Summary
Background:
Older adults with advanced cancer are at high risk for treatment toxicity. Geriatric assessment (GA) evaluates aging-related domains and guides management. We examined whether a GA intervention can reduce serious toxicity in older patients with advanced cancer receiving high risk treatment (e.g., chemotherapy).
Methods:
In this cluster randomized trial, we enrolled patients aged ≥ 70 with incurable solid tumors or lymphoma and at least one impaired GA domain starting a new treatment regimen. Community oncology practice clusters across the United States (n=40) were randomized to intervention (oncologists received a tailored GA summary and management recommendations) or usual care. The primary outcome was the proportion of patients who experienced any grade 3–5 toxicity (NCI’s Common Terminology Criteria for Adverse Events V4.0) over 3 months. Practice staff prospectively captured toxicities; blinded oncology clinicians reviewed medical records to verify. Secondary outcomes included overall survival (OS), treatment intensity, and GA outcomes.
Trial Registration:
Findings:
From 2014–19, we enrolled 718 patients. Age (mean 77·2 years), sex (43·3% female), number of impaired GA domains (median 4·5/8), and treatment (chemotherapy 88·2%) did not differ by arm. More patients in intervention were Black versus other race (11·5% vs 3·3%, p<0·01) and had prior chemotherapy (30·8% vs 22·7%, p=0·02). A lower proportion of patients in intervention experienced grade 3–5 toxicity (177/349; 50·7%) than in usual care (263/369; 71·2%); relative risk (RR) was 0·74 (95% CI: 0·64–0·86; p<0·001). While more patients in intervention received reduced intensity treatment at cycle 1, OS was not different by arm at 6 months. Patients in intervention experienced fewer falls over 3 months (11·7% vs 20·7%; RR 0·58, p<0·01) and had more medications discontinued (mean adjusted difference 0·14, p=0·02).
Interpretation:
A GA intervention for older patients with advanced cancer reduced serious toxicity from cancer treatment without compromising OS.
Funding:
National Cancer Institute
Introduction
Communication of treatment tolerability is essential to informed and shared decision-making between patients, their families, and their oncologists. Clinical trials capture clinician-reported toxicity as measured by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) to assess tolerability. Tolerability data derived from clinical trials are particularly important to guide clinical decision making when treatment is palliative, prognosis is poor, and multiple treatment options are available.
Adults aged 70 years and older with aging-related conditions are underrepresented in clinical trials that have established the standard of care for treatment of advanced cancer.1 Aging-related conditions (i.e., disability, comorbidity, and geriatric syndromes) are highly prevalent in older patients cared for by community oncologists.2 Older patients often state that their goals for treatment of their advanced cancers include minimizing risk of toxicities and maximizing function and quality of life.3,4 Many older adults assert that they would forgo intensive treatments if such treatments posed a significant risk to their independence.5 Because therapeutic clinical trials do not often address the endpoints most valued by older adults,6 interventions are needed to guide clinical decision-making for this vulnerable population at high risk for adverse outcomes.
The American Society of Clinical Oncology (ASCO), the Cancer and Aging Research Group (CARG), and the International Society of Geriatric Oncology (SIOG) all recommend integration of geriatric assessment (GA) into oncology clinical care.2 GA utilizes patient-reported and objective measures to evaluate aging-related domains (e.g., function, cognition, comorbidity). Based on studies demonstrating that GA can identify older adults at highest risk of serious toxicity from chemotherapy, an ASCO guideline recommends that all older adults receiving chemotherapy undergo GA.2 Nevertheless, despite studies demonstrating feasibility,2 enhanced communication,3,7 and improved patient and caregiver satisfaction,3 implementation of GA and GA-guided management remains uncommon,8,9 in part because of limited data demonstrating benefits on cancer-specific outcomes.
To our knowledge, this study is the first nationwide cluster randomized clinical trial to evaluate whether providing a GA summary with management recommendations (i.e., a GA intervention) to community oncologists can improve clinical outcomes of older adults with advanced cancer. We hypothesized that the GA intervention would lower serious toxicity from high-risk cancer treatments through improved decision-making.
Methods
Study design
In this cluster randomized trial, “Geriatric Assessment for Patients 70 years and older (GAP70+; NCT02054741),” we randomized community oncology practices to intervention or usual care (Figure 1). We recruited practices from the University of Rochester NCI Community Oncology Research Program (UR NCORP) Research Base network. NCORP is a national network in the United States that brings cancer clinical trials and care delivery studies to people in their communities (https://ncorp.cancer.gov/about/). NCORP Community Affiliates (i.e., networks of community oncology practices) receive NCI funding to enroll patients onto cancer clinical trials and care delivery studies coordinated by NCI-funded NCORP Research Bases. Community oncology practices in the United States are generally not physically located at an academic or medical teaching institution or hospital. The UR NCORP Research Base developed practice clusters in collaboration with the individual community oncology practices. Practice clusters were comprised of NCORP-affiliated community oncology practices that had overlap between any participating study team members (e.g., oncologist or coordinator, Figure 1). Participating practice clusters represent a large geographic area across the United States (Supplemental Table 1). While the UR Research Base coordinated study activities, University of Rochester did not enroll participants. The University of Rochester (Rochester, NY, USA) and all participating practice clusters obtained approval from their institutional review boards. The study protocol and measures are available on https://www.mycarg.org/.
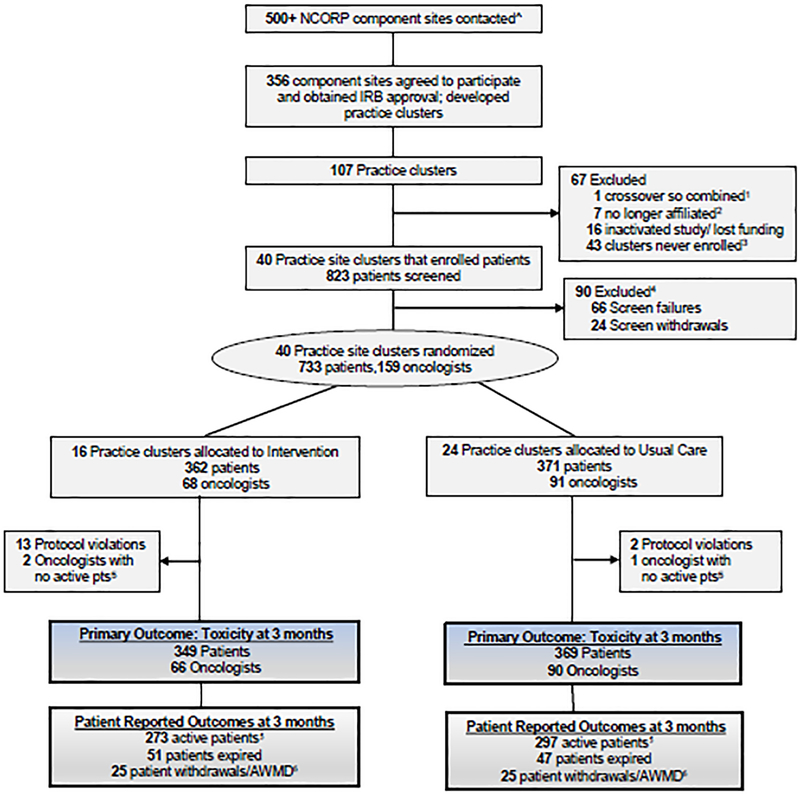
Figure 1. CONSORT flow diagram for the GAP-70+ Study 1.
One cluster was combined with another cluster due te oncologist crossover
2: Sites are no longer associaed with their respective NCORP or with the University of Rochester Research Base
3: Clusters that mainatained IRB approval but never actually enrolled any participants
4: Patients who were screened but either failed screening eligibility or withdrew prior to completing the baseline visit.
5: A patient is considered active if they complete all or some PROs; this includes patients that have a missed visit due to illness, hospitalization, or scheduling.
6: Includes patients who withdrew or were active with missing data (AWMD) (e.g. entered hospice and no longer completed study procedures).
Footnote: ^Practice clusters were built from community oncology practices that expressed interest in study participation. Practice clusters were comprised of community oncology practices that had overlap between any participating study team members. If an oncologist, coordinator, or research nurse or any other research study staff worked at multiple community practices those practices would be grouped into a cluster. Due to this crossover, multiple community oncology practices could be in one practice cluster. Practice clusters varied in size.
Participants
Only patients of enrolled oncologists were eligible to participate.8 Patient eligibility criteria included age ≥70 years, at least one GA domain impairment other than polypharmacy,2,3 an incurable advanced solid tumor or lymphoma (i.e., stage III or IV), ability to provide informed consent independently or via a health care proxy, and an understanding of English. Patients were eligible if they planned to start a new cancer treatment regimen with a high risk of toxicity within 4 weeks. Because patients were required to have incurable cancers, treatment was to be initiated for palliative intent, with the presumed goals of prolonging survival or reducing symptoms rather than cure. Eligible regimens had to include at least one chemotherapy agent or have a >50% prevalence of grade 3–5 toxicity as determined by the primary oncologist with review and approval by a clinical team blinded to study arm at the Research Base.10 Oncologists selected the specific treatment regimen, dosing, and schedules. For those regimens that did not include a chemotherapy agent, clinician investigators on the study team (SM, MM) blinded to arm verified that the regimen had a >50% prevalence of grade 3–5 toxicity after review of published data and drug labels. Patients provided written informed consent.
Randomization and blinding (masking)
Practice clusters were randomized to one of the two study arms by means of a computer–generated randomization table. UR NCORP Research Base statisticians (EC, HX) provided oversight for all randomization procedures. Prior accrual records from UR NCORP studies were used to stratify practice clusters as high or low accruing sites. Because this study evaluated a model of care, participants and staff at the community oncology clinics were not blinded. Other than the statisticians who completed the analyses, all Research Base investigators were blinded to the assignment. Further, blinding was preserved among the clinical team members centrally reviewing treatment and toxicity data.
Procedures
For the intervention arm, the study team developed the GA summary and GA-guided management recommendations for the GA intervention, including cancer treatment considerations (e.g., dose reduction in cycle one with escalation as tolerated), through literature review, guidelines, and expert consensus.2,11 Patients in both arms underwent a GA that evaluated eight domains using patient-reported and objective measures prior to starting the new treatment regimen.2,11 Patients had the option of completing the patient-reported GA measures at home or in the office. Practice staff (i.e., research coordinators) reviewed the measures for completion and administered the objective cognition and physical performance tests. At practices randomized to the intervention arm, staff generated a tailored GA summary and management recommendations using a web-based platform. At study entry, oncologists in the intervention practices received a brief training about GA and were told that they had autonomy for how they wished to use GA for their enrolled patients. Training provided an overview of how the GA summary could be used to guide treatment decisions and how recommendations could be used to guide management of aging-related conditions.2 For usual care, oncologists received alerts for significantly impaired scores on depression and cognitive screening tests; a GA summary and recommendations were not provided. GA outcome measures were completed at 4–6 week, 3-month, and 6-month follow-up visits. We have previously described the GA intervention in a separate report, which demonstrated benefits for improved communication.3
Outcomes
In both arms, coordinators prospectively captured and assessed the frequency and severity of all grade 3–5 toxicities using NCI CTCAE V4 for the primary outcome over three months or until the regimen was discontinued. They confirmed grading of the toxicity with the patient and treating oncologist; the oncologist also confirmed the relationship of the observed toxicity with treatment decisions. The Research Base received all medical records. A Research Base team, blinded to study arm, led by an oncologist (MM), reviewed toxicity grading by comparing data forms with the medical record. If discrepancies were identified, practice staff reviewed and resolved them.
We examined the effects of GA on treatment intensity and survival as secondary outcomes. At UR NCORP, two blinded clinicians (MM, MF, or AM) reviewed each enrolled patient’s medical record and treatment regimen and used guidelines and clinical trials to determine standard dosing. We evaluated the proportion of patients who received a reduced intensity regimen (e.g., lower dose or omission of an agent compared to standard) at cycle one. Subsequently, we calculated the Relative Dose Intensity (RDI)12 (i.e., the ratio of the total dose actually delivered to standard dose [not planned dose]) over the first three months of treatment. Coordinators captured survival up to one year after registration. As an exploratory aim, we examined the effects of the intervention on GA outcomes over three months.
Statistical analysis
The primary outcome measure for this study was the proportion of participants who experienced grade 3–5 toxicity within three months of starting a new treatment regimen. We determined the trial sample size using data about toxicity and intra-cluster correlation (ICC) among seven different sites from a CARG multicenter study.13 This design provided 80% power to detect a 13% reduction in the proportion of participants who experienced any grade 3–5 chemotherapy toxicity within three months of treatment initiation, assuming a two-sided significance level of 0.05 and an ICC of 0.10. Accounting for a drop-out rate of 10% between consent and registration, the targeted accrual was 700 participants. All eligible participants were included in analyses. We originally aimed for participation of 16 practice clusters. Since recruitment was initially slower than anticipated, we allowed more practices to participate (as specified by the protocol) but did not adjust the total sample size.
Descriptive statistics were used to evaluate demographics, GA results, clinical information, and outcome measures. Bivariate analyses were performed to compare between-arm differences in patient characteristics, treatments, and outcome measures using Chi-square tests for categorical variables and t-tests for continuous variables.
For the primary outcome, we applied generalized linear mixed model (GLMM) methodology to account for the cluster randomized study design. The proportion of patients who experienced any grade 3–5 toxicity within three months was the response, and arm was the fixed effect. Practices were included as a random effect independent of residual error. Estimation was performed using the Residual Pseudo Likelihood procedure, assuming a binary distribution and log link. Using the fitted model, we provided risk ratio estimates comparing the proportion of patients who experienced toxicity between the arms. We also examined the proportion of patients in each arm who experienced any grade 3–5 toxicity in stratified analyses by cancer treatment history and cancer type and calculated risk ratio estimates for these subgroups.
Secondary outcomes included survival and intensity of treatment. We determined the effect of the intervention on six-month and one-year survival using the Cox Shared Frailty Model that included practices as a random effect and report adjusted hazards ratio (aHR) from the model. To evaluate the proportion of patients who experienced reduced treatment intensity in the first cycle, we used a similar GLMM approach as with the primary outcome. RDI was analyzed with a linear mixed model (LMM), with RDI as the response, arm as the fixed effect, and practices as a random effect.
To assess the effect of the intervention on GA outcomes over time (exploratory aims), we used longitudinal LMMs. The model was adjusted for arm and baseline value as fixed effects and practices as a random effect independent of the within-subject random effects, and it was fit via restricted maximum likelihood (REML). An “unstructured” correlation matrix was used for the repeated measures from the same subject. When a mixed effect model did not converge, the linear or generalized linear model without practice random effect were conducted.
SAS version 9.4 (SAS Institute, Cary, NC) was used for analysis. A two-sided p-value <0.05 was deemed statistically significant. The University of Rochester Wilmot Cancer Institute’s Data and Safety Monitoring Committee reviewed the trial yearly. The trial is registered on clinicaltrials.gov: NCT02054741.
Role of the funding source
Other than providing feedback on study design during reviews, the funder of the study (i.e., NCI) had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Participant Characteristics
From July 2014 to March 2019, 40 practice clusters (16 intervention and 24 usual care) enrolled participants, including 156 oncologists and 718 eligible patients (Figure 1). Patients had a mean age of 77·2 years (range 70–96) and 43·3% were female. While most baseline characteristics were similar (Table 1), more patients in the intervention arm were Black (11·5% vs 3·3%) or other race (7·4% vs 2·4%) and less were Non-Hispanic White (80·5% vs 94·0%) than in the usual care arm (p<0.01). More patients in the intervention arm had prior chemotherapy (30·8% vs 22·7%, p=0·02) and had gastrointestinal cancers; lung cancer was more prevalent in the usual care arm. The mean number of GA domain impairments was 4·5 (standard deviation 1·6) and was not different between the arms. Patients in the intervention arm had a lower prevalence of impaired physical performance (90·0% vs 96·2%, p<0·01), but a higher prevalence of impaired social support (31·8% vs 22·6%, p<0·01) and cognitive impairment (40·1% vs 32·8%, p=0·04) (Table 1). Baseline data for oncologists8 were previously published.
Table 1:
Patient Characteristics by Study Arm*
| All patients | Intervention arm | Usual care arm | P values | |
|---|---|---|---|---|
| (N=718) | (N=349) | (N=369) | ||
| Age (mean (standard deviation)) | 77·2 (5·4) | 77·2 (5·7) | 77·2 (5·2) | 0·98 |
| 70–79 | 494 (68·8%) | 244 (69·9%) | 250 (67·8%) | 0·61 |
| 80–89 | 204 (28·4%) | 94 (26·9%) | 110 (29·8%) | |
| ≥90 | 18 (2·5%) | 10 (2·9%) | 8 (2·2%) | |
| Missing | 2 (0·3%) | 1 (0·3%) | 1 (0·3%) | |
| Gender | 0·35 | |||
| Male | 405 (56·4%) | 203 (58·2%) | 202 (54·7%) | |
| Female | 311 (43·3%) | 145 (41·5%) | 166 (45·0%) | |
| Missing | 2 (0·3%) | 1 (0·3%) | 1 (0·3%) | |
| Race/Ethnicity | <0·01 | |||
| Non-Hispanic White | 628 (87·5%) | 281 (80·5%) | 347 (94·0%) | |
| Black | 52 (7·2%) | 40 (11·5%) | 12 (3·3%) | |
| Others | 35 (4·9%) | 26 (7·4%) | 9 (2·4%) | |
| Missing | 3 (0·4%) | 2 (0·6%) | 1 (0·3%) | |
| Marital Status | 0·32 | |||
| Single, Never Married | 17 (2·4%) | 11 (3·2%) | 6 (1·6%) | |
| Married/ Domestic Partnership | 449 (62·5%) | 212 (60·7%) | 237 (64·2%) | |
| Separated/ Widowed/ Divorced | 250 (34·8%) | 125 (35·8%) | 125 (33·9%) | |
| Missing | 2 (0·3%) | 1 (0·3%) | 1 (0·3%) | |
| Education | 0·67 | |||
| <High school | 111 (15·5%) | 58 (16·6%) | 53 (14·4%) | |
| High school graduate | 244 (34·0%) | 119 (34·1%) | 125 (33·9%) | |
| Some college or above | 361 (50·3%) | 171 (49·0%) | 190 (51·5%) | |
| Missing | 2 (0·3%) | 1 (0·3%) | 1 (0·3%) | |
| Income | 0·16 | |||
| ≤$50,000 | 371 (51·7%) | 189 (54·2%) | 182 (49·3%) | |
| >$50,000 | 190 (26·5%) | 94 (26·9%) | 96 (26·0%) | |
| Decline to answer | 155 (21·6%) | 65 (18·6%) | 90 (24·4%) | |
| Missing | 2 (0·3%) | 1 (0·3%) | 1 (0·3%) | |
| Cancer type | <0·01 | |||
| Breast | 56 (7·8%) | 19 (5·4%) | 37 (10·0%) | |
| Gastrointestinal | 246 (34·2%) | 132 (38·0%) | 114 (30·8%) | |
| Genitourinary | 109 (15·2%) | 56 (16·0%) | 53 (14·4%) | |
| Gynecological | 43 (6·0%) | 29 (8·3%) | 14 (3·8%) | |
| Lung | 180 (25·1%) | 64 (18·3%) | 116 (31·4%) | |
| Lymphoma | 46 (6·4%) | 23 (6·6%) | 23 (6·2%) | |
| Other | 38 (5·3%) | 26 (7·4%) | 12 (3·3%) | |
| Cancer stage | 0·11 | |||
| Stage III | 77 (10·7%) | 42 (12·0%) | 35 (9·5%) | |
| Stage IV | 628 (87·5%) | 304 (87·1%) | 324 (87·8%) | |
| Others | 13 (1·8%) | 3 (0·9%) | 10 (2·7%) | |
| Prior chemotherapy | 185 (26·6%) | 104 (30·8%) | 81 (22·7%) | 0·02 |
| Number of Impaired Geriatric Assessment Domains** (mean (SD)) | 4·5(1·6) | 4·6(1·6) | 4·4(1·5) | 0·23 |
| Physical performance domain impairment | 669 (93·2%) | 314 (90·0%) | 355 (96·2%) | <0·01 |
| Polypharmacy domain impairment | 584 (81·3%) | 287 (82·2%) | 297 (80·5%) | 0·65 |
| Comorbidity domain impairment | 484 (67·5%) | 236 (67·6%) | 248 (67·4%) | 0·90 |
| Functional status domain impairment | 412 (57·5%) | 200 (57·3%) | 212 (57·6%) | 0·96 |
| Nutrition domain impairment | 439 (61·1%) | 211 (60·5%) | 228 (61·8%) | 0·71 |
| Cognition domain impairment | 261 (36·4%) | 140 (40·1%) | 121 (32·8%) | 0·04 |
| Social support domain impairment | 194 (27·1%) | 111 (31·8%) | 83 (22·6%) | <0·01 |
| Psychological status domain impairment | 205 (28·6%) | 107 (30·7%) | 98 (26·6%) | 0·22 |
Missing data for any variable <5%; p-values included since this is a cluster randomized trial
See Table 3 for Domain Definitions
Grade 3–5 Toxicity
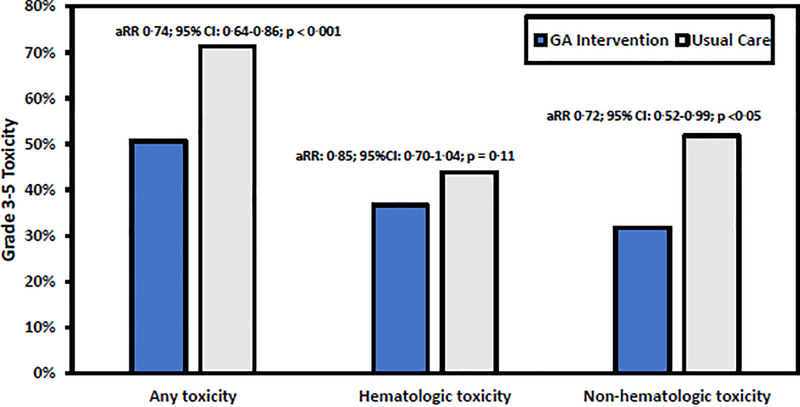
In 718 evaluable patients, the prevalence of patients experiencing any grade 3–5 toxicity was 61·3% (440/718) within three months of starting a new treatment regimen; of these, 0·7% (n=5) experienced a grade 5 toxicity (i.e., death). A lower proportion of patients in the intervention arm experienced grade 3–5 toxicity (177/349; 50·7%) than patients in usual care (263/369; 71·3%). The GA intervention reduced the risk of toxicity (adjusted risk ratio [aRR] 0·74; 95% CI: 0·64–0·86; p<0·001; clustering effect [CE] p=0·16) (Figure 2). The proportion of patients who experienced a grade 3–5 toxicity was lower in the GA intervention arm compared to usual care when stratifying by history of prior chemotherapy and cancer type (Supplemental Figure 1). In additional sensitivity stratified analyses, we evaluated the robustness of the results with respect to covariates with imbalance between arms. Supplemental Figure 2 shows that direction of the treatment effect was consistent across all categories and the GA intervention was favored for all subgroups.
Figure 2.
Prevalence of any Grade 3–5 CTCAE Toxicity over 3 Months by Study Arm
Footnote:
Abbreviations: GA: Geriatric Assessment; aRR: adjusted Risk Ratio
From all grade 3–5 non-hematologic toxicities (n=867), the most common were fatigue/generalized weakness (94/867; 10·8%), electrolyte imbalance (90/867; 10·4%), gastrointestinal distress (86/867; 9.9%), infection (67/867; 7·7%), and hypovolemia/dehydration (64/867; 7·4%). The proportion of patients with any grade 3–5 non-hematologic toxicity was lower in the intervention arm (111/349; 31·8%) than in usual care (191/369; 51·8%), with a lower risk of non-hematologic toxicity for patients in intervention (aRR 0·72; 95% CI: 0·52–0·99; p=0·045; CE p<0·01) (Figure 2). From all grade 3–5 hematologic toxicities (n=857), the most common were decreased neutrophil count (210/857; 24·5%), decreased lymphocyte count (188/857; 21·9%), and anemia (187/857; 21·8%). While a lower proportion of patients experienced grade 3–5 hematologic toxicity (128/349; 36·7%) in the intervention arm than in usual care (162/369; 43·9%), there was no statistically significant reduction in hematologic toxicity risk (aRR: 0·85; 95% CI: 0·70–1·04; p=0·11; CE p=0·36) (Figure 2).
Treatment Decisions and Clinical Care
Table 2 includes the prevalence of the most common regimens by arm. Chemotherapy regimens most commonly included taxanes and/or platinum agents. There were differences in chemotherapy treatment patterns (p<0·01) between arms; a higher proportion of patients in GA intervention received less intense combinations. A higher proportion of patients in the intervention arm received single agent chemotherapy (79/349; 22·6% vs 68/369; 18·4%), chemotherapy plus other agents (e.g., monoclonal antibodies) (85/349; 24·4% vs 66/369; 17·9%), and non-chemotherapy regimens compared to usual care (44/349; 12·6% vs 41/369; 11·1%). A higher proportion of patients in usual care received doublet chemotherapy (141/349; 40·4% vs 194/369, 52·6%). Planned use of G-CSF prophylaxis was similar between arms.
Table 2:
Common Treatment Regimens Received at Cycle One
| Treatment regimen | All patients (N=718) | GA arm (N=349) | Usual care arm (N=369) |
|---|---|---|---|
| Lung cancer regimens | N=180 | N=64 | N= 116 |
| Pemetrexed- carboplatin +/− pembrolizumab | 66 (36%) | 13 (20%) | 53 (46%) |
| Paclitaxel- carboplatin +/− monoclonal antibody | 36 (20%) | 20 (30%) | 16 (14%) |
| Carboplatin- etoposide | 20 (10%) | 5 (8%) | 15 (13%) |
| Carboplatin- nab paclitaxel | 17 (9%) | 7 (11%) | 10 (9%) |
| Gastro-intestinal cancers regimens | N=246 | N=132 | N= 114 |
| FOLFOX +/− bevacizumab | 65 (26%) | 25 (19%) | 40 (35%) |
| Gemcitabine- nab paclitaxel | 44 (18%) | 24 (18%) | 20 (18%) |
| Capecitabine | 23 (9%) | 21(16%) | 2 (2%) |
| FOLFIRI +/− bevacizumab | 18 (7%) | 12 (9%) | 6 (5%) |
| FOLFIRINOX +/− bevacizumab | 9 (4%) | 3 (2%) | 6 (5%) |
| Genito-urinary cancers regimens | N=109 | N=56 | N= 53 |
| Abiraterone +/− prednisone | 35 (32%) | 22 (39%) | 13 (25%) |
| Docetaxel +/− prednisone | 32 (29%) | 19 (34%) | 13 (25%) |
| Enzalutamide +/− prednisone | 13 (12%) | 3 (5%) | 10 (19%) |
| Gemcitabine carboplatin | 11 (10%) | 3 (5%) | 8 (15%) |
| Breast Cancer regimens | N= 56 | N= 19 | N= 37 |
| Palbociclib+ AI | 18 (32%) | 6 (32%) | 12 (32%) |
| Paclitaxel +/− trastuzumab | 8 (14%) | 1 (5%) | 8 (22%) |
| Gemcitabine carboplatin +/− trastuzumab | 5 (9%) | 2 (11%) | 3 (8%) |
| Capecitabine | 4 (7%) | 0 (0%) | 4 (11%) |
| Lymphoma Regimens | N=46 | N=23 | N= 23 |
| BR | 18 (39%) | 7 (30%) | 11(48%) |
| R-CHOP | 9 (20%) | 5 (22%) | 4(17%) |
| Gynecological cancers regimens | N=43 | N=29 | N= 14 |
| Paclitaxel carboplatin | 19 (44%) | 10 (35%) | 9 (60%) |
This table only included commonly received regimens at cycle one
Abbreviations: AI, aromatase inhibitors; BR, bendamustine/rituximab; FOLOFOX, 5-fluorouracil/ leucovorin/ oxaliplatin; FOLFIRI, 5-fluorouracil/ leucovorin/ irinotecan; FOLFIRINOX/ 5-fluorouracil/ leucovorin/ oxaliplatin/ irinotecan; R-CHOP, rituximab/ cyclophosphamide/ doxorubicin/ prednisone/ vincristine
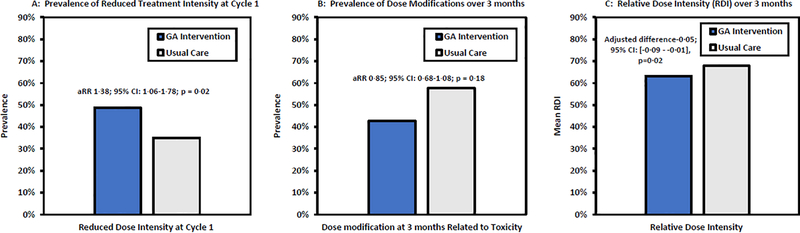
Figure 3 depicts differences in treatment intensity. A higher proportion of patients in intervention received treatment at a reduced dose intensity than standard at cycle one (170/349; 48·7%) compared to usual care (129/369; 35·0%). The intervention was associated with a higher likelihood of receiving reduced intensity treatment (aRR 1·38; 95% CI: 1·06–1·78; p=0·02; CE p=0·03). There were more dose reductions due to toxicity over three months in the usual care (57·7%; 213/369) than in the intervention arm (42·7%; 149/349), but the difference was not statistically significant (aRR 0·85; 95% CI: 0·68–1·08; p=0·18; CE p=0·02). Patients in the intervention arm had a lower RDI over three months than those in usual care (0·63 vs 0·68 in n=641 patients; adjusted between-arm difference −0·05; 95% CI: [−0·09 - −0·01], p=0·02).
Figure 3.
Treatment Intensity by Study Arm
Footnote:
Abbreviations: GA: Geriatric Assessment; aRR: adjusted Risk Ratio
Overall Survival
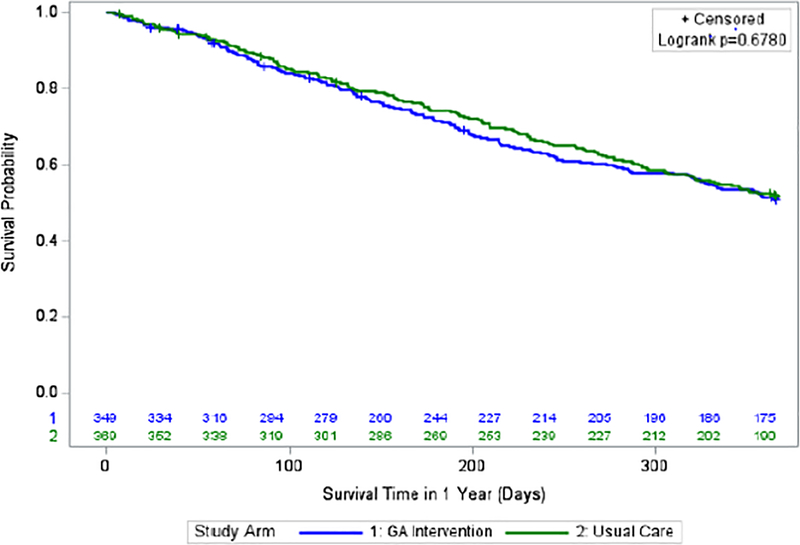
The proportion of patients alive at six months was similar for the intervention arm compared to usual care (250/349, 71·6% vs 275/369, 74·5%; p=0·38). There were no survival differences between arms at six months (adjusted hazard ratio [aHR] 1·13; 95% CI: 0·85–1·50; p=0·39; CE p=0·04) or one year (aHR 1·05; 95% CI 0·85–1·29; p=0·68; CE p=0·05) (Figure 4).
Figure 4:
Survival over 1 year by Study Arm
Geriatric Assessment-Guided Management Recommendations and Outcomes
Table 3 includes the prevalence of GA-guided management recommendations considered by oncologists in the intervention arm. Frequent toxicity checks, adjusting cancer treatment schedule or dosing, reviewing medications for duplications or interactions, providing education materials on aging-related conditions, and referrals to relevant disciplines (i.e., social worker, nutritionist) were among the most common recommendations selected by oncologists.
Table 3:
| Domains | Tools | Descriptions | Definitions of impairment | Prevalence of the most common GA-guided management recommendations chosen by oncologists in the intervention arm |
|---|---|---|---|---|
| Physical performance (n=314/349 impaired in intervention arm) |
Timed “Up and Go” | Assess mobility over 3 meters; longer time indicates worse performance | > 13·5 seconds | - Conduct frequent toxicity checks (86·0%) - Provide fall counselling hand-out/information (86·0%) - Provide information on exercise and exercise prescription (83·.4%) - Provide hand-out on energy conservation (82·5%) - Medication Review: minimize psychoactive meds including those used for supportive care (36·6%); minimize duplicative medications (47·8%) - Treatment modification: consider modification of treatment dose or choice. Examples: 1) consider single agent rather than doublet therapy if appropriate (33·4%): 2) modify dosage (e.g., 20% dose reduction with escalation as tolerated)(46·8%); 3) modify treatment regimen (e.g., use an option with demonstrated safety and efficacy in older and/or frail adults)(49·4%) - Referrals: refer to 1) physical therapist (outpatient or home-based depending on eligibility for home care) (23·6%); 2) occupational therapist (11·1%); 3) aide services (14·3%); 4) personal emergency response information (19·7%); 5) vision specialist if difficulties (12·1%) - Physical Examination: check orthostatic blood pressure (29·3%) and decrease or eliminate blood pressure meds if blood pressure is low or low normal (21·3%) |
| Short Physical Performance Battery | Assess balance, gait speed, and strength; higher score indicates better performance (range 0–12 points) | ≤ 9 points | ||
| Falls History | Assess the number of falls | Any history of falls in the prior 6 months | ||
| OARS Physical Health | Assess any limitation in activities (e.g. climbing several flights of stairs, walking more than a mile) as a result of his/her health (options: a lot, a little, not at all) | If the patient answered any question as “a lot” | ||
| Functional status (n=200/349 impaired in intervention arm) |
Activities of Daily Living (ADL) | Assess difficulty with the following 6 activities: bathing, dressing, eating, getting in and out of bed/chairs, walking, toileting (options: yes/no) | Any deficit (yes) | - Conduct frequent toxicity checks (86·5%) - Provide fall counselling hand-out/information (85·0%) - Provide information on exercise and exercise prescription (84·5%) - Provide hand-out on energy conservation (81·0%) - Medication Review: minimize psychoactive meds including those used for supportive care (37·0%); minimize duplicative medications (51·5%) - Treatment modification: consider modification of treatment dose or choice. Examples: 1) consider single agent rather than doublet therapy if appropriate (36·0%): 2) modify dosage (e.g., 20% dose reduction with escalation as tolerated)(49·0%); 3) modify treatment regimen (e.g., use an option with demonstrated safety and efficacy in older and/or frail adults)(53·0%) - Referrals: refer to 1) physical therapist (outpatient or home-based depending on eligibility for home care) (26·5%); 2) occupational therapist (13·0%); 3) aide services (16·0%); 4) personal emergency response information (22·5%); 5) vision specialist if difficulties (13·5%) - Physical Examination: check orthostatic blood pressure (28·0%) and decrease or eliminate blood pressure meds if blood pressure is low or low normal (20·0%) |
| Instrumental ADLs | Assess independence in the following 7 activities: using the telephone, transportation, shopping, preparing meals, doing housework, taking medicine, managing money (options: without help, with some help, completely unable to) | Any deficit (with some help or completely unable to) | ||
| Comorbidity (n=236/349 impaired in intervention arm) |
OARS Comorbidity | Assess the presence of 13 illnesses (e.g. other cancer or leukemia, arthritis, glaucoma) as well as hearing and visual impairments, and how much each problem interferes with his/her activities (options: not at all, somewhat, a great deal) | Patient answered “yes” to 3 illnesses OR answered that 1 illness interferes “a great deal” (including eyesight and hearing) | - Initiate direct communication (written, electronic, or phone) with patient’s primary care physician about the plan for the patient’s cancer (85·2%) - Modify treatment choices if applicable to the individual patient. Examples: 1) History of diabetes - avoid neurotoxic agents if another option is equivalent (19·1%); 2) History of heart failure - minimize volume of agents and/or administer treatments at slower infusion rate (11·9%); 3) History of renal impairment-adjust as appropriate (19·1%) - Modify dosage or schedule if there is concern about how the patient will tolerate therapy or if there is a concern about worsening of comorbidities (47·9%) - Provide smoking cessation counseling if the patient currently smokes (0·04%) |
| Cognition (n=140/349 impaired in intervention arm) |
Blessed Orientation-Memory-Concentration | Assess orientation, memory, and concentration using 6 items and scores are weighted; higher score indicates worse performance (range 0–28 points) | ≥ 11 points | - Provide explicit and written instructions for appointments, medications, and treatment (74·3%) - Medication review - minimize psychoactive and high risk medications (63·6%) - Assess decision-making capacity and elicit health care proxy information and input if the patient lacks decision-making capacity (62·9%) - Cancer treatment decision - modify treatment choice (consider starting with single agent with escalation to doublet if standard at second cycle - depending on tolerance) (48·6%) - Give patient/family member handout on delirium risk counseling (22·9%) - Referral: refer to clinician experienced in memory care (21·4%) |
| Mini Cog | Assess word recall and clock drawing based on 3 items; lower score indicates worse performance (range 0–5 points) | 0 words recalled OR 1–2 recalled words + abnormal clock drawing test | ||
| Nutrition (n=211/349 impaired in intervention arm) |
Body Mass Index | Divide weight in kilograms by height in meters squared | < 21 kg/m | - Conduct frequent toxicity checks (91·0%) - Give Nutrition hand-out (80·1%) - Give mucositis hand-out (63·0%) - Cancer Treatment: 1) use caution with highly emetogenic regimens and use another option if appropriate (64·0%); 2) utilize aggressive anti-emetic therapy (72·5%) - Referrals: refer to: 1) Nutritionist/Clinical Dietician (44·1%); 2) dentist if poor dentition or denture issues (1·0%); 3) speech and swallow if difficulty with swallowing (0·05%) |
| Weight loss | Assess change in weight over 6 months | > 10% change in weight from 6 months ago | ||
| Mini Nutrition Assessment | Assess nutritional status using 6 items; lower score is worse (range 0–14 points). | ≤ 11 points | ||
| Social Support (n=111/349 impaired in intervention arm) |
Medical Social Support | Assess the presence of social support using 4 items (“someone to help if you were confined to bed, someone to take you to the doctor if needed, someone to prepare your meals if you were unable to do it yourself, someone to help you with daily chores if you were sick.” Options: none of the time, a little of the time, some of the time, most of the time, all of the time) | Patient answers any one of questions as “some of the time, a little of time, none of the time” | - Confirm documented health care proxy is in medical record (70·3%) - Modify treatment choice and/or dosage (60·4%) - Provide referral or information on 1) Social worker via on-site or visiting nurse services (45·9%); 2) visiting nurse service or home health aide (if meets criteria) (15·3%); 3) transportation or ride services (19·8%); 4) medical insurance advising, advocacy, and negotiation (17·1%); 5) legal assistance for economic and social needs (0·05%); 6) community resource mobilization (25·2%) |
| Polypharmacy (n=287/349 impaired in intervention arm) |
Medications | Assess the number of regularly scheduled medications, presence of high risk medication, or kidney function | 5 regularly scheduled prescription medications (OR Any high risk medication OR creatinine clearance<60) | - Ask patient to bring in prescribed, over-the counter medications, and supplements to review at the next visit (55·1%) - Contact primary care provider to help reduce regimen complexity (28·6%) - Reduce medicines solely used for hypertension or diabetes if appropriate (including dose and number of medications) (20·6%) - Consult the pharmacist who fills the patient’s scripts to synchronize medication refills whenever possible (18·1%) - Have pharmacist meet with the patient to evaluate drug interactions and medication counseling (20·6%) - Recommend pillbox and/or medication calendar (42·9%) - Provide hand out on polypharmacy (77·7%) |
| Psychological status (n=107/349 impaired in intervention arm) |
Geriatric Depression Scale | Assess depression using 15 items; higher score is worse (range 0–15 points) | ≥ 5 points |
- Provide written or verbal communication with primary care physician (41·1%) - Referral: refer to 1) counseling or psychotherapy (18·7%); 2) social work (39·3%); 3) spiritual counseling or Chaplaincy services (16·8%); 4) psychiatry if severe symptoms or if already on medications which are not adequate (10·3%); 5) palliative care if other physical and/or cancer symptoms are present (22·4%). - Initiate pharmacologic therapy if appropriate in conjunction with primary care provider (16·8%) - Provide linkage to community resources (such as support groups and local/national buddy or volunteer programs) (25·2%) |
| Generalized Anxiety Disorder-7 item scale | Assess anxiety using 7 items; higher score is worse (range 0–21 points) | ≥ 10 points |
Abbreviations: ADL, Activity of Daily Living; OARS, Older American Resources and Services; TSH, thyroid stimulating hormone.
References for measures can be found in Mohile et al. Practical assessment and management of vulnerabilities in older patients receivng chemotherapy: ASCO guideline for geriatric oncology. Journal of Clinical Oncology. 2018 Aug 1;36(22):2326–2347. Oncologists were provided a list of the management recommendations to choose from.
A lower proportion of patients experienced a new fall over three months in the intervention arm (35/298, 11·7%) compared to usual care (68/329, 20·7%) (Table 4). Adjusting for a history of baseline falls, patients in the intervention arm had a lower risk of experiencing a new fall (aRR 0·58; 95% CI: 0·40–0·84; p<0·01). Further, a greater number of medications was discontinued in the intervention arm compared to usual care before starting the new treatment regimen (mean difference=0·14 meds; 95% CI: 0·03–0·25; p=0·02). We did not detect any significant between-arm differences for other GA domains over three months (Table 4).
Table 4:
Effect of the Geriatric Assessment (GA) Intervention on GA Outcomes
| Outcomes | Range | Overall between-arm difference (GA-usual care) or risk ratio | P value | P value for site clustering effect |
|---|---|---|---|---|
| Instrumental Activities of Daily Living scores over 3 months * | 0–14 | −0·13 (−0·58–0·31) | 0·50 | 0·28 |
| Short Physical Performance Battery scores over 3 months * | 0–12 | −0·33 (−0·80–0·14) | 0·15 | 0·36 |
| OARS physical health subscale scores over 3 months * | 0–20 | −0·24 (−1·15–0·65) | 0·55 | 0·28 |
| Geriatric Depression Scale scores over 3 months * | 0–15 | −0·04 (−0·52–0·43) | 0·84 | 0·20 |
| Number of prescription medications discontinued prior to starting cancer treatment regimen | 0–11 | 0·10 (0–0·20) | 0·03 | N/A |
| Number of overall medications (prescription and non-prescription) discontinued prior to starting cancer treatment regimen | 0–13 | 0·14 (0·03–0·25) | 0·02 | N/A |
| Any fall over 3 months | 0–1 | risk ratio 0·58 (0·40–0·84) | <0·01 | N/A |
Abbreviation: OARS, Older Americans Resources and Services;
Higher scores indicate better health except for depression scale and medications;
Measures were analyzed using linear mixed models adjusted for baseline values;
Polypharmacy was analyzed using adjusted linear regression models;
Any fall over 3 months was analyzed using generalized linear regression model (binary distribution with log link) adjusted for baseline values;
N/A, models with practice site random effect did not converge.
Discussion
The GAP70+ trial is the first large nation-wide cluster randomized trial to demonstrate that providing a GA summary with GA-guided management recommendations to community oncologists significantly reduces serious treatment toxicity in vulnerable patients aged 70 and over with advanced cancer. The trial met its primary endpoint; the GA intervention reduced the risk of serious toxicity by over 20%. In the intervention arm, more patients received reduced treatment intensity at cycle one (i.e., primary dose reduction), indicating an impact on treatment decisions. Patients in the intervention also had fewer falls and more medications discontinued, reducing polypharmacy. Importantly, reduced dose intensity in the intervention arm did not compromise survival, which was similar between groups at six months and one year. The GAP70+ results are significant because weighing the risks and benefits of cancer treatment in vulnerable older adults is challenging, largely because they are disproportionately underrepresented in randomized clinical trials that establish the standards for cancer treatment.6 Therefore, vulnerable older patients with advanced cancer often receive treatments that have greater risks than benefits. This study demonstrates that simply providing information about health status through a GA summary tied to management recommendations can improve up-front decision- making for treatment and optimize clinically significant outcomes. The GA intervention improved outcomes that are important to older adults with cancer—serious treatment-related toxicity, falls, and polypharmacy.6
The GAP70+ trial is the first to enroll over 700 older patients with advanced cancer who were at high risk for adverse outcomes from palliative cancer treatment. The mean number of impaired GA domains was >4, indicating a high prevalence of aging-related conditions and frailty. The ASCO geriatric oncology guideline2 and systematic reviews9,14 highlight that older adults with aging-related conditions receiving treatment for advanced cancer are at high risk of toxicity, lower rates of treatment completion, and early mortality. The high prevalence of adverse outcomes shortly after starting treatment in this population offers an opportunity for utilization of models of care and management that improve decision making regarding cancer treatments. GAP70+ demonstrates that, when available, community oncologists will utilize GA information to personalize treatment decisions for vulnerable older patients with advanced cancer; the GA intervention led to different treatment patterns (e.g. a higher prevalence of single versus doublet chemotherapy) and reduced intensity treatment at cycle one.
Evidence has increasingly revealed that GA-guided management improves clinical outcomes for older patients with cancer.2,15 Randomized pilot studies have suggested that GA integration into oncology care is feasible,16 can reduce treatment toxicity,17 and can improve quality of life.18 In a comparative study of two cohorts, older adults receiving chemotherapy who underwent geriatrician co-management were over four times more likely to complete cancer treatment.19 A RCT enrolling vulnerable older adults with colorectal cancer receiving adjuvant or first-line chemotherapy found that a GA intervention improved treatment completion, quality of life, and mobility.20 We completed an independent clinical trial in older adults showing that this same GA-guided intervention improved communication about aging-related conditions and enhanced satisfaction for both older patients with advanced cancer and their caregivers.3 The GAP70+ trial is unique: it is the first nation-wide cluster randomized study to show that a multi-component GA intervention delivered in community oncology practices can lower the risk of serious toxicity in older patients with advanced cancer and aging-related conditions receiving palliative treatment.
GA can improve clinical outcomes in two ways: 1) by influencing treatment decisions and 2) by guiding interventions supported by the geriatrics’ literature. A systematic review of 35 studies found that GA influenced oncologists’ treatment plans in a median of 28% of patients (8–54%) and guided non-oncologic interventions in 72% (range 26–100%); there was a trend towards improvements in treatment completion (75% of studies) and reductions in toxicity (55% of studies).9 In a large prospective observational study, older patients with breast cancer who were fit as defined by GA were more likely to receive adjuvant chemotherapy.4 In a sample of 321 older adults receiving palliative chemotherapy, 25% experienced a primary dose reduction; older age and comorbidity were associated with primary dose reduction.21 In randomized clinical trials, older and/or frail patients with advanced colorectal22 and gastric cancers23 randomized to reduced intensity chemotherapy experienced lower toxicity and similar survival as those who received standard dosing. In another randomized clinical trial, treatment allocation guided by GA reduced treatment toxicity without compromising survival in older patients with advanced lung cancer.24 Therapeutic clinical trials should further examine tailored dosing strategies and utilize GA as an essential component of the study design.
The GAP70+ trial is also the first to show that a GA intervention can lower the risk of falls and reduce polypharmacy. Both falls and polypharmacy are more common in older patients with cancer and can increase the risk of adverse clinical outcomes such as functional impairment, hospitalizations, and mortality; further, polypharmacy increases the risk of falling. 25 Consistent with other research,25 the prevalence of falls in our patients receiving usual care was high, with over 20% experiencing a new fall within three months of starting a new cancer treatment. Evidence-based GA-guided recommendations for falls prevention26 were provided to the vast majority of participants in the intervention arm, since impairment in the physical performance domain was highly prevalent. Deprescribing high risk medications (e.g., benzodiazepines) may also have reduced serious treatment-related toxicities and falls.27
Other randomized clinical trials studying GA for patients with cancer will add to knowledge about other clinical outcomes, populations, and models for guiding aging-appropriate care.2 Several other large randomized controlled studies presented at the 2020 ASCO annual meeting demonstrated benefits of GA-guided interventions on clinical outcomes in older adults.2,28 A GA-guided intervention led by a nurse practitioner at an academic cancer center in the United States demonstrated reduced toxicity for older patients receiving chemotherapy in a clinical trial randomized at the patient level. Another randomized trial demonstrated benefits of geriatrician co-management on health-related quality of life and health care utilization for older patients with cancer in Australia. These ongoing trials will add valuable information to guide clinical care in older patients with cancer who are more fit (i.e., without clinically significant aging-related conditions), who are receiving cancer treatment for curative-intent, and who have specific tumor types. The GAP70+ study is unique in that our intervention was delivered by community oncology practice staff to older adults with aging-related conditions and advanced cancer at high risk of adverse outcomes from cancer treatment. Future research should build upon these efficacy studies to evaluate implementation strategies for aging-sensitive interventions that integrate GA- and GA-guided management into oncology clinical care.
This study has limitations. The intervention was conducted only at a single time point, although the intervention influenced outcomes for three months. The low intensity of the intervention and delivery by oncologists rather than geriatricians may have limited the ability to improve GA outcomes beyond falls and polypharmacy. Integrated and longitudinal co-management between oncologists and geriatricians may provide even greater benefits for functional and quality of life outcomes due to better adherence to recommendations. Access to geriatricians, however, is limited in many places, which could prevent implementation of co-management models.29 Because survival was a secondary aim and only captured for one year, the study was not designed to examine non-inferiority between the arms; further research is required to evaluate the effects of GA interventions on survival as a primary aim and for tumor control. Oncologists determined the risk of toxicity of regimens for eligibility which may lead to bias. To reduce potential bias, the blinded clinical team at the Research Base reviewed toxicity risk for all regimens.10 Future research could consider incorporating standardized tools such as the MAX2 index to determine risk of toxicity.30 Imbalances in patient characteristics due to potential selection bias from differing practice characteristics inherent to cluster randomization may have influenced results. Some characteristics, such as receipt of prior chemotherapy, may have increased the prevalence of toxicity in the intervention arm. Others, such as impaired physical performance, may have increased toxicity in the usual care arm. Further, less accrual from individual practices randomized to the usual care arm may reflect differences in care patterns. However, in an analysis of baseline data, oncologists’ characteristics were not associated with decision to provide chemotherapy to vignette patients, suggesting that oncologists may make decisions similarly.8 Differential response rates for patient reported outcomes (PROs) and missing data may have influenced results. For example, a higher prevalence of PROs completed in the usual care arm may have led to a higher reporting of falls. A strength of this study, however, is that the response rates for the patient reported outcomes (PROs) was high in both arms (Figure 1). This study enrolled a heterogeneous group of older patients with advanced cancer receiving palliative treatments for various cancer types with a high risk of toxicity, consistent with the population who is cared for in community oncology practices. Nevertheless, stratified analyses demonstrated benefits across history of previous treatment, cancer type, and treatment type. Thus, these results appear to be relevant to the majority of older adults with aging-related conditions and advanced cancer, a significant strength.
In conclusion, the GAP70+ trial is the first nation-wide cluster randomized clinical trial to demonstrate that GA and GA-guided management, when integrated into oncology care, can significantly reduce treatment toxicity, falls, and polypharmacy in older patients with advanced cancer receiving treatment. While a higher proportion of patients in the intervention received reduced intensity treatment at cycle one, survival was not different by arm. GA and GA-guided management should be considered as the standard of care for older patients with advanced cancer and aging-related conditions starting a new treatment regimen with a high risk of toxicity.
Supplementary Material
Research in Context.
Evidence before this study
Adults aged 70 years and older with aging-related conditions are underrepresented in the trials that have established the standard of care for treatment of advanced cancer. Aging-related conditions (i.e., disability, comorbidity, and geriatric syndromes) are highly prevalent in older patients with advanced cancer who are cared for in community oncology practices. Geriatric assessment (GA) utilizes patient-reported and objective measures to evaluate aging-related domains (e.g., function). GA can guide cancer treatment decisions as well as management recommendations for aging-related conditions. To develop a multi-component GA intervention for community oncology practices, we conducted a Delphi consensus study with geriatric oncology experts in the United States. Further, the American Society of Clinical Oncology (ASCO) facilitated a systematic review of the literature: Using PubMed, the search included the words “geriatric assessment” and “humans” and clinical trial, phase II” or “clinical trial, phase III” or controlled clinical trial” or “meta-analysis” from 2005 to September 2017. Of 70 records, 10 relevant abstracts were identified and reviewed by the panel. Only two publications provided evidence included in the systematic review; both were pilot studies with small sample sizes. The published literature showed a dearth of interventions to improve tolerability outcomes of older patients with advanced cancer receiving treatment.
Added value of this study
Interventions are needed to guide clinical decision-making for older patients with advanced cancer and aging-related conditions who are at high risk for adverse outcomes. We hypothesized that providing a GA summary with management recommendations (i.e., a GA intervention) to community oncologists would lower serious toxicity from high-risk cancer treatments through improved decision-making. The GA intervention reduced the risk of serious toxicity in older patients with advanced cancer and aging-related conditions. In the intervention arm, more patients had reduced treatment intensity at cycle one (i.e., primary dose reduction) indicating an effect on treatment decision-making; patients in this arm also experienced fewer falls and had more medications discontinued, reducing polypharmacy. Reduced dose intensity in the intervention arm did not compromise survival, which was similar between groups.
Implications of all the available evidence
The GAP70+ trial is the first nation-wide cluster randomized trial to demonstrate that GA and GA-guided management, when integrated into oncology care, can reduce treatment toxicity, falls and polypharmacy in older patients with advanced cancer receiving treatment. GA and GA-guided management should be considered as the standard of care for older patients with advanced cancer and aging-related conditions starting a new treatment regimen with a high risk of toxicity.
Acknowledgments:
This study received funding from the National Cancer Institute (R01CA177592, U01CA233167 SGM and UG1CA189961 to KMM), the National Institute on Aging (K24AG056589 to SGM and K76AG064394 to AM, R33AG059206 to WD, SGM), and KL2TR001999 from the National Center for Advancing Translational Sciences (RFD). KPL is supported by the National Cancer Institute (K99CA237744) and Wilmot Cancer Institute Research Fellowship Award. We would like to thank Susan Rosenthal, M.D., for editing and Charles Heckler, Ph.D., Joseph J. Guido, M.S., and Javier Bautista, M.S., M.B.A., for their help with data analysis and management. We would like to thank the geriatric oncology research staff, the University of Rochester NCI Community Oncology Research Base staff, and the Cancer and Aging Research Group. We would also like to thank the patients, community oncologists, and staff who participated in the study.
Declaration of Interests:
Dr. Loh reports receiving consultant fees from Pfizer and Seattle Genetics and honoraria from Pfizer. Dr. Dunne received honoraria for consulting from Exelixis Inc. Dr. Wildes reports research funding from Janssen and receiving consultant fees from Seattle Genetics and Carevive. All other authors declare no competing interests.
Table of Author Names for Pubmed Listing
- Supriya G.
Mohile
- Mostafa R.
Mohamed
- Huiwen
Xu
- Eva
Culakova
- Kah Poh
Loh
- Allison
Magnuson
- Marie A.
Flannery
- Spencer
Obrecht
- Nikesha
Gilmore
- Erika
Ramsdale
- Richard
Dunne
- Tanya
Wildes
- Sandy
Plumb
- Amita
Patil
- Megan
Wells
- Lisa
Lowenstein
- Michelle
Janelsins
- Karen
Mustian
- Judith O.
Hopkins
- Jeffrey
Berenberg
- Navin
Anthony
- William
Dale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.BrintzenhofeSzoc K, Krok-Schoen JL, Canin B, et al. The underreporting of phase III chemotherapeutic clinical trial data of older patients with cancer: A systematic review. J Geriatr Oncol 2020; 11(3): 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018; 36(22): 2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohile SG, Epstein RM, Hurria A, et al. Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncol 2020; 6(2): 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battisti NML, Reed MWR, Herbert E, et al. Bridging the Age Gap in breast cancer: Impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer 2021; 144: 269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346(14): 1061–6. [DOI] [PubMed] [Google Scholar]
- 6.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 2021; 71(1): 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenstein LM, Volk RJ, Street R, et al. Communication about geriatric assessment domains in advanced cancer settings: “Missed opportunities”. J Geriatr Oncol 2019; 10(1): 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohile SG, Magnuson A, Pandya C, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw 2018; 16(3): 301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 2018; 9(5): 430–40. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed MR, Kyi K, Mohile SG, et al. Prevalence of and factors associated with treatment modification at first cycle in older adults with advanced cancer receiving palliative treatment. J Geriatr Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw 2015; 13(9): 1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shayne M, Culakova E, Wolff D, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer 2009; 115(22): 5319–28. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011; 29(25): 3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014; 25(2): 307–15. [DOI] [PubMed] [Google Scholar]
- 15.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014; 32(24): 2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattar S, Alibhai SMH, Brennenstuhl S, et al. Health status, emergency department visits, and oncologists’ feedback: An analysis of secondary endpoints from a randomized phase II geriatric assessment trial. J Geriatr Oncol 2019; 10(1): 169–74. [DOI] [PubMed] [Google Scholar]
- 17.Nadaraja S, Matzen LE, Jorgensen TL, et al. The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: A randomized parallel-group clinical trial. J Geriatr Oncol 2020; 11(3): 488–95. [DOI] [PubMed] [Google Scholar]
- 18.Puts MTE, Sattar S, Kulik M, et al. A randomized phase II trial of geriatric assessment and management for older cancer patients. Support Care Cancer 2018; 26(1): 109–17. [DOI] [PubMed] [Google Scholar]
- 19.Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015; 112(9): 1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund CM, Vistisen KK, Olsen AP, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Brit J Cancer 2021; 124(12): 1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajra A, Klepin HD, Feng T, et al. Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older with solid tumors. Journal of geriatric oncology 2015; 6(2): 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011; 377(9779): 1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall PS, Swinson D, Cairns DA, et al. Efficacy of Reduced-Intensity Chemotherapy With Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer The GO2 Phase 3 Randomized Clinical Trial. Jama Oncol 2021; 7(6): 869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corre R, Greillier L, Le Caer H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol 2016; 34(13): 1476–83. [DOI] [PubMed] [Google Scholar]
- 25.Sattar S, Haase K, Kuster S, et al. Falls in older adults with cancer: an updated systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer 2021; 29(1): 21–33. [DOI] [PubMed] [Google Scholar]
- 26.Force USPST, Grossman DC, Curry SJ, et al. Interventions to Prevent Falls in Community-Dwelling Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(16): 1696–704. [DOI] [PubMed] [Google Scholar]
- 27.Barlow A, Prusak ES, Barlow B, Nightingale G. Interventions to reduce polypharmacy and optimize medication use in older adults with cancer. J Geriatr Oncol 2021; 12(6): 863–71. [DOI] [PubMed] [Google Scholar]
- 28.Soto-Perez-de-Celis E, Aapro M, Muss H. ASCO 2020: The Geriatric Assessment Comes of Age. Oncologist 2020; 25(11): 909–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie GAG, Bullock AF, Greenley SL, Lind MJ, Johnson MJ, Pearson M. Implementation of geriatric assessment in oncology settings: A systematic realist review. J Geriatr Oncol 2021; 12(1): 22–33. [DOI] [PubMed] [Google Scholar]
- 30.Extermann M, Bonetti M, Sledge GW, O’Dwyer PJ, Bonomi P, Benson AB 3rd. MAX2--a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer 2004; 40(8): 1193–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.