Abstract
Monocytes are innate immune cells that develop in the bone marrow and are continually released into circulation, where they are poised to enter tissues in response to homeostatic or inflammatory cues. Monocytes are highly plastic cells that can differentiate in tissues into a variety of monocyte-derived cells to replace resident tissue macrophages, promote inflammatory responses, or resolution of inflammation. As such, monocytes can support tissue homeostasis as well as productive and pathogenic immune responses. Recent work shows previously unappreciated heterogeneity in monocyte development and differentiation in the steady state and during infectious, autoimmune, and inflammatory diseases. Monocyte-derived cells can differentiate via signals from cytokines, pattern recognition receptors or other factors, which can influence development in the bone marrow or in tissues. An improved understanding of these monocyte-derived cells and the signals that drive their differentiation in distinct inflammatory settings could allow for targeting these pathways in pathological inflammation.
Keywords: Monocytes, myelopoiesis, inflammation
Introduction
In the past decade, we have come to understand monocyte development, differentiation, and homeostasis in much greater detail. Much of this fundamental work has been in the mouse system, which is infinitely tractable with sophisticated genetic, cell labeling and tracking techniques. However, recent studies in human systems have given us new insights into these same processes. Several excellent recent reviews have been published on monocyte development, differentiation, and homeostasis in the steady state [1–3]. Here, we focus on these processes during infection and inflammation, highlighting signals that lead to alterations in these programs during infectious, inflammatory, and autoimmune diseases, which can lead to changes in progenitor production of monocytes and in monocyte differentiation in tissues.
Blood monocytes comprise at least three populations of cells, typically defined by cell surface receptor expression: the “classical” or “inflammatory” monocytes (defined as Ly6Chi in mouse and CD14++CD16- in humans), the “non-classical” or “patrolling” monocytes (Ly6Clo in mouse, CD14+CD16++ in humans), and the “intermediate” monocytes. Both mouse and human monocytes share high CCR2 and intermediate CX3CR1 expression on classical, inflammatory monocytes, and low CCR2 and high CX3CR1 expression on non-classical, patrolling monocytes [4–6]. Classical monocytes are the major population, comprising ~85% of blood monocytes, and give rise to patrolling monocytes through an intermediate monocyte transition [7,8]. Classical monocytes are also the principal monocyte population that differentiate into various macrophage and monocyte-derived dendritic cell (DC) populations. Therefore, classical monocytes are highly plastic cells on which we will focus.
Monocyte development and heterogeneity
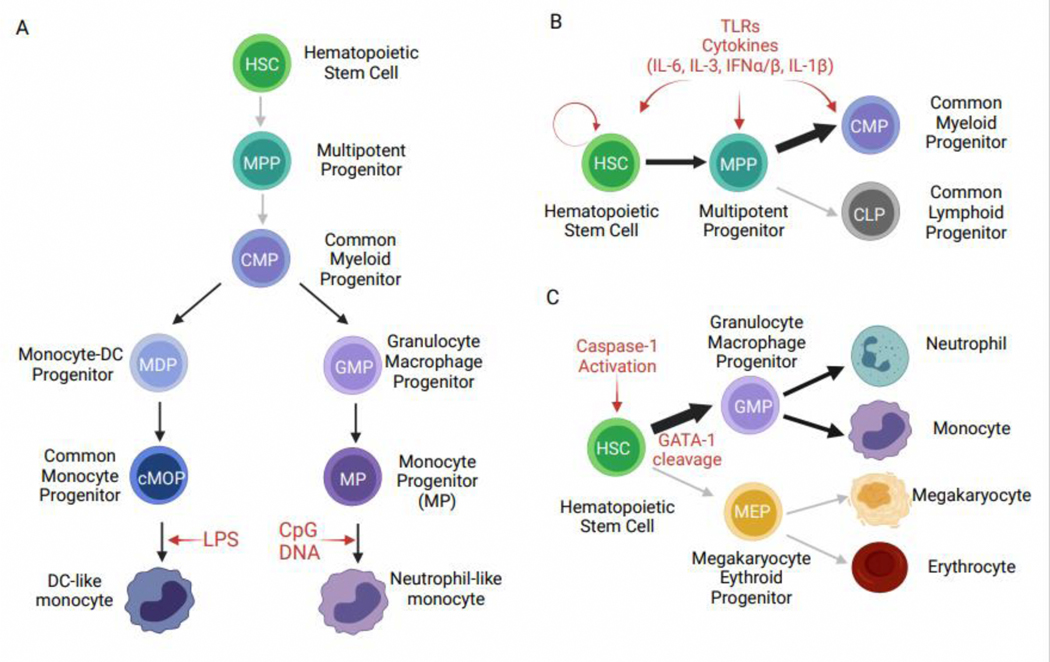
Myelopoiesis in the bone marrow (BM) begins with committed myeloid progenitors (CMPs) that generate monocytes, neutrophils, and dendritic cells. It was originally thought that a simple linear pathway leads from common myeloid progenitors (CMPs) to classical monocytes via granulocyte macrophage progenitors (GMPs), monocyte dendritic cell progenitors (MDPs), and finally a restricted common monocyte progenitor (cMoP). Work by Yanez et al. showed that monocyte development in the BM proceeds via two parallel pathways during homeostasis—one more closely related to neutrophils, and one more closely related to DCs [9]. These pathways diverge at the common myeloid progenitor (CMP), with some monocytes developing in a CMP→GMP→MP→monocyte trajectory, while other monocytes develop in a CMP→MDP→cMOP→monocyte trajectory (Figure 1A). In vitro differentiation and in vivo adoptive transfer studies coupled with gene expression analyses showed that these pathways give rise to highly related, yet distinct Ly6ChiCCR2hi classical monocytes. GMP-derived monocytes express higher levels of genes typically associated with neutrophils and have been termed neutrophil-like monocytes, while MDP-derived monocytes express genes involved in antigen presentation and DC function and have been termed DC-like monocytes [9]. MHCII+CD209a+ monocytes in the blood had previously been identified by Menezes et al. as progenitors of monocyte-derived DCs [10] and are likely the same population of MDP-derived monocytes characterized by Yanez et al. [9]. A recent study using clonal barcoding of hematopoietic progenitors followed by in vitro and in vivo differentiation and single cell (sc)RNA-Seq of progeny also supports these two routes of monocyte differentiation [11]. scRNA-Seq studies of human peripheral blood myeloid cells have also supported heterogeneity within the CD14hi classical monocyte population [12,13], though whether similar parallel monocyte development pathways exist in humans remains to be determined.
Figure 1: Bone marrow myelopoiesis during inflammation.
A) Development of DC-like and neutrophil-like monocytes in the bone marrow. HSCs and MPPs generate CMPs that are committed to the myeloid lineage, at which point monocyte development pathways diverge. CMPs can differentiate into MDPs that generate cMOPs, that in turn make DC-like monocytes. CMPs can also differentiate into GMPs that generate MPs, which then make neutrophil-like monocytes. In vivo treatment with LPS promotes the development of DC-like monocytes, whereas treatment with CpG DNA promotes the development of neutrophil-like monocytes. B) Emergency myelopoiesis promotes myelopoiesis over lymphopoiesis in response to infection or inflammation. This can be via direct signals, such as TLRs, on HSCs, MPPs, or CMPs or via indirect signals, such as cytokines made by other cells or progenitors themselves. C) In a zebrafish model, caspase-1 activation in HSCs caused the cleavage of GATA-1, a key transcription factor promoting megakaryocyte and erythrocyte development, leading to increased output of monocytes and neutrophils and reduced megakaryocytes and erythrocytes.
Monocyte development in the bone marrow during inflammation or infection
The process of emergency myelopoiesis induces preferential myeloid over lymphoid development, yielding increased monocytes and neutrophils to rapidly respond to pathogens. A variety of signals can promote emergency myelopoiesis, including both cytokines and direct sensing of pathogen products through pattern recognition receptors such as Toll-like receptors (TLRs) (Figure 1B). These signals can act on cells as early as hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs), as well as cells already committed to the myeloid lineage, such as CMPs (reviewed in [14–16]). All hematopoietic stem and progenitor cells express some TLRs that can directly drive macrophage differentiation in vitro, though in vivo TLRs can have both direct and indirect effects via cytokines [17–23]. TLR signaling in vivo differentially induces monocyte expansion downstream of GMPs and MDPs, giving rise to GMP-derived monocytes after LPS treatment and MDP-derived monocytes after CpG DNA treatment (Figure 1A, Table 1) [9]. However, whether this effect is direct or indirect remains an open question. More recently, the inflammasome family of innate sensors has been implicated in driving emergency myelopoiesis through mature IL-β release during injury [24]. Interestingly, Tyrkalska et al. demonstrated that caspase-1 cleavage of GATA-1, a key erythroid lineage determining factor, in HSCs promoted myelopoiesis over erythropoiesis during chronic infection in a zebrafish model (Figure 1C) [25]. Thus, infection and inflammation can be sensed in the BM to shape myeloid output.
Table 1:
Mouse monocyte differentiation during inflammation
| Inflammatory Environment | Cell type | Tissue | Signal or Mechanism | Example Markers | Functions and/or outcomes | Ref. |
|---|---|---|---|---|---|---|
| LPS in vivo | “Neutrophil-like” monocyte | BM, blood, spleen | LPS (unknown direct or indirect) | Ly6Chi, CCR2hi, CD115+, Elane, Prtn3, Ctsb, Serpinb1a, MPO | Primary granule proteases, microbicidal response | [9] [11] |
| CpG DNA in vivo | “DC-like” monocyte | BM, blood, spleen | CpG DNA (unknown direct or indirect) | Ly6Chi, CCR2hi, CD115+, CD11c+ MHCIIhi, CD86hi, CD74, Flt3, CD209a | Antigen presentation genes | [9] [11] |
| GM-CSF | CD209a+ moDC monocyte progenitor | BM, blood | High levels of PU.1 | CD115+,Ly6Chi, MHCII+, CD209a+, FcγRIII+, PU.1hi | DC-related genes and functions | [10] |
| LPS, L.m. infection | iNOS+ macrophage monocyte progenitor | BM, blood | Low levels of PU.1 | CD115+, Ly6Chi, FcgRIII+, CD209a,MHCII-,PU.1lo | Microbicidal response | [10] |
| Acute injury to bone marrow | HSCs → Myeloid differentiation | BM | Il-1β downstream of inflammasome activation | Mac-1, CD16/32, PU.1, GM-CSFR, M-CSFR, CD18 | Leads to Mac-1+Gr-1int pregranulocytes/ monocytes | [24] |
| Caspase-1 inhibition of mouse HSCs in vitro; Overexpression of ASC & Caspa, S. Tminfection* | HSCs → GMP → MDPs → monocytes (and neutrophils) in mouse and zebrafish models; | Mouse BM; Zebrafish larvae | Caspase-1 cleavage of GATA-1 | N/A | Myelopoiesis over erythropoiesis | [25] |
| L.m. infection | Tip-DCs | Spleen | Require NK cell-derived IFNγ | CD11bint, CD11cint, Mac-3hi, TNF, iNOS, MHCII | Bacterial clearance, host survival | [26] [27] [28] |
| T. gondii infection | cMOP→ Ly6Chiregulatory monocytes | SI lamina propria | lipid mediator PGE2, Require IFNγ produced by NK cells | MHCII, Sca-1, CX3CR1++ | Controls neutrophilic inflammation in the gut | [38] [39] |
| Bleomycin-induced lung fibrosis | GMP→SatMs | Lung | Unknown | CD115, myeloperoxidase and neutrophil elastase | Promote pathogenic fibrosis | [41] |
| Chronic TLR7/TLR9 (SLE-MAS model), P. yoelii infection | Inflammatory hemophagocytes (iHPCs) | BM, spleen | Cell-intrinsic TLR7/9 & IRF5 (MAS), MyD88 & Unc93b1 (P. yoelii) | SpiC, CD11bhi, CD31hi, PDL2 | Hemophagocytosis, contribute to anemia & thrombocytopenia in SLE-MAS disease model | [42] |
| Helicobacter hepaticusinduced colitis | Inflammatory macrophages | LI lamina propria | Require IRF5 | CD11c+, MHCII+ | Promote pathogenic intestinal inflammation | [35] |
| Chikungunya virus infection | Inflammatory macrophages | Lymph node | Require IRF5 | CD11c+, MHCII+ | Disrupt lymph node structure & B cell response | [29] |
| Atherosclerosis | Inflammatory macrophages | Aortic plaque | Require IRF5 | CD11c+, MHCII+ | Lesion development | [32] |
| DSS-induced colitis | Regulatory monocytes | BM, spleen, blood | GM-CSF and IL-3 | Ly6ChiYm1+ | Promote tissue repair | [40] |
| Nod2-driven inflammation | Patrolling monocytes | BM, Vasculature | Muramyl dipeptide, unknown direct vs indirect | CD115+Ly6CloCD43+LFA1+CX3CR1+ Nr4a1 | Crawling on endothelium | [48] |
| Models of Lupus-like disease | Patrolling monocytes | Kidney vasculature | TLR7/9 & MyD88-dependent require Nod2 | CD115+,Ly6Clo, CD43hi, F4/80lo, Nr4a1, Cebpb, | Drive early glomerulonephritis | [49] [50] |
| TLR7-driven inflammation | Patrolling monocytes | Vasculature | Dll1-Notch2 signaling | CD115+,Ly6Clo, CD43+, CD11c+, Nr4a1, Pou2f2 | Crawling on endothelium, Endothelial repair | [51] [52] |
Zebrafish
Unique monocyte differentiation fates during inflammation and signals driving these fates
Under homeostatic conditions, monocytes migrate into tissues and differentiate into macrophages or specialized monocyte populations depending on environmental signals or can remain as a monocyte reservoir. During infection or inflammation, monocytes rapidly enter inflamed tissues, and work by several groups has identified unique monocyte fates with specialized functions in different inflammatory settings. Here, we highlight several monocyte-derived populations defined recently and discuss signals identified to drive their differentiation. Although the terminology defining these cell populations (e.g. monocyte vs. monocyte-derived macrophage vs. monocyte-derived DCs) often differs depending on the biological context, it is important to note that some of these populations may overlap due to a lack of consistent markers distinguishing these cells. Thus, it is important to keep an open mind when comparing cells described by different laboratories.
Early work often focused on classical monocytes entering inflamed tissues and becoming bactericidal, inflammatory macrophages. Pioneering work from Eric Pamer’s lab defined the role of the chemokine receptor CCR2 in releasing mature classical monocytes from the BM into circulation during infection, increasing available blood monocytes for recruitment into tissues [26]. They used Listeria monocytogenes infection to define signals for monocyte release from the BM and monocyte differentiation in the spleen required for control of this infection. Monocytes upregulate inflammatory markers during infection and participate in bacterial clearance (Table 1). These cells were initially called Tip-DCs (TNF iNOS-producing DCs) [27,28] and have since been identified in many infectious and inflammatory settings [29]. Interestingly, this splenic monocyte differentiation process required NK cell-derived interferon (IFN) γ [30]. More recently, the circulating precursors that differentiate into these iNOS+ inflammatory macrophages during Listeria infection were identified as a specific classical monocyte subpopulation [10] (Table 1), but whether these monocytes include or overlap with the GMP-derived monocytes described by Yanez et al. [9] has not been investigated.
In multiple inflammatory settings, monocytes differentiate into CD11c+MHCII+ inflammatory macrophages that promote inflammation, similar to the Tip-DCs described above. Interestingly, these macrophages can have a protective or pathogenic role, depending on the situation. Several studies found that a commonality in these CD11c+MHCII+ macrophages is the dependence on the transcription factor IRF5 for their differentiation from monocytes. During pathogenic chikungunya virus infection, monocytes required IRF5 to differentiate into iNOS+ cells in the lymph nodes draining the site of infection, and these cells disrupted protective virus-specific B cell responses [31]. In atherosclerosis, extravasation and differentiation of monocytes to CD11chi macrophages occurs at sites of plaque formation where they contribute to lesion development. IRF5 deficiency skewed monocyte differentiation away from pathogenic CD11chi macrophages towards CD206+ macrophages suggested to be of a M2 (anti-inflammatory, tissue repair) phenotype, thereby reducing aortic lesion size [32]. Likewise, in a model of obesity-associated metabolic dysfunction, IRF5-deficient mice showed increased M2 macrophage number in subcutaneous white adipose tissue compared to control mice, ameliorating metabolic dysfunction [33]. These studies are reminiscent of earlier in vitro findings that IRF5 promotes M1 (antimicrobial, inflammatory) and represses M2 fate [34]. More recently, using a Helicobacter hepaticus-induced colitis model, Corbin et al. found that myeloid IRF5 deficiency protected mice from pathogenic intestinal inflammation [35]. The investigators used a combination of mixed BM chimeras and scRNA-Seq to demonstrate that IRF5 was a critical factor in differentiation of Ly6Chi monocytes into pathogenic CD11chi macrophages in the inflamed colon. Although the upstream signals and receptors were not elucidated, it is clear that IRF5 promoted the differentiation of pathogenic monocytes/macrophages in this model. Together, these studies show IRF5 is a key regulator of monocyte differentiation into CD11c+MHCII+ macrophages during inflammation. Although the signals inducing IRF5 activation were not identified in many of these studies, previous work has shown that in vitro GM-CSF promotes IRF5 expression in macrophages [34], and TLR signaling is the best characterized pathway leading to IRF5 signaling during inflammatory responses [36,37].
In addition to providing pathogen clearance functions during infection, monocyte-derived cells can also protect against immunopathology. During Toxoplasma gondii infection in the gut, unique regulatory monocytes appear in the small intestinal lamina propria that produce the lipid mediator PGE2 and repress local neutrophilic inflammation [38]. Interestingly, these regulatory monocytes depend upon specific conditioning of cMOPs in the BM that develop into Ly6Chi monocytes expressing MHCII, Sca-1 and high levels of CX3CR1 [39]. Similar to monocyte-derived Tip-DCs during Listeria infection, these regulatory monocytes depend upon IFNγ, although they are not pro-inflammatory. Whether this difference is due to IFNγ acting on a different cell (cMOP vs. Ly6Chi monocyte), location (BM vs. spleen), or in combination with other soluble factors is not yet clear. In the DSS-induced colitis model, Ikeda et al. also identified a Ly6ChiYm1+ monocyte that expands in the BM, is recruited to the inflamed colon, and promotes tissue repair [40]. These cells are reminiscent of the cells described by Grainger et al. in T. gondii infection [38], although the signals from the injured intestine were not identified in this study. Thus, cytokines produced during tissue inflammation can alter BM myelopoiesis to dampen excessive inflammation and may have different effects in distinct infections.
Monocytes can also differentiate into cells that promote tissue pathology. Segregated-nucleus-containing atypical monocytes (SatM) promoted fibrosis following airway exposure to bleomycin [41]. SatMs have some similarities to neutrophil-like monocytes seen in the steady state [9] in that they expressed neutrophil granule proteins, such as myeloperoxidase and neutrophil elastase, and they differentiated in the BM from GMPs. Unlike neutrophil-like monocytes, SatMs developed via a dedicated progenitor without a Ly6Chi monocyte stage. However, like all monocytes, SatMs expressed CD115 and by gene expression analysis, clustered more closely with Ly6Chi monocytes than neutrophils. Thus, similar to T. gondii infection [38], during chemically-induced lung fibrosis, the tissue state is relayed to the BM to affect monocyte differentiation in a specific manner. Whether these SatMs promote fibrosis in tissues other than the lung and develop in response to diverse stimuli, and what signals feed back to the BM to influence myelopoiesis and promote this fate, remain to be determined.
During sustained systemic inflammation, we identified a unique monocyte differentiation pathway for macrophages specialized for hemophagocytosis [42]. We first identified inflammatory hemophagocytes (iHPCs) in a mouse model of the autoimmune disease systemic lupus erythematosus (SLE) driven by transgenic overexpression of TLR7 [43], which develop severe anemia and thrombocytopenia reminiscent of Macrophage Activation Syndrome (MAS) [42]. iHPCs differentiated from Ly6Chi monocytes and were identified in multiple blood-rich organs. Interestingly, iHPCs correlated with anemia and thrombocytopenia in this lupus-like MAS model, and depletion of Ly6Chi monocytes led to a rescue from MAS. Similar to inflammatory macrophages discussed above, IRF5 participated in the differentiation of iHPCs downstream of TLR7 signaling. iHPCs were also associated with anemia in a model of severe malarial anemia, where signaling through the adaptor MyD88 and the chaperone UNC93b1 was required for iHPC differentiation, implicating endosomal TLR signaling as an important initiating signal in this monocyte differentiation process [42]. Together, these findings suggest differentiated monocytes promote pathological hemophagocytosis both in autoimmunity and infection.
Another monocyte fate preferentially seen during inflammation is the monocyte-derived DC (moDC), originally defined in humans by monocyte differentiation in the presence of GM-CSF and IL-4. In the past, many mouse monocyte-derived populations have been called moDCs due to the upregulation of CD11c and MHCII on cells during bacterial and viral infections, including the Tip-DCs discussed above. However, the recent finding that CD11b+ classical cDC2s express Ly6C in many inflammatory situations calls into question many previous descriptions of moDCs (reviewed in [44]). Even excluding these newly defined cDC2s, whether moDCs should be called DCs remains controversial. To some, a strict definition of a DC requires the ability to migrate in a CCR7-dependent manner from tissues to lymph nodes via lymphatics, where the cells prime naïve T cells. Following this view, moDCs are not DCs [45]. To others, moDCs are monocyte-derived cells, developmentally distinct from the classical DC (cDC) lineage that stimulate activated or effector T cells in tissues via MHCII [44]. By this definition, many monocyte-derived cells in tissues could be termed moDCs if they can locally present antigens to T cells. Further discussion of moDCs is beyond the scope of this review.
As previously discussed, classical monocytes differentiate into patrolling monocytes during homeostasis, a process that is accelerated during TLR7 and TLR9-mediated inflammation as well as by Nod2 signaling [46–48]. This process also occurs in models of lupus-like disease, where patrolling monocytes increase in the blood and accumulate in the kidney in a TLR7/9 and MyD88-dependent manner, driving the development of glomerulonephritis, a common complication of lupus [49]. This is supported by scRNA-Seq studies of kidney leukocytes in individuals with lupus nephritis [50]. During TLR7-driven inflammation, Notch2 was required for patrolling monocyte differentiation, though the Notch ligands contributing to patrolling monocyte differentiation were not identified [51,52]. In the absence of Notch2, TLR7 signaling drives classical monocyte differentiation to moDCs and F4/80+MHCII+ macrophages [52], highlighting the differentiation choices of monocytes in different inflammatory settings and the signals that balance those pathways.
Conclusions
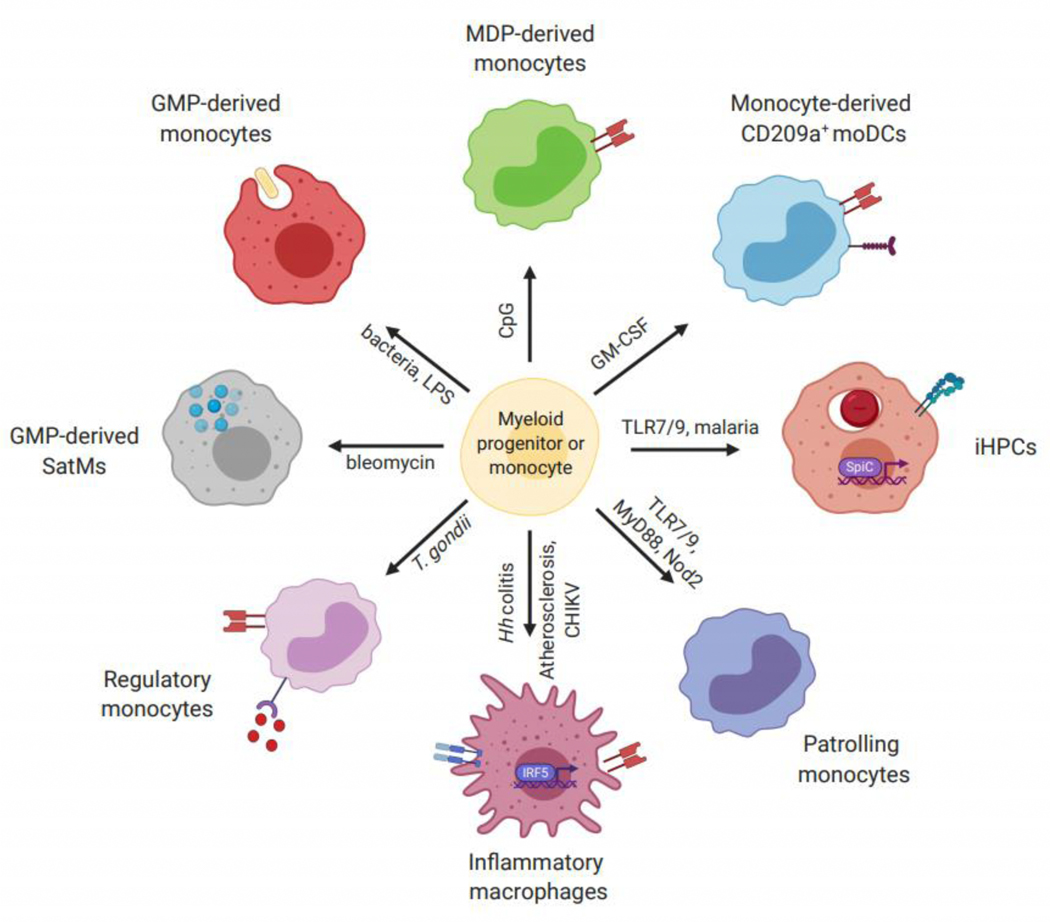
As highlighted here, in recent years we have begun to appreciate that there is heterogeneity in classical, inflammatory monocytes both during homeostasis and during inflammation. This heterogeneity is seen in monocyte development in the bone marrow, in blood monocyte populations, and in differentiation of monocytes once they enter tissues. The inflammatory contexts of monocyte differentiation we have reviewed here vary widely, including a variety of infections, autoimmune diseases, and other pathologies (Figure 2). While monocyte differentiation fates can promote protective or pathogenic immune responses, some common themes emerge. These include the conditioning of monopoiesis in the bone marrow in response to infection or inflammation in distal tissues, a common role for IRF5 in promoting monocyte to inflammatory macrophage differentiation, and an awareness that disrupting one monocyte differentiation pathway can promote differentiation down an alternative pathway.
Figure 2: Monocyte fates induced by inflammation.
A variety of inflammatory signals can act on myeloid progenitor cells or monocytes to induce differentiation into specialized monocyte-derived populations. Signals can act in a direct (e.g. Toll-like receptors) fashion or through indirect mechanisms (e.g. cytokines) to promote these cell fates. Different combinations of markers and gene expression patterns allow for the identification of monocyte-derived cell populations. TLR, Toll-like receptor; Hh colitis, Helicobater hepaticus-induced colitis; CHIKV, chikungunya virus; T. gondii, Toxoplasma gondii; MDP, Monocyte-DC Progenitor; GMP, Granulocyte Macrophage Progenitor; SatM, segregated-nucleus-containing atypical monocyte; iHPC, inflammatory hemophagocyte; moDC, monocyte-derived dendritic cell.
The work reviewed here is most likely the tip of the iceberg in defining monocyte differentiation pathways during inflammation. Although it appears that monocyte-derived populations share some functional and developmental similarities across different inflammatory contexts, much work remains to better understand the development, localization, and function of these cells. As we define new specialized monocyte fates during specific infections or diseases, it will be important to compare them to previously defined populations in the literature, regardless of whether we call them monocytes, macrophages, or moDCs. If not, we will end up with an overabundance of overlapping monocyte-derived populations. Understanding the signals that drive differentiation of these distinct populations will help classify these monocyte-derived cells and will bring greater clarity to the field. Additional effort should go into defining monocyte differentiation during specific disease states in humans and relating these to the populations and pathways identified in mouse models. In particular, monocyte-derived cells in inflamed target tissues in autoimmunity, such as the joints in rheumatoid arthritis and the intestines in inflammatory bowel disease, have been characterized by cell surface markers and cytokine production. New work characterizing these cells by scRNA-Seq should be viewed not only through the lens of pathogenic functions of these cells, but also related to monocyte differentiation signals and pathways that could be targeted therapeutically [50,53,54].
Highlights.
Monocyte differentiation varies widely during inflammation resulting in protective or pathological functions
Emergency myelopoiesis can be induced by direct or indirect signals
Monocyte differentiation may be conditioned in the bone marrow by signals from tissues
IRF5 is implicated in inflammatory macrophage differentiation
Patrolling monocyte differentiation is accelerated by TLR7 and Notch2 signaling
Acknowledgments
This work was supported by the National Institutes of Health [1F32HL154700 (SLO), 5T32 AR007108 (SPC), T32 HD007233 (SPC), 1F32HL156516 (SPC), R01AR076242 (JAH), R01AI150178 (JAH), R21AI154841 (JAH)] and the Lupus Research Alliance [704821 (JAH)].
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures were created with BioRender.com.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References1
- 1.Trzebanski S, Jung S: Plasticity of monocyte development and monocyte fates. Immunol. Lett 2020, 227:66–78. [DOI] [PubMed] [Google Scholar]
- 2.Wolf AA, Yáñez A, Barman PK, Goodridge HS: The Ontogeny of Monocyte Subsets. Front Immunol 2019, 10:57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilliams M, Mildner A, Yona S: Developmental and Functional Heterogeneity of Monocytes.Immunity 2018, 49:595–613. [DOI] [PubMed] [Google Scholar]
- 4.Passlick B, Flieger D, Ziegler-Heitbrock HW: Identification and characterization of a novel monocyte subpopulation in human peripheral blood.Blood 1989, 74:2527–2534. [PubMed] [Google Scholar]
- 5.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties.Immunity 2003, 19:71–82. [DOI] [PubMed] [Google Scholar]
- 6.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al. : Comparison of gene expression profiles between human and mouse monocyte subsets.Blood 2010, 115:e10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC: Nonclassical Monocytes in Health and Disease.Annu. Rev. Immunol 2019, 37:439–456. [DOI] [PubMed] [Google Scholar]
- 8.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, et al. : The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med 2017, 214:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yáñez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, Salomonis N, Grimes HL, Goodridge HS: Granulocyte-Monocyte Progenitors and MonocyteDendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47:890–902.e4. **The authors use scRNA-Seq and adoptive transfer studies to identify two different classical monocyte populations in mouse blood with distinct differentiation pathways from myeloid progenitor cells. These monocyte development pathways had distinct responses to LPS and CpG DNA treatment in vivo.
- 10.Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, Patel R, Gautier EL, Hugues S, Longhi MP, et al. : The Heterogeneity of Ly6C(hi) Monocytes Controls Their Differentiation into iNOS(+) Macrophages or Monocyte-Derived Dendritic Cells.Immunity 2016, 45:1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinreb C, Rodriguez-Fraticelli A, Camargo FD, Klein AM: Lineage tracing on transcriptional landscapes links state to fate during differentiation.Science 2020, 367:eaaw3381. *This study confirms the findings of Yáñez et al. that multiple classical monocyte populations exist in mouse using lineage tracing of individual hematopoietic progenitor clones with both in vitro and in vivo differentiation.
- 12. Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. : Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors.Science 2017, 356:eaah4573. *The authors used scRNA-Seq to define heterogeneity within human blood monocytes, reflecting what had been identified in mouse systems.
- 13.Dutertre C-A, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, et al. : Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells.Immunity 2019, doi: 10.1016/j.immuni.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Boettcher S, Manz MG: Sensing and translation of pathogen signals into demand-adapted myelopoiesis.Curr. Opin. Hematol 2016, 23:5–10. [DOI] [PubMed] [Google Scholar]
- 15.Schultze JL, Mass E, Schlitzer A: Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation.Immunity 2019, 50:288–301. [DOI] [PubMed] [Google Scholar]
- 16.Sezaki M, Hayashi Y, Wang Y, Johansson A, Umemoto T, Takizawa H: Immuno-Modulation of Hematopoietic Stem and Progenitor Cells in Inflammation.Front Immunol 2020, 11:585367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW: Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006, 24:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buechler MB, Teal TH, Elkon KB, Hamerman JA: Cutting edge: Type I IFN drives emergency myelopoiesis and peripheral myeloid expansion during chronic TLR7 signaling.J. Immunol 2013, 190:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buechler MB, Akilesh HM, Hamerman JA: Cutting Edge: Direct Sensing of TLR7 Ligands and Type I IFN by the Common Myeloid Progenitor Promotes mTOR/PI3K-Dependent Emergency Myelopoiesis.J. Immunol 2016, 197:2577–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yáñez A, Hassanzadeh-Kiabi N, Ng MY, Megías J, Subramanian A, Liu GY, Underhill DM, Gil ML, Goodridge HS: Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce.Eur. J. Immunol 2013, 43:2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sioud M, Floisand Y, Forfang L, Lund-Johansen F: Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J. Mol. Biol 2006, 364:945–954. [DOI] [PubMed] [Google Scholar]
- 22.De Luca K, Frances-Duvert V, Asensio MJ, Ihsani R, Debien E, Taillardet M, Verhoeyen E, Bella C, Lantheaume S, Genestier L, et al. : The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia 2009, 23:2063–2074. [DOI] [PubMed] [Google Scholar]
- 23.Zhao JL, Ma C, O’Connell RM, Mehta A, Diloreto R, Heath JR, Baltimore D: Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis.Cell Stem Cell 2014, 14:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner J-M, Will B, et al. : Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal.Nat. Cell Biol 2016, doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tyrkalska SD, Pérez-Oliva AB, Rodríguez-Ruiz L, Martínez-Morcillo FJ, Alcaraz-Pérez F, Martínez-Navarro FJ, Lachaud C, Ahmed N, Schroeder T, Pardo-Sánchez I, et al. : Inflammasome Regulates Hematopoiesis through Cleavage of the Master Erythroid Transcription Factor GATA1.Immunity 2019, 51:50–63.e5. **While many studies have focused on how TLR signaling regulates emergency myelopoiesis, this paper shows an exciting role for inflammasomes and caspase-1 in regulating this process via cleavage of GATA1.
- 26.Serbina NV, Pamer EG: Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. 2006, 7:311–317. [DOI] [PubMed] [Google Scholar]
- 27.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG: TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19:59–70. [DOI] [PubMed] [Google Scholar]
- 28.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG: Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 2003, 19:891–901. [DOI] [PubMed] [Google Scholar]
- 29.Xiong H, Pamer EG: Monocytes and infection: modulator, messenger and effector. Immunobiology 2015, 220:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S-J, Liang H-E, Reizis B, Locksley RM: Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells.Immunity 2008, 29:819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy MK, Reynoso GV, Winkler ES, Mack M, Diamond MS, Hickman HD, Morrison TE: MyD88-dependent influx of monocytes and neutrophils impairs lymph node B cell responses to chikungunya virus infection via Irf5, Nos2 and Nox2.PLoS Pathog 2020, 16:e1008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seneviratne AN, Edsfeldt A, Cole JE, Kassiteridi C, Swart M, Park I, Green P, Khoyratty T, Saliba D, Goddard ME, et al. : Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis.Circulation 2017, 136:1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalmas E, Toubal A, Alzaid F, Blazek K, Eames HL, Lebozec K, Pini M, Hainault I, Montastier E, Denis RGP, et al. : Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity.Nat. Med 2015, 21:610–618. [DOI] [PubMed] [Google Scholar]
- 34.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA: IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses.Nat. Immunol 2011, 12:231–238. [DOI] [PubMed] [Google Scholar]
- 35. Corbin AL, Gomez-Vazquez M, Berthold DL, Attar M, Arnold IC, Powrie FM, Sansom SN, Udalova IA: IRF5 guides monocytes toward an inflammatory CD11c+ macrophage phenotype and promotes intestinal inflammation.Sci Immunol 2020, 5:eaax6085. **This study used bone marrow chimeras together with bulk and scRNA-Seq to define the role of IRF5 in promoting pathogenic CD11c+ inflammatory macrophage differentiation during Helicobacter hepaticus-induced colitis.
- 36.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S-I, Honda K, Ohba Y, Mak TW, et al. : Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors.Nature 2005, 434:243–249. [DOI] [PubMed] [Google Scholar]
- 37.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT: The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling.J. Biol. Chem 2005, 280:17005–17012. [DOI] [PubMed] [Google Scholar]
- 38.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser IDC, Belkaid Y: Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection.Nat. Med 2013, 19:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Askenase MH, Han S-J, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su X-Z, et al. : Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection.Immunity 2015, 42:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda N, Asano K, Kikuchi K, Uchida Y, Ikegami H, Takagi R, Yotsumoto S, Shibuya T, Makino-Okamura C, Fukuyama H, et al. : Emergence of immunoregulatory Ym1+Ly6Chi monocytes during recovery phase of tissue injury.Sci Immunol 2018, 3:eaat0207. [DOI] [PubMed] [Google Scholar]
- 41. Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, et al. : Identification of an atypical monocyte and committed progenitor involved in fibrosis.Nature 2017, 541:96–101. *The authors defined a pro-fibrotic monocyte in the lung in a mouse model of bleomycin-induced lung damage. Signals from the lung conditioned a unique monocyte development pathway in the bone marrow to produce these SatM monocytes.
- 42. Akilesh HM, Buechler MB, Duggan JM, Hahn WO, Matta B, Sun X, Gessay G, Whalen E, Mason M, Presnell SR, et al. : Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes.Science 2019, 363:eaao5213. **This study defined a novel population of macrophages differentiated from classical monocytes in response to TLR7 or TLR9 signaling that are specialized for phagocytosis of red blood cells in a model of lupus-associated Macrophage Activation Syndrome and a model of malarial anemia. These inflammatory hemophagocytes were pathogenic in contributing to severe anemia and thrombocytopenia.
- 43.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S: Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 2007, 27:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coillard A, Segura E: Antigen presentation by mouse monocyte-derived cells: Re-evaluating the concept of monocyte-derived dendritic cells.Molecular Immunology 2021, 135:165–169. [DOI] [PubMed] [Google Scholar]
- 45.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis E Sousa C: Dendritic Cells Revisited.Annu. Rev. Immunol 2021, 39:131–166. [DOI] [PubMed] [Google Scholar]
- 46.Santiago-Raber M-L, Baudino L, Alvarez M, van Rooijen N, Nimmerjahn F, Izui S : TLR7/9-mediated monocytosis and maturation of Gr-1(hi) inflammatory monocytes towards Gr-1(lo) resting monocytes implicated in murine lupus.J. Autoimmun 2011, 37:171–179. [DOI] [PubMed] [Google Scholar]
- 47.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F: Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal.Cell 2013, 153:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lessard A-J, LeBel M, Egarnes B, Préfontaine P, Thériault P, Droit A, Brunet A, Rivest S, Gosselin J: Triggering of NOD2 Receptor Converts Inflammatory Ly6Chigh into Ly6Clow Monocytes with Patrolling Properties.Cell Rep 2017, 20:1830–1843. [DOI] [PubMed] [Google Scholar]
- 49. Kuriakose J, Redecke V, Guy C, Zhou J, Wu R, Ippagunta SK, Tillman H, Walker PD, Vogel P, Häcker H: Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis.J. Clin. Invest 2019, 129:2251–2265. **This study shows that patrolling monocytes accumulate in the kidneys of three different mouse models of lupus with glomerulonephritis, and defines a requirement for MyD88, TLR7, and TLR9 in this process.
- 50. Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, Chicoine A, Eisenhaure TM, Jonsson AH, Li S, et al. : The immune cell landscape in kidneys of patients with lupus nephritis.Nat. Immunol 2019, 20:902–914. **This paper uses scRNA-Seq to define leukocyte populations from biopsies of individuals with lupus nephritis. They show clusters of patrolling monocytes not seen in control biopsies and differentiation states of these cells into phagocytic and anti-inflammatory populations.
- 51.Gamrekelashvili J, Giagnorio R, Jussofie J, Soehnlein O, Duchene J, Briseño CG, Ramasamy SK, Krishnasamy K, Limbourg A, Kapanadze T, et al. : Regulation of monocyte cell fate by blood vessels mediated by Notch signalling.Nat Commun 2016, 7:12597–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gamrekelashvili J, Kapanadze T, Sablotny S, Ratiu C, Dastagir K, Lochner M, Karbach S, Wenzel P, Sitnow A, Fleig S, et al. : Notch and TLR signaling coordinate monocyte cell fate and inflammation.Elife 2020, 9. *The authors build on their previous work and show here that Notch2 signaling synergizes with TLR7 signaling to promote patrolling monocyte differentiation from classical moncoytes. In the absence of Notch2, alternative monocyte differentiation pathways occur.
- 53.Martin JC, Chang C, Boschetti G, Ungaro R, Giri M, Grout JA, Gettler K, Chuang L-S, Nayar S, Greenstein AJ, et al. : Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy.Cell 2019, 178:1493–1508.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Goodman SM, Tabechian D, Hughes LB, Salomon-Escoto K, et al. : Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry.Nat. Immunol 2019, 20:928–942. [DOI] [PMC free article] [PubMed] [Google Scholar]