Graphical abstract
Dunaliella salina and its isolated Zeaxanthin mitigate counteract age related dementia via attenuating levels of inflammatory mediators in the brain, i.e. interlekin-1β and inducible nitric oxide synthase, as well as brain Aβ protein and myelin base protein. D. salina overcomes brain aging and thereby repairs age-related dementia, both for its modulating function in attenuating the Aβ protein and neurotransmitters (serotonin, norepinephrine & dopamine).

Keywords: Dunalialla salina, Aging, Dementia, Amyloid-β protein, Neurotransmitters
Highlights
-
•
D. salina as well as its isolated zeaxanthin showed marked recovery of the D-gal-induced effect on the escape latency time.
-
•
D. salina exerted an amelioration in the brain Aβ contents and an increase in the brain 5-HT, NE and DOP levels.
-
•
These effects were confirmed by histopathological increase in number of viable neurons in both cerebral cortex and hippocampus.
Abstract
Age-related deterioration of sensorimotor and cognitive abilities suggests that the brain undergoes regressive alterations with aging that compromise its function. Thus, the present study was designed to assess the efficacy of Dunaliella salina in counteracting D-galactose (D-gal)-induced dementia brain aging and its modulatory role in attenuating amyloid β (Aβ) protein and neurotransmitters. Aging associated dementia was generated by injection of D-gal (200 mg/kg; i.p) of rats for 8 weeks. D. salina biomass (250 mg/kg), polar (30 mg/kg), its carotenoid (30 mg/kg) fractions as well as the isolated zeaxanthin (250 μg/kg) were given orally simultaneously with D-gal for additional two weeks. Twenty-four hours after the last treatment dose; behavioral, biochemical and histopathological assessment were performed. Results showed that oral treatment of motor deficit rats with D. salina biomass and its isolated polar and carotenoid fractions showed amelioration in the motor coordination assessed by the rotarod test and in the memory and learning capabilities evaluated by Morris water maze test. D. salina also showed a reduction in brain levels of inflammatory indicators viz. interlekin-1β and inducible nitric oxide synthetase as well as brain contents of Aβ protein and myelin base protein. Likewise, oral treatment with D. salina biomass and its isolated polar and carotenoid fractions exhibited an increase in the rats’ brain neurotransmitters and their metabolites. Furthermore, histopathological investigations have confirmed all of these results. Our findings suggest that D. salina overcomes brain aging and thereby repairs age-related dementia, both for its modulating function in attenuating the Aβ protein and neurotransmitters.
1. Introduction
Dementia is one of the most debilitating geriatric diseases with intellectual capacities such as comprehension, memory, and judgement. Lower cortical functions are also affected, such as language, thought, and the capability to obey instructions. Extensive pesticide exposure is one of the underlying reasons from cognitive decline and dementia [1]. Alzheimer's disease (AD) is considered to be the most prevalent type of dementia that affects people older than 60 years old [2]. Amyloid plaques and neurofibrillary tangles are pathological and histological hallmarks of AD. Amyloid-beta (Aβ) level dysregulation contributes to the emergence of senile plaques containing depositions of Aβ [3,4]. Aβ is a complicated biological substance that interacts with a variety of receptors and, in essence, alternates between non-physiological and physiological depositions in neurons. Synaptic injury, mitochondrial irregularities, inflammatory reactions, hormonal variations, and cell cycle abnormalities are all linked to AD [5,6].
On the other hand, age-related memory deficiency is often known to be associated with elevated redox homeostasis in the aging brain [7,8]. Moreover, senescence is the reason for both short-term and long-term memory dysfunctions due to a reduction in cholinergic activity [9,10]. Reduced levels of brain biogenic amines have also been specifically associated with age-related memory impairments in senescent rats [11]. Apparently, dopamine (DA) [12], 5-hydroxytryptamine i.e. seratonin (5-HT) and norepinephrine (NE) have a pivotal role in learning and memory [13]. Researchers have been designing various drug molecules based on structural alterations found in both brain tissues of patients and animal models with AD [6].
However, there is no actual therapy for AD, medication therapies are available only to overcome the disease’s cognitive symptoms and protect against further cognitive deterioration. Scientists worldwide are focusing on discovering improved medications, prevention measures, and eventually a cure [3]. Recently, Aβ has become a potential therapeutic target in drug development for overcoming age-related dementia. Several clinical trials have been performed targeting this protein; some study lines are currently under review. Low Aβ levels enhance long-term hippocampal potentiation and improve memory, suggesting its novel beneficial, modulatory function in memory and neurotransmission [14]. There are many therapeutic strategies being studied for the treatment of AD, among of these strategies are amyloid-based therapies using herbal-based anti-amyloid approach.
Therefore, the objective of the current investigation was to verify hypotheses in senescent rats using the D-galactose (D-Gal) experimental model of aging. The goal was also extended to assess the efficacy of Dunaliella salina; a type of halophilic green microalgae that contains potential bioactive compounds for novel biomedical and pharmaceutical applications, in diminishing D-gal-induced dementia and brain aging and its modulatory role in attenuating Aβ protein and neurotransmitters viz. DA, 5-HT and NE as well as their metabolites; 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA) and dihydroxyphenylacetic acid (DOPAC) and their critical role in learning and memory.
2. Material and methods
2.1. Cultivation of Dunaliella salina and preperation of algal fractions
Dunaliella salina was isolated from Egyptian Salts and Minerals Company salt deposition basins and grown in a vertical photo bioreactor with a capacity of 4000 L on Bold nutritional medium (Table 1). Using a basket centrifuge, algal biomass was extracted by centrifugation at 2000 rpm for 15 min. Samples were rinsed, dried in a 50 °C oven, crushed into a homogeneous powder. Moreover, the carotenoid fraction was obtained by maceration of dried finely crushed algal biomass in hexane, ethyl acetate (80:20) until exhaustion, yielding the carotenoid fraction after filtration. The residue was left to dry before being extracted with 70 % methanol until it was exhausted, yielding the polar fraction. The two fractions were dried, then stored in dark bottles in the refrigerator for further analysis [15].
Table 1.
Boldʼs Nutrient Composition.
| Macroelements | Concentration |
|---|---|
| Urea | 0.3 g/L |
| K2HPO4 | 0.075 g/L |
| KHPO4 | 0.175 g/L |
| MgSO4(7H2O) | 0.075 g/L |
| Na Cl | 0.025 g/L |
| CaCl2 (2H2O) | 0.025 g/L |
| Microelements | |
| ZnSO4(7H2O) | 8.8 mg/L |
| MnCl2(4H2O) | 0.44 mg/L |
| MoO3 | 0.071 mg/L |
| CuSO4 (5H2O) | 1.57 mg/L |
| H3BO3 | 11.42 mg/L |
| EDTA | 50 mg/L |
| KOH | 31 mg/L |
| Fe SO4 (7H2O) | 4.98 mg/L |
| Co(NO3)2.6H2O | 0.49 mg/L |
| H2SO4 | 1 μL/L |
One milliliter of micronutrient solution was added to the culture medium. The culture was harvested by centrifugation at 4000 rpm, dried at 50°C and then grounded into homogeneous fine powder.
2.2. Isolation, purification and identification of zeaxanthin
The algal extract was put to a glass column that had been dry-packed with silica gel and eluted with hexane and ethyl acetate in increasing amounts. Sub-columns for similar fractions were kept for zeaxanthin (ZH) separation [16].
2.3. Docking study
The MOE homology modeling tool was used to create the 3D structures of the principal elements, -carotene and zeaxanthin (ZH). MOE 2015.10 software was used to prepare ligands and proteins prior to docking simulation. The Molecular Builder application was used to create 3D structures of the isolated chemicals. Finally, the ligands were translated to their 3D structures and used as MOE-docking input files. For docking, the proteins Tau-Tubulin Kinase 1 (4BTK) and Acetylcholinesterase (4M0E) were retrieved from the Research Collaboratory for Structural Bioinformatics PDB (https://www.rcsb.org/). Using the Triangular Matching docking approach, the two ligands were docked independently into the active sites of both proteins, and 30 conformations of each Ligand protein complex were created with docking score (S). Each complex's interactions were examined, and its 3D posture was captured [17].
2.4. Pharmacological study
2.4.1. Animals
Adult male albino Wistar rats (weight 200–250 g), collected from the animal house colony of National Research Centre (NRC, Egypt), were maintained at a controlled temperature of 24 ± 1 °C with a 12–12 h light-dark cycle (light cycle, 07:00–19:00). They were given unlimited access to water and normal food. This study has been approved by the ethics committees of the NRC. All procedures and experiments were carried out in accordance with the national and international ethics guidelines. This study used the earliest scientifically justified endpoint to prevent pain or distress in the experimental animals.
2.4.2. Chemicals
Sigma–Aldrich (St. Louis, Missouri, USA) provided the D-Galactose (D-Gal). All additional compounds were obtained from well-known commercial sources and were of analytical grade.
2.4.3. Experimental design
In rats, age-related dementia was generated by injecting D-Gal (200 mg/kg/day) intraperitoneally for eight weeks. They were randomly allocated into six groups; each group contains six rats. A group served as a negative control group, whereas another group served as a positive control group, receiving D-Gal for eight weeks. The remaining four groups were given D-Gal then received D. salina biomass (250 mg/kg) (BDS), polar (30 mg/kg) (PDS), its carotenoid (30 mg/kg) fractions (CDS) as well as the isolated zeaxanthin (250 μg/kg) (ZH) orally simultaneously with D-gal for additional two weeks. The doses were calculated according to the yields of the fractions and the isolated compound; ZH [18].
After the treatments, behavioral assessment was carried out, then the animals were sacrificed and decapitated. Brain tissues were isolated, rinsed and kept at −80 °C until biochemical assays were performed. Another brain tissues were then fixed in 10 % formalin for further histopathological examination. The following flowchart clarifies the experimental protocol.
2.4.4. Behavioral assessment
2.4.4.1. Spontaneous motor coordination
The rotarod (Ugo Basile model 7700, Veresi, Italy) was used to assess motor performance and learning. The rotarod consists of a circular rod turning at a constant or increasing speed. Vertical barriers are used to separate the animals from one another. Animals placed on the rotating rod try to remain on it rather than fall onto a platform some 30 cm below.
For two successive days with intervals of 10−15 min, testing comprises of four trials/ day. Every rat was located on the rod, which increased in speed from 4 to 40 rpm over the course of 300 s. When the animals fell off or the time limit was reached, the trials were called off. Up to five rats were tested simultaneously. Rats were taken out of the apparatus when the last rat fell. Latency to fall served indicates motor coordination [19].
2.4.4.2. Spatial memory and learning measured by Morris water maze (MWM)
The Morris water maze test is one of the most popular and established behavioral tests to evaluate rodents’ spatial learning and memory, which was originally invented by Richard G. Morris in 1983 [20]. A circular tank (diameter 1 m) filled with water was utilized to test the MWM. In the target quadrant's center, a platform was submerged beneath the water's surface. A video camera set overhead monitored the rats' swimming activities, which was automatically recorded by a video tracking system. When the animal is launched from diverse, random locations around the outside of the tank, it must learn to employ distal signals to take a direct path to the hidden platform. If no proximal cues are available, the use of distal signals is the most effective way to accomplish this. Most protocols begin at one of four points: N, S, E, or W. Animals are put through a series of daily trials with a semi-random or random set of start locations. The most popular start position settings are those in which the four positions are employed semi-randomly, with one trial per day from each of the four positions. Rats were allowed to rest for 1 h before putting the animals in the MWM. The entire experiment took four days in a row. The room was set up in such a way that the animal being examined was unable to view the experimenter while being tested and the room lighting should be indirect. The platform was kept visible on the first day of the experiment by keeping the water transparent and putting a flag on the platform to promote visibility; the positions of the platform and rat dumping in the pool were modified for each trial. Animals were trained five times to reach the platform, with a 20-minute gap between trials. The water which was kept at temperatures between 22−25 °C, was made opaque by adding milk to the pool on the second and third days, and the platform was painted white to hide it. For all trails, the testing method was performed with the platform in a fixed quadrant of the pool about four centimeters below the water level. The time the animal took to get to the platform was then recorded. Only one testing event was necessary for each animal on the final day of the trial. The platform was removed from the pool and the animal was dropped into the pool from a single fixed position. The time the rats spent in the target quadrant (the quadrant where the platform was retained) was then calculated [21].
2.4.5. Brain tissues biochemical analysis
Brain tissues were homogenized in PBS to obtain 20 % homogenate. The supernatant was used to determine the concentrations of interleukin-1 (IL-1) (RayBiotech, USA), inducible nitric oxide synthase (iNOS) (RayBiotech, USA), acetylcholine (Ach) (Biovision, USA), amyloid protein (Aβ1−42) (My Bioscource, USA), and myelin basic protein (MBP) (RayBiotech, USA) using Elisa kits.
2.4.6. Brain tissue levels of monoamines
Each brain tissue was thoroughly homogenized in 75 % aqueous HPLC grade methanol (10 % w/v). Brain monoamines were detected by HPLC and the method described by Pagel et al. [22] was conducted [22].
2.4.7. Histopathological examination
Two brains from each group were fixed in 10 % neutral buffered formalin for at least 72 h, washed, dehydrated, and embedded in paraffin. Afterwards, brains were sectioned coronally at sections of 4 μm thick. Sections were stained with hematoxylin and eosin (H&E) for routine histopathological examination. Five sections per group were examined using a binocular Olympus CX31 microscope (UK). For quantitative analysis, five sections per group were examined. In order to assess the brain injury and neuronal loss that could be demonstrated in the cerebral cortex and hippocampus, the normal surviving neurons were counted in the cerebral cortex and CA1 region of hippocampus in an area of 1um2, as described by [23], with some modifications [23]. Then the data was statistically analyzed. Additionally, gliosis was assessed in ten random high power microscopic fields per group, according to the number of glial cells. Mild gliosis indicates few number of glial cells in the field; moderate gliosis indicates larger number of glial cells and diffuse gliosis indicates intense number of glial cells associated with sparse normal neurons.
2.4.8. Statistical analysis
Values are presented as means ± standard error of the means (SE). One-way analysis of variance (ANOVA) followed by Tukey test for multiple comparisons were conducted for comparisons between different groups. However, the spatial memory and learning test measured by MWM two-way ANOVA was used. Graphpad Prism software, version 7 (USA) was utilized to perform these statistical tests whereas, the difference was considered significant when p < 0.05.
3. Results
3.1. Docking study
The molecular docking of the two compounds under investigation on TTK1 showed notable binding affinities as estimated through the estimated free energies of binding recorded by MOE homology modeling tool which estimated -5.962 kcal/mol for β-carotene and -6.406 kcal/mol for zeaxanthin. However, they both showed high affinities towards acetylcholinesterase active site as defined by the estimated free energies of binding. β-carotene recorded free estimated energy of binding of -7.687 kcal/mol whereas zeaxanthin recorded -6.142 kcal/mol (Figs. 1&2 ).
Fig. 1.
Virtual 2-dimensional (A) and 3-dimentional (B) interaction of β-carotene and 2-dimensional (C) and 3-dimentional (D) interaction of zeaxanthin with the active site of TauTubulin Kinase 1 (TTK1).
Fig. 2.
Virtual 2-dimensional (A) and 3-dimentional (B) interaction of β-carotene and 2-dimensional (C) and 3-dimentional (D) interaction of zeaxanthin with the active site of Acetylcholinesterase.
3.2. Behavioral variations
3.2.1. Motor coordination assessment using rotarod
Rats’ motor coordination has been declined dramatically in the group injected with D-gal by about 38 % thus indicating an impairment in the motor coordination in these animals. Oral treatment of motor deficit rats with D. salina biomass and its isolated polar, carotenoid fractions and zeaxanthin showed an elevation in the motor coordination levels by about 8.5 %, 15.9 %, 24.8 % and 36.4 %, respectively (Fig. 3).
Fig. 3.
Effect of D. salina biomass and its isolated polar and carotenoid fractions as well as the separated zeaxanthin on locomotor activity in dementia-induced rats.
Data was expressed as mean ± SEM, n= 6 rats/group.
a Significantly different from the normal control.
b Significantly different from the AD group at P < 0.05.
3.2.2. Memory and learning assessment by MWM
3.2.2.1. The mean escape latency time (MELT)
There were no major variations among groups in the MELT on the first day of training. However, on the second and third days of the experiment, D-gal markedly augmented the MELT of the rats when compared with the normal rats. Treatment of the D-gal-injected rats with ZH resulted in a prominent reduction in the MELT with respect to D-gal group on the second day of the experiment. However, on the third day experiment, D. salina biomass and its isolated polar and carotenoid fractions as well as zeaxanthin showed marked recovery of the D-gal-induced effect on the escape latency time (Table 2).
Table 2.
Effect of D. salina biomass and its isolated polar and carotenoid fractions on the mean escape latency time in Morris water maze in dementia-induced rats.
| GROUPS | Acquisition Phase MWM |
||
|---|---|---|---|
| Mean Escape Latency time (sec) |
|||
| 1st Day | 2nd Day | 3rd Day | |
| Normal | 29.47 ± 4.24 | 28.05 ± 1.63 | 26.25 ± 1.83 |
| D-Gal | 52. 45. ± 5.52a | 40.6 ± 2.59a | 38.9 ± 3.61a |
| D-Gal + BDS | 40.04 ± 2.22 | 37.45 ± 2.71 | 27.7 ± 1.47b |
| D-Gal + PDS | 37.64 ± 5.53 | 34.75 ± 2.02 | 26.55 ± 1.44b |
| D-Gal + CDS | 29.92 ± 4.18b | 31.00 ± 1.31b | 21.35 ± 2.61b |
| D-Gal + ZH | 28.89 ± 3.32b | 29.56 ± 2.01b | 23.88 ± 1.54b |
Data was expressed as mean ± SEM, n = 6 rats/group.
Significantly different from the normal control on the corresponding day at P < 0.05.
Significantly different from the AD group on the corresponding day at P < 0.05.
3.2.2.2. The time spent on the target
Intraperitoneal D-gal injection dramatically lowered the mean time spent by rats in the goal quadrant by 47 % suggesting decreased learning and memory functions. However, treatment of animals with D. salina biomass and its isolated polar, carotenoid fractions and zeaxanthin led to a substantial surge in mean time spent in the goal quadrant by 59 %, 69 %, 79 % and 78 %, respectively, when compared with the D-gal group, suggesting improvement in learning and memory functions (Fig. 4).
Fig. 4.
Effect of D. salina biomass and its isolated polar and carotenoid fractions as well as the separated zeaxanthin on mean time spent in the quadrant in dementia-induced rats.
Data was expressed as mean ± SEM, n= 6 rats/group.
a Significantly different from the normal control.
b Significantly different from the AD group at P < 0.05.
3.3. Brain biochemical levels
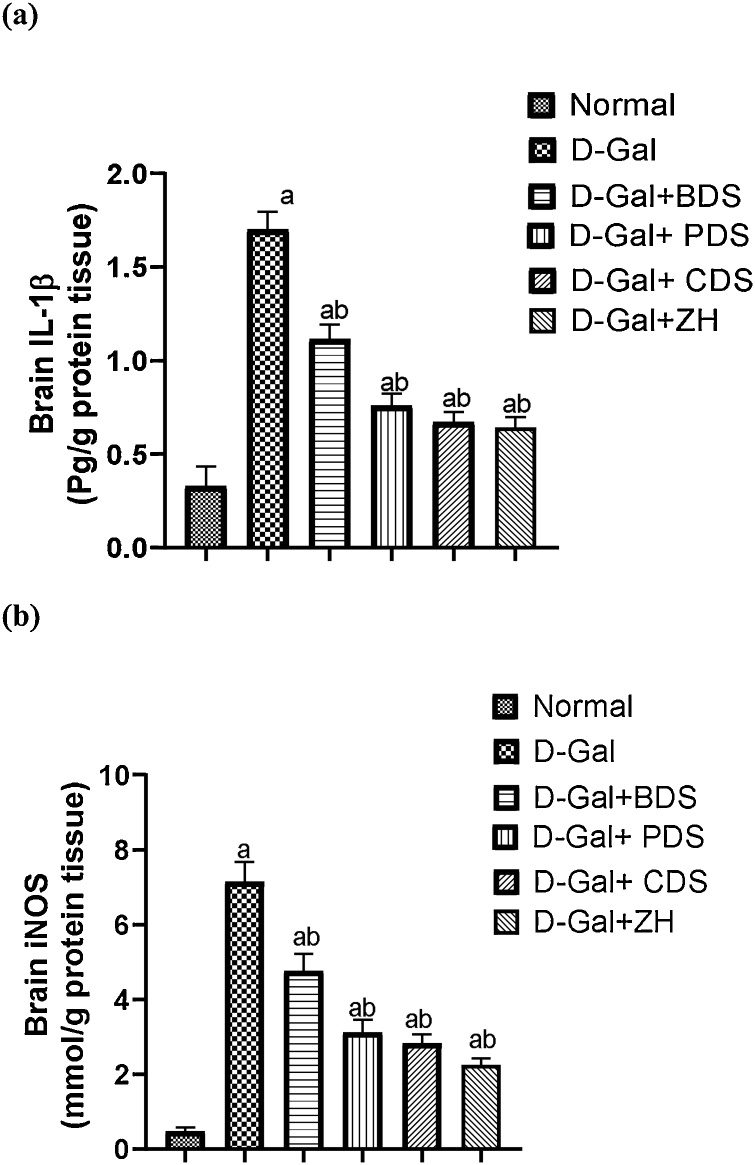
Age-related dementia is associated with prominent alterations in the brain contents of IL-1β and iNOS demonstrated by their elevations reaching about 5 and 15 folds, respectively, compared with normal group. Treatment of D-gal induced dementia in rats with D. salina biomass and its isolated polar, carotenoid fractions and zeaxanthin showed a reduction in these inflammatory indicators by 34.4 %, 55.3 %, 60.6 % and 62 %, respectively and 33.3 %, 56.4 %, 60.2 % and 67 %, respectively, compared to untreated rats (Fig. 5a & b).
Fig. 5.
Effect of D. salina biomass and its isolated polar and carotenoid fractions as well as the separated zeaxanthin on brain IL-1β (a) and iNOS (b) levels in dementia-induced rats.
Data was expressed as mean ± SEM, n= 6 rats/group.
a Significantly different from the normal control.
b Significantly different from the AD group at P < 0.05.
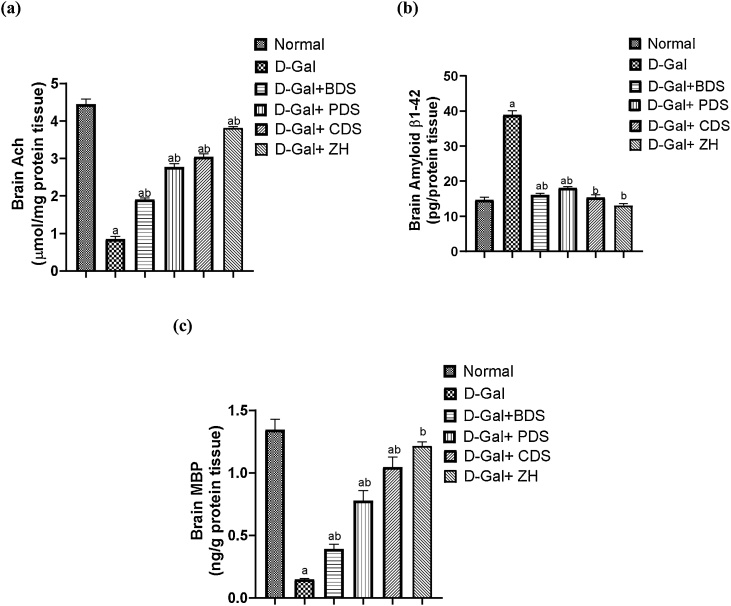
Similarly, brain Ach, Aβ and MBP contents have been altered in dementia-induced rats evidenced by dramatic elevation of Aβ reaching 2.6 folds and decline of Ach and MBP by about 81 % and 89 %, respectively with respect to normal rats. On the other hand, treatment with D. salina biomass and its isolated polar and carotenoid fractions as well as the separated compound; ZH, exerted an elevation of brain Ach levels by reaching 2.2, 3.3, 3.6 and 4.5 folds, respectively (Fig. 6a). Moreover, oral treatment with D. salina biomass and their fractions as well as ZH showed an amelioration in the brain Aβ contents by 58.7 %, 53.5 %, 60.4 % and 66.4 %, respectively (Fig. 6b) and exaggeration in the brain MBP contents by reaching 2.6, 5.26, 7 and 8.2 folds, respectively (Fig. 6c).
Fig. 6.
Effect of D. salina biomass and its isolated polar and carotenoid fractions as well as the separated zeaxanthin on brain Ach (a), Aβ (b) and MBP (c) contents in dementia-induced rats.
Data was expressed as mean ± SEM, n= 6 rats/group.
a Significantly different from the normal control.
b Significantly different from the AD group at P < 0.05.
3.4. Brain neurotransmitters and their metabolites
Dementia in rats was related with a noticeable reduction in brain neurotransmitters levels viz. 5-HT, NE and DA as well as elevation in their metabolites viz. 5-HIAA and DOPAC. Oral treatment of rats with D. salina biomass and its isolated polar, carotenoid fractions and ZH exhibited an increase in the brain 5-HT, NE and DA levels. However, carotenoid fractions and ZH showed a decrease in the metabolites 5-HIAA and DOPAC with no significance alteration in HVA with respect to the AD group (Table 3).
Table 3.
Effect of D. salina biomass and its isolated polar and carotenoid fractions on the brain neurotransmitters levels in dementia-induced rats.
| GROUPS | Neurotransmitter content (ug/g tissue) |
Metabolites contents (ug/g tissue) |
||||
|---|---|---|---|---|---|---|
| 5-HT | NE | DOP | 5-HIAA | HVA | DOPAC | |
| Normal | 0.32 ± 0.02 | 0.21 ± 0.002 | 0.859 ± 0.005 | 0.33 ± 0.04 | 0.104 ± 0.004 | 0.090 ± 0.003 |
| D-Gal | 0.14 ± 0.012a | 0.15 ± 0.009a | 0.281 ± 0.006a | 0.66 ± 0.01a | 0.098 ± 0.004 | 0.132 ± 0.004a |
| D-Gal + BDS | 0.17 ± 0.01a | 0.19 ± 0.006ab | 0.324 ± 0.008ab | 0.63 ± 0.02a | 0.861 ± 0.02 | 0.120 ± 0.03a |
| D-Gal + PDS | 0.2 ± 0.02ab | 0.17 ± 0.004ab | 0.454 ± 0.003ab | 0.56 ± 0.03a | 0.972 ± 0.06 | 0.109 ± 0.009a |
| D-Gal + CDS | 0.26 ± 0.006ab | 0.20 ± 0.01b | 0.580 ± 0.02ab | 0.45 ± 0.01ab | 0.898 ± 0.03 | 0.099 ± 0.04ab |
| D-Gal + ZH | 0.23 ± 0.005ab | 0.18 ± 0.02b | 0.670 ± 0.01ab | 0.48 ± 0.01ab | 0.980 ± 0.04 | 0.091 ± 0.03ab |
Data was expressed as mean ± SEM, n = 6 rats/group.
a Significantly different from the normal control.
b Significantly different from the AD group at P < 0.05.
3.5. Histopathological alterations
The results of quantitative analysis of surviving normal surviving neurons recorded in the cerebral cortex and CA1 hippocampal region of normal and treated groups are illustrated in Table 4.
Table 4.
Quantitative analysis of surviving neurons recorded in the cerebral cortex and CA1 hippocampal regions of normal and treated groups.
| Gliosis | Number of surviving neurons in CA1 region of hippocampus (count/μm2) | Number of surviving cerebral cortical neurons (count/μm2) | Group |
|---|---|---|---|
| Normal glial cell | 30.70 ± 1.49 | 33.71 ± 1.40 | Normal |
| Diffuse | 17.00 ± 1.19a | 7.50 ± 0.84a | D-gal |
| Moderate | 23.57 ± 2.29ab | 12.40 ± 0.60ab | D-Gal + BDS |
| Moderate | 25.00 ± 2.90ab | 13.70 ± 1.39 ab | D-Gal + PDS |
| Mild | 27.14 ± 3.60b | 26.50 ± 1.91ab | D-Gal + CDS |
| Mild | 29.28 ± 2.11b | 28.50 ± 2.25b | D-Gal + ZH |
Data was expressed as mean ± SEM.
aSignificantly different from the normal control.
bSignificantly different from the AD group at P < 0.05.
Brain sections from normal rats revealed normal histological structure of the cerebral cortex and hippocampus. The cerebral cortical neurons have large cell bodies and nuclei with single prominent nuclei (Fig. 7a). Similarly, the neurons of CA1, CA2 and CA3 regions of the hippocampus appeared normal (Figs. 8a & 2 b). On the contrary, Significant decrease of number of normal cerebral cortical and CA1 hippocampal neurons was recorded in D-Gal group (Table 4). Heterologous degeneration of the entorhinal cortical neurons, that extends to the hippocampus, was clearly evident in D-Gal group. The degenerated neurons appeared with shrunken cell bodies and intensely stained eosinophilic cytoplasm as well as a small pyknotic nuclei (Fig. 7b). Glial response with diffuse gliosis and presence of reactive astrocytes with more distinct eosinophilic cytoplasm and large eccentric nuclei were clearly demonstrated in the frontal cortex (Fig. 7c) of this group. Marked pathological alterations were demonstrated in the amygdala of this group, characterized by neuronal degeneration and diffuse gliosis. Deposition of amyloid plaques with eosinophilic core surrounded by astrocytes and microglia cells was demonstrated in the cerebral cortex (Fig. 7d). Cerebral amyloid angiopathy, with deposition of pink amyloid in the wall of cortical blood vessels, was also demonstrated (Fig. 7e). The hippocampus revealed degeneration of neuronal cells of CA1 and CA2 regions (Figures 8c & 8d). In comparison to D-Gal group, mild improvement with relative increase of normal cerebral cortical and CA1 hippocampal neurons was recorded in D-Gal + BDS and D-Gal + PDS groups (Table 4). Decreased number of degenerated neurons was demonstrated in the cerebral cortex of D-gal-BDS group, in which neuronal degeneration with neuronophagia was frequent (Fig. 7f). Hippocampus of this group revealed degeneration of pyramidal neurons associated with activation of astrocytes (Fig. 8e & f). Decreased number of degenerated neurons associated with moderate was demonstrated in both cerebral cortex (Fig. 7h) and hippocampus (Fig. 8g & h) of D-Gal + PDS group. On the other side, much better amelioration was demonstrated in D-gal-CDS and D-gal-ZH group, with significant increase of number of surviving neurons in the cerebral cortex and CA1 hippocampal region, with non-significant difference between them (Table 4). Few degenerated neurons and mild gliosis were demonstrated in the cerebral cortex (Fig. 7i & j, respectively) and hippocampus (Fig. 8i & j and k & l, respectively).
Fig. 7.
Effect of D. salina biomass and its isolated polar and carotenoid fractions on brain histopathological alterations associated with dementia induced in rats.
Brain tissue of, (a) normal rats showing normal cerebral cortical neurons with large cell bodies and nuclei with single prominent nuclei normal (black arrows), (b,c,d,e) D-gal group showing decreased number of normal neurons and increased number of degenerated neurons, which appeared with shrunken cell bodies and intensely stained eosinophilic cytoplasm as well as a small pyknotic nuclei (red arrows) in addition to activation of microglia (arrow head) (b), diffuse gliosis and presence of reactive astrocytes with more distinct eosinophilic cytoplasm and large eccentric nuclei (black arrow) (c), deposition of amyloid plaques with eosinophilic core surrounded by astrocytes and microglia (black arrow) (d) and cerebral amyloid angiopathy (black arrow) (e), (f) D-gal-BDS group showing degeneration of cerebral cortical neurons (black arrow) associated with neuronophagia (red arrow), (g) D-gal-PDS group showing decreased number of degenerated neurons associated with mild gliosis (arrow), (h) D-gal-CDS group showing pronounced decrease of degenerated neurons (black arrows), and (i) D-gal-ZH group showing sparse degenerated neurons (black arrow). (Stain H&E; Scale bar=100μm).
Fig. 8.
Effect of D. salina biomass and its isolated polar and carotenoid fractions on hippocampus histopathological alterations associated with dementia induced in rats.
Hippocampus of, (a, b) normal rats showing normal neurons (black arrows), (c, d) D-gal group showing increased number of degenerated neurons (black arrows), (e, f) D-gal-BDS group showing degeneration of hippocampal pyramidal neurons (black arrow) associated with activation of astrocytes (red arrow), (g, h) D-gal-PDS group showing decreased number of degenerated neurons associated with mild gliosis (black arrow for degenerated neurons and red arrow for astrocyte), (I, j) D-gal-CDS group showing pronounced decrease of degenerated neurons (black arrow), and (k, l) D-gal-ZH group showing sparse degenerated neurons (black arrow). (Stain H&E; Scale bar=100μm).
4. Discussion
A marked symptom in the progression of AD is the impairment of motor abilities. Several studies have shown a marked decrease in motor function in patients throughout the progression of the disease. In addition, motor impairments can be used in predicting the onset and the outcome of AD [24]. Thus, motor deficits are an important sign to evaluate AD. In the present study, D-gal induced dementia was associated with a marked motor deficiency evidenced by latency falling time in the rotarod apparatus.
Likewise, MWM test results revealed that prolonged D-gal administration damaged rat memory and learning capabilities. Such outcomes in different literatures are in line with previous findings [25,26]. It has been reported that the aging process is related to memory deficiency. The deterioration in memory associated with age is said to be due to elevation in the redox homeostasis imbalance i.e. elevated oxidative stress in the aged brain [5,27], which plays a crucial part in the development and progression of AD [8]. Recent studies have shown a major reduction in the memory abilities of senescence in aged rats. These rats demonstrated spatial deterioration of memory, which was evidenced by increasing latency time in locating the submerged platform.
Acetylcholine plays a crucial part in cognitive processes, and the cholinergic system has been linked to various types of dementia, including Alzheimer's disease [28]. Cholinergic transmission deficits have the ability to affect many aspects of cognition and behavior, including cortical and hippocampal information processing. Disruption of cholinergic inputs to the cortex can affect attention and the ability to use instructional signals for ongoing behavior decision-making [29]. On the other hand, treatment with D. salina biomass and its isolated polar and carotenoid fractions as well as zeaxanthin, restored brain Ach levels when compared with the AD-induced groups of rats. These results have been confirmed by the docking study which presented β-carotene and zeaxanthin have obvious affinity towards acetylcholinesterase which prevent the breakdown of Ach thus increase its levels.
In hippocampal memory formation, Aβ peptides, like Aβ, play a significant physiological role [30]. There is a balance between the development and elimination of Aβ in young brains and under normal conditions, which preserves Aβ at steady state. However, in aging, there are disruptions in the formation of Aβ that contribute to Aβ(1–42) accumulation and to the formation of senile plaque [31]. Interestingly, it has been noticed that very low levels of Aβ can play a role in neural progress and cholinergic neurotransmission regulation [32]. Besides, in reaction to oxidative conditions, neurons overexpress Aβ to diminish the effects of oxidative stress. It also appears that, in addition to neurotoxicity, Aβ(1–42) in higher levels reduces blood flow throughout the cerebral system and accelerates neuronal dysfunction [33]. Remarkably, treatment of D-gal induced dementia in rats with D. salina biomass and its fractions exerted an amelioration in the brain Aβ(1–42) contents indicating the protective role of the microalgae against age-related dementia. Previous study has attributed the modulatory role of D. salina towards AD to the reduction in the levels of DNA adducts. It has been documented that D. salina is considered to have a high carotenoid content. In addition, in both in vitro and in vivo studies, D. salina extracts have been shown to contain high levels of antioxidant activities. These studies have shown that the ameliorative effect of D. salina is due to the content of 9-cis b-carotene [34]. The docking study also has confirmed all the previous results and displayed that both ligands; β-carotene and zeaxanthin have noticeable affinity towards Tau-Tubulin Kinase 1 and acetylcholinesterase.
Conversely, MBP; the basic protein in myelin sheath of the central nervous system, significantly inhibits its fibrillary assembly as a novel Aβ chaperone. It has been also proposed to have a major role in intracellular signaling by interactions with membrane actin and tubulin [35]. The MBP in the white matter of rats’ brain in each group was observed. In D-gal group MBP content was decreased as a result of white matter damage which cause chronic white matter deficiency [36]. The results of the current study also indicated that D. salina could increase the low MBP content caused by myelin sheath damage, decrease the damage caused by inadequate cerebral perfusion to the myelin sheath, and play a protecting role of the white matter.
Interestingly, a number of studies in AD patients and animal models of AD pathology have documented myelin loss and breakdown of MBP. AD risk factors; for instant aging are associated with this loss of myelin and an elevation of Aβ peptides [37]. In comparison, regions of white matter abundantly supplied with MBP i.e. corpus callosum, striatum, show very little deposition of Aβ. Taken together, these results indicate an inverse association between MBP and Aβ levels. Whether MBP may actually affect in vivo accumulation of Aβ, however, remains uncertain [38].
It has been demonstrated that carotenoids such as zeaxanthin can improve the neural efficiency. Hence, increasing central zeaxanthin levels could boost neural performance, both speeding up neural conduction and reducing the probability of cross-talking between axons at the same time [39]. Carotenoids have been shown to be more effective at defending liposomes from lipid peroxidation than any existing carotenoid [40].
Age-related decline in the amount of biogenic amines in brain tissue, i.e. NA, DA, and 5-HT as well as elevation in their metabolites viz. 5-HIAA and DOPAC were also observed in the current research which have been tightly related to cognitive processes such as concentration and learning. Previous studies have shown that brain neurotransmitters levels have decreased by age [8]. In the initial stages of AD, serotonergic dysfunction is most pronounced and 5-HT activity has been decreased and its metabolite has been found in the post-mortem AD brain. The temporal cortex of patients with AD had reduced 5-HT reuptake sites as well as decreased 5-HT and metabolite levels. These disturbances have been observed in AD in both post-synaptic and pre-synaptic 5-HT systems [41].
Administration of D-gal has extensively damaged multiple neurotransmitter systems viz. serotonergic and dopaminergic systems. The role of 5-HT and its receptors in various aspects of memory and learning functions has also been described in preclinical and clinical studies. Interestingly, the current study has shown that oral treatment of rats with D. salina biomass and its isolated polar, carotenoid fractions and ZH exhibited an increase in the brain levels of 5-HT, NE and DA. Moreover, salina biomass and its isolated polar fraction showed a slight decrease in the neurotransmission metabolites not yet significant, however, carotenoid fractions and ZH showed a marked reduction in the metabolites 5-HIAA and DOPAC.
Furthermore, age-related dementia in the current study has displayed dramatic incrimination in the brain contents of IL-1β and iNOS. Previous studies have shown that over-expression of the immune-modifying cytokine IL-1β produced by microglia and astrocytes covering Aβ plaques occurs compared to age-matched controls in the AD brain and in animal models of AD [42]. Similarly, it has been demonstrated that iNOS as key Aβ action mediators [43]. Treatment of D-gal induced dementia in rats with D. salina biomass and its isolated polar and carotenoid fractions showed a reduction in these inflammatory indicators compared to untreated rats. All of these outcomes has been confirmed by the histopathological examinations.
5. Conclusion
From all these findings, new sights have been clarified that D. salina and its fractions particularly its zeaxanthin containing carotenoids as well as the isolated zeaxanthin could constitute a new therapeutic opportunity for learning and memory alterations as a multi-targeting approach in neuroprotection, neuroregeneration, and other rational perspectives in novel drug development are highlighted by general antioxidant and anti-inflammatory pathways. Furthermore, D. salina counteracts brain aging due to its modulatory role in attenuating Aβ protein and neurotransmitters and their metabolites and thus repair age-related dementia.
Data availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgement
The authors also are grateful to Dr. Azza Hassan, Department of Pathology, Faculty of Veterinary Cairo University, Egypt, for her contribution in histopathological examinations.
Handling Editor: DR. Aristidis Tsatsakis
Contributor Information
Farouk K. El-Baz, Email: fa_elbaz@hotmail.com.
Gehad A. Abdel Jaleel, Email: gehad_abougharam@yahoo.com.
References
- 1.Aloizou A.-M., Siokas V., Vogiatzi C., Peristeri E., Docea A.O., Petrakis D., et al. Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. doi: 10.1016/j.toxlet.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Duong S., Patel T., Chang F. Dementia: what pharmacists need to know. Can. Pharm. J. (Ott) 2017;150(2):118–129. doi: 10.1177/1715163517690745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S.K., Srivastav S., Yadav A.K., Srikrishna S., Perry G. Overview of alzheimer’s disease and some therapeutic approaches targeting aβ by using several synthetic and herbal compounds. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/7361613. 7361613-7361613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., et al. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review) Mol. Med. Rep. 2019;19(4):2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubandru M., Margina D., Tsitsimpikou C., Goutzourelas N., Tsarouhas K., Ilie M., et al. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food and Chem. Toxicol.: an Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;61:209–214. doi: 10.1016/j.fct.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Kandimalla R., Reddy P.H. Therapeutics of neurotransmitters in alzheimer’s disease. J. Alzheimer’s Dis.: JAD. 2017;57(4):1049–1069. doi: 10.3233/JAD-161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietá Dias C., Martins de Lima M.N., Presti-Torres J., Dornelles A., Garcia V.A., Siciliani Scalco F., et al. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146(4):1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Haider S., Saleem S., Perveen T., Tabassum S., Batool Z., Sadir S., et al. Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordr) 2014;36(3) doi: 10.1007/s11357-014-9653-0. 9653-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha R., Rizvi S.I. Age-dependent decline in erythrocyte acetylcholinesterase activity: correlation with oxidative stress. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. 2009;153(3):195–198. doi: 10.5507/bp.2009.032. [DOI] [PubMed] [Google Scholar]
- 10.Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011;219(2):197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.H., Hwang I.K., Choi J.H., Yoo K.Y., Park O.K., Huh S.O., et al. Age-dependent changes in calretinin immunoreactivity and its protein level in the gerbil hippocampus. Neurochem. Res. 2010;35(1):122–129. doi: 10.1007/s11064-009-0037-2. [DOI] [PubMed] [Google Scholar]
- 12.Peters R. Ageing and the brain. Postgrad. Med. J. 2006;82(964):84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meneses A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 1999;23(8):1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 14.Puzzo D., Privitera L., Leznik E., Fà M., Staniszewski A., Palmeri A., et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci.: Off. J. Soc. Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Baz F., Abdel Jaleel G., Saleh D., Hussein R. Protective and therapeutic potentials of <i>Dunaliella salina</i> on aging-associated cardiac dysfunction in rats. Asian Pac. J. Trop. Biomed. 2018;8(8):403–410. [Google Scholar]
- 16.El-Baz F.K., Hussein R.A., Saleh D.O., Abdel Jaleel G.A.R. Zeaxanthin isolated from Dunaliella salina microalgae ameliorates age associated cardiac dysfunction in rats through stimulation of retinoid receptors. Mar. Drugs. 2019;17(5) doi: 10.3390/md17050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Baz F.K., Saleh D.O., Abdel Jaleel G.A., Hussein R.A., Hassan A. Heamatococcus pluvialis ameliorates bone loss in experimentally-induced osteoporosis in rats via the regulation of OPG/RANKL pathway. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.109017. [DOI] [PubMed] [Google Scholar]
- 18.El-Baz F.K., Saleh D.O., Abdel Jaleel G.A., Hussein R.A. Attenuation of age-related hepatic steatosis by Dunaliella salina microalgae in senescence rats through the regulation of redox status, inflammatory indices, and apoptotic biomarkers. Adv. Pharm. Pharm. Sci. 2020;2020:3797218. doi: 10.1155/2020/3797218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter R.J., Morton J., Dunnett S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001 doi: 10.1002/0471142301.ns0812s15. Chapter 8: Unit 8.12. [DOI] [PubMed] [Google Scholar]
- 20.Morris R.G. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12(2):239–260. [Google Scholar]
- 21.Bromley-Brits K., Deng Y., Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011;(53) doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagel P., Blome J., Wolf H.U. High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J. Chromatogr. B Biomed. Sci. Appl. 2000;746(2):297–304. doi: 10.1016/s0378-4347(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 23.Khalil M.N.A., Choucry M.A., El Senousy A.S., Hassan A., El-Marasy S.A., El Awdan S.A., et al. Ambrosin, a potent NF-kappabeta inhibitor, ameliorates lipopolysaccharide induced memory impairment, comparison to curcumin. PLoS One. 2019;14(7):e0219378. doi: 10.1371/journal.pone.0219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner J.M., Sichler M.E., Schleicher E.M., Franke T.N., Irwin C., Löw M.J., et al. Analysis of motor function in the Tg4-42 mouse model of alzheimer’s disease. Front. Behav. Neurosci. 2019;13(107) doi: 10.3389/fnbeh.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenawy S., Hegazy R., Hassan A., El-Shenawy S., Gomaa N., Zaki H., et al. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: protective role of metformin and saxagliptin. PLoS One. 2017;12(8):e0183565. doi: 10.1371/journal.pone.0183565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehman S.U., Shah S.A., Ali T., Chung J.I., Kim M.O. Anthocyanins reversed D-Galactose-Induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017;54(1):255–271. doi: 10.1007/s12035-015-9604-5. [DOI] [PubMed] [Google Scholar]
- 27.Bagheri M., Joghataei M.T., Mohseni S., Roghani M. Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2011;95(3):270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Maurer S.V., Williams C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017;8(1489) doi: 10.3389/fimmu.2017.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: targeting the cholinergic system. Curr. Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Osta A., Alberini C.M. Amyloid beta mediates memory formation. Learn. Mem. 2009;16(4):267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baranello R.J., Bharani K.L., Padmaraju V., Chopra N., Lahiri D.K., Greig N.H., et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 2015;12(1):32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadigh-Eteghad S., Sabermarouf B., Majdi A., Talebi M., Farhoudi M., Mahmoudi J. Amyloid-beta: a crucial factor in alzheimer’s disease. Med. Princ. Pract. 2015;24(1):1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cioffi F., Adam R.H.I., Broersen K. Molecular mechanisms and genetics of oxidative stress in alzheimer’s disease. J. Alzheimer’s Dis.: JAD. 2019;72(4):981–1017. doi: 10.3233/JAD-190863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Baz F.K., Aly H.F., Abd-Alla H.I. The ameliorating effect of carotenoid rich fraction extracted from Dunaliella salina microalga against inflammation- associated cardiac dysfunction in obese rats. Toxicol. Rep. 2019;7:118–124. doi: 10.1016/j.toxrep.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou-Yang M.-H., Van Nostrand W.E. The absence of myelin basic protein promotes neuroinflammation and reduces amyloid β-protein accumulation in Tg-5xFAD mice. J. Neuroinflammation. 2013;10 doi: 10.1186/1742-2094-10-134. 134-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang K., Shen L., Niu T., Zhao Y., Fu J., Cao Y. Naomaitai ameliorated brain damage in rats with vascular dementia by PI3K/PDK1/AKT signaling pathway. Evid. Based Complement. Altern. Med. 2019;2019:2702068. doi: 10.1155/2019/2702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papuć E., Rejdak K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2018;16(2):345–351. doi: 10.5114/aoms.2018.76863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rossi P., Buggia-Prévot V., Clayton B.L., Vasquez J.B., van Sanford C., Andrew R.J., et al. Predominant expression of Alzheimer’s disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol. Neurodegener. 2016;11(1):59. doi: 10.1186/s13024-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renzi-Hammond L.M., Bovier E.R., Fletcher L.M., Miller L.S., Mewborn C.M., Lindbergh C.A., et al. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients. 2017;9(11) doi: 10.3390/nu9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahl W., Sies H. Bioactivity and protective effects of natural carotenoids. Biochimica et Biophysica Acta (BBA) – Mol. Basis of Dis. 2005;1740(2):101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Šimić G., Babić Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milošević N., et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter J.M., Kwan J., Malek-Ahmadi M., Maarouf C.L., Kokjohn T.A., Belden C., et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One. 2012;7(5):e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros R., Prediger R.D.S., Passos G.F., Pandolfo P., Duarte F.S., Franco J.L., et al. Connecting TNF-α signaling pathways to iNOS expression in a mouse model of alzheimer’s disease: relevance for the behavioral and synaptic deficits induced by amyloid β protein. J. Neurosci. 2007;27(20):5394–5404. doi: 10.1523/JNEUROSCI.5047-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.