Abstract
In vertebrates and invertebrates, the insulin/insulin-like growth factor 1 (IGF1) signaling (IIS) cascade is highly conserved and plays a vital role in many different physiological processes. Among the many tissues that respond to IIS in mosquitoes, the fat body has a central role in metabolism, lifespan, reproduction, and innate immunity. We previously demonstrated that fat body specific expression of active Akt, a key IIS signaling molecule, in adult Anopheles stephensi and Aedes aegypti activated the IIS cascade and extended lifespan. Additionally, we found that transgenic females produced more vitellogenin (Vg) protein than non-transgenic mosquitoes, although this did not translate into increased fecundity. These results prompted us to further examine how IIS impacts immunity, metabolism, growth and development of these transgenic mosquitoes. We observed significant changes in glycogen, trehalose, triglycerides, glucose, and protein in young (3–5 d) transgenic mosquitoes relative to non-transgenic sibling controls, while only triglycerides were significantly changed in older (18 d) transgenic mosquitoes. More importantly, we demonstrated that enhanced fat body IIS decreased both the prevalence and intensity of Plasmodium falciparum infection in transgenic An. stephensi. Additionally, challenging transgenic An. stephensi with Gram-positive and Gram-negative bacteria altered the expression of several antimicrobial peptides (AMPs) and two anti-Plasmodium genes, nitric oxide synthase (NOS) and thioester complement-like protein (TEP1), relative to non-transgenic controls. Increased IIS in the fat body of adult female An. stephensi had little to no impact on body size, growth or development of progeny from transgenic mosquitoes relative to non-transgenic controls. This study both confirms and expands our understanding of the critical roles insulin signaling plays in regulating the diverse functions of the mosquito fat body.
Keywords: insulin signaling, Anopheles stephensi, mosquito, metabolism, innate immunity
Graphical Abstract
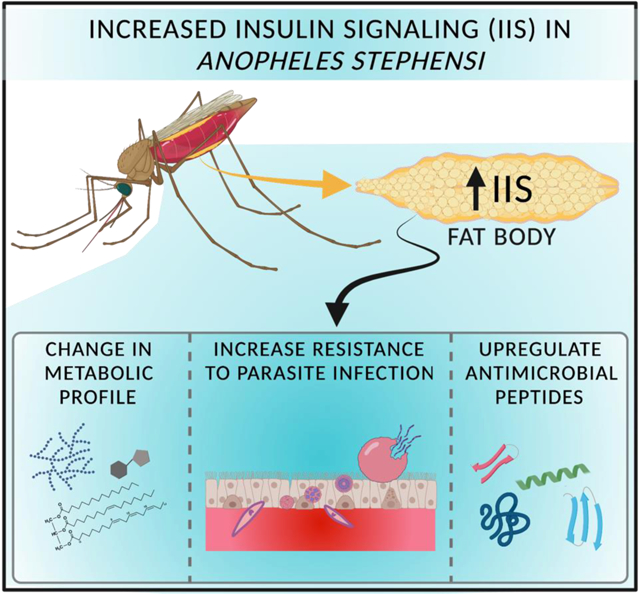
1. Introduction
Malaria continues to be a major problem worldwide with an estimated 229 million cases and more than 400,000 deaths annually (World Health Organization, 2020). While the widespread distribution of long-lasting insecticide treated bednets has improved the situation, increasing drug and insecticide resistance will likely limit these gains unless new control strategies can be identified. The insulin/insulin growth factor 1 signaling (IIS) cascade serves as a central regulator of a variety of physiologies critical to vectorial capacity, including innate immunity, reproduction, growth, metabolism and lifespan (Ahlers et al., 2019; Antonova et al., 2012; Hun et al., 2019; Ling et al., 2017; Ling and Raikhel, 2021; Sharma et al., 2019). As such, IIS is an attractive target for reducing parasite infections in the mosquito vector and limiting parasite transmission. IIS functions in a tissue-specific manner to control cellular metabolism based on the requirements of each tissue (Antonova et al., 2012; Li et al., 2020; Nandi et al., 2004; Saltiel and Kahn, 2001). In insects, insulin-like peptides (ILPs) are primarily expressed in the medial neurosecretory cells (MNCs) located in the pars intercerebralis of the head. However, the expression of ILPs has also been characterized in a variety of other insect tissues, including the midgut, ovary, glial cells, fat body and imaginal discs (Das and Arur, 2017; Lin and Smagghe, 2019; Marquez et al., 2011; Nässel et al., 2013; Riehle et al., 2006). When secreted, ILPs activate the IIS cascade in a range of tissues, including the midgut, ovaries and fat body, to elicit specific physiological effects. The insect fat body is particularly interesting as IIS in this tissue has been shown to play a fundamental role in energy metabolism, reproduction, and lifespan (Arrese and Soulages, 2010; Bai et al., 2012; Li et al., 2019). The insect fat body is also the primary site of transcriptionally induced humoral immune responses against a variety of pathogens (Ali Mohammadie Kojour et al., 2020; Lemaitre and Hoffmann, 2007; Wang et al., 2018), although the role of IIS on fat body immunity remains unclear.
Prior studies in Drosophila melanogaster revealed IIS regulation of immune pathways (DiAngelo et al., 2009; Dionne, 2014; Dionne et al., 2006; Hotamisligil, 2017; Rynes et al., 2012). For example, activation of FOXO in a non-tissue specific manner resulted in strong induction of antimicrobial peptides in both larvae and adults (Becker et al., 2010; Guo et al., 2014). Mutations in chico, the Drosophila insulin receptor substrate orthologue, were associated with increased infection resistance and a reduced bacterial load when flies were challenged (Libert et al., 2008; McCormack et al., 2016). Drosophila FOXO regulates antimicrobial peptide (AMP) expression downstream of target of rapamycin (TOR) and is essential for controlling AMP gene expression during starvation (Becker et al., 2010; Varma et al., 2014). Activation of the Toll signaling pathway in larval Drosophila fat body has been shown to disrupt IIS by preventing the phosphorylation of Akt and limiting fat body expression of dilp6 (Roth et al., 2018; Suzawa et al., 2019). In mosquitoes, the IIS cascade similarly contributes to innate defenses against pathogens. Pakpour et al. (2012) and Surachetpong et al. (2011) determined that components of the blood meal including transforming growth factor-β1 and insulin activated the IIS and MAPK signaling pathways in Anopheles stephensi, a key vector of Plasmodium that has become an emerging threat in Africa (Sinka et al., 2020). This led to increased mosquito susceptibility to infection with the human malaria parasite Plasmodium falciparum. Conversely, mosquitoes provisioned with human insulin-like growth factor (IGF)-1 in the bloodmeal exhibited reduced susceptibility to P. falciparum (Drexler et al., 2013). Moreover, overexpression of an active form of Akt, a key activator of the IIS pathway, in the midgut of An. stephensi completely blocked P. falciparum development (Corby-Harris et al., 2010). Further work revealed that this parasite-blocking phenotype resulted from increased synthesis of mitochondrially-derived reactive oxygen and nitrogen species and an inhospitable environment for developing parasites (Luckhart et al., 2013). Surprisingly, midgut expression of the IIS inhibitor PTEN was also associated with reduced susceptibility of An. stephensi to P. falciparum, although in this case improved midgut epithelial integrity and possibly the depletion of pantothenate, the precursor of coenzyme A required by the parasite, led to parasite death (Hauck et al., 2013; Souvannaseng et al., 2018). In addition to the effects of IIS manipulation on parasite infection, manipulation of An. stephensi ILP levels altered mosquito susceptibility to P. falciparum infection. Specifically, knockdown of An. stephensi ilp3 or ilp4 decreased the prevalence and intensity of P. falciparum infection and increased anti-parasite gene expression (Pietri et al., 2015). When An. stephensi were provisioned with ILP4, both prevalence and intensity of parasite infection were significantly increased, whereas provisioning of ILP3 significantly decreased infection prevalence (Pietri et al., 2016).
In addition to its role in insect host defense, IIS is an important regulator of nutrient metabolism in the fat body where a majority of insect energy regulation and nutrient storage occurs. ILPs and IIS induce fat body cells to convert glucose to glycogen, and to switch from catabolic to anabolic lipid and protein metabolism. In D. melanogaster, knockout of DILPs, individually or in combination, suggested that DILPs 2, 3, 5, 6 and 7 work together to regulate carbohydrate and lipid metabolism (Grönke et al., 2010; Semaniuk et al., 2018; Zhang et al., 2018). Slaidina et al. (2009) showed that under larval starvation conditions, expression of dilps 2, 3 and 5 were suppressed, whereas the fat body specific dilp6 was induced. In adult Drosophila, increased activity of the insulin receptor in the fat body led to significantly increased triglyceride levels and fat cell numbers, whereas increased activity of dFOXO led to reduced adipocyte numbers (DiAngelo et al., 2009). In the kissing bug Rhodnius prolixus, knockdown of Rhopr-ILP led to increased circulating lipids and carbohydrates, but decreased fat body carbohydrates (Defferrari et al., 2016). In the mosquito Aedes aegypti, ILP3 injection following decapitation was associated with significantly increased glycogen and lipid stores and reduced circulating trehalose levels (Brown et al., 2008). Disruption of ILP7 in Ae. aegypti via CRISPR/Cas9 led to increased triglyceride levels and decreased glycogen levels, while disruption of ILP8 had the opposite effects (Ling et al., 2017). The complexity of these phenotypes highlight the fact that additional studies on the role of mosquito IIS regulation of nutrient metabolism are needed.
In our previous studies, we genetically engineered an active form of the IIS kinase Akt into An. stephensi and Ae. aegypti mosquitoes under the regulation of the fat body and bloodmeal specific vitellogenin (Vg) promoter (Arik et al., 2015). We found that these TG mosquitoes survived significantly longer than NTG sibling controls, likely due to decreased expression of ilp2 in the brain and increased expression of the putative insulin binding protein imp-l2. We also found that TG mosquitoes produced significantly more Vg transcript and protein than NTG controls, although we surprisingly did not observe any changes to female fecundity (Arik et al., 2015; Hun et al., 2019). Although egg production was not increased, female mosquitoes may have provisioned their progeny with additional reserves, in turn providing them with increased resources during embryogenesis or they may have simply reallocated this excess protein to other uses. In this study, we attempted to address the role of fat body IIS on innate immunity, metabolism and progeny fitness using Vg-Akt transgenic An. stephensi.
2. Methods:
2.1. Mosquito culture
An. stephensi (Indian strain) mosquitoes were reared as described previously (Corby-Harris et al. 2010). Hemizygous transgenic (TG) mosquito lines were outcrossed with wild type colony mosquitoes each generation to enhance genetic diversity, resulting in a 50:50 mix of hemizygous TG and non-transgenic (NTG) sibling mosquitoes reared under identical conditions. TG and NTG mosquitoes were separated at the pupal stage using an Olympus SZX10 fluorescent stereomicroscope (Olympus, Tokyo, Japan) and appropriate filters for detecting enhanced green fluorescent protein (EGFP). For line maintenance and experiments, female mosquitoes were provided human blood (American Red Cross; IBC protocol 2010–014) via artificial membrane feeders.
2.2. Metabolic assays
To determine nutrient reserves for TG and NTG mosquitoes, both young (3–5 d) and old (18 d) mosquitoes were collected and allowed to mate for 48 h. Mosquitoes were then provided a blood meal via an artificial membrane feeder for 45 minutes. Whole bodies of two blood fed female TG and NTG An. stephensi mosquitoes were collected at various time points (non-blood fed or NBF, 24, 36, 48 and 72 h) post blood meal (PBM) and homogenized in phosphate buffered saline (PBS; 50 mM NaCl, 50 mM sodium phosphate buffer, pH 7.2). For the 18 d mosquito metabolic assays, all females were provided with a blood meal every 2 d until 18 d post-emergence. Samples were then collected as described for young mosquitoes.
Glucose, glycogen, trehalose, triglycerides and proteins were extracted from two mosquitoes following the modified micro-separation protocol (Telang and Wells, 2004; Zhou et al., 2004). Once fractionated, the glucose, glycogen, trehalose, triglyceride, and protein extracts were frozen at −80°C until the nutrients were quantified as described below. Three biological replicates of each experiment using unique cohorts of mosquitoes were conducted. To quantify triglycerides, a vanillin reagent assay (Van Handel, 1985a; Van Handel, 1985b) was used with the following modifications. A stock solution of triolein (1.0 mg/ml, Sigma-Aldrich, St. Louis, MO) was used to generate a standard curve from 0–200 μg. Frozen lipid fractions were dried by chloroform removal at 65°C. Next, 100 μl of 95% sulfuric acid was added to the dried sample and the resuspended sample vortexed and heated at 100°C for 10 min. Samples were cooled for 10 min at room temperature, 2 ml of vanillin reagent was added, and samples vortexed. The samples were developed in darkness for 15 min, and 100 μl of sample was added to a 96 well plate in triplicate. Absorbance at 525 nm was recorded using a spectrophotometer (Multiskan Go by Thermo Scientific, Waltham, MA) against a blank reagent control.
Glycogen was quantified using a modified anthrone-based assay (Van Handel, 1985a; Van Handel, 1985b). A 1.0 mg/ml glycogen stock (Thermo Scientific, Waltham, MA) was diluted to generate a 0–200 μg standard curve. At the final step of the micro-separation the glycogen sample was precipitated to form a pellet and dried. The glycogen pellet was solubilized with 200 μl of 25% ethanol and vortexed. Subsequently, 2 ml of anthrone reagent was added, the sample vortexed again, and then incubated for 20 min at 90°C in the dark to protect the reaction from light. Samples were cooled at 4°C for 15 min followed by vortexing. Finally, 100 μl of each sample or standard was added to a 96 well plate in triplicate. Absorbance at 625 nm was recorded spectrophotometrically (Multiskan Go by Thermo Scientific, Waltham, MA).
For glucose determination, solvent from the glucose fraction was evaporated in a heating block and the resulting residue was heated with anthrone reagent (Van Handel, 1985a; Van Handel, 1985b). Subsequently, 2 ml of anthrone reagent was added, the samples vortexed and heated at 90°C for 20 min This step releases glucose units, which react with anthrone to generate a green substrate in the dark. Samples were cooled for 15 min at 4°C, vortexed, and 100 μl of each solution was added to a 96 well plate in triplicate. Absorbance at 625 nm was recorded spectrophotometrically (Multiskan Go by Thermo Scientific, Waltham, MA) against a blank reagent control and glucose concentrations were determined against the standard curve.
To determine trehalose levels, 25% ethanol (0.2 ml) and 1M HCl (25 μl) were added to the solvent tube, the samples vortexed and then heated at 90°C for 7 min. Next, 75 μl of 1M NaOH was added to each tube, vortexed, and then heated at 90°C for 7 min. This ensured that free glucose and fructose would not lead to an overestimation of trehalose concentration. Finally, 2 ml of anthrone reagent was added, vortexed, and the sample heated at 90°C for 20 min. Samples and standards were cooled at 4°C for 15 min, vortexed and 100 μl of each solution was added to a 96 well plate in triplicate. Absorbance at 625 nm was recorded spectrophotometrically (Multiskan Go by Thermo Scientific, Waltham, MA).
To quantify protein, we used the BCA protein assay reagent kit (Thermo Scientific, Waltham, MA). A 1.0 mg/ml solution of bovine serum albumin was diluted to a 0–200 μg final concentration to generate the standard curve. Both samples and standards were treated according to the manufacturer’s protocol. Tubes were heated at 60°C for 30 min and then cooled to room temperature. Absorbance at 562 nm was recorded spectrophotometrically (Multiskan Go by Thermo Scientific, Waltham, MA) against a blank reagent control. All nutrient assays were reported as μg per individual mosquito.
2.3. Growth rate measurement
To determine the impact of fat body IIS and increased Vg titers (Arik et al., 2015) on the growth and development of An. stephensi progeny we performed three crosses: (1) TG female crossed with wild type male (F-TG), (2) TG male crossed with wild type female (M-TG), and (3) wild type female crossed with wild type male (WT). The F-TG cross was performed to examine how increased Vg protein titers in TG females affected progeny fitness. The M-TG and WT crosses were controls where no additional Vg was provided to the developing embryos by the female. Previously, we reported that engineered, myristoylated (activated) An. stephensi Akt (myrAkt) was expressed in the fat body at 24 h PBM, reaching its maximal expression at 48 h and decreasing by 72 h (Arik et al., 2015). We also showed that TG An. stephensi had significantly higher Vg protein levels at 24 h PBM relative to the NTG controls (Arik et al., 2015; Hun et al., 2019). Although we did not observe significant differences in egg production during the first and second reproductive cycles (Arik et al. 2015), the resulting offspring may have been provisioned with additional Vg, leading to improved larval fitness. To determine whether increased Vg abundance influenced progeny fitness during the first and second reproductive cycles, fifty 3–5 d old adult female mosquitoes were allowed to mate for 4 h and then separated into two groups. One group was provided with a single blood meal, while the other group received two blood meals. The second blood meal was provided at 48 h following the first blood meal to ensure that myrAkt was expressed in the fat body at the beginning of the second reproductive cycle. Mosquitoes were offered blood via an artificial membrane feeder for 45 min and fully engorged females were separated into individual cages (10 cm × 8.5 cm × 14.8 cm) with an oviposition cup. Non-fed or partially fed females were discarded. All cages were checked for eggs 72 h PBM. Eggs from individual females were hatched and mosquitoes reared to the pupal stage. Pupae were collected daily until no surviving progeny remained. TG and NTG pupae were separated using an Olympus SZX10 fluorescent stereomicroscope (Olympus, Waltham, MA) using EGFP filters and the number of TG and NTG pupae recorded. Emerging adults were collected daily, and the number of male and female mosquitoes recorded at the time of collection.
2.4. Weight and body size measurement
A total of 20 TG and 20 NTG F1 progeny were frozen overnight at −80°C in 50 ml conical tubes (VWR) and wet weight was recorded (Satorius: ED1245, Vernon Hills, IL). The same mosquitoes were subsequently dried overnight in an incubator at 50°C and weighed again to determine dry weight. As a proxy for body size we measured the wing length of both male and female progeny. The wings were mounted on slides and its length (from the axillary incision to the wing tip) and maximum width were measured using a light microscope (Olympus, Waltham, MA) and an ocular micrometer calibrated with a micrometer scale at a fixed optical zoom.
2.5. Bacterial challenge assay
To test for inducible expression of key antimicrobial genes, five TG and five NTG female mosquitoes were collected at various time points after a blood meal (NBF, 24 h, 36 h, 48 h PBM). To induce an immune response, we challenged mosquitoes from each time point against Gram-negative Escherichia coli or Gram-positive Bacillis subtilis bacteria by pricking each mosquito in the thorax with a needle dipped in bacterial culture (OD600 = 0.5). Sham inoculation (buffer only) and no inoculation controls were also included. To minimize temporal or human bias, mosquitoes were alternately inoculated with different treatments. Following inoculation, mosquitoes were given 4 h to develop a response to the bacterial challenge prior to total RNA isolation. The assay was replicated with three unique cohorts of mosquitoes.
2.6. RNA extraction and qPCR assays
We performed qPCR to assess antimicrobial peptide transcript expression in TG and NTG mosquitoes. Total RNA was isolated from pools of five whole bodies or abdominal body walls from female An. stephensi mosquitoes using the RNeasy kit and QIAcube (Qiagen, Germantown, MD) per the manufacturer’s protocol. Following insolation, total RNA samples were treated with RNAse-free DNAse 1 (Thermo Scientific Waltham, MA) to eliminate genomic DNA contamination. Total RNA concentrations were determined using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA) and diluted to a final concentration of 250 ng/μl for cDNA synthesis using the cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA) per the manufacturer’s protocol. The integrity of the cDNA samples was validated by performing a reverse-transcriptase PCR amplification of actin using Gotaq Green Master Mix (Promega, Madison, WI) and An. stephensi actin-specific primers.
The cDNA samples were used as templates for quantitative real time PCR (qPCR) on a Bio-Rad CFX Real-Time System Thermocycler (Bio-Rad, Hercules, CA) to assess the transcript abundance of various immune genes using specific primer pairs as shown in Table S1. A 20 μl qPCR reaction was assembled using 2X Sybr Green reaction mixture prepared according to the manufacturer’s protocol. Samples were loaded in triplicate onto 96 well plates and the following cycling parameters were used: 95°C for 10min, [95°C for 15 sec, 55°C for 30 sec, 72°C for 40 sec] (36 cycles), 95°C for 60 sec. qPCR data for target genes were normalized to the S7 ribosomal control (ΔCt). Fold change expression levels between TG and NTG mosquitoes were calculated using the 2^(−ΔΔCt) method. Three biological replicates from unique cohorts of mosquitoes were used for these assays. Data were analyzed by post-hoc two-way ANOVA to determine differences between TG and NTG control groups. P-values were deemed significant at P ≤ 0.05.
2.7. Bacterial challenge mortality assay
TG and NTG female mosquitoes (n=150) were allowed to mate with wild type males for 48 h before receiving a blood meal. Engorged females were separated into new containers for each group (TG and NTG). Prior to the bloodmeal (NBF) and 12 and 24 h PBM, TG and NTG female mosquitoes (n=25 each) were challenged against E. coli or B. subtilis cultures at 0.5 OD600. Challenged mosquitoes included E. coli- or B. subtilis-inoculated, PBS-inoculated and non-injected controls. To eliminate any potential time bias, pricking was performed by two people alternating between TG and NTG mosquitoes and bacteria or buffer control. Once pricked, mosquitoes were allowed to recover for 4 h, provided sugar water ad libitum, and maintained in a temperature and humidity-controlled chamber to minimize desiccation. Dead mosquitoes were counted and removed daily to determine TG and NTG mosquito mortality. Survival curves were analyzed using the Log- Rank (Mantel-Cox) test. These experiments were replicated with three biologically distinct cohorts of mosquitoes.
2.8. Plasmodium falciparum infection studies
Cultures of P. falciparum NF54 were initiated at 1% parasitemia in 10% heat-inactivated human serum, and 6% washed human red blood cells (RBCs) in RPMI 1640 with HEPES (Gibco, Thermo Scientific Waltham, MA) and hypoxanthine. Stage V gametocytes were evident by day 15 and exflagellation was evaluated on the day prior to and the day of mosquito feeding. For our assays, 5-day old female TG and NTG An. stephensi were fed on a mature gametocyte culture diluted with human erythrocytes and heat-inactivated serum. On day 10, midguts from infected females were dissected in PBS and stained with 1% mercurochrome/PBS to visualize P. falciparum oocysts. Oocysts were counted for each midgut. The mean number of oocysts per midgut (infection intensity) and percentages of infected mosquitoes (infection prevalence or presence of at least one oocyst) were calculated from all dissected mosquitoes. Experiments were independently replicated with three cohorts of 60–120 mosquitoes. Data were pooled for analysis as one-way ANOVA determined that infections in control groups were not significantly different among replicates. Infection prevalence and intensity data were analyzed by Fisher’s exact test, Mann–Whitney test, or nonparametric ANOVA (Kruskal–Wallis) followed by Dunn’s multiple comparisons test to determine differences between TG and NTG control. P-values were deemed significant when P ≤ 0.05.
3. Results
3.1. Increased IIS in the fat body increased early glucose levels and later nutrient storage following the blood meal in young An. stephensi
We previously demonstrated that myrAkt protein expression in the An. stephensi fat body was initially observed at 24 h PBM, with FOXO phosphorylation evident at 36 h PBM, and myrAkt expression reaching maximal levels at 48 h PBM (Arik et al., 2014). In this work we assessed the impact of increased fat body IIS on An. stephensi nutrient metabolism by quantifying glucose, glycogen, triglycerides, trehalose and protein levels in young (3–5 days) and older (18 days) mosquitoes at various time points during a reproductive cycle. Young TG mosquitoes exhibited significantly increased glucose levels early in the reproductive cycle (24 h PBM, P<0.01; 36 h PBM, P<0.0008) relative to NTG controls (Fig. 1A). Metabolic storage molecules were significantly increased later in the reproductive cycle. Specifically, glycogen levels were significantly elevated in TG mosquitoes at 48 h (P<0.01) and 72 h (P<0.005) PBM relative to NTG controls (Fig. 1B), while triglyceride levels were significantly higher in TG mosquitoes at 48 h (P<0.001) and 96 h (P<0.0003) PBM (Fig. 1D). Interestingly, levels of trehalose (P<0.01) and protein (P<0.003) in TG mosquitoes were both significantly higher at 72 h PBM (Fig. 1C and E) relative to NTG controls but did not differ at other timepoints. In contrast to younger mosquitoes where dramatic energy changes were observed in TG mosquitoes, in older mosquitoes (18 days), only triglyceride levels were significantly higher in TG mosquitoes at 24 h (P<0.03) and 36 h (P<0.002) PBM (Fig. S1D). No significant differences in glucose, glycogen, trehalose, or protein were observed between TG and NTG controls at any time points (Fig. S1A–C, E). It should be noted that older mosquitoes, both TG and NTG, trended towards reduced levels of glucose, trehalose, and glycogen, suggesting modest but not significant reductions in energy reserves as mosquitoes aged.
Fig.1. Impact of increased fat body IIS on the nutrient stores of young mosquitoes.
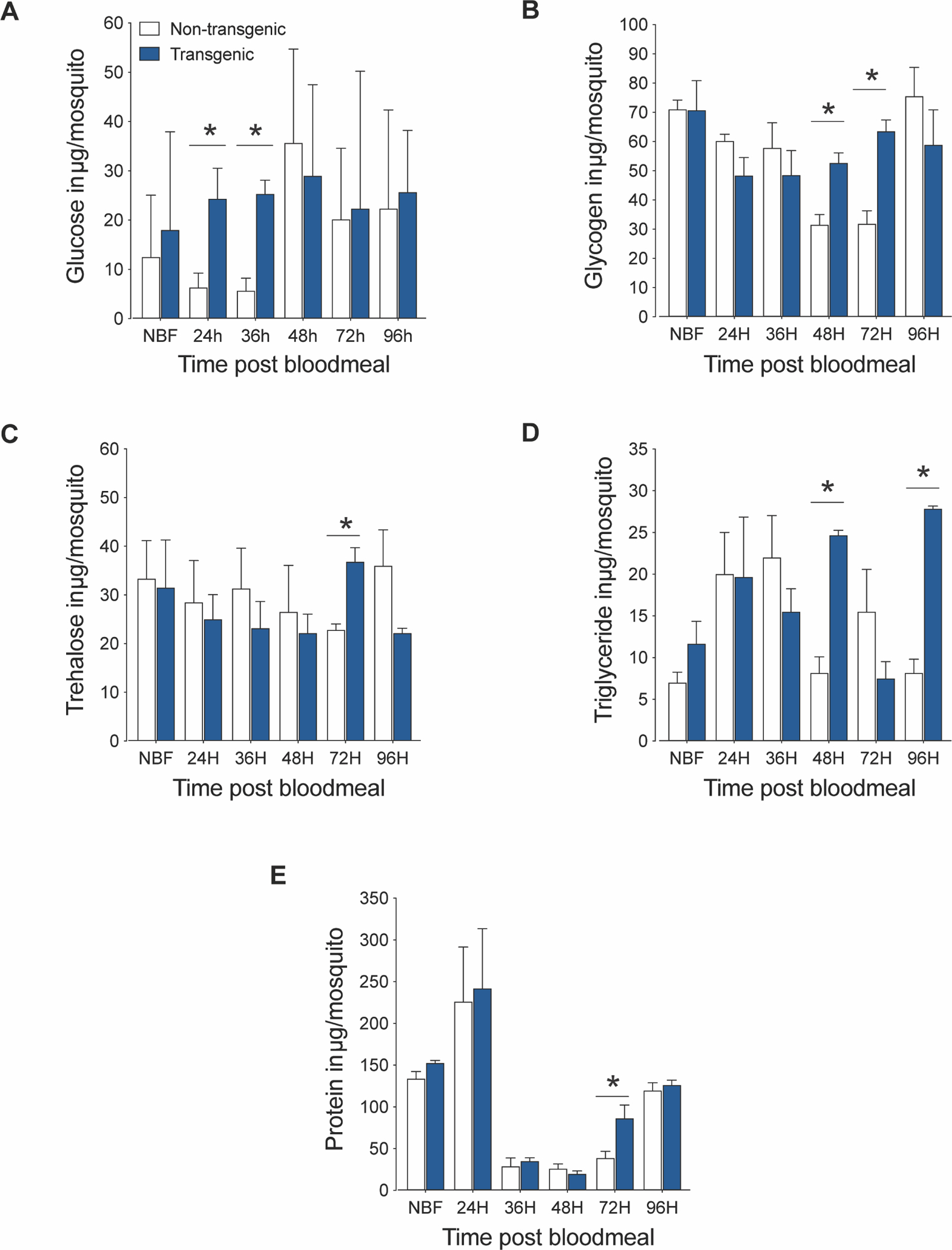
Increased IIS in the fat body during a reproductive cycle significantly increased glucose, glycogen, trehalose, and triglyceride levels in young (3–5 days) TG female An. stephensi. Glucose (A), glycogen (B), trehalose (C), and triglycerides (D) were extracted and assayed from at least three unique cohorts of mosquitoes. Asterisks (*) indicate a significant difference (P<0.05) versus NTG controls.
3.2. Increased fat body IIS did not affect the growth and development of An. stephensi progeny
Previously, we demonstrated that increased IIS in the fat body led to increased synthesis of Vg proteins in TG An. stephensi and Ae. aegypti, but this did not result in an increase in egg production during the first two reproductive cycles (Arik et al., 2015). To assess whether increased Vg production leads to increased provisioning of developing eggs and in turn increased progeny fitness, we performed three different crosses (F-TG, M-TG, and WT per above Methods). We examined the number of eggs, pupae and the number of adults (males and females) from all three crosses that received either a single blood meal or a second meal 48 h after the first ensuring transgene expression throughout the second reproductive cycle (Arik et al., 2015). Female An. stephensi provided a single blood meal showed no differences in either the total number of eggs or average number of eggs per female (Fig. 2A–B). While F-TG (P<0.03) and M-TG (P<0.03) crosses generated significantly higher numbers of pupae per female relative to WT controls (Fig. 2C), this did not translate into significantly increased numbers of emerged adults per female (Fig. 2D). Furthermore, there were no differences in the percentage of eggs from each individual female that survived to adulthood (Fig. 2E), in the total number of males or females produced among these three crosses, or in the observed sex ratios (Fig. 2F and G).
Fig.2. Increased fat body IIS does not affect the development of progeny.
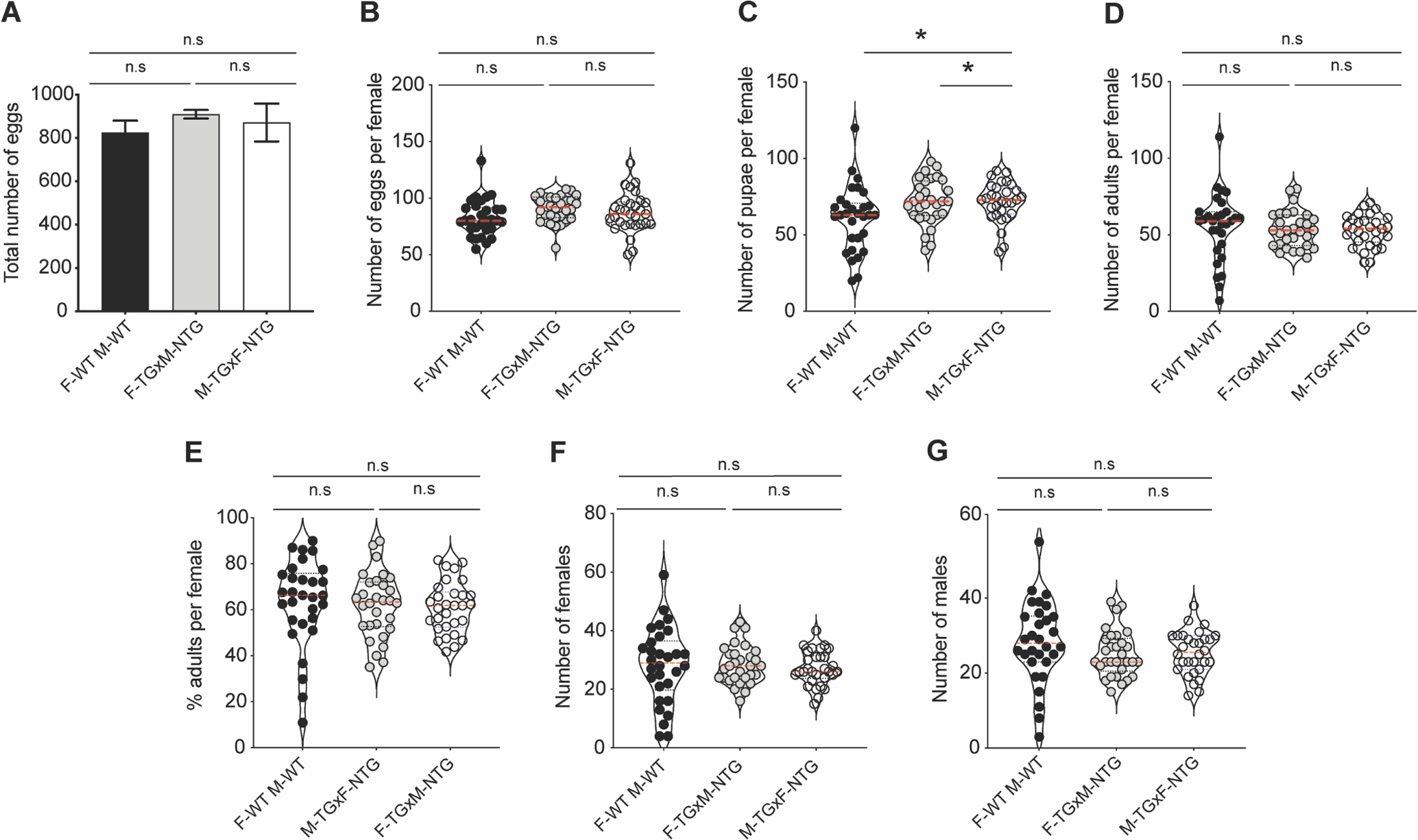
Three crosses were (F-WT X M-WT; F-TG X M-WT and F-WT X M-TG) and the impact on their progeny examined. The total number of eggs (A), number of eggs per female (B), number of progeny successfully pupating per female (C), number of progeny reaching adult emergence (D), percentage of eggs from each individual female that survived to adulthood (E) and the ratio of females (F) and males (G) were assayed from three different cohorts of ten females per replicate (n=3). Asterisks (*) indicate a significant difference from the NTG controls (P<0.05), while n.s indicates no (significant) difference.
In our previous study, we demonstrated that myrAkt transgene transcript expression was first observed at 12 h after a blood meal, with initial protein expression occurring at 24 h and maximal expression observed by 48 h (Arik et al., 2015). Thus, during the first blood feeding of transgenic mosquitoes, IIS is not increased during the first third of the reproductive cycle, which includes the initiation of vitellogenesis. To ensure that myrAkt protein was present at the beginning of the second reproductive cycle, we provided a second blood meal 48 h after the initial bloodmeal to the F-TG cross. This allowed for the initiation of the next reproductive cycle while significant levels of myrAkt protein were present. We again observed a significant increase in that the number of pupae produced by the F-TG cross (P<0.03) relative to the WT control (Fig. S2C). However, we did not observe any differences in the total number of eggs (Fig. S2A), the number of eggs per female (Fig. S2B), the number of adults per mosquito (Fig. S2D), or the total number of females and males (Fig. S2A and B) among the three crosses. Furthermore, there were no differences in males and females produced nor were there any differences in sex ratios (Fig. S2E and F) among the three crosses. In summary, the expression of myrAkt in the An. stephensi fat body did not affect the number of progeny that successfully developed to adulthood or their sex ratios.
We also considered the possibility that increased nutrient availability during embryo provisioning could lead to increased growth and adult body size. To assess the impact on progeny body size, we measured wing length as a proxy for body size, as well as wet and dry weights from all three crosses. We observed no significant differences in wing length, wet weight or dry weight among TG, NTG and WT mosquitoes for any of the three crosses (Fig. S3).
3.3. Bacterial challenge upregulated immune gene expression in transgenic An. stephensi relative to non-transgenic controls
The mosquito fat body plays a key role in innate immunity as the primary source of antimicrobial peptides (Bian et al., 2005; Kokoza et al., 2010). To assess whether fat body IIS functions in the regulation of innate immunity in An. stephensi, we measured transcript expression of various immune genes including Defensin (Def), Gambicin (Gam), Cercropin (Cec), Thioester-containing protein 1 (TEP1), Anopheles Plasmodium-responsive leucine-rich repeat 1 (APL1), and the transmembrane peptidoglycan (PGN) Recognition Protein LC (PGRP-Lc) following three different treatments: (1) unmanipulated (no inoculation), (2) injured (saline control), and (3) Gram-positive or Gram-negative bacterial challenge at various time points prior to and following a blood meal (NBF, 24, 36, 48 h PBM) in TG and NTG mosquitoes. When challenged with E. coli, TG mosquitoes exhibited increased transcript expression of Def at 24 h PBM (Fig. 3M; P< 0.001), Gam in NBF (Fig. 3N; P<0.0007) and at 24 h PBM (Fig. 3N; P<0.02), Cec in NBF, 24, 36 and 48 h PBM (Fig. 3O; P<0.001, P< 0.02, P<0.02 and P<0.01) and TEP1 at 36 h PBM (Fig. 3P; P<0.02) relative to NTG controls. We did not observe any differences in PGRP-Lc (Fig. 3Q) and APL1 (Fig. 3R) expression levels in E. coli-challenged TG relative to NTG controls. Importantly, there were no significant differences in Def, Gam, Cec, TEP1, APL1, and PGRP-Lc transcript levels between TG and NTG mosquitoes in unmanipulated (Fig. 3A–F) and injured (Fig. 3G–L) An. stephensi demonstrating that increased antimicrobial peptide expression was specific to the presence of bacteria. When TG and NTG mosquitoes were challenged with B. subtilis, we observed significantly increased expression of Def at 24 h PBM (Fig. 4M; P<0.01), Gam at 36 h PBM (Fig. 4N; P<0.01), TEP1 at 48 h PBM (Fig. 4P; P<0.02), and PGRP-Lc at 48 h PBM (Fig. 4Q; P<0.04) in TG mosquitoes relative to NTG controls. No significant differences were observed for Cec (Fig. 4O) and APL1 (Fig. 4R) expression. As with E. coli, there were no significant differences in expression between unmanipulated (Fig. 4A–F) or injured (Fig. 4G–L) TG and NTG at any time point PBM. Finally, we also report the ΔCt values relative to S7 ribosomal control to demonstrate the relative abundance of antimicrobial peptide transcript in the mosquito (Fig. S4 – E. coli; Fig. S5 - B. subtilis). Notably, E. coli challenge resulted in increased expression of Gam in both TG and NTG mosquitoes relative to the sham injected controls, while Cec levels were only increased relative to the sham injected controls in TG mosquitoes (Fig. S4). In contrast, B. subtilis challenge led to significant increases in Def and Cec levels in both TG and NTG mosquitoes (Fig. S5). Importantly, ΔCt values greater than 10, as seen for many TEP1, APL1 and PGPLC samples in the non-injected and sham injected treatments, suggest minimal transcript expression.
Fig. 3. Impact of increased fat body IIS on antimicrobial peptide expression following an E. coli challenge.
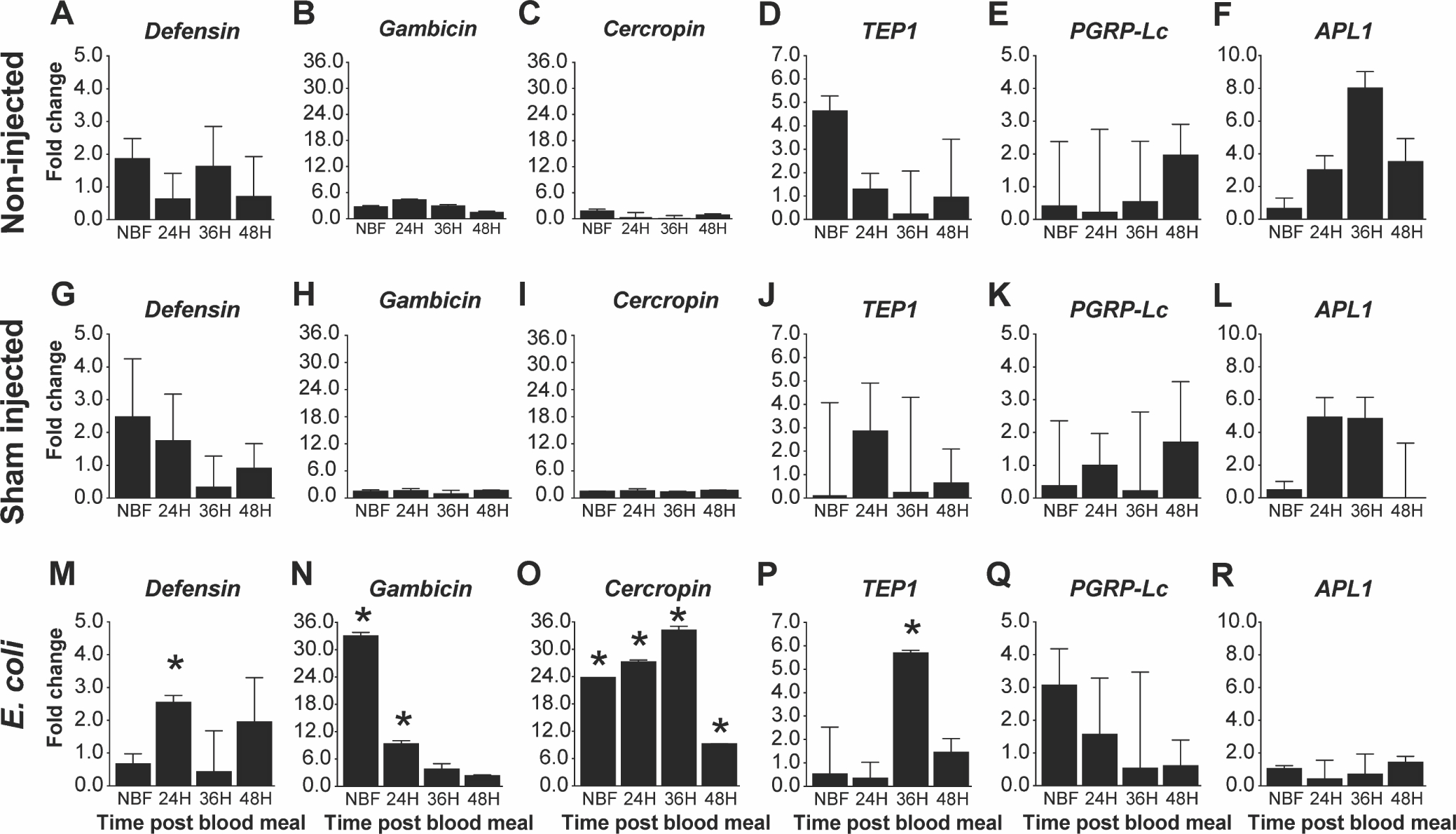
Graphs represent fold-change (2^(−ΔΔCt)) of TG An. stephensi transcript expression for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1) relative to NTG sibling controls. Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with E. coli at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Transcript expression in TG mosquitoes were normalized against NTG sibling controls at each specific time points to calculate the fold change. Three biological replicates were conducted. Error bars indicate SEM and an asterisks (*) represents a significant difference (P<0.05) between the TG and NTG samples.
Fig. 4. Impact of increased fat body IIS on antimicrobial peptide expression following a B. subtilis challenge.
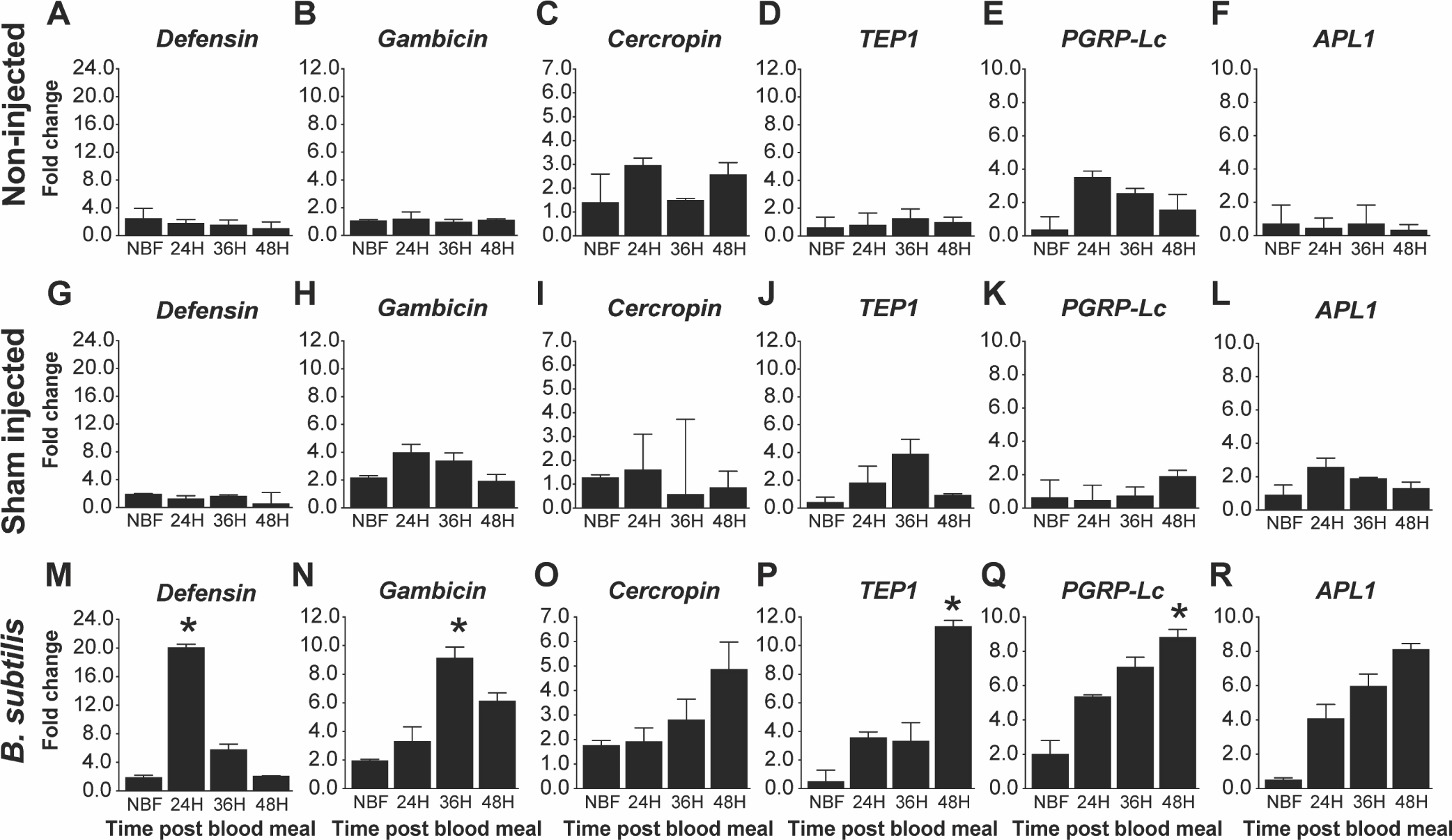
Graphs represent fold-change (2^(−ΔΔCt)) of TG An. stephensi transcript expression for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1) relative to NTG sibling controls. Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with B. subtilis at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Transcript expression in TG mosquitoes were normalized against NTG sibling controls at each specific time points to calculate the fold change. Three biological replicates were conducted. Error bars indicate SEM and an asterisks (*) represents a significant difference (P<0.05) between the TG and NTG samples.
3.4. Increased fat body IIS partially rescued An. stephensi survival following bacteria challenge
The marked increase in antimicrobial peptide transcript expression in myrAKT transgenic An. stephensi led us to test whether survivorship following bacterial challenge might be improved in TG relative to NTG mosquitoes. Survivorship was examined under three treatments: unmanipulated, injured, or E. coli- or B. subtilis-challenged, with these treatments delivered prior to blood feeding (NBF) or at 12 h or 24 h PBM. These time points were selected because, in our previous work, we found that myrAKT transgene expression in An. stephensi was initiated at 12 h PBM and increased in expression up to 48 h PBM. As would be expected, unmanipulated mosquitoes survived longer than injured mosquitoes, which in turn survived longer than mosquitoes challenged with E. coli or B. subtilis (Figs. 5A, 5B and 5C; S6A, B and C). Survivorship of TG and NTG mosquitoes challenged with E. coli or B. subtilis prior to blood feeding was not different in any of the three biological replicates (Figs. 5B and S6B). This was expected since the transgene is not expressed in mosquitoes in the absence of a bloodmeal. However, at 12 h and 24 h PBM, when myrAKT expression was increasing, survival of TG mosquitoes challenged with E. coli was significantly increased (P<0.03 to P<0.0006) relative to NTG controls (Fig.5B and 5C and S6B and C). As with E. coli challenge, TG and NTG NBF mosquitoes challenged with B. subtilis showed no differences in survival (Figs. 5B and S6B). However, survival of TG mosquitoes was significantly increased following challenge with B. subtilis at 24 h PBM (P<0.021 to P<0.011) in 2 out of 3 independent experiments (Fig. 5C and Fig. S6C), and marginally increased (P<0.1) at both 12 h and 24 h for all other biological replicates of the B. subtilis treatments (Fig.5B and C; S6B and C). Collectively, the increased survival of TG mosquitoes challenged with both Gram-negative and Gram-positive bacteria, coupled with the increased expression of antimicrobial peptides, provides strong evidence that fat body IIS plays an important role in regulating host defenses that are critical to survival following immune challenge.
Fig. 5. Survival of TG and NTG mosquitoes following E. coli or B. subtilis infection.
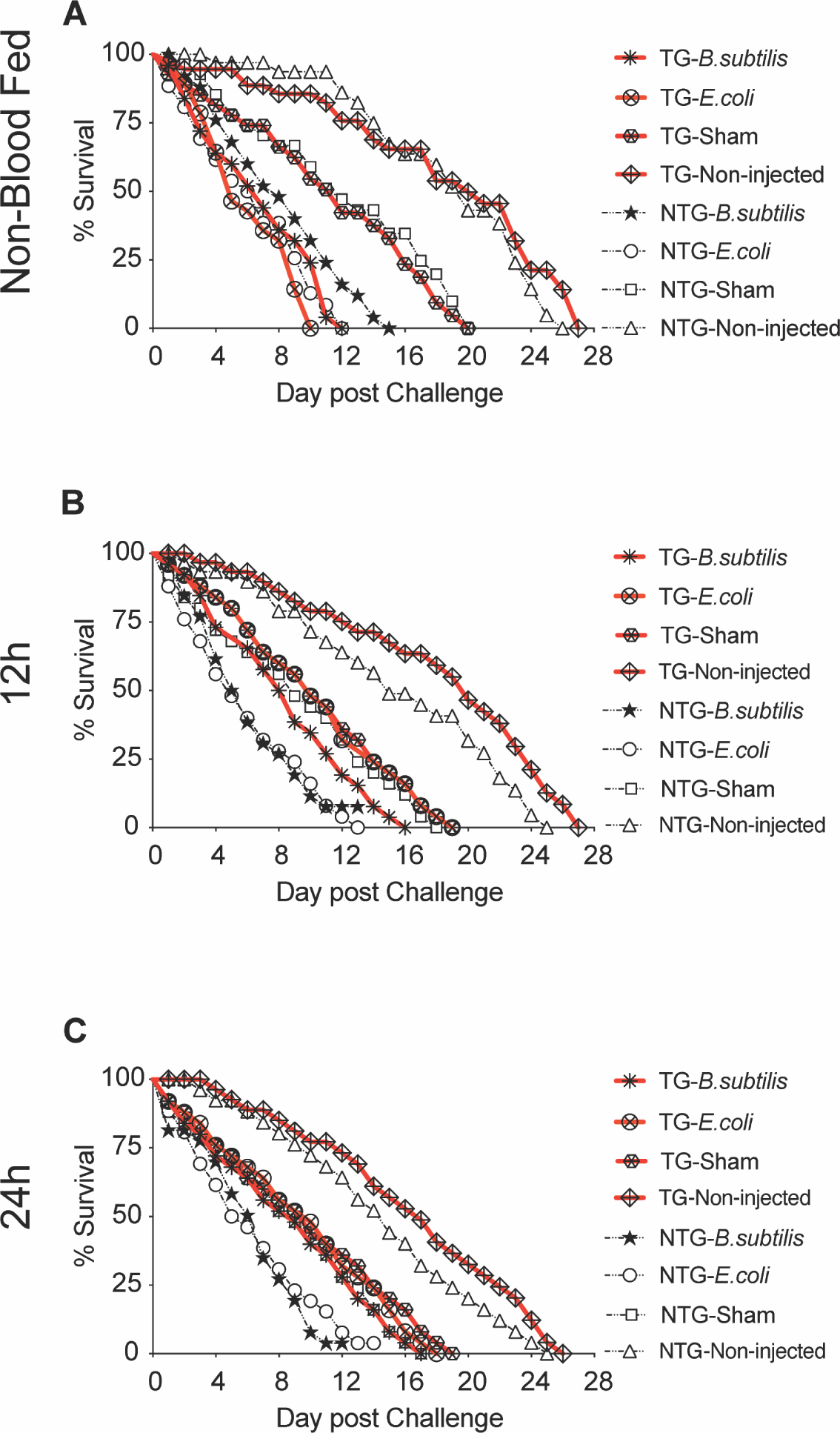
TG and NTG sibling mosquitoes (n=25/treatment) were challenged with either E. coli or B. subtilis and maintained until the final mosquito perished to establish a survival curve. Non-injected and sham injected mosquitoes were used as controls. Mosquitoes were challenged prior to blood feeding, when no transgene was present and 12 and 24 h post-bloodmeal after transgene induction. All treatments are shown on the graphs (A, B and C). This figure is a representative assay and survival curves from two additional biological replicates can be found in supplementary figures (Supp. Figs 6). P values were calculated using the Log-Rank (Mantel-Cox) test and reflect comparisons between matched TG and NTG controls at alpha = 0.05.
3.5. Increased fat body IIS reduced the prevalence and intensity of P. falciparum infection
Increased fat body myrAKT activity and IIS in TG mosquitoes relative to NTG mosquitoes resulted in significant reductions in both the percentage of mosquitoes infected with P. falciparum (infection prevalence) as well as the number of oocysts per infected mosquito midgut (infection intensity, zeroes not included; Fig. 6). Specifically, the percentage of mosquitoes with one or more oocysts was significantly decreased, from an average of 55% (n=127) in NTG mosquitoes to 22% (n=115) in TG mosquitoes (P<0.0001; Fig. 6A). Furthermore, infection intensity was reduced by 33.5%, from an average of 1.71 oocysts/midgut (n=70) in NTG controls to 1.22 oocysts/midgut (n=23) in TG mosquitoes (P<0.0001; Fig. 6B).
Fig. 6. Resistance of TG and NTG mosquitoes to P. falciparum infection.
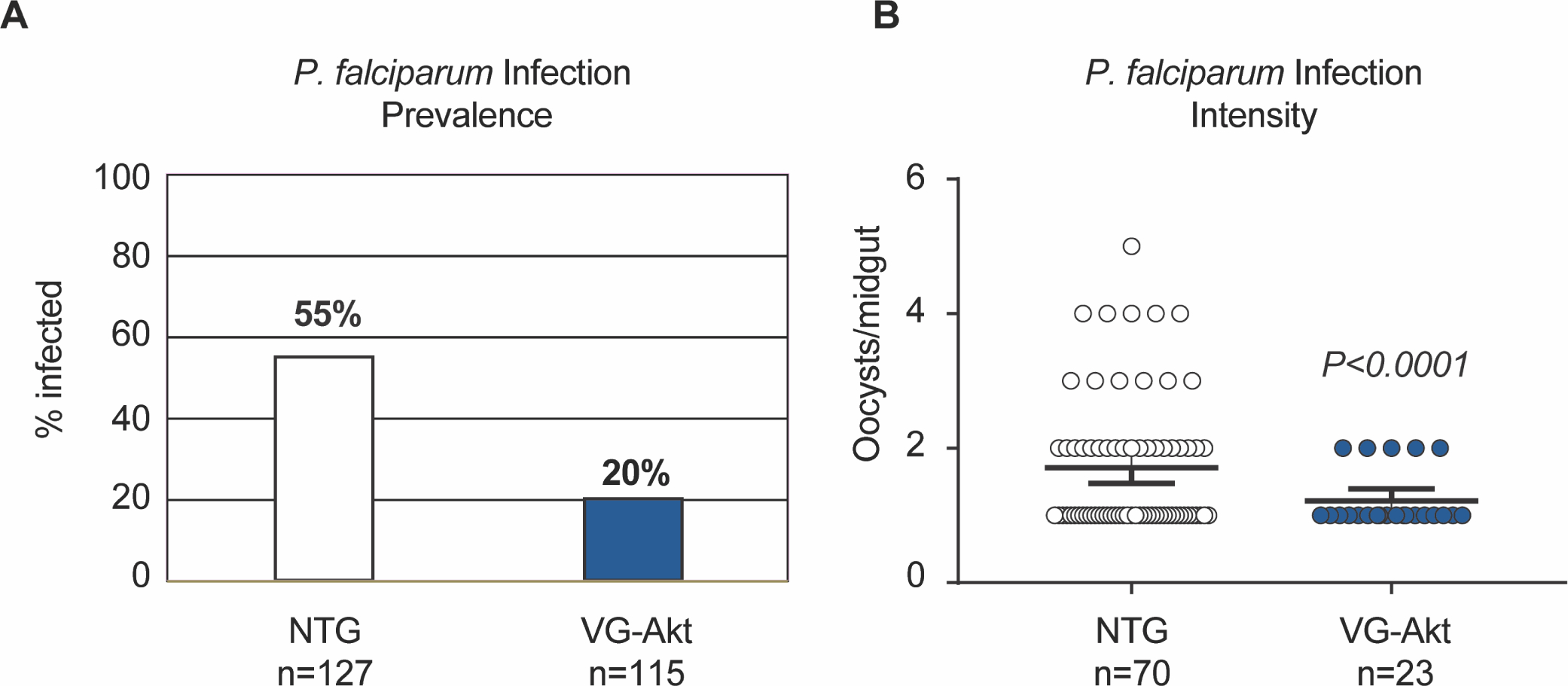
TG and NTG mosquitoes were provided with an artificial blood meal containing P. falciparum NF54 gametocytes. Ten days after infection, the midguts were dissected and the numbers of P. falciparum oocysts were counted. A) Infection prevalence was defined as the percentage of mosquitoes that had at least one oocyst in the midgut. B) Infection intensity was defined as the total number of oocysts per midgut. The black line represents the mean number of oocysts per midgut ± SEM. Three replicates were conducted using unique biological cohorts.
3.6. Increased fat body IIS was associated with increased NOS mRNA expression
Studies have shown that NOS expression in An. stephensi is transcriptionally activated following infection by Plasmodium, thereby limiting infection with both mouse malaria parasites and the human parasite P. falciparum (Drexler et al., 2014; Lim et al., 2005; Luckhart et al., 2013; Luckhart et al., 1998; Peterson et al., 2007; Surachetpong et al., 2011). Based on the reduction of the P. falciparum above in TG relative to NTG An. stephensi, we measured NOS transcript expression in whole bodies and abdominal walls of TG and NTG NBF mosquitoes and at 24, 36, and 48 h PBM (Fig. 7). In these experiments, TG mosquitoes exhibited significantly increased NOS expression in the abdomen at 24 h (P=0.03) and 36 h (P=0.03) PBM relative to NTG controls (Fig. 7D). Similarly, in whole body, NOS expression was significantly higher in TG mosquitoes at 24 h (P=0.003) and 36 h (P=0.0008) PBM as compared to NTG controls (Fig. 7B). These results are consistent with a variety of studies demonstrating that manipulation of mosquito IIS leads to increased AsNOS gene expression in An. stephensi (Drexler et al., 2014; Luckhart et al., 2013; Pietri et al., 2015). Collectively, these data suggest that NO, in addition to increased antimicrobial peptides, contribute to significantly reduced P. falciparum infection observed in TG relative to NTG mosquitoes.
Fig. 7. Fat body IIS increased AsNOS gene expression in An. stephensi.
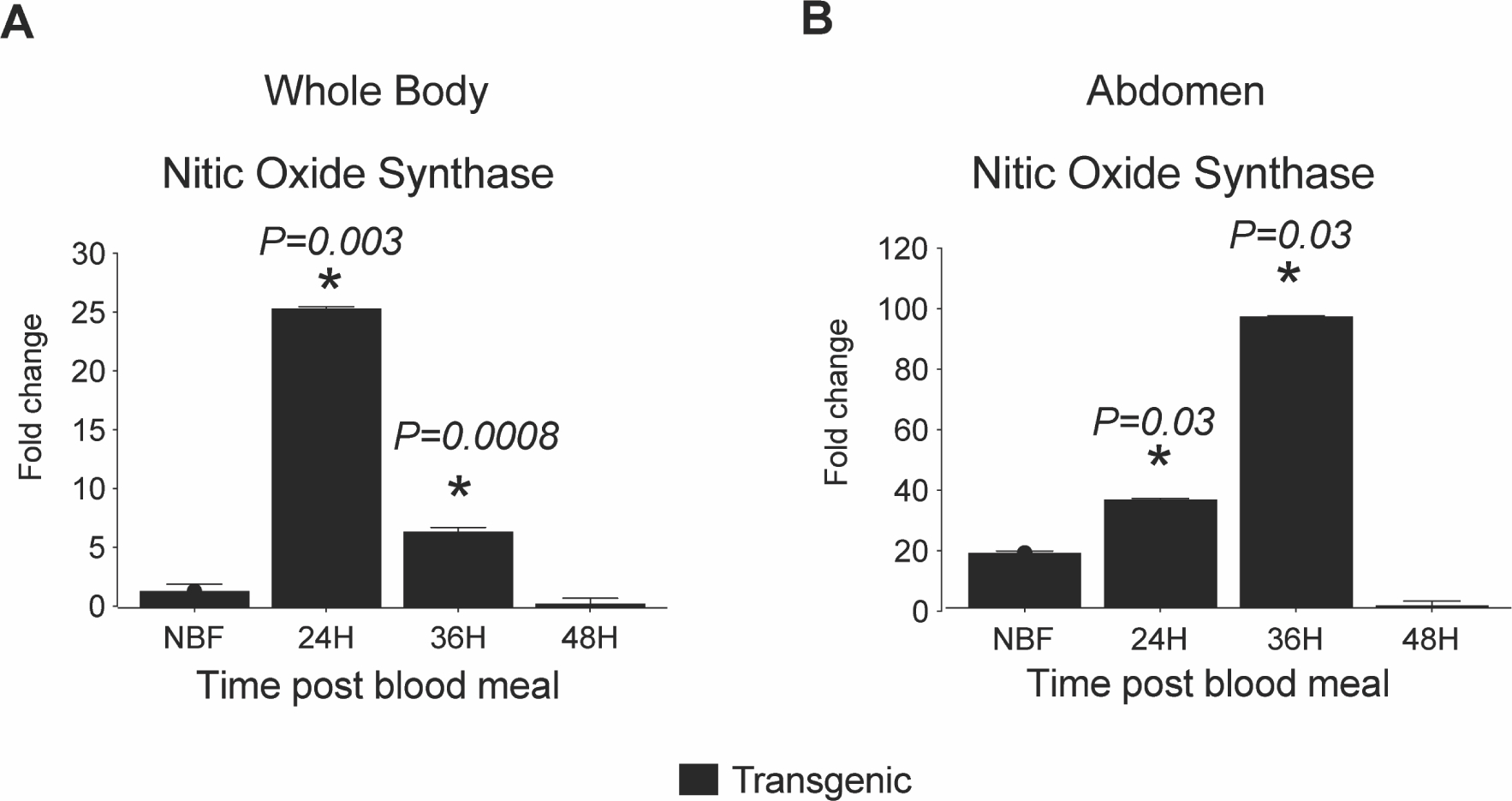
Graph represents the mean (ΔCt) mRNA expression of selected immune genes in RNA samples prepared from a pool of 5: (A) whole body mosquitoes, and (B) abdominal wall (of mosquito abdomen) at different time points for both TG and NTG mosquitoes. Graphs represent means ± SEMs of fold change in the expression of AsNOS for: (C) whole body mosquitoes, and (D) abdominal wall (of mosquito abdomen). Pairwise comparisons of treatments and matched controls were analyzed by Student’s t-test; significant and marginally significant p-values are shown, n = 5.
4. Discussion
The IIS cascade is present in all multicellular organisms and regulates diverse physiological functions including growth, development, fecundity, metabolic homeostasis, immunity and lifespan in a tissue dependent manner (Antonova-Koch et al., 2013; Sharma et al., 2019). In our previous work, we demonstrated that myrAkt-dependent IIS activation in the mosquito fat body led to a significant extension of lifespan relative to NTG controls (Arik et al., 2015). We reported that this lifespan extension was likely due to reduced expression of ILP2 in the head and increased expression of imp-L2 (Hun et al., 2019). Furthermore, we observed that adult female mosquitoes produced significantly more Vg protein, although this did not translate to an increase in fecundity (Arik et al., 2015). In this study, we explored the impact of fat body specific IIS on mosquito metabolism, innate immune response, and whether increased Vg expression had any impact on the growth and development of progeny.
As expected, IIS in the mosquito fat body was a key regulator of metabolism in the mosquito. During the first half of the reproductive cycle in young TG mosquitoes, glucose levels were increased relative to NTG controls, but metabolic stores of glycogen and triglycerides were minimally changed. During the second half of the reproductive cycle, in contrast, we observed increased levels of both storage molecules. In D. melanogaster, ablation of MNCs in early larval stages resulted in increased hemolymph sugar levels (Rulifson et al., 2002). However, this phenotype could be abolished by ubiquitously overexpressing dilp2 (Rulifson et al., 2002). Another study in D. melanogaster showed that overexpression of dilp6 in the adult fat body suppressed dilp2 expression in the head (Bai et al. 2012). This ultimately extended lifespan and increased levels of triglycerides, glycogen and trehalose, similar to our observations in the latter half of the reproductive cycle. Increased fat storage and lipid turnover have been shown to positively impact lifespan in D. melanogaster (Katewa et al., 2012; Longo and Mattson, 2014). In our previous study, we observed that mosquitoes with increased fat body IIS exhibited significantly reduced ILP2 expression in the head (Hun et al., 2019), which could explain the metabolic changes we observed in TG mosquitoes in a manner consistent with observations in D. melanogaster. While the high level of triglycerides observed in older mosquitoes could result from the conversion of carbohydrates into stored fat and improved lipid turnover, this metabolism could also contribute to extended lifespans of TG mosquitoes (Arik et al., 2015). In contrast to younger mosquitoes, we did not see marked changes in the metabolism of older TG and NTG mosquitoes with the exception of 24 h to 36 h PBM when triglyceride levels were increased in TG mosquitoes. These older mosquitoes had modestly reduced levels of glucose, trehalose, and triglycerides and markedly reduced levels of glycogen compared to younger mosquitoes, which likely represents the ongoing depletion of these resources as mosquitoes aged. Thus, increased fat body IIS in the TG mosquitoes may not have sufficient capacity to influence resource levels in older mosquitoes.
In addition to controlling metabolism, fat body IIS plays an important role in regulating the innate immune responses. Specifically, E. coli challenge induced Def and Gam expression in both TG and NTG mosquitoes relative to non-injected and sham-injected controls (Fig. 4S), whereas B. subtilis challenge induced the expression of both Def and Cec (Fig. 5S). Since AMPs are synthesized on demand in response to an immune challenge, it is likely that transcript titers provide a reasonable estimate of the AMP immune response (Jovanovic et al., 2015; Liu et al., 2016). Importantly, increased fat body IIS in TG mosquitoes further induced expression of AMPs and TEP1 following bacterial challenge (Figs. 3 and 4) relative to NTG controls. TG mosquitoes challenged with E. coli increased Def, Gam Cec and TEP1 expression relative to NTG controls, while TG mosquitoes challenged with B. subtilis had greater expression of Def, Gam, TEP1, and PGRP-Lc compared to NTG controls. These inductions occurred after TG mosquitoes blood fed (24–48 h PBM), which corresponded to expression of the myrAkt under control of the Vg promoter (Arik et al., 2015). Previous studies in D. melanogaster indicated that the Toll pathway antagonized insulin signaling downstream of phosphoinositide 3-kinase (PI3K) as evidenced by a reduction in Akt phosphorylation (DiAngelo et al., 2009). In TG mosquitoes Akt myristylation circumvents the need for PI3K phosphorylation of phosphatidylinositol (3,4,5)-trisphosphate and thus may evade this inhibition by the Toll pathway. In Bombyx mori, it was also demonstrated that increased FOXO expression or decreased insulin receptor expression led to an increase in defensin transcript (BmDefB) in the fat body, although no immune challenge was performed (Zhang et al., 2018). We did not observe significant differences in immune gene expression between TG and NTG control groups (i.e., non- or sham-injected), only in bacterially challenged mosquitoes, which may explain this difference. Further, our IIS manipulation was fat body specific, whereas in B. mori overexpression of FOXO (baculovirus) and suppression of the insulin receptor (RNAi) were systemic. Surprisingly, Gam and Cec transcript levels were abundant in NBF TG mosquitoes relative to NTG controls following E. coli challenge, suggesting a low level of transgene expression in NBF TG mosquitoes. In support of this, we previously detected very low levels of myrAkt transcript in carcasses prior to blood feeding, although no protein was detected (Arik et al., 2015).
Because TG mosquitoes exhibited significantly increased expression of AMPs and other immune genes, we expected that this would increase the survival of bacterially challenged TG mosquitoes relative to NTG controls. Indeed, our survival analysis of challenged TG and NTG mosquitoes indicated that TG mosquitoes had significantly increased survival following challenge with E. coli or B. subtilis at 12 h and 24 h PBM. As anticipated, mosquitoes that did not receive a bloodmeal, and thus no transgene expression or increased fat body IIS, did not survive any better than NTG sibling controls. This provides compelling evidence that the significantly improved survival of TG mosquitoes was in fact due to increased fat body IIS. In D. melanogaster, both the Toll and Imd pathways are required to maximize resistance against Gram-negative Pseudomonas aeruginosa, which suppresses AMP gene expression (Apidianakis et al., 2005; Lau et al., 2003). Ae. aegypti engineered to overexpress Cec-A and Def-A under the Vg promoter were highly resistant to P. aeruginosa infection (Kokoza et al., 2010). in this light, our data suggest that increased fat body IIS is linked to improved innate immune responses against both Gram-positive and Gram-negative bacteria, likely through increased expression of AMPs in response to immune challenge.
In addition to enhancing survival in response to bacterial challenge, we also demonstrated that increased fat body IIS had a profound effect on P. falciparum infection. Our previous work on midgut IIS in An. stephensi has demonstrated that IIS and the various signaling pathways it interacts with in this tissue can significantly reduce P. falciparum infection prevalence and intensity (Luckhart and Riehle, 2020). For example, increased expression of active Akt in the midgut of An. stephensi completely blocked P. falciparum infection (Corby-Harris et al., 2010) by disrupting mitochondrial function (Luckhart et al., 2013). Conversely, midgut overexpression of the IIS inhibitor PTEN also increased resistance to P. falciparum, although via a different mechanism (Hauck et al., 2013). More recently, we demonstrated that IIS and JNK signaling can influence the synthesis of coenzyme A through the rate limiting enzyme pantothenate kinase (Simão-Gurge et al., 2021; Souvannaseng et al., 2018), presumably limiting the availability of endogenous pantothenate essential for P. falciparum sporogony. However, these studies focused on the mosquito midgut, the first barrier the parasite must physically cross and interact with for an extended period. It was unclear whether IIS in this fat body would have an impact on P. falciparum development. Here we observed significant reductions in both the prevalence and intensity of P. falciparum infection in our TG mosquitoes, indicates that IIS can influence parasite development both locally (midgut) and systemically (fat body). While increase AMP expression in the fat body could disrupt parasite development, other mechanism may also be at play. We observed that TG mosquitoes exhibited significantly higher levels of NOS expression in both the whole body and the abdominal body wall at 24 h and 36 h PBM. Moreover, we found that TEP1 mRNA expression was significantly higher in TG mosquitoes at 36 h and 48 h PBM when challenged with E. coli and B. subtilis, respectively. Since TEP1 binds and kills P. berghei and P. falciparum during the midgut stages (Blandin and Levashina, 2004), it is has been suggested that TEP1 expression is induced in the fat body to replenish circulating titers (Gupta et al., 2009; Volohonsky et al., 2017). Combined, our results are consistent with published studies associating NOS and TEP1 with reduced Plasmodium infection (Drexler et al., 2014; Luckhart et al., 2013).
ILPs and IIS are key regulators of reproduction, both in ovarian follicle cells for the synthesis of 20-hydroxyecdysone and in the fat body for the synthesis of Vg (Sharma et al., 2019; Arik et al., 2009). While we observed significant increases in both Vg transcript and protein levels in TG mosquitoes (Arik et al., 2015), this did not appear to directly affect mosquito fecundity. In this work we explored whether observed increases in Vg titers in TG mosquitoes conferred a fitness advantage to the progeny of male or female mosquitoes. Despite the fact that we observed a significant increase in pupae from TG mosquitoes, there were no significant increases in the number of male or female adults resulting from these crosses. Similar outcomes have been observed in Caenorhabditis elegans, D. melanogaster, and several species of ants and bees. In C. elegans, mutation of the age-1 gene resulted in lifespan extension with no associated fitness cost or loss of fecundity (Johnson, 2003; Walker et al., 2000). Mutation of Indy and daf-2 genes in C. elegans extended lifespan without any associated change in reproduction (Gems et al., 1998; Kenyon et al., 1993; Marden et al., 2003). Similarly, overexpression of dFOXO in the pericerebral fat body in D. melanogaster extended lifespan without reducing fecundity (Hwangbo et al., 2004). Moreover, a study by Buch et al. (2008) demonstrated that fruit flies with ablated insulin-producing cells at the post-larval stage are long-lived and have normal fecundity when kept on a protein-rich diet (Buch et al., 2008). Our data also showed no differences between the wet weights, dry weights and wing lengths of male or female progeny among all three crosses. Thus, even though TG female mosquitoes synthesized additional Vg, this did not confer a fitness advantage to the progeny, suggesting that excess Vg was likely reallocated by the female mosquito for other uses. Interestingly, Vg, lipophorin and Apolipophorin III have been implicated in the innate immune response against Plasmodium parasites (Mendes et al., 2008; Rono et al., 2010). Rono et al. (2010) demonstrated that RNAi knockdown of Vg led to a reduction in the infection intensity of Plasmodium berghei in Anopheles gambiae mosquitoes. Although these data are counter to our observations of reduced oocyst numbers in the presence of increased Vg, other factors, including timing of the Vg increase, increased fat body IIS and increased AMP synthesis likely play a role in these complex interactions. Nevertheless, additional studies to elucidate the interactions between Vg, IIS on anti-Plasmodium responses in the mosquito are warranted.
In this study, we demonstrated that increased fat body IIS in An. stephensi plays central and conserved roles in maintaining energy balance, resulting in higher levels of stored energy while changing the profile of circulating carbohydrates. We also demonstrated that fat body IIS is an important regulator of mosquito innate immunity conferring increased resistance to bacterial challenge and P. falciparum infection. This is accomplished through the upregulation of AMPs and other components of the innate immune response. Combined with our previous studies demonstrating the key role fat body IIS plays in adult survival we have begun to unravel the complex signaling regulations of diverse physiologies in the fat body of adult mosquitoes.
Supplementary Material
Fig. 1S. Impact of increased fat body IIS on the nutrient stores of old mosquitoes. Increased IIS in the fat body during a reproductive cycle significantly triglyceride levels in old (18 day) TG female An. stephensi, but had no effects on other nutrients. Glucose (A), glycogen (B), trehalose (C), and triglycerides (D) were extracted and assayed from at least three unique cohorts of mosquitoes. Asterisks indicate a significant difference (P<0.05) versus NTG controls.
Fig. 2S. Increased fat body IIS does not affect the development of progeny. Three crosses were (F-WT X M-WT; F-TG X M-WT and F-WT X M-TG) and the impact on their progeny examined. The total number of eggs (A), number of eggs per female (B), number of progeny successfully pupating per female (C), number of progeny reaching adult emergence (D) and the ratio of females (E) and males (F) were assayed from three different cohorts of ten females per replicate (n=3). Asterisks indicate a significant difference from the NTG controls (p<0.05), while N.S indicates no (significant) difference.
Fig. 3S. IIS in the fat body had no impact on the body size of TG An. stephensi progeny. Female (A) and male (B) average wing length were assayed from three different cohorts of ten mosquitoes per biological replicate (n = 3). Red lines indicate mean wing length and one-way ANOVA with Multiple comparisons was used to assess significance. No significant (n.s.) difference was observed among any of the treatments. Similarly, IIS in the fat body has no significant impact on wet weight (C, D) or dry weight (E, F) of female (C, E) or male (D, F) An. stephensi (n = 3).
Fig. 4S. Impact of increased fat body IIS on antimicrobial peptide expression following a E. coli challenge. Graphs represent AMP transcript expression relative to RPS7 expression (ΔCt) of TG and NTG An. stephensi for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1). Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with E. coli at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Three biological replicates were conducted. Error bars indicate SEM and ΔCt values greater than 10 suggest minimal transcript expression of the AMP. Lower ΔCt values equal greater transcript levels.
Fig. 5S. Impact of increased fat body IIS on antimicrobial peptide expression following a B. subtilis challenge. Graphs represent AMP transcript expression relative to RPS7 expression (ΔCt) of TG and NTG An. stephensi for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1). Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with B. subtilis at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Three biological replicates were conducted. Error bars indicate SEM and ΔCt values greater than 10 suggest minimal transcript expression of the AMP. Lower ΔCt values equal greater transcript levels.
Fig. 6S. Survival analysis under E. coli and B. subtilis challenges. Biological replicates two and three of bacterial challenge survival assays. All assays were replicated as described for Fig. 5.
Highlights.
Fat body IIS increased mosquito survival and AMP levels after bacterial challenge.
Fat body IIS significantly reduced malaria parasite infection prevalence and intensity.
Mosquitoes with increased fat body IIS had increased nutrient storage.
Acknowledgement:
We would like to thank Jenet Soto-Shoumaker for the maintenance of the wild type, non-transgenic and transgenic mosquito lines.
Funding Sources:
This work was supported by the National Institutes of Health (Grant numbers R21AI1225823, R56AI118926 and R56AI129420).
Abbreviations
- AMP
Antimicrobial Peptide
- DILPs
Drosophila Insulin-Like Peptides
- EGFP
Enhanced Green Fluorescent Protein
- FOXO
Forkhead Box O
- IGF-1
Insulin-Like Growth Factor-1
- INR
Insulin Receptor
- ILP
Insulin-Like Peptide
- IIS
Insulin/Insulin Growth Factor 1 Signaling
- MNC
Medial Neurosecretory Cells
- Myr
Myristoylation
- NOS
Niitric Oxide Synthase
- NTG
Non-Transgenic
- PBM
Post-Bloodmeal
- PI3K
Phosphoinositide 3-Kinase
- PTEN
Phosphatase and Tensin Homolog
- PTTH
Prothoracicotropic Hormone
- TEP1
Thioester Complement-Like Protein 1
- TG
Transgenic
- VG
Vitellogenin
- WT
Wild-Type
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlers LR, Trammell CE, Carrell GF, Mackinnon S, Torrevillas BK, Chow CY, Luckhart S, Goodman AG, 2019. Insulin potentiates JAK/STAT signaling to broadly inhibit flavivirus replication in insect vectors. Cell Rep. 29, 1946–1960. e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Mohammadie Kojour M, Han YS, Jo YH, 2020. An overview of insect innate immunity. Entomol. Res. 50, 282–291. [Google Scholar]
- Antonova-Koch Y, Riehle MA, Arik AJ, Brown MR, 2013. Insulin-like peptides. Handbook of Biologically Active Peptides 267. [Google Scholar]
- Antonova Y, Arik AJ, Moore W, Riehle MA, Brown MR, 2012. Insulin-like peptides: structure, signaling, and function, Insect Endocrinol. Elsevier, pp. 63–92. [Google Scholar]
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, Davis RW, Rahme LG, 2005. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc. Natl. Acad. Sci. U.S.A. 102, 2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arik AJ, Hun LV, Quicke K, Piatt M, Ziegler R, Scaraffia PY, Badgandi H, Riehle MA, 2015. Increased Akt signaling in the mosquito fat body increases adult survivorship. FASEB J. 29, 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arik AJ, Rasgon JL, Quicke KM, and Riehle MA, 2009. Manipulating insulin signaling to enhance mosquito reproduction: A possible genetic drive mechanism? BMC Physiology. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL, 2010. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Kang P, Tatar M, 2012. Drosophila insulin‐like peptide‐6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin‐like peptide‐2 from the brain. Aging Cell 11, 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M, 2010. FOXO-dependent regulation of innate immune homeostasis. Nature 463, 369–373. [DOI] [PubMed] [Google Scholar]
- Bian G, Shin SW, Cheon H-M, Kokoza V, Raikhel AS, 2005. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 102, 13568–13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Levashina EA, 2004. Thioester-containing proteins and insect immunity. Mol. Immunol. 40, 903–908. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR, 2008. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 105, 5716–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ, 2008. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 7, 321–332. [DOI] [PubMed] [Google Scholar]
- Corby-Harris V, Drexler A, De Jong LW, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis EE, Brown JM, Luckhart S, 2010. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 6, e1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Arur S, 2017. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Develop. 84, 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defferrari MS, Orchard I, Lange AB, 2016. Identification of the first insulin-like peptide in the disease vector Rhodnius prolixus: involvement in metabolic homeostasis of lipids and carbohydrates. Insect Biochem. Mol. Biol. 70, 148–159. [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ, 2009. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 20853–20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne M, 2014. Immune-metabolic interaction in Drosophila. Fly 8, 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS, 2006. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 16, 1977–1985. [DOI] [PubMed] [Google Scholar]
- Drexler A, Nuss A, Hauck E, Glennon E, Cheung K, Brown M, Luckhart S, 2013. Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi. J. Exp. Biol. 216, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler AL, Pietri JE, Pakpour N, Hauck E, Wang B, Glennon EK, Georgis M, Riehle MA, Luckhart S, 2014. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 10, e1004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL, 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genet. 150, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L, 2010. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Karpac J, Tran SL, Jasper H, 2014. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 156, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C, 2009. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, Liu D, Blacutt J, Riehle MA, Luckhart S, 2013. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microb. Infect. 15, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, 2017. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47, 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hun LV, Luckhart S, Riehle MA, 2019. Increased Akt signaling in the fat body of Anopheles stephensi extends lifespan and increases lifetime fecundity through modulation of insulin-like peptides. J. Insect Physiol. 118, 103932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gersham B, Tu M-P, Palmer M, Tatar M, 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566. [DOI] [PubMed] [Google Scholar]
- Johnson TE, 2003. Advantages and disadvantages of Caenorhabditis elegans for aging research. Exp. Gerontol. 38, 1329–1332. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, Rodriguez EH, Fields AP, Schwartz S, Raychowdhury R, 2015. Dynamic profiling of the protein life cycle in response to pathogens. Science 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, Kapahi P, 2012. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 16, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Shin SW, Okafor N, Zou Z, Raikhel AS, 2010. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 107, 8111–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GW, Goumnerov BC, Walendziewicz CL, Hewitson J, Xiao W, Mahajan-Miklos S, Tompkins RG, Perkins LA, Rahme LG, 2003. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa. Infect. Immun. 71, 4059–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J, 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743. [DOI] [PubMed] [Google Scholar]
- Li D, Chen X, Zhu F, Chen K, 2020. Insulin-like peptides in model insects. Invertebrate Survival Journal, 186–195. [Google Scholar]
- Li S, Yu X, Feng Q, 2019. Fat body biology in the last decade. Annu. Rev. Entomol. 64, 315–333. [DOI] [PubMed] [Google Scholar]
- Libert S, Chao Y, Zwiener J, Pletcher SD, 2008. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol. Immunol. 45, 810–817. [DOI] [PubMed] [Google Scholar]
- Lim J, Gowda DC, Krishnegowda G, Luckhart S, 2005. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect. Immun. 73, 2778–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Smagghe G, 2019. Roles of the insulin signaling pathway in insect development and organ growth. Peptides 122, 169923. [DOI] [PubMed] [Google Scholar]
- Ling L, Kokoza VA, Zhang C, Aksoy E, Raikhel AS, 2017. MicroRNA-277 targets insulin-like peptides 7 and 8 to control lipid metabolism and reproduction in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 114, E8017–E8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Raikhel AS, 2021. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Beyer A, Aebersold R, 2016. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP, 2014. Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Giulivi C, Drexler AL, Antonova-Koch Y, Sakaguchi D, Napoli E, Wong S, Price MS, Eigenheer R, Phinney BS, 2013. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog. 9, e1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA, 2020. Midgut mitochondrial function as a gatekeeper for malaria parasite infection and development in the mosquito host. Front. Cell. Infect. Microbiol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R, 1998. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 95, 5700–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH, Rogina B, Montooth KL, Helfand SL, 2003. Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. Proc. Natl. Acad. Sci. U.S.A. 100, 3369–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S, 2011. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Compar. Endocrinol. 173, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S, Yadav S, Shokal U, Kenney E, Cooper D, Eleftherianos I, 2016. The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun. Ageing 13, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes AM, Schlegelmilch T, Cohuet A, Awono-Ambene P, De Iorio M, Fontenille D, Morlais I, Christophides GK, Kafatos FC, Vlachou D, 2008. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog. 4, e1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Kitamura Y, Kahn CR, Accili D, 2004. Mouse models of insulin resistance. Physiol. Rev. 84, 623–647. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Kubrak OA, Liu Y, Luo J, Lushchak OV, 2013. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 4, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, Luckhart S, 2012. Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect. Immun. 80, 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TM, Gow AJ, Luckhart S, 2007. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic. Biol. Med. 42, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri JE, Pakpour N, Napoli E, Song G, Pietri E, Potts R, Cheung KW, Walker G, Riehle MA, Starcevich H, 2016. Two insulin-like peptides differentially regulate malaria parasite infection in the mosquito through effects on intermediary metabolism. Biochem. J. 473, 3487–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri JE, Pietri EJ, Potts R, Riehle MA, Luckhart S, 2015. Plasmodium falciparum suppresses the host immune response by inducing the synthesis of insulin-like peptides (ILPs) in the mosquito Anopheles stephensi. Dev. Comp. Immunol. 53, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR, 2006. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27, 2547–2560. [DOI] [PubMed] [Google Scholar]
- Rono MK, Whitten MM, Oulad-Abdelghani M, Levashina EA, Marois E, 2010. The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 8, e1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SW, Bitterman MD, Birnbaum MJ, Bland ML, 2018. Innate immune signaling in Drosophila blocks insulin signaling by uncoupling PI (3, 4, 5) P3 production and Akt activation. Cell Rep. 22, 2550–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R, 2002. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120. [DOI] [PubMed] [Google Scholar]
- Rynes J, Donohoe CD, Frommolt P, Brodesser S, Jindra M, Uhlirova M, 2012. Activating transcription factor 3 regulates immune and metabolic homeostasis. Mol. Cell. Biol. 32, 3949–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR, 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806. [DOI] [PubMed] [Google Scholar]
- Semaniuk UV, Gospodaryov DV, Feden’ko KM, Yurkevych IS, Vaiserman AM, Storey KB, Simpson SJ, Lushchak O, 2018. Insulin-like peptides regulate feeding preference and metabolism in Drosophila. Front. Physiol. 9, 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Nuss AB, Gulia-Nuss M, 2019. Insulin-like peptide signaling in mosquitoes: The road behind and the road ahead. Front. Endocrinol. 10, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão-Gurge RM, Thakre N, Strickland J, Isoe J, Delacruz LR, Torrevillas BK, Rodriguez AM, Riehle MA, Luckhart S, 2021. Activation of Anopheles stephensi pantothenate kinase and coenzyme A biosynthesis reduces infection with diverse Plasmodium species in the mosquito host. Biomolecules 11, 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M, Pironon S, Massey N, Longbottom J, Hemingway J, Moyes C, Willis K, 2020. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. U.S.A. 117, 24900–24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P, 2009. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell 17, 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannaseng L, Hun LV, Baker H, Klyver JM, Wang B, Pakpour N, Bridgewater JM, Napoli E, Giulivi C, Riehle MA, Luckhart S, 2018. Inhibition of JNK signaling in the Asian malaria vector Anopheles stephensi extends mosquito longevity and improves resistance to Plasmodium falciparum infection. PLoS Pathog. 14, e1007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surachetpong W, Pakpour N, Cheung KW, Luckhart S, 2011. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 14, 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa M, Muhammad NM, Joseph BS, Bland ML, 2019. The toll signaling pathway targets the insulin-like peptide Dilp6 to inhibit growth in Drosophila. Cell Rep. 28, 1439–1446. e1435. [DOI] [PubMed] [Google Scholar]
- Telang A, Wells MA, 2004. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 50, 677–685. [DOI] [PubMed] [Google Scholar]
- Van Handel E, 1985a. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc 1, 299–301. [PubMed] [Google Scholar]
- Van Handel E, 1985b. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1, 302–304. [PubMed] [Google Scholar]
- Varma D, Bülow MH, Pesch Y-Y, Loch G, Hoch M, 2014. Forkhead, a new cross regulator of metabolism and innate immunity downstream of TOR in Drosophila. J. Insect Physiol. 69, 80–88. [DOI] [PubMed] [Google Scholar]
- Volohonsky G, Hopp A-K, Saenger M, Soichot J, Scholze H, Boch J, Blandin SA, Marois E, 2017. Transgenic expression of the anti-parasitic factor TEP1 in the malaria mosquito Anopheles gambiae. PLoS Pathog. 13, e1006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ, 2000. Evolution of lifespan in C. elegans. Nature 405, 296–297. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Chang M-M, Wang X-L, Zheng A-H, Zou Z, 2018. The immune strategies of mosquito Aedes aegypti against microbial infection. Dev. Comp. Immunol. 83, 12–21. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2020. World malaria report 2020: 20 years of global progress and challenges, World malaria report 2020: 20 years of global progress and challenges.
- Zhang J, Yang W, Xu J, Yang W, Li Q, Zhong Y, Cao Y, Yu X-Q, Deng X, 2018. Regulation of antimicrobial peptide genes via insulin-like signaling pathway in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 103, 12–21. [DOI] [PubMed] [Google Scholar]
- Zhou G, Flowers M, Friedrich K, Horton J, Pennington J, Wells MA, 2004. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J. Insect Physiol. 50, 337–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1S. Impact of increased fat body IIS on the nutrient stores of old mosquitoes. Increased IIS in the fat body during a reproductive cycle significantly triglyceride levels in old (18 day) TG female An. stephensi, but had no effects on other nutrients. Glucose (A), glycogen (B), trehalose (C), and triglycerides (D) were extracted and assayed from at least three unique cohorts of mosquitoes. Asterisks indicate a significant difference (P<0.05) versus NTG controls.
Fig. 2S. Increased fat body IIS does not affect the development of progeny. Three crosses were (F-WT X M-WT; F-TG X M-WT and F-WT X M-TG) and the impact on their progeny examined. The total number of eggs (A), number of eggs per female (B), number of progeny successfully pupating per female (C), number of progeny reaching adult emergence (D) and the ratio of females (E) and males (F) were assayed from three different cohorts of ten females per replicate (n=3). Asterisks indicate a significant difference from the NTG controls (p<0.05), while N.S indicates no (significant) difference.
Fig. 3S. IIS in the fat body had no impact on the body size of TG An. stephensi progeny. Female (A) and male (B) average wing length were assayed from three different cohorts of ten mosquitoes per biological replicate (n = 3). Red lines indicate mean wing length and one-way ANOVA with Multiple comparisons was used to assess significance. No significant (n.s.) difference was observed among any of the treatments. Similarly, IIS in the fat body has no significant impact on wet weight (C, D) or dry weight (E, F) of female (C, E) or male (D, F) An. stephensi (n = 3).
Fig. 4S. Impact of increased fat body IIS on antimicrobial peptide expression following a E. coli challenge. Graphs represent AMP transcript expression relative to RPS7 expression (ΔCt) of TG and NTG An. stephensi for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1). Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with E. coli at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Three biological replicates were conducted. Error bars indicate SEM and ΔCt values greater than 10 suggest minimal transcript expression of the AMP. Lower ΔCt values equal greater transcript levels.
Fig. 5S. Impact of increased fat body IIS on antimicrobial peptide expression following a B. subtilis challenge. Graphs represent AMP transcript expression relative to RPS7 expression (ΔCt) of TG and NTG An. stephensi for five antimicrobial peptides (AMPs) and the thioester containing protein 1 (TEP1). Total RNA was isolated from TG and NTG An. stephensi pools (n=5 females per pool) that were either not inoculated (non-injected), inoculated with buffer only (sham injected), or inoculated with B. subtilis at various time points after a bloodmeal (0, 24, 36 or 48 h) to stimulate transgene expression. Three biological replicates were conducted. Error bars indicate SEM and ΔCt values greater than 10 suggest minimal transcript expression of the AMP. Lower ΔCt values equal greater transcript levels.
Fig. 6S. Survival analysis under E. coli and B. subtilis challenges. Biological replicates two and three of bacterial challenge survival assays. All assays were replicated as described for Fig. 5.


