Summary
SARS Coronavirus‐2 is one of the most widespread viruses globally during the 21st century, whose severity and ability to cause severe pneumonia and death vary. We performed a comprehensive systematic review of all studies that met our standardised criteria and then extracted data on the age, symptoms, and different treatments of Covid‐19 patients and the prognosis of this disease during follow‐up. Cases in this study were divided according to severity and death status and meta‐analysed separately using raw mean and single proportion methods. We included 171 complete studies including 62,909 confirmed cases of Covid‐19, of which 148 studies were meta‐analysed. Symptoms clearly emerged in an escalating manner from mild‐moderate symptoms, pneumonia, severe‐critical to the group of non‐survivors. Hypertension (Pooled proportion (PP): 0.48 [95% Confident interval (CI): 0.35–0.61]), diabetes (PP: 0.23 [95% CI: 0.16–0.33]) and smoking (PP: 0.12 [95% CI: 0.03–0.38]) were highest regarding pre‐infection comorbidities in the non‐survivor group. While acute respiratory distress syndrome (PP: 0.49 [95% CI: 0.29–0.78]), (PP: 0.63 [95% CI: 0.34–0.97]) remained one of the most common complications in the severe and death group respectively. Bilateral ground‐glass opacification (PP: 0.68 [95% CI: 0.59–0.75]) was the most visible radiological image. The mortality rates estimated (PP: 0.11 [95% CI: 0.06–0.19]), (PP: 0.03 [95% CI: 0.01–0.05]), and (PP: 0.01 [95% CI: 0–0.3]) in severe‐critical, pneumonia and mild‐moderate groups respectively. This study can serve as a high evidence guideline for different clinical presentations of Covid‐19, graded from mild to severe, and for special forms like pneumonia and death groups.
Keywords: ARDS, Covid‐19, critical, death, laboratory, mild, pneumonia, radiology, SARS‐CoV‐2, symptomatology
Abbreviations
- AKI
Acute kidney injury
- ALT
Alanine aminotransferase
- ANOVA
Analysis of variance
- APTT
Activated partial thromboplastin time
- ARDS
Acute respiratory distress syndrome
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CD
Cannot determine
- CI
Confidence interval
- CK
Creatine kinase
- CKRT
Continuous kidney replacement therapy
- Covid‐19
Coronavirus disease 2019
- CRP
C‐reactive protein
- DBP
Diastolic blood pressure
- DIC
Disseminated intravascular coagulation
- ECMO
Extracorporeal membrane oxygenation
- ESR
Erythrocyte sedimentation rate
- FiO2
Fraction of inspired oxygen
- GGO
Ground‐glass opacification
- HR
Heart rate
- IL‐6, IL12
Interleukin‐6, Interleukin‐12
- IMV
Intermittent mandatory ventilation
- ISI
Institute for Scientific Information
- LDH
Lactate dehydrogenase
- MERS
Middle East respiratory syndrome
- NA
Not applicable
- NIH
National Institutes of Health
- NIV
Non‐invasive ventilation
- NR
Not reported
- PaCO2
Partial pressure of carbon dioxide
- PLT
Platelet
- pO2
Partial pressure of oxygen
- PP
Pooled proportion
- PT
Prothrombin time
- RNA
Ribonucleic acid
- RT‐PCR
Real‐time polymerase chain reaction
- SARS
Severe acute respiratory syndrome
- SBP
Systolic blood pressure
- TNF‐α
Tumor necrosis factor‐ α
- WBCs
White blood cells
- WHO
World Health Organization
- WHO GHL
WHO Global Health Library
- WoS
Web of Science
1. INTRODUCTION
In late December of 2019, the World Health Organization (WHO) China Country Office received a report about several patients having pneumonia of unknown etiology. 1 After, new cases started appearing rapidly with common symptoms such as fever, cough and dyspnea with less common presentation consisting of headache, sore throat and runny nose. The cause was later attributed to coronavirus 2019 (Covid‐19). With the majority of cases being located in Wuhan, China, the transmission mechanism was mainly presumed to be from exposure to the unique seafood and meat of the Wuhan market.
This severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a positive‐sense single‐stranded RNA virus closely related in structure to SARS‐CoV and the same family as MERS‐CoV. 2 According to WHO, the most common symptoms included low‐grade fever, dry cough, and fatigue. More serious Covid‐19 illness more frequently caused shortness of breath, persistent chest pain, dizziness, anorexia and high‐grade fever. 3 This sharp variation in symptoms may be due to the difference in reception of the virus among humans, which brings us difficulty understanding how it behaves and anticipates its development and then controlling and treating the disease. 4 Thus, the number of confirmed Covid‐19 cases has exceeded 125 million, with 2.5 million mortalities worldwide. 5
It is currently recommended that people with severe symptoms be provided with intensive care, ventilators and respiratory support. Corticosteroid analogues like dexamethasone are utilised to reduce mortality due to the low effectiveness of current antivirals and antibiotics prescribed to Covid‐19 patients. 6 , 7 , 8 Remdesvir, 9 bamlanivimab plus etesevimab, 10 inhaled interferon‐beta, 11 baricitinib, 12 tocilizumab or sarilumab 13 , 14 have shown clinical benefits in treatment of Covid‐19 along with their safety in various randomised clinical trials. The mechanisms they based on in their actions mainly depend on reducing the cytokine storm increased by SARS‐CoV‐2, including IL‐6, IL‐12, TNF‐α, and complement fragments C3a and C5a. 15 The study aims to systematically review the published literature on the Covid‐19 to estimate the frequency of clinical symptoms by comparing different clinical presentations based on the severity and living status of the cases. We also reveal the complications that appeared after admission to the hospital, clinical outcome, different methods of treatment, and prognosis tendency.
2. METHODS
2.1. Search strategy and selection criteria
Our systematic review and meta‐analysis used the global terms of Covid‐19 since January 2020. We selected this specific date because no publication has been published for the SARS‐CoV‐2 of the Wuhan outbreak before that time. The search strategy was conducted on the following seven network databases on 6 March 2020: PubMed, EMBASE, SCOPUS, Web of Science (WoS), Google Scholar, Cochrane Library and WHO Global Health Library (WHO GHL). No restrictions on the origin of cases or filters of age, gender, ethnicity, language, type of publication and human were applied. The entire strategy is shown in Table S1. The exported databases' results were incorporated into Endnote version X9.0 program to remove detected duplications. After the full‐text screening stage, we have done a manual search on 13 March 2020. The manual search method included searching by references reported in related full‐text or reviews, by citations of these articles, or even by conducting a simple search in PubMed or Google Scholar. The study’s protocol has been published in PROSPERO with ID CRD42020167929. The report of the findings was based on PRISMA guidelines published in 2009. 16 , 17 , 18 Our steps of the systematic review were reported in PRISMA checklist version 2020 (Table S2).
2.2. Study eligibility criteria and study selection
We conducted the selection phase in two stages: firstly, we screened the studies included after removing duplicates through their titles and abstracts. Then, we checked for full‐text eligibility of the included studies from the first stage. The study’s inclusive criteria included all possible case reports, case series, and epidemiological observational studies containing any medical information or clinical investigation of confirmed SARS‐CoV‐2 cases, regardless of the country and time of examination. We also included non‐English articles that were written in Chinese. Criteria of exclusion were other original studies such as books, reviews, theses, conference papers, and articles without available full text. Two independent authors were assigned for each step, and the discussion was held to resolve the conflicts with the help of the third senior author to reach the final consensus.
2.3. Data extraction and quality assessment
Two independent authors were chosen to extract distinguished items of the prepared data extracted from each included full‐text article and qualified them. Then the discussion was undertaken to solve any discrepancies between these two by the third reviewer. We prepared the sheets by Excel, Microsoft Office program 2016. It contained the information of the cited references of eligible studies included, the place and time that the study was preformed, name of the journal, year of publication, country of the first author, city of recruited patients, country of recruited patients, the continent of a recruited patient, study design (case reports, case series study or cross‐sectional study), sample size, the follow‐up periods of the confirmed cases with Covid‐19, used confirmation test for Covid‐19 infected patient, the demographic characteristics of the patients involving the age, race, gender, the characteristics of included Covid‐19 patients, the method of laboratory diagnosis, clinical samples; duration of onset symptom (e.g., fever, cough, muscle ache) and hospitalisation, type of used therapy (antiviral, antibiotic or supportive therapies) and clinical outcome (live, discharge or death).
Regarding the lab diagnostic form for Covid‐19 infection, we have confirmed the cases that have followed the WHO 2019 guideline. 19 For infection confirmation, the positive results must be processed by the SARS‐CoV‐2 Real‐Time RT‐PCR test. The confirmed diagnosis of SARS‐CoV‐2 infection was recorded from the included studies. We then collected the clinical manifestations, laboratory values, and the history of these cases, especially those who demonstrated symptoms associated with comorbidities. The last confirmed follow‐up time or the last date of clinical death has been recorded.
All included articles were evaluated for study quality. We divided the articles into two types for various method studies: Case report/case series/case‐control studies and observation cohort/Cross‐sectional studies. We used Study Quality Assessment Tools of the National Institutes of Health (NIH) for included studies. 20 There are nine items to extract for case reports/case series. For observation cohort/cross‐sectional studies, there are 13 items. Each item was rated as one or 0/NA‐not applicable/NR‐not reported/CD‐cannot determine. The final score will be calculated as a percentage with equal points for each item. The scoring thresholds are those over 75% ‘good’ quality, those between 75% and 43% ‘fair’, and below 43% ‘poor’. Two reviewers independently extracted the assessment. Discrepancies were discussed and resolved by a third reviewer. These assessment data were incorporated into a separate excel sheet.
2.4. Data analysis
Our strategy data synthesis targeted to conduct a qualitative synthesis using systematic review and quantitative one using R software version 3.6.2 software (https://www.r‐project.org/). The meta‐analysis was conducted for case series, and observation studies that have equal or more than five included Covid‐19 patients in each dataset. Ultimately, the values of the variables in five various sets (mild to moderate, severe to critical, pneumonic only, death and survival/discharged patients) were based on the authors’ severity classification in each study with the exclusion of ‘children’ studies. 21 , 22 , 23 , 24 The meta‐analysis of the results of CT‐Scan has been combined for all groups and for all (less than five patients) studies to understand the most common presentation the patients` can show in this imaging examination. Each group was analysed independently using the single pooled proportion (PP) or raw mean inverse variance random effect size method of meta‐analysis with the recommended restricted maximum likelihood tau method. 25 , 26 We have chosen these estimate methods as there is no direct comparison between these groups in most of the included studies. We have not reported the outcomes, which have <1 publication that reported on them. Moreover, we have visualised the different effect estimates of severity groups (mild‐moderate vs. severe‐critical) and living status groups (survivors vs. non‐survivors) by bar graphs with 95% CI error bars.
The summary of the categorical findings was written in percentages with 95% confidence interval (CI) with logit transformation measured for the suite of signs, symptoms, and outcomes. In comparison, the continuous variables were reported using means (95% CI) with log transformed means. A chi‐squared test was used for normally distributed data for categorical variables; on the other hand, Student t‐test and variance (ANOVA) were used or continuous ones. Using either the fixed or random effect size model was depending on the fact of heterogeneity. We assessed the heterogeneity using Cochran Q and I 2 ‐statistic tests. It was considered significant if p value < 0.1. When I 2 ‐statistic 26–50, heterogeneity evaluation was recognised as low. The I 2 = 51%–75% was considered moderate and high if I 2 > 75%. The random model was used if high to moderate heterogeneity (I 2 > 50%) was detected. We assessed the publication bias and meta‐regression analyses if any of the measured outcomes have >10 publications. 27 , 28 , 62
2.5. Subgroup and sensitivity analysis
We performed a subgroup meta‐analysis for adult studies (≥16 years old participants) for our outcome of interests which was determined after our primary analysis to prove the consistency of results throughout our analysis severity groups. To validate our results, we conducted a secondary comparative meta‐analysis (mild‐moderate vs. severe‐critical) and (survivor vs. non‐survivor) using odds ratios (OR) for dichotomous variables and standardised mean difference (SMD) for continuous ones with 95% CI. We also performed a meta‐regression analysis to estimate the impact of age and number of males factors on our outcome of interests if they reported in >10 publications in one of the divided groups.
3. RESULTS
3.1. Systematic search and screening results
Our literature searches and review of reference lists initially identified 4,309 records through seven database searches. After removing 1,999 duplicates by Endnote software version X9.0, we continued to exclude 2,082 irrelevant articles by screening titles and abstracts. Out of the 228 articles in the full‐text review stage, there were 85 articles excluded, and after including 28 articles from the manual search, there were 171 final eligible full‐text records. Reasons for exclusion at the full‐text stage were irrelative articles, not original, overlapped, non‐extractable data or no full‐text available, and animal articles. In summary, 148 included publications for both systematic review and meta‐analysis, based on the data of 62,949 confirmed patients (Figure 1 ).
FIGURE 1.
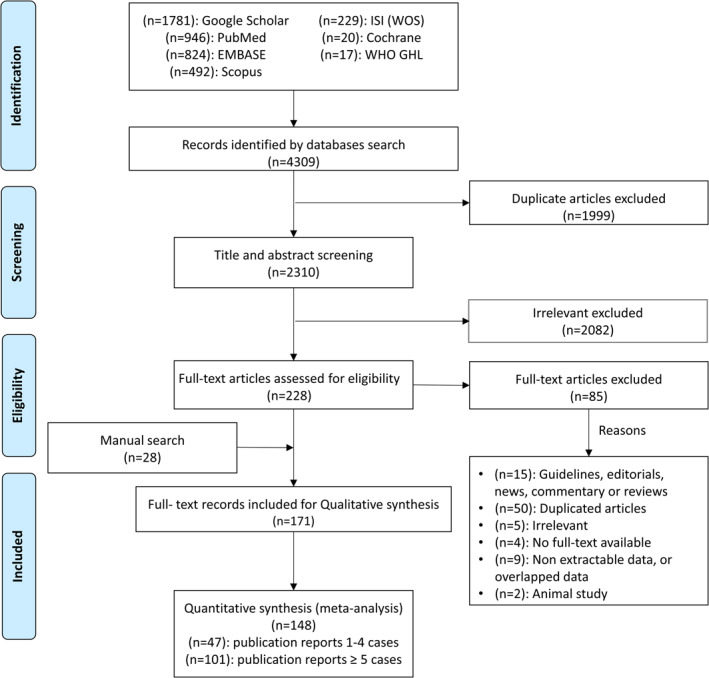
Flow diagram of the article selection procedure. WOS, Web of Science
3.2. Characteristics of included studies
Among the 171 included publications shown in Tables S3 and S4, there were 52 case reports (30.59%), 37 case series (21.76%), 79 cross‐sectional studies (46.47%), and two case‐control studies (1.18%). One hundred and sixty studies (93.57%) were performed in Western Pacific region with predominance of China (n = 146), followed by South Korea (n = 6), Singapore (n = 3), Vietnam (n = 2), Australia (n = 1), Japan (n = 1) and Taiwan (n = 1). Other regions only had the entire 11 studies, namely the European Region withfour studies per country (Germany, United Kingdom, France and Italy), Region of the Americas with four (two in the United States, one in Canada, and one in Brazil), South‐East Asia Region with three (two in Thailand and one in Nepal). All data were collected by the retrospective method in 2020. One hundred and 55 eligible studies were written in English, and the rest were in Chinese. Most studies were conducted in adults (93.57%), whereas only 24 patients were conducted in children. Notably, seven studies included pregnant.
3.3. History of Covid‐19 patients:
The history of Covid‐19 patients is summarised in Table 1. The variables were classified by illness severity including mild to moderate, severe to critical and pneumonia. In general, hypertension, smoking, and diabetes were the most common comorbidities. On detailed analysis, they accounted for mild to moderate group 0.17 [95% CI: 0.1–0.26, p < 0.01], 0.1 [95% CI: 0.06–0.17, p = 0.01], and 0.08 [95% CI: 0.05–0.11, p = 0.01]; for severe to critical group, 0.32 [95% CI: 0.24–0.4, p < 0.01], 0.15 [95% CI: 0.06–0.32, p = 0.01], and 0.19 [95% CI: 0.16–0.22, p = 0.61]; for pneumonia group, 0.15 [95% CI: 0.1–0.22, p < 0.01], 0.06 [95% CI: 0.03–0.12, p = 0.01] and 0.11 [95% CI: 0–0.16, p = 0.01], respectively. Table S5 presents the comorbidities of survivor and non‐survivor COVID‐19 patients. Notably, hypertension accounted 0.48 [95% CI: 0.35–0.61, p < 0.01], 0.04 [95% CI: 0.01–0.26, p < 0.01]; diabetes 0.23 [95% CI: 0.16–0.33, p = 0.01], 0.04 [95% CI: 0.01–0.12, p = 0.01]; and smoking 0.12 [95% CI: 0.03–0.38, p = 0.06], 0.05 [95% CI: 0.02–0.13, p = 0.03] for non‐survivor compared to survivor respectively.
TABLE 1.
Meta‐analysis summary of history of Covid‐19 patients according to severity status
| Variable | Mild to moderate | Severe to critical | Pneumonia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled single proportion (95% CI) | N of studies | p value | Total | Pooled single proportion (95% CI) | N of studies | p value | Total | Pooled single proportion (95% CI) | N of studies | p value | Total | |
| Cardiovascular disease | 0.06 (0.03–0.14) | 8 | 0.01 | 661 | 0.12 (0.06–0.22) | 15 | 0.22 | 347 | 0.06 (0.03–0.1) | 13 | 0.01 | 2296 |
| Cerebrovascular disease | 0.01 (0.01–0.02) | 7 | 0.8 | 1536 | 0.08 (0.04–0.13) | 8 | 0.04 | 414 | 0.06 (0.04–0.08) | 9 | 0.37 | 700 |
| Chronic kidney disease | 0.02 (0.01–0.03) | 9 | 0.2 | 1671 | 0.06 (0.03–0.1) | 9 | 0.02 | 522 | 0.03 (0.02–0.05) | 8 | 0.42 | 720 |
| Chronic liver disease | 0.04 (0.02–0.07) | 4 | 0.18 | 426 | 0.05 (0.02–0.1) | 5 | 0.89 | 153 | 0.05 (0.03–0.08) | 7 | 0.53 | 472 |
| COPD a | 0.01 (0.01–0.02) | 9 | 0.71 | 1674 | 0.09 (0.06–0.13) | 11 | 0.15 | 488 | 0.03 (0.02–0.07) | 11 | 0.01 | 1987 |
| Coronary heart disease | 0.07 (0.02–0.25) | 5 | 0.01 | 1371 | 0.17 (0.04–0.47) | 4 | 0.01 | 269 | 0.05 (0.02–0.09) | 2 | 0.65 | 172 |
| Respiratory system disease | 0.03 (0–0.16) | 2 | <0.01 | 1143 | 0.15 (0.12–0.2) | 3 | 0.22 | 275 | 0.05 (0.03–0.07) | 8 | 0.7 | 608 |
| Digestive system disease | ‐ | ‐ | ‐ | ‐ | 0.03 (0.01–0.09) | 2 | 0.66 | 102 | 0.06 (0.04–0.11) | 5 | 0.21 | 388 |
| Endocrine system disease | 0.04 (0.01–0.27) | 2 | 0.04 | 224 | 0.13 (0.03‐0.45) | 3 | <0.01 | 160 | 0.07 (0.03‐0.13) | 6 | <0.01 | 535 |
| Diabetes | 0.08 (0.05–0.11) | 16 | 0.01 | 2382 | 0.19 (0.16–0.22) | 20 | 0.61 | 819 | 0.11 (0–0.16) | 21 | 0.01 | 2849 |
| Hypertension | 0.17 (0.1–0.26) | 16 | <0.01 | 2382 | 0.32 (0.24–0.4) | 19 | <0.01 | 767 | 0.15 (0.1–0.22) | 21 | <0.01 | 2790 |
| Malignancy | 0.02 (0.01–0.05) | 9 | <0.01 | 1703 | 0.08 (0.04–0.15) | 12 | <0.01 | 589 | 0.05 (0.03–0.08) | 13 | <0.01 | 1212 |
| Immunodeficiency | 0 (0–0.01) | 2 | 0.6 | 1169 | 0.06 (0–0.62) | 3 | <0.01 | 203 | 0.04 (0.01–26) | 5 | <0.01 | 1246 |
| Current smoking | 0.10 (0.06–0.17) | 7 | 0.01 | 1364 | 0.15 (0.06–0.32) | 8 | 0.01 | 407 | 0.06 (0.03–0.12) | 7 | 0.01 | 519 |
| External smoking | 0.03 (0.01–0.07) | 4 | 0.01 | 1158 | 0.06 (0.03–0.1) | 3 | 0.83 | 313 | 0.05 (0.03–0.08) | 2 | 0.99 | 281 |
| Never smoking | 0.88 (0.83–0.91) | 3 | 0.2 | 1167 | 0.6 (0.21–0.89) | 2 | <0.01 | 223 | 0.04 (0.02–0.08) | 4 | 0.4 | 272 |
Note: p‐value of heterogeneity is significant when <0.1.
COPD: Chronic obstructive lung disease.
3.4. Vital signs
A meta‐analysis of a summary of vital signs in Covid‐19 patients according to severity and death status is summarised in Table S6. Variables related to blood pressure, heart rate, body mass index (BMI) and temperature were quite similar among different severities of Covid‐19. While the respiratory rate was highest in the pneumonia group (26.2 breaths per minute [95% CI: 21.14–31.26, p < 0.01]), the value of SpO2 in the mild to moderate group was the highest (97.03% [95% CI: 96.48–97.58, p = 0.08]). In terms of survivor status, variables of blood pressure and BMI were not reported. In the non‐survivor group, the pooled mean heart rate (90.84 Bpm [95% CI: 88.05–93.63, p = 0.42]) and temperature (38.56°C [95% CI: 38.43–38.69, p = 0.46]) was worse, but the respiratory rate was lower (23.34 breaths per minute [95% CI: 20.81–25.88, p = 0.09]) despite the differences not being high.
3.5. Covid‐19 symptoms
Fever was the most common general symptom demonstrated at highest rate in the death group (rate: 0.87 [95% CI: 0.77–0.86, p = 0.02]) followed by severe to critical (0.84 [95% CI: 0.77–0.89, p < 0.0001]), pneumonia (0.82 [95% CI: 0.77–0.86, p < 0.0001]), mild to moderate (0.79 [95% CI: 0.72–0.86, p < 0.0001]), and survival groups (0.64 [95%CI: 0.13–0.95, p < 0.0001]). The second most common systemic symptom was fatigue documented as following: death group (0.66 [95%CI: 0.42–0.84, p < 0.0001]), survivor group (0.53 [95% CI 0.38–0.66, p = 0.001]), severe to critical group (0.4 [95% CI: 0.27–0.54, p < 0.0001]), mild to moderate group (0.25 [95% CI: 0.15–0.39, p < 0.0001], and pneumonia (0.21 [95% CI: 0.13–0.32, p < 0.0001]). As regarding to the most appearing respiratory symptom, cough was at top of the list in all groups with the pooled percentages of 0.73 [95% CI: 0.53–0.87, p = 0.1] (non‐survivor group), 0.65 [95% CI: 0.55–0.74, p < 0.0001] (severe to critical group), 0.59 [95% CI: 0.49–0.67, p < 0.0001] (mild to moderate group), and 0.57 [95% CI: 0.48–0.64, p < 0.0001] (pneumonia). Dyspnea accounted 0.48 [95% CI: 0.77–1.88, p = 0.0068], 0.35 [0.21–0.51, p< 0.0001], and chest pain accounted 0.73 [95% CI: 0.53–0.87, p = 0.0032], 0.06 [95% CI: 0.02–0.15, p = 0.02] were more associated with death and severe groups respectively. Concerning the GIT symptoms, anorexia may be considered as a bad sign among the death group accounting 0.71 [95% CI: 0.4–0.9, p = 0.01) while 0.2 [95% CI: 0.07–0.44, p = 0.01] for the critically ill group. The further details of other symptoms were described in Figure 2, Table 2 and Table S7.
FIGURE 2.
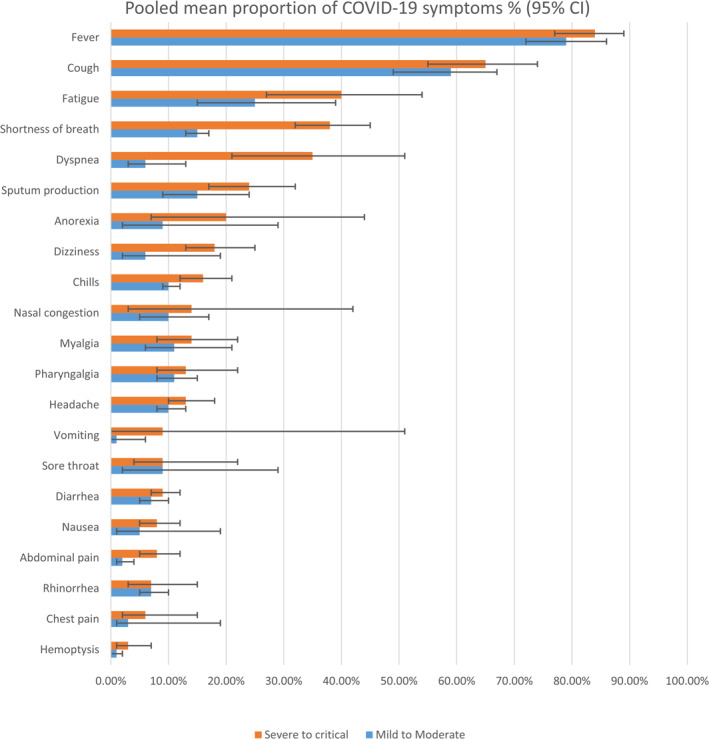
The pooled proportion of clinical symptoms on severity grading
TABLE 2.
Meta‐analysis summary of radiological investigation of Covid‐19 patients
| Variable | Pooled single proportion (95% CI) | Number of studies | Heterogeneity (I2) | p value | Total |
|---|---|---|---|---|---|
| Bilateral GGO a | 0.68 (0.59–0.75) | 62 | 91.8% | <0.01 | 5028 |
| Consolidation | 0.25 (0.16–0.36) | 37 | 95.4% | <0.01 | 4355 |
| Crazy‐paving | 0.36 (0.24–0.49) | 27 | 94.5% | <0.01 | 3653 |
| Enlarged mediastinal or hilar lymph nodes | 0.15 (0.07–0.28) | 18 | 94.4% | <0.01 | 2639 |
| GGO with consolidation | 0.42 (0.33–0.52) | 41 | 87.7% | <0.01 | 1849 |
| Left lung involvement | 0.45 (0.3–0.61) | 28 | 77.8% | <0.01 | 947 |
| Multiple mottling GGO | 0.65 (0.49–0.78) | 21 | 89% | <0.01 | 671 |
| Multiple peripheral GGO | 0.62 (0.44–0.78) | 24 | 94.9% | <0.01 | 1484 |
| Bilateral involvement lesions | 0.69 (0.6–0.77) | 38 | 88.8% | <0.01 | 3772 |
| Unilateral involvement lesions | 0.35 (0.27–0.45) | 41 | 92.7% | <0.01 | 5463 |
| Pure GGO | 0.62 (0.54–0.69) | 68 | 92.2% | <0.01 | 8951 |
| Unilateral GGO | 0.21 (0.15–0.29) | 27 | 75.6% | <0.01 | 1224 |
| Pericardial effusion | 0.05 (0.03–0.08) | 6 | 46.1% | 0.1 | 397 |
| Pleural effusion | 0.07 (0.05–0.11) | 27 | 86% | <0.01 | 3113 |
| Pneumothorax | 0.36 (0.01–0.97) | 4 | 93.8% | <0.01 | 337 |
| Right lung involvement | 0.6 (0.42–0.76) | 21 | 82.7% | <0.01 | 896 |
Note: p‐value of heterogeneity is significant when <0.1
GGO: Ground glass opacities.
3.6. Laboratory findings of Covid‐19
Table S8, Figure 3 describes the laboratory findings of Covid‐19 patients according to severity. Generally, the sodium, potassium, pH, pO2 and HB findings were similar among severity classifications. Elevated troponin was only reported in the severe to critical group with 11.98 pg/mL [95% CI: 3.59–39.96, p < 0.01]. Comparing with mild to moderate and pneumonia patients, the findings of Glucose, lactate, CK, LDH, D‐dimer, C‐ CRP, procalcitonin of severe to critical individuals were the highest. By contrast, while myoglobin and serum ferritin was the highest among pneumonia patients, the mild to moderate group had the highest value of pO2/FiO2. For coagulation tests, the results of PT and APTT were similar among the three groups: mild to moderate, severe to critical, and pneumonia. Regarding the test of liver function, ALT and AST of severe to critical group obtained the highest value with 41.98 U/L [95% CI: 28.71–61.39, p < 0.01] and 47.17 U/L [95% CI: 28.24–78.78, p < 0.01], respectively; bilirubin among classifications was relatively similar. For findings of kidney function, the severe to the critical group showed higher values with 88.84 μmol/L [95% CI: 62.33–126.62, p < 0.01] of creatinine, and 6.12 mmol/L [95% CI: 4.96–7.54, p < 0.01] of urea. In terms of blood cells, while only monocytes showed similar results among the three groups, the value of lymphocytes 1.18 × 10⁹ cells/L [95% CI: 1.09–1.27, p < 0.01], and platelet 191.61 × 10⁹ cells/L [95% CI: 173.11–212.09, p < 0.01] was the highest in mild to moderate group, neutrophil 5.05 × 10⁹ cells/L [95% CI: 4.22–6.04, p < 0.01], and total WBCs 6.48 × 10⁹cells/L [95% CI: 5.74–7.33, p < 0.01] was the highest in severe to critical.
FIGURE 3.
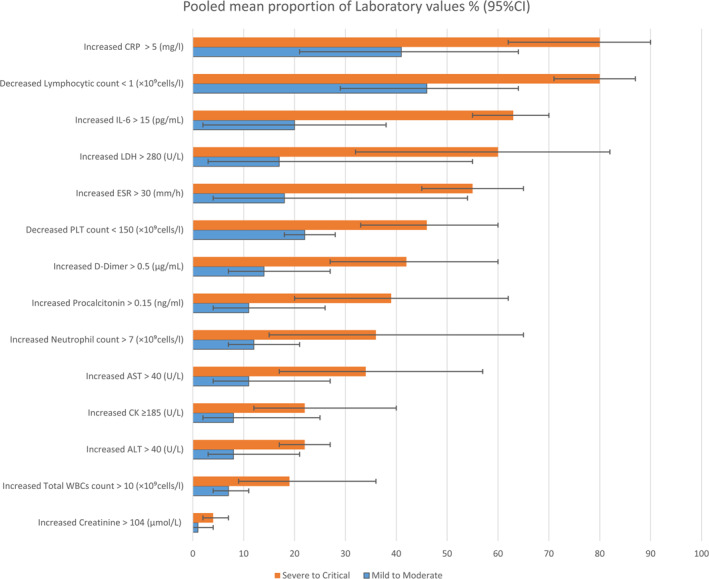
The pooled proportion of laboratory values on severity grading. CRP, C‐reactive protein
For laboratory values of Covid‐19 were presented in Table S9 and Figure 4 with a comparison between survivors and non‐survivors. In general, non‐survivors have worse laboratory values in most tests. However, the value of some findings was similar in the comparison of survivors and non‐survivor, including sodium level, PaCO2, lactate, coagulation tests (PT, APTT), bilirubin level and haemoglobin. By contrast, the non‐survivor group had indicators of decreased pO2, albumin, lymphocyte and platelet values in the non‐survivor group. Several variables only reported in non‐survivor namely pO2/FiO2, pH, glucose, myoglobin, IL‐6 and monocyte with 189.12 mmHg [95% CI: 152.09–235.17, p = 0.62], 7.37 [95% CI: 7.24–7.5, p < 0.01], 9.42 mmol/L [95% CI: 8.47–10.49, p = 0.85], 142.75 U/L [95% CI: 77.62–262.52, p < 0.01], 223.97 pg/mL [95% CI: 88.68–565.67, p < 0.01], 0.37 × 10⁹cells/L [95% CI: 0.3–0.46, p = 0.05], respectively.
FIGURE 4.
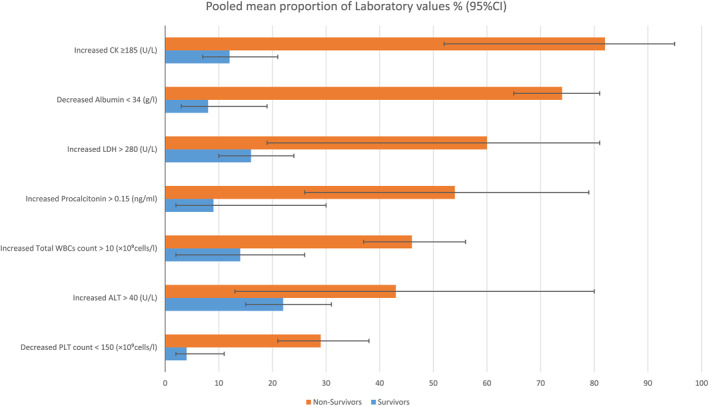
The pooled proportion of laboratory values according to death status
3.7. Radiological CT findings of Covid‐19
Regarding the CT imaging findings of Covid‐19, we demonstrated the most typical presentation was bilateral GGO 0.68 [95% CI: 0.59–0.75, p < 0.01] with multiple mottling pattern 0.65 [95% CI: 0.49–0.78, p < 0.01] which most of lesions bilaterally involved 0.69 [95% CI: 0.6–0.77, p < 0.01]. Right lung (0.6 [95% CI: 0.42–0.76, p < 0.01]) are more affected than left (0.45 [95% CI: 0.3–0.61, p < 0.01]). For pleural and pericardial effusion were very rare 0.07 [95% CI: 0.05–0.11, p < 0.01] and 0.05 [95% CI: 0.03–0.08, p = 0.1], accordingly (Table 2).
3.8. Covid‐19 treatments
Some specific treatments have been applied to COVID‐19 infections in the meta‐analysis of included studies. The results are summarised in Table S10. Generally, antiviral was the most common recipe, followed by antibiotics. Compared to the survivor group, all treatments were applied with higher frequency in non‐survivor group. ECMO therapy was only reported within death group (0.02 [95% CI: 0.00–0.07, p = 0.46]). Among patients with severe to critical, common treatments were antiviral (0.89 [95% CI: 0.74–0.96, p < 0.01]), antibiotic (0.87 [95% CI: 0.7–0.95, p < 0.01]), oxygen therapy (0.85 [95% CI: 0.48–0.97, p < 0.01]), corticosteroid (0.64 [95% CI: 0.53–0.73, p < 0.01]), and immunoglobulin therapy (0.61 [95% CI: 0.45–0.74, p < 0.01]). Antiviral, antibiotic, nasal cannula, and corticosteroid were four popular therapies in mild to moderate group with mean pooled value: 0.87 [95%: 0.58–0.97, p < 0.01], 0.5 [95%: 0.14–0.87, p < 0.01], 0.14 [95%: 0.00–0.84, p < 0.0001], and 0.13 [95%: 0.03–0.41, p < 0.01], respectively. Also, CKRT were unavailable in this group. Similarly, pneumonia group used therapies of antiviral (0.92 [95% CI: 0.85–0.96, p < 0.01]), antibiotic (0.84 [95% CI: 0.48–0.97, p < 0.01]), nasal cannula (0.56 [95% CI: 0.02–0.98, p < 0.0001]) and immunoglobulin therapy (0.35 [95% CI: 0.25–0.47, p < 0.01]) as common treatments.
3.9. Covid‐19 complications
Vigorous complications have been found to be more attributed to severe Covid‐19 patients than other groups. In our single proportion meta‐analysis, ARDS showed to be highly associated among different severity groups, accounting for 0.49 [95% CI: 0.29–0.78, p < 0.01] in severe to critical, 0.23 [95% CI: 0.09–0.45, p < 0.01] in pneumonia and 0.04 [95% CI: 0.01–0.05, p = 0.06] in mild to moderate group. Myocardial injury and septic shock have also been reported mainly in severe to critical patients, with 0.23 [95% CI: 0.11–0.34, p = 0.06] and 0.22 [95% CI: 0.04–0.26, p < 0.01], respectively. AKI was the most common in pneumonia group, with 0.08 [95% CI: 0.02–0.3, p < 0.01]; followed by severity to critical group, with 0.24 [95% CI: 0.06–0.3, p < 0.01] and 0.03 [95% CI: 0–0.03, p = 0.08] in mild to moderate group. DIC was the most uncommon complication. Regarding clinical outcomes, most of patients having ARDS have been alive (0.37 [95% CI: 0.02–0.39, p < 0.01]), compared to 0.17 [95% CI: 0.01–0.18, p = 0.04] in patients with AKI and 0.11 [95% CI: 0.05–0.16, p = 0.09] myocardial injury. The death was mostly observed in the group of myocardial injury, taking up 0.81 [95% CI: 0.04–0.85, p < 0.01], followed by ARDS and AKI (Table S11).
3.10. Subgroup and sensitivity analysis results
Our subgroup analysis showed consistency results among all groups through comorbidities, symptoms, laboratory findings, vital signs, and complications by removing ‘adult and children’ studies which are Jiuling/2020/China, 29 Tang/2020/China, 24 Yu‐Huan Xu/2020/China, 21 Qian/2020/China, 30 Wu/2020/China, 31 Zhang/2020/China, 32 Hu/2020/China, 33 Liu/2020/China 34 and Tian/2020/China. 35 Details were visualised in Table S12. The meta‐regression analysis presented in Appendix 1 revealed a higher incidence of either cardiovascular disease, hypertension or diabetes in mild‐moderate, and pneumonia groups in older people with higher mean CRP and lower mean lymphocytic count. Our secondary comparative meta‐analysis results were included in Appendix 2.
3.11. Covid‐19 death outcomes
Table S13 presents the mortality rate by severity using a single proportion pool. The death rate happened commonly in severe critical, followed by pneumonia and mild to moderate group. The single proportion pooled rate was 0.11 [95% CI: 0.06–0.19, p < 0.01], 0.03 [95% CI: 0.01–0.05, p < 0.01] and 0.01 [95% CI: 0–0.3, p < 0.01], respectively.
3.12. Quality assessment
Most studies in this review were ‘fair’ in terms of bias. The entire two case‐control studies were fair. Among the 90 case reports/case series studies, 53 studies obtained ‘good’ quality, 34 studies matched the ‘fair’, and the rest three studies were in ‘poor’ quality. The overall rating based on the 13 criteria among observation cohort/cross‐sectional studies showed that 19 of the studies were of ‘good’ quality, up to 50 studies were of ‘fair’ quality, and only 10 studies were of ‘poor’ quality. However, none of these studies were excluded from our study (Tables S3 and S4).
4. DISCUSSION
Since Covid‐19 has dramatically impacted every aspect of human life, it is necessary to know the nature of this disease in detail. Many studies and case reports have been published in major journals for patients with/without travel history, whether from China or other countries. These reports gave clues about unsolved questions, including disease progression and expected outcomes, as well as potential predisposing factors. We aimed to delineate the clinical features, complications, laboratory diagnosis, treatment methods and clinical outcomes. Thus, we can predict a better management option through studying these items for each severity group.
In general, our systematic review (SR) has divided the severity of Covid‐19 disease into five categories: mild to moderate, severe to critical, pneumonia, survivor and death groups. We believe that these different datasets can remove the heterogeneity resulted from combining them into a single Covid‐19 group and help in better understanding the diversity of Covid‐19. We have included 171 different full‐texts, of whom 148 have entered the final analysis between January and March 2020, which is considered higher relatively similar to previous SRs Covid‐19 articles (Figure 1, Tables S3 and S4). 36 , 37
Subsequently, we proved in our study that hypertension and diabetes were the most common comorbidities associated with a more frequent higher mortality rate, which was consistent with other reports of Covid‐19. 38 , 39 , 40 , 41 In detail, the patients with severe to critical and death group always had a higher mean pooled comorbidity rate of hypertension were 0.32 and 0.48, respectively, compared to mild to moderate patients 0.17. For diabetes, the measures were 0.19 and 0.23 compared to 0.08, respectively. Furthermore, we estimated ORs for diabetes to be 0.31 for mild‐moderate versus severe‐critical and 0.06 for survivor versus non‐survivor groups and for hypertension 0.51 and 0.02 respectively (Figure S8 and S13 in Appendix 2). Moreover, smoking was found to be a considerable risk factor for severity 0.15 and death 0.12 mean pooled proportion (PP) of current smokers compared to 0.1 for mild disease, which the PP reached 0.88. Reddy et al. 42 has estimated the pooled risk ratio (RR) of smoking to progress to severe or critical Covid‐19 disease to be 1.31 and for hospital mortality 1.26 in patients with smoking history. Furthermore, cerebrovascular and cardiovascular could help in a poorer prognosis of disease linked with critical illness (PP: 0.08, 0.12) in and for non‐survivors (PP: 0.18, 0.23) in contrast to a mild‐moderate group which showed the least association (PP: 0.01, 0.06) respectively. The RR has calculated in another SR study to be 2.38 and 2.25 for mortality and 1.88 and 2.25 for severity accordingly (Tables 1 and S5). 43 , 44
The findings of vital signs presented that most indicators were not a large range of differences among severity groups apart from SpO2. Our study cannot support the notion that tachypnea and tachycardia have a solid relation to severe disease and death. However, results from other studies showed that tachyarrhythmia is among severity levels was higher. 45 SBP and DBP were observed as significant indicators for mortality in Covid‐19 but not in our study. 46 The best marker for severeness was SpO2, with a mean estimated at 92.36 in critically severe compared to 97.02 mild patients. Moreover, the same study was counted as a risk factor for mortality which agreed with our study findings [ raw mean: 85.76 for mortality compared to 89.9 for survival] (Table S6). 46
The emergence of symptoms was noticed in an escalating manner in the group of mild and moderate symptoms, pneumonia, severe and critical to the group of non‐survivors. The major clinical features noted were fever and cough, consistent with other SRs. 47 , 48 Besides, we detected that dyspnea, chest pain and anorexia were relatively ominous clinical signs for the seriousness of the disease and mortality. These findings were indeed the same as the recent WHO classification of Covid‐19 severity. Alizadehsani et al. 49 has estimated the significant value of the p‐value of anorexia as a risk factor for mortality to be 0.04 in a prospective study compared to healthy individuals. Regarding respiratory imaging features, bilateral GGO involvement with multiple mottling is the most common radiological feature in our SR., with a little higher proportion of lesions seen on the right lung. These findings resemble imaging features of other reports (Tables 2, 3 and S7). 36 , 50
TABLE 3.
Meta‐analysis summary of Covid‐19 patients' symptoms according to severity status
| Variable | Mild to moderate | Severe to critical | Pneumonia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled single proportion (95% CI) | No. of studies | p value | Total | Pooled single proportion (95% CI) | No. of studies | p value | Total | Pooled single proportion (95% CI) | No. of studies | p value | Total | ||
| Systemic symptoms | |||||||||||||
| Chills | 0.10 (0.09–0.12) | 3 | 0.6 | 1285 | 0.16 (0.12–.21) | 3 | 0.39 | 278 | ‐ | ‐ | ‐ | ‐ | |
| Dizziness | 0.06 (0.02–0.19) | 6 | <0.01 | 902 | 018 (0.13–0.25) | 5 | 0.11 | 241 | 0.09 (0.05–0.15) | 5 | 0.03 | 433 | |
| Enlargement of lymph nodes | 0.004 (0–0.03) | 2 | 0.08 | 1143 | 0.01 (0–0.04) | 2 | 0.38 | 223 | ‐ | ‐ | ‐ | ‐ | |
| Fatigue | 0.25 (0.15–0.39) | 14 | <0.01 | 2700 | 0.40 (0.27–0.54) | 15 | <0. 01 | 645 | 0.21 (0.13–0.32) | 17 | <0. 01 | 2759 | |
| Fever | 0.79 (0.72–0.86) | 22 | <0.01 | 3485 | 0.84 (0.77–0.89) | 23 | <0.01 | 1054 | 0.82 (0.77–0.86) | 32 | <0.01 | 3843 | |
| <37.3 | 0.26 (0.09–0.56) | 4 | <0.01 | 1771 | 0.34 (0.13–0.64) | 4 | <0. 01 | 371 | 0.20 (0.12–0.31) | 6 | <0. 01 | 857 | |
| 37.3–38 | 0.38 (0.24–0.54) | 7 | <0.01 | 2025 | 0.31 (0.21–0.44) | 7 | <0. 01 | 434 | 0.31 (0.26–0.37) | 7 | 0.02 | 891 | |
| 38.1–39 | 0.27 (0.20–0.35) | 7 | <0.01 | 2025 | 0.29 (0.18–0.42) | 7 | <0.01 | 434 | 0.32 (0.29–0.36) | 6 | 0 | 777 | |
| >39 | 0.08 (0.03–0.23) | 5 | <0.01 | 1657 | 0.09 (0.04–0.22) | 5 | 0.04 | 245 | 0.12 (0.05–0.25) | 6 | <0. 01 | 777 | |
| Headache | 0.10 (0.08–0.13) | 14 | 0.06 | 2135 | 0.13 (0.10–0.18) | 16 | 0.17 | 642 | 0.09 (0.06–0.14) | 19 | <0. 01 | 2974 | |
| Myalgia | 0.11 (0.06–0.21) | 9 | <0.01 | 1183 | 0.14 (0.08–0.22) | 12 | 0.01 | 354 | 0.18 (0.13–0.23) | 15 | <0. 01 | 874 | |
| Respiratory symptoms | |||||||||||||
| Chest pain | 0.03 (0.01–0.19) | 5 | <0.01 | 1006 | 0.06 (0.02–0.15) | 7 | 0.02 | 290 | 0.05 (0.02–0.10) | 10 | <0. 01 | 1680 | |
| Cough | 0.59 (0.49–0.67) | 23 | <0. 1 | 3380 | 0.65 (0.55–0.74) | 25 | <0. 01 | 990 | 057 (0.48–0.64) | 34 | <0. 01 | 3979 | |
| Dyspnea | 0.06 (0.03–0.13) | 14 | <0.01 | 1871 | 0.35 (0.21–0.51) | 15 | <0. 01 | 543 | 0.20 (0.12–0.33) | 14 | <0. 01 | 1433 | |
| Haemoptysis | 0.01 (0–0.02) | 3 | 0.22 | 1171 | 0.03 (0.01–0.07) | 3 | 0.51 | 236 | ‐ | ‐ | ‐ | ‐ | |
| Nasal congestion | 0.10 (0.05‐0.17) | 5 | <0.01 | 1365 | 0.14 (0.03–0.42) | 3 | <0.01 | 259 | 0.20 (0.11–0.34) | 7 | <0.01 | 1536 | |
| Rhinorrhoea | 0.07 (0.05–0.10) | 2 | 0.57 | 339 | 0.07 (0.03–015) | 3 | 0.46 | 90 | 0.06 (0.01–0.19) | 4 | <0.01 | 1311 | |
| Shortness of breath | 0.15 (0.13–0.17) | 4 | 0.27 | 986 | 0.38 (0.32–0.45) | 4 | 0.14 | 201 | 0.15 (0.08–0.27) | 10 | <0.01 | 1597 | |
| Sore throat | 0.09 (0.02–0.29) | 7 | <0. 01 | 1878 | 0.09 (0.04–0.22) | 9 | 0.03 | 357 | 0.07 (0.04–0.12) | 12 | <0.01 | 2024 | |
| Sputum production | 0.15 (0.09–0.24) | 12 | <0.01 | 2293 | 0.24 (0.17–0.32) | 13 | <0.01 | 496 | 0.16 (0.11–0.24) | 17 | <0.01 | 2184 | |
| Stuffy and runny nose | ‐ | ‐ | ‐ | ‐ | 0.19 (0.07–0.41) | 2 | 0.36 | 23 | 0.09 (0.02–0.27) | 2 | 0.07 | 142 | |
| Digestive symptoms | |||||||||||||
| Abdominal pain | 0.02 (0.01–0.04) | 4 | 0.65 | 452 | 0.08 (005–0.12) | 4 | 0.41 | 237 | 0.08 (0.05–0.12) | 4 | 0.41 | 340 | |
| Anorexia | 0.09 (0.02–0.29) | 6 | 0.01 | 1206 | 0.2 (0.07–0.44) | 6 | 0.01 | 322 | 0.11 (0.05–0.25) | 7 | 0.01 | 695 | |
| Diarrhoea | 0.07 (0.05–0.1) | 18 | 0.01 | 2282 | 0.09 (0.07–0.12) | 19 | 0.2 | 797 | 0.09 (0.06–0.12) | 24 | 0.01 | 1974 | |
| Nausea | 0.05 (0.01–0.19) | 5 | 0.01 | 923 | 0.08 (0.05–0.12) | 6 | 0.45 | 278 | 0.12 (0.08–0.17) | 4 | 0.11 | 51 | |
| Pharyngalgia | 0.11 (0.08–0.15) | 7 | 0.07 | 723 | 0.13 (0.08–0.22) | 7 | 0.02 | 276 | 0.08 (0.03–0.2) | 4 | 0.11 | 330 | |
| Vomiting | 0.01 (0–0.06) | 4 | 0.01 | 841 | 0.09 (0–0.51) | 5 | 0.51 | 225 | 0.06 (0.04–0.08) | 5 | 0.58 | 416 | |
Note: p‐value of heterogeneity is significant when <0.1.
Consequently, our analysis has broadly distinguished the laboratory investigation results in terms of severity and mortality. Firstly, we realised that Covid‐19 didn’t largely affect the hepatic function with a slight or no increase of liver enzymes in most cases. However, secondary end liver damage may be seen in dead individuals with 74% with hypoalbuminemia, 43% with increased ALT, and 53.84 in the raw mean of AST. Peishan et al. 51 has measured them to be −4, 5.07, 14.26, respectively, in mean difference with significant outcomes (p‐value <0.05). Overall, Covid‐19 patients have an increase in CK, CRP, procalcitonin, LDH, Urea, creatinine, D‐dimer, PT, ESR, IL‐6, neutrophils, and total WBCs and a decrease in pO2/FiO2, pO2, lymphocyte, and PLT values in critical and non‐survivor groups compared to other groups. A similar pattern of laboratory features has been identified in previous SRs (Tables S8 and S9, Figures 3 and 4). 39 , 50
Considerably, although we found in our analysis, the most common treatment used was antiviral and antibiotic drugs. The increasing rate of using corticosteroids, ECMO, NIV, CKRT, MIV, immunoglobulin and oxygen therapy was distinctly witnessed in severity and mortality groups as rescue treatments. The efficacy of these treatments was discussed in a network configuration by Siemieniuk et al. 52 who proved that increased corticosteroid use in mechanical ventilation could reduce fatality risk (Table S10). For the impact of anti‐hypertensive drugs (ACEIs/ARBs), several authors found that ACEI/ARB use has no association with increased in‐hospital severity or mortality. 53 , 54 We only included a single study that reported the incidence among Covid‐19 discharged/death patients. But it doesn`t showed any association to the mortality (p‐value > 0.99). 55 Even better, Zhang et al. suggested lower all‐cause mortality in ACEI/ARB using inpatient compared with non‐users. 56 The explanation could be that ACEI or ARB therapy relates to decreased peak viral load, improved CD3 and CD4 count, and lower levels of IL‐6 in peripheral blood, hence lower the rate of severe diseases. 57
Many studies have shown that various complications, including multisystem organ failure, including acute respiratory, cardiac, renal failure and even mortality, are in line with current reports. 58 , 59 , 60 , 61 , 63 Our results also showed ARDS, heart injury, septic, AKI were common complications in non‐survivor and severe to critical and occurred much less often in the mild disease and survivor groups (Table S11).
Our outcomes of interest are restricted to adults‐only studies, which showed symmetry with our previous findings in Table S12. However, heterogeneity was still high for non‐specified reasons. Most of the previous SRs reported the same heterogeneity even with including only adults into their analysis system. 47 It indicated that the Covid‐19 behaviour pattern was so hard to be predicted. However, our study can present a new approach for classifying this disease through different datasets of severity, pneumonia, and dying patients.
4.1. Limitations of our study
The studies included in our review were heterogeneous. Some included studies lack a detailed patient history, particularly in clinical features or criteria of suspicion. Values missing due to incomplete data presented in some results can be considered a limitation in our SR. However, our severity grading has reduced heterogeneity. Besides, uncontrollable factors of different ages, gender, ethnicity, comorbidities and people’s normal physiological and psychological response to multiple genetic variants of SARS‐CoV‐2 in most of the included studies could play a role. The rapid rise in recent publications of Covid‐19 could be lost in our search after the exact dates we set. However, the main point of our study is to reproduce the epidemiology and clinical symptoms of Covid‐19 with more accessible key evidence of severity shaped by the environment based on a meta‐analysis.
5. CONCLUSION
Covid‐19 has been established in various severity forms and characteristic groups such as pneumonia and fatal cases, which are distinguished in our data. The current study provides a comprehensive guide for the health professionals on what to expect from Covid‐19 patients. It will help to develop suspicious criteria and a more effective screening tool for patients' contacts. The findings shed light on the possible involvement of the gastrointestinal tract in the pathogenesis, with more attention given to any related symptoms that may precede fever and cough so far. Updated suspicion criteria should be made with associated cost‐benefit‐oriented screening protocols and prompt isolation of the confirmed patients.
CONFLICT OF INTERESTS
We declare that there is no conflict of interests while conducting this study.
AUTHOR CONTRIBUTIONS
Author Le Huu Nhat Minh, Ali Ahmed‐Fouad Abozaid, Nam Xuan Ha, Loc Le Quang, Nguyen Tien Huy have given substantial contributions to the conception and the design of the manuscript, author Le Huu Nhat Minh, Ali Ahmed‐Fouad Abozaid, Nguyen Dang Kien acquisition, analysis, and interpretation of the data. All authors have participated in drafting the manuscript, while author Nguyen Tien Huy revised it critically. All authors read and approved the final version of the manuscript.
Supporting information
Supplementary material 1
Supplementary material 2
Supplementary material 3
ACKNOWLEDGMENT
We would like to thank Professor Paul Griffiths, as well as the Editors and Reviewers of the Journal Review in Medical Virology for their insightful comments which improved our manuscript. No funding was received for this article.
Minh LHN, Abozaid AA‐F, Ha NX, et al. Clinical and laboratory factors associated with coronavirus disease 2019 (Covid‐19): a systematic review and meta‐analysis. Rev Med Virol. 2021;31(6):e2288. 10.1002/rmv.2288
Le Huu Nhat Minh, Ali Ahmed‐Fouad Abozaid, Nam Xuan Ha, and Loc Le Quang equally contributed to the work.
REFERENCES
- 1. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Emergencies Preparedness Team . What Are the Symptoms of COVID‐19? World Health Organization; 2020. Accessed March 17, 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/question‐and‐answers‐hub/q‐a‐detail/coronavirus‐disease‐covid‐19#:%E2%88%BC:text=symptoms [Google Scholar]
- 4. Koyama T, Platt D, Parida L. Variant analysis of SARS‐CoV‐2 genomes. Bull World Health Organ. 2020;98:495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Emergency Response Team . COVID‐19 Weekly Epidemiological Update, 9 March 2021. World Health Organization; 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐‐‐10‐march‐2021 [Google Scholar]
- 6. WHO Emergencies Preparedness Team . Are There Treatments for COVID‐19? World Health Organization. 2020. Accessed March 18, 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/question‐and‐answers‐hub/q‐a‐detail/coronavirus‐disease‐covid‐19#:~:text=protect [Google Scholar]
- 7. Brotherton H, Usuf E, Nadjm B, et al. Dexamethasone for COVID‐19: data needed from randomized clinical trials in Africa. Lancet Glob Health. 2020;8:e1125‐e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lester M, Sahin A, Pasyar A. The use of dexamethasone in the treatment of COVID‐19. Annals of Medicine and Surgery, 2020;56:218–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324:1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2021;325:632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta‐1a (SNG001) for treatment of SARS‐CoV‐2 infection: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2021;9:196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N. Engl J Med. 2021;384:795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hermine O, Mariette X, Tharaux P‐L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID‐19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Internal Medicine. 2021;181:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon AC, Mouncey PR, Al‐Beidh F, et al. Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N. Engl J Med, 2021;384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: time for a paradigm change. Rev Med Virology, 2020;30(5):e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1‐e34. [DOI] [PubMed] [Google Scholar]
- 17. Minh LHN, Loc LQ, Ahmed‐Fouad Abozaid A, et al. Clinical aspect of novel Coronavirus (2019‐nCoV): a systematic review and meta‐analysis. PROSPERO CRD42020167929; 2020. [Google Scholar]
- 18. Tawfik GM, Dila KAS, Mohamed MYF, et al. A step by step guide for conducting a systematic review and meta‐analysis with simulation data. Trop Med Health. 2019;47:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO Headquarters . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. World Health Organization; 2020. https://www.who.int/publications/i/item/10665‐331501 [Google Scholar]
- 20. NHLBI . Study Quality Assessment Tools; National Heart, Lung, and Blood Institute; 2018. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools [Google Scholar]
- 21. Xu Y‐H, Dong J‐H, An W‐M, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect. 2020;80:394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thrombosis Haemostasis. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langan D, Higgins JP, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random‐effects meta‐analyses. Res Synthesis Methods. 2019;10:83‐98. [DOI] [PubMed] [Google Scholar]
- 26. Giang HTN, Banno K, Minh LHN, et al. Dengue hemophagocytic syndrome: a systematic review and meta‐analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virology. 2018;28:e2005. [DOI] [PubMed] [Google Scholar]
- 27. Huedo‐Medina TB, Sánchez‐Meca J, Marín‐Martínez F, Botella J. Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193‐206. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 29. Mannan SB, Elhadad H, Loc TTH, et al. Prevalence and associated factors of asymptomatic leishmaniasis: a systematic review and meta‐analysis. Parasitol Int. 2021;81:102229. doi: 10.1016/j.parint.2020.102229 [DOI] [PubMed] [Google Scholar]
- 30. Cheng J, Huang C, Zhang G, et al. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Chin J Tuberc Respir Dis. 2020;43:E027‐E027. https://pubmed.ncbi.nlm.nih.gov/32118390/ [DOI] [PubMed] [Google Scholar]
- 31. Qian G‐Q, Yang N‐B, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: a retrospective, multi‐centre case series. QJM . Int J Med. 2020;113:474‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Z, Wu W, Jin Y & Pan A Key points of clinical and CT imaging features of 2019 novel coronavirus (2019‐nCoV) imported pneumonia based on 21 cases analysis. Available at SSRN 3543610 2020.
- 33. Zhang J, Yang S, Xu Y, et al. Epidemiological and Clinical Characteristics of COVID‐19 Infection outside Wuhan, China: A Multicenter Study; 2020. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546040 [Google Scholar]
- 34. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei Y, Lu Y, Xia L, et al. Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: an epidemiological study from a fever clinic in Wuhan, China. J Med Virology. 2020;92:2758‐2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID‐19) CT findings: a systematic review and meta‐analysis. J Am Coll Radiology. 2020;17:701‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galbadage T, Peterson BM, Awada J, et al. Systematic review and meta‐analysis of sex‐specific COVID‐19 clinical outcomes. Front Med. 2020;7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben‐Shoshan M. COVID‐19 and comorbidities: a systematic review and meta‐analysis. PGM (Postgrad Med). 2020;132:749‐755. [DOI] [PubMed] [Google Scholar]
- 40. Ebrahimi M, Malehi AS, Rahim F. COVID‐19 patients: a systematic review and meta‐analysis of laboratory findings, comorbidities, and clinical outcomes comparing medical staff versus the general population. Osong Public Health Res Perspect. 2020;11:269‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia–a systematic review, meta‐analysis, and meta‐regression. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020;14:395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID‐19 pneumonia: a systematic review, meta‐analysis and meta‐regression. J Renin‐Angiotensin‐Aldosterone Syst JRAAS. 2020:21(2):1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID‐19 severity: a systematic review and meta‐analysis. J Med Virology. 2021;93:1045‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID‐19–systematic review, meta‐analysis, and meta‐regression. J Stroke Cerebrovasc Dis. 2020;29:104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID‐19: systematic review and meta‐analysis. Am J Emerg Med, 2020;46:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shafi AM, Shaikh SA, Shirke MM, Iddawela S, Harky A. Cardiac manifestations in COVID‐19 patients—a systematic review. J Cardiac Surg. 2020;35:1988‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Du M, Zhao J, Yin X, Zhang N, Zheng G. The Impact of Vital Signs on the Death of Patients with New Coronavirus Pneumonia: A Systematic Review and Meta‐Analysis; 2020. medRxiv. doi: 10.1101/2020.09.17.20196709 [DOI] [Google Scholar]
- 48. Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS‐CoV‐2; COVID‐19): a systematic review and meta‐analysis of 148 studies from 9 countries. PloS One, 2020;15:e0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. da Rosa Mesquita R, Junior LCFS, Santana FMS, et al. Clinical manifestations of COVID‐19 in the general population: systematic review. Wien Klin Wochenschr. 2020;133:377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alizadehsani R, Alizadeh Sani Z, Behjati M, et al. Risk factors prediction, clinical outcomes, and mortality in COVID‐19 patients. J Med Virology. 2021;93:2307‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Trav Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qiu P, Zhou Y, Wang F, et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID‐19 deceased: a systematic review and meta‐analysis. Aging Clin Exp Res, 2020;32:1869‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid‐19: living systematic review and network meta‐analysis. BMJ. 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid‐19. N. Engl J Med. 2020;382:2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiology. 2020;5:825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen M, Fan Y, Wu X, et al. Clinical Characteristics and Risk Factors for Fatal Outcome in Patients with 2019‐coronavirus Infected Disease (COVID‐19) in Wuhan. China; 2020. [Google Scholar]
- 57. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circulation Res. 2020;126:1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9:757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. SeyedAlinaghi S, Afsahi AM, MohsseniPour M, et al. Late complications of COVID‐19; a systematic review of current evidence. Arch Acad Emerg Med; 2021;9(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kunutsor SK, Laukkanen JA. Renal complications in COVID‐19: a systematic review and meta‐analysis. Ann Med. 2020;52:345‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID‐19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta‐analysis. Expet Rev Respir Med. 2020;14:1149‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID‐19: systematic review and meta‐analysis. Can J Kidney Health Dis. 2020;7:2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ninh TT, Dong V, Loc LQ, et al. COVID‐19 induced immunosuppression leading to secondary infection in a non‐HIV patient. Minerva Respir Med. 2021;60:(2). doi: 10.23736/s2784-8477.21.01914-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3


