ, Wood NW, Rothwell JC, Bhatia KP. Different patterns of electrophysiological deficits in manifesting and non-manifesting carriers of the DYT1 gene mutation. Brain. 2003;126:2074–2080. doi: 10.1093/brain/awg209. [DOI] [PubMed] [Google Scholar]
- 70.Huang YZ, Trender-Gerhard I, Edwards MJ, Mir P, Rothwell JC, Bhatia KP. Motor system inhibition in dopa-responsive dystonia and its modulation by treatment. Neurology. 2006;66:1088–1090. doi: 10.1212/01.wnl.0000214304.03105.f4. [DOI] [PubMed] [Google Scholar]
- 71.Gilio F, Currà A, Inghilleri M, Lorenzano C, Suppa A, Manfredi M, et al. Abnormalities of motor cortex excitability preceding movement in patients with dystonia. Brain. 2003;126:1745–1754. doi: 10.1093/brain/awg188. [DOI] [PubMed] [Google Scholar]
- 72.Kojovic M, Pareés I, Kassavetis P, Palomar FJ, Mir P, Teo JT, et al. Secondary and primary dystonia: Pathophysiological differences. Brain. 2013;136:2038–2049. doi: 10.1093/brain/awt150. [DOI] [PubMed] [Google Scholar]
- 73.Stinear CM, Byblow WD. Task-dependent modulation of silent period duration in focal hand dystonia. Mov Disord. 2005;20:1143–1151. doi: 10.1002/mds.20514. [DOI] [PubMed] [Google Scholar]
- 74.Ganos C, Ferrè ER, Marotta A, Kassavetis P, Rothwell J, Bhatia KP, et al. Cortical inhibitory function in cervical dystonia. Clin Neurophysiol. 2018;129:466–472. doi: 10.1016/j.clinph.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Rona S, Berardelli A, Vacca L, Inghilleri M, Manfredi M. Alterations of motor cortical inhibition in patients with dystonia. Mov Disord. 1998;13:118–124. doi: 10.1002/mds.870130123. [DOI] [PubMed] [Google Scholar]
- 76.Kang JS, Terranova C, Hilker R, Quartarone A, Ziemann U. Deficient homeostatic regulation of practice-dependent plasticity in writer's cramp. Cereb Cortex. 2011;21:1203–1212. doi: 10.1093/cercor/bhq204. [DOI] [PubMed] [Google Scholar]
- 77.Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. A reflection on plasticity research in writing dystonia. Mov Disord. 2014;29:980–987. doi: 10.1002/mds.25908. [DOI] [PubMed] [Google Scholar]
- 78.Padovani A, Benussi A, Cantoni V, Dell'Era V, Cotelli MS, Caratozzolo S, et al. Diagnosis of mild cognitive impairment due to Alzheimer's disease with transcranial magnetic stimulation. J Alzheimers Dis. 2018;65:221–230. doi: 10.3233/JAD-180293. [DOI] [PubMed] [Google Scholar]
- 79.Benussi A, Alberici A, Ferrari C, Cantoni V, Dell'Era V, Turrone R, et al. The impact of transcranial magnetic stimulation on diagnostic confidence in patients with Alzheimer disease. Alzheimers Res Ther. 2018;10:94. doi: 10.1186/s13195-018-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terranova C, Rizzo V, Cacciola A, Chillemi G, Calamuneri A, Milardi D, et al. Is there a future for non-invasive brain stimulation as a therapeutic tool? Front Neurol. 2018;9:1146. doi: 10.3389/fneur.2018.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noda Y, Silverstein WK, Barr MS, Vila-Rodriguez F, Downar J, Rajji TK, et al. Neurobiological mechanisms of repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex in depression: A systematic review. Psychol Med. 2015;45:3411–3432. doi: 10.1017/S0033291715001609. [DOI] [PubMed] [Google Scholar]
- 82.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 83.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the Hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 86.O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Reply regarding “efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial”. Biol Psychiatry. 2010;67:e15–e17. doi: 10.1016/j.biopsych.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 87.Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry. 2017;74:143–152. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 88.Corlier J, Wilson A, Hunter AM, Vince-Cruz N, Krantz D, Levitt J, et al. Changes in functional connectivity predict outcome of repetitive transcranial magnetic stimulation treatment of major depressive disorder. Cereb Cortex. 2019;29:4958–4967. doi: 10.1093/cercor/bhz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammoud MZ, Milad MR. Symptom changes in posttraumatic stress disorder and major depressive disorder after transcranial magnetic stimulation: Mechanisms of where and how in the brain. Biol Psychiatry. 2018;83:200–202. doi: 10.1016/j.biopsych.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 90.Hanlon CA, Dowdle LT, Henderson JS. Modulating neural circuits with transcranial magnetic stimulation: Implications for addiction treatment development. Pharmacol Rev. 2018;70:661–683. doi: 10.1124/pr.116.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou DD, Wang W, Wang GM, Li DQ, Kuang L. An updated meta-analysis: Short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. J Affect Disord. 2017;215:187–196. doi: 10.1016/j.jad.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 92.Hasan A, Wobrock T, Guse B, Langguth B, Landgrebe M, Eichhammer P, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22:857–864. doi: 10.1038/mp.2016.161. [DOI] [PubMed] [Google Scholar]
- 93.Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373–380. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- 94.Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, et al. Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain. 2015;156:1601–1614. doi: 10.1097/j.pain.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Charalambous CC, Bowden MG, Adkins DL. Motor cortex and motor cortical interhemispheric communication in walking after stroke: The roles of transcranial magnetic stimulation and animal models in our current and future understanding. Neurorehabil Neural Repair. 2016;30:94–102. doi: 10.1177/1545968315581418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diehl CD, Schwendner MJ, Sollmann N, Oechsner M, Meyer B, Combs SE, et al. Application of presurgical navigated transcranial magnetic stimulation motor mapping for adjuvant radiotherapy planning in patients with high-grade gliomas. Radiother Oncol. 2019;138:30–37. doi: 10.1016/j.radonc.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 97.Bungert A, Antunes A, Espenhahn S, Thielscher A. Where does TMS stimulate the motor cortex? combining electrophysiological measurements and realistic field estimates to reveal the affected cortex position. Cereb Cortex. 2017;27:5083–5094. doi: 10.1093/cercor/bhw292. [DOI] [PubMed] [Google Scholar]
- 98.Hallett M, di Iorio R, Rossini PM, Park JE, Chen R, Celnik P, et al. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clin Neurophysiol. 2017;128:2125–2139. doi: 10.1016/j.clinph.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salinas FS, Franklin C, Narayana S, Szabó CÁ, Fox PT. Repetitive transcranial magnetic stimulation educes frequency-specific causal relationships in the motor network. Brain Stimul. 2016;9:406–414. doi: 10.1016/j.brs.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, et al. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- 101.Parks NA. Concurrent application of TMS and near-infrared optical imaging: Methodological considerations and potential artifacts. Front Hum Neurosci. 2013;7:592. doi: 10.3389/fnhum.2013.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maniglia M, Trotter Y, Aedo-Jury F. TMS reveals inhibitory extrastriate cortico-cortical feedback modulation of V1 activity in humans. Brain Struct Funct. 2019;224:3399–3408. doi: 10.1007/s00429-019-01964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nassi JJ, Lomber SG, Born RT. Corticocortical feedback contributes to surround suppression in V1 of the alert primate. J Neurosci. 2013;33:8504–8517. doi: 10.1523/JNEUROSCI.5124-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 105.Bergmann TO, Karabanov A, Hartwigsen G, Thielscher A, Siebner HR. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: Current approaches and future perspectives. Neuroimage. 2016;140:4–19. doi: 10.1016/j.neuroimage.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Curtin A, Tong SB, Sun JF, Wang JJ, Onaral B, Ayaz H. A systematic review of integrated functional near-infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS) studies. Front Neurosci. 2019;13:84. doi: 10.3389/fnins.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev. 2016;64:175–184. doi: 10.1016/j.neubiorev.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 108.Rogasch NC, Sullivan C, Thomson RH, Rose NS, Bailey NW, Fitzgerald PB, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open-source TESA software. Neuroimage. 2017;147:934–951. doi: 10.1016/j.neuroimage.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 109.Farzan F, Vernet M, Shafi MM, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography. Front Neural Circuits. 2016;10:73. doi: 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Lara LIN, Tik M, Woletz M, Frass-Kriegl R, Moser E, Laistler E, et al. High-sensitivity TMS/fMRI of the human motor cortex using a dedicated multichannel MR coil. Neuroimage. 2017;150:262–269. doi: 10.1016/j.neuroimage.2017.02.062. [DOI] [PubMed] [Google Scholar]
- 111.Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, et al. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest Radiol. 1998;33:336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Bungert A, Chambers CD, Phillips M, Evans CJ. Reducing image artefacts in concurrent TMS/fMRI by passive shimming. Neuroimage. 2012;59:2167–2174. doi: 10.1016/j.neuroimage.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 113.Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: From ‘virtual lesions’ to functional-network accounts of cognition. Cortex. 2009;45:1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baeken C, Marinazzo D, Everaert H, Wu GR, van Hove C, Audenaert K, et al. The impact of accelerated HF-rTMS on the subgenual anterior cingulate cortex in refractory unipolar major depression: Insights from 18FDG PET brain imaging. Brain Stimul. 2015;8:808–815. doi: 10.1016/j.brs.2015.01.415. [DOI] [PubMed] [Google Scholar]
- 115.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: A new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sommer M, Norden C, Schmack L, Rothkegel H, Lang N, Paulus W. Opposite optimal current flow directions for induction of neuroplasticity and excitation threshold in the human motor cortex. Brain Stimul. 2013;6:363–370. doi: 10.1016/j.brs.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, et al. Column-based model of electric field excitation of cerebral cortex. Hum Brain Mapp. 2004;22:1–14. doi: 10.1002/hbm.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krieg TD, Salinas FS, Narayana S, Fox PT, Mogul DJ. PET-based confirmation of orientation sensitivity of TMS-induced cortical activation in humans. Brain Stimul. 2013;6:898–904. doi: 10.1016/j.brs.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11:230–238. doi: 10.1002/pon.562. [DOI] [PubMed] [Google Scholar]
- 120.Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37. doi: 10.1016/j.biopsych.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boes AD, Prasad S, Liu HS, Liu Q, Pascual-Leone A, Caviness VS, Jr, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cash RFH, Weigand A, Zalesky A, Siddiqi SH, Downar J, Fitzgerald PB, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry 2020: S0006–S3223(20)31668–1. [DOI] [PubMed]
- 124.Cash RFH, Cocchi L, Lv J, Fitzgerald PB, Zalesky A. Functional magnetic resonance imaging-guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry. 2021;78:337–339. doi: 10.1001/jamapsychiatry.2020.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chouinard PA, van der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- 126.Valero-Cabré A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: A 14C–2DG tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- 127.Vidal-Piñeiro D, Martín-Trias P, Falcón C, Bargalló N, Clemente IC, Valls-Solé J, et al. Neurochemical modulation in posteromedial default-mode network cortex induced by transcranial magnetic stimulation. Brain Stimul. 2015;8:937–944. doi: 10.1016/j.brs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 128.Tik M, Hoffmann A, Sladky R, Tomova L, Hummer A, de Lara LN, et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289–296. doi: 10.1016/j.neuroimage.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 129.Quentin R, Elkin Frankston S, Vernet M, Toba MN, Bartolomeo P, Chanes L, et al. Visual contrast sensitivity improvement by right frontal high-beta activity is mediated by contrast gain mechanisms and influenced by Fronto-parietal white matter microstructure. Cereb Cortex. 2016;26:2381–2390. doi: 10.1093/cercor/bhv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson M, Darby RR, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry. 2019;86:749–758. doi: 10.1016/j.biopsych.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang SG, Wang Q, Kong XZ, Deng W, Yang X, Li XJ, et al. White matter abnormalities in major depression biotypes identified by diffusion tensor imaging. Neurosci Bull. 2019;35:867–876. doi: 10.1007/s12264-019-00381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhong Y, Wang C, Gao WJ, Xiao Q, Lu DL, Jiao Q, et al. Aberrant resting-state functional connectivity in the default mode network in pediatric bipolar disorder patients with and without psychotic symptoms. Neurosci Bull. 2019;35:581–590. doi: 10.1007/s12264-018-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2014;85(Pt 3):961–970. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381–404. doi: 10.1016/j.neubiorev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 136.Barker AT, Shields K. Transcranial magnetic stimulation: Basic principles and clinical applications in migraine. Headache. 2017;57:517–524. doi: 10.1111/head.13002. [DOI] [PubMed] [Google Scholar]
- 137.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 138.Ramos-Estebanez C, Merabet LB, Machii K, Fregni F, Thut G, Wagner TA, et al. Visual phosphene perception modulated by subthreshold crossmodal sensory stimulation. J Neurosci. 2007;27:4178–4181. doi: 10.1523/JNEUROSCI.5468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luber B, Stanford AD, Bulow P, Nguyen T, Rakitin BC, Habeck C, et al. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex. 2008;18:2077–2085. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 141.Sale MV, Nydam AS, Mattingley JB. Stimulus uncertainty enhances long-term potentiation-like plasticity in human motor cortex. Cortex. 2017;88:32–41. doi: 10.1016/j.cortex.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 142.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 143.Funke K, Benali A. Modulation of cortical inhibition by rTMS - findings obtained from animal models. J Physiol. 2011;589:4423–4435. doi: 10.1113/jphysiol.2011.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hui J, Zomorrodi R, Lioumis P, Salavati B, Rajji TK, Chen R, et al. Pharmacological mechanisms of interhemispheric signal propagation: A TMS-EEG study. Neuropsychopharmacology. 2020;45:932–939. doi: 10.1038/s41386-019-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang A, Thickbroom G, Rodger J. Repetitive transcranial magnetic stimulation of the brain: Mechanisms from animal and experimental models. Neuroscientist. 2017;23:82–94. doi: 10.1177/1073858415618897. [DOI] [PubMed] [Google Scholar]
- 146.Lenz M, Galanis C, Müller-Dahlhaus F, Opitz A, Wierenga CJ, Szabó G, et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. 2016;7:10020. doi: 10.1038/ncomms10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 148.Roelfsema PR, Holtmaat A. Control of synaptic plasticity in deep cortical networks. Nat Rev Neurosci. 2018;19:166–180. doi: 10.1038/nrn.2018.6. [DOI] [PubMed] [Google Scholar]
- 149.Wilkinson ST, Holtzheimer PE, Gao S, Kirwin DS, Price RB. Leveraging neuroplasticity to enhance adaptive learning: The potential for synergistic somatic-behavioral treatment combinations to improve clinical outcomes in depression. Biol Psychiatry. 2019;85:454–465. doi: 10.1016/j.biopsych.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang AD, Hong I, Boddington LJ, Garrett AR, Etherington S, Reynolds JN, et al. Low-intensity repetitive magnetic stimulation lowers action potential threshold and increases spike firing in layer 5 pyramidal neurons in vitro. Neuroscience. 2016;335:64–71. doi: 10.1016/j.neuroscience.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 151.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang FR, Zhang Y, Wang L, Sun P, Luo XW, Ishigaki Y, et al. Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer's disease model mice. Neuropharmacology. 2015;97:210–219. doi: 10.1016/j.neuropharm.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 153.Weise D, Mann J, Rumpf JJ, Hallermann S, Classen J. Differential regulation of human paired associative stimulation-induced and Theta-burst stimulation-induced plasticity by L-type and T-type Ca2+ channels. Cereb Cortex. 2017;27:4010–4021. doi: 10.1093/cercor/bhw212. [DOI] [PubMed] [Google Scholar]
- 154.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duffau H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. 2006;13:885–897. doi: 10.1016/j.jocn.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 156.May A, Hajak G, Gänssbauer S, Steffens T, Langguth B, Kleinjung T, et al. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- 157.Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning——revisited. PLoS ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 159.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Philip NS, Barredo J, van’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83:263–272. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shafi MM, Westover MB, Fox MD, Pascual-Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur J Neurosci. 2012;35:805–825. doi: 10.1111/j.1460-9568.2012.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sack AT. Transcranial magnetic stimulation, causal structure-function mapping and networks of functional relevance. Curr Opin Neurobiol. 2006;16:593–599. doi: 10.1016/j.conb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 163.Lenz M, Vlachos A. Releasing the cortical brake by non-invasive electromagnetic stimulation? rTMS induces LTD of GABAergic neurotransmission. Front Neural Circuits. 2016;10:96. doi: 10.3389/fncir.2016.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat Neurosci. 2013;16:838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Antonietti A, Monaco J, D'Angelo E, Pedrocchi A, Casellato C. Dynamic redistribution of plasticity in a cerebellar spiking neural network reproducing an associative learning task perturbed by TMS. Int J Neural Syst. 2018;28:1850020. doi: 10.1142/S012906571850020X. [DOI] [PubMed] [Google Scholar]
- 166.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 167.Sack AT, Cohen Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. J Cogn Neurosci. 2009;21:207–221. doi: 10.1162/jocn.2009.21126. [DOI] [PubMed] [Google Scholar]
- 168.Luber BM, Davis S, Bernhardt E, Neacsiu A, Kwapil L, Lisanby SH, et al. Reprint of "Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention". Neuroimage. 2017;151:65–71. doi: 10.1016/j.neuroimage.2017.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009;2:234–237. doi: 10.1016/j.brs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 170.Opitz A, Legon W, Rowlands A, Bickel WK, Paulus W, Tyler WJ. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage. 2013;81:253–264. doi: 10.1016/j.neuroimage.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 171.Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, et al. Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Hum Brain Mapp. 2005;26:100–109. doi: 10.1002/hbm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gomez LJ, Dannhauer M, Koponen LM, Peterchev AV. Conditions for numerically accurate TMS electric field simulation. Brain Stimul. 2020;13:157–166. doi: 10.1016/j.brs.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gomez-Tames J, Hamasaka A, Laakso I, Hirata A, Ugawa Y. Atlas of optimal coil orientation and position for TMS: A computational study. Brain Stimul. 2018;11:839–848. doi: 10.1016/j.brs.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 174.Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50:1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: Locus of excitation. J Physiol. 1993;460:201–219. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tay G, Chilbert M, Battocletti J, Sances A, Swiontek T, Kurakami C. Measurement of magnetically induced current density in saline in vivo. Images of the Twenty-First Century. Proceedings of the Annual International Engineering in Medicine and Biology Society 1989: 1167–1168.
- 177.Yunokuchi K, Kato R, Yoshida H, Tamari Y, Saito M. Study on the distributions of induced electric field in an inhomogeneous medium exposed a pulsed magnetic field. Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol. 20 Biomedical Engineering Towards the Year 2000 and Beyond (Cat. No. 98CH36286) 1998, 6: 3294–3297.
- 178.Lisanby SH, Luber B, Schroeder C, Osman M, Finck D, Jalinous R, et al. 333. Intracerebral measurement of rTMS and ECS induced voltage in vivo. Biol Psychiatry. 1998;43:100. [Google Scholar]
- 179.Bohning DE, Pecheny AP, Epstein CM, Speer AM, Vincent DJ, Dannels W, et al. Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport. 1997;8:2535–2538. doi: 10.1097/00001756-199707280-00023. [DOI] [PubMed] [Google Scholar]
- 180.Aberra AS, Wang BS, Grill WM, Peterchev AV. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020;13:175–189. doi: 10.1016/j.brs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ruohonen J, Karhu J. Navigated transcranial magnetic stimulation. Neurophysiol Clin. 2010;40:7–17. doi: 10.1016/j.neucli.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 182.Puonti O, Saturnino GB, Madsen KH, Thielscher A. Value and limitations of intracranial recordings for validating electric field modeling for transcranial brain stimulation. Neuroimage. 2020;208:116431. doi: 10.1016/j.neuroimage.2019.116431. [DOI] [PubMed] [Google Scholar]
- 183.Huang Y, Liu AL, Lafon B, Friedman D, Dayan M, Wang XY, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife. 2017;6:e18834. doi: 10.7554/eLife.18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Htet AT, Saturnino GB, Burnham EH, Noetscher GM, Nummenmaa A, Makarov SN. Comparative performance of the finite element method and the boundary element fast multipole method for problems mimicking transcranial magnetic stimulation (TMS) J Neural Eng. 2019;16:024001. doi: 10.1088/1741-2552/aafbb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Makarov SN, Noetscher GM, Raij T, Nummenmaa A. A quasi-static boundary element approach with fast multipole acceleration for high-resolution bioelectromagnetic models. IEEE Trans Biomed Eng. 2018;65:2675–2683. doi: 10.1109/TBME.2018.2813261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nummenmaa A, McNab JA, Savadjiev P, Okada Y, Hämäläinen MS, Wang RP, et al. Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stimul. 2014;7:80–84. doi: 10.1016/j.brs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Geeter ND, Dupré L, Crevecoeur G. Modeling transcranial magnetic stimulation from the induced electric fields to the membrane potentials along tractography-based white matter fiber tracts. J Neural Eng. 2016;13:026028. doi: 10.1088/1741-2560/13/2/026028. [DOI] [PubMed] [Google Scholar]
- 188.Klooster DC, de Louw AJ, Aldenkamp AP, Besseling RM, Mestrom RM, Carrette S, et al. Technical aspects of neurostimulation: Focus on equipment, electric field modeling, and stimulation protocols. Neurosci Biobehav Rev. 2016;65:113–141. doi: 10.1016/j.neubiorev.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 189.de Lucia M, Parker GJ, Embleton K, Newton JM, Walsh V. Diffusion tensor MRI-based estimation of the influence of brain tissue anisotropy on the effects of transcranial magnetic stimulation. Neuroimage. 2007;36:1159–1170. doi: 10.1016/j.neuroimage.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 190.Yokota T, Maki T, Nagata T, Murakami T, Ugawa Y, Laakso I, et al. Real-time estimation of electric fields induced by transcranial magnetic stimulation with deep neural networks. Brain Stimul. 2019;12:1500–1507. doi: 10.1016/j.brs.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 191.Stenroos M, Koponen LM. Real-time computation of the TMS-induced electric field in a realistic head model. Neuroimage. 2019;203:116159. doi: 10.1016/j.neuroimage.2019.116159. [DOI] [PubMed] [Google Scholar]
- 192.Herwig U, Schönfeldt-Lecuona C, Wunderlich AP, von Tiesenhausen C, Thielscher A, Walter H, et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 2001;108:123–131. doi: 10.1016/s0925-4927(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 193.Fleming MK, Sorinola IO, Newham DJ, Roberts-Lewis SF, Bergmann JH. The effect of coil type and navigation on the reliability of transcranial magnetic stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20:617–625. doi: 10.1109/TNSRE.2012.2202692. [DOI] [PubMed] [Google Scholar]
- 194.Neggers SF, Langerak TR, Schutter DJ, Mandl RC, Ramsey NF, Lemmens PJ, et al. A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage. 2004;21:1805–1817. doi: 10.1016/j.neuroimage.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 195.Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul. 2020;13:206–214. doi: 10.1016/j.brs.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 196.Cash RFH, Cocchi L, Lv J, Wu YM, Fitzgerald PB, Zalesky A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Hum Brain Mapp. 2021;42:4155–4172. doi: 10.1002/hbm.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cash RFH, Cocchi L, Anderson R, Rogachov A, Kucyi A, Barnett AJ, et al. A multivariate neuroimaging biomarker of individual outcome to transcranial magnetic stimulation in depression. Hum Brain Mapp. 2019;40:4618–4629. doi: 10.1002/hbm.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, 3rd, Rao H, Deng ZD, et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human Primates. Nat Neurosci. 2014;17:1130–1136. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]