Abstract
Circulating tumor cells (CTCs) are malignant cells separate from primary tumors, which can migrate through the peripheral blood, colonize other tissues, and lead to the formation of metastases. The first description of CTCs dates back to 1869 when Thomas Ashworth recognized malignant cells similar to the ones of the primary tumor in the blood vessels of an autopsied patient with metastatic cancer. Currently, CTCs have been identified in various types of cancer and have been recognized for their clinical value in the prediction of prognosis, diagnosis of minimal residual diseases, assessment of tumor sensitivity to anticancer drugs, and personalization of therapies.
However, research about these topics has several limitations, principally the rarity of CTCs in bloodstream and their heterogeneous characteristics, which makes detection and isolation difficult. As a result of these limitations, current studies are focused on improvement of isolation and characterization techniques to achieve better sensitivity in clinical applications. This review covers the methods of CTC isolation and detection and current research progression on CTC in different cancer types. The clinical applications, limitations, and perspectives of CTCs are also discussed.
Keywords: Circulating tumor cells, Cancer, Liquid biopsy, Micrometastases, Metastasis
9.1. Introduction
Mechanisms of cancer progression and spreading have been a focus of surgeons and researchers for more than 100 years. These investigations have formed the basis that made possible the recent insights gained in these processes. Circulating tumor cells (CTCs) are malignant cells that disseminate from the primary tumor, circulate in the peripheral blood, and have the potential of colonizing other tissues, eventually leading to metastasis. Metastasis is the principal cause of cancer-related death, and it is accepted that it occurs when the microenvironment shows appropriate conditions for implantation and growth of CTCs in secondary sites, forming tumors in distant organs [1].
CTCs have been isolated from patients with various types of cancer and are recognized to be useful in increasing our understanding of tumor progression and metastasis, besides prognosis, monitoring of recurrences, therapeutic responses, and drug resistance mechanisms. Although clinical applications are limited by poor detection of CTCs using current isolation processes, frequently caused by the heterogeneous immunophenotypic features of CTCs, promising steps are being taken in the use of CTCs for personalized anticancer therapies [2].
9.2. Historical Background
The first morphological description of CTCs dates back to 1869 when the Australian physician Thomas Ashworth recognized malignant cells similar to the ones of the primary tumor in the blood vessels of an autopsied patient with metastatic cancer [3]. Later, the American surgeon William Halsted extended this theory to the lymphatic system and incorporated it into his practice by performing resections of axillary lymph nodes in breast cancer surgeries [4].
In 1889, the British surgeon Stephen Paget proposed the “seed and soil” theory of metastasis in which he compared selected tumoral cells with a seed that dislocates via the bloodstream and reaches specific distant organs that form the soils for sowing [5]. Paget examined more than 900 autopsy records of patients with several types of cancer. He observed discrepancies between the relative blood supply and the frequency of metastases in certain organs, and he also found that visceral and bone metastasis did not occur randomly, but followed distinct patterns. This discarded the belief of that time that metastasis was an outcome of fortuity, and he concluded that certain tumor cells (the “seeds”) have specific affinity for the environment of certain organs (the “soil”), and metastases arise only when the seed and soil are compatible. Paget’s observations still hold true today.
During the twentieth century, James Ewing suggested that mechanical factors prompted metastatic dissemination as a result of the anatomical disposition of the vascular system [6]. Subsequently, Weiss observed some differences between regional metastasis and organ distant metastases, as the clinical data were reviewed on site prevalence of metastases of diverse human malignancies [7]. The regional involvement could be related to anatomical or mechanical factors, such as the efferent venous circulation or lymphatic drainage to regional lymph nodes. In contrast, metastases to distant organs of several types of cancers were more site specific [8].
In 1980, Ian Hart and Isaiah Fidler supported the “seed and soil” hypothesis to explain the non-random pattern of cancer metastasis, when they documented the selective nature of metastasis in an assay of experimental metastasis of B16 melanoma in syngeneic mice [9]. The results showed that despite some occurrence of mechanical arrest of tumor cells in the capillary niche of distant organs, subsequent proliferation and growth into metastatic lesions were defined by specific organ cells (Fig. 9.1).
Fig. 9.1.

Timeline of the historical discovery of CTCs
9.3. Detection Methods
The first methods for detection of CTCs in peripheral blood of patients with cancer were reported in the twentieth century, using filtration and sedimentation techniques. Using a filtration approach, Salgado et al. showed that tumor cells were commonly present in peripheral blood and in blood drained from tumor sites at surgery [10]. The protocol consisted of collecting blood samples in a syringe containing heparin followed by centrifugation. After this, the cells were incubated with a hemolyzing agent (streptolysin O) to destroy red blood cells and some white blood cells and then filtered through a Millipore filter. Finally, the cells were fixed and stained by the Papanicolaou technique. On the other hand, Alexander and Spriggs used a method to concentrate white cells by sedimentation followed by searching for the tumor cells [11]. In this technique, patient blood was mixed with a solution of dextran and heparin to sediment the erythrocytes and the fluid centrifuged for 10 min and then spread on slides to allow May-Grunwald-Giemsa staining. After this, the slides were examined under the microscope to detect unusual cells.
Today, almost a century later since the report of CTCs by Ashworth [3], numerous specific and sensitive technologies have emerged to detect CTCs in the bloodstream. However, the current methods used are an improvement over the first methods described. Detection techniques can be classified into two main groups according to the CTC properties based on biological or physical properties (Fig. 9.2).
Fig. 9.2.
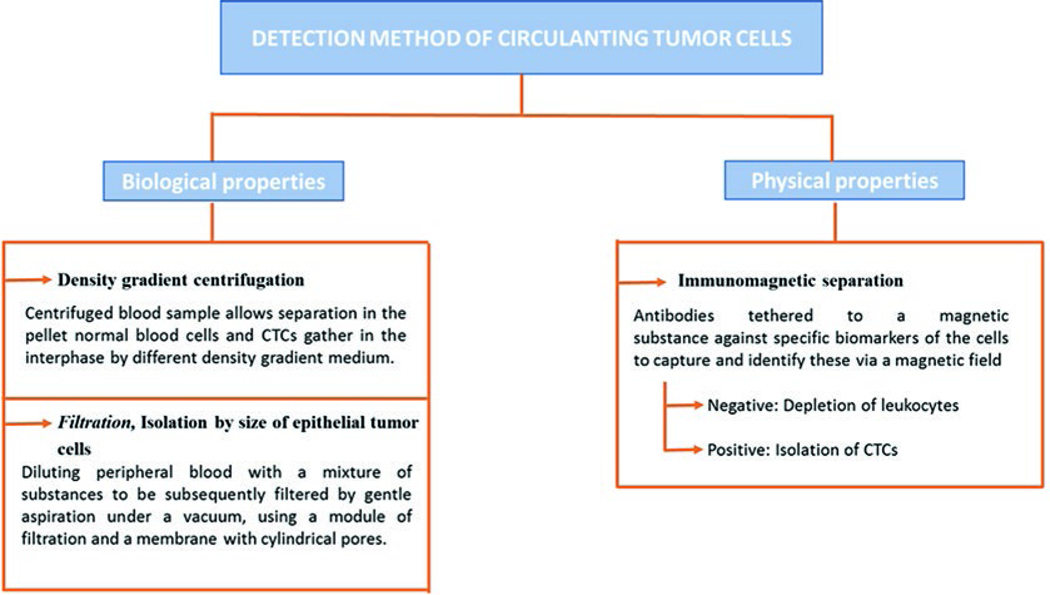
Summary of detection methods of CTCs
9.3.1. Biological Properties
9.3.1.1. Density Gradient Centrifugation
The low density of CTCs (<1.077 g/mL) allows them to be distinguished from erythrocytes, leukocytes, and platelets. Centrifugation of blood samples allows separation in the pellet with normal blood cells and CTCs gathering at the interphase on differential step density gradients. Additional analyses can be performed in these CTCs by integrated cell culture (ICC) and polymerase chain reaction (PCR) [12].
9.3.1.2. Filtration
In 2000, Vona et al. [13] presented a new technique called “isolation by size of epithelial tumor cells (ISET)” in order to detect and count CTCs in peripheral blood samples of patients with carcinomas. In addition, this technique allows the immunocytological and molecular characterization of the cellular population. The method consists of diluting peripheral blood with a mixture of substances (saponin, paraformaldehyde, ethylenediamine tetraacetic acid (EDTA), and bovine serum albumin) to be subsequently filtered by gentle aspiration under vacuum, using a module of filtration and a calibrated polycarbonate mem-brane with 8-μm-diameter cylindrical pores. Soon after, the isolated cells can be stained with hematoxylin and eosin (H&E) or May-Grunwald-Giemsa and studied by immunostaining, laser microdissection, and/or fluorescence in situ hybridization (FISH).
9.3.2. Physical Properties
The first immunomagnetic separation of CTCs was developed by Racila et al. [14]. At present, it is the most used technique due to its effectiveness for both detection and isolation. Also, it is the only method approved by the Food and Drug Administration (FDA) for this purpose. The assay combines immunomagnetic enrichment with flow cytometry and immunohistochemistry, using magnetic beads labeled with antibodies against specific target cell antigens, which can be used to identify the cells via a magnetic field. For the immunomagnetic separation phase, two approaches are available, although both selection procedures can be combined [15].
9.3.2.1. Negative Selection
This approach involves depletion of leukocytes using anti-CD45-labeled magnetic beads, with the CTCs being the non-selected cells.
9.3.2.2. Positive Selection
In this approach, the isolation of CTCs is achieved using magnetic beads labeled with antibodies against CTC surface proteins such as epithelial cell adhesion molecule (EpCAM), epithelial specific antigen (HEA), and anti-cytokeratin peptides.
In order to obtain more accurate results, modifications to this technique have been described by several authors; an example is the microchip technology developed by Nagrath et al. in the USA [16]. This method has the advantages of high-throughput processing, low shear, and efficient isolation with no requirement of pre-labeling or processing of the samples [17]. This technology consists of the interaction of target CTCs with antibody (EpCAM)-coated microposts under precisely controlled laminar flow conditions [16]. Additionally, Deng et al. showed that the use of anti-cytokeratin antibodies in combination with the anti-EpCAM antibodies enhances assay sensitivity significantly [18].
9.4. Research of Circulating Tumor Cells in Several Types of Cancers
Research in cancer is focused in the development of therapeutic targets and early identification of metastases to reduce mortality rates [19] In this case, the identification of CTCs was a big scientific breakthrough that has been studied in several types of cancer [2]. The principal studies performed are summarized in Table 9.1.
Table 9.1.
Principal studies of CTCs in several types of cancer
| Reference | Type of cancer | Method of CTC detection | n Groupsa | Main conclusion |
|---|---|---|---|---|
| [22] | Metastatic, colorectal | CellSearch system | n = 109 ≤3 CTCs/7.5 mL >3 CTCs/7.5 mL | Patients with less CTCs had shorter free and overall survival |
| [23] | Resectable lung cancer | ISET method | n = 208 | The number of CTCs was an independent prognostic factor for overall survival |
| [24] | Metastatic, prostate | CellSearch system | n = 231 >5 CTC/7.5 mL <5 CTC/7.5 mL | CTC count was the best predictor of prognosis; patients with more CTCs had shorter overall survival rates |
| [25] | Melanoma | n = 87 | CTCs were identified in 29% of patients with primary melanoma and 62.5% with metastatic melanoma patients | |
| [26] | Metastatic sarcoma | ISET method | n = 11 | All patients showed CTCs |
| [27] | Locally advanced head and neck cancer | ISET method | n = 83 <6.5 CTC/mL ≥6.5 CTC/ mL | CTCs were identified in 94% of patients and higher counts were strongly correlated with survival and response to treatment |
CTCs circulating tumor cells, ISET isolation by size of epithelial tumor cells
Some studies have categorized patients into high and low CTC count
9.4.1. Breast Cancer
The first clinical study using the CellSearch System to identify and determine the prognostic significance of CTCs was performed in women with metastatic breast cancer (MBC). Patients (n = 117) were separated in two groups according to the number of CTCs before the chemotherapeutic treatment: (1) fewer than 5 CTCs/7.5 mL of whole blood and (2) higher than 5 CTCs/7.5 mL of whole blood. The results showed that the number of CTCs in patients with MBC is an independent survival predictor, due to subjects with higher counts of CTCs having shorter progression-free survival and overall survival rates than the patients with fewer CTCs [20].
9.4.2. Colorectal Cancer
Colorectal malignant neoplasms are the second cause of cancer-related deaths. Development of chemotherapeutic agents for specific targets has been investigated with the identification of CTCs [21]. Cohen et al. used EpCAM isolated magnetically to characterize CTCs from patients with metastatic colorectal cancer and later analyzed their prognostic significance [22]. The authors classified the patients into two groups based on CTC levels (≤3 CTCs /7.5 mL of blood and >3 CTCs/7.5 mL of blood) and showed that patients with more than 3 CTCs had shorter median progression-free survival and overall survival, than those patients with lower CTC counts.
9.4.3. Lung Cancer
In 2011, the American Association for Cancer Research published a study that identified CTCs in approximately half of patients with resectable lung cancer (n = 208). This used the ISET method associated with cytologic analyses and correlated the presence and number of CTCs with clinicopathological features and survival. Patients showing 50 or more CTCs in blood had worse overall and disease-free-survival (DSF), independently of clinical stage, and presented higher risk of recurrence and cancer-related death. The number of CTCs was a significant and independent prognostic factor of overall survival, and it was proposed as a new prognostic biomarker [23].
9.4.4. Prostate Cancer
The relationship between CTCs and survival in patients affected by prostate cancer was studied initially by De Bono et al. in 2008 [24]. The prospective research employed the CellSearch System to detect and count CTCs in blood samples of subjects with progressive disease in different stages: (1) before treatment, (2) at the start of a new line of chemotherapy, and (3) monthly thereafter. The assay patients were categorized into either unfavorable or favorable groups according to the number of CTCs: >5 CTCs/7.5 mL or <5 CTCs/7.5 mL, respectively. The analysis showed that the CTC count was an accurate predictor of prognosis, given that patients with unfavorable counts before and after treatment had shorter overall survival rates than those with favorable counts.
9.4.5. Melanoma
In 2010, the first study of CTC identification was published concerning 87 patients with cutaneous melanoma by ISET method, followed by CTC characterization by immunohistochemistry (S-100, melanosome (HMB45), MART-1/Melan-A) and reverse transcription polymerase chain reaction (RT-PCR). The methodology included a control group of healthy volunteers with melanocytic nevi and non-melanoma skin lesions. CTCs were identified in 29% of patients with primary melanoma and in 62.5% of metastatic melanoma patients, while in the control group CTCs were not detected [25].
9.4.6. Sarcomas
Recently Chinen et al. performed for the first time the isolation, identification, and characterization of CTCs in sarcoma patients [26]. The study included blood samples from 11 patients with high-grade and metastatic sarcoma isolated by ISET. CTCs were identified in all of the cases by cytomorphology and characterized by double immunostaining with vimentin or pan-cytokeratin and CD45 antibodies. The number of CTCs identified varied from 2 to 48/8 mL of blood, with the highest number of cells found in the case of an epithelioid sarcoma and the lowest in an osteoblastic osteosarcoma. The ISET technique was limited to study epithelial malignancies, and thus this research showed the sensitivity of this method applied to sarcomas, a group of neoplasms with frequent metastasis and poor prognosis.
9.4.7. Head and Neck Cancer
A prospective study evaluating 83 patients affected by head and neck cancer demonstrated the prognostic role of CTCs in this malignancy through the ISET method. The study evaluated blood samples of patients diagnosed with non-metastatic locally advanced head and neck squamous cell carcinoma, treated with curative surgical resection plus adjuvant radiotherapy, or by a non-surgical strategy (radiotherapy and/or chemotherapy). Patients were sorted according to the count of CTCs at baseline (<6.5/mL versus ≥6.5/mL). CTCs were detected in 94% of the patients (n = 78) and significantly correlated with prognosis and response to treatment. The 2-year overall survival was 85.6% versus 22.9% (HR, 0.18; 95%CI, 0.06–0.49; P< 0.0001), revealing the prognostic potential of CTCs in head and neck cancer [27].
9.5. Clinical Applications and Limitations
Cancer-related death is usually provoked by dissemination, resulting in regional and distant metastases that could develop years after the removal of the primary tumor, despite the fact that tumor spread may not be evident at the time of the primary diagnosis. For example, many patients affected by breast cancer with negative axillary lymph nodes develop local or distant metastases. This could be explained by the presence of CTCs, which would represent the hematogenous phase of metastasis [28, 29].
Imaging has been the gold standard for disease monitoring in cancer. Combined with these traditional methods, CTC quantification must represent an alternative approach that could reveal micrometastases earlier than is currently possible, thereby improving the monitoring of disease status [30]. In this regard, Bud et al. compared CTC quantification to traditional radiologic assessment and suggested that CTC calculation is a reproducible tool that could be used earlier in the course of disease compared to imaging evaluation [31]. It has been proposed that CTCs play a crucial role for developing metastases. For this reason, monitoring CTCs may provide valuable information for treatment and, in the future, could be used as a real-time “liquid biopsy” [32, 33].
Until now, most of the studies of CTCs and prognosis using the CellSearch System (https://www.cellsearchctc.com/) for quantification have applied a cutoff value ≥5 CTCs/7.5 mL for categorization, as proposed by Cristofanilli et al. [20]. Prognostic correlations have been observed such that those few patients with very high levels of CTCs had a markedly short survival time. In one-third of MBC patients, CTCs were not detected, which constitutes a positive prognostic factor relative to patients with ≥1 CTCs/7.5 mL at baseline and during treatment [34]. In MBC, the prognostic properties of CTCs were shown to be robust during therapy by Hayes and colleagues [35].
Further than quantification, the evaluation of CTCs represents an accessible source of molecular information about the tumor, through the presence of treatment-relevant biomarkers (e.g., multidrug resistance proteins) [36].
Despite promising findings of several studies, CTC assessment still has not provided information on specific staging of disease, or in the guiding of adjuvant treatment. Pesta et al. proposed that analysis of the CTCs should provide information useful for the management of cancer patients, fulfilling the objectives of predictive, preventive, and personalized medicine (PPPM) [30]. However, the diagnostic value of CTC analysis is still not sufficient for clinical use. A three-step method to study CTCs was proposed to achieve specific uses for clinical practice. The first step is monitoring of treatment efficacy of cancer patients. The second one is to characterize the captured CTCs at the molecular level for the targeted treatment. The third stage is the culture of CTCs for use in a chemosensitivity assay. These steps would allow researchers to recognize and respond to changes in the phenotype of cancer cells during disease progression and introduce PPPM assisted by CTC analysis, into clinical practice.
While the clinical relevance of sequential CTC counts during treatment for use as an early response evaluation marker has been clearly demonstrated, the value of CTC characterization to guide treatment decisions in the clinic remains to be investigated [33].
There are three main limitations for CTC isolation. The first, and probably the most significant, is the rarity of CTCs in the bloodstream, since approximately ~1–100 CTCs per 109 blood cells are detected in cancer patients, and a significant quantity of normal hematological cells, such as erythrocytes and leukocytes, have to be eliminated to obtain pure CTCs. Secondly, there is an apparent absence of CTCs in some patients. Finally, there are morphological and genetic heterogeneities of CTCs, even when they are disseminated from the same primary tumor. This latter property may make the isolation of CTCs difficult and the utility could be limited as the testing of drug response using such cells may differ from that of the primary tumor [2, 37–39].
It has been proposed that these limitations may be linked [40]. Studies have found that the lack or small number of CTCs in peripheral blood may actually be an issue of detection. It is known that most of the time, tumor cells require epithelial-to-mesenchymal transition (EMT) for major invasion and subsequent dissemination. When EMT occurs, CTCs suffer phenotypic changes, such as loss of expression of epithelial markers, and they acquire more mesenchymal-like phenotypes, which enables them to invade and survive in blood vessels and to invade other organs [41, 42].
Considering that CTCs are rare in peripheral blood and that CTCs with EMT may lose expression of EpCAM, this would result in the missing of EpCAM-negative CTCs in detection procedures based on use of the EpCAM antibody. In some cases, this could mean missing all of the CTCs in patients. This is an important limitation of the more extended and accepted technologies of CTC detection that are based in the presence of EpCAM in tumoral cells [2, 43, 44].
Following this hypothesis of EpCAM expression heterogeneity in CTCs, Hyun et al. [45] developed a model of EMT-induced MCF-7 breast cancer cells in order to study the physical and molecular characters of these cells. They showed that EMT-induced breast cancer cells have low levels of EpCAM expression. By RT-PCR and Western blotting, they observed that EpCAM mRNA was substantially reduced in MCF-7 cells, which indicates that EMT induction may result in decreased EpCAM expression levels. Also, they used a novel EpCAM-independent isolation system that demonstrated efficient isolation of CTCs regardless of heterogeneous EpCAM expression in breast cancer patient blood samples. This approach is called parallel multi-orifice flow fractionation (p-MOFF), which is a chip for high-throughput size-based CTC separation and was developed by the same research group in 2013 [46].
Hamilton et al. studied cell lines of primary, metastatic, and CTCs of small cell lung carcinoma (SCLC) and treated these in vitro with topotecan and epirubicin [37]. This showed that the CTC cell lines presented considerably more chemosensitivity than permanent SCLC cell lines, which suggests that response to second-line chemotherapy in SCLC patients may overestimate the effect on resident SCLC lesions and metastases. Chemosensitivity of CTCs compared to primary and metastatic tumors has been recently studied since, in some malignancies like SCLC, a decline in the CTC count during or after treatment could not reflect the response to chemotherapy of the permanent cells (from primary and metastatic tumors). Consequently, the detection of CTCs may facilitate a paradigm shift from treatment, based only on primary tumor features to a treatment that considers the molecular characteristics of CTCs [2]. Development of new technologies that overcome limitations of the more traditional techniques of CTC isolation will increase the understanding of CTC biology and association with prognosis and treatments, which is crucial in developing clinical applications. The principal clinical applications of circulating tumor cells are summarized in Table 9.2.
Table 9.2.
Main clinical applications of detection and study of CTCs
|
• Monitoring disease status |
|
• Detection of micrometastases |
|
• Prognosis factor |
|
• Pharmacological studies (e.g., drug resistance) |
|
• Planning of personalized treatments |
9.6. Conclusion
Evidence shows that CTCs have an important clinical value in early diagnosis of metastasis, as a predictor of prognosis, for monitoring of treatment and development of targeted treatment approaches. However, there are several challenges ahead, principally the rarity of CTCs in bloodstream, their heterogeneous characteristics, and cell loss during isolation techniques. These factors make difficult the validation of a specific application of CTCs to improve survival rates in patients. Additional studies are needed to clarify the knowledge and to achieve better isolation techniques to pave the way for successful clinical applications in cancer patients.
Acknowledgments
This work was supported in part by NIH grants (R01DE028351 and R03DE028387) and CURS Summer Scholars to Y. Teng. M. M. Galvis is the federal Brazilian scholarship (FAPESP) awardee.
Contributor Information
Marisol Miranda Galvis, Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA, USA.
Celeste Sánchez Romero, Molecular Pathology, Faculty of Dentistry, Universidad de la República (UDELAR), Montevideo, Uruguay.
Thiago Oliveira Bueno, Clinical Oncology Department, A.C. Camargo Cancer Center, São Paulo, Brazil Marisol Miranda Galvis and Celeste Sánchez Romero contributed equally..
Yong Teng, Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA, USA Department of Biochemistry and Molecular Biology.
References
- 1.Cen P, Ni X, Yang J, Graham DY, Li M (2012) Circulating tumor cells in the diagnosis and management of pancreatic cancer. Biochim Biophys Acta 1826(2):350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyun KA, Jung HI (2014) Advances and critical concerns with the microfluidic enrichments of circulating tumor cells. Lab Chip 14(1):45–56 [DOI] [PubMed] [Google Scholar]
- 3.Ashworth TR (1869) A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australian Med J 14:146–147 [Google Scholar]
- 4.Cotlar AM, Dubose JJ, Rose DM (2003) History of surgery for breast cancer: radical to the sublime. Curr Surg 60(3):329–337 [DOI] [PubMed] [Google Scholar]
- 5.Paget S (1989) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 8(2):98–101 [PubMed] [Google Scholar]
- 6.Ewing J (1924) Neoplastic diseases, a treatise on tumors. Can Med Assoc J 14(5):466 [Google Scholar]
- 7.Weiss L (2000) Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev 19(3–4):I–XI, 193–383 [PubMed] [Google Scholar]
- 8.Sugarbaker EV (1979) Cancer metastasis: a product of tumor-host interactions. Curr Probl Cancer 3(7):1–59 [DOI] [PubMed] [Google Scholar]
- 9.Hart IR, Fidler IJ (1980) Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 40(7):2281–2287 [PubMed] [Google Scholar]
- 10.Salgado I, Hopkirk JF, Long RC, Ritchie AC, Ritchie S, Webster DR (1959) Tumour cells in the blood. Can Med Assoc J 81:619–622 [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander RF, Spriggs AI (1960) The differential diagnosis of tumour cells in circulating blood. J Clin Pathol 13:414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ (2013) Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv 31(7):1063–1084 [DOI] [PubMed] [Google Scholar]
- 13.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K et al. (2000) Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 156(1):57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW et al. (1988) Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 95(8):4589–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira MM, Ramani VC, Jeffrey SS (2016) Circulating tumor cell technologies. Mol Oncol 10(3):374–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L et al. (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450(7173):1235–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B et al. (2015) A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods 12(7):685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF (2008) Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res 10(4):R69. 10.1186/bcr2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Cancer Report: International Agency for Research on Cancer (2020). https://www.iarc.fr/cards_page/world-cancer-report/
- 20.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al. (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791 [DOI] [PubMed] [Google Scholar]
- 21.El Zouhairi M, Charabaty A, Pishvaian MJ (2011) Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest Cancer Res 4(1):15–21 [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY et al. (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26(19):3213–3221 [DOI] [PubMed] [Google Scholar]
- 23.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Flejou JF et al. (2011) Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 17(4):827–835 [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H et al. (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14(19):6302–6309 [DOI] [PubMed] [Google Scholar]
- 25.De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A et al. (2010) Application of a filtration-and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 130(10):2440–2447 [DOI] [PubMed] [Google Scholar]
- 26.Chinen LT, Mello CA, Abdallah EA, Ocea LM, Buim ME, Breve NM et al. (2014) Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: a window on sarcoma-cell invasion. Onco Targets Ther 7:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bueno TBA, Nicolau U, Abdallah E, Silva V, Fonseca V, Calsavara V et al. (2019) Prognostic impact of baseline circulating tumor cells (CTCs) detected by the isolation by size of epithelial tumor cells (ISET) in locally advanced head and neck squamous cell carcinoma (LAHNSCC): results of a prospective study. J Clin Oncol 37(15_suppl):6061–6061 [Google Scholar]
- 28.Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128(11):2527–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leong SP, Tseng WW (2014) Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: clinical significance and biologic implications. CA Cancer J Clin 64(3):195–206 [DOI] [PubMed] [Google Scholar]
- 30.Pesta M, Kulda V, Narsanska A, Fichtl J, Topolcan O (2015) May CTC technologies promote better cancer management? EPMA J 6(1):1. 10.1186/s13167-014-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC et al. (2006) Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409 [DOI] [PubMed] [Google Scholar]
- 32.Pantel K, Alix-Panabieres C (2013) Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res 73(21):6384–6388 [DOI] [PubMed] [Google Scholar]
- 33.Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, Sleijfer S (2016) The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castration-resistant prostate cancer. Cancer Treat Rev 46:42–50 [DOI] [PubMed] [Google Scholar]
- 34.Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M et al. (2012) High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol 23(3):618–624 [DOI] [PubMed] [Google Scholar]
- 35.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC et al. (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224 [DOI] [PubMed] [Google Scholar]
- 36.Hayashi N, Nakamura S, Tokuda Y, Shimoda Y, Yagata H, Yoshida A et al. (2012) Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol 17(2):96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton G, Rath B, Holzer S, Hochmair M (2016) Second-line therapy for small cell lung cancer: exploring the potential role of circulating tumor cells. Transl Lung Cancer Res 5(1):71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS et al. (2013) Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep 3:1259. 10.1038/srep01259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Gregory SG, Garcia-Blanco MA, Armstrong AJ (2015) Using circulating tumor cells to inform on prostate cancer biology and clinical utility. Crit Rev Clin Lab Sci 52(4):191–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ et al. (2015) The detection of EpCAM(+) and EpCAM(−) circulating tumor cells. Sci Rep 5:12270. 10.1038/srep12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564 [DOI] [PubMed] [Google Scholar]
- 42.Gorges TM, Pantel K (2013) Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother 62(5):931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B et al. (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13(3):920–928 [DOI] [PubMed] [Google Scholar]
- 44.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F et al. (2012) Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 9(1):016003. 10.1088/1478-3975/9/1/016003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI et al. (2016) Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7(17):24677–24687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyun KA, Kwon K, Han H, Kim SI, Jung HI (2013) Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens Bioelectron 40(1):206–212 [DOI] [PubMed] [Google Scholar]


