Summary
Light is an important regulator of daily human physiology in providing time‐of‐day information for the circadian clock to stay synchronised with the 24‐hr day. The coronavirus disease 2019 (COVID‐19) pandemic led to social restrictions in many countries to prevent virus spreading, restrictions that dramatically altered daily routines and limited outdoor daylight exposure. We previously reported that sleep duration increased, social jetlag decreased, and mid‐sleep times delayed during social restrictions (Global Chrono Corona Survey, N = 7,517). In the present study, we investigated in the same dataset changes in wellbeing and their link to outdoor daylight exposure, and sleep–wake behaviour. In social restrictions, median values of sleep quality, quality of life, physical activity and productivity deteriorated, while screen time increased, and outdoor daylight exposure was reduced by ~58%. Yet, many survey participants also reported no changes or even improvements. Larger reductions in outdoor daylight exposure were linked to deteriorations in wellbeing and delayed mid‐sleep times. Notably, sleep duration was not associated with outdoor daylight exposure loss. Longer sleep and decreased alarm‐clock use dose‐dependently correlated with changes in sleep quality and quality of life. Regression analysis for each wellbeing aspect showed that a model with six predictors including both levels and their deltas of outdoor daylight exposure, sleep duration and mid‐sleep timing explained 5%–10% of the variance in changes of wellbeing scores (except for productivity). As exposure to daylight may extenuate the negative effects of social restriction and prevent sleep disruption, public strategies during pandemics should actively foster spending more daytime outdoors.
Keywords: circadian rhythms, light–dark cycle, resilience, screen time, sleep–wake behaviour
1. INTRODUCTION
An adaptation to Earth’s rotation is essential for survival, and has fostered the evolution of endogenous, circadian clocks, that synchronize (entrain) to cyclic environmental cues (zeitgebers) (Aschoff & Pohl, 1978). Light–dark cycles (LD) are the dominant zeitgeber for the human clock as shown in laboratory and real‐life studies (Pilz et al., 2018; Stothard et al., 2017; Wright et al., 2013). Individuals entrain differently to LD cycles, earlier or later, depending on the clock’s characteristics and zeitgeber strength. Circadian clocks adapt a stable relationship to the zeitgeber (“phase of entrainment” or chronotype), which range from extremely early (“larks”) to extremely late (“owls”), with the majority of individuals (“doves”) falling in between (Roenneberg et al., 2019). Chronotype depends on genes, age and sex (Roenneberg et al., 2004; Roenneberg et al., 2019), on geographic location (Leocadio‐Miguel et al., 2017) and zeitgeber strength (i.e. the maximum and minimum of the LD cycle) (Pilz et al., 2018).
In industrialised societies, people predominantly live inside and artificially illuminate the night, which weakens zeitgeber strength, thereby delaying chronotype in most people. Early schedules expose especially late chronotypes to a mismatch between circadian and social time (Wittmann et al., 2006), which is quantified as difference between mid‐sleep times on work and work‐free days. This social jetlag (SJL) has been linked to health‐risk behaviours and diseases (Mota et al., 2019; Wittmann et al., 2010).
Besides acting as a zeitgeber for the circadian clock, light promotes alertness, mood, vitality, cognitive function, and social interactions (Gaggioni et al., 2014; Partonen & Lonnqvist, 2000). Morning light can compensate for cognitive deficits, e.g. attention‐deficit hyperactivity disorder in adults (Korman, Palm, et al., 2020; Rybak et al., 2006). Light therapy is widely used to treat depression and mood disorders, e.g. seasonal affective disorder (SAD) (Sit et al., 2018; Wirz‐Justice et al., 2005). Light therapy is thought to activate dopaminergic (Kim et al., 2017), adrenergic (Bowrey et al., 2017), and serotonergic (Li, 2018) pathways that are directly linked to affect, emotion, mood, and melatonin production. The effects of light depend on time‐of‐day, duration, intensity, and its wavelength (Marshall, 2016; Wirz‐Justice et al., 2005). Integration of light over the day is also important (Leocadio‐Miguel et al., 2017). Notably, artificial light is mostly orders of magnitude lower compared to outdoor daylight. Light‐at‐night, especially blue light, alerts, suppresses melatonin levels (usually rising after dusk), and delays the circadian clock (Duffy & Czeisler, 2009). Light‐at‐night gains relevance with increased blue‐light bulbs and screens and is considered harmful to health (Marshall, 2016).
Social restrictions during the coronavirus disease 2019 (COVID‐19) pandemic were often associated with robust changes in outdoor daylight exposure (OLE), daily behaviour, and sleep. Although sleep worsened in many individuals, changes were positive at the population level: sleep duration increased and SJL decreased significantly (Gao & Scullin, 2020; Korman, Tkachev, et al., 2020; Leone et al., 2020; Wright et al., 2020). The more under‐slept and misaligned individuals were before social restrictions, the more they increased sleep duration or decreased SJL during social restrictions (Korman, Tkachev, et al., 2020). Sleep quality was not uniformly affected (Gao & Scullin, 2020; Kocevska et al., 2020; Leone et al., 2020). Notably, sleep quality improved in individuals who suffered from clinical insomnia prior to the pandemic (Kocevska et al., 2020), while it did not change in a USA sample (Gao & Scullin, 2020). These positive changes may reflect decreased social time pressure due to home offices, elimination of commutes or more relaxed work schedules (Korman, Tkachev, et al., 2020) and contrast reports of the pandemic’s negative impact on psychological and psychiatric wellbeing (Ozamiz‐Etxebarria et al., 2020), challenging mental health services worldwide (Thome et al., 2020).
It was proposed that managing sleep, along with stress, anxiety, and symptoms of depression is important during social restrictions (Altena et al., 2020). In the present study, we examined associations between changes in OLE, sleep–wake behaviour, and various aspects of wellbeing (sleep quality, quality of life, physical activity, screen time and productivity) in the same participants as in (Korman, Tkachev, et al., 2020). We expected the changes in wellbeing parameters to be cross‐correlated. We also hypothesised that the magnitude of the decrease in OLE during social restrictions would be associated with negative changes in wellbeing, particularly, in sleep quality, physical activity, and quality‐of‐life aspects, known to be related to OLE. In addition, we expected that changes in sleep duration and timing would be associated with those in wellbeing and OLE during social restrictions.
2. METHODS
The internet‐based Global Chrono Corona Survey (GCCS) was approved by the Ariel University Human Research Ethics Committee of the Faculty of Health Sciences (AU‐HEA‐MK‐20200629). Survey participants provided electronic consent to participate in the study. We collected data using the SoGoSurvey platform (Herndon, VA, USA), a cloud‐based platform for creation and distribution of multilingual surveys. The GCCS was translated into 10 languages (English, German, Hebrew, Arabic, Hindi, Japanese, Italian, Portuguese, Russian, and Spanish) by an international network of colleagues (see Acknowledgements). Recruitment methods included digital advertisements at universities, academic and non‐academic social networks, and email‐based approaches. Participation in the survey was anonymous.
The GCCS contained 40–54 items (for specifics of their implementation, see Korman, Tkachev, et al., 2020) concerning their daily behaviours and lifestyle separately for the time before social restrictions (preSocialRestriction [preSR]) and during restrictions (inSocialRestriction [inSR]). Each section included questions about current work/study status, whether working from home or/and shift/night work. The core GCCS questions were about the sleep times on work and on work‐free days, habitual use of alarm clock, and typical mealtimes (not evaluated for this report). To avoid misunderstandings, participants were explicitly and repeatedly reminded to use the 24‐hr time format.
The following measures of daily behaviour were calculated for each individual (please, avoid possible confusion of the acronyms for SD [sleep duration] and SR [social restrictions] in the present paper with the commonly used abbreviations in the sleep field for sleep deprivation and sleep restriction):
Mean sleep duration over the week (SD) was calculated as a weighted average of the sleep duration on workdays (SDW, assuming 5 workdays) and work‐free days (SDF), SD = (5* SDW + 2* SDF)/7).
Individual mid‐sleep time (MST) was defined as the mid‐point of sleep on free days (MSF) corrected for sleep deficit accumulated over the workweek (usually abbreviated MSFsc). MST is an indicator of the phase of entrainment or chronotype (Roenneberg, Pilz, et al., 2019).
SJL was calculated as the difference between mid‐point of sleep on free and workdays.
For individual deltas (ΔSD, ΔMST, ΔSJL), we subtracted the respective preSR values from those inSR.
Additionally, participants indicated their average OLE separately for workdays and work‐free days as falling into one of the following categories: <30 min, 30–60 min, 1–2 hr, 2–3 hr, 3–4 hr, 4–5 hr, 5–6 hr, 6–7 hr, >7 hr. Categorical answers were transformed to numerical values using the mid‐point of the category interval: 15 min, 45 min, …, 390 min, and 450 min (for >7 hr), respectively. Individual mean OLE over the week was calculated as a weighted average of OLE on workdays (5 days assumed) and work‐free days.
The GCCS also queried subjective wellbeing parameters using 5‐item Likert scales: Sleep‐Quality (got worse–got better), Quality‐of‐Life (got worse–got better), Physical‐Activity (increased–decreased), Screen‐Time (increased–decreased) and Productivity (got worse–got better), inSR compared to preSR. Participants’ responses were coded as: very negative (−2), negative (−1), no change (0), positive (+1), or very positive (+2) changes. We considered increased Screen‐Time as deleterious change, consistent with the extensive literature linking the use of light‐emitting devices to negative effects on sleep and circadian health (Chang et al., 2015).
2.1. Study participants
Demographic data were published previously (Korman, Tkachev, et al., 2020). In total, 11,431 respondents (aged ≥18 years) from 40 countries completed the GCCS during the first wave of SR (April 4 to May 16, 2020). The highest response rates (>200 respondents/country) were from Portugal, Italy, USA, UK, Germany, Israel, India, Russia, Japan, and Brazil. Exclusion criteria were a COVID‐19 diagnosis (1.1%), shift/night workers (16.3%), extreme sleep durations (<3 hr and >14 hr; 3.1%) and missing/invalid data (8.3%). To correct for the over representation of young (aged 18–22 years) participants from Russia compared with the other two leading countries (Japan and India), we randomly excluded 656 participants from Russia (5.7%). To this end, a uniform random selection procedure (rand function in Excel) was applied to a subgroup of Russian participants in the age group 18–22 years (N original = 1,006, N final = 350). The final sample included 7,517 participants (68.2% female), all under SR on the day of response. On average, participants had been under SR for 32.7 ± 9.1 days (range 10–59 days), presumably allowing full adjustment to new schedules. In all, 80% of respondents worked or studied both preSR and inSR. InSR, 66% worked from home (preSR, 11%). Full sociodemographic data can be found in the Supplementary Material (Table S1).
2.2. Data handling and statistical analysis
Data were pre‐processed as published (Korman, Tkachev, et al., 2020). We used non‐parametric data analyses due to the non‐normal distribution or homoscedastic nature of the behavioural data. One‐sample Wilcoxon tests (separate for each wellbeing parameter) were used to assess significant in Δscores and the Kruskal–Wallis H test (one‐way analysis of variance [ANOVA] on ranks) to assess significant differences between the three categories (negative, no change, or positive) in each of the Δscores (Sleep‐Quality, Quality‐of‐Life, Physical‐Activity, Screen‐Time, and Productivity) (Δ = qualitative change). Significant Kruskal–Wallis tests with η2 > 0.01 were followed up by pairwise Dunn test comparisons with Bonferroni corrections to determine which wellbeing categories differed. Spearman’s rank correlation analysis was performed to assess associations between Δscores, behaviours and OLE; p values were corrected for multiple comparisons where appropriate (cross‐correlation tests). Mann–Whitney U tests, using Glass rank biserial correlation as a measure of effect size (r g = 2[M1–M2]/N1+N2, where M1, M2 = mean ranks and N1, N2 = group sizes), compared between ad hoc groups (e.g. sex groups). Multiple linear regressions for six predictors (OLE, ΔSD, and ΔST and the actual values of OLE, SD, and ST inSR) of Δscores were performed with criterion Probability‐of‐F‐to‐enter ≤0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS®), version 26 (IBM Corp., Armonk, NY, USA) and R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria); p < 0.05 defined significance.
3. RESULTS
3.1. Changes in wellbeing during social restrictions
Reported impairments outnumbered reported improvements inSR (Figure 1a–e); medians differed significantly from “no change” for all queried aspects of wellbeing (separate one‐sample Wilcoxon tests, see Table S2). Changes in most wellbeing aspects (Δscores) were mostly negative: Quality‐of‐Life (49.6%), Physical‐Activity (51.0%), Productivity (66.8%) and Screen‐Free‐Time (74.3%). Notably, more participants reported no change in Sleep‐Quality (42.8%) than worsening (34.2%) or improving (23.0%).
FIGURE 1.
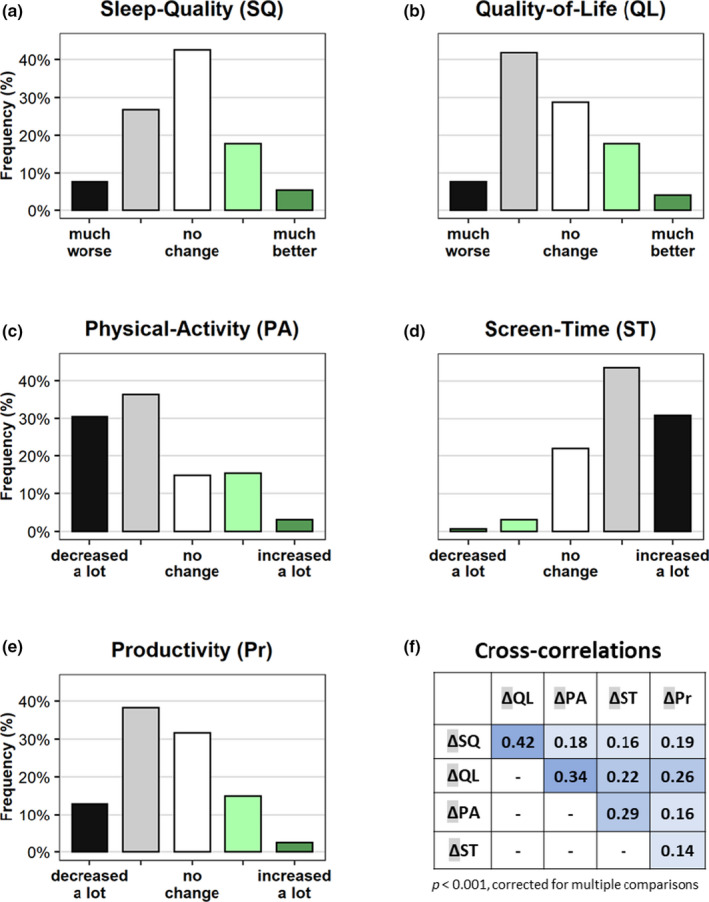
Subjective changes induced by social restrictions in five aspects of wellbeing: (a) Sleep‐Quality (SQ), (b) Quality‐of‐Life (QL), (c) Physical‐Activity (PA), (d) Screen‐Time (ST) and (e) Productivity (Pr) compared to the period before social restrictions. Black, very negative; grey, negative; white, no change; light green, positive; dark green, very positive changes. (f) Spearman’s bivariate correlations between Δscores in wellbeing, p values corrected for multiple comparisons. All p < 0.001. Colour‐coded by strength of correlation [Colour figure can be viewed at wileyonlinelibrary.com]
Women had more negative ΔSleep‐Quality, ΔQuality‐of‐Life, and ΔScreen‐Time scores than men (Mann–Whitney U tests between sex groups: p < 0.001, r g = 0.06; p = 0.033, r g = 0.03; p < 0.001, r g = 0.06, respectively), but the effect sizes of the differences between sexes were negligible (r g < 0.1). No sex differences were found for ΔPhysical‐Activity and ΔProductivity. The Δscores were age independent, with the only exclusion for Screen‐Time: older participants reported smaller increase in inSR (Spearman’s ρ = 0.222, p < 0.001).
All Δscores cross‐correlated (Figure 1f); the strongest links (ρ > 0.3) were found between the ΔSleep‐Quality and ΔQuality‐of‐Life, and between ΔPhysical‐Activity and ΔQuality‐of‐Life (Figure 1f).
3.2. Changes in OLE during social restrictions
The median weekly OLE was reduced from 1 hr 47 min (interquartile range [IQR] 2 hr 07 min) to 45 min (IQR 1 hr 15 min) inSR (Z = −63.47, p < 0.001, r = 0.73; Wilcoxon signed ranks test, Figure 2a–c). More than 70% of the sample reported less OLE inSR (47% reported reductions >1 hr; Figure 2b, grey bars; reported increases in OLE, green bars). Consistent decreases in OLE were obtained across workdays and free days (see Table S3). The ΔOLE was −72 ± 112 min. The ΔOLE correlated with age, with larger losses in young people (Spearman’s ρ = 0.15, p < 0.001), but there were no differences between the sexes.
FIGURE 2.
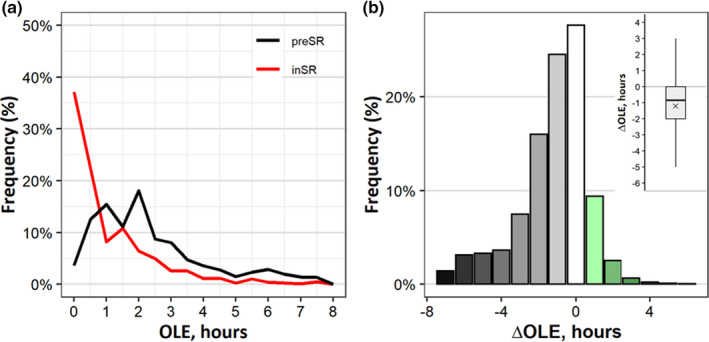
Outdoor light exposure (OLE) and its changes due to social restriction: (a) Distributions of OLE preSR (black line) and inSR (red line), percentage of total group. (b) The distribution of changes in light exposure (ΔOLE) in 1‐hr colour‐coded bins; white bars represent no change (±30 min change); green bars, gains; grey bars, losses. The inset in the upper right corner, boxplot of individual ΔOLE (hr). Positive values, increase; negative values, decrease in OLE. Whiskers, maximum and minimum values; box boundaries, 75th and 25th percentiles; line through the box, median; ×mark, mean; inSR, inSocialRestriction; preSR, preSocialRestriction [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Relationships between OLE, daily behaviour, and wellbeing
As previously reported (Korman, Tkachev, et al., 2020), SD increased by 15 min, MST delayed by 34 min, and SJL decreased by 30 min inSR. We examined associations between Δscores with changes in SD, MST, SJL and OLE, but also with their respective values.
3.3.1. Outdoor light exposure and wellbeing
Greater individual losses in OLE were associated with negative Δscores (Spearman’s ρ values: ΔSleep‐Quality (ρ = 0.16), ΔQuality‐of‐Life (ρ = 0.21), ΔPhysical‐Activity (ρ = 0.32), ΔScreen‐Time (ρ = 0.26); all significant at p < 0.001 Bonferroni corrected), except ΔProductivity.
To further explore these relationships, including whether they imply dose dependency, we analysed the association between ΔOLE and three post hoc subgroups (participants with negative, no change, or positive change in score in each wellbeing aspect) using Kruskal–Wallis H tests (see full analyses in the Table S4). Negative ΔOLE was associated with negative Δscores in four out of five wellbeing aspects: ΔSleep‐Quality, ΔQuality‐of‐Life, ΔPhysical‐Activity, and ΔScreen‐Time (H = 224, η 2 = 0.029; H = 288, η 2 = 0.038; H = 595, η 2 = 0.079; and H = 338, η 2 = 0.048, respectively, Figure 3). Kruskal–Wallis tests were followed by pairwise Dunn test comparisons between subgroups (results were Bonferroni corrected). The same four aspects also showed significant differences between the negative–positive and negative–no change subgroups. Only for ΔQuality‐of‐Life was the difference between the no change–positive pair significant.
FIGURE 3.
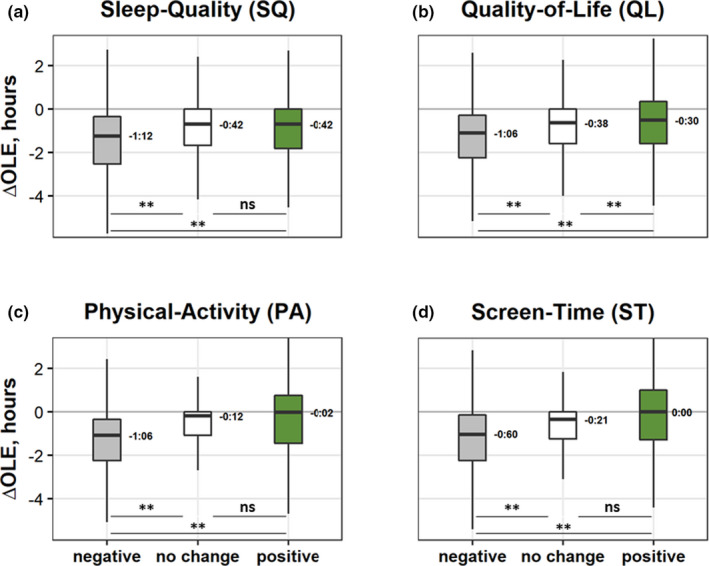
Boxplots of changes in outdoor daylight exposure (OLE), ΔOLE (hr), in the four aspects of wellbeing with significant Kruskal–Wallis tests, by subgroups: (a) ΔSleep‐Quality (negative, grey, N = 2,566; no change, white, N = 3,212; and positive, green, N = 1,730); (b) ΔQuality‐of‐Life (negative, N = 3,724; no change, N = 2,153; and positive, N = 1,629); (c) ΔPhysical‐Activity (negative, N = 5,016; no change, N = 1,109; and positive, N = 1,381); (d) ΔScreen‐Time (negative change, more screen time, negative N = 5,578; no change, N = 1,644; and positive, N = 1,629). Box boundaries, 75th and 25th percentiles; line through the box, median; numbers, values of the medians; whiskers, maximum and minimum values. **, significant (p < 0.001) pairwise Dunn’s test comparisons with Bonferroni corrections; ns, non‐significant [Colour figure can be viewed at wileyonlinelibrary.com]
Negative ΔOLE was also associated with both later MST inSR (ρ = 0.23) and larger ΔMST (ρ = 0.16). The ΔOLE was independent of SD inSR, ΔSD, SJL inSR, and ΔSJL.
3.3.2. Sleep duration and wellbeing
Lengthening of sleep (positive ΔSD) was linked to increased Sleep‐Quality (ρ = 0.21) and Quality‐of‐Life (ρ = 0.11); longer SD inSR correlated with larger increases in Sleep‐Quality (ρ = 0.13; all significant at p < 0.001 Bonferroni corrected).
The ΔSD and ΔSleep‐Quality, as well as ΔSD and ΔQuality‐of‐Life showed significant associations (Kruskal–Wallis H tests; H = 308, η 2 = 0.041 and H = 86, η 2 = 0.012, respectively; Figure 4). While none of negative subgroups of Δscores showed a gain in sleep duration, the positive subgroups of Sleep‐Quality and Quality‐of‐Life did. All three subgroups differed from each other (see pairwise comparisons in Figure 4), showing a dose‐dependent relationship from negative to positive changes in both of the wellbeing aspects with ΔSD (note the strong gains in SD associated with positive change, the median was 32 and 21 min for Sleep‐Quality and Quality‐of‐Life, respectively).
FIGURE 4.
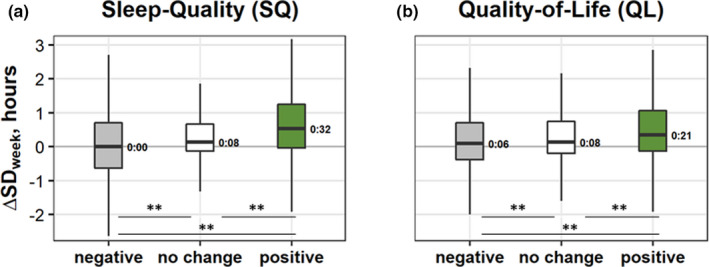
Boxplots of changes in sleep duration (SD), ΔSD (hr), in the two wellbeing aspects with significant Kruskal–Wallis tests, by subgroups (negative, grey; no change, white; and positive, green): (a) ΔSleep‐Quality (negative, N = 2,566; no change, N = 3,212; and positive, N = 1,730) and (b) ΔQuality‐of‐Life (negative, N = 3,724; no change, N = 2,153; and positive, N = 1,629). Box boundaries,75th and 25th percentiles; line through the box, median; numbers, values of the medians; whiskers; maximum and minimum values; **, significant (p < 0.001) pairwise Dunn’s test comparisons with Bonferroni corrections [Colour figure can be viewed at wileyonlinelibrary.com]
3.3.3. Mid‐sleep time and wellbeing
Greater individual delay in ΔMST negatively correlated with Δscores in three out of five aspects (ΔSleep‐Quality, ρ = −0.15; ΔPhysical‐Activity, ρ = −0.10; ΔScreen‐Time, ρ = −0.17). Moreover, later MST inSR negatively correlated with Δscores in four out of five aspects (ΔSleep‐Quality, ρ = −0.14; ΔQuality‐of‐Life, ρ = −0.12; ΔPhysical‐Activity, ρ = 0.14; ΔScreen‐Time, ρ = −0.23). All Spearman correlations were significant at p < 0.001 and corrected for multiple comparisons.
Changes in MST (ΔMST) were associated with ΔSleep‐Quality and ΔScreen‐Time (Kruskal–Wallis H tests: H = 343, η 2 = 0.045 and H = 132, η 2 = 0.017, respectively; Figure 5). The subgroup with negative change in ΔSleep‐Quality showed larger ΔMST (median = 45 min) than no change or positive change subgroups (both medians <30 min). Similar results were obtained for ΔScreen‐Time: the subgroup with negative change shifted to significantly later MST (median was 30 min compared to 10 min for no change and 15 min for positive subgroups). Note that the pairwise comparisons between the no change and the positive subgroups were non‐significant.
FIGURE 5.
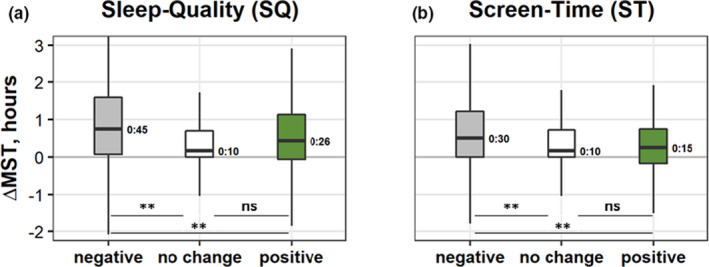
Boxplots of changes in mid‐sleep time (MST), ΔMST (hr), in the two wellbeing aspects with significant Kruskal–Wallis tests, by subgroups (negative, grey; no change, white; and positive, green): (a) ΔSleep‐Quality (negative, N = 2,566; no change, N = 3,212; and positive, N = 1,730) and (b) ΔScreen‐Time (negative change, more screen time, negative N = 5,578; no change, N = 1,644; and positive, N = 1,629). Box boundaries, 75th and 25th percentiles; line through the box, median; numbers; values of medians; whiskers, maximum and minimum values. **, significant (p < 0.001) pairwise Dunn’s test comparisons with Bonferroni corrections; ns, non‐significant [Colour figure can be viewed at wileyonlinelibrary.com]
3.3.4. Contributions of OLE and daily behaviour to changes in wellbeing
A series of multiple regressions were conducted to examine the extent to OLE and daily behaviour parameters explained the variance in Δscores in wellbeing. The model included a set of six predictors: ΔOLE, ΔSD, ΔMST, in addition to the actual values of OLE, SD and MST inSR. This model explained between 5.6%–10% of the variance in Δscores: 5.6% (ΔQuality‐of‐Life), 8.9% (ΔSleep‐Quality), 9.1% (ΔScreen‐Time) and 10% (ΔPhysical‐Activity; Table 1). OLE inSR and ΔOLE were the leading predictors: ΔOLE alone explained 3.2% of the variance for ΔQuality‐of‐Life, 5.6% for ΔScreen‐Time, and 2.1% for ΔPhysical‐Activity. Actual duration of OLE inSR alone explained 7.1% of the variance for ΔPhysical‐Activity. Changes in individual’s SD (ΔSD) and MST (ΔMST) inSR were the main predictors of variance for ΔSleep‐Quality (explaining 4% and 3.2%, respectively). None of the predictors accounted for >1% change in the variance in ΔProductivity.
TABLE 1.
Multiple linear regressions for six predictors of changes in wellbeing categories. (Green background, predictors that were responsible for >1% change in the variance in the wellbeing change scores)
| Wellbeing category | Predictor | Adjusted R 2 | R 2 change | Standardised coefficients β | t statistic |
|---|---|---|---|---|---|
| ΔSleep‐quality | ΔSD | 0.040 | 0.040 | 0.190 | 13.883** |
| ΔMST | 0.071 | 0.032 | −0.136 | −10.719** | |
| ΔOLE | 0.086 | 0.014 | 0.102 | 8.205** | |
| MST | 0.088 | 0.002 | −0.054 | −4.197** | |
| SD | 0.089 | 0.001 | 0.035 | 2.589* | |
| OLE | 0.089 | 0.001 | 0.190 | 2.514* | |
| Model | 0.089 | ||||
| ΔQuality‐of‐life | ΔOLE | 0.032 | 0.032 | 0.123 | 9.728** |
| ΔSD | 0.043 | 0.012 | 0.116 | 10.255** | |
| OLE | 0.049 | 0.006 | 0.080 | 6.279** | |
| ΔMST | 0.054 | 0.005 | −0.055 | −4.252** | |
| MST | 0.055 | 0.001 | −0.040 | −3.109* | |
| Model | 0.055 | ||||
| ΔPhysical‐activity | OLE | 0.071 | 0.071 | 0.181 | 14.642** |
| ΔOLE | 0.092 | 0.021 | 0.154 | 12.458** | |
| MST | 0.095 | 0.003 | −0.051 | −4.042** | |
| SD | 0.099 | 0.004 | 0.065 | 5.935** | |
| ΔMST | 0.100 | 0.001 | −0.030 | −2.399* | |
| Model | 0.100 | ||||
| ΔScreen‐time | ΔOLE | 0.055 | 0.056 | 0.164 | 13.194** |
| MST | 0.083 | 0.027 | −0.134 | −10.536** | |
| OLE | 0.089 | 0.006 | 0.088 | 7.062** | |
| ΔST | 0.091 | 0.002 | −0.054 | −4.268** | |
| ΔSD | 0.091 | 0.001 | 0.024 | 2.134* | |
| Model | 0.091 |
MST, mid‐sleep time; OLE, outdoor daylight exposure inSocialRestriction (inSR) and their respective deltas relative to preSocialRestriction (preSR); SD, sleep duration.
0.001 < p < 0.05.
p < 0.001.
3.4. Effects of alarm clock use on wellbeing
To assess the impact of alarm clock use inSR on wellbeing Δscores, we selected a group of participants who worked/studied both preSR and inSR, used an alarm clock on workdays preSR, and worked/studied from home inSR. This group (N = 4,135) was then subdivided into those who stopped using an alarm clock inSR (Alarm/NoAlarm; N = 1,539 [37%]) and those who continued to use alarm clock inSR (Alarm/Alarm; N = 2,596 [63%]). On average, the Alarm/NoAlarm group had higher ΔSleep‐Quality and ΔQuality‐of‐Life scores (Mann–Whitney tests; Z = 3.53, p < 0.001, and Z = 3.04, p < 0.001, respectively) but lower ΔProductivity scores (Z = −5.06, p < 0.001) compared to the Alarm/Alarm group. There were no significant differences between the groups in ΔPhysical‐Activity and ΔScreen‐Time scores. The two groups were similar in age and sex composition (Table S5).
4. DISCUSSION
As part of preventing infections with COVID‐19, governments around the world imposed drastic restrictions on their citizens’ freedom to move. These social restrictions represented a global experiment that changed OLE, social time pressures, and many aspects of daily routines. Our previously published findings of the GCCS study (Korman, Tkachev, et al., 2020) showed that participants slept longer and later inSR with a concomitant decrease in SJL. In the present study, we show the importance of changes in OLE and sleep–wake behaviour linked to changes in wellbeing during the period of social restrictions. Our most important findings are summarised in Figure 6.
FIGURE 6.
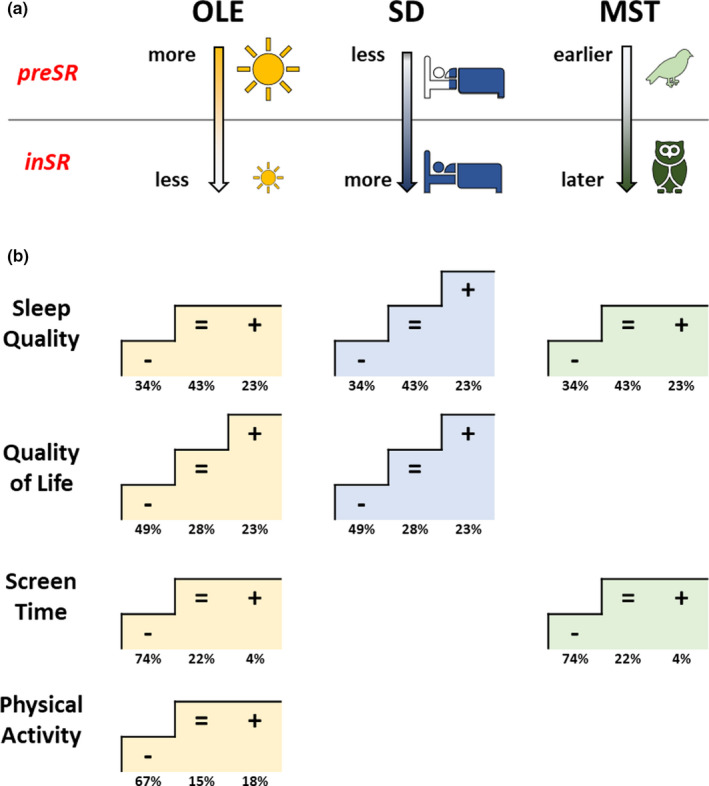
Changes in outdoor light exposure (OLE), sleep duration (SD) and mid‐sleep time (MST) in relation to Δscores of wellbeing aspects. (a) PreSR–inSR directions of change in OLE, SD and MST parameters: participants were exposed to less OLE and slept longer and later inSR. (b) Four aspects of wellbeing (Sleep‐Quality, Quality‐of‐Life, Screen‐Time, and Physical‐Activity) that significantly correlated with changes in OLE, SD and MST, by wellbeing Δscore subgroups: negative change (−), no change (=), positive change (+); numbers, % of total. The “staircases” show which subgroups within each wellbeing aspect were significantly different from each other in terms of respective changes in OLE (yellow), SD (blue) and MST (green). inSR, inSocialRestriction; preSR, preSocialRestriction [Colour figure can be viewed at wileyonlinelibrary.com]
Social restrictions impaired all aspects of wellbeing, with sleep quality, quality of life, physical activity, and productivity deteriorating and screen time increasing in their medians. Yet, many GCCS participants also reported no changes or even improvements. Notably, more participants reported no changes in Sleep‐Quality (43%) than deteriorations or improvements (34% and 23%, respectively). This is consistent with previous reports of large scale studies (Florea et al., 2021; Gao & Scullin, 2020; Kocevska et al., 2020; Leone et al., 2020) and a recent meta‐analysis that found that sleep problems of people from the general population during the COVID‐19 pandemic affected ~32% (Jahrami et al., 2021). Thus, longer sleep and less SJL (as reported for the same sample by Korman, Tkachev, et al., 2020) seem not directly linked to sleep quality. However, analyses of individuals show that those reporting deteriorations in Sleep‐Quality and Quality‐of‐Life also reported smaller gains in sleep duration and used alarm clocks more often. Although causalities in this association remain untested, it is plausible that social restrictions affect quality of life through stress mechanisms (Gao & Scullin, 2020; Ozamiz‐Etxebarria et al., 2020), thereby preventing longer sleep despite relaxed social time pressure. Altogether, relief from social time pressure during social restrictions allows both longer sleep (Korman, Tkachev, et al., 2020) and waking without an alarm clock, thereby improving sleep quality and quality of life (both scores were higher in the subgroup that stopped using an alarm clock inSR).
Notably, deteriorations in Sleep‐Quality, Quality‐of‐Life, Physical‐Activity and Screen‐Time during the pandemic were associated with higher losses in weekly OLE. As decreased weekly OLE is predominantly caused by the social restrictions rather than merely associated with them, it is fair to presume a causal positive influence of OLE on many aspects of wellbeing. A combination of decreased OLE and increased Screen‐Time has predictably powerful effects on circadian timing. They combine decrease in zeitgeber strength and more light after sunset, and both these effects individually delay the circadian phase (MST inSR) in most individuals (Moderie et al., 2017). An important methodological limitation of the present study in this respect is that it is unknown during what time of day the changes in screen time took place. An increase in the Screen‐Time/OLE ratio has been suggested to exacerbate myopia during the recent pandemic (Wong et al., 2021).
The division of participants into subgroups reflecting their wellbeing changes (negative, no change, positive) strongly indicates that the loss of OLE during the pandemic actually mediates changes in wellbeing, especially as the changes in Quality‐of‐Life were dose‐dependent (Figure 3b). Changes in Sleep Duration lead to similar dose‐dependent changes in Sleep‐Quality and Quality‐of‐Life (Figure 4a,b). Regression analysis performed for each aspect of wellbeing showed that a multiple predictor model including both deltas and absolute values of OLE and daily behaviour parameters explained 5%–10% of the variance in wellbeing change scores, excluding the productivity aspect (Table 1). OLE inSR and ΔOLE were the main predictors in four aspects of wellbeing. Changes in individual sleep‐duration and ‐timing (chronotype) inSR, were the main predictors for changes in Sleep‐Quality (explaining 4% and 3.2%, respectively).
Depressive symptoms have been shown to have increased during the COVID‐19 pandemic (Ettman et al., 2020; Fancourt et al., 2021); this may well be associated to the reduced OLE, as seen in SAD (Wirz‐Justice et al., 2020). A 1‐hr morning walk in the open air can improve mood in SAD, as well as the conventional artificial bright light therapy (Wirz‐Justice et al., 1996).
It is important that the present data were collected during the first wave of COVID‐19 in all participating countries and a small number of participants who had COVID‐19 during the data collection period were excluded from the analysis (see Methods). Therefore, the present study reflects the impact of social restrictions on wellbeing and daily behaviour rather than the consequences of viral infections. Since then, millions have contracted COVID‐19 and many continue to suffer from its long‐term effects that frequently include sleep problems (Jahrami et al., 2021). Our present study has several limitations, including possible selection bias, absence of data about existing medical conditions, medication use and sleep/circadian disorders (described in the first publication of the GCCS study by Korman, Tkachev, et al., 2020). Nonetheless, the large sample size, ethnic and geographic diversity, homogeneity in the time of response to the survey (first wave of COVID‐19‐related restrictions), reduce the risk of systematic bias.
Sufficient OLE and sleep are important determinants of resilience (Cloonan et al., 2021), and our present results show that this holds also for pandemics. Positive effects of daylight go beyond the effects through the eye’s retina: daylight upregulates vitamin D production and bone health (Wirz‐Justice et al., 2020) and has disinfectant properties including against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) disinfectant properties (Ratnesar‐Shumate et al., 2020). A recent study found that sunlight exposure increased COVID‐19 recovery rates (Asyary & Veruswati, 2020); another study showed that high pulse dose of vitamin D significantly reduced inflammatory markers in patients with COVID 19 without side‐effects (Lakkireddy et al., 2021). Hence, exposure to daylight is not only a factor of resilience during the pandemic, but also a probable remediating factor. In summary, strategies to improve wellbeing under social restrictions and to accelerate COVID‐19 recovery should actively foster spending more daytime outdoors and keeping good sleep hygiene.
CONFLICT OF INTEREST
All authors declare no competing interest.
AUTHOR CONTRIBUTIONS
MK and TR designed research; MK, VT and TR performed research; MK, TR, CR, YK, SK, DG, and VK contributed translations of the GCCS to different languages and advertised the study in their countries; MK, VT and TR analysed data; MK, VT, CR, YK, SK, DG, VK and TR wrote the paper.
Supporting information
Tables S1–S5
Korman, M. , Tkachev, V. , Reis, C. , Komada, Y. , Kitamura, S. , Gubin, D. , Kumar, V. , & Roenneberg, T. (2022). Outdoor daylight exposure and longer sleep promote wellbeing under COVID‐19 mandated restrictions. Journal of Sleep Research, 31, e13471. 10.1111/jsr.13471
DATA AVAILABILITY STATEMENT
We included all the data needed for the evaluation of the conclusions in the Results section or in the Supplementary Information file. Additional data related to this article may be requested from the authors.
REFERENCES
- Altena, E. , Baglioni, C. , Espie, C. A. , Ellis, J. , Gavriloff, D. , Holzinger, B. , Schlarb, A. , Frase, L. , Jernelöv, S. , & Riemann, D. (2020). Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. Journal of Sleep Research, 29(4), e13052. 10.1111/jsr.13052 [DOI] [PubMed] [Google Scholar]
- Aschoff, J. , & Pohl, H. (1978). Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften, 65(2), 80–84. 10.1007/BF00440545 [DOI] [PubMed] [Google Scholar]
- Asyary, A. , & Veruswati, M. (2020). Sunlight exposure increased Covid‐19 recovery rates: A study in the central pandemic area of Indonesia. The Science of the Total Environment, 729, 139016. 10.1016/j.scitotenv.2020.139016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey, H. E. , James, M. H. , & Aston‐Jones, G. (2017). New directions for the treatment of depression: Targeting the photic regulation of arousal and mood (PRAM) pathway. Depress Anxiety, 34(7), 588–595. 10.1002/da.22635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A.‐M. , Aeschbach, D. , Duffy, J. F. , & Czeisler, C. A. (2015). Evening use of light‐emitting eReaders negatively affects sleep, circadian timing, and next‐morning alertness. Proceedings of the National Academy of Sciences of the United States of America, 112(4), 1232–1237. 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan, S. A. , Taylor, E. C. , Persich, M. R. , Dailey, N. S. , & Killgore, W. D. (2021). Sleep and Resilience during the COVID‐19 Pandemic. [Google Scholar]
- Duffy, J. F. , & Czeisler, C. A. (2009). Effect of light on human circadian physiology. Sleep Medicine Clinics, 4(2), 165–177. 10.1016/j.jsmc.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettman, C. K. , Abdalla, S. M. , Cohen, G. H. , Sampson, L. , Vivier, P. M. , & Galea, S. (2020). Prevalence of depression symptoms in US adults before and during the COVID‐19 pandemic. JAMA Network Open, 3(9), e2019686. 10.1001/jamanetworkopen.2020.19686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt, D. , Steptoe, A. , & Bu, F. (2021). Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID‐19 in England: A longitudinal observational study. Lancet Psychiatry, 8(2), 141–149. 10.1016/s2215-0366(20)30482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea, C. , Topalidis, P. , Hauser, T. , Angerer, M. , Kurapov, A. , Beltran Leon, C. A. , Soares Brandão, D. , & Schabus, M. (2021). Sleep during COVID‐19 lockdown: A cross‐cultural study investigating job system relevance. Biochemical Pharmacology, 191, 114463. 10.1016/j.bcp.2021.114463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioni, G. , Maquet, P. , Schmidt, C. , Dijk, D. J. , & Vandewalle, G. (2014). Neuroimaging, cognition, light and circadian rhythms. Frontiers in Systems Neuroscience, 8, 126. 10.3389/fnsys.2014.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, C. , & Scullin, M. K. (2020). Sleep health early in the coronavirus disease 2019 (COVID‐19) outbreak in the United States: Integrating longitudinal, cross‐sectional, and retrospective recall data. Sleep Medicine, 73, 1–10. 10.1016/j.sleep.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrami, H. , BaHammam, A. S. , Bragazzi, N. L. , Saif, Z. , Faris, M. , & Vitiello, M. V. (2021). Sleep problems during the COVID‐19 pandemic by population: A systematic review and meta‐analysis. Journal of Clinical Sleep Medicine, 17(2), 299–313. 10.5664/jcsm.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Jang, S. , Choe, H. K. , Chung, S. , Son, G. H. , & Kim, K. (2017). Implications of circadian rhythm in dopamine and mood regulation. Molecules and Cells, 40(7), 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocevska, D. , Blanken, T. F. , Van Someren, E. J. W. , & Rösler, L. (2020). Sleep quality during the COVID‐19 pandemic: not one size fits all. Sleep Medicine, 76, 86–88. 10.1016/j.sleep.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman, M. , Palm, D. , Uzoni, A. , Faltraco, F. , Tucha, O. , Thome, J. , & Coogan, A. N. (2020). ADHD 24/7: Circadian clock genes, chronotherapy and sleep/wake cycle insufficiencies in ADHD. The World Journal of Biological Psychiatry, 21(3), 156–171. 10.1080/15622975.2018.1523565 [DOI] [PubMed] [Google Scholar]
- Korman, M. , Tkachev, V. , Reis, C. , Komada, Y. , Kitamura, S. , Gubin, D. , Kumar, V. , & Roenneberg, T. (2020). COVID‐19‐mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Scientific Reports, 10(1), 22225. 10.1038/s41598-020-79299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkireddy, M. , Gadiga, S. G. , Malathi, R. D. , Karra, M. L. , Raju, I. S. S. V. P. M. , Ragini, Chinapaka, S. , Baba, K. S. S. S. , & Kandakatla, M. (2021). Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Scientific Reports, 11(1), 10641. 10.1038/s41598-021-90189-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Leocadio‐Miguel, M. A. , Louzada, F. M. , Duarte, L. L. , Areas, R. P. , Alam, M. , Freire, M. V. , Fontenele‐Araujo, J. , Menna‐Barreto, L. , & Pedrazzoli, M. (2017). Latitudinal cline of chronotype. Scientific Reports, 7(1), 5437. 10.1038/s41598-017-05797-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone, M. J. , Sigman, M. , & Golombek, D. A. (2020). Effects of lockdown on human sleep and chronotype during the COVID‐19 pandemic. Current Biology, 30(16), R930–R931. 10.1016/j.cub.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , & Li, X. (2018). The antidepressant effect of light therapy from retinal projections. Neuroscience Bulletin, 34(2), 359–368. 10.1007/s12264-018-0210-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, J. (2016). Light in man's environment. Eye, 30(2), 211–214. 10.1038/eye.2015.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderie, C. , Van der Maren, S. , & Dumont, M. (2017). Circadian phase, dynamics of subjective sleepiness and sensitivity to blue light in young adults complaining of a delayed sleep schedule. Sleep Medicine, 34, 148–155. 10.1016/j.sleep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Mota, M. C. , Silva, C. M. , Balieiro, L. C. T. , Gonçalves, B. F. , Fahmy, W. M. , & Crispim, C. A. (2019). Association between social jetlag food consumption and meal times in patients with obesity‐related chronic diseases. PLoS ONE, 14(2), e0212126. 10.1371/journal.pone.0212126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozamiz‐Etxebarria, N. , Idoiaga Mondragon, N. , Dosil Santamaría, M. , & Picaza Gorrotxategi, M. (2020). Psychological symptoms during the two stages of lockdown in response to the COVID‐19 outbreak: An investigation in a sample of Citizens in Northern Spain. Frontiers in Psychology, 11, 1491. 10.3389/fpsyg.2020.01491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partonen, T. , & Lonnqvist, J. (2000). Bright light improves vitality and alleviates distress in healthy people. Journal of Affective Disorders, 57(1–3), 55–61. 10.1016/S0165-0327(99)00063-4 [DOI] [PubMed] [Google Scholar]
- Pilz, L. K. , Levandovski, R. , Oliveira, M. A. B. , Hidalgo, M. P. , & Roenneberg, T. (2018). Sleep and light exposure across different levels of urbanisation in Brazilian communities. Scientific Reports, 8(1), 11389. 10.1038/s41598-018-29494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar‐Shumate, S. , Williams, G. , Green, B. , Krause, M. , Holland, B. , Wood, S. , Bohannon, J. , Boydston, J. , Freeburger, D. , Hooper, I. , Beck, K. , Yeager, J. , Altamura, L. A. , Biryukov, J. , Yolitz, J. , Schuit, M. , Wahl, V. , Hevey, M. , & Dabisch, P. (2020). Simulated sunlight rapidly inactivates SARS‐CoV‐2 on surfaces. The Journal of Infectious Diseases, 222(2), 214–222. 10.1093/infdis/jiaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg, T. , Kuehnle, T. , Pramstaller, P. P. , Ricken, J. , Havel, M. , Guth, A. , & Merrow, M. (2004). A marker for the end of adolescence. Current Biology. 14(24), R1038–R1039. [DOI] [PubMed] [Google Scholar]
- Roenneberg, T. , Pilz, L. K. , Zerbini, G. , & Winnebeck, E. C. (2019). Chronotype and social jetlag: A (self‐) critical review. Biology, 8(3), 54. 10.3390/biology8030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg, T. , Winnebeck, E. C. , & Klerman, E. B. (2019). Daylight saving time and artificial time zones ‐ A battle between biological and social times. Frontiers in Physiology, 10, 944. 10.3389/fphys.2019.00944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak, Y. E. , McNeely, H. E. , Mackenzie, B. E. , Jain, U. R. , & Levitan, R. D. (2006). An open trial of light therapy in adult attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry, 67(10), 1527–1535. 10.4088/JCP.v67n1006 [DOI] [PubMed] [Google Scholar]
- Sit, D. K. , McGowan, J. , Wiltrout, C. , Diler, R. S. , Dills, J. J. , Luther, J. , Yang, A. , Ciolino, J. D. , Seltman, H. , Wisniewski, S. R. , Terman, M. , & Wisner, K. L. (2018). Adjunctive bright light therapy for bipolar depression: A randomized double‐blind placebo‐controlled trial. American Journal of Psychiatry, 175(2), 131–139. 10.1176/appi.ajp.2017.16101200 [DOI] [PubMed] [Google Scholar]
- Stothard, E. R. , McHill, A. W. , Depner, C. M. , Birks, B. R. , Moehlman, T. M. , Ritchie, H. K. , Guzzetti, J. R. , Chinoy, E. D. , LeBourgeois, M. K. , Axelsson, J. , & Wright, K. P. (2017). Circadian entrainment to the natural light‐dark cycle across seasons and the weekend. Current Biology, 27(4), 508–513. 10.1016/j.cub.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome, J. , Deloyer, J. , Coogan, A. N. , Bailey‐Rodriguez, D. , da Cruz e Silva, O. A. B. , Faltraco, F. , Grima, C. , Gudjonsson, S. O. , Hanon, C. , Hollý, M. , Joosten, J. O. , Karlsson, I. , Kelemen, G. , Korman, M. , Krysta, K. , Lichterman, B. , Loganovsky, K. , Marazziti, D. , Maraitou, M. , … Fond‐Harmant, L. (2020). The impact of the early phase of the COVID‐19 pandemic on mental‐health services in Europe. The World Journal of Biological Psychiatry, Online ahead of print. 10.1080/15622975.2020.1844290 [DOI] [PubMed] [Google Scholar]
- Wirz‐Justice, A. , Benedetti, F. , Berger, M. , Lam, R. W. , Martiny, K. , Terman, M. , & Wu, J. C. (2005). Chronotherapeutics (light and wake therapy) in affective disorders. Psychological Medicine, 35(7), 939–944. [DOI] [PubMed] [Google Scholar]
- Wirz‐Justice, A. , Graw, P. , Kräuchi, K. , Sarrafzadeh, A. , English, J. , Arendt, J. , & Sand, L. (1996). ‘Natural’ light treatment of seasonal affective disorder. Journal of Affective Disorders, 37(2), 109–120. 10.1016/0165-0327(95)00081-X [DOI] [PubMed] [Google Scholar]
- Wirz‐Justice, A. , Skene, D. J. , & Münch, M. (2020). The relevance of daylight for humans. Biochemical Pharmacology, 191, 114304. 10.1016/j.bcp.2020.114304 [DOI] [PubMed] [Google Scholar]
- Wittmann, M. , Dinich, J. , Merrow, M. , & Roenneberg, T. (2006). Social jetlag: Misalignment of biological and social time. Chronobiology International, 23(1–2), 497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- Wittmann, M. , Paulus, M. , & Roenneberg, T. (2010). Decreased psychological well‐being in late ‘chronotypes’ is mediated by smoking and alcohol consumption. Substance Use and Misuse, 45(1–2), 15–30. 10.3109/10826080903498952 [DOI] [PubMed] [Google Scholar]
- Wong, C. W. , Tsai, A. , Jonas, J. B. , Ohno‐Matsui, K. , Chen, J. , Ang, M. , & Ting, D. S. W. (2021). Digital screen time during COVID‐19 pandemic: Risk for a further myopia boom? American Journal of Ophthalmology, 223, 333–337. 10.1016/j.ajo.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, K. P. , Linton, S. K. , Withrow, D. , Casiraghi, L. , Lanza, S. M. , Iglesia, H. D. L. , Vetter, C. , & Depner, C. M. (2020). Sleep in university students prior to and during COVID‐19 stay‐at‐home orders. Current Biology, 30(14), R797–R798. 10.1016/j.cub.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, K. P. , McHill, A. W. , Birks, B. R. , Griffin, B. R. , Rusterholz, T. , & Chinoy, E. D. (2013). Entrainment of the human circadian clock to the natural light‐dark cycle. Current Biology: CB, 23(16), 1554–1558. 10.1016/j.cub.2013.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Data Availability Statement
We included all the data needed for the evaluation of the conclusions in the Results section or in the Supplementary Information file. Additional data related to this article may be requested from the authors.


