Abstract
The mechanical properties of guard cell (GC) walls are important for stomatal development and stomatal response to external stimuli. However, the molecular mechanisms of pectin synthesis and pectin composition controlling stomatal development and dynamics remain poorly explored. Here, we characterized the role of two Arabidopsis (Arabidopsis thaliana) galacturonosyltransferases, GAUT10 and GAUT11, in plant growth, stomatal development, and stomatal dynamics. GAUT10 and GAUT11 double mutations reduced pectin synthesis and promoted homogalacturonan (HG) demethylesterification and demethylesterified HG degradation, resulting in larger stomatal complexes and smaller pore areas, increased stomatal dynamics, and enhanced drought tolerance of plants. In contrast, increased GAUT10 or GAUT11 expression impaired stomatal dynamics and drought sensitivity. Genetic interaction analyses together with immunolabeling analyses suggest that the methylesterified HG level is important in stomatal dynamics, and pectin abundance with the demethylesterified HG level controls stomatal dimension and stomatal size. Our results provide insight into the molecular mechanism of GC wall properties in stomatal dynamics, and highlight the role of GAUT10 and GAUT11 in stomatal dimension and dynamics through modulation of pectin biosynthesis and distribution in GC walls.
Galacturonosyltransferases, GAUT10 and GAUT11 are involved in stomatal development and dynamics through participation in pectin synthesis and distribution in guard cell walls.
Introduction
Stomata are formed by pairs of uniquely differentiated guard cells (GCs) in the epidermis, which regulate gas and water exchange between plants and the environment. Stomata undergo repeated and reversible opening and closing in response to various external environmental changes and intracellular signals. For example, darkness, elevated concentrations of CO2, drought, and abscisic acid promote stomatal closure, whereas light and reduced concentrations of CO2 induce stomatal opening (Webb et al., 2001; Kim et al., 2010; Kollist et al., 2014). These processes are achieved by reversible expansion and contraction of GCs, which is controlled by dynamic balance between intracellular turgor pressure and the physical resistance of the GC wall. Adequate GC wall elasticity and strength are needed to ensure the functional demands of stomatal dynamics (Cosgrove, 2016). Thus, understanding the molecular mechanisms of synthesis, modification, deposition, and degradation of GC wall components is fundamental to exploring the relationship between GC structure and stomatal function (Rui et al., 2016, 2018; Rui and Anderson, 2016; Woolfenden et al., 2018; Yi et al., 2018).
The plant cell wall is 3D and consists of celluloses, hemicelluloses, pectins, and structural proteins, which are assembled into a rigid, flexible, and dynamically organized network (Somerville et al., 2004; Voiniciuc et al., 2018a). Pectins are the most complicated polysaccharide in dicots and nongraminaceous monocots primary cell walls, accounting for 50% of the Arabidopsis (Arabidopsis thaliana) leaf cell walls (Ridley et al., 2001; Jones et al., 2005; Vogel, 2008). Among the three major pectin polysaccharide types (homogalacturonan [HG], rhamnogalacturonans I (RG-I) and RG-II) (Mohnen, 2008; Caffall and Mohnen, 2009). HG, a linear homopolymer of α-1,4-linked galacturonic acid, accounts for ∼65% of pectins (Atmodjo et al., 2013). The HG backbone is synthesized in the Golgi apparatus in a highly methylesterified form by galacturonosyltransferases (GAUTs) and pectin methyltransferases, respectively, and then is secreted into the apoplast and demethylesterified in the cell wall by pectin methylesterases (PMEs) (Zablackis et al., 1995; Sterling et al., 2001; Mouille et al., 2007; Mohnen, 2008; Driouich et al., 2012; Atmodjo et al., 2013). PME inhibitors inhibit the activity of PMEs by direct binding (Jolie et al., 2010; Sénéchal et al., 2015). Pectins with blockwise demethylesterification tend to crosslink with Ca2+, leading to wall stiffening and flexibility, whereas those with random demethylesterification are more susceptible to degradation by polygalacturonases (PGs) or pectate lyases (PLs), thus contributing to wall loosening (Xiao et al., 2014; Hocq et al., 2017; Rui et al., 2018).
GC walls are rich in pectins and have a profound effect on stomatal function. Pectin modification and degradation in GC walls are important in stomatal development and dynamics in response to different stimuli. HG methyltransferase QUASIMODO2 mutation reduced cell adhesion in the cotyledon, with more detached GC pairs (Du et al., 2020). PMEs, PME6, and PME34 are required for normal maintenance of pectin methylesterification in GC walls. pme6 mutation caused impaired stomatal dynamics to elevated CO2 and drought sensitivity (Negi et al., 2013; Amsbury et al., 2016), and pme34 showed a defect in stomatal aperture during heat stress conditions via altering PME and PG activity (Huang et al., 2017; Wu et al., 2017). PG INVOLVED IN EXPANSION3 (PGX3), an endogenous wall-degrading pectinase, is essential for pore dimensions and stomatal dynamics by modulating pectin molecular mass and abundance (Rui et al., 2017). Pectin degradation, particularly in the middle lamella, can facilitate pore formation between two sister GCs during the last stomatal developmental step (Rui et al., 2019). Polar stiffening contributed by pectins in GCs leads to more efficient stomatal response to altered turgor pressure, and loss of polar stiffening by PG treatment leads to a large decrease in pore aperture under any given pressure (Carter et al., 2017). Furthermore, treatment with arabinanase and feruloyl esterase impeded stomatal opening (Jones et al., 2003). However, the underlying molecular mechanisms of pectin modification and degradation in GC walls that modulate stomatal development and dynamics still remain poorly understood. Moreover, which pectin composition, the methylesterified HG or demethylesterified HG, plays the primary role in determination of stomatal development or stomatal dynamics is not known.
GAUTs function in cell wall pectin biosynthesis, by transferring GalA from uridine-diphosphate-GalA (UDP-GalA) onto HG acceptors (Sterling et al., 2006; Caffall et al., 2009). GAUTs with confirmed HG:GalA transferase activity, such as GAUT1:GAUT7 complex, GAUT4 and GAUT11, have been reported to function in plant cell wall pectin biosynthesis (Atmodjo et al., 2011; Amos et al., 2018; Biswal et al., 2018; Voiniciuc et al., 2018b). Other GAUTs with putative GAUT activity regulate cell wall properties in Arabidopsis. For example, GAUT8 and Irregular Xylem8 (IRX8)/GAUT12 directly affect cell adhesion and cell wall integrity (Bouton et al., 2002; Leboeuf et al., 2005; Orfila et al., 2005; Peña et al., 2007; Persson et al., 2007). GAUT10 is auxin responsive and regulates sugar-mediated root meristem maintenance (Pu et al., 2019). Pollen-expressed GAUT13 and GAUT14 function redundantly in pollen tube shape and growth (Wang et al., 2013), and GAUT5, GAUT6, and GAUT7 are also required for HG synthesis in growing pollen tubes and HG deposition at the pollen tube apex (Lund et al., 2020). However, their functions in HG biosynthesis in GC walls are elusive, and whether or how pectin synthesis would affect pectin abundance and distribution in GC walls, thus influencing stomatal development and dynamics, remain unexplored.
We identified that closely related GAUT10 and GAUT11 are highly expressed in GCs and involved in pectin synthesis in GC walls. They function redundantly and play major roles in plant development, stomatal size and dimension, and stomatal dynamics to environmental changes.
Results
Identification of GAUT10 and GAUT11 in GCs
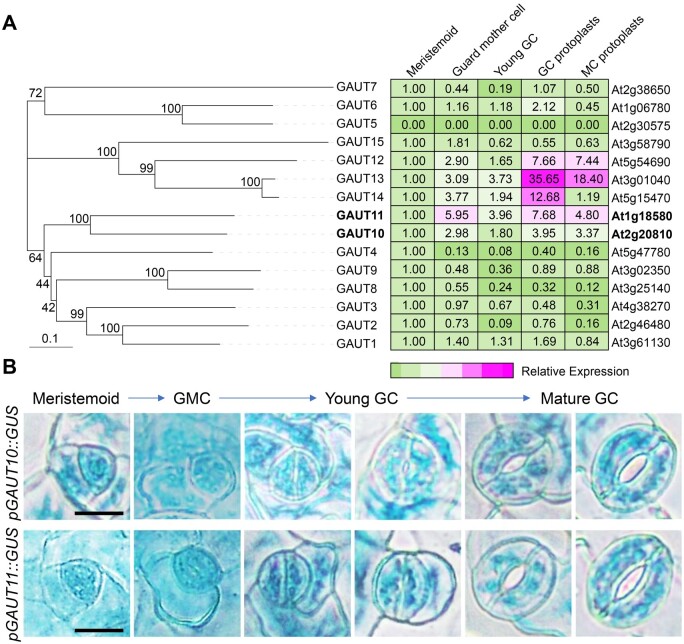
To determine whether pectin level or pectin abundance in GC walls influences stomatal function, we mainly focused on GAUTs that are highly expressed in GCs. There are 15 GAUT members in Arabidopsis (Caffall et al., 2009). Expression-level analyses in GC linage cells from the public dataset (Winter et al., 2007) showed that GAUT10, GAUT11, GAUT12, GAUT13, and GAUT14 were highly expressed in guard mother cells, young and mature GCs (Figure 1A). GAUT12, GAUT13, and GAUT14 were reported to be involved in pollen tube growth and shape (Persson et al., 2007; Wang et al., 2013; Hao et al., 2014). We then focused on GAUT10 and GAUT11, which are closely related homologous genes (Figure 1A).
Figure 1.
Closely related homologous genes GAUT10 and GAUT11 are highly expressed in GCs. A, Phylogenetic analysis of GAUT proteins in Arabidopsis and relative expression of GAUTs in stomatal lineage cells based on the eFP Browser (Winter et al., 2007). Phylogenetic tree was constructed by MEGA X with the neighbor-joining method. The tree is drawn to scale, with branch lengths measured in genetic distance. For each gene, the expression level in meristemoids was set as 1.0 (green), and the expression levels in other cell types are relative to meristemoids. Different colors represent the abundance of transcripts, from low (green) to high (magenta). B, GUS staining of stomatal lineage cells at several stages of the stomatal development in pGAUT10::GUS and pGAUT10::GUS transgenic plants. Bars = 10 µm.
To confirm that GAUT10 and GAUT11 are expressed in GCs, we generated transgenic plants expressing the GUS reporter gene controlled by the GAUT10 or GAUT11 promoter, respectively. GAUT10 and GAUT11 showed similar and broad expression patterns in embryo, cotyledons, hypocotyls, vascular strands, rosette leaves, roots, flowers, and siliques (Supplemental Figure S1A). At the cellular level, both GAUT10 and GAUT11 were strongly expressed in the stomatal lineage cells during GC development, including meristemoids, guard mother cells, and both young and mature GCs (Figure 1B; Supplemental Figure S1B), consistent with the microarray data (Figure 1A). These results confirm that GAUT10 and GAUT11 are greatly expressed in GCs and suggest their possible involvement in stomatal biology.
GAUT10 and GAUT11 localize in Golgi and participate in pectin synthesis
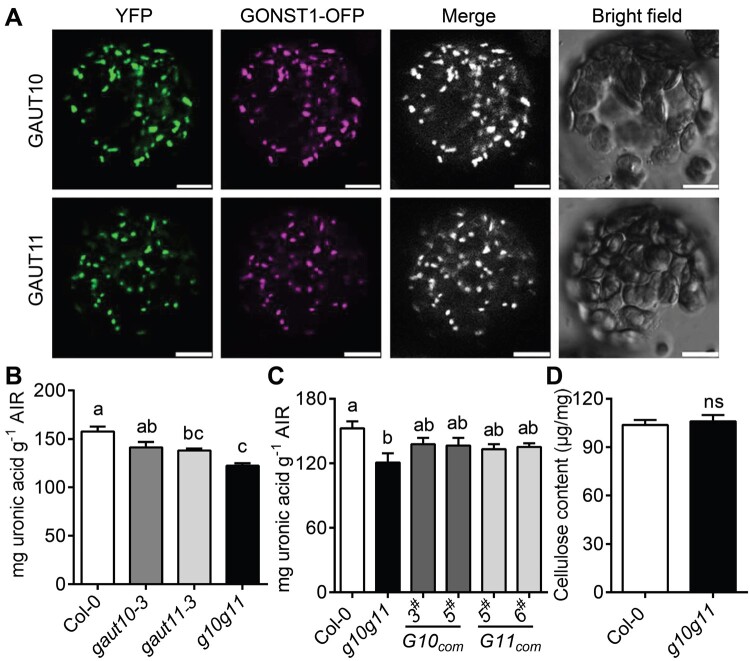
GAUT10 and GAUT11 belong to the glycosyltransferase family, which are considered as Golgi-localized type II membrane protein (Sterling et al., 2001, 2006; Yin et al., 2010). To determine the subcellular localizations of GAUT10 and GAUT11, the GAUT10 and GAUT11 CDS (coding sequence) fused with a YFP tag at the C-terminus were transiently expressed in Nicotiana benthamiana epidermal cells. YFP fluorescence overlapped well with the Golgi-localized marker GONST1 (Baldwin et al., 2001), with punctuate distribution pattern in the transiently expressed epidermal cells and mesophyll cell (MC) protoplasts (Figure 2A; Supplemental Figure S1C). These results confirmed that GAUT10 and GAUT11 localize to the Golgi apparatus where pectin synthesis is known to take place (Sterling et al., 2001), and are thus consistent with the possible roles of GAUT10 and GAUT11 in pectin biosynthesis.
Figure 2.
GAUT10 and GAUT11 are Golgi-localized GAUTs and participate in pectin biosynthesis. A, YFP-tagged GAUT10 and GAUT11 fusion proteins are co-localized with GONST1-OFP (Golgi apparatus marker) in Golgi in MC protoplasts of N. benthamiana leaves. The signals were visualized with a laser confocal microscope. Bars = 10 µm. B, Uronic acid measurements in leaves from 4-week-old Col-0, gaut10-3, gaut11-3, and g10g11 plants. Values are means ± se (three biological replicates). P < 0.05, one-way ANOVA and Tukey’s test. C, Uronic acid measurements in GAUT10 or GAUT11 complementation lines. Values are means ± se (three biological replicates). P < 0.05, one-way ANOVA and Tukey’s test. D, Cellulose content measurements by Updegraff method from leaves of 4-week-old Col-0 and g10g11 plants. Values are means ± se (two biological replicates). No significant difference (ns), P ≥ 0.05; Student’s t test.
To elucidate that GAUT10 and GAUT11 function as GAUTs in pectin biosynthesis, two T-DNA insertion mutants, gaut10-3 (SALK_092577) (Pu et al., 2019) and gaut11-3 (SAIL_567_H05; Voiniciuc et al., 2018b), were ordered from the Arabidopsis Biological Resource Center (ABRC; www.arabidopsis.org; Supplemental Figure S2A). Reverse transcription-quantitative PCR (RT-qPCR) analyses revealed that gaut10-3 was a knockout mutant, while gaut11-3 was a knock-down mutant (Supplemental Figure S2, B and C; Supplemental Table S1). A gaut10-3gaut11-3 (g10g11) double mutant was then generated and pectin content in the single and double mutant plants was determined. In this assay, alcohol-insoluble residue (AIR) was extracted from the rosette leaves and used for uronic acid content determination. gaut11-3 exhibited a reduced total uronic acid level compared to Col-0, consistent with a previous study (Voiniciuc et al., 2018b), while gaut10-3 exhibited a uronic acid level comparable to Col-0. However, g10g11 double mutant had much lower amount of total uronic acid level compared to Col-0 (Figure 2B). These results suggest that GAUT10 and GAUT11 function redundantly in pectin biosynthesis.
To further determine their effects on pectin synthesis, the constructs containing GAUT10 or GAUT11 genomic DNA driven by their native promoter were introduced into the g10g11 mutants, respectively. Two independent transgenic lines expressing GAUT10 or GAUT11 (G10com3# and G10com5#, G11com5# and G11com6#), whose transcript levels were comparable or greater to that of the wild-type (WT; Supplemental Figure S2, D and E), were selected for pectin level analyses. Expression of GAUT10 or GAUT11 rescued the reduced uronic acid level of g10g11 plants (Figure 2C), suggesting that GAUT10 and GAUT11 are functional GAUTs and involved in pectic HG synthesis. When GAUT10 or GAUT11 were overexpressed in Col-0 (G10OE4# and G10OE13#, G11OE7# and G11OE13#; Supplemental Figure S2, F and G), the pectin levels detected by this method were not significantly increased (Supplemental Figure S3A). Because cellulose is a major cell wall structural component and is considered to be closely interacted with pectins (Broxterman and Schols, 2018), we also examined cellulose content in the g10g11 plants by Updegraff method (Rui and Anderson, 2016). The cellulose content was not changed in the g10g11 cell wall (Figure 2D). These results demonstrate the redundant role of GAUT10 and GAUT11 in pectic HG synthesis.
GAUT10 and GAUT11 are required for plant growth and development
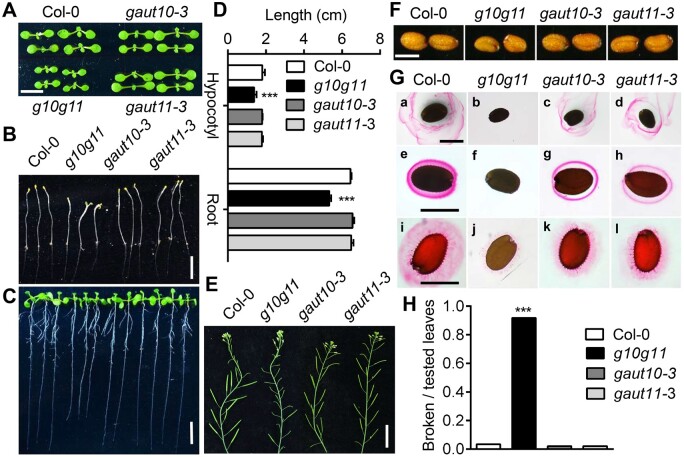
We determined the plant morphology of gaut10-3, gaut11-3, and g10g11. The gaut10-3 and gaut11-3 single mutants did not show any obviously altered morphologies at our growth conditions, such as plant size at both seedling and adult stages either in the half strength Murashige and Skoog (1/2 MS) medium (1% sucrose) or soil, plant height, primary root length, and seed size, compared to Col-0 (Figure 3). However, g10g11 plants were slightly smaller than the WT at both seedling and adult stages, with no substantial difference in plant growth rate, plant height, and leaf number compared to Col-0 (Figure 3A; Supplemental Figure S4, A and B). g10g11 had slightly shorter primary root length at normal growth conditions and shorter hypocotyl length in darkness, the flowering time was similar to Col-0 but the fertility was severely impaired (Figure 3, B–E), suggesting GAUT10 and GAUT11 mutations inhibit plant growth. Furthermore, the seed size and seed germination rate of g10g11 were substantially reduced (Figure 3F; Supplemental Figure S4C), and Ruthenium Red staining analyses showed both soluble mucilage and adherent mucilage were almost undetectable in the g10g11 seeds, compared to the reduced mucilage levels observed in the gaut10-3 and gaut11-3 single mutants (Figure 3G), further supporting the functional redundancy between GAUT10 and GAUT11 in pectin synthesis. Additionally, when the expanded leaves were bending down 180°, around 80% of leaf petioles or midribs were broken in the g10g11 double mutant, however, these were almost intact in Col-0 (Figure 3H). These results suggest the essential role of GAUT10 and GAUT11 in plant growth, and the growth inhibition in g10g11 plants may be due to the reduced pectin level, which changes the cell wall expansion and flexibility.
Figure 3.
GAUT10 and GAUT11 function in plant development. A, Eight-day-old seedlings of Col-0, gaut10-3, gaut11-3, and g10g11. Bar = 5 mm. B, Etiolated g10g11 seedlings (6-d-old) exhibited shorter hypocotyls. Bar = 1 cm. C, Light-grown g10g11 seedlings (9-d-old) exhibited shorter primary root length. Bar = 1 cm. D, Statistical analyses of hypocotyl and primary root length in (B) and (C). Values are means ± se (n ≥ 30 seedlings per genotype per experiment). Three independent experiments; ***P < 0.001 compared to Col-0, Student’s t test. E, g10g11 plants exhibited reduced fertility compared to Col-0 plants. Bar = 2 cm. F, Mature seeds of Col-0, gaut10-3, gaut11-3, and g10g11 plants. Bars = 200 µm. G, Ruthenium red staining of mucilage surrounding the seeds of Col-0, gaut10-3, gaut11-3, and g10g11. Seeds were stained directly ([a] to [d]) or after imbibition for 1 h in water ([e] to [h]) or EDTA ([i] to [l]). Bars = 200 µm. H, Statistical analyses of broken petioles for brittleness of g10g11 mutant leaves. Fully expanded leaves of 4- to 5-week-old plants were bent by 180° so that the adaxial surface of the leaf was parallel with the soil. Broken leaf petioles were considered brittle. Values are means ± se (n ≥ 30, ***P < 0.001 compared to Col-0, Student’s t test).
GAUT10 and GAUT11 mutations result in a larger stomatal complex and smaller pore area
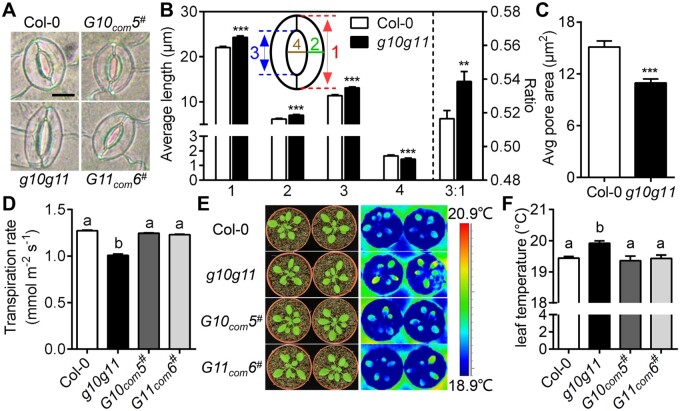
GAUT10 and GAUT11 are highly expressed in the stomatal lineage cells (Figure 1; Supplemental Figure S1B), we then assessed the role of GAUT10 and GAUT11 in stomata. No significant differences in stomatal density and index were found in mature g10g11 leaves under normal growth conditions (Supplemental Figure S4, D and E). However, g10g11 mutant plants exhibited a larger stomatal complex size in both mature leaves and cotyledons (Figure 4A; Supplemental Tables S2 and S3). In particular, the stomatal pore length and the ratio of pore length to stomatal complex length, as well as stomatal complex length and GC width, were all significantly greater in g10g11 than in Col-0 controls (Figure 4B). In addition, g10g11 exhibited a reduced pore width under normal conditions, resulting in smaller pore area and reduced transpiration rate (Figure 4, B–D). Consistent with these phenotypes, leaf temperature of g10g11 plants was higher than Col-0 controls (Figure 4, E and F). These phenotypes were all recovered to the WT level in the GAUT10 or GAUT11 complementation lines (Figure 4; Supplemental Table S2). However, in the GAUT10- or GAUT11-overexpression lines, stomatal size, and dimension did not differ from that of Col-0 (Supplemental Table S4). These results suggest that GAUT10 and GAUT11 influence GC size and stomatal pore dimension.
Figure 4.
GAUT10 and GAUT11 control stomatal size and pore dimension. A, Stomata in 3- to 4-week-old Col-0, g10g11, G10com5#, and G11com6# plants. Bars = 10 µm. B, Measurements of stomatal complex length (1), GC width (2), pore length (3), pore width (4), and the ratio of pore length to stomatal complex length (3:1) in 3- to 4-week-old Col-0 and g10g11 plants. Values are means ± se (n ≥ 60 stomata from at least six plants per genotype, ***P < 0.001; **P < 0.01; Student’s t test). C, Measurements of stomatal pore area in 3- to 4-week-old Col-0 and g10g11 plants under normal growth conditions. Values are means ± se (n ≥ 50 stomata from at least six plants per genotype, ***P < 0.001; Student’s t test). D, Measurements of leaf transpiration rate (mmol m−2 s−1) using a LI-6400XT gas exchange system in 3- to 4-week-old Col-0 and g10g11 plants. Values are means ± se, P < 0.05, one-way ANOVA, and Tukey’s test. E, Thermal imaging of well-watered Col-0, g10g11, G10com5#, and G11com6# plants. F, Statistical analysis of leaf temperature from thermal images of Col-0, g10g11, G10com5#, and G11com6# plants. Error bars indicate ± se, n ≥ 6, P < 0.05, one-way ANOVA, and Tukey’s test.
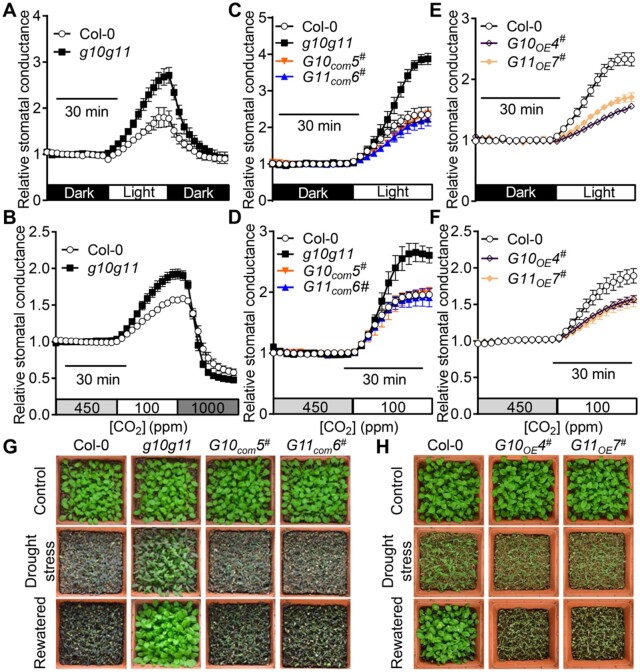
Changes in GAUT10 and GAUT11 expression alter stomatal dynamics to environmental changes
We next determined the stomatal conductance changes in intact leaves of 3- to 4-week-old WT and g10g11 mutant plants in response to changes in light intensity and CO2 concentrations. Light and reduced [CO2] treatments promoted stomatal opening and thus increased stomatal conductance, and further shifts to darkness and elevated [CO2] facilitate stomatal closing in the Col-0 controls (Figure 5, A and B; Supplemental Figure S5, A and B). In contrast, the increases of stomatal conductance during light and reduced [CO2] treatments were greater in the g10g11 mutant plants, and further stomatal closure induced by darkness and high [CO2], respectively, in g10g11 was also faster (Figure 5, A and B; Figure S5, A–D). These results suggest that g10g11 stomata are more sensitive and have a larger range of motions in response to environmental changes in [CO2] shifts, and dark to light transitions. G10com3#, G10com5#, G11com5#, and G11com6# complementation lines behaved similar to WT with respect to stomatal dynamics to these environmental changes (Figure 5, C and D; Supplemental Figure S5, E and F), suggesting that GAUT10 and GAUT11 mutations cause the g10g11 stomatal hypersensitivity. To further test whether altered stomatal dynamics in the g10g11 mature leaves was due to the changes in stomatal dimension, we measured stomatal dynamics in the GAUT10- and GAUT11-overexpression lines, in which stomatal size and dimension were not significantly different from that in Col-0 (Supplemental Table S4). Interestingly, G10OE4#, G10OE13#, G11OE7#, and G11OE13#-overexpression lines showed greatly impaired stomatal dynamic response to [CO2] shifts and dark to light transitions (Figure 5, E and F; Supplemental Figure S5, G and H), suggesting that the stomatal dynamics correspond with GAUT10 and GAUT11 expression, but not with stomatal dimension changes.
Figure 5.
GAUT10 and GAUT11 are involved in stomatal dynamics and drought performance. A and B, Relative stomatal conductance of 3- to 4-week-old Col-0 and g10g11 mutant in response to shifts in light intensity or CO2 concentrations. Values are means ± se, n ≥ 3 leaves per genotype per experiment. Experiments are repeated 3 times. C–F, Relative stomatal conductance of 3- to 4-week-old Col-0, g10g11, G10com5#, G11com6#, G10OE4#, and G11OE7# plants in response to shifts in light intensity or CO2 concentrations. Values are means ± se, n ≥ 3 leaves per genotype per experiment. Experiments are repeated 3 times. G and H, Drought performance of 3-week-old Col-0, g10g11, G10com5#, G11com6#, G10OE4#, and G11OE7# plants under drought stresses and water recovery. Images were obtained before drought (top), drought stress for 10 d (middle) in (G) and 8 d in (H), and 2 d after re-watering (bottom).
Because g10g11 displayed reduced transpiration rate and increased stomatal response to environmental stimuli, we then performed drought stress assays of g10g11, and GAUT10 or GAUT11 complementation and overexpression lines. g10g11 double mutant plants showed enhanced drought tolerance during exposure to drought stresses and water recovery (Figure 5G), matching well with the reduced stomatal pore area and increased stomatal dynamics (Figures 4C and 5, A and B). GAUT10 or GAUT11 expression rescued the drought insensitive phenotypes (Figure 5G), and GAUT10 or GAUT11 overexpression lines were more sensitive to drought stresses (Figure 5H). These results suggest that GAUT10 and GAUT11 are essential for stomatal behavior and drought performance. These results also suggest that pectin synthesis by GAUT10 and GAUT11 modulates stomatal dynamics. The reduced pectin level might accelerate cell wall expansion during stomatal opening, whereas excess pectin level limits cell wall expansion.
GAUT10 and GAUT11 modulate HG de-methylesterification and demethylesterified HG abundance in GCs
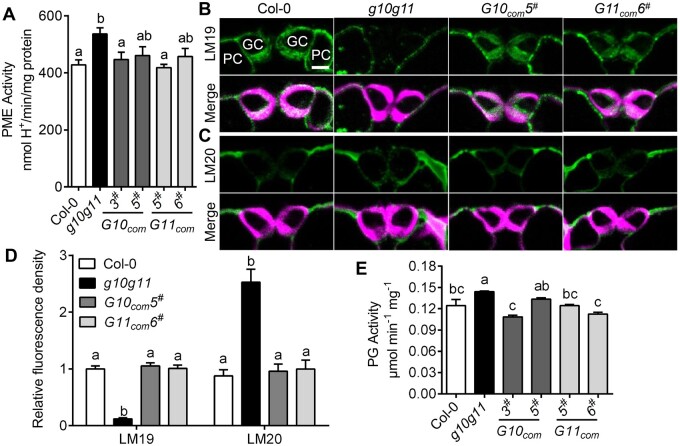
GC pectin methylesterification status affects GC wall stiffness and stomatal dynamics (Amsbury et al., 2016). To explore whether reduced pectin synthesis changes the pectin methylesterification degree in the g10g11 cell walls, we measured total PME activity in the leaves of 3- to 4-week-old Col-0, g10g11, and GAUT10 or GAUT11 complementation plants. g10g11 mutant plants exhibited greater PME activity than Col-0, while GAUT10 or GAUT11 complementation lines exhibited comparable PME activity as Col-0 (Figure 6A). These results suggest that GAUT10 and GAUT11 mutations increase pectin demethylesterification. To further determine that GAUT10 and GAUT11 function in GC walls, we performed immunohistochemical assays using specific antibodies that recognize different HG forms in GC walls of Col-0, g10g11, G10com5#, and G11com6# plants. Calcofluor White staining, which binds to beta-1,3 and beta-1,4 glucan chains, showed that cell wall thickness and structure were not changed in g10g11 (Figure 6, B and C). LM19 and LM20 recognize demethylesterified HG and highly methylesterified HG, respectively. The WT GC walls were rich in demethylesterified pectins indicated by LM19 binding, and had much lower amount of methylesterified HG indicated by LM20 binding (Figure 6, B–D). Interestingly, in contrast, the immunolabeling pattern was altered in g10g11 GC walls, in which LM19 binding was much weaker and LM20 binding was brighter than in Col-0 (Supplemental Figure 6, B–D). Moreover, G10com5# and G11com6# exhibited WT-like pectin distribution patterns (Figure 6, B–D). Control sections that were only incubated with secondary antibodies (without primary antibodies) showed a very low level of fluorescence in the green channel (Supplemental Figure S6). These results suggest that GAUT10 and GAUT11 mutations alter the pectin methylesterification degree in GC walls.
Figure 6.
GAUT10 and GAUT11 modulate pectin demethylesterification and abundance in GC walls. A, PME activity in the leaves of 4-week-old Col-0, g10g11, G10com3#, G10com5#, G11com5#, and G11com6# plants. Values are means ± se, three biological replicates; P < 0.05, one-way ANOVA, and Tukey’s test. B, Immunolabeling of demethylesterified HG (labeled with LM19) of GC walls from WT, g10g11, G10com5#, and G11com6# plants visualized by a confocal microscope. Top panels, LM19 labeling; bottom panels, LM19 labeling (green) merged with Calcofluor White signal (magenta).PC, pavement cell. Bars = 5 µm. C, Immunolabeling of highly methylesterified HG (labeled with LM20) of GC walls from WT, g10g11, G10com5#, and G11com6# plants. (Top), LM20 labeling; bottom, LM20 labeling (green) merged with Calcofluor White signal (magenta). Bars = 5 µm. D, LM19 and LM20 immunolabeling fluorescence intensity in the GC walls of 3- to 4-week-old Col-0, g10g11, G10com5#, and G11com6# plants. Values are means ± se and letters represent significant difference (n ≥ 20 GCs per genotype; P < 0.05, one-way ANOVA and Tukey’s test). E, PG activity in the leaves of 4-week-old Col-0, g10g11, G10com3#, G10com5#, G11com5#, and G11com6# plants. Values are means ± se, three biological replicates; P < 0.05, one-way ANOVA, and Tukey’s test.
Demethylesterified HG can be degraded by HG-degrading enzyme PGs (Xiao et al., 2014; Rui et al., 2017). The very reduced demethylesterified HG in g10g11 GC walls (Figure 6B) may be resulted from the reduced HG synthesis, or also the contribution of demethylesterified HG degradation. We then measured PG activity in g10g11. Surprisingly, the total PG activity was significantly increased in g10g11 and its level was recovered to the WT-like level in GAUT10 or GAUT11 complementation lines (Figure 6E). These results indicate that demethylesterified HG degradation contributes to the extremely reduced demethylesterified HG level in g10g11. Demethylesterified HG degradation together with the reduced HG level might lead to efficient binding of LM20 on highly methylesterified HG and brighter LM20 fluorescence in g10g11 than Col-0 (Figure 6, B and C). Similar phenomena were observed in the PGX3 overexpression plants (Rui et al., 2017). These results suggest that GAUT10 and GAUT11 modulate not only pectin biosynthesis, but also HG de-methylesterification and degradation in GC walls, providing molecular evidence for the altered stomatal dynamics in the g10g11 mutant.
PME6 mutation rescues the increased stomatal dynamics in g10g11
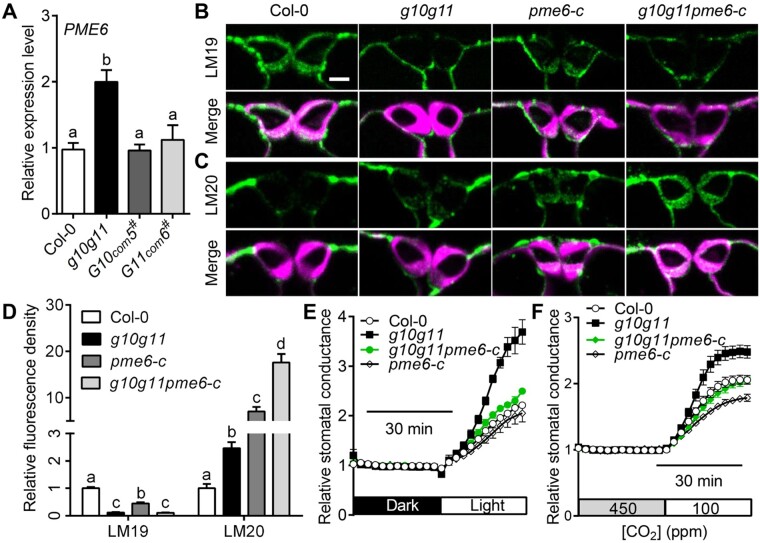
PME6 and PME34 are vital PMEs that are highly expressed in GCs and crucial for stomatal movement (Amsbury et al., 2016; Huang et al., 2017). We, therefore, examined PME6 and PME34 expression in Col-0, g10g11, and GAUT10 or GAUT11 complementation lines. RT-qPCR analyses showed that PME6 expression was significantly greater in g10g11, and recovered to a similar level as Col-0 in the GAUT10 or GAUT11 complementation lines (Figure 7A). However, PME34 expression was not altered in g10g11 (Supplemental Figure S7A). These results suggest that PME6 upregulation may be a cause for the increased PME activity and stomatal dynamics in g10g11.
Figure 7.
PME6 mutation rescues the stomatal hypersensitivity of g10g11. A, PME6 expression level in Col-0, g10g11, GAUT10, or GAUT11 complementation plants. Values are means ± se, three biological replicates; P < 0.05, one-way ANOVA, and Tukey’s test. B, Immunolabeling of demethylesterified HG (LM19) of GC walls from Col-0, g10g11, pme6-c, and g10g11pme6-c plants. Top, LM19 labeling; bottom, LM19 labeling (green) merged with Calcofluor White signal (magenta). Bars = 5 µm. C, Immunolabeling of highly methylesterified HG (LM20) of GC walls from Col-0, g10g11, pme6-c, and g10g11pme6-c plants. Top panels, LM20 labeling; bottom panels, LM20 labeling (green) merged with Calcofluor White signal (magenta). Bars = 5 µm. D, LM19 and LM20 immunolabeling fluorescence intensity in the GC walls of 3- to 4-week-old Col-0, g10g11, pme6-c, and g10g11pme6-c plants. Values are means ± se, and letters represent significant difference (n ≥ 20 GCs per genotype; P < 0.05, one-way ANOVA and Tukey’s test). E and F, Relative stomatal conductance in 3- to 4-week-old Col-0 controls, g10g11, pme6-c, and g10g11pme6-c plants in response to shifts in light intensity or CO2 concentrations. Experiments are repeated 3 times. Values are means ± se, n ≥ 3 leaves per genotype per experiment.
To prove this, we generated a new pme6 allele, pme6-c, using a CRISPR-Cas9 system (Ma et al., 2015). pme6-c had a 648-bp deletion in the PME6 genomic DNA, which prevented normal PME6 translation (Supplemental Figure S8, A and B). Immunolabeling assays showed that pme6-c had a reduced level of demethylesterified pectin indicated by LM19 binding and a greater level of highly methylesterified pectin by LM20 binding compared with Col-0 (Figure 7, B–D), consistent with a previous study (Amsbury et al., 2016). The methylesterified and demethylesterified pectin levels in g10g11 were less than those in pme6-c (Figure 7, B–D), in accordance with the reduced pectin synthesis in g10g11 (Figure 2B). The demethylesterified HG was still extremely reduced in g10g11pme6-c GC walls, similar to g10g11. However, a strong distribution of highly methylesterified pectins was observed in g10g11pme6-c GC walls, more than that in pme6-c (Figure 7, B–D), suggesting that PME6 mutation increases the methylesterified HG level in g10g11 GC walls.
Interestingly, PME6 mutation complemented the increased stomatal dynamic response in g10g11 to CO2 changes, as well as dark to light transition (Figure 7, E and F; Supplemental Figure S9, A and B). Because GAUT10 and GAUT11 mutations altered the stomatal dimension and size, we also observed stomatal morphology in g10g11pme6-c plants. g10g11pme6-c plants had a stomatal complex size similar to g10g11 (Supplemental Table S5), in agreement with a previous study that pme6 mutation did not alter the stomatal dimension (Amsbury et al., 2016). These results suggest that PME6 upregulation is the main cause for the increased stomatal dynamics in g10g11, but not a major contribution for stomatal size and dimension. These findings demonstrate that GAUT10 and GAUT11 control GC wall flexibility, at least partially, through PME6 modulation on pectin methylestrification to mediate stomatal dynamics.
PGX3 mutation cannot restore stomatal dimension and dynamics in g10g11
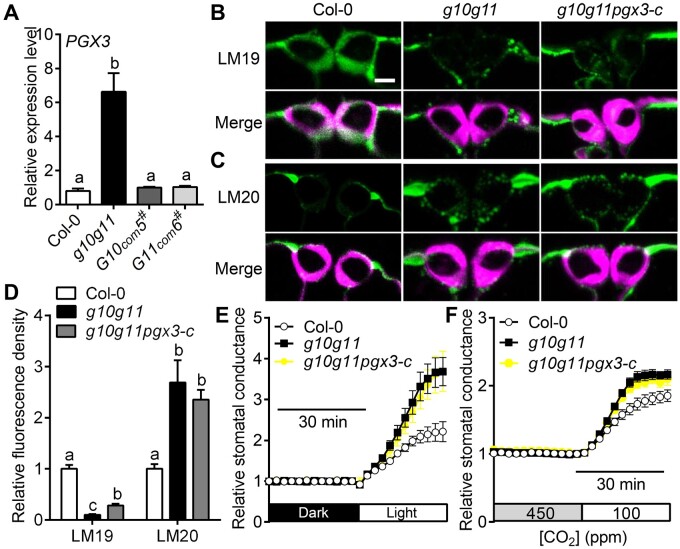
PME6 mutation rescued g10g11 stomatal dynamics, but not the stomatal size and dimension (Figure 7; Supplemental Table S5), and g10g11 exhibited greater PG activity (Figure 6E), indicating that increased PG activity and demethyesterified pectin degradation may contribute to stomatal size and dimension changes. To determine which enzyme contributes to the increased PG activity, we first analyzed several PG genes that were reported to regulate plant growth and development. RT-qPCR analyses showed that the expression levels of PGX2, PGX3, ADPG2, and QRT2 genes were increased in g10g11 (Figure 8A; Supplemental Figure S7B), indicating that upregulation of these PGs could be partially responsible for the increased PG activity in g10g11, which may degrade the demethylesterified HG in g10g11 GC walls. PGX3 modulates stomatal pore dimensions and stomatal dynamics, whose overexpression led to a larger stomatal size in cotyledons and accelerated stomatal opening (Rui et al., 2017), similar to g10g11 (Figures 4 and 5). We then generated a PGX3 mutation by a CRISPR–Cas9 system in the g10g11 background (Wang et al., 2015), in which 460 bp was deleted in the PGX3 genomic DNA, resulting in a mutated form of PGX3 (Supplemental Figure S8, A and B). The phenotypic characterization of the isolated pgx3-c mutant by crossing with Col-0 showed that the mutated PGX3 gene was dysfunctional, in which stomatal dimension in cotyledons was reduced and cotyledon shape was changed (Supplemental Figure S10), consistent with a previous study (Rui et al., 2017). Immunolabeling assays showed that PGX3 mutation slightly increased LM19 fluorescence in the g10g11 GC walls (Figure 8, B–D), demonstrating that PGX3 upregulation contributes to demethylesterified HG degradation and extremely reduced demethylesterified HG level in g10g11 GC walls. However, the g10g11pgx3-c triple mutant showed comparable [CO2] or light-induced stomatal opening compared with g10g11 (Figure 8, E and F; Supplemental Figure S9, C and D), suggesting that PGX3 upregulation is not the major reason for the increased stomatal dynamics in g10g11. Furthermore, g10g11pgx3-c triple mutant exhibited a similar stomatal morphology in both mature leaves and cotyledons (Supplemental Tables S3 and S5). Since PGX3 regulation of stomatal morphology is only in cotyledons and the total PG activity was not significantly decreased in g10g11pgx3-c (Supplemental Figure S11C), these results indicate that there could be other PGs or functional redundancy in PGs, such as PGX2, ADPG2, and QRT2 identified here, which are responsible for the increased PG activity in the g10g11 GC walls and stomatal morphology.
Figure 8.
PGX3 mutation cannot restore the hypersensitive stomatal dynamics of g10g11. A, PGX3 expression level in 3-week-old rosette leaves from Col-0, g10g11, G10com5#, and G11com6# plants. Values are means ± se, three biological replicates; P < 0.05, one-way ANOVA, and Tukey’s test. B, Immunolabeling of demethylesterified HG (LM19) of GC walls from Col-0, g10g11, and g10g11pgx3-c plants. Top panels, LM19 labeling; bottom panels, LM19 labeling (green) merged with Calcofluor White signal (magenta). Bars = 5 µm. C, Immunolabeling of highly methylesterified HG (LM20) of GC walls from Col-0, g10g11, and g10g11pgx3-c plants. Top panels, LM20 labeling; bottom panels, LM20 labeling (green) merged with Calcofluor White signal (magenta). Bars = 5 µm. D, LM19 and LM20 immunolabeling fluorescence intensity in the GC walls of 3- to 4-week-old Col-0, g10g11, and g10g11pgx3-c plants. Values are means ± se and letters represent significant differences (n ≥ 20 GCs per genotype; P < 0.05, one-way ANOVA and Tukey’s test). E and F, Relative stomatal conductance in 3- to 4-week-old Col-0, g10g11 and g10g11pgx3-c plants in response to shifts in light intensity or CO2 concentrations. Experiments are repeated 3 times. Values are means ± se, n ≥ 3 leaves per genotype per experiment.
Discussion
GC development involves differential cell divisions and cell wall thickening. As the major cell wall constituents, the pectin composition determines cell wall mechanical properties, which play a crucial role in regulating cell growth and movement in various plant physiological and developmental processes. In this study, we identify that two GAUTs GAUT10 and GAUT11, are involved in GC pectin synthesis and essential for stomatal development and stomatal dynamics in Arabidopsis (Figure 9).
Figure 9.
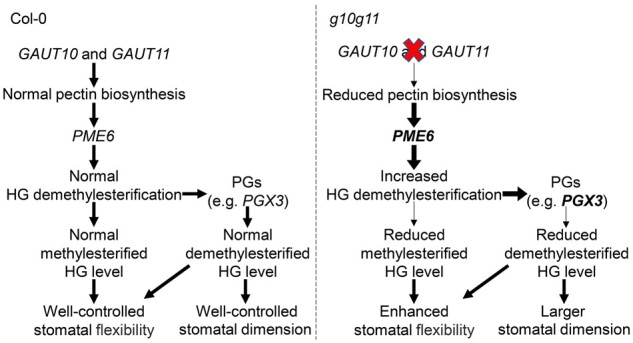
Model of GAUT10 and GAUT11 in regulation of stomatal development and dynamics. Pectin is synthesized by GAUTs (GAUT10 and GAUT11) in a highly methylesterified form in Golgi apparatus and then delivered to the GC wall. Highly methylesterified HG is demethylesterified by PMEs (e.g. PME6), demethylesterified HG can either be cross-linked with Ca2+ or be degraded by PGs (e.g. PGX3), which coordinate to control cell wall property. In Col-0 plants, the mechanical properties of GC wall are maintained at normal levels due to the normal amounts of methylesterified and demethylesterified HG, allowing well-controlled stomatal development and dynamics. In g10g11 mutant, reduced pectin synthesis promotes PME6 expression and HG demethylesterfication, which is in favor of PGs upregulation and PG activity on demethylesterfied HG degradation, finally leading to reduced methylesterified and demethylesterfied HG levels in GC walls. The reduced pectins together with the reduced demethylesterfied HG level loosen the cell wall and increase cell expansion, leading to enlarged stomatal dimension and size. A reduction in pectin synthesis combined with a major contribution of reduced methylesterfied HG level and minor contribution of demethylesterfied HG level leads to increased stomatal flexibility. PME6 and PGX3 in bold mean their expressions are activated. The thick arrows indicate the processes are enhanced and the thin arrows indicate the processes are reduced.
GAUT1-related proteins are key players in the synthesis of pectins in plant primary cell walls (Mohnen, 2008; Caffall and Mohnen, 2009). Defects in cell wall structures have been observed in several reported GAUT1-related mutants, such as gaut13gaut14 heterozygous (Wang et al., 2013), irx8 (Persson et al., 2007), qua1 (Bouton et al., 2002), gatl5 (Kong et al., 2013), and gaut5−/−gaut6−/−gaut7+/− mutants (Lund et al., 2020), resulting in defective plant growth phenotypes. GAUT10 and GAUT11 belong to the GAUT family, and are essential for sugar-mediated root meristem maintenance and release of seed coat mucilage (Voiniciuc et al., 2018b; Pu et al., 2019), respectively. We show that GAUT10 and GAUT11 play important roles in plant growth and development. The g10g11 double mutant showed reduced pectin level and exhibited growth retardation, with slightly smaller plant size, shorter hypocotyl, and primary root, reduced fertility, and smaller seed size. g10g11 also exhibited fragile leaf blades. However, no obvious growth defects were found in the gaut10-3 and gaut11-3 single mutants at our growth conditions (Figure 3), suggesting the functional redundancy of GAUT10 and GAUT11 in these processes. Considering that GAUT10 and GAUT11 exhibited consistent expression patterns in various tissues at different plant development stages and their involvement in pectin synthesis (Figure 2B;Supplemental Figure S1A), and the phenotypes were rescued by GAUT10 or GAUT11 expression (Figure 2C), we conclude that GAUT10 and GAUT11 have functional overlap in multiple physiological and developmental processes through regulation of cell wall physical properties and are required for plant growth and development.
High expression of GAUT10 and GAUT11 in the stomatal lineage cells (Figure 1) indicates their involvement in pectin deposition in GC walls during stomatal development. The g10g11 double mutant exhibited an increased stomatal dimension compared with Col-0 (Figure 4, A and B) and these phenotypes were recovered by GAUT10 or GAUT11 expression (Supplemental Table S2), suggesting that the reduced pectin level may loosen the cell wall and increase cell expansion, leading to a larger stomatal size in g10g11 (Figure 4A). pme6 mutation did not affect stomatal morphological phenotypes in g10g11 (Supplemental Table S5), suggesting that the methylesterified HG level is not the main reason for the increased stomatal size and dimension. There is increasing evidence that the degradation of demethylesterified pectins by HG-degrading enzymes (e.g. PGs and PLs) and GC pressurization make contributions to pore initiation and enlargement (Rui et al., 2017, 2019). g10g11 had increased total PME and PG activities (Figure 6, A and E), and increased expression of PME6 and PGs (Supplemental Figure S7), which may contribute to the increased stomatal size and pore enlargement. Knocking out PGX3, whose overexpression produced a larger stomatal dimension in cotyledons as in g10g11 (Rui et al., 2017), slightly increased demethyesterified HG level in GC wall (Figure 8, B–D), but did not restore g10g11 stomatal morphology in either cotyledons or mature leaves (Supplemental Tables S3 and S5). Considering that the genes encoding PMEs, PGs, and PLs exist in large families (Kim et al., 2006; González-Carranza et al., 2007; McCarthy et al., 2014; Sénéchal et al., 2014) and several PG encoding genes, such as PGX2, ADPG2, and QRT2, were upregulated in g10g11 (Supplemental Figure S7B), ablation of one gene might not be enough to have a significant effect on stomatal dimension in the g10g11 background, in which the pectin level was reduced. Higher-order mutations of PGs and PLs in the g10g11 background need to be further generated and investigated in the future. The uronic acid levels in GAUT10- or GAUT11-overexpression lines were not obviously increased in our assay, possibly because the uronic acid assays we used are not sensitive enough to detect the small increase of pectin content in the overexpressed plants, since overexpression of GAUT10 and GAUT11 had opposite effect on stomatal behavior and drought performance (Figure 5). GAUT10 or GAUT11 overexpression may have increased pectin content in a very low amount that is not detectable by the assay we used here, and therefore enhanced the cell wall stiffness very slightly, which lead to similar stomatal size in the GAUT10- or GAUT11-overexpressing and Col-0 plants (Supplemental Table S4).
Decrease in pectin synthesis and abnormal stomatal morphology may influence stomatal behavior under various environments. g10g11 mutants showed an increased stomatal dynamic response to external stimuli such as CO2 and light intensity changes (Figure 5, A and B; Supplemental Figure S5, A–D), while GAUT10 or GAUT11 overexpression showed reduced ability to respond to these environmental changes (Figure 5, E and F; Supplemental Figure S5, G and H). Consistent with this, g10g11 plants showed enhanced drought tolerance, whereas GAUT10 or GAUT11-overexpressing plants were sensitive to drought stresses (Figure 5, G and H). The opposite stomatal behavior and drought performance correspond with GAUT10 and GAUT11 expression levels, suggesting the essential role of GAUT10 and GAUT11 and pectin abundance in stomatal dynamics. The simultaneous loss of GAUT10 and GAUT11 promoted HG demethylestrification and degradation, which were caused by the increased PME and PG activity and enzyme expression (Figure 6, A and E; Supplemental Figure S7). Therefore, the more flexible g10g11 stomatal movement could be the combined result of these multiple factors. The synergistic actions of PME and PG activity are important for the maintenance of cell wall integrity, PME activity is spatially and temporally modulated in response to environmental changes, HG demethylesterification provides substrates for HG-degrading enzymes (PGs and PLs), and releases protons that promote PG activity, resulting in demethylesterified HG degradation and cell wall loosening (Moustacas et al., 1991; Hocq et al., 2017). Increased PME activity accompanied by an increased PG activity has been reported to promote pectin degradation and stomatal dynamics in both feeding assays and genetic mutants. For example, treatment with both PME and PG enzymes promoted wider stomatal opening during fusicoccin application, and reversed the impaired stomatal movement caused by arabinan degradation (Jones et al., 2003, 2005). A pme34 mutant had greatly increased PME activity under a lethal heat-stress treatment and also an irregular increase of PG activity, which allows pectin degradation and increased stomatal opening (Huang et al., 2017; Wu et al., 2017). Similar to heat stress, reduced pectin synthesis in g10g11 may also be a stress for plants, which produces a feedback that promotes upregulation of PMEs and random HG demethylesterification, leading to the enhanced PG activity on demethylesterified HGs degradation and GC wall loosening, thus increasing the stomatal dynamic response to environmental changes (Figure 5, A and B; Supplemental Figure S5, A–D). Further mutation of PGX3 increased the demethylesterifed HG level in g10g11, suggesting that PGs act downstream of PMEs to regulate pectin modification in cell walls. Our results together with these previous findings suggest that coordination of PME and PG are important in regulating GC wall flexibility and stomatal response.
A pme6 mutant had increased levels of highly methylesterified pectin and impaired stomatal dynamics (Amsbury et al., 2016). Knocking out PME6 in g10g11 not only enhanced the methylesterified HG level indicated by LM20 in GC walls, but also greatly restored the increased stomatal dynamics (Figure 7). Together with that demethylesterified HG was still absent in the GC walls of g10g11pme6-c (Figure 7, B–D), these results suggest that the rescue of stomatal dynamics in g10g11pme6-c mutant may be mainly due to the increase of highly methylesterified HG level in GC walls (Figure 7, C and D). PGX3 overexpression showed increased stomatal dynamics (Rui et al., 2017), similar to the g10g11 mutant (Figure 5, A and B; Supplemental Figure S5, A–D), whose mutation in g10g11 increased demethylesterified HG level but did not recover the increased stomatal dynamics (Figure 8). These findings support the previous studies that HG demethylesterification helps increase cell wall elasticity and elevated HG methylesterification inhibits cell growth and movement (Peaucelle et al., 2011; Amsbury et al., 2016; Jonsson et al., 2021). This further suggests the key determinant role of the methylesterified HG level in regulation of stomatal dynamics at the genetic level. Our results also suggest that the increased stomatal dynamics in g10g11 are not associated with stomatal morphological changes (Figure 5; Supplemental Tables S2 and S4). In the GAUT10 or GAUT11 overexpressing plants, stomatal dimension, and PME activity were not changed (Supplemental Figure S3B; Supplemental Table S4), but stomatal dynamics were impaired (Figure 5, E and F; Supplemental Figure S5, G and H). It is possible that excessive pectin can regulate the GC wall matrix and embed more pectin polymers in the cell wall frame, thus changing the cell wall properties and limiting the stomatal response to stimuli.
Mutations of GAUT10 and GAUT11 resulted in reduced pectin synthesis and enhanced HG demethylesterification in g10g11 mutant plants (Figures 2, B and 6, A). Demethylesterified HG can be degraded by pectin-degrading enzymes and pectin/PLs (Xiao et al., 2014; Hocq et al., 2017; Rui et al., 2018), our experimental results showed that PG activity was increased in g10g11 (Figure 6E). Thus the reduced pectin synthesis and degradation of demethylated pectin led to the extremely reduced demethylesterified HG level (LM19) in g10g11 (Figure 6, B and D). g10g11 should have reduced level of methylesterified HG in GCs; however, our immunolabeling assay showed brighter LM20 labeling (Figure 6, C and D). Similar phenomena have been reported in other studies, in which overexpression of PGX3 also led to enhanced LM20 labeling (Rui et al., 2017), and a higher degree of HG methylesterification was observed in the PGX1AT cell walls (Phyo et al., 2017). The brighter LM20 labeling in g10g11 may be caused by the extremely low level of demethylesterified HG, which lead to the highly methylesterified HG being more accessible to LM20 for efficient labeling, or that highly methylesterified HG could not be degraded by pectin degrading enzymes. A recent report has shown that Arabidopsis PL gene PL LIKE12 (PLL12) is required for normal stomatal dynamic by modulating GC wall modulus and turgor pressure and fine-tuning the levels of calcium crosslinked pectin (Chen et al., 2021). Although both PG and PL enzymes degrade demethylesterified HG, PLL12, and PGX3 alter stomatal dynamics differently, and the substrate specificity between PG and PL might be different (Rui et al., 2017; Chen et al., 2021). In this study, we did not examine whether expression levels of PL genes and calcium crosslinked pectin levels were changed in the g10g11 mutant. It is worthwhile to explore whether PL genes play a role in stomatal function of g10g11 mutant in the future.
In conclusion, our findings identify two components that regulate HG synthesis and modulate stomatal development and dynamics, through modulation of PME6 and PGs expression (Figure 9). In Col-0 plants, methylesterified and demethylesterified HG in GC walls is maintained at normal levels, which cause well-controlled stomatal development and dynamics. Mutations of GUAT10 and GAUT11 reduce pectin synthesis, which increases the expression levels of PME6 and PGs, thus leading to the reduced methylesterified and demethylesterfied HG levels in GC walls. The reduced demethylesterfied HG level loosens the cell wall and increases cell expansion, leading to enlarged stomatal dimension and size. A reduction of pectin synthesis, together with reduced methylesterfied and demethylesterfied HG levels leads to increased stomatal flexibility. Our findings also provide insight into the mechanisms of pectin synthesis, pectin modification, and degradation in GC walls to regulate stomatal development and dynamics. Additionally, we show that the methylesterfied HG is important in stomatal dynamics but not in stomatal development, such as stomatal size and dimension.
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) mutant seeds (gaut10-3 and gaut11-3 [Col-0 background]), were ordered from the ABRC (www.Arabidopsis.org). Surface-sterilized seeds were sown on 1/2MS plates (0.5 g/L MES, 2.2 g/L MS salts, 1% [w/v] sucrose, and 0.8% [w/v] agar, pH 5.6) or pots containing a mixture of perlite, vermiculite, and peat, and kept at 4°C in dark for 3–5 d. Plants were grown in a well-controlled growth room at 21°C with 60% relative humidity under long-day conditions (16-h light/8-h dark).
Phylogenetic analyses and gene expression profile analyses
Arabidopsis GAUT sequences were obtained from Araport11 (Cheng et al., 2017). Alignments were performed using the MUSCLE method, and phylogenetic tree was constructed by MEGA X using the neighbor-joining method (Kumar et al., 2018). Tree reliability was evaluated by the bootstrap method (2,500 replicates). For expression profile analyses of GAUTs in stomatal lineage cells, the microarray data were extracted from the eFP Browser (Winter et al., 2007). For each GAUT, its expression level in meristemoids was set as 1, and the heat map was constructed by TBtools (Chen et al., 2020).
RNA isolation and gene expression analyses
Rosette leaves from 3- to 4-week-old Arabidopsis were used to isolate RNA for transcript analyses. Leaf tissues were ground to a fine powder in liquid nitrogen for total RNA extraction by Trizol regent (Simgen, Hangzhou, China). Genomic DNA was removed by DNase I (TaKaRa, Shiga, Japan) treatment and first-strand cDNAs were synthesized from 2 µg DNase-treated RNA using the Reverse Transcription kit (Promega, Madison, WI, USA). Real-time PCR was performed using a CFX96 TM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. ACTIN7 was used as an internal control.
GUS expression and staining
The spatial expression patterns of GAUT10 and GAUT11 were examined by GUS expression analyses. About 2-kb fragment upstream of the start codon was used to generate the pGAUT10::GUS and pGAUT10::GUS constructs. For GUS staining, plant tissues were immersed in buffer containing 50 mM sodium phosphate, pH 7.2, 0.2% (v/v) Triton X-100, 2 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, and cyclohexylammonium salt (X-Gluc), and incubated at 37°C in the dark for 2–16 h. Tissues were decolorized in 70% ethanol for several times, and images were captured using a microscope (TS100, Nikon, Japan).
Measurement of stomatal complex and pore size
To measure the stomatal complex size and pore dimension, abaxial epidermal layers from 3- to 4-week-old leaves were imaged by a microscope (TS100, Nikon, Japan). Stomatal pore area, stomatal pore length, GC pair height, and GC width were measured by ImageJ software. At least 60 stomata were counted.
PME activity assay
PME activity assay was carried out according to a previous study (Richard et al., 1994). Rosette leaves of 3- to 4-week-old plants were ground to a fine powder in liquid nitrogen, then 200 µL PME extraction buffer (0.1 M citrate acid, 0.2 M Na2HPO4, and 1 M NaCl, pH 5.0) was added. The homogenate was incubated on ice for 1 h and the supernatant was collected by centrifugation at 4°C with 12,000 rpm for 15 min. Protein extracts were incubated in 1 mL of substrate solution containing 0.5% citrus pectins (Sigma-Aldrich, St Louis, MO, USA), 0.2 M NaCl, and 0.002% methyl red, pH 6.8, at 37°C for 1 h. The absorbance of the solution was measured by a spectrophotometry at 525 nm. A calibration curve was obtained by replacing the protein extracts with 5–30 µL of 0.01 M HCl to 1 mL of substrate solution. PME activity (nmol H+/min/mg protein) was calculated based on this standard curve.
PG activity assay
Protein was extracted according to a previous study (Xiao et al., 2014). Briefly, leaf tissues were ground into a fine powder in liquid nitrogen and then mixed with extraction buffer containing 50 mM Tris–HCl (pH 7.5), 3 mM EDTA, 2.5 mM DTT, 2 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and 1 M NaCl, and 10% (v/v) glycerol. The homogenized materials were incubated at 4°C for 1 h and the supernatant was collected by centrifugation at 20,000 g and 4°C for 30 min. PG activity was measured by the increase in reducing end groups from polygalacturonicacid (poly-GalUA) (Sigma-Aldrich) by a spectrophotometer. The sample solution (50 µL) was incubated with 180 µL of 0.1% (w/v) poly-GalUA (in 40 mM sodium acetate buffer, pH 4.4) at 37°C for 10 min. The reaction was stopped by the addition of 1.2 mL of 0.1 M borate buffer (pH 9.0) and 240 µL of 1% 2-cyanoacetamide (Sigma-Aldrich) and heated at 100°C for 10 min. The solution absorbance was measured by a spectrophotometer at 276 nm (Honda et al., 1980). A blank was obtained in the same way by adding sodium acetate buffer solution instead of protein extracts. d-galacturonic acid (Sigma-Aldrich) was used as a standard.
Preparation of AIR and uronic acid assays
Rosette leaves of 3- to 4-week-old plants were ground into a fine powder in liquid nitrogen. The powder was washed twice with 70% (v/v) ethanol and then twice with chloroform/methanol (1:1, v/v). Samples were dried by a vacuum dryer for 2 h and stored at 4°C. Uronic acid content was determined as described (Blumenkrantz and Asboe-hansen, 1973) with modifications. Briefly, samples were mixed with 1 mL concentrated sulfuric acid containing 0.0125 M sodium tetraborate (Sigma-Aldrich) and boiled for 5 min in a 100°C water bath. After cooling to room temperature, the background absorbance was read at 520 nm using a spectrophotometer. About 20 µL of 0.15% (w/v) m-hydroxydiphenyl (Sigma-Aldrich) dissolved in 0.5% (w/v) NaOH was added and incubation at room temperature for 5 min, and then the absorbance was measured at 520 nm again. A calibration curve was obtained by adding 20–100 µg/mL UDP-GalA instead of AIR.
Cellulose content measurement
Cellulose contents were measured as described (Rui and Anderson, 2016). Rosette leaves of 3- to 4-week-old plants were incubated in 80% ethanol overnight at 65°C, and then incubated in acetone at room temperature overnight. Samples were air dried in the fume hood for 4 d and ground into powder. The powder was boiled in a solution containing acetic acid:nitric acid:water (8:2:1) for 30 min. The pellets were collected by centrifuging 12,000 rpm for 5 min and resuspended in 1 mL of 67% sulfuric acid. Fifty microliters of the resuspended samples were incubated with 1 mL of 0.2% anthrone in concentrated sulfuric acid, the absorbance of the solution was measured by spectrophotometry at 620 nm.
Stomatal dynamic analyses
Stomatal conductance in response to CO2 concentration shifts and dark to light transitions was analyzed in intact leaves of 4-week-old plants using a portable gas-exchange system (LI-6400XT, Li-Cor) as reported (Hu et al., 2010). For plant response to CO2 changes, leaves were stabilized at 450 ppm CO2, followed by 100 ppm to stimulate stomatal opening, and then shifted to 1,000 ppm to promote stomatal closure. For dark to light transitions, leaves were equilibrated at 450 ppm CO2 under darkness, shifted to 150 μmol m−2 s−1 light intensity, and then back to darkness again.
Immunolabeling assay
Immunolabeling assay of GC walls was performed as described (Amsbury et al., 2016). About 3- to 4-week-old leaves were trimmed into 3 mm squares and fixed in 4% paraformaldehyde. Samples were then dehydrated in an ethanol series and wrapped in resin-filled gelatin capsules (Electron Microscopy China), and polymerized for 7 d at 37°C. Sections (2-µm thick) were sliced on an ultramicrotome with a glass knife.
For immunolabeling with LM19 (PlantProbes, UK) and LM20 (PlantProbes, UK) antibodies, sections were blocked in phosphate-buffered saline solution (PBS, pH 7.2) with 3% BSA (w/v) for 1 h, and then incubated with a 1:10 dilution primary antibody in PBS buffer with 3% BSA at room temperature for 5 h. Samples were washed with PBS buffer 3 times, then incubated with anti-rat-lgG coupled to fluorescein isothiocyanate (FITC) at a 1:100 dilution in PBS buffer containing 3% BSA in the dark for 1 h, and washed again with PBS buffer 3 times. Samples were counterstained with 0.25% (w/v) Calcofluor White solution (Sigma-Aldrich) for 5 min before observation using a Leica confocal with a 488-nm excitation laser and a 520–550 nm emission filter for FITC signals and a 405 nm excitation laser and a 455 nm emission filter for Calcofluor White signals, the excitation intensity and gains set in the experiment are consistent for all samples.
Statistics
All statistical analyses (Student’s t test, one-way ANOVA, and Tukey’s test) in this study were performed using GraphPad PRISM version 8.0 software.
Accession numbers
Sequence data of genes covered in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: GAUT10 (AT2G20810), GAUT11 (AT1G18580), PME6 (AT1G23200), and PGX3 (AT1G48100).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression pattern and subcellular localization of GAUT10 and GAUT11.
Supplemental Figure S2. Gene structure and transcript analyses of GAUT10 and GAUT11.
Supplemental Figure S3. Total uronic acid content and PME activity are not changed in GAUT10 or GAUT11 overexpression lines.
Supplemental Figure S4. Phenotypic characterization of g10g11 mutant.
Supplemental Figure S5. GAUT10 and GAUT11 modulate stomatal dynamics in response to light and CO2 changes.
Supplemental Figure S6. Control images for immunolabeling in GC walls.
Supplemental Figure S7. Expression levels of PME and PG genes in the g10g11 mutant.
Supplemental Figure S8. Schematic of gene structure and transcript analysis of PME6 and PGX3.
Supplemental Figure S9. PME6 mutation rescues stomatal dynamic response to CO2 and light changes in g10g11.
Supplemental Figure S10. pgx3-c mutant showed defects in seedling development and cotyledon stomatal development.
Supplemental Figure S11. Mutation of PME6 or PGX3 does not affect the increased PME or PG activity in the g10g11 mutant.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. Measurement of stomatal dimensions in g10g11 and GAUT complementation lines.
Supplemental Table S3. Measurement of stomatal dimensions in cotyledons of Col-0, g10g11, and g10g11pgx3-c plants.
Supplemental Table S4. Measurement of stomatal dimensions in Col-0 and GAUT overexpression lines.
Supplemental Table S5. Measurement of stomatal dimensions in mature leaves of Col-0, g10g11, pme6-c, g10g11pme6-c, and g10g11pgx3-c plants.
Supplementary Material
Acknowledgments
The authors thank Dr Zhuqing Zhou (Huazhong Agricultural University) for providing the ultramicrotome.
Funding
This work was supported by grants from the National Natural Science Foundation (31970730, 31771552) and the National Key Research and Development Program of China (2016YFD0100606).
Conflict of interest statement. The authors declare no conflicts of interest.
H.H. conceived and designed the research. H.G., C.X., Q.L., R.L., and Z.Y. performed the experiments. H.H. and H.G. analyzed the data, and wrote the paper. X.Y. discussed the data.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is Honghong Hu (huhh@mail.hzau.edu.cn).
References
- Amos RA, Pattathil S, Yang JY, Atmodjo MA, Urbanowicz BR, Moremen KW, Mohnen D (2018) A two-phase model for the non-processive biosynthesis of homogalacturonan polysaccharides by the GAUT1:GAUT7 complex. J Biol Chem 293: 19047–19063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE (2016) Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol 26: 2899–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA 3rd, Orlando R, Scheller HV, Mohnen D (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan: galacturonosyltransferase complex. Proc Natl Acad Sci USA 108: 20225–20230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TC, Handford MG, Yuseff MI, Orellana A, Dupree P (2001) Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell 13: 2283–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal AK, Atmodjo MA, Li M, Baxter HL, Yoo CG, Pu Y, Lee YC, Mazarei M, Black IM, Zhang JY, et al. (2018) Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat Biotechnol 36: 249–257 [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-hansen G (1973) New methed for quantitative determination of uronic acids. Anal Biochem 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F, Lahaye M, Höfte H, Truong HN (2002) QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14: 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman SE, Schols HA (2018) Interactions between pectin and cellulose in primary plant cell walls. Carbohydr Polym 192: 263–272 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D (2009) Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol Plant 2: 1000–1014 [DOI] [PubMed] [Google Scholar]
- Carter R, Woolfenden H, Baillie A, Amsbury S, Carroll S, Healicon E, Sovatzoglou S, Braybrook S, Gray JE, Hobbs J, et al. (2017) Stomatal opening involves polar, not radial, stiffening of guard cells. Curr Biol 27: 2974–2983 e2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13: 1194–1202 [DOI] [PubMed] [Google Scholar]
- Chen Y, Li W, Turner JA, Anderson CT (2021) PECTATE LYASE LIKE12 patterns the guard cell wall to coordinate turgor pressure and wall mechanics for proper stomatal function in Arabidopsis. Plant Cell doi: 10.1093/plcell/koab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD (2017) Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J 89: 789–804 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2016) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot 67: 463–476 [DOI] [PubMed] [Google Scholar]
- Driouich A, Follet-Gueye ML, Bernard S, Kousar S, Chevalier L, Vicre-Gibouin M, Lerouxel O (2012) Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front Plant Sci 3: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Kirui A, Huang S, Wang L, Barnes WJ, Kiemle SN, Zheng Y, Rui Y, Ruan M, Qi S, et al. (2020) Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell 32: 3576–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA (2007) Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J Exp Bot 58: 3719–3730 [DOI] [PubMed] [Google Scholar]
- Hao Z, Avci U, Tan L, Zhu X, Glushka J, Pattathil S, Eberhard S, Sholes T, Rothstein GE, Lukowitz W, et al. (2014) Loss of Arabidopsis GAUT12/IRX8 causes anther indehiscence and leads to reduced G lignin associated with altered matrix polysaccharide deposition. Front Plant Sci 5: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocq L, Pelloux J, Lefebvre V (2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci 22: 20–29 [DOI] [PubMed] [Google Scholar]
- Honda S, Matsuda Y, Takahashi M, Kakehi K, Ganno S (1980) Fluorimetric determination of reducing carbohydrates with 2-cyanoacetamide and application to automated analysis of carbohydrates as borate complexes. Anal Chem 52: 1079–1082 [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordstrom M, Bohmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Wu HC, Wang YD, Liu CH, Lin CC, Luo DL, Jinn TL (2017) PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol 174: 748–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolie RP, Duvetter T, Van Loey AM, Hendrickx ME (2010) Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr Res 345: 2583–2595 [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ (2005) A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta 221: 255–264 [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100: 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, Lathe RS, Kierzkowski D, Routier-Kierzkowska AL, Hamant O, Bhalerao RP (2021) Mechanochemical feedback mediates tissue bending required for seedling emergence. Curr Biol 31: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Kim J, Shiu SH, Thoma S, Li WH, Patterson SE (2006) Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol 7: R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MR (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Kong Y, Zhou G, Abdeen AA, Schafhauser J, Richardson B, Atmodjo MA, Jung J, Wicker L, Mohnen D, Western T, et al. (2013) GALACTURONOSYLTRANSFERASE-LIKE5 is involved in the production of Arabidopsis seed coat mucilage. Plant Physiol 163: 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboeuf E, Guillon F, Thoiron S, Lahaye M (2005) Biochemical and immunohistochemical analysis of pectic polysaccharides in the cell walls of Arabidopsis mutant QUASIMODO 1 suspension-cultured cells: implications for cell adhesion. J Exp Bot 56: 3171–3182 [DOI] [PubMed] [Google Scholar]
- Lund CH, Stenbaek A, Atmodjo MA, Rasmussen RE, Moller IE, Erstad SM, Biswal AK, Mohnen D, Mravec J, Sakuragi Y (2020) Pectin synthesis and pollen tube growth in Arabidopsis involves three GAUT1 Golgi-anchoring proteins: GAUT5, GAUT6, and GAUT7. Front Plant Sci 11: 585774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, et al. (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8: 1274–1284 [DOI] [PubMed] [Google Scholar]
- McCarthy TW, Der JP, Honaas LA, dePamphilis CW, Anderson CT (2014) Phylogenetic analysis of pectin-related gene families in Physcomitrella patens and nine other plant species yields evolutionary insights into cell walls. BMC Plant Biol 14: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11: 266–277 [DOI] [PubMed] [Google Scholar]
- Mouille G, Ralet MC, Cavelier C, Eland C, Effroy D, Hematy K, McCartney L, Truong HN, Gaudon V, Thibault JF, et al. (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50: 605–614 [DOI] [PubMed] [Google Scholar]
- Moustacas AM, Nari J, Borel M, Noat G, Ricard J (1991) Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. Biochem J 279: 351–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Moriwaki K, Konishi M, Yokoyama R, Nakano T, Kusumi K, Hashimoto-Sugimoto M, Schroeder JI, Nishitani K, Yanagisawa S, et al. (2013) A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr Biol 23: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfila C, Sørensen SO, Harholt J, Geshi N, Crombie H, Truong HN, Reid JS, Knox JP, Scheller HV (2005) QUASIMODO1 is expressed in vascular tissue of Arabidopsis thaliana inflorescence stems, and affects homogalacturonan and xylan biosynthesis. Planta 222: 613–622 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H (2011) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Zhong R, Zhou GK, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye ZH (2007) Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C (2007) The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell 19: 237–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo P, Wang T, Xiao C, Anderson CT, Hong M (2017) Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: insights from solid-state NMR. Biomacromolecules 18: 2937–2950 [DOI] [PubMed] [Google Scholar]
- Pu Y, Walley JW, Shen Z, Lang MG, Briggs SP, Estelle M, Kelley DR (2019) Quantitative early auxin root proteomics identifies GAUT10, a galacturonosyltransferase, as a novel regulator of root meristem maintenance. Mol Cell Proteomics 18: 1157–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L, Qin LX, Gadal P, Goldberg R (1994) Molecular cloning and characterisation of a putative pectin methylesterase cDNA in Arabidopsis thaliana (L.). FEBS Lett 355: 135–139 [DOI] [PubMed] [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Rui Y, Anderson CT (2016) Functional analysis of cellulose and xyloglucan in the walls of stomatal guard cells of Arabidopsis. Plant Physiol 170: 1398–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Chen Y, Kandemir B, Yi H, Wang JZ, Puri VM, Anderson CT (2018) Balancing strength and flexibility: how the synthesis, organization, and modification of guard cell walls govern stomatal development and dynamics. Front Plant Sci 9: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Chen Y, Yi H, Purzycki T, Puri VM, Anderson CT (2019) Synergistic pectin degradation and guard cell pressurization underlie stomatal pore formation. Plant Physiol 180: 66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Xiao C, Yi H, Kandemir B, Wang JZ, Puri VM, Anderson CT (2017) POLYGALACTURONASE INVOLVED IN EXPANSION3 functions in seedling development, rosette growth, and stomatal dynamics in Arabidopsis thaliana. Plant Cell 29: 2413–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Yi H, Kandemir B, Wang JZ, Puri VM, Anderson CT (2016) Integrating cell biology, image analysis, and computational mechanical modeling to analyze the contributions of cellulose and xyloglucan to stomatal function. Plant Signal Behav 11: e1183086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, L'Enfant M, Domon JM, Rosiau E, Crépeau MJ, Surcouf O, Esquivel-Rodriguez J, Marcelo P, Mareck A, Guérineau F, et al. (2015) Tuning of pectin methylesterification: pectin methylesterase inhibitor 7 modulates the processive activity of co-expressed pectin methylesterase 3 in a pH-dependent manner. J Biol Chem 290: 23320–23335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Wattier C, Rustérucci C, Pelloux J (2014) Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. J Exp Bot 65: 5125–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. (2004) Toward a systems approach to understanding plant cell walls. Science 306: 2206–2211 [DOI] [PubMed] [Google Scholar]
- Sterling JD, Atmodjo MA, Inwood SE, Kumar Kolli VS, Quigley HF, Hahn MG, Mohnen D (2006) Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc Natl Acad Sci USA 103: 5236–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling JD, Quigley HF, Orellana A, Mohnen D (2001) The catalytic site of the pectin biosynthetic enzyme alpha-1,4-galacturonosyltransferase is located in the lumen of the Golgi. Plant Physiol 127: 360–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J (2008) Unique aspects of the grass cell wall. Curr Opin Plant Biol 11: 301–307 [DOI] [PubMed] [Google Scholar]
- Voiniciuc C, Engle KA, Günl M, Dieluweit S, Schmidt MHW, Yang JY, Moremen KW, Mohnen D, Usadel B (2018b) Identification of key enzymes for pectin synthesis in seed mucilage. Plant Physiol 178: 1045–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Pauly M, Usadel B (2018a) Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol 176: 2590–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang W, Wang YQ, Liu YY, Wang JX, Zhang XQ, Ye D, Chen LQ (2013) Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant 6: 1131–1148 [DOI] [PubMed] [Google Scholar]
- Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ (2015) Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Larman MG, Montgomery LT, Taylor JE, Hetherington AM (2001) The role of calcium in ABA-induced gene expression and stomatal movements. Plant J 26: 351–362 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An "Electronic Fluorescent Pictograph" browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfenden HC, Baillie AL, Gray JE, Hobbs JK, Morris RJ, Fleming AJ (2018) Models and mechanisms of stomatal mechanics. Trends Plant Sci 23: 822–832 [DOI] [PubMed] [Google Scholar]
- Wu HC, Huang YC, Stracovsky L, Jinn TL (2017) Pectin methylesterase is required for guard cell function in response to heat. Plant Signal Behav 12: e1338227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Somerville C, Anderson CT (2014) POLYGALACTURONASE INVOLVED IN EXPANSION1 functions in cell elongation and flower development in Arabidopsis. Plant Cell 26: 1018–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Rui Y, Kandemir B, Wang JZ, Anderson CT, Puri VM (2018) Mechanical effects of cellulose, xyloglucan, and pectins on stomatal guard cells of Arabidopsis thaliana. Front Plant Sci 9: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Chen H, Hahn MG, Mohnen D, Xu Y (2010) Evolution and function of the plant cell wall synthesis-related glycosyltransferase family 8. Plant Physiol 153: 1729–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.