Abstract
Background
Immunotherapy with immune checkpoint inhibitors (ICIs) recently became the standard treatment for patients with advanced non‐small cell lung cancer (NSCLC). Here, we present the first results of a real‐world observational study on the effectiveness of ICI monotherapy in patients with advanced NSCLC treated at a single academic center in a Central and Eastern European (CEE) country.
Materials and Methods
Overall, 66 consecutive patients with advanced NSCLC treated with ICIs in everyday clinical practice, either with first‐line pembrolizumab (26 patients) or second‐line atezolizumab, nivolumab, or pembrolizumab (40 patients), from August 2015 to November 2018, were included. All data were retrieved from a hospital lung cancer registry, in which the data is collected prospectively.
Results
Included patients had a median age of 64 years, most were male (55%), 6% were in performance status ≥2, and 18% had controlled central nervous system metastases at baseline. In first‐line, the median progression‐free survival (mPFS) was 9.3 months, while the median overall survival (mOS) was not reached. The 1‐year overall survival (OS) was 62%. In second‐line, the mPFS and mOS were 3.5 months and 9.9 months, respectively, with a 1‐year OS of 35%. In the overall population, adverse events of any grade were recorded in 79% of patients and of severe grade (3–4) in 12% of patients.
Conclusion
The first real‐world outcomes of NSCLC immunotherapy from a CEE country suggest comparable effectiveness to those observed in clinical trials and other real‐world series, mainly coming from North America and Western European countries. Further data to inform on the real‐world effectiveness of immunotherapy worldwide are needed.
Implications for Practice
Immunotherapy is a standard treatment of advanced non‐small cell lung cancer (NSCLC). The real‐world data on immunotherapy are still limited. This article presents the first data on the effectiveness of mono‐immunotherapy with immune checkpoint inhibitors for patients with advanced NSCLC treated at a single academic center in a Central and Eastern European country. The survival rates and toxicity are comparable to those achieved in randomized clinical trials and other real‐world series, coming mainly from North American and Western European countries. There is a pressing need to gather further data on the effectiveness of immunotherapy in everyday practice worldwide.
Keywords: Advanced non‐small cell lung cancer, Immunotherapy, Real‐world outcome, Central and Eastern European region
Short abstract
Immunotherapy has become the standard treatment for advanced non‐small cell lung cancer (NSCLC). This article presents the first real‐world results on the effectiveness of immune checkpoint inhibitor treatment for advanced NSCLC from a single academic center from Central and Eastern European country.
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide. Over 2 million new cases are diagnosed each year and result in more than 1.7 million deaths [1]. The most common form is non‐small cell lung cancer (NSCLC). Nearly half of patients are diagnosed at an advanced stage of disease, which leads to poor 5‐year overall survival (OS). The observed 5‐year OS in Europe is 13% and has not changed much in the last few decades [2]. Until this decade, chemotherapy was the standard of care and, in fact, the only systemic treatment option for advanced NSCLC. The median overall survival (mOS) of patients on chemotherapy was up to 13.4 months [3].
The development of targeted therapies redefined the treatment paradigm for patients with oncogene‐driven NSCLC. All pivotal clinical trials, performed in advanced epidermal growth factor receptor (EGFR)‐ or anaplastic lymphoma kinase (ALK)‐positive NSCLC, demonstrated improved response rates and progression‐free survival in patients treated with targeted therapy compared with standard chemotherapy [3]. Moreover, with the sequential use of ALK‐targeted agents, median overall survival of more than 7 years has been observed in patients treated in routine clinical practice [4]. Consequently, predictive biomarker testing and targeted therapies have been introduced into standard care for patients with advanced NSCLC [5, 6]. Currently, international guidelines recommend testing for EGFR mutations, ALK and ROS1 rearrangements, and BRAF mutations for all patients with advanced non‐squamous NSCLC [6]. In our country, reflex testing for particular predictive biomarkers has been adopted in accordance with European guidelines as soon as they were published. However, access to targeted therapies for NSCLC followed a slower pace, similarly to other Central and Eastern European (CEE) countries [7, 8]. This is supported by recently published data reporting a lag time of 14 months between approval of new anticancer drugs by the European Medicines Agency and a positive national reimbursement decision in Slovenia [9]. Despite significant improvements, the benefits of targeted therapies are still restricted to a small proportion of patients with advanced NSCLC who harbor targetable driver mutations.
The unmet need for effective therapies in patients with NSCLC without a targetable oncogene was hoped to be met by immuno‐oncology. The immune checkpoint inhibitors (ICIs) atezolizumab, nivolumab, and pembrolizumab were first studied in second‐line treatment of advanced NSCLC. Substantial improvements in mOS rates of up to 13.8 months were observed compared with up to 9.6 months achieved with docetaxel [10, 11, 12, 13]. Even more impressive results were achieved in the first‐line setting. In the pivotal trial of pembrolizumab monotherapy in a patient population with programmed death–ligand 1 (PD‐L1) ≥50%, a mOS of 30 months was observed [14, 15]. To further improve treatment results, the combination of two previous first‐line standards, chemotherapy and immunotherapy, was studied in unselected patient populations and further added efficacy compared with chemotherapy alone while retaining a reasonable safety profile [16]. These significant improvements are indeed coupled with new immune‐related adverse events (AEs). But even though treatment‐related AEs are recorded in the majority of patients, more than 60%, only approximately 15% experience severe AEs (grade 3 or 4), treatment discontinuation, or, very rarely, fatal toxicity [17].
Although immunotherapy represents a new treatment standard for advanced NSCLC, many questions still need to be answered to ensure its optimal use in everyday clinical practice [18]. It is vital to evaluate how the impressive efficacy data and favorable safety profile reported in randomized clinical trials (RCTs) translate into everyday clinical practice effectiveness. A substantial gap in outcomes between patients included in clinical trials and those treated in the real‐world scenario was observed, particularly in cancer populations of older patients with comorbidities [19]. Clinical trials tend to include younger patients in good performance status (PS) with minimal comorbidity, thus making real‐world data for populations of advanced NSCLC, who tend to be older and with multiple comorbidities, most interesting [20, 21]. There are many published studies on the real‐world effectiveness of immunotherapy with ICIs in advanced NSCLC (Tables 1 and 2). Most of them provide reassuring data for second‐line mono‐immunotherapy, including also patients with poor prognostic characteristics (Table 2). However, data in the first‐line setting are limited and not as reassuring, especially when compared with the pivotal trial (Table 1). Of note, real‐world data published so far mainly involve patients from North America and Western Europe, similar to those included in pivotal trials. To our knowledge, no real‐world data on immunotherapy outcomes in NSCLC for CEE countries, which are still facing a gap in cancer control, have been published so far [22].
Table 1.
First‐line immunotherapy outcomes in the pivotal RCTs, real‐world studies, and present study
| First author, year | Study design (country) | Drug | Patients, n | Age median (range) | Gender: males, n (%) | PS ≥ 2, n (%) | CNS metastases, n (%) | Median PFS, mo | Median OS, mo | 1‐year OS, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Reck, 2016 & 2019 [11, 12] | RCT, multicentric | Pem | 154 | 64.5 (33–90) | 92 (59.7) | 1 (0.6) | 18 (11.7) | 10.3 | 30.0 | 70.3 |
| Ksienski, 2019 [34] | Observational, multicentric (Canada) | Pem | 141 | 70.0 (41–91) a | 97 (51.1) a | 65 (34.2) a | 26 (13.7) a | 3.7 a | 24.3 | ND |
| Velcheti, 2019 [39] | Observational, multicentric (U.S.) | Pem | 188 | 72.0 (46–84) | 90 (47.9) | 0 | 23 (12.2) | 6.8 | 19.1 | 60.4 |
| Amrane, 2019 [24] | Observational, multicentric (France) | Pem | 108 | 67.0 (37–87) | 70 (64.8) | 25 (23.1) | 19 (17.6) | 10.1 | 15.2 | ND |
| Tamiya, 2019 [32] | Observational, multicentric (Japan) | Pem | 213 | 71 (39–91) | 176 (82.6) | 41 (19.2) | ND | 8.3 | 17.8 | ND |
| Tambo, 2020 [40] | Observational, multicentric (Japan) | Pem | 95 | 72.0 (51–89) | 71 (74.7) | 21 (22.1) | ND | 6.1 | NR | 58.3 |
| Current study | Observational, single center (Slovenia) | Pem | 26 | 65.5 (39–78) | 16 (62.0) | 3 (11.0) | 4 (15.0) | 9.3 | NR | 62.0 |
Reported for 190 patients, of which 49 were treated in second‐line setting.
Abbreviations: CNS, central nervous system; ND, no data; NR, not reached; OS, overall survival; Pem, pembrolizumab; PFS, progression‐free survival; PS, performance status; RCT, randomized clinical trial.
Table 2.
Second‐line immunotherapy outcomes in the pivotal RCTs, real‐world studies, and present study
| First author, year | Study design (country) | Drug | Patients, n | Age, median (range), yr | Gender: male, n (%) | PS ≥ 2, n (%) | CNS metastases, n (%) | Median PFS, mo | Median OS, mo | 1‐year OS, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Brahmer, 2015 [8] | RCT, multicentric | Nivo | 135 a | 62.0 (39–85) | 111 (82.0) | 2 (1.5) | 9 (6.6) | 3.5 | 9.2 | 42.0 |
| Borghaei, 2015 [7] | RCT, multicentric | Nivo | 292 b | 61.0 (37–84) | 151 (51.7) | 0 | 34 (11.6) | 2.3 | 12.2 | 51.0 |
| Herbst, 2015 [9] | RCT, multicentric | Pem | 344 c | 63.0 (56–69) | 212 (61.6) | 3 (0.9) | 56 (16.3) | 3.9 | 10.4 | 43.2 |
| Rittmeyer, 2016 [10] | RCT, multicentric | Atezo | 425 | 63.0 (33–82) | 261 (61.4) | 0 | 85 (10.0) d | 2.8 | 13.8 | 55.0 |
| Brustugun, 2016 [41] | Observational, single center (Norway) | Nivo | 58 | 64.6 (32–88) | 28 (48.3) | 14 (24.1) | 0 | 4.0 d | 11.7 | 50.0 |
| Schouten, 2017 [42] | Observational, multicentric (Netherlands) | Nivo | 248 | 63.0 (29–84) | 136 (54.8) | 40 (16.1) | 56 (22.6) | 2.6 | 10.0 | ND |
| Dudnik, 2018 [26] | Observational, multicentric (Israel) | Nivo | 260 | 67.0 (41–99) | 176 (67.7) | 139 (53.5) | 55 (21.1) | 2.8 | 5.9 | ND |
| Manrique, 2018 [43] | Observational, multicentric (Spain) | Nivo | 188 | 58.0 (45–81) | 144 (76.6) | 19 (10.1) | 42 (22.3) | 4.8 | 12.8 | 55.0 |
| Lin, 2018 [27] | Observational, single center (Taiwan) | Nivo, Pem | 74 | 62.1 (34–87) | 43 (58.1) | 36 (48.6) | 33 (44.6) | 1.8 | 7.9 | 46.0 |
| Kobayashi, 2018 [44] | Observational, multicentric (Japan) | Nivo | 142 | 67.0(34–85) | 106 (74.6) | 23 (16.2) | 27 (19.0) | 1.9 | ND | ND |
| Geier, 2018 [45] | Observational, multicentric (France) | Nivo | 259 | 62.0 (29–85) | 187 (72.2) | 15 (5.8) | 53 (20.5) | 2.3 | 11.0 | 47.9 |
| Juergens, 2018 [46] | Observational, multicentric (Canada) | Nivo | 472 | 66.0 (36–92) | 203 (43.0) | 42 (8.9) | 62 (13.1) | 3.5 e | 12.0 | 50.0 |
| Fujimoto, 2018 [47] | Observational, multicentric (Japan) | Nivo | 613 | 66.9 (ND) | 433 (71) | 141 (23) | ND | ND | ND | 54 |
| Montana, 2018 [28] | Observational, multicentric (France) | Nivo | 98 | 65.5 (42–85) | 70 (71.4) | 39 (39.7) | ND | 1.8 | 6.3 | ND |
| Almazan, 2019 [48] | Observational, multicentric (Spain) | Nivo | 221 | 64.5 (NR) | 185 (83.7) | 30 (13.8) | 22 (10.0) | 5.3 | 9.7 | ND |
| Khozin, 2019 [49] | Observational, multicentric (U.S.) | Nivo, Pem | 1,344 f | 69.0 (61–75) | 747 (55.6) | ND | ND | ND | 8.0 | 39.0 |
| Khozin, 2019 [50] | Observational, multicentric (U.S.) | Atezo, Nivo, Pem | 5,257 g | 69.0 (62–76) | 2,819 (53.6) | ND | ND | 3.2 | 9.3 | ND |
| Ahn, 2019 [29] | Observational, single centre (Korea) | Nivo, Pem | 155 | 64.0 (35–58) | 113 (72.9) | 34 (21.9) | 61 (39.4) | 3.0 | 10.2 | ND |
| Crino, 2019 [51] | Observational, multicentric (Italy) | Nivo | 371 a | 68.0 (31–91) | 298 (80.3) | 22 (5.9) | 37 (9.9) | 4.2 | 7.9 | 39.0 |
| El Karak, 2019 [38] | Observational, (Lebanon) | Nivo, Pem | 110 | 66.0 (ND) | 75 (68.2) | ND | 17 (15.5) | 4.0 | 8.1 | ND |
| Grossi, 2019 [33] | Observational, multicentric (Italy) | Nivo | 1,588 b | 66.0 (27–89) | 1,029 (64.8) | 108 (6.8) | 409 (25.7) | 3.0 | 11.3 | 48.0 |
| Morita, 2020 [52] | Observational, multicentric (Japan) | Nivo | 901 h | 67.0 (30–90) | 651 (72.3) | 157 (17.4) | 201 (22.3) | 2.1 | 14.6 | 54.3 |
| Figueiredo, 2020 [31] | Observational, multicentric (Portugal) | Nivo | 219 | 64.0 (37–83) | 154 (70.3) | 29 (13.2) | ND | 4.9 | 13.2 | 56.5 |
| Weis, 2020 [53] | Observational, single centre (U.S.) | Atezo | 43 | 67.2 (ND) | 23 (53.5) | 9 (20.9) | ND | 2.0 | 6.5 | ND |
| Nivo | 81 | 64.3 (ND) | 39 (48.2) | 21 (28.4) | ND | 2.2 | 8.4 | ND | ||
| Martin, 2020 [37] | Observational, multicentric (Argentina) | Nivo | 109 | 65.0 (56–72) | 63 (57.8) | 17 (15.6) | ND | 6.1 | 12.1 | ND |
| Barlesi, 2020 [30] | Observational, multicentric (France) | Nivo | 1,420 i | 66.0 (35–91) | 986 (69.4) | 241 (17.1) | 282 (19.9) | 2.8 | 11.2 | 48.6 |
| Current study | Observational, single center (Slovenia) | Atezo, Nivo, Pem | 40 | 63.0 (42–77) | 20 (50.0) | 1 (3.0) | 8 (20.0) | 3.5 | 9.9 | 35.0 |
Only squamous cell lung cancer.
Only nonsquamous cell lung cancer.
cohort treated with pembrolizumab 2 mg/kg.
Reported for atezolizumab and docetaxel cohort combined.
Time to treatment failure.
227 (16.9%) patients in first line.
1,329 (25.3%) patients in first line.
38 (4.2%) patients in first line.
Four (0.3%) patients in first line.
Abbreviations: Atezo, atezolizumab; CNS, central nervous system; ND, no data; NR, not reached; Nivo, nivolumab; OS, overall survival; Pem, pembrolizumab; PFS, progression‐free survival; PS, performance status; RCT, randomized clinical trial.
Here, we report the first results of a real‐world observational study evaluating treatment outcomes for patients with advanced NSCLC treated with ICI monotherapy, either in first‐ or second‐line setting at a single academic center in a Central and Eastern European country.
Patients, Materials, and Methods
Patients
The study included consecutive patients with pathologically confirmed advanced NSCLC treated with ICI monotherapy between August 2015 and November 2018 in routine clinical practice at a single academic center in Slovenia. Patients received pembrolizumab in the first‐line setting if they had a PD‐L1 expression ≥50%, or atezolizumab, nivolumab, or pembrolizumab in the second‐line setting. PD‐L1 testing was mandatory before first‐line but not second‐line therapy and was performed on formalin‐fixed, paraffin‐embedded histology samples or cytospins by using PD‐L1 monoclonal antibodies (22C3 clone by DAKO, Glostrup, Denmark or SP263 clone by Ventana/Roche, Oro Valley, AZ).
Nivolumab was available within a compassionate use program already in August 2015, whereas atezolizumab and pembrolizumab were available only after granted the marketing authorization by the European Medicines Agency and national reimbursement. Pembrolizumab, both for first‐ and second‐line, was reimbursed in August 2017, and second‐line atezolizumab was reimbursed in May 2018. Included patients may have had controlled central nervous system (CNS) disease, with or without previous CNS irradiation. All patients were free of corticosteroid treatment. All included patients were routinely tested for EGFR mutations and ALK rearrangement and, after 2016, also for ROS1 rearrangements. None of the included patients had a known driver oncogene, and none had known autoimmune disease. All patients are treated and routinely followed at a single institution, where clinicians are encouraged to record and grade all AEs by Common Terminology Criteria for Adverse Events, version 4 and to evaluate the objective response rate (ORR) according to RECIST 1.1 [23, 24].
Data Collection
Data were retrieved from the hospital‐based lung cancer registry, which prospectively collects comprehensive demographics, pathological and molecular characteristics, and treatment and survival data of all patients with lung cancer diagnosed and treated at the center. The hospital registry includes approximately 600 new patients with lung cancer per year, representing nearly half of all newly diagnosed patients with lung cancer in Slovenia. Patients consent to collecting data in the frame of the lung cancer registry at the time of diagnosis or start of treatment. All data were collected anonymously. For this study, progression and survival status were updated, and the data were retrieved in December 2019.
Outcome Measures and Statistical Analysis
Patient and treatment characteristics were analyzed using descriptive statistics. Treatment outcomes are presented separately for patients treated in the first‐line or second‐line setting. The Kaplan Meier method was used to estimate progression‐free survival (PFS) and OS. PFS was calculated from the date of the first immunotherapy dose until tumor progression or death from any cause, whichever occurred first. OS was calculated from the date of the first immunotherapy dose until death from any reason. Median follow‐up time was calculated using the reverse Kaplan‐Meier estimator. Statistical analyses were generated using R Studio, R version 3.5.3, with package survminer (R Foundation, Vienna) [25, 26].
Results
Patients and Treatments
Overall, 66 patients were treated with ICIs consecutively during the study period from August 2015 to November 2018. Among these, 26 patients were treated with ICIs in the first‐line and 40 in the second‐line setting.
The included 66 patients had a median age of 64 years (range, 39–78 years), 55% (36/66) of patients were male, 82% (54/66) were current or former smokers. The majority, 83% (55/66), of patients had adenocarcinoma. Most, 94 % (62/66), of patients were in a good PS of 0–1. CNS metastases were present in 18% (12/66) of patients at baseline. Baseline demographics and clinical characteristics by line of therapy are displayed in Table 3.
Table 3.
Baseline characteristics of patients included in the study
| All patients(n = 66) | First‐line(n = 26) | Second‐line(n = 40) | |
|---|---|---|---|
| Median age (range), yr | 64.0 (39–78) | 65.5 (39–78) | 63.0 (42–77) |
| Gender | |||
| Female | 30 (45) | 10 (38) | 20 (50) |
| Male | 36 (55) | 16 (62) | 20 (50) |
| Smoking status | |||
| Former or current smoker | 54 (82) | 20 (77) | 34 (85) |
| Nonsmokers | 12 (18) | 6 (23) | 6 (15) |
| ECOG performance status | |||
| 0–1 | 62 (94) | 23 (89) | 39 (97) |
| 2 or more | 4 (6) | 3 (11) | 1 (3) |
| Histology | |||
| Adenocarcinoma | 55 (83) | 21 (81) | 34 (85) |
| Squamous cell carcinoma | 8 (12) | 2 (8) | 6 (15) |
| NOS or other | 3 (5) | 3 (12) | 0 (0) |
| PD‐L1 expression | |||
| 0% | 19 (29) | 0 (0) | 19 (48) |
| 1%–49% | 7 (11) | 0 (0) | 7 (18) |
| 50% or more | 36 (55) | 26 (100) | 10 (25) |
| Unknown | 4 (6) | 0 (0) | 4 (10) |
| CNS metastasis | 12 (18) | 4 (15) | 8 (20) |
Data presented as n (%), unless otherwise noted.
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; PD‐L1, programmed death–ligand 1.
In the first‐line setting, all 26 patients were treated with pembrolizumab monotherapy (200 mg every 3 weeks) and had the required PD‐L1 expression ≥50%. In the second‐line setting, 10% (4/40) of patients received atezolizumab (1,200 mg every 3 weeks), 58% (23/40) of patients received nivolumab (3 mg/kg every 2 weeks), and 33% (13/40) of patients received pembrolizumab (2 mg/kg every 3 weeks). In this setting, only 25% (10/40) of patients had a PD‐L1 expression ≥50%, while most, 48% (19/40), of patients had PD‐L1 expression <1%; PD‐L1 expression was not known in 10% (4/40) of patients.
Treatment Outcomes
In the first‐line setting, an ORR was achieved in 46% (12/26) of patients (four complete responses [CR] and eight partial responses [PR]), with a further 27% (7/26) of patients achieving stable disease (SD). Median (m)PFS in the first‐line cohort was 9.3 months (95% confidence interval [CI], 3.5–NR; Fig. 1), and median OS has not yet been reached (95% CI, 7.1–NR; Fig. 1). The 1‐year OS rate was 62% (95% CI, 45%–83%). The median follow‐up (FU) time was 19.9 months.
Figure 1.
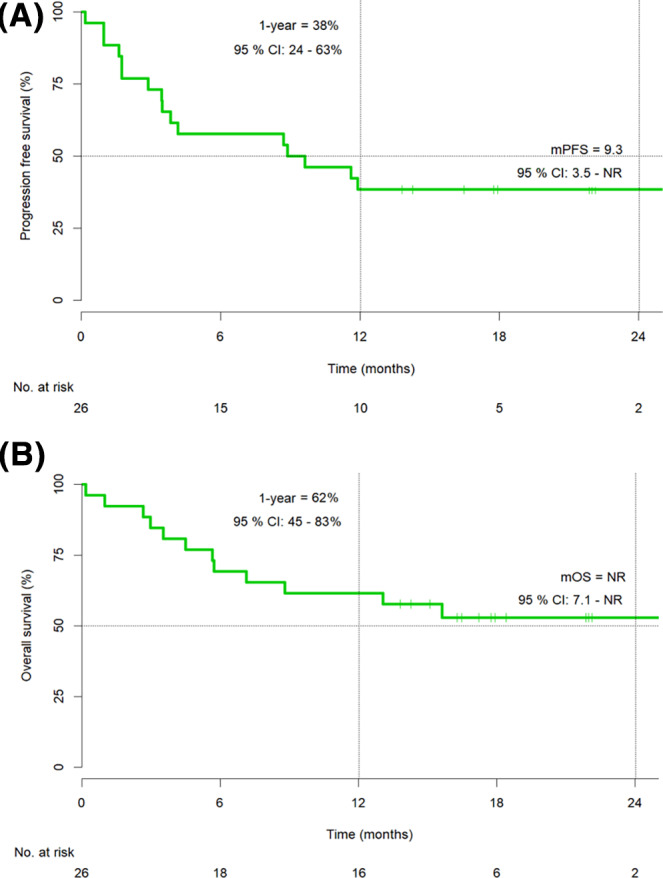
Progression‐free survival (A) and overall survival (B) in the first‐line cohort (n = 26).
Abbreviations: CI, confidence interval; mOS, median overall survival; mPFS, median progression‐free survival; NR, not reached.
In the second‐line setting, an ORR was achieved in 25% (10/40) of patients (one CR and nine PR), with a further 23% (9/40) of patients achieving SD. Median PFS was 3.5 months (95% CI, 1.9–6.6; Fig. 2), and median OS was 9.9 months (95% CI, 4.9–16.1; Fig. 2). The 1‐year OS rate was 35% (95% CI, 23%–53%). The median FU time was 34.2 months.
Figure 2.
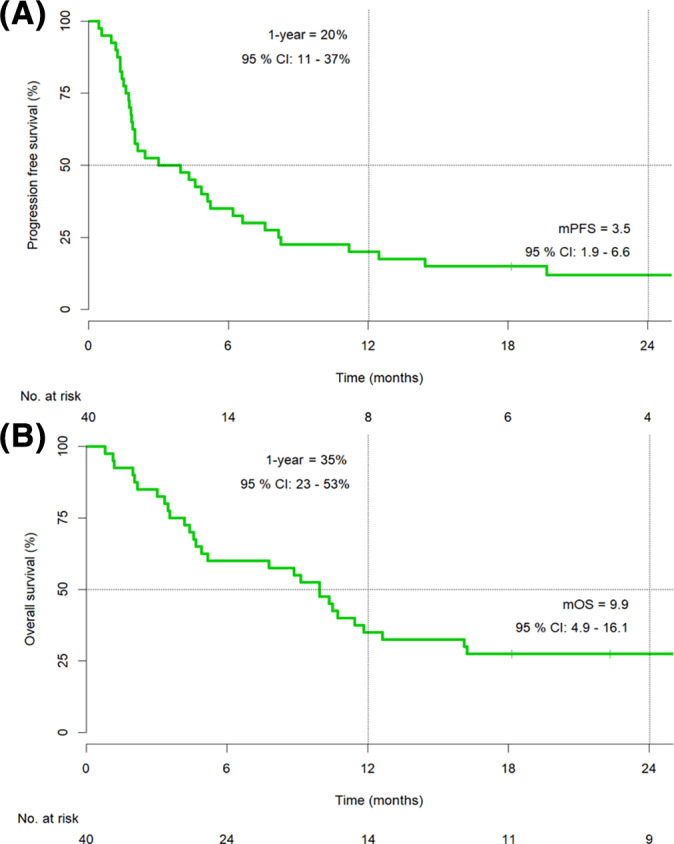
Progression‐free survival (A) and overall survival (B) in the second‐line cohort (n = 40).
Abbreviations: CI, confidence interval; mOS, median overall survival; mPFS, median progression‐free survival.
Safety
Because of the small sample size and because no difference was expected in the AE profile among first‐ and second‐line ICI treatment, safety outcomes are presented for the whole group of patients [17]. Treatment‐related AEs were recorded in 79% (52/66) of all patients (Table 4). The most common AEs were fatigue, skin disorders, hypo/hyperthyroidism, and hepatotoxicity, each occurring in more than 15% of patients. However, AEs were severe, grades 3 or 4, only in 12% (8/66) of patients. Treatment with systemic corticosteroid was required in 17% (11/66) of patients, and permanent treatment discontinuation due to AE occurred in 14% (9/66) of patients. Of the nine patients who discontinued immunotherapy because of toxicity, the two patients with PR had durable remissions. After immunotherapy discontinuation, the first patient was in remission for an additional 5 months, and the second patient was still in remission for more than 26 months at data cut‐off. No treatment‐related death was recorded.
Table 4.
Adverse events in the whole cohort of patients (n = 66)
| Adverse event, n (%) | Any grade | G 1–2 | G 3–4 |
|---|---|---|---|
| Any | 52 (79) | 44 (67) | 8 (12) |
| Fatigue | 25 (38) | 25 (38) | 0 (0) |
| Skin disorders | 19 (29) | 18 (27) | 1 (2) |
| Hypo‐/hyperthyroidism | 11 (17) | 11 (17) | 0 (0) |
| Hepatotoxicity | 12 (18) | 10 (15) | 2 (3) |
| Diarrhea | 3 (5) | 3 (5) | 0 (0) |
| Colitis | 3 (5) | 1 (2) | 2 (3) |
| Diabetes mellitus | 2 (3) | 0 (0) | 2 (3) |
| Other a | 13 (20) | 9 (14) | 4 (7) |
| Led to corticosteroid treatment | 11 (17) | ||
| Led to discontinuation | 9 (14) | ||
| Led to death | 0 (0) |
Other adverse event G1–2: Myalgia, arthralgia, stomatitis, anaemia, agranulocytosis. Other adverse event G 3–4: infection, mesenteritis, Cushing syndrome, agranulocytosis.
Abbreviation: G, grade.
Discussion
The present study provides the first results of an observational study evaluating the effectiveness and safety of mono‐immunotherapy with ICIs in a real‐world collective of patients with advanced NSCLC treated at an academic center in Central and Eastern Europe. The reported treatment outcomes for the second‐line setting (mPFS of 3.5 months, mOS of 9.9 months, and 1‐year OS of 35%) are similar to those published in RCTs and multiple real‐world studies (Table 2). However, the results for the first‐line setting (mPFS of 9.3 months, mOS not yet reached, and 1‐year OS of 62%) are not so reassuring. However, the so far published real‐world data on first‐line immunotherapy are limited, and the median follow‐up of patients in real‐world series is still short (Table 1), making any comparison difficult.
The real‐world evidence on upfront immunotherapy in advanced NSCLC is limited because of the short time since regulatory approval of pembrolizumab in first‐line treatment. Moreover, only one study from Europe has been published, making any additional European real‐world data most needed [27]. The herein reported 1‐year OS of 62% is broadly similar to those in other published real‐world studies. The pivotal RCT KEYNOTE‐024, comparing first‐line pembrolizumab with chemotherapy in advanced NSCLC with PD‐L1 ≥ 50%, reported a remarkable mOS of 30.0 months and 1‐year OS of 70% that have yet not been reproduced in routine clinical practice (Table 1). However, the present and other real‐world outcomes compare better to those reported in the subgroup of patients with a PD‐L1 ≥ 50% participating in the KEYNOTE‐042, with a mOS of 20.0 months [28]. Of note, the KEYNOTE‐042 trial was conducted in a larger number of centers and regions, including centers from CEE and Latin America, thus representing a broader and more global population of patients. The comparable outcomes in real‐world series were achieved even though most of them also included patients with poor PS ≥2, often representing well above 20% of patients, whereas these were excluded from participation in KEYNOTE‐024. As already mentioned, median FU is still very short in most real‐world series and is much shorter than in KEYNOTE‐024 [15]. Therefore, further evaluation of more extensive real‐world series with longer follow‐up is awaited.
The real‐world evidence on immunotherapy use in second‐line is more extensive, with reports including more than thousands of patients and providing a better insight into ICI monotherapy outcomes in routine clinical practice (Table 2). The herein reported 9.9‐month mOS is in line with those in RCTs, with mOS ranging from 9.2 to 13.8 months, and within the broad ranges of individual real‐world studies, reporting mOS from 5.9 to 14.6 months (Table 2). As expected, shorter mOS was reported in cohorts with a high share of patients with poorer prognostic factors, such as PS ≥2, exceeding 50% of patients in some series, and brain metastases, sometimes exceeding 40% of patients [29, 30, 31, 32]. Both PS ≥2 and symptomatic brain metastases were proven as independent predictors of poor survival in a large prospective observational trial evaluating effectiveness of second‐line monotherapy with nivolumab in France [33]. In our series only 6% of patients had PS of ≥2, but nearly a fifth (18%) had CNS metastases at baseline.
The herein observed safety of immunotherapy treatment, with most (79%) of patients experiencing an AE but being severe only in 12% of patients, is consistent with data from pivotal mono‐immunotherapy RCT trials in NSCLC and a recent meta‐analysis of immunotherapy AEs in RCTs in solid tumors [10, 11, 12, 13, 14, 15, 17]. Interestingly, the discontinuation rate in our cohort of patients (14%) is higher than the up to 8% reported in the abovementioned references, possibly suggesting inadequate AE management with decreasing expertise from clinical trials. In fact, before initiating nivolumab compassionate use program, there were no clinical trials with ICIs for advanced NSCLC running at our institution. No new safety concerns and no treatment‐related deaths were noticed in our observational study. Our findings fall within the significant variability reported in the frequency of AE in real‐world studies, with the highest being very similar to those observed herein and the lowest reporting any AE in only approximately a third of patients [33, 34, 35, 36, 37]. Thus, although the available real‐world evidence is reassuring in confirming a favorable tolerability profile for ICI monotherapy in everyday clinical practice, a word of caution still needs to be in place when acknowledging the possible underreporting of adverse events outside the clinical trial setting.
The abundance of real‐world evidence on ICI outcomes in NSCLC is limited to North America, Western Europe, and the Asian‐Pacific region, mainly Japan. These data are unlikely generalizable to other parts of the world, where access and availability to cancer care vary significantly. CEE is still a region with a lack of financial and human resources and with substantially higher cancer mortality to incidence ratio than Western Europe [22]. Ensuring access to immunotherapy, with its indications expanding to almost all cancer types, represents a challenge to all health care systems and mandates the evaluation of immunotherapy real‐world outcomes. Real‐world data are most needed for regions with lack of resources and limited participation in clinical trials, such as CEE countries. In those countries, it might happen that novel drugs are first being used in routine clinical practice without previous expertise and skills gained within clinical trials, thus hampering their safe transfer to everyday clinical practice [38, 39]. Although some CEE countries improved their participation in clinical trials during the last decade, this was not the case for smaller countries such as Slovenia [40]. According to our knowledge, there are no published data on real‐world immunotherapy outcomes in patients with NSCLC treated in our region. Only two real‐world data sets are from regions less often represented in RCT, namely Argentina and Lebanon, with favorable results [41, 42]. Hopefully, our report will trigger the publication of more real‐world data from regions with a lower development index.
Nevertheless, a few limitations apply to our study. First, our cohort of patients is small, with 66 patients included altogether and only 26 patients in the first‐line setting. Second, despite the mFU for second‐line therapy reaching almost 3 years, the mFU for first‐line therapy is only approximately 1.5 years. Consequently, no subgroup analysis was possible in this report because of the small number of patients and relatively short follow‐up. Third, our report is limited to only one academic center in the country. Indeed, the real‐world data collected at the national level would provide better insight and more reassuring data.
Conclusion
The first results of our observational trial on the effectiveness of mono‐immunotherapy with ICIs in the first‐ and second‐line treatment of advanced NSCLC at a single center in a Central and Eastern European country are in line with pivotal clinical trials and other real‐world reports. Our results indicate effective and safe use of mono‐immunotherapy with ICIs in routine clinical practice; however, for any firm conclusions, more data derived from larger collectives of patients with longer follow‐up times are needed. Further real‐world studies, especially of immunotherapy in the first‐line setting, either as monotherapy or combined with chemotherapy, are most needed to inform their effectiveness in routine clinical practice. This urge is greater in health care systems and countries traditionally underrepresented in pivotal trials, such as the Central and Eastern European countries.
Author Contributions
Conception/design: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Provision of study material or patients: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Collection and/or assembly of data: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Data analysis and interpretation: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Manuscript writing: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Final approval of manuscript: Marija Ivanović, Lea Knez, Ana Herzog, Mile Kovačević, Tanja Cufer
Disclosures
Marija Ivanović: AstraZeneca, Merck Sharp & Dohme, Pfizer, Roche, (C/A), Astellas Pharma, AstraZeneca, Janssen, Lek, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, Takeda (H); Lea Knez: Lek, Novartis, Pfizer (H); Tanja Cufer: Boehringer Ingelheim, Takeda (C/A), AstraZeneca, Merck Sharp & Dohme, Pfizer, Roche (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors thank all medical doctors included in providing a standard of care for patients with lung cancer at University Clinic Golnik, especially to pathologist Izidor Kern, M.D., and oncologists Katja Mohorčič, M.D., and Urška Janžič, M.D. We also thank everyone from the Hospital Registry Department and Department for Clinical Research, especially Tjaša Brus Pičman and Nina Rošič.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Francisci S, Minicozzi P, Pierannunzio D et al. Survival patterns in lung and pleural cancer in Europe 1999–2007: Results from the EUROCARE‐5 study. Eur J Cancer 2015;51:2242–2253. [DOI] [PubMed] [Google Scholar]
- 3. Cufer T, Knez L. Update on systemic therapy of advanced non‐small‐cell lung cancer. Expert Rev Anticancer Ther 2014;14:1189–1203. [DOI] [PubMed] [Google Scholar]
- 4. Duruisseaux M, Besse B, Cadranel J et al. Overall survival with crizotinib and next‐generation ALK inhibitors in ALK ‐positive non‐small‐cell lung cancer (IFCT‐1302 CLINALK): A French nationwide cohort retrospective study. Oncotarget 2017;8:21903–21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerr KM, Bubendorf L, Edelman MJ et al. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non‐small‐cell lung cancer. Ann Oncol 2014;25:1681–1690. [DOI] [PubMed] [Google Scholar]
- 6. Planchard D, Popat S, Kerr K et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018;29(suppl 4):iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 7. Ryska A, Buiga R, Fakirova A et al. Non‐small cell lung cancer in countries of Central and Southeastern Europe: Diagnostic procedures and treatment reimbursement surveyed by the Central European Cooperative Oncology Group. The Oncologist 2018;23:e152–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cufer T, Ciuleanu TE, Berzinec P et al. Access to novel drugs for non‐small cell lung cancer in Central and Southeastern Europe: A Central European Cooperative Oncology Group analysis. The Oncologist 2020;25:e598–e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janzic U, Knez L, Janzic A et al. Time to access to novel anticancer drugs and the correlation with ESMO‐Magnitude of Clinical Benefit Scale in Slovenia. Expert Rev Pharmacoecon Outcomes Res 2019;19:717–723. [DOI] [PubMed] [Google Scholar]
- 10. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 13. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1–positive non–small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 15. Reck M, Rodríguez–Abreu D, Robinson AG et al. Updated analysis of KEYNOTE‐024: Pembrolizumab versus platinum‐based chemotherapy for advanced non–small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37:537–546. [DOI] [PubMed] [Google Scholar]
- 16. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 17. Magee DE, Hird AE, Klaassen Z et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta‐analysis of randomized clinical trials. Ann Oncol 2020;31:50–60. [DOI] [PubMed] [Google Scholar]
- 18. Planchard D, Popat S, Kerr K et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. European Society for Medical Oncology Web site, 2020. Available at https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer. Accessed November 30, 2020. [DOI] [PubMed]
- 19. Templeton AJ, Booth CM, Tannock IF. Informing patients about expected outcomes: The efficacy‐effectiveness gap. J Clin Oncol 2020;38:1651–1654. [DOI] [PubMed] [Google Scholar]
- 20. Di Maio M, Perrone F, Conte P. Real‐world evidence in oncology: Opportunities and limitations. The Oncologist 2020;25:e746–e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin S, Pazdur R, Sridhara R. Re‐Evaluating eligibility criteria for oncology clinical trials: Analysis of investigational new drug applications in 2015. J Clin Oncol 2017;35:3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vrdoljak E, Bodoky G, Jassem J et al. Cancer control in Central and Eastern Europe: Current situation and recommendations for improvement. The Oncologist 2016;21:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Cancer Institute, National Institutes of Health. Common Terminology Criteria for Adverse Events v4.0. Bethesda, MD: U.S. National Institutes of Health, May 28, 2009.
- 24. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team . A language and environment for statistical computing. 2020.Available at https://www.r-project.org/. Accessed November 30, 2020.
- 26. Alboukadel Kassambara MK. survminer: Drawing Survival Curves using “ggplot2”. R package version 0.4.3. 2018. Available at https://cran.r-project.org/package=survminer. Accessed November 30, 2020.
- 27. Amrane K, Geier M, Corre R et al. First‐line pembrolizumab for non–small cell lung cancer patients with PD‐L1 ≥50% in a multicenter real‐life cohort: The PEMBREIZH study. Cancer Med 2020;9:2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mok TSK, Wu YL, Kudaba I et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 29. Dudnik E, Moskovitz M, Daher S et al. Effectiveness and safety of nivolumab in advanced non‐small cell lung cancer: The real‐life data. Lung Cancer 2018;126:217–223. [DOI] [PubMed] [Google Scholar]
- 30. Lin SY, Yang CY, Liao BC et al. Tumor PD‐L1 expression and clinical outcomes in advanced‐stage non‐small cell lung cancer patients treated with nivolumab or pembrolizumab: Real‐world data in Taiwan. J Cancer 2018;9:1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montana M, Garcia ME, Ausias N et al. Efficacy and safety of nivolumab in patients with non‐small cell lung cancer: A retrospective study in clinical practice. J Chemother 2019;31:90–94. [DOI] [PubMed] [Google Scholar]
- 32. Ahn BC, Pyo KH, Xin CF et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non‐small cell lung cancer treated with anti‐PD‐1 therapy in real‐world practice. J Cancer Res Clin Oncol 2019;145:1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barlesi F, Dixmier A, Debieuvre D et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: Preliminary results from the real‐world EVIDENS study. Oncoimmunology 2020;9:1744898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Figueiredo A, Almeida MA, Almodovar MT et al. Real‐world data from the Portuguese Nivolumab Expanded Access Program (EAP) in previously treated non small cell lung cancer (NSCLC). Pulmonology 2020;26:10–17. [DOI] [PubMed] [Google Scholar]
- 35. Tamiya M, Tamiya A, Hosoya K et al. Efficacy and safety of pembrolizumab as first‐line therapy in advanced non‐small cell lung cancer with at least 50% PD‐L1 positivity: A multicenter retrospective cohort study (HOPE‐001). Invest New Drugs 2019;37:1266–1273. [DOI] [PubMed] [Google Scholar]
- 36. Grossi F, Genova C, Crinò L et al. Real‐life results from the overall population and key subgroups within the Italian cohort of nivolumab expanded access program in non‐squamous non–small cell lung cancer. Eur J Cancer 2019;123:72–80. [DOI] [PubMed] [Google Scholar]
- 37. Ksienski D, Wai ES, Croteau N et al. Pembrolizumab for advanced nonsmall cell lung cancer: Efficacy and safety in everyday clinical practice. Lung Cancer 2019;133:110–116. [DOI] [PubMed] [Google Scholar]
- 38. Food and Drug Administration . Drug trials snapshots summary report 2015–2019. U.S. Food and Drug Administration Web site, 2020. Available at https://www.fda.gov/media/143592/download. Accessed November 30, 2020.
- 39. Shulman LN, Palis BE, McCabe R et al. Survival as a quality metric of cancer care: Use of the National Cancer Data Base to assess hospital performance. J Oncol Pract 2018;14:e59–e72. [DOI] [PubMed] [Google Scholar]
- 40. Udvaros I, Toth BE. Central and Eastern Europe, the growing opportunistic location to perform oncology clinical trials. SGS 2016. Available at https://www.sgs.com/en/news/2016/12/oncology‐clinical‐trials‐in‐central‐and‐eastern‐europe. Accessed May 26, 2021. [Google Scholar]
- 41. Martin C, Lupinacci L, Perazzo F et al. Efficacy and safety of nivolumab in previously treated patients with non–small‐cell lung cancer: Real world experience in Argentina. Clin Lung Cancer 2020;31:e380–e387. [DOI] [PubMed] [Google Scholar]
- 42. El Karak F, Gh Haddad F, Eid R et al. Lung cancer and immunotherapy: A real‐life experience from second line and beyond. Future Oncol 2019;15:3025–3032. [DOI] [PubMed] [Google Scholar]
- 43. Areses Manrique MC, Mosquera Martínez J, García González J et al. Real world data of nivolumab for previously treated non‐small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res 2018;7(3):404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kobayashi K, Nakachi I, Naoki K et al. Real‐world efficacy and safety of nivolumab for advanced non–small‐cell lung cancer: a retrospective multicenter analysis. Clin Lung Cancer 2018;19(3):e349–e358. [DOI] [PubMed] [Google Scholar]
- 45. Geier M, Descourt R, Corre R et al. Real life second‐line nivolumab in advanced non‐small cell lung cancer: A French observational multicenter study of 259 patients (ABCT‐IMMUNOBZH). Cancer Reports and Rev 2018;2(5):1–6. [Google Scholar]
- 46. Juergens RA, Mariano C, Jolivet J et al. Real‐world benefit of nivolumab in a canadian non‐small‐cell lung cancer cohort. Curr Oncology 2018;25(6):384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fujimoto D, Yoshioka H, Kataoka Y et al. Efficacy and safety of nivolumab in previously treated patients with non‐small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018;119:14–20. [DOI] [PubMed] [Google Scholar]
- 48. Merino Almazán M, Duarte Pérez JM, Marín Pozo JF et al. A multicentre observational study of the effectiveness, safety and economic impact of nivolumab on non‐small‐cell lung cancer in real clinical practice. Int J of Clin Pharm 2019;41:(1):272–279. [DOI] [PubMed] [Google Scholar]
- 49. Khozin S, Carson KR, Zhi J et al. Real‐world outcomes of patients with metastatic non‐small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following U.S. regulatory approval. Oncologist 2019;24(5):648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khozin S, Miksad RA., Adami J et al. Real‐world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non–small cell lung cancer. Cancer 2019;125(22):4019–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crinò L, Bidoli P, Delmonte A et al. Italian Cohort of Nivolumab Expanded Access Program in Squamous Non‐Small Cell Lung Cancer: Results from a Real‐World Population. Oncologist 2019;24(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morita R, Okishio K, Shimizu J et al. Real‐world effectiveness and safety of nivolumab in patients with non‐small cell lung cancer: A multicenter retrospective observational study in Japan. Lung Cancer 2020;140:8–18. [DOI] [PubMed] [Google Scholar]
- 53. Weis TM, Hough S, Reddy HG et al. Real‐world comparison of immune checkpoint inhibitors in non‐small cell lung cancer following platinum‐based chemotherapy. J Oncol Pharm Pract 2020;26(3):564–571. [DOI] [PubMed] [Google Scholar]


