Abstract
Durvalumab (IMFINZI®), a fully human monoclonal antibody against programmed cell death-ligand 1 (PD-L1), is approved for use in combination with etoposide and either carboplatin or cisplatin for the first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC). In the pivotal phase III CASPIAN trial in previously untreated adults with ES-SCLC, the addition of durvalumab to chemotherapy for up to 4 cycles followed by maintenance durvalumab was associated with a significantly longer overall survival and a favourable hazard ratio for progression-free survival compared with chemotherapy alone for up to 6 cycles. A higher proportion of patients in the durvalumab plus chemotherapy group had an objective response compared with the chemotherapy alone group. The efficacy of durvalumab was also sustained with longer follow-up. Durvalumab in combination with etoposide and either carboplatin or cisplatin had a manageable tolerability profile in patients with ES-SCLC. Given the available evidence, durvalumab in combination with etoposide and either carboplatin or cisplatin represents a valuable treatment option for the first-line treatment of patients with ES-SCLC, and is an accepted standard of care option in this setting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-021-00843-0.
Plain Language Summary
Small cell lung cancer (SCLC) is the most aggressive form of lung cancer; extensive-stage (ES) disease, which accounts for about two-thirds of all SCLC, is associated with high relapse rates and a poor prognosis. Expression of programmed cell death-ligand 1 (PD-L1) on both tumour cells and tumour-associated immune cells is an adaptive immune response that helps tumour cells avoid detection and subsequent elimination by the immune system. Durvalumab (IMFINZI®) is a fully human monoclonal antibody against PD-L1, which blocks the interaction of PD-L1 with its receptors, thus enhancing anti-tumour immune responses. When used in combination with chemotherapy (etoposide and either carboplatin or cisplatin) in adults with untreated ES-SCLC, durvalumab prolonged overall survival compared with chemotherapy alone; the improvements in overall survival were also maintained with additional follow-up. The tolerability profile of durvalumab in combination with chemotherapy was manageable in patients with ES-SCLC. Durvalumab in combination with chemotherapy is an effective and valuable treatment option for previously untreated patients with ES-SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-021-00843-0.
| Digital Features for this Adis Drug Evaluation can be found at 10.6084/m9.figshare.16583855 |
Durvalumab: clinical considerations in ES-SCLC
| A fully human monoclonal antibody that selectively blocks the interaction of PD-L1 with its receptors, PD-1 and CD80 |
| Significantly prolongs overall survival when added to etoposide plus either carboplatin or cisplatin; benefits were sustained with longer follow-up |
| Manageable tolerability profile |
Introduction
Small cell lung cancer (SCLC), characterized by rapid proliferation, high growth fraction and early development of metastases, is the most aggressive form of lung cancer and accounts for ≈ 15% of cases [1, 2]. About two-thirds of all SCLC cases present with extensive-stage (ES) disease, which for more than three decades has typically been treated with etoposide plus either carboplatin or cisplatin as standard first-line treatment. Despite initially high response rates to chemotherapy, most patients relapse within 6 months of completing treatment; prognosis for these patients is poor with a 2-year survival rate of < 5% [3].
The treatment paradigm of many solid tumours has been revolutionized following the emergence of immune checkpoint inhibitors targeting the programmed cell death receptor-1 (PD-1)/PD-ligand 1 (PD-L1) pathway, which has an important role in immune regulation. PD-L1 is overexpressed in many solid tumours and is associated with a poor prognosis in some types of cancer [4].
Durvalumab (IMFINZI®) is a fully human monoclonal antibody against PD-L1 that is widely approved in combination with etoposide and either carboplatin or cisplatin for the first-line treatment of patients with ES-SCLC (Sect. 6). This article provides an overview of the pharmacological properties of durvalumab and reviews the clinical data relevant to its use in ES-SCLC. Durvalumab is also approved in the USA [5], EU [6], Japan [7], China [8] and many other countries as monotherapy for the treatment of stage III, unresectable non-SCLC (NSCLC) in adults whose disease has not progressed following platinum-based chemoradiation therapy and, in the EU, whose tumours express PD-L1 on ≥ 1% of tumour cells [6]. However, discussion of this indication is outside the scope of this review.
Pharmacodynamic Properties of Durvalumab
Expression of PD-L1 on tumour cells and tumour-associated immune cells in the tumour microenvironment can be induced by inflammatory signals (e.g. IFN-gamma) [5, 6] and is an adaptive immune response that helps tumours evade detection and elimination by the immune system [6]. By interacting with its receptors, PD-1 and CD80 (B7.1), PD-L1 inhibits T-cell function and activation, and reduces cytotoxic T-cell activity, proliferation and cytokine production [5, 6].
Durvalumab, a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody, selectively blocks the interaction of PD-L1 with both PD-1 and CD80 [5, 6], thereby enhancing anti-tumour immune responses and increasing T-cell activation [6]. Durvalumab is not associated with induction of antibody-dependent cell-mediated cytotoxicity [5, 6]. In co-engrafted human tumour and immune cell xenograft mouse models, PD-L1 blockade with durvalumab was associated with a decrease in tumour size [5].
Because of the potential interference with the pharmacodynamic activity and efficacy of durvalumab, the use of systemic corticosteroids or immunesuppressants [with the exception of physiological doses of systemic corticosteroids (i.e. ≤ 10 mg/day prednisone or equivalent)] before starting durvalumab is not recommended; these agents can be used after initiating durvalumab or to manage immune-related adverse reactions (Sect. 5) [6].
There is potential for immunogenicity with therapeutic proteins [5]. However, treatment-emergent anti-drug antibodies or neutralizing antibodies were not detected in evaluable patients (n = 201) who received durvalumab plus chemotherapy in the phase III CASPIAN trial in previously untreated adults with ES-SCLC [5, 6, 9].
Pharmacokinetic Properties of Durvalumab
The pharmacokinetics of durvalumab monotherapy have been studied in more than 2900 patients with solid tumours treated with doses ranging from 0.1 to 20 mg/kg administered intravenously once every 2, 3 or 4 weeks [6]. Durvalumab exhibited greater than dose-proportional exposure at doses < 3 mg/kg and dose-proportional exposure at doses ≥ 3 mg/kg every 2 weeks; steady state was reached at ≈ 16 weeks. The geometric mean steady-state volume of distribution in patients who received durvalumab monotherapy ≥ 10 mg/kg every 2 weeks was 5.6 L [5, 6].
Clearance of durvalumab decreases over time, with a mean maximal reduction of ≈ 23% from baseline values and a resultant geometric mean steady-state clearance of 8.2 mL/h at day 365; the decrease in steady-state clearance is not considered clinically relevant [5, 6]. Based on baseline clearance, the geometric mean terminal half-life of durvalumab was ≈ 18 days. Durvalumab is primarily eliminated by protein catabolism via the reticuloendothelial system or target-mediated disposition [6]. There were no clinically meaningful differences between the pharmacokinetic properties of durvalumab when used as monotherapy or in combination with chemotherapy [5, 6].
The pharmacokinetics of durvalumab were not affected to a clinically relevant extent by age (19–96 years), body weight (31–149 kg), sex, albumin levels, lactate dehydrogenase levels, creatinine levels, soluble PD-L1, tumour type, race, mild or moderate kidney impairment, mild hepatic impairment or ECOG/WHO performance status [5, 6]. The effects of severe kidney impairment and moderate or severe hepatic impairment on the pharmacokinetics of durvalumab are unknown [5, 6], although a change in hepatic function is not expected to influence durvalumab exposure as IgG monoclonal antibodies are not primarily cleared via hepatic pathways [6].
Results of population pharmacokinetics and exposure-response analyses based on the interim analysis of CASPIAN (Sect. 4) support the use of a fixed dose of durvalumab (1500 mg) in combination with etoposide plus either carboplatin or cisplatin for treatment-naïve patients with ES-SCLC [10]. There was no meaningful impact of exposure or body weight (> 30 kg) on efficacy or safety in patients treated with a fixed dose of durvalumab, and no clinically meaningful exposure-response relationship between durvalumab pharmacokinetics and progression-free survival (PFS), overall survival (OS) or safety endpoints [10].
Metabolic drug-drug interactions are not expected with durvalumab because of the pathways involved in its elimination; however, no formal pharmacokinetic interaction studies have been conducted with durvalumab [6].
Therapeutic Efficacy of Durvalumab
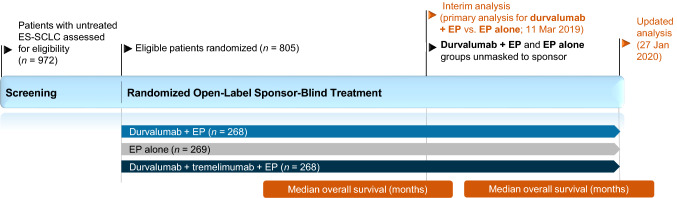
This section focuses on the efficacy of durvalumab in combination with etoposide plus either carboplatin or cisplatin in adults with untreated ES-SCLC, as evaluated in the open-label (unmasked to investigators and patients, but not to sponsor) multicentre, phase III CASPIAN trial (Fig. 1) [11].
Fig. 1.
Trial design of the randomized, open-label, sponsor-blind, multicentre, phase III CASPIAN trial in adults with untreated extensive-stage small cell lung cancer [11, 12]. Efficacy results for durvalumab plus EP versus EP alone are reported in the animated figure (available online); results from the durvalumab plus tremelimumab plus EP arm (unapproved combination) are omitted. EP platinum-etoposide, ES-SCLC extensive-stage small cell lung cancer, HR hazard ratio
Supplementary file1 Video (MP4 8106 kb)
CASPIAN enrolled patients (n = 805) aged ≥ 18 years (≥ 20 years in Japan) with treatment-naïve histologically or cytologically documented ES-SCLC, a WHO performance status score of 0 or 1, measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST) [version 1.1], suitability for first-line platinum-based chemotherapy, life expectancy of ≥ 12 weeks from the study start, and adequate organ and marrow function. Patients with active or previous autoimmune or inflammatory disorders; uncontrolled, concurrent illness or active infections; or a history of radiotherapy to the chest or planned consolidation chest radiotherapy were excluded [11].
Randomization was stratified according to planned platinum (carboplatin or cisplatin) [11]. Eligible patients were randomized in a 1:1:1 ratio (in blocks of six) to receive durvalumab (1500 mg) plus chemotherapy [which consisted of etoposide 80–100 mg/m2 (administered on days 1–3 of each 21-day cycle) plus the investigator’s choice of either carboplatin area under the curve 5–6 mg/mL/min or cisplatin 75–80 mg/m2 (administered on day 1 of each cycle)], durvalumab plus tremelimumab (75 mg) plus chemotherapy, or chemotherapy alone (up to 6 cycles every 3 weeks plus optional prophylactic cranial irradiation after discontinuation of chemotherapy). Patients treated with immunotherapy received four cycles of platinum-etoposide plus durvalumab, with or without tremelimumab, every 3 weeks followed by maintenance durvalumab every 4 weeks. All drugs were administered intravenously. Treatment continued until unacceptable toxicity, other discontinuation criteria were met, or disease progression, although continuation was permitted after disease progression provided there was evidence of clinical benefit [11].
The two primary endpoints were OS, assessed in the intent-to-treat population, for durvalumab plus chemotherapy versus chemotherapy alone and for durvalumab plus tremelimumab plus chemotherapy versus chemotherapy alone [11]. Secondary endpoints included PFS, objective response rate (ORR; unconfirmed) [both investigator-assessed according to RECIST version 1.1], OS rate at 18 months, PFS rates at 6 months and 12 months, and safety. A hierarchical multiple-testing procedure with a gatekeeping strategy was used across the primary OS analyses and secondary PFS analyses (i.e. PFS was only tested if both OS primary analyses achieved significance), and the study was considered positive if either of the OS analysis results achieved statistical significance [11].
At the time of the planned interim analysis (data cut-off 11 March 2019), OS with durvalumab plus chemotherapy versus chemotherapy alone met the prespecified threshold for statistical significance (Table 1); therefore, the independent data monitoring committee recommended unmasking these groups to the sponsor and the interim analysis was considered to be the final result in terms of formal statistical testing for this comparison, despite ongoing follow-up [11]. Discussion in this section will be limited to the approved combination of durvalumab plus chemotherapy, with no further discussion of the findings for the combination of durvalumab plus tremelimumab plus chemotherapy, which did not meet the predefined statistical significance threshold at the time of either the interim analysis or the updated (final) analysis [11, 12].
Table 1.
Efficacy of durvalumab in combination with etoposide plus either cisplatin or carboplatin in treatment-naïve patients with extensive-stage small cell lung cancer in the CASPIAN trial
| Treatment | OSa (months) | PFSb (months) | ORRc (% of pts) |
|---|---|---|---|
| Planned interim analysis (data cut-off 11 March 2019) [11] | |||
| DUR+EP (n = 268) | 13.0d | 5.1d | 79 |
| EP (n = 269) | 10.3d | 5.4d | 70 |
| HR/OR (95% CI) | HR 0.73 (0.59–0.91)e* | HR 0.78 (0.65–0.94)f | OR 1.64 (1.11–2.44) |
| Updated analysis (data cut-off 27 January 2020) [12] | |||
| DUR+EP (n = 268) | 12.9d | 5.1d | 79 |
| EP (n = 269) | 10.5d | 5.4d | 71 |
| HR/OR (95% CI) | HR 0.75 (0.62–0.91)** | HR 0.80 (0.66–0.96)f | OR 1.61 (1.09‒2.40) |
DUR durvalumab, EP platinum (investigator’s choice of either carboplatin or cisplatin) plus etoposide, HR hazard ratio, OR odds ratio, ORR objective response rate, OS overall survival, PFS progression-free survival
*p = 0.0047, **p = 0.0032 (nominal) vs. EP
aPrimary endpoint; defined as time from randomization to death from any cause
bSecondary endpoint; defined as time from randomization to the date of objective disease progression or death from any cause in the absence of progression
cUnconfirmed ORR (secondary endpoint); defined as proportion of patients with a complete response or partial response on ≥ 1 visit
dMedian value
eMet the boundary for declaring statistical significance (i.e. p < 0.0178)
fPFS could not be tested for significance within the multiple-testing procedure due to the hierarchical study design
At baseline, demographics and disease characteristics were generally similar between the durvalumab plus chemotherapy (n = 268) and chemotherapy (n = 269) treatment groups [11]. Across both groups, the majority of patients were male (70%), White (84%), aged < 65 years (60%; median age 63 years), and had stage IV disease (90%) and a WHO performance status of 1 (65%). A total of 10% of patients had brain or CNS metastases and 39% of patients had liver metastases [11].
At the data cut-off for the interim analysis, the median duration of follow-up for OS was 14.2 months and 336 deaths had occurred (155 and 181 events in the durvalumab plus chemotherapy and chemotherapy alone groups; 62.6% maturity) [11]. The addition of durvalumab to chemotherapy with platinum-etoposide in the first-line treatment of ES-SCLC significantly extended OS compared with chemotherapy alone with a hazard ratio (HR) of 0.73 (95% CI 0.59–0.91; p = 0.0047); the median OS was 2.7 months longer in the durvalumab plus chemotherapy group than in the chemotherapy alone group (Table 1) [11]. An OS benefit of durvalumab plus chemotherapy over chemotherapy alone (i.e. HR < 1) was also observed across all prespecified subgroups based on planned platinum, age, sex, WHO performance status, smoking status, brain or CNS metastases, disease stage at diagnosis, race and region. The 12- and 18-month OS rates with durvalumab plus chemotherapy were 54% and 34% (vs. 40% and 25% with chemotherapy alone) [11].
At the time of the interim analysis, 226 and 233 patients in the durvalumab plus chemotherapy and chemotherapy alone groups had disease progression or died [11]. Median PFS was similar across the durvalumab plus chemotherapy and chemotherapy alone groups, but the risk of progression or death was 22% lower with durvalumab plus chemotherapy than with chemotherapy alone (Table 1) [11]. PFS could not be formally tested for significance within the multiple-testing procedure at the interim analysis because of the hierarchical design of CASPIAN. The 6- and 12-month PFS rates in the durvalumab plus chemotherapy group were 45% and 18% (vs. 46% and 5% in the chemotherapy alone group) [11].
A higher proportion of patients in the durvalumab plus chemotherapy group had an unconfirmed objective response compared with the chemotherapy alone group (Table 1) [11]. Amongst patients who had a confirmatory scan no sooner than 4 weeks after the initial response, the confirmed ORR was 68% and 58% in the durvalumab plus chemotherapy and chemotherapy alone groups; the median duration of confirmed response was 5.1 months in each group. In patients with a confirmed response in the durvalumab plus chemotherapy and chemotherapy alone groups, the estimated percentages of patients remaining in response at 6 months were 39% and 34%, and at 12 months were 23% and 6% [11].
In a preplanned subgroup analysis of patients recruited in Japan (n = 34; median follow-up 12.5 months), the addition of durvalumab to chemotherapy (n = 18) compared with chemotherapy alone (n = 16) was associated with an HR for OS of 0.77 [(95% CI 0.26–2.26); median OS not reached vs. 15.2 months] and an estimated OS rate at 12 months of 72.2% versus 60.2% [13]. In the durvalumab plus chemotherapy and chemotherapy alone groups, median PFS was 4.5 and 4.7 months (HR 0.90; 95% CI 0.43–1.89) and the confirmed ORR was 89% and 69% (odds ratio 3.64; 95% CI 0.65–28.75) [13].
The addition of durvalumab to platinum-etoposide in CASPIAN improved survival outcomes without detrimentally impacting patient-reported outcomes (PROs), as assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30) [version 3] and its lung cancer module, Quality of Life Questionnaire-Lung Cancer 13 (QLQ-LC13) [14]. For the key prespecified disease-related symptoms [cough, dyspnoea and chest pain (as measured by QLQ-LC13) and fatigue and loss of appetite (QLQ-C30)], numerical reductions in the symptom burden over 12 months or until disease progression were observed in both the durvalumab plus chemotherapy and chemotherapy alone groups. A between-group difference (nominal p = 0.009) in favour of durvalumab plus chemotherapy was evident for the improvement of appetite loss from baseline. The time to deterioration for global health status/quality of life (QoL), all functioning scales (cognitive, emotional, physical, role and social) and all QLQ-C30 and QLQ-LC13 symptom scales was longer (i.e. HR < 1) in the durvalumab plus chemotherapy group than in the chemotherapy alone group [14].
At the data cut-off for the updated analysis (27 January 2020), the median duration of follow-up for OS was 25.1 months and 441 deaths had occurred across the two treatment groups (210 and 231 events in the durvalumab plus chemotherapy and chemotherapy alone groups; 82.1% maturity) [12]. The updated analysis of OS with 11 additional months of follow-up indicated a sustained OS improvement with durvalumab plus chemotherapy versus chemotherapy alone that was consistent with the results of the interim analysis (Table 1) [12]. An OS benefit with durvalumab plus chemotherapy versus chemotherapy alone (i.e. HR < 1) was consistently observed across all prespecified subgroups [12], as well as post hoc subgroups defined by the extent of disease (thoracic only or any extra-thoracic disease [15] and presence or absence of liver metastases [12]) at baseline. The 24-month OS rate was higher in the durvalumab plus chemotherapy group (22.2%) than in the chemotherapy alone group (14.4%) [12].
At the time of the updated analysis, median PFS was similar in the durvalumab plus chemotherapy and chemotherapy alone groups (Table 1); the 24-month PFS rate was higher in the durvalumab plus chemotherapy group (11.0%) than in the chemotherapy alone group (2.9%) [12]. Consistent with the interim analysis, the unconfirmed ORR was higher with durvalumab plus chemotherapy than with chemotherapy alone (Table 1) and the confirmed ORR was 68% and 58%, respectively. The estimated proportion of patients in the durvalumab plus chemotherapy group remaining in response at 24 months was 13.5% (vs. 3.9% in the chemotherapy alone group) [12].
Tolerability of Durvalumab
Durvalumab in combination with etoposide and either carboplatin or cisplatin had a manageable tolerability profile in patients with ES-SCLC treated in the CASPIAN trial [11, 12], including the subgroup of patients enrolled in Japan [13]. The safety profile of this regimen was consistent with the well-established safety profiles of the individual agents [11, 12]. Discussion in this section focuses on data from the updated analysis (data cut-off 27 January 2020) with the longer duration of exposure [12]. The safety population in CASPIAN comprised 265 patients in the durvalumab plus chemotherapy group and 266 patients in the chemotherapy alone group. The median total duration of exposure to durvalumab and to platinum-etoposide in the durvalumab plus chemotherapy group was 28.0 weeks and 12.1 weeks, while the median total duration of exposure to platinum-etoposide was 19.0 weeks in the chemotherapy alone group [12].
Adverse events (AEs) of any cause and any grade occurred in 98% and 97% of patients in the durvalumab plus chemotherapy and chemotherapy alone groups [12]. The most frequently reported AEs of any cause and any grade are shown in Table 2; neutropenia and anaemia were the most common AEs in both treatment groups [12]. Grade ≥ 3 AEs of any cause occurred in 65% of patients in both the durvalumab plus chemotherapy and chemotherapy alone groups; the most common (≥ 5% incidence in either treatment group) events were neutropenia (24% in the durvalumab plus chemotherapy group vs. 33% in the chemotherapy alone group), anaemia (9% vs. 18%), thrombocytopenia (6% vs. 10%), decreased neutrophil count (6% vs. 6%), leukopenia (6% vs. 5%) and febrile neutropenia (5% vs. 6%) [12]. Withholding durvalumab for grade 2 or 3 adverse reactions until grade ≤ 1 or baseline should be considered for non-immune-mediated adverse reactions, and discontinuation is recommended for grade 4 reactions (decision should be based on clinical judgement and accompanying clinical signs and symptoms for grade 4 laboratory abnormalities) [6].
Table 2.
Adverse events of any cause and any grade occurring in ≥10% of patients in either treatment group in the updated analysis of the CASPIAN trial (safety population) [12]
| Adverse event |
DUR + EP (n
= 265) [% of pts] |
EP (n = 266) [% of pts] |
|---|---|---|
| Neutropenia | 42 | 47 |
| Anaemia | 38 | 47 |
| Nausea | 34 | 33 |
| Alopecia | 32 | 34 |
| ↓ appetite | 18 | 17 |
| Fatigue | 18 | 17 |
| Constipation | 17 | 19 |
| Asthenia | 16 | 15 |
| Thrombocytopenia | 15 | 20 |
| Vomiting | 15 | 17 |
| Cough | 13 | 7 |
| Dyspnoea | 12 | 11 |
| Leukopenia | 11 | 12 |
| Diarrhoea | 11 | 11 |
| ↓ neutrophil count | 10 | 12 |
| Hyponatraemia | 10 | 5 |
| Hyperthyroidism | 10 | < 1 |
DUR durvalumab, EP platinum (investigator’s choice of either carboplatin or cisplatin) plus etoposide, pts patients, ↓ decreased
In the durvalumab plus chemotherapy and chemotherapy alone groups, serious AEs (any cause) were reported in 32% and 36% of patients; those most commonly (≥ 2% incidence) reported in the durvalumab plus chemotherapy group were febrile neutropenia (5%), pneumonia, anaemia and pancytopenia (2% each) [12]. AEs leading to discontinuation of treatment were reported in 10% and 9% of patients in the durvalumab plus chemotherapy and chemotherapy alone groups [most commonly (≥ 1% incidence in either treatment group) acute kidney injury and sudden death (1% each) in durvalumab plus chemotherapy recipients, and acute kidney injury (2%), thrombocytopenia, neutropenia and deafness (1% each) in chemotherapy alone recipients] [12]
Treatment-related AEs (any grade) occurred in 89% and 90% of patients in the durvalumab plus chemotherapy and chemotherapy alone groups [12]. Grade 3 or 4 treatment-related AEs occurred in 46% and 52% of patients in the durvalumab plus chemotherapy and chemotherapy alone groups and treatment-related serious AEs were reported in 13% and 19% of patients. Treatment-related AEs leading to treatment discontinuation were reported in 6% and 5% of patients in the durvalumab plus chemotherapy and chemotherapy alone groups [12].
Death because of AEs of any cause occurred in 13 recipients (5%) of durvalumab plus chemotherapy (including six deaths related to treatment comprising cardiac arrest, dehydration, hepatotoxicity, interstitial lung disease, pancytopenia and sepsis) and 15 recipients (6%) of chemotherapy alone (including two deaths related to treatment comprising pancytopenia and thrombocytopenia/haemorrhage) [12].
Given the mechanism of action of durvalumab, immune-mediated AEs may occur [5, 6]. In CASPIAN, immune-mediated AEs were reported in 20% of durvalumab plus chemotherapy recipients and 3% of chemotherapy alone recipients, and were mostly low grade (grade 1 or 2); grade 3 or 4 immune-mediated AEs occurred in 5% and < 1% of patients in the respective treatment groups [12]. The most common (≥ 5% incidence in either treatment group) any-grade immune-mediated AEs were hypothyroid events (9% in the durvalumab plus chemotherapy group vs. 1% in the chemotherapy alone group) and hyperthyroid events (5% vs. 0%). Death because of immune-mediated AEs occurred in two patients in the durvalumab plus chemotherapy group (hepatotoxicity and interstitial lung disease) and one patient in the platinum-etoposide group (pneumonitis) [12].
Patients should be monitored closely for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions and should be evaluated at the start of treatment and periodically during treatment for abnormal tests (e.g. liver, thyroid, renal) [5, 6]. Suspected immune-mediated adverse reactions should be adequately assessed to confirm aetiology and exclude alternate causes. In general, durvalumab should be withheld (for grade 2 or 3 reactions, depending on the specific immune-mediated AE) or permanently discontinued (for grade 4 or recurrent grade 3 reactions requiring systemic immunosuppressive treatment); for many immune-mediated adverse reactions, concomitant systemic corticosteroid therapy is recommended until improvement to grade ≤ 1, with subsequent tapering over ≥ 1 month [5, 6].
Severe or life-threatening infusion-related reactions may occur with durvalumab treatment, and patients should be monitored for signs and symptoms of these reactions [5, 6]. In the CASPIAN trial, infusion-related reactions (including urticaria) were reported in 1.9% of durvalumab plus chemotherapy recipients at the time of the interim analysis [6]. Based on the severity of the infusion-related reaction, interruption or slowing the rate of infusion (for grade 1 or 2 events) or permanent discontinuation (for grade 3 or 4 events) of durvalumab is recommended [5, 6].
Dosage and Administration of Durvalumab
Durvalumab is approved in the USA [5], EU [6], Japan [7], China [16] and many other countries globally for use in combination with etoposide and either carboplatin or cisplatin for the first-line treatment of adult patients with ES-SCLC. In the USA and EU, the recommended dosage of durvalumab for the treatment of patients with ES-SCLC is 1500 mg in combination with chemotherapy every 3 weeks (21 days) for 4 cycles, followed by 1500 mg every 4 weeks as monotherapy, until disease progression or unacceptable toxicity [5, 6]. Durvalumab is administered as an intravenous infusion over 60 minutes prior to chemotherapy on the same day. For patients with a body weight ≤ 30 kg, weight-based dosing, equivalent to durvalumab 20 mg/kg in combination with chemotherapy every 3 weeks for 4 cycles followed by 20 mg/kg every 4 weeks as monotherapy, is advised until weight increases to > 30 kg [5, 6]. Reducing or escalating the dose of durvalumab is not recommended, although delaying or discontinuing durvalumab treatment for adverse reactions is advised. There are no data on the use of durvalumab in pregnant women. Based on animal studies and its mechanism of action, durvalumab may cause foetal harm if used during pregnancy [5, 6]. Women of childbearing potential should be advised to use effective contraception during treatment and for at least 3 months after the last dose of durvalumab [5, 6].
Local prescribing information should be consulted for detailed information regarding recommended dose modifications for adverse reactions, warnings and precautions and use in special populations.
Place of Durvalumab in the Management of Extensive-Stage SCLC
Recommendations for the use of durvalumab in combination with etoposide and either carboplatin or cisplatin are included in the NCCN [17] and ESMO [18] clinical practice guidelines for SCLC. In the USA, the preferred regimens to be used as primary therapy for ES-SCLC are durvalumab in combination with etoposide and either carboplatin or cisplatin, or atezolizumab in combination with etoposide plus carboplatin [17]. Guidelines from the American Society of Clinical Oncology and the American College of Chest Physicians have not yet been updated to reflect the approval of durvalumab in this indication [19, 20]. In the EU, the use of durvalumab in combination with platinum and etoposide or atezolizumab in combination with carboplatin and etoposide is strongly recommended in treatment-naïve patients with ES-SCLC (defined as stage IV or III SCLC not eligible for treatment of curative intent) with a PS of 0–1 and no contraindications for immunotherapy [18].
The approval of durvalumab was based on data from the planned interim analysis of the multicentre phase III CASPIAN trial (Sect. 4). At the time of this analysis, durvalumab in combination with etoposide plus either carboplatin or cisplatin was associated with a significantly prolonged OS, a favourable HR for PFS, and a higher ORR compared with chemotherapy alone in treatment-naïve patients with ES-SCLC. The OS benefit with durvalumab plus chemotherapy versus chemotherapy alone was also observed across all prespecified subgroups (Sect. 4). Results of the updated analysis of CASPIAN were consistent with the interim findings, indicating sustained effects (Sect. 4). The addition of durvalumab to platinum-etoposide improved clinical outcomes without adversely impacting PROs and was associated with delayed worsening of patient-reported symptoms, global health status/QoL and functioning domains, compared with platinum-etoposide alone (Sect. 4).
Durvalumab in combination with chemotherapy had a manageable tolerability profile in treatment-naïve patients with ES-SCLC in the CASPIAN trial; the overall safety profile of the durvalumab plus chemotherapy regimen was consistent with the known safety profiles of the individual agents (Sect. 5). The most commonly reported grade 3 or 4 AEs with durvalumab plus chemotherapy were haematological in nature. Immune-mediated AEs, most commonly hypothyroid and hyperthyroid events, were reported; these AEs were mostly low grade and manageable with standard treatment guidelines (Sect. 5). The incidence of infusion-related reactions was low. Monitoring for signs and symptoms of immune-mediated AEs and infusion-related reactions is warranted (Sect. 5).
Durvalumab is currently the only immunotherapy approved for the first-line treatment of ES-SCLC that provides the flexibility of combining with either cisplatin or carboplatin (plus etoposide). Furthermore, durvalumab offers the convenience of 4-weekly administration during the maintenance phase of treatment, while atezolizumab is administered every 3 weeks during the maintenance phase [18]. Patient-related factors and the toxicity profiles are important considerations when choosing between carboplatin and cisplatin. For example, cisplatin may be preferred when taking age (< 70 years), toxicity profile (mainly non-haematological toxicities, e.g. nausea and vomiting) and performance status into account [18].
Brain metastases are common and associated with poor clinical outcomes in patients with ES-SCLC. However, the role of prophylactic cranial irradiation in patients with ES-SCLC treated with immunotherapy is not well defined, due to the paucity of data [18]; further research in this area would be of interest.
While research to identify biomarkers that can be used to inform treatment decisions in patients with SCLC is underway, there are currently no validated biomarkers with either prognostic or predictive relevance [18]. Further biomarker research is warranted.
To conclude, given the available evidence, durvalumab in combination with etoposide plus either carboplatin or cisplatin is a valuable treatment option for the first-line treatment of patients with ES-SCLC, and is an accepted standard of care option in this setting.
| Data Selection Durvalumab: 75 records identified | |
|---|---|
| Duplicates removed | 14 |
| Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 34 |
| Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 7 |
| Cited efficacy/tolerability articles | 5 |
| Cited articles not efficacy/tolerability | 15 |
| Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were durvalumab, Imfinzi, small cell lung cancer. Records were limited to those in English language. Searches last updated 6 Sep 2021. | |
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
During the peer review process, the manufacturer of durvalumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Zaina T. Al-Salama is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
The manuscript was reviewed by: M. Fukudo, Department of Pharmacy, Sapporo Medical University Hospital, Sapporo, Japan; K. Leventakos, Division of Medical Oncology, Mayo Clinic, Rochester, MN, USA; J. Rivera Concepcion, Medical Oncology, Mayo Clinic, Rochester, MN, USA; N. Tsoukalas, Oncology Department, 401 General Military Hospital, Athens, Greece.
The original version of this article was revised due to a retrospective Open Access order.
Change history
12/6/2021
A Correction to this paper has been published: 10.1007/s11523-021-00863-w
References
- 1.Oronsky B, Reid TR, Oronsky A, et al. What's new in SCLC? A review. Neoplasia. 2017;19(10):842–847. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7(1):1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Zimmermann S, Parikh K, et al. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94(8):1599–1622. doi: 10.1016/j.mayocp.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AstraZeneca. IMFINZI® (durvalumab) injection, for intravenous use: US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761069s029lbl.pdf. Accessed 6 Sep 2021.
- 6.AstraZeneca. IMFINZI (durvalumab): EU summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf. Accessed 6 Sep 2021.
- 7.AstraZeneca Co. Ltd. Imfinzi® intravenous infusion 120mg / Imfinzi® intravenous Infusion 500mg: Japanese prescribing information. 2020. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/670227_4291443A1023_1_04. Accessed 6 Sep 2021.
- 8.AstraZeneca. Imfinzi approved in China for the treatment of unresectable, stage III non-small cell lung cancer based on the phase III PACIFIC trial [media release]. 12 Dec 2019. https://www.astrazeneca.com/media-centre/press-releases/2019/imfinzi-approved-in-china-for-the-treatment-of-unresectable-stage-iii-non-small-cell-lung-cancer-based-on-the-phase-iii-pacific-trial-12122019.html.
- 9.Ozguroglu M, Goldman JW, Reinmuth N, et al. First-line durvalumab plus platinum-etoposide in extensive-stage (ES)-SCLC: safety, pharmacokinetics (PK) and immunogenicity in CASPIAN [abstract no. LBA2] Ann Oncol. 2019;30(Suppl 11):xi66. doi: 10.1093/annonc/mdz453.003. [DOI] [Google Scholar]
- 10.Zheng Y, Jin D, Guan Y, et al. Population pharmacokinetics and exposure-response with durvalumab plus platinum-etoposide in ES-SCLC: results from CASPIAN [abstract no. P48.21 plus poster] J Thorac Oncol. 2021;16(3 Suppl):S508. doi: 10.1016/j.jtho.2021.01.891. [DOI] [Google Scholar]
- 11.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 12.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Nishio M, Saito H, et al. First-line durvalumab plus platinum-etoposide in extensive-stage small-cell lung cancer: CASPIAN Japan subgroup analysis. Int J Clin Oncol. 2021;26(6):1073–1082. doi: 10.1007/s10147-021-01899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman JW, Garassino MC, Chen Y, et al. Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study. Lung Cancer. 2020;149:46–52. doi: 10.1016/j.lungcan.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Reinmuth N, Dvorkin M, Garassino MC, et al. First-line durvalumab plus platinum-etoposide in ES-SCLC: exploratory analyses based on extent of disease in CASPIAN [abstract no. P48.03 plus poster] J Thorac Oncol. 2021;16(3 Suppl):S500. doi: 10.1016/j.jtho.2021.01.873. [DOI] [Google Scholar]
- 16.AstraZeneca. Imfinzi approved in China for the treatment of extensive-stage small cell lung cancer [media release]. 19 Jul 2021. https://www.astrazeneca.com/media-centre/press-releases/2021/imfinzi-approved-in-china-for-extensive-stage-sclc.html.
- 17.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: small cell lung cancer (version 3.2021). 2021. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed 6 Sep 2021.
- 18.Dingemans AC, Fruh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(7):839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Clin Oncol. 2015;33(34):4106–4111. doi: 10.1200/JCO.2015.63.7918. [DOI] [PubMed] [Google Scholar]
- 20.Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e400S–19S. doi: 10.1378/chest.12-2363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.