Abstract
Background
In geriatric oncology, polypharmacy is often assessed during a comprehensive geriatric assessment. Previous studies about its association with survival among patients with colorectal cancer (CRC) were inconclusive and had high risk for indication bias.
Patients and Methods
A cohort study was conducted with 3,239 patients with CRC, aged ≥65 years, who were recruited in Germany between 2003 and 2016, while being hospitalized for CRC surgery. We defined polypharmacy as the concurrent use of five or more drugs, and excessive polypharmacy (EPP) as concurrent use of eight or more drugs. Cox proportional hazards regression models were performed to assess the associations of polypharmacy with 5‐year overall (OS), CRC‐specific (CSS), and non‐cancer‐specific survival (NCS) with rigorous adjustment for morbidity to minimize indication bias (e.g., for cancer stage, functional status, and 13 common diseases/conditions).
Results
The prevalence of polypharmacy was 54.7% and that of EPP was 24.2%. During up to 5 years of follow‐up, 1,070 participants died, among whom 615 died of CRC and 296 died of other causes than cancer. EPP was statistically significantly associated with poorer up‐to‐5‐year OS (hazard ratio [HR], 1.23; 95% confidence interval [CI], 1.02–1.47) and CSS (HR, 1.31; 95% CI, 1.03–1.68). HR point estimate for NCS was higher than 1 (1.22) but not statistically significant.
Conclusion
Polypharmacy was very common and EPP was a weak risk factor for mortality in this large cohort of older patients with CRC. Clinical trials are needed to address the causality of this relationship because older patients with CRC might benefit from deprescribing drugs without an indication.
Implications for Practice
The results of this study support the hypothesis that excessive polypharmacy, defined as use of eight or more concurrently used active substances, has a negative impact on the prognosis of older patients with colorectal cancer (CRC). This study suggests to oncologists that performing a medication review for older patients with CRC with eight drugs or more is indicated (especially when a broader comprehensive geriatric assessment is being performed). Such a medication review should not only focus on reducing the number of medications (by deprescribing drugs without an indication) but also check the appropriateness of indicated drugs for older patients with cancer.
Keywords: Geriatric oncology, Colorectal cancer, Polypharmacy, Survival, Comprehensive Geriatric Assessment
Short abstract
Excessive polypharmacy, defined as the concurrent use of eight or more drugs, is becoming more common, especially in the older population. This article evaluates the association of polypharmacy with overall survival in large cohort patients with colorectal cancer.
Introduction
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers worldwide, accounting for more than 1.9 million incident cases and more than 900,000 deaths in 2020 [1], and thus is a disease with great public health relevance [2]. As more than half of patients with CRC are diagnosed after the age of 70 and the 5‐year relative survival rate for all stages of CRC combined has recently increased to surpass 60% [1, 3, 4], pharmacological care of older and often multimorbid patients with CRC is increasingly a challenge for the caring physicians but is also an area with enormous potential for improvements in the general health and survival of older patients with CRC.
The assessment of polypharmacy, which is most commonly defined as the concomitant use of five or more medications [5], is incorporated in most comprehensive geriatric assessment (CGA) tools in order to tackle clinical complexity in frail older patients [6]. Polypharmacy is at least as frequently seen in older patients with cancer as in the general older population. In previous studies, the prevalence of polypharmacy varied between 5.6% and 96% in older cancer populations [7, 8]. The wide range can be explained by different cancer populations and disparate polypharmacy definitions [7].
Patients with cancer are particularly prone to unintended consequences of polypharmacy because they often receive chemotherapy and symptom‐relieving agents, which may entail additional risk for drug‐drug interactions and unwanted adverse drug reactions (ADRs) [9]. Consequently, studies with older patients with cancer observed associations of polypharmacy with frailty, diminished physical and cognitive function, postoperative complications, lengthened hospital stay, treatment‐related toxicities, and premature mortality [7, 8, 10, 11, 12, 13].
Three studies with patients with CRC specifically addressed the associations of polypharmacy with overall survival (OS) so far [14, 15, 16]. Except for one study, which defined using an uncommonly extreme cutoff of ≥8 drugs to define polypharmacy [14], results were not statistically significant. In addition, the results were not adjusted or were poorly adjusted for comorbidity and therefore may have been vulnerable to indication bias [17, 18].
Given the inconclusiveness and the high risk of indication bias of previous studies, we aimed to evaluate the association of polypharmacy with OS in a large cohort of patients with CRC with comprehensive adjustment for potential confounders. Moreover, we widened the spectrum of endpoints by additionally investigating CRC‐specific‐survival (CSS), non‐cancer‐specific survival (NCS), and chemotherapy‐related ADRs.
Materials and Methods
Study Design and Population
This study has a cohort study design because only the patients with CRC aged ≥65 years (cases) from the ongoing, population‐based Darmkrebs: Chancen der Verhütung durch Screening (DACHS) case‐control study were included. The DACHS study recruits CRC cases in 22 hospitals and randomly selects control participants with no history of colorectal cancer in the Rhine‐Neckar‐Heilbronn area in Germany. Details of the DACHS study design have been described elsewhere [19, 20, 21]. Briefly, patients with a histologically confirmed first diagnosis of CRC (International Classification of Diseases, 10th Revision [ICD‐10], codes C18–C20) [22], aged at least 30 years, and able to speak German are eligible to participate. The DACHS study was approved by the ethics committees of Heidelberg University and the state medical boards of Baden‐Württemberg and Rhineland‐Palatinate. All participants sign a written informed consent.
At baseline, shortly after CRC surgery in the collaborating hospitals, trained study nurses carry out personal interviews with the study participants. Information on sociodemographic and lifestyle factors, medical history, and drug use is collected using a standardized questionnaire. Moreover, comorbidities, last medication, and functional status are extracted from patients’ hospital discharge letters or hospital records. Comorbidities are coded with the ICD‐10 coding algorithm validated by Quan et al. [23] and drugs are coded according to a German adaption of the World Health Organization's Anatomical Therapeutic Chemical (ATC) code (2019 version) [24].
Detailed information on the participants’ CRC treatment (chemotherapy and/or radiotherapy), chemotherapy‐related ADRs, and recurrence history is further gathered from questionnaires sent to gastroenterologists in the outpatient setting 3 years after diagnosis. Vital status and the cause of death of deceased patients are ascertained from population registries. The outlined and further time‐points of data collection during the course of the DACHS study are illustrated in a study timeline in supplemental online Figure 1.
Inclusion and Exclusion Criteria
We included patients with CRC of the DACHS study recruited between 2003 and 2016 in order to have follow‐up of at least 3 years (the end of available mortality follow‐up was February 2020). We further restricted the analyses to patients 65 years and older because the CGA, of which polypharmacy is a part, is being performed in older patients with cancer only and 65 years is an often‐used cutoff to define older age. Further exclusions were made for patients with CRC not having received any surgery (mostly patients with stage IV disease with very poor survival prognosis who cannot be cured anymore by surgery), without documentation of discharge medication in the hospital release records or lost to follow‐up with respect to mortality, leaving 3,239 patients for the survival analyses (supplemental online Fig. 2). For analyses on chemotherapy‐related ADRs, patients without adjuvant/neoadjuvant chemotherapy or 3‐year follow‐up information from gastroenterologists were further excluded, leaving 1,209 patients for those analyses.
Medication Assessment and Definition of Polypharmacy
We reviewed the drugs recorded in the discharge letters and additionally took the patients’ self‐reported medications at baseline into consideration to achieve as complete drug information as possible. To avoid double counting of self‐reported medications and drugs from discharge letters, we counted prescriptions with the same ATC codes to the fourth level, which represents duplicates of chemical or pharmacological substances of similar functions [25, 26], once. We used the most common polypharmacy definition, which counts all concurrently used drugs and defines use of five or more medications as polypharmacy [5]. We further defined the concurrent use of eight or more drugs as excessive polypharmacy. In addition, we applied a modified polypharmacy definition and counted only potentially clinically relevant active substances. Details of the modified definition have been described elsewhere [27]. In brief, we counted combination drugs based on the number of active substances rather than from the perspective of pill number. For example, a pill that contains a beta‐blocker (bisoprolol) and a thiazide diuretic (hydrochlorothiazide) was counted as two active substances (details of ATC codes of combination drugs are presented in supplemental online Table 1). Furthermore, we did not count drugs that are known to be safe, that is, food supplements, homeopathic or anthroposophical drugs, some herbal drugs, and nonsystematically acting drugs if they have no known ADRs other than local reactions. In extension of the previously published modified polypharmacy definition, three drug classes were additionally excluded because they were prescribed to most patients with CRC during or shortly after CRC surgery for short‐term use. If antithrombotic agents, nonsteroidal anti‐inflammatory drugs, and drugs against peptic ulcer disease were listed in the discharge letters but were not mentioned in the interview with the patients, it was assumed that they were prescribed for short‐term use in the context of the CRC surgery and were not counted. The full list of excluded ATC codes or ATC code groups for the modified polypharmacy definition is shown in supplemental online Table 2.
Ascertainment of Chemotherapy‐Related Adverse Drug Reactions
Chemotherapy‐related ADRs were collected from patients’ gastroenterologists in the outpatient setting via questionnaires sent at 3‐year follow‐up. From a list of documented ADRs, we selected those with at least 100 cases to have adequate statistical power. The neurological and gastrointestinal ADRs had this case number and were defined as separate, dichotomized dependent outcomes (occurred yes/no). The Common Terminology Criteria for Adverse Events grades were not available.
Ascertainment of Overall, CRC‐Specific, and Non‐Cancer‐Specific Survival
Information about the vital status, date, and cause of death of study participants was collected via inquiry at local population registries. The ICD‐10 codes of the causes of death were verified by death certificates. Overall, CRC‐specific, and non‐cancer‐specific survival are defined as time from CRC hospitalization to death from any cause, from CRC, or from cause of death other than cancers, respectively, or end of follow‐up (February 2020).
Statistical Analyses
A dichotomous polypharmacy variable (≥5 vs. <5 drugs) was used in analyses for associations of baseline characteristics with polypharmacy. Two variable sets of multivariable logistic regression models were used to assess the associations of patient characteristics with the dichotomous variable for polypharmacy, with one adjusted for age and sex and the other comprising all variables shown in Table 1.
Table 1.
Associations of the baseline characteristics of older patients with CRC with polypharmacy
| Variables | Total n | Polypharmacy | ||
|---|---|---|---|---|
| n (%) a | Age‐ and sex‐adjusted, OR (95% CI) | Multivariable, OR (95% CI) b | ||
| Sex | ||||
| Female | 1,334 | 785 (58.9) | Ref | Ref |
| Male | 1,905 | 987 (51.8) | 0.79 (0.69–0.92) | 0.54 (0.43–0.69) |
| Age at CRC diagnosis | ||||
| Per 1‐year increase | — | — | 1.04 (1.03–1.05) | 1.00 (0.99–1.02) |
| Years of schooling | ||||
| ≤9 | 2,318 | 1,332 (57.5) | Ref | Ref |
| 10–11 | 486 | 235 (48.4) | 0.68 (0.56–0.83) | 0.77 (0.60–0.99) |
| ≥12 | 435 | 205 (47.1) | 0.71 (0.58–0.87) | 0.89 (0.69–1.17) |
| Year of CRC diagnosis | ||||
| 2003–2007 | 1,098 | 558 (50.8) | Ref | Ref |
| 2008–2012 | 1,223 | 676 (55.3) | 1.19 (1.01–1.41) | 1.40 (1.13–1.74) |
| 2013–2016 | 918 | 538 (58.6) | 1.34 (1.12–1.60) | 1.67 (1.32–2.11) |
| Tumor location | ||||
| Colon | 2,088 | 1,162 (55.7) | Ref | Ref |
| Rectum | 1,151 | 610 (53.0) | 0.97 (0.84–1.13) | 1.21 (1.00–1.45) |
| Tumor stage | ||||
| I | 769 | 431 (56.1) | Ref | Ref |
| II | 1,082 | 606 (56.0) | 0.95 (0.79–1.15) | 0.99 (0.78–1.25) |
| III | 985 | 526 (53.4) | 0.87 (0.71–1.05) | 1.17 (0.87–1.58) |
| IV | 403 | 209 (51.9) | 0.88 (0.68–1.12) | 1.22 (0.85–1.74) |
| (Neo‐) adjuvant chemotherapy | ||||
| Yes | 1,211 | 586 (48.4) | 0.75 (0.65–0.87) | 0.81 (0.63–1.05) |
| No | 2,028 | 1,186 (58.5) | Ref | Ref |
| BMI, kg/m2 | ||||
| <25 | 1,197 | 562 (47.0) | Ref | Ref |
| 25–29.9 | 1,417 | 803 (56.7) | 1.62 (1.38–1.90) | 1.21 (0.99–1.46) |
| ≥30 | 625 | 407 (65.1) | 2.45 (1.99–3.01) | 1.28 (0.99–1.66) |
| Lifetime physical activity, MET‐hours/week | ||||
| T1 | 1,079 | 588 (54.5) | Ref | Ref |
| T2 | 1,080 | 607 (56.2) | 1.06 (0.89–1.26) | 0.90 (0.73–1.12) |
| T3 | 1,080 | 577 (53.4) | 1.05 (0.88–1.25) | 0.94 (0.75–1.18) |
| Smoking status | ||||
| Never‐smoker | 1,466 | 793 (54.1) | Ref | Ref |
| Former smoker | 1,473 | 832 (56.5) | 1.36 (1.15–1.60) | 1.23 (1.01–1.51) |
| Current smoker | 300 | 147 (49.0) | 1.07 (0.82–1.38) | 1.20 (0.87–1.66) |
| Lifetime alcohol consumption | ||||
| None | 603 | 349 (57.9) | Ref | Ref |
| T1 | 873 | 497 (56.9) | 1.06 (0.85–1.31) | 1.22 (0.93–1.59) |
| T2 | 883 | 447 (50.6) | 0.94 (0.75–1.19) | 1.14 (0.85–1.53) |
| T3 | 880 | 479 (54.4) | 1.21 (0.94–1.55) | 1.31 (0.96–1.80) |
| Red meat consumption | ||||
| <1 time/week | 259 | 139 (53.7) | Ref | Ref |
| 1 time/week | 735 | 393 (53.5) | 1.02 (0.77–1.37) | 1.30 (0.90–1.89) |
| Multiple times per week | 2,245 | 1,240 (55.2) | 1.19 (0.91–1.56) | 1.29 (0.90–1.83) |
| Processed meat consumption | ||||
| <1 time/week | 406 | 219 (53.9) | Ref | Ref |
| 1 time/week | 426 | 217 (50.9) | 0.91 (0.69–1.20) | 0.90 (0.64–1.27) |
| Multiple times per week | 2,407 | 1,336 (55.5) | 1.17 (0.94–1.45) | 1.10 (0.83–1.45) |
| Functional status | ||||
| Excellent | 629 | 276 (43.9) | Ref | Ref |
| Fair | 1,214 | 603 (49.7) | 1.32 (1.06–1.65) | 1.45 (1.11–1.88) |
| Poor | 1,396 | 893 (64.0) | 2.25 (1.78–2.85) | 1.72 (1.31–2.26) |
| Comorbidity | ||||
| Hypertension | ||||
| No | 984 | 238 (24.2) | Ref | Ref |
| Yes | 2,255 | 1,534 (68.0) | 6.44 (5.42–7.64) | 4.87 (4.00–5.93) |
| Cardiac insufficiency | ||||
| No | 2,560 | 1,240 (48.4) | Ref | Ref |
| Yes | 679 | 532 (78.4) | 3.47 (2.84–4.25) | 1.66 (1.30–2.13) |
| Acute coronary syndrome | ||||
| No | 2,845 | 1,444 (50.8) | Ref | Ref |
| Yes | 394 | 328 (83.3) | 4.78 (3.62–6.30) | 1.89 (1.36–2.64) |
| History of myocardial infarction | ||||
| No | 2,640 | 1,258 (47.7) | Ref | Ref |
| Yes | 599 | 514 (85.8) | 6.81 (5.33–8.70) | 3.84 (2.88–5.12) |
| History of stroke | ||||
| No | 2,849 | 1,485 (52.1) | Ref | Ref |
| Yes | 390 | 287 (73.6) | 2.40 (1.89–3.05) | 1.74 (1.31–2.31) |
| Atrial fibrillation | ||||
| No | 2,816 | 1,449 (51.5) | Ref | Ref |
| Yes | 423 | 323 (76.4) | 2.80 (2.20–3.56) | 1.94 (1.46–2.57) |
| COPD | ||||
| No | 2,933 | 1,545 (52.7) | Ref | Ref |
| Yes | 306 | 227 (74.2) | 2.61 (2.00–3.42) | 2.51 (1.82–3.46) |
| Type II diabetes mellitus | ||||
| No | 2,403 | 1,123 (46.7) | Ref | Ref |
| Yes | 836 | 649 (77.6) | 3.98 (3.32–4.79) | 2.70 (2.19–3.34) |
| Depression | ||||
| No | 2,985 | 1,588 (53.2) | Ref | Ref |
| Yes | 254 | 184 (72.4) | 2.34 (1.75–3.13) | 2.53 (1.79–3.58) |
| Chronic pain | ||||
| No | 1,274 | 591 (46.4) | Ref | Ref |
| Yes | 1,965 | 1181 (60.1) | 1.66 (1.44–1.92) | 1.40 (1.17–1.67) |
| Gastrointestinal illness | ||||
| No | 2,531 | 1,363 (53.9) | Ref | Ref |
| Yes | 708 | 409 (57.8) | 1.17 (0.98–1.38) | 1.06 (0.86–1.31) |
| Anemia | ||||
| No | 2,896 | 1,560 (53.9) | Ref | Ref |
| Yes | 343 | 212 (61.8) | 1.25 (0.99–1.58) | 1.10 (0.83–1.48) |
| Hypothyroidism | ||||
| No | 3,072 | 1,649 (53.7) | Ref | Ref |
| Yes | 167 | 123 (73.7) | 2.33 (1.63–3.32) | 2.06 (1.36–3.12) |
Values in bold are statistically significant (p < .05).
The column n (%) was calculated using the imputation data set 1.
Effect estimates of a multivariable model comprising all variables shown in this table.
Abbreviations: —, no data; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; MET, metabolic equivalent of task; OR, odds ratio; Ref, reference.
Furthermore, associations of polypharmacy (analyzed as a variable with three categories: 0–4 drugs as reference group; 5–7 drugs; and ≥ 8 drugs) with up‐to‐5‐year OS, CSS, and NCS were assessed with Cox proportional hazards models. The follow‐up time was limited to 5 years because drug exposure may change during longer follow‐up and in order to have comparable mortality follow‐up times between 3 and 5 years for all years of recruitment in the DACHS study. Nevertheless, we carried out sensitivity analyses with unlimited follow‐up time (between 3 and 17 years) for the main analyses. The proportional hazards assumption was assessed for the main determinants and all covariates by adding time‐dependent interaction terms. Only smoking status, tumor stage, and whether neoadjuvant/adjuvant chemotherapy was received violated the assumption, and their interaction terms with follow‐up time were added to the models. The polypharmacy variables did not violate the proportional hazards assumption in analyses with restricted or unrestricted follow‐up time for all three outcomes (OS, CSS, and NCS). Associations of polypharmacy (analyzed as a variable with three categories: 0–4 drugs as reference group; 5–7 drugs; and ≥ 8 drugs) with chemotherapy‐related ADRs were evaluated with multivariable logistic regression models because the time between baseline and ADR assessment was almost the same for all study participants.
Three variable sets for multivariable logistic/Cox proportional regression models were used in survival analyses and analyses on chemotherapy‐related ADRs. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, tumor location and stage, year of CRC diagnosis, neoadjuvant/adjuvant chemotherapy (not included in models on chemotherapy‐related ADRs), years of school education, smoking status, body mass index, lifetime physical activity, lifetime alcohol consumption, and red and processed meat consumption. Model 3 additionally included the functional status and comorbidity (13 common diseases/conditions as shown in Table 1). As functional status was recorded by different instruments in medical records from the various hospitals, (American Society of Anesthesiologists Physical Status Classification System, the Eastern Cooperative of Oncology Group [ECOG], and the Karnofsky performance status), a harmonized indicator variable for functional status was created as previously prescribed [28]. Furthermore, restricted cubic splines were applied to graphically illustrate the association of an increasing number of concomitantly used drugs and the outcomes without relying on the arbitrary cut‐point of five drugs to define polypharmacy.
Subgroup analyses were carried out for subgroups defined by age (65–74/≥75 years), sex, tumor location (colon/rectum), neoadjuvant/adjuvant chemotherapy (Yes/No), tumor stage (I, II/III/IV), and functional status (excellent/fair/poor).
Multiple imputation was performed to impute missing covariate data using the Markov Chain Monte Carlo [29] technique with 200 burn‐in iterations, and 20 data sets were generated. All covariates needed for model 3 were used as the imputation model. All analyses were conducted with the SAS software, version 9.4 (SAS Institute, Cary, NC). Statistical tests were two‐tailed, with a significance level (α) of .05.
Results
Cross‐Sectional Analyses
We included 3,239 patients for cross‐sectional analyses and analyses on survival endpoints (supplemental online Fig. 2). The mean age of the included study participants was 75.0 years (SD, 6.5 years) at baseline, and 1,334 (41.2%) were female. The distribution of the number of medications with the clinically oriented, modified definition of polypharmacy (Fig. 1) and the simple count of all drugs as the traditional polypharmacy definition (supplemental online Fig. 3) were both right‐skewed. According to the modified polypharmacy definition, the mean (SD) number of concurrently used drugs was 5.3 (3.3) and the maximum was 20 drugs. According to the traditional polypharmacy definition, the mean (SD) number of concurrently used drugs was 6.2 (3.2) and the maximum was 19 drugs. Overall, with the modified polypharmacy definition, 54.7% of the study participants concomitantly used ≥5 medications and 24.2% used ≥8 medications. With the traditional definition of polypharmacy, these two proportions were 66.3% and 31.7%, respectively. In the following, the results for the modified polypharmacy definition are reported in the article and the corresponding, similar results for the traditional polypharmacy definition are shown in supplemental online Tables 3–6 and supplemental online Figure 4.
Figure 1.
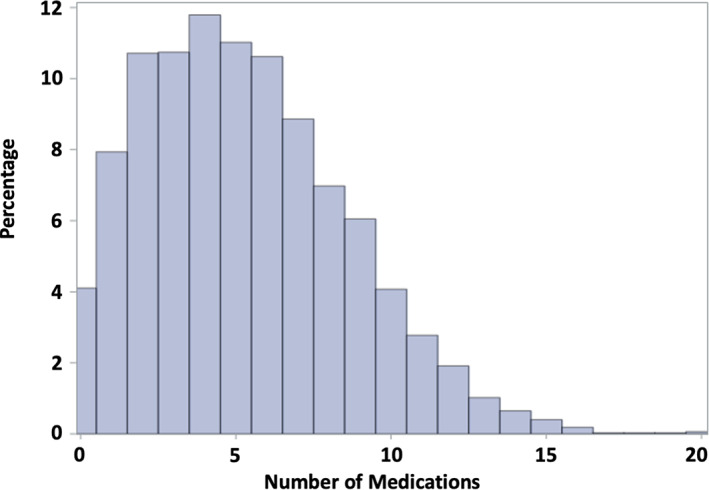
Distribution of number of medications.
The baseline characteristics of the study population and their associations with polypharmacy (≥5 medications) are shown in Table 1. With increasing calendar years of CRC diagnosis, the prevalence of polypharmacy increased. Male patients had a 46% lower chance to be exposed to polypharmacy than female patients. The significant association of age with polypharmacy in the age‐ and sex‐adjusted model disappeared after including other stronger determinants of polypharmacy, such as comorbidity and functional status, in the full model. The number of years of school education was inversely associated with polypharmacy. Patients with rectal cancer had a statistically significantly 21% higher odds of polypharmacy compared with those with colon cancer. Tumor stage and (neo‐)adjuvant chemotherapy were not associated with polypharmacy. For lifestyle factors, participants who were former smokers had 23% and current smokers 20% higher odds of polypharmacy (the latter not statistically significant). Poorer functional status was significantly associated with a 72% higher chance for polypharmacy. With respect to comorbidity, subjects with hypertension, cardiac insufficiency, acute coronary syndrome, history of myocardial infarction, history of stroke, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, depression, chronic pain, and hypothyroidism had a significantly increased odds of polypharmacy.
Longitudinal Analyses
During up to 5 years of follow‐up for OS, 1,070 participants died, among whom 615 died of CRC as the primary cause of death (57.5%) and 296 had a cause of death other than cancer (27.7%). In model 1 and 2, the associations of polypharmacy with colorectal cancer–specific and non‐cancer‐specific mortality were very different: no or weak associations were observed with colorectal cancer–specific mortality, whereas the association of polypharmacy with non‐cancer‐specific mortality was strong and statistically significant (Table 2). With additional adjustment for comorbidity and functional status in model 3, the hazard ratios (HRs) and 95% confidence intervals (CIs) for CSS (HR, 1.31; 95% CI, 1.03–1.68 for ≥8 drugs vs. 0–4 drugs) and NCS (HR, 1.22; 95% CI, 0.87–1.71 for ≥8 drugs vs. 0–4 drugs) became comparable. The HRs and 95% CIs for OS (HR, 1.23; 95% CI, 1.02–1.47 for ≥8 drugs vs. 0–4 drugs) and the association with CSS remained statistically significant. The described changes in the results for polypharmacy from model 2 to model 3 were likewise observed in the dose‐response analyses (Fig. 2). The restricted cubic spline curves for model 3 show a flat line at the null effect value of HR = 1 for the point estimates of the associations of the number of drugs with all outcomes up to 5–6 medications. After this cutoff, the curves show statistically significantly, monotonically worsening overall, colorectal cancer–specific, and non‐cancer‐specific survival with an increasing number of drugs, and the associations became more profound after the cutoff of eight medications.
Table 2.
The associations of polypharmacy with up to 5‐year overall, CRC‐specific, and non‐cancer‐specific survival in older patients with CRC
| Survival outcomes | Total n | Cases, n (%) | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Overall survival | |||||
| 0–4 drugs | 1,467 | 430 (29.3) | Ref | Ref | Ref |
| 5–7 drugs | 988 | 326 (33.0) | 1.09 (0.94–1.26) | 1.16 (1.00–1.35) | 1.07 (0.91–1.26) |
| ≥8 drugs | 784 | 314 (40.1) | 1.32 (1.14–1.53) | 1.44 (1.24–1.68) | 1.23 (1.02–1.47) |
| CRC‐specific survival | |||||
| 0–4 drugs | 1,432 | 276 (19.3) | Ref | Ref | Ref |
| 5–7 drugs | 964 | 185 (19.2) | 0.98 (0.81–1.18) | 1.03 (0.86–1.25) | 1.05 (0.86–1.30) |
| ≥8 drugs | 749 | 154 (20.6) | 1.08 (0.88–1.31) | 1.24 (1.01–1.53) | 1.31 (1.03–1.68) |
| Non‐cancer‐specific survival | |||||
| 0–4 drugs | 1,432 | 90 (6.3) | Ref | Ref | Ref |
| 5–7 drugs | 964 | 91 (9.4) | 1.41 (1.06–1.90) | 1.41 (1.05–1.90) | 1.02 (0.74–1.40) |
| ≥8 drugs | 749 | 115 (15.4) | 2.14 (1.62–2.83) | 2.15 (1.62–2.87) | 1.22 (0.87–1.71) |
Values in bold are statistically significant (p < .05).
Adjusted for age and sex.
Adjusted for age, sex, tumor stage, tumor location, year of CRC diagnosis, neoadjuvant/adjuvant chemotherapy, year of schooling, smoking status, body mass index, lifetime physical activity, lifetime alcohol consumption, red meat consumption, and processed meat consumption.
Adjusted for variables of model 2, functional status, and comorbidity.
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio.
Figure 2.
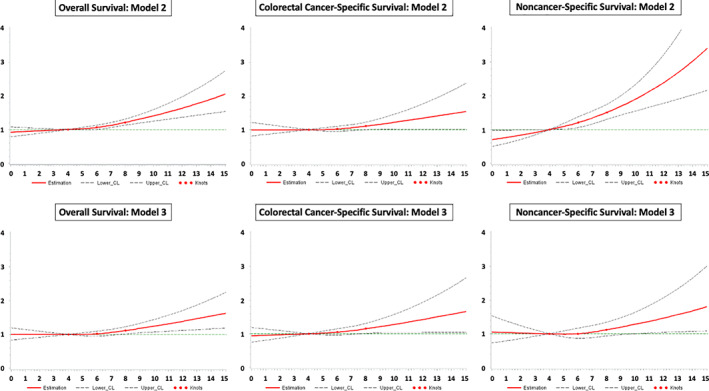
Dose‐response curves for the associations of the number of drugs with overall, colorectal cancer–specific, and non‐cancer‐specific survival in model 2 (without adjustment for functional status and comorbidity) and model 3 (full model). The dose‐response curves for model 3 were obtained from the imputation data set 1.Abbreviation: CL, confidence limit.
In sensitivity analyses, using unlimited follow‐up time of up to 17 years, 1,618 participants died, among whom 747 died of CRC as the primary cause of death (46.2%) and 578 had a cause of death other than cancer (35.7%). The results of sensitivity analyses were similar to those obtained in the main analyses with limited follow‐up of up to 5 years (supplemental online Table 7). Due to the higher statistical power by more events, the association of polypharmacy with OS was estimated with a higher precision but the point estimate did not change (HR, 1.23; 95% CI, 1.06–1.43 for ≥8 drugs vs. 0–4 drugs). The association between polypharmacy and CSS became weaker but remained statistically significant (HR, 1.26; 95% CI, 1.00–1.58 for ≥8 drugs vs. 0–4 drugs). In contrast, the association of polypharmacy with NCS became stronger and statistically significant in the fully adjusted model (HR, 1.35; 95% CI, 1.06–1.72 for ≥8 drugs vs. 0–4 drugs).
In subgroup analyses, a substantially stronger association (HR > 1.35) of excessive polypharmacy with all‐cause mortality than that in the total cohort was observed among patients with rectal cancer, patients receiving chemotherapy, with stage III cancer, and excellent functional status (Table 3). However, the differences in the strata were not very pronounced. Except for patients with rectal cancer and with stage III cancer, all subgroup results lacked statistical significance attributed to the reduced statistical power.
Table 3.
Subgroup analyses for the associations of polypharmacy with up‐to‐5‐year OS in older patients with CRC
| Variable | Total n | Cases, n (%) | HR (95% CI) a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–4 drugs | 5–7 drugs | ≥8 drugs | 0–4 drugs | 5–7 drugs | ≥8 drugs | 0–4 drugs | 5–7 drugs | ≥8 drugs | |
| Age, yr | |||||||||
| 65–74 | 848 | 491 | 313 | 209 (24.7) | 129 (26.3) | 99 (31.6) | Ref | 1.07 (0.84–1.37) | 1.32 (0.96–1.80) |
| ≥75 | 619 | 497 | 471 | 221 (35.7) | 197 (39.6) | 215 (45.7) | Ref | 1.05 (0.85–1.30) | 1.22 (0.97–1.54) |
| Sex | |||||||||
| Female | 549 | 435 | 350 | 166 (30.2) | 143 (32.9) | 144 (41.1) | Ref | 0.92 (0.71–1.19) | 1.27 (0.95–1.69) |
| Male | 918 | 553 | 434 | 264 (28.8) | 183 (33.1) | 170 (39.2) | Ref | 1.15 (0.93–1.41) | 1.16 (0.91–1.47) |
| Tumor location | |||||||||
| Colon | 926 | 616 | 546 | 290 (31.3) | 194 (31.5) | 218 (39.9) | Ref | 0.94 (0.77–1.14) | 1.23 (0.98–1.54) |
| Rectum | 541 | 372 | 238 | 140 (25.9) | 132 (35.5) | 96 (40.3) | Ref | 1.40 (1.07–1.83) | 1.36 (0.97–1.89) |
| Chemotherapy | |||||||||
| No chemotherapy | 841 | 628 | 558 | 200 (23.8) | 167 (26.6) | 205 (36.7) | Ref b | 0.87 (0.64–1.18) b | 1.17 (0.75–1.38) b |
| Chemotherapy | 624 | 360 | 225 | 229 (36.7) | 159 (44.2) | 108 (48.0) | Ref b | 1.38 (0.98–1.95) b | 1.42 (0.92–2.20) b |
| Tumor stage | |||||||||
| I and II | 812 | 568 | 465 | 144 (17.7) | 105 (18.5) | 130 (28.0) | Ref | 0.84 (0.64–1.11) | 1.02 (0.75–1.38) |
| III | 454 | 296 | 223 | 133 (29.3) | 120 (40.5) | 110 (49.3) | Ref | 1.22 (0.91–1.63) | 1.50 (1.09–2.08) |
| IV | 192 | 120 | 89 | 152 (79.2) | 99 (82.5) | 70 (78.7) | Ref | 1.06 (0.78–1.42) | 1.13 (0.79–1.62) |
| Functional status | |||||||||
| Excellent | 297 | 135 | 80 | 58 (19.5) | 36 (26.7) | 24 (30.0) | Ref | 1.34 (0.81–2.21) | 1.73 (0.93–3.23) |
| Fair | 423 | 261 | 202 | 118 (27.9) | 67 (25.7) | 74 (36.6) | Ref | 1.04 (0.73–1.49) | 1.33 (0.89–1.98) |
| Poor | 280 | 287 | 303 | 105 (37.5) | 122 (42.5) | 134 (44.2) | Ref | 1.23 (0.92–1.64) | 1.08 (0.79–1.49) |
Values in bold are statistically significant (p < .05).
Adjusted for age, sex, tumor stage, tumor location, year of CRC diagnosis, neoadjuvant/adjuvant chemotherapy, year of schooling, smoking status, body mass index, lifetime physical activity, lifetime alcohol consumption, red meat consumption, processed meat consumption, functional status, and comorbidity except stratifying factor.
Data are shown as OR (95% CI).
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; OR, odds ratio.
Among 961 subjects who received chemotherapy after hospital discharge and with available information on chemotherapy‐related ADRs, 465 (48.4%) simultaneously used five or more medications and 175 (18.2%) used eight or more medications. Statistically significant associations of polypharmacy were not seen with neurological (odds ratio [OR], 0.95; 95% CI, 0.64–1.41 for ≥8 drugs vs. 0–4 drugs) or gastrointestinal ADRs (OR, 1.00; 95% CI, 0.75–1.33 for ≥8 drugs vs. 0–4 drugs) in the main model 3 (supplemental online Table 8).
Discussion
In this large‐scale cohort study of patients with CRC, an increasing number of medications upon hospital discharge after CRC surgery was significantly associated with poorer up‐to‐5‐year OS. The strengths of the associations of polypharmacy with CSS and NCS were similar compared with that with OS, but the association with NCS was not statistically significant. Furthermore, no statistically significant associations were observed between polypharmacy and chemotherapy‐related gastrointestinal and neurological ADRs.
Like four of the previous studies with patients with CRC [15, 30, 31, 32], we also defined polypharmacy as concomitant use of five medications or more, and its prevalence in our study population (66.3% when counting all drugs and 54.7% when counting only clinically relevant drugs) was comparable to that reported by the other studies (prevalence range: 25.8%–71.2%) [15, 30, 31, 32]. In this way, we are confident that the reporting quality of medication information in our study was at least as good as that in previous studies.
Three studies with patients with CRC (n = 114, n = 178, and n = 1,528) specifically addressed the associations of polypharmacy with overall survival so far [14, 15, 16]. It is unclear for the two small studies from Ommundsen et al. [14, 16] whether part of the recruited patients with CRC were the same. The earlier publication reported a statistically significant association (OR, 2.20; 95% CI, 1.10–4.30) for a comparison of users of ≥8 and < 8 drugs [14], and the later publication observed no significant association for a comparison of users of ≥6 and < 6 drugs (HR, 1.5; 95% CI, 0.8–2.7) [16]. The analysis in the large Surveillance, Epidemiology, and End Results Medicare database (n = 1,528 patients with CRC and n = 1,595 patients with breast cancer) observed statistically significant associations for the comparisons of users of 5–10 drugs (HR, 1.27; 95% CI, 1.00–1.61) and ≥ 11 drugs (HR, 1.75; 95% CI, 1.34–2.28) with users of 0–4 drugs when patients with CRC and patients with breast cancer were combined. In distinct analysis, the statistical power was insufficient, and polypharmacy was not significantly associated with overall mortality among patients with CRC or those with breast cancer; the HRs were not reported. For patients with CRC, the study only observed a statistically significant association of polypharmacy with the outcome emergency room visits [15]. A few further CRC cohort studies have addressed associations of polypharmacy with clinical outcomes other than survival, but most of them reported null results attributed to insufficient statistical power [15, 30, 31, 32, 33, 34]. Of note, the results of previous studies on the associations of polypharmacy and clinical outcomes were not or poorly adjusted for comorbidity and therefore may have been vulnerable to indication bias [17, 18].
Compared with the result of a published meta‐analysis of 18 previous cohort studies with older patients with cancer with various cancer types [7], the association of polypharmacy with all‐cause mortality (risk ratio, 1.37; 95% CI, 1.25–1.50) was similar to the HR and 95% CI of model 2 from our study with older patients with CRC (HR, 1.44; 95% CI, 1.24–1.68 for ≥8 drugs vs. 0–4 drugs). Fifteen out of the 18 studies included in the meta‐analysis and the model 2 analysis from our study were not adjusted for comorbidity. When we additionally adjusted for comorbidity and functional status, which are important potential confounders modeling the morbidity level, the association of polypharmacy with all‐cause mortality was remarkably attenuated but remained statistically significant (HR, 1.23; 95% CI, 1.02–1.47 for ≥8 drugs vs. 0–4 drugs). Thus, after comprehensive correction for confounding by indication in our study, the excess risk by polypharmacy was approximately halved from 44% to 23% for patients using ≥8 drugs. In addition, we did not observe any increased mortality for users of 5–7 drugs (HR, 1.07; 95% CI, 0.91–1.26 in comparison with 0–4 drugs). We estimate that approximately half of the excess risk for the association of polypharmacy and all‐cause mortality in older patients with cancer reported from previous studies might just be the result of indication bias. The main reason why most of previous studies insufficiently adjusted for comorbidity is that the objectives of these studies were to assess polypharmacy as a prognostic factor and not as a risk factor. Polypharmacy is often incorporated into CGAs that aim to predict adverse health outcomes in frail patients with cancer. For these prognostic studies, adjustment for potential confounders is generally not needed. However, when assessing the research question whether polypharmacy is a risk factor for adverse health outcomes in frail patients with cancer, proper adjustment for potential confounders is crucial.
Besides comorbidity, we observed that the functional status is an important potential confounder that needs to be taken into account. We observed that participants with poorer functional status also had higher odds of polypharmacy, and the association was still statistically significant after additionally adjusting for lifestyle factors and comorbidities (OR, 1.72; 95% CI, 1.31–2.26 for poor functional status vs excellent functional status). This finding is in agreement with previous studies, which investigated the associations between patient characteristics and polypharmacy in older patients with cancer and observed that poorer ECOG performance status was significantly associated with polypharmacy [35, 36]. Interestingly, in our cohort, age was not associated with polypharmacy any more in the full model, in which we included more clinically relevant variables than age, like the functional status and 13 diseases/conditions. Thus, we are confident that a sufficient adjustment for the health status was made and residual confounding was minimized as much as possible.
Another aspect of causality assessment is a dose‐response relationship. An important finding of our study is that such a monotonic increase in mortality was only observed for the number of drugs used and all survival outcomes after the number of drugs surpassed 5–6 drugs and that the associations started to become more profound with eight drugs or more. This may indicate that the oversight of the potential for ADRs and drug‐drug interactions of up to the number of seven drugs by physicians works well for older patients with CRC. However, with eight drugs and more, the medication management for older patients with CRC becomes more and more complex and risks by ADRs and drug‐drug interactions may be overseen or occur because there are unknown interactions [9].
Drug‐drug interactions with chemotherapy and a higher severity of chemotherapy‐related ADRs are of special concern among oncologists who need to decide about the initiation of chemotherapy for certain older patients with CRC with polypharmacy. We did not find any significant associations of polypharmacy with neurological and gastrointestinal chemotherapy‐related ADRs. However, there is much more research needed in this area because our investigation was the first of its kind and had the limitation of low number of events, which prohibited us to address rarer ADRs (e.g., hematological ADRs).
We acknowledge that there are some further limitations in our study despite its unique strengths. We already stated the reasons why we think that under‐reporting of drugs and residual confounding are no big issues in our study. However, we had no information on prescription changes over time and medication adherence of study participants. The resulting inaccuracy of polypharmacy classification has most likely led to an underestimation of effect estimates. Another aspect that could have led to an underestimation is the healthy‐user/sick‐stopper bias because a new‐user design was not possible to apply in this study [37]. Besides, our study was conducted in Germany and the generalizability of its results to other countries may be limited.
Nevertheless, taken together, our study suggests that performing a medication review for older patients with CRC with five drugs or more is indicated and could be included in a broader CGA. Such a medication review should not only focus on reducing the number of medications (by deprescribing drugs without an indication) but also check the appropriateness of indicated drugs for older adults. Several tools to assess such potentially inappropriate medication for older patients with cancer are available [38, 39, 40, 41].
Conclusion
In this large cohort study of older patients with CRC, polypharmacy was very common and concurrent use of eight or more drugs was a weak risk factor for up‐to‐5‐year all‐cause and colorectal cancer–specific mortality. No statistically significant associations were observed between polypharmacy and NCS as well as chemotherapy‐related gastrointestinal and neurological ADRs, and larger studies with higher statistical power are needed to address these clinical endpoints. Although some aspects of our results suggest a causal relationship of polypharmacy with all‐cause mortality of patients with CRC (temporality, low risk of confounding, and dose‐response relationship), causality needs to be addressed in randomized clinical trials comparing conduction of a comprehensive medication review and usual care. We think that such trials are needed in the field of geriatric oncology. Although the results of such trials are being awaited, our results underline the need for the conduction of medication reviews for older patients with CRC with excessive polypharmacy (≥8 drugs) as part of the CGA.
Author Contributions
Conception/design: Li‐Ju Chen, Ben Schöttker
Provision of study material or patients: Li‐Ju Chen, Ben Schöttker
Collection and/or assembly of data: Jenny Chang‐Claude, Michael Hoffmeister, Hermann Brenner
Data analysis and interpretation: Li‐Ju Chen, Ben Schöttker
Manuscript writing: Li‐Ju Chen, Thi Ngoc Mai Nguyen, Jenny Chang‐Claude, Michael Hoffmeister, Hermann Brenner, Ben Schöttker
Final approval of manuscript: Li‐Ju Chen, Thi Ngoc Mai Nguyen, Jenny Chang‐Claude, Michael Hoffmeister, Hermann Brenner, Ben Schöttker
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Illustration of study timeline and collection of data in the DACHS study
Figure S2. Flow chart of the study population
Figure S3. Distribution of number of medications when counting all drugs without exclusions
Figure S4. Dose‐response curves for the associations of the number of drugs (all counted without exclusions) with overall, colorectal cancer specific, and non‐cancer specific survival in model 2 (without adjustment for functional status and comorbidity) and model 3 (full model)a
Table S1 List of ATC codes or ATC code groups of combination drugs containing 2 or 3 active substances for the modified polypharmacy definition
Table S2. List of ATC codes or ATC code groups excluded from the count of drugs for the modified polypharmacy definition
Table S3. Associations of the baseline characteristics of older colorectal cancer patients with polypharmacy (traditional definition).
Table S4. The associations of polypharmacy (traditional definition) with up to 5‐year overall, colorectal cancer specific, and non‐cancer specific survival in older CRC patients
Table S5. Subgroup analyses for the associations of polypharmacy (traditional definition) with up to 5‐year overall survival in older colorectal cancer patients
Table S6. The association of polypharmacy (traditional definition) with chemotherapy‐related neurological and gastrointestinal adverse drug reactions (ADRs) in older CRC patients
Table S7. The associations of polypharmacy (modified definition) with overall, colorectal cancer specific, and non‐cancer specific survival in older CRC patients during up to 17 years of follow‐up
Table S8. The association of polypharmacy (modified definition) with chemotherapy‐related neurological and gastrointestinal adverse drug reactions (ADRs) in older CRC patients
Acknowledgments
We thank Ute Handte‐Daub, Ansgar Brandhorst, and Petra Bächer for their technical assistance. We also thank the study participants and interviewers who assisted in the data collection. We thank the following clinics and institutions: Chirurgische Universitätsklinik Heidelberg, Klinik am Gesundbrunnen Heilbronn, St. Vincentiuskrankenhaus Speyer, St. Josefskrankenhaus Heidelberg, Chirurgische Universitätsklinik Mannheim, Diakonissenkrankenhaus Speyer, Krankenhaus Salem Heidelberg, Kreiskrankenhaus Schwetzingen, St. Marienkrankenhaus Ludwigshafen, Klinikum Ludwigshafen, Stadtklinik Frankenthal, Diakoniekrankenhaus Mannheim, Kreiskrankenhaus Sinsheim, Klinikum am Plattenwald Bad Friedrichshall, Kreiskrankenhaus Weinheim, Kreiskrankenhaus Eberbach, Kreiskrankenhaus Buchen, Kreiskrankenhaus Mosbach, Enddarmzentrum Mannheim, Kreiskrankenhaus Brackenheim, and Krebsregister Rheinland‐Pfalz, Mainz. The work was supported by grants from the German Research Council (grant numbers BR 1704/6‐1, BR 1704/6‐3, BR 1704/6‐4, BR 1704/6‐6, CH 117/1‐1, HO 5117/2‐1, HE 5998/2‐1, KL 2354/3‐1, RO 2270/8‐1, BR 1704/17‐1), the German Federal Ministry of Education and Research (grant numbers 01KH0404, 01GS08181, 01ER0814, 01ER0815, 01ER1505A, 01ER1505B), and the Ministry of Science, Research, and Arts of Baden‐Wuerttemberg. Open access funding enabled and organized by Projekt DEAL.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. World Health Organization International Agency for Research . Global Cancer Observatory. Available at http://gco.iarc.fr/. Accessed February 7, 2021.
- 2. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:913–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert Koch Institut . Zentrum für Krebsregisterdaten: Colorectal cancer. Available at https://www.krebsdaten.de/Krebs/EN/Content/Cancer_sites/Colorectal_cancer/colorectal_cancer_node.html. Accessed February 7, 2021.
- 4. Siegel RL, Miller KD, Goding Sauer A et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 5. Masnoon N, Shakib S, Kalisch‐Ellett L et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017;17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sergi G, De Rui M, Sarti S et al. Polypharmacy in the elderly: Can comprehensive geriatric assessment reduce inappropriate medication use? Drugs Aging 2011;28:509–518. [DOI] [PubMed] [Google Scholar]
- 7. Chen LJ, Trares K, Laetsch DC et al. Systematic review and meta‐analysis on the associations of polypharmacy and potentially inappropriate medication with adverse outcomes in older cancer patients. J Gerontol A Biol Sci Med Sci 2021;76:1044–1052. [DOI] [PubMed] [Google Scholar]
- 8. Sharma M, Loh KP, Nightingale G et al. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol 2016;7:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maggiore RJ, Dale W, Gross CP et al. Polypharmacy and potentially inappropriate medication use among older adults with cancer undergoing chemotherapy: Impact on chemotherapy‐related toxicity and hospitalization during treatment. J Am Geriatr Soc 2014;62:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohamed MR, Ramsdale E, Loh KP et al. Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: A systematic review and meta‐analysis. The Oncologist 2020;25:e94–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pujara D, Mansfield P, Ajani J et al. Comprehensive geriatric assessment in patients with gastric and gastroesophageal adenocarcinoma undergoing gastrectomy. J Surg Oncol 2015;112:883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner JP, Shakib S, Singhal N et al. Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer 2014;22:1727–1734. [DOI] [PubMed] [Google Scholar]
- 13. van Abbema D, van Vuuren A, van den Berkmortel F et al. Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. J Geriatr Oncol 2017;8:176–184. [DOI] [PubMed] [Google Scholar]
- 14. Ommundsen N, Wyller TB, Nesbakken A et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. The Oncologist 2014;19:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karuturi MS, Holmes HM, Lei X et al. Potentially inappropriate medications defined by STOPP criteria in older patients with breast and colorectal cancer. J Geriatr Oncol 2019;10:705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ommundsen N, Nesbakken A, Wyller TB et al. Post‐discharge complications in frail older patients after surgery for colorectal cancer. Eur J Surg Oncol 2018;44:1542–1547. [DOI] [PubMed] [Google Scholar]
- 17. Joffe MM. Confounding by indication: The case of calcium channel blockers. Pharmacoepidemiol Drug Saf 2000;9:37–41. [DOI] [PubMed] [Google Scholar]
- 18. Salas M, Hofman A, Stricker BH. Confounding by indication: An example of variation in the use of epidemiologic terminology. Am J Epidemiol 1999;149:981–983. [DOI] [PubMed] [Google Scholar]
- 19. Brenner H, Chang‐Claude J, Seiler CM et al. Protection from colorectal cancer after colonoscopy: A population‐based, case‐control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 20. Hoffmeister M, Jansen L, Rudolph A et al. Statin use and survival after colorectal cancer: The importance of comprehensive confounder adjustment. J Natl Cancer Inst 2015;107:djv045. [DOI] [PubMed] [Google Scholar]
- 21. Maalmi H, Walter V, Jansen L et al. Relationship of very low serum 25‐hydroxyvitamin D(3) levels with long‐term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol 2017;32:961–971. [DOI] [PubMed] [Google Scholar]
- 22.ICD‐10: International statistical classification of diseases and related health problems. World Health Organization. Available at https://apps.who.int/iris/handle/10665/42980. Accessed October 16, 2019.
- 23. Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 24.ATC‐Klassifikation für den deutschen Arzneimittelmarkt WIdO – Wissenschaftliches Institut der AOK. Available at https://www.wido.de/publikationen‐produkte/arzneimittel‐klassifikation/. Accessed November 21, 2019.
- 25. Lim CM, Aryani Md Yusof F, Selvarajah S et al. Use of ATC to describe duplicate medications in primary care prescriptions. Eur J Clin Pharmacol 2011;67:1035–1044 [DOI] [PubMed] [Google Scholar]
- 26. WHO Collaborating Centre for Drug Statistics Methodology ‐ ATC/DDD Index 2021. Available at https://www.whocc.no/atc_ddd_index/. Accessed April 7, 2021.
- 27. Schöttker B, Saum KU, Muhlack DC et al. Polypharmacy and mortality: New insights from a large cohort of older adults by detection of effect modification by multi‐morbidity and comprehensive correction of confounding by indication. Eur J Clin Pharmacol 2017;73:1041–1048. [DOI] [PubMed] [Google Scholar]
- 28. Boakye D, Walter V, Jansen L et al. Magnitude of the age‐advancement effect of comorbidities in colorectal cancer prognosis. J Natl Compr Canc Netw 2020;18:59–68. [DOI] [PubMed] [Google Scholar]
- 29. Yuan YC. Multiple imputation for missing data: Concepts and NewDevelopment (Version 9.0). SAS Institute Inc. Available at https://support.sas.com/rnd/app/stat/papers/multipleimputation.pdf. Accessed October 17, 2019. [Google Scholar]
- 30. Fagard K, Casaer J, Wolthuis A et al. Value of geriatric screening and assessment in predicting postoperative complications in patients older than 70 years undergoing surgery for colorectal cancer. J Geriatr Oncol 2017;8:320–327. [DOI] [PubMed] [Google Scholar]
- 31. Kristjansson SR, Jordhøy MS, Nesbakken A et al. Which elements of a comprehensive geriatric assessment (CGA) predict post‐operative complications and early mortality after colorectal cancer surgery? J Geriatr Oncol 2010;1:57–65. [Google Scholar]
- 32. Samuelsson KS, Egenvall M, Klarin I et al. Preoperative geriatric assessment and follow‐up of patients older than 75 years undergoing elective surgery for suspected colorectal cancer. J Geriatr Oncol 2019;10:709–715. [DOI] [PubMed] [Google Scholar]
- 33. Antonio M, Carmona‐Bayonas A, Saldaña J et al. Factors predicting adherence to a tailored‐dose adjuvant treatment on the basis of geriatric assessment in elderly people with colorectal cancer: A prospective study. Clin Colorectal Cancer 2018;17:e59–e68. [DOI] [PubMed] [Google Scholar]
- 34. Lee YH, Oh HK, Kim DW et al. Use of a comprehensive geriatric assessment to predict short‐term postoperative outcome in elderly patients with colorectal cancer. Ann Coloproctol 2016;32:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morio K, Maeda I, Yokota I et al. Risk factors for polypharmacy in elderly patients with cancer pain. Am J Hosp Palliat Care 2019;36:598–602. [DOI] [PubMed] [Google Scholar]
- 36. Prithviraj GK, Koroukian S, Margevicius S et al. Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. J Geriatr Oncol 2012;3:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. J Gen Intern Med 2011;26:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fick DM, Semla TP, Steinman M et al. American Geriatrics Society 2019 updated AGS beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674–694. [DOI] [PubMed] [Google Scholar]
- 39. O'Mahony D, O'Sullivan D, Byrne S et al. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015;44:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pazan F, Weiss C, Wehling M. The FORTA (Fit fOR The Aged) List 2018: Third version of a validated clinical tool for improved drug treatment in older people. Drugs Aging 2019;36:481–484. [DOI] [PubMed] [Google Scholar]
- 41. Renom‐Guiteras A, Meyer G, Thürmann PA. The EU(7)‐PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015;71:861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Illustration of study timeline and collection of data in the DACHS study
Figure S2. Flow chart of the study population
Figure S3. Distribution of number of medications when counting all drugs without exclusions
Figure S4. Dose‐response curves for the associations of the number of drugs (all counted without exclusions) with overall, colorectal cancer specific, and non‐cancer specific survival in model 2 (without adjustment for functional status and comorbidity) and model 3 (full model)a
Table S1 List of ATC codes or ATC code groups of combination drugs containing 2 or 3 active substances for the modified polypharmacy definition
Table S2. List of ATC codes or ATC code groups excluded from the count of drugs for the modified polypharmacy definition
Table S3. Associations of the baseline characteristics of older colorectal cancer patients with polypharmacy (traditional definition).
Table S4. The associations of polypharmacy (traditional definition) with up to 5‐year overall, colorectal cancer specific, and non‐cancer specific survival in older CRC patients
Table S5. Subgroup analyses for the associations of polypharmacy (traditional definition) with up to 5‐year overall survival in older colorectal cancer patients
Table S6. The association of polypharmacy (traditional definition) with chemotherapy‐related neurological and gastrointestinal adverse drug reactions (ADRs) in older CRC patients
Table S7. The associations of polypharmacy (modified definition) with overall, colorectal cancer specific, and non‐cancer specific survival in older CRC patients during up to 17 years of follow‐up
Table S8. The association of polypharmacy (modified definition) with chemotherapy‐related neurological and gastrointestinal adverse drug reactions (ADRs) in older CRC patients


