Abstract
Purpose
. The concept of lockdown in relation to COVID-19 is thought to have an indirect impact on the quality of life and well-being of the elderly due to its consequences on the physical, psychological, and cognitive health of individuals. However, previous published studies on this subject are limited in terms of methodological approach used, including the absence of pre-confinement status and the type of experimental design, which is often cross-sectional. The present study proposes a longitudinal design with pre-confinement measures. It assesses changes in quality of life, perceived health, and well-being by comparing the period before lockdown (T1 = December 2019), three months after the start of the first lockdown (T2 = June 2020), and during the second lockdown (T3 = January 2021) due to COVID-19.
Materials and Methods
. This study is conducted with a group of 72 healthy elderly persons. They completed an electronic (online) survey assessing personal factors, activities, and participation as well as responding to the EuroQol-5D and Warwick-Edinburgh Mental Well-being Scale.
Results
. A decrease in quality of life, perceived health and well-being was observed between T1 and T2 and between T1 and T3, but no difference was reported between the two lockdown periods. The variables associated with these changes included energy level, level of happiness, physical activity, change in medical condition, memory difficulties, level of perceived isolation and age.
Conclusion
. This study will help to target variables that may have a deleterious effect on older adults for consideration in future confinement settings and for preventive purposes.
Keywords: Elderly, Quality of life, Well-being, Perceived health, Lockdown, COVID-19
1. Introduction
As of September 27th, 2021, COVID-19 has infected 231,931,655 people and has caused 4766,874 deaths worldwide (Worldometer, 2021). Among these, 1598,807 Canadians have contracted the virus and 27,620 have died, whereas in Québec there have been 408,462 cases and 11,356 deaths. People aged 60 years and over represent 16.6% and 20.1% of cases in Canada and Quebec respectively. However, they account for 93.8% and 97.1% of deaths (Gouvernement du Québec, 2021; Government of Canada, 2021). Since the start of the pandemic, COVID-19 was therefore considered a geriatric health emergency (Tyrrell & Williams, 2020). As in several developed countries, the Government of Canada instituted social distancing and confinement measures to protect individuals at higher risk of infection and secondary complications from COVID-19, particularly among the elderly (Malone et al., 2020). In Canada, the City of Montreal, the second-most populous city (> 2 million inhabitants) in the country, and the most populous city in the Canadian province of Quebec, was considered in maximum “red alert” (red zone) with more restrictive measures than other regions in the province and over a longer period of time (i.e., restriction and closure of non-essential services and stronger bans on gatherings). A first lockdown was implemented between March and June 2020, followed by an inter-wave period from June to October 2020, and a second lockdown between October 2020 and June 2021 (Institut national de santé publique du Québec [INSPQ], 2021).
These types of situations affect personal well-being due to changes in the environment. Changes in the environment, such as lockdown, are likely to present a barrier and directly affect many dimensions of life (body structures and functions, activities, and participation), as coded in the International Classification of Functioning, Disability and Health (ICF), with impact on a person's health, quality of life and well-being (McDougall, Wright, & Rosenbaum, 2010). Indeed, recent studies revealed that changes in the environment and routine (e.g., shopping from a distance, changes in physical activity, confinement, etc.) may induce feelings of uncertainty. Stress in particular, and stress of lockdowns due to COVID-19 and other illnesses, have been linked to “indirect” or “secondary” impacts on the ICF dimension of body structure of a person, such as physical health problems (e.g., increased cardiovascular disease and falls), the presence of sleep disturbances (Bauer et al., 2020), greater prevalence of geriatric disorders and hospitalizations (Sepúlveda-Loyola et al., 2020). COVID-19 lockdown also decreases physical activity (Callow et al., 2020; Goethals et al., 2020) which correlates with functional ability, independence, activity performance, intimacy, mental health, psychological health, and physical vigor (Vagetti et al., 2014), all elements of the ICF that are found to affect well-being.
Like the benefits of physical fitness, social participation is linked to better cognitive functioning, including more efficient memory and executive function (Evans, Martyr, Collins, Brayne, & Clare, 2019). A clear definition of social participation is still lacking, and proposed definitions have an overlap with participation and activity as defined by the ICF. Social participation is an organized process in which individuals are characterized by specific, collective, conscious, and voluntary actions, which ultimately leads to self-actualization and achievement of goals (Dehi Aroog & Mohammadi Shahboulaghi, 2020). Levasseur and colleagues performed a content analysis of 43 articles with original definitions of social participation in older adults. They defined social participation as “a person's involvement in social activities that provide social interactions within his/her community or society.” (Levasseur, Richard, Gauvin, & Raymond, 2010). In the context of a lockdown, activities with others are likely to decrease and social participation may be affected (Ammar et al., 2020). Moreover, a lower level of social participation would predict cognitive decline (Zunzunegui, Alvarado, Del Ser, & Otero, 2003). Similarly, isolation has been associated with impaired semantic memory, perceptual speed, and visuospatial abilities, as well as an increased risk of dementia (Wilson et al., 2007). These cognitive impacts may also be intrinsically linked to social distancing and confinement (Ammar et al., 2020). It should be noted, however, that these studies did not specify the duration of isolation, nor the frequency or decrease of social interactions, and were conducted in social isolation, not during a lockdown such as the ones experienced due to COVID-19.
Confinement has also been associated with several psychological consequences and, as a result, a decrease in well-being. Well-being is defined as the absence of negative emotions (depression, anxiety, etc.), as well as the presence of positive emotions and moods (happiness, contentment, accomplishment, etc.). A recent literature review of ten studies reports that up to 47.2% of older adults experienced depressive symptoms during COVID-19 lockdown (Sepúlveda-Loyola et al., 2020), especially if they are dissatisfied with their social life, have lost a loved one, are financially troubled, are female, and are older (Bauer et al., 2020). In addition, suicidal ideation and self-harm reportedly increased in the Canadian adult population during COVID-19 lockdown (Daly et al., 2020). Sepúlveda-Loyola et al. (2020) also point out that more than 49.7% of elderly people reported anxiety symptoms during COVID-19 lockdown. These could even persist for up to six months after a period of lockdown (Jeong et al., 2016). Also, a longer duration of confinement could exacerbate post-traumatic stress symptoms (Brooks et al., 2020). Finally, in relation to the previous sections, reduced physical activity secondary to lockdown would also have a negative impact on depressive symptoms, feelings of isolation, stress, and poorer mental health (Bauer et al., 2020). These consequences would in turn lead to a decrease in overall life satisfaction, an important dimension of well-being (Benke, Autenrieth, Asselmann, & Pané-Farré, 2020). Many of the said impacts consequent to lockdown and confinement are important factors associated with quality of life, which could be defined by the degree of satisfaction or dissatisfaction experienced during the subjective and multidimensional (physical, psychological, and social) assessments of positive (e.g., mobility, functional abilities, contentment) and negative (e.g., fatigue, pain, sadness) dimensions (Farquhar, 1995; WHOQOL Group, 1995).
A change in environment is useful in protecting against health problems directly related to infection by reducing the risk of transmission, such as lockdowns. However, conversely it might have an indirect impact on quality of life, perceived health, and well-being in older adults due to its physical, cognitive, and psychological consequences, among other things, as revealed by the aforementioned studies. To the best of our knowledge, these observational and population-based studies did not compare the studied group to a control (non-confined) group or to the state of the participants before these periods of confinement. In addition, they were cross-sectional and not longitudinal, limiting the interpretation of long-term effects of lockdown. Thus, it is difficult to determine whether it is the lockdown itself or its indirect effects that caused the multiple changes in people's health.
1.1. Objectives and hypotheses
In addition to surveying many dimensions of an individuals’ health status, the present study assesses the quality of life, perceived health, and well-being in a group of healthy and active elderly both before the lockdown (T1- December 2019) due to COVID-19 and then at two additional measurement times over the course of a full year (T2- June 2020 and T3- January 2021). T2 and T3 were three months after the start of the first lockdown and during the second lockdown due to COVID-19, respectively.
A decrease in quality of life, perceived health and well-being is expected between the pre-lockdown period and the two lockdown periods and it is expected that these three main measures will be more reduced during the second period of lockdown (T3). A second exploratory objective, based on dimensions classified using the ICF model, will be to identify the body functions and structures as well as activities and participation of the participants that will be most susceptible to changes of the main outcomes before and during the second period of lockdown. We hypothesized that a greater decrease in quality of life, perceived health and well-being will be associated with the presence of a fragile medical condition, a lower level of physical activity, the presence of functional limitations, the presence of cognitive problems, a lower energy level, the absence of feelings of happiness, feeling more confined, feeling more isolated, as well as having a lower education and being older.
2. Material and methods
2.1. Participants
A sample of 104 healthy, active elderly persons were recruited for the study conducted in a public art museum. Specifically, they were VIP members of the Montreal Museum of Fine Arts (MMFA) who had recently participated in another study (conducted in December 2019) and who had agreed to be re-contacted for future studies. The study conducted in December 2019 aimed to explore the effects of a visit to the Montreal Museum of Fine Arts (MMFA) on mobility, cognition, well-being in a group of healthy seniors. Within two months of this project, a lockdown following COVID19 was announced in Québec, Canada. Being fortunate to have very recent pre-COVID data, we proposed to follow these individuals over time for a year. Before their visit, the participants completed the same electronic survey and questionnaires (CESAM), quality of life, perceived health, and well-being that we used at T2 and T3. Thus, these participants formed a perfect baseline to assess the effects of the lockdowns. We believe that the results of that study which evaluated the feasibility of assessing mobility and cognition using technology in a museum did not influence the results of the present study which is mainly using questionnaires.
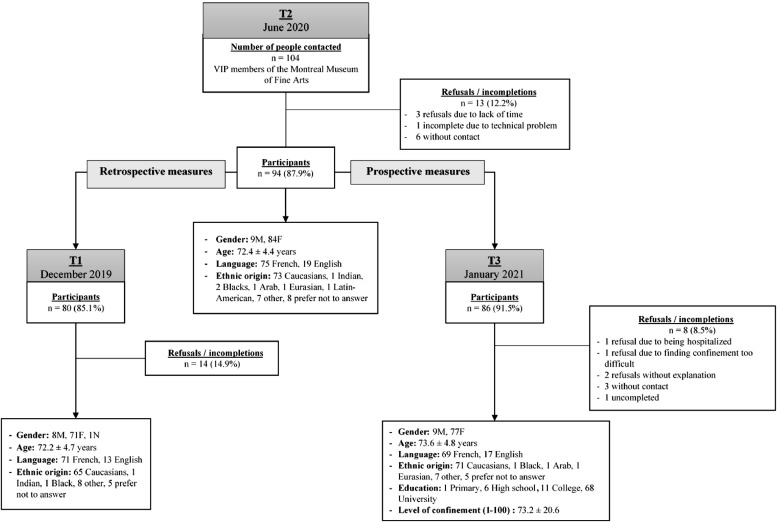
The inclusion criteria were as follows: be over 60 years old, be able to move around the museum independently for a visit of about an hour (with rest if necessary), be French-speaking or English-speaking, be literate, be able to consent and be able to acquire and use a device (smartphone, mobile, laptop or computer) to access the electronic survey. Those with mobility problems preventing them from walking around a museum or with reduced mobility tolerance were excluded. In June 2020, after three months of COVID-19 lockdown, these 104 VIP members were contacted (from the original sample) and 94 agreed to answer the electronic survey. In January 2021, during the second lockdown period, the 94 participants were called and 86 completed the survey again. For 80 of these participants, we had the retrospective results for the same survey before the COVID-19 pandemic (December 2019). This first survey time-point was identified as T1. June 2020 (after 3-month lockdown) and January 2021 (one year after T1) were identified as T2 and T3, respectively. In fact, the participants assessed in the study conducted in December 2019 (T1-no pandemic) were retrospectively invited to participate in the present study during lockdowns at T2 and T3. Some of these participants who did not complete the CESAM and/or the questionnaires at T1 despite two reminders December 2019 (phone call or email) agreed to complete them at T2 and T3. The flowchart of participants is summarized in Fig. 1 . This study was approved by the Ethics Committees of the Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal (CRIR) and University of Montreal (# CRIR-1432–0819).
Fig. 1.
Flowchart of recruitment.
2.2. Instrument
During the three data collections (T1, T2 and T3), an individual link to the survey was made available on a secure web platform and was sent to the participants by email via a data management software (REDCap). First, the consent form was presented to the participant on the web platform or by email. The survey was only made available if the individual consented to participate in the study. The electronic survey, called the CESAM (Beauchet et al., 2020b) took an average of 15 min to complete and was self-administered.
The first section of the T1 survey asked participants about personal factors (e.g., age, gender, ethnicity) as well as body structure, functions, and their activities and participation. These questions included an assessment of their overall health, including functional abilities (whether or not they need help with certain tasks), medication, physical activity, presence of falls, psycho-emotional state (e.g., feeling unhappy, full of energy), as well as problems with sight, hearing, memory, attention, and concentration. During the T2 phase, questions more specific to the confinement were added regarding the participants’ medical history (e.g., medical condition, urgent medical consultations, diagnosis of COVID-19, hospitalizations) and about changes in the practice of physical activity between the periods before and during lockdown. The answers were of binary form (yes-no), were on scales between two to five forced choices (e.g., to answer the question “How do you feel today?”, participants chose between happy, not happy, and neither one nor the other) or were open-ended (e.g., what activities the participants did). At T3, questions on level of education, perceived isolation during confinement (three forced choices on feeling excluded, lonely, and isolated from others) and perceived degree of confinement (an open response and a scale of 1–100 where 100 is feeling completely confined) were added.
The second section of the survey, which was the same for all T1, T2 and T3 timeline measurements, assessed the quality of life and the perception of health with the validated EuroQol-5D questionnaire (EQ-5D; Brooks & EuroQol Group, 1996). Quality of life was reported by participants according to five dimensions: mobility, self-care, activities of daily living, pain/discomfort, and anxiety/depression. These dimensions were assessed using a Likert scale (1 = no problem to 5 = disability) and their total score was calculated, with a maximum of 25 indicating maximum difficulties. Therefore, a higher score indicates more difficulties and a lower quality of life (Beauchet, Bastien, Mittelman, Hayashi, & Hau Yan Ho, 2020a). A sixth and additional question (EQ-6) evaluated perceived health on a scale from 0 to 100, where 100 indicates the best health imaginable and a better quality of life. The EQ-5D has moderate test-retest reliability (ICC = 0.69), inter-rater agreement (ICC = 0.57; Janssen, Birnie, Haagsma, & Bonsel, 2008), and convergent validity with the World Health Organization (WHO)’s Well-being Questionnaire (WHO-5; ICC = 0.53; Janssen et al., 2013).
The third section of the survey, also equivalent between the three measurements, was composed of items from the validated Warwick-Edinburgh Mental Well-being Scale (WEMWBS; Stewart-Brown & Janmohamed, 2008). The WEMWBS has 14 items (e.g., “I feel optimistic about the future”, “I feel happy”, etc.) aimed at assessing the feeling of well-being. Responses are graded on a five-point Likert scale (never, rarely, sometimes, often, always). The total score of well-being was calculated between a minimum of 14 and a maximum of 70. A score of 70 indicates a high level of well-being. The WEMWBS has excellent internal consistency (α = 0.91), good test-retest reliability (ICC = 0.83), moderate and strong correlations with seven other life satisfaction and well-being questionnaires (r = 0.53 to 0.77) as well as a low social desirability bias (r = 0.18 to 0.35; Tennant et al., 2007).
2.3. Statistical analyses
Descriptive statistics were used to describe the sample. For a repeated measures design including three variables, with a significance level of 0.05 and sufficient statistical power (0.80), it is estimated that a sample of 44 participants would make it possible to detect a large effect size (d = 0.8) on the main outcomes. The first objective, which aimed to determine the effect of the lockdown on the changes in quality of life and well-being, was evaluated using repeated measures ANOVAs and pairwise comparisons with Bonferroni correction. Changes were calculated as the difference between values at T2 (after three months of the first confinement) and T1 (before confinement), T3 (during the second period of confinement) and T1, as well as T3 and T2. Normality was assumed due to the large sample but was also verified because both parametric and their equivalent non-parametric tests gave identical results for quality of life (EQ-5D total score) and well-being (WEMWBS total score). This was not the case for change in perception of health (EQ-6) between T1, T2 and T3. No transformations were done, but non-parametric Friedman and Wilcoxon tests were used. Finally, a comparison was done between repeated-measures ANOVA (which includes all participants regardless of missing data) and a mixed linear model (which does not include participants with missing data) to determine if the missing data was missing at random or not. The similar results between the two tests suggested that all missing data could be considered missing at random, therefore using repeated-measure ANOVA was acceptable, which in turn means that even though we did not screen for missing data at T1, this did not affect the outcomes.
The second objective aimed at exploring variables explaining the changes (determined by T3-T1) in quality of life, perception of health and well-being. The changes in the scores between T1 and T3 were used as dependent variables. The independent variables, listed by the ICF categorization, scores and periods are summarized in Table 1 . These variables were selected based on what was previously found in publications on quality of life and well-being. The first independent variable, the presence of medical conditions, was computed by attributing one point if the answer was yes and two points if the answer was no for the following criteria: if the participant had a fall in the past year, had been hospitalized, had used emergency services, had been diagnosed with COVID-19 and if they had problems with their sight or hearing. Additional points were given for the number of medications taken (1 = 10 or more, 2 = 5 to 9, 3 = 1 to 4, 4 = 0). A lower score indicates more severe medical conditions.
Table 1.
Independent variables according to the International Classification of Functioning, Disability and Health (ICF).
| ICFCategorization | Variables | Scores | Periods |
|---|---|---|---|
|
Body Structure and Function |
Medical conditions (5 items at 2 levels; 1 item at 4 levels) | 6 (worst) – 14 (best) | T1, T3 |
| Change in medical conditions | T3-T1 (- means decline) | ||
| Functional limitations (12 items at 2 levels) | 12 (worst) – 24 (best) | T1, T3 | |
| Change in functional limitations | T3-T1 (- means decline) | ||
| Memory problems before lockdown (2 levels) | 1 (yes) – 2 (no) | T1 | |
| Cognitive difficulties during lockdown (3 items at 2 levels) | 3 (worst) – 6 (best) | T3 | |
| Feeling of happiness (2 levels) | 1 (happy) – 2 (not happy) | T1, T3 | |
| Change in feeling of happiness | T3-T1 (-+ means decline) | ||
| Perceived energy level (2 levels) | 1 (high) – 2 (low) | T1, T3 | |
| Activities | PA level (10 levels) | 1 (any) – 10 (very active) | T3 |
| PA weekly frequency (4 levels) | 1 (never) – 4 (very often) | T3 | |
| PA weekly hours (4 levels) | 1 (very little) – 4 (a lot) | T3 | |
| Participation | Perceived degree of confinement | 0 (no) – 100 (fully) | T3 |
| Perceived degree of isolation (3 levels) | 3 (never) – 9 (often) | T3 | |
|
Personal Factors |
Age (years) | T1, T2, T3 | |
| Level of Education (2 levels: University / College or lower) | T3 |
Note. T1 = December 2019; T2 = June 2020; T3 = January 2021; PA = physical activity.
Another independent variable, functional limitations, was computed by adding one point if the answer was yes and two points if the answer was no for the following: incontinence, needing help with walking, eating, using the phone, taking public transportation, managing medication, handling finances, answering the questionnaire, grooming, bathing, getting dressed, as well as using home-help services. A lower score indicates more functional difficulties.
A third independent variable, cognitive problems during lockdown, was calculated by the sum of one point if the answer was yes and two points if the answer was no for memory, attention, and concentration problems. A lower score indicates more cognitive difficulties.
Three more independent variables were computed. Change in medical conditions was calculated as the difference between the scores at T3 and T1 (T3-T1). A negative change implies a worsening of medical conditions. Change in functional limitations was also computed as the difference between T1 and T3. A negative change indicates an increase in limitations. Finally, change in happiness was calculated between T1 and T3, where a positive score means a decrease in feelings of happiness. Other variables used to explore the three main outcomes are presented in Table 1.
Student's T-tests were used to evaluate how the dichotomous independent variables (i.e., presence of memory problems at T1, high energy level at T3, feelings of happiness at T1 and T3) influenced the dependent variables (quality of life, perception of health and well-being). Feelings of happiness at T1 and T3 was measured on a 3-point scale (happy, unhappy and neither) but was recalculated into two groups: happy and not happy (which includes those who answered neither). The continuous independent variables (i.e., medical conditions at T1 and T3, change in medical conditions, functional limitations at T1 and T3, change in functional limitations, cognitive problems at T3, and change in feelings of happiness, physical activity (PA) level, weekly frequency and hours at T3, age, education, perceived degree of confinement at T3 and isolation at T3) were correlated with each dependent variable using Pearson's correlation.
The independent variables which had resulted in a significant T-test, which correlated significantly (p < .05), or approached this level, with quality of life, perception of health or well-being were analyzed in separate ascending multiple regressions. The independent variables were introduced in the models to predict these changes using stepwise multiple regressions with p-values entering and exiting the model at 0.10 and 0.15 respectively.
The analyses were done using SPSS 25 and a critical alpha threshold of 0.05. Effect sizes (Cohen's d and Kendall's W) were calculated and interpreted using Cohen's criteria (1992) as cited in Field (2018).
3. Results
3.1. Descriptive statistics
The process of recruitment is shown in Fig. 1. In June 2020, the information of the 104 participants of the study conducted in December 2019 was accessible. These 104 potential participants for the current study were screened for acceptance or refusal to participate in future studies following the one they did in 2019. These participants were not screened for completion or incompletion of the questionnaire before being contacted at T2 in June 2020. Therefore, we did not acknowledge at that time that 14 participants had not completed the questionnaire at T1 since the data was under analysis. To add, the statistical analyses were not done until after data from all three periods was collected, therefore we did not decide how to proceed with missing data before contacting participants at T2. Nonetheless, these missing data did not affect our results.
Thus, as shown in Fig. 1, ninety-four VIP members of the MMFA participated in June 2020 (T2). Of the 94 VIP members, 86 (91.5%) participated during the second period of confinement in January 2021 (T3) and 80 (85.1%) of those assessed at T2 responded to the survey during their visit to the museum in December 2019 (T1). A total of 8 participants didn't entirely completed the questionnaire either at T1 and or T3. As stated in the section Instrument, the questionnaire consisted of three sections. The first section was on personal and descriptive factors, body structure, functions, activities and participation (e.g. age, gender, ethnicity, health, functional abilities, cognitive difficulties, psycho-emotional state, etc.). The second section, based on the EuroQol-5D, assessed quality of life and perception of health. The third section was based on the Warwick-Edinburgh Mental Well-being Scale and assessed well-being. The eight participants who did not complete the entire questionnaire had missing data for at least one of the three main outcomes at T1 and/or T3. As stated above, this missing data did not affect the results for objective 1 comparing the three main outcomes between T1, T2 and T3. These eight participants were not included in the analyses of the second objective which aimed to identify the variables associated to the changes found in the first objective. Overall, seventy-two participants have provided data at both T1 and T3 periods for any of the three outcomes. They were all 65 years and older. Overall, seventy-two participants have provided data at both T1 and T3 periods for any of the three outcomes. During recruitment and to increase the number of participants at three times, the research ethics committee allowed us to follow up three times with the participants by e-mail or telephone. If there was no response after these two reminders, we were obliged to stop the reminders. Despite these strategies, of the 94 participants at T2, 15 had missing data for quality of life, 15 had missing data for perceived health, and 17 had missing data for well-being at T1 (this includes the 14 participants who did not complete the questionnaire). Of the 94 participants, one had missing data for quality of life, six had missing data for perceived health, and 2 had missing data for well-being at T2. At T3, 8 had missing data for quality of life, 11 had missing data for perceived health, and 11 had missing data for well-being. These missing data include those who did not complete the questionnaire, and those who missed or skipped an item.
At T2 and T3, all participants who agreed to future studies were contacted up to three times, after which a message was left indicating where to contact us if they were interested in participating in our study. Those who did not answer were considered incompletions. As the survey was done online and could only be answered once, it was hard to control for participants missing or skipping a question. However, as explained above, all missing data was missing at random, therefore this did not affect the results. Their characteristics are presented in Table 2 .
Table 2.
Descriptive statistics (N = 72).
| M | SD | Min | Max | n | % | ||
|---|---|---|---|---|---|---|---|
| Age | 72.4 | 4.8 | 65 | 87 | |||
| Perceived degree of confinement on a scale from 1 to 100a | 74.5 | 20.4 | 5 | 100 | |||
| Language | English | 12 | 16.7 | ||||
| French | 60 | 83.3 | |||||
| Gender | Female | 65 | 90.3 | ||||
| Male | 7 | 9.7 | |||||
| Ethnic origin | Caucasian | 53 | 73.6 | ||||
| Other | 11 | 15.3 | |||||
| I prefer not to answer | 8 | 11.1 | |||||
| Level of educationa | University | 57 | 79.2 | ||||
| College or lower | 15 | 20.8 |
Note. aAssessed at T3 (January 2021).
3.2. Quality of life
The quality of life of the participants decreased over time (ANOVA Greenhouse-Geisser; F(1.9, 129.2) = 12.7, p < .001) with significant increase in scores between T1 and T2 (6.2 ± 0.2 vs 7.2 ± 0.3; p < .001, η2 = 0.198; see Table 3 ) and T1 and T3 (6.2 ± 0.2 vs 7.4 ± 0.3, p < .001; η2 = 0.197). The slight increase observed between T2 and T3 was not significant (p = .402). Both effect sizes were medium to large (r < 0.5; Field, 2018). The average changes in quality of life are shown in Table 2. For T1 and T3, they ranged from −3 (improvement) to 10 (deterioration) over 25 with a mean value of 1.1(2.3).
Table 3.
Changes in Quality of Life, Perceived Health, and Well-Being Between the Period Before Lockdown (T1), Three Months After the First Lockdown (T2) and During the Second Lockdown (T3)
| Quality of life (n=71) | Scores | T1 vs T2 | T1 vs T3 | T2 vs T3 | |||||||
| T1 | T2 | T3 | p | η2 | p | η2 | p η2 | ||||
| Mean (SD) | 6.2 (1.4) | 7.2 (2.2) | 7.4 (2.6) | .000 | .198 | .004 | .197 | .402 .010 | |||
| Min-Max | 5-10 | 5-15 | 5-16 | ||||||||
| Changes (SD) | 0.9 (1.9) | 0.2 (1.8) | |||||||||
| Min-Max | -3-7 | -6-6 | |||||||||
| 1.1 (2.3) -3-10 |
|||||||||||
| Perceived health* (n=67) |
Scores | T1 vs T2 | T1 vs T3 | T2 vs T3 | |||||||
| T1 | T2 | T3 | p | r | p | r | p r | ||||
| Mean (SD) | 85.3 (11.6)) | 81.2 (14.1) | 79.2 (17.0) | .008 | .302 | .001 | .351 | .164 .170 |
|||
| Min-Max | 40-100 | 30-100 | 20-100 | ||||||||
| Changes (SD) | -4.0 (13.6) | -2.6 (15.4) | |||||||||
| Min-Max | -53-36 | -55-55 | |||||||||
| -6.3 (15.3) -64-39 |
|||||||||||
| Well-being (n=66) | Scores | T1 vs T2 | T1 vs T3 | T2 vs T3 | |||||||
| T1 | T2 | T3 | p | η2 | p | η2 | p η2 | ||||
| Mean (SD) | 57.5 (6.9) | 53.3 (7.5) | 53.1 (8.3) | .000 | .413 | .001 | .353 | .630 .004 | |||
| Min-Max | 35-69 | 35-66 | 37-69 | ||||||||
| Changes (SD) Min-Max |
-4.7 (6.1) -24-9 |
0.1 (4.9) -15-14 |
|||||||||
| -4.5 (6.5) -25-7 |
|||||||||||
Note. * Friedman and Wilcoxon tests, other ANOVA (see text). Right part: p value for the Contrasts and η2 effect size. r : effect size for non parametric statistics.
3.3. Perception of health
The data for perception of health were not normally distributed so Friedman and Wilcoxon tests were used. The participants perceived their health as less good (decreased) over time (Friedman; χ2 (2) = 10.3, p = .006) with scores decreasing significantly between T1 and T2 (85.3 ± 1.4 vs 81.3 ± 1.7, p = .008, W = 0.213; r = 0.302; see Table 3) and T1 and T3 (85.3 ± 1.4 vs 79.2 ± 17.0, p = .001, W = 0.283; r = 0.351). Both effect sizes were small to medium (W < 0.3; Field, 2012). Scores between T2 and T3 decreased but not significantly (p = .164). The changes of perceived of health on a scale from 1 to 100 were on average −6.3 ± 15.3 for T1 to T3 with a range from −64 (decrease) to 39 (increase) over 100 meaning that for some participants, their perception of health has improved.
3.4. Well-being
Well-being also decreased over time (ANOVA Greenhouse-Geisser; F(1.8, 118.9) = 30.8, p < .001). The participants’ scores decreased significantly between T1 and T2 (57.5 ± 6.9 vs 53.3 ± 7.5, p < .001; η2 = 0.413; see Table 3), as well as between T1 and T3 (57.5 ± 6.9 vs 53.1 ± 8.3, p < .001; η2 = 0.353). These results show large effects (r > 0.5; Field, 2018). There was no significant change between T2 and T3 (p = .630). On average, well-being decreased by 4.5 ± 6.5 between T1 and T3 with values ranging from −25 (decrease well-being) to 7 (increase) over 70, the maximal score.
3.5. Variables associated to the outcome measures
Student's T-tests (see Table 4 ) and Pearson's (or Spearman's) correlations (see Table 5 ) were conducted between the computed changes in quality of life, perception of health, and well-being between T1 and T3 and independents variables. Only the change between T1 and T3 was analyzed because there was no difference between the main outcomes at T2 and T3. This allowed to explore the changes during a longer period. For change in quality of life, correlations were found with physical activity level (r = −0.308) and functional limitations at T1 (r = −0.263). For the changes in perception of health, the highest correlation was also with physical activity level (r = −0.462), followed by change in feeling of happiness (r = −0.348), change in medical condition (r = 0.295), change in functional limitations (r = 0.283), level of education (r = 0.268), and perceived degree of isolation (r = 0.253). Independent T tests (see Table 4) also revealed those with (vs. those without) without memory problems at T1 (t = −2.52, p = .014), those who did not (vs. those who did) feel happy at T3 (t = 2.74, p = .008), as well as those without (vs. those with) a high energy level at T1 (t = 2.23, p = .045) and at T3 (t = 3.27, p = .002) had a decline in perceived health. For change in well-being, energy level at T3 (t = 2.57, p = .013), feelings of happiness at T3 (t = 2.29, p = .025), an increase in functional disabilities (r = 0.220) and perception of isolation (r = −0.415) significantly influenced the scores.
Table 4.
T-tests between independent variables before and during the second confinement (T3) due to COVID-19 and changes in quality of life, perception of health and well-being.
| Explanatory variables | Changes in quality of life | Changes in perception of health | Changes in well-being | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) |
M (SD) |
t (df) |
p | d | n (%) |
M (SD) |
t (df) |
p | d | n (%) |
M (SD) |
t (df) |
p | d | ||
| Memory problems before lockdown | Yes | 13 (18.1) |
2.08 (3.38) |
1.69 (70) |
.096 | .0421 |
11 (15.3) |
−16.6 (25.5) |
−2.52 (68) |
.014 |
.615 | 9 (12.5) |
−5.67 (6.65) |
−0.528 (65) |
.563 |
.201 |
| No | 59 (81.9) |
.92 (1.93) |
59 (81.9) |
−4.36 (11.9) |
58 (80.6) |
−4.31 (6.49) |
||||||||||
| Energy level before lockdown | Yes | 59 (81.9) |
.85 (1.89) |
−1.60 (13.73) |
.133 |
.692 |
58 (80.6) |
−3.64 (11.73) |
2.23 (12.18) |
.045 |
1.079 |
55 (76.4) |
−4.20 (6.11) |
.790 (65) |
.433 |
.251 |
| No | 13 (18.1) |
2.38 (3.36) |
12 (16.7) |
−19.00 (23.24) |
12 (16.7) |
−5.83 (8.11) |
||||||||||
| Energy level during lockdown | Yes | 39 (54.2) |
.77 (1.53) |
−1.39 (46.71) |
.173 |
.346 |
39 (54.2) |
−1.05 (10.74) |
3.27 (47.05) |
.002 |
.830 |
38 (52.8) |
−2.79 (5.99) |
2.57 (65) |
.013 |
.632 |
| No | 33 (45.8) |
1.55 (2.89) |
31 (45.8) |
−12.84 (17.64) |
29 (40.3) |
−6.72 (6.50) |
||||||||||
| Happiness before lockdown | Happy | 52 (72.2) |
1.33 (2.23) |
1.22 (70) |
.227 |
.311 | 52 (74.3) |
−6.54 (14.43) |
−0.247 (68) |
.806 |
.064 | 49 (73.1) |
−3.76 (5.97) |
1.56 (65) |
.125 |
.404 |
| Not happy | 20 (27.8) |
0.60 (2.37) |
18 (25.7) |
−5.50 (17.97) |
18 (26.9) |
−6.50 (7.50) |
||||||||||
| Happiness during lockdown | Happy | 37 (51.4) |
0.78 (1.66) |
−1.30 (55.83) |
.200 |
.313 | 37 (52.9) |
−1.76 (12.18) |
2.74 (68) |
.008 |
.649 | 34 (50.7) |
−2.76 (5.89) |
2.29 (65) |
.025 |
.559 |
| Not happy | 35 (48.6) |
1.49 (2.75) |
33 (47.1) |
−11.33 (16.94) |
33 (49.3) |
−6.27 (6.65) |
||||||||||
Note. Student's T-tests show a significant difference in changes in quality of life, perception of health and well-being between those who have a high energy level before and during confinement and those who don't.
SD = standard deviation; t = Student's T-test; df = degrees of freedom; d = Cohen's d.
*p < 0.05. ⁎⁎p < 0.01. ⁎⁎⁎p < 0.001.
Table 5.
Correlations between independent variables and changes in quality of life, perception of health and well-being.
| ICF | Variables | Change in quality of life | Change in perception of health | Change in well-being | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | r | p | N | r | p | N | r | p | ||
| Body structure and function | Medical conditions before lockdown | 64 | .006 | .480 | 62 | .047 | .357 | 59 | .016 | .453 |
| Change in medical conditions | 64 | .011 | .467 | 62 | .295* | .010 | 59 | .056 | .336 | |
| Functional limitations before lockdown | 64 | −0.263* | .018 | 62 | .035 | .393 | 59 | −0.016 | .452 | |
| Change in functional limitations | 64 | −0.111 | .154 | 62 | .283* | .013 | 59 | .220* | .047 | |
| Cognitive difficulties during lockdown | 64 | −0.193 | .064 | 62 | .084 | .259 | 59 | .078 | .279 | |
| Change in feeling of happiness | 64 | .161 | .103 | 62 | −0.348** | .003 | 59 | .023 | .432 | |
| Activities | PA level | 64 | −0.308⁎⁎ | .007 | 62 | .462⁎⁎ | .000 | 59 | .183 | .083 |
| PA weekly frequency | 64 | .166 | .096 | 62 | .111 | .195 | 59 | .044 | .371 | |
| PA weekly hours | 64 | −0.064 | .307 | 62 | .159 | .109 | 59 | −0.009 | .473 | |
| Participation | Perceived degree of confinement | 64 | .122 | .167 | 62 | −0.003 | .490 | 59 | .101 | .224 |
| Perceived degree of isolation | 64 | .027 | .415 | 62 | −0.253* | .024 | 59 | −0.415** | .001 | |
| Personal Factors |
Age at T1 | 64 | .041 | .374 | 62 | −0.075 | .282 | 59 | −0.205 | .060 |
| Education | 64 | .008 | .474 | 62 | .268* | .018 | 59 | .057 | .333 | |
Note. r = Pearson's correlation; ρ = Spearman's correlation.
p < 0.05. ⁎⁎p < 0.01.
3.6. Change in quality of life
The results of the regression (see Table 6 ) showed that four predictors accounted for 26.7% of the variance (R 2 = 0.267, F(4,59) = 6.736, p < .001). Considering that the EQ-5D scale is inversed (a higher score means lower quality of life), a bigger decrease in quality of life between the period before lockdown and during the second lockdown was explained by a lower energy level at T1 by 10.8% (β = 0.304, p = .031), a lower level of physical activity at T3 by 9.7% (β = −0.416, p = .005), feeling happy at T1 by 6.3% (β = −0.291, p = .019), and a higher weekly frequency of physical activity at T3 by 4.5% (β = 0.384, p = .003).
Table 6.
Stepwise multiple regression models for change in quality of life, perceived health, and well-being between before lockdown (T1) and during the second lockdown (T3) due to COVID-19.
| B | SE | β | t | p | R2 change | Model adjusted R2 | ||
|---|---|---|---|---|---|---|---|---|
| Change in quality of life as dependent variable | .267 | |||||||
| Energy level before lockdown | 1.724 | 0.779 | 0.304 | 2.212 | 0.031* | .108 | ||
| PA level during lockdown | −0.039 | 0.014 | −0.416 | −2.894 | 0.005⁎⁎ | .097 | ||
| Feeling happy before lockdown | −1.387 | 0.577 | −0.291 | −2.403 | 0.019⁎⁎ | .063 | ||
| PA weekly frequency during lockdown | 1.044 | 0.337 | 0.384 | 3.099 | 0.003⁎⁎ | .045 | ||
| Change in perceived health as dependent variable | .447 | |||||||
| PA level during lockdown | .258 | .070 | .365 | 3.675 | .001⁎⁎ | .214 | ||
| Change in feelings of happiness | −9.946 | 2.806 | −0.338 | −3.544 | .001⁎⁎ | .117 | ||
| Change in medical conditions | 10.080 | 4.125 | .235 | 2.443 | .018* | .067 | ||
| Memory problems before lockdown | 12.092 | 4.120 | .289 | 2.935 | .005⁎⁎ | .057 | ||
| Feeling isolated during lockdown | −1.809 | .886 | −0.209 | −2.043 | .046* | .038 | ||
| Change in well-being as dependent variable | .264 | |||||||
| Feeling isolated during lockdown | −1.213 | .406 | −0.349 | −2.989 | .004⁎⁎ | .173 | ||
| Age | −0.360 | .157 | −0.261 | −2.295 | .026* | .067 | ||
| Energy level during lockdown | −3.844 | 1.528 | −0.296 | −2.516 | .015* | .063 |
Note. SE = standard error of B; PA = physical activity.
*p < .05. **p < .01.
3.7. Change in perception of health
The regression (see Table 6) revealed that four predictors accounted for 44.7% of change in perceived health between before confinement and during the second lockdown (R 2 = 0.447, F(5,56) = 10.861, p < .001). Particularly, those who perceived a bigger decline in their health, had a lower level of physical activity at T3 (21.4%, β = 0.365, p = .001), changing from feeling happy to not happy between T1 and T3 (11.7%, β = −0.338, p = .001), an increase in medical conditions between T1 and T3 (6.7%, β = 0.235, p = .018), memory problems at T1 (5.7%, β = 0.289, p = .005), and feeling more isolated at T3 (3.8%, β = −0.209, p = .046).
3.8. Change in well-being
The regression (see Table 6) revealed that three predictors explained 26.4% of change in well-being between the period before lockdown and during the second lockdown (R 2 = 0.264, F(3,55) = 7.944, p < .001). More specifically, a bigger decline in well-being was explained by feeling more isolated at T3 by 17.3% (β = −0.349, p = .004), being older by 6.7% (β = −0.261, p = .026), and having a lower energy level at T3 by 6.3% (β = −0.296, p = .015).
4. Discussion
4.1. Changes in quality of life, perception of health and well-being
The main purpose of this study was to evaluate long-term changes in quality of life, perceived health, and well-being during confinement due to COVID-19 among older, healthy, active VIP members of the Montreal Museum of Fine Arts (MMFA) who form a representative subgroup of active elderly persons living in a developed and populous city. The hypothesis of this study was partly confirmed where the results showed a decrease of quality of life, perceived health, and well-being among participants between the period before lockdown (T1) and after three months of the first confinement (T2 = June 2020), as well as before and during the second confinement (T3 = January 2021; one year after T1). These findings suggest that even healthy and active older adults were significantly and negatively impacted in their daily life by a lockdown. Our results comply with prior studies that showed that quality of life (Siette et al., 2021), perceived health (Ferreira, Pereira, da Fé Brás, & Ilchuk, 2021) and well-being (Prati, 2020) were negatively affected during COVID-19 quarantine.
Nonetheless, the results of the present study did not show a difference between the two periods of lockdown that covers approximatively nine months (T2 and T3) for our three outcomes. Therefore, our revised hypothesis is that older and active adults of our cohort experienced an adaptation period between the first and second quarantines due to COVID-19. Although, some restrictions remained through T3, however somewhat less severe than T1 (e.g., some activities did not restart, restrictions on social contacts), life did not come back to how it was before the pandemic, so the elderly may have adapted to this situation, which could explain why the T3 results were not worse nor better than T2. This is somewhat consistent with previous studies, although they did not produce results on a timeline of a year of lockdown, nor did they provide pre-lockdown status of their participants for comparison. In fact, Chaudhuri et al. (2021) showed that during the COVID-19 lockdown, well-being assessed using the General Health Questionnaire declined for about 54 days before meeting an adaptation period which was followed by improvement and thus followed a U shape. This adaptation period was explained by use of resilience and coping mechanisms by their participants. Moreover, a previous study in Spain showed that the older population adapted better to the lockdown (e.g., kept routines, used coping mechanisms to reduce fear, were more resilient) than younger adults (Morales-Vives, Dueñas, Vigil-Colet, & Camarero-Figuerola, 2020). The theory of “cognitive reserve” could also affect the lack of change between the two lockdowns. This theory assumes that individual differences in flexibility and adaptability of neural networks allow some older people, particularly those with higher levels of education, to better manage and adapt to changes in their environment (Steffener & Stern, 2012). Our participants were highly educated but also very active prior to the confinement, which may explain the significant drop of their quality of life at the first confinement. Then, their greater cognitive reserve may have contributed to an adaptation between both confinements, allowing them to better adapt to this longer context. This reserve may have favored and supported spontaneous reorganization in the months following the first confinement, but future studies are needed to confirm this hypothesis.
4.2. Predictors of change in quality of life, perception of health and well-being
A secondary and exploratory goal of this study was to explore the predictors of change in quality of life, perceived health, and well-being between the period before lockdown (T1 = December 2019) and during the second lockdown (T3 = January 2021). This allowed the researchers to identify some characteristics of the people surveyed that place them at risk to have a decrease in these three areas of life. In fact, lockdown due to COVID-19 caused changes in physical, social, and attitudinal environments which affect one's functioning as described in the WHO's ICF classification (McDougall et al., 2010; WHO, 2001). The predictors found in the present study resemble some of the components described in the ICF framework of COVID-19 outcome measures (Patel et al., 2020) as well as contextual factors that can influence these components: body structure and function (feeling happy at T1, change in happiness between T1 and T3, lower energy level at T1 and T3, increase in medical conditions from T1 to T3, functional limitations at T1, an increase in functional limitations between T1 and T3, memory difficulties at T1), activities (physical activity level and weekly frequency at T3), participation (perceived degree of isolation at T3), and personal factors (age, education). Other variables related to the ICF framework were not significant predictors in our study: body structure and function (presence of medical conditions, and cognitive difficulties at T3), activities (weekly hours of physical activity), and participation (perceived degree of confinement).
4.3. Quality of life
In the present study, quality of life was associated with the level of physical activity during lockdown. More specifically, we found that individuals who did less exercise during the confinement (despite a higher frequency of physical activity per week than others) reported a poorer quality of life. A meta-analysis (Park, Sun Han, & Kang, 2014) of 18 studies supports this result and highlights the link between exercise and improvement in quality of life in healthy adults 65 years and over. Namely, physical activity enhances physical functioning (Canuto Wanderley et al., 2015), and, therefore, older adults can live more independently (Svantesson, Jones, Wolbert, & Alricsson, 2015). To explain our contrasting findings, about the decrease in the level of physical activities despite higher frequency of practice per week, our hypothesis is that active older participants may have had exercised frequently before lockdown, but their activities changed during the lockdown. For example, participants who took part in physical activities five to seven times a week often stopped doing group activities (e.g., badminton, volleyball, Pilates, pickleball, etc.) and did more hours per week of solo activities than the others (e.g., walking, stretching, cross-country skiing, etc.). It is possible that the type of activity or the social experience associated with said activity explains the change in quality of life. For example, a meta-analysis found that moderate levels of physical activity with high physical, mental, and social demands are associated with better functional abilities needed for activities of daily living (Roberts, Phillips, Cooper, Gray, & Allan, 2021).
In addition to physical activity, the perceived quality of life was also associated with the level of happiness before lockdown. This finding revealed that participants who reported a greater decrease in their quality of life were those who also felt happier before this period. Therefore, we hypothesize that those who reported higher happiness before the pandemic noticed a bigger loss during the confinement periods compared to those with lower level of happiness before lockdown who did not experience a significant deterioration in their condition. This result is not surprising since happiness is strongly associated with quality of life, a greater decrease in the level of happiness may be associated with greater decrease in perceived quality of life. Thus, Veenhonven (2001) mentioned a relationship between happiness and certain aspects of quality of life such as freedom, autonomy, physical security, social participation, and personal relationships, all things that were lost during the COVID-19 stay-at-home orders, especially for those participants who highly enjoyed these aspects of their life before confinement.
The level of energy before lockdown was also found to be a good predictor of quality of life where the participants with lower level of energy before lockdown where those with a higher decreased in their perceived quality of life. Previous studies report that fatigue (low mood, tiredness, lethargy, unfocused mental state, uncomfortable bodily state, etc.) has negative repercussions on emotions, daily activities, health, and, therefore, quality of life (Hockey, 2013).
4.4. Perception of health
In the present study, a bigger decrease in perceived health was predicted by a lower level of physical activity at the second lockdown, which is consistent with previous studies (Eifert, Wideman, Oberlin, & Labban, 2014; Jakobsson, Malm, Furberg, Ekelund, & Svensson, 2020; Jiménez-Pavón, Carbonell-Baeza, & Lavie, 2020; Kwaśniewska, Bielecki, & Drygas, 2004; Roberts et al., 2021). A study by Veenhonven (2001) also revealed that happiness was related to self-perceived health, and that a change in health is associated to a parallel change in happiness. This relationship supports our finding which suggests that changing from feeling happy to not happy was also among the predictors of a decrease of perceived health.
Not surprisingly, our findings also indicate that a worsening medical condition between T1 and T3 was related to a decline in self-reported health. A longitudinal study showed that a higher number of medical conditions was associated with worse perceived health due to more difficulty performing activities of daily living (Barile et al., 2013). Moreover, self-reported health status and medically diagnosed conditions were correlated in a previous study (Bush et al., 2011) where the conditions that affect perceived health the most were mobility issues, sleep problems, contentment with one's health, as well as difficulty with everyday tasks such as cleaning, washing, or shopping (Lindgren, Svärdsudd, & Tibblin, 1994).
In addition, the present study showed that older participants who perceived a higher decrease in perceived health also reported memory problems before the first lockdown. A previous study reported an association between memory difficulties and worsened self-perceived health due to more limitations on activities of daily living and due to the fear of developing dementia (Montejo, Montenegro, Fernández, & Maestú, 2012). Lower memory capacity has also been linked to a decline in physical and self-perceived health, especially for those people between 75 and 87 years old (Nelson, Jacobucci, Grimm, & Zelinski, 2020). Accordingly, we hypothesize that those with troubles with their memory before the stay-at-home orders may have had worsened memory in the last year and, being more cognitively vulnerable, had therefore worsened perceived health.
Finally, we found that participants whose perceived health diminished during the lockdown due to COVID-19 also felt more isolated. Social isolation and loneliness are common among older adults, but the stay-at-home orders linked with lockdown magnified the risks. A better perception of health in the elderly is related to having access to good social resources and opportunities for social participation (León, Mangin, & Ballesteros, 2020), as well as having a higher number of social activities (Gilmour, 2012), which was quite limited during lockdown.
4.5. Well-being
As supported by the present study, previous studies have shown that lockdown due to COVID-19 caused social isolation, which in turn is detrimental to well-being because of an increase in anxiety and depression, as well as a decrease in sleep quality (Sepúlveda-Loyola et al., 2020) and group activities (INSPQ, 2020). Well-being in seniors is dependent on social interaction and support, which were lacking during the pandemic. The COVID-19 lockdown also exacerbated some risk factors of social isolation in the elderly, such as loss of loved ones and grieving, ageism, insecurity, loss of mobility due to lockdown, less opportunities for social participation, etc. (INSPQ, 2020). Our participants are generally a more active group than other older adults, it is therefore possible that those who were more socially active prior to the lockdown had the biggest change in environment (now being confined at home with less interactions) and noticed a stronger impact on their well-being. Keeping a good level of social interaction could counter the loneliness brought on by the lockdown (Macdonald & Hülür, 2021). However, this is difficult since many elderly persons live alone and use fewer types of technologies (i.e., online) to communicate (INSPQ, 2020).
We also found that those who reported lower energy during the second lockdown had a higher decrease in perceived health. To our knowledge, no study has been done on feeling energetic in a healthy older population. However, there seems to be a link between feeling energetic and both mental and physical quality of life in people with dystonia (Soeder et al., 2009) and spinal cord injury (Wijesuriya, Tran, Middleton, & Craig, 2012). Similarly, taking this into consideration, it is possible that those who felt less energetic before the lockdown became more lethargic during the pandemic and therefore their well-being was more affected than others.
Age was another variable associated with well-being. This relationship has been consistent during the COVID-19 lockdown in other studies, where younger adults reported worse well-being (Pieh, Probst, Budimir, & Humer, 2021). However, our results showed that a bigger decline in well-being was associated with participants who were older. It is possible that our older participants have more risk factors of low well-being caused by the pandemic itself instead of their age. For example, the oldest “old persons” have more chance of being widowed, are living alone, and having smaller social networks, and generally this puts them at risk of social isolation and poor well-being (Courtin and Knapp, 2015) during lockdown. They may also have more functional limitations and medical conditions preventing them from engaging in physical activity which helps to increase their well-being (Stathi, Fox, & McKenna, 2002).
4.6. Limitations
This study has at least three potential limitations. First, as a longitudinal study, we cannot confirm that the findings are solely based on confinement. It is possible that the changes in quality of life, perceived health, and well-being are explained by other aspects of life. There was also a difference in sample size between the three times (85.11% of 94 participants answered the survey at T1 and 91.49% at T3), although in each case the sample sizes met the criteria for statistical analyses. Those who did not answer all three surveys (8.9%) were not considered in the analyses, so it is possible that some information was lost between the three measures. Second, the generalization of the results is limited. The participants are all VIP members of the Montreal Museum of Fine Arts and therefore represent a specific subgroup of well-educated, healthy, and active older population. Furthermore, they may have a higher socioeconomic status (e.g., they can afford membership costs) and may be more cognitively, physically, and socially active than other older adults. The sample was also composed of mostly women (89.4%), which limits the generalization to the older male population. Third, even though the EuroQol-5D and WEMWBS are validated questionnaires, they are only comprised of 6 and 14 items respectively. Thus, it is plausible that they do not measure all aspects of quality of life and well-being.
4.7. Conclusions
The COVID-19 pandemic has greatly affected people worldwide. To prevent the spread of and complications caused by the virus, and especially to protect vulnerable populations such as the elderly, governments implemented lockdowns and stay-at-home orders. However, this study shows that the COVID-19 lockdown has had negative effects on older adults’ quality of life, perceived health, and well-being. In the event that other periods of confinement are implemented in the future or in various situations where the elderly must be isolated, for various reasons (i.g., loss of mobility) and, from a prevention perspective, the people susceptible of experiencing a decrease in quality of life, perceived health and well-being must be specifically targeted. Among them, those who reported changes in their level of energy and happiness related to a confinement as well as those who reported changes in their level of practice of physical activities should be given more attention. The elderly, for whom a prevention intervention might also be important, are the oldest ones, those who reported feeling isolated, as well as those who suffered from diverse medical problems as well as those with a premorbid cognitive vulnerability (i.e. memory impairments).
In the context of clinical practice with an elderly clientele, our study suggests that physical activity should be recommended to people who are isolated in order to increase their quality of life and a more positive perception of their health. It is therefore suggested to encourage good lifestyle habits and to prescribe physical activities, such as walking or other non-sedentary activities appreciated by the person. In addition, in a situation of isolation, the medical, cognitive, and psychological conditions of the elderly person should be closely monitored and addressed in order to intervene when necessary and with the objective of promoting a better quality of life and well-being.
Finally, we believed that future studies are needed to see if the secondary effects to confinement are long lasting, or if quality of life, perceived health and well-being will increase once seniors can again participate in their usual activities. Nonetheless, our findings will allow for future interventions to target which issues are most likely to affect the older population in any confinement or isolation context, given that older adults may be more often isolated, as they are living alone, form smaller social networks, and have decreased mobility.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
E. Colucci: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. S. Nadeau: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – original draft, Visualization, Supervision, Project administration. J. Higgins: Methodology, Writing – review & editing. E. Kehayia: Methodology, Writing – review & editing. T. Poldma: Methodology, Writing – review & editing. A. Saj: Methodology, Writing – review & editing. E. de Guise: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
None.
Acknowledgements
First, we would like to thank the Montreal Museum of Fine Arts for allowing this project to take place. We would also like to thank Dr. Olivier Beauchet for giving us the permission to use the CESAM survey, and Kevin Gallery for his help in using it. We also would like to show our appreciation for the help of Nancy Azevedo and Catherine Gagnon in collecting data. Lastly, we would like to thank all of the Montreal Museum of Fine Arts VIP members for participating in this study.
References
- Ammar A., Chtourou H., Boukhris O., Trabelsi K., Masmoudi L., Brach M., et al. COVID-19 home confinement negatively impacts social participation and life satisfaction: A worldwide multicenter study. International Journal of Environmental Research and Public Health. 2020;17(17):6237–6254. doi: 10.3390/ijerph17176237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile J.P., Thompson W.W., Zack M.M., Krahn G.L., Horner-Johnson W., Bowen S.E. Multiple chronic medical conditions and health-related quality of life in older adults, 2004–2006. Preventing Chronic Disease. 2013;10(162):1–11. doi: 10.5888/pcd10.120282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L.L., Seiffer B., Deinhart C., Atrott B., Sudeck G., Hautzinger M., et al. Associations of exercise and social support with mental health during quarantine and social-distancing measures during the COVID-19 pandemic: A cross-sectional survey in Germany. medRxiv. 2020 doi: 10.1101/2020.07.01.20144105. [DOI] [Google Scholar]
- Beauchet O., Bastien T., Mittelman M., Hayashi Y., Hau Yan Ho A. Participatory art-based activity, community-dwelling older adults and changes in health condition: Results from a pre–post intervention, single-arm, prospective and longitudinal study. Maturitas. 2020;134:8–14. doi: 10.1016/j.maturitas.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Beauchet O., Cooper-Brown L., Hayashi Y., Galery K., Vilcocq C., Bastien T. Effects of “Thursdays at the Museum” at the Montreal Museum of Fine Arts on the mental and physical health of older community dwellers: The art-health randomized clinical trial protocol. Trials. 2020;21(1):709. doi: 10.1186/s13063-020-04625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke C., Autenrieth L.K., Asselmann E., Pané-Farré C.A. Lockdown, quarantine measures, and social distancing: Associations with depression, anxiety and distress at the beginning of the COVID-19 pandemic among adults from Germany. Psychiatry Research. 2020;293 doi: 10.1016/j.psychres.2020.113462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R., EuroQol Group EuroQol: The current state of play. Health Policy (Amsterdam, Netherlands) 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., et al. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. The Lancet. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow D.D., Arnold-Nedimala N.A., Jordan L.S., Pena G.S., Won J., Woodard J.L., et al. The mental health benefits of physical activity in older adults survive the COVID-19 pandemic. The American Journal of Geriatric Psychiatry. 2020;28(10):1046–1057. doi: 10.1016/j.jagp.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuto Wanderley F.A., Oliveira N.L., Marques E., Moreira P., Oliveira J., Carvalho J. Aerobic versus resistance training effects on health-related quality of life, body composition, and function of older adults. Journal of Applied Gerontology. 2015;34:NP143–NP165. doi: 10.1177/0733464812468502. [DOI] [PubMed] [Google Scholar]
- Daly Z., Slemon A., Richardson C.G., Salway T., McAuliffe C., Gadermann A.M., et al. Psychiatry Research. 2020;295 doi: 10.1016/j.psychres.2020.113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehi Aroogh M., Mohammadi Shahboulaghi F. Social participation of older adults: A Concept Analysis. International Journal of Community-Based Nursing and Midwifery. 2020;8(1):55–72. doi: 10.30476/IJCBNM.2019.82222.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert E.K., Wideman L., Oberlin D.J., Labban J. The relationship between physical activity and perceived health status in older women: Findings from the Woman's College Alumni Study. Journal of Women & Aging. 2014;26(4):305–318. doi: 10.1080/08952841.2014.906878. [DOI] [PubMed] [Google Scholar]
- Evans I.E.M., Martyr A., Collins R., Brayne C., Clare L. Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer's Disease. 2019;70(s1):S119–S144. doi: 10.3233/JAD-180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. Elderly people's definitions of quality of life. Social Science & Medicine. 1995;41(10):1439–1446. doi: 10.1016/0277-9536(95)00117-P. [DOI] [PubMed] [Google Scholar]
- Ferreira L.N., Pereira L.N., da Fé Brás M., Ilchuk K. Quality of life under the COVID-19 quarantine. Quality of Life Research. 2021;30(5):1389–1405. doi: 10.1007/s11136-020-02724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. 5th ed. SAGE Publishing; London: 2018. Discovering statistics using r; pp. 72–91. 122-31. [Google Scholar]
- Gilmour H. Social participation and the health and well-being of Canadian seniors (publication no 82-003-X) Statistics Canada. 2012;23(4) https://www.researchgate.net/profile/Heather-Gilmour-2/publication/232607486_Social_participation_and_the_health_and_well-being_of_Canadian_seniors/links/09e415086d6c6ca8c4000000/Social-participation-and-the-health-and-well-being-of-Canadian-seniors.pdf Catalogue no. 82-003-XPE. [PubMed] [Google Scholar]
- Goethals L., Barth N., Guyot J., Hupin D., Celarier T., Bongue B. Impact of home quarantine on physical activity among older adults living at home during the COVID-19 pandemic: Qualitative interview study. Journal of Medical Internet Research. 2020;22(5):1–5. doi: 10.2196/19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvernement du Québec. (2021,. 27 September). Data on COVID-19 in Québec. https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/situation-coronavirus-in-quebec.
- Gouvernment of Canada. (2021,. 27 September). COVID-19 daily epidemiology update.https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html.
- Hockey R. Cambridge University Press; Cambridge: 2013. The psychology of fatigue: Work, effort and control. [DOI] [Google Scholar]
- Institut National de Santé Publique du Montréal, Québec . 2020. Tackling social isolation and loneliness among seniors in a pandemic context (publication no 3033)https://www.inspq.qc.ca/sites/default/files/publications/3033-social-isolation-loneliness-seniors-pandemic-covid19.pdf [Google Scholar]
- Institut national de santé publique du Montréal, Québec . 2021. Ligne du temps COVID-19 au québec.https://www.inspq.qc.ca/covid-19/donnees/ligne-du-temps 12 février) [Google Scholar]
- Jakobsson J., Malm C., Furberg M., Ekelund U., Svensson M. Physical activity during the coronavirus (COVID-19) pandemic: Prevention of a decline in metabolic and immunological functions. Front Sports Active Living. 2020;2(57) doi: 10.3389/fspor.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen M.F., Birnie E., Haagsma J.A., Bonsel G.J. Comparing the standard EQ-5D three-level system with a five-level version. Value in Health. 2008;11(2):275–284. doi: 10.1111/j.1524-4733.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- Janssen M.F., Pickard A.S., Golicki D., Gudex C., Niewada M., Scalone L., et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3 Lacross eight patient groups: A multi-country study. Quality of Life Research. 2013;22(7):1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Yim H.W., Song Y.-J., Ki M., Min J.-A., Cho J., et al. Mental health status of people isolated due to Middle East Respiratory Syndrome. Epidemiology and Health. 2016;38 doi: 10.4178/epih.e2016048. e2016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Pavón D., Carbonell-Baeza A., Lavie C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Progress in Cardiovascular Diseases. 2020;63:386–388. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaśniewska M., Bielecki W., Drygas W. Sociodemographic and clinical determinatnts of quality of life in urban population of Poland. Central European Journal of Public Health. 2004;12(2):63–68. [PubMed] [Google Scholar]
- León L.P., Mangin J.P.L., Ballesteros S. Psychosocial determinants of quality of life and active aging. A structural equation model. International Journal of Environmental Research and Public Health. 2020;17(17):6023. doi: 10.3390/ijerph17176023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur M., Richard L., Gauvin L., Raymond E. Inventory and analysis of definitions of social participation found in the aging literature: Proposed taxonomy of social activities. Social Science & Medicine. 2010;71(12):2141–2149. doi: 10.1016/j.socscimed.2010.09.041. Epub 2010 Oct 19. PMID: 21044812; PMCID: PMC3597625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A.M., Svärdsudd K., Tibblin G. Factors related to perceived health among elderly people: The Albertina Project. Age and Ageing. 1994;23(4):328–333. doi: 10.1093/ageing/23.4.328. [DOI] [PubMed] [Google Scholar]
- Macdonald B., Hülür G. Well-being and loneliness in swiss older adults during the COVID-19 pandemic: The role of social relationships. The Gerontologist. 2021;61(2):240–250. doi: 10.1093/geront/gnaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M.L., Hogan T.M., Perry A., Biese K., Bonner A., Pagel P., et al. COVID-19 in older adults: Key points for emergency department providers. Journal Of Geriatric Emergency Medicine. 2020;1(4):1–11. [Google Scholar]
- McDougall J., Wright V., Rosenbaum P. The ICF model of functioning and disability: Incorporating quality of life and human development. Developmental Neurorehabilitation. 2010;13(3):204–211. doi: 10.3109/17518421003620525. [DOI] [PubMed] [Google Scholar]
- Montejo P., Montenegro M., Fernández M.A., Maestú F. Memory complaints in the elderly: Quality of life and daily living activities. A population based study. Archives of Gerontology and Geriatrics. 2012;54(2):298–304. doi: 10.1016/j.archger.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Morales-Vives F., Dueñas J.M., Vigil-Colet A., Camarero-Figuerola M. Psychological variables related to adaptation to the COVID-19 lockdown in Spain. Frontiers in Psychology. 2020;11:565634. doi: 10.3389/fpsyg.2020.565634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N.A., Jacobucci R., Grimm K.J., Zelinski E.M. The bidirectional relationship between physical health and memory. Psychology and Aging. 2020;35(8):1140–1153. doi: 10.1037/pag0000579. [DOI] [PubMed] [Google Scholar]
- Park S.H., Sun Han K., Kang C.-.B. Effects of exercise programs on depressive symptoms, quality of life, and self-esteem in older people: A systematic review of randomized controlled trials. Applied Nursing Research. 2014;27(4):219–226. doi: 10.1016/j.apnr.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Patel K., Straudi S., Sien N.Y., Frayed N., Melvin J.L., Sivan M. Applying the WHO ICF framework to the outcome measures used in the evaluation of long-term clinical outcomes in coronavirus outbreaks. International Journal of Environmental Research and Public Health. 2020;17(18):6476. doi: 10.3390/ijerph17186476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieh C., Probst T., Budimir S., Humer E. Diminished well-being persists beyond the end of the COVID-19 lockdown. General Hospital Psychiatry. 2021;70:137–138. doi: 10.1016/j.genhosppsych.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati G. Mental health and its psychosocial predictors during national quarantine in Italy against the coronavirus disease 2019 (COVID-19) Anxiety, Stress & Coping. 2020;34(2):145–156. doi: 10.1080/10615806.2020.1861253. [DOI] [PubMed] [Google Scholar]
- Roberts C.E., Phillips L.H., Cooper C.L., Gray S., Allan J.L. Effect of different types of physical activity on activities of daily living in older adults: Systematic review and meta-analysis. Journal of Aging and Physical Activity. 2021;25(4):653–670. doi: 10.1123/japa.2016-0201. [DOI] [PubMed] [Google Scholar]
- Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., Ganz F., Torralba R., Oliveira D.V., et al. Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. The Journal of Nutrition Health and Aging. 2020;24:938–947. doi: 10.1007/s12603-020-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J., Dodds L., Seaman K., Wuthrich V., Johnco C., Earl J., et al. The impact of COVID-19 on the quality of life of older adults receiving community-based aged care. Australasian Journal on Ageing. 2021;40(1):84–89. doi: 10.1111/ajag.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeder A., Kluger B.M., Okun M.S., Garvan C.W., Soeder T., Jacobson C.E., et al. Mood and energy determinants of quality of life in dystonia. Journal of Neurology. 2009;256(6):996. doi: 10.1007/s00415-009-5060-3. [DOI] [PubMed] [Google Scholar]
- Stathi A., Fox K.R., McKenna J. Physical activity and dimensions of subjective well-being in older adults. Journal of Aging and Physical Activity. 2002;10:76–92. doi: 10.1123/japa.15.4.382. [DOI] [PubMed] [Google Scholar]
- Steffener J., Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2012;1822(3):467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R., Hiller L., Fishwick R., et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5 doi: 10.1186/1477-7525-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svantesson U., Jones J., Wolbert K., Alricsson M. Impact of physical activity on the self-perceived quality of life in non-frail older adults. Journal of Clinical Medicine Research. 2015;7(8):585–593. doi: 10.14740/jocmr2021w. http://dx.doi.org/10.14740/jocmr2021w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R., Hiller L., Fishwick R., Platt S., Joseph S., Weich S., et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): Development and UK validation. Health and Quality of Life Outcomes. 2007;5(63) doi: 10.1186/1477-7525-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell C.J., Williams K.N. The paradox of social distancing: Implications for older adults in the context of COVID-19. Psychological Trauma. 2020;12(S1):S214–S216. doi: 10.1037/tra0000845. https://dx.doi.org/10.1037/tra0000845S214. [DOI] [PubMed] [Google Scholar]
- Vagetti G.C., Barbosa Filho V.C., Moreira N.B., Oliveira V., Mazzardo O., Campos W. Association between physical activity and quality of life in the elderly: A systematic review. Brazilian Journal of Psychiatry. 2014;36:76–88. doi: 10.1590/1516-4446-2012-0895. [DOI] [PubMed] [Google Scholar]
- Veenhoven R. Salute e qualità dell vida. Centro Scientifico Editore; Torino, Italia: 2001. Quality-of-life and happiness: Not quite the same; pp. 67–95. [Google Scholar]
- WHOQOL Group The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Social Science & Medicine. 1995;41(10):1403–1409. doi: 10.1016/0277-9536(95)00112-K. [DOI] [PubMed] [Google Scholar]
- Wijesuriya N., Tran Y., Middleton J., Craig A. Impact of fatigue on the health-related quality of life in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2012;93(2):319–324. doi: 10.1016/j.apmr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Wilson R.S., Krueger K.R., Arnold S.E., Schneider J.A., Kelly J.F., Barnes L.L., et al. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. (2001). Classification internationale du fonctionnement, du handicap et de la santé : CIF. https://apps.who.int/iris/handle/10665/42418.
- Worldometer. (2021,. 27 September). COVID-19 coronavirus pandemic.https://www.worldometers.info/coronavirus/.
- Zunzunegui M.-.V., Alvarado B.E., Del Ser T., Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. The Journals of Gerontology: Series B. 2003;58(2):S93–S100. doi: 10.1093/geronb/58.2.S9. [DOI] [PMC free article] [PubMed] [Google Scholar]