Abstract
This article describes the initial study on the simultaneous determination of multiclass antibiotic residues in imported and local frozen poultry specimens, including turkey gizzard and muscle tissues, and chicken muscle tissues, commonly consumed in Ogun State, Nigeria. Minced tissues were treated with phosphate buffer adjusted to pH 7 that was cleaned using C18 SPE-column (Supelclean™) cartridge. For the determination of six antibiotic residues including fluoroquinolones, sulfonamides, and macrolides, a solid-phase extraction method was used, followed by extract analysis using high-performance liquid chromatography–diode array detection (HPLC–DAD). The coefficient of determination (R2) for the external standards for all the analytes ranged between 0.963 and 0.999. The limit of detection (LOD) and quantification (LOQ) ranged between 5.37 – 55.4 μg/kg, and 17.9–185 μg/kg, respectively. Enrofloxacin, sulfadimethoxine, sulfamerazine, and tylosin showed high concentration levels in the frozen poultry beyond acceptable maximum residue limits (MRLs). The six drugs considered in this study were present at higher concentrations in domestic chicken tissues than the permissible level. This suggests that farmers do not observe the cessation period before poultry birds previously treated with antibiotics are sold to consumers thus exposing them to potentially hazardous antibiotic residues.
Keywords: Antibiotic residues, Food contamination, Solid-phase extraction, High-performance liquid chromatography, Poultry tissues
Antibiotic residues; Food contamination; Solid-phase extraction; High-performance liquid chromatography; Poultry tissues.
1. Introduction
Nigeria consumes a substantial amount of antibiotics through poultry production, and it is one of the five countries with the highest projected increase in antimicrobial drugs and growth hormones use (163%) by 2030 [1, 2, 3]. Nigeria is Africa's leading egg producer and fourth largest poultry meat producer, with 454 billion tons of poultry meat produced annually and 180 million laying birds producing 3.8 million eggs each year [4, 5]. Ogun State, the present study area, has the highest proportion of laying birds in Nigeria [6], and it is the main point of entry for imported poultry meat into the country. Nigeria has a developing economy with an estimated 212 million people, a poultry sector worth more than $3.3 billion, and an annual chicken consumption of over 1.5 million metric tons. Of this total, only 30% is produced locally, with the remaining imported through various informal channels. Besides the negative impacts on the poultry sector and the economy, these imported poultry products are not subjected to any quality and safety screenings for antibiotic residues and other chemical contaminants at the points of entry. Similarly, poultry products produced by farmers in the country are hardly screened for antibiotic residues and other harmful toxins by oversight federal and state agencies. Thus, monitoring antibiotic residues in food products becomes crucially important to guarantee food safety and to promote regulatory oversight over imported and locally produced food supplies. Nigeria, unlike many other countries, currently lacks surveillance and monitoring programme and report for all antibiotic classes that are approved or prohibited for usage, as well as aggressive national action plan to address the associated violative health effects. However, efforts have recently been made under the National Action Plan to ensure responsible administration and application of antibiotics in animal production [7]. Meanwhile, development partners in Ghana, Tanzania, Uganda and Zambia are tackling antimicrobial resistance through the Commonwealth Partnerships for Antimicrobial Stewardship (AMS) with increased funding and political activity [8, 9].
Fluoroquinolones are a class of synthetic antibiotics that are primarily used to treat and prevent bacterial infections [3, 10, 11]. Given the safety concerns posed by these antibiotics, many of them are prohibited as veterinary drugs for livestock production by several countries including USA, Australia, China, EU member countries, UK, and Canada [12, 13, 14]. However, to safeguard the public health and minimize the potential exposure to antibiotic residues from food products, maximum residue limits (MRLs) have been established by the USA Food and Drug Administration [15] and European Union (Commission Decision 37/2010/CE [16]. Statutory surveillance programs operated by different agencies to monitor and report chemical residues in foods have also been established by some developed countries [17].
Monitoring veterinary drug residues, especially antibiotics in foods meant for human consumption is one method of preventing potential harm to consumers, particularly if large doses are ingested. Antibiotic residues in foods of animal origin have been reported to cause allergic reactions, particularly in hypersensitive individuals, and may compromise their immune systems [18, 19]. Furthermore, the presence of these residues at sub-therapeutic levels in foodstuffs for longer periods has led to the appearance of bacterial strains that are resistant to drugs used in human medicine [20]. These problems which arise as a result of poor management practices, incorrect use of veterinary drugs such as overdose, inappropriate use times and failure to follow label instructions, and non-observance of withdrawal periods prior to slaughtering for meat consumption or laying of eggs, may leave residues of drugs in tissues and eggs at concentrations that may be harmful to human health [21, 22, 23, 24]. Food safety is currently under threat as a result of dynamic risks prevalent with the combination of chemical contaminants. As a result, it is imperative to assess target and non-target antibiotic residues in food products to ensure safety [25].
Antibiotic residues have been reported in poultry meat in the area under study, but no comprehensive data on the occurrence, distribution patterns and health implications of widely used antibiotics have been documented. Data of enrofloxacin, sulfadimethoxine, sulfamerazine, sulfamethoxazole, sulfamoxole, and tylosin used in this paper are available in a data collections article and in line with the format of such data articles no interpretation, extensive discussion or conclusion were included [26]. The present manuscript is therefore an original interpretation of those data. According to Oyedeji et al. [26], the data highlighted a wide range of potential safety disclosures as well as human health risk assessments into the antibiotic concentrations and exposures in the examined food products. Given the life-threatening effects associated with antibiotic residues in foodstuffs, the aim of this study was to provide a comprehensive survey and monitoring of antibiotic residues commonly used in poultry production, using solid-phase extraction for the pre-concentration and clean-up step prior to determination by high-performance liquid chromatography coupled to a diode array detector. The veterinary drugs considered in the present study were chosen due to their low cost and known use as growth promoters in poultry production.
We report the results of the first surveillance investigation aimed at detecting and quantifying multiclass veterinary antibiotic residues in imported and locally produced frozen poultry meat widely consumed in Ogun State, Nigeria. This work is expected to provide a framework for the simultaneous analysis of fluoroquinolones, sulfonamides, and macrolides in poultry tissue samples, allowing for the establishment of appropriate drug residue regulatory limits as well as the assessment of potential human health risks associated with long-term dietary consumption of antibiotics through poultry products.
2. Materials and methods
2.1. Real samples
Refrigerated meat products, including muscle and gizzard tissues of chicken and turkey, and table-size live chickens (broilers and cockerels) and laying birds, were purchased from local sales outlets and commercial farming stores in specific areas across Ogun State, Nigeria. A total of 150 frozen muscle and gizzard tissues, and chicken muscle tissue cuts upon purchase from the different outlets were kept individually in iced plastic bags to avoid cross-contamination. The samples were transported to the laboratory within 4 h of purchase, and stored in the freezer at 0 °C prior to extraction. One hundred and fifty cockerels, broilers and laying chickens, aged 3–8 months and with an average weight of 3.6 kg were sampled. The chickens were slaughtered, de-feathered and cut into parts. Prior to extraction, the diced pieces were packed in aluminum foil and stored in a freezer after being thoroughly cleaned with ultrapure water.
2.2. Antibiotic standards and reagents
Antibiotic standards (enrofloxacin, sulfadimethoxine, sulfamerazine, sulfamoxole, sulfamethoxazole, and tylosin), methanol (HPLC grade ≥99.9%), ammonium hydroxide, and phosphate buffer were purchased from Fisher Scientific, Leicestershire, United Kingdom. Acetonitrile (Fisher Scientific, Leicestershire, United Kingdom), and formic acid (Fisher Scientific, Leicestershire, United Kingdom) were used. An Integral 10 Elix Milli-Q system with an LC (Biopak) polisher (Massachusetts, USA) was used to produce the deionized water. Standard solutions containing 500 μg/mL of each antibiotic were prepared by precisely measuring and diluting 2.5 mg stock in 5 mL methanol:water (50:50 v/v) and later stored in a freezer maintained at 4 °C. From the stock, working solutions were prepared. Further information on each standard is presented in Figure 1.
Figure 1.
Target chemical compounds (antibiotics) considered in this study.
2.2.1. Equipment
Waring laboratory blender (Thomas Scientific, Swedesboro, USA), Vortex mixer-VM18 (Schiltern Scientific, Beds, UK), Centrifuge - ROTOFIX 32A Benchtop centrifuge (Thomas Scientific, Swedesboro, USA), C18 SPE-column (Supelclean™) (Sigma-Aldrich (St. Louis, MO, USA), Nitrogen evaporator - 6 Position N-Evap (Thomas Scientific, Swedesboro, USA), Acrodisc syringe filters (GHP membrane, diam. 25 mm, pore size 0.45 μm), (Sigma-Aldrich, St. Louis, MO, USA), HPLC system (Agilent 1200 series, Software – Agilent ChemStation Version B.040.01) SP1 (Agilent Technologies, Germany), and Chromatographic column - XTerra MS C18, 125Å (4.6 × 100 mm, 3.5 μm) from Waters Corporation, Milford Massachusetts, USA.
2.3. Solid-phase extraction (SPE) and clean-up
The method of extraction, screening, and analytical procedures for antibiotic residues in fresh and frozen poultry products have been previously reported [26]. The tissues were carefully excised from the poultry cuts and blended using a laboratory blender before drug extraction. A 2 g sample of homogenized meat was taken for antibiotic residues’ extraction using the modified method of Shunli et al. [27]. The homogenate was transferred to a 50 mL stainless centrifuge tube that had been previously washed. 10 mL of phosphate buffer solution (0.01 M adjusted to pH 7.0) was added and allowed to remain at room temperature for 15 min before vortex mixing for about 20 s and centrifuging for 5 min at 3500 rpm. The supernatant was transferred to a new flask, and the extraction procedure was performed twice more. The aggregated extracts were subsequently passed through a C18 SPE column preconditioned with 2 mL each of methanol and deionized water. The aliquot was then rinsed with a 3 mL mixture of water and methanol (4:1, v/v), and the constituents were eluted with 2 mL of 10% ammonium hydroxide:methanol (1:19, v/v). The collected filtrates were dried with N2 and heated to 40 °C before being reconstituted in 1 mL of phosphate buffer and filtered through 0.45 μm syringe filters prior to injecting into the HPLC. To give sufficient identifying information, analytes were monitored at detection wavelengths of 275, 270, 257, and 245 nm using a diode array detector (DAD). Furthermore, to optimize the HPLC response, sample extracts were spiked with 50 ng/mL standards mix and ran using the LC system.
2.3.1. Chromatographic conditions
The mobile phase consisted of ultrapure water (A) and acetonitrile (B) prepared by adding 1 mL of formic acid solution into 1 L of A and B that were mixed using the pump in gradient mode as follows 5% B (0 min), 5–30% B (6 min) and 30–70% B (12 min). The flow rate was 1.2 mL/min, injection volume of 10 μL and a column temperature of 40 °C.
2.3.2. Analyte quantification and figures of merit
The calibration curves for antibiotic standards were produced by plotting the peak area versus the retention time. The average concentration values were calculated after triplicate determinations. Each standard was used to quantify the analyte concentration in spiked samples, and the calibration curves were produced using an average concentration range of 0–1000 ng/mL. The limit of detection (LOD) and limit of quantification (LOQ) for each standard were established using the calibration curves and corresponding linear regression equation, considering that the HPLC response y correlates linearly with the concentration of the standard using the model (Eqn 1):
| y = bx + a | (Eqn. 1) |
The sensitivity of b, as well as LOD and LOQ, can be expressed using the equation. Hence, the following equations (Eqs. (2) and (3)) can be used to mathematically express the LOD and LOQ, respectively:
| (Eqn. 2) |
| (Eqn. 3) |
where, sa = the standard deviation of instrument response, which is calculated by the standard deviation of either the y-intercepts of the regression lines or the y residuals, and b = the calibration curve's slope. In instances where sample concentrations were above the upper limit of the calibration curve, samples were diluted and re-analyzed.
2.3.3. Accuracy and precision
The accuracy of extraction was assessed by means of recovery tests performed by adding known concentration of reference standards to free chicken samples without history of antibiotic medication. Samples were spiked at three different levels (25, 50 and 100 μg/kg) considering the MRL for the different antibiotics, and in triplicate. The spiked samples were left in the dark for 12 h after agitation in a swirling water bath at 10 °C for 30 min. The percentage recovery (%R) of analytes was calculated as expressed in Eq. (4) below:
| (Eqn. 4) |
where CF is the concentration detected in the spiked sample, CU is the concentration detected in the sample before the spiking, and CA is the truly added concentration. The percent bias was determined by comparing the results of the analyses of the spiked samples. Reproducibility of analytical results was assessed by re-running a randomly chosen sample after every 10 analyses.
2.4. Ethics approval
This study was approved by the Ethics Review Committee of the University of South Africa School of Science under Ethics Number 2017/SSR-ERC/012 & 2017/SSR-EC/010.
3. Results
3.1. Instrument calibration results for the six antibiotic standards
The model (Table 1) shows the limit of detection (LOD) and quantitation (LOQ) for the various standards ranged from 5.37 – 55.4 and 17.9–185 μg/kg, respectively suggesting the suitability of the model for the detection and quantification of the drugs since the LOQ values are below the maximum allowed residue limits for the drugs in poultry meat.
Table 1.
Calibration results for six antibiotic standards.
| Antibiotics | Linear equation | R2 | LOD | LOQ | MRL (μg/kg) | y-Absolute | %y-intercept |
|---|---|---|---|---|---|---|---|
| SUM | y = 0.0200x + 0.8996 | 0.999 | 9.23 | 30.8 | 100 | 0.857 ± 0.062 | 99.9 |
| SMZ | y = 0.0019x + 0.4875 | 0.996 | 55.4 | 185 | 100 | 0.460 ± 0.035 | 99.9 |
| SDX | y = 0.0244x + 0.1471 | 0.999 | 5.37 | 17.9 | 100 | 0.145 ± 0.044 | 100 |
| ENR | y = 0.0178x + 0.4708 | 0.999 | 5.78 | 19.3 | 100 | 0.467 ± 0.043 | 99.9 |
| TYL | y = 0.0039x + 0.1864 | 0.999 | 8.39 | 27.9 | 200 | 0.183 ± 0.011 | 100 |
| SMX | y = 0.0183x + 0.5757 | 0.999 | 8.46 | 28.2 | 100 | 0.613 ± 0.052 | 99.9 |
SUM – Sulfamoxole, SMZ – Sulfamerazine, SDX - Sulfadimethoxine, ENR – Enrofloxacin, TYL - Tylosin, SMX – Sulfamethoxazole.
3.2. Order of elution of standard antibiotics
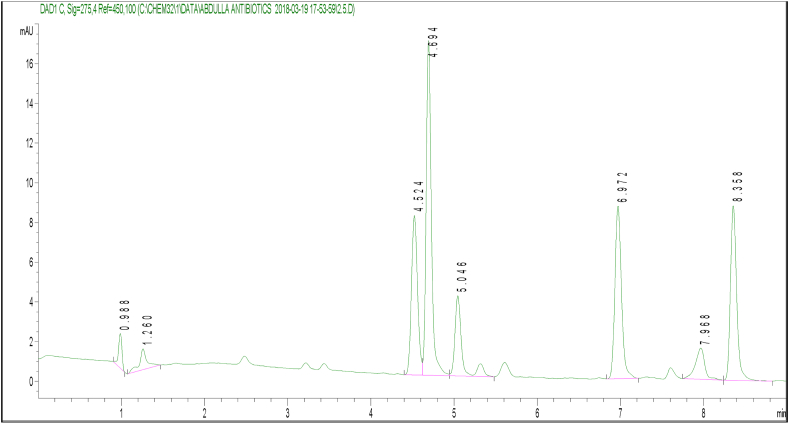
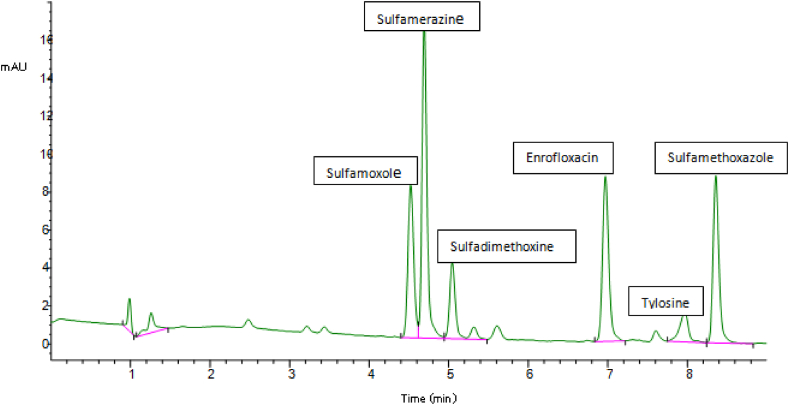
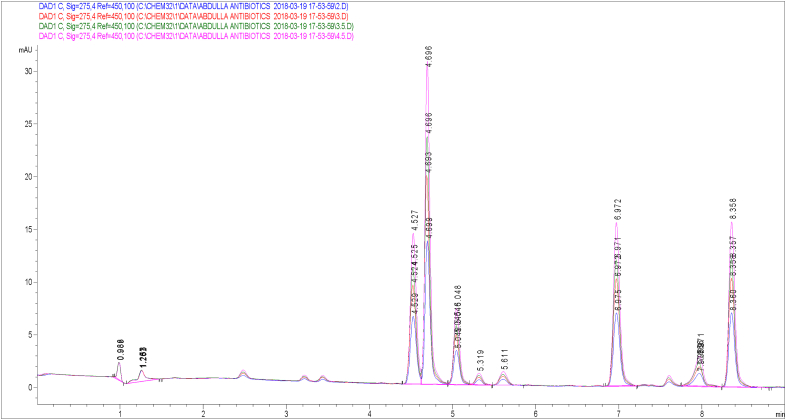
After optimizing the chromatographic conditions, the target antibiotics were eluted individually from the column and the retention time was identified. Following that, a mixed standard of the various antibiotics was prepared with a concentration range of 0–500 ng/mL. The 6 targeted antibiotics eluted according to their retention times were sulfamoxole (SUX), sulfamerazine (SMR), sulfadimethoxine (SDM), enrofloxacin (ENR), tylosin (TYL), and sulfamethoxazole (SMZ) (Figures 2 and 3). To derive the linear equations presented in Table 1, a 15-point calibration curve was produced using the standard's retention time and the integrated peak area of the chromatograms. Sample extracts were spiked with 50 ng/mL standard mix to improve the signals of the analytes, and highlight strong peaks. The target antibiotics were determined using the peak areas, while the spiked values were thereafter deducted from corresponding concentration values. Figure 4 is an overlay of extract chromatograms with those of the standards at one of the wavelengths. The elution order for the different antibiotics beside their polarity is strictly dependent on the column type and other chromatographic conditions including solvent combination and gradient [28, 29]. The order of elution observed in the present study was, however, consistent with the work of Sajjid et al. [30] that used a similar column, though with differences in retention time that were attributable to the other chromatographic conditions.
Figure 2.
Elution profile of the six antibiotic residues with retention time (RT).
Figure 3.
Elution profile of the six antibiotic residues.
Figure 4.
An overlay of extract chromatograms with those of the standards.
3.3. Accuracy of the extraction of antibiotics
The results for the accuracy of the extraction method for the different analytes are shown in Table 2. The percent mean recovery for all the antibiotics was greater than 80%.
Table 2.
Recovery data for antibiotic standards added to sample blanks.
| Standard | Added amount (μg/kg) | Recoverya (%) | RSD (%) | Biasb (%) |
|---|---|---|---|---|
| SUM | 25 | 101.50 | 0.89 | 0.01 |
| 50 | 102.23 | 0.50 | 0.02 | |
| 100 | 88.74 | 0.65 | -0.11 | |
| SMZ | 25 | 81.52 | 0.75 | -0.18 |
| 50 | 101.03 | 0.16 | 0.01 | |
| 100 | 96.93 | 0.92 | -0.03 | |
| SDX | 25 | 101.34 | 0.59 | 0.01 |
| 50 | 82.52 | 0.56 | -0.17 | |
| 100 | 80.71 | 0.64 | -0.19 | |
| ENR | 25 | 101.25 | 0.59 | 0.01 |
| 50 | 80.61 | 0.72 | -0.19 | |
| 100 | 81.80 | 0.73 | -0.18 | |
| TYL | 25 | 99.45 | 0.36 | -0.01 |
| 50 | 89.52 | 0.44 | -0.10 | |
| 100 | 86.77 | 0.61 | -0.13 | |
| SMX | 25 | 86.63 | 0.37 | -0.13 |
| 50 | 82.88 | 0.89 | -0.17 | |
| 100 | 97.16 | 0.98 | -0.03 |
Mean of triplicate determinations.
Bias = (measured concentration – nominal concentration/nominal concentration x 100).
3.4. Distribution pattern of antibiotic residues in imported frozen poultry products
The distribution of the six antibiotic residues in different frozen poultry tissues is as shown in Table 3. The highest enrofloxacin concentration was found in frozen turkey gizzard, with a mean concentration of 66.0 ± 34.4 μg/kg and a detection frequency of 62.5 percent. Enrofloxacin, however, showed significantly higher detection frequency even at lower concentrations. The frozen chicken had a higher mean concentration of enrofloxacin than frozen turkey muscle with detection frequencies of 50.0 and 30.7%, respectively. The mean concentration of enrofloxacin in the various tissues was less than the antibiotic's acceptable maximum residue limit (MRL) of 100 μg/kg [17]. Furthermore, the maximum concentration of enrofloxacin detected in frozen turkey gizzard was 190 μg/kg, which was higher than the MRL. Sulfadimethoxine was appreciably present in frozen turkey muscle with a mean concentration of 6590 ± 530 μg/kg, and it was in 23.1% of the samples that had a maximum concentration of 1006 μg/kg, and were above the 100 μg/kg MRL in all the tissues that were analyzed. The frozen chicken muscle tissue yielded 1650 ± 0.0 μg/kg mean concentration of sulfadimethoxine, which exceeded the MRL; this concentration was determined in only one sample representing 2.0% of the entire samples analyzed. Sulfadimethoxine, on the other hand, was not detected in any gizzard tissues of frozen turkey samples investigated.
Table 3.
Distribution of antibiotic residues (μg/kg) in imported frozen turkey and chicken cuts (n = 50).
| Antibiotics residue | Turkey |
Chicken Muscle |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle |
Gizzard |
||||||||
| Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | |
| ENR | 30.0 ± 16.3a∗ | 30.8 | 40.0 | 66.0 ± 34.4a | 62.5 | 190 | 36.0 ± 10.3a | 50.0 | 60.0 |
| SDX | 6590 ± 5300a | 23.1 | 9580 | 0.00 | 0.00 | 0.00 | 1640 ± 0.00b | 2.00 | 0.00 |
| SMZ | 91.8 ± 69.9a | 69.2 | 180 | 253 ± 152a | 75.0 | 951 | 185 ± 114a | 90.9 | 1172 |
| SMX | 37.0 ± 14.1a | 30.8 | 32.8 | 20.6 ± 10.9a | 37.5 | 32.8 | 20.6 ± 0.00a | 18.2 | 0.00 |
| SUM | 39.2 ± 26.8a | 38.5 | 67.4 | 3.28 ± 0.00a | 12.5 | 0.00 | 17.3 ± 8.43a | 36.4 | 33.7 |
| TYL | 6650 ± 1010a | 46.2 | 20300 | 32.6 ± 0.00a | 12.5 | 0.00 | 426 ± 292a | 27.3 | 932 |
ENR – Enrofloxacin, SDX - Sulfadimethoxine, SMZ – Sulfamerazine, SMX – Sulfamethoxazole, SUM – Sulfamoxole, TYL – Tylosin.
Means across the row with same superscript are not different (p < 0.05).
However, sulfamerazine was detected in all the tissue samples with a detection frequency more than 65.0% and variable mean concentrations. The frozen turkey gizzard samples recorded the highest mean concentration of sulfamerazine, and it was detected in 75.0% of the samples, with the maximum concentration being 956 μg/kg. Moreover, sulfamerazine was found in high concentrations in frozen chicken muscle tissues, with a mean concentration of 185 ± 114 μg/kg. The drug (sulfamerazine) was in 90.9% of the frozen chicken muscle with a maximum concentration of 1180 μg/kg. Frozen chicken muscle had the least average amount of sulfamerazine (91.8 ± 69.9 μg/kg), this was present in 69.2% of the samples. The maximum sulfamerazine concentration determined in the frozen turkey muscle was 209 μg/kg.
The average concentrations of sulfamethoxazole in frozen turkey muscle, gizzard and chicken muscle were 37.0 ± 14.1, 20.6 ± 10.9 and 20.6 ± 0.0 μg/kg, respectively. Their percentage occurrences in the three matrices were 30.8, 37.5 and 18.2% in that order while the maximum concentrations in the matrices were 53.4, 42.5 and 20.6 μg/kg in frozen turkey muscle, gizzard and chicken muscle, respectively. Both the mean and maximum concentrations were below the 100 μg/kg MRL.
On the other hand, sulfamoxole occurred in all the matrices at 38.5, 36.4 and 12.5% in decreasing order for frozen turkey muscle, chicken muscle and turkey gizzard. The maximum concentration of sulfamoxole in the frozen turkey muscle, chicken muscle and turkey gizzard were 70.7, 36.9 and 3.28 μg/kg, respectively. Tylosin had an average concentration of 6650 ± 1010, 426 ± 292 and 32.6 ± 0.0 μg/kg in 45.2, 27.3 and 12.5% of frozen turkey gizzard, chicken muscle and turkey gizzard, respectively. The maximum concentrations for this drug in the three matrices were 20300, 1010 and 32.6 μg/kg, respectively. Frozen turkey had the highest average concentration of tylosin among the matrices, and it occurred in the highest number of samples compared to others. The mean concentration of tylosin in frozen turkey muscle was about 26 times the level in the chicken muscle and 75 times the level in the turkey gizzard. The mean and maximum concentration of tylosin in frozen turkey and chicken muscle were above the 200 μg/kg MRL.
The mean concentration levels of the drugs in the different matrices were, however, not significantly different (p < 0.05) but beyond the allowed maximum residue limit (MRL) except in the instances of enrofloxacin, sulfamethoxazole and sulfamoxole in the various tissues. Tylosin and sulfadimethoxine were the most common antibiotics in frozen turkey muscle. Sulfamerazine had a small mean concentration, but occurred in more samples than the other drugs, and it also had a high maximum concentration. Sulfamoxole, sulfamethoxazole and enrofloxacin were also present in a few samples of frozen turkey muscle at low mean and maximum levels. Frozen turkey gizzard had sulfamerazine in large amounts, and in a large number of samples with a high maximum concentration. Frozen chicken muscle had high mean concentrations of sulfamerazine, sulfadimethoxine, and tylosin although at a lower percentage of occurrences in the samples, except sulfamerazine that was present in more than 90.0% of the samples. The maximum concentrations for these common drugs in the frozen chicken muscle were also high. Enrofloxacin, sulfamoxole and sulfamethoxazole had low mean and maximum concentrations; and poorly distributed, except enrofloxacin.
3.5. Distribution of antibiotic residues in laying birds
Antibiotic residues in the different tissues of laying birds are as shown in Table 4. Enrofloxacin had the highest mean value of 371 ± 139 μg/kg and above the MRL, and this occurred in the liver. About 61.5% of the liver samples analyzed contained enrofloxacin with a maximum concentration of 1170 μg/kg. Enrofloxacin had a mean concentration of 287 ± 29.0 μg/kg in the muscle, even though only 37.5% of the samples contained the drug. Among the three matrices, gizzard had the least mean enrofloxacin concentration and the least percentage of samples (33.3%) containing the drug. The mean enrofloxacin in the gizzard was 56.7 ± 17.5 μg/kg.
Table 4.
Distribution of antibiotic residues (μg/kg) in laying birds tissues (n = 50).
| Antibiotics residue | Layers Muscle |
Layers Liver |
Layers Gizzard |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | |
| ENR | 287 ± 29.0a | 37.5 | 100 | 371 ± 139b | 61.5 | 1140 | 56.7 ± 17.5c | 33.3 | 100 |
| SDX | 1120 ± 526a | 25.0 | 1050 | 3750 ± 2180a | 46.2 | 14100 | 2690 ± 1190a | 38.89 | 8840 |
| SMZ | 234 ± 181a | 37.5 | 566 | 277 ± 109a | 30.8 | 508 | 744 ± 364a | 61.15 | 3370 |
| SMX | 256 ± 234a | 25.0 | 469 | 2010 ± 1440a | 38.5 | 7680 | 538 ± 363a | 16.67 | 1200 |
| SUM | 268 ± 253a | 25.0 | 708 | 2250 ± 692b | 38.5 | 3720 | 183 ± 135c | 23.5 | 573 |
| TYL | 3760 ± 2320a | 50.0 | 9890 | 4492 ± 1380a | 30.8 | 7440 | 1700 ± 500a | 50.0 | 421 |
ENR – Enrofloxacin, SDX - Sulfadimethoxine, SMZ – Sulfamerazine, SMX – Sulfamethoxazole, SUM – Sulfamoxole, TYL – Tylosin.
Sulfadimethoxine, with 46.2% occurrence had the highest mean concentration of 3750 ± 2180 μg/kg and a maximum concentration value of 14400 μg/kg in the muscle. The gizzard had a mean concentration of 2690 ± 1190 μg/kg for sulfadimethoxine. 38.9% of the gizzard samples showed the presence of sulfadimethoxine and with a maximum concentration of 9120 μg/kg. The least mean concentration of sulfadimethoxine, 1120 ± 526 μg/kg, occurred in the muscle and with only 25.0% of the samples. Also, sulfamerazine occurred in the highest amount in the gizzard with a mean concentration of 744 ± 364 μg/kg and in 61.2% of the samples with a maximum concentration of 3380 μg/kg. The muscle and the liver had mean concentrations of 234 ± 181 and 277 ± 109 μg/kg, respectively and these occurred in 37.5 and 30.8% of the muscle and liver, respectively. The maximum concentration of sulfamerazine in the muscle and liver was 595 and 569 μg/kg, respectively.
The liver of layers had the highest mean concentration of sulfamethoxazole with 38.5% detection frequency. The gizzard and the tissue followed the liver distantly with maximum concentrations of 1240 and 491 μg/kg for the gizzard and the tissue, and detection frequencies of 16.7 and 25.0%, respectively. Sulfamoxole occurred most in the liver with a mean concentration of 2250 ± 691 μg/kg, and this was in 38.5% of analyzed liver samples. Sulfamoxole also occurred in the muscle and gizzard but at lower mean concentrations compared with the levels in the liver. It occurred in 25.0 and 23.5% of the muscle and liver, with mean concentrations of 268 ± 254 and 183 ± 135 μg/kg, respectively. The muscle and liver tissues of layers had high concentrations of tylosin at 3760 ± 2320 and 4492 ± 1383 μg/kg, with occurrence in 50.0 and 30.8% of the muscle and liver samples, respectively. The highest individual concentration of tylosin was in the liver at the concentration of 2073 μg/kg while the muscle had a concentration of 10190 μg/kg. The gizzard had the least mean concentration for tylosin (1702 ± 500 μg/kg), occurring in 50.0% of the samples.
The mean concentrations of the six antibiotics in the three matrices were not significantly different at 95% confidence limit except for sulfamoxole, and were above the MRL excluding enrofloxacin in the gizzard that had 56.7 ± 17.5 μg/kg. Tylosin, sulfadimethoxine and enrofloxacin were the three most available antibiotics in layers’ muscle as shown by their mean concentrations. However, sulfamethoxazole, sulfamerazine and sulfamoxole were the least available drugs as indicated in Table 4. All of the six antibiotics had high mean concentrations in the liver with values above 200 μg/kg. Tylosin, sulfadimethoxine, sulfamoxole, sulfamethoxazole and enrofloxacin showed very high mean concentrations, occurring above 90% in all the liver samples. Except for sulfadimethoxine, tylosin and sulfamerazine all the other drugs occurred below the MRL in the gizzard and were present at between 16.7 and 33.3% of the gizzard samples.
3.6. Distribution of antibiotics in broiler chickens
The detailed distribution of antibiotic residues in different tissues of broiler chickens is as shown in Table 5. The highest mean concentration of enrofloxacin (1660 ± 210 μg/kg) was in 25.0% of liver samples that had a maximum concentration of 1870 μg/kg. Only one broiler muscle sample had 1320 ± 0.0 μg/kg of enrofloxacin. The least mean amount of enrofloxacin (478 ± 202 μg/kg) among the three matrices was in the gizzard, even though it occurred in more samples compared with the other matrices (33.3%). Sulfadimethoxine occurred in 50.0% of gizzard samples and with the highest mean concentration of 10522 ± 5050 μg/kg. The maximum concentration of sulfadimethoxine in the gizzard was 17400 μg/kg. Twenty-five percent (25.0%) of broiler liver samples had a mean concentration of 4860 ± 3320 μg/kg and the maximum concentration encountered was 8170 μg/kg. The least mean concentration and percentage occurrence of sulfadimethoxine was in the broiler muscle at 171 ± 0.0 μg/kg and 2.0%, respectively. All of the concentrations are above the allowed MRL for the various drugs in the different tissues.
Table 5.
Distribution of antibiotic residues (μg/kg) in broiler chicken tissues (n = 50).
| Antibiotics residue | Broilers Muscle |
Broilers Liver |
Broilers Gizzard |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | |
| ENR | 1320 ± 0.00a | 16.7 | 0 | 1660 ± 210a | 25.0 | 420 | 478 ± 202a | 33.3 | 405 |
| SDX | 171 ± 0.00a | 16.7 | 0 | 4860 ± 3320a | 25.0 | 6630 | 10500 ± 5050a | 50.0 | 16700 |
| SMZ | 98.7 ± 109a | 33.3 | 155 | 168 ± 120a | 37.5 | 385 | 293 ± 153a | 66.7 | 648 |
| SMX | 304 ± 98.3a | 33.3 | 196 | 622 ± 356a | 37.5 | 1130 | 359 ± 219a | 33.3 | 437 |
| SUM | 59.6 ± 3.27a | 66.7 | 121 | 461 ± 417a | 50.0 | 1669 | 329 ± 0.00a | 16.7 | 0 |
| TYL | 631 ± 343a | 50.0 | 1180 | 7380 ± 3710a | 50.0 | 16600 | 955 ± 405a | 33.3 | 810 |
ENR – Enrofloxacin, SDX - Sulfadimethoxine, SMZ – Sulfamerazine, SMX – Sulfamethoxazole, SUM – Sulfamoxole, TYL - Tylosin.
The gizzard with a mean concentration of 293 ± 153 μg/kg that occurred in 66.7% of samples and with a maximum concentration of 750 μg/kg was the matrix with the highest mean concentration of sulfamerazine. Broiler liver followed the gizzard in sulfamerazine concentration with a mean concentration of 168 ± 120 μg/kg, and this occurred in 37.5% of the samples. The maximum concentration of sulfamerazine in the liver was more than the maximum level for the gizzard, notwithstanding the higher mean value for the gizzard. Sulfamerazine in the liver was about twice the level in the muscle, that had a mean concentration of 98.7 ± 109 μg/kg, and this was in 33.3% of the samples. Sulfamethoxazole occurred most in the liver of broilers with a mean concentration of 622 ± 357 μg/kg and was in 37.5% of the liver samples. 1330 μg/kg was the maximum concentration of sulfamethoxazole in broiler liver. Broiler gizzard ranked next to the liver concerning sulfamethoxazole and with a mean concentration of 359 ± 219 μg/kg, and this occurred in 33.3% of the gizzard samples with a maximum concentration of 578 μg/kg. The least mean concentration of sulfamethoxazole was in broiler muscle (305 ± 98.4 μg/kg), occurring in 33.3% of the samples with a maximum concentration of 403 μg/kg.
The liver had the highest mean concentration of sulfamoxole at 461 ± 417 μg/kg and was present in 50.0% of the samples. About 329 ± 0.0 μg/kg of sulfamoxole was the concentration in only one gizzard sample that equaled 2.0% of the samples. The least mean concentration of sulfamoxole (59.6 ± 3.27 μg/kg) was in the muscle, and it occurred in 66.7% of the samples. The maximum concentration of sulfamoxole in an individual sample was in the liver (1710 μg/kg), followed by the gizzard (329 μg/kg) while the least was in the broiler muscle at a concentration of 121 μg/kg. Tylosin also occurred with the highest mean concentration in the liver (7380 ± 3710 μg/kg) and in 50.0% of the liver samples. The highest measured amount of tylosin in the broiler liver was 7980 μg/kg. Tylosin was in 33.3% of gizzard samples with a mean concentration of 955 ± 405 μg/kg, about seven times below the amount recorded for the liver. The maximum concentration of tylosin in the gizzard for an individual sample was 1360 μg/kg. Broiler muscle had the least mean tylosin concentration in 50.0% of the samples and with a maximum concentration of 1260 μg/kg.
The mean concentrations for all the drugs in all of the matrices were not significantly different at 95% confidence limit and above the allowed MRL except sulfamerazine that was 98.7 ± 109 μg/kg in the broiler muscle. Tylosin, enrofloxacin and sulfamethoxazole were the essential drugs in the broiler muscle while sulfamoxole and sulfamerazine had concentrations below 100 μg/kg. The liver had higher concentrations of the six antibiotics compared with the other matrices. Tylosin, sulfadimethoxine, enrofloxacin, sulfamethoxazole and sulfamoxole, in that order were the important antibiotics in the liver, and they occurred in high amounts. Broiler gizzard had sulfadimethoxine, tylosin, sulfamerazine, enrofloxacin and sulfamethoxazole as the main antibiotics while sulfamoxole occurred least and in the least number of samples.
3.7. Distribution of antibiotics residues in cockerel chickens
The distributions of six antibiotics residues including enrofloxacin, sulfadimethoxine, sulfamerazine, sulfamethoxazole, sulfamoxole and tylosin were determined in parts of cockerel chickens as indicated in Table 6. Enrofloxacin occurred in all the matrices in at least 50.0% of the samples. The gizzard and liver had almost the same mean concentrations of enrofloxacin. The drug occurred in 50.0 and 71.4% of liver and gizzard samples, respectively, with maximum concentrations of 280 and 676 μg/kg that were above the MRL. Cockerel muscle had a lower mean concentration of enrofloxacin at 77.6 ± 48.7 μg/kg, and this occurred in 50.0% of the samples. The maximum concentration of enrofloxacin in the cockerel muscles was 220 μg/kg. Cockerel muscle had a very high mean concentration of sulfadimethoxine (2280 ± 1550 μg/kg), and this was in 50.0% of the samples with 6800 μg/kg being the maximum concentration. Only one cockerel gizzard sample had sulfadimethoxine (2800 ± 0.0 μg/kg) but in an amount far below that determined in the muscle but above that in the liver. The least mean concentration of sulfadimethoxine was in the liver, and it was 382 ± 0.0 μg/kg with 2.0% occurrence.
Table 6.
Distribution of antibiotic residues (μg/kg) in cockerel chicken tissues (n = 50).
| Antibiotics residue | Cockerel Muscle |
Cockerel Liver |
Cockerel Gizzard |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | Mean ± SD | Detection frequency (%) | Range | |
| ENR | 77.5 ± 48.7a | 50.0 | 210 | 175 ± 105a | 50.0 | 210 | 122 ± 76.97a | 71.4 | 400 |
| SDX | 2280 ± 1560a | 50.0 | 6630 | 381 ± 0.00a | 2.00 | 0 | 2800 ± 0.00a | 2.00 | 0 |
| SMZ | 88.4 ± 22.8a | 50.0 | 107 | 275 ± 49.2a | 50.0 | 98.4 | 168 ± 121a | 57.1 | 533 |
| SMX | 851 ± 0.00a | 2.00 | 0 | 9.68 ± 0.00a | 2.00 | 0 | 86.2 ± 10.9a | 28.6 | 21.8 |
| SUM | 234 ± 219a | 25.0 | 438 | 72.2 ± 4.79a | 50.0 | 9.58 | 8.90 ± 5.62a | 28.6 | 11.2 |
| TYL | 1038 ± 379a | 62.5 | 2150 | 3670 ± 0.00a | 25.0 | 0 | 481 ± 285a | 57.1 | 1230 |
ENR – Enrofloxacin, SDX - Sulfadimethoxine, SMZ – Sulfamerazine, SMX – Sulfamethoxazole, SUM – Sulfamoxole, TYL - Tylosin.
Sulfamerazine occurred in 50.0% of cockerel muscle and the liver but with a higher mean concentration of 275 ± 49.2 μg/kg for the drugs in the liver. 324 μg/kg was the maximum concentration of sulfamerazine in cockerel liver while cockerel muscle had 144 μg/kg as its maximum but with a mean concentration of 88.4 ± 22.8 μg/kg. Cockerel gizzard came after the liver with a mean concentration of 168 ± 121 μg/kg for sulfamerazine but in 57.1% of analyzed samples. The maximum concentration of sulfamerazine in the gizzard was 562 μg/kg. On the other hand, sulfamethoxazole occurred in the least number (2.0%) of cockerel muscle samples compared with the other matrices, but it had the highest concentration of the drug 851 ± 0.0 μg/kg. Cockerel gizzard had 86.2 ± 10.9 μg/kg sulfamethoxazole and was present in 28.6% of the samples. The maximum concentration of sulfamethoxazole in cockerel gizzard was 413 μg/kg. The least concentration of sulfamethoxazole was in the liver (9.68 ± 0.0 μg/kg), and 2.0% of the liver samples had the drug.
Sulfamoxole was highest in cockerel muscle with a mean concentration of 234 ± 219 μg/kg, and this occurred in only 25.0% of the samples with a maximum concentration of 453 μg/kg. The liver had 72.2 ± 4.79 μg/kg sulfamoxole, and it was present in only 50.0% of the samples and with a maximum concentration of 76.9 μg/kg. The least concentration of sulfamoxole was in the gizzard (8.90 ± 5.62 μg/kg), and was present in only 28.6% of the gizzards. The maximum sulfamoxole in the gizzard was 31.3 μg/kg. The highest concentration of tylosin (3670 μg/kg) was found in 2.0% of the liver samples. Next to the liver in tylosin was the muscle and it was found in 62.5% of the liver samples. The maximum concentration of tylosin in the muscle was 2390 μg/kg. Cockerel gizzard had the lowest mean tylosin concentration and in 57.1% of the gizzard samples. The maximum tylosin concentration in the gizzard was 1260 μg/kg. The mean concentration of all the drugs in the different matrices were, however, not different (p < 0.05) and above the acceptable MRL in the cases of enrofloxacin and sulfamerazine in the muscle, sulfamethoxazole and sulfamoxole in the liver and gizzard. Sulfadimethoxine, as indicated in Table 6, appeared to be the most prevalent among the six antibiotics in cockerel muscle. Tylosin and sulfamethoxazole were next to sulfadimethoxine in prevalence while sulfamerazine and enrofloxacin had mean concentrations below 100 μg/kg. Enrofloxacin, sulfadimethoxine, sulfamerazine and tylosin were the main residues in cockerel liver while sulfamethoxazole and sulfamoxole were present at below 10 and 100 μg/kg, respectively.
4. Discussion
Naeem et al. and Aslam et al. [31, 32] had documented enrofloxacin in poultry tissues from Lahore and Faisalabad, Pakistan, respectively. Enrofloxacin with a concentration range of 3.10 and 364 μg/kg and 55–92% violation against the MRL had been reported [30] for different boiler chicken tissues while Aslam et al. [32] reported a mean concentration of 208 ± 55 and 527 ± 84 μg/kg for the muscle and liver, and 58.5 and 71.2% above the MRL, respectively. The observations from the works of Naeem et al. and Aslam et al. [31, 32] are consistent with the results from the present study for the different tissues analyzed for enrofloxacin. Meanwhile, Weiss et al. [33] and Roudaut & Fournet [34] had in different studies reported the absence of enrofloxacin in Italian and French poultry tissues, respectively but concluded that exposure to antibiotics is still higher in the poultry compared to other livestock sectors, and also that their results might not be guaranteed for citizens in low poultry producing countries due to lower control frequency arising from trade imbalance. These observations are in agreement with the report by Klein et al. [35] on global antibiotic consumption between 2000 and 2015 that confirmed an increase in the consumption of broad-spectrum agents like the fluoroquinolones and macrolides in low- and medium-income countries like Nigeria with clear implications in adverse drug-related events. Enrofloxacin is, however, not permitted for use in animals from which eggs are produced for human consumption [36] and contrary to this, it was found in different tissues of laying birds in the present study. Heavy usage of enrofloxacin in poultry has been linked with increased Salmonella infections [37].
Sulfadimethoxine, sulfamerazine, sulfamoxole and sulfadiazine had at various times been determined in poultry tissues including turkey. Sulfadimethoxine was found at 115–456 μg/kg in different chicken muscles from a local market in Romania [38] while Matea et al. [39] in an earlier study, however, did not find sulfadimethoxine in chicken samples from the area. Machado et al. [40] and Zotou & Vasiliadou [41] in the analyses of chicken muscle samples from Alfenas, Brazil and Greece, respectively did not detect any of the sulfonamides in the tissues in their studies.
Chicken samples from Baoding, China did not contain sulfadiazine [42], sulfamerazine and sulfadiazine were also absent in chicken samples from Taiwan [43] and likewise, samples from Romania [38]. The aforementioned reports are in contrast to those obtained in the present study where sulfamerazine, sulfamoxole and sulfadiazine were found in varying concentrations and in some instances at levels above the MRLs in the different tissues [26]. More so, the documented data article highlights the potential health risks associated with poultry products smuggled across the border into Nigeria as well as those produced domestically in terms of antibiotic residues. The differences in results could be attributed to different management practices adopted by poultry farmers in different areas. Sulfonamides, in general, are not acceptable for use in birds that lay eggs, and their concentration in uncooked edible tissues according to the code of federal regulations of the U.S. food and drug administration (FDA) should not exceed 100 μg/kg [44]. These assertions are greatly at variance with results from this study.
Residues of tylosin have been found in the liver and muscle of laying hens and turkeys at above the MRLs [45]. Costa et al. [46] reported that tylosin administered to chickens affects meat quality, by causing changes in the protein and fat content of meat. Tylosin was assayed in this study and it was above the 200 μg/kg MRL [44] in all the samples except frozen turkey gizzard. Tylosin, though registered for use in the management of chronic respiratory disease in chickens and infectious sinusitis in turkeys, has, however, been used as a growth-promoting agent in poultry [47]. The high concentration of tylosin encountered in the different tissues of poultry in this study could be an indication of its use for growth-promoting rather than therapeutic purposes.
There are considerable worldwide concerns about the presence of enhanced antibiotic and growth hormones residues in poultry products due to their potentially toxic health effects. Multiple reports throughout the world have documented varying amounts of antibiotic residues in poultry meats [48, 49, 50, 51, 52]. The presence and concentration of antibiotic residues in poultry tissues could be affected by a variety of factors, including the type of antibiotics, feed ingredients, drug administration dosages, testing method, differing maximum residue limits (MRLs) for antibiotics, and poultry health stressors [53]. Although the MRLs vary from country to country, however, Nigeria does not have any regulations governing the maximum residue limits in poultry products imported into the country. Numerous people in Nigeria believe that imported poultry products contain excessive levels of antibiotic or hormone residues, which could lead to life-threatening challenges. Equally, poultry products produced in the country are rarely tested for antibiotic residues and other chemical contaminants, and therefore may not be of the highest quality standards. There are numerous factors that contribute to antibiotic overuse and relatively high occurrence and prevalence in poultry meats. These include farmers' arbitrary and inappropriate use of veterinarian pharmaceuticals, the limited period between antibiotic administration and discontinuation, and the absence of aggressive surveillance mechanisms for monitoring antibiotics administered by farmers and poultry products imported into the country.
5. Conclusions
This study established the presence of six antibiotic residues in three matrices of poultry tissues using a previously validated solid phase extraction procedure and HPLC-DAD. The LOQ obtained for all the analytes were below their respective maximum residue limits of 100 μg/kg, thus making the method suitable for their quantification. The antibiotics were present in all the matrices in varying amount. Mean concentrations in some instances were below the acceptable limits while it far exceeded the established MRLs in other matrices. Except enrofloxacin and sulfamoxole in laying birds tissues, the concentration of the various drugs in the different matrices were not significantly different at p = 0.005. The maximum drug levels and the percentage of samples containing the drugs in most of the matrices suggested that farmers do not observe the cessation period before birds are sold thus compromising the health of consumers. To safeguard the safety of our food supplies, governmental agencies and university researchers should aggressively monitor poultry products for violative antibiotic residues in poultry meats and tissues. Nationwide monitoring strategies for veterinary pharmaceuticals are recommended, and government laboratories are required to develop rapid, sensitive, and reliable screening and confirmatory methodologies for all antibiotic classes that are approved or prohibited for usage.
Declarations
Author contribution statement
Abdulrasaq O. Oyedeji, Akan B. Williams, Nsikak U. Benson: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Titus A. M. Msagati: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Tertiary Education Trust Fund of Nigeria.
Data availability statement
Data associated with this study is available at Chemical Data Collections (Elsevier) at https://doi.org/10.1016/j.cdc.2019.100312.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Covenant University Centre for Research, Innovation and Discovery (CUCRID), Covenant University, for the publication support. Dr. M. Vimbhai and G. K. Temesgen of the University of South Africa (UNISA), Florida, South Africa is acknowledged. The authors are grateful to the anonymous reviewers and editor for their invaluable suggestions.
References
- 1.Van Boeckel T.P., Charles B.C., Gilbert M., et al. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Boeckel T.P., Pires J., Silvester R., et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365(eaaw1944):1–5. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 3.Oyedeji A.O., Msagati T.A.M., Williams A.B., Benson N.U. Determination of Antibiotic residues in frozen poultry by a solid-phase dispersion method using liquid chromatography-triple quadrupole mass spectrometry. Toxicol. Rep. 2019;6:951–956. doi: 10.1016/j.toxrep.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agbota S. 2017. How smuggling’s hurting Nigeria’s N1trn poultry industry.http://sunnewsonline.com/how-smugglings-hurting-nigerias-n1trn-poultry-industry/ Available at: [Google Scholar]
- 5.Food and Agriculture Organization (FAO) 2018. Africa Sustainable Livestock, 2050. Livestock and Livelihoods Spotlight Nigeria. Cattle and Poultry Sectors.http://www.fao.org/3/CA2149EN/ca2149en.pdf CA2149EN/1/10.18. [Google Scholar]
- 6.Adebowale O.O., Adeyemo O.K., Awoyomi O., Dada R., Adebowale O. Antibiotic use and practices in commercial poultry laying hens in Ogun State Nigeria. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 2016;69(1):41–45. [Google Scholar]
- 7.Tsokar D.K. 2019. Nigeria, FAO to Enhance Monitoring of Antibiotic Use, abuse in plants/animals with guidelines.http://www.fao.org/nigeria/news/detail-events/en/c/1253970/ Retrieved. [Google Scholar]
- 8.Tuck C. Commonwealth Antimicrobial Stewardship Partnerships Announced. The Pharmaceutical Journal. 2019 https://www.pharmaceutical-journal.com/news-and-analysis/news/commonwealth-antimicrobial-stewardship-partnerships-announced/20206157.article [press release] Available at: Accessed. [Google Scholar]
- 9.Simpkin V.L., Renwick M.J., Ruth K.R., Mossialos E. Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps. J. Antibiot. (Tokyo) 2017;70:1087–1096. doi: 10.1038/ja.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aufartova J., Brabcova I., Torres-Padron M.E., et al. Determination of fluoroquinolones in fishes using microwave-assisted extraction combined with ultra-high-performance liquid chromatography and fluorescence detection. J. Food Compos. Anal. 2017;56:140–146. [Google Scholar]
- 11.Martins M.T., Barreto F., Hoff R.B., et al. Multiclass and multi-residue determination of antibiotics in bovine milk by liquid chromatography–tandem mass spectrometry: combining efficiency of milk control and simplicity of routine analysis. Int. Dairy J. 2016;59:44–51. [Google Scholar]
- 12.Moema D., Nindi M.M., Dube S. Development of a dispersive liquid–liquid microextraction method for the determination of fluoroquinolones in chicken liver by high- performance liquid chromatography. Analyitca Chimica Acta. 2012;730:80–86. doi: 10.1016/j.aca.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Viceo J.A., Rosales-Conrado N., Guillen-Casla V., et al. Fluoroquinolone antibiotic determination in bovine milk using capillary liquid chromatography with diode array and mass spectrometry detection. J. Food Compos. Anal. 2012;28:99–106. [Google Scholar]
- 14.Gao S., Jin H., You J., Ding Y., Zhang N., Wang Y. Ionic liquid-based homogeneous liquid–liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J. Chromatogr. A. 2011;1218(41):7254–7263. doi: 10.1016/j.chroma.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 15.USDA. USDA . USDA Veterinary Drug MRL; 2012. International Maximum Residue Level Database.http://www.mrldatabase.com/default.cfm?selectvetdrug=1 Available online: Accessed on. [Google Scholar]
- 16.ECR European Union Commission Regulation No. 37/2010 on Pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Comm. 2010;L15:1. [Google Scholar]
- 17.Kang J.W., Park H.-C., Gedi V., et al. Veterinary drug residues in domestic and imported foods of animal origin in the Republic of Korea. Food Add. Contam. Part B Surveill. 2015;8(2):106–112. doi: 10.1080/19393210.2014.1001795. [DOI] [PubMed] [Google Scholar]
- 18.Andon A., Martinez-Larranaga M.R., Ares I., Martinez M.A. In: Veterinary Toxicology. third ed. Gupta R.C., editor. Elsevier; Amsterdam: 2018. Regulatory aspects for the drugs and chemicals used in food-producing animals in the European union; pp. 103–131. (Basic and Clinical Principles). [Google Scholar]
- 19.Okocha R.C., Olatoye I.O., Adedeji O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Publ. Health Rev. 2018;39(21) doi: 10.1186/s40985-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Companyo R., Jimenez V., Rubies A., Centrich F., Guiteras J. Development and validation of a multiclass method for the analysis of antibiotic residues in eggs by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2011;1218(11):1443–1451. doi: 10.1016/j.chroma.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Darko G., Mensah J.K., Dapaah S.S., Odei J. Estimated dietary exposure to veterinary residues in chicken and eggs. Int. J. Flow Control. 2015;2(16):1–8. [Google Scholar]
- 22.Ahmed M.B., Sree Y.H., Abdel-Fattah S.M., Hassan N.S., Saad M.M. Determination of tylosin, spiramycin, and erythromycin residues in Egyptian buffaloes’ meat by Thin-Layer Chromatography – Bioautography. J. Planar Chromatogr. Mod. TLC. 2013;26(5):409–416. [Google Scholar]
- 23.Alaboudi A., Basha E.A., Musallam I. Chlortetracycline and sulfanilamide residues in table eggs: prevalence, distribution between yolk and white and effect of refrigeration and heat treatment. Food Control. 2013;33(1):281–286. [Google Scholar]
- 24.Lehotay S.J., Lightfield A.R., Geis-Asteggiante L., et al. Development and validation of a streamlined method designed to detect residues of 62 veterinary drugs in bovine kidney using ultrahigh- performance liquid chromatography – tandem mass spectrometry. Drug Test. Anal. 2012;4(1):75–90. doi: 10.1002/dta.1363. [DOI] [PubMed] [Google Scholar]
- 25.Farre´ M., Barcelo D., Barcelo D. Analysis of emerging contaminants in food. Trends Anal. Chem. 2013;43:240–253. [Google Scholar]
- 26.Oyedeji A.O., Msagati T.A.M., Williams A.B., Benson N.U. ” Chemical Data Collections; 2019. Solid-Phase Extraction and High Performance Liquid Chromatography with Diode Array Detection Method for the Determination of Antibiotic Residues in Poultry Tissues. 100312. [Google Scholar]
- 27.Shunli J., Tengfei L., Wen Y., et al. A hollow porous molecularly imprinted polymer as a sorbent for the extraction of 7 macrolide antibiotics prior to their determination by HPLC-MS/MS. Microchimica Acta. 2013;185(203) doi: 10.1007/s00604-018-2728-3. [DOI] [PubMed] [Google Scholar]
- 28.Patyra E., Przeniosło-Siwczynska M., Kwiatek K. Determination of sulfonamides in feeds by high-performance liquid chromatography after fluorescamine precolumn derivatization. Molecules. 2019;24(452) doi: 10.3390/molecules24030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Combs M.T., Ashraf-Khorassani M., Taylor L.T. Method development for the separation of sulfonamides by supercritical fluid chromatography. J. Chromatogr. Sci. 1997;35(4):176–180. [Google Scholar]
- 30.Sajid M., Na N., Safdar M., et al. Rapid trace level determination of sulfonamide residues in honey with online extraction using short C18 column by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A. 2013;1314:173–179. doi: 10.1016/j.chroma.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Naeem M., Khan K., Rafiq S. Determination of residues of quinolones in poultry products by high-performance liquid chromatography. J. Appl. Sci. 2006;6(2):373–379. [Google Scholar]
- 32.Aslam R., Kouser N., Jaevd I., et al. Determination of enrofloxacin in commercial broiler using high-performance liquid chromatography. Indust. J. Food Prop. 2016;19:2463–2470. [Google Scholar]
- 33.Weiss C., Conte A., Milandri C., et al. Veterinary drugs residue monitoring in Italian poultry: current strategies and possible developments. Food Control. 2007;18(9):1068–1076. [Google Scholar]
- 34.Roudaut B., Fournet I. Surveillance of veterinary drug residues in poultry meat and eggs. Bulletin Epidémiologique, Animal Health and Nutrition. 2017;77:37–41. [Google Scholar]
- 35.Klein E.Y., Van Boeckel T.P., Martinez E.M., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. U. S. A. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EMEA . 2010. European Medicines Agency) “Reflection Paper on the Use of Macrolides, Lincosamides and Streptogramins (MLS) in Food-Producing Animals in the European Union: Development of Resistance and Impact on Human and Animal Health.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099151.pdf Accessed. [Google Scholar]
- 37.Morales-Barrera E., Calhoun N., Lobato-Tapia J.L., et al. Risks involved in the use of enrofloxacin for Salmonella enteritidis or Salmonella Heidelberg in commercial poultry. Front. Veter. Sci. 2016;3(72):1–7. doi: 10.3389/fvets.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitescu C.L., Nicolau A.I., Csuma A., Moisoiu C. Simultaneous analysis of four sulfonamides in chicken muscle tissue by HPLC. Food Addit. Contam. 2011;28(8):1013–1020. doi: 10.1080/19440049.2011.577098. [DOI] [PubMed] [Google Scholar]
- 39.Matea C.T., Bele C., Dulf F. Determination of sulfonamides in chicken meat by solid-phase extraction and high-performance liquid chromatography. Bulletin Ube Coll. 2007;63l:563–568. [Google Scholar]
- 40.Machado S.C., Landin-Silva M., Maia P.P., Rath S., Martins I. QuEChERS-HPLC-DAD method for sulphonamides in chicken breast. Braz. J. Pharm. Sci. 2013;49(1):155–166. [Google Scholar]
- 41.Zotou A., Vasiliadou C. LC of sulfonamide residues in poultry muscle and eggs extracts using fluorescence precolumn derivatization and monolithic silica column. J. Separ. Sci. 2010;33(1):11–22. doi: 10.1002/jssc.200900461. [DOI] [PubMed] [Google Scholar]
- 42.Sun H., Hao Y., Wu X. A rapid and effective method for simultaneous determination of residual sulfonamides and sarafloxacin in pork and chicken muscle by high-performance liquid chromatography with accelerated solvent extraction – solid-phase extraction cleanup. J. Chromatogr. Separ. Tech. 2012;3(154) [Google Scholar]
- 43.Fuh M.-R., Chu S.-Y. Quantitative determination of sulfonamide in meat by solid-phase extraction and capillary electrophoresis. Anal. Chim. Acta. 2003;499(1-2):215–221. [Google Scholar]
- 44.USDA. U.S. Food & Drug Administration CFR . 2018. Code of federal regulations title 21.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm? cfrpart=556 Available online: Accessed. [Google Scholar]
- 45.Vandenberge V., Delezie E., Delahaut P., et al. Transfer of flubendazole and tylosin from feed at cross-contamination levels to various poultry matrices. Poultry Sci. 2012;91(9):2351–2360. doi: 10.3382/ps.2012-02265. [DOI] [PubMed] [Google Scholar]
- 46.Costa A.I.A., Teldeschi E., Gerritzen M.A., et al. Influence of flock treatment with the antibiotic tylosin on poultry meat quality: results of a preliminary experiment. NJAS-Wagen. 2007;54(3):269–278. [Google Scholar]
- 47.N. A. Botsoglou and D. J. Fletouris, “Antimicrobial drugs,” In: Drug Residues in Foods. Pharmacology, Food Safety, and Analysis. Marcel Dekker, New York, NY, USA, pp. 27-115..
- 48.Panzenhagen P.H.N., Aguiar W.S., Gouvêa R., de Oliveira A.M.G., Barreto F., Pereira V.L.A., Aquino M.H.C. Investigation of enrofloxacin residues in broiler tissues using ELISA and LC-MS/MS. Food Addit. Contam. 2016;33(4):639–643. doi: 10.1080/19440049.2016.1143566. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Qiu W., Li Y., Liu L. Antibiotic residues in poultry food in Fujian Province of China. Food Addit. Contam. B. 2020;13:177–184. doi: 10.1080/19393210.2020.1751309. [DOI] [PubMed] [Google Scholar]
- 50.Jammoul A., El Darra N. Evaluation of antibiotics residues in chicken meat samples in Lebanon. Antibiotics. 2019;8:69. doi: 10.3390/antibiotics8020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S., Zhang Q., Chen M., Zhang X., Liu P. Determination of veterinary drug residues in food of animal origin: sample preparation methods and analytical techniques. J. Liq. Chromatogr. Relat. Technol. 2020;43(17-18):701–724. [Google Scholar]
- 52.Sha L., Tang X., Liu D., Xu Y., Ding Y., Ding F. Detection and quantitation of lomefloxacin and pefloxacin residues in the organ tissues and eggs of laying hens. J. Food Protect. 2018;81(5):810–814. doi: 10.4315/0362-028X.JFP-17-422. [DOI] [PubMed] [Google Scholar]
- 53.Mohammadzadeh M., Montaseri M., Hosseinzadeh S., Majlesi M., Berizi E., Zare M., Derakhshan Z., Ferrante M., Conti G.O. Antibiotic residues in poultry tissues in Iran: a systematic review and meta-analysis. Environ. Res. 2021;204(Part B):112038–112222. doi: 10.1016/j.envres.2021.112038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study is available at Chemical Data Collections (Elsevier) at https://doi.org/10.1016/j.cdc.2019.100312.