Abstract
Background
Adjuvant therapy for patients with cervical cancer (CC) with intermediate‐risk factors remains controversial. The objectives of the present study are to assess the prognoses of patients with early‐stage CC with pathological intermediate‐risk factors and to provide a reference for adjuvant therapy choice.
Materials and Methods
This retrospective study included 481 patients with stage IB–IIA CC. Cox proportional hazards regression analysis, machine learning (ML) algorithms, Kaplan‐Meier analysis, and the area under the receiver operating characteristic curve (AUC) were used to develop and validate prediction models for disease‐free survival (DFS) and overall survival (OS).
Results
A total of 35 (7.3%) patients experienced recurrence, and 20 (4.2%) patients died. Two prediction models were built for DFS and OS using clinical information, including age, lymphovascular space invasion, stromal invasion, tumor size, and adjuvant treatment. Patients were divided into high‐risk or low‐risk groups according to the risk score cutoff value. The Kaplan‐Meier analysis showed significant differences in DFS (p = .001) and OS (p = .011) between the two risk groups. In the traditional Sedlis criteria groups, there were no significant differences in DFS or OS (p > .05). In the ML‐based validation, the best AUCs of DFS at 2 and 5 years were 0.69/0.69, and the best AUCs of OS at 2 and 5 years were 0.88/0.63.
Conclusion
Two prognostic assessment models were successfully established, and risk grouping stratified the prognostic risk of patients with CC with pathological intermediate‐risk factors. Evaluation of long‐term survival will be needed to corroborate these findings.
Implications for Practice
The Sedlis criteria are intermediate‐risk factors used to guide postoperative adjuvant treatment in patients with cervical cancer. However, for patients meeting the Sedlis criteria, the choice of adjuvant therapy remains controversial. This study developed two prognostic models based on pathological intermediate‐risk factors. According to the risk score obtained by the prediction model, patients can be further divided into groups with high or low risk of recurrence and death. The prognostic models developed in this study can be used in clinical practice to stratify prognostic risk and provide more individualized adjuvant therapy choices to patients with early‐stage cervical cancer.
Keywords: Cervical cancer, Intermediate‐risk factor, Adjuvant therapy, Sedlis criteria, Prediction model, Machine learning algorithm
Short abstract
Adjuvant treatment for patients with cervical cancer remains controversial. This article assesses the prognosis of early‐stage cervical cancer with pathological intermediate‐risk factors and provides a reference for choice of adjuvant therapy.
Introduction
Every year, more than 500,000 women are diagnosed with cervical cancer (CC), and more than 300,000 deaths occur due to this disease worldwide [1]. Although cervical screening strategies have decreased the incidence of CC, data from global population‐based CC registries have revealed that 5‐year survival has improved only slightly in recent decades [2]. Furthermore, nearly 90% of deaths from CC occur in developing and low‐resource countries [3]. The prognosis of patients with CC is closely related to the clinical staging system determined by the International Federation of Gynecology and Obstetrics (FIGO). Radical hysterectomy with pelvic lymphadenectomy is the preferred surgical plan for patients with early CC [4]. Surgical risk factors and lymph node status were first included in the FIGO staging system in 2018 [5]. In addition to lymph node status, other pathological risk indicators widely recognized to affect survival and recurrence in CC include parietal infiltration and marginal positivity [6, 7, 8, 9]. Furthermore, the Gynecologic Oncology Group (GOG) defined lymphovascular space invasion (LVSI), stromal invasion (SI), and tumor size as “Sedlis criteria,” which are intermediate‐risk factors used to guide adjuvant treatment decisions [10].
For patients with early‐stage CC who meet the Sedlis criteria, controversies remain regarding adjuvant therapy after surgical treatment. The European Society for Medical Oncology clinical practice guidelines for CC recommend that patients with intermediate‐risk do not need further adjuvant therapy (evidence level II, B) [3]. The FIGO CC report recommends that postoperative radiotherapy is required, but chemotherapy is not recommended, if a patient exhibits any two of the following risk factors: tumor size more than 4 cm, LVSI, and deep SI [5]. The National Comprehensive Cancer Network clinical practice guidelines for CC recommend pelvic external beam radiation therapy (category 1) with or without concurrent platinum‐containing chemotherapy (category 2B for chemotherapy) for lymph node–negative, postsurgery patients who were diagnosed at stage IA2, IB1, or IIA1 and have large primary tumors, deep SI, and/or LVSI [11].
Therefore, evaluation of intermediate‐risk factors and adjuvant therapy remains controversial, and potential intermediate‐risk factors for both recurrence and survival may include factors beyond the Sedlis criteria. Patients with early‐stage CC typically have a favorable prognosis, and radiotherapy is often associated with considerable adverse effects during adjuvant therapy [12]. Thus, participating clinicians must identify the suitable management method after surgery to avoid overtreatment. At present, there are no published survival prediction models for predicting overall survival (OS) or disease‐free survival (DFS) of patients with early‐stage CC by pathological intermediate‐risk factors.
The objective of our study is to establish prognostic evaluation models and stratify the prognostic risk in patients with CC with intermediate‐risk factors. Our results provide a more individualized reference for planning postoperative adjuvant treatment in clinical practice.
Materials and Methods
Patients
After the study obtained approval from the Ethical Committee of Qilu Hospital of Shandong University (protocol number 2018 066) and received a waiver for informed consent, 481 patients with CC who had received treatment at Qilu Hospital of Shandong University between January 2005 and December 2016 were included in our study. All patients met the following inclusion criteria: (a) stage IB–IIA CC according to the 2009 FIGO staging system [13]; (b) primary treatment by radical or modified radical hysterectomy and pelvic lymphadenectomy. The exclusion criteria were (a) lymph node metastasis, parametrial involvement, or positive resection margin after surgery; (b) the presence of other primary malignant tumors; (c) insufficient medical records.
Predictors and Endpoints
The following clinical characteristics were included: age, FIGO stage (2009), histology, histological grade, LVSI, SI, tumor size, adjuvant therapy after surgery, and survival and recurrence information. Recurrence and death were defined as the primary outcomes of this study. DFS and OS were described as the time interval from surgery to the first evidence of any recurrence and death or last follow‐up. The tumor size was measured by clinical palpation.
Model Development
The workflow of this study is presented in Figure 1. Each characteristic was estimated using univariable Cox survival analysis for both DFS and OS, and results were described as hazard ratios (HRs), with associated 95% confidence intervals (CIs), and p values. After selection of risk factors, the age, pathological risk factors as defined in the Sedlis criteria (LVSI, SI, and tumor size), and adjuvant treatment methods were entered into a multivariable Cox proportional hazards regression analysis to construct intermediate‐risk prediction models. The time‐dependent receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were used to evaluate the discrimination ability of the model. Nomogram lists were developed to predict the risk of 2‐ and 5‐year DFS, as well as 2‐ and 5‐year OS. We then calculated the risk score of each patient according to the nomogram lists, selecting the median risk score as the cutoff value. Patients were divided into low‐risk and high‐risk groups according to risk score. A heatmap was generated based on the distribution of risk factors in the two groups. The Kaplan‐Meier method with the log‐rank test was used to compare the abilities of the traditional Sedlis criteria and the new risk groups from the developed model to distinguish prognoses.
Figure 1.
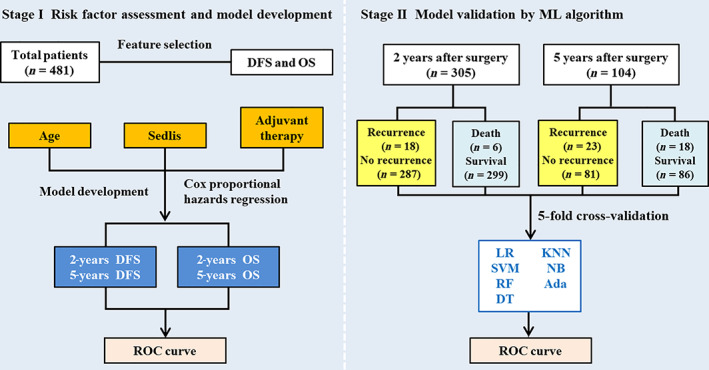
The workflow of this study. Abbreviations: Ada, AdaBoost; DFS, disease‐free survival; DT, decision tree; KNN, k‐nearest neighbor; LR, logistic regression; ML, machine learning; NB, naïve Bayes; OS, overall survival; RF, random forest; ROC, receiver operating characteristic; SVM, support vector machine.
Model Validation
Currently, machine learning (ML) is often used in the development and validation of prediction models in clinical research [14]. We divided patients into four groups according to whether there was recurrence or death within 2 and 5 years of the primary surgery. ML algorithms, including logistic regression (LR), support vector machine (SVM), random forest (RF), decision tree, k‐nearest neighbor, naïve Bayes, and AdaBoost, were used for model validation. Fivefold cross‐validation was applied for each algorithm. All patients were randomly partitioned into five equal‐sized subsamples. Four subsamples were used in training data, and the final subsample was selected as the validation data for testing. AUCs were calculated over multiple rounds of cross‐validation to assess the models.
Statistical Analysis
The descriptive statistical analysis, univariate and multivariate Cox proportional hazard regression analysis, nomogram lists, ROC analysis, and log‐rank test were conducted with R (version 3.6.1). The ML algorithms were conducted in Python (version 3.6.4) using the machine learning library scikit‐learn (version 0.19.1).
Results
Patient Characteristics
Patient characteristics are reported in Table 1. In 481 patients with early‐stage CC, 344 (71.5%) women were diagnosed at stage IB1, 94 (19.5%) at stage IB2, 25 (5.2%) at stage IIA1, and 18 (3.7%) at stage IIA2. Most patients underwent laparotomy with radical hysterectomy (n = 385, 80%), and 379 (80.9%) patients received adjuvant therapy after surgery. After a median follow‐up period of 31 months (range, 9–145 months), 35 (7.3%) patients experienced recurrences, and 20 (4.2%) died. The 2‐year DFS and OS were 94.1% and 98.0%, respectively, and the 5‐year DFS and OS were 77.9% and 82.7%, respectively.
Table 1.
Characteristics of patients
| Characteristic | Total (n = 481) | Recurrence (n = 35, 7.3%) | Death (n = 20, 4.2%) |
|---|---|---|---|
| Age, years | |||
| ≤40 | 137 (28.5) | 5 (14.3) | 2 (10.0) |
| >40 | 344 (71.5) | 30 (85.7) | 18 (90.0) |
| FIGO stage (2009) | |||
| IB1 | 344 (71.5) | 24 (68.6) | 15 (75.0) |
| IB2 | 94 (19.5) | 9 (25.7) | 4 (20.0) |
| IIA1 | 25 (5.2) | 2 (5.7) | 1 (5.0) |
| IIA2 | 18 (3.7) | 0 (0.0) | 0 (0.0) |
| Operation method | |||
| Laparotomy | 385 (80.0) | 30 (85.7) | 18 (90.0) |
| Laparoscopy | 96 (20.0) | 5 (14.3) | 2 (10.0) |
| Histology | |||
| Squamous | 390 (81.1) | 9 (25.7) | 5 (25.0) |
| Nonsquamous | 91 (18.9) | 26 (74.3) | 15 (75.0) |
| Histological grade | |||
| I (well differentiated) | 41 (8.5) | 2 (5.7) | 13 (65.0) |
| II (moderately differentiated) | 152 (31.6) | 9 (25.7) | 5 (25.0) |
| III (poorly differentiated) | 288 (59.9) | 24 (68.6) | 2 (10.0) |
| LVSI | |||
| No | 376 (778.2) | 27 (77.1) | 16 (80.0) |
| Yes | 105 (21.8) | 8 (22.9) | 4 (20.0) |
| Stromal invasion | |||
| Superficial 1/3 | 134 (27.9) | 7 (20.0) | 4 (20.0) |
| Middle 1/3 | 185 (38.5) | 9 (25.7) | 4 (20.0) |
| Deep 1/3 | 162 (33.7) | 19 (54.3) | 12 (60.0) |
| Tumor size, cm | |||
| <2 | 102 (21.4) | 5 (14.3) | 2 (10.0) |
| ≥2 | 280 (58.2) | 17 (48.6) | 10 (50.0) |
| ≥4 | 58 (12.1) | 7 (20.0) | 5 (25.0) |
| ≥5 | 40 (8.3) | 6 (17.1) | 3 (15.0) |
| Adjuvant therapy | |||
| None | 92 (19.1) | 8 (22.9) | 5 (25.0) |
| Chemotherapy | 222 (46.2) | 14 (40.0) | 6 (30.0) |
| Radiotherapy | 20 (4.2) | 2 (5.7) | 1 (5.0) |
| Chemotherapy and radiotherapy | 147 (30.6) | 11 (31.4) | 8 (40.0) |
Values are presented as n (%).
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion.
Predictor Assessment of DFS and OS
As shown in Table 2, there was a significant association between patients older than 40 years and postoperative recurrence (HR 2.60, 95% CI 1.02–6.78, p = .046), whereas the association between this age group and death was weaker (HR 3.95, 95% CI 0.92–17.02, p = .065). Furthermore, the FIGO stage (2009), operation method, histology, histological grade, LVSI, SI, tumor size, and adjuvant therapy were not significantly associated with either DFS or OS.
Table 2.
Univariable Cox proportional hazards regression analysis for DFS and OS
| Characteristic | DFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, years | .046 | .065 | ||
| ≤40 | Reference | Reference | — | |
| >40 | 2.63 (1.02–6.78) | 3.95 (0.92–17.02) | ||
| FIGO stage (2009) | .989 | .933 | ||
| IB1 | Reference | — | Reference | — |
| IB2 | 1.06 (0.49–2.28) | .891 | 0.70 (0.23–2.10) | .519 |
| IIA1 | 1.29 (0.30–5.44) | .733 | 1.06 (0.14–8.02) | .956 |
| IIA2 | — | — | — | — |
| Operation method | .350 | .374 | ||
| Laparotomy | Reference | Reference | ||
| Laparoscopy | 1.61 (0.60–4.32) | 2.02 (0.43–9.45) | ||
| Histology | .378 | .640 | ||
| Squamous | Reference | Reference | ||
| Nonsquamous | 0.71 (0.33–1.52) | 0.79 (0.29–2.16) | ||
| Histological grade | .561 | .648 | ||
| I (well differentiated) | Reference | — | Reference | — |
| II (moderately differentiated) | 1.47 (0.35–6.23) | .600 | 0.73 (0.16–3.22) | .672 |
| III (poorly differentiated) | 0.99 (0.21–4.60) | .992 | 0.49 (0.10–2.54) | .398 |
| LVSI | .385 | .551 | ||
| No | Reference | Reference | ||
| Yes | 1.42 (0.64–3.14) | 1.40 (0.47–4.20) | ||
| Stromal invasion | .130 | .202 | ||
| Superficial 1/3 | Reference | — | Reference | — |
| Middle 1/3 | 1.04 (0.39–2.78) | .946 | 0.82 (0.21–3.30) | .784 |
| Deep 1/3 | 2.02 (0.85–4.82) | .111 | 2.04 (0.66–6.33) | .218 |
| Tumor size, cm | .126 | .239 | ||
| <2 | Reference | — | Reference | — |
| ≥2 | 1.24 (0.46–3.35) | .678 | 1.82 (0.40–8.30) | .441 |
| ≥4 | 2.29 (0.73–7.23) | .157 | 3.88 (0.75–20.02) | .105 |
| ≥5 | 3.09 (0.94–10.13) | .063 | 4.00 (0.67–23.96) | .129 |
| Adjuvant therapy | .822 | .363 | ||
| None | Reference | — | Reference | — |
| Chemotherapy | 0.71 (0.30–1.69) | .435 | 0.49 (0.15–1.60) | .237 |
| Radiotherapy | 0.72 (0.15–3.43) | .682 | 0.52 (0.06–4.50) | .555 |
| Chemotherapy and radiotherapy | 0.97 (0.39–2.41) | .942 | 1.21 (0.40–3.70) | .740 |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; LVSI, lymphovascular space invasion; OS, overall survival.
Model Development of DFS and OS
Multivariable Cox proportional hazards regression used age, LVSI, SI, tumor size, and adjuvant treatment method to develop prediction models for DFS and OS. The two models are shown as nomogram lists in Figure 2. The risk score for DFS = 100 × (age > 40 years) + 66.8 × (LVSI [+]) + 3.5 × middle 1/3 invasion +62.8 × deep 1/3 invasion +14.4 × (tumor size ≥2 cm) + 79.5 × (tumor size ≥4 cm) + 98.4 × (tumor size ≥5 cm) + 66.8 × no adjuvant treatment +12.2 × chemotherapy +19.7 × chemotherapy and radiotherapy. The risk score for OS = 100 × (age > 40 years) + 51.9 × (LVSI [+]) + 13.3 × superficial 1/3 invasion +54.8 × deep 1/3 invasion +37.1 × (tumor size ≥2 cm) + 99.3 × (tumor size ≥4 cm) + 77.6 × (tumor size ≥5 cm) + 80.5 × no adjuvant treatment +10.4 × chemotherapy +60.3 × chemotherapy and radiotherapy.
Figure 2.
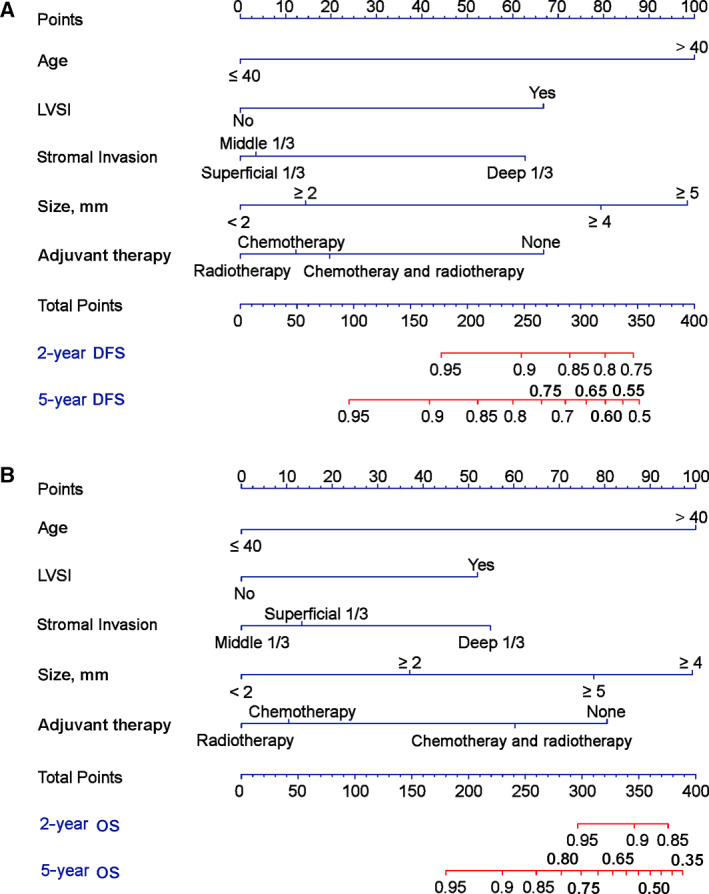
Nomogram lists of risk prediction models for DFS (A) and OS (B). Abbreviations: DFS, disease‐free survival; LVSI, lymphovascular space invasion; OS, overall survival.
ROC curves for both models are shown in supplemental online Figure 1. The recurrence model yielded an AUC of 0.74 in 2‐year DFS and 0.66 in 5‐year DFS (supplemental online Fig. 1A). The death model yielded an AUC of 0.87 in 2‐year OS and 0.69 in 5‐year OS (supplemental online Fig. 1B). The time‐dependent ROC curve is shown in supplemental online Figure 1C. To determine the effect of risk score on clinical outcome, we divided the cohort over the median risk score. In the recurrence model, patients with a risk score higher than 167 points were placed in the high risk of recurrence group (supplemental online Fig. 2A). In the death model, patients with a risk score higher than 194 points were placed in the high risk of death group (supplemental online Fig. 2B). The survival and recurrence time of each patient is shown in supplemental online Figure 2C, 2D. We compared the distribution of prognostic factors in the two groups by heatmap (supplemental online Fig. 2E, 2F), in which the red color indicates higher significance. Patients in the high‐risk groups were older, had larger tumors, and had more diagnoses of positive LVSI and deep of cervical invasion.
Comparison of the Traditional Sedlis Criteria Groups and New Risk Groups
Patient distribution according to the traditional Sedlis criteria groups and the new risk groups defined in this study are shown in Table 3. The Kaplan‐Meier analysis showed that the Sedlis criteria could not distinguish DFS or OS in patients with CC (p > .05; Fig. 3A–3D) and that high‐risk groups for both recurrence and death were significantly associated with poor DFS (p = .001; Fig. 3E) and OS (p = .011; Fig. 3F).
Table 3.
The information of patients in traditional Sedlis criteria groups and new risk groups
| Groups according to Sedlis criteria | Total (n = 481) | Recurrence (n = 35, 7.3%) | Death (n = 20, 4.2%) |
|---|---|---|---|
| Sedlis criteria (detailed) | |||
| None | 337 (70.1) | 22 (62.9) | 13 (65.0) |
| LVSI + Deep 1/3 | 32 (6.7) | 3 (8.6) | 1 (5.0) |
| LVSI + Middle 1/3 + Tumor size ≥2 cm | 39 (8.1) | 3 (8.6) | 2 (10.0) |
| LVSI + Superficial 1/3 + Tumor size ≥5 cm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Middle or deep 1/3 + Tumor size ≥4 cm | 73 (15.2) | 7 (20.0) | 4 (20.0) |
| Sedlis criteria | |||
| No | 337 (70.1) | 22 (62.9) | 13 (65.0) |
| Yes | 144 (29.9) | 13 (37.1) | 7 (35.0) |
| Risk group of recurrence | |||
| Low‐risk group | 224 (46.6) | 7 (20.0) | 4 (20.0) |
| High‐risk group | 257 (53.4) | 28 (80.0) | 16 (80.0) |
| Risk group of death | |||
| Low‐risk group | 248 (51.6) | 10 (28.6) | 4 (20.0) |
| High‐risk group | 233 (48.4) | 25 (71.4) | 16 (80.0) |
Values are presented as n (%).
Abbreviation: LVSI, lymphovascular space invasion.
Figure 3.
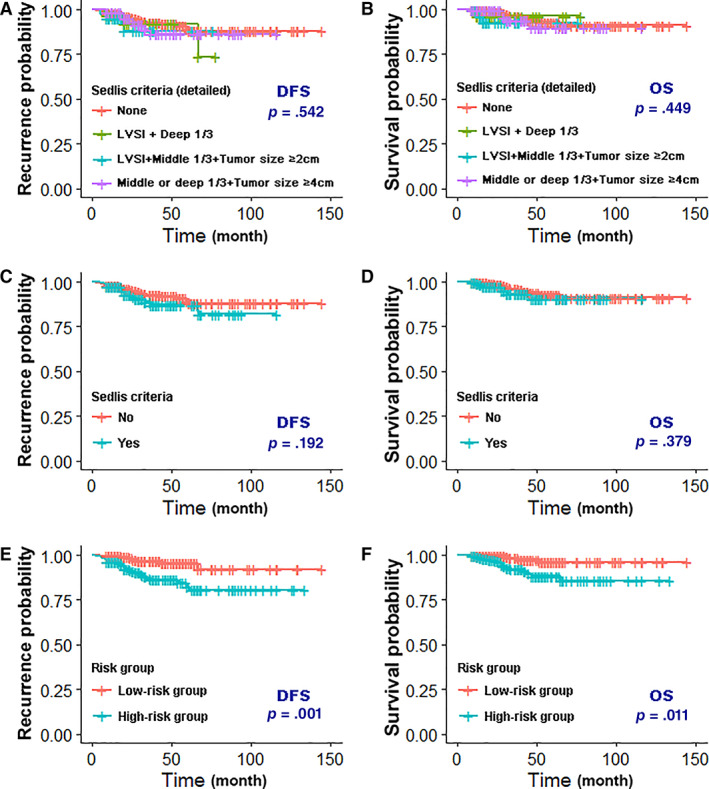
Kaplan‐Meier analysis of the Sedlis criteria (detailed) with DFS (A) and OS (B); Sedlis criteria with DFS (C) and OS (D); and risk group with DFS (E) and OS (F). Abbreviations: DFS, disease‐free survival; LVSI, lymphovascular space invasion; OS, overall survival.
Model Training and Validation Based on ML Algorithms
For further validation, ML algorithms were applied to verify the discrimination ability of the two models. Figure 4 shows the results from the fivefold cross‐validation. The AUC of the 2‐year DFS prediction model ranged from 0.61 to 0.69, with the highest AUC produced by the SVM algorithm (Fig. 4A). In the 5‐year DFS model, the AUC ranged from 0.64 to 0.69, and the LR calculation yielded the highest AUC (Fig. 4B). The best AUC was obtained for the 2‐year OS prediction model, which ranged from 0.84 to 0.88 (Fig. 4C), and the AUC of the 5‐year OS prediction model ranged from 0.60 to 0.63 (Fig. 4D). Overall, the LR and SVM algorithms had good discrimination compared with the other five algorithms in the fivefold cross‐validation process.
Figure 4.
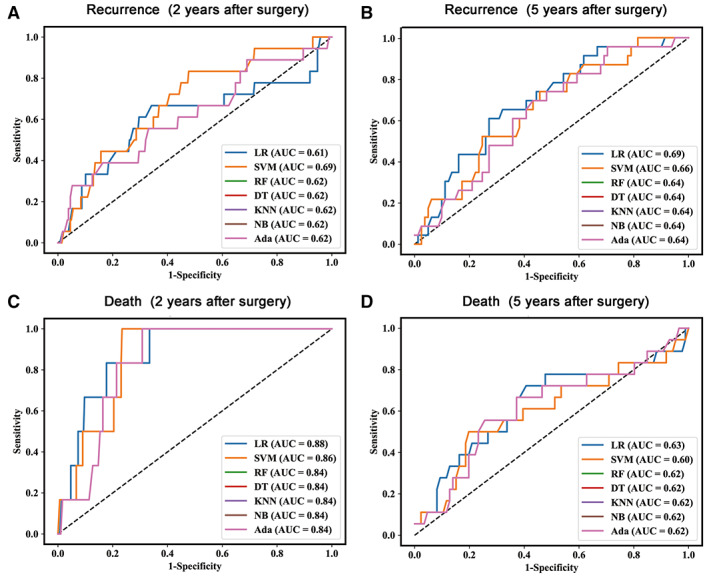
ROC curves of the ML‐based validation of patient recurrence occurred within 2 years after surgery (A) and 5 years after surgery (B); and patient death occurred within 2 years after surgery (C) and 5 years after surgery (D). Abbreviations: Ada, AdaBoost, AUC, area under the receiver operating characteristic curve; DT, decision tree; KNN, k‐nearest neighbor; LR, logistic regression; NB, naïve Bayes; RF, random forest; SVM, support vector machine.
Discussion
The present paper describes the development of prediction models for DFS and OS in patients with early‐stage CC based on pathological intermediate‐risk factors and postoperative adjuvant therapy. The prediction models were constructed by age, LVSI, depth of SI, tumor size, and postoperative adjuvant therapy. We found significant differences in DFS and OS between patients in the high‐risk and low‐risk groups and showed that risk grouping can be used to stratify the prognostic risk of early‐stage CC in patients with intermediate‐risk factors. Finally, we used ML algorithms to validate the models. Our results suggest that the favorable prognostic risk assessment model could be used in clinical practice as a potential postoperative evaluation tool for patients with early‐stage CC.
The Sedlis criteria, which were proposed by the GOG through a prospective study, are currently widely used and include three factors (LVSI, deep of SI, and tumor size) [10]. A retrospective study of CC showed that although 50% of recurrences in the study occurred in patients who did not meet the criteria, all recurrences occurred in patients with two or more pathological intermediate‐risk factors [15]. These results show that the risk factors in the Sedlis criteria need to be further optimized to accurately evaluate the prognoses of patients with early‐stage CC.
In the U.S., the median age of patients with CC at diagnosis is 47 years, and almost 50% of patients are diagnosed at ages below 35 years [16]. In addition, in developing nations such as South Africa, more than 25% of patients with CC are diagnosed between the ages of 40 and 49 [17]. These data suggest that a significant proportion of patients with CC are young adults. Moreover, the univariable survival analysis performed in the present study shows that women older than 40 years were more likely to be diagnosed with a recurrence. Therefore, we included age as one of the risk factors and constructed the multivariable Cox proportional hazards regression models with other intermediate‐risk factors. In a population‐based study, after a 7‐year follow‐up, the advanced‐stage disease was more likely to be diagnosed among women aged 50 years and older (2.2–2.5 times the diagnosis rate of patients aged 21–34 years) [18]. Thus, for older patients with intermediate‐risk factors, the adjuvant treatment plan after the primary surgery needs to be carefully discussed.
The intent of primary surgery for early‐stage CC is therapeutic and diagnostic, and surgical staging may provide pathological evidence for subsequent adjuvant treatment [19]. As lymph node status and surgical risk factors have been included in the FIGO staging system for CC [5], more pathological and surgical factors should be considered for the patient's prognosis, such as histology, grade, LVSI, SI, tumor size, and operation method. In our study, all patients underwent radical or modified radical hysterectomy and pelvic lymphadenectomy to complete surgical staging. However, the univariable Cox proportional hazards regression analysis indicted that none of the above risk factors were significantly associated with DFS or OS. On the one hand, this result reflects the low sensitivity of these intermediate‐risk factors (LVSI, SI, and tumor size), which is in line with the previous study [15]. On the other hand, selecting the adequate adjuvant therapy may improve the prognosis of patients [20]. The multivariable Cox model assesses the relationship between the different combinations of intermediate‐risk factors and prognoses comprehensively, and adjuvant therapy was included in the development process of the model. Several studies have shown that radiotherapy decreases the incidence of local recurrence but has little effect on OS [21, 22, 23]. Our results show that radiotherapy can reduce the incidence of both recurrence and death in patients without high‐risk pathological factors. Moreover, recent research has suggested that concurrent chemoradiotherapy can confer further benefits to patients [24, 25, 26, 27]. It remains to be determined whether concurrent chemoradiotherapy is necessary for patients with intermediate‐risk factors.
Tumor size in our study was obtained by preoperative clinical palpation, which is consistent with the Sedlis criteria, but measuring errors are unavoidable with this method. In a study by Ryu et al., tumor size was measured by abdominopelvic computed tomography (CT) or magnetic resonance imaging (MRI), which seem to be more accurate methods for identifying tumor size than clinical palpation. In high‐income countries, MRI and CT have been increasingly used in the clinical staging of CC [28]. Furthermore, in the 2018 FIGO cancer report, imaging findings could be used for clinical stage assignment, which reflects the importance of the imaging evaluation [5].
The Sedlis criteria are used in clinical practice for patients with early‐stage CC without pathological high‐risk factors. Therefore, patients with lymph node metastasis, parametrial involvement, or positive resection margin were not included in this study. The strength of our study is to develop two prognostic evaluation models for patients with early‐stage CC through a unique combination of intermediate‐risk factors and an adjuvant therapy plan. Basing on the weighted risk factors in nomogram lists, these models can provide individualized predictions for each patient. Prognostic risk groups calculated by these models perform better than the traditional Sedlis criteria. The limitations of the present study include its retrospective nature and small sample size. Histology types (cervical squamous cell carcinoma and adenocarcinoma) were not separately modeled for prognosis in our study. Because the patients with CC in our study were classified as stage IB–IIA, according to the 2009 FIGO classification system, the postoperative adjuvant treatment rate was higher than in other studies. Moreover, we did not set up an independent validation cohort to validate the models. Instead, we chose ML algorithms by fivefold cross‐validation to verify the predictive abilities of the models. Prospective validation will compensate for the above research limitations.
Conclusion
We developed two intermediate‐risk factor models that can predict DFS and OS in patients with early‐stage CC after initial surgical treatment. Risk grouping completed the prognostic risk stratification of patients. These models may provide more individualized predictions and provide a reference for adjuvant therapy choice. Prospective validation in future studies will be needed to improve the predictive abilities of the above models.
Author Contributions
Conception/design: Ran Chu, Yue Zhang, Xu Qiao, Lin Xie, Wei Chen, Beihua Kong, Kun Song
Provision of study materials or patients: Beihua Kong, Kun Song
Collection and/or assembly of data: Yue Zhang, Lin Xie, Ying Zhao, Yintao Xu, Zeng Yuan, Xiaolin Liu, Aijun Yin, Zhiwen Wang, Qing Zhang, Xingsheng Yang, Xuantao Su
Data analysis and interpretation: Ran Chu, Lin Xie
Manuscript writing: Ran Chu, Yue Zhang
Final approval of manuscript: Ran Chu, Yue Zhang, Xu Qiao, Lin Xie, Wei Chen, Ying Zhao, Yintao Xu, Zeng Yuan, Xiaolin Liu, Aijun Yin, Zhiwen Wang, Qing Zhang, Xingsheng Yang, Xuantao Su, Beihua Kong, Kun Song
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Acknowledgments
This work was supported by the National Key Technology R&D Program of China (grant number 2019YFC1005200 and 2019YFC1005204), the National Natural Science Foundation of China (grant number U1806202), the Taishan Scholar Youth Project of Shandong Province (grant number tsqn201812130), and the Research Leader Studio of Jinan (grant number 2019GXRC049).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Weir HK, Carreira H et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marth C, Landoni F, Mahner S et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28(suppl 4): iv72–iv83. [DOI] [PubMed] [Google Scholar]
- 4. Landoni F, Maneo A, Cormio G et al. Class II versus class III radical hysterectomy in stage IB‐IIA cervical cancer: A prospective randomized study. Gynecol Oncol 2001;80:3–12. [DOI] [PubMed] [Google Scholar]
- 5. Bhatla N, Aoki D, Sharma DN et al. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143:22–36. [DOI] [PubMed] [Google Scholar]
- 6. Park JY, Kim DY, Kim JH et al. Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early‐stage cervical cancer. Gynecol Oncol 2010;117:53–58. [DOI] [PubMed] [Google Scholar]
- 7. Wright JD, Grigsby PW, Brooks R et al. Utility of parametrectomy for early‐stage cervical cancer treated with radical hysterectomy. Cancer 2007;110:1281–1286. [DOI] [PubMed] [Google Scholar]
- 8. Aoki Y, Sasaki M, Watanabe M et al. High‐risk group in node‐positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol 2000;77:305–309. [DOI] [PubMed] [Google Scholar]
- 9. Xia X, Xu H, Wang Z et al. Analysis of prognostic factors affecting the outcome of stage IB‐IIB cervical cancer treated by radical hysterectomy and pelvic lymphadenectomy. Am J Clin Oncol 2016;39:604–608. [DOI] [PubMed] [Google Scholar]
- 10. Sedlis A, Bundy BN, Rotman MZ et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–183. [DOI] [PubMed] [Google Scholar]
- 11. Koh WJ, Abu‐Rustum NR, Bean S et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:64–84. [DOI] [PubMed] [Google Scholar]
- 12. Ryu SY, Kim MH, Nam BH et al. Intermediate‐risk grouping of cervical cancer patients treated with radical hysterectomy: A Korean Gynecologic Oncology Group Study. Br J Cancer 2014;110:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103–104. [DOI] [PubMed] [Google Scholar]
- 14. Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur Heart J 2017;38:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryu SY, Park SI, Nam BH et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors. Int J Radiat Oncol Biol Phys 2011;79:794–799. [DOI] [PubMed] [Google Scholar]
- 16. Waggoner SE. Cervical cancer. Lancet 2003;361:2217–2225. [DOI] [PubMed] [Google Scholar]
- 17. Olorunfemi G, Ndlovu N, Masukume G et al. Temporal trends in the epidemiology of cervical cancer in South Africa (1994–2012). Int J Cancer 2018;143:2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fedewa SA, Cokkinides V, Virgo KS et al. Association of insurance status and age with cervical cancer stage at diagnosis: National Cancer Database, 2000–2007. Am J Public Health 2012;102:1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray HJ. Primary management of early stage cervical cancer (IA1‐IB) and appropriate selection of adjuvant therapy. J Natl Compr Can Netw 2008;6:47–52. [DOI] [PubMed] [Google Scholar]
- 20. Cohen PA, Jhingran A, Oaknin A et al. Cervical cancer, Lancet 2019;393:169–182. [DOI] [PubMed] [Google Scholar]
- 21. Atkovar G, Uzel O, Ozşahin M et al. Postoperative radiotherapy in carcinoma of the cervix: Treatment results and prognostic factors. Radiother Oncol 1995;35:198–205. [DOI] [PubMed] [Google Scholar]
- 22. Hasselle MD, Rose BS, Kochanski JD et al. Clinical outcomes of intensity‐modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2011;80:1436–1445. [DOI] [PubMed] [Google Scholar]
- 23. Uno T, Ito H, Itami J et al. Adjuvant pelvic irradiation in patients with pathologic T2b carcinoma of the uterine cervix. Int J Gynecol Cancer 2002;12:187–191. [DOI] [PubMed] [Google Scholar]
- 24. Peters WA 3rd, Liu PY, Barrett RJ 2nd et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. J Clin Oncol 2000;18:1606–1613. [DOI] [PubMed] [Google Scholar]
- 25. Eifel PJ, Winter K, Morris M et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para‐aortic irradiation for high‐risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90‐01. J Clin Oncol 2004;22:872–880. [DOI] [PubMed] [Google Scholar]
- 26. Stehman FB, Ali S, Keys HM et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: Follow‐up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 2007;197:503.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trifiletti DM, Swisher‐McClure S, Showalter TN et al. Postoperative chemoradiation therapy in high‐risk cervical cancer: Re‐evaluating the findings of gynecologic oncology group study 109 in a large, population‐based cohort. Int J Radiat Oncol Biol Phys 2015;93:1032–1044. [DOI] [PubMed] [Google Scholar]
- 28. Viswanathan AN, Creutzberg CL, Craighead P et al. International brachytherapy practice patterns: A survey of the Gynecologic Cancer Intergroup (GCIG). Int J Radiat Oncol Biol Phys 2012;82:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.


