Abstract
Background
Black patients tend to develop coronary artery disease at a younger age than other groups. Previous data on racial disparities in outcomes of myocardial infarction (MI) have been inconsistent and limited to older populations. Our objective was to investigate racial differences in the outcome of MI among young and middle‐aged patients and the role played by socioeconomic, psychosocial, and clinical differences.
Methods and Results
We studied 313 participants (65% non‐Hispanic Black) <61 years old hospitalized for confirmed type 1 MI at Emory‐affiliated hospitals and followed them for 5 years. We used Cox proportional‐hazard models to estimate the association of race with a composite end point of recurrent MI, stroke, heart failure, or cardiovascular death after adjusting for demographic, socioeceonomic status, psychological, and clinical risk factors. The mean age was 50 years, and 50% were women. Compared with non‐Black patients, Black patients had lower socioeconomic status and more clinical and psychosocial risk factors but less angiographic coronary artery disease. The 5‐year incidence of cardiovascular events was higher in Black (35%) compared to non‐Black patients (19%): hazard ratio (HR) 2.1, 95% CI, 1.3 to 3.6. Adjustment for socioeconomic status weakened the association (HR 1.3, 95% CI, 0.8–2.4) more than adjustment for clinical and psychological risk factors. A lower income explained 46% of the race‐related disparity in outcome.
Conclusions
Among young and middle‐aged adult survivors of an MI, Black patients have a 2‐fold higher risk of adverse outcomes, which is largely driven by upstream socioeconomic factors rather than downstream psychological and clinical risk factors.
Keywords: cardiovascular disease, prognosis, risk factors, socioeconomic position
Subject Categories: Cardiovascular Disease, Race and Ethnicity, Risk Factors, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- MIMS2
Myocardial Infarction and Mental Stress Study 2
- SES
socioeconomic status
Clinical Perspective
What Is New?
In young and middle‐aged survivors of myocardial infarction, we demonstrate that Black patients have more than a 2‐fold risk of developing adverse cardiovascular events compared with non‐black patients.
While a multitude of factors contribute to these disparities, socioeconomic status indicators are major drivers of these differences.
What Are the Clinical Implications?
These results underscore the importance of social determinants of health for this at‐risk population, and highlight the need to intervene in this area in order to mitigate racial disparities in the outcome of early‐onset myocardial infarction.
The Black population in the United States has worse cardiovascular health and higher rates of cardiovascular morbidity and mortality compared with other racial groups. 1 Of further concern is the fact that downward trends in cardiovascular‐related mortality in the past 4 to 5 decades have been less pronounced in Black individuals compared to other groups, leading to an increase in disparity over time. 2 , 3 Understanding and eliminating such health inequalities has long been recognized as a national priority. 4 Black adults in the United States overall have a more unfavorable cardiometabolic risk factor profile than their White counterparts, but whether these risk factors fully explain race‐related disparities is controversial. 5 , 6 , 7 Many studies have evaluated the contribution of low socioeconomic status (SES) to health inequalities by race across medical conditions and healthcare settings. 5 , 8 However, disentangling the effect of race from that of SES has proven to be challenging. Nearly every indicator of SES is highly related to race, with US Black individuals bearing a disproportionate burden of poverty and other indicators of social disadvantage in comparison with White individuals. 9
This issue is especially unresolved among individuals with coronary heart disease. Few studies have had sufficient numbers of Black participants and detailed socioeconomic and clinical information to evaluate the influence of both race and SES on myocardial infarction (MI). 10 , 11 , 12 , 13 , 14 , 15 In a large study of hospitalized patients with MI, the excess mortality in Black patients compared with White patients was observed only among patients younger than 65 years of age, and differences in mortality by race diminished as age increased. 16 This phenomenon, known as “racial crossover,” has been reported before in population studies and has been attributed to higher mortality among high‐risk Black individuals who never reach the oldest ages. 17 , 18 , 19 This earlier study, however, lacked information on SES. To date, few studies have focused on the young and middle‐aged post‐MI population to understand the reasons behind the high risk of Black patients and race‐related differences in outcomes. Black individuals tend to be disadvantaged socioeconomically, but they also have more cardiometabolic risk factors and more psychosocial stressors compared with other groups. 20 The relative importance of all these factors in explaining race differences in the risk of adverse outcomes among young patients with MI is currently unexplored.
In this study we sought to investigate race differences in the outcome of MI among young and middle‐aged survivors of MI, and the relative role played by SES, traditional risk factors, and psychosocial factors. We were especially interested in the relative importance of SES versus other individual patient characteristics including psychosocial and traditional clinical risk factors in explaining racial disparities in MI outcomes. Our hypothesis was that a more adverse socioeconomic and psychosocial profile among Black patients would play a key role in explaining differences in the outcomes after MI between Black and non‐Black patients.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
Between August 2012 and March 2016, a total of 313 adult men and women were enrolled from the MIMS2 (Myocardial Infarction and Mental Stress Study 2), a prospective cohort study of patients 18 to 60 years of age with a documented history of MI in the previous 8 months at Emory‐affiliated hospitals in Atlanta, Georgia. 21 MI case diagnosis (type 1) was verified with medical record review based on standard criteria of troponin elevation, symptoms of ischemia, and changes in the ECG or other evidence of myocardial necrosis. 22 Exclusion criteria included unstable angina, acute coronary syndrome or decompensated heart failure in the previous week, severe comorbid medical or psychiatric disorder that could interfere with the study assessments, pregnancy or breastfeeding, or the use of immunosuppressant or psychotropic medications other than antidepressants. Each participant underwent an assessment protocol that included a blood draw, measured height and weight, and clinic tests of myocardial perfusion imaging. A research nurse obtained sociodemographic, medical history and body measurements, and participants completed standardized questionnaires on behavioral, social, and health status information. After the baseline visit, patients were followed for 5 years for adverse events, including cardiovascular death, recurrent (type 1) MI, stroke, and heart failure hospitalization. All events were independently adjudicated. The Emory University Institutional Review Board approved the protocol, and all participants provided written informed consent.
Baseline Study Measures
Demographic and SES information included sex, race/ethnicity, age, educational attainment, employment status and income. Race/ethnicity was self‐reported. Participants who self‐reported as neither Black nor White were few; thus they were grouped together as “non‐Black.” Educational attainment was assessed as years of education and dichotomized as <12 or ≥12 years. Annual household income was categorized as <$35 000, $35 000 to $75 000, and >$75 000. Body mass index (BMI) was calculated as measured weight divided by the square of measured height (kg/m2). History of cardiovascular risk factors was ascertained by chart review and by standardized questionnaires and included history of smoking, diabetes mellitus, hypertension, and dyslipidemia. Characteristics of the index MI were abstracted from the medical records and included type of MI (ST‐elevation myocardial infarction [STEMI] versus non‐ST‐elevation myocardial infarction [NSTEMI]), left ventricular ejection fraction, preventive medication use (eg, aspirin, beta blockers) and angiographic data, the latter obtained from the coronary angiogram associated with the index MI. CAD severity was quantified using the Gensini Score. 23
We obtained 6 scales of psychological characteristics with known association with cardiovascular disease or prognosis. Current depressive symptoms were assessed with the Beck Depression Inventory, a 21‐item self‐administered scale. 24 PTSD symptoms were assessed using the civilian version of the PTSD Symptom Checklist, a 17‐item scale. 25 Trait anxiety was measured with the State‐Trait Anxiety Inventory. 26 To measure trait anger symptoms, we used the Spielberger's State‐Trait Anger Expression Inventory 27 ; to measure hostility, we administered the Cook‐Medley Hostility Scale, 28 and to assess general perceived stress, we used the Perceived Stress Scale. 29
Outcomes
Participants were followed prospectively for adverse cardiovascular outcomes for a median time of 5 years after the baseline visit. Follow‐up information was collected through patient contacts, medical record review, and by querying the Social Security Death Index. Patients were contacted at their ≈3‐ and 5‐year anniversary from their initial visit. If hospitalizations or procedures were reported, patients' physicians were contacted, and hospital records were obtained. Follow‐up was virtually complete, with only 5 (1.6%) patients lost to follow‐up. Ascertained cardiovascular events included cardiovascular death, recurrent MI (type 1), stroke, and heart failure hospitalization. All events were adjudicated by consensus by study investigators (A.J.S., A.A.Q., V.V.), who were blinded to other study data. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI), cardiac arrhythmia (including cardiac resuscitation), or heart failure. The end point of the study was a composite outcome of adverse cardiovascular events, including cardiovascular death, recurrent MI, stroke, or hospitalization for heart failure.
Statistical Analysis
We calculated descriptive statistics of the sample. Next, we used cumulative incidence plots and Cox proportional hazards models to derive hazard ratios (HR) and 95% CI for the association between race (Black versus non‐Black patients) and adverse outcomes. Pre‐defined covariates were included in a sequential fashion to the unadjusted model to assess the impact of covariate adjustment on the estimate for race. 30 First, we added demographic variables, including age and sex, followed by the addition of SES factors including education, income and employment status, and then psychosocial factors. Because virtually all the psychological factors were related to race, to avoid model overfitting, we constructed a global psychological distress measure integrating the six scales of psychological characteristics (symptoms of depression, anxiety, anger, perceived general stress, PTSD, and hostility) using similar methodology previously followed by us and others. 31 , 32 , 33 Individuals were ranked on each of the 6 psychological measures; then all ranks were averaged for each participant to obtain a global psychological distress score. 31 We also ran additional models where psychosocial factors were included as separate variables rather than in the aggregated score. Lastly, we added to the model baseline traditional risk factors and clinical characteristics, including smoking, history of hypertension, dyslipidemia, diabetes mellitus, and heart failure, left ventricular ejection fraction, BMI, and type of MI. This sequence was selected because SES and psychological factors were considered more “proximal” to race than traditional/clinical factors in the relationship to outcome, and potentially in the causal pathway between race and traditional risk factors. However, because we were interested in comparing the effect of SES versus traditional/clinical risk factors on the estimate for race, we also inverted the order of the adjustment factors in the sequential models. Finally, we tested for interactions between race and SES variables, including race‐by‐income, race‐by‐education, and race‐by‐employment status interactions. The assumption of proportionality for the Cox proportional hazards regression model was assessed graphically and formally tested with the Schoenfeld residuals test.
The proportion of missing covariate data ranged between 0% and 11%. To avoid loss of information and possible bias due to these missing covariate values, multiple imputation was performed for the primary analysis with 50 imputations using Markov Chain Monte Carlo equations with SAS PROC MI. Imputed regression estimates were then combined using SAS PROC MIANALYZE.
Lastly, we performed a mediation analysis with bootstrapping (1000 bootstrap samples and a 95 % CI to test the statistical effects of SES on the association of race with major adverse cardiac events [SPSS PROCESS macro version 2.16.3]). This method uses an ordinary least squares or logistic regression‐based path framework to estimate direct and indirect effects and produces CIs from bias‐corrected bootstrap samples. Out of our 3 SES indicators, we chose income as the primary marker of SES in our mediation analysis because it may be a more sensitive indicator relative to educational attainment and employment, especially among Black individuals. Compared with White individuals, Black individuals receive less income and are less likely to be employed at the same education levels. 20 All other statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and significance level was set at α=0.05, two tailed.
Results
Baseline Characteristics
A total of 205 black and 108 non‐Black participants were enrolled in the study. Table 1 shows descriptive characteristics of the analytic sample by race. There were no differences in age, but Black patients were more often female, less likely to be married, and had a more adverse socioeconomic profile, including lower income, lower education and lower likelihood to be employed. Black participants had more traditional cardiovascular risk factors than non‐Black participants, including a higher BMI and a more frequent history of hypertension and diabetes mellitus. Black patients were also more likely to have a history of heart failure, but there were no differences by race in type of MI and left ventricular ejection fraction; Black patients were less likely than their non‐Black counterparts to have obstructive CAD. Differences were also noted for use of preventive cardiac medications, with Black patients being less likely to be taking aspirin and statins. When psychological factors were compared by race, Black patients had a worse psychological risk profile for virtually all measures, and especially for depression, PTSD, and hostility scores, compared with non‐Black patients. Descriptive characteristics of clinical risk factors stratified by income category appear in Table S1.
Table 1.
Descriptive Characteristics of Participants Stratified by Race (N=313) in the Myocardial Infarction and Mental Stress 2 Study (MIMS2) at Baseline
| Variable | Black Participants (n=205) | Non‐Blacks Participants (n=108) |
|---|---|---|
| Demographics | ||
| Age, y, mean (SD) | 50 (7) | 51 (6) |
| Age <50 y, % | 42 | 31 |
| Female, % | 56 | 36 |
| Married/living with partner, % | 30 | 65 |
| Income, % | ||
| <$35 000/y | 64 | 27 |
| $35 000–$75 000/y | 28 | 25 |
| >$75 000/y | 8 | 48 |
| Education >12 y, % | 52 | 73 |
| Employed, % | 38 | 64 |
| Cardiovascular risk factors | ||
| BMI (kg/m2), mean (SD) | 32 (8) | 30 (7) |
| Ever smoker, % | 58 | 49 |
| History of hypertension, % | 88 | 69 |
| History of dyslipidemia, % | 81 | 79 |
| History of diabetes mellitus, % | 37 | 21 |
| Prior MI to index MI, % | 24 | 16 |
| History of stroke, % | 6 | 3 |
| History of CABG, % | 18 | 25 |
| History of PTCA, % | 69 | 70 |
| Comorbidities | ||
| Congestive heart failure, % | 14 | 3 |
| Peripheral artery disease % | 2 | 3 |
| Chronic obstructive pulmonary disease, % | 7 | 7 |
| Chronic kidney disease, % | 5 | 3 |
| Coronary angiography and electrocardiography results | ||
| Gensini severity score, mean (SD) | 37 (43) | 49 (46) |
| Obstructive CAD (stenosis ≥70%), % | 81 | 91 |
| 3‐Vessel disease (at ≥70%), % | 11 | 18 |
| LV ejection fraction, mean (SD) | 51 (12) | 51 (12) |
| LV ejection fraction ≤35%, % | 15 | 14 |
| ST‐segment elevation MI, % | 26 | 36 |
| Medication use | ||
| Beta‐blocker, % | 86 | 83 |
| Statin, % | 81 | 92 |
| Aspirin, % | 77 | 91 |
| P2Y12 inhibitors, % | 65 | 79 |
| ACE inhibitors, % | 50 | 42 |
| Anti‐diabetics, % | 32 | 20 |
| Antidepressants, % | 16 | 20 |
| Laboratory values during index MI | ||
| Maximum troponin (ng/L), mean (SD) | 35 (60) | 23 (45) |
| Hemoglobin A1c (%), mean (SD) | 7 (2) | 6 (2) |
| Total cholesterol (mg/dL), mean (SD) | 175 (50) | 176 (50) |
| HDL (mg/dL), mean (SD) | 43 (13) | 42 (16) |
| Triglycerides (mg/dL), mean (SD) | 143 (121) | 169 (118) |
| Psychosocial risk factors | ||
| Beck Depression inventory, mean (SD) | 14 (11) | 10 (9) |
| PTSD Symptom Checklist, mean (SD) | 34 (15) | 28 (13) |
| Anger Expression Inventory, mean (SD) | 31 (12) | 29 (14) |
| Anxiety State Inventory, mean (SD) | 37 (13) | 35 (13) |
| Perceived Stress Scale, mean (SD) | 17 (9) | 15 (9) |
| Hostility Scale, mean (SD) | 0.2 (1) | −0.3 (1) |
| Composite distress score, mean (SD) | 151 (63) | 125 (65) |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; HDL, high‐ density lipoprotein; LV, left ventricular; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; PTSD, post‐traumatic stress disorder; and SD, standard deviation.
Race and Adverse Cardiovascular Outcomes
During a median follow up of 5 years, 71 of 205 (35%) Black and 20 of 108 (18%) non‐Black patients developed a composite study end point. In addition to the primary end point, Black patients had a higher rate of events for each individual component than non‐Black patients (Figure 1). The cumulative incidence of adverse cardiovascular events was significantly higher in Black compared with non‐Black patients, with an unadjusted HR of 2.1, 95% CI, 1.3–3.6, P=0.004 (Figure 2).
Figure 1. Composite outcome and individual outcomes by race.
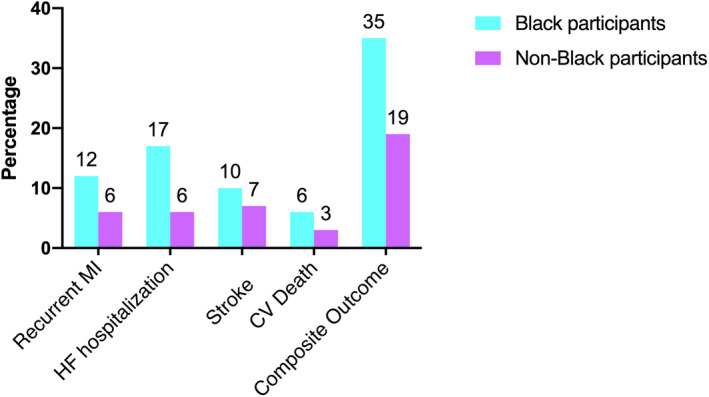
CV indicates cardiovascular; HF, heart failure; and MI, myocardial infarction.
Figure 2. Cumulative incidence for the association between race and adverse cardiovascular outcomes (composite endpoint of recurrent MI, heart failure hospitalization, stroke, and cardiovascular death).
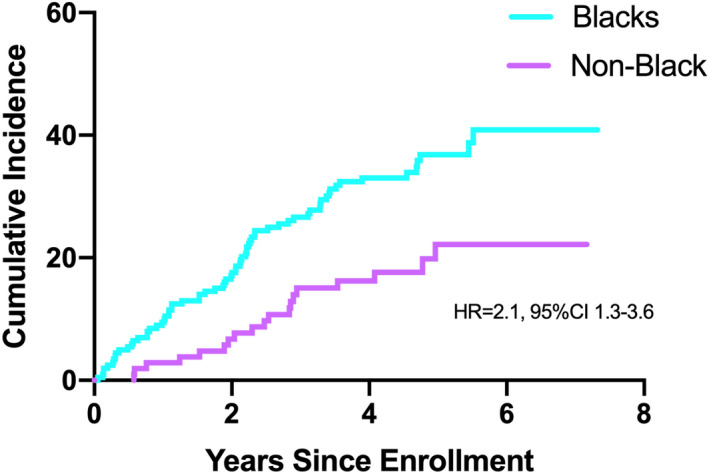
MI indicates myocardial infarction.
As shown in Figure 3, in sequential, nested multivariable models, addition of demographic variables did not affect the estimate by race (HR, 2.2, 95% CI, 1.3–3.6). Addition of SES variables induced a substantial attenuation of the differences in outcome by race (HR, 1.3, 95% CI, 0.8–2.4). Addition of the composite psychological distress index to the model did not further attenuate the difference in outcome by race (HR 1.4, 95% CI, 0.8–2.5). Including psychosocial factors as separate variables in the model provided fairly similar results (data not shown); the HR for race was 1.5 (95% CI, 0.8–2.8). Lastly, addition of clinical risk factors including smoking history, BMI, history of hypertension, history of diabetes mellitus, history of heart failure, left ventricular ejection fraction and type of MI, contributed further to explain the residual risk, bringing the estimate for race close to the null (HR 1.1, 95% CI, 0.6–1.9).
Figure 3. Forest plot for nested, sequential models for the association of race with adverse cardiovascular events (composite end point of recurrent MI, heart failure hospitalization, stroke, and cardiovascular death).
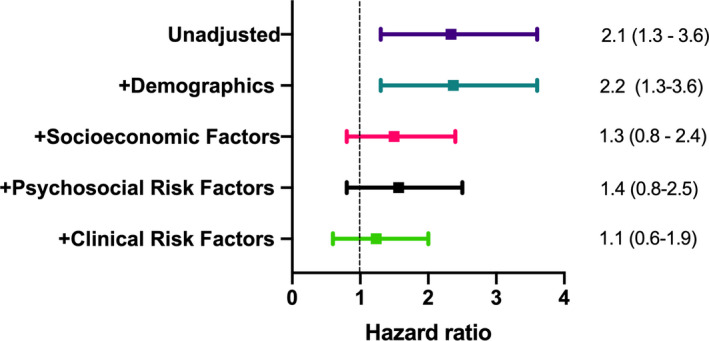
Hazard ratio analysis of Black vs non‐Black patients. Demographic factors: age and sex. Socioeconomic factors: education, income and employment. Psychosocial factors: composite distress score. Clinical risk factors: smoking, BMI, History of hypertension, history of diabetes mellitus, history of dyslipidemia, history of heart failure, left ventricular ejection fraction, and type of MI. BMI indicates body mass index; and MI, myocardial infarction.
Next, we compared the impact of the order of the adjustment factors in the sequential models after the demographics model (Table 2). Addition of SES variables first, heavily attenuated differences in outcome by race (HR, 1.3, 95% CI, 0.8–2.4), with a percent effect explained of 82%. In comparison, addition of clinical risk factors first, attenuated the effect to a lesser extent (HR 1.6, 95% CI, 0.9–2.7), with a percent effect explained of 55%. Lastly, addition of both SES and clinical risk factors together, brought the estimate for race close to the null (HR 1.1, 95% CI, 0.6–1.9), with a percent effect explained of 92%. There were no significant interactions between race and SES variables, including race‐by‐income, race‐by‐education or race‐by‐employment status interactions.
Table 2.
Comparative Models for the Association of Race With Cardiovascular Events (Composite End Point of Recurrent MI, Heart Failure Hospitalization, Stroke, and Cardiovascular Death)
| HR (95% CI), Black vs Non‐Black Participants | Percent Effect Explained* | |
|---|---|---|
| Model 1: Adjusted for demographic variables (age and sex) | 2.2 (1.3–3.6) | … |
| Model 2: SES first: Adjusted for demographic variables+socioeconomic factors (education, income & employment) | 1.3 (0.8–2.4) | 82% |
| Model 3: Clinical factors first: Adjusted for demographic variables+clinical risk factors (smoking history, BMI, history of hypertension, history of diabetes mellitus, history of heart failure, history of dyslipidemia, left ventricular ejection fraction and type of MI) | 1.6 (0.9–2.7) | 55%* |
| Model 4: Both SES and clinical factors: Adjusted for demographic variables and both socioeconomic and clinical factors (all variables in Models 2 and 3) | 1.1 (0.6–1.9) | 92% |
The percent effect explained was derived by calculating percent change in the hazard ratio. BMI indicates body mass index; HR, hazard ratio; and MI, myocardial infarction.
Compared to Model 1.
Mediation Analysis
To quantify the effect of SES in the pathway linking Black race to major adverse cardiac events, we performed formal mediation analysis using income as a representative measure of SES. As shown in Figure 4, lower income significantly mediated the association of Black race with major adverse cardiac events by 45.7% (indirect effect/total effect).
Figure 4. Mediation analysis.
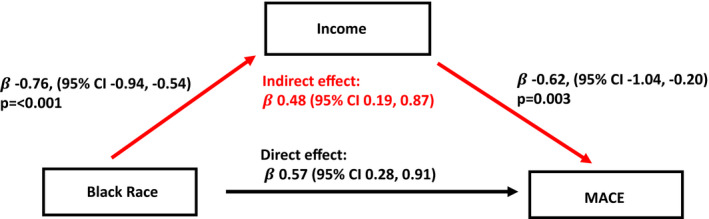
Mediation analysis linking Black race and major adverse cardiac events through income (as marker of SES). Indirect effect =−0.76×−0.62. This pathway accounted for 45.7% of the total effect (indirect effect/(indirect effect+direct effect)×100).
Discussion
In this sample of young and middle‐aged men and women with recent MI, Black MI survivors had a more than a 2‐fold increased risk of adverse cardiovascular outcomes over 5 years of follow‐up, and the excess risk was driven more by SES than by clinical risk factors. Psychological factors did not contribute to the disparity once SES factors were accounted for. The combination of SES and clinical risk factors explained most of the excess risk for Black patients with a much greater contribution of SES than clinical risk factors. A lower SES represented the dominant explanation for race‐related differences in outcome in this study; a lower income explained almost 50% of the disparity. These results highlight the importance of SES as a determinant of health among young and middle‐aged survivors of a MI and advance our understanding of the high risk for adverse outcomes faced by Black patients.
In the United States, race and SES are highly connected. However, no previous study has examined whether SES explains race‐related outcome differences after an early‐onset MI in younger individuals. Two previous studies found that SES explained a worse outcome after MI among Black than non‐Black patients in older populations. 10 , 34 A third study evaluated the relationship between race, area‐level SES (measured by zip code–level median household income from Census data), and life expectancy among Medicare beneficiaries who were hospitalized with MI, 6 and found that both Black race and low area‐level SES were independent predictors of shorter life expectancy after acute MI. The authors found that post‐MI life expectancy was shorter for Black patients than White patients across all SES levels only in patients between 65 and 75 years of age. After multivariable adjustment, only younger Black patients (<68 years) had shorter life expectancies than their White counterparts, whereas older Black patients had longer life expectancies than Whites. Thus, even though this sample was limited to patients aged ≥65 years, it highlights the fact that younger Black patients are at a disproportionately higher risk after an MI. This study also found that the largest White‐Black gap in life expectancy occurred in younger patients living in high and medium‐SES areas. In our study we found no evidence of interaction between race and SES, but we used individual‐level SES rather than area‐level SES. Consistent with our results, in another study of older patients, socioeconomic and social factors were the most important characteristics differentiating White and Black patients after an MI, and characteristics associated with Black race, including SES and social factors, but not race itself, were associated with mortality risk after MI. 34
In an effort to understand racial disparities in outcomes after MI, our study integrated robust psychological measures as these can be important mediators in the pathway connecting SES and cardiovascular outcomes. 35 , 36 , 37 Although psychological stress is a known risk factor for incident cardiovascular disease, including MI, 38 , 39 , 40 much of the previous work related to the role of psychological stress in health disparities by race has been limited to single domains of stress, such as discrimination, or to general perceived stress. Using comprehensive measures of psychological distress, we found that psychological disturbances did not contribute to disparities in outcome by race after accounting for socioeconomic factors.
An important implication of our findings is that understanding the importance of social determinants of health in relation to traditional clinical risk factors is needed if we are to overcome existing disparities in outcomes. 41 Although clinical interventions that address traditional risk factors may decrease the risk for both Black and non‐Black patients after an MI, they are unlikely to eliminate racial disparities in cardiovascular disease without concomitant interventions that address upstream SES disadvantage. Our study suggests that this may be especially true among younger patients with MI, a group in which disparities in outcome by race after an MI are largest. Addressing SES inequalities is therefore urgently needed to improve the outcomes of younger Black patients with coronary heart disease. Policy changes or interventions targeted at upstream social determinants should be prioritized, along with risk factor control, in order to ameliorate health disparities.
The present findings should be interpreted in the context of potential limitations. First, the MIMS2 study included study participants from a single institution; therefore the results may not be generalizable throughout the country. However, the location of our study within the Atlanta metropolitan area allowed us to enroll an urban patient population with large representation of young Black patients. Second, because this was an observational study, race may be a proxy of unmeasured characteristics that differ by race. However, our study collected variables in multiple domains, including SES, psychological distress, and clinical risk factors, and the combination of these factors explained outcome differences by race almost completely. Third, this study relied on self‐identified racial categories; thus, contributions of genetically determined components of race/ethnicity to outcomes could not be determined. Nonetheless, in the context of racial disparities in health outcomes and social determinants of health, self‐identified race is more relevant to consider than genetic ancestry. 42 Indeed, our results support the notion that genetic factors do not play a large role in mortality difference by race, given that the latter was largely explained by socioeconomic characteristics, which are potentially modifiable. Lastly, we did not have information on health insurance and insurance coverage for medications.
There are also important strengths to this study. To our knowledge, this is one of a few studies of race‐based differences in the outcome of MI among younger patients and the first study to examine a complex set of patient characteristics, including individual‐level SES indicators, a comprehensive psychological assessment, and detailed clinical data in explaining inequalities in outcome by race. The large number of young Black patients, the nearly equal numbers of men and women, and the broad portfolio of SES and psychological assessments make this study unique and well‐suited to explore this question.
Conclusions
In a cohort of young and middle‐aged post‐MI patients, we demonstrate that Black patients have more than a 2‐fold risk of developing adverse cardiovascular events compared to non‐Black patients. While a multitude of factors contribute to these disparities, SES indicators are major drivers of these differences. Our results underscore the importance of social determinants of health for this at‐risk population, and highlight the need to intervene in this area in order to mitigate racial disparities in the outcome of early‐onset MI.
Sources of Funding
We would like to acknowledge the support of the Byron William Jr, MD Fellowship Fund. This work was supported by the National Institutes of Health (P01HL101398, R01HL109413, R01HL109413‐02S1, UL1TR000424, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, and THL130025A, TL1TR002382). The authors of this article are solely responsible for the content of this paper. The funding agency had no role in the design and conduct of this study, in the collection, analysis, interpretation of the data, or in the preparation, review, or approval of this manuscript.
Disclosures
None.
Supporting information
Table S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020828
For Sources of Funding and Disclosures, see page 8.
References
- 1. Carnethon M, Pu J, Howard G, Albert M, Anderson C, Bertoni A, Mujahid M, Palaniappan L, Taylor H Jr, Willis M, et al. American Heart Association Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 2. Van Dyke M, Greer S, Odom E, Schieb L, Vaughan A, Kramer M, Casper M. Heart disease death rates among blacks and whites aged≥ 35 years—United States, 1968–2015. MMWR Surveill Summ. 2018;67:1. DOI: 10.15585/mmwr.ss6705a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhry SI, Khan RF, Chen J, Dharmarajan K, Dodson JA, Masoudi FA, Wang Y, Krumholz HM. National trends in recurrent ami hospitalizations 1 year after acute myocardial infarction in medicare beneficiaries: 1999–2010. J Am Heart Assoc. 2014;3:e001197. DOI: 10.1161/JAHA.114.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AfHRa Q. National Healthcare Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [Google Scholar]
- 5. Richardson WC, Berwick D, Bisgard J, Bristow L, Buck C, Cassel C; Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 6. Bucholz EM, Ma S, Normand S‐LT, Krumholz HM. Race, socioeconomic status, and life expectancy after acute myocardial infarction. Circulation. 2015;132:1338–1346. DOI: 10.1161/CIRCULATIONAHA.115.017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamad R, Penko J, Kazi DS, Coxson P, Guzman D, Wei PC, Mason A, Wang EA, Goldman L, Fiscella K, et al. Association of low socioeconomic status with premature coronary heart disease in US adults. JAMA Cardiol. 2020;5:899–908. DOI: 10.1001/jamacardio.2020.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geiger HJ. Racial and ethnic disparities in diagnosis and treatment: a review of the evidence and a consideration of causes. Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003:417. [PubMed] [Google Scholar]
- 9. Bulatao RA, Anderson NB. Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC: National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 10. Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. DOI: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding J, Roux AVD, Nieto FJ, McNamara RL, Hetmanski JB, Taylor HA Jr, Tyroler HA. Racial disparity in long‐term mortality rate after hospitalization for myocardial infarction: the Atherosclerosis Risk in Communities Study. Am Heart J. 2003;146:459–464. DOI: 10.1016/S0002-8703(03)00228-X. [DOI] [PubMed] [Google Scholar]
- 12. Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Raffeld M, et al. Gene‐expression profiles in hereditary breast cancer. N Engl J Med. 2001;344:539–548. DOI: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 13. Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. DOI: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Napan S, Kashinath R, Orig M, Kadri S, Khadra S. Racial difference in cardiovascular outcomes following percutaneous coronary intervention in a public health service patient population. J Invasive Cardiol. 2010;22:168–173. [PubMed] [Google Scholar]
- 15. Iribarren C, Tolstykh I, Somkin CP, Ackerson LM, Brown TT, Scheffler R, Syme L, Kawachi I. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165:2105–2113. DOI: 10.1001/archinte.165.18.2105. [DOI] [PubMed] [Google Scholar]
- 16. Manhapra A, Canto JG, Vaccarino V, Parsons L, Kiefe CI, Barron HV, Rogers WJ, Weaver WD, Borzak S. Relation of age and race with hospital death after acute myocardial infarction. Am Heart J. 2004;148:92–98. DOI: 10.1016/j.ahj.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17. Corti M‐C, Guralnik JM, Ferrucci L, Izmirlian G, Leveille SG, Pahor M, Cohen HJ, Pieper C, Havlik RJ. Evidence for a black‐white crossover in all‐cause and coronary heart disease mortality in an older population: the North Carolina EPESE. Am J Public Health. 1999;89:308–314. DOI: 10.2105/AJPH.89.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao L, Robert SA. Examining the racial crossover in mortality between African American and white older adults: a multilevel survival analysis of race, individual socioeconomic status, and neighborhood socioeconomic context. J Aging Res. 2011;2011:1–8. DOI: 10.4061/2011/132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pollard K, Scommegna P. The health and life expectancy of older blacks and hispanics in the United States. PRB. 2013;28:1–8. [Google Scholar]
- 20. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status and health: complexities, ongoing challenges and research opportunities. Ann N Y Acad Sci. 2010;1186:69. DOI: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, et al. Mental stress–induced‐myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137:794–805. DOI: 10.1161/CIRCULATIONAHA.117.030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. DOI: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 23. Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164:547–552.e541. DOI: 10.1016/j.ahj.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beck AT, Steer RA, Brown GK. BDI_II. Beck Depression Inventory–II. Second Edition. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 25. Blanchard EB, Jones‐Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther. 1996;34:669–673. DOI: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 26. Spielberger CD, Gorsuch RL, Lushene RE. State‐Trait Anxiety (STAI) manual. Palo Alto, CA: Psychologists Press; 1970. [Google Scholar]
- 27. Spielberger CD. State‐Trait Anger Expression Inventory: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 28. Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook‐Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. DOI: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 29. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30. Greenland S, Pearce N. Statistical foundations for model‐based adjustments. Annu Rev Public Health. 2015;36:89–108. DOI: 10.1146/annurev-publhealth-031914-122559. [DOI] [PubMed] [Google Scholar]
- 31. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;133:1341–1350. DOI: 10.1161/CIRCULATIONAHA.115.018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Lima BB, Kim JH, Garcia EV, et al. The relation of psychosocial distress with myocardial perfusion and stress‐induced myocardial ischemia. Psychosom Med. 2019;81:363. DOI: 10.1097/PSY.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pimple P, Lima BB, Hammadah M, Wilmot K, Ramadan R, Levantsevych O, Sullivan S, Kim JH, Kaseer B, Shah AJ, et al. Psychological distress and subsequent cardiovascular events in individuals with coronary artery disease. J Am Heart Assoc. 2019;8:e011866. DOI: 10.1161/JAHA.118.011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Graham GN, Jones PG, Chan PS, Arnold SV, Krumholz HM, Spertus JA. Racial disparities in patient characteristics and survival after acute myocardial infarction. JAMA Netw Open. 2018;1:e184240. DOI: 10.1001/jamanetworkopen.2018.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, et al. Stress‐associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol. 2019;73:3243–3255. DOI: 10.1016/j.jacc.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Y Acad Sci. 2010;1186:146–173. DOI: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 37. Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status–health gradient. Ann N Y Acad Sci. 2010;1186:174–189. DOI: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 38. Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐Amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 39. Cummings DM, Kirian K, Howard G, Howard V, Yuan Y, Muntner P, Kissela B, Redmond N, Judd SE, Safford MM. Consequences of comorbidity of elevated stress and/or depressive symptoms and incident cardiovascular outcomes in diabetes: results from the reasons for geographic and racial differences in stroke (REGARDS) study. Diabetes Care. 2016;39:101–109. DOI: 10.2337/dc15-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart RAH, Colquhoun DM, Marschner SL, Kirby AC, Simes J, Nestel PJ, Glozier N, O’Neil A, Oldenburg B, White HD, et al. Persistent psychological distress and mortality in patients with stable coronary artery disease. Heart. 2017;103:1860–1866. DOI: 10.1136/heartjnl-2016-311097. [DOI] [PubMed] [Google Scholar]
- 41. Spertus J. Broadening our understanding of survival after myocardial infarction: the association of neighborhood with outcomes. Circulation. 2010;121:348–350. DOI: 10.1161/CIR.0b013e3181d0b9c0. [DOI] [PubMed] [Google Scholar]
- 42. Doubeni CA, Simon M, Krist AH. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021;325:627–628. DOI: 10.1001/jama.2020.26188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


