Abstract
Introduction:
Systemic inflammation has been increasingly implicated in the pathogenesis of Alzheimer’s disease (AD), yet the mechanistic and temporal specificity of this relationship is poorly understood. We aimed to characterize the cross-sectional and longitudinal associations between peripheral inflammatory biomarkers, cognition, and Aβ deposition in oldest-old cognitively unimpaired (CU) adults.
Methods:
A large sample of 139 CU older adults (mean age (range) = 85.4 (82–95)) underwent neuropsychological testing, Pittsburgh compound-B (PiB)-PET imaging and structural MRI. Hierarchical regression models examined associations between circulating inflammatory biomarkers (Interleukin-6 (IL-6), soluble Tumor Necrosis Factor receptors 1 and 2 (sTNFr1 and sTNFr2), soluble cluster of differentiation 14 (sCD14), C-reactive protein (CRP)), cognition, and global and regional Aβ deposition at baseline and over follow-up. Indices of preclinical disease, including pathologic Aβ status and hippocampal volume, were incorporated to assess conditional associations.
Results:
At baseline evaluation, higher concentrations of IL-6 and sTNFr2 were associated with greater global Aβ burden in those with lower hippocampal volume. In longitudinal models, IL-6 predicted subsequent conversion to MCI and both IL-6 and CRP predicted greater change in global and regional Aβ deposition specifically among participants PiB-positive at baseline. These relationships withstood adjustment for demographic factors, anti-hypertensive medication use, history of diabetes, heart disease, APOE ε4 carrier status, and white matter lesions.
Discussion:
In a large prospective sample of CU adults aged 80 and over, peripheral inflammatory biomarkers were associated with and predictive of the progression of Aβ deposition. This was specific to those with biomarker evidence of preclinical AD at baseline, supporting recent evidence of disease-state-dependent differences in inflammatory expression profiles. Chronic, low-level systemic inflammation may exacerbate the deposition of Aβ pathology among those with emerging disease processes, and place individuals at a higher risk of developing clinically significant cognitive impairment.
Keywords: Systemic inflammation, amyloid-beta, cognition, preclinical Alzheimer’s disease, Pittsburgh compound-B PET
1.0. INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent cause of dementia and is pathologically defined in the brain by aggregated amyloid-beta (Aβ) plaques and hyperphosphorylated tau tangles (NFTs). These neurodegenerative processes commence many years before clinical symptoms manifest, with Aβ plaques considered the first detectable change in the preclinical stage of AD (Jack et al., 2018; Sperling et al., 2011). Along with Aβ and NFTs, it is now widely recognized that both the onset and the progression of AD likely involves a complex network of processes that interact to provoke a cycle of cellular dysfunction, injury, and death (Musiek and Holtzman, 2015; Wang et al., 2017). The development of timely and targeted interventions requires an enhanced understanding of the mechanisms that predict or perpetuate these neurodegenerative changes, particularly early in the course of disease onset. While inflammation has been increasingly implicated in AD pathogenesis (Canter et al., 2016; Heneka et al., 2015), the mechanistic and temporal specificity of this relationship is not well understood.
The acute inflammatory response to brain injury or infection is a well-established and adaptive defense system. Mediated by microglial cells, the inflammatory cascade serves to restore tissue health and benefit the affected neural environment (Calsolaro and Edison, 2016; Rubio-Perez and Morillas-Ruiz, 2012). However, in AD, perturbations in the inflammatory response occur. Abundant animal work shows that the presence and accumulation of Aβ causes microglial cells to remain in a state of chronic activation, resulting in prolonged pro-inflammatory signaling that exacerbates the neurodegenerative processes observed in AD, including the generation and progression of Aβ species (for review, see Bronzuoli et al., 2016; Dá Mesquita et al., 2016; Heneka et al., 2015; Kinney et al., 2018; Spangenberg and Green, 2017). Consistent with this, translational work in humans shows that activated microglia localize to Aβ plaques in post-mortem tissue (Calsolaro and Edison, 2016; Strauss et al., 1992), and in vivo evaluations using Positron Emission Tomography (PET) imaging confirm that neuroinflammatory signaling is elevated among those with AD (Chandra et al., 2019) as well as those in the preclinical and prodromal phases (Bradburn et al., 2019; Chandra et al., 2019; Parbo et al., 2018; Zou et al., 2020). While the precise mechanisms remain a matter of debate, these studies suggest that neuroinflammatory processes are initiated early in the disease course and may peak or become particularly relevant during distinct timepoints of disease development.
The pathogenic role of inflammation in AD may not be restricted to immune cells originating in the brain, with several lines of evidence supporting the dynamic involvement of peripheral inflammatory processes. Vascular risk factors that result in a sustained, pro-inflammatory state (e.g., hypertension, midlife obesity, insulin resistance, and high cholesterol) represent well-established risk factors for AD (Barnes and Yaffe, 2011; Kamer et al., 2008; Kivipelto et al., 2001; Welty et al., 2016). Moreover, hypertension (Hughes et al., 2014b), arterial stiffness (Hughes et al., 2014a), elevated triglycerides (Choi et al., 2016), and genetic markers of cholesterol transport (Hughes et al., 2014b) have independently been associated with elevated cortical Aβ burden in preclinical and prodromal populations. Efforts to characterize the relationship between peripheral inflammatory biomarkers and Aβ have largely involved symptomatic populations (Brosseron et al., 2014; Lai et al., 2017; Saleem et al., 2015). However, elevations in systemic inflammatory signaling may emerge or contribute to AD pathogenesis well before symptom manifestation.
Recent hypotheses propose that systemic inflammatory processes may modify the course of disease progression, in part, by acting as an accelerator, hastening or exacerbating ongoing neurodegenerative processes (Dionisio-Santos et al., 2019; Eikelenboom et al., 2012; Wang et al., 2017; Yasuno et al., 2017). Indeed, elevated levels of peripheral pro-inflammatory biomarkers including Interleukin-6 (IL-6), C-reactive protein (CRP), and soluble cluster of differentiation 14 (sCD14), predict cognitive decline (Beydoun et al., 2019; Bradburn et al., 2018;) and incident dementia (Darweesh et al., 2018; Pase et al., 2020). Elevated soluble Tumor Necrosis Factor receptor levels are associated with a higher risk of progression from mild cognitive impairment (MCI) to dementia (Buchhave et al., 2010; Diniz et al., 2010), and acute inflammatory events restricted to the periphery predict cognitive deficits (Liu et al., 2018) and hasten the trajectory of cognitive decline among those with advanced AD (Holmes et al., 2009; Simone and Tan, 2011). Mechanistically, this is supported by rodent models showing that chronically activated or primed microglial cells exhibit an enhanced sensitivity to subsequent inflammatory signaling, including from cells that originate in the periphery. Moreover, the neuroinflammatory response to Aβ aggregates includes the active transport of peripheral immune cells into the brain (Calsolaro and Edison, 2016; Dá Mesquita et al., 2016; Heneka et al., 2015; Unger et al., 2020), which intensifies the neuroinflammatory drive and further promotes the progression of neurotoxic Aβ (Heneka et al., 2015; Kyrkanides et al., 2011; MacPherson et al., 2017). Despite the emerging significance of peripheral inflammation in disease onset and progression, there is an absence of longitudinal studies and work conducted in non-demented samples using in vivo measures of Aβ burden (Janelidze et al., 2018; Magalhães et al., 2018). Thus, the potential impact of systemic inflammatory processes on the pathogenesis and progression of Aβ is not well understood, particularly prior to the onset of clinical symptoms - a time when targeted interventions may be most efficacious.
We aimed to characterize the cross-sectional and longitudinal associations between peripheral inflammatory biomarkers, cognition, and global and regional Aβ deposition in cognitively asymptomatic older adults. Using the 2018 National Institute of Aging and Alzheimer’s Association (NIA-AA) classification guidelines (Jack et al., 2018), we further assessed whether these relationships differed between study participants with and without biomarker evidence of preclinical disease by distinguishing those with elevated Aβ deposition (PiB-negative/PiB-positive) and evidence of neurodegeneration (hippocampal atrophy). Given mechanistic findings from animal models of a feedforward relationship between pro-inflammatory processes and Aβ, we anticipated that the association between peripheral inflammatory markers and Aβ burden would be magnified among individuals already exhibiting biomarker evidence of preclinical AD. Finally, we assessed whether peripheral inflammatory markers predicted cognitive decline and the longitudinal progression of Aβ deposition.
2.0. MATERIALS AND METHODS
2.1. Participants and Procedures
Participants were a subsample of the Ginkgo Evaluation of Memory Study (GEMS; 2000–2008)(Hughes et al., 2014a, 2014b; Lopez et al., 2014; Mathis et al., 2013), which began in September 2000 and concluded with a final visit between October 2007 and March 2008 (Hughes et al., 2014a, 2014b; Lopez et al., 2014). During the final visit, participants completed a neuropsychological assessment and a blood draw was obtained (Hughes et al., 2014b). A mean (SD) of 10 (3) months after the GEMS close-out visit, a subsample of 197 non-demented participants from the Pittsburgh site were subsequently recruited to participate in the GEMS Imaging Sub-Study and underwent brain magnetic resonance imaging (MRI) and PET using the Aβ ligand Pittsburgh compound-B (PiB) (Supplementary Figure 1). Study details have been previously described (Mathis et al., 2013). Exclusionary criteria for the GEMS Imaging Sub-Study include dementia at the GEMS closeout visit (in 2008) or contraindications for neuroimaging (Mathis et al., 2013; Zhao et al., 2018). Of the 197 participants, 34 were classified as having MCI at baseline, 11 were excluded for technical issues with the PiB-PET (n = 3) or MRI scans (n = 8), four were excluded due to dementia diagnosis at baseline, four were excluded due to absence of fluid data, and five participants with C-reactive protein (CRP) values exceeding 10 mg/L were excluded from all analyses, leaving 139 participants eligible for analysis. A subset of 90 participants from the GEMS Imaging Sub-Study who remained non-demented underwent follow-up neuroimaging and cognitive assessment as part of a prospective observational study. The mean (SD) interval between baseline and follow-up PiB-PET imaging was 1.8 (0.5) years (Hughes et al., 2014a).
2.2. Assessments
2.2.1. Magnetic Resonance Imaging
MRI data were collected using a GE Signa 1.5 T scanner and a standard head coil (Price et al., 2005). A high-resolution T1-weighted volumetric spoiled gradient recalled sequence (SPGR) was acquired (0.937 X 0.937mm) in either the sagittal or coronal orientation with the following parameters: TE/TR = 5/25; flip angle = 40 degrees; slice thickness = 1.2mm/0mm interslice (Price et al., 2005). Using an atlas-based segmentation approach in FSL, hippocampal regions of interest (ROIs) were defined on the reference brain (MNI) and transformed to fit each participant’s anatomical image (Lopez et al., 2014). Total bilateral hippocampal volume was normalized to total intracranial volume (ICV), which was computed using FMRIB’s Brain Extraction Tool (Lopez et al., 2014). White matter hyperintensities were segmented using a fuzzy-connectedness algorithm on each T2-weighted fluid-attenuated inversion recovery (FLAIR) image (Wu et al., 2006). The volume of white matter hyperintensities was estimated and calculated as a proportion of the ICV.
2.2.2. Positron Emission Tomography (PET)
Using a Siemens/CTI ECAT HR+ scanner, PiB-PET data was acquired for 20 minutes (4 × 5 minute frames) and began 50 minutes after participants were injected with 15 ± 1.5 mCi of PiB in 3-dimensional imaging mode (2.4 mm slice width, 63 planes) (Lopez et al., 2014; Snitz et al., 2013). PET data were reconstructed using filtered back-projection, with a final PET image resolution of ~6 mm (transverse and axial) (Lopez et al., 2014; Mathis et al., 2013; Snitz et al., 2013). PiB retention was scaled to the injected dose and body mass of each participant to yield standardized uptake values (SUV) (Snitz et al., 2013). An SUV ratio (SUVR) was calculated using PiB SUV in the cerebellar grey matter as the reference region (Snitz et al., 2013).
Image coregistration and regional sampling of the PiB-PET data were performed for the full baseline sample (Lopez et al., 2014; Mathis et al., 2013) and the repeat imaging subsample (Cohen et al., 2013; Rosario et al., 2011) as previously described. Following PiB-PET data acquisition, PET images were co-registered to MR images to facilitate region of interest (ROI) segmentation. Among participants that completed repeated PiB-PET imaging, manual ROIs were hand drawn using various anatomical criteria by trained raters, based on each individual participants’ structural MR images. The SUVRs derived from 6 bilateral ROI’s (anterior cingulate gyrus, anterior ventral striatum, frontal cortex, lateral temporal cortex, parietal cortex, precuneus) were averaged on a voxel-weighted basis to provide a continuous composite measure of global PiB-PET retention (mean SUVR) (Lopez et al., 2014), which served as the primary dependent variable in statistical models. A sparse k-means clustering algorithm was applied to define PiB positivity as previously described (Cohen et al., 2013), with participants considered PiB positive if the partial volume corrected global SUVR was > 1.64. The term preclinical AD used in the present study is a biomarker-based classification from the NIA-AA guidelines to characterize those with abnormal AD biomarkers (e.g., PiB positivity, hippocampal atrophy) without evidence of cognitive impairment (Jack et al., 2018; Sperling et al., 2011). Given the protracted nature of AD pathogenesis, this classification framework is meant to capture early disease processes in those without cognitive symptoms, and identify those at greatest risk for decline (Brookmeyer et al., 2018; Jack et al., 2018; Sperling et al., 2014).
2.2.3. Neuropsychological Assessment
All participants underwent a comprehensive neuropsychological assessment at baseline evaluation and follow-up, which included measures of visual (Rey-Osterrieth Complex Figure Test (REY-O)) and verbal (California Verbal Learning Test (CVLT)) memory (for further assessment details, see Snitz et al., 2013). Cognitive adjudication was completed by the GEMS Cognitive Diagnostic Center, which was blind to the PiB-PET results and considered current and historical serial cognitive assessments obtained during the course of the GEMS trial (Mathis et al., 2013; Snitz et al., 2013). Performance on 1 to 3 cognitive tests that exceeded 1.5 standard deviations below age- and education-adjusted means, and evidence of decline, were required for a diagnosis of MCI (Lopez et al., 2018, 2014; Snitz et al., 2013). Performance on immediate and delayed trials of the REY-O and CVLT served as continuous outcome variables in statistical models, along with cognitive status at follow-up (CU vs. MCI).
2.2.4. Inflammatory Blood Assays
Participants were asked to fast and to avoid exercise and alcohol for 12 hours prior to blood draw. Blood samples were collected at the GEMS closeout visit between October 2007 and March 2008 and plasma was frozen and stored at −70°C until analyzed (Hughes et al., 2014b). Soluble cluster of differentiation 14 (sCD14), C-reactive protein (CRP), and Interleukin-6 (IL-6) were measured in thawed plasma samples using an enzyme-linked immunosorbant assay (ELISA) kit according to the manufacturer’s instructions (R&D Systems)(Buchhave et al., 2010; Diniz et al., 2010). The soluble Tumor Necrosis Factor receptors 1 (sTNFr1) and 2 (sTNFr2) were measured using a Multiplex Panel (Millipore). Soluble forms of TNF receptors have been validated as sensitive and reliable indicators of activation of the TNF-α system (Diniz et al., 2010; Kreuzer et al., 1996; McCoy and Tansey, 2008). Relative to TNF-α, TNF receptors have longer half-lives and show greater stability over time, providing reliable measurements of TNF-α signaling activity (Aderka et al., 1998, 1992; Wajant, H, Pfizenmaier, K, Scheurich, 2003).
2.2.5. Apolipoprotein genotyping
Apolipoprotein (APOE) genotyping of 3 major allelic forms of APOE (ε2, ε3, ε4) was performed on isolated DNA from plasma samples collected at the GEMS closeout visit (Hughes et al., 2014b; Mathis et al., 2013). Frozen plasma samples were thawed and analyzed using an immunoturbidimetric procedure developed by Kamiya Biomedical Company (Hughes et al., 2014b) and a binary variable was created distinguishing non-carriers from heterozygous and homozygous APOE ε4 carriers.
2.2.6. Covariates
Assessment for history of cardiovascular disease was conducted, and presence of self-reported history of myocardial infarction, congestive heart failure, coronary revascularization procedures, angina pectoris and peripheral vascular disease was recorded. Variables were also calculated categorizing participants based on the presence of diabetes and use of anti-hypertensive medication (0 = present, 1 = present) at baseline. Participants completed the Center for Epidemiological Studies Depression (CES-D) scale (Radloff, 1977) at baseline, a 20-item self-report measure of depressive symptomology.
2.3. Statistical Analysis
Sample characteristics were assessed continuously and categorically by PiB-positive/PiB-negative status using two-sample t-tests, chi-squared tests, and Mann-Whitney U Tests. Hierarchical linear regression models were used to evaluate the association between each inflammatory biomarker and cognitive performance on immediate and delayed trials of verbal (CVLT) and visual memory (REY-O) tasks. Statistical significance was set at P < 0.05, and all tests were 2-tailed (Rothman, 1990). To characterize the relationship between inflammatory biomarkers and baseline Aβ deposition, hierarchical linear regression analyses were conducted using a continuous measure of global PiB-PET retention (SUVR) as the primary dependent variable. Neuroimaging indices of preclinical AD were incorporated to assess conditional associations between inflammatory biomarkers and global Aβ deposition. Normalized hippocampal volume, an established biomarker of neurodegeneration, served as a continuous predictor and moderator. Thus, along with covariates, hierarchical models included each inflammatory biomarker and hippocampal volume as independent predictors, and their interaction product. Significant synergistic relationships were assessed for additional moderation by PiB status (PiB-positive/PiB-negative). The Johnson-Neyman technique was employed to interrogate interaction effects (Hayes, 2018).
Hierarchical linear models were constructed to assess whether inflammatory biomarkers predicted the longitudinal progression of Aβ deposition. The primary dependent variable was change in global Aβ deposition, calculated as the difference in global Aβ deposition between baseline PiB-PET (T1) and follow-up (T2). Baseline PiB status was included as a dichotomous predictor and moderator to assess whether the relationship between each inflammatory biomarker and Aβ progression was greater in magnitude among participants exhibiting biomarker evidence of preclinical AD. In models with significant main or interaction effects on global PiB-PET retention, we executed secondary analyses on manually-defined ROI’s to evaluate the potential regional specificity of these associations. Multivariable logistic regression models were constructed to determine whether each inflammatory biomarker was associated with odds of diagnostic conversion to MCI at follow-up. All statistical models adjusted for age, sex, years of education, duration of time (weeks) between blood draw and baseline image acquisition, and duration between consecutive PiB-PET scans (in longitudinal models). Significant terms in demographically-adjusted models were subsequently evaluated in conservatively-adjusted models controlling for additional Aβ-relevant factors including history of heart disease, diabetes, use of anti-hypertensive medication, depressive symptoms (CES-D), APOE ε4 allele, and white matter hyperintensities. Analyses were conducted in SPSS, version 26 (IBM Corp., version 26, 2019).
3.0. RESULTS
3.1. Baseline Sample Characteristics
The cross-sectional sample consisted of 139 CU older adults (mean [SD] age, 85.4 [2.8] years, range 82 – 95; 60 women (43%); 14.8 [2.7] years of education). There were 27 APOE ε4 carriers (19.4%; 8 participants missing genotype data) and 64 (47%) were designated as PiB-positive (Table 1). A series of planned comparisons determined that the PiB-positive participants did not differ from PiB-negative participants in age, years of education, time from blood draw to PET imaging, history of heart disease, diabetes, use of anti-hypertensive medications or proportion of female participants. Compared to the PiB-negative group, there were greater CES-D scores and more APOE ε4 carriers among PiB-positive participants. Concentrations of IL-6, CRP, sCD14, sTNFr1 and sTNFr2 were similar between groups.
Table 1.
Baseline demographic, clinical, and biomarker characteristics.
| Total Sample N = 139 |
PiB− N = 75 |
PiB+ N = 64 |
|
|---|---|---|---|
| M, SD or % (N) | M, SD or % (N) | M, SD or % (N) | |
| Age, y | 85.36 ± 2.84 | 85.51 ± 2.85 | 85.19 ± 2.85 |
|
| |||
| Sex (Female) | 43.2% (60) | 42.7% (32) | 43.8% (28) |
|
| |||
| Education, y | 14.79 ± 2.65 | 14.73 ± 2.73 | 14.86 ± 2.57 |
|
| |||
| APOE ε4+ | 19.4% (27) | 9.3% (7) | 31.3% (20) |
|
| |||
| Heart Disease | 15.1% (21) | 13.3% (10) | 17.2% (11) |
|
| |||
| Diabetes | 5.2% (7) | 4% (3) | 6.3% (4) |
|
| |||
| Anti-Hypertensive Medications | 66.9% (93) | 66.7% (50) | 67.2% (43) |
|
| |||
| MMSE | 28.04 ± 1.67 | 28.23 ± 1.64 | 27.82 1.69 |
|
| |||
| CES-D | 4.10 ± 4.30 | 3.35 ± 4.42 | 4.98 ± 4 |
|
| |||
| sCD14 | 1367.61 ± 256.47 | 1331.68 ± 226.35 | 1409.71 ± 283.84 |
|
| |||
| CRP | 1.71 ± 1.55 | 1.70 ± 1.48 | 1.73 ± 1.65 |
|
| |||
| sTNFr1 | 1275.85 ± 371.05 | 1322.9 ± 350.51 | 1220.81 ± 389.31 |
|
| |||
| sTNFr2 | 6286.28 ± 1692.24 | 6316.07 ± 1719. 64 | 6251.37 ± 1672.42 |
|
| |||
| IL-6 | 2.56 ± 1.54 | 2.55 ± 1.50 | 2.58 ± 1.58 |
|
| |||
| Duration blood draw to PET, weeks | 88.84 ± 42.12 | 86.28 ± 27.88 | 91.82 ± 54.37 |
Abbreviations: APOE= apolipoprotein; sCD14 = soluble cluster of differentiation 14; CES-D = Center for Epidemiological Studies Depression; CRP = C-reactive protein; IL-6 = Interleukin-6; MMSE = Mini Mental Status Exam; sTNFr1 = soluble Tumor Necrosis Factor receptor 1; sTNFr2 = soluble Tumor Necrosis Factor receptor 2
3.2. Cross-sectional Associations between Inflammatory Biomarkers and Cognition
Hierarchical linear regression models were employed to explore the cross-sectional relationships between peripheral inflammatory biomarkers, cognition, hippocampal volume, and Aβ deposition. IL-6 was inversely associated with immediate (β (df) = −0.16 (138); p = 0.039) and delayed (β (df) = −0.16 (138); p = 0.035) verbal memory performance in demographically-adjusted models. Similarly, elevated sTNFr2 was associated with reduced immediate (β (df) = −0.201 (138); p = 0.014) and delayed (β (df) = −0.188 (138); p = 0.025) verbal memory performance. Neither biomarker was related to visual memory performance, and there were no associations between CRP, sCD14, or sTNFr1 and memory indices.
3.3. Inflammatory Biomarkers, Aβ deposition, and hippocampal atrophy
Elevated levels of sCD14 predicted greater global PiB SUVR (β = 0.18; t = 2.03; p = 0.045) after adjusting for age, sex, education, and time from blood draw to imaging. This relationship was attenuated in conservatively-adjusted models (β = 0.107; t = 1.14; p = 0.258). There were no main effects of sTNFr1 (β = −0.059; t = −0.645; p = 0.52), sTNFr2 (β = 0.050; t = 0.545; p = 0.59); IL-6 (β = 0.024; t = 0.28; p = 0.78), CRP (β = 0.011; t = 0.122; p = 0.90) or hippocampal volume (β = −0.097; t = −1.063; p = 0.290) on global PiB SUVR.
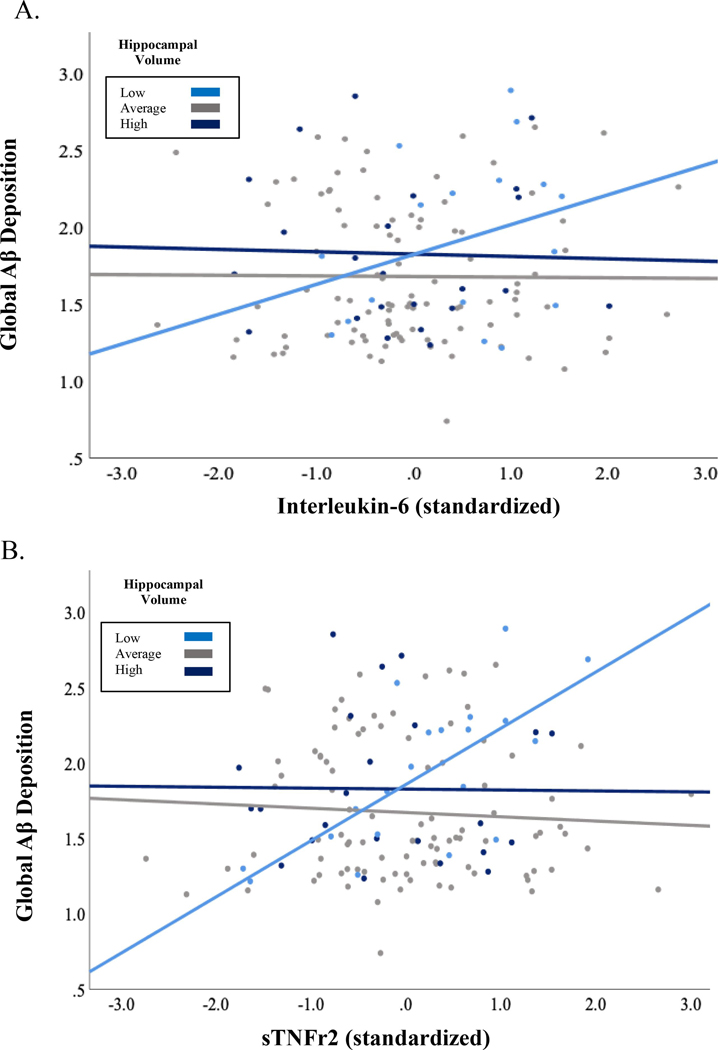
We observed a conditional association between IL-6 and Aβ deposition, such that higher concentrations of IL-6 predicted greater global PiB binding specifically among those with smaller normalized hippocampal volume (B (df) −6.12 (138); SE = 2.85; p = 0.033; R2 change = 0.033) in demographically-adjusted models. The relationship between sTNFr2 and global PiB SUVR was also moderated by hippocampal volume (B (df) = −.001 (138); SE = .001; p = 0.027; R2 change = 0.036) (Figure 1). Interrogating these relationships using the Johnson-Neyman technique revealed that global PiB binding remained stable across varying levels of cytokine expression in participants exhibiting minimal hippocampal atrophy, while higher levels of both inflammatory markers (IL-6 and sTNFr2) predicted greater global PiB SUVR specifically among those with smaller hippocampal volume. The interaction between each biomarker (IL-6 and sTNFr2) and hippocampal volume on global PiB SUVR remained significant after additional adjustment for history of heart disease, diabetes, use of anti-hypertensive medication, white matter hyperintensities, APOE ε4 status, depressive symptoms and time from blood draw to PiB-PET acquisition (IL-6: (B (df) −7.24 (138); SE = 3.12; p = 0.022; R2 change = 0.037; sTNFr2: (B (df) −.001 (138); SE = .001; p = 0.022; R2 change = 0.037). Observed interactions were not moderated by PiB status (p > 0.05).
Figure 1.
A) The relationship between IL-6 and global Aβ was moderated by hippocampal volume, such that higher levels of IL-6 predicted elevated Aβ burden among those with smaller hippocampal volume; B) Interaction between sTNFr2 and hippocampal volume on global Aβ. Hippocampal volume stratified by 1 standard deviation for visualization purposes. Low = > 1 SD below the mean (N = 20), average = within 1 SD (N = 96) high = > 1 SD above the mean (N = 23).
3.4. Longitudinal Sample Characteristics
Seventy-nine participants completed follow-up imaging and comprehensive assessments two years later (mean [SD] age at baseline 85.3 [3] years, 32 women (43.2%)). Five participants missing APOE genotype data were excluded from analyses (see Table 2 for sample characteristics). Participants who did not complete neuroimaging at T2 did not differ from the 74 participants that returned for follow-up in terms of age, sex, education, and concentrations of inflammatory biomarkers. Those who were not recruited for follow up imaging were more likely to be PiB-positive at baseline (χ2 = 7.85; p = .006).
Table 2.
Longitudinal sample characteristics.
| Total Sample N = 74 |
PiB− N = 47 |
PiB+ N = 27 |
|
|---|---|---|---|
| M, SD or % (N) | M, SD or % (N) | M, SD or % (N) | |
| Age, y | 85.34 ± 2.97 | V 85.28 ± 2.62 | 85.44 ± 3.56 |
|
| |||
| Sex (Female) | 43.2% (32) | 38.3% (18) | 51.9% (14) |
|
| |||
| Education, y | 14.99 ± 2.70 | 15.2 ± 2.78 | 14.56 ± 2.56 |
|
| |||
| ApoE ε4+ | 17.6% (13) | 8.5% (4) | 33.3% (9) |
|
| |||
| Heart Disease | 13.5% (10) | 14.9% (7) | 11.1% (3) |
|
| |||
| Diabetes | 4.1% (3) | 4.3% (2) | 3.7% (1) |
|
| |||
| Anti-hypertensive medication, % yes | 66.2% (49) | 66% (31) | 66.7% (18) |
|
| |||
| CES-D | 3.62 ± 4.27 | 2.96 ± 4.38 | 4.78 ± 3.9 |
|
| |||
| sCD14 | 1381.1 ± 277.59 | 1360.86 ± 298.6 | 1416.32 ± 237.79 |
|
| |||
| CRP | 1.83 ± 1.79 | 1.8 ± 1.56 | 1.97 ± 2.17 |
|
| |||
| sTNFr1 | 1278.34 ± 363.31 | 1317 ± 374.37 | 1211.06 ± 339.46 |
|
| |||
| sTNFr2 | 6231.58 ± 1623.94 | 6388.65 ± 1815. 94 | 5958.18 ± 1203.3 |
|
| |||
| IL-6 | 2.67 ± 1.74 | 2.70 ± 1.62 | 2.60 ± 1.96 |
|
| |||
| Duration blood draw to PET, weeks | 82.12 ± 28.10 | 84.78 ± 33.21 | 77.48 ± 14.19 |
|
| |||
| Duration, consecutive scans (weeks) | 96.59 ± 26.89 | 90.92 ± 23.12 | 106.48 ± 30.33 |
Abbreviations: APOE= apolipoprotein; sCD14 = soluble cluster of differentiation 14; CES-D = Center for Epidemiological Studies Depression; CRP = C-reactive protein; IL-6 = Interleukin-6; MMSE = Mini Mental Status Exam; sTNFr1 = soluble Tumor Necrosis Factor receptor; sTNFr2 = soluble Tumor Necrosis Factor receptor 2
3.5. Inflammatory Biomarkers and Change in Cognition
Among the 74 participants CU at baseline, 22 met diagnostic criteria for MCI at the 24-month follow-up. IL-6 predicted subsequent cognitive status, such that elevated concentrations of IL-6 conferred a higher risk of diagnostic conversion to MCI (95% CI) = 2.14 (1.12; 4.09); p = 0.021) after adjusting for age, sex, years of education, history of heart disease, use of anti-hypertensive medications, APOE carrier status, and duration from blood draw to PET imaging. There was not a significant relationship between CRP, sCD14, sTNFr1 or sTNFr2 and diagnostic status at follow-up, and inflammatory biomarkers did not predict change in continuous measures of memory performance.
3.6. Inflammatory Biomarkers and Change in Global Aβ Deposition
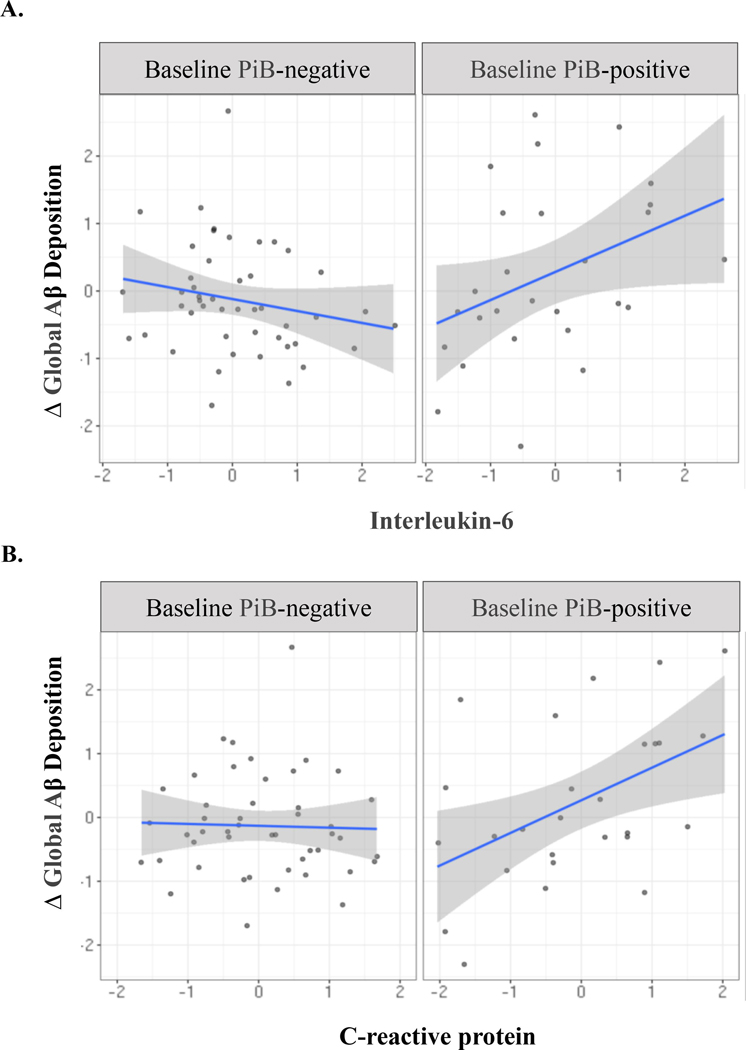
At baseline, 27 of 74 participants (36.5%) were PiB-positive compared with 41 of 74 (55.4%) at follow-up. All subjects that were PiB-positive at baseline evaluation remained PiB-positive at follow-up. Over the 24-month follow up, 66 subjects demonstrated an increase and 8 a decrease in global PiB SUVR. Baseline PiB status was independently associated with subsequent change in Aβ deposition, as PiB-positive participants exhibited a greater longitudinal increase in global PiB SUVR. Moreover, there was an interaction between PiB status and IL-6 (Figure 2), such that elevated concentrations of IL-6 predicted a subsequent increase in global PiB SUVR specifically among those classified as PiB-positive at T1 (Table 3). There was also a synergistic relationship between CRP and baseline PiB status on change in global PiB SUVR (Table 4). For both biomarkers, interaction terms remained significant in conservatively-adjusted models. In sensitivity models excluding the 8 participants that exhibited a decrease in global PiB SUVR over follow-up, the IL-6 term remained significant while the CRP term was attenuated. sCD14, sTNFr1 and sTNFr2 were not associated with change in global Aβ deposition and interaction terms were non-significant.
Figure 2.
All variables standardized; Δ global Aβ reflects change in global PiB-PET retention from T1 to T2. Abbreviations: Δ = change; Aβ = β-amyloid; PiB = Pittsburgh compound-B
Table 3.
Parameter estimates from hierarchical linear regression models of PiB status, IL-6 and their interaction product as predictors of change in global and regional Aβ deposition from baseline to follow-up.
| Region | Baseline PiB Status |
Interleukin-6 |
PiB Status X Interleukin-6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | p-value | Estimate | Standard Error | p-value | Estimate | Standard Error | t-value | p-value | R2 change | |
| Global PiB SUVR | 0.099 | 0.049 | 0.049 | 0.057 | 0.094 | 0.544 | 0.49 | 0.184 | 2.65 | 0.011 | 0.095 |
| Frontal Cortex | 0.190 | 0.062 | 0.003 | 0.006 | 0.119 | 0.958 | 0.341 | 0.243 | 1.40 | 0.167 | 0.026 |
| Anterior Cingulate Gyrus | 0.197 | 0.073 | 0.010 | 0.245 | 0.140 | 0.086 | 0.732 | 0.275 | 2.67 | 0.010 | 0.090 |
| Precuneus Cortex | 0.043 | 0.053 | 0.417 | 0.117 | 0.101 | 0.254 | 0.48 | 0.201 | 2.39 | 0.02 | 0.077 |
| Parietal Cortex | 0.047 | 0.054 | 0.379 | 0.059 | 0.102 | 0.569 | 0.61 | 0.17 | 3.12 | 0.003 | 0.130 |
| Anterior Ventral Striatum | 0.069 | 0.044 | 0.123 | −0.11 | 0.084 | 0.18 | 0.12 | 0.17 | 0.69 | 0.495 | 0.007 |
| Lateral Temporal Cortex | 0.09 | 0.048 | 0.073 | −0.022 | 0.091 | 0.81 | 0.49 | 0.18 | 2.75 | 0.008 | 0.101 |
Data from moderation models adjusting for age, sex, years of education, duration (weeks) from blood draw to imaging, history of heart disease, iabetes, current anti-hypertensive medication use, duration between consecutive PiB-PET scans, depressive symptoms, APOE ε4, and white matter hyperintensities. Abbreviations: PiB = Pittsburgh compound-B; SUVR = standardized uptake value ratio
Table 4.
Parameter estimates from hierarchical linear regression models of PiB status, CRP and their interaction product as predictors of change in global and regional Aβ deposition from baseline to follow-up.
| CRP |
PiB Status X CRP |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | Estimate | Standard Error | p-value | Estimate | Standard Error | t-value | p-value | R2 change |
| Global PiB SUVR | 0.074 | 0.047 | 0.125 | 0.203 | 0.091 | 2.223 | 0.030 | 0.067 |
| Frontal Cortex | 0.041 | 0.061 | 0.501 | 0.173 | 0.120 | 1.439 | 0.156 | 0.027 |
| Anterior Cingulate Gyrus | 0.083 | 0.073 | 0.262 | 0.181 | 0.145 | 1.246 | 0.218 | 0.022 |
| Precuneus Cortex | 0.098 | 0.051 | 0.061 | 0.239 | 0.098 | 2.451 | 0.018 | 0.078 |
| Parietal Cortex | 0.056 | 0.052 | 0.286 | 0.173 | 0.102 | 1.694 | 0.096 | 0.043 |
| Anterior Ventral Striatum | 0.002 | 0.044 | 0.959 | 0.089 | 0.088 | 1.014 | 0.315 | 0.015 |
| Lateral Temporal Cortex | 0.089 | 0.045 | 0.053 | 0.217 | 0.086 | 2.522 | 0.015 | 0.081 |
Models adjusted for age, sex, years of education, duration (weeks) from blood draw to imaging, history of heart disease, diabetes, current anti-hypertensive medication use, duration between consecutive PiB-PET scans, depressive symptoms, APOE ε4, and white matter hyperintensities. Abbreviations: CRP = C-reactive protein; PiB = Pittsburgh compound-B; SUVR = standardized uptake value ratio
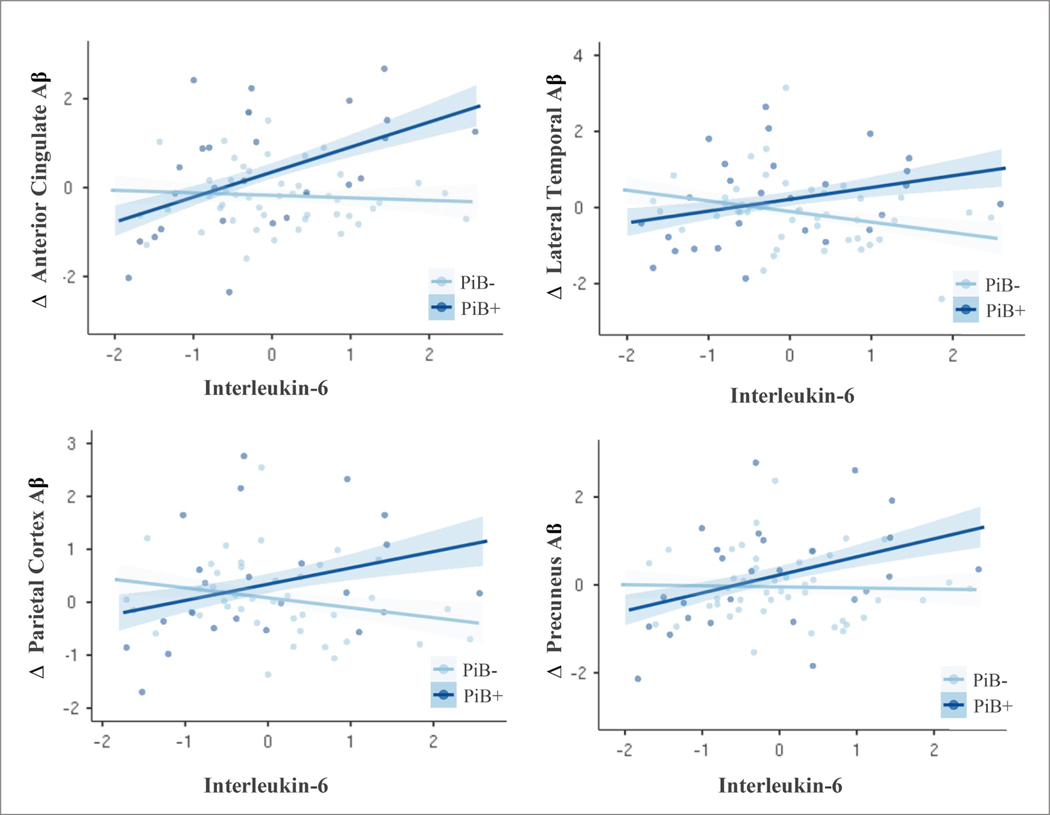
3.7. Inflammatory Biomarkers and Change in Regional Aβ Deposition
We explored the potential regional specificity of these relationships in secondary linear regression models within the 6 manually-defined ROI’s. Baseline PiB-positive status was an independent predictor of change in regional PiB-PET retention in the frontal cortex and anterior cingulate gyrus. The relationship between IL-6 and change in regional Aβ deposition was moderated by PiB-positivity, such that higher concentrations of IL-6 predicted increased PiB SUVR accumulation in the anterior cingulate cortex, parietal cortex, precuneus and lateral temporal cortex specifically among those PiB-positive at baseline (Figure 3). Interaction terms did not reach statistical significance in the anterior ventral striatum or frontal cortex. The relationship between CRP and change in regional PiB SUVR also varied as a function of PiB-PET status, specifically in the precuneus and lateral temporal cortex. Parameter estimates for main effect and interaction terms can be found in Table 3 and Table 4. All models adjusted for demographic factors, duration from blood draw to PET imaging, time between consecutive PiB-PET scans, history of heart disease, anti-hypertensive medication use, CES-D, white matter hyperintensities and APOE ε4 carrier status.
Figure 3.
Scatterplot of associations between Interleukin-6 (log transformed and z-scored) and change in standardized regional PiB-PET retention from T1 to T2. Data stratified by baseline PiB-PET status (PiB-/PiB+). Individual scatterplots depict significant interaction effects for ROI’s, showing that elevated IL-6 predicts greater regional amyloid accumulation among those with pathologic Aβ status at baseline, while change in PiB-PET retention remained stable across varying levels of cytokine expression among those that were PiB-negative. Abbreviations: Δ = change; PiB = Pittsburgh compound-B
4.0. DISCUSSION
Evolving perspectives of AD etiology have begun to consider the entire biological system, including the critical impact of peripheral health, on disease onset and progression (Dá Mesquita et al., 2016; Eikelenboom et al., 2012; Wang et al., 2017). Although systemic inflammation is increasingly implicated in disease risk, how these inflammatory processes impact the pathogenesis of AD, and when they may first start to take effect, is poorly understood (Canter et al., 2016; Cao and Zheng, 2018; Heneka et al., 2015). In a prospective sample of 139 CU adults aged 80 and over, we found that select peripheral pro-inflammatory markers were associated with and predictive of the longitudinal progression of Aβ deposition. This was specific to those with biomarker evidence of preclinical AD, supporting recent hypotheses regarding disease-state-dependent differences in inflammatory expression profiles (Brosseron et al., 2014, Parbo et al., 2018). Chronic, low-level systemic inflammation may exacerbate the deposition of Aβ pathology among those with emerging disease processes, and place individuals at a higher risk of developing clinically significant cognitive impairment.
The premise of the present study was largely informed by animal models, which have identified a feedforward loop between Aβ and inflammatory signaling. While the cumulative impact of inflammatory processes appears detrimental, the relationship between inflammation and Aβ may vary over the course of disease development as some evidence suggests that the pro-inflammatory cascade may, temporarily, be neuroprotective (Femminella et al., 2019; Heneka et al., 2015). Indeed, microglial activation may initially help to target Aβ pathology by increasing phagocytosis, promoting the breakdown and clearance of neurotoxic Aβ species, and stimulating the release of protective trophic factors (Fan et al., 2015; Heneka et al., 2015; Ramesh et al., 2013; Wang et al., 2015). However, prolonged exposure to Aβ aggregates results in microglial priming, typified by a sustained and exaggerated response to subsequent inflammatory signaling, including from cells that originate in the periphery (Calsolaro and Edison, 2016; Dionisio-Santos et al., 2019; Heneka et al., 2015). Through numerous pathways, this neuroinflammatory cascade has been shown to potentiate the generation and progression of Aβ, including upregulating the aberrant cleavage of the amyloid precursor protein and compromised clearance of Aβ species (Bronzuoli et al., 2016; Dá Mesquita et al., 2016; Heneka et al., 2015; Kinney et al., 2018). Thus, chronic exposure to Aβ initiates a prolonged and amplified inflammatory response that, in turn, promotes Aβ pathogenesis and proliferates the pro-inflammatory drive.
In the current study, we attempted to approximate or estimate this phenotypic shift in CU older adults by distinguishing those exhibiting elevated levels of Aβ deposition measured by PiB-PET and evidence of neurodegeneration in the form of hippocampal atrophy (Jack et al., 2018). In cross-sectional models, the association between inflammatory biomarkers and global Aβ deposition varied as a function of hippocampal volume, such that elevated levels of both IL-6 and sTNFr2 predicted greater Aβ deposition specifically among those with smaller hippocampal volumes. Participants with greater hippocampal atrophy may be further along the preclinical continuum, suggesting prolonged exposure of brain-resident immune cells to neurodegenerative processes and, potentially, an enhanced sensitivity to peripheral pro-inflammatory signaling. sTNFr1 and sTNFr2 regulate TNF-α signaling (Idriss and Naismith, 2000; Perry et al., 2001). While sTNFr1 largely mediates pro-inflammatory and apoptotic signaling pathways, sTNFr2 is thought to promote tissue homeostasis and cell survival and proliferation (McCoy and Tansey, 2008; Montgomery and Bowers, 2012). In the present study, elevated sTNFr2 was associated with poorer immediate and delayed verbal memory performance, which converges with recent work showing an inverse relationship between sTNFr2 and cognitive performance in a community-dwelling sample (Gao et al., 2016). Peripheral sTNFr1 and sTNFr2 are both elevated in those with MCI and AD (Lai et al., 2017), and sTNFr2 has been found to predict conversion from MCI to dementia (Buchhave et al., 2010). Moreover, higher sTNFr2 has been associated with reduced hippocampal (Schmidt et al., 2016) and total brain volume (Jefferson et al., 2007) in CU samples, and levels of both sTNFr1 and sTNFr2 correlate with Aβ40 and β-site amyloid precursor protein-cleaving enzyme-1 activity (Buchhave et al., 2010). While functionally neuroprotective, higher peripheral concentrations of sTNFr2 may reflect abnormalities in the TNF signaling system that may contribute to cognitive deficits, brain atrophy, and Aβ. We also observed a direct association between sCD14 and cortical Aβ in demographically-adjusted models. This complements recent work showing prospective associations between plasma sCD14, total brain atrophy, and incident dementia (Pase et al., 2020), and suggests a potential mechanistic link between sCD14 and AD risk.
Efforts to characterize the relationship between inflammation and Aβ pathology have largely involved cognitively impaired samples and focused on associations with neuroinflammatory signaling. To date, very few studies have examined associations between peripheral pro-inflammatory biomarkers and Aβ pathology measured in the brain, particularly in CU samples (Janelidze et al., 2018; Walker et al., 2018; Yasuno et al., 2017). One study found sex-specific associations between midlife CRP and Aβ deposition 22 years later (Walker et al., 2018), and another showed a higher percentage of peripheral cytotoxic T cells (CD8+) among those with elevated cortical amyloid in a small sample of CU older adults (Yasuno et al., 2017). These studies provide preliminary evidence of a relationship between peripheral inflammation and Aβ in non-demented elders. We extend this literature by investigating associations longitudinally and interrogating the moderating impact of preclinical disease status.
Our findings suggest that systemic inflammation may predict the longitudinal progression of Aβ, and that the pathogenic effects may be amplified among those with emerging or underlying disease processes. In longitudinal models, higher concentrations of circulating IL-6 predicted subsequent conversion to MCI and both IL-6 and CRP predicted greater global Aβ accumulation over the course of two years. This was specific to those with biomarker evidence of preclinical AD, such that positive associations between IL-6, CRP, and change in Aβ deposition were exclusively observed among participants PiB-positive at baseline evaluation. These findings underscore the complex temporal dynamics of the relationship between inflammation and AD highlighted in recent reports (Femminella et al., 2019; Yasuno et al., 2017), and provide translational evidence in humans of a potential feedforward loop between Aβ and peripheral inflammatory processes. In participants PiB-positive at baseline, CRP and IL-6 also predicted focal Aβ accumulation in several ROIs including the precuneus, anterior cingulate gyrus, and lateral temporal cortex. These regions overlap with those recently associated with neuroinflammatory signaling in a preclinical sample (Zou et al., 2020) and are among the brain areas showing structural and functional alterations associated with CRP and IL-6 in older adulthood (Dev et al., 2017; Gu et al., 2017; Magalhães et al., 2018; Marsland et al., 2015; Walker et al., 2020; Zhang et al., 2016).
The present findings support an emerging hypothesis that systemic inflammatory processes may contribute to disease progression, in part, by accelerating or amplifying ongoing neurodegenerative processes. Complex and elaborate pathways exist that allow for peripheral immune cells to infiltrate the CNS and influence inflammatory processes in the brain (for review, see Capuron and Miller, 2011). This dynamic peripheral immune-brain communication is thought to be exacerbated under pathological conditions. In AD, the neuroinflammatory response to Aβ includes the expression of signaling molecules and pro-inflammatory mediators that modulate the permeability of the blood brain barrier and facilitate the migration of peripheral immune cells into the brain (Calsolaro and Edison, 2016; Heppner et al., 2015; MacPherson et al., 2017; Unger et al., 2020; Wang et al., 2015). Thus, the early deposition of Aβ alters local immune cell signaling, permitting pro-inflammatory cells that proliferate in a state of chronic inflammation to more easily infiltrate the CNS and advance disease pathogenesis.
Systemic inflammatory processes may have additive or even distinct neuromodulatory effects in AD. In humans, elevations in peripheral inflammatory biomarkers (Bradburn et al., 2019; Darweesh et al., 2018) and conditions that result in low-grade, chronic inflammation (Barnes and Yaffe, 2011; Kivipelto et al., 2001; Rethorst et al., 2014; Welty et al., 2016) increase risk of AD onset. Moreover, acute inflammatory events restricted to the periphery (e.g., acute infection, injury) hasten cognitive decline and predict the onset of neuropsychiatric symptoms in populations with clinically manifest AD (Hall et al., 2013; Holmes et al., 2009; Simone and Tan, 2011). These studies demostrate the capacity for peripheral inflammatory processes to modify the clinical course of AD, although the underlying mechanisms are not well understood. Our findings in a large sample of CU adults aged 80 and over expand this work and suggest that low-grade, systemic inflammation may perpetuate early disease progression via impacts on Aβ deposition. Moreover, systemic inflammation may differentially impact those with emerging disease processes, rendering those with preclinical AD particularly susceptible to the pathogenic effects of peripheral inflammatory processes.
Our findings withstood adjustment for several factors known to contribute to Aβ pathogenesis, including APOE ε4 status and history of diabetes and heart disease. The influence of inflammatory factors on Aβ deposition may reflect a pathway that is mechanistically distinct or independent of the impact of cardiovascular risk factors. However, the present study used binary variables to reflect vascular risk factors which may lack the sensitivity to detect these relationships relative to continuous measures. Moreover, inflammatory biomarkers were obtained at a single time-point and may not be representative of the inflammatory milieu at the time of follow-up imaging. While these markers have been shown to have moderate to high stability over time (Alley et al., 2007; Clendenen et al., 2010; Epstein et al., 2013), future studies using repeated measurements are needed to confirm the longitudinal validity of these findings.
Though this study provides novel insights into the potential role of peripheral pro-inflammatory signaling in AD-related neuropathology, these findings should be interpreted within the context of several limitations. The current study focused on several inflammatory biomarkers that have been previously implicated in AD, although other potentially relevant biomarkers such as 1L-1 and IL-18 were not evaluated here. Future studies using a comprehensive panel of inflammatory mediators including pro- and anti-inflammatory cytokines and chemokines are needed to further characterize cellular signatures and predictors of AD pathogenesis. Fewer participants (especially those PiB-positive at baseline) underwent follow-up imaging, although participants who completed sequential neuroimaging did not significantly differ demographically from the baseline sample. In our cross-sectional models, PiB status did not moderate the conditional relationships between inflammatory biomarkers and global Aβ deposition as a function of hippocampal volume. While we may have been inadequately powered to detect 3-way interactions, hippocampal atrophy is non-specific to AD and may reflect other underlying disease processes (Jack et al., 2014; Mormino et al., 2014). Moreover, we did not distinguish between MCI subtypes (e.g., amnestic, multidomain MCI) at follow-up, and are unable to make causal or mechanistic inferences regarding the relationship between elevated IL-6 and subsequent diagnostic conversion to MCI. Along with Aβ, analysis of other in vivo indices of AD-related pathologies, including cerebral hypometabolism and tauopathy, may reveal additional cellular pathways linking systemic inflammation to AD (Jack et al., 2018).
In addition, we evaluated a well-characterized cohort of non-demented older adults aged 80 and over. This advanced age group represents the fastest growing segment of the population in many countries, including the United States (Roberts et al., 2018), necessitating identification of factors that might contribute to decline in this population. Our study suggests involvement of low-grade, systemic inflammation in Aβ pathogenesis, although our ability to generalize these findings to younger samples of older adults is limited. Moreover, the presence of Aβ pathology without corresponding clinical symptoms in this age range suggests the existence of protective factors that may not be present to a similar degree in the broader population. Further investigation in elderly samples with a younger age distribution, including those falling within the median age of onset for AD, and involving a longer follow-up duration will improve the generalizability of our findings.
Taken together, our findings suggest that low-grade, systemic inflammation may precipitate or perpetuate Aβ deposition in the absence of cognitive symptoms among individuals aged 80 and over, and may differentially impact those with emerging disease processes. Future longitudinal studies involving a broader panel of inflammatory measures and encompassing multiple AD-related biomarkers, including both Aβ and tau pathology, are needed to more precisely identify cellular signatures of preclinical AD and characterize the potential scope of neuropathological change associated with peripheral inflammation.
Supplementary Material
HIGHLIGHTS.
IL-6 predicts conversion from cognitively unimpaired to mild cognitive impairment
IL-6, CRP associated with longitudinal change in amyloid-β in non-demented elders
Systemic inflammation differentially predicts amyloid-β based on preclinical disease
Those with preclinical Alzheimer’s disease more susceptible to systemic inflammation
Acknowledgments
Source of Funding: This work was supported by National Institutes of Health grants P50 AG005133, R37 AG025516, and P01 AG025204; and a National Institute of Mental Health T32 postdoctoral training grant (recipient: L.E. Oberlin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D, 1992. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J. Exp. Med 175, 323–329. 10.1084/jem.175.2.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderka D, Sorkine P, Abu-Abid S, Lev D, Setton A, Cope AP, Wallach D, Klausner J, 1998. Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J. Clin. Invest 101, 650–659. 10.1172/JCI694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L, 2007. Three-year change in inflammatory markers in elderly people and mortality: The Invecchiare in Chianti study. J. Am. Geriatr. Soc 55, 1801–1807. 10.1111/j.1532-5415.2007.01390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Weiss J, Obhi HK, Beydoun HA, Dore GA, Liang H, Evans MK, Zonderman AB, 2019. Cytokines are associated with longitudinal changes in cognitive performance among urban adults. Brain. Behav. Immun 80, 474–487. 10.1016/j.bbi.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn S, Murgatroyd C, Ray N, 2019. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev 50, 1–8. 10.1016/j.arr.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Bradburn S, Sarginson J, Murgatroyd CA, 2018. Association of peripheral interleukin-6 with global cognitive decline in non-demented adults: A meta-analysis of prospective studies. Front. Aging Neurosci 10.3389/fnagi.2017.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzuoli MR, Iacomino A, Steardo L, Scuderi C, 2016. Targeting neuroinflammation in Alzheimer’s disease. J. Inflamm. Res 10.2147/JIR.S86958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, Kawas CH, Corrada MM, 2018. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dement. 14, 121–129. 10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseron F, Krauthausen M, Kummer M, Heneka MT, 2014. Body Fluid Cytokine Levels in Mild Cognitive Impairment and Alzheimer’s Disease: a Comparative Overview. Mol. Neurobiol 10.1007/s12035-014-8657-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O, 2010. Soluble TNF receptors are associated with Aβ metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol. Aging 31, 1877–1884. 10.1016/j.neurobiolaging.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Calsolaro V, Edison P, 2016. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 12, 719–732. 10.1016/j.jalz.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Canter RG, Penney J, Tsai LH, 2016. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 10.1038/nature20412 [DOI] [PubMed] [Google Scholar]
- Cao W, Zheng H, 2018. Peripheral immune system in aging and Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences. Mol. Neurodegener 10.1186/s13024-018-0284-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2011. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther 10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Valkimadi PE, Pagano G, Cousins O, Dervenoulas G, Politis M, 2019. Applications of amyloid, tau, and neuroinflammation PET imaging to Alzheimer’s disease and mild cognitive impairment. Hum. Brain Mapp 40, 5424–5442. 10.1002/hbm.24782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Byun MS, Yi D, Choe YM, Sohn BK, Baek HW, Lee JH, Kim HJ, Han JY, Yoon EJ, Kim YK, Woo JI, Lee DY, 2016. Association Between Serum Triglycerides and Cerebral Amyloidosis in Cognitively Normal Elderly. Am. J. Geriatr. Psychiatry 24, 604–612. 10.1016/j.jagp.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Clendenen TV, Arslan AA, Lokshin AE, Idahl A, Hallmans G, Koenig KL, Marrangoni AM, Nolen BM, Ohlson N, Zeleniuch-Jacquotte A, Lundin E, 2010. Temporal reliability of cytokines and growth factors in EDTA plasma. BMC Res. Notes 3. 10.1186/1756-0500-3-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, Nebes RD, Saxton JA, Snitz B, DeKosky S, Williamson J, Lopez OL, Price JC, Mathis CA, Klunk WE, 2013. Classification of amyloid-positivity in controls: Comparison of visual read and quantitative approaches. Neuroimage 71, 207–215. 10.1016/j.neuroimage.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dá Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F, 2016. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A, 2018. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s Dement. 14, 1450–1459. 10.1016/j.jalz.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Dev SI, Moore RC, Soontornniyomkij B, Achim CL, Jeste DV, Eyler LT, 2017. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Int. J. Geriatr. Psychiatry 32, 341–349. 10.1002/gps.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonça VA, Gattaz WF, Forlenza OV, 2010. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimer’s Dis 22, 1305–1311. 10.3233/JAD-2010-100921 [DOI] [PubMed] [Google Scholar]
- Dionisio-Santos DA, Olschowka JA, O’Banion MK, 2019. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflammation 16, 1–13. 10.1186/s12974-019-1453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Hoozemans JJM, Veerhuis R, Van Exel E, Rozemuller AJM, Van Gool WA, 2012. Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer’s disease. Alzheimer’s Res. Ther 10.1186/alzrt118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Bream JH, Jacobson LP, Martínez-Maza O, Birmann BM, 2013. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol. Biomarkers Prev 22, 2009–2015. 10.1158/1055-9965.EPI-13-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Okello AA, Brooks DJ, Edison P, 2015. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 138, 3685–3698. 10.1093/brain/awv288 [DOI] [PubMed] [Google Scholar]
- Femminella GD, Dani M, Wood M, Fan Z, Calsolaro V, Atkinson R, Edginton T, Hinz R, Brooks DJ, Edison P, 2019. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 92, E1331–E1343. 10.1212/WNL.0000000000007133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Camous X, Lu YX, Lim ML, Larbi A, Ng TP, 2016. Novel inflammatory markers associated with cognitive performance: Singapore Longitudinal Ageing Studies. Neurobiol. Aging 39, 140–146. 10.1016/j.neurobiolaging.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Gu Y, Vorburger R, Scarmeas N, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, Brickman AM, 2017. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain. Behav. Immun 65, 150–160. 10.1016/j.bbi.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Wiechmann AR, Johnson LA, Edwards M, Barber RC, Winter AS, Singh M, O’Bryant SE, 2013. Biomarkers of vascular risk, systemic inflammation, and microvascular pathology and neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimer’s Dis 35, 363–371. 10.3233/JAD-122359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2018. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regession Approach. Guilford Press; 3–4. [Google Scholar]
- Heneka MT, Carson MJ, Khoury J. El, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP, 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci 16, 358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH, 2009. Systemic inflammation and disease progression in alzheimer disease. Neurology 73, 768–774. 10.1212/WNL.0b013e3181b6bb95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Kuller LH, Barinas-Mitchell EJM, McDade EM, Klunk WE, Cohen AD, Mathis CA, DeKosky ST, Price JC, Lopez OL, 2014a. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol. 71, 562–568. 10.1001/jamaneurol.2014.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Lopez OL, Evans RW, Kamboh MI, Williamson JD, Klunk WE, Mathis CA, Price JC, Cohen AD, Snitz BE, DeKosky ST, Kuller LH, 2014b. Markers of cholesterol transport are associated with amyloid deposition in the brain. Neurobiol. Aging 35, 802–807. 10.1016/j.neurobiolaging.2013.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss HT, Naismith JH, 2000. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech 50, 184–195. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, Senjem ML, Gunter JL, Lowe V, Gregg BE, Pankratz VS, Petersen RC, 2014. Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology 82, 1605–1612. 10.1212/WNL.0000000000000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattsson N, Stomrud E, Lindberg O, Palmqvist S, Zetterberg H, Blennow K, Hansson O, 2018. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 91, e867–e877. 10.1212/WNL.0000000000006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF, Lipinska I, Kathiresan S, Benjamin EJ, Decarli C, 2007. Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology 68, 1032–1038. 10.1212/01.wnl.0000257815.20548.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ, 2008. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimer’s Dement. 4, 242–250. 10.1016/j.jalz.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT, 2018. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissien A, 2001. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. Br. Med. J 322, 1447–1451. 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer KA, Rockstroh JK, Sauerbruch T, Spengler U, 1996. A comparative study of different enzyme immunosorbent assays for human tumor necrosis factor-. J. Immunol. Methods 195, 49–54. 10.1016/0022-1759(96)00090-7 [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Tallents RH, Miller JNH, Olschowka ME, Johnson R, Yang M, Olschowka JA, Brouxhon SM, O’Banion MK, 2011. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J. Neuroinflammation 8. 10.1186/1742-2094-8-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, Carvalho AF, Herrmann N, 2017. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 88, 876–882. 10.1136/jnnp-2017-316201 [DOI] [PubMed] [Google Scholar]
- Liu X, Yu Y, Zhu S, 2018. Inflammatory markers in postoperativedelirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS One 13. 10.1371/journal.pone.0195659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Chang YF, Klunk WE, Mathis C, Price J, Aizenstein HJ, Snitz B, Cohen AD, DeKosky ST, Ikonomovic M, Ilyas Kamboh M, Kuller LH, 2018. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology 90, E1920–E1928. 10.1212/WNL.0000000000005549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Klunk WE, Mathis C, Coleman RL, Price J, Becker JT, Aizenstein HJ, Snitz B, Cohen A, Ikonomovic M, McDade E, Dekosky ST, Weissfeld L, Kuller LH, 2014. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology 83, 1804–1811. 10.1212/WNL.0000000000000977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson KP, Sompol P, Kannarkat GT, Chang J, Sniffen L, Wildner ME, Norris CM, Tansey MG, 2017. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol. Dis 102, 81–95. 10.1016/j.nbd.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães TNC, Weiler M, Teixeira CVL, Hayata T, Moraes AS, Boldrini VO, dos Santos LM, de Campos BM, de Rezende TJR, Joaquim HPG, Talib LL, Forlenza OV, Cendes F, Balthazar MLF, 2018. Systemic Inflammation and Multimodal Biomarkers in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Mol. Neurobiol 55, 5689–5697. 10.1007/s12035-017-0795-9 [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DCH, Sheu LK, Krajina K, Manuck SB, 2015. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain. Behav. Immun 48, 195–204. 10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Kuller LH, Klunk WE, Snitz BE, Price JC, Weissfeld LA, Rosario BL, Lopresti BJ, Saxton JA, Aizenstein HJ, McDade EM, Kamboh MI, Dekosky ST, Lopez OL, 2013. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann. Neurol 73, 751–761. 10.1002/ana.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG, 2008. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflammation. 10.1186/1742-2094-5-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SL, Bowers WJ, 2012. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J. Neuroimmune Pharmacol 10.1007/s11481-011-9287-2 [DOI] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, 2014. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 71, 1379–1385. 10.1001/jamaneurol.2014.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM, 2015. Three dimensions of the amyloid hypothesis: Time, space and “wingmen.” Nat. Neurosci 10.1038/nn.4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Perera S, Snitz BE, Mathis CA, Price J, Williamson JD, DeKosky ST, Klunk WE, Lopez OL, 2017. Association of brain amyloid-β with slowgait in elderly individuals without dementia influence of cognition and apolipoprotein e ϵ4 genotype. JAMA Neurol. 74, 82–90. 10.1001/jamaneurol.2016.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbo P, Ismail R, Sommerauer M, Stokholm MG, Hansen AK, Hansen KV, Amidi A, Schaldemose JL, Gottrup H, Brændgaard H, Eskildsen SF, Borghammer P, Hinz R, Aanerud J, Brooks DJ, 2018. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study. Neurobiol. Dis 117, 211–216. 10.1016/j.nbd.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Pase MP, Himali JJ, Beiser AS, DeCarli C, McGrath ER, Satizabal CL, Aparicio HJ, Adams HHH, Reiner AP, Longstreth WT, Fornage M, Tracy RP, Lopez O, Psaty BM, Levy D, Seshadri S, Bis JC, 2020. Association of CD14 with incident dementia and markers of brain aging and injury. Neurology 94, e254–e266. 10.1212/WNL.0000000000008682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Wiener H, Acton R, Go RCP, 2001. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol. Aging 22, 873–883. 10.1016/S0197-4580(01)00291-3 [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA, 2005. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab 25, 1528–1547. 10.1038/sj.jcbfm.9600146 [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ramesh G, Maclean AG, Philipp MT, 2013. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013. 10.1155/2013/480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Bernstein I, Trivedi MH, 2014. Inflammation, obesity, and metabolic syndrome in depression: Analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES). J. Clin. Psychiatry 75, e1428–e1432. 10.4088/jcp.14m09009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA, 2018. The population 65 years and older in the United States: 2016. Am. Community Surv Reports ACS-38. [Google Scholar]
- Rosario BL, Weissfeld LA, Laymon CM, Mathis CA, Klunk WE, Berginc MD, James JA, Hoge JA, Price JC, 2011. Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage 55, 933–941. 10.1016/j.neuroimage.2010.12.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- Rubio-Perez JM, Morillas-Ruiz JM, 2012. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J 10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Herrmann N, Swardfager W, Eisen R, Lanctot KL, 2015. Inflammatory Markers in Mild Cognitive Impairment: A Meta-Analysis. J. Alzheimer’s Dis 47, 669–679. 10.3233/JAD-150042 [DOI] [PubMed] [Google Scholar]
- Simone MJ, Tan ZS, 2011. The Role of Inflammation in the Pathogenesis of Delirium and Dementia in Older Adults: A Review. CNS Neurosci. Ther 10.1111/j.1755-5949.2010.00173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Lopez OL, Kuller LH, Saxton J, Singhabahu DM, Klunk WE, Mathis CA, Price JC, Ives DG, Cohen AD, McDade E, DeKosky ST, 2013. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology 80, 1378–1384. 10.1212/WNL.0b013e31828c2fc8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg EE, Green KN, 2017. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain. Behav. Immun 61, 1–11. 10.1016/j.bbi.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K, 2014. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 10.1016/j.neuron.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH, 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B, 1992. Detection of interleukin-6 and α2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab. Investig 66, 223–230. [PubMed] [Google Scholar]
- Unger MS, Li E, Scharnagl L, Poupardin R, Altendorfer B, Mrowetz H, Hutter-Paier B, Weiger TM, Heneka MT, Attems J, Aigner L, 2020. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain. Behav. Immun 10.1016/j.bbi.2020.05.070 [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P, 2003. Tumor Necrosis Factor Signaling Pathways. Cell Death Differ. 10, 45–65. 10.1016/B978-0-12-394447-4.30048-7 [DOI] [PubMed] [Google Scholar]
- Walker KA, Gross AL, Moghekar AR, Soldan A, Pettigrew C, Hou X, Lu H, Alfini AJ, Bilgel M, Miller MI, Albert MS, Walston J, 2020. Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of nondemented older adults. Brain. Behav. Immun 10.1016/j.bbi.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Windham BG, Brown CH, Knopman DS, Jack CR, Mosley TH, Selvin E, Wong DF, Hughes TM, Zhou Y, Gross AL, Gottesman RF, 2018. The Association of Mid-and Late-Life Systemic Inflammation with Brain Amyloid Deposition: The ARIC-PET Study. J. Alzheimer’s Dis 66, 1041–1052. 10.3233/JAD-180469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gu BJ, Masters CL, Wang YJ, 2017. A systemic view of Alzheimer disease - Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol 10.1038/nrneurol.2017.111 [DOI] [PubMed] [Google Scholar]
- Wang WY, Tan MS, Yu JT, Tan L, 2015. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med 3, 1–15. 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty FK, Alfaddagh A, Elajami TK, 2016. Targeting inflammation in metabolic syndrome. Transl. Res 167, 257–280. 10.1016/j.trsl.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, Aizenstein HJ, 2006. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. - Neuroimaging 148, 133–142. 10.1016/j.pscychresns.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Kazui H, Kajimoto K, Ihara M, Morita N, Taguchi A, Yamamoto A, Matsuoka K, Takahashi M, Nakagawara J, Tsuji M, Iida H, Kishimoto T, Nagatsuka K, 2017. Mutual effect of cerebral amyloid β and peripheral lymphocytes in cognitively normal older individuals. Int. J. Geriatr. Psychiatry 32, e93–e99. 10.1002/gps.4660 [DOI] [PubMed] [Google Scholar]
- Zhang H, Sachdev PS, Wen W, Crawford JD, Brodaty H, Baune BT, Kochan NA, Slavin MJ, Reppermund S, Kang K, Trollor JN, 2016. The relationship between inflammatory markers and voxel-based gray matter volumes in nondemented older adults. Neurobiol. Aging 37, 138–146. 10.1016/j.neurobiolaging.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tudorascu DL, Lopez OL, Cohen AD, Mathis CA, Aizenstein HJ, Price JC, Kuller LH, Kamboh MI, DeKosky ST, Klunk WE, Snitz BE, 2018. Amyloid β deposition and suspected non-Alzheimer pathophysiology and cognitive decline patterns for 12 years in oldest old participants without dementia. JAMA Neurol. 75, 88–96. 10.1001/jamaneurol.2017.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Tao S, Johnson A, Tomljanovic Z, Polly K, Klein J, Razlighi QR, Brickman AM, Lee S, Stern Y, Kreisl WC, 2020. Microglial activation, but not tau pathology, is independently associated with amyloid positivity and memory impairment. Neurobiol. Aging 85, 11–21. 10.1016/j.neurobiolaging.2019.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.