Abstract
Low-intensity cognitive behaviour therapy including behavioural activation is an evidence-based treatment for depression, a condition frequently co-occurring with autism. The feasibility of adapting low-intensity cognitive behaviour therapy for depression to meet the needs of autistic adults via a randomised controlled trial was investigated. The adapted intervention (guided self-help) comprised materials for nine individual sessions with a low-intensity psychological therapist. Autistic adults (n = 70) with depression (Patient Health Questionnaire-9 score ⩾10) recruited from National Health Service adult autism services and research cohorts were randomly allocated to guided self-help or treatment as usual. Outcomes at 10-, 16- and 24-weeks post-randomisation were blind to treatment group. Rates of retention in the study differed by treatment group with more participants attending follow-up in the guided self-help group than treatment as usual. The adapted intervention was well-received, 86% (n = 30/35) of participants attended the pre-defined ‘dose’ of five sessions of treatment and 71% (25/35) attended all treatment sessions. The findings of this pilot randomised controlled trial indicate that low-intensity cognitive behaviour therapy informed by behavioural activation can be successfully adapted to meet the needs of autistic people. Evaluation of the effectiveness of this intervention in a full scale randomised controlled trial is now warranted.
Keywords: adults, autism, cognitive behaviour therapy, depression
Introduction
Autism spectrum disorder (ASD), thought to affect 1% of the UK adult population (Brugha et al., 2011), is characterised by qualitative impairments in social communication and a stereotyped, repetitive or restricted pattern of activities, behaviours and interests (American Psychiatric Association, 2013). High rates of co-occurring mental health conditions are reported across the life-span in ASD (e.g. Joshi et al., 2013; Kim et al., 2000; Simonoff et al., 2008) including depression. Depression, characterised by persistent sadness and low mood with a range of accompanying physical, emotional and behavioural symptoms, is a debilitating mental health condition (Whiteford et al., 2013). There is evidence that autistic 1 people are disproportionately affected by depression (Rai et al., 2018) with an estimated current and life-time adult prevalence of 23% and 37%, respectively (Hollocks et al., 2018). Effective treatments for depression exist. ‘Low-intensity’ cognitive behaviour therapy (CBT), to include behavioural activation (BA), is recommended as an evidence-based psychological treatment for mild–moderate depression (CG90, National Institute for Health and Care Excellence (NICE), 2009) in adults. Low intensity typically refers to low usage of specialist therapist time (Bower & Gilbody, 2005) via the provision of evidence-based information which can be accessed independently (self-help) or with the support of a mental health worker (guided self-help (GSH)), or by the cost-effective use of specialist therapist time such as group interventions.
Accessing mainstream psychological therapies can be difficult for autistic people due to communication and neurocognitive differences. In addition, many therapists have not been trained in working with autistic people (Cooper et al., 2018). Clinical guidance for psychosocial interventions for co-occurring mental health problems in autism state that the recommended intervention for the specific disorder in the general population should be offered with adaptations made to meet the needs of autistic people (NICE CG 142, 170). Where the recommended intervention is CBT, strategies to adapt CBT to meet the needs of autistic people have been identified in the NICE clinical guidance for autism in adults (NICE CG 142). Adapted CBT has been found to be an effective treatment for anxiety co-occurring with autism (see reviews by White et al., 2018; Weston, Hodgekins, & Langdon, 2016). A review by White et al. (2018) highlight that there have been few studies investigating depression. Studies of combined anxiety and depression treatment (McGillivray & Evert, 2014; Sizoo & Kuiper, 2017) have reported no convincing evidence of an effect of treatment group on depression measures, although sample sizes have been small. There has been a study of an adapted group CBT protocol for depression (Santomauro et al., 2016) reporting a reduction in depression scores of 20 autistic adolescents. The authors urge caution in the light of a small sample size and lack of control group at follow-up. A study of adults (n = 42) randomised to mindfulness-based stress reduction (MBSR) or treatment as usual (TAU) reported a significant effect of MBSR on depression measures (Spek et al., 2013). These studies suggest some preliminary evidence in support of cognitive-behavioural interventions for depression adapted for autism. However, there have been no adult studies with an exclusive focus on depression investigating autism adaptations to low-intensity CBT for depression.
Low-intensity CBT may offer benefits for the treatment of depression co-occurring with autism for a number of reasons. First, BA is the recommended treatment model for low-intensity CBT for depression. BA, informed by learning theory, is an approach developed by Martell et al. (2001) which aims to change an individual’s behavioural repertoire to increase opportunities to access positive reinforcement and thereby reduce depression. These aims are primarily achieved through activity scheduling, whereby the individual plans and schedules specified activities in their weekly routine based on a detailed analysis of their current behaviour. People are encouraged to become more aware of the triggers for low mood and consequences of a range of behaviours and to then use this information to make changes in line with individual goals. BA may be highly suited as an intervention for depression in autistic adults. First, a restricted, repetitive, stereotyped pattern of behaviour, interests and activities is a core characteristic of autism. It is possible that when depressed, autistic people do not easily generate shifts in routines, behaviours and thought patterns towards activities and actions that may offer increased access to pleasure and a sense of achievement, and thus reduce depression. This tendency towards restricted activities and interests, including repetitive thinking, may form part of the behavioural maintenance cycle of depression symptoms in autism with some research evidence in support of this (e.g. Gotham et al., 2014). Second, the well-documented executive function differences in autism (Demetriou et al., 2018) mean that generating and implementing novel planned behaviour and activity can be compromised in autistic people. To this end, BA adapted for the needs of autistic people may be helpful in broadening behavioural and thought repertoires to ultimately improve depressed mood.
In addition, adaptations to CBT to meet the needs of autistic people recommend the use of written information with visual aids (NICE, 2012). The provision of written information is at the core of a low-intensity intervention. Adapting the written information to meet the needs of autistic people means that low-intensity psychological therapists who do not have extensive experience in working with autistic adults have less elements of their therapeutic practice to adapt, that is, some of the key adaptations are delivered through the materials. This may confer some advantages to a low-intensity intervention over individual talking therapy which is solely verbally mediated. In a survey of CBT therapists, confidence in adapting practice for autistic people was significantly associated with level of therapist training (Cooper et al., 2018). Low-intensity interventions were developed with the aim of disseminating evidence-based practice to mental health professionals from a range of backgrounds. This may be highly pertinent to autistic adults who can receive support and healthcare from a range of professionals.
The present study, developed in response to a themed call from the Health Technology Assessment (HTA) panel of the National Institute of Health Research (NIHR) (HTA 14/43), had the following aims:
Develop a low-intensity intervention for depression based on NICE guidance for adults, adapted for autism, including training materials for low-intensity psychological therapists;
Investigate the feasibility and patient and healthcare worker acceptability of the low-intensity intervention;
Estimate the rate of recruitment and retention for a fully powered randomised controlled trial (RCT);
Identify the most appropriate outcome measure for a fully powered RCT.
Methods
Study design
The feasibility study comprised a randomised controlled trial, with a nested qualitative study. Detailed information about the study methods is available in the published protocol (Russell et al., 2017). In brief, participants were randomised to one of two groups: (1) an adapted low-intensity CBT intervention for depression GSH or (2) TAU. The nested qualitative study will be reported in full elsewhere. Ethical approval for the study was granted by WALES Research Ethics Committee (REC 3) (Integrated Research Application System (IRAS) project ID: 191558) and permissions to carry out the study were obtained from the National Health Service (NHS) trusts involved. Trial registration was assigned (ISRCTN54650760).
Participants
Eligible participants were adults (⩾18 years old) with a diagnosis of ASD and current depression defined as Patient Health Questionnaire-9 (PHQ-9) score ⩾10. Excluded were those with the following: a risk of suicide such that clinical need exceeded a low-intensity intervention, current alcohol or substance-use dependence, untreated epilepsy, a history of psychosis or who had received ⩾6 sessions of individual CBT in the last 6 months. Also excluded were individuals who were unable to understand the study materials due to language or literacy levels.
Recruitment
Potential participants were introduced to the study by clinicians in two NHS adult autism services or by letter to autistic adults registered on an NHS research opportunity (Bristol) and a UK autism cohort study, across two geographical regions of England (Bristol, Bath and North East Somerset; and Northumberland, Tyne and Wear). The recruitment procedure is described in full in the published protocol (Russell et al., 2017). In brief, those interested in the study who gave permission to be contacted by the research team were provided with full study information and if they expressed an interest had a telephone screening according to the inclusion and exclusion criteria. The PHQ-9 (Kroenke & Spitzer, 2002) could be completed as part of the telephone screening or by return post if preferred. The PHQ-9 is a reliable and valid nine-item self-report measure of depression commonly used in primary care settings. A PHQ-9 score ⩾10 conferred eligibility for the study which was further confirmed at a face-to-face meeting. The Clinical Interview Schedule-Revised (CIS-R) (Lewis et al., 1992) was administered at this meeting to capture current depression. The CIS-R is a widely used, well-validated diagnostic instrument which generates International Classification of Diseases (ICD-10) psychiatric diagnoses. Fully informed consent in writing was obtained from eligible participants willing to take part in the study. A series of open questions about participation in the study ensured consent was fully informed.
Randomisation
Eligible consenting participants were randomised to receive (1) GSH or (2) TAU. Allocation was concealed from recruiting researchers through use of a remote computerised randomisation service. Allocation was stratified by site (Bristol, Bath and North East Somerset or Newcastle, Tyne and Wear NHS regional centre) and minimised by depression severity (mild–moderate: PHQ-9 score between 10 and 15 or moderate–severe: PHQ-9 score between 16 and 27) and current use of antidepressant medication (yes or no). Participants were informed about the outcome of randomisation by the trial manager.
Intervention
GSH comprised materials for nine weekly sessions which participants could work through with the support of a ‘coach’, that is, a low-intensity psychological therapist. The intervention (see Russell et al., 2017), based on BA, was tailored to meet the needs of autistic people. The feedback of two autistic adults informed two iterations of the session materials. The materials had a consistent structure and format. Visual images were used to supplement written accounts of psychological principles. Emotional literacy and executive function difference were supported throughout. The aim of the intervention was to facilitate learning about links between situations, behaviours and feelings, and use this learning to schedule activities promoting positive feelings. The initial session was an orientation to GSH, the role of the ‘coach’ and an opportunity for the coach to learn about individualised needs for autism specific adaptations. Individual goal-setting for the intervention was completed at the end of this session. Sessions 2 and 3 focused on mapping daily situations and behaviours using a visual map and diary sheet. Session 4 took an educational approach to noticing and rating positive feelings. Session 5 brought together information about situations, behaviours and feelings. This information was used to schedule activities to increase opportunities for positive mood which were also in line with an individual’s goals and Maslow’s hierarchy of needs. An individual’s needs for support and prompts to implement the activity schedule plans were also discussed. Recording information about situations, behaviours and feelings using the map and/or diary sheets was a consistent between-session task. Sessions 6–8 were spent refining activity scheduling to extend to novel situations and novel behaviours if appropriate. Session 9 was a review of treatment principles and goals. To simulate routine clinical practice, the PHQ-9 and General Anxiety Disorder Questionnaire (GAD-7) were administered at the start of each session. The intervention was delivered face-to-face, weekly in outpatient clinic settings and was intended to be delivered across 10 weeks. However, participant and/or coach availability as a result of factors such as illness, holidays and educational/work commitments meant that 16 weeks presented a more realistic time-frame for delivery of the intervention. Each session lasted between 30 and 45 min, with the exception of the initial planning session which could last up to 90 min. The final two sessions could be delivered by telephone according to patient preference. Engagement with five sessions constituted a treatment ‘dose’ as the main therapeutic principles had been presented at that stage.
GSH coaches were graduate level psychologists with foundation knowledge of CBT who attended 15 h of training in the intervention and working with autistic people. Coaches received weekly supervision (1 h in duration) from the research clinical psychologists who had designed the intervention (A.R., S.B. and K.C.). A manual for the coaches accompanied the session materials.
TAU
There were no constraints on TAU. Participants randomised to TAU were signposted to local psychological therapy treatment services, and this was also communicated to participants’ GPs by letter, along with their PHQ-9 score.
Data collection and outcome measurement
Outcomes were measured at 10-, 16- and 24-weeks post-randomisation by researchers blind to treatment group. Self-report outcome measures were ordinarily completed via a computerised survey at a face-to-face meeting, remotely using an electronic link or by post according to individual preference. Where follow-up was not face-to-face, the interview measure of depression (see below) was conducted by telephone.
There is some evidence about the reliability and validity of two self-report measures to screen and identify depression in the autistic population (Cassidy et al., 2018) but no information about whether measures are sensitive to change. Therefore, one of the objectives of this feasibility study was to identify the most appropriate outcome measure, and hence three depression measures were included and no primary outcome measure specified. Qualitative interviews about the experience of being in the study and the experience of the intervention were conducted by telephone after the 10-weeks follow-up.
Depression measures
PHQ-9 (Kroenke & Sptizer, 2002): This is a reliable (Cronbach’s α = 0.83–0.94) and valid nine-item self-report measure of depression commonly used in primary care settings. To the authors’ knowledge, the psychometric properties of the scale have not been investigated with the autistic population.
Beck Depression Inventory-II (BDI-II) (Beck et al., 1996): This is a widely used, 21-item self-report measure of depression found to be reliable (Cronbach’s α = 0.92) for outpatients. A validation study of 50 young autistic people reported good internal consistency on the BDI-II (Cronbach’s α = 0.90) (Gotham et al., 2015).
GRID-Hamilton Rating Scale for Depression (GRID-HAM-D-17) (Hamilton, 1960; Williams et al., 2008): This is a 17-item clinician administered interview which has been found to be reliable and valid in the general population but to our knowledge has not been investigated in the autistic population. GRID-HAM-D interviews were audio recorded with participant consent for the purposes of inter-rater reliability. Interviewers were trained in GRID-HAM-D administration using face-to-face training with demonstrations and role-plays. The first six HAM-D interviews by each interviewer were subject to a second independent rating (excluding items 8 and 9 which require face-to-face assessment, that is, observation of psychomotor retardation and agitation) to establish reliability of each assessor. To establish reliability across the study, a random sample (20%) of GRID-HAM-D recordings were independently rated.
Other measures
The feasibility of capturing a broad range of outcomes was considered due to the range and frequency of co-occurring conditions associated with autism. The measures are described in more detail in the published protocol (Russell et al., 2017) and include well-validated measures of anxiety (General Anxiety Disorder-7, Spitzer et al., 2006; Cronbach’s α = 0.92); obsessive compulsive symptoms (Obsessive Compulsive Inventory-Revised (OCI-R), Foa et al., 2002) – excellent internal consistency for use of the OCI-R has been reported in autistic adults (Cronbach’s α = 0.92); Cadman et al. (2015); positive and negative affect (Positive and Negative Affect Schedule (PANAS), Crawford & Henry, 2004) (positive scale Cronbach’s α = 0.89, negative scale Cronbach’s α = 0.85); the Work and Social Adjustment Scale (WSAS) (Mundt et al., 2002) (Cronbach’s α = 0.7–0.94); quality of life and health (EQ-5D-5L: Herdman et al., 2011 and 12-Item Short Form Health Survey (SF-12): Ware et al., 1996) were completed at each outcome point.
We also assessed the feasibility of data collection on statutory health and voluntary service use with a self-report questionnaire. We also accessed GP and secondary care records for information about health care consultations and prescriptions. These data would inform the collection of resource use information needed for an economic evaluation alongside a large-scale trial. Adverse events were captured using a standardised operating procedure and reported to the study Data Monitoring and Ethics Committee (DMEC).
Sample size
As this was a feasibility study not designed to test effectiveness, there was no formal sample size calculation. Therefore, we aimed to recruit 70 participants to inform decision-making about the practical issues of conducting a fully powered RCT.
Statistical analysis
Analysis and reporting of this trial was in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines. A pre-defined analysis plan was agreed with the DMEC. All analyses were conducted in Stata version 15.
The characteristics of those randomised were described using appropriate descriptive statistics. The primary interest was in estimating recruitment and retention rates for a large-scale trial. Therefore, we calculated the following: (1) the proportion of autistic adults consenting to the study; (2) the proportion completing the baseline assessment and entering the randomised phase; (3) for those in the intervention group, the number of GSH sessions attended and the proportion completing five or more sessions; (4) the proportion completing follow-up assessments at 10-, 16- and 24-weeks post-randomisation. The completeness of data for each outcome measure and time point was compiled.
To identify the most appropriate outcome measure for a fully powered RCT, we sought to evaluate two widely used self-report measures of depression against a clinician administered, interview measure of depression (HAM-D). We considered the interview measure (HAM-D) as the benchmark measure of depression. To ensure reliability of the HAM-D as the benchmark depression measure, we examined inter-rater agreement between continuous scores using Intra-Cluster Correlation (ICC) from a two-way mixed effect, repeated measures analysis of variance (ANOVA) model (observations are random, outcome measure instrument is fixed) for 20% of all HAM-D interview recordings. We then examined the sensitivity to change (defined as a binary outcome of at least 50% improvement in symptoms of the HAM-D at time point of measurement of the primary outcome compared to baseline) using Receiver Operating Characteristic (ROC) curve analyses separately for the two self-report depression measures.
We also compared the continuous scores on the depression outcome measures only (HAM-D, PHQ-9 and BDI-II) between groups. The data on service use were described using appropriate descriptive statistics including completeness of data and source of data (self-report or medical records where applicable).
Results
Feasibility of recruitment
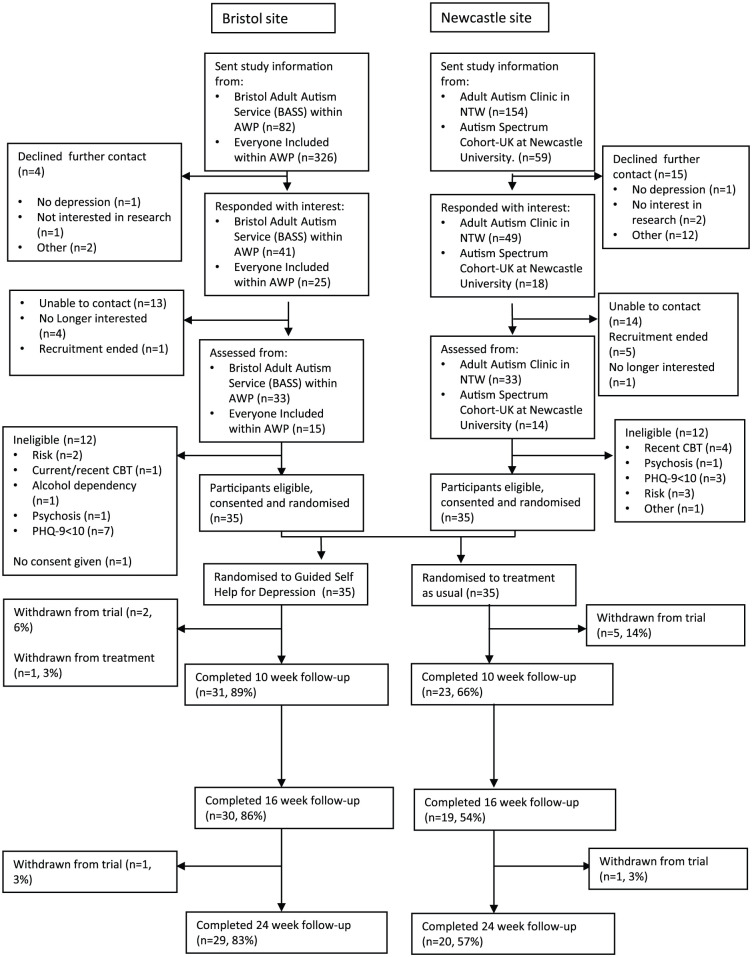
Participant recruitment began in October 2016; the final participant was randomised in September 2017, and the final follow-up measures completed in March 2018. Recruitment via the four recruitment pathways varied (Supplemental Material 1); in Bristol NHS adult autism service, 27% (n = 22/82) of patients sent study information were randomised, compared with 18% (n = 28/154) in the North-East NHS autism service. Differences in clinic procedures such as pre-clinic screening, post-diagnostic groups and use of a retrospective clinic list might account for this variation. In terms of the other recruitment pathways, 4% (n = 13/326) and 12% (n = 7/59) of people from the Bristol NHS research opportunity and UK autism cohort study, respectively, who were sent information about the study were randomised. Across all recruitment pathways, in total, 21% (n = 133/621) of autistic adults introduced to the study expressed interest in contact with the research team and of these 71% (95/133) completed the screening for potential eligibility for the study.
Twenty-five percent (n = 24/95) of adults who were interested in the study and assessed were not eligible (see Figure 1); 40% (n = 10/25) had a PHQ-9 score of less than 10, 20% (n = 5/25) were assessed to be at risk of suicide and 40% (n = 10/25) due to other reasons. One participant did not give consent (n = 1/95, 1%). Seventy participants were randomised: 35 to GSH and 35 to TAU.
Figure 1.
CONSORT diagram from recruitment onwards.
Baseline characteristics
Participants in the GSH and TAU groups were similar in terms of demographic characteristics and study measures at baseline (Table 1 numerical denominators vary across measures due to missing data). Levels of depression were relatively severe; the mean PHQ-score was 15.8 (SD: 4.1, n = 70), with 55% (n = 38/69) of participants meeting ICD-10 criteria for a primary diagnosis of a moderate or severe depressive episode. Sixty-seven percent (n = 35/52) had been depressed for 2 years or more, and 46% of the participants (n = 32/70) self-reported taking antidepressant medication. The majority of participants in both groups had prior experience of psychological therapy.
Table 1.
Participant characteristics, demographic information and symptom measures at baseline.
| TAU (n = 35) mean (SD), N completed, or n/N completed (%) | GSH (n = 35) mean (SD), N completed, or n/N completed (%) | |
|---|---|---|
| Gender: male | 27/35 (77%) | 24/34 (69%) |
| Age in years: mean, SD, n | 40.2 (12.6), 28 | 35.3 (13.6), 29 |
| Ethnicity: White | 33/35 (94%) | 33/34 (94%) |
| Accommodation type: owner occupied | 11/31 (35%) | 14/34 (41%) |
| Residential status: primary tenant/leaseholder | 17/30 (55%) | 11/33 (32%) |
| Education: GCSE and above | 28/31 (90%) | 33/34 (97%) |
| Employment status: paid or voluntary employment or training (full- or part-time) | 17/32 (53%) | 15/33 (45%) |
| Financial support/stress: moderate/significant financial stress | 17/31 (55%) | 11/33 (33%) |
| Relationship status: single | 23/31 (74%) | 19/34 (56%) |
| Currently taking other medication for mental health | 5/28 (18%) | 7/32 (22%) |
| Experience of psychological/talking therapy: at least one experience | 21/29 (75%) | 20/29 (70%) |
| Depression measures | ||
| PHQ-9 score | 16.5 (4.8), 35 | 15.0 (3.2), 35 |
| BDI-II score | 32.0 (11.5), 31 | 29.9 (8.8), 33 |
| HAM-D score | 17.6 (6.9), 29 | 17.4 (5.6), 34 |
| CIS-R score | 29.4 (11.0), 35 | 30.5 (8.6), 34 |
| Depression duration | ||
| Less than 2 weeks | 1/24 (4%) | 0/28 |
| Between 2 weeks and 6 months | 5/24 (21%) | 3/28 (11%) |
| Between 6 months and 1 year | 3/24 (13%) | 1/28 (4%) |
| Between 1 and 2 years | 0 | 4/28 (14%) |
| Two years or more | 15/24 (63%) | 20/28 (71%) |
| CIS-R categories (ICD-10) | ||
| Primary diagnosis | ||
| No diagnosis identified | 2/35 (6%) | 1/34 (3%) |
| Generalised anxiety disorder – mild | 0/35 (0%) | 1/34 (3%) |
| Mixed anxiety and depressive disorder | 1/35 (3%) | 1/34 (3%) |
| Specific (isolated) phobia | 2/35 (6%) | 0/34 (0%) |
| Agoraphobia | 1/35 (3%) | 0/34 (0%) |
| Generalised anxiety disorder | 4/35 (11%) | 4/34 (12%) |
| Panic disorder | 2/35 (6%) | 1/34 (3%) |
| Mild depressive episode | 5/35 (14%) | 6/34 (18%) |
| Moderate depressive episode | 13/35 (37%) | 16/34 (47%) |
| Severe depressive episode | 5/35 (14%) | 4/34 (12%) |
| Secondary diagnosis | ||
| No diagnosis identified | 3/35 (9%) | 1/34 (3%) |
| Mixed anxiety and depressive disorder (mild) | 2/35 (6%) | 2/34 (6%) |
| Mixed anxiety and depressive disorder | 12/35 (34%) | 7/34 (21%) |
| Specific (isolated) phobia | 1/35 (3%) | 2/34 (6%) |
| Social phobia | 2/35 (6%) | 5/34 (15%) |
| Agoraphobia | 1/35 (3%) | 1/34 (3%) |
| Generalised anxiety disorder | 12/35 (34%) | 12/34 (35%) |
| Panic disorder | 2/35 (6%) | 4/34 (12%) |
| Other outcome measures | ||
| GAD-7 score | 12.9 (4.4), 32 | 12.4 (4.5), 34 |
| OCI-R score | 31.2 (15.4), 29 | 29.0 (13.7), 34 |
| WSAS score | 25.8 (8.9), 32 | 24.8 (7.8), 33 |
| SF-12 normalised physical function score | 43.4 (11.3), 30 | 41.8 (9.0), 32 |
| SF-12 normalised mental health score | 29.6 (10.0), 30 | 30.5 (7.0), 32 |
| EQ-5D-5L score | 0.605 (0.210), 31 | 0.621 (0.214), 33 |
TAU: treatment as usual; SD: standard deviation; GSH: guided self-help; PHQ-9: Patient Health Questionnaire-9; BDI-II: Beck Depression Inventory-II; CIS-R: Clinical Interview Schedule-Revised; GAD-7: General Anxiety Disorder Questionnaire; OCI-R: Obsessive Compulsive Inventory-Revised; WSAS: Work and Social Adjustment Scale; HAM-D: Hamilton Rating Scale for Depression; GCSE: General Certificate of Secondary Education; SF-12: 12-Item Short Form Health Survey; ICD: International Classification of Diseases.
Feasibility of the GSH intervention
Eleven percent (n = 4/35) of participants randomised to GSH withdrew from treatment. Of these, two participants did not attend any treatment sessions and withdrew from the study, one participant attended two GSH sessions and withdrew from the study and one participant attended five GSH sessions but did not withdraw from the study, completing all outcome measures. Sixty-three percent (n = 20/32) of participants who started GSH did so within 2 weeks of randomisation. Ninety-one percent (n = 32/35) of participants attended at least one session of GSH, and 71% (n = 25/35) attended all nine sessions. One participant was unable to attend any sessions due to ill-health but did not withdraw from the treatment or the study. The mean number of treatment sessions was 7.6 (SD: 2.9, n = 35) with 86% (n = 30/35) completing the pre-specified ‘dose’ of treatment, that is, five sessions. By 10 weeks, 31% (10/32) had completed the intervention, by 16 weeks, 73% (23/32) had completed the intervention and by 24 weeks, 84% (27/32) had completed treatment. Information about reasons for spacing of appointments and rate of attendance was not systematically collected. However, participant and coach availability due to holidays or ill-health, adverse events such as homelessness, organisational difficulties and the impact of depression on behavioural motivation were factors which affected attendance and pacing of appointments such that 16 weeks presented a more practicable treatment window.
The intervention was delivered face-to-face for most participants. A minority accessed the intervention via Skype (n = 3/32, 9%) due to difficulties attending face-to-face treatment for health and childcare reasons.
TAU
Of those participants in TAU who attended follow-up and provided information across the duration of the study, 15 (63%) were prescribed antidepressant medication which is a small increase from the percentage (n = 18 (51%)) reporting this at baseline, 9 (38%) were offered primary care mental health support and 1 participant received support from secondary mental health care services.
Adverse events
Four adverse events were reported during the course of the study, one participant in the TAU group and three in the GSH group. Adverse events comprised involvement in a road traffic accident, medical investigations, a period of temporary homelessness and increased vulnerability due to deterioration in housing. None were related to the intervention or were considered serious adverse events.
Rate of retention
Figure 1 shows the flow of participants through the study. Seventeen percent (n = 6/35) of participants allocated to TAU and 9% (n = 3/35) of participants allocated to GSH withdrew from the trial. The majority (n = 5/6) of participants withdrawing from TAU did so soon after randomisation. The timing of withdrawals in the GSH group was shortly after randomisation (n = 2) and before the final follow-up (n = 1).
The rate of retention in the study differed by treatment group (see Table 2). Follow-up was higher in the GSH group at 10-, 16- and 24-weeks follow-up, with just 54% of the TAU group completing 16-weeks outcome measures compared to 86% in the GSH group.
Table 2.
Rates of retention by treatment group.
| Follow-up time point | Overall % (95% confidence interval, n) | Treatment as usual (95% confidence interval, n) | Guided self-help (95% confidence interval, n) |
|---|---|---|---|
| 10 weeks | 77% (66%–86%, n = 54) | 66% (48%–80%, n = 23) | 89% (73%–96%, n = 31) |
| 16 weeks | 70% (58%–80%, n = 49) | 54% (38%–70%, n = 19) | 86% (70%–94%, n = 30) |
| 24 weeks | 70% (58%–80%, n = 49) | 57% (40%–73%, n = 20) | 83% (66%–92%, n = 29) |
In respect of mode of follow-up, 75% (n = 46/61) participants attended a face-to-face meeting, 21% (n = 13/61) opted to complete self-report follow-up measures remotely using an electronic link (21.3%, n = 13/61) or by post (3.3%, n = 2/61). Those completing measures at baseline and follow-up generally completed the depression measures and the full set of secondary outcome measures.
Acceptability of depression measures and choice of primary outcome
Given that only 31% of the participants had completed the intervention by 10-weeks post-randomisation, it would be more appropriate to measure the primary outcome at a later time point for a large-scale trial. Therefore, the acceptability of the three depression outcome measures (PHQ-9, BDI-II and HAM-D) was assessed at 16 weeks when 72% of the GSH participants had completed treatment. At this time point, 78% (n = 38/49) of participants completed both the PHQ-9 and HAM-D, while 69% (n = 34/49) of participants completed the BDI-II.
Table 3 shows the mean scores on the depression and other outcome measures by group at 10-, 16- and 24-weeks post-randomisation.
Table 3.
Mean scores and standard deviation on outcome measures by treatment group.
| TAU |
GSH |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 weeks |
16 weeks |
24 weeks |
10 weeks |
16 weeks |
24 weeks |
|||||||
| Mean (SD) n |
Min–Max | Mean (SD) n |
Min–Max | Mean (SD) n |
Min–Max | Mean (SD) n |
Min–Max | Mean (SD) n |
Min–Max | Mean (SD) n |
Min–Max | |
| Patient Health Questionnaire (PHQ-9) | 14.1 (6.5) n = 23 |
1–23 | 12.9 (6.6) n = 17 |
3–23 | 15.2 (6.4) n = 20 |
5–27 | 11.0 (5.3) n = 31 |
3–25 | 9.4 (5.9) n = 28 |
0–23 | 10.1 (6.1) n = 28 |
0–23 |
| Beck Depression Inventory-II (BDI-II) | 28.2 (11.8) n = 23 |
5–47 | 27.6 (13.6) n = 16 |
4–44 | 29.6 (13.7) n = 20 |
4–53 | 23.2 (11.4) n = 31 |
5–49 | 18.7 (10.3) n = 28 |
0–43 | 20.3 (11.8) n = 28 |
0–40 |
| Hamilton Rating Scale for Depression (HAM-D) | 13.2 (6.0) n = 17 |
3–24 | 14.5 (7.3) n = 18 |
4–27 | 14.5 (8.0) n = 18 |
3–37 | 13.6 (5.7) n = 27 |
1–26 | 10.5 (6.2) n = 29 |
0–24 | 12.3 (7.1) n = 26 |
1–27 |
| Obsessive Compulsive Inventory-Revised (OCI-R) | 27.3 (18.3) n = 20 |
3–66 | 23.3 (16.4) n = 17 |
2–57 | 25.7 (16.5) n = 17 |
2–57 | 23.0 (13.9) n = 29 |
4–49 | 20.0 (12.0) n = 28 |
3–54 | 22.1 (13.7) n = 27 |
4–55 |
| Positive and Negative Affect Schedule (PANAS): positive score | 19.6 (6.7) n = 23 |
11–38 | 20.5 (7.9) n = 17 |
10–41 | 17.9 (5.4) n = 19 |
10–30 | 22.5 (8.4) n = 31 |
10–40 | 25.0 (9.5) n = 27 |
10–44 | 22.9 (9.1) n = 27 |
10–40 |
| Positive and Negative Affect Schedule (PANAS): negative score | 28.0 (7.2) n = 23 |
14–37 | 26.5 (8.6) n = 17 |
11–41 | 26.6 (8.8) n = 18 |
11–39 | 24.6 (7.7) n = 31 |
11–41 | 21.9 (7.2) n = 27 |
10–38 | 24.3 (8.0) n = 28 |
13–43 |
| General Anxiety Disorder Questionnaire (GAD-7) | 11.8 (5.4) n = 23 |
1–20 | 10.6 (5.7) n = 17 |
1–20 | 12.3 (5.5) n = 20 |
0–20 | 8.8 (5.3) n = 31 |
1–19 | 8.3 (4.5) n = 28 |
1–19 | 8.5 (5.0) n = 28 |
1–19 |
| Work and Social Adjustment Scale (WSAS) | 22.5 (9.7) n = 22 |
6–37 | 23.5 (9.9) n = 17 |
5–34 | 24.6 (7.8), (31.0, 20) | 5–36 | 24.1 (9.2) n = 29 |
5–39 | 18.0 (9.8) n = 27 |
0–34 | 19.2 (6.9) n = 28 |
8–31 |
| SF-12 normalised physical function score | 45.2 (11.0) n = 22 |
11–16.7 | 42.8 (11.8) n = 16 |
23.8–63.3 | 37.8 (14.3), (48.6, 17) | 15.4–64 | 42.4 (9.4) n = 28 |
19.7–56.2 | 41.5 (9.0) n = 27 |
16–57.9 | 44.3 (9.8) n = 26 |
18–62.8 |
| SF-12 normalised mental health score | 29.7 (12.4) n = 22 |
9.7–53.3 | 30.5 (11.7) n = 16 |
14.4–56.5 | 28.9 (10.4), (31.8, 17) | 14–45.8 | 31.6 (9.2) n = 28 |
19–49.3 | 35.6 (10.0) n = 27 |
24.5–56.8 | 33.2 (10.5) n = 26 |
16.4–58.5 |
| EQ-5D-5L score | 0.590 (0.215) n = 22 |
0.193–0.879 | 0.660 (0.189) n = 16 |
0.174–0.879 | 0.535 (0.223), (0.801, 20) | 0.036–0.837 | 0.606 (0.202) n = 29 |
0.087–0.879 | 0.691 (0.236) n = 28 |
0.107–1.00 | 0.713 (0.185) n = 27 |
0.200–0.906 |
TAU: treatment as usual; GSH: guided self-help; SD: standard deviation; ASD: autism spectrum disorder; SF-12: 12-Item Short Form Health Survey.
Range reported as maximum–minimum. Clinical range – PHQ-9: ⩾10; BDI-II: ⩾14; HAM-D: ⩾7; OCI-R: ⩾29 suggested as optimal cut-off for ASD (Cadman et al., 2015); GAD-7: ⩾10; WSAS: ⩾20 indicative of moderate–severe functional impairment.
The sensitivity to change of the self-report measures of depression (PHQ-9 and BDI-II) with the observer-rated HAM-D was assessed. At the beginning of the study, 14 HAM-D assessments administered by two raters were double-rated independently with inter-rater agreement on total HAM-D scores very high (>90%), with some variability in inter-rater agreement at the individual item level (e.g. 37.5% on depressed mood and 87.5% for insomnia). Additional raters were trained in HAM-D administration across the duration of the study, and inter-rater agreement on the 28% of the HAM-D administrations assessed at the end of the study indicated poor reliability. Only one pair of assessors had a Cohen’s kappa of more than 0.8 (range 0.1–0.8), which had been pre-specified as acceptable. Given these data, the planned sensitivity to change analyses of the two self-reported depression measures was considered inappropriate.
Feasibility of data collection on resource use
Rates of completion of the questionnaire were at 80% for the majority of items (see Supplemental Materials 2 and 3). During the study, participants used antidepressant medication (43%–66%), attended an NHS outpatient or community mental health team clinic for mental health problems (11%–49%) and/or visited their GP (26%–46%). Twenty-nine percent of participants also used other medication for mental health conditions. Participants did not report any overnight stays in an NHS hospital, or visits to a private hospital or clinic due to mental health problems, and rarely attended an A&E department, an out-of-hours clinic or NHS walk-in centre. Twenty-three percent of participants had counselling or talking therapy during the trial outside of the trial intervention. Participants used a variety of different types of help for mental health problems including support groups and received help around or outside of the home. Twenty-seven percent of participants took time off work due to mental health problems. Many participants attended a social group (24%) or drop-in service (26%) for autistic adults. Participants rarely had a home visit from any other professional (for any medical condition). Thirty percent of participants paid NHS prescription charges, and 43% received disability payments. Participants did not come into contact with the criminal justice system (either as a victim or as a potential suspect).
It was possible to compare data from self-reported questionnaires and electronic medical records (EMRs) only for those participants from the Bristol region due to issues with collecting EMR data from the North-East site. There was a lack of concordance between the self-reported and the EMR data for those variables where both sources of information were available (Supplemental Material 3).
Discussion
It was feasible to develop and implement a low-intensity intervention for depression for autistic adults and recruit the planned number of participants into a RCT. Rates of retention differed across the study according to treatment group. Noticeably, fewer people remained in the TAU group of the study with the majority withdrawing directly after randomisation. It was difficult to engage these participants in the qualitative interview study to ascertain the reasons why. One participant did agree to be interviewed and stated the outcome of randomisation was the reason for withdrawal. More than 70% of the participants had prior experience of talking therapy which may account for the potential lack of clinical equipoise. Participants in the qualitative study talked about wanting to try something ‘new’ (Russell et al., in press). The findings of the qualitative study indicated that participants understood the information provided about the trial and the randomisation procedure.
Depression in this sample of autistic adults was relatively severe and persistent. The majority of participants had depression for 2 years or more. The relative severity of the depression in this sample raises questions about the appropriateness of a low-intensity intervention. A number of individuals were assessed as not eligible to take part in the study because of the severity of illness and were referred for higher intensity treatment. Taken together, these findings highlight the range of clinical need in this group.
There was good engagement with the adapted low-intensity intervention and the majority of participants attended all sessions. Rates of withdrawal from the treatment group are similar to those reported by other trials of psychological interventions with autistic adults (e.g. Langdon et al., 2016). Sixteen weeks presented a realistic window for treatment delivery. The therapist GSH intervention, with nine individual sessions of up to 45 min could be construed as at the upper limit of ‘low intensity’. In addition to session duration, coaches made practical adjustments to accommodate the needs of individual participants such as appointment reminders and flexibility around timings and frequency of sessions. The findings of the qualitative interview study (Horwood, Cooper, Harvey, Evans, & Russell, 2018; Russell et al., in press) indicated that the intervention was well-received by participants.
The set of measures used in the study captured a broad range of outcomes with the aim of characterising the sample and the feasibility of investigating the potential for co-occurring conditions to influence treatment outcome in a larger study. In the GSH group, the majority of participants completed the full set of measures, but the high rate of attrition in the TAU group impacts on our ability to learn from these. To our knowledge, this is the first adult study of a psychological intervention using a randomised trial design with TAU as a comparator. Measures to improve participant engagement with future trials of similar design may benefit from co-production of study information and newsletters as well as including incentives for follow-up.
In respect of findings related to measurement in this study, two main issues were identified. First, it was not possible to conduct the planned analyses to identify which of the two self-report measures of depression was most sensitive to change. Poor inter-rater reliability of the ‘benchmark’ interview measure was at the root of this. The expert raters commented that valid administration of the HAM-D in autistic adults was difficult if interviewers were relatively new to the client group. Open questions did not always work well, in particular questions about emotional states. Less-experienced interviewers lacked the confidence to be more directive at interview, for example, follow-up with direct/closed or forced-choice prompts to elicit the information needed to rate items. The two self-report measures of depression were well-aligned. There was some anecdotal evidence from researchers that participants preferred the format of the BDI-II with item sets of descriptive statements from which to choose. Researchers described how some participants struggled to complete the PHQ-9 with the need to rate their experiences using temporal categories requiring a degree of judgement, for example, distinguishing between ‘not at all’ and ‘several’ days. Two studies have reported the BDI-II to be an adequate screening tool for depression in autism (Cederlund et al., 2010; Gotham et al., 2015). A systematic review of the use of depression measures in autistic adults (Cassidy et al., 2018) found that one study investigating psychometric properties of depression measures met eligibility criteria for the review. This study (Gotham et al., 2015) reported the BDI-II to have good internal consistency, adequate sensitivity and specificity and good convergent validity when administered to autistic people. The psychometric properties of the PHQ-9 for use in autistic adults have not been investigated. There were no studies reporting the sensitivity to change of any measures. A number of clinical trials with autistic children and adults have reported treatment effect sizes to vary according to rater. The results of a meta-analysis report how informant and clinician ratings show medium–large effect sizes when compared with small effects of treatment as reported by patients themselves (Weston & Hodgekins, 2016). Issues such as alexithymia (e.g. Berthoz & Hill, 2005) and the co-existence of multiple problems may mean discriminating change at the individual symptom level is difficult. Nonetheless, symptom change is reported across a number of measures including the depression measures in the present study, suggesting that the use of self-report in adults who are able to do so may be viable as part of a larger treatment study. On the basis of the research evidence and the findings of this study, we conclude that the BDI-II presents as the most suitable outcome measure for a future randomised controlled trial.
Second, there was a discrepancy between self-report and EMR reports about a number of variables including antidepressant prescriptions and primary and secondary healthcare attendance. Discrepancies were not in a single direction. It is likely that practice records are the most accurate records of primary care attendance and antidepressant medication. However, the interface between primary and secondary care in respect of the latter may make it more difficult to accurately capture prescriptions.
This study was not powered to detect differences between the treatment groups. However, review of scores on the main measures indicates that the intervention merits further investigation. In conclusion, it was feasible to develop a low-intensity cognitive-behavioural intervention adapted for the needs of autistic people which was time-limited, structured, protocol-driven and used the principles and elements of BA including functional analysis, attending to behaviours that increase positive affect, activity scheduling and consolidation of learning to sustain treatment gains. Differences to standard treatment comprised greater therapist flexibility about location, timing and spacing of appointments, a longer introductory session to build working alliance, increased awareness of and literacy about positive mood via visual material, use of spatial cognition to link behavioural antecedents and consequences and linking behaviours and positive affect to personal needs to encourage self-regulation and self-care. These adaptations to standard treatment are in line with those recommended by UK clinical guidance (NICE CG142) for adapting psychosocial treatments for autism and also align well with the outline of candidate mechanisms of action for therapeutic intervention proposed in the Research Domain Criteria (RDoC) matrix (e.g. White et al., 2018). The intervention was well-received, and it was feasible to run a clinical trial with a randomised design. We now need evidence from a fully powered, large-scale trial to inform guidance as to whether this low-intensity psychological intervention is an effective treatment for autistic adults with co-occurring depression.
Supplemental Material
Supplemental material, AUT889272_Supplemental_material_1 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism
Supplemental material, AUT889272_Supplemental_material_2 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism
Supplemental material, AUT889272_Supplemental_material_3 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism
Acknowledgments
We would like to acknowledge the support of the West of England and Northeast and North Cumbria Clinical Research Networks. We would also like to acknowledge the work of the Trial Steering Committee and Data Monitoring and Ethics Committee chaired by Dr Biza Stenfert-Kroese and Professor Alan Montgomery, respectively.
Results of a survey of the UK autism community highlighted that ‘Autism’, ‘on the Autism Spectrum’ and ‘Autistic people’ are preferred terms to describe autism. Here on in, the terms autism and autistic people will be used (Kenny et al., 2016).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme (HTA 14/43/02). This study was also supported by the NIHR Bristol Biomedical Research Centre (BRC-1215-2011). This study was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration (BRTC), a UKCRC Registered Clinical Trials Unit (CTU) which, as part of the Bristol Trials Centre, is in receipt of NIHR CTU support funding. The views expressed in this article are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health and Social Care.
Trial registration: Trial Registration Information: assigned 28/09/2016 http://www.isrctn.com/ISRCTN54650760
ORCID iDs: Ailsa Russell  https://orcid.org/0000-0002-8443-9381
https://orcid.org/0000-0002-8443-9381
Kate Cooper  https://orcid.org/0000-0002-8216-5567
https://orcid.org/0000-0002-8216-5567
Barry Ingham  https://orcid.org/0000-0002-7268-2288
https://orcid.org/0000-0002-7268-2288
Supplemental material: Supplemental material for this article is available online.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Berthoz S., Hill E. L. (2005). The validity of using self-reports to assess emotion regulation abilities in adults with autism spectrum disorder. European Psychiatry, 20(3), 291–298. [DOI] [PubMed] [Google Scholar]
- Bower P., Gilbody S. (2005). Stepped care in psychological therapies: Access, effectiveness and efficiency: Narrative literature review. The British Journal of Psychiatry, 186(1), 11–17. [DOI] [PubMed] [Google Scholar]
- Brugha T. S., McManus S., Bankart J., Scott F., Purdon S., Smith J., … Meltzer H. (2011). Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry, 68(5), 459–465. [DOI] [PubMed] [Google Scholar]
- Cadman T., Spain D., Johnston P., Russell A., Mataix-Cols D., Craig M., … Wilson C. E. (2015). Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: What does self-report with the OCI-R tell us? Autism Research, 8(5), 477–485. [DOI] [PubMed] [Google Scholar]
- Cassidy S. A., Bradley L., Bowen E., Wigham S., Rodgers J. (2018). Measurement properties of tools used to assess depression in adults with and without autism spectrum conditions: A systematic review. Autism Research, 11(5), 738–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederlund M., Hagberg B., Gillberg C. (2010). Asperger syndrome in adolescent and young adult males. Interview, self-and parent assessment of social, emotional, and cognitive problems. Research in Developmental Disabilities, 31(2), 287–298. [DOI] [PubMed] [Google Scholar]
- Cooper K., Loades M. E., Russell A. (2018). Adapting psychological therapies for autism. Research in Autism Spectrum Disorders, 45, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. R., Henry J. D. (2004). The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 43(3), 245–265. [DOI] [PubMed] [Google Scholar]
- Demetriou E. A., Lampit A., Quintana D. S., Naismith S. L., Song Y. J. C., Pye J. E., … Guastella A. J. (2018). Autism spectrum disorders: A meta-analysis of executive function. Molecular Psychiatry, 23(5), 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E. B., Huppert J. D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P. M. (2002). The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychological Assessment, 14(4), 485–496. [PubMed] [Google Scholar]
- Gotham K., Bishop S. L., Brunwasser S., Lord C. (2014). Rumination and perceived impairment associated with depressive symptoms in a verbal adolescent-adult ASD sample. Autism Research, 7(3), 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K., Unruh K., Lord C. (2015). Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism, 19(4), 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A Rating Scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman M., Gudex C., Lloyd A., Janssen M. F., Kind P., Parkin D., … Badia X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20(10), 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks M. J., Lerh J. W., Magiati I., Meiser-Stedman R., Brugha T. S. (2018). Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychological Medicine, 49, 559–572. [DOI] [PubMed] [Google Scholar]
- Horwood J., Cooper K., Harvey H., Evans L., Russell A. (2018). Qualitative evaluation of a low-intensity psychological intervention for depression in adults with autism: The Autism Depression Trial (ADEPT) [Conference session]. International Society for Autism Research, Rotterdam. https://insar.confex.com/insar/2018/webprogram/Paper27708.html. [Google Scholar]
- Joshi G., Wozniak J., Petty C., Martelon M. K., Fried R., Bolfek A., … Caruso J. (2013). Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: A comparative study. Journal of Autism and Developmental Disorders, 43(6), 1314–1325. [DOI] [PubMed] [Google Scholar]
- Kenny L., Hattersley C., Molins B., Buckley C., Povey C., Pellicano E. (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. [DOI] [PubMed] [Google Scholar]
- Kim J. A., Szatmari P., Bryson S. E., Streiner D. L., Wilson F. J. (2000). The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism, 4(2), 117–132. [Google Scholar]
- Kroenke K., Spitzer R. L. (2002). The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals, 32(9), 509–515. [Google Scholar]
- Langdon P. E., Murphy G. H., Shepstone L., Wilson E. C., Fowler D., Heavens D., … Mullineaux L. (2016). The People with Asperger syndrome and anxiety disorders (PAsSA) trial: A pilot multicentre, single-blind randomised trial of group cognitive–behavioural therapy. BJPsych Open, 2(2), 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G., Pelosi A. J., Araya R., Dunn G. (1992). Measuring psychiatric disorder in the community: A standardized assessment for use by lay interviewers. Psychological Medicine, 22(2), 465–486. [DOI] [PubMed] [Google Scholar]
- Martell C. R., Addis M. E., Jacobsen N. S. (2001). Depression in context: Strategies for guided action. W. W. Norton. [Google Scholar]
- McGillivray J. A., Evert H. T. (2014). Group cognitive behavioural therapy program shows potential in reducing symptoms of depression and stress among young people with ASD. Journal of Autism and Developmental Disorders, 44(8), 2041–2051. [DOI] [PubMed] [Google Scholar]
- Mundt J. C., Marks I. M., Shear M. K., Greist J. M. (2002). The Work and Social Adjustment Scale: A simple measure of impairment in functioning. The British Journal of Psychiatry, 180(5), 461–464. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2009). Depression in adults: Recognition and management [Clinical Guidelines 90 (CG90)]. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2012). Autism spectrum disorder in adults: Recognition and management [Clinical Guidelines 142 (CG142)]. [Google Scholar]
- Rai D., Heuvelman H., Dalman C., Culpin I., Lundberg M., Carpenter P., Magnusson C. (2018). Association between autism spectrum disorders with or without intellectual disability and depression in young adulthood. JAMA Network Open, 1(4), e181465–e181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A., Cooper K., Barton S., Ensum I., Gaunt D., Horwood J., … Rai D. (2017). Protocol for a feasibility study and randomised pilot trial of a low-intensity psychological intervention for depression in adults with autism: The Autism Depression Trial (ADEPT). BMJ Open, 7(12), Article e019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A., Gaunt D., Cooper K., Horwood J., Barton S., Ensum I., … Wiles N. (in press). Guided self-help for depression in autistic adults: The ADEPT feasibility RCT. Health Technology Assessment 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomauro D., Sheffield J., Sofronoff K. (2016). Depression in adolescents with ASD: A pilot RCT of a group intervention. Journal of Autism and Developmental Disorders, 46(2), 572–588. [DOI] [PubMed] [Google Scholar]
- Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Sizoo B. B., Kuiper E. (2017). Cognitive behavioural therapy and mindfulness based stress reduction may be equally effective in reducing anxiety and depression in adults with autism spectrum disorders. Research in Developmental Disabilities, 64, 47–55. [DOI] [PubMed] [Google Scholar]
- Spek A. A., Van Ham N. C., Nyklíček I. (2013). Mindfulness-based therapy in adults with an autism spectrum disorder: A randomized controlled trial. Research in Developmental Disabilities, 34(1), 246–253. [DOI] [PubMed] [Google Scholar]
- Spitzer R. L., Kroenke K., Williams J. B., Löwe B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Ware J. E., Jr., Kosinski M., Keller S. D. (1996). A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. [DOI] [PubMed] [Google Scholar]
- Weston L., Hodgekins J., Langdon P. E. (2016). Effectiveness of cognitive behavioural therapy with people who have autistic spectrum disorders: A systematic review and meta-analysis. Clinical Psychology Review, 49, 41–54. [DOI] [PubMed] [Google Scholar]
- White S. W., Simmons G. L., Gotham K. O., Conner C. M., Smith I. C., Beck K. B., Mazefsky C. A. (2018). Psychosocial treatments targeting anxiety and depression in adolescents and adults on the autism spectrum: Review of the latest research and recommended future directions. Current Psychiatry Reports, 20(10), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford H. A., Degenhardt L., Rehm J., Baxter A. J., Ferrari A. J., Erskine H. E., … Burstein R. (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet, 382(9904), 1575–1586. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Kobak K. A., Bech P., Engelhardt N., Evans K., Lipsitz J., … Kalali A. (2008). The GRID-HAMD: Standardization of the Hamilton Depression Rating Scale. International Clinical Psychopharmacology, 23(3), 120–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, AUT889272_Supplemental_material_1 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism
Supplemental material, AUT889272_Supplemental_material_2 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism
Supplemental material, AUT889272_Supplemental_material_3 for The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: The Autism Depression Trial study – a pilot randomised controlled trial by Ailsa Russell, Daisy M Gaunt, Kate Cooper, Stephen Barton, Jeremy Horwood, David Kessler, Chris Metcalfe, Ian Ensum, Barry Ingham, Jeremy R Parr, Dheeraj Rai and Nicola Wiles in Autism