Abstract
Recent insights about the transport mechanisms involved in the in and out of calcium ions in plant organelles, and their role in the regulation of cytosolic calcium homeostasis in different signaling pathways.
The transport of Ca2+ across the membranes of subcellular compartments contributes to cytosolic Ca2+ homeostasis as well as environmental and developmental responses.
Introduction
Plants are continuously subjected to environmental changes, such as fluctuating light, the day/night cycle, oscillating temperatures, water availability, and interaction with other organisms. Often these variations can be adverse, being stressful and thus affecting plant growth, development and, in several cases, causing major yield losses in agriculture. Especially, plants cannot move to a more comfortable environment to survive and they, thus, need to continuously monitor, and possibly anticipate, any upcoming stress (Zhu, 2016).
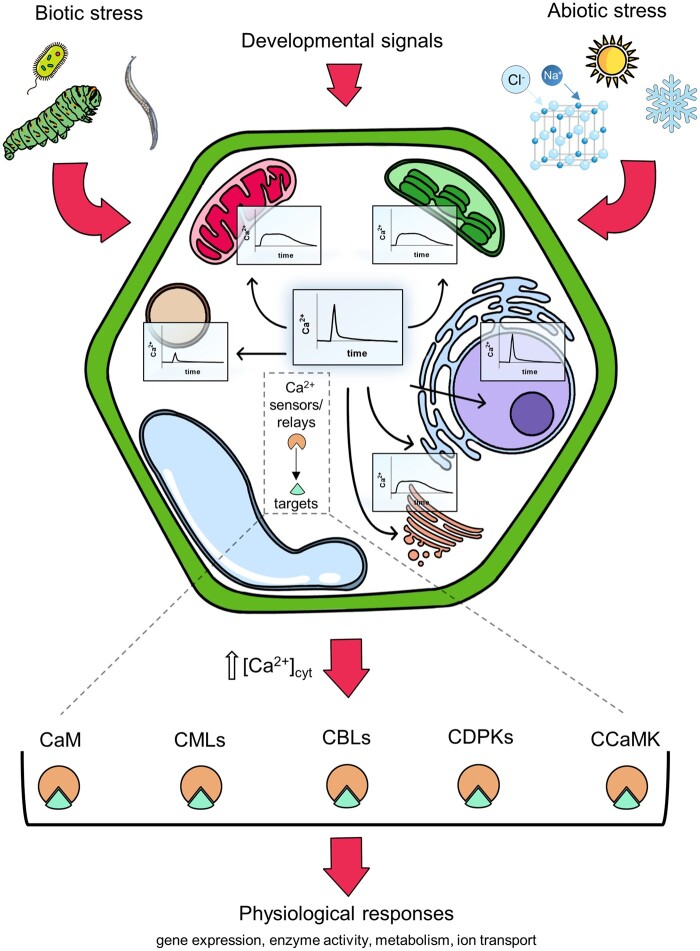
Plants’ early responses to stress often occur in a time frame of seconds or a few minutes in different cell compartments, and in these cases, they mainly rely on quick changes of ions’ concentrations (e.g. Ca2+, H+, K+, NO3−, Cl−) which are dependent upon their movements across membranes (Stephan et al., 2016; Behera et al., 2018; Costa et al., 2018; Demes et al., 2020; Martí Ruiz et al., 2020). Among the ions, it is well-accepted that calcium (Ca2+) plays a key role in many signal transduction pathways (Trewavas and Malhó, 1998; Sanders et al., 2002; Dodd et al., 2010; Kudla et al., 2010, 2018; Tian et al., 2020). At rest, in plant cells, the cytosolic free Ca2+ concentration ([Ca2+]cyt) is in the range of 100–200 nM (Trewavas, 1999; Logan and Knight, 2003; Stael et al., 2012; Jezek and Blatt, 2017), but it can rise steeply (often too low µM concentrations) in response to the perception of different stimuli (Knight et al., 1997; Ranf et al., 2008). Importantly, the stimulus-induced increase in [Ca2+]cyt is not a “digital signal,” but an “analog” one that, depending on the stimulus type and magnitude, assumes a peculiar dynamic often dubbed “cytosolic Ca2+ signature” (Sanders et al., 2002; Kudla et al., 2010, 2018; Tian et al., 2020). The model predicts that different signatures activate different Ca2+ sensors (e.g. Calmodulin [CaM], CaM-like proteins [CMLs], calcineurin β-like proteins [CBLs]), and Ca2+ relays (e.g. Ca2+-dependent protein kinases [CDPKs], or CaM-dependent protein kinase [CCaMK]) that trigger precise and tailored responses, altering gene expression and metabolism (Figure 1; reviewed in Sanders et al., 2002; McCormack et al., 2005; DeFalco et al., 2009; Hamel et al., 2014; Edel et al., 2017; Kudla et al., 2018; Lenzoni et al., 2018; Poovaiah and Du, 2018; Liu et al., 2020; Tang et al., 2020). The understanding of how the Ca2+ signature is generated and shaped to assume its “analog nature” is a key aspect to dissect the roots of Ca2+ signaling, which is traceable to its movement across membranes. Thus, important questions are (1) how do plant cells generate specific Ca2+ signatures? (2) which are the mechanisms for their tight regulation? A simple answer is that generation and shaping of cytosolic Ca2+ signatures is dependent on a coordinate and intertwined activity of Ca2+ permeable channels, Ca2+ transporters, and Ca2+ buffers (Sanders et al., 2002; McAinsh and Pittman, 2009; Michard et al., 2011; Costa et al., 2017; Behera et al., 2018; Wudick et al., 2018; Hilleary et al., 2020; Tian et al., 2020). Whereas it is reported that the major source of cytosolic Ca2+ is the apoplast and that the movement of Ca2+ across the plasma membrane (PM) plays a major role in the generation of cytosolic Ca2+ transients (Gao et al., 2004; Stael et al., 2012; Costa et al., 2018; Tian et al., 2019; Lopez-Hernandez, 2020; Tian et al., 2020), increasing evidence demonstrates that subcellular compartments are involved in the shaping of the signatures, working as Ca2+ sinks (Qudeimat et al., 2008; Costa et al., 2010; Loro et al., 2012; Nomura et al., 2012; Bonza et al., 2013; Nomura and Shiina, 2014; Wagner et al., 2015; Loro et al., 2016; Lenglet et al., 2017; Behera et al., 2018; Corso et al., 2018, Teardo et al., 2019; Hilleary et al., 2020), as well as Ca2+ sources (Wang et al., 2010; Zhu et al., 2010; Tian et al., 2014, Shkolnik et al., 2018). Interestingly, some compartments (i.e. chloroplast and nucleus) can also generate their own organellar Ca2+ signature (Xiong et al., 2004; Loro et al., 2016; Sello et al., 2016; 2018; Kelner et al., 2018; Leitão et al., 2019) adding a additional level of complexity to Ca2+ signaling (Figure 1).
Figure 1.
Biotic and abiotic stress as well as developmental stimuli can trigger cytosolic and organellar [Ca2+] increases. Cytosolic Ca2+ transients are decoded by different Ca2+ sensors and relays, such as CaM, CMLs, CBLs proteins, CDPKs, and CCaMK that trigger precise and tailored responses altering gene expression and metabolism.
ADVANCES
Genetically encoded sensors allow the in vivo analysis of Ca2+ dynamics in different subcellular compartments.
Clear in vivo evidence demonstrated that subcellular compartments accumulate and release Ca2+ ions.
The alteration of Ca2+ transport in the subcellular compartments alters cytosolic Ca2+ signatures with downstream effects on gene regulation and sensitivity to stress.
Direct and indirect evidence supports the role of the ER as both a sink and source of Ca2+.
Indirect evidence supports the role of the vacuole as both a sink and source of Ca2+.
In this update, we will provide readers with an evidence that subcellular compartments experience their own Ca2+ transients in response to different stimuli, with particular attention to describing those cases where their role in the shaping of cytosolic Ca2+ dynamics and in the regulation of downstream responses was experimentally observed. We will give examples to demonstrate that cytosolic and organellar Ca2+ are directly intertwined, as well as that dysfunctions in the transport of other ions can lead to altered cytosolic Ca2+ signatures and visible phenotypes. For simplicity, we will divide our update into four different fields of cellular signaling: (1) plant responses to abiotic stimuli, (2) plant responses to biotic stimuli, (3) stomatal movements, and (4) plant developmental processes. We will not discuss the chloroplast Ca2+ signatures observed in responses to light–dark transitions and in response to heat stress (Sai and Johnson, 2002; Loro et al., 2016; Sello et al., 2016; Frank et al., 2019; Lenzoni and Knight, 2019; Martí Ruiz et al., 2020), as those two mechanisms are extensively covered in the update by He et al. (2021) of this Focus Issue.
Contribution of organelles in the shaping of cytosolic calcium increases in response to salt and osmotic stresses
Plants are particularly sensitive to abiotic stress, and their perception elicits (1) characteristic [Ca2+]cyt increase, (2) activation of protein kinases and phosphatases, (3) modulation of gene expression, and (4) hormone biosynthesis (Gong et al., 1998; van Der Luit et al., 1999; Knight and Knight, 2001; Zhu, 2002; 2016; Tracy et al., 2008; Peleg and Blumwald 2011; Krebs et al., 2012; Yuan et al., 2014; Choi et al., 2014a, 2014b; Wagner et al., 2019). In this section, we will detail recent evidence showing the involvement of different subcellular compartments in the shaping of cytosolic Ca2+ signature in response to abiotic stress, focusing on salt and osmotic stress, and reporting, when available, the consequent downstream effects on the plant’s physiology.
Salt and osmotic stresses are stimuli that induce in plants a quick [Ca2+]cyt increase with dose-dependent as well as tissue-specific responses (Kiegle et al., 2000). The application of these stresses has been efficiently exploited to design genetic screenings that were instrumental in identifying salt- and osmosensors as well as key components involved in the signal transduction (Yuan et al., 2014; Jiang et al., 2019; Chen et al., 2020). Such genetic screenings were based on Arabidopsis (Arabidopsis thaliana) seedlings expressing the genetically encoded Ca2+ sensor aequorin targeted to the cytosol (Box 1; Figure 2). Moreover, the exposure to salt and osmotic stress of Arabidopsis seedlings expressing genetically encoded Ca2+ sensors localized in different subcellular compartments (Box 1; Figure 2; reviewed in Stael et al., 2012; Costa et al., 2018), highlighted that besides the cytosol, also chloroplasts, nongreen plastids, mitochondria, endoplasmic reticulum (ER), Golgi apparatus (GA), and nuclei experience Ca2+ transients (Xiong et al., 2004; Loro et al., 2012; Ordenes et al., 2012; Bonza et al., 2013; Sello et al., 2016; Huang et al., 2017; Corso et al., 2018; Kelner et al., 2018; Teardo et al., 2019). In most of the reported cases, the analyzed subcellular compartments showed Ca2+ transients that followed the [Ca2+]cyt increase, therefore, behaving as sinks and not as sources of Ca2+ (reviewed in Stael et al., 2012; Costa et al., 2018). This result allows us to hypothesize a primary role for subcellular compartments in the shaping of the cytosolic Ca2+ signature rather than in its generation (McAinsh and Pittman, 2009).
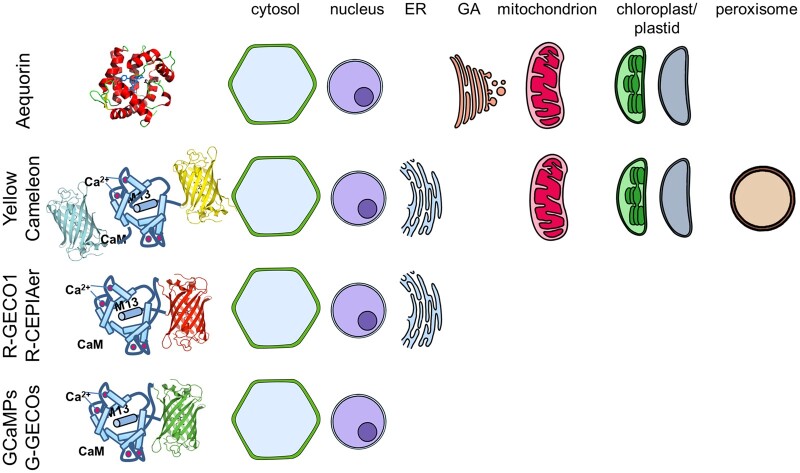
Box 1 Genetically encoded Ca2+ indicators
Genetically Encoded Calcium Indicators (GECIs) allow a non-invasive monitoring of free Ca2+ concentrations [Ca2+] in different subcellular compartments. In plants, the two mainly used GECIs have been aequorin and Cameleon.
Aequorin enables monitoring of Ca2+ dynamics by photon emission measurements in transformed plants after reconstitution of the aequorin holoenzyme with the exogenously applied prosthetic group coelenterazine (Knight et al., 1991). Due to its low quantum yield, aequorin suffers from poor spatial resolution. Aequorin has been targeted to the cytosol, nucleus, endoplasmic reticulum (ER), the Golgi apparatus, mitochondria, chloroplasts, and apoplast (reviewed in Costa et al., 2018). To improve the spatial resolution the use of fluorescent Ca2+ sensors was exploited. The ratiometric Ca2+ reporter Cameleon was the first fluorescent GECI expressed in plant cells (Allen et al., 1999). Cameleon exploits the Förster Resonance Energy Transfer (FRET) property occurring between one fluorescent protein that acts as a donor (e.g. CFP) that, when excited, transfers absorbed energy to a second fluorescent protein, the acceptor (e.g. YFP or cpVenus). The efficiency of FRET depends on the donor-acceptor distance which is dependent upon the CaM-M13 Ca2+-dependent interaction. The CaM-M13 sensor is sandwiched between the two fluorophores (Miyawaki et al., 1997). With Cameleon, the readout is the ratio between acceptor and donor fluorescence emissions which reduces artefacts due to the expression level of the sensor or focus changes. Cameleon has been targeted to the cytosol, nucleus, ER, mitochondria, chloroplasts, and peroxisomes (Costa et al., 2018).
Intensiometric GFP-based Ca2+ sensors have also been developed and successfully used in plants. We can cite GCaMP3, GCaMP6, and the green and red variants of GECO1 or CEPIA (Zhao et al., 2011; Costa et al., 2018; Kelner et al., 2018; Luo et al., 2020). All these GECIs rely on a change in the sensor quantum yield, measured as a change of the intensity of the emitted fluorescence which depends upon the amount of Ca2+ bound to the sensory domain. A change of [Ca2+] affects the CaM conformation which is transmitted to a circularly permuted variant of green (e.g. cpGFP) or red (e.g. cpmApple) fluorescent proteins. Intensiometric biosensors exhibit a higher signal change compared to a FRET-based sensor, but a change of their expression level could be misinterpreted as a change in free Ca2+ concentration (Costa et al., 2018). GCaMPs, R-GECO1 and R-CEPIA have been targeted to the cytosol, nucleus, and ER (Kelner et al., 2018; Luo et al., 2020).
Figure 2.
Overview of organelle-targeted genetically encoded Ca2+ indicators.
Organellar calcium in salt stress responses
Plants expressing genetically-encoded Ca2+ indicators (GECIs) targeted to different subcellular compartments (i.e. aequorin, Cameleon, R-GECO1, and G-GECO1.2; Knight et al., 1991; Miyawaki et al., 1997; Zhao et al., 2011; Box 1; Figure 2 ) were instrumental to show that salt stress induces a transient accumulation of Ca2+ in the cytosol, ER, chloroplasts/plastids and nucleus (Bonza et al., 2013; Sello et al., 2016; Huang et al., 2017; Corso et al., 2018; Kelner et al., 2018; Teardo et al., 2019). Some pieces of evidence support the role of ER, chloroplasts, and vacuoles in the dampening and/or shaping of the cytosolic Ca2+ increase.
The genetic ablation of the ER-localized Arabidopsis Ca2+/cation transporter AtCCX2 (ccx2-knockout [KO] mutant) leads to a reduced salt-induced [Ca2+]cyt increase, and less salt-tolerant plants compared to wild-type. Remarkably, CCX2 overexpression (CCX2-OX) has the opposite effect, with an exacerbation of the [Ca2+]cyt transient maximum and plants more tolerant to salt stress compared to wild-type (Corso et al., 2018). Thus, a link between the magnitude of the Ca2+ signature and plant tolerance was proposed. These data highlight the involvement of AtCCX2 and, therefore, of the ER, in the regulation of cytosolic Ca2+ in response to salt stress (Corso et al., 2018). The study of AtCCX2 topology in the ER membrane, as well as Ca2+ transport analyses in isolated ER fractions from wild-type and mutant plants, should allow us to better define the AtCCX2 mode of action and its physiological function.
The role of chloroplasts in the regulation of [Ca2+]cyt increase in response to salt stress (e.g. NaCl) was also demonstrated. This evidence came from a genetic screen of Arabidopsis mutants defective in salt stress-induced [Ca2+]cyt elevation that allowed the identification of the chloroplast-localized glycosyl-transferase QUASIMODO1 (AtQUA1), a protein involved in the stacking of thylakoids grana. Compared to the wild-type, the qua1-4 mutant exhibited a dramatically greater increase in [Ca2+]cyt under NaCl treatment (Zheng et al., 2017), and this effect was linked to the activity of the chloroplast Ca2+ sensor Calcium Sensing Receptor (CAS; Han et al., 2003; Nomura et al., 2008; 2012 ). There was no measurement of [Ca2+] in the chloroplast stroma or thylakoid lumen of the qua1-4 mutant, thus, we lack experimental evidence that the alteration of [Ca2+]cyt is directly dependent on a deregulated chloroplast Ca2+ handling. Nevertheless, in independent studies, by using Arabidopsis plants expressing the Ca2+ sensor aequorin directed to the chloroplasts/plastids stroma (Box 1; Figure 2), it has been shown that these compartments transiently accumulate Ca2+ in response to salt stress (Sello et al., 2016; Teardo et al., 2019). At the present time, knowledge about the molecular players (Ca2+-permeable channels or transporters in the outer and/or inner membrane) involved in the chloroplast Ca2+ accumulation in response to salt stress is rather limited. The Arabidopsis glutamate receptor-like channel AtGLR3.4 is permeable to Ca2+ (Vincill et al., 2012; Box 2; Figure 3), and besides being localized in the PM (Meyerhoff et al., 2005; Vincill et al., 2012) it was also reported to be present in chloroplasts (Teardo et al., 2011). Two Arabidopsis independent AtGLR3.4 mutant alleles, atglr3.4-1 and atglr3.4-2, showed an impaired NaCl-induced [Ca2+]cyt increase and a more salt-sensitive phenotype during seed germination and postgermination growth compared to wild-type plants (Cheng et al., 2018). However, the fact that data regarding the Ca2+ dynamics in the chloroplasts/plastids of these mutants are lacking, does not allow clear conclusions to be drawn. In addition, no other evidence has supported the chloroplast localization of GLR3.4, thus this result might require validation.
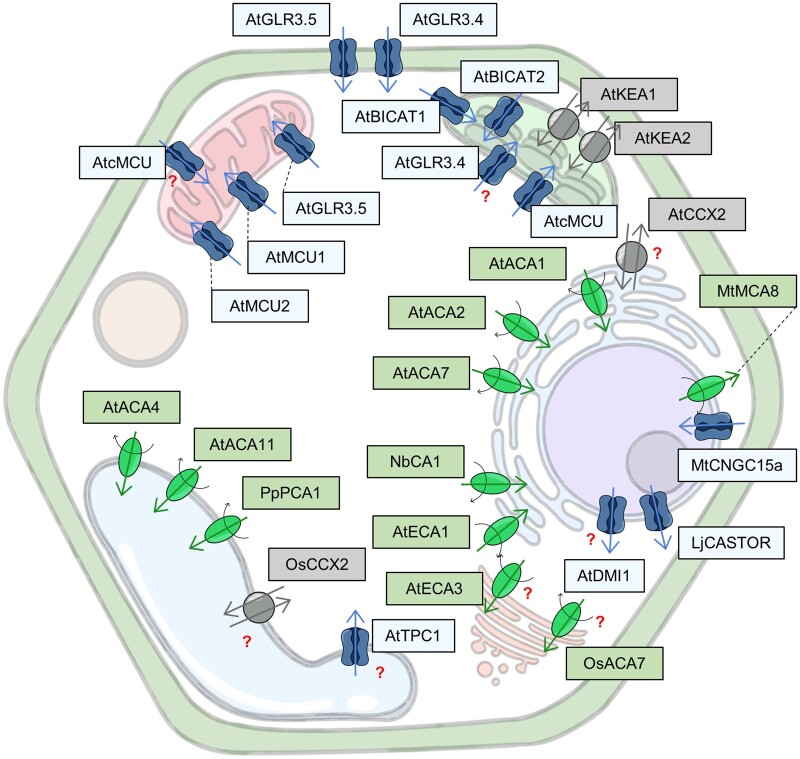
Box 2 Ca2+ transport systems in plants.
Even though plants do not possess canonical Ca2+ channels, they are equipped with Ca2+-permeable channels that establish a hydrophilic path for Ca2+ diffusion down its electrochemical gradient. Different Ca2+-permeable channel families are localized at the PM, facilitating Ca2+ influx from the apoplast, but can be also resident at the inner membranes, such as those surrounding the ER, GA, mitochondria, chloroplasts, vacuole, and possibly peroxisomes.
Ca2+-extruding systems actively remove Ca2+ from the cytosol either by transporting it out of the cell across the PM or into internal organelles. These transporters include Ca2+ pumps and Ca2+/cation antiporters (CaCAs).
Ca2+-ATPases are the major active transport system that ensures the compartmentalization of Ca2+. They are members of the P-type ATPases superfamily that use the energy of ATP hydrolysis to pump Ca2+ from the cytoplasm to the apoplast or into intracellular compartments. Plant cells possess two types of Ca2+-pumping ATPase, belonging to subgroups PIIA-type (ER-type Ca2+-ATPase) and PIIB-type (auto-inhibited Ca2+-ATPase) that have been found at the tonoplast, PM, ER, Golgi, nucleus, and perhaps also at the plastid envelope. While the autoinhibitory regulative mechanism of PIIB-type members has been extensively studied and characterized, very little is known about the regulation of those belonging to PIIA-type subfamily in plants.
The CaCA superfamily comprises cation/Ca2+ exchangers (CCX) and Ca2+/proton exchangers (CAX) that derive the energy needed to mediate active Ca2+ transport from the electrochemical gradient of protons across membranes facing the cytosol.
While Ca2+ pumps are high affinity and low-capacity systems, exchangers are characterized by low-affinity but high-capacity Ca2+ sequestration. The coordinated action of both extrusion mechanisms covers a wide range of physiological cytosolic Ca2+ concentrations.
A detailed analysis of different Ca2+ transport mechanisms across plant membranes has been extensively reviewed in Demidchik et al. (2018).
Figure 3.
Channels and transporters localized in subcellular compartments reported to have a role in plant developmental processes and stress responses linked to the regulation of Ca2+ transport across membranes. The red question marks indicate that further studies are required. Channels are represented in blue, pumps in green, and cotransporters in grey. At, Arabidopsis thaliana; Lj, Lotus japonicus; Mt, Medicago truncatula; Nb, Nicotiana benthamiana; Pp, Physcomitrium patens; Os, Oryza sativa.
The role of intracellular compartments in the shaping of [Ca2+]cyt transients in response to salt stress was also found in the moss Physcomitrium patens (formerly Physcomitrella patens), where the mRNA coding for the tonoplast-localized PIIB-type Ca2+-ATPase (Box 2; Figure 3) PCA1 was shown to be upregulated by NaCl treatment. PCA1 loss-of-function mutants (ΔPCA1) exhibit a sustained elevated [Ca2+]cyt in response to salt, and an enhanced salt susceptibility and higher cell death rates upon treatment. Remarkably, the altered Ca2+ response in the ΔPCA1 lines corresponded with the altered expression level of stress-induced genes, suggesting the disturbance of a stress-associated signaling pathway (Qudeimat et al., 2008). Although direct evidence of an impaired Ca2+ accumulation in the vacuole in the ΔPCA1 was not reported, the well-defined role of PIIB-type Ca2+-ATPases in the transport of Ca2+ out of the cytosol (Box 2) strongly supports a direct role of PCA1 in the restoration of prestimulus [Ca2+]cyt, with the vacuole working as a cytosolic Ca2+ capacitor/buffer (Qudeimat et al., 2008). In Arabidopsis, the two tonoplast-localized PIIB-type Ca2+-ATPase isoforms AtACA4 and AtACA11 (Box 2, Figure 3) have a role in the regulation of [Ca2+]cyt in leaf epidermal cells in response to bacterial flagellin (flg22; see next section; Hilleary et al., 2020), and in the salicylic acid (SA)-dependent programmed cell death pathway (Boursiac et al., 2010), but so far, in plants, no data are available regarding their role in salt stress, even if ACA4 conferred protection against osmotic stress such as high NaCl, KCl, and mannitol when expressed in yeast cells (Geisler et al., 2000). Nevertheless, a key role of the vacuole in the regulation of [Ca2+]cyt in response to this stress was shown in Arabidopsis. Choi et al. (2014b), by challenging Arabidopsis seedling root tip cells with 100 mM NaCl, demonstrated the existence of a long-distance root-to-shoot cytosolic Ca2+ wave responsible for the induction of the expression of stress-regulated genes in the shoot. The role of the vacuole in this process was evidenced by the fact that the ablation of the tonoplast-localized two-pore channel AtTPC1 (Peiter et al., 2005) strongly dampened the speed of the Ca2+ wave, whereas its overexpression boosted it (Choi et al., 2014b). Importantly, upon salt stress, the tpc1-KO mutant did not show the upregulation of stress-related marker genes in the shoot such as the Multi-Stress-Responsive Zinc-Finger Protein (ZAT12) and the CaM-Related Touch 2 (TCH2; Choi et al., 2014a, 2014b). Despite some controversy about a direct involvement of AtTPC1 in the release of Ca2+ from the vacuole (Ranf et al., 2008; Beyhl et al., 2009; Gradogna et al., 2009; Lenglet et al., 2017, Hedrich et al., 2018, Jaślan et al., 2019), these results demonstrate the importance of this compartment in the plant response to salt stress and in the overall strategy that plants adopt to cope with it. Of note, Dindas et al. (2021) have recently proposed, through a modeling approach, that the involvement of AtTPC1 in the long-distance signaling is not due to its direct involvement in the release of Ca2+ from the vacuole, but rather depends on its capacity to transport K+ which is regulated by the vacuolar membrane potential. Thus, in accordance with Jezek and Blatt (2017), the cytosolic Ca2+ signature could be intimately connected with the flux of ions other than Ca2+.
Defining the importance of the subcellular compartments in the shaping of cytosolic Ca2+ signatures in response to salt stress was achieved by an orthogonal approach. Thanks to the expression, in Arabidopsis plants, of the rat Ca2+-binding protein parvalbumin (PV) fused to either a nuclear export (PV-NES) or a nuclear localization sequence (NLS-PV), the cytosolic or nucleosolic Ca2+ were selectively buffered (Huang et al., 2017). When both cytosolic and nuclear Ca2+ levels were monitored in root cells by means of the Cameleon Ca2+ sensor (YC3.6; Box 1; Figure 2), both compartments experienced salt- and osmotic-induced [Ca2+] increases. Buffering of nuclear Ca2+ prevented the nuclear Ca2+ increase but not the cytosolic one, whereas buffering of cytosolic Ca2+ did not affect the nuclear Ca2+ rise. In both cases, in response to salt stress, the length of the primary root was impaired, and the expression of several abiotic stress-induced genes was deregulated, especially when nuclear Ca2+ was buffered (Huang et al., 2017). This latter result strongly supports the idea that the alteration of cellular Ca2+ signatures, in particular at the nuclear level, has a direct effect on the regulation of gene transcription and is in accordance with the demonstration that Ca2+ can regulate gene expression via CDPK and CaM binding transcription factors (CAMTA; Reddy et al., 2011; Dubiella et al., 2013; Gao et al., 2013; Whalley and Knight, 2013; Lenzoni and Knight, 2019; Liu et al., 2020).
Organellar calcium in osmotic stress responses
Salt imposes on plant cells both ionic and osmotic stress, but this latter is per se detrimental for plant growth (Xiong and Zhu, 2002; Yang and Guo, 2018). Both hyperosmotic and hypoosmotic stress elicit [Ca2+]cyt increases (Yuan et al., 2014; Basu and Haswell, 2020). Similar to what occurred with salt stress (see above), the Arabidopsis ccx2-KO or CCX2-OX plants showed a smaller and a higher [Ca2+]cyt increase respectively compared to the wild-type when subjected to both hyperosmotic and hypoosmotic shocks. Moreover, in response to the same stress, the ER of the ccx2-KO accumulated more Ca2+ than the wild-type. Overall, these data demonstrate that the AtCCX2 transporter is involved in the regulation of Ca2+ transport between the ER and cytosol (Figure 3) in response to both hyperosmotic and hypoosmotic stress (Corso et al., 2018). Interestingly, even if localized to the tonoplast, the Oryza sativa (rice) CCX2 (OsCCX2; Figure 3) that mediates Ca2+/cation transport in yeast, is also upregulated in response to drought stress (Yadav et al., 2015). However, at present, no information about its putative role in the regulation of intracellular Ca2+ homeostasis has been reported.
Being triggered by a drop in the soil water potential, hydrotropism can be considered as a response to a sort of osmotic stress (Dietrich, 2018; Fromm, 2019). The involvement of the ER in the regulation of Ca2+ signaling was recently highlighted in the hydrotropic response of Arabidopsis roots. The inhibition of AtECA1, a PIIA-type Ca2+-ATPase (Box 2; Figure 3), by the Myc-Interacting Zinc-Finger Protein 1 (AtMIZ1) determined a slow asymmetric rise of [Ca2+]cyt in the elongation zone of root tip cells which was required for the root bending toward areas of higher water potential. The Arabidopsis eca1-KO and the pharmacological inhibition of AtECA1 by cyclopiazonic acid in wild-type seedlings produced a higher [Ca2+]cyt increase and exacerbated the root bending response, demonstrating the key role of the ER in this signaling pathway (Shkolnik et al., 2018). Remarkably, by using Arabidopsis seedlings expressing an ER-localized Cameleon Ca2+ sensor (CRT-D4ER; Bonza et al., 2013; Box 1; Figure 2), it was shown that the hydrotropic stimulus triggered a decrease in ER luminal [Ca2+], supporting a role for the ER as a Ca2+ source in this process (Shkolnik et al., 2018). This latter result is thus a demonstration of direct involvement of the ER in the generation of a [Ca2+]cyt transient through the inhibition of a Ca2+ pump and not through the opening of Ca2+-permeable channels. A better understanding of how PIIA-type Ca2+-ATPases are fine-tuned is a key issue that will deserve attention in the near future.
A recent report demonstrated that the lack of the so-called AtcMCU, a member of the mitochondrial calcium uniporter (MCU) family (Baughman et al., 2011; De Stefani et al., 2011; Stael et al., 2012; Box 2; Figure 3) localized both in mitochondria and chloroplasts (Teardo et al., 2019), reduced the stromal Ca2+ accumulation in response to hyperosmotic stress, with a consequent higher rise of the [Ca2+]cyt peak compared to the wild-type (Teardo et al., 2019). The lack of activation of mitogen-activated protein kinase 3 and 6 (MAPK3/6) and a deregulated gene expression pattern in the cmcu-KO plants, suggested the hypothesis that AtcMCU is required for a retrograde chloroplast signaling in response to the osmotic stress (Teardo et al., 2019). On the other hand, the AtcMCU presence in mitochondria (Teardo et al., 2019) would suggest that, in the cmcu-KO, the hyperosmotic induced Ca2+ dynamics could be altered also in this compartment, but at the present time, data to support this hypothesis are lacking. Interestingly, AtcMCU did not seem to be involved in the transport of Ca2+ into chloroplasts during salt stress thus suggesting its stress-specific role in the osmotic response (Teardo et al., 2019).
The possibility that plastids/chloroplasts represent a hub for the regulation of cytosolic Ca2+ dynamics in response to osmotic stress is sustained by another recent study as well. The lack of two plastidial envelope-localized K+ exchange antiporters (KEA) , AtKEA1 and AtKEA2 (Figure 3), impaired the rapid hyperosmotic-induced [Ca2+]cyt increase (Stephan et al., 2016). At present, the mechanism responsible for the alteration of the cytosolic Ca2+ signature in the kea1/kea2 mutant is not understood, and a study of stromal Ca2+ dynamics is required.
Based on the cited works, it is evident that ER, vacuole, and chloroplasts/plastids are involved in the response to different abiotic stresses by contributing to shaping the cytosolic Ca2+ dynamics, and that proper nuclear Ca2+ dynamics are important for the regulation of gene transcription. Despite the recent identification of some molecular players involved in the Ca2+ accumulation in mitochondria (Wagner et al., 2015; Teardo et al., 2017; Selles et al., 2018), and the proposed role of this organelle in the salt stress response through retrograde signaling (Vanderauwera et al., 2012), there are no direct demonstrations that an impaired Ca2+ accumulation in this organelle can affect the cytosolic Ca2+ signature in response to abiotic stress.
Even less is known about a possible role of the other compartments, such as the GA, where the Arabidopsis PIIA-type Ca2+-ATPase AtECA3 and the rice PIIB-type Ca2+-ATPase OsACA7 have been localized (Figure 3; Mills et al., 2008; Singh et al., 2014). Interestingly, yeast cells expressing the OsACA7 are less sensitive to salt tolerance (Singh et al., 2014). However, since a clear role of these two pumps in planta has not been reported, further research in this direction is required.
Contribution of organelles in the shaping of cytosolic calcium increases in responses to biotic stimuli
One of the earliest events occurring in different plant biotic interactions is a rise in cytosolic and nuclear [Ca2+] (Blume et al., 2000; Oldroyd and Downie, 2006). Plant biotic interactions can be beneficial, as in the case of symbiosis with Rhizobia and Mycorrhiza, or detrimental as in those with a wide range of pathogens (e.g. Pseudomonas syringae). In this section, we will briefly touch on the role played by the nuclear Ca2+ in symbiotic interactions, and then we will address more extensively recent evidence of how organellar Ca2+ homeostasis is involved in the regulation of cytosolic Ca2+ signatures in response to pathogen elicitors.
Symbiotic interactions have been mainly studied in Leguminosae in which a clear role of nuclear Ca2+ signaling was reported (reviewed in Oldroyd and Downie, 2006; Charpentier, 2018). It is well known that the perception of nodulation (NOD) and mycorrhizal (MYC) factors trigger characteristic nuclear Ca2+ oscillations that are then transduced by the nuclear-localized Ca2+- and CaM-dependent Ser/Thr protein kinase (CCaMK; reviewed in Charpentier and Oldroyd, 2013). As the symbiosis interaction topic and the role played by nuclear Ca2+ is so vast, we redirect the readers to a recent review (e.g. Charpentier, 2018). Nonetheless, it is worth noting that one of the major discoveries in the field, made by Charpentier et al. (2016), was the identification of the Medicago truncatula cyclic nucleotide-gated channel 15 (MtCNGC15) as one of the Ca2+-permeable channels involved in the generation of NOD-induced nuclear Ca2+ oscillations (Box 2; Figure 3). Of note, MtCNGC15, being specifically localized in the nuclear envelope (NE), presumably promotes the release of Ca2+ from the ER directly into the nucleoplasm (Charpentier et al., 2016) even if a Ca2+ depletion from NE was not reported. Previously, it was also demonstrated that the Medicago PIIA-type Ca2+-ATPase MtMCA8 is targeted to the inner NE membrane (Figure 3) and is essential for symbiotic Ca2+ oscillations, suggesting its role in efficient Ca2+ reloading from the nucleoplasm (Capoen et al., 2011). A mathematical modeling approach predicted that besides the need for a Ca2+-permeable channel (i.e. MtCNGC15) and a Ca2+-ATPase (i.e. MtMCA8), the generation of nuclear Ca2+ oscillations requires the activity of a Ca2+-activated K+ channel to counterbalance the movement of positive charges. The Doesn't Make Infections 1 (MtDMI1) channel is permeable to K+, is localized at the NE, and is, therefore, presumably the component needed to counterbalance the charge caused by the influx of Ca2+ into the nucleoplasm (Granqvist et al., 2012). The same group later demonstrated that Ca2+ can diffuse from the nucleus into the cytosol (Kelner et al., 2018), but no physiological function has been ascribed to this [Ca2+]cyt rise.
Pathogen-associated molecular patterns (PAMPs) are recognized by cell surface receptors (pattern-recognition receptors PRR) and activate an array of basal defense responses (PAMP-triggered immunity, PTI; Zipfel and Oldroyd, 2017; Zhang et al., 2020). In response to PAMPs, the plant immune system relies on two parallel signal transduction pathways based on MAPK and Ca2+ signaling leading to transcriptional reprogramming (Boudsocq et al., 2010). In Arabidopsis, the PAMP flagellin (flg22 peptide) induces a quick [Ca2+]cyt increase in leaves and roots (Ranf et al., 2008; Yuan et al., 2017; Hilleary et al., 2020; Emonet et al., 2021). The flg22-induced [Ca2+]cyt rise is suggested to be mediated by different classes of PM-localised Ca2+-permeable channels with evidence supporting a role of both members of glutamate receptor-like channels (GLR) and cyclic nucleotide-gated channels (CNGC) families (Kwaaitaal et al., 2011; Tian et al., 2019; Bjornson et al., 2021). Nonetheless, in guard cells, the flg22-induced [Ca2+]cyt increase is, at least partially, dependent on members of the reduced hyperosmolality-induced [Ca2+]i increase channels (OSCA) family (AtOSCA1.3 and 1.7; Thor et al., 2020) suggesting that multiple families of Ca2+ channels contribute to the overall pattern-induced Ca2+ response in Arabidopsis.
Direct involvement of the subcellular compartments in the generation of the flg22-induced [Ca2+]cyt has been predicted (Ma et al., 2017), but experimental evidence is still lacking. Nevertheless, by using Arabidopsis plants expressing chloroplast-localized aequorin, it was shown that they transiently accumulate Ca2+ in the stroma in response to flg22 and that this stromal Ca2+ transient temporally follows the [Ca2+]cyt increase (Nomura et al., 2012). Similar to what was observed in response to salt stress (see the previous section), the lack of the chloroplast Ca2+ sensor AtCAS affected flg22-induced chloroplast Ca2+ dynamics, and such a CAS-dependent mechanism was involved in transcriptional reprogramming through retrograde signaling (Nomura et al., 2012). Neither clues about the mechanism of Ca2+ transport across chloroplasts’ membranes, nor consequent impairment of the cytosolic Ca2+ signature were reported. The fact that the cmcu-KO had an impaired H2O2-induced chloroplast Ca2+ accumulation (Teardo et al., 2019) makes this channel a putative candidate to mediate the Ca2+ influx in response to flg22, but there is no direct evidence for this as yet.
The impairment of Ca2+ transport across the tonoplast was also shown to impact the cytosolic Ca2+ signature in response to flg22 (Hilleary et al., 2020). The dissection of cytosolic Ca2+ dynamics by using the ultrasensitive Ca2+ sensor Cameleon YC-Nano 65 (Horikawa et al., 2010; Choi et al., 2014b) in epidermal leaf cells of Arabidopsis plants lacking the two tonoplast-localized PIIB-type Ca2+-ATPase AtACA4 and AtACA11 (Figure 3), revealed that this double KO mutant exhibited higher basal [Ca2+]cyt and a larger flg22-induced Ca2+ increase compared to the wild-type. This was accompanied by an upregulation of the defense regulator gene CBP60g and a higher resistance to Pst bacterial challenge at 1 d postinoculation (Hilleary et al., 2020). No attempts to measure vacuole Ca2+ dynamics were carried out. However, the aca4/aca11 mutant phenotype was complemented when the PIIB-type PM localised-Ca2+-ATPase AtACA8, was (mis)targeted to the tonoplast of the mutant. This latter result strongly supports the idea that the observed phenotype of aca4/aca11 was directly dependent on the impairment of Ca2+ import into the vacuole lumen. Very similar observations were made in the Arabidopsis aca1/aca2/aca7 triple KO mutant lacking three ER-localized PIIB-type Ca2+-ATPases. In comparison to wild-type, the triple mutant showed (1) a higher [Ca2+]cyt in leaf cells at rest, (2) an increased magnitude and duration of the [Ca2+]cyt transient induced by flg22 treatment, (3) smaller rosettes, and (4) a high frequency of leaf lesions (Ishka et al., 2021). Similar to the aca4/aca11 mutant, the lesions phenotype of aca1/aca2/aca7 was suppressed by the expression of a transgene encoding NahG, an enzyme that degrades SA (Ishka et al., 2021). Once again these results demonstrate the importance of the fine-tuning of the cytosolic Ca2+ homeostasis both at rest and during the recovery of the prestimulus [Ca2+]cyt, a process mediated by active Ca2+ transporters present in the tonoplast and ER, extruding Ca2+ out of the cytosol (Qudeimat et al., 2008; Costa et al., 2017; Hilleary et al., 2020; Ishka et al., 2021; Box 2; Figure 3).
Besides Arabidopsis, Nicotiana tabacum plants have also been used as a model for several studies investigating the role of cytosolic and organellar Ca2+ in the response to pathogen elicitors. N. tabacum plants are particularly sensitive to cryptogein, a 10-kDa protein secreted by the oomycete Phytophthora cryptogea, that induces a hypersensitive response in Nicotiana tabacum (var. Xanthi) plants and systemic acquired resistance against various pathogens (Bourque et al., 2002). Experiments using cells of Nicotiana tabacum var. Xanthi expressing aequorin localized to the cytosol, chloroplast, and mitochondria showed that cryptogein was effective in inducing specific Ca2+ signatures in each compartment with chloroplasts clearly showing a Ca2+ increase that peaked after the cytosolic one (Manzoor et al., 2012). Interestingly, a pharmacological approach indicated that inositol trisphosphate (IP3) could play a role in the cryptogein-induced Ca2+ signaling, suggesting its possible contribution in promoting a Ca2+ release from intracellular Ca2+ stores (e.g. vacuole or ER; Manzoor et al., 2012). Indeed, the silencing of the N. tabacum ER-localised PIIB-type Ca2+-ATPase NbCA1 (Figure 3) accelerated the cryptogein-induced cell death in leaf cells (Zhu et al., 2010). Importantly, by using a genetically encoded Ca2+ sensor (Case 12) the authors showed that the downregulation of NbCA1 resulted in an altered cryptogein-induced cytosolic Ca2+ signature, with an increase of amplitude and duration of Ca2+ spikes. These findings suggest that the NbCA1 is involved in the Ca2+ efflux pathway that controls cell death during plant innate immune response (Zhu et al., 2010). In addition, other proteinaceous elicitors including flg22 were shown to induce in N. tabacum cells, cytosolic, and nuclear Ca2+ elevations, with this latter apparently dependent on [Ca2+]cyt, IP3, and reactive active oxygen species (ROS; Lecourieux et al., 2005).
The involvement of Ca2+ transport across ER in plant-pathogen interactions was also reported by studying OsXA10, a R gene for resistance to bacterial blight in rice. The OsXA10 protein is localized at the ER membrane where it is assembled as hexamers potentially working as a Ca2+ transporter (Tian et al., 2014). It was shown that the expression of OsXA10 in different systems (e.g. rice, Nicotiana benthamiana, and mammalian HeLa cells) triggered programmed cell death in a mechanism that is dependent on the release of Ca2+ from the ER (Tian et al., 2014). In leaf epidermal cells of N. benthamiana, the expression of the OsXA10-mCherry fusion disturbed the Ca2+ homeostasis, inducing a Ca2+ depletion of the ER accompanied by a [Ca2+]cyt increase. Similar results were also obtained by expressing the OsXA10-RFP fusion in human HeLa cells (Tian et al., 2014). Noticeably, the expression of OsXA10 variants shown to be able to abolish the ER Ca2+ depletion also abolished the programmed cell death in N. benthamiana and HeLa cells as well as the disease resistance in rice (Tian et al., 2014) strongly supporting, in this specific case, the role of ER as a source of Ca2+ in the triggering of a [Ca2+]cyt increase and promoting cell death.
Whereas the OsXA10 protein has an apparent direct role in the plant response to pathogens through the regulation of Ca2+ homeostasis in the ER, other indirect evidence highlighted the importance of proper Ca2+ homeostasis of this intracellular compartment in response to biotic stress. In Arabidopsis, the ER-localized Ca2+ binding protein calreticulin 3 (AtCRT3) is essential for the correct maturation of the EF-Tu receptor (EFR; Saijo et al., 2009) which recognizes the bacterial elicitor elf18 on the cell surface (Zipfel et al., 2006). Although Ca2+ measurements in the ER lumen of the Atcrt3 mutant are lacking, this result provides indirect evidence about the critical role of ER Ca2+ homeostasis in plant immunity.
In conclusion, the involvement of ER, vacuole, and chloroplasts in the regulation of cytosolic Ca2+ homeostasis/Ca2+ transients induced in response to pathogen elicitors has been clearly demonstrated. In contrast, even if N. tabacum plants treated with cryptogein showed an accumulation of Ca2+ in the mitochondrial matrix (Manzoor et al., 2012), evidence of a role for mitochondrial Ca2+ elevations in this physiological context is still missing. However, the fact that a splice variant of the Arabidopsis AtGLR3.5 was localized to the inner mitochondrial membrane and that the glr3.5 mutant showed accelerated leaf senescence (Teardo et al., 2015), allows speculation about a role of mitochondrial Ca2+ transport in the plant responses to biotic stress. On the other hand, the glr3.5 showed a mild phenotype regarding the mitochondrial Ca2+ uptake in response to leaf wounding and no mutant showing a strong impairment in the transport of Ca2+ has been reported yet. The isolation of such a line would represent a good tool to further investigate any possible role of this compartment in plant-pathogen interactions as well as other responses.
Contribution of organelles in the regulation of calcium homeostasis in stomatal movements
Stomata are made of a pair of guard cells present mainly in the leaf epidermis, and through them, plants acquire the CO2 needed for photosynthesis and release O2. When stomatal pores are open, plants, besides exchanging gases, lose water via transpiration, and, therefore, to prevent an excessive loss of water with consequent plant wilting, the opening of the pores must be finely controlled (Taiz et al., 2014).
Stomata open in response to light and close in response to drought stress, elevated CO2, ozone, low humidity, pathogen attack, and other stimuli (reviewed in Kim et al., 2010). The importance of stomata in plant physiology has elected guard cells as a highly developed model system for dissecting signal transduction mechanisms in plants, and for elucidating how individual signaling mechanisms can interact within a network in a single cell (Kim et al., 2010). Indeed, stomatal guard cells represent, together pollen tubes and root hairs (see below) cell models in which Ca2+ signaling has been deeply investigated (reviewed in Kim et al., 2010; Jezek and Blatt, 2017).
The opening and closing of stomata are mechanistically regulated by the coordinated movement across plasma and vacuolar membranes of ions (e.g. K+, Cl−) and molecules (e.g. malate, glucose), which by determining the osmotically driven movement of water, affect cells turgor (Pandey et al., 2007; Wang et al., 2014; Demes et al., 2020; Flütsch et al., 2020a, 2020b; Cubero-Font and De Angeli, 2021). The passage of ions across membranes is dependent upon the activity of different ion channels, the gating of which depends on several actors (e.g. membrane potential, phosphorylation, cytosolic pH, cytosolic Ca2+; reviewed in Jezek and Blatt, 2017). In response to drought, plants synthesize the hormone abscisic acid (ABA) that induces stomatal closure through both Ca2+-dependent and Ca2+-independent signaling pathways (MacRobbie, 1992; Laanemets et al., 2013; Huang et al., 2019). Guard cells were among the first cell types where in vivo analyses of cytosolic Ca2+ dynamics were studied by using firstly, Ca2+ sensitive dyes and later, genetically encoded Ca2+ sensors (McAinsh et al., 1995; Allen et al., 1999; Garcia-Mata et al., 2003). Years of intense studies have shown that ABA, PM hyperpolarization, ROS, external Ca2+, among other stimuli, activate PM Ca2+ permeable channels leading to cytosolic Ca2+ increases (Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003). Interestingly, in guard cells, cytosolic Ca2+ increases often occur in the form of repetitive Ca2+ oscillations whose frequency, duration, amplitude, and transient number, which are linked to fluctuations in the membrane voltage and in ions’ fluxes, regulate the stomatal aperture (Allen et al., 2001; Minguet-Parramona et al., 2016). Experimental evidence demonstrated that in several cases the generation of cytosolic Ca2+ transients and oscillations were dependent upon both an influx of Ca2+ from the apoplast (Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001), and its release from internal stores (Garcia-Mata et al., 2003). Molecules like nitric oxide (NO), cyclic ADP-ribose (cADPR), IP3, and inositol hexakisphosphate (IP6) were shown to activate endomembrane channels, thus promoting Ca2+ release from internal stores (Alexandre et al., 1990; Muir and Sanders, 1996; Wu et al., 1997; Leckie et al., 1998; Grabov and Blatt, 1999; Garcia-Mata et al., 2003; Lemtiri-Chlieh et al., 2003; reviewed in Jezek and Blatt, 2017). Of note, ABA activates the Ca2+ permeable channels at the PM (Hamilton et al., 2000; Pei et al., 2000, Murata et al., 2001; Kwak et al., 2003) and the consequent influx of Ca2+ into the cytosol stimulates the Ca2+ release from intracellular stores, a process described as Ca2+-induced Ca2+ release (CICR; Grabov and Blatt, 1998, 1999). While computational and modeling approaches have indicated that the release of Ca2+ from internal stores may account for more than 95% of the Ca2+ entering the cytosol during Ca2+ transients increase (Chen et al., 2012; Wang et al., 2014; Minguet-Parramona et al., 2016), the molecular identity of channels or transporters mediating this Ca2+ release lags behind.
Since the slow vacuolar ion channel coded by the AtTPC1 gene (Peiter et al., 2005) is permeable to both K+ and Ca2+ and its activity is modulated by cytosolic Ca2+, AtTPC1 was one of the main candidates predicted to be involved in the CICR process (Hedrich and Neher, 1987; Ward and Schroeder, 1994; Peiter et al., 2005; Gradogna et al., 2009; Dindas et al., 2021). Although data showed that AtTPC1 was required for the inhibition of the light-induced stomatal opening in the presence of external ABA (Peiter et al., 2005), it was later demonstrated that the tpc1 null mutant closes stomata like the wild-type in response to ABA, and Methyl Jasmonate (MeJA), but not in response to external Ca2+ (Islam et al., 2010). Interestingly, when cytosolic Ca2+ was monitored in Arabidopsis guard cells expressing the Cameleon Ca2+ sensor (Box 1, Figure 2), tpc1 did not show any difference in the Ca2+, ABA, and MeJA induced cytosolic Ca2+ oscillations compared to the wild-type (Islam et al., 2010). Thus, apparently, in guard cells a direct contribution of AtTPC1 in the CICR process is negligible.
On the other hand, the importance of the transport of ions across the tonoplast for the proper regulation of cytosolic Ca2+ dynamics has been demonstrated. The Arabidopsis De-Etiolated 3 mutant (det3) lacking the subunit C of the vacuolar H+-ATPase showed, in guard cells, altered cytosolic Ca2+ dynamics in response to external Ca2+ and ROS (i.e. H2O2) compared to wild-type (Allen et al., 2000). To be precise, whereas in the wild-type external Ca2+ and H2O2 induced characteristic cytosolic Ca2+ oscillations, the det3 mutant showed sustained cytosolic Ca2+ increases and did not close the stomata (Allen et al., 2000). Conversely, in the same mutant, ABA was effective in inducing stomatal closure, showing a wild-type pattern of cytosolic Ca2+ oscillations (Allen et al., 2000). Thus, it is plausible that the det3 mutant, which is defective in H+ transport across the tonoplast, also presents an impaired Ca2+ uptake in the subcellular compartments such as the vacuole and possibly the ER (Schumacher et al., 1999; Allen et al., 2000). The demonstration that several Ca2+ transport systems, including CAXs and Ca2+-ATPases (Box 2) catalyze a Ca2+/H+ exchange (Bonza and De Michelis, 2011; Demidchik et al., 2018) supports the prediction that defects in H+ transport can also affect Ca2+ transport and its homeostasis (Hills et al., 2012; Wang et al., 2014; Jezek and Blatt, 2017; Dindas et al., 2021). At present, to the best of our knowledge, no attempts to investigate vacuolar and ER Ca2+ dynamics during stomatal movements have been made. As a consequence, the analysis of Ca2+ dynamics in these two compartments is an important challenge for future research. Available mutants like the Arabidopsis aca4/aca11, and aca1/aca2/aca7 (Hilleary et al., 2020; Ishka et al., 2021) already represent an important genetic resource to perform analyses in this direction. In support of this, analyses of stomatal conductance carried out in these two mutants showed kinetics that slowed significantly on repeated CO2 elevations compared to the wild-type. These results underpin an important role played by the pools of endomembrane Ca2+ in a sort of a “carbon memory” of stomatal responsiveness to light and CO2 that ultimately affects photosynthesis and water use by the plant (Jezek et al., 2021). Besides ER and vacuole, analyses of nuclear, mitochondrial, peroxisomal, and chloroplasts Ca2+ dynamics have been carried out in guard cells, but their contribution to the regulation of stomatal movements is still missing. Stimuli, such as hyperosmotic and hypoosmotic stress also induce, besides a [Ca2+]cyt increase, nuclear and mitochondrial [Ca2+] rises (Loro et al., 2012) and PM hyperpolarization triggers cytosolic and peroxisomal Ca2+ increases (Costa et al., 2010). Of note, it was demonstrated that the Arabidopsis cmcu-KO mutant failed to close the stomata in response to the osmotic stress and lost water faster compared to the wild-type under drought stress (Teardo et al., 2019). Unfortunately, analyses of cytosolic and chloroplasts Ca2+ dynamics in cmcu-KO guard cells are lacking. On the other hand, the dual localization of cMCU to both chloroplasts and mitochondria would also require the analysis of mitochondrial Ca2+ dynamics. As an intriguing component of the chloroplast Ca2+ regulatory network, the Ca2+ sensing protein CAS localized in chloroplast thylakoid membranes has been associated with guard cell dynamics. In particular, the lack of AtCAS activity in Arabidopsis, prevents the cytosolic Ca2+ increase in guard cells and the stomatal closure induced by the administration of external Ca2+ (Nomura et al., 2008; Weinl et al., 2008). Nevertheless, the channels and/or Ca2+ transporters involved in this mechanism are unknown.
The large size of guard cells’ chloroplasts and the fact that their imaging is relatively easy, allowed Ca2+ imaging analyses in single organelles (Loro et al., 2016). This analysis showed that the transition from high-to-low-intensity blue light (needed to excite the Cameleon Ca2+ sensor; Box 1, Figure 2), induces a transient Ca2+ increase in the stroma. Interestingly, the stromal [Ca2+] rise was characterized by two components: (1) a series of Ca2+ spiking superimposed to (2) a gradual sustained Ca2+ increase (Loro et al., 2016). These Ca2+ spikes were dependent on the availability of cytosolic Ca2+ and were not synchronized between individual chloroplasts of the same cell. In contrast, the gradual sustained Ca2+ increase occurred independently of cytosolic Ca2+, suggesting an intraorganellar Ca2+ release (Loro et al., 2016). At present, the molecular identity of the channels and/or transporters involved both in the generation of the sustained and transient stromal Ca2+ increases are not known, but the Arabidopsis AtBICATs, and AtcMCU channels are obvious candidates (Figure 3; Frank et al., 2019, Teardo et al., 2019).
In the future, the combination of modeling, in vivo imaging, and genetics will be instrumental to better define the role played by the subcellular compartments in the stomatal movements.
Contribution of organellar/cytosolic calcium homeostasis in plant developmental processes
As reported above organellar Ca2+ dynamics have been mainly studied in relation to plant responses to both abiotic and biotic stresses. Less is known about their functions during developmental processes, and only a few cases have reported on the roles of different subcellular compartments in the regulation of cytosolic Ca2+ dynamics during development. Nevertheless, plants have at least two well-known systems where a precise spatiotemporal regulation of [Ca2+]cyt is required for the execution of the proper growth processes: pollen tubes (PTs) and root hairs (RHs; Schoenaers et al., 2017). While PTs provide for water-independent propagation of species, RHs confer an increase in root surface area which enhances nutrient and water uptake and better anchor plants to the soil. PTs and RHs are filamentous cells that grow unidirectionally by depositing flexible cell wall material at one side of the cell and exploiting turgor pressure as the driving force for elongation, a process also referred to as tip growth (Schiefelbein et al., 1993; Feijó et al., 2004). Although these tip-growing systems are very similar, they also display differences in their growth-related molecular machineries (Feijó et al., 2004).
Role of organellar calcium in directing pollen tube tip growth
Flowering plant reproduction comprises several sequential steps from pollination to fertilization. PTs growth is essential for reproduction, and they have long been considered outstanding models for cell biology for a variety of reasons. One important feature of PTs is their prominent dependence on ion dynamics to promote and regulate growth (Michard et al., 2017). PTs appropriately adjust turgor pressure by adapting to changes in external osmolarity (Hill et al., 2012). In 1963, it was revealed that Ca2+ is essential for in vitro PTs cultures (Brewbaker and Kwack, 1963), and the relationship between [Ca2+] and PTs growth has been extensively examined. By using both Ca2+ sensitive dyes or genetically encoded Ca2+ sensors, it was evident that a tip-focused [Ca2+]cyt gradient occurred in growing PTs showing regular oscillations that correlate with growth (Holdaway-Clarke et al., 1997; Iwano et al., 2009; Michard et al., 2011). It was also shown that the perturbation of the tip [Ca2+] gradient may affect the growth as well as the viability of PTs (Feijó et al., 2001; Zhang et al., 2007; Winship et al., 2017). As in other cell systems, there is clear evidence that the dynamics of Ca2+ signals in PTs are largely controlled by Ca2+ influx and Ca2+ efflux through the PM (Box 2). Just to cite some examples, GLRs (Michard et al., 2011; Wudick et al., 2018), CNGCs (Frietsch et al., 2007; Gao et al., 2016; Pan et al., 2019), and isoforms of PIIB-Ca2+-ATPase (Schiøtt et al., 2004; Ishka et al., 2021) were demonstrated to be required for the proper PT elongation and growth. In a few cases, it was also demonstrated that growth defects were linked to deregulated tip [Ca2+] oscillations (Michard et al., 2011, Chen et al., 2015).
Knowledge about the role of subcellular compartments in the regulation of the tip-Ca2+ dynamics is rather limited, but some research has highlighted a potential role of ER and mitochondrial Ca2+ in this process (e.g. Holdaway-Clarke et al., 1997; Colaço et al., 2012; Ishka et al., 2021). Of interest, was the demonstration that the treatment of PTs with caffeine reversibly stops their growth and dissipates the tip-Ca2+ gradient (Pierson et al., 1996; Diao et al., 2018). In mammalian cells, caffeine is known to promote the release of Ca2+ from the ER through the activation of IP3 receptors (IP3Rs; Taylor and Tovey, 2010; Foskett et al., 2007). Thus, it is reasonable to hypothesize that its effect on pollen could depend on a disturbance of cytosolic Ca2+, dependent on a Ca2+ release from the ER. However, no IP3Rs have been identified in superior plants (Edel et al., 2017), and no caffeine-dependent ER-Ca2+ release has been reported so far. Nonetheless, the role of Ca2+ transport across the ER membrane in the regulation of PTs growth was first highlighted by Iwano et al. (2009) who analyzed Ca2+ dynamics in the cytosol and ER lumen of Arabidopsis PTs by using Cameleon Ca2+ sensors localized in the two compartments (YC4.6 and YC3.6 in the ER and in the cytosol, respectively; Iwano et al., 2009). The administration of cyclopiazonic acid that inhibits the activity of ER-localised PIIA-type Ca2+-ATPases triggered the PTs’ growth arrest and an ER luminal [Ca2+] decrease (Iwano et al., 2009). This result allowed the authors to ascribe a role of ER in the fine regulation of the tip-focused [Ca2+]cyt gradient (Iwano et al., 2009). To go deeper inside the ER/cytosolic Ca2+ relationships during PTs growth, simultaneous observations of Ca2+ dynamics in these two compartments would be required. Whereas there is no genetic evidence that the lack of the ER PIIA-type Ca2+-ATPase AtECA1 activity compromises pollen development and plant fertility, a very recent work showed that the ablation of three ER-localized PIIB-type Ca2+-ATPases, AtACA1, 2, and 7 strongly compromised pollen fertility, with a decrease in pollen transmission efficiency (Ishka et al., 2021). Single knockout mutants did not show any phenotype demonstrating the existence of functional redundancy (Ishka et al., 2021). Neither cytosolic nor ER Ca2+ dynamics were however analyzed.
As reported in the previous sections, plant mitochondria accumulate Ca2+ possibly through the activity of the MCU complex (MCUC; Wagner et al., 2015; Teardo et al., 2017), but a demonstration that in planta this complex represents the main route for Ca2+ import is still missing. Nevertheless, in Arabidopsis, there are six MCU isoforms (Stael et al., 2012) and experimental evidence supports a role for AtMCU1 and AtMCU2 in the regulation of pollen germination. Selles et al. (2018) showed that AtMCU1 and AtMCU2 are expressed in the vegetative cell and in the germinated PTs but were not detectable in the embryo sac. Remarkably, pollen grains germination and PTs elongation were reduced in vitro in mcu2-KO mutants and this ultimately led to the limited paternal transmission of mcu2-KO to the progeny. No reproductive phenotype could be detected in mcu1-KO mutants. These results tend to suggest that mitochondrial Ca2+ homeostasis is an additional player in pollen germination and PTs growth. However, no measurements of both cytosolic and mitochondrial Ca2+ were carried out in the PTs of these mutant lines, thus the conclusion that the observed phenotype is directly linked to a deregulated Ca2+ transport could not be drawn yet. Similar to what was suggested for the ER, simultaneous analysis of Ca2+ dynamics during PTs growth in the two compartments would be desirable.
Another link in support of a role played by the Ca2+ handling by subcellular compartments in the regulation of PT growth came from the indirect evidence that tonoplast-localized Ca2+ sensors are required to control the developmental process. In Arabidopsis, the calcineurin B-like proteins AtCBL2 and AtCBL3 are significantly expressed in pollen and PTs. AtCBL2 and AtCBL3 were described as interacting with the AtCIPK12 at the tonoplast, with evident phenotypic consequences at the level of vacuolar morphology and PTs’ polar growth upon mutation or overexpression of CBL2/3 or CIPK12 (Steinhorst et al., 2015). Interestingly, Tang et al. (2020) demonstrated that seedlings of the Arabidopsis cbl2/cbl3 mutant were severely stunted under low K+ conditions, a phenotype due to an impairment in K+ remobilization from vacuoles. Therefore, it is tempting to speculate that a similar mechanism could be present in PTs. Because AtCBL2 and AtCBL3 are attached to the tonoplast facing the cytosolic side, it is conceivable that they are sensitive to changes in the cytosolic Ca2+ in the tonoplast microdomain (Knight et al., 1996). At present, there is no evidence that the Ca2+ regulation of the CBL2/3-CIPK12 complex in the tonoplast is released from the vacuole, but this is a suggested hypothesis that may require further research.
Role of organellar calcium in directing root hair tip growth
RHs are tubular extensions from the surface of root epidermal cells (Schoenaers et al., 2017). This “hairlike” morphology is essential for its role in nutrient and water uptake, plant anchoring in the soil, and microbial interactions (Véry and Davies, 2000; Charpentier and Oldroyd, 2013). In RHs, the tip [Ca2+] gradient (Wymer et al., 1997; Monshausen et al,. 2007; 2008; Candeo et al., 2017) is maintained by an influx of extracellular Ca2+ through hyperpolarization-activated plasma membrane-localized Ca2+ channels (Véry and Davies, 2000), and CNGCs (Brost et al., 2019). Indeed, similarly to PTs, [Ca2+]cyt has emerged as a key component controlling the RHs growth and its perturbation alters the RHs’ proper developmental program (Monshausen et al., 2008). It is conceivable that as in PTs, also in RHs the organellar Ca2+ transport can impinge on the cytosolic Ca2+ dynamics. However, at present, the information available regarding their possible contribution is very scarce. Attempting a comparison with PTs, we might predict that in RHs, the ER and mitochondria Ca2+ transport could also affect the developmental process. Only a few pieces of evidence support a possible role of mitochondria in the RHs development. Wang et al. (2010) demonstrated that treatments of Arabidopsis RHs with latrunculin B or jasplakinolide, which depolymerize and polymerize actin filaments respectively, decreased the mitochondrial inner membrane potential, triggered a release of Ca2+ from the mitochondria, and induced a [Ca2+]cyt increase in the RHs (Wang et al., 2010). Moreover, the authors highlighted the presence of a [Ca2+] gradient in mitochondria from the tip to the base of the RH that was disrupted by the drugs. Even if it is suggestive of a role of mitochondria in the regulation of [Ca2+]cyt in RHs development, genetic evidence in support of this hypothesis is lacking. Teardo et al. (2017) analyzed the roots of seedlings of the mcu1-KO and AtMCU1 overexpressing plants without reporting any abnormalities in the RHs development. However, Arabidopsis has six MCU isoforms and at least two of them (AtMCU1 and AtcMCU) are expressed in RHs (source: eFP browser; Winter et al., 2007) and all are expressed in roots (source: Geneinvestigator; Hruz et al., 2008; Klepikova et al., 2016), therefore the absence of clear RHs phenotypes might be due to functional redundancy. The generation of higher order mcu mutants should be pursued to analyze their involvement in mitochondrial Ca2+ regulation during RHs development.
Role of calcium in root tip growth
Primary root tip growth is another plant development program that seems to be subject to the regulation of [Ca2+]cyt dynamics by subcellular compartments. Leitão et al. (2019) showed that in Arabidopsis, nuclear Ca2+ signaling is elemental for proper primary root development, including meristem development and auxin homeostasis. The genetic ablation of the NE localized AtDMI1, a functional analog of MtDMI1 in root symbioses thus a putative ion channel permeable to K+ that counterbalances the charge caused by the influx of Ca2+ into the nucleus (Granqvist et al., 2012), was able to modulate the nuclear Ca2+ signatures, and primary root development (Leitão et al., 2019; Figure 3). However, the molecular identity of the Ca2+ permeable channel responsible for the generation of the nuclear Ca2+ transient is still unknown. Based on structural homology analyses, AtDMI1 can be classified as a BK channel (Kim et al., 2019). Interestingly, the LjCASTOR BK channel of Lotus japonicus, when expressed in HEK293 cells as a truncated form, lacking the N-terminus and two transmembrane domains, was shown to be both permeable to and regulated by Ca2+ (Kim et al., 2019). Based on this piece of evidence we might speculate that AtDMI1 could also be permeable to Ca2+ but this needs to be experimentally demonstrated. Nevertheless, it would be of major interest to directly monitor the Ca2+ in the NE to have direct proof that this compartment, in continuity with the ER, is responsible for the observed nucleoplasm Ca2+ transient.
Conclusions
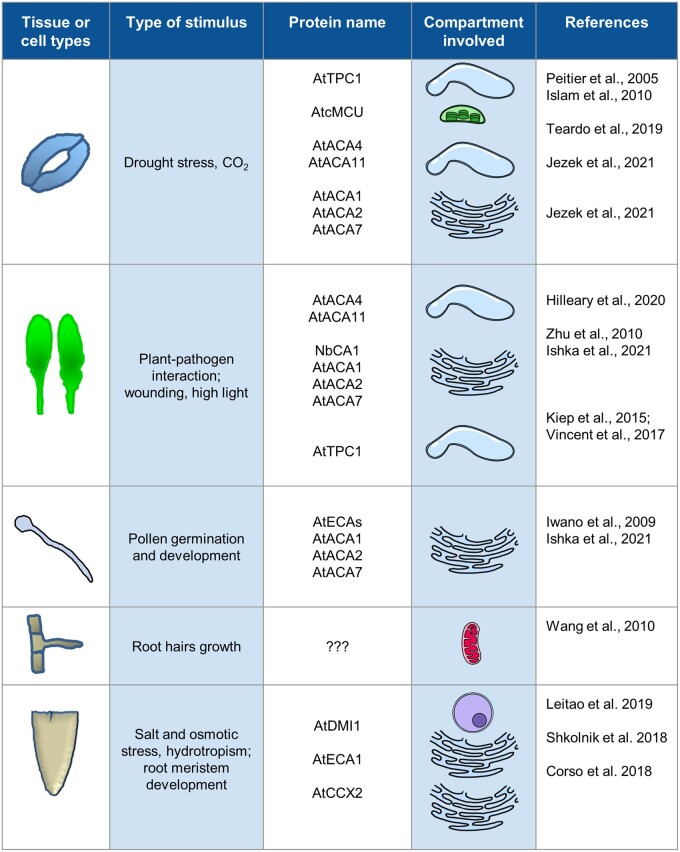
Since the publication of the seminal review from McAinsh and Pittman (2009) in which the authors provided and discussed the first available evidence about the role of subcellular compartments in the regulation of the cellular Ca2+ homeostasis in plant cells, much progress has been made. In this Update, we have recapitulated the experimental proofs collected in recent years supporting the role of the intracellular compartments in the generation and shaping of [Ca2+]cyt increases observed in response to environmental and developmental stimuli (Figure 4). Forward and reverse genetic approaches coupled to the use of imaging tools have now shed light on key roles played by vacuole, chloroplast/plastid, and ER/NE in the shaping of cytosolic Ca2+ dynamics in different physiological/stressful conditions. In some cases, channels and transporters directly involved in the movement of Ca2+ across the membranes of these compartments were also identified (Box 2; Figure 3). However, despite this increased knowledge, understanding of the role played by mitochondria in the regulation of cytosolic Ca2+ dynamics is still limited. Equally, the organelles’ contribution in Ca2+-dependent stomatal movements needs experimental confirmation (see “Outstanding questions”).
Figure 4.
Summary of the Ca2+ transport mechanisms localized in the subcellular compartments of plant cells involved in the regulation of cytosolic Ca2+ dynamics for which a physiological response in different plant tissues/cell types was demonstrated.
Outstanding questions
How are the Ca2+ transport systems of different subcellular compartments regulated?
Does mitochondrial Ca2+ transport impinge on cytosolic dynamics in the responses to stress or in developmental processes?
What is the role of AtcMCU in mitochondria?
What is the role of GA in Ca2+ signaling?
What is the role of AtECA3 and OsACA7 in the Golgi apparatus?
Which are the organellar Ca2+ transport systems involved in the regulation of stomatal movements?
Can the design of new genetic screenings help to identify new Ca2+ transporters of subcellular compartments?
Much work needs to be done. Forward genetic screening based on the use of recently developed organelle localized Ca2+ sensors (Box 1; Figure 2), could provide a valuable strategy for the identification of additional players involved in the accumulation and release of Ca2+ from internal stores. Moreover, besides using model plants, the identification and the definition of the physiological role of intracellularly localized Ca2+ permeable channels and transporters should be extended to important crop species such as rice, for which preliminary results are already available (Singh et al., 2014).
Acknowledgments
This update is dedicated to the memory of our friend and colleague Hillel Fromm who passed away on 12 October 2020. We apologize to all authors of papers not mentioned in this manuscript due to space limitations.
Funding
This work was supported by Piano di Sviluppo di Ateneo 2019 (University of Milan; to A.C.), by Ministero dell’Istruzione, dell’Università e della Ricerca Fondo per Progetti di ricerca di Rilevante Interesse Nazionale 2017 (grant no. PRIN 2017ZBBYNC; to M.C.B.).
Conflict of interest statement. The authors declare no conflict of interest.
A.C. conceived the project and wrote the main body of the article. F.R. wrote the part on developmental processes. M.G. prepared Figures 1 and 3. M.C.B. prepared Box 2. All authors made comments on the manuscript, which were integrated by A.C. All authors contributed to the article and approved the submitted version.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Alex Costa (alex.costa@unimi.it).
References
- Alexandre J, Lassalles JP, Kado RT (1990) Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-trisphosphate. Nature 343: 567–570 [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 28: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Basu D, Haswell ES (2020) The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr Biol 30: 2716–2728 [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Zhaolong X, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Morris RJ, Schwarzländer M, Costa A (2018) Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 30: 2704–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhl D, Hortensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R (2009) The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J 58: 715–723 [DOI] [PubMed] [Google Scholar]
- Bjornson M, Pimprikar P, Nürnberger C, Zipfel (2021) The transcriptional landscape of Arabidopsis thaliana pattern-triggered immunity. Nat Plants 7: 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonza MC, De Michelis MI (2011) The plant Ca2+-ATPase repertoire: biochemical features and physiological functions. Plant Biol (Stuttg) 13: 421–430 [DOI] [PubMed] [Google Scholar]
- Bonza MC, Loro G, Behera S, Wong A, Kudla J, Costa A (2013) Analyses of Ca2+ accumulation and dynamics in the endoplasmic reticulum of Arabidopsis root cells using a genetically encoded Cameleon sensor. Plant Physiol 163: 1230–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque S, Lemoine R, Sequeira-Legrand A, Fayolle L, Delrot S, Pugin A (2002) The elicitor cryptogein blocks glucose transport in tobacco cells. Plant Physiol 130: 2177–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF (2010) Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol 154: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50: 859–865 [Google Scholar]
- Brost C, Studtrucker T, Reimann R, Denninger P, Czekalla J, Krebs M, Fabry B, Schumacher K, Grossmann G, Dietrich P (2019) Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J 99: 910–923 [DOI] [PubMed] [Google Scholar]
- Candeo A, Doccula FG, Valentini G, Bassi A, Costa A (2017) Light sheet fluorescence microscopy quantifies calcium oscillations in root hairs of Arabidopsis thaliana. Plant Cell Physiol 58: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoen W, Sun J, Wysham D, Otegui MS, Venkateshwaran M, Hirsch S, Miwa H, Downie JA, Morris RJ, Ané JM, et al. (2011) Nuclear membranes control symbiotic calcium signaling of legumes. Proc Natl Acad Sci USA 108: 14348–14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M (2018) Calcium signals in the plant nucleus: origin and function. J Exp Bot 69: 4165–4173 [DOI] [PubMed] [Google Scholar]
- Charpentier M, Oldroyd GE (2013) Nuclear calcium signaling in plants. Plant Physiol 163: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M, Sun J, Vaz Martins T, Radhakrishnan GV, Findlay K, Soumpourou E, Thouin J, Vèry AA, Sanders D, Morris RJ, et al. (2016) Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 352: 1102–1105 [DOI] [PubMed] [Google Scholar]
- Chen K, Gao J, Sun S, Zhang Z, Yu B, Li J, Xie C, Li G, Wang P, Song C-P, et al. (2020) BONZAI proteins control global osmotic stress responses in plants. Curr Biol 30: 4815–4825 [DOI] [PubMed] [Google Scholar]
- Chen J, Gutjahr C, Bleckmann A, Dresselhaus T (2015) Calcium signaling during reproduction and biotrophic fungal interactions in plants. Mol Plant 8: 595–611 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang X, Sun T, Tian Q, Zhang WH (2018) Glutamate receptor Homolog 3.4 is involved in regulation of seed germination under salt stress in Arabidopsis. Plant Cell Physiol 59: 978–988 [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G (2014a) Identification of a plant receptor for extracellular ATP. Science 343: 290–294 [DOI] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014b) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaço R, Moreno N, Feijó JA (2012) “On the fast lane”: mitochondria structure, dynamics and function in growing pollen tubes. J Micros 247: 106–118 [DOI] [PubMed] [Google Scholar]
- Corso M, Doccula FG, de Melo JRF, Costa A, Verbruggen N (2018) Endoplasmic reticulum-localized CCX2 is required for osmotolerance by regulating ER and cytosolic Ca2+ dynamics in Arabidopsis. Proc Natl Acad Sci USA 115: 3966–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J 62: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Luoni L, Marrano CA, Hashimoto K, Köster P, Giacometti S, De Michelis MI, Kudla J, Bonza MC (2017) Ca2+-dependent phosphoregulation of the plasma membrane Ca2+-ATPase ACA8 modulates stimulus-induced calcium signatures. J Exp Bot 68: 3215–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Navazio L, Szabo I (2018) The contribution of organelles to plant intracellular calcium signalling. J Exp Bot 69: 4175–4193 [DOI] [PubMed] [Google Scholar]
- Cubero-Font P, De Angeli A (2021) Connecting vacuolar and plasma membrane transport networks. New Phytol 229: 755–762 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA (2009) Breaking the code: Ca2+ sensors in plant signalling. Biochem J 425: 27–40 [DOI] [PubMed] [Google Scholar]
- Demes E, Besse L, Cubero-Font P, Satiat-Jeunemaitre B, Thomine S, De Angeli A (2020) Dynamic measurement of cytosolic pH and [] uncovers the role of the vacuolar transporter AtCLCa in cytosolic pH homeostasis. Proc Natl Acad Sci USA 117: 15343–15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220: 49–69 [DOI] [PubMed] [Google Scholar]
- Diao M, Qu X, Huang S (2018) Calcium imaging in Arabidopsis pollen cells using G-CaMP5. J Integr Plant Biol 60: 897–906 [DOI] [PubMed] [Google Scholar]
- Dietrich D (2018) Hydrotropism: how roots search for water. J Exp Bot 69: 2759–2771 [DOI] [PubMed] [Google Scholar]
- Dindas J, Dreyer I, Huang S, Hedrich R, Roelfsema MRG (2021) A voltage-dependent Ca2+-homeostat operates in the plant vacuolar membrane. New Phytol. doi: 10.1111/nph.17272. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel KH, Marchadier E, Brownlee C, Kudla J, Hetherington AM (2017) The evolution of Calcium-based signalling in plants. Curr Biol 27: R667–R679 [DOI] [PubMed] [Google Scholar]
- Emonet A, Zhou F, Vacheron J, Heiman CM, Dénervaud Tendon V, Ma KW, Schulze-Lefert P, Keel C, Geldner N (2021) Spatially restricted immune responses are required for maintaining root meristematic activity upon detection of bacteria. Curr Biol 31:1012–1028 [DOI] [PubMed] [Google Scholar]
- Feijó JA, Costa SS, Prado AM, Becker JD, Certal AC (2004) Signalling by tips. Curr Opin Plant Biol 7: 589–598. [DOI] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Holdaway-Clarke T, Cordeiro MS, Kunkel JG, Hepler PK (2001) Cellular oscillations and the regulation of growth: the pollen tube paradigm. Bioessays 23: 86–94 [DOI] [PubMed] [Google Scholar]
- Flütsch S, Nigro A, Conci F, Fajkus J, Thalmann M, Trtílek M, Panzarová K, Santelia D (2020a) Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth. EMBO Rep 21: e49719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flütsch S, Wang Y, Takemiya A, Vialet-Chabrand SRM, Klejchová M, Nigro A, Hills A, Lawson T, Blatt MR, Santelia D (2020b) Guard cell starch degradation yields glucose for rapid stomatal opening in Arabidopsis. Plant Cell 32: 2325–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO (2007) Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Happeck R, Meier B, Hoang MTT, Stribny J, Hause G, Ding H, Morsomme P, Baginsky S, Peiter E (2019) Chloroplast-localized BICAT proteins shape stromal calcium signals and are required for efficient photosynthesis. New Phytol 221: 866–880 [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H (2019) Root plasticity in the pursuit of water. Plants (Basel) 8: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M, Sheen J, et al. (2013) Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog 9: e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF (2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C (2004) Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol 134: 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Frangne N, Gomès E, Martinoia E, Palmgren MG (2000) The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124:1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, van der Luit AH, Knight R, Trewavas AJ (1998) Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol 116: 429–437 [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradogna A, Scholz-Starke J, Gutla PV, Carpaneto A (2009) Fluorescence combined with excised patch: measuring calcium currents in plant cation channels. Plant J 58: 175–182 [DOI] [PubMed] [Google Scholar]
- Granqvist E, Wysham D, Hazledine S, Kozlowski W, Sun J, Charpentier M, Martins TV, Haleux P, Tsaneva-Atanasova K, Downie JA, et al. (2012) Buffering capacity explains signal variation in symbiotic calcium oscillations. Plant Physiol 160: 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Sheen J, Séguin A (2014) Ancient signals: comparative genomics of green plant CDPKs. Trends Plant Sci 19: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM (2003) A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425: 196–200 [DOI] [PubMed] [Google Scholar]
- He J, Rössner N, Hoang MTT, Alejandro S, Peiter E (2021) Transport, functions, and interaction of calcium and manganese in plant organellar compartments. Plant Physiol 187: 1940–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Mueller TD, Becker D, Marten I (2018) Structure and function of TPC1 Vacuole SV channel gains shape. Mol Plant 11: 764–775 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neher E (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329: 833–836 [Google Scholar]
- Hill AE, Shachar-Hill B, Skepper JN, Powell J, Shachar-Hill Y (2012) An osmotic model of the growing pollen tube. PLoS One 7: e36585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R, Paez-Valencia J, Vens C, Toyota M, Palmgren M, Gilroy S (2020) Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc Natl Acad Sci USA 117: 18849–18857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Yamada Y, Matsuda T, Kobayashi K, Hashimoto M, Matsu-ura T, Miyawaki A, Michikawa T, Mikoshiba K, Nagai T (2010) Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat Methods 7: 729–732 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008:420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Luo J, Ning T, Cao W, Jin X, Zhao H, Wang Y, Han S (2017) Cytosolic and nucleosolic calcium signaling in response to osmotic and salt stresses are independent of each other in roots of Arabidopsis seedlings. Front Plant Sci 8: 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Waadt R, Nuhkat M, Kollist H, Hedrich R, Roelfsema MRG (2019) Calcium signals in guard cells enhance the efficiency by which abscisic acid triggers stomatal closure. New Phytol 224: 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishka MR, Brown E, Rosenberg A, Romanowsky S, Davis J, Choi W-G, Harper JF (2021) Arabidopsis Ca2+-ATPases 1, 2, and 7 in the endoplasmic reticulum contribute to growth and pollen fitness. Plant Physiol 185: 1966–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y (2010) Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol 51: 302–311 [DOI] [PubMed] [Google Scholar]
- Iwano M, Entani T, Shiba H, Kakita M, Nagai T, Mizuno H, Miyawaki A, Shoji T, Kubo K, Isogai A, et al. (2009) Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol 150: 1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaślan D, Dreyer I, Lu J, O'Malley R, Dindas J, Marten I, Hedrich R (2019) Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat Commun 10: 2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Silva-Alvim FAL, Hills A, Donald N, Ishka MR, Shadbolt J, He B, Lawson T, Harper JF, Wang Y, et al. (2021) Guard cell Ca2+-ATPases underpin a ‘carbon memory’ of photosynthetic assimilation. that impacts on water use efficiency. Nat Plants in press. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zhou X, Tao M, Yuan F, Liu L, Wu F, Wu X, Xiang Y, Niu Y, Liu F, et al. (2019) Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 572: 341–346 [DOI] [PubMed] [Google Scholar]
- Kelner A, Leitao N, Chabaud M, Charpentier M, de Carvalho-Niebel F (2018) Dual color sensors for simultaneous analysis of calcium signal dynamics in the nuclear and cytoplasmic compartments of plant cells. Front Plant Sci 9: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zeng W, Bernard S, Liao J, Venkateshwaran M, Ane JM, Jiang Y (2019) Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations. Nat Commun 10: 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepikova AV, Kasianov AS, Gerasimov ES, Logacheva MD, Penin AA (2016) A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J 88: 1058–1070 [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6: 262–267 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1997) Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J 12:1067–1078 [DOI] [PubMed] [Google Scholar]
- Krebs M, Held K, Binder A, Hashimoto K, Den Herder G, Parniske M, Kudla J, Schumacher K (2012) FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J 69: 181–192 [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K (2018) Advances and current challenges in calcium signaling. New Phytol 218: 414–431 [DOI] [PubMed] [Google Scholar]
- Kwaaitaal M, Huisman R, Maintz J, Reinstädler A, Panstruga R (2011) Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem J 440: 355–365 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Brandt B, Li J, Merilo E, Wang YF, Keshwani MM, Taylor SS, Kollist H, Schroeder JI (2013) Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol 163: 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM (1998) Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 15837–15842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux D, Lamotte O, Bourque S, Wendehenne D, Mazars C, Ranjeva R, Pugin A (2005) Proteinaceous and oligosaccharidic elicitors induce different calcium signatures in the nucleus of tobacco cells. Cell Calcium 38: 527–538 [DOI] [PubMed] [Google Scholar]
- Leitão N, Dangeville P, Carter R, Charpentier M (2019) Nuclear calcium signatures are associated with root development. Nat Commun 10: 4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100: 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet A, Jaslan D, Toyota M, Mueller M, Muller T, Schonknecht G, Marten I, Gilroy S, Hedrich R, Farmer EE (2017) Control of basal jasmonate signalling and defence through modulation of intracellular cation flux capacity. New Phytol 216: 1161–1169 [DOI] [PubMed] [Google Scholar]
- Lenzoni G, Knight MR (2019) Increases in absolute temperature stimulate free calcium concentration elevations in the chloroplast. Plant Cell Physiol 60: 538–548 [DOI] [PubMed] [Google Scholar]
- Lenzoni G, Liu J, Knight MR (2018) Predicting plant immunity gene expression by identifying the decoding mechanism of calcium signatures. New Phytol 217: 1598–1609 [DOI] [PubMed] [Google Scholar]
- Liu KH, Diener A, Lin Z, Liu C, Sheen J (2020) Primary nitrate responses mediated by calcium signalling and diverse protein phosphorylation. J Exp Bot 71: 4428–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Knight MR (2003) Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol 133: 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Hernandez F, Tryfona T, Rizza A, Yu XL, Harris MOB, Webb AAR, Kotake T, Dupree P (2020) Calcium binding by arabinogalactan polysaccharides is important for normal plant development. Plant Cell 32: 3346–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro G, Drago I, Pozzan T, Schiavo FL, Zottini M, Costa A (2012) Targeting of Cameleons to various subcellular compartments reveals a strict cytoplasmic/mitochondrial Ca2+ handling relationship in plant cells. Plant J 71: 1–13 [DOI] [PubMed] [Google Scholar]
- Loro G, Wagner S, Doccula FG, Behera S, Weinl S, Kudla J, Schwarzlander M, Costa A, Zottini M (2016) Chloroplast-specific in vivo Ca2+ imaging using Yellow Cameleon fluorescent protein sensors reveals organelle-autonomous Ca2+ signatures in the stroma. Plant Physiol 171: 2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Chen L, Huang F, Gao P, Zhao H, Wang Y, Han S (2020) Intraorganellar calcium imaging in Arabidopsis seedling roots using the GCaMP variants GCaMP6m and R-CEPIA1er. J Plant Physiol 246–247:153127. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhao Y, Berkowitz GA (2017) Intracellular Ca2+ is important for flagellin-triggered defense in Arabidopsis and involves inositol polyphosphate signaling. J Exp Bot 68: 3617–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC (1992) Calcium and ABA-induced stomatal closure. Phil Trans R Soc Lond B338: 5–18 [Google Scholar]
- Manzoor H, Chiltz A, Madani S, Vatsa P, Schoefs B, Pugin A, Garcia-Brugger A (2012) Calcium signatures and signaling in cytosol and organelles of tobacco cells induced by plant defense elicitors. Cell Calcium 51: 434–444 [DOI] [PubMed] [Google Scholar]
- Martí Ruiz MC, Jung HJ, Webb AAR (2020) Circadian gating of dark-induced increases in chloroplast- and cytosolic-free calcium in Arabidopsis. New Phytol 225: 1993–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb A, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytosolic free Calcium. Plant Cell 7: 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E, Tsai YC, Braam J (2005) Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci 10: 383–389 [DOI] [PubMed] [Google Scholar]
- Meyerhoff O, Müller K, Roelfsema MR, Latz A, Lacombe B, Hedrich R, Dietrich P, Becker D (2005) AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta 222: 418–427 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu L-H, Obermeyer G, Feijó JA (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijó JA (2017) Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173: 91–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE (2008) ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir SR, Sanders D (1996) Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett 395: 39–42 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53: 988–998 [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, Furuichi T, Takebayashi K, Sugimoto T, Sano S, et al. (2012) Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun 3:926. [DOI] [PubMed] [Google Scholar]
- Nomura H, Shiina T (2014) Calcium signaling in plant endosymbiotic organelles: mechanism and role in physiology. Mol Plant 7: 1094–1104 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Downie JA (2006) Nuclear calcium changes at the core of symbiosis signalling. Curr Opin Plant Biol 9: 351–357 [DOI] [PubMed] [Google Scholar]
- Ordenes VR, Moreno I, Maturana D, Norambuena L, Trewavas AJ, Orellana A (2012) In vivo analysis of the calcium signature in the plant Golgi apparatus reveals unique dynamics. Cell Calcium 52: 397–404 [DOI] [PubMed] [Google Scholar]
- Pan Y, Chai X, Gao Q, Zhou L, Zhang S, Li L, Luan S (2019) Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev Cell 48: 710–725 [DOI] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581: 2325–2336 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Peleg Z., Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14: 290–295 [DOI] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174: 160–173 [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Du L (2018) Calcium signaling: decoding mechanism of calcium signatures. New Phytol 217: 1394–1396 [DOI] [PubMed] [Google Scholar]
- Qudeimat E, Faltusz AM, Wheeler G, Lang D, Holtorf H, Brownlee C, Reski R, Frank W (2008) A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc Natl Acad Sci USA 105:19555–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Wunnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Reddy AS, Ali GS, Celesnik H, Day IS (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23: 2010–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai J, Johnson CH (2002) Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. Plant Cell 14:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Schulze-Lefert P (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28: 3439–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Galway M, Masucci J, Ford S (1993) Pollen tube and root-hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Plant Physiol 103: 979–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaers S, Balcerowicz D, Vissenberg K (2017) Molecular mechanisms regulating root hair tip growth: a comparison with pollen tubes. InObermeyer G, Feijó J, eds, Pollen Tip Growth. Springer, Cham. 10.1007/978-3-319-56645-0_9 [Google Scholar]
- Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J (1999) The Arabidopsis det3 mutant reveals a central role for the vacuolar H+-ATPase in plant growth and development. Genes Dev 15: 3259–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selles B, Michaud C, Xiong TC, Leblanc O, Ingouff M (2018) Arabidopsis pollen tube germination and growth depend on the mitochondrial calcium uniporter complex. New Phytol 219: 58–65 [DOI] [PubMed] [Google Scholar]
- Sello S, Moscatiello R, Mehlmer N, Leonardelli M, Carraretto L, Cortese E, Zanella FG, Baldan B, Szabo I, Vothknecht UC, et al. (2018) Chloroplast Ca2+ fluxes into and across thylakoids revealed by thylakoid-targeted aequorin probes. Plant Physiol 177: 38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sello S, Perotto J, Carraretto L, Szabo I, Vothknecht UC, Navazio L (2016) Dissecting stimulus-specific Ca2+ signals in amyloplasts and chloroplasts of Arabidopsis thaliana cell suspension cultures. J Exp Bot 67: 3965–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik D, Nuriel R, Bonza MC, Costa A, Fromm H (2018) MIZ1 regulates ECA1 to generate a slow, long-distance phloem-transmitted Ca2+ signal essential for root water tracking in Arabidopsis. Proc Natl Acad Sci USA 115: 8031–8036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kanwar P, Yadav AK, Mishra M, Jha SK, Baranwal V, Pandey A, Kapoor S, Tyagi AK, Pandey GK (2014) Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS J 281: 894–915 [DOI] [PubMed] [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63: 1525–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst L, Mähs A, Ischebeck T, Zhang C, Zhang X, Arendt S, Schültke S, Heilmann I, Kudla J (2015) Vacuolar CBL-CIPK12 Ca2+-sensor-kinase complexes are required for polarized pollen tube growth. Curr Biol 25: 1475–1482 [DOI] [PubMed] [Google Scholar]
- Stephan AB, Kunz HH, Yang E, Schroeder JI (2016) Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc Natl Acad Sci USA 113: E5242–E5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E, Møller IM, Murphy A (2014) Plant Physiology and Development, Ed 6. Sinauer Associates, Sunderland, CT [Google Scholar]
- Tang RJ, Zhao FG, Yang Y, Wang C, Li K, Kleist TJ, Lemaux PG, Luan S (2020) A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments. Nat Plants 6: 384–393 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC (2010) IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2: a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, De Bortoli S, Costa A, Behera S, Wagner R, Lo Schiavo F, Formentin E, Szabo I (2015) Alternative splicing-mediated targeting of the Arabidopsis GLUTAMATE RECEPTOR3.5 to mitochondria affects organelle morphology. Plant Physiol 167: 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, Moscatiello R, Cortese E, Vicario M, Festa M, Maso L, De Bortoli S, Calì T, Vothknecht UC, Formentin E, et al. (2019) A chloroplast-localized mitochondrial calcium uniporter transduces osmotic stress in Arabidopsis. Nat Plants 5: 581–588 [DOI] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, Wagner S, Formentin E, Behera S, De Bortoli S, Larosa V, Fuchs P, Lo Schiavo F, Raffaello A, et al. (2017) Physiological characterization of a plant mitochondrial Calcium uniporter in vitro and in vivo. Plant Physiol 173: 1355–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teardo E, Formentin E, Segalla A, Giacometti GM, Marin O, Zanetti M, Lo Schiavo F, Zoratti M, Szabo I (2011) Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasma membrane. Biochim Biophys Acta 1807: 359–367 [DOI] [PubMed] [Google Scholar]
- Thor K, Jiang S, Michard E, George J, Scherzer S, Huang S, Dindas J, Derbyshire P, Leitão N, DeFalco TA, et al. (2020) The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 585: 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu Q, Li L, Staskawicz BJ, Luan S (2019) A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572:131–135 [DOI] [PubMed] [Google Scholar]
- Tian W, Wang C, Gao Q, Li L, Luan S (2020) Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants 6: 750–759 [DOI] [PubMed] [Google Scholar]
- Tian D, Wang J, Zeng X, Gu K, Qiu C, Yang X, Zhou Z, Goh M, Luo Y, Murata-Hori M, et al. (2014) The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell 26: 497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy FE, Gilliham M, Dodd AN, Webb AA, Tester M (2008) NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ 31: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ (1999) Le calcium, c'est la vie: Calcium makes waves. Plant Physiol 120: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Malhó R (1998) Ca2+ signalling in plant cells: the big network! Curr Opin Plant Biol 1: 428–433 [DOI] [PubMed] [Google Scholar]
- van Der Luit AH, Olivari C, Haley A, Knight MR, Trewavas AJ (1999) Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiol 121: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S, Vandenbroucke K, Inzé A, van de Cotte B, Mühlenbock P, De Rycke R, Naouar N, Van Gaever T, Van Montagu MC, Van Breusegem F (2012) AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 109: 20113–20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Davies JM (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 59: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Behera S, De Bortoli S, Logan DC, Fuchs P, Carraretto L, Teardo E, Cendron L, Nietzel T, Fussl M, et al. (2015) The EF-Hand Ca2+ binding protein MICU choreographs mitochondrial Ca2+ dynamics in Arabidopsis. Plant Cell 27: 3190–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Steinbeck J, Fuchs P, Lichtenauer S, Elsässer M, Schippers JHM, Nietzel T, Ruberti C, Van Aken O, Meyer AJ, et al. (2019) Multiparametric real-time sensing of cytosolic physiology links hypoxia responses to mitochondrial electron transport. New Phytol 224: 1668–1684 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hills A, Blatt MR (2014) Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiol 164: 1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu Y, Ling Y, Zhang H, Liu P, Baluska F, Samaj J, Lin J, Wang Q (2010) Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs. BMC Plant Biol 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J (2008) A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol 179: 675–686 [DOI] [PubMed] [Google Scholar]
- Whalley HJ, Knight MR (2013) Calcium signatures are decoded by plants to give specific gene responses. New Phytol 197: 690–693 [DOI] [PubMed] [Google Scholar]
- Winship LJ, Rounds C, Hepler PK (2017) Perturbation analysis of calcium, alkalinity and secretion during growth of lily pollen tubes. Plants (Basel) 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua NH (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278: 2126–2130 [DOI] [PubMed] [Google Scholar]
- Wudick MM, Michard E, Oliveira Nunes C, Feijó JA (2018) Comparing plant and animal glutamate receptors: common traits but different fates? J Exp Bot 69: 4151–4163 [DOI] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]
- Xiong TC, Jauneau A, Ranjeva R, Mazars C (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signalling. Plant J 40: 12–21 [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25: 131–139 [DOI] [PubMed] [Google Scholar]
- Yadav AK, Shankar A, Jha SK, Kanwar P, Pandey A, Pandey GK (2015) A rice tonoplastic calcium exchanger, OsCCX2 mediates Ca2+/cation transport in yeast. Sci Rep 5: 17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217: 523–539 [DOI] [PubMed] [Google Scholar]
- Yuan P, Jauregui E, Du L, Tanaka K, Poovaiah BW (2017) Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr Opin Plant Biol 38: 173–183 [DOI] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Zhang J, Coaker G, Zhou JM, Dong X (2020) Plant immune mechanisms: from reductionistic to holistic points of view. Mol Plant 13: 1358–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu J, Chena Z, Lina J (2007) In vitro germination and growth of lily pollen tubes is affected by calcium inhibitor with reference to calcium distribution. Flora 202: 581–588 [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, et al. (2011) An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liao C, Zhao S, Wang C, Guo Y (2017) The glycosyltransferase QUA1 regulates chloroplast-associated Calcium signaling during salt and drought stress in Arabidopsis. Plant Cell Physiol 58: 329–341 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Caplan J, Mamillapalli P, Czymmek K, Dinesh-Kumar SP (2010) Function of endoplasmic reticulum calcium ATPase in innate immunity-mediated programmed cell death. EMBO J 29: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Oldroyd GE (2017) Plant signalling in symbiosis and immunity. Nature 543: 328–336 [DOI] [PubMed] [Google Scholar]