Abstract
Background
Klebsiella pneumoniae (K. pneumoniae) causes community-acquired and hospital-acquired pneumonia. The mortality rates of invasive infections caused by hypervirulent K. pneumoniae (HvKP) are extremely high. However, the microbiological characteristics and clinical manifestations of K. pneumoniae in AnHui province still remain unclear.
Purpose
To show the high prevalence of HvKP infections regarding clinical characteristics and antimicrobial resistance in Anhui province.
Patients and Methods
A retrospective analysis was conducted to study the clinical data of 115 strains of K. pneumoniae from July 2019 to March 2020 in The First Affiliated Hospital of AnHui Medical University. The virulence genes, capsular types, carbapenemase genes, and molecular subtypes of these hypervirulent isolates were detected.
Results
Overall, 59.1% (68/115) cases were HvKP infections, mainly from the department of intensive care unit (ICU, n=14, 20.6%) and the department of respiratory and critical care (n=13, 19.1%). K2 was the most prevalent capsular serotype (n=26), followed by K1 (n=21). The results of MLST identification of 68 strains showed that ST23 (n=15, 22.1%) was the most common type of ST, followed by ST11 and ST65 (n=12, 17.6%), ST86 (n=9, 13.2%), and ST412 (n=6, 8.8%). Among 68 hvKP strains, 12 isolates were carbapenem resistant, and all except two harboured KPC.
Conclusion
The high incidence of carbapenemase producing HvKP in the Anhui province, especially the higher mortality of HvKP, should be paid more attention. Meanwhile, epidemiological surveillance and clinical treatment strategies should be continuously determined and implemented.
Keywords: Klebsiella pneumoniae, virulence genes, capsular types, antimicrobial resistance
Introduction
Klebsiella pneumoniae was first isolated in 1893 by Friedlander, from the lung tissues of patients with lobar pneumonia. It is a Gram-negative bacterium with a thick capsule, and easily causes community and nosocomial infections. After the first report of hypervirulent K. pneumoniae (HvKP) by Chinese researchers in Taiwan in 1986, reports on HvKP have continued to emerge. In most cases, high mucilage often indicates that the bacteria have strong virulence. Unlike common K. pneumoniae, HvKP can arouse severe metastatic infections such as liver abscesses, endophthalmitis, and bacteraemia in young and healthy populations. Previous studies have demonstrated that K. pneumoniae rates were 5% among healthy Korean adults.1,2 Data from studies in areas where HvKP is endemic indicates that its prevalence can reach 12–45%.3–5 In China, the prevalence rates of HvKP infections are about 8.33–73.9%, and the mortality rate is even as high as 60%.6–8 Colonization of the gastrointestinal system further promotes community transmission.9 Analyses of the Human Microbiome Project in the United States shows about 4% colonization rate for K. pneumoniae in faecal samples.10 However, the rate of K. pneumoniae colonization in hospitalized patients was 19–38%.11,12 At present, consistent with the string test positive for high viscosity is high appraisal main virulence K. pneumonia bacteria method, but it is still controversial that mucous is necessarily a high virulence. Therefore, it is inaccurate to define whether the strain is a highly virulent one only by positive “string test”.13,14 The virulence plasmid pLVPK, which carries the virulence genes rmpA and rmpA2, plays an important role in the virulence of HvKP. Therefore, when rmpA, rmpA2, and string test were all positive, the strain is defined as HvKP.15 Our study also defined HvKP according to such criteria. In general, strains that are both highly virulent and resistant are rare. However, owing to the abuse of antibacterial drugs, a growing number of studies have reported the presence of multidrug-resistant (MDR) the carbapenemase producing HvKP, which is understandably detrimental.7,16–19 In this study, we collected 115 K. pneumoniae strains that were “string test” positive in the First Affiliated Hospital of Anhui Medical University from July 2019 to March 2020, and screened 68 strains of HvKP to further explore the molecular biological characteristics of HvKP and antimicrobial resistance to lay a foundation for subsequent scientific research and clinical treatment.
Materials and Methods
Clinical K. pneumoniae Isolates and Patients’ Data Collection
We collected 115 consecutive patients at The First Affiliated Hospital of Anhui Medical University who were infected K. pneumoniae and showed positive string test. All specimens were numbered from 1 to 115 and stored at −80°C. Some clinical data and microbiological data related to the specimens could be obtained from the electronic or paper medical records of the hospital and the microbiological database. The inpatient information included demographic characteristics, clinical manifestations, microbiological reports, post-admission treatment, outcomes, and prognosis. Each patient in this study was admitted to the hospital after signing an informed consent.
Determination of Hypervirulent Phenotype
Phenotypic identification of K. pneumoniae relies on the classical “string test”.8 The strain taken from the refrigerator of −80°C was inoculated on an agar plate and cultured overnight in the incubator at 37°C until colony formation. Using a bacterial inoculation ring for stretching, a positive string test that formed mucoviscous string >5 mm was considered as the hypervirulent phenotype of K. pneumoniae.
Capsular Serotyping and Determination of Virulence Genes rmpA, rmpA2
All strains were grown overnight on agar plates, the genomic DNA was extracted. Then, polymerase chain reaction (PCR) was used to amplify virulence genes rmpA and rmpA2 and capsular serotype genes as previously described.20–22 The primer sequences used are shown in I note you uploaded the file 07_Jul_2020_63.xlsx. Please advise what this is, is this to be published with your manuscript or was this requested by the reviewer? 1. The reaction mixture was prepared as follows: initial denaturation at 95°C for 3 min; followed by 35 cycles of 95°C for 30 s, 52°C for 30 s, and 72°C for 3 min and a final extension at 72°C for 10 min. Agarose gel electrophoresis and sequencing were used to analyse the PCR products. The capsular serotypes of 12 strains carbapenemase producing HvKP were detected by wzi gene sequencing. The results were submitted to http://bigsdb.pasteur.fr.
Susceptibility Testing and KPC Gene Identification
The agar dilution method was used to identify and test antimicrobial susceptibility. The antimicrobial agents included ceftazidime, ceftriaxone, cefepime, cefotaxime, piperacillin-tazobactam, cefoperazone-sulbactam, imipenem, meropenem, amikacin, gentamicin, ciprofloxacin, levofloxacin, and aztreonam. Broth microdilution method was used for drug sensitivity tests of tigecycline and colistin (since there was no MIC breakpoints of these two drugs on CLSI, we referred to it of EScherichia coli on EUCAST). K. pneumoniae 700,603 was selected as the positive control group for antimicrobial susceptibility testing, and Escherichia coli 25,922 was used as the negative control. The Clinical and Laboratory Standards Institute (CLSI) guidelines and CLSI breakpoints or European Committee on Antimicrobial Susceptibility Testing criteria (version 10.0, http://www.eucast.org/clinicalbreakpoints/), respectively (CLSI 2021), can be referred to for specific experimental methods and result analysis. Moreover, Table 1 showed the identification of KPC gene, including KPC-2, NDM-1, VIM-1, IMP-1 and OXA-48.
Table 1.
Primer Sequence About Virulence and Resistance Associated Genes
| Prime Name | Sequence | |
|---|---|---|
| rmpA | Forward | ACTGGGCTACCTCTGCTTCA |
| Reverse | CGCACCAGTAATTCCAACAG | |
| rmpA2 | Forward | CTTTATGTGCAATAAGGATGTT |
| Reverse | CCTCCTGGAGAGTAAGCATT | |
| K1 | Forward | GGTGCTCTTTACATCATTGC |
| Reverse | GCAATGGCCATTTGCGTTAG | |
| K2 | Forward | GGAGCCATTTGAATTCGGTG |
| Reverse | TCCCTAGCACTGGCTTAAGT | |
| K5 | Forward | GCCACCTCTAAGCATATAGC |
| Reverse | CGCACCAGTAATTCCAACAG | |
| K20 | Forward | CCGATTCGGTCAACTAGCTT |
| Reverse | GCACCTCTATGAACTTTCAG | |
| K54 | Forward | CATTAGCTCAGTGGTTGGCT |
| Reverse | GCTTGACAAACACCATAGCAG | |
| KPC-2 | Forward | ATGTCACTGTATCGCCGTCT |
| Reverse | TTTTCAGAGCCTTACTGCCC | |
| NDM-1 | Forward | GGTTTGGCGATCTGGTTTTC |
| Reverse | CGGAATGGCTCATCACGATC | |
| VIM-1 | Forward | AAATTCCGGTCGGAGAGGTC |
| Reverse | AATGCGCAGCACCAGGATAG | |
| IMP-1 | Forward | GGAATAGAGTGGCTTAATTCTCC |
| Reverse | GGTTTAATAAAACAACCACC | |
| OXA-48 | Forward | GCGTGGTTAAGGATGAACAC |
| Reverse | CATCAAGTTCAACCCAACCG | |
Multilocus Sequence Typing (MLST)
MLST typing was carried out for all HvKP strains screened according to the MLST website (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html) with seven housekeeping genes (gapA, infB, mdh, phoE, pgi, rpoB, and tonB). We have submitted new alleles and STs that have not been previously reported.
Pulsed-Field Gel Electrophoresis (PFGE)
All isolated strains were subjected to PFGE. XBa I (TaKaRa, Lot# AIF2232A) was used as the restriction endonuclease, and Salmonella H9812 as the marker for DNA size of PFGE electrophoresis. All strains were prepared by gelatinization, enzymatic digestion, and electrophoresis. Then, the PFGE images were processed by BioNumerics software and the tree diagram were drawn. The similarity coefficient of the strains in the similarity analysis matrix >80% were of the same PFGE type.23
Statistical Analysis
Data analysis was performed using SPSS software (version 23.0; IBM Corporation, Armonk, NY, USA). If the continuous variables followed a normal distribution, they were indicated using mean±standard deviation (SD). Chi-square or Fisher exact tests were used to analyse categorical variables. When P<0.05, difference between the two groups was considered statistically significant.
Results
HvKP Isolates Clinical Characteristics
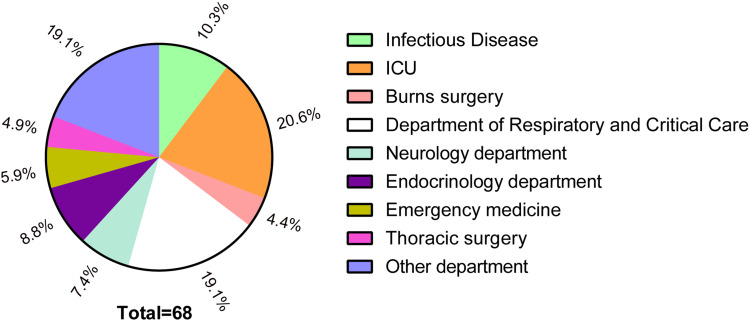
Among 115 strains, 59.1% (68/115) isolates were HvKP, and 40.9% (47/115) were non-HvKP. Figure 1 shows the department source distribution of 68 HvKP isolates: 20.6% (n=14) were from the ICU and 19.1% (n=13) were from the Department of Respiratory and Critical Care. Table 2 summarizes the clinical characteristics of HvKP patients and non-HvKP patients. In the HvKP group, there were 43 (63.2%) male and 25 (36.8%) female patients. The mean age was 57.01±19.00 years. Among all 68 community-acquired HvKP isolates, 41 patients (60.33%) had pulmonary disease; 33 patients (48.5%) were attached to invasive equipment; and 21 (30.9%), 18 (26.5%), and 8 (11.8%) patients, respectively, had hypertension, diabetes, and liver abscess.
Figure 1.
Distribution percentage of 68 strains of HvKP.
Table 2.
Clinical and Microbiological Characteristics of HvKP Isolates
| Characteristics | NO.(%) of Isolates | P value | |
|---|---|---|---|
| HvKP (n=68) | Non-HvKP (n=47) | ||
| Demographic characteristics | |||
| Male sex | 43 (63.2) | 35 (74.5) | 0.205 |
| Age (years) (mean ± SD) | 57.01 ± 19.00 | 57.26 ± 16.20 | 0.944 |
| K serotypes | |||
| K1 | 21/68 (30.9) | 2/47 (4.3) | 0.000 |
| K2 | 26/68 (38.2) | 9/47 (19.1) | 0.029 |
| K5 | 12/68 (17.6) | 4/47 (8.5) | 0.164 |
| K20 | 0/68 (0.0) | 2/47 (4.3) | 0.086 |
| K57 | 8/68 (11.8) | 1/47 (2.1) | 0.059 |
| Antimicrobial susceptibility | |||
| Ceftazidime | 13/68 (19.1) | 12/47 (25.5) | 0.093 |
| Ceftriaxone | 14/68 (20.6) | 15/47 (32.0) | 0.028 |
| Cefepime | 13/68 (19.1) | 11/47 (23.4) | 0.030 |
| Cefotaxime | 14/68 (20.6) | 15/47 (32.0) | 0.028 |
| Piperacillin-tazobactam | 12/68 (17.6) | 9/47 (19.1) | 0.273 |
| Cefoperazone-sulbactam | 13/68 (19.1) | 10/47 (21.3) | 0.254 |
| Imipenem | 12/68 (17.6) | 10/47 (21.3) | 0.168 |
| Meropenem | 12/68 (17.6) | 10/47 (21.3) | 0.168 |
| Amikacin | 12/68 (17.6) | 6/47 (12.8) | 0.872 |
| Gentamicin | 13/68 (19.1) | 10/47 (21.3) | 0.254 |
| Ciprofloxacin | 15/68 (22.1) | 14/47 (29.8) | 0.082 |
| Levofloxacin | 14/68 (20.6) | 13/47 (27.7) | 0.088 |
| Aztreonam | 15/68 (22.1) | 14/47 (29.8) | 0.082 |
| Tigecycline | 0/68 (0) | - | - |
| Colistin | 0/68 (0) | - | - |
| Carbapenemase | 12/68 (17.6) | 11/47 (23.4) | 0.448 |
| Diseases | |||
| Cancer | 7 (10.3) | 12 (25.5) | 0.031 |
| Liver abscess | 8 (11.8) | 1 (0.02) | 0.059 |
| Hypertension | 21 (44.7) | 15 (32.0) | 0.907 |
| Diabetes | 18 (26.5) | 8 (17.0) | 0.234 |
| Pulmonary disease | 19 (28.0) | 12 (25.5) | 0.775 |
| Invasive equipment | 33 (48.5) | 21 (44.7) | 0.684 |
Capsular Serotyping of HvKP and Non-HvKP
Thus far, more than 100 serotypes of K. pneumoniae have been reported, most of which are closely associated with the types of infection and severity of disease progression.24–26 Common clinical capsular serotypes include K1, K2, K5, K20, and K54. We detected the capsular serotypes of the collected clinical isolates, and the results are shown in Table 2. Among HvKP isolates, the most common capsular serotype was K2 (n=26, 38.2%), followed by K1 (n=21, 30.9%). Among non-HvKP isolates too, the most common serotype was K2 (n=9, 19.1%), followed by K1 (n=2, 4.3%). Obviously, both K1 and K2 serotypes were higher in HvKP than in non-HvKP (P<0.05).
Antimicrobial Resistance Between HvKP and Non-HvKP
The sensitivity and drug resistance of 15 antimicrobial agents among HvKP and non-HvKP isolates are shown in Table 2. The resistance rate of cephalosporins in the non-HvKP group was higher than that of the HvKP group. All HvKP are sensitive to tigecycline and colistin. There were 23 carbapenemase producing isolates (12 strains in the HvKP group and 11 strains in the non-HvKP group) out of all 115 clinical strains.
Prevalence of Carbapenemase Producing HvKP
The demographic, microbiological, and clinical characteristics of the 12 carbapenemase producing HvKP patients are shown in Table 3. Nine patients developed pneumonia, and three patients experienced sepsis. Most of the 12 carbapenemase producing HvKP strains contained at least three genes encoding carbapenemase producing isolates. Consistent with domestic reports,27,28 the results of this study showed that ST11 was the dominant serotype in carbapenemase producing HvKP isolates.
Table 3.
Clinical and Microbiological Characteristics of Carbapenemase Producing HvKP Isolates
| Strain4 | Strain7 | Strain16 | Strain29 | Strain32 | Strain34 | Strain35 | Strain37 | Strain38 | Strain51 | Strain55 | Strain68 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||||||||
| Gender | Male | Male | Male | Male | Female | Female | Male | Male | Male | Male | Female | Male |
| Age | 56 | 52 | 46 | 54 | 53 | 33 | 29 | 81 | 55 | 50 | 60 | 44 |
| Microbiological characteristics | ||||||||||||
| Virulence-associated features | ||||||||||||
| String test | + | + | + | + | + | + | + | + | + | + | + | + |
| rmpA | + | + | + | + | + | + | + | + | + | + | + | + |
| rmpA2 | + | + | + | + | + | + | + | + | + | + | + | + |
| Cps genotype | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K14/K64 | K2 | No-K-type | K14/K64 |
| MLST | ST11 | ST11 | ST11 | ST11 | ST11 | ST11 | ST11 | ST11 | ST11 | ST25 | ST11 | ST11 |
| Resistance-associated features | ||||||||||||
| KPC-2 | + | + | + | + | - | + | + | + | + | - | + | + |
| NDM-1 | - | + | + | + | + | - | + | + | + | + | + | + |
| VIM-1 | + | + | + | + | + | + | + | + | + | - | + | + |
| IMP-1 | - | - | - | - | - | - | - | - | - | - | - | - |
| OXA-48 | + | + | + | + | + | + | + | + | + | + | + | + |
| Clinical characteristics | ||||||||||||
| Specimen type | Sputum | Secretions | Sputum | Blood | Venous catheter | Sputum | Sputum | Sputum | Sputum | Sputum | Sputum | Puncture fluid |
| Infection type | Pneumonia | Pneumonia | Pneumonia | Pneumonia | Pneumonia | Pneumonia | Sepsis | Sepsis | Pneumonia | Pneumonia | Pneumonia | Abdominal infection |
| Clinical outcomes | Survived | Survived | Survived | Survived | Survived | Survived | Died | Died | Died | Survived | Survived | Survived |
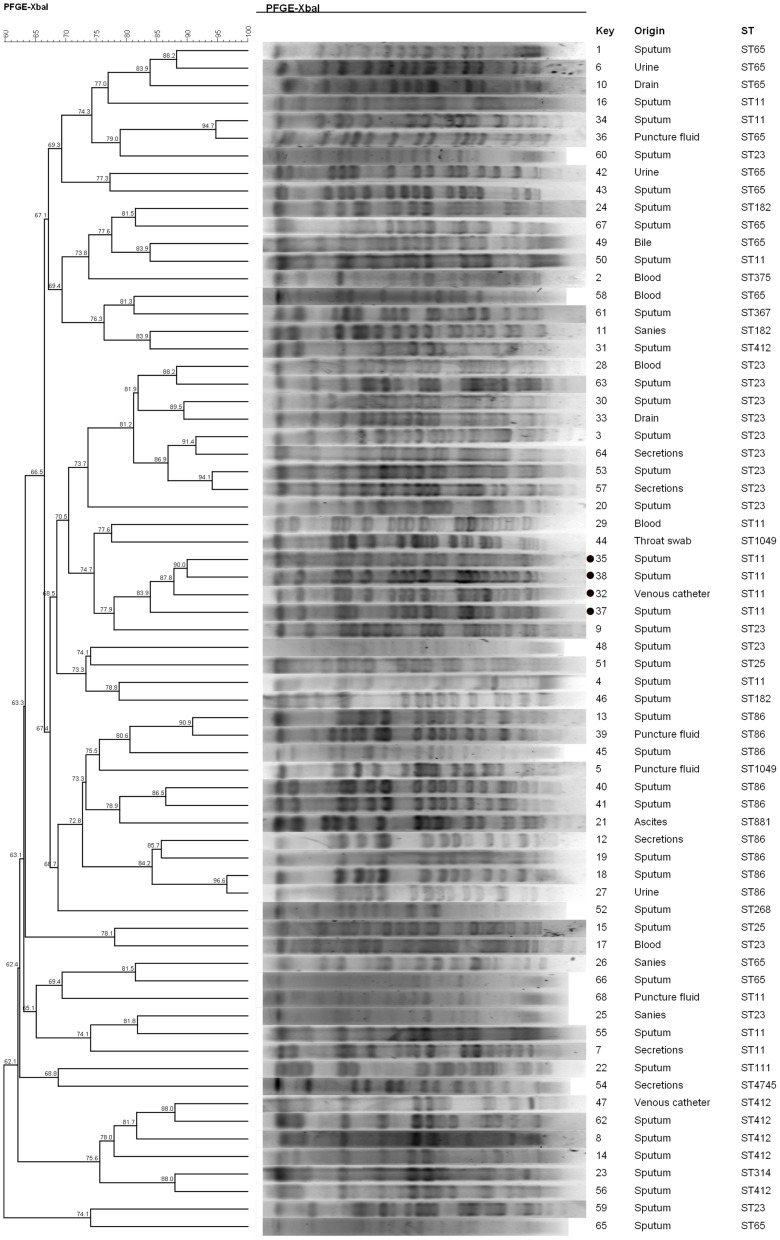
MLST Genotyping and PFGE
MLST identification of 68 strains showed that ST23 (n=15, 22.1%) was the most common type of ST, followed by ST11 and ST65 (n=12, 17.6%), ST86 (n=9, 13.2%), and ST412 (n=6, 8.8%). Homology analysis of the 68 strains of HvKP was conducted by PFGE. After XBaI digestion and BioNumerics software processing, a tree diagram was obtained (Figure 2). Briefly, 40 groups of different PFGE bands were detected in 68 strains of HvKP, indicating that the strain was highly polymorphic. Strain No. 32, 35, 37, and 38 of the 12 strains of carbapenemase producing HvKP were highly homologous, and most likely originated from the same strain or was caused by nosocomial infection.
Figure 2.
Pulsed field gel electrophoresis (PFGE) cluster analysis of 68 strains of HvKP from different sources. Four highly homologous isolates from carbapenemase producing HvKP have been marked with a black circle to the serial number left.
Discussion
Klebsiella pneumoniae is one of the three most common causes of Gram-negative hospital-acquired infections (HAI, 10.2%), second only to Pseudomonas aeruginosa (11.5%) and Escherichia coli (10.4%).29 Klebsiella pneumoniae infections can occur in any individual and thrive in different regions of the body. The mortality rate of bacteraemia caused by Klebsiella pneumoniae is about 20–26%.30 This life-threatening bacterial infection has been emerging in many countries of the world and has become a major threat to host health.31
HvKP was defined on the basis of positive virulence genes rmpA and rmpA2 and a positive string test. As is known to all, ~ 200kb virulence plasmid is an important characteristic of HvKP, which contains many virulence coding genes, such as rmpA and rmpA2 mentioned above and siderophore (aerobactin and salmochelin). Nassif et al demonstrated that the aerobactin and myxoid phenotype were associated with type K1 and K2.32,33 Ye et al found that all detected strains contained iuc, iro, rmpA and rmpA2 genes in their study of 40 pyogenic liver abscess specimens.34 RmpA/rmpA2 gene and siderophores cluster are considered to be more important in invasive infections caused by Klebsiella pneumoniae.35 Some studies have found that iroB, iucA, peg-344, rmpA, and rmpA2 are the most accurate molecular markers for differentiating between HvKP and classical Kp strains.36 Indeed, in addition to rmpA and rmpA2, iucA (encoding aerobactin) has been demonstrated to be one of the most accurate genetic markers for identifying HvKP and has not been studied in the present work.
The top three units from where the 68 HvKP strains were isolated were the Department of ICU (n=14, 20.6%), Department of Respiratory Medicine and Critical Care (n=13, 19.1%), and the Department of Infectious Disease (n=7, 10.3%), poor physical quality and low immunity of the patient. Thus, these departments should be paid more attention to, to control HvKP infections. Consistent with previous studies, our results show that neither age nor sex is associated with HvKP.37 However, K. pneumoniae is highly aggressive and has been linked to infections in healthy young people.38–41 Studies have shown that HvKP infection is more prone to metastatic infection than non-HvKP infections.42,43 But our study shows that patients with non-HvKP infection are more susceptible to tumours than those who are infected with HvKP (25.5% vs 10.3%, p=0.031). Other diseases or invasive equipment possibly have no association with HvKP.
Our study showed that HvKP was associated with K1 and K2 expression (p<0.05, Table 2), which is consistent with previous research.8 Liu et al suggested that the resistance of K1 and K2 capsular serotypes to phagocytosis might be one of the causes, thus providing favourable conditions for the colonization and growth of bacteria.22 Different from other literature reports,44 our results showed that the most common capsular serotype in HvKP strain was K2 (26/68, 38.2%), followed by K1 (21/68, 30.9%). However, in general, these two serotypes are predominant.
Carbapenems including imipenem, meropenem, and ertapenem are currently considered the most effective antibiotics in the treatment of Gram-negative bacilli infection. However, owing to the abuse of antibacterial drugs, especially carbapenem antibiotics, multidrug-resistant K. pneumoniae strains are constantly emerging, making it difficult to control these infections.45 The high virulence and antimicrobial resistance of K. pneumoniae vary to a large extent.46 In our study, 12 of the 23 carbapenemase producing strains were HvKP isolates, and 3 of these 12 individuals eventually died. The differences in carbapenemase producing isolates between the HvKP and non-HvKP group showed no statistical significance (17.6% vs 23.4%, p=0.448). We speculate that this is because of the insufficient number of clinical specimens. Regarding the rare co-existence of high virulence and resistance of K. pneumoniae, some scholars47 consider that HvKP has no resistance plasmids or that the resistance genes vanish when carrying virulence-related plasmids and genes. Further research studies are needed to verify these suggestions. The distribution and prevalence of ST types vary greatly from region to region. For example, ST23 is the most prevalent in Wuhan, accounting for 21.7%, while ST11 is the most prevalent in Zhejiang, accounting for 25%.8 In our study, 11 of the 12 carbapenemase producing HvKP strains were ST11 clones, which indicated that this strain might have an epidemic phenomenon over a certain period of time. The source and collection time of these strains were variable, and the circulation of hospital personnel or airborne transmission further increased the possibility of infections in patients. Therefore, hand hygiene of medical staff and infection prevention and control in hospitals are paramount.
Carbapenemase producing HvKP has caused fatal infections,16,19 which requires immediate action in case of multidrug-resistant infections. However, there are few studies on this strain in Anhui province. Our research highlights the high prevalence of HvKP infections in the Anhui province, including clinical characteristics and antimicrobial resistance. These severe infections caused by HvKP and the continuous occurrence of carbapenemase producing HvKP nudge us to improve clinical awareness, infection prevention, and treatment strategies.
Conclusion
Klebsiella pneumoniae (K. pneumoniae) causes community-acquired and hospital-acquired pneumonia. Our research shows the microbiological characteristics and clinical manifestations of K. pneumoniae in AnHui province, and the high incidence of carbapenemase producing HvKP, emphasizing the importance of monitoring, prevention and identifying clinical treatment strategies.
Acknowledgments
We sincerely thank Zhou Liu (Clinical Laboratory of the Second Affiliated Hospital of Anhui Medical University) and Kaili Sun (The First Affiliated Hospital of University of Science and Technology of China) for technical support.
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant no. 81973983), the National Science and Technology Major Project (grant no. 2017ZX10204401), the Borrowing and Transferring Subsidy Project in 2019, Hefei (grant no. J2019Y04), the Collaborative Tackling and Public Health Collaborative Innovation Project in Anhui Province (grant no. GXXT-2020-018), the Joint Construction Project of Clinical Medicine University and Hospital (grant no. 2021lcxk006), and the Natural Science Research Project of Universities in Anhui Province (grant no. KJ2020A0176).
Data Sharing Statement
Data can be made available through contact with the corresponding author (Professor Jiabin Li).
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki, and the protocols applied in this study were approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University, China.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Chung DR, Lee H, Park MH, et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31(4):481–486. doi: 10.1007/s10096-011-1334-7 [DOI] [PubMed] [Google Scholar]
- 2.Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. doi: 10.1186/1471-2180-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Gu Y, Li X, et al. Identification and Characterization of NDM-1-producing Hypervirulent (Hypermucoviscous) Klebsiella pneumoniae in China. Ann Lab Med. 2019;39(2):167–175. doi: 10.3343/alm.2019.39.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan Y, Zhou M, Jian Z, et al. Prevalence of pks gene cluster and characteristics of Klebsiella pneumoniae-induced bloodstream infections. J Clin Lab Anal. 2019;33(4):e22838. doi: 10.1002/jcla.22838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Shi J, Guo J. High prevalence of hypervirulent Klebsiella pneumoniae infection in the genetic background of elderly patients in two teaching hospitals in China. Infect Drug Resist. 2018;11:1031–1041. doi: 10.2147/IDR.S161075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Ping Y, Li L, et al. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10(3):208–213. doi: 10.3855/jidc.6697 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553–560. doi: 10.1016/j.jinf.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi: 10.1128/AAC.01127-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choby JE, Howard‐Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae – clinical and molecular perspectives. J Intern Med. 2020;287(3):283–300. doi: 10.1111/joim.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan S, Kong HH, Segre JA. Species-level analysis of DNA sequence data from the NIH human microbiome project. PLoS One. 2012;7(10):e47075. doi: 10.1371/journal.pone.0047075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorrie CL, Mirceta M, Wick RR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017;65(2):208–215. doi: 10.1093/cid/cix270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin RM, Cao J, Brisse S, et al. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. Msphere. 2016;1(5). doi: 10.1128/mSphere.00261-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo TA, Olson R, Macdonald U, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82(6):2356–2367. doi: 10.1128/IAI.01667-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcantar-Curiel MD, Giron JA. Klebsiella pneumoniae and the pyogenic liver abscess: implications and association of the presence of rpmA genes and expression of hypermucoviscosity. Virulence. 2015;6(5):407–409. doi: 10.1080/21505594.2015.1030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YT, Chang HY, Lai YC, et al. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene. 2004;337:189–198. doi: 10.1016/j.gene.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Lin D, Chan EW, et al. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother. 2016;60(1):709–711. doi: 10.1128/AAC.02173-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M, Fu Y, Fang Y, et al. High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect Drug Resist. 2019;12:641–653. doi: 10.2147/IDR.S191892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan H, Lou Y, Zeng L, et al. Infections caused by carbapenemase-producing Klebsiella pneumoniae: microbiological characteristics and risk factors. Microb Drug Resist. 2019;25(2):287–296. doi: 10.1089/mdr.2018.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Li XY, Wan LG, et al. Virulence and transfer ability of resistance determinants in a novel Klebsiella pneumoniae sequence type 1137 in China. Microb Drug Resist. 2014;20(2):150–155. doi: 10.1089/mdr.2013.0107 [DOI] [PubMed] [Google Scholar]
- 20.Fang CT, Lai SY, Yi WC, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45(3):284–293. doi: 10.1086/519262 [DOI] [PubMed] [Google Scholar]
- 21.Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52(12):4377–4380. doi: 10.1128/JCM.02316-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JC, Koh TH, Lee N, et al. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 2014;6:21. doi: 10.1186/1757-4749-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku YH, Chuang YC, Chen CC, et al. Klebsiella pneumoniae isolates from meningitis: epidemiology, virulence and antibiotic resistance. Sci Rep. 2017;7(1):6634. doi: 10.1038/s41598-017-06878-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyres KL, Wick RR, Gorrie C, et al. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom. 2016;2(12):e000102. doi: 10.1099/mgen.0.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafat C, Messika J, Barnaud G, et al. Hypervirulent Klebsiella pneumoniae, a 5-year study in a French ICU. J Med Microbiol. 2018;67(8):1083–1089. doi: 10.1099/jmm.0.000788 [DOI] [PubMed] [Google Scholar]
- 26.Catalan-Najera JC, Barrios-Camacho H, Duran-Bedolla J, et al. Molecular characterization and pathogenicity determination of hypervirulent Klebsiella pneumoniae clinical isolates serotype K2 in Mexico. Diagn Microbiol Infect Dis. 2019;94(3):316–319. doi: 10.1016/j.diagmicrobio.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Ho PL, Cheung YY, Wang Y, et al. Characterization of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from a healthcare region in Hong Kong. Eur J Clin Microbiol Infect Dis. 2016;35(3):379–385. doi: 10.1007/s10096-015-2550-3 [DOI] [PubMed] [Google Scholar]
- 28.Sun K, Chen X, Li C, et al. Clonal dissemination of multilocus sequence type 11 Klebsiella pneumoniae carbapenemase - producing K. pneumoniae in a Chinese teaching hospital. Apmis. 2015;123(2):123–127. doi: 10.1111/apm.12313 [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Venkatachalam I, Tee NW, et al. Prevalence of healthcare-associated infections and antimicrobial use among adult inpatients in Singapore acute-care hospitals: results from the first national point prevalence survey. Clin Infect Dis. 2017;64(suppl_2):S61–S67. doi: 10.1093/cid/cix103 [DOI] [PubMed] [Google Scholar]
- 30.Tan TY, Ong M, Cheng Y, Ng L. Hypermucoviscosity, rmpA, and aerobactin are associated with community-acquired Klebsiella pneumoniae bacteremic isolates causing liver abscess in Singapore. J Microbiol Immunol Infect. 2019;52(1):30–34. doi: 10.1016/j.jmii.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 31.Struve C, Roe CC, Stegger M, et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. Mbio. 2015;6(4):e00630. doi: 10.1128/mBio.00630-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassif X, Sansonetti PJ. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun. 1986;54(3):603–608. doi: 10.1128/iai.54.3.603-608.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassif X, Fournier JM, Arondel J, Sansonetti PJ. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun. 1989;57(2):546–552. doi: 10.1128/iai.57.2.546-552.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye M, Tu J, Jiang J, et al. Clinical and genomic analysis of liver abscess-causing Klebsiella pneumoniae identifies new liver abscess-associated virulence genes. Front Cell Infect Mi. 2016;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt KE, Wertheim H, Zadoks RN, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance inKlebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci. 2015;112(27):E3574–E3581. doi: 10.1073/pnas.1501049112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo TA, Olson R, Fang C-T, et al. Identification of biomarkers for differentiation of Hypervirulent Klebsiella pneumoniae from Classical K. pneumoniae. J Clin Microbiol. 2018;56(9). doi: 10.1128/JCM.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Liu W, Cui Q, et al. Prevalence and detection of Stenotrophomonas maltophilia carrying metallo-beta-lactamase blaL1 in Beijing, China. Front Microbiol. 2014;5:692. doi: 10.3389/fmicb.2014.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect Dis. 2010;10:307. doi: 10.1186/1471-2334-10-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomakova DK, Hsiao CB, Beanan JM, et al. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31(6):981–989. doi: 10.1007/s10096-011-1396-6 [DOI] [PubMed] [Google Scholar]
- 40.Jung SW, Chae HJ, Park YJ, et al. Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea. Epidemiol Infect. 2013;141(2):334–340. doi: 10.1017/S0950268812000933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brisse S, Fevre C, Passet V, et al. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4(3):e4982. doi: 10.1371/journal.pone.0004982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YT, Huang YW, Huang HH, et al. In vivo evolution of tigecycline-non-susceptible Klebsiella pneumoniae strains in patients: relationship between virulence and resistance. Int J Antimicrob Agents. 2016;48(5):485–491. doi: 10.1016/j.ijantimicag.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 43.Keynan Y, Karlowsky JA, Walus T, Rubinstein E. Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand J Infect Dis. 2007;39(9):828–830. doi: 10.1080/00365540701266763 [DOI] [PubMed] [Google Scholar]
- 44.Hao Z, Duan J, Liu L, et al. Prevalence of community-acquired, hypervirulent Klebsiella pneumoniae isolates in Wenzhou, China. Microb Drug Resist. 2020;26(1):21–27. doi: 10.1089/mdr.2019.0096 [DOI] [PubMed] [Google Scholar]
- 45.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bialek-Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–1820. doi: 10.3201/eid2011.140206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58(2):225–232. doi: 10.1093/cid/cit675 [DOI] [PubMed] [Google Scholar]