Abstract
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects the competence of academic performance and social wellness in children and adults. The causes of ADHD are unclear. Both genetic and environmental factors contribute to the development of ADHD. The behavioral impairments in ADHD are associated with epigenetic changes in genes that are important for neurodevelopment. Among environmental causes of ADHD, the neurotoxin methylmercury (MeHg) is associated with an increased risk for ADHD. Developing children are susceptible to neurotoxic effects of prenatal MeHg exposure. Human epidemiology studies have shown that prenatal MeHg exposure could invoke epigenetic changes in genes that are involved in ADHD. In addition, the pathogenesis of ADHD involves dopaminergic system, which is a target of developmental MeHg exposure. MeHg-induced alterations in the dopaminergic system have a profound impact on behavioral functions in adults. As a trace level of MeHg (around nM) can induce long-lasting behavioral alterations, potential mechanisms of MeHg-induced functional changes in the dopaminergic system may involve epigenetic mechanisms. Here, we review the relevant evidence on developmental MeHg exposures and the risk for ADHD. We also point out research gaps in understanding environmental causes of ADHD.
Keywords: mercury, DNA methylation, dopamine, attention, hyperactivity
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects children’s learning ability, social behavior and emotional wellness [1]. Children and adults with ADHD suffer challenges in academic performance, social communication and emotional control. There is no curable treatment for the disease [2]. The symptoms of ADHD include inattention, hyperactivity–impulsivity or both. The estimated incidence of ADHD is 5% in children and 2.5% in adults worldwide [3]. The increased social awareness of ADHD and the great social and economic burden inflicted on the patients call for a better understanding and treatment of the disease [4].
Currently, clinical management of ADHD relies on stimulants including methylphenidate and amphetamine; however, their efficacy is a subject for debate. For example, a recent review pointed out that there is a great uncertainty regarding the effect of long-term treatment with the dopamine agonist amphetamine in adults with ADHD [5]. Furthermore, nearly 10% of patients did not respond to either amphetamine or methylphenidate [6]. In addition, while ADHD mostly afflicts children, many of them show persistent symptoms in adulthood. The causes for ADHD are multifactorial. Both environmental and genetic factors are involved in the development of ADHD [7, 8].
Methylmercury (MeHg) is an organic form of mercury species that naturally occurs in human environment [9]. Human exposure to MeHg comes from eating fish animals that absorb and biomagnify MeHg produced from aquatic microorganisms [10]. The most notable toxic target of MeHg is the brain [11]. Developing fetus is vulnerable to MeHg’s neurotoxicity. As an internal exposure marker of MeHg [12], blood mercury in asymptomatic mothers can cause a long-lasting damage to fetal neurodevelopment [13]. MeHg can form a complex with the amino acid cysteine. The MeHg–cysteine complex is a structural mimicry of the amino acid methionine. Therefore, the complex can take a free pass into the brain through transporters for methionine [14, 15]. MeHg can disrupt cellular redox balance, leading to a cascade of toxic effects [16, 17]. Although there is an uncertainty on the adverse effects of fish eating on the neurobehavioral functions, environmental MeHg exposure can alter DNA methylation levels, an important mechanism in the epigenetic regulation of gene expression [18].
Recent research suggests that environmental MeHg exposure may contribute to the development of ADHD [19]. However, we are far from understanding the causal link between MeHg and ADHD. Herein, we attempt to summarize the current available evidence in support of the pathogenic role of MeHg in the development of ADHD. Although most evidence is indirect, it provides an important base and impetus for future studies. We focused on mechanistic roles of epigenetic effects of MeHg exposure and risk for ADHD, particularly on the modulation of dopaminergic neurotransmission by MeHg. We did not include the heritable effects of MeHg toxicity, genetic susceptibility to MeHg toxicity or significance of latent MeHg effects in age-related diseases, as these subjects were discussed elsewhere [20–23].
ADHD, Dopamine, and MeHg Toxicity
The development of ADHD involves structural and functional alterations in the developing brain. These alterations are believed to be outcomes of deviation of ‘normal’ brain development [3]. The clinical diagnostic criteria are based on symptoms of learning and social behaviors that are manifested in typical ADHD patients. However, it has to be pointed out that clinical ADHD patients only represent those whose apparent behavior and cognitive developments deviate from ‘normality.’ There is still a proportion of people that exhibit mild and subclinical symptoms of ADHD [24]. The development of ADHD involves multi-systems of neurotransmission in the brain. Dopaminergic neurotransmission plays an important role in the development of ADHD [25].
Dopaminergic neurotransmission is involved in brain functions including reward system, motor control and emotion regulation [26]. The efficacy of dopamine neurotransmission stimulants in mitigating ADHD suggests that the normal dopaminergic neurotransmission may have been disrupted in ADHD patients [27]. Dopamine synthesis takes several steps, among which tyrosine hydroxylase (TH) is the rate-limiting enzyme to produce dopamine (Fig. 1). Intracellular dopamine is packaged into synaptic vesicles to be released into the synaptic cleft for neurotransmission. Extrasynaptic dopamine levels are regulated and can be transported back into presynaptic neurons by dopamine transporters (DATs).
Figure 1:
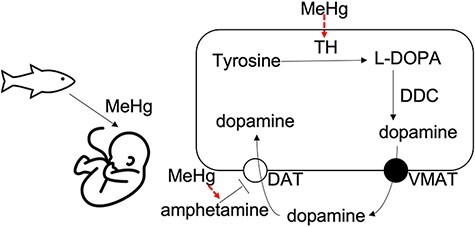
Potential impacts of developmental MeHg exposures on dopamine neurotransmission. The developing brain of fetus is susceptible to environmental exposure to neurotoxins. The primary pathway for dopamine synthesis involves several enzymes including TH and DDC. For the dopamine neurotransmission, MeHg exposure can alter the epigenetic regulation of the TH gene and potentiate the effect of dopamine neurotransmission agonists such as amphetamine [55–58]. TH, tyrosine hydroxylase; L-DOPA, L-3,4-dihydroxyphenylalanine; DDC, DOPA decarboxylase; VMAT, vesicular monoamine transporter 2; DAT, dopamine transporter
Studies have shown that the expression of DATs is epigenetically regulated, which is linked to the risk for ADHD [28–30]. For example, in a case–control study on risk factors for ADHD, alterations in DAT-1 expression were linked to ADHD. The study did not find a significant change in overall DNA methylation levels in the promoter region of the dat-1 gene; however, a possible change in methylation levels in several individual sites of the dat-1 region was proposed [28]. The importance of dat-1 epigenetics in the prognosis of ADHD was also demonstrated in a clinical study showing that the methylation status in the promoter region of dat-1 can predict the treatment outcomes of ADHD with methylphenidate, particularly on oppositional and hyperactive-impulsive symptoms [29]. Furthermore, in a recent investigation on the epigenetics of dat-1 in ADHD, it is showed that the methylation level in the dat-1 gene significantly changed in ADHD patients, which was not only related to the severity of ADHD symptoms but also had a predictive value for clinical prognosis [30].
The neurotoxicity of MeHg is mediated via several well-established mechanisms including oxidative stress, mitochondria toxicity and disruption of calcium homeostasis [31–33]. However, it is still unclear whether MeHg exposures at the environmental relevant level invoke the same mechanisms to alter neuronal functions. Recent studies suggest that mechanisms of toxicity induced by the environmental level of MeHg involve epigenetic regulations [34–38], which also play key roles in the transgenerational effects of MeHg [39, 40]. Further, we have recently shown that environmentally relevant exposures of developing human neurons from pluripotent stem cells cause subtle and persistent effects on both neuronal differentiation and neuronal gene expression [41, 42].
MeHg exposure can alter dopamine-mediated neurotransmission [43–50], which can be attributed to MeHg-induced effects on intracellular and mitochondrial calcium regulation [51]. A recent study showed that a dopamine-mediated neurobehavior in Caenorhabditis elegans was changed long after cessation of MeHg exposure, suggesting that mechanisms other than calcium signaling are also involved in MeHg-induced alterations in dopaminergic neurotransmission [52]. Given that dopamine-mediated neurobehaviors can be altered by environmental chemicals and the effects are transgenerational [39, 53, 54], studies on the role of epigenetics in MeHg toxicity and its implication for the risk of ADHD are timely and meritorious. In one such in vitro study, it was shown that MeHg exposure (1 nM) can repress the expression of TH. The study further investigated methylation status at the promoter region of the TH gene and showed that tri-methylation of histone H3 lysine 27 was significantly increased following MeHg exposure (1 nM) [55]. The importance of dopamine systems in MeHg’s toxicity was corroborated in the C. elegans study showing that reduced swimming speeds following MeHg exposure were modulated by the homolog of the TH gene [52]. Furthermore, a recent study showed that the effect of MeHg on neurobehavior functions invoked mechanisms of sperm epimutation, a heritable change in differential DNA methylation regions [39].
The importance of dopamine metabolism in MeHg’s neurotoxicity and its implication in ADHD was also supported by behavioral studies in rodents. A study in female rats showed that their behavioral sensitivity to d-amphetamine was increased following developmental exposure to MeHg [56]. In another study with male rats exposed to MeHg during adolescent development, it was shown that the effects of MeHg exposure on adult neurobehaviors including attention and memory were augmented by the dopamine agonist, d-amphetamine [57]. A follow-up study concluded that adolescence was vulnerable to MeHg and d-amphetamine, and the effect persisted in adulthood [58]. These studies provide important bases for the involvement of dopamine neurotransmission system in behavioral toxicity of MeHg, particularly in the behaviors related to ADHD [57]. However, a direct link between MeHg and ADHD via epigenetic mechanisms remains scarce.
Environmental MeHg Exposure and ADHD
Developing brains are especially sensitive to MeHg toxicity. Several large cohort studies investigated prenatal and postnatal MeHg exposures and neurodevelopmental outcomes in children. In the Seychelles Child Development Study, although the study did not reveal any significant adverse associations between MeHg exposure and a series of neurobehavioral outcomes [59], significant adverse associations between scholastic achievement and postnatal MeHg exposure were noted particularly in males [60]. Importantly, the study showed that some measures of neurodevelopmental tests were improved rather than adversely affected. This is in contrast with the conclusion of another large cohort study that showed that developmental MeHg exposure adversely affects neurobehavioral functions including attention, memory and verbal functions [61, 62]. The subtle effects of environmental levels of MeHg on neurobehavioral functions in these studies suggest that nutritional factors and co-exposed neurotoxins in the fish may have compounded functional measures of developing brain [63, 64]. In addition, the integrity of epigenetic regulation in the developing nervous system is extremely susceptible to environmental exposures [65, 66]. The potential role of epigenetic alterations by MeHg exposure may have contributed a significant effect in the observed neurofunctional measures (for more on this, see these reviews on MeHg-induced epigenetic alterations [67–69]).
MeHg exposure has been described as a risk factor for ADHD, given that the developing nervous system is most sensitive to the neurotoxicity of MeHg [70–72]; indeed, several studies have shown that mercury exposure, in the form of thimerosal (a mercury-based vaccine preservative), may be positively related to increased occurrence of behavior phenotype of ADHD [73, 74]. In addition, the association between prenatal MeHg exposure and the risk of ADHD was shown in a prospective cohort study in the Canadian Arctic and other cross-sectional studies [19, 75, 76]. These studies suggested that cord blood mercury was associated with higher scores of attention problems and scores of the Disruptive Behavior Disorders Rating Scale with ADHD. However, another cohort study in New Bedford, MA, reported an opposite conclusion, namely, that low mercury level is associated with ADHD behaviors [77]. The frequency of fish consumption is positively related to body mercury levels [78]. Intriguingly, an inverse relationship between mercury levels and risk for the behaviors of ADHD was shown in the cohort study in New Bedford, MA. This effect is probably modified by the level of fish consumption, which provides nutritional factors that are of benefit to brain development. For instance, the incidence of ADHD was reported to be decreased in groups eating the Mediterranean diet, and fish is an important component of the diet [79]. The apparent inconsistency in the conclusions from these studies may also reflect the inherent difference in the populations, such as diet and genetic variations.
In addition, neuronal differentiation and migration in the developing brain require numerous epigenetic modifications to ensure proper regulation of gene expressions and integrated function [80]. Epigenetic regulations in the developing brain are susceptible to environmental exposures [67]. MeHg exposure at trace levels could induce long-lasting and transgenerational epigenetic effects [39, 40]; however, what is less understood is how environmental MeHg exposure might alter epigenetic regulations in neuronal cells in vivo and its significance in behavioral outputs of ADHD. Apparently, a mechanistic understanding of MeHg exposure through eating fish and the risk for ADHD may suffice to generate a new hypothesis for future investigations on epigenetic factors that contribute to environmental influences on brain development [18, 81]. Furthermore, understanding epigenetic mechanisms of MeHg’s toxicity is helpful in identifying vulnerable targets and refining measures of developmental outcomes in human studies [82].
Studies on human epigenetic alterations following MeHg exposure used epigenetic markers in blood cells or saliva to infer possible epigenetic influences of MeHg on the brain [18, 81, 83, 84]. Recent epidemiology studies demonstrate that prenatal exposure to MeHg alters epigenetic markers in several genes that are involved in the regulation of neurodevelopment [18, 85]. For example, in the Nutrition Cohort 2 of the Seychelles Child Development Study, prenatal MeHg exposure was associated with increased levels of DNA methylation at the cytosine of CG dinucleotides located at gene-expression regulation sites [18]. The affected genes include brain-derived neurotropic factor (BDNF), glucocorticoid receptor (NR3C1) and glutamate receptor subunit NR2B (GRIN2B), all of which had been shown to be involved in the development of ADHD in other independent studies [86–88]. Increased methylation levels of NR3C1 associated with prenatal mercury exposure were also reported in another human study showing that the methylation level of NR3C1 was increased in those with an average mercury level of 0.17 µg/g compared with the reference mercury level of 0.01 µg/g [85].
It has been recognized that perinatal MeHg exposure adversely affects neurobehavior development [13]. A birth cohort by Maccani et al. showed that prenatal mercury exposure increased the risk for poorer quality of movement, poorer self-regulation and increased signs of physiologic stress [89]. In addition, toenail mercury tertiles are associated with 339 CpG loci, with an average methylation difference of >0.125. The study also showed that the prenatal mercury level in the toenails of infants is associated with several genes with altered methylation levels, which include transcription elongation regulator 1-like (TCERG1L), a possible factor involved in ADHD [90]. Because significant changes in the methylation level of many other genes were also noted in the study, it is difficult to conclude the alteration of methylation level in TCERG1L is a direct effect of mercury.
A recent new study carried out in Spain revealed that postnatal mercury exposure was associated with an increased risk for ADHD. The study also showed that boys were more vulnerable than girls to these effects [91]. The study further demonstrated that the polymorphism in BDNF modified the association between mercury and behaviors of ADHD. The sex-specific effect on DNA methylation levels following prenatal mercury exposure was also reported in a cohort study in Japan [92]. Specifically, the study showed that hyper-methylation in one locus of the gene of haloacid dehalogenase-like hydrolase domain-containing protein 1 (HDHD1) within the transcriptional regulation site was only noted in boys. Furthermore, the temporal changes of epigenetic effects related to prenatal mercury exposure were shown in a study on prenatal mercury exposure and neurocognitive effects [93]. The study further showed that alterations in DNA methylation levels induced by prenatal mercury exposure varied from early to mid-childhood, suggesting that the interaction between mercury and DNA methylation regulation may have been compounded by other factors during development and that the observed changes were indirectly caused by mercury. Methylation of cytosine at CG dinucleotides can be oxidized to hydroxy-methylation, which can independently regulate gene expression [94]. The DNA hydroxy-methylation level was lower in those with higher prenatal mercury levels, which was attenuated from early childhood to mid-childhood [84]. Another investigation of newborns on global DNA methylation level and prenatal mercury exposure showed that the methylation level in the gene of transcription elongation factor A (SII) N-terminal and central domain containing 2 (TCEANC2) was associated with cord blood mercury levels [95]. The TCEANC2 gene is a known risk factor for sporadic Parkinson’s disease [96]; however, the implication of the epigenetic changes to neurobehavioral functions in developing children is unknown.
As mentioned before, the effects of prenatal mercury exposure on the epigenetic markers can be modulated by nutritional factors as well as other toxins. For example, the association between DNA methylation level and prenatal mercury was modified by in utero exposure to arsenic [97]. Another neurotoxin that coexists with MeHg in fish [98], namely polychlorinated biphenyls, also had a significant effect on DNA methylation profiles in blood leukocytes [99]. In addition, nutritional elements also affect global DNA methylation levels. One of the mechanisms of MeHg toxicity is the inhibition of the activity of enzymes requiring selenium as a cofactor [9]. Studies have shown that maternal blood selenium is associated with global DNA methylation levels in both pregnant mothers and newborns [83]. Consequently, the disruption of selenoprotein activity and synthesis by MeHg can interfere with DNA methylation of developing brain. Furthermore, prenatal mercury exposure can induce changes in micro RNA profiles in the placenta and cervix, respectively [100, 101], which may lead to altered regulation of epigenetics during fetal development. Taking together, these studies provide important clues on how developmental MeHg exposure alters brain functions and potential effects on the epigenetic control of genes associated with neurotransmission. Several important questions regarding epigenetic effects of MeHg need to be answered. The first is what is the mechanism of MeHg-induced alterations of the DNA methylation level. Finding a mechanistically trackable DNA methylation marker following MeHg exposure will facilitate the research on biological markers that reflect MeHg toxicity. Secondly, what are the epigenetic programs that modulate the development of dopaminergic neurotransmission. This will help to elucidate the critical developmental window that is vulnerable to the adverse effects of environmental factors such as MeHg. Lastly, as human association studies revealed many DNA methylation loci that can be modified MeHg exposure and exhibit a sex-specific pattern, what is the significance of the epigenic alterations induced by MeHg in behavioral outputs. The recent study on the epigenetic effects of MeHg and neurobehavior outcomes in the model organism zebrafish provides an important base for the understanding of these questions [39].
Conclusion
ADHD is one of the most common neurodevelopmental disorders. Although the pathogenesis of ADHD is not fully understood, exposure to environmental contaminants is associated with the disease. Current evidence suggests that epigenetic regulatory mechanisms such as DNA methylation are a target of environmental MeHg exposure. The investigations on the link between DNA methylation and prenatal MeHg exposure have shown that MeHg exposure may be associated with the pathogenesis of ADHD. Although MeHg exposure is associated with ADHD, the behavioral impacts of MeHg-induced epigenetic alterations warrant further investigations. Furthermore, MeHg exposure may be associated with the epigenetic regulation of genes involved in dopamine metabolism. Developmental MeHg exposure alters the pharmacological effects of dopamine agonists through the interaction with dopaminergic system. These studies provide important clues on how developmental MeHg exposure alters brain functions and its effects on the epigenetic control of genes associated with dopaminergic neurotransmission. However, further studies on the role of MeHg exposure in epigenetic alterations are needed to better understand the association between MeHg and ADHD.
The multifactorial nature of the causes for ADHD suggests that MeHg exposure can significantly alter neurobehavioral outcomes in animal models. As epigenetic marker is particularly susceptible to environmental factors, further investigations on the epigenetic effects of MeHg will shed new insights into the mechanisms of environmental causes of ADHD.
Funding
This work was supported by the National Institutes of Health to MA and ABB (NIEHS R01ES007331).
Contributor Information
Tao Ke, Department of Molecular Pharmacology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Room 209, Bronx, NY 10461, USA.
Alexey A Tinkov, World-Class Research Center “Digital Biodesign and Personalized Healthcare”, IM Sechenov First Moscow State Medical University (Sechenov University), Moscow 119435, Russia; Laboratory of Ecobiomonitoring and Quality Control, Yaroslavl State University, Yaroslavl 150003, Russia.
Antoly V Skalny, World-Class Research Center “Digital Biodesign and Personalized Healthcare”, IM Sechenov First Moscow State Medical University (Sechenov University), Moscow 119435, Russia; Laboratory of Medical Elementology, K.G. Razumovsky Moscow State University of Technologies and Management, Moscow 109004, Russia.
Aaron B Bowman, School of Health Sciences, Purdue University, West Lafayette, IN 47907-2051, USA.
Joao B T Rocha, Department of Biochemistry and Molecular Biology, Federal University of Santa Maria, Santa Maria, RS 97105-900, Brazil.
Abel Santamaria, Laboratorio de Aminoácidos Excitadores, Instituto Nacional de Neurología y Neurocirugía, Mexico City 14269, Mexico.
Michael Aschner, Department of Molecular Pharmacology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Room 209, Bronx, NY 10461, USA.
Conflict of interest statement
All authors edited and approved the manuscript. MA has recently consulted on mercury’s neurotoxicity. The other authors declare no conflict of interest.
References
- 1. Austerman J. ADHD and behavioral disorders: assessment, management, and an update from DSM-5. Cleve Clin J Med 2015;82:S2–7. [DOI] [PubMed] [Google Scholar]
- 2. Gavin B, McNicholas F. ADHD: science, stigma and service implications. Ir J Psychol Med 2018;35:169–72. [DOI] [PubMed] [Google Scholar]
- 3. Faraone SV, Asherson P, Banaschewski T. et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 4. Beyens I, Valkenburg PM, Piotrowski JT. Screen media use and ADHD-related behaviors: four decades of research. Proc Natl Acad Sci USA 2018;115:9875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castells X, Blanco-Silvente L, Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;8:Cd007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hechtman L. Treatment of ADHD in patients unresponsive to methylphenidate. J Psychiatry Neurosci 2011;36:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosang GM, Lichtenstein P, Ronald A. et al. Association of genetic and environmental risks for attention-deficit/hyperactivity disorder with hypomanic symptoms in youths. JAMA Psychiatry 2019;76:1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Froehlich TE, Anixt JS, Loe IM. et al. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 2011;13:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nogara PA, Oliveira CS, Schmitz GL. et al. Methylmercury’s chemistry: from the environment to the mammalian brain. Biochim Biophys Acta Gen Subj 2019;1863:129284. [DOI] [PubMed] [Google Scholar]
- 10. Villar E, Cabrol L, Heimbürger-Boavida LE. Widespread microbial mercury methylation genes in the global ocean. Environ Microbiol Rep 2020;12:277–87. [DOI] [PubMed] [Google Scholar]
- 11. Antunes Dos Santos A, Appel Hort M, Culbreth M. et al. Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol 2016;38:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. You SH, Wang SL, Pan WH. et al. Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children. Int J Hyg Environ Health 2018;221:697–703. [DOI] [PubMed] [Google Scholar]
- 13. Tatsuta N, Nakai K, Sakamoto M. et al. Methylmercury exposure and developmental outcomes in Tohoku study of child development at 18 months of age. Toxics 2018;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aschner M, Eberle NB, Goderie S. et al. Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res 1990;521:221–8. [DOI] [PubMed] [Google Scholar]
- 15. Aschner M, Clarkson TW. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol 1989;64:293–7. [DOI] [PubMed] [Google Scholar]
- 16. Ni M, Li X, Yin Z. et al. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 2010;116:590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caito S, Aschner M. Neurotoxicity of metals. Handb Clin Neurol 2015;131:169–89. [DOI] [PubMed] [Google Scholar]
- 18. Cediel Ulloa A, Gliga A, Love TM. et al. Prenatal methylmercury exposure and DNA methylation in seven-year-old children in the Seychelles Child Development Study. Environ Int 2021;147:106321. [DOI] [PubMed] [Google Scholar]
- 19. Boucher O, Jacobson SW, Plusquellec P. et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ Health Perspect 2012;120:1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andreoli V, Sprovieri F. Genetic aspects of susceptibility to mercury toxicity: an overview. Int J Environ Res Public Health 2017;14:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Julvez J, Grandjean P. Genetic susceptibility to methylmercury developmental neurotoxicity matters. Front Genet 2013;4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamm C, Ceccatelli S. Mechanistic insight into neurotoxicity induced by developmental insults. Biochem Biophys Res Commun 2017;482:408–18. [DOI] [PubMed] [Google Scholar]
- 23. Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect 2002;110:851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Floros O, Axelsson J, Almeida R. et al. Vulnerability in executive functions to sleep deprivation is predicted by subclinical attention-deficit/hyperactivity disorder symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging 2021;6:290–8. [DOI] [PubMed] [Google Scholar]
- 25. Kirley A, Hawi Z, Daly G. et al. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology 2002;27:607–19. [DOI] [PubMed] [Google Scholar]
- 26. Hirano S. Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol Med 2021;27:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pitzianti MB, Spiridigliozzi S, Bartolucci E. et al. New insights on the effects of methylphenidate in attention deficit hyperactivity disorder. Front Psychiatry 2020;11:531092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, Chen XT, Luo M. et al. Multiple epigenetic factors predict the attention deficit/hyperactivity disorder among the Chinese Han children. J Psychiatr Res 2015;64:40–50. [DOI] [PubMed] [Google Scholar]
- 29. Ding K, Yang J, Reynolds GP. et al. DAT1 methylation is associated with methylphenidate response on oppositional and hyperactive-impulsive symptoms in children and adolescents with ADHD. World J Biol Psychiatry 2017;18:291–9. [DOI] [PubMed] [Google Scholar]
- 30. Adriani W, Romano E, Pucci M. et al. Potential for diagnosis versus therapy monitoring of attention deficit hyperactivity disorder: a new epigenetic biomarker interacting with both genotype and auto-immunity. Eur Child Adolesc Psychiatry 2018;27:241–52. [DOI] [PubMed] [Google Scholar]
- 31. Farina M, Aschner M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim Biophys Acta Gen Subj 2019;1863:129285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ke T, Gonçalves FM, Gonçalves CL. et al. Post-translational modifications in MeHg-induced neurotoxicity. Biochim Biophys Acta Mol Basis Dis 2019;1865:2068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramanathan G, Atchison WD. Ca2+ entry pathways in mouse spinal motor neurons in culture following in vitro exposure to methylmercury. Neurotoxicology 2011;32:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilsner JR, Lazarus AL, Nam DH. et al. Mercury-associated DNA hypomethylation in polar bear brains via the LUminometric Methylation Assay: a sensitive method to study epigenetics in wildlife. Mol Ecol 2010;19:307–14. [DOI] [PubMed] [Google Scholar]
- 35. Cao M, Song F, Yang X. et al. Identification of potential long noncoding RNA biomarker of mercury compounds in zebrafish embryos. Chem Res Toxicol 2019;32:878–86. [DOI] [PubMed] [Google Scholar]
- 36. Bjørklund G, Pivina L, Dadar M. et al. Mercury exposure, epigenetic alterations and brain tumorigenesis: a possible relationship? Curr Med Chem 2020;27:6596–610. [DOI] [PubMed] [Google Scholar]
- 37. Pamphlett R, Kum Jew S, Cherepanoff S. Mercury in the retina and optic nerve following prenatal exposure to mercury vapor. PLoS One 2019;14:e0220859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rahman MA, Rahman MS, Uddin MJ. et al. Emerging risk of environmental factors: insight mechanisms of Alzheimer’s diseases. Environ Sci Pollut Res Int 2020;27:44659–72. [DOI] [PubMed] [Google Scholar]
- 39. Carvan MJ 3rd, Kalluvila TA, Klingler RH. et al. Mercury-induced epigenetic transgenerational inheritance of abnormal neurobehavior is correlated with sperm epimutations in zebrafish. PLoS One 2017;12:e0176155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bose R, Onishchenko N, Edoff K. et al. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol Sci 2012;130:383–90. [DOI] [PubMed] [Google Scholar]
- 41. Prince LM, Neely MD, Warren EB. et al. Environmentally relevant developmental methylmercury exposures alter neuronal differentiation in a human-induced pluripotent stem cell model. Food Chem Toxicol 2021;152:112178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neely MD, Xie S, Prince LM. et al. Single cell RNA sequencing detects persistent cell type- and methylmercury exposure paradigm-specific effects in a human cortical neurodevelopmental model. Food Chem Toxicol 2021;154:112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tiernan CT, Edwin EA, Hawong HY. et al. Methylmercury impairs canonical dopamine metabolism in rat undifferentiated pheochromocytoma (PC12) cells by indirect inhibition of aldehyde dehydrogenase. Toxicol Sci 2015;144:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dreiem A, Shan M, Okoniewski RJ. et al. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicol Teratol 2009;31:312–7. [DOI] [PubMed] [Google Scholar]
- 45. Daré E, Fetissov S, Hökfelt T. et al. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn Schmiedebergs Arch Pharmacol 2003;367:500–8. [DOI] [PubMed] [Google Scholar]
- 46. Giménez-Llort L, Ahlbom E, Daré E. et al. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol 2001;9:61–70. [DOI] [PubMed] [Google Scholar]
- 47. Castoldi AF, Blandini F, Randine G. et al. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2‘,4,4’,5,5ʹ-hexachlorobiphenyl (PCB153). Brain Res 2006;1112:91–8. [DOI] [PubMed] [Google Scholar]
- 48. Ke T, Santamaria A, Rocha JBT. et al. The role of human LRRK2 in methylmercury-induced inhibition of microvesicle formation of cephalic neurons in Caenorhabditis elegans. Neurotox Res 2020;38:751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ke T, Tsatsakis A, Santamaría A. et al. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 2020;77:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farina M, Aschner M, da Rocha JBT. The catecholaminergic neurotransmitter system in methylmercury-induced neurotoxicity. Adv Neurotoxicol 2017;1:47–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuan Y, Atchison WD. Methylmercury-induced increase of intracellular Ca2+ increases spontaneous synaptic current frequency in rat cerebellar slices. Mol Pharmacol 2007;71:1109–21. [DOI] [PubMed] [Google Scholar]
- 52. Ke T, Prince LM, Bowman AB. et al. Latent alterations in swimming behavior by developmental methylmercury exposure are modulated by the homolog of tyrosine hydroxylase in Caenorhabditis elegans. Neurotoxicol Teratol 2021;85:106963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Torres Valladares D, Kudumala S, Hossain M. et al. Caenorhabditis elegans as an in vivo model to assess amphetamine tolerance. Brain Behav Evol 2020;95:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith LL, Ryde IT, Hartman JH. et al. Strengths and limitations of morphological and behavioral analyses in detecting dopaminergic deficiency in Caenorhabditis elegans. Neurotoxicology 2019;74:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Go S, Kurita H, Matsumoto K. et al. Methylmercury causes epigenetic suppression of the tyrosine hydroxylase gene in an in vitro neuronal differentiation model. Biochem Biophys Res Commun 2018;502:435–41. [DOI] [PubMed] [Google Scholar]
- 56. Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to D-amphetamine and pentobarbital in adult rats. Neurotoxicol Teratol 2001;23:45–55. [DOI] [PubMed] [Google Scholar]
- 57. Kendricks DR, Boomhower SR, Newland MC. Methylmercury, attention, and memory: baseline-dependent effects of adult d-amphetamine and marginal effects of adolescent methylmercury. Neurotoxicology 2020;80:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boomhower SR, Newland MC. d-Amphetamine and methylmercury exposure during adolescence alters sensitivity to monoamine uptake inhibitors in adult mice. Neurotoxicology 2019;72:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Wijngaarden E, Thurston SW, Myers GJ. et al. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol Teratol 2017;59:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davidson PW, Leste A, Benstrong E. et al. Fish consumption, mercury exposure, and their associations with scholastic achievement in the Seychelles Child Development Study. Neurotoxicology 2010;31:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Debes F, Budtz-Jørgensen E, Weihe P. et al. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol 2006;28:536–47. [DOI] [PubMed] [Google Scholar]
- 62. Rice DC. Identification of functional domains affected by developmental exposure to methylmercury: Faroe Islands and related studies. Neurotoxicology 2000;21:1039–44. [PubMed] [Google Scholar]
- 63. Stahl LL, Snyder BD, Olsen AR. et al. Contaminants in fish tissue from US lakes and reservoirs: a national probabilistic study. Environ Monit Assess 2009;150:3–19. [DOI] [PubMed] [Google Scholar]
- 64. Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J Nutr 2007;137:2805–8. [DOI] [PubMed] [Google Scholar]
- 65. Kundakovic M, Jaric I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes (Basel) 2017;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wallace DR, Taalab YM, Heinze S. et al. Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cells 2020;9:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Raciti M, Ceccatelli S. Epigenetic mechanisms in developmental neurotoxicity. Neurotoxicol Teratol 2018;66:94–101. [DOI] [PubMed] [Google Scholar]
- 68. Culbreth M, Aschner M. Methylmercury epigenetics. Toxics 2019;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ijomone OM, Ijomone OK, Iroegbu JD. et al. Epigenetic influence of environmentally neurotoxic metals. Neurotoxicology 2020;81:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamada R, Arimura K, Osame M. Maternal-fetal mercury transport and fetal methylmercury poisoning. Met Ions Biol Syst 1997;34:405–20. [PubMed] [Google Scholar]
- 71. Martins AC, Ke T, Bowman AB. et al. New insights on mechanisms underlying methylmercury-induced and manganese-induced neurotoxicity. Curr Opin Toxicol 2021;25:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ke T, Santamaria A, Rocha JBT. et al. Cephalic neuronal vesicle formation is developmentally dependent and modified by methylmercury and sti-1 in Caenorhabditis elegans. Neurochem Res 2020;45:2939–48. [DOI] [PubMed] [Google Scholar]
- 73. Geier DA, Kern JK, Homme KG. et al. The risk of neurodevelopmental disorders following Thimerosal-containing Hib vaccine in comparison to Thimerosal-free Hib vaccine administered from 1995 to 1999 in the United States. Int J Hyg Environ Health 2018;221:677–83. [DOI] [PubMed] [Google Scholar]
- 74. Geier DA, Kern JK, Homme KG. et al. A cross-sectional study of the relationship between infant Thimerosal-containing hepatitis B vaccine exposure and attention-deficit/hyperactivity disorder. J Trace Elem Med Biol 2018;46:1–9. [DOI] [PubMed] [Google Scholar]
- 75. Skogheim TS, Weyde KVF, Engel SM. et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ Int 2021;152:106468. [DOI] [PubMed] [Google Scholar]
- 76. He B, Wang Y, Li S. et al. A cross-sectional survey of preschool children: exploring heavy metal exposure, neurotransmitters, and neurobehavioural relationships and mediation effects. Ecotoxicol Environ Saf 2021;220:112391. [DOI] [PubMed] [Google Scholar]
- 77. Sagiv SK, Thurston SW, Bellinger DC. et al. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch Pediatr Adolesc Med 2012;166:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Caito SW, Jackson BP, Punshon T. et al. Editor’s highlight: variation in methylmercury metabolism and elimination status in humans following fish consumption. Toxicol Sci 2018;161:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ríos-Hernández A, Alda JA, Farran-Codina A. et al. The Mediterranean diet and ADHD in children and adolescents. Pediatrics 2017;139:e20162027. [DOI] [PubMed] [Google Scholar]
- 80. Gapp K, Woldemichael BT, Bohacek J. et al. Epigenetic regulation in neurodevelopment and neurodegenerative diseases. Neuroscience 2014;264:99–111. [DOI] [PubMed] [Google Scholar]
- 81. Leung YK, Ouyang B, Niu L. et al. Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 2018;13:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Basu N, Goodrich JM, Head J. Ecogenetics of mercury: from genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem 2014;33:1248–58. [DOI] [PubMed] [Google Scholar]
- 83. Weyde KVF, Olsen AK, Duale N. et al. Gestational blood levels of toxic metal and essential element mixtures and associations with global DNA methylation in pregnant women and their infants. Sci Total Environ 2021;787:147621. [DOI] [PubMed] [Google Scholar]
- 84. Cardenas A, Rifas-Shiman SL, Godderis L. et al. Prenatal exposure to mercury: associations with global DNA methylation and hydroxymethylation in cord blood and in childhood. Environ Health Perspect 2017;125:087022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Appleton AA, Jackson BP, Karagas M. et al. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017;12:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dorval KM, Wigg KG, Crosbie J. et al. Association of the glutamate receptor subunit gene GRIN2B with attention-deficit/hyperactivity disorder. Genes Brain Behav 2007;6:444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nguyen HTN, Kato H, Sato H. et al. Positive effect of exogenous brain-derived neurotrophic factor on impaired neurite development and mitochondrial function in dopaminergic neurons derived from dental pulp stem cells from children with attention deficit hyperactivity disorder. Biochem Biophys Res Commun 2019;513:1048–54. [DOI] [PubMed] [Google Scholar]
- 88. van der Meer D, Hoekstra PJ, Bralten J. et al. Interplay between stress response genes associated with attention-deficit hyperactivity disorder and brain volume. Genes Brain Behav 2016;15:627–36. [DOI] [PubMed] [Google Scholar]
- 89. Maccani JZ, Koestler DC, Lester B. et al. Placental DNA methylation related to both infant toenail mercury and adverse neurobehavioral outcomes. Environ Health Perspect 2015;123:723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Neale BM, Medland S, Ripke S. et al. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010;49:906–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lozano M, Murcia M, Soler-Blasco R. et al. Exposure to mercury among 9-year-old children and neurobehavioural function. Environ Int 2021;146:106173. [DOI] [PubMed] [Google Scholar]
- 92. Nishizawa-Jotaki S, Sakurai K, Eguchi A. et al. Association between mercury in cord serum and sex-specific DNA methylation in cord tissues. J Dev Orig Health Dis 2021;12:124–31. [DOI] [PubMed] [Google Scholar]
- 93. Cardenas A, Rifas-Shiman SL, Agha G. et al. Persistent DNA methylation changes associated with prenatal mercury exposure and cognitive performance during childhood. Sci Rep 2017;7:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Etchegaray JP, Chavez L, Huang Y. et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat Cell Biol 2015;17:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bakulski KM, Lee H, Feinberg JI. et al. Prenatal mercury concentration is associated with changes in DNA methylation at TCEANC2 in newborns. Int J Epidemiol 2015;44:1249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boros FA, Török R, Vágvölgyi-Sümegi E. et al. Assessment of risk factor variants of LRRK2, MAPT, SNCA and TCEANC2 genes in Hungarian sporadic Parkinson’s disease patients. Neurosci Lett 2019;706:140–5. [DOI] [PubMed] [Google Scholar]
- 97. Cardenas A, Koestler DC, Houseman EA. et al. Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics 2015;10:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cleary BM, Romano ME, Chen CY. et al. Comparison of recreational fish consumption advisories across the USA. Curr Environ Health Rep 2021;8:71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Georgiadis P, Gavriil M, Rantakokko P. et al. DNA methylation profiling implicates exposure to PCBs in the pathogenesis of B-cell chronic lymphocytic leukemia. Environ Int 2019;126:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sanders AP, Burris HH, Just AC. et al. Altered miRNA expression in the cervix during pregnancy associated with lead and mercury exposure. Epigenomics 2015;7:885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li Q, Kappil MA, Li A. et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 2015;10:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]


