Abstract
Objectives
High-resolution magnetic resonance imaging has the potential of characterising arterial wall changes after endovascular mechanical thrombectomy. The purpose of this study is to evaluate high-resolution magnetic resonance imaging features of large intracranial arteries following mechanical thrombectomy.
Methods
Patients who presented with acute ischaemic stroke due to large vessel occlusion and underwent mechanical thrombectomy were prospectively recruited. Subjects underwent high-resolution magnetic resonance imaging within 24 hours of the procedure. Magnetic resonance imaging sequences included whole brain T1 pre and post-contrast black-blood imaging, three-dimensional T2, contrast-enhanced magnetic resonance angiography and susceptibility-weighted imaging. Arterial wall enhancement was objectively assessed after normalisation with the pituitary stalk. The contrast ratio of target vessels was compared with non-affected reference vessels.
Results
Twenty patients with 22 target vessels and 20 reference vessels were included in the study. Sixteen patients were treated with stentriever with or without aspiration, and four with contact aspiration only. Significantly higher arterial wall enhancement was identified on the target vessel when compared to the reference vessel (U = 22.5, P < 0.01). The stentriever group had an 82% increase in the contrast ratio of the target vessel (x̄ = 0.75 ± 0.21) when compared to the reference vessel (x̄ = 0.41 ± 0.13), whereas the contact aspiration group had a 64% increase of the contrast ratio difference between target (x̄ = 0.62 ± 0.07) and reference vessels (x̄ = 0.38 ± 0.12). Approximately 65% of patients in the stentriever group had a positive parenchymal susceptibility-weighted imaging versus 25% in the contact aspiration group. There was no statistically significant correlation between susceptibility-weighted imaging volume and the percentage increase in the contrast ratio (rs = 0.098, P = 0.748).
Conclusions
This prospective pilot study used the objective quantification of arterial wall enhancement in determining arterial changes after mechanical thrombectomy. Preliminary data suggest that the use of stentrievers is associated with a higher enhancement as compared to reperfusion catheters.
Keywords: Mechanical thrombectomy, signal intensity, stroke, contact aspiration, endothelial damage
Introduction
The acute stroke management guidelines from the American Heart Association recommend the use of mechanical thrombectomy (MT) to treat acutely selected patients with large vessel occlusion. 1 The use of MT has resulted in significant decreased mortality and disability after large vessel occlusion, with a number needed to treat of 2.6 for one additional patient to achieve improvement by at least one level on the modified Rankin scale at 90 days. 2 There are several devices and techniques that have been approved for MT. The two main approaches use stentrievers and contact aspiration to achieve clot retrieval. 3 These techniques have similar efficacy and safety in achieving recanalisation. 4 However, there is increasing evidence that endovascular manipulation with these devices may result in damage to the endothelial inner layer of the arterial wall. Two small case series suggest that MT results in intracranial arterial wall thickening and enhancement, consistent with inflammation.5,6 As an increasing number of stroke patients undergo recanalisation with MT procedures, it is important to understand better the impact of these devices on the arterial wall.
Human and animal data suggest that mechanical stresses of stentriever devices and/or aspiration catheters against the intimal wall may lead to inflammation and subsequent permanent vascular changes such as intracranial atherosclerosis.7–9 High-resolution (HR) magnetic resonance imaging (MRI) is a promising non-invasive technique that has been used to characterise intracranial arteries.10–12 The aim of this pilot study is to characterise the presence of arterial wall enhancement (AWE) after MT. AWE may be a surrogate biomarker of inflammation and possibly endothelial damage.
Materials and methods
Study design and patient recruitment
This pilot study was designed to determine the feasibility of a HR-MRI protocol in detecting changes of the arterial wall after MT. The study protocol was approved by our local institutional review board. Potential subjects were prospectively identified after MT for inclusion in the study. Informed consent was obtained from the patient or legal representative after the procedure. HR-MRI was acquired within 24 hours of MT. Inclusion criteria were: (a) age 18 years or greater diagnosed with acute ischaemic stroke; and (b) MT of a large intracranial artery (middle cerebral artery (MCA), internal carotid artery (ICA), basilar and/or vertebral and P1 segment of the posterior cerebral artery (PCA)). Exclusion criteria were: (a) placement of a permanent stent or other implant in the target artery; (b) patient unable to undergo HR-MRI with a 3T MRI due to claustrophobia or unstable medical condition; (c) contraindication to MRI and/or intravenous gadolinium; and (d) an estimated glomerular filtration rate less than 30 mL/min/1.73 m2.
Clinical data collection and High Resolution-MRI
Basic demographic data, clinical comorbidities, presentation, technical details about the MT and outcome were collected from electronic medical records. Within 24 hours after the MT procedure, patients underwent a 45-minute 3T brain MRI with contrast (MAGNETOM Skyra, Siemens; 20-channel head coil). The HR-MRI protocol included the following standardised sequences for the detection of intracranial wall abnormalities: T1 pre and post-contrast black-blood imaging, three-dimensional (3D) T2, contrast-enhanced (CE) magnetic resonance angiography (MRA) and susceptibility-weighted imaging (SWI). Technical parameters are presented in Supplementary Table 1. T1 post-contrast and CE-MRA sequences were obtained immediately after contrast was noted in the aorta (0.1 mmol/kg gadobutrol (Gadavist, Bayer Pharmaceuticals)). All images were analysed with the picture archiving communication system (PACS, Carestream Vue). Two neuroradiologists with at least 5 years of experience, and blinded to the MT technique and recanalisation outcomes performed the objective quantification of enhancement. First, the brain area affected by the stroke and the target vessel that underwent MT were identified. The target vessel was co-registered on both pre and post-contrast T1-weighted sequences using the inbuilt co-registration functionality of PACS. CE-MRA and 3D T1-weighted SPACE images were used as a reference to exclude the vessel lumen and determine the arterial wall boundaries. 3D T2-weighted sequences were used to identify artefacts such as cerebrospinal fluid, meninges and surrounding veins. A two-dimensional (2D) region of interest (ROI) of the arterial wall was drawn on T1 CE images to select the arterial wall segment localised at the site of endovascular clot retrieval (Figure 1). As previously described, signal intensity (SI) values on the arterial wall were quantified and normalised to the pituitary stalk. 13 A reference vessel wall was also sampled in a contralateral vascular territory. For strokes in the anterior circulation, the M1 segment of the contralateral MCA or supraclinoid segment of the contralateral ICA were used as the reference vessel, whereas for strokes comprising the basilar, the reference vessel was the M1 segment of the right MCA. If the target vessel was located in the PCA, the contralateral PCA was used as the reference vessel. If the both PCAs were accessed in attempting to achieve recanalisation, the M1 segment of the right MCA was used as the reference vessel. Normalisation of SI with the pituitary stalk was performed as previously described: the mean/maximal SI value of the arterial wall was divided by the mean/maximal SI value of the pituitary stalk (Supplementary Figure 1). Thus, the artery-to-pituitary stalk contrast ratio (CR) was calculated for the target and reference arteries as follows: CR = (SI artery/SI stalk).
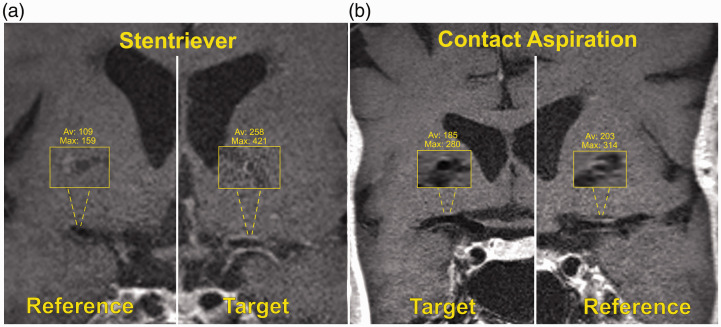
Figure 1.
(a) High-resolution magnetic resonance imaging (HR-MRI) coronal view of a patient who underwent mechanical thrombectomy (MT) with a stentriever. Inlets show the cross-sectional views of the reference (right middle cerebral artery (MCA)) and target (left MCA) vessels. Note the signal intensity (SI) difference between target and reference vessels (max/mean 421/258 vs. 159/109). (b) Coronal view of a patient who underwent MT with contact aspiration: inlets of the target (right MCA) and reference (left MCA) vessels show minimal SI difference (max/mean 280/185 vs. 314/203).
In order to determine the presence of intra-parenchymal haemorrhage post MT, SWI sequences were analysed on PACS. SWI has a high sensitivity in detecting microbleeds and in characterising petechial haemorrhage after MT. 14 The semi-automated livewire segmentation tool with an active contour model was used to segment the SWI positive signal slabs. 15 Only SWI located in the territory of the target artery was quantified and included in the analysis.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as frequency and percentage. SI measurements from the arterial walls were dichotomised in two separate categories: target vessel and reference vessel. The sample groups were analysed by use of a stentriever device versus no use of a stentriever. Distributions of values for CR were tested for normality using the Shapiro–Wilks method. For normally distributed variables, Student’s t tests were used to compare means. For non-normally distributed variables, a Mann–Whitney U test was used. Pearson correlations were used to examine relationships between normally distributed variables and Spearman correlations were used to examine relationships between non-normally distributed variables. For the categorical data comparison, we used Fisher’s exact test due to a small sample size. The receiver operating characteristic (ROC) curve was plotted to find the best CR cut-off. A two-sided test with a P value less than 0.05 was considered significant. All statistical analyses were performed with SPSS Statistics 25.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8.0.0 (GraphPad, San Diego, CA, USA).
Results
Patient characteristics and MT outcomes
Fifty-six patients underwent MT during the duration of this study (August 2018 to December 2018). Thirty-six patients were excluded due to a contraindication for MRI (n = 10); use of an implant in the target artery (n = 2); unstable medical condition or claustrophobia (n = 11); and did not consent for the study (n = 13). Twenty patients with 22 target vessels and 20 reference vessels were included in the study. Baseline characteristics are depicted in Table 1. The mean age was 69.9 ± 15.5 years, and 11 (55%) were women. Twelve patients (60%) were treated with a combined approach stentriever plus aspiration catheter, four (20%) with stentriever only, and four (20%) with contact aspiration only. The analysis of AWE was performed in two groups: stentriever with or without aspiration catheter and contact apiration only. This is the standard practice at our institution and interventionalists may choose to use stentriever and aspiraton versus contact aspiration only. The mean number of recanalisation attempts was 2.6 ± 1.9 and successful recanalisation (thrombolysis in cerebral infarction (TICI) 2b–3) was achieved in 95% of cases (Supplementary Table 2). Eleven patients (55%) underwent monitored anaesthesia care (MAC) and nine patients (45%) underwent general anaesthesia. MRIs were obtained under 24 hours in all 20 patients (μ = 9.6 ± 6.9 hours).
Table 1.
Baseline characteristics.
| Variable | Patients, N = 20 (%)a |
|---|---|
| Age, years (mean ± SD) | 69.9 ± 15.5 |
| Women | 11 (55) |
| Caucasian | 19 (95) |
| Diabetes | 2 (10) |
| Hypertension | 13 (65) |
| Atrial fibrillation | 9 (45) |
| Hyperlipidemia | 5 (25) |
| Prior stroke | 0 (0) |
| mRS prior to stroke (mean ± SD) | 0.3 ± 0.7 |
| 0–2 | 19 (95) |
| 3–5 | 1 (5) |
| NIHSS at admission (mean ± SD) | 16.9 ± 8.7 |
| 0–15 | 8 (40) |
| ≥16 | 12 (60) |
| IV tPA | 8 (40) |
| ASPECTS (mean ± SD) | 9 ± 1.2 |
| 7 | 3 (15) |
| 8 | 4 (20) |
| 9 | 3 (15) |
| 10 | 10 (50) |
| Procedural anaesthesia | |
| MAC | 9 (45) |
| General anaesthesia | 11 (55) |
| MT technique | |
| Contact aspiration | 4 (20) |
| Contact aspiration + stentriever | 12 (60) |
| Stentriever only | 4 (20) |
| Attempts (mean ± SD) | 2.6 ± 1.9 |
| MT target vesselb | |
| ICA | 2 (9.1) |
| MCA | 16 (72.7) |
| BA | 2 (9.1) |
| PCA | 2 (9.1) |
| TICI | |
| 2a | 1 (5) |
| 2b–3 | 19 (95) |
| LKW to recanalisation (hours) | 8.9 ± 5.4 |
| Puncture to recanalisation (min) | 48.7 ± 45.8 |
| Time from MT to MRI (hours) | 9.6 ± 6.9 |
| mRS at discharge (mean ± SD) | 1.95 ± 2.2 |
| 0–2 | 14 (70) |
| 3–5 | 3 (15) |
| 6 (death) | 3 (15) |
| NIHSS at discharge (mean ± SD) | 3.94 ± 7.3 |
| 0–15 | 16 (80) |
| ≥16 | 1 (5) |
| CR† | 0.58 ± 0.22 |
| <0.55 | 18 (42.9) |
| ≥0.55 | 24 (57.1) |
IV tPA: intravenous tissue plasminogen activator; ASPECTS: Alberta score program early CT score; BA: basilar artery; CR: artery-to-pituitary stalk contrast ratio; ICA: internal carotid artery; LKW: last known well; MAC: monitored anaesthesia care; MCA: middle cerebral artery; MRI: magnetic resonance imaging; mRS: modified Rankin scale; MT: mechanical thrombectomy; NIHSS: National Institute of Health Stroke Scale; PCA: posterior cerebral artery; SD: standard deviation; TICI: thrombolysis in cerebral infarction recanalisation score.
aValues are presented as number and percentage (%) unless specified otherwise.
bTwo patients had bilateral target vessels that underwent MT (one patient bilateral PCAs, one patient bilateral MCAs), whereas only one contralateral artery was considered the reference vessel for all cases. Therefore, the total number of CR measurements is 42 (22 thrombectomy vessels + 20 reference vessels).
Arterial wall enhancement
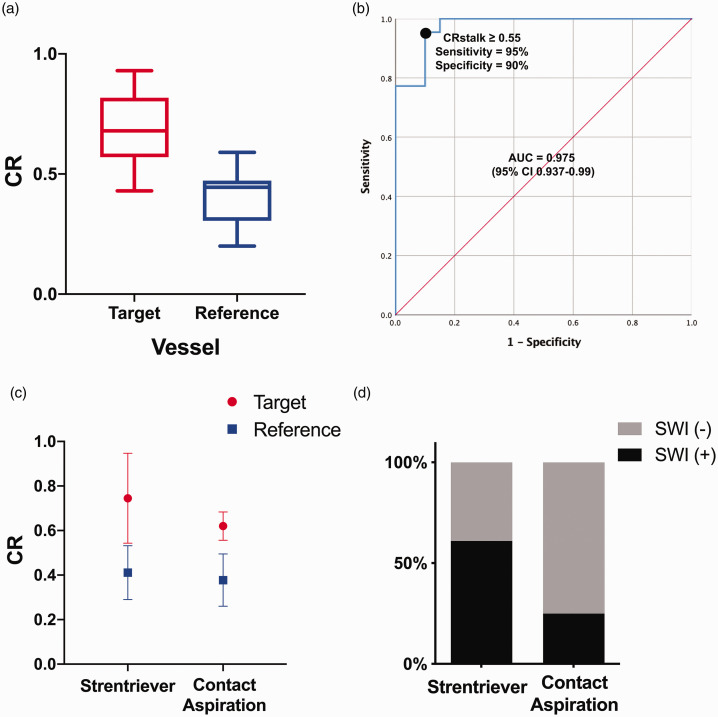
Target vessels enhanced more than reference vessels, mean/max SI of target vessels = 386/473.8 versus mean/max SI of reference vessels = 202.9/260.1 A Shapiro–Wilks test showed a departure from normality of the CR values of the target vessels (W = 0.84, P = 0.002) and a normal distribution for the reference vessels (W = 0.96, P = 0.47). A Mann–Whitney U test showed that the CR was significantly higher in the arterial wall of target vessels (Mdn 0.69) subjected to MT compared with reference vessels (Mdn 0.45) (U = 22.50, P < 0.01) (Figure 2(a)). Therefore, a ROC curve was plotted to define the best CR cut-off using the DeLong method to discriminate between target and reference vessels (Figure 2(b)). 16 A CR of 0.55 or greater reached a sensitivity of 95% and a specificity of 90% in identifying AWE of target vessels (area under the curve (AUC) 0.975, 95% confidence interval (CI) 0.93–0.99). Multiple binary logistic regressions were performed to assess the association between clinical/procedural variables and AWE based on this cut-off (Table 2). The use of a stentriever device showed the highest odds ratio (OR) on determining AWE after MT (OR 1.43, P = 0.65).
Figure 2.
(a) Tukey’s boxplot comparing the contrast ratio (CR) of the target (x̄ = 0.72 ± 0.19) versus reference vessels (x̄ = 0.40 ± 0.11). (b) Receiver operating characteristic (ROC) curve of CR to discriminate between target and reference vessels. (c) Vessels treated with stentriever enhanced more than vessels treated with contact aspiration when compared to reference vessels: stentriever target vessel (x̄ = 0.75 ± 0.20) versus reference vessel (x̄ = 0.41 ± 0.12); contact aspiration target vessel (x̄ = 0.62 ± 0.06) versus reference vessel (x̄ = 0.37 ± 0.11). (d) Susceptibility-weighted imaging (SWI) by mechanical thrombectomy (MT) technique.
Table 2.
Binary logistic regression analysis for enhancing target vessels (CR ≥0.55).
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age | 1.01 (0.97–1.06) | 0.52 |
| Women | 0.94 (0.28–3.23) | 0.93 |
| Hypertension | 1.27 (0.36–4.54) | 0.71 |
| Atrial fibrillation | 0.71 (0.21–2.44) | 0.59 |
| Hyperlipidemia | 1.17 (0.28–4.95) | 0.83 |
| mRS baseline | 0.86 (0.36–2.02) | 0.70 |
| NIHSS admission | 1.03 (0.96–1.10) | 0.47 |
| ASPECTS | 1.21 (0.69–2.08) | 0.50 |
| IV tPA | 0.63 (0.18–2.20) | 0.46 |
| Stentrievera | 1.43 (0.31–6.70) | 0.65 |
| MCA | 0.18 (0.02–1.62) | 0.13 |
| Attempts | 1.08 (0.78–1.48) | 0.65 |
| TICI 2c–3 | 1.40 (0.41–4.79) | 0.59 |
| LKW to recanalisation | 1.01 (0.89–1.13) | 0.98 |
| Puncture to recanalisation | 1.00 (0.99–1.02) | 0.54 |
| mRS discharge | 1.11 (0.84–1.49) | 0.45 |
| NIHSS discharge | 0.99 (0.91–1.09) | 0.96 |
ASPECTS: Alberta score program early CT score; IV tPA: intravenous tissue plasminogen activator; MCA: middle cerebral artery; mRS: modified Rankin scale; NIHSS: National Institute of Health Stroke Scale; TICI: thrombolysis in cerebral infarction recanalisation score; LKW: last known well; OR: odds ratio; CI: confidence interval.
aIncludes both primary stentriever and stentriever plus contact aspiration MT techniques.
Multiple Pearson coefficients were calculated with the continuous variables previously included in the binary logistic regressions to analyse target vessels. The CR values of the reference vessel for both stentriever (x̄ = 0.41 ± 0.12) and contact aspiration (x̄ = 0.37 ± 0.11) were very similar. The stentriever group had an 82% increase in CR of the target vessel (x̄ = 0.75 ± 0.21) when compared to the reference vessel (x̄ = 0.41 ± 0.13), whereas the contact aspiration group had a 64% increase of the CR difference between target (x̄ = 0.62 ± 0.07) and reference vessels (x̄ = 0.38 ± 0.12) (Figure 2(c)).
Haemorrhagic transformation
Eleven (65%) patients who underwent MT with a stentriever had positive findings on SWI versus one (25%) in the contact aspiration group, P = 0.272 with a Fisher’s exact test (Figure 2(d)). Volumes ranged between 0.24 and 61.4 mL (x̄ = 4.28 ± 4.74). One outlier with a large post-MT haemorrhage was excluded from the analysis as this was considered a complication with intraprocedural contrast extravasation. No statistically significant correlation was found when comparing CR of the target vessel and the presence of SWI changes (rs = 0.098, P = 0.74).
Discussion
This prospective pilot study quantified the degree of AWE as a surrogate marker of endothelial damage after MT. Objective quantification of enhancement demonstrated that all the target vessels had increased contrast enhancement after MT as opposed to reference vessels. Stentrievers produced more enhancement than contact aspiration catheters.
Previous studies have shown that increased contrast enhancement of vessels after MT occurs after intimal injury.6,17 Truong et al. analysed the pattern of wall enhancement with 7T MRI on seven patients who underwent MT. 17 Vessel wall enhancement in all cases correlated with the deployment zone of the stentriever rather than the location of the thrombus. The manipulation of target vessels by MT devices produces intimal injury, but the extent of this injury is unknown and the impact on different layers of the arterial wall is unclear. 18 The presence of vascular injuries varies from subintimal dissection to intramural haematoma that can progress to occlusion of the treated vessel. 19 Factors that may contribute to the magnitude of morphological changes induced by thrombectomy devices include the design of the device, the number of device passes and the diameter of the target vessel.8,20
Thrombectomy techniques and/or devices generate different stresses within the target vessel. Stentrievers were associated with a higher AWE (386.1) in comparison to contact aspiration catheters (202.9). Likewise, the change in CR percentage between the target vessel/reference vessel in the stentriever group is higher than the contact aspiration group: 82% versus 64%. An in-vivo study by Teng et al. also demonstrated in a swine model an decreased density of endothelial cells in vessels treated with stentrievers as opposed to contact aspiration catheters. 21 The authors suggest that mechanical disruption of the blood–brain barrier, loss of large-vessel intermediate filament compliance as a functional unit, and the resultant inflammatory responses initiated at the endothelial level remain potential sources of secondary neuronal injury. Moreover, increased release of inflammatory mediators due to endothelial damage may have an unfavourable effect on the downstream brain tissue, which can lead to increased infarct volume. 22 Peschillo et al. also studied the swine model in comparing stentrievers with contact aspiration. 7 Tissue samples from the vessels treated with stentriever showed almost complete loss of endothelium, thickening of the internal elastic lamina and degeneration of the elastic fibres of the bordering lamina media and adventitia. In contrast, tissue samples of the vessels treated with contact aspiration had a clear integral internal elastic lamina and uninterrupted endothelial lining.
MT data suggest that larger stentrievers are associated with higher first-pass reperfusion as opposed to smaller stentrievers. 23 In most cases, the radial force applied to the wall depends on the diameter of the stentriever. Some stentrievers are specifically aimed to exert a high radial force intended to achieve a better integration of the clot within the stent structure. 24 Increased radial force may lead to more extensive damage of the inner layers of the arterial wall and even to contrast extravatation. Renu et al. documented increased contrast enhacement with a greater number of device passes. 25 Contact aspiration has also evolved into larger bore catheters. Evidence suggests that large bore catheters are more effective at ingesting the entire clot, with a decreased risk of fragmentation and distal emboli. 26 This approach of ‘larger is better’ may lead to secondary endothelial damage, inflammation and greater disruption of the blood–brain barrier. 27 The objective quantification of contrast enhancement of the arterial wall proposed by our study could be used in future outcome imaging studies aimed at determining arterial wall injury. Recanalisation defined by the TICI score is only one of the pieces of the puzzle of arterial occlusion. Arterial wall damage after MT may lead to secondary stroke injury through disruption of the blood–brain barrier and release of inflammatory mediators.
Limitations
The main limitation of our study is its small sample size. A larger sample would provide more robust evidence about the difference of techniques and devices used in MT. The other limitation is that no follow-up imaging was performed to observe any dynamic changes. A third limitation is that the presence of a subjacent atherosclerotic or inflammatory arteriopathy cannot be completely discarded as a possible contributor to vessel enhancement. However, the analysis of a reference vessel controls at least for a central nervous system arteriopathy. In addition, the parenchymal haemorrhage volumes were calculated using SWI. Gradient-based images are well recognised to result in ‘blooming’ artefact, and this likely overestimated the parenchymal haemorrhage volume. Finally, the long-term clinical outcomes with these HR-MRI findings have not been sufficiently studied to determine whether there is a meaningful clinical consequence.
Conclusion
Target arteries that underwent MT have increased contrast enhancement when compared to non-target reference vessels. This enhancement is more pronounced when stentrievers are used, as opposed to the use of aspiration catheters for contact aspiration.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_19714009211017782 for High-resolution vessel wall imaging after mechanical thrombectomy by Sami Al Kasab, Girish Bathla,AlbertoJorge A Roa, Ryan Sabotin, Ashrita Raghuram, Wu Chaorong, David M Hasan, Tanya N Turan, Rano Chatterjee and Edgar A Samaniego in The Neuroradiology Journal
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Pilot Research grant from the Society of Vascular and Interventional Neurology, granted to Edgar Samaniego. The study was supported by the Clinical and Translational Science Award grant funded from the National Institutes of Health (UL1TR002537).
Supplemental material: Supplementary material for this article is available online.
ORCID iDs
Alberto Varon https://orcid.org/0000-0001-8447-4067
Edgar A Samaniego https://orcid.org/0000-0003-2764-2268
References
- 1.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 3.Samaniego EA, Roa JA, Limaye K, et al. Mechanical thrombectomy: emerging technologies and techniques. J Stroke Cerebrovasc Dis 2018; 27: 2555–2571. [DOI] [PubMed] [Google Scholar]
- 4.Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER Randomized Clinical Trial. JAMA 2017; 318: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power S, Matouk C, Casaubon LK, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke 2014; 45: 2330–2334. [DOI] [PubMed] [Google Scholar]
- 6.Abraham P, Scott Pannell J, Santiago-Dieppa DR, et al. Vessel wall signal enhancement on 3-T MRI in acute stroke patients after stent retriever thrombectomy. Neurosurg Focus 2017; 42: E20. [DOI] [PubMed] [Google Scholar]
- 7.Peschillo S, Diana F, Berge J, et al. A comparison of acute vascular damage caused by ADAPT versus a stent retriever device after thrombectomy in acute ischemic stroke: a histological and ultrastructural study in an animal model. J Neurointerv Surg 2017; 9: 743–749. [DOI] [PubMed] [Google Scholar]
- 8.Gory B, Bresson D, Kessler I, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol 2013; 34: 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurre W, Perez MA, Horvath D, et al. Does mechanical thrombectomy in acute embolic stroke have long-term side effects on intracranial vessels? An angiographic follow-up study. Cardiovasc Intervent Radiol 2013; 36: 629–636. [DOI] [PubMed] [Google Scholar]
- 10.Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013; 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakih R, Roa JA, Bathla G, et al. Detection and quantification of symptomatic atherosclerotic plaques with high-resolution imaging in cryptogenic stroke. Stroke 2020; STROKEAHA120031167. [DOI] [PubMed] [Google Scholar]
- 12.Yun SY, Heo YJ, Jeong HW, et al. Spontaneous intracranial vertebral artery dissection with acute ischemic stroke: high-resolution magnetic resonance imaging findings. Neuroradiol J 2018; 31: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roa JA, Zanaty M, Osorno-Cruz C, et al. Objective quantification of contrast enhancement of unruptured intracranial aneurysms: a high-resolution vessel wall imaging validation study. J Neurosurg 2020; 134(3): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CC, Kwan GNC, Hapugoda S, et al. Susceptibility weighted imaging in acute cerebral ischemia: review of emerging technical concepts and clinical applications. Neuroradiol J 2017; 30: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roa JA, Fakih R, Zanaty M, et al. Quantitative assessment of ventriculostomy-related hemorrhage: a volume-based classification system to predict new neurological symptoms. Oper Neurosurg (Hagerstown) 2020; 20(2): 198–205. [DOI] [PubMed]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 17.Truong M, Bloch KM, Andersen M, et al. Subacute vessel wall imaging at 7-T MRI in post-thrombectomy stroke patients. Neuroradiology 2019; 61: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peschillo S, Tomasello A, Diana F, et al. Comparison of subacute vascular damage caused by ADAPT versus stent retriever devices after thrombectomy in acute ischemic stroke: histological and ultrastructural study in an animal model. Interv Neurol 2018; 7: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin NS, Benavides S, Starkman S, et al. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke 2010; 41: 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marosfoi MG, Korin N, Gounis MJ, et al. Shear-activated nanoparticle aggregates combined with temporary endovascular bypass to treat large vessel occlusion. Stroke 2015; 46: 3507–3513. [DOI] [PubMed] [Google Scholar]
- 21.Teng D, Pannell JS, Rennert RC, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke 2015; 46: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haussen DC, Al-Bayati AR, Grossberg JA, et al. Longer stent retrievers enhance thrombectomy performance in acute stroke. J Neurointerv Surg 2019; 11: 6–8. [DOI] [PubMed] [Google Scholar]
- 24.Akpinar CK, Ozdemir AO, Gurkas E, et al. Favorable first-pass recanalization rates with NeVa thrombectomy device in acute stroke patients: initial clinical experience. Interv Neuroradiol 2020; 27(1): 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renu A, Laredo C, Lopez-Rueda A, et al. Vessel wall enhancement and blood–cerebrospinal fluid barrier disruption after mechanical thrombectomy in acute ischemic stroke. Stroke 2017; 48: 651–657. [DOI] [PubMed] [Google Scholar]
- 26.Ospel JM, Volny O, Jayaraman M, et al. Optimizing fast first pass complete reperfusion in acute ischemic stroke – the BADDASS approach (BAlloon guiDe with large bore Distal Access catheter with dual aspiration with Stent-retriever as Standard approach). Expert Rev Med Devices 2019; 16: 955–963. [DOI] [PubMed] [Google Scholar]
- 27.Luby M, Hsia AW, Nadareishvili Z, et al. Frequency of blood–brain barrier disruption post-endovascular therapy and multiple thrombectomy passes in acute ischemic stroke patients. Stroke 2019; 50: 2241–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-neu-10.1177_19714009211017782 for High-resolution vessel wall imaging after mechanical thrombectomy by Sami Al Kasab, Girish Bathla,AlbertoJorge A Roa, Ryan Sabotin, Ashrita Raghuram, Wu Chaorong, David M Hasan, Tanya N Turan, Rano Chatterjee and Edgar A Samaniego in The Neuroradiology Journal