Abstract
Background
Pathological conditions with ongoing inflammatory processes result in specific heat signatures at the affected body parts; infrared thermography (IRT) detects these changes and can be utilied in screening such conditions. The modern devices are advanced and their non contact, convenient and precise readings can aid in multiple medical sub fields. Orthopaedics as a broad entity has witnessed utilisation of this technology for different indications and the present scoping review was done to assess these established indications and further scope of its utility.
Method
ology: A Medline search was done on April 26, 2021 with specific keywords for studies of any design in English language discussing the usage of thermography in Orthopaedics. Animal studies, conference abstracts, systematic reviews, e-posters, case reports, book chapters, and studies describing the use of thermography in non-Orthopaedic patients were excluded.
Results
Total number of hits were 1380. 43 studies including case series and case control studies were included in the review. The subfields or indications described were pain/arthritis, Charcot's foot/neuropathic ulcers, infections associated with diabetic feet and arthroplasties, reflex sympathetic dystrophy, carpal tunnel syndrome, sports medicine, paediatric orthopaedics, spine, ergonomics and compartment syndrome.
Conclusion
IRT has been described to be effective in orthopaedic conditions with specific heat signatures and this can assess the trend of the ongoing inflammatory process as well as response to a particular treatment. Additionally, it can specifically determine the exact loci of the pathology for targeted interventions.
Keywords: Infrared thermography, IRT, Orthopaedics, Periprosthetic infection, Arthritis, Sports medicine
1. Introduction
Infrared thermography (IRT) is a non-contact tool that can detect even subtle changes in human body temperature.1 Any disease or localized condition that activates the inflammatory cascade can provide specific heat signatures at the affected part or area of the body, which IRT can pick up.2 These patterns have shown to be persistent, reproducible, and IRT has been utilized in settings of inflammatory conditions like rheumatoid arthritis, soft tissue injuries, surgical site infections, and monitoring surgical wound healing after various abdominal, plastic and thoracic surgeries.3
Modern-day devices are portable cameras with precision and can be used by untrained individuals, which have dispelled previous limitations of extensive usage. The basic principle of IRT is that it records the infrared radiation emitted from a surface or object and converts that energy into heat signatures.1,4 It can then provide color-coded thermal maps or images that can be compared with non affected surfaces or body parts to screen for a specific condition. Invented in the 1960s as a technology for the night vision cameras, the technology has expanded to various fields like civil engineering and medicine.5,6
Thermal images represent hyperemia due to localized disease, and the persistent changes in chronic conditions demonstrate non-restoration of normalcy. With the establishment of standardized procedures and guidelines in terms of patient position and preparation as well as reference values of healthy subjects, the technology has witnessed improved accuracy, and multiple research have been witnessed in the different fields of medicine and surgery like ophthalmology, obstetrics, plastic surgery, etc.6,7
In orthopaedics as well, there have been reports of the utilisation of IRT in various disorders like osteoarthritis, rheumatoid arthritis, carpal tunnel syndrome, soft tissue injuries and tendinopathy, periprosthetic joint infections (after total knee replacements), diabetic foot infections, Charcot arthropathy, and spine disorders. The present review was done to identify and briefly discuss the applications and effectiveness of IRT in orthopaedics.
2. Methodology
2.1. Search strategy
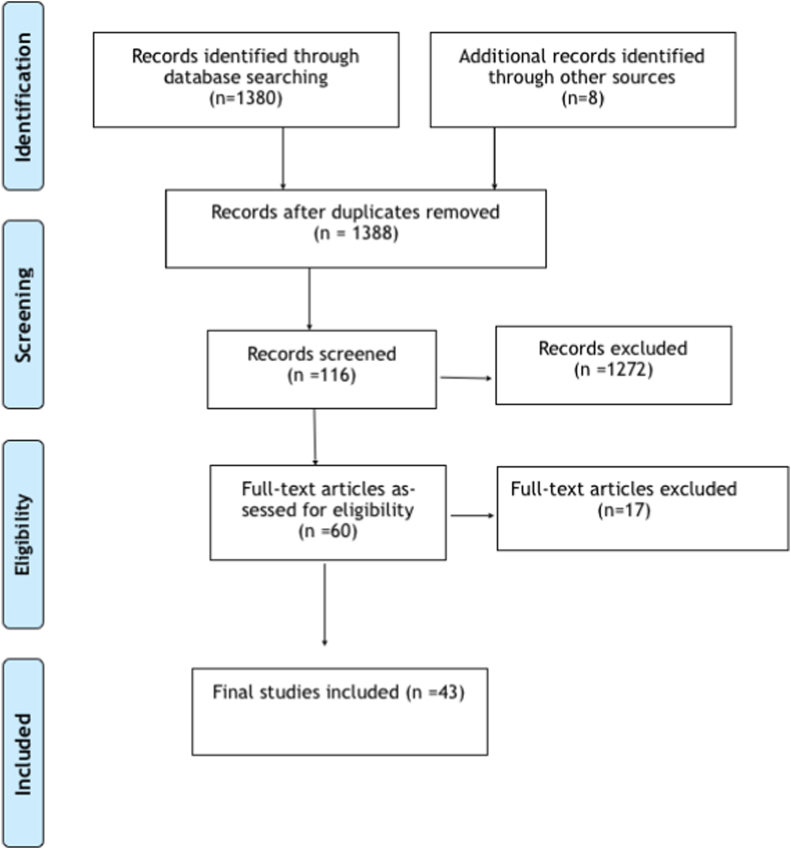
A Medline database search was conducted on April 26, 2021 with the combinations of keywords, as shown in Table 1. This yielded 1380 hits, out of which 43 were included in the current review as per our inclusion and exclusion criteria.
Table-1.
Search strategy followed in the Medline (via PubMed) database.
| Sl no. | Search description | Results |
|---|---|---|
| 1 | (((Thermography) OR (Thermal imaging)) OR (Telethermographic)) AND (Orthopaedics) | 393 |
| 2 | (((Thermography) OR (Thermal imaging)) OR (Telethermographic)) AND (Arthroplasty) | 60 |
| 3 | (((Thermography) OR (Thermal imaging)) OR (Telethermographic)) AND (trauma) | 1469 |
| 4 | (((Thermography) OR (Thermal imaging)) OR (Telethermographic)) AND (Orthopaedic infection) | 23 |
| 5 | (((Thermography) OR (Thermal imaging)) OR (Telethermographic)) AND (spine) | 326 |
| 6 | #1 OR #2 OR #3 OR #4 OR #5 | 1380 |
2.2. Selection of studies (inclusion and exclusion criteria)
Studies of any design in the English language discussing the usage of thermography in
Orthopaedics were screened. Animal studies, conference abstracts, posters, systematic reviews, case reports, book chapters, and studies describing the use of thermography in non-Orthopaedic patients were excluded, as shown in Fig. 1.
Fig. 1.
Flowchart depicting selection of studies.
2.3. Data collection
All the hits were screened independently by two authors based on title and/or abstract for inclusion. The full text of all the screened articles was read, and the relevance was assessed.
Discussions among the authors resolved discrepancies. A secondary search was also done from the bibliography list of all selected articles.
3. Results
The total hits from the search were 1380, out of which after removing duplicates and studies which were unrelated to IRT in orthopaedics, 108 studies were assessed. Full texts of 71 out of 108 studies were read, and finally, 43 studies were included. These studies described the utility of IRT in orthopaedic sub-fields like pain and arthritis (5), Charcot foot and neuropathic ulcers (6), infections in diabetic foot and joint arthroplasty (3); reflex sympathetic dystrophy (2), Carpal tunnel syndrome (5), sports medicine (6), paediatric orthopaedics (7), spine (5), and miscellaneous including ergonomics, elbow pain and compartment syndrome (4) (Table 2).
Table 2.
Review of relevant Literature of application of Infrared thermography in Orthopaedics.
| NO. | AUTHOR | YEAR | TYPE OF STUDY | NUMBER OF PATIENTS | INDICATION FOR IRT | CONCLUSION |
|---|---|---|---|---|---|---|
| 1 | Denoble et al. | 2010 | Case control study | 30 | Knee osteoarthritis | Skin temperature of the patellar region measured using IRT can be used as an indicator of knee osteoarthritis severity |
| 2 | Spalding et al. | 2008 | Case control study | 22 | Rheumatoid arthritis | Heat distribution index calculated using IRT, greater than 1.30 celsius correlated well with clinically assessed active arthritis |
| 3 | Jones et al. | 2018 | Case control study | 79 | Rheumatoid arthritis | IRT imaging may be useful in assessing larger joints but it is not an effective modality for assessing small joints of hands in RA patients |
| 4 | Freidrich et al. | 2020 | Case control study | 133 | Post operative knee pain | IRT is useful in localising the pathological sites in postoperative knee pain |
| 5 | Wu et al. | 2009 | Case control study | 53 | Coccygodynia | IRT is a useful tool to objectively monitor the disease activity in patients with coccygodynia |
| 6 | Dallimore et al. | 2020 | – | 32 | Charcot's arthropathy | IRT can be used confidently and reliably in clinical and research settings to assess skin temperature in patients with Charcot foot |
| 7 | Armstrong et al. | 1997 | Cohort study | 39 | Charcot's arthropathy | Increased temperatures as detected using IRT strongly correlated with the location of arthropathy and IRT can also be used to predict future ulcerations |
| 8 | Armstrong et al. | 1996 | Cohort study | 25 | Neuropathic ulcers | IRT may be used to look for the effectiveness of foot “off-loading” with therapeutic footwear and insoles, and also to monitor wound healing and inflammation in the diabetics with neuropathic ulcers |
| 9 | Armstrong et al. | 2007 | RCT | 225 | Neuropathic ulcers | IRT can be used to detect high temperature gradients between the feet in diabetic patients at high risk for ulcerations, and it is helpful in predicting and preventing the development of ulcers in these patients |
| 10 | Ghosh et al. | 2020 | Case control study | 30 | Neuropathic ulcers | IRT may have a role in guiding the duration of offloading therapy, in predicting ulcer healing, in determining complete healing, and in predicting the recurrence of ulcers in diabetic patients |
| 11 | Lavery et al. | 2004 | RCT | 85 | Neuropathic ulcers | At-home, daily self-monitoring of foot temperatures using IRT maybe an effective supplementary tool to prevent lower extremity complications such as ulcers and amputations in high risk diabetic patients |
| 12 | Hazenberg et al. | 2014 | – | 38 | Diabetic foot infections | IRT imaging in combination with photographic imaging of the foot can be used for home-monitoring to facilitate early diagnosis of foot infections in high risk diabetic patients |
| 13 | Romano et al. | 2012 | Case control study | 55 | Periprosthetic knee infections after TKR | IRT imaging produced a quantifiable and reproducible thermographic pattern of surgical site healing in patients with uncomplicated TKR, whereas, in infected patients, there was an elevated mean differential temperature of >1 °C |
| 14 | Romano et al. | 2013 | Case control study | 70 | Periprosthetic knee infections after TKR | IRT is a reliable tool to diagnose peri-prosthetic knee infections |
| 15 | Gulevich et al. | 1997 | Case control study | 185 | Reflex sympathetic dystrophy | Stress IRT is a sensitive and specific indicator of RSD |
| 16 | Hassan et al. | 2003 | Case control study | – | Reflex sympathetic dystrophy | IRT can diagnose painful sites due to neuropathic dysfunction and also monitor the treatment response |
| 17 | Papez et al. | 2009 | Case control study | 119 pathological hands | Carpal tunnel syndrome | IRT is useful as a screening tool in populations with high ergonomic risks for CTS |
| 18 | Zivcak et al. | 2011 | Case control study | 14 pathological hands, 120 normal hands | Carpal tunnel syndrome | IRT is a promising method in the diagnosis of CTS |
| 19 | Baic et al. | 2017 | Case control study | 30 | Carpal tunnel syndrome | It is possible to use IRT in the diagnosis of CTS as well as monitoring the healing process after surgery in patients with CTS |
| 20 | Maxel et al. | 2014 | Case control study | 11 | Carpal tunnel syndrome | IRT can be a suitable candidate for early, non-invasive, and reliable diagnosis of CTS |
| 21 | Bargiel at al | 2021 | Case control study | 80 | Carpal tunnel syndrome | Dynamic IRT can be useful in confirming CTS diagnosis; however, presently it serves scientific purposes mainly and has limited clinical applications |
| 22 | Gomez-Carmona et al. | 2020 | Case control study | 24 | Sports injury | IRT based injury prevention program can reduce injuries, injury severity, and loss of sports days by early identification of players at risk |
| 23 | Corte et al. | 2019 | Case control study | 28 | Sports injury | A promising catalyst to conduct a rigorous RCT to examine if IRT can actually contribute to muscle injury prevention programme was provided by their study |
| 24 | Al-Nakhil et al. | 2012 | Case control study | – | Sports injury | Use of IRT to detect delayed onset of muscle soreness in its early stages can be helpful in reducing the incidence of injuries in sports setting |
| 25 | Oliviera et al. | 2016 | Case control study | 19 | Ankle injuries | IRT being safe, non-invasive, quick, and cost effective can be used for the grading of ankle sprain lesions in any setting, although future studies with wider sample size are required to establish the significance |
| 26 | Mangine et al. | 1987 | Case control study | 17 | Patellar tendinitis | IRT is useful as a non-invasive and objective method to detect soft tissues inflammation around the patellar tendon and to differentiate this from other knee pathologies |
| 27 | Yang et al. | 2014 | Case control study | 20 | Knee ligament injuries | IRT can be used as a supportive tool in the diagnosis of knee ligamentous injuries, especially in the evaluation of a medial collateral ligament injury |
| 28 | Curkovic et al. | 2015 | Case control study | 19 | Paediatric forearm fractures |
IRT may be used as a tool in future in the follow-up of paediatric forearm fractures and decreasing the number of radiographic scans required for the same |
| 29 | Saxena et al. | 2007 | – | 6 with digit amputations, 42 with extremity infections |
Paediatric digit amputations, re-implantation and extremity infections | IRT provides immediate information about the success of revascularisation and viability of the re-implanted digits. IRT is also helpful in localising a suspected area of extremity infection, and in the early detection of surgical site infections |
| 30 | Seuser et al. | 2018 | Case control study | 22 | Children with hemophilia | IRT is a useful tool for the early detection of joint inflammation in children with severe hemophilia, and is helpful in prevention of joint overloading and bleeding |
| 31 | De Salis et al. | 2018 | Case control study | 11 | Children with osteogenesis imperfect | IRT may prove to be a novel tool in the detection and monitoring of vertebral fractures in children with OI; however, further studies with larger sample size are required to verify the same |
| 32 | Silva et al. | 2012 | Case control study | 51 | Children with extremity trauma | Further evaluation with larger cohorts are necessary to prove that IRT can optimally pinpoint a site of injury, and results of this are encouraging for the same |
| 33 | Reed et al. | 2020 | Case control study | 105 | Children with wrist injuries | IRT may be useful as a screening or diagnostic adjunct in children with wrist sprain/fracture, and also for differentiation between the two |
| 34 | Owen et al. | 2017 | Case control study | 30 | Children with acute limp | IRT is potentially helpful in the diagnosis of acute limp in children, although studies with larger sample size are required for reproducing the same results |
| 35 | Kwok et al. | 2017 | Case control study | 30 | Adolescent idiopathic scoliosis |
IRT seems to be a feasible tool to be included in the school scoliosis screening program, although further studies are required for the validation of same |
| 36 | Dragan et al. | 2002 | Case control study | 403 | Adolescent idiopathic scoliosis |
IRT is very useful in the eavaluation of cases of adolescent idiopathic scoliosis, especially for monitoring the conservative treatment course |
| 37 | Lubkowska et al. | 2020 | Case control study | 40 | Scoliosis | IRT may be used as a supportive tool for school scoliosis screening; however, more research is required to establish its reliability and accuracy |
| 38 | Alfieri et al. | 2019 | Case control study | 57 | Low back pain | There was a higher superficial temperature in the lumbar region in individuals with low back pain, and IRT is an interesting method to assess these patients. |
| 39 | Zaproudina et al. | 2006 | Case control study | 85 | Low back pain | IRT may be a useful adjunct in the evaluation and documentation of autonomic dysfunctions in low back pain patients |
| 40 | Lasanen et al. | 2017 | Cohort study | 14 | Work ergonomics | IRT can detect temperature differences due to the differential muscle activation in different postures, and help in the planning of better work ergonomics |
| 41 | Alexandre et al. | 2016 | Case control study | 4 | Work ergonomics | IRT is a powerful tool to detect and prevent ergonomic injuries due to the use of lead aprons by medical staff, although future studies with larger sample size are required to establish its significance |
| 42 | Katz et al. | 2008 | Case control study | 164 | Compartment syndrome | IRT holds promise as a tool for the early detection of acute compartment syndrome in trauma patients |
| 43 | Gabrhel et al. | 2017 | Case control study | 43 | Elbow pain | IRT is a highly sensitive tool for diagnosing a case of epicondylitis which commonly causes elbow pain. It can supplement radiological scans in the diagnosis and also provide information on the acuteness of morphological changes |
4. Discussion
4.1. Pain and arthritis
Arthritis represents a plethora of joint pathologies which can present clinically as joint pain, swelling, redness, stiffness, and increased temperature in acute stages. Osteoarthritis (OA) and Rheumatoid arthritis (RA) are the two most common causes which are highly prevalent, and subtle changes in the joint surface temperature, whether increased or decreased, can be an indirect indicator of exacerbation or reduction of the undergoing inflammatory process associated with these; this can also be objectively measured using IRT.3 Fokam et al. described IRT as a reliable, easy, cheap, non-invasive, and radiation-free technique to assess skin temperature to aid in the diagnosis and therapeutic follow-up of arthritis, and the underlying inflammation. They also concluded that IRT imaging of the inflamed joint surface also correlates with the intensity of joint pain. Factors such as camera quality being used, ambient temperature, distance between the joint and the camera, angle of measurement, variability of the blood flow, and defining the region of interest on which temperature is to be measured must be carefully looked after to ensure better reproducibility of IRT.8
Denoble et al. compared IRT findings in 15 females with symptomatic OA knee with 15 age-matched normal controls. IRT presented an objective measure of the ongoing inflammation process, so the surface temperature can indicate the inter-relationship of structural knee damage (osteoarthritis) and the inflammation process causing it.9 Spalding et al. demonstrated a significant difference (p < 0.001) between the Heat Distribution Index (HDI) of normal and arthritic joints. HDI values from 18 wrists and 9 MCP joints (2nd to 5th joints) of 17 patients with active arthritis were obtained and compared to data from 10 wrists and 5 MCP regions as controls. It was determined that HDI value of greater than 1.3 °C correlated significantly with clinically assessed active arthritis.10
On the contrary, Jones et al. found no significant relationship between joint temperature and disease activity, using factors such as CRP (p = 0.6), ESR (p = 0.5), Health assessment questionnaire (p = 0.056), and swollen joints (p = 0.2) in RA patients.11 Frieddrich et al. looked for the role of surface IRT imaging in the diagnosis and management of postoperative knee pain in 133 patients who underwent ACL reconstructions, knee arthroscopies, and total knee arthroplasties. Thermographic imaging of the affected knee at defined regions of interest was done, and contralateral knees were used as control. Clinical evaluations were repeated again after subcutaneous 2% scandicain at thermographically defined sites of pathologies. All patients had a reduction in pain and better mobility. It was concluded that thermography can help detect exact site of pathology in persistent postoperative knee pain and accordingly management can be done.12 Wu et al. assessed local physiological responses after manual treatment and short wave diathermy in 53 cases of coccydynia with IRT. Pretreatment assessments with a 0 to 10 numeric pain rating scale (NPRS) and IRT of the buttock area including the coccyx were done with follow-ups at 12 weeks. There was significant improvement at 12 weeks, as compared to the pretreatment levels (p < 0.05); temperature decrease and NPRS improvement (p < 0.01, r = 0.67) also correlated.13
Overall, IRT seems to have a potential role in different aspects of arthritis and pain; it can assess the trend of ongoing inflammatory process as well as response to a particular treatment. Additionally, it can specifically determine the loci of the pathology for targeted interventions. Its usefulness in small joints pathologies is inconclusive, and further research is needed to elaborate and substantiate the current evidence.
4.2. Charcot foot and neuropathic ulcers
Charcot's foot is a limb-threatening complication most commonly seen in diabetics with peripheral neuropathy. Dallimore et al. recruited 32 adults with Charcot neuropathy and infrared dermal thermometry was performed by 2 independent raters at 10 anatomical sites of the feet using both touch and non-touch techniques. They concluded that it can be used in clinical settings with good to excellent intra-rater and inter-rater relative reliability for both the techniques.14 Nube et al. quantified the temperature changes in Charcot's arthropathy; in acute phases there is increased local temperature with lower temperatures later on. The temperature variations due to treatment modalities can also be documented with IRT, which can monitor the progress of Charcot's arthropathy and study its pathogenesis and effects of management.15 Armstrong et al. measured skin temperature in 39 diabetics with acute Charcot's arthropathy undergoing treatment to monitor the prognosis. There was a steady decrease in temperatures of the foot during the casting regimen and with the resolution of acute arthropathy. The elevated temperatures strongly correlated with the exact location of arthropathy, and the increase in temperature gradients over time was predictive of the future development of neuropathic ulcers.16 Armstrong et al. also evaluated the skin temperatures at the site of plantar ulcers in 25 diabetic patients before, during, and after healing. Contralateral feet were used as controls, and the skin temperature gradient on the affected side was found to be higher by an average of 6.9 °F at the start; the parameters were largest at the ulceration sites. Subjects with higher degrees of neuropathy also had larger gradients, and IRT imaging can monitor the effectiveness of the off-loading therapy, wound inflammation, and healing.17 Armstrong et al. also studied 225 diabetics in 2 groups at high risk for ulceration to evaluate the efficacy of in-home self-measured skin temperature using an infrared probe to limit ulceration; 1 group performed dermal thermometry of 6 foot sites twice daily apart from the standard therapeutic care. A difference of more than 4 °F was considered significant for the hospital visit. Subjects with IRT had 1/3 rd lesser ulceration. There was a significantly longer time to develop ulcers, and patients who ulcerated had 4.8 times greater temperature difference at the site of ulceration in the week before ulceration compared with the patients who did not develop ulcers. Therefore, higher temperature gradients are predictors of neuropathic ulceration, and self-monitoring can help patients modify their activities to prevent ulceration.18 Ghosh et al. evaluated the role of IRT in 30 patients with one-sided neuropathic ulcers by measuring the skin temperatures of corresponding sites on both feet. The average and ulcer temperatures were significantly higher by 1.2 °C and 3.1 °C respectively. At the time of ulcer healing, the temperature gradient normalized; however, it lowered but persisted between ulcer and the corresponding site in the normal foot for a month after healing. An average temperature gradient of ≥ 1 °C between the two feet at any time indicated impaired healing. An increasing gradient was predictive of ulcer recurrence. Overall, IRT imaging had a role in predicting ulceration, determining the end period of ulcer healing, and guiding the off-loading duration. Serial monitoring can also predict recurrence.19 Lavery et al. divided 173 patients into 3 treatment groups; 1 (n = 58) received standard therapy (physician evaluation every 8 weeks, education on foot complications, self-care practices and use of orthoses), 2 (n = 48) additionally received special training to conduct a structured foot examination twice a day apart, and 3 (n = 59) were taught infrared dermal thermometry to record temperatures of 6 sites in each foot. Patients in groups 1 and 2 had 4.37 and 4.71 times more risks of developing ulcers versus group 3. IRT can serve as an early warning in the prevention of foot ulcers in high-risk diabetic patients.20
4.3. Thermography in infections
4.3.1. Diabetic foot
These patients are more prone to infections, and IRT imaging can theoretically detect temperature changes in the early stages of inflammation. Home monitoring can screen the early stages of infection in diabetics to intervene early for better outcomes.18,20,21 Hazenberg et al. assessed 38 diabetic patients with foot-related infections and complications. Before initiating treatment, photographs of the plantar surface and temperature from 6 plantar loci of each foot were measured. Clinical assessment of the feet for signs of infection was also done, patients with PEDIS (Perfusion-Extent-Depth-Infection-Sensation) grade 2 or more were included. A temperature difference of >2.2 °C was defined as a hotspot. Diagnosis was sensitive (>90%) but not very specific (<25%); when combined with photographic assessment, specificity increased to > 79%, signifying that the method was valid and reliable.22
However, the temperatures can be confounded by associated neuropathy/vasculopathy in diabetics limiting IRT's role.22 Thermography cannot predict the severity and the ultimate outcome.23
4.3.2. Arthroplasties
Periprosthetic infections could be devastating after hip and knee replacements, which commonly lead to revision surgeries and inferior outcomes in these patients.24 MSIS (Musculoskeletal infection society) criteria currently considered gold standard for the diagnosis involve invasive tests and histopathology.25 Thermography may prove to be an effective, non-invasive, rapid, and affordable modality in such cases. Scheidt et al. in a review of IRT for early diagnosis of periprosthetic joint infections concluded that it was useful in the peri-operative monitoring for these patients following arthroplasties. The temperature is locally measured which is free from the influence of a concurrent infection of any other system of the body, which can otherwise affect the general blood parameters. However, IRT may be used as a supplement only, and it cannot replace the present criteria.26 Romano et al. used IRT to obtain reference values for the physiological thermographic pattern of normal wound healing after uncomplicated TKRs. These were compared with the values obtained in 15 patients with periprosthetic infections. In normal patients, the mean differential temperature (MDT) gradually fell to zero at 90 days. The MDT in infections ranged between 1.1 and 2.5 °C.
IRT imaging produces an accurate pattern of surgical site healing, and an elevated MDT can be used for the indirect diagnosis of infections.2
For late septic failures, Romano et al. studied 70 patients, 36 scheduled for revisions for infection and 34 with other implant-related issues (34), after 1 year of primary surgery. There was an average difference of 1.9 °C between the affected knee and the normal knee in infected cases; only 0.3 °C in the aseptic cases. IRT imaging is a prospective tool to diagnose late periprosthetic infections and differentiate them from aseptic failures.27
Overall, IRT can screen infections at an early stage, and in combination with already established diagnostic protocols, it could prove to be a game-changer in terms of early interventions and better outcomes. The usage can be expanded to infections in other subfields of orthopaedics like trauma, where the scope is immense, especially in developing countries with heavy footfalls.
4.4. Reflex sympathetic dystrophy
Reflex sympathetic dystrophy (RSD) is a disorder that occurs as an aftermath of major or minor trauma and is characterized by chronic pain, swelling, skin changes, hyperthermia, or hypothermia at the injury site. Friedman presented a series of 6 cases of RSD in which IRT was able to detect localized temperature fluctuations and gave diagnostic clues in patients with subtle symptoms.28
Gulevich et al. also found IRT to be sensitive (93%) and specific (89%) for diagnosing RSD with a positive predictive value of 90% and negative predictive value of 94%.29 Hassan et al. studied IRT in RSD patients who received standard pain care and physical therapy along with placebo tablets in one group and experimental drug in another group, for 5 weeks alternatively. Temperatures on the painful sides were more than the normal sides by at least 1 °C. After the cold stimulation test, thermal recovery time was longer on the painful side, and the authors concluded that IRT coan diagnose painful sites due to neuropathic dysfunction apart from monitoring the treatment response.30
4.5. Carpal tunnel syndrome
Another application of IRT has been described in the Carpal tunnel syndrome (CTS); its diagnosis is usually made clinically (Tinel's sign, Phalen's test), but subjective variations and indeterminate results of electrodiagnostic tests at times can hinder diagnosis.
Papez et al. analyzed the IRT pattern, using artificial neural networks, of the dorsal and palmar sides of 132 healthy and 119 hands with CTS. They found that the dorsal side of the hand is more important than the palmar side for thermographical diagnosis of CTS, and patterns in the cases and controls differed significantly.31 Zivcak et al. did IRT imaging of predefined points on the dorsal side and found that CTS patients had higher temperatures in the distal phalanges than the wrists; it was opposite in the normal hands. They concluded that CTS hands have significantly different thermal patterns, and IRT being a non-invasive and painless modality can screen CTS.32 Baic et al. analyzed the role of IRT in the monitoring of CTS patients postoperatively. The mean temperature difference between patients and controls was significantly lower at 4 weeks than pre-operatively; the temperature got closer to the control group. IRT was found effective in monitoring the recovery process post-surgery.33 Maxel et al. used IRT imaging for both the participants’ hands in their study, before and after cold stimulation. CTS patients showed abnormal variation and no thermographic symmetry before and after the test; their hands showed a greater temperature gradient compared to the normal sides. It was concluded that thermal response in CTS could be easily detected using IRT.34 However, its corroboration with the actual recovery was not looked into and needed evaluation. Bargiel et al. concluded that IRT is not helpful in the objective assessment of recovery postoperatively. They found a significant difference in the thermographic patterns of hands of cases and controls, and when a cold stimulus was given to the affected hands, the temperature returned to normal faster in the control group, although operated cases were symptoms free. So, thermographic readings in the postoperative period did not corroborate with the symptomatic relief they had after surgery.35
Overall the usefulness of IRT as a potential diagnostic tool for CTS is deliberated; it may be utilized as an addendum to the clinical signs and tests. Its role in monitoring recovery is inconclusive; although the thermal patterns progress towards normalcy, but clinically the symptoms may not correlate with them.
4.6. Sports medicine
High-level sports require intense training, which predisposes sportspersons to injuries, leading to absence from sports.36 Thermal asymmetry can identify areas of physical stress in professional athletes and can be utilized in guiding the training regimes to minimize significant injuries.37
In a study on 24 professional soccer players using the IRT injury prevention model, Gomez-Carmona et al. showed a decrease in injuries compared to the previous conventional program (6 versus 15). Additionally, injury severity and days of absence due to injuries were also lower.38
Corte et al. performed IRT imaging of both lower limbs 2 days after the games in 28 professional soccer players in the 2nd season and used the 1st season players as controls. In cases with more than 0.4 °C of asymmetry between the 2 lower limbs, injury prevention protocol was initiated. There was a significant difference between the 2 seasons (11 vs 4).39 Delayed onset muscle soreness (DOMS) can occur in athletes who train beyond the normal limits and can result in injuries; manifests as pain and tenderness40 Al-Nakhli et al. did IRT imaging at pre-exercise, 24 h, and 48 h post-exercise, in 41 subjects. There was a significant temperature difference between day 1 and day 2 (p < 0.01) in the exercised arm without any changes in the unexercised arms. VAS readings and the skin temperature measurements on day 2 also correlated. Increased temperatures 24 h post exercise is an indicator of increased blood flow due to inflammation and underlying muscle fibre damage, leading to soreness.41 In athletes, this muscle soreness can be detected at an early stage using IRT which can contain the injuries.42
Ankle sprains can result in a significant loss of productive time, especially in sportspersons. It is important to differentiate between the grades of ankle sprains; severe grades can lead to residual disability, recurrence, and decreased functionality.43,44 Oliveira et al. in a prospective study, concluded that IRT in the setting of acute ankle sprain injuries can be a potential tool for grading and guiding the management.45 Ioannou et al. found a temperature increase between participants based on the ankle sprain severity, and IRT as a novel modality can assess the soft tissue stress resulting from exercise or injury and estimate the magnitude of soft tissue damage.46
The patellar tendon is prone to overuse, and tendinitis in athletes repetitively performing stressful activities is common. Mangine et al. evaluated 17 patients with patellar tendinitis by IRT. 14 subjects had a specific thermal abnormality in the tendon region while the peri-patellar regions were normal. 12 subjects demonstrated focal “hot spots” while 2 subjects had focal “cold spots.” A significant mean temperature difference was observed between the symptomatic and asymptomatic knees. In the follow-up period, 5 subjects showed a correlation between thermal asymmetry and specific palpated pain sites. Therefore, IRT can diagnose patellar tendinitis objectively and monitor rehabilitation results in these cases.47
Medial collateral ligament (MCL) injury is a common ligament injury resulting due to valgus stress on the knee. Yang et al. compared temperatures of 20 MCL injuries at the middle of patella, superolateral, superomedial, inferomedial, inferolateral, medial, and lateral zones with the unaffected sides. Except for the lateral and inferolateral regions, all other regions on the affected side showed a significant increase in temperature (p < 0.05). They concluded that IRT can be used as an adjunct in diagnosing MCL injury of the knee even in the incipient stage.48
4.7. Paediatric orthopaedics
Forearm fractures comprise 35% of all paediatric fractures.49,50 Curkovic et al. studied the role of IRT in the follow-up of 19 patients managed conservatively. It was found that the temperature difference between the healthy and affected forearm was the highest (0.8–2 °C) on day 7, which corroborated with the inflammatory phase of fracture healing. The temperature difference was lowest (0–0.7 °C) on day 21. Thermographic pattern correlated well with the radiological pattern of healing, and it was concluded that IRT can also be utilized in tracking union in fractures at other superficial areas like knee, tibia & elbow and reduce X-rays.51 Saxena et al. studied the role of IRT in 6 paediatric patients undergoing re-implantation of digits and in 42 paediatric patients with extremity abscess, gangrene, and wound infections (42). In cases with partial amputations, a temperature differential of 2.2–2.8 °C was found after surgery which reduced after 48 h and returned to normal after 3 months. In cases of a complete digit amputation, the initial differential decrease of 2.2–2.8 °C persisted for 96 h; reduction was observed on the 12th-day post-surgery. The use of IRT imaging enabled the authors to easily assess the viability of the re-implanted digits and the success of revascularisation in an objective way. IRT was also used in determining the levels of amputations and recognizing amputation stumps at risk for re-infection. The authors concluded that IRT is a valuable diagnostic tool, especially in children with an excellent acceptance and compliance due to its non-contact nature.52 Seuser et al. studied IRT findings, Hemophilia Joint Health Scores (HJHS), and did clinical examination to elicit tender points in 10 asymptomatic children with severe hemophilia with no history of any joint bleed; compared with age-matched controls. There was no significant difference in HJHS; however, clinical examination for tender points was significant (69 silent symptoms in the hemophilia group compared to 25 in the control group). IRT changes were more in the hemophiliacs (found in 82 cases vs 32 controls). The authors concluded that IRT can detect signs of inflammation in the pre-clinical stage in these asymptomatic cases and prevent overloading and bleeding in joints.53 De Salis et al. studied 11 children of osteogenesis imperfect (OI) with vertebral fractures; did X-ray, DEXA, and IRT of the back to detect surface skin temperature (SST) changes. A metallic disc was placed on the skin as a marker before the scans. The adjacent skin surface was used as a comparative reference. There was a significant thermal asymmetry between the skin over the fractured vertebra and the adjacent skin surface. Thus, IRT can detect and monitor vertebral fractures in OI children; however, further studies with more sample size are recommended.54
Silva et al. evaluated the role of IRT in localising pain and/or fracture; the warmest area was compared to the site of pain/fracture on radiographs. IRT findings corroborated with the areas of pain in 73% of patients; it only identified fractures in 7/11 cases. Although the results were inconclusive, they were encouraging enough to study a larger cohort.55 Reed et al. conducted IRT of both the forearms in 40 children (19 fractures and 21 sprains) presenting with wrist injuries. Imaging was done in both in neutral, and 45° extended position. All cases showed increased mean temperature on the injured wrist compared to the contralateral side; however, this was significant only for fractures. Therefore, IRT may be helpful in differentiating fractures from a sprain in paediatric wrist injuries.56 Owen et al. used IRT in 30 children presenting with acute limp and found that the median skin temperature at the affected lower limb was higher (greater in fractures than the other pathologies). IRT detected a hotspot in the lower knee of a patient at the initial presentation when x-rays did not show any pathology. After 2 weeks radiographs depicted a toddler's fracture of the right tibia. This highlights the potential benefit of IRT in the early diagnosis of these types of cases.57
4.8. Spine
Adolescent idiopathic scoliosis (AIS) is a three-dimensional deformity of the spine that appears and progresses during school-going children's rapid growth phase.58 Kwok et al. used IRT in 17 scoliotic subjects and compared them with the normal subjects. There was a significant thermal asymmetry between the right and left sides of the trapezius (p = 0.04), latissimus dorsi (p = 0.00), and quadratus lumborum (p = 0.01) only in the scoliotic subjects. Thus, IRT can be incorporated in school scoliosis screening programs; however, further studies are required for validation.59
Dragan et al. found that the maximum difference in the average temperature between the left and right sides did not exceed 0.5 °C in normal individuals. It also did not exceed over 4.3 °C between the upper and bottom parts of the spine. As the degree of spine deformity increases, the temperature difference between sides of the primary curvature increases, and IRT can monitor the treatment course.60 Lubkowska et al. showed that parts of the upper body (chest, abdomen, and back) were warmer by about 4 °C than the lower body parts (thigh, shank) in both scoliotic and normal children no thermal asymmetry existed between the right and left sides normally. In the scoliotic group, thermal asymmetry was present, and a strong correlation of the angle of trunk rotation (ATR) with the chest temperature and the temperature difference was also found. IRT was termed a non-invasive method for scoliosis screening, and the areas mentioned above should be assessed.61
Low back pain is perhaps the most common complaint seen in an orthopaedic outpatient department, which can potentially lead to significant morbidity and decreased productivity. Alfieri et al.
evaluated regions of the right and left paravertebral muscles and L4-L5 ligament and found that temperatures were significantly elevated (p < 0.001) in individuals with low back pain. Pain perception additionally correlated with the temperature at the evaluated site (p = 0.001). IRT seemed to be promising in objectively analyzing low back pain.62 Zaproudina et al. on analysis of the IRT findings at the plantar surface of feet found that the temperature changes correlated with the LBP intensity (p = 0.00) and concluded that IRT imaging can be an objective indicator of the pain and autonomic disturbances in LBP patients.63
Overall, IRT can be utilized as a screening tool for scoliosis. Since it is non-invasive and easy, compliance in children is also a non-issue. It can also be utilized in monitoring the deformity correction. It can provide an objective assessment of the symptoms for low backache and can potentially be used in prognosticating.
4.9. Miscellaneous
4.9.1. Ergonomics
Traditional desktop work can lead to prolonged sitting in abnormal postures causing musculoskeletal fatigue and pain. IRT can be utilized in assessing the thermal patterns in individual back muscles to guide better posture and improve ergonomics. Lasanen et al. conducted IRT imaging, surface electromyography (SEMG) of the back region, and neck pain severity evaluation using neck disability index (NDI) in 14 female participants. Initial recordings were done when the participants worked at their traditional workstations. Second measurements were done after 1-month of usage of furniture, which enabled sitting upright. Mean absolute deviation (MAD) was significantly reduced (p < 0.05) while working in an upright posture during the day. SEMG also demonstrated a significant reduction in muscle activity of left neck extensor, right trapezius, left and right rhomboids, and NDI value was significantly lower with upright posture. The authors concluded that IRT can be potentially used to detect differential muscle activation in different postures and help in better ergonomics.64 Alexandre et al. studied the impact of wearing lead aprons during surgery on body mechanics, which can alter posture, and cause muscle fatigue. 4 health care workers underwent IRT; at rest, after 3 h in surgery without lead aprons, and after wearing aprons for 3 h. Trapezius, pectoralis major, deltoid, paraspinal muscles of the lumbar region, and the hamstrings were analyzed. There was a temperature increase of 0.55–0.95 °C after wearing lead aprons in all the muscle groups (maximum in the lumbar region). IRT proved to help detect and prevent ergonomic injuries.65
4.9.2. Compartment syndrome
It is a serious complication occurring in trauma patients that may lead to permanent disability or death if diagnosed late.66, 67, 68 Even in patients who do not develop compartment syndrome, abnormal pressures can be recorded, reducing the reliability of its diagnosis.69 Katz et al. analyzed the IRT recordings of both the legs of these patients and found that patients who subsequently developed compartment syndrome had a significant difference (p < 0.01) in temperature of proximal and distal surfaces of legs, compared to those without compartment syndrome. In unilateral cases, there was a significant temperature difference (p < 0.01) compared to the normal contralateral leg. They concluded that this difference between the thigh and foot regions (thigh-foot index), detected by IRT, holds promise for the early diagnosis of compartment syndrome in trauma patients.70
4.9.3. Elbow pain
It can be caused due to many reasons, although epicondylitis of lateral and medial sides is the most common. Gabrhel et al. studied 43 patients with elbow pain and found that sensitivity of IRT in the clinical diagnosis of elbow pain was 91% compared to 71% of the sonogram. They concluded that IRT imaging is highly sensitive in cases of elbow epicondylitis and is a potential adjunct to established imaging such as USG,MRI.71
5. Conclusion
IRT has been described to be effective in orthopaedic conditions with specific heat signatures and this can assess the trend of the ongoing inflammatory process as well as response to a particular treatment. Additionally, it can specifically determine the exact loci of the pathology for targeted interventions. In practice, infrared thermography can be utilized in isolation as well as in combination with other parameters, e.g, ESR and C reactive protein in periprosthetic infections, to screen or diagnose a prospective case early, so as to initiate adequate treatment, be it debridement or targeted antibiotic therapy. In other scenarios like assessment of bone union and related complications like non unions, thermography can pick up the periodic trends in heat signatures, which can indicate whether or not a certain case requires early response (surgical or medical) to improve the union chances. As the technology will further improve and the cost benefit aspect improves, we will definitely see an uprise in utilisation of this novel device in the various sub-fields of orthopaedics on a wider scale.
Conflict of interest
The authors declare no conflict of interest and nil funding
Contributor Information
Prasoon Kumar, Email: drprasoonksingh@gmail.com.
Ankit Gaurav, Email: ankitgaurav1994@yahoo.com.
Rajesh Kumar Rajnish, Email: duktiraj@gmail.com.
Siddhartha Sharma, Email: sids82@gmail.com.
Vishal Kumar, Email: drkumarvishal@gmail.com.
Sameer Aggarwal, Email: drsameer35@yahoo.co.in.
Sandeep Patel, Email: sandeepdrpatelortho@gmail.com.
References
- 1.Tattersall G.J. Infrared thermography: a non-invasive window into thermal physiology. Comp Biochem Physiol Mol Integr Physiol. 2016;202:78–98. doi: 10.1016/j.cbpa.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Romanò C.L., Logoluso N., Dell'Oro F., Elia A., Drago L. Telethermographic findings after uncomplicated and septic total knee replacement. Knee. 2012;19(3):193–197. doi: 10.1016/j.knee.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Ring E.F., Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012;33(3):R33–R46. doi: 10.1088/0967-3334/33/3/R33. [DOI] [PubMed] [Google Scholar]
- 4.Rogalski A. History of infrared detectors. Opto-Electronics. 2012;20:279–308. Rev. [Google Scholar]
- 5.Meola C. Infrared thermography in the architectural field. Sci World J. 2013;2013:323948. doi: 10.1155/2013/323948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahiri B.B., Bagavathiappan S., Jayakumar T., Philip J. Medical applications of infrared thermography: a review. Infrared Phys Technol. 2012;55(4):221–235. doi: 10.1016/j.infrared.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uematsu S. Symmetry of skin temperature comparing one side of the body to other. Thermology. 1986;1:4–7. [Google Scholar]
- 8.Fokam D., Lehmann C. Clinical assessment of arthritic knee pain by infrared thermography. J Basic Clin Physiol Pharmacol. 2018 Oct 31;30(3) doi: 10.1515/jbcpp-2017-0218. [DOI] [PubMed] [Google Scholar]
- 9.Denoble A.E., Hall N., Pieper C.F., Kraus V.B. Patellar skin surface temperature by thermography reflects knee osteoarthritis severity. Clin Med Insights Arthritis Musculoskelet Disord. 2010 Jan;3 doi: 10.4137/CMAMD.S5916. CMAMD-S5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spalding S.J., Kwoh C.K., Boudreau R., et al. Three-dimensional and thermal surface imaging produces reliable measures of joint shape and temperature: a potential tool for quantifying arthritis. Arthritis Res Ther. 2008 Feb;10(1):1–9. doi: 10.1186/ar2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones B., Hassan I., Tsuyuki R.T., Dos Santos M.F., Russell A.S., Yacyshyn E. Hot joints: myth or reality? A thermographic joint assessment of inflammatory arthritis patients. Clin Rheumatol. 2018 Sep;37(9):2567–2571. doi: 10.1007/s10067-018-4108-0. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich D., Köhne M. Thermography in persistent postoperative pain after knee surgery. Orthopaedic J Sports Med. 2020 May 28;8(5_suppl 4) 2325967120S00330. [Google Scholar]
- 13.Wu C.L., Yu K.L., Chuang H.Y., Huang M.H., Chen T.W., Chen C.H. The application of infrared thermography in the assessment of patients with coccygodynia before and after manual therapy combined with diathermy. J Manipulative Physiol Therapeut. 2009 May 1;32(4):287–293. doi: 10.1016/j.jmpt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Dallimore S.M., Puli N., Kim D., Kaminski M.R. Infrared dermal thermometry is highly reliable in the assessment of patients with Charcot neuroarthropathy. J Foot Ankle Res. 2020 Dec;13(1) doi: 10.1186/s13047-020-00421-z. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nubé V.L., McGill M., Molyneaux L., Yue D.K. From acute to chronic: monitoring the progress of Charcot's arthropathy. J Am Podiatr Med Assoc. 2002 Jul;92(7):384–389. doi: 10.7547/87507315-92-7-384. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong D.G., Lavery L.A. Monitoring healing of acute Charcot's arthropathy with infrared dermal thermometry. J Rehabil Res Dev. 1997 Jul 1;34(3):317. [PubMed] [Google Scholar]
- 17.Armstrong D.G., Lavery L.A. Monitoring neuropathic ulcer healing with infrared dermal thermometry. J Foot Ankle Surg. 1996 Jul 1;35(4):335–338. doi: 10.1016/s1067-2516(96)80083-4. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong D.G., Holtz-Neiderer K., Wendel C., Mohler M.J., Kimbriel H.R., Lavery L.A. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007 Dec 1;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A., Ray S., Garg M.K., Chowdhury S., Mukhopadhyay S. The role of infrared dermal thermometry in the management of neuropathic diabetic foot ulcers. Diabet Med. 2021 Apr;38(4) doi: 10.1111/dme.14368. [DOI] [PubMed] [Google Scholar]
- 20.Lavery L.A., Higgins K.R., Lanctot D.R., et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007 Jan 1;30(1):14–20. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 21.Lavery L.A., Higgins K.R., Lanctot D.R., et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004 Nov 1;27(11):2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 22.Hazenberg C.E., van Netten J.J., van Baal S.G., Bus S.A. Assessment of signs of foot infection in diabetes patients using photographic foot imaging and infrared thermography. Diabetes Technol Therapeut. 2014 Jun 1;16(6):370–377. doi: 10.1089/dia.2013.0251. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong D.G., Lipsky B.A., Polis A.B., Abramson M.A. Does dermal thermometry predict clinical outcome in diabetic foot infection? Analysis of data from the SIDESTEP∗ trial. Int Wound J. 2006 Dec;3(4):302–307. doi: 10.1111/j.1742-481X.2006.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozic K.J., Kurtz S.M., Lau E., et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010 Jan;468(1):45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvizi J., Tan T.L., Goswami K., et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018 May 1;33(5):1309–1314. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 26.Scheidt S., Rüwald J., Schildberg F.A., et al. A systematic review on the value of infrared thermography in the early detection of periprosthetic joint infections. J Orthoped Trauma Surg. 2020 Aug;158(4):397–405. doi: 10.1055/a-0969-8675. [DOI] [PubMed] [Google Scholar]
- 27.Romanò C.L., D'Anchise R., Calamita M., Manzi G., Romanò D., Sansone V. Value of digital telethermography for the diagnosis of septic knee prosthesis: a prospective cohort study. BMC Muscoskel Disord. 2013 Dec;14(1):1–7. doi: 10.1186/1471-2474-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman M.S. The use of thermography in sympathetically maintained pain. Iowa Orthop J. 1994;14:141. [PMC free article] [PubMed] [Google Scholar]
- 29.Gulevich S.J., Conwell T.D., Lane J., et al. Stress infrared telethermography is useful in the diagnosis of complex regional pain syndrome, type I (formerly reflex sympathetic dystrophy) Clin J Pain. 1997 Mar 1;13(1):50–59. doi: 10.1097/00002508-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Hassan M., Hattery D., Chernomordik V., et al. Vol. 2. IEEE; 2003 Sep 17. Infrared thermographic imaging for the assessment of temperature asymmetries in reflex sympathetic dystrophy; pp. 1102–1105. (InProceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439)). [Google Scholar]
- 31.Papež B.J., Palfy M., Mertik M., Turk Z. Infrared thermography based on artificial intelligence as a screening method for carpal tunnel syndrome diagnosis. J Int Med Res. 2009 May;37(3):779–790. doi: 10.1177/147323000903700321. [DOI] [PubMed] [Google Scholar]
- 32.Živcák J., Madarasz L., Hudak R. In2011 IEEE 12th International Symposium on Computational Intelligence and Informatics (CINTI) IEEE; 2011 Nov 21. Application of medical thermography in the diagnostics of Carpal tunnel syndrome; pp. 535–539. [Google Scholar]
- 33.Baic A., Kasprzyk T., Rżany M., et al. Can we use thermal imaging to evaluate the effects of carpal tunnel syndrome surgical decompression? Medicine. 2017 Sep;96(39) doi: 10.1097/MD.0000000000007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxel X., Bodnar J.L., Stubbe L. Detection of carpal tunnel syndrome by infrared thermography. Mechanics & Industry. 2014;15(5):363–370. [Google Scholar]
- 35.Bargiel P., Czapla N., Prowans P., et al. Thermography in the diagnosis of carpal tunnel syndrome. Open Med. 2021 Jan 1;16(1):175–182. doi: 10.1515/med-2021-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekstrand J., Hägglund M., Waldén M. Epidemiology of muscle injuries in professional football (soccer) Am J Sports Med. 2011;39:1226–1232. doi: 10.1177/0363546510395879. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Cuevas I., Marins J.C.B., Lastras J.A., et al. Classification of factors influencing the use of infrared thermography in humans: a review. Infrared Phys Technol. 2015;71:28–55. doi: 10.1016/j.infrared.2015.02.007. [DOI] [Google Scholar]
- 38.Gómez-Carmona P., Fernández-Cuevas I., Sillero-Quintana M., Arnaiz-Lastras J., Navandar A. Infrared thermography protocol on reducing the incidence of soccer injuries. J Sport Rehabil. 2020 Mar 17;29(8):1222–1227. doi: 10.1123/jsr.2019-0056. [DOI] [PubMed] [Google Scholar]
- 39.Côrte A.C., Pedrinelli A., Marttos A., Souza I.F., Grava J., Hernandez A.J. Infrared thermography study as a complementary method of screening and prevention of muscle injuries: pilot study. BMJ Open Sports Exerc Med. 2019 Jan 1;5(1) doi: 10.1136/bmjsem-2018-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrofsky J., et al. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Therapeut. 2011;13:645–655. doi: 10.1089/dia.2011.0002. [DOI] [PubMed] [Google Scholar]
- 41.MacIntyre D.L., Reid W.D., McKenzie D.C. Delayed muscle soreness: the inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Al-Nakhli H.H., Petrofsky J.S., Laymon M.S., Berk L.S. The use of thermal infra-red imaging to detect delayed onset muscle soreness. JoVE: JoVE. 2012;(59) doi: 10.3791/3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swenson D.M., Yard E.E., Fields S.K., Comstock R.D. Patterns of recurrent injuries among US high school athletes, 2005–2008. Am J Sports Med. 2009;37(8):1586–1593. doi: 10.1177/0363546509332500. [DOI] [PubMed] [Google Scholar]
- 44.Gerber J.P., Williams G.N., Scoville C.R., Arciero R.A., Taylor D.C. Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot Ankle Int. 1998;19(10):653–660. doi: 10.1177/107110079801901002. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira J., Vardasca R., Pimenta M., Gabriel J., Torres J. Use of infrared thermography for the diagnosis and grading of sprained ankle injuries. Infrared Phys Technol. 2016 May 1;76:530–541. [Google Scholar]
- 46.Ioannou S. Functional infrared thermal imaging: a contemporary tool in Soft tissue Screening. Sci Rep. 2020 Jun 9;10(1):1–9. doi: 10.1038/s41598-020-66397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangine R.E., Siqueland K.A., Noyes F.R. The use of thermography for the diagnosis and management of patellar tendinitis. J Orthop Sports Phys Ther. 1987 Oct;9(4):132–140. doi: 10.2519/jospt.1987.9.4.132. [DOI] [PubMed] [Google Scholar]
- 48.Yang H., Park H., Lim C., Park S., Lee K. Infrared thermal imaging in patients with medial collateral ligament injury of the knee-A retrospective study. J Pharmacopuncture. 2014 Dec;17(4):50. doi: 10.3831/KPI.2014.17.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rennie L., Court-Brown C.M., Mok J.Y., BeattieTF The epidemiology of fractures in children. Injury. 2007;38(8):913–922. doi: 10.1016/j.injury.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Oskam J., Kingma J., Klasen H.J. Fracture of the distal forearm: epidemiological developments in the period 1971–1995. Injury. 1998;29(5):353–355. doi: 10.1016/s0020-1383(97)00212-x. [DOI] [PubMed] [Google Scholar]
- 51.Ćurković S., Antabak A., Halužan D., Luetić T., Prlić I., Šiško J. Medical thermography (digital infrared thermal imaging–DITI) in paediatric forearm fractures–A pilot study. Injury. 2015 Nov 1;46:S36–S39. doi: 10.1016/j.injury.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 52.Saxena A.K., Willital G.H. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr. 2008 Jul 1;167(7):757–764. doi: 10.1007/s00431-007-0583-z. [DOI] [PubMed] [Google Scholar]
- 53.Seuser A., Kurnik K., Mahlein A.K. Infrared thermography as a non-invasive tool to explore differences in the musculoskeletal system of children with hemophilia compared to an age-matched healthy group. Sensors. 2018 Feb;18(2):518. doi: 10.3390/s18020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Salis A.F., Saatchi R., Dimitri P. Evaluation of high resolution thermal imaging to determine the effect of vertebral fractures on associated skin surface temperature in children with osteogenesis imperfecta. Med Biol Eng Comput. 2018 Sep;56(9):1633–1643. doi: 10.1007/s11517-018-1806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva C.T., Naveed N., Bokhari S., et al. Early assessment of the efficacy of digital infrared thermal imaging in pediatric extremity trauma. Emerg Radiol. 2012 Jun;19(3):203–209. doi: 10.1007/s10140-012-1027-2. [DOI] [PubMed] [Google Scholar]
- 56.Reed C., Saatchi R., Burke D., Ramlakhan S. Infrared thermal imaging as a screening tool for paediatric wrist fractures. Med Biol Eng Comput. 2020 Jul;58:1549–1563. doi: 10.1007/s11517-020-02167-z. [DOI] [PubMed] [Google Scholar]
- 57.Owen R., Ramlakhan S., Saatchi R., Burke D. Development of a high-resolution infrared thermographic imaging method as a diagnostic tool for acute undifferentiated limp in young children. Med Biol Eng Comput. 2018 Jun;56(6):1115–1125. doi: 10.1007/s11517-017-1749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigo M.D., Villagrasa M., Gallo D. A specific scoliosis classification correlating with brace treatment: description and reliability. Scoliosis. 2010 Dec 1;5(1):1. doi: 10.1186/1748-7161-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwok G., Yip J., Yick K.L., et al. Postural screening for adolescent idiopathic scoliosis with infrared thermography. Sci Rep. 2017 Oct 31;7(1):1–8. doi: 10.1038/s41598-017-14556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dragan S., Konik H., Prastowski A., Orzechowski W. Application of thermography in diagnostics and prognostication of scoliosis treatment. Acta Bioeng Biomech. 2002;4(1):63–70. [Google Scholar]
- 61.Lubkowska A., Gajewska E. Temperature distribution of selected body surfaces in scoliosis based on static infrared thermography. Int J Environ Res Publ Health. 2020 Jan;17(23):8913. doi: 10.3390/ijerph17238913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alfieri F.M., Lima A.R., Battistella L.R. Superficial temperature and pain tolerance in patients with chronic low back pain. J Bodyw Mov Ther. 2019 Jul 1;23(3):583–587. doi: 10.1016/j.jbmt.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Zaproudina N., Ming Z., Hänninen O.O. Plantar infrared thermography measurements and low backpain intensity. J Manipulative Physiol Therapeut. 2006 Mar 1;29(3):219–223. doi: 10.1016/j.jmpt.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Lasanen R., Malo M.K., Airaksinen O., Karhu J., Töyräs J., Julkunen P. Infrared thermography reveals effect of working posture on skin temperature in office workers. Int J Occup Saf Ergon. 2018 Jul 3;24(3):457–463. doi: 10.1080/10803548.2017.1336299. [DOI] [PubMed] [Google Scholar]
- 65.Alexandre D., Prieto M., Beaumont F., Taiar R., Polidori G. Wearing lead aprons in surgical operating rooms: ergonomic injuries evidenced by infrared thermography. J Surg Res. 2017 Mar 1;209:227–233. doi: 10.1016/j.jss.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Frink M., Klaus A.K., Kuther G., et al. Long term results of compartment syndrome of the lower limb in polytraumatised patients. Injury. 2007;38:607–613. doi: 10.1016/j.injury.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Heemskerk J., Kitslaar P. Acute compartment syndrome of the lower leg: retrospective study on prevalence, technique, and outcome of fasciotomies. World J Surg. 2003;27:744–747. doi: 10.1007/s00268-003-6691-7. [DOI] [PubMed] [Google Scholar]
- 68.Olson S.A., Glasgow R.R. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13:436–444. doi: 10.5435/00124635-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Prayson M.J., Chen J.L., Hampers D., et al. Base- line compartment pressure measurements in isolated lower extremity fractures without clinical compartment syndrome. J Trauma. 2006;605:1037–1040. doi: 10.1097/01.ta.0000215444.05928.2f. [DOI] [PubMed] [Google Scholar]
- 70.Katz L.M., Nauriyal V., Nagaraj S., et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008 Jun 1;36(6):1756–1761. doi: 10.1097/CCM.0b013e318174d800. [DOI] [PubMed] [Google Scholar]
- 71.Gabrhel J., Popracová Z., Tauchmannova H., Ammer K. The role of infrared thermal imaging and sonography in the assessment of patients with a painful elbow. Thermol Int. 2017;27(2):58–66. [Google Scholar]