Abstract
Both mitogen-activated protein kinases and cyclin-dependent kinases play a role in hyphal development in Candida albicans. Using an oligonucleotide probe-based screen, we have isolated a new member of the Cdc2 kinase subfamily, designated Crk1 (Cdc2-related kinase). The protein sequence of Crk1 is most similar to those of Saccharomyces cerevisiae Sgv1 and human Pkl1/Cdk9. In S. cerevisiae, CRK1 suppresses some, but not all, of the defects associated with an sgv1 mutant. Deleting both copies of CRK1 in C. albicans slows growth slightly but leads to a profound defect in hyphal development under all conditions examined. crk1/crk1 mutants are impaired in the induction of hypha-specific genes and are avirulent in mice. Consistent with this, ectopic expression of the Crk1 kinase domain (CRK1N) promotes filamentous or invasive growth in S. cerevisiae and hyphal development in C. albicans. The activity of Crk1 in S. cerevisiae requires Flo8 but is independent of Ste12 and Phd1. Similarly, Crk1 promotes filamentation through a route independent of Cph1 and Efg1 in C. albicans. RAS1V13 can also activate filamentation in a cph1/cph1 efg1/efg1 double mutant. Interestingly, CRK1N produces florid hyphae in ras1/ras1 strains, while RAS1V13 generates feeble hyphae in crk1/crk1 strains.
Candida albicans is the fungus most frequently identified from clinical isolates. It can cause a variety of opportunistic infections, including deadly systemic candidiasis in immunocompromised patients (for a review, see reference 52). C. albicans is capable of dramatic morphological switching between budding yeast growth and filamentous hyphal growth. Both growth forms coexist in infected tissues. Because mutant strains defective in morphological switching are much less virulent than wild-type strains (14, 22, 35, 39), competence to perform the switch has been linked with pathogenicity in C. albicans. Hyphal cells have been suggested to aid in adhesion and penetration of epithelial or endothelial cell layers to facilitate the infection (24).
C. albicans cells are able to respond to and integrate a large variety of environmental signals during their morphological development. Serum, nitrogen starvation, high temperature, and neutral pH, for example, promote hyphal development (51). Hyphal development is also accompanied by transcriptional induction of many hypha-specific genes, such as ECE1, HWP1, and HYR1 (5, 7, 59). A conserved mitogen-activated protein (MAP) kinase pathway has been shown to regulate hyphal development; mutations in Cst20 (PAK), Hst7 (MEK), Cek1 (MAP kinase), and Cph1 (a transcription factor) partially block hyphal colony formation on certain hypha-inducing media (15, 31, 34, 36). The Cek1 MAP kinase pathway functions in parallel with Efg1, a member of a family of basic-helix-loop-helix proteins important for developmental processes in several fungi (39, 60). Efg1 may function downstream of Tpk2, the catalytic subunit of protein kinase A (PKA), in hyphal development (57). Furthermore, a C. albicans Ras protein has been shown to be required for serum-induced hyphal differentiation (20). The complicated nature of dimorphic regulation is underscored by the discovery of more signaling pathways necessary for proper hyphal development. A two-component histidine kinase, Cos1/Nik1, and a Hog1 MAP kinase are involved in hyphal morphogenesis (2, 15, 48, 49, 58). More recently, we have found a G1 cyclin-dependent kinase (Cdk) to be important for hyphal development under specific hypha-inducing conditions and for transcription of hypha-specific genes (42). Negative regulators of hyphal development have also been identified. The deletion of TUP1, which encodes a global transcriptional corepressor, causes hyperfilamentation under yeast growth conditions (9). Considering that C. albicans cells can respond to a large number of extracellular signals and growth conditions in monitoring hyphal development, they are likely to utilize many parallel signal transduction pathways to integrate these signals.
Many of the regulatory components for dimorphic switching are conserved in filamentous fungi despite their enormous diversity in size and shape and their genetic distance. For example, elements of the same conserved MAP kinase pathway involved in hyphal development in C. albicans are also required for filamentous growth in other fungi (45). In Saccharomyces cerevisiae, the switch from a unicellular yeast growth to a pseudohyphal growth upon nitrogen starvation depends on this MAP kinase pathway. Four protein kinases, Ste20, Ste11, Ste7, and Kss1, function in sequence to activate the transcriptional factors Ste12 and Tec1 (12, 37, 44, 46). Similarly, the same MAP kinase pathway is necessary for filamentous growth and virulence in the corn smut Ustilago maydis (6) and for appressorium formation and virulence in the rice fungus Magnaporthe grisea (65). Cyclic AMP (cAMP)/PKA is another conserved signal transduction pathway important for filamentous growth in several fungi (45). In S. cerevisiae, changing the level of intracellular cAMP either by an activated allele of RAS2 GTPase or by the G protein α subunit homologue Gpa2 affects the amount of filamentation (23, 32, 43). The cAMP-mediated signal transduction in filamentous growth is independent of the Kss1 MAP kinase pathway; instead it requires Flo8, a transcriptional regulatory necessary for pseudohyphal growth (38, 53, 55). The cAMP/PKA pathway is also important for filamentous growth, virulence, and mating in the human pathogen Cryptococcus neoformans (3). In addition to the MAP kinase and cAMP/PKA pathways, the Cdk Cdc28 has been shown to regulate filamentous growth in S. cerevisiae (1, 19, 41). Depending on its associated cyclins, Cdc28 plays different roles in filamentous growth (1, 19, 41).
Here we report the identification of another protein kinase in the same Cdc2 subfamily as MAP kinases and Cdks. We have designated it Crk1, for Cdc2-related kinase. Disruption of both copies of CRK1 in C. albicans leads to defective hyphal formation under all conditions examined. Furthermore, crk1/crk1 mutants fail to induce hypha-specific genes and are avirulent in mice. Consistent with mutant phenotypes, the ectopic expression of a CRK1 catalytic domain promotes the formation of hyphal colonies under conditions suited for yeast growth. Expression of the Crk1 catalytic domain in S. cerevisiae and C. albicans mutants defective in components of known filamentation signaling pathways suggested that Crk1 can activate filamentous growth via a route independent of the filamentation MAP kinase pathway and that of Phd1/Efg1. The relationship between Ras1 and Crk1 in hyphal development is also discussed.
MATERIALS AND METHODS
Strains and culture conditions.
The C. albicans and S. cerevisiae strains used in this study are listed in Tables 1 and Table 2, respectively. Yeast strains were routinely grown on YPD medium or on SD medium for selection of prototropic strains (56). Synthetic low-ammonia medium (SLAD) was used for observing pseudohyphal colony formation of S. cerevisiae (23). Invasive growth of S. cerevisiae was examined as described by Roberts and Fink (54) except that uracil-deficient synthetic complete medium (SC−Ura) was used instead of YPD medium. Transformation of S. cerevisiae was performed as described by Ito et al. (29). C. albicans strains were cultured as described previously (42). Ura− C. albicans strains were selected on 5-fluoro-orotic acid (FOA)-containing medium (8). The protoplasting method of Kurtz et al. (33) was used for C. albicans transformation. Cell and colony morphologies were photographed as described by Loeb et al. (42).
TABLE 1.
C. albicans strains used
| Strain | Genotype | Reference |
|---|---|---|
| SC5314 | CRK1/CRK1 URA3/URA3 | 21 |
| CAI4 | ura3::1 imm434/ura3::1 imm434 | 21 |
| CAW1 | crk1::hisG-URA3-hisG/CRK1 ura3::1 imm434/ura3::1 imm434 | This study |
| CAW2 | crk1::hisG/crk1 ura3::1 imm434/ura3::1 imm434 | This study |
| CAW3 | crk1::hisG/crk::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | This study |
| CAW4 | crk1::hisG/crk1::hisG ura3::1 imm434/ura3::1 imm434 | This study |
| CAW5 | crk1::hisG/crk1::hisG ura3::1 imm434/ura3::1 imm434 (pYPB1-ADHpt) | This study |
| CAW6 | crk1::hisG/crk1::hisG ura3::1 imm434/ura3::1 imm434 (pYPBCRK1) | This study |
| CAW7 | crk1::hisG/crk1::hisG ura3::1 imm434/ura3::1 imm434 (pYPBCRK1N) | This study |
| JKC97 | cst20::hisG/cst20::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | 31 |
| JKC129 | hst7::hisG/hst7::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | 31 |
| JKC19 | cph1::hisG/cph1::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | 36 |
| HLC52 | efg1::hisG/efg1::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | 39 |
| HLC54 | cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG-URA3-hisG ura3::1 imm434/ura3::1 imm434 | 39 |
| Ras1-2/ras1-3 | ras1Δ::hisG/ras1Δ::hph-URA3-hph ura3::1 imm434/ura3::1 imm434 | 20 |
TABLE 2.
S. cerevisiae strains used
| Strain | Genotype | Reference or source |
|---|---|---|
| KMG58-7Aa | MATa sgv1 ura3 leu2 trp1 | 28 |
| YPH499a | MATa ura3-53 lys2-801 ade2-101 trp1-63 his3-200 leu2-1 cir+ | 64 |
| L5528 | MATa ura3-52 his3::hisG | 37 |
| HLY367 | MATa ste7::LEU2 ura3-52 leu2::hisG | 37 |
| HLY362 | MATa ste12::LEU2 ura3-52 leu2::hisG | 37 |
| HLY2000 | MATa tec1::HIS3 ura3-52 | Derived from L6149 |
| 44 | ||
| HLY850 | MATa flo8::hisG ura3-52 | 38 |
| CG31 | MATa/α ura3-52/ura3-52 | 23 |
| HLY351 | MATa/α ste7::LEU2/ste7::LEU2 ura3-52/ura3-52 leu2::hisG/leu2::hisG | 37 |
| HLY352 | MATa/α ste12::LEU2/ste12::LEU2 ura3-52/ura3-52 leu2::hisG/leu2::hisG | 37 |
| HLY2002 | MATa/α tec1::HIS3/tec1::HIS3 ura3-52/ura3-52 | Derived from L6149 |
| 44 | ||
| HLY852 | MATa/α flo8::hisG/flo8::hisG ura3-52/ura3-52 | 38 |
| L6235 | MATa/α his3::hisG/his3::hisG leu2::hisG/leu2::hisG ura3-52/ura3-52 phd1::hisG-URA3-hisG/ phd1::hisG-URA3-hisG ste12::LEU2/ste12::LEU2 (pRS314, B2552) | 39 |
| L6235b | MATa/α phd1::hisG/phd1::hisG ste12::LEU2/ste12::LEU2 ura3-52/ura3-52 | 39 |
Not Σ strain.
Cloning and sequencing of CRK1.
Two oligonucleotides, 5′AAAATTTGTGAC(or T)TTTGGTTTA and 5′TCTTGCTAAACCA, were synthesized according to a nucleotide sequence that encodes KICDFGLAR, a conserved region in the subdomain VII of Cdc2-related protein kinases. The two oligonucleotides were annealed to each other, and the two ends were labeled by blunt-end filling in with Klenow enzyme in the presence of [α-32P]dATP (Amersham). The oligonucleotide was used as a hybridization probe to screen a C. albicans genomic library inserted in λGEM12 phage (Promega) (10). The hybridization was performed at 40°C in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1× Denhart solution–100 μg of yeast tRNA/ml–0.05% sodium pyrophosphate. The membrane was washed with 6× SSC at room temperature. Sixty-five positive λ plaques were isolated. The recombinant λ DNA was digested with restriction enzymes and analyzed by Southern hybridization using the same oligonucleotide probe. The 65 λ phage clones were classified into 13 groups. Two groups had restriction patterns of the known MAP kinase genes CEK1 and MKC1 (50, 63). The inserts from the other putative λ phage clones were released by digestion with BamHI and cloned into the BamHI site of pBSK (Stratagene). Two clones, pBSZS1 and pBSZS2, had much stronger hybridization signals than the others and were analyzed in detail. pBSZS2 was found to contain a gene for a new MAP kinase (J. Chen et al., unpublished data). A 9-kb BamHI fragment in pBSZS1 was digested with EcoRI, PstI, and Bal31 and then subcloned into pBSK for sequence analysis. Nucleotide sequences of the DNA fragment that hybridized to the probe were determined by the dideoxy-chain termination method using Sequenase (U.S. Biochemical) and [α-35S]dATP (Amersham). Protein sequence comparisons were conducted by using the BLAST algorithm of Altschul et al. (4). Plasmid pBSZS1 contained a DNA sequence with a 2,241-bp open reading frame, corresponding to a protein of 746 amino acids, designated Crk1.
Plasmid and C. albicans strain construction.
A 4-kb SacI fragment containing the entire coding region of CRK1 was subcloned from pBSZS1 into the SacI site of a pUC19 vector, generating plasmid pUC19CRK1 (Table 3). The internal 2-kb EcoRV-XhoI fragment in plasmid pUC19CRK1 was replaced with a 4.8-kb SalI-ScaI hisG-URA3-hisG fragment from plasmid pCUB6 (21) (see Fig. 3A), generating plasmid pUC19CRK1URA3. SacI-digested pUC19CRK1URA3 DNA (see Fig. 3A) was used to transform Candida ura3/ura3 strain CAI4 (21) to produce CRK1/crk1 and crk1/crk1 strains (Table 1).
TABLE 3.
Plasmids used
| Plasmid | Description | Reference |
|---|---|---|
| pYPB1-ADHpt | C. albicans ADH1 promoter and ADH1 terminator on a C. albicans URA3/ARS/2μm vector | 13 |
| pYPBCRK1 | 2.25-kb full-length CRK1 in pYPB1-ADHpt | This study |
| pYPBCRK1N | 1.1-kb Crk1 kinase domains CRK1N in pYPB1-ADHpt | This study |
| BES119CRK1N | 1.1-kb CRK1N in BES119 | 20, this study |
| PQF145.2 | RAS1V13 in BES119 | 20 |
| pVT102U | S. cerevisiae ADH1 promoter and S. cerevisiae ADH1 terminator in S. cerevisiae URA3/2μm vector | 62 |
| pVTUCRK1 | Full-length CRK1 in pVT102U | This study |
| pVTUCRK1N | Crk1 kinase domains CRK1N in pVT102U | This study |
| pVTUHACRK1 | CRK1-HA in pVT102U | This study |
| pVTUHACRK1N | CRK1N-HA in pVT102U | This study |
| pVTUSGV1 | Full-length SGV1 in pVT102U | This study |
| pVTUSGV1N | Sgv1 kinase domains SGV1N in pVT102U | This study |
| pUC19CRK1 | 4-kb SacI CRK1 genomic fragment in pUC19 | This study |
| pUC19CRK1URA3 | crk1::hisG-URA3-hisG in pUC19 | This study |
| pBSKZS1 | 9-kb BamHI CRK1 genomic fragment in pBSK | This study |
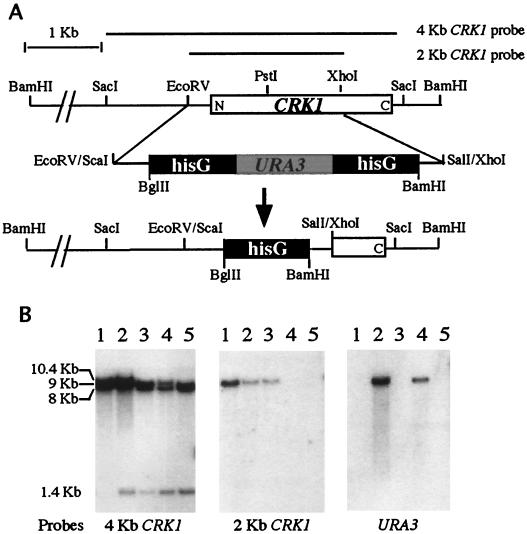
FIG. 3.
Disruption of the C. albicans CRK1 gene. (A) Restriction map and disruption strategy for CRK1. (B) Southern analysis of transformants with the CRK1 disruption construct. Genomic DNA from the recipient strain (lane 1, CAI4), a heterozygote transformant (lane 2, CAW1), an FOAr/ura3 derivative of CAW1 (lane 3, CAW2), a homozygote transformant (lane 4, CAW3), and an FOAr/ura3 derivative of CAW3 (lane 5, CAW4) were digested with BamHI. The Southern blot on the left was probed with the 4-kb SacI fragment shown in panel A. The BamHI site in the hisG-URA3-hisG sequence generated two new hybridization fragments of 10.4 and 1.4 kb from the original 9-kb wild-type BamHI fragment. The 10.4-kb crk1::hisG-URA3-hisG fragment became an 8-kb crk1::hisG fragment after selection on an FOA plate to loop out the URA3 and one copy of hisG. This size difference between crk1::hisG and crk1::hisG-URA3-hisG is evident in lane 4, where the doublet represents fragments of 10.4 and 8 kb, respectively. The Southern blot in the middle was probed with the 2-kb EcoRV-XhoI fragment shown in panel A. The EcoRV-XhoI region was replaced with the hisG-URA3-hisG sequence in the deletion construct. Therefore, the 2-kb probe is expected to hybridize only to the 9-kb BamHI fragment from the wild-type CRK1 locus. Homozygous crk1/crk1 mutants do not contain the 9-kb BamHI fragment. The Southern blot on the right was probed with C. albicans URA3.
For complementation and ectopic expression in S. cerevisiae and C. albicans, several plasmids carrying the CRK1 or SGV1 gene under regulation of the ADH1 promoter were constructed (Table 3). Full-length CRK1 and CRK1N (truncated CRK1, encoding just the 11 kinase domains of Crk1) were generated by PCR. The primers used for synthesis of CRK1 and CRK1N were 5′GTCGGATCCAT GTCTGTTATTGCTGGCCAT, 5′GCTAAGCTTACATAGATTTGTGTCC, and 5′GCTAAGCTTTATCAATTTCGTGAC. CRK1 and CRK1N PCR products were digested with BamHI and HindIII and cloned into the BamHI-HindIII site of pVT102U (URA3, 2μm) (62), generating S. cerevisiae expression plasmids pVTUCRK1 and pVTUCRK1N, respectively. CRK1 and CRK1N PCR products were also cloned into the EcoRV site of plasmid pYPB1-ADHpt (C. albicans URA3 and ARS) (13), generating C. albicans expression plasmids pYPBCRK1 and pYPBCRK1N.
For the kinase assay, a synthetic linker containing a hemagglutinin (HA) coding sequence was fused in frame to the N terminus of CRK1 and CRK1N in plasmids pVTUCRK1 and pVTUCRK1N, generating plasmids pVTUHACRK1 and pVTUHACRK1N, respectively. The linker sequence was 5′GAGCTCATGGCTTACCCATACGATGTTCCAGATTACGCTAGCGGATCCATG. Full-length SGV1 and SGV1N, encoding just the kinase domain, were generated by PCR. The primers used for synthesis of SGV1 and SGV1N were 5′GTCGGATCCATGAGTGATAATGGTTCCCCC, 5′CTGGAGCTCTTAATATCAGCTTCA, and 5′CTGGAGCTCCGTAATTAGCCACGAGGC. SGV1 and SGV1N PCR products were digested with BamHI and SacI and then cloned into the BamHI-SacI site of pVT102U, generating expression plasmids pVTUSGV1 and pVTUSGV1N, respectively. The BamHI-HindIII CRK1N fragment from pVTUCRK1N was inserted into BES119 (20) to generate plasmid BES119CRK1N.
Southern and Northern analyses.
Methods for DNA isolation and Southern blot hybridization were as previously described (11). Total RNA extraction and Northern blot hybridization were performed as described in Current Protocols in Molecular Biology (26). DNA probes were labeled with the Bethesda Research Laboratories random-primer labeling kit and [α-32P]dATP (Amersham); 4-kb SacI and 2-kb EcoRV-XhoI CRK1 fragments from pUC19CRK1, a 1.4-kb XbaI-ScaI URA3 fragment from pUR3 (30), and a ClaI-SalI ACT1 fragment from plasmid p1595/3 (18) were used as probes. C. albicans ECE1 and HWP1 PCR products were used for probing Northern blots. The primers used were 5′GCCATCCACCATGCTCC and 5′GTGCTACTGAGCCGGCATCTC for ECE1 and 5′TGCTCCAGGTACTGAATCCGC and 5′GGCAGATGGTTGCATGAGTGG for HWP1. The 2-kb CRK1 fragment (see Fig. 3) was used as a probe in Northern hybridization. The sizes of mRNAs on Northern blots correlated with the expected lengths based on information from the C. albicans genome database.
Kinase assays.
For kinase assays, plasmids pVTUHACRK1 and pVTUHACRK1N were introduced into YPH499. Extract preparation, immunoprecipitation, and kinase assays of immune complexes were performed as described elsewhere (61, 64). For each immunoprecipitation, 2.5 mg of protein total extract was used, with 1 μg of myelin basic protein (MBP; Sigma) or histone H1 protein (Sigma) as a substrate.
Bioassay for response to α-factor.
A halo bioassay was performed as described by Irie et al. (28). In short, 0.1 ml of overnight culture (107 cells) was mixed with 5 ml of 0.7% soft agar and spread onto a YPD plate. Whatman paper disks (6 mm in diameter) were placed on the nascent lawn. Different quantities (0, 0.5, and 5 μg) of synthetic α-factor (Sigma) were dotted onto each disk in 5-μl aliquots. Photographs were taken after 48 h.
Virulence studies.
The virulence of C. albicans strains was tested as described by De Bernardis et al. (16). C. albicans strains were grown on SD−Ura plates for 48 h at 30°C. The cells were suspended in physiological saline solution and counted in a hemacytometer. Following quantitation, cells were adjusted to densities of 5 × 107 and 5 × 106 cells/ml. Each C. albicans strain was tested for virulence by injecting 0.1 ml of cells (5 × 106 and 5 × 105 cells) into the lateral tail veins of ICR male mice (18 to 21 g each; Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China). Eight mice were injected for each strain. Surviving mice were observed daily after infection with C. albicans.
Nucleotide sequence accession number.
The GenBank accession number for the CRK1 nucleotide sequence is U92261.
RESULTS
Cloning of protein kinase gene CRK1.
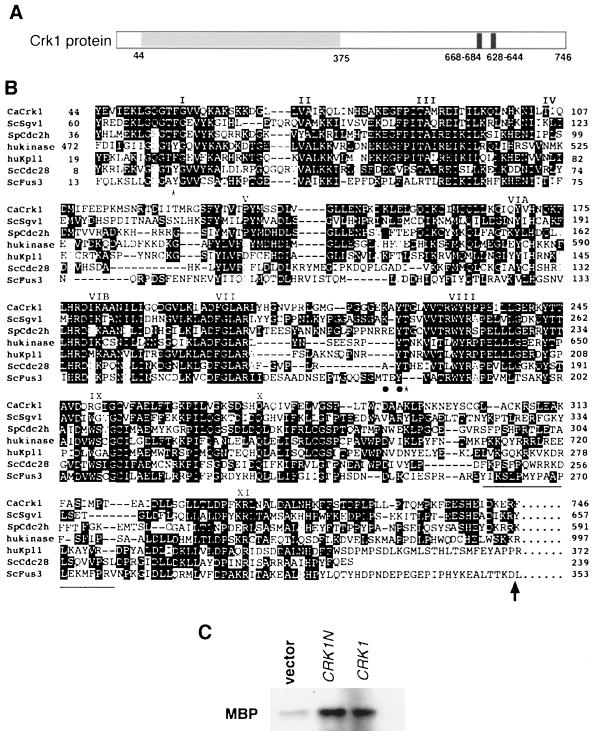
We used an oligonucleotide probe-based screen to clone putative protein kinases in the Cdc28/Cdc2 subfamily from a C. albicans genomic library. The oligonucleotide sequence was designed from a region in the kinase subdomain VII, which is conserved among all MAP kinases and Cdks (Fig. 1B; Materials and Methods). Four putative kinase genes were cloned: one for a new MAP kinase (Chen et al., unpublished); two known MAP kinase genes, CEK1 and MKC1 (50, 63); and a novel gene that encodes a 746-amino-acid predicted protein (Fig. 1). The amino-terminal half of the coding sequence contains all 11 kinase catalytic domains that are highly conserved among members of the Cdc2 subfamily (Fig. 1B) (27). It shares the highest similarity with S. cerevisiae Sgv1 (28) (47% identical and 63% similar). A Schizosaccharomyces pombe Cdc2-like gene ranked second in our BLAST search, with 46% identity and 61% similarity. Two human kinases, a sequence of cDNA isolated from brain tissue and a PITALRE kinase (Pk11/Cdk9) (17, 25), also gave comparable scores in the search. In addition, the C. albicans kinases have an insertion common to all members of the Cdc2 branch of the kinase family (27) (Fig. 1B, underlined region between X and XI). Thus, we designated the C. albicans protein Crk1, for Cdc2-related kinase. However, Crk1 lacks some of the conserved residues that are known to be important for Cdk functions (47), including the highly conserved regulatory residue Tyr15 (Fig. 1B, Tyr19 for Cdc28), whose phosphorylation state modulates the kinase activity, and the conserved PSTAIRE sequence (Fig. 1B, domain III) important for interacting with the cell cycle-regulated cyclins. It also lacks the conserved MEK phosphorylation site TXY at the L12 region between subdomains VII and VIII of all MAP kinases (66). Very few similarities outside the kinase domains exist between Crk1 and the other four kinases, except for a short sequence immediately following subdomain XI which shows significant similarity between Crk1 and Sgv1 (Fig. 1B).
FIG. 1.
Comparison of the Crk1 sequence to sequences of other Cdc2-related kinases. (A) Diagram of predicted functional domains in Crk1. The shaded region contains the conserved kinase domain, as shown in panel B. Potential nuclear localization sequences (based on the PSORT program) near the carboxyl terminus are also indicated. (B) Sequence alignment of C. albicans Crk1 (CaCrk1) kinase domains with those of S. cerevisiae Sgv1 (ScSgv1), an S. pombe Cdc2 homologue (SpCdc2h; accession no. AB004534), a human Ser/Thr kinase (hukinase; accession no. AB020711), the human PITALRE kinase HuKp11/Cdk9, S. cerevisiae Cdc28, and S. cerevisiae Fus3. Subdomains are labeled according to Hanks et al. (27). Shaded residues represent identities among these kinases. Conserved phosphorylation sites in Cdc28 and Fus3 are indicated with asterisks and dots, respectively. Underlined sequences denote the insertion unique for the Cdc2 branch of the kinases. The arrow indicates the ending position of Crk1N and Sgv1N. (C) Kinase activity associated with Crk1 and Crk1N immunocomplexes. Yeast cell extracts were immunoprecipitated with anti-HA antibodies. Immunocomplexes were assayed for the ability to phosphorylate MBP (2-h exposure).
We tagged full-length Crk1 and the Crk1 catalytic domain (designated Crk1N) with the HA epitope and expressed both in S. cerevisiae Crk1 and Crk1N proteins were then precipitated with anti-HA antibodies and protein A-conjugated agarose beads. Protein kinase activity was assayed with either histone H1 or MBP as the substrate. MBP was phosphorylated by both Crk1 and Crk1N immunocomplexes, whereas the control showed a significantly reduced level of MBP phosphorylation (Fig. 1C). Histone H1, however, was not phosphorylated by either Crk1 or Crk1N immunocomplexes (data not shown). The human PITAIRE kinase Kpl1/Cdk9 also prefers MBP to histone H1 as the substrate in in vitro assays (25). The result of our immunoprecipitation kinase assay is consistent with the deduced protein sequence of Crk1 being a protein kinase.
CRK1 suppresses the hypersensitive pheromone-induced growth arrest phenotype of S. cerevisiae sgv1 mutants.
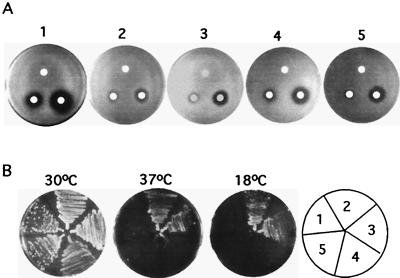
As shown in Fig. 1, Crk1 is most similar to S. cerevisiae Sgv1 in protein sequence. SGV1 was isolated in a mutant screen for suppressors that could repress hyperadaptation from pheromone-induced growth arrest in GPA1Val50 cells (28). GPA1 encodes the α subunit of the G protein for pheromone receptors in S. cerevisiae. It also plays a positive role in promoting recovery from pheromone-induced growth arrest. The effect of sgv1 on recovery from pheromone treatment is not specific to the GPA1Val50 mutation, as sgv1 mutants in otherwise wild-type strains are more sensitive to pheromone-induced growth arrest (28). The same sgv1 mutation also causes temperature-sensitive and cold-sensitive growth phenotypes, consistent with the fact that SGV1 is essential for vegetative growth (28).
To test whether CRK1 can complement S. cerevisiae sgv1 mutants, CRK1 and CRK1N were cloned into an S. cerevisiae expression vector under the regulation of a constitutive ADH1 promoter. The constructs were transformed into a MATa sgv1 strain to test whether CRK1 could complement sgv1. sgv1 mutants were hypersensitive to pheromone and produced a large halo ring around the disks containing α-factor as observed previously (28). The hypersensitive growth arrest by pheromone in sgv1 mutants was complemented by expression of either SGV1 or SGV1N (Fig. 2A). Similarly, both CRK1 and CRK1N were able to partially suppress the hypersensitive pheromone-induced growth arrest phenotype in sgv1 mutants, based on results of the halo assay (Fig. 2A). However, neither CRK1 nor CRK1N suppressed the temperature-sensitive or cold-sensitive growth defect of sgv1 (Fig. 2B).
FIG. 2.
Suppression of S. cerevisiae sgv1 mutants by C. albicans Crk1. (A) Pheromone-induced growth arrest assay. Haploid S. cerevisiae sgv1 mutants were transformed with vector (pVTU) (1), SGV1 (pVTUSGV1) (2), SGV1N (pVTUSGV1N) (3), CRK1 (pVTUCRK1) (4), and CRK1N (pVYUCRK1N) (5). Approximately 107 cells were plated on each YPD plate. Sterile filter disks were placed on the nascent cell lawns; α-factor in the amounts of 0 ng (top), 50 ng (left), and 500 ng (right) was added to the disks. Plates were incubated for 2 days at 30°C. (B) Effect of temperature on growth. The strains used for panel B were used to test growth properties at 37 and 18°C. Cells were streaked onto SC−Ura plates, which were incubated for 5 days at 30°C, 7 days at 37°C, and 7 days at 18°C.
Chromosomal deletion of CRK1 in C. albicans.
To elucidate cellular functions of CRK1, we deleted CRK1 in C. albicans. Part of the CRK1 coding region was replaced by URA3 with two flanking sequences of hisG for gene deletion by homologous recombination (Fig. 3A). A sequential gene disruption strategy was used to delete both copies of CRK1 in C. albicans as described by Fonzi and Irwin (21). Of 185 transformants from the first round of transformation, 90% had the hisG-URA3-hisG insertion at the CRK1 locus, based on Southern hybridizations (Fig. 3B, lane 2). The pattern of Southern hybridization with the 4-kb CRK1 probe is consistent with integration of the crk1::hisG-URA3-hisG construct at the CRK1 locus. After growth selection on FOA to remove the URA3 marker, the second copy of CRK1 was deleted by another round of transformation with the same crk1::hisG-URA3-hisG construct; 16 out of 78 transformants displayed the homologous recombination at the second copy of the CRK1. This was determined by the loss of the wild-type CRK1 gene shown by Southern hybridization (Fig. 3B, middle, lane 3).
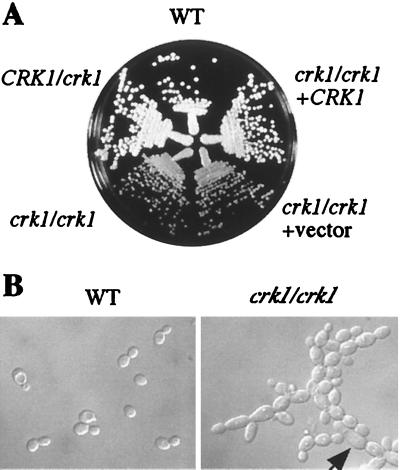
The ability to obtain homozygous crk1/crk1 mutants suggests that Crk1 is not essential for cell viability. However, we observed that all crk1/crk1 homozygous mutants grew slightly slower than the wild-type parental strain. The wild-type strain had a doubling time of 1.5 h in YPD at 30°C, while the crk1/crk1 strains required 2.2 h for each doubling under the same conditions. On solid YPD medium, crk1/crk1 mutant strains produced slightly smaller wild-type colonies, than and the difference in colony size was more evident at 22°C (Fig. 4A). The low growth rate was caused by the CRK1 deletion, as the phenotype was reversed by reintroducing wild-type CRK1 on an autonomous replicating plasmid into the crk1/crk1 strain (Fig. 4A).
FIG. 4.
Effects of CRK1 disruption on cell growth. (A) crk1/crk1 strains grow slower than wild type. Wild-type (WT; SC5314), CRK1/crk1 (CAW1), crk1/crk1 (CAW3), crk1/crk1 carrying a vector (CAW5), and crk1/crk1 carrying CRK1 (CAW6) were grown on a YPD plate for 5 days at 22°C. (B) Comparison of cell morphologies. Wild-type (SC5314) and crk1/crk1 (CAW3) cells were grown in YPD medium at 22°C for 15 h and photographed.
Deletion of both copies of CRK1 also had a subtle effect on cell morphology. crk1/crk1 cells are larger than wild-type cells (Fig. 4B), a phenotype similar to that of the S. cerevisiae sgv1 mutant at the restrictive temperature (28). In addition, crk1/crk1 cells tend to form chains whereas wild-type cells detach after cytokinesis (Fig. 4B). The phenotypes of cell morphology and incomplete cell-cell separation were enhanced at 22°C. About 4% of crk1/crk1 cells showed an abnormally elongated morphology (Fig. 4B). The morphological defect of crk1/crk1 was reversed by retransformation with the wild-type CRK1 gene (data not shown).
Crk1 is necessary for hyphal development.
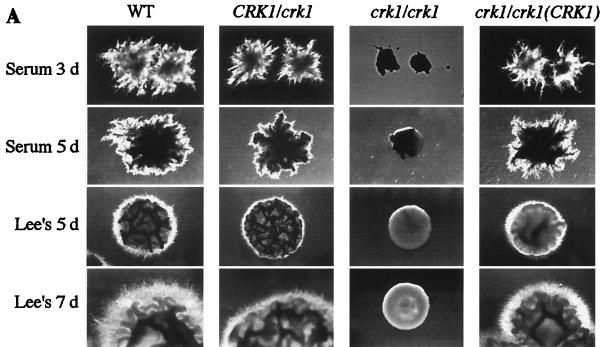
Deletion of CRK1 caused a profound defect in hyphal development on all solid hypha-inducing media tested (Fig. 5A). On serum-containing agar medium, crk1/crk1 strains produced mostly round cells, with a very low percentage of stunted hyphal cells in the initial hours, whereas wild-type strains produced long hyphae. After 3 days of incubation, the wild-type strains generated florid hyphal colonies, whereas crk1/crk1 strains produced round colonies (Fig. 5A, top row). The colonies remained round even after a longer incubation time (Fig. 5A). The heterozygous strain CRK1/crk1 produced intermediate hyphal colonies (Fig. 5A). The defective hyphal growth was most likely caused by the CRK1 deletion, since reintroducing a wild-type CRK1 gene on an autonomous replicating plasmid rescued the mutant phenotype (Fig. 5A). On solid Lee's medium, crk1/crk1 strains also failed to develop hyphal colonies after 5 days (Fig. 5A, third row). On Lee's medium, the wild-type strain produced highly filamentous hyphal colonies and the heterozygous strain made intermediate hyphal colonies. We consistently observed that the defect in hyphal development persisted after 7 days. crk1/crk1 strains were also defective in developing hyphal colonies on all other solid media that we tried, including Spider medium and SLAD medium (data not shown).
FIG. 5.
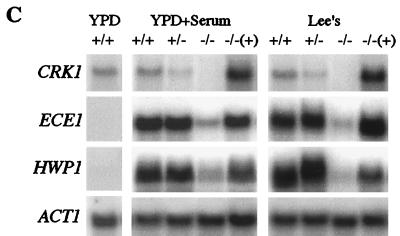
crk1/crk1 strains are defective in hyphal development. (A) crk1/crk1 strains cannot develop hyphal colonies. Ura+ strains, including wild type (WT; SC5314), CRK1/crk1 (CAW1), crk1/crk1 (CAW3), and crk1/crk1 carrying ectopically expressed CRK1 (CAW6), were plated at a density of about 50 colonies per plate on solid serum-containing medium and solid Lee's medium. Colonies were photographed after incubation at 37°C for the days (d) indicated. (B) crk1/crk1 strains are impaired in hyphal filament formation in liquid media. Overnight cultures of the four Ura+ strains used for panel A were diluted in YPD medium containing 10% serum or modified Lee's medium for hyphal induction. Cells were photographed after incubation at 37°C for the time indicated. (C) crk1/crk1 mutants are defective in induction of hypha-specific genes. RNA from wild-type cells grown in YPD at 30°C for 6 h was used as a control for gene expression in the yeast growth form (left). RNA from cells of the same Ura+ strains induced for 3.5 h by serum (panel B, top row) and induced for 6 h in Lee's medium (panel B, middle row) were subjected to Northern analysis, as shown in panel C, middle and right gels, respectively. Northern probes are as indicated. The image for the CRK1 Northern blot was obtained after 3 days of exposure. Images for ECE1-, HWP1-, and ACT1-probed filters were obtained after 3 h of exposure. Transcript levels were quantified with a PhosphorImager.
The defect in hyphal filament formation associated with crk1/crk1 mutants was also observed in all liquid media examined. Serum, in combination with a temperature shift to 37°C, is one of the most effective hypha-inducing conditions and therefore tends to be a more stringent test for cell elongation than growth on solid medium. Wild-type cells form germ tubes within 1 h of incubation (not shown). Longer hyphae are usually observed after 3.5 h (Fig. 5B). crk1/crk1 strains, on the other hand, generated a mixture of mostly round yeast cells and some short pseudohypha-like cells, as well as a limited number of hyphal cells (about 5%) (Fig. 5B, first row). The homozygous strain was impaired in serum-induced hyphal development regardless of the duration of induction. crk1/crk1 mutants were also defective in hyphal formation in Lee's medium at pH 7. After 6 h of incubation in Lee's medium, the wild type made long hyphal cells whereas the crk1/crk1 mutants made mostly round cells mixed with occasional long cells (about 5%) (Fig. 5B, second row). Wild-type strains produced mycelia after 15 h of growth, whereas the crk1/crk1 strain generated clusters of mostly round cells, with some hyphal cells surrounding the clusters (Fig. 5B, third row). These hyphal cells produced in Lee's medium were different from the long crk1/crk1 cells seen in YPD at 22°C (Fig. 4B) in that hyphal cells were longer and thinner. Furthermore, hypha-specific transcripts were undetectable by Northern blotting in crk1/crk1 cells under yeast growth conditions (not shown).
The existence of occasional long hyphal cells in crk1/crk1 strains suggested that Crk1 might not be directly responsible for the polarization of actin cytoskeleton. Rather, it might be involved in the transcriptional regulation of hypha-specific genes necessary for filamentation and cell elongation. Therefore, we examined the ability of Crk1 to induce transcription of the hypha-specific genes ECE1 (extent of cell elongation) and HWP1 (hyphal wall protein) by Northern analysis. Expression of the ECE1 transcript has been found to directly correlate with the extent of cell elongation regardless of the conditions or media used for hyphal induction (5, 7, 59), making it a suitable marker for this study. Overnight cultures were diluted into YPD plus 10% serum at 37°C or Lee's medium at 37°C for hyphal induction. ECE1 expression was dramatically induced in wild-type cells after 3 h in serum or 6 h in Lee's medium (Fig. 5C). The ECE1 transcript was equally induced in the heterozygous mutant and the wild-type strain (Fig. 5C). However, ECE1 expression in both hyphal induction conditions was severely reduced in the crk1/crk1 strains. Compared to the wild type, the level of ECE1 expression in the crk1/crk1 mutant was 7-fold lower in YPD-serum medium and 10-fold lower in Lee's medium (Fig. 5C). The defect in transcriptional induction of hypha-specific genes was not limited to ECE1. The expression of HWP1, which encodes a hyphal wall protein (5, 7, 59), was similarly affected in the crk1/crk1 strain. The level of HWP1 in the crk1/crk1 strain was 8-fold lower than that in wild-type cells in YPD serum medium and 12-fold lower than wild-type transcription in Lee's medium. Therefore, Crk1 is required for normal induction of the hypha-specific transcriptional program.
The defect in hyphal development observed in the crk1/crk1 mutant was caused by deleting CRK1. Introducing a wild-type CRK1 into the crk1/crk1 mutant restored its competence in producing hyphal colonies (Fig. 5A) and hyphal filaments in liquid hypha-inducing media (Fig. 5B). The CRK1 gene also complemented the defect in the hypha-specific transcriptional program, as both ECE1 and HWP were expressed in the CRK1-transformed crk1/crk1 strains (Fig. 5C).
crk1/crk1 is avirulent in mice.
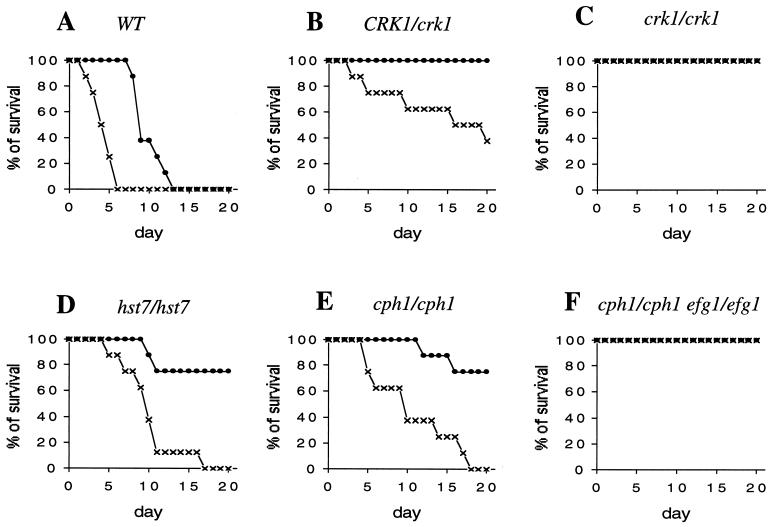
The dimorphic transition ability of C. albicans has been linked with its pathogenicity in mice (22, 35, 39). Here we assessed the virulence of crk1/crk1 mutant strains by the intravenous injection of mice (Materials and Methods). Injection of mice with wild-type C. albicans cells is fatal. Injection with an inoculum of 5 × 106 cells caused all mice to die in 6 days, and a smaller inoculum of 5 × 105 cells killed all mice in 13 days (Fig. 6A). The heterozygotic CRK1/crk1 mutant strain was less virulent than the wild-type strain despite being capable of hyphal development (Fig. 5A and B). All mice survived for over 20 days after injection with 5 × 105 cells, and 60% survived after 10 days with an inoculum of 5 × 106 cells (Fig. 6B). The crk1/crk1 cells were avirulent at both inoculum sizes: all mice survived for more than 20 days after injection with 5 × 106 or 5 × 105 crk1/crk1 cells (Fig. 6C). This is comparable to the virulence level of the cph1/cph1 efg1/efg1 double mutant (Fig. 6F), which has been previously shown to be avirulent with a similar inoculum size (39). We also used cph1/cph1 and hst7/hst7 single mutants as controls in our experiments. The two strains showed similar survival curves and were slightly less virulent than the wild type (Fig. 6E and D) (39). In comparison, the heterozygotic CRK1/crk1 mutant was less virulent than either cph1/cph1 or hst7/hst7 strains.
FIG. 6.
Virulence assay. ICR male mice were injected with wild-type (WT; SC5314; A), CRK1/crk1 (CAW1; B), crk1/crk1 (CAW3; C), hst7/hst7 (JKC129; D), cph1/cph1 (JKC19; E), and cph1/cph1 efg1/efg1 (HLC54; F) strains. The mice were injected with 5 × 105 (●) and 5 × 106 (×) C. albicans cells. Mice injected with either crk1/crk1 cells or cph1/cph1 efg1/efg1 cells all survived for more than 20 days.
CRK1N promotes invasive or filamentous growth in S. cerevisiae through Flo8 but not through the filamentation MAP kinase pathway or Phd1.
Many of the regulatory components of dimorphic transition are conserved between C. albicans and S. cerevisiae. Furthermore, several C. albicans hyphal regulatory proteins were identified by their ability to promote pseudohyphal growth in S. cerevisiae (36, 60). Therefore, we decided to use S. cerevisiae as an initial step to investigate potential regulatory targets of Crk1.
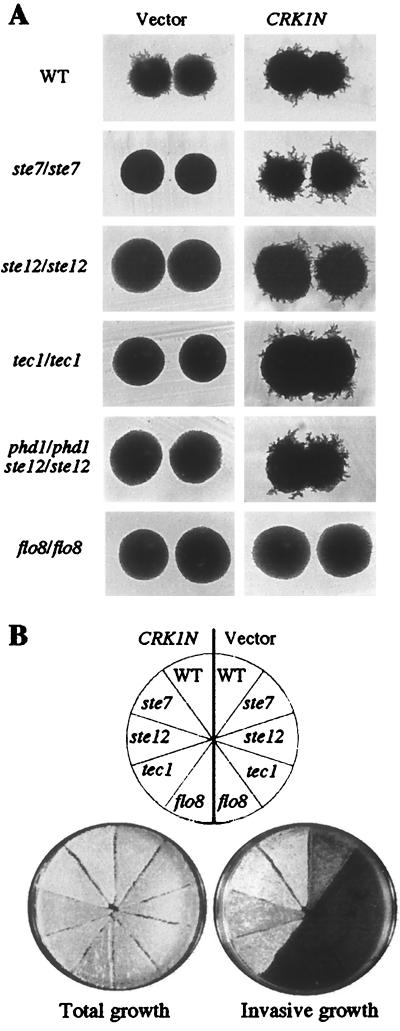
Ectopic expression of the Crk1 catalytic domain (CRK1N) in S. cerevisiae promoted pseudohyphal growth in diploids (Fig. 7A and Table 3). In addition, it enhanced invasive growth in haploid S. cerevisiae (Fig. 7B), a phenomenon that shares many features and regulatory components with pseudohyphal growth. Ectopic expression of full-length Crk1 did not alter the level of filamentation (Table 3) or invasive growth (not shown), suggesting that the noncatalytic domain is potentially inhibitory to Crk1 activity, at least in S. cerevisiae. Nevertheless, ectopic expression of CRK1N in S. cerevisiae mutations in components of known signaling pathways could be used to dissect the pathway with which Crk1 is associated.
FIG. 7.
CRK1N stimulated filamentous and invasive growth in S. cerevisiae. (A) Colony morphologies of isogenic wild-type (WT; CG31), ste7/ste7 (HLY351), ste12/ste12 (HLY352), tec1/tec1 (HLY2002), phd1/phd1 ste12/ste12 (L6235), and flo8/flo8 (HLY852) strains carrying vector (left) or CRK1N (right) grown on SLAD at 30°C for 4 days. (B) Total and invasive growth of wild-type (L5528), ste7 (HLY367), ste12 (HLY362), tec1 (HLY2000), and flo8 (HLY850) strains carrying a vector (left) or CRK1N (right) after 5 days of growth on SC−Ura.
One of the pathways for invasive or filamentous growth in S. cerevisiae is the Kss1-mediated MAP kinase pathway (37, 44). Stimulation of the filamentation MAP kinase pathway is achieved through Ste7, which activates the MAP kinase Kss1, thereby eliminating the inhibitory activity of Kss1 on the transcriptional factor Ste12, leading to the activation of Ste12 (44), which in turn is necessary for the pseudohyphal transcriptional program. Ectopic expression of CRK1N bypassed this requirement for the MAP kinase pathway in both filamentous and invasive growth (Fig. 7). CRK1N promoted filamentation in diploid ste7/ste7, ste12/ste12, and tec1/tec1 strains under nitrogen starvation conditions (Fig. 7A). Quantification of the percentage of pseudohyphal colonies in various strains showed that CRK1N increased the magnitude of filamentation as well as the percentage of pseudohyphal colonies (Table 4). CRK1N also suppressed the defect in invasive growth in haploid ste7, ste12, and tec1 mutants (Fig. 7B). S. cerevisiae PHD1 has been suggested to function in a pathway parallel to Ste12 during pseudohyphal growth (39). We found that CRK1N bypassed the requirement for filamentous growth in a ste12/ste12 phd1/phd1 double mutant. Our result suggests that Crk1 activates filamentous growth via a third pathway, independent of the Phd1 and the MAP kinase pathways.
TABLE 4.
Filament formation on SLAD medium
| Plasmid | Filament formationa
|
|||||
|---|---|---|---|---|---|---|
| CG31 (WT) | HLY351 (ste7/ste7) | HLY352 (ste12/ste12) | HLY2002 (tec1/tec1) | HLY852 (flo8/flo8) | HLY1873b (phd1/phd1 ste12/ste12) | |
| Vector | +++ (80) | + (10) | + (10) | ++ (10) | + (50) | |
| − (20) | − (90) | − (100) | − (90) | − (90) | − (50) | |
| CRK1 | +++ (80) | + (20) | + (10) | ++ (10) | ++ (50) | |
| − (20) | − (80) | − (100) | − (90) | − (90) | + (50) | |
| CRK1N | +++++ (80) | +++++ (50) | ++++ (40) | +++++ (50) | ++ (10) | +++++ (50) |
| − (20) | + (50) | + (60) | + (50) | − (90) | + (50) | |
−, smooth colonies; +, filamentous colonies. The level of cell elongation is indicated by the number of +'s. The percentage of filamentous or smooth colonies in total colonies counted is given in parentheses.
The cAMP PKA pathway is another pathway implicated in filamentous or invasive growth. Increasing the level of intracellular cAMP promotes filamentous growth (53, 55). Function of the cAMP pathway in filamentous or invasive growth requires the transcriptional regulator Flo8 (53, 55), which is necessary for both invasive growth and filamentous growth (38). We found that mutations in FLO8 blocked CRK1N-promoted filamentous growth (Fig. 7A and Table 4). flo8 also blocked CRK1N-promoted haploid invasive growth (Fig. 7B). Therefore, Crk1-stimulated filamentous or invasive growth in S. cerevisiae requires Flo8.
Ectopic expression of CRK1N promotes hyphal growth under conditions favorable for yeast growth.
While complementing the hypha-defective phenotype in crk1/crk1 mutants, we observed that the ectopic expression of CRK1 enhanced hyphal growth under conditions that are otherwise favorable for the yeast form of growth. The ectopic expression of CRK1 allowed formation of visible hyphal colonies after 3 days of growth on solid YPD medium (Fig. 8A). No filaments were observed in the wild-type control strain grown under the same conditions until day 5 (Fig. 8A). We also observed that the expression of the catalytic domain of Crk1 promoted more filamentous growth than Crk1 (Fig. 8A), indicating that Crk1N might be more active than Crk1.
FIG. 8.
Ectopic expression of CRK1- or CRK1N-promoted filamentation under yeast growth conditions. (A) Colony phenotypes of wild-type (SC5314), crk1/crk1 with vector (CAW5), crk1/crk1 with ectopic expression of CRK1 (CAW6), and CRK1N (CAW7) strains grown on YPD medium at 30°C for 3 days. (B) Induction of ECE1 transcription by ectopic expression of CRK1 and CRK1N. Wild-type (SC5314), CRK1/crk1 (CAW1), crk1/crk1 carrying a vector (V; CAW5), crk1/crk1 carrying CRK1 (CAW6), and crk1/crk1 carrying CRK1N (CAW7) strains were grown in YPD at 30°C for 6 h, and total RNA was extracted for Northern analysis. The Northern blot was probed with CRK1, ECE1, and ACT1 and exposed for 3 days (for the CRK1 and ECE1 transcripts) and 3 h (for ACT1).
The ectopic expression of CRK1 or CRK1N also promoted the expression of hypha-specific genes under yeast growth conditions. Northern analysis demonstrated that the levels of CRK1 and CRK1N expression from plasmids were higher than from the endogenous chromosomal copies (Fig. 8B). While the ECE1 transcript was not detectable at 30°C in YPD in a wild-type strain, it was detected in strains overexpressing either CRK1 or CRK1N. However, the level of ECE1 transcript in CRK1- and CRK1N-expressing strains was about 20- to 30-fold lower than that of wild-type hyphal cells (compare Fig. 8B to Fig. 5C). This may explain why the ectopic expression of CRK1N did not generate hyphal cells in liquid YPD at 30°C (not shown).
CRK1 can promote hyphal development through a pathway that is independent of Cph1 and Efg1 in C. albicans.
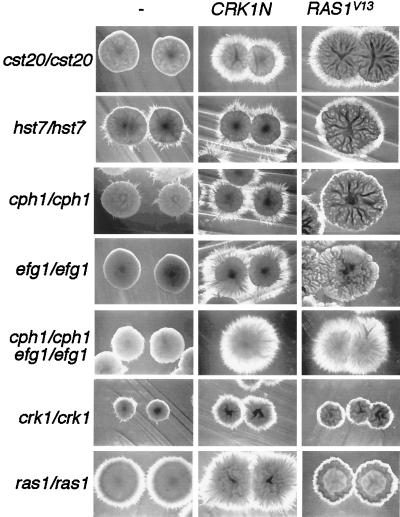
To address the function of Crk1 in the context of known C. albicans signaling components, we expressed CRK1N in various C. albicans strains defective in the filamentation MAP kinase pathway and the parallel pathway Efg1. CRK1N suppressed the hyphal formation defect in cst20/cst20 strains (Fig. 9). The effect of CRK1N was evident after 3 days but more obvious after a longer incubation. Although Cst20 is supposedly in the same pathway as Hst7 and Cph1, the filament-promoting activity of Crk1N was much weaker in hst7/hst7 and cph1/cph1 strains than in the cst20/cst20 strain. As shown in Fig. 9, hst7/hst7 and cph1/cph1 colonies were more filamentous than cst20/cst20 colonies, but with the expression of CRK1N, they were less filamentous than the cst20/cst20 strain. This indicated that the signaling pathway from Cst20 to Hst7 might not be linear. We also showed that CRK1N could partially suppress the defect of hyphal development in the efg1/efg1 mutants (Fig. 9). Surprisingly, when transformed with CRK1N, efg1/efg1 cph1/cph1 double mutants generated more hyphal filaments than either efg1/efg1 or cph1/cph1 single mutants with CRK1N (Fig. 9). Comparable to this observation, RAS1V13 promoted dramatic hyphal formation in efg1/efg1 cph1/cph1 double mutant. Subtle differences existed between CRK1N- and RAS1V13-activated filamentation. CRK1N-promoted hyphal filamentation was most evident around the initial streaks and well-separated single colonies, whereas RAS1V13-activated filamentation was seen throughout the streak regardless of the colony density. Interestingly, the expression of RAS1V13 in either MAP kinase pathway-defective strains or efg1/efg1 strains led to the formation of large wrinkled sheet-like colonies (Fig. 9). The fact that both CRK1N and RAS1V13 generated more filaments in the double mutant than in each of the single mutants indicated that there might be complicated negative regulation by Cph1 and Efg1 on filamentation. The phenotypes of CRK1N and RAS1V13 in the efg1/efg1 cph1/cph1 strain suggested that they both could activate hyphal development through pathways independent of Efg1 and Cph1.
FIG. 9.
Functional relationship of Crk1 with the filamentation MAP kinase pathway, Efg1, and Ras1 in C. albicans hyphal development. The C. albicans mutant strains indicated on the left (described in Table 1) were transformed with CRK1N (BES119CRK1N) and RAS1V13 (pQF145.2). Both genes are under the control of the MAL2 promoter. The C. albicans transformants were grown on an SC−Ura+sucrose (2%) plate containing 50 mM succinate at pH 5 for 4 days at 30°C (20). Well-separated colonies near the edge of each steak were photographed.
To further examine the relationship between Ras1 and Crk1, CRK1N and RAS1V13 were expressed in C. albicans ras1/ras1 and crk1/crk1 mutants, respectively. The ras1/ras1 mutant produced fewer hyphae than the wild type (not shown), and CRK1N dramatically enhanced filament formation in the ras1/ras1 strain (Fig. 9). On the other hand, although RAS1V13 suppressed the hyphal formation defect in crk1/crk1 strains, the level of filamentation produced by RAS1V13 in the crk1/crk1 strain was much lower than that of CRK1N in the ras1/ras1 strain (Fig. 9).
DISCUSSION
A new member of the Cdc2-related protein kinase family with a regulatory carboxyl terminus.
We have cloned a novel gene, CRK1, which encodes a Ser/Thr kinase with a catalytic domain highly conserved among kinases of the Cdk subfamily. The kinase domain of Crk1 is most similar to those of the S. cerevisiae protein Sgv1 and three other Cdc2-related protein kinases. In addition to being similar in sequence, Crk1 may also share overlapping functions with Sgv1, because CRK1 partially suppresses the hypersensitive pheromone-induced growth arrest phenotype in sgv1 mutants. This suppression is specific to sgv1, as neither Crk1 nor Crk1N promotes adaptation to pheromone induction in wild-type strains. However, Crk1 and Sgv1 also have nonoverlapping functions. First, Crk1 cannot complement the conditional growth defect of sgv1. Second, overexpression of full-length Sgv1 or its catalytic domain does not promote invasive or filamentous growth in S. cerevisiae (Chen, unpublished observation). Third, Sgv1 is essential for viability in S. cerevisiae whereas Crk1 is dispensable in C. albicans. Since the deletion of SGV1 leads to lethality in S. cerevisiae, it is impossible to address whether sgv1/sgv1 mutants will block pseudohyphal growth. All of these findings indicate that functional differences exist between Crk1 and Sgv1. Similar to Crk1, the Ras proteins from S. cerevisiae and C. albicans also have functional differences; S. cerevisiae Ras proteins are essential, whereas the C. albicans Ras1 protein is not. The functional differences between Crk1 and Sgv1 may reflect variation in substrate specificity. The substrate specificity is likely to be defined by a region in the catalytic domain of Crk1 and Sgv1 since the activity to promote invasive/filamentous growth and the ability to complement the conditional growth in sgv1 are supported by the catalytic domains of Crk1 and Sgv1, respectively.
All four kinases, Crk1, Sgv1, the uncharacterized S. pombe Cdc2-like protein, and the predicted protein from a human brain cDNA, have a long noncatalytic carboxyl domain. For Crk1, the catalytic domain alone seems to be more active than the complete protein. Therefore, the noncatalytic domain may function as an inhibitor to the kinase activity. One possible mechanism of Crk1 activation is to unfold the inhibitory domain and thus expose the catalytic domain during the dimorphic switch. Although the noncatalytic domains of these four kinases are not similar, it is still possible that the mechanisms for their regulation are similar, but the regulators of the noncatalytic domain are different in each organism.
Role of Crk1 in hyphal development in C. albicans.
Crk1 is required for hyphal development under all hypha-inducing conditions investigated. Deleting CRK1 dramatically impaired hyphal formation under various hypha-inducing conditions, whereas the ectopic expression of its catalytic domain promoted hyphal colony formation even under conditions favorable for yeast form growth. Crk1 is probably not directly responsible for changes in cytoskeleton that are necessary for the polarized growth during hyphal development. Rather, several lines of evidence support its role in regulating the transcriptional program of hypha-specific genes. First, crk1/crk1 mutants are severely impaired in the induction of two hypha-specific genes. Second, the catalytic domain of Crk1 can induce the expression of hyphal genes under yeast growth conditions when hyphal genes are normally undetectable. Third, the ectopic expression of the Crk1 catalytic domain in S. cerevisiae promoted invasive growth, a phenomenon caused by the expression of a cell wall protein, Flo11 (40). Flo11 is necessary for invasive growth, and its expression is regulated by transcription factors required for invasive/pseudohyphal growth. Finally, the Crk1 sequence predicts two conserved basic bipartite nuclear localization sequences at the carboxyl terminus (Fig. 1), suggesting a nuclear function. Taken together, these findings indicate that Crk1 plays a role in regulating the hyphal transcriptional program. This could be achieved by its phosphorylation of some transcription factor(s) or regulator(s) important for hyphal development.
The substrate directly phosphorylated and controlled by Crk1 during C. albicans hyphal development is not known. Our studies of S. cerevisiae suggest that Crk1 acts independently of Ste12 and Phd1, which correspond to Cph1 and Efg1 in C. albicans. Consistent with this, CRK1N can suppress the hyphal development defect of cph1/cph1 efg1/efg1 double mutants in C. albicans. Thus, Crk1 promotes filamentation through a pathway independent of Cph1 and Efg1. The invasive/pseudohyphal growth-promoting activity of Crk1 in S. cerevisiae is blocked by Flo8, which is necessary for the cAMP-mediated signaling (53, 55). The sequence and functional conservation between the Ras proteins from C. albicans and S. cerevisiae suggests that C. albicans Ras1 may act in the cAMP pathway (20). Based on experiments in S. cerevisiae, C. albicans Ras1 has been suggested to function upstream of the Cph1 and Phd1 pathways (20). The phenotypes of RAS1V13 in mutant strains defective in the MAP kinase pathway or in EFG1 are supportive of this view; mutations in either pathway dramatically reduce the activity of RAS1V13 in filamentation. However, Ras1 can also activate hyphal filament formation through additional routes, as RAS1V13 generates florid hyphal filaments in efg1/efg1 cph1/cph1 double mutants. It is interesting that CRK1N and RAS1V13 have similar patterns of suppression in mutants of these two pathways. Further epistasis studies show that CRK1N can promote dramatic filamentation in ras1/ras1 strains, whereas RAS1V13 shows weaker suppression in crk1/crk1 strains. It is tempting to suggest that Crk1 might be one of the downstream targets of Ras1 in hyphal development. However, a linear model of signal transduction may not be adequate to explain the Ras1/cAMP-mediated regulation of hyphal development. Therefore, epistasis experiments alone might not be able to dissect the complicated network involved in hyphal development.
Integrative regulation of multiple pathways for hyphal development.
Our data suggest that Ras1 and Crk1 regulate additional pathways independent of Cph1 and Efg1, and all of these pathways converge to regulate a common set of hypha-specific genes. This is reminiscent of the pseudohyphal development program in S. cerevisiae, where the Kss1 MAP kinase pathway and the cAMP-regulated pathway converge on the promoter of FLO11 (53, 55). Since both crk1/crk1 and efg1/efg1 mutants are unable to induce hyphal development under similar in vitro hyphal growth conditions, it is unlikely that each pathway responds to a particular hypha-inducing condition; rather, each pathway may respond to a specific signal in a hypha-inducing condition. The strong activation for any one pathway may be enough to reach a threshold required for hyphal development, but in many cases, integrated inputs from more than one pathway may be required to reach the threshold required for hyphal development. A defect in any one of the signaling pathways will reduce the total integrated inputs and hamper hyphal development. This integrative regulation may be necessary for fine-tuning of the signaling system, such as in rapid response versus sustained activation. Alternatively, multiple pathways could be used by C. albicans cells to sense subtle differences in the growth conditions of its native host environment. Our defined laboratory media, which have been chosen for its all or no hyphal growth property, may fail to mimic the subtle differences in growth conditions in the host.
Virulence.
The crk1/crk1 is avirulent under the conditions used in our investigation. The reduced growth rate of crk1/crk1 may contribute to the reduced virulence. The reduced virulence could also be due to the impaired ability of crk1/crk1 strains to undergo hyphal formation, as many mutants defective in hyphal formation have been shown to be less virulent or avirulent compared to the wild type (14, 22, 35, 39). Alternatively, there may be other targets of Crk1 that are not involved in hyphal growth but are required for virulence. Given its role in virulence, uncovering the proteins regulated by Crk1 should reveal new targets for antifungal drug development.
ACKNOWLEDGMENTS
We thank J. D. Loeb and S. Lane for critical reading of the manuscript. We thank W. Fonzi, A. Brown, K. Matsumoto, and G. Fink for kindly providing reagents.
This work was supported by grants from the Chinese National Natural Science Foundation (grant 39625009) and Shanghai Scientific and Technological Development Foundation (grant 97QMA1409) to J.C. and from the Burroughs Wellcome Fund (BWF0462) and NIH (GM-55155) to H.L.
REFERENCES
- 1.Ahn S, Acurio A, Kron S. Regulation of G2/M progression by the STE MAP kinase pathway in budding yeast filamentous growth. Mol Biol Cell. 1999;10:3301–3316. doi: 10.1091/mbc.10.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alex L A, Korch C, Selitrennikoff C P, Simon M I. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci USA. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alspaugh J A, Perfect J R, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Bailey D A, Feldmann P J, Bovey M, Gow N A, Brown A J. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banuett F, Herskowitz I. Identification of Fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 1994;8:1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- 7.Birse C E, Irwin M Y, Fonzi W A, Sypherd P S. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–3655. doi: 10.1128/iai.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 9.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wang Q, Fu Z, Zhou S, Fonzi W A. Tca1, the retrotransposon-like element of Candida albicans, is a degenerate and inactive element. J Bacteriol. 1998;180:3657–3662. doi: 10.1128/jb.180.14.3657-3662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J Y, Fonzi W A. A temperature-regulated, retrotransposon-like element from Candida albicans. J Bacteriol. 1992;174:5624–5632. doi: 10.1128/jb.174.17.5624-5632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook J G, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- 13.Cormack B P, Bertram G, Egerton M, Gow N A, Falkow S, Brown A J. Yeast-enhanced green fluorescent protein (yEGFP)a reporter of gene expression in Candida albicans. Microbiology. 1997;143:303–311. doi: 10.1099/00221287-143-2-303. [DOI] [PubMed] [Google Scholar]
- 14.Corner B E, Magee P T. Candida pathogenesis: unraveling the threads of infection. Curr Biol. 1997;7:R691–R694. doi: 10.1016/s0960-9822(06)00357-5. [DOI] [PubMed] [Google Scholar]
- 15.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Bernardis F, Muhlschlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Falco G, Giordano A. CDK9 (PITALRE): a multifunctional cdc2-related kinase. J Cell Physiol. 1998;177:501–506. doi: 10.1002/(SICI)1097-4652(199812)177:4<501::AID-JCP1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Delbruck S, Ernst J F. Morphogenesis-independent regulation of actin transcript levels in the pathogenic yeast Candida albicans. Mol Microbiol. 1993;10:859–866. doi: 10.1111/j.1365-2958.1993.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 19.Edgington N P, Blacketer M J, Bierwagen T A, Myers A M. Control of Saccharomyces cerevisiae filamentous growth by cyclin-dependent kinase Cdc28. Mol Cell Biol. 1999;19:1369–1380. doi: 10.1128/mcb.19.2.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale C A, Bendel C A, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M K. Linkage of adhesion, filamentous growth and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 23.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 24.Gow N A. Germ tube growth of Candida albicans. Curr Top Med Mycol. 1997;8:43–55. [PubMed] [Google Scholar]
- 25.Grana X, De Luca A, Sang N, Fu Y, Claudio P P, Rosenblatt J, Morgan D O, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg M E. Preparation and analysis of RNA, Chapter 4. In: Ausubel F M, Brent R, Kinston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley Interscience; 1987. [Google Scholar]
- 27.Hanks S K, Quinn A M, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 28.Irie K, Nomoto S, Miyajima I, Matsumoto K. SGV1 encodes a CDC28/cdc2-related kinase required for a G alpha subunit-mediated adaptive response to pheromone in S. cerevisiae. Cell. 1991;65:785–795. doi: 10.1016/0092-8674(91)90386-d. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly R, Miller S M, Kurtz M B, Kirsch D R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987;7:199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubler E, Mosch H U, Rupp S, Lisanti M P. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J Biol Chem. 1997;272:20321–20323. doi: 10.1074/jbc.272.33.20321. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz M B, Cortelyou M W, Kirsch D R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986;6:142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A, Brown A J, Thomas D Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol. 1997;7:539–546. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Kohler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Styles C A, Fink G R. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Styles C A, Fink G R. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 40.Lo W S, Dranginis A M. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:161–171. doi: 10.1091/mbc.9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loeb J D, Kerentseva T A, Sepulveda-Becerra M, Pan T, Liu H. Saccharomyces cerevisiae G1 cyclins are differentially involved in invasive and pseudohyphal growth independent of the filamentation MAP kinase pathway. Genetics. 1999;153:1535–1546. doi: 10.1093/genetics/153.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeb J D, Sepulveda-Becerra M, Hazan I, Liu H. A G1 cyclin is necessary for maintenance of filamentous growth in Candida albicans. Mol Cell Biol. 1999;19:4019–4027. doi: 10.1128/mcb.19.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorenz M C, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 1997;16:7008–7018. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madhani H D, Fink G R. Combinatorial control required for the specificity of yeast MAPK signaling. Science. 1997;275:1314–1317. doi: 10.1126/science.275.5304.1314. [DOI] [PubMed] [Google Scholar]
- 45.Madhani H D, Fink G R. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- 46.Madhani H D, Styles C A, Fink G R. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 47.Marcote M J, Knighton D R, Basi G, Sowadski J M, Brambilla P, Draetta G, Taylor S S. A three-dimensional model of the Cdc2 protein kinase: localization of cyclin- and Suc1-binding regions and phosphorylation sites. Mol Cell Biol. 1993;13:5122–5131. doi: 10.1128/mcb.13.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monge R A, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sanchez M, Nombela C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagahashi S, Mio T, Ono N, Yamada-Okabe T, Arisawa M, Bussey H, Yamada-Okabe H. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology. 1998;144:425–432. doi: 10.1099/00221287-144-2-425. [DOI] [PubMed] [Google Scholar]
- 50.Navarro-Garcia F, Sanchez M, Pla J, Nombela C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197–2206. doi: 10.1128/mcb.15.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odds F C. Morphogenesis in Candida albicans. Crit Rev Microbiol. 1985;12:45–93. doi: 10.3109/10408418509104425. [DOI] [PubMed] [Google Scholar]
- 52.Odds F C. Pathogenesis of Candida infections. J Am Acad Dermatol. 1994;31:S2–S5. doi: 10.1016/s0190-9622(08)81257-1. [DOI] [PubMed] [Google Scholar]
- 53.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts R L, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 55.Rupp S, Summers E, Lo H J, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 57.Sonneborn A, Bockmuhl D P, Gerads M, Kurpanek K, Sanglard D, Ernst J F. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol. 2000;35:386–396. doi: 10.1046/j.1365-2958.2000.01705.x. [DOI] [PubMed] [Google Scholar]
- 58.Srikantha T, Tsai L, Daniels K, Enger L, Highley K, Soll D R. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology. 1998;144:2715–2729. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 59.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline- and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 60.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tedford K, Kim S, Sa D, Stevens K, Tyers M. Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates. Curr Biol. 1997;7:228–238. doi: 10.1016/s0960-9822(06)00118-7. [DOI] [PubMed] [Google Scholar]
- 62.Vernet T, Dignard D, Thomas D Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 63.Whiteway M, Dignard D, Thomas D Y. Dominant negative selection of heterologous genes: isolation of Candida albicans genes that interfere with Saccharomyces cerevisiae mating factor-induced cell cycle arrest. Proc Natl Acad Sci USA. 1992;89:9410–9414. doi: 10.1073/pnas.89.20.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J, Xia Z, Cao Z, Ao S. Phosphorylation of Pho4 protein by the Pho85-Pap1 kinase complex. Acta Biochim Biophys Sin. 1998;30:63–69. [PubMed] [Google Scholar]
- 65.Xu J R, Hamer J E. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 66.Zhang F, Strand A, Robbins D, Cobb M H, Goldsmith E J. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]