Abstract
Background
Although several risk schemes have been proposed to predict new‐onset atrial fibrillation (AF), clinical prediction models specific for Asian patients were limited. In the present study, we aimed to develop a clinical risk score (Taiwan AF score) for AF prediction using the whole Taiwan population database with a long‐term follow‐up.
Methods and Results
Among 7 220 654 individuals aged ≥40 years without a past history of cardiac arrhythmia identified from the Taiwan Health Insurance Research Database, 438 930 incident AFs occurred after a 16‐year follow‐up. Clinical risk factors of AF were identified using Cox regression analysis and then combined into a clinical risk score (Taiwan AF score). The Taiwan AF score included age, male sex, and important comorbidities (hypertension, heart failure, coronary artery disease, end‐stage renal disease, and alcoholism) and ranged from −2 to 15. The area under the receiver operating characteristic curve of the Taiwan AF scores in the predictions of AF are 0.857 for the 1‐year follow‐up, 0.825 for the 5‐year follow‐up, 0.797 for the 10‐year follow‐up, and 0.756 for the 16‐year follow‐up. The annual risks of incident AF were 0.21%/year, 1.31%/year, and 3.37%/year for the low‐risk (score −2 to 3), intermediate‐risk (score 4 to 9), and high‐risk (score ≥10) groups, respectively. Compared with low‐risk patients, the hazard ratios of incident AF were 5.78 (95% CI, 3.76–7.75) for the intermediate‐risk group and 8.94 (95% CI, 6.47–10.80) for the high‐risk group.
Conclusions
We developed a clinical AF prediction model, the Taiwan AF score, among a large‐scale Asian cohort. The new score could help physicians to identify Asian patients at high risk of AF in whom more aggressive and frequent detections and screenings may be considered.
Keywords: atrial fibrillation, incidence, Taiwan AF score
Subject Categories: Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities Study
- CHARGE‐AF
Cohorts for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation
- FHS
Framingham Heart Study
- NHIRD
National Health Insurance Research Database
Clinical Perspective
What Is New?
The Taiwan atrial fibrillation score was derived from 7 220 654 patients with 438 930 incident atrial fibrillation events and included age, male sex, and important comorbidities (hypertension, heart failure, coronary artery disease, end‐stage renal disease, and alcoholism) and ranged from −2 to 15.
What Are the Clinical Implications?
The annual risks of incident atrial fibrillation were 0.21%/year, 1.31%/year, and 3.37%/year for the low‐risk (score −2 to 3), intermediate‐risk (score 4–9), and high‐risk (score ≥10) groups, respectively.
The new score could help physicians to identify Asian patients at high risk of atrial fibrillation in whom more aggressive and frequent detections and screenings may be considered.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia that is associated with increased risk of mortality, heart failure, ischemic stroke, and dementia. 1 The prevalence of AF is expected to rise substantially during the next few decades because of the aging population, improved public awareness, and better diagnostic tools. 2 , 3 Although the incidence and prevalence of AF are generally lower for Asian patients compared with White patients, 4 the prevalence rates of AF in Asian countries will continuously increase in parallel to that of Western countries. For example, from year 2020 to 2050, the prevalence rates of AF are projected to increase from 1.51% to 4.0% in Taiwan and from 2.1% to 5.4% in South Korea. 1 , 5 Therefore, the overall burden of patients with AF will largely grow in Asian regions, and how to identify patients at risk of AF is important.
Several risk schemes have been proposed to predict new‐onset AF, including the FHS (Framingham Heart Study) score, 6 the ARIC (Atherosclerosis Risk in Communities Study) score, 7 and the CHARGE‐AF (Cohorts for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation) score. 8 However, these scoring schemes were derived in White populations and may not be fully applied to Asian patients. Although the C2HEST score has been proposed to predict incident AF for Asian patients, there were only 921 incident AF events among 471 446 Chinese patients after a mean follow‐up of 4.1 years. 9 The incidence of AF was around 0.5/1000 person‐years in the study from which the C2HEST score was developed and is lower than that reported from Taiwan (1.51/1000 person‐years) and South Korea (1.77/1000 person‐years). 1 , 5 This previous study may be limited by selected population from certain hospitals of 1 single China province. 9 In the present study, we aimed to develop a clinical risk score (Taiwan AF score) for AF prediction using the whole Taiwan population database with long‐term follow‐up.
METHODS
Database
The authors declare that all supporting data are available within the article and its online supplementary files. This study used data from the National Health Insurance Research Database (NHIRD) provided by Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. The National Health Insurance system is a mandatory universal health insurance program that was launched on March 1, 1995, and that offers comprehensive medical care coverage to all Taiwanese residents. NHIRD consists of detailed healthcare data from >23 million enrollees, representing >99% of Taiwan’s population, from January 1, 1996, to December 31, 2016. In this cohort data set, the patients’ original identification numbers have been encrypted to protect their privacy, but the encrypting procedure was consistent so that a linkage of the claims belonging to the same patient was feasible within the National Health Insurance database and can be followed continuously. The descriptions about Taiwan NHIRD have been reported in our previous studies. 1 , 10 , 11
Study Population
From January 1, 2000, to December 31, 2000, a total of 7 220 654 patients aged ≥40 years without a past history of cardiac arrhythmias were identified from the NHIRD. Information about important comorbid conditions of each individual was retrieved from the NHIRD based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes (Table S1). The diagnostic accuracies of important comorbidities in NHIRD, such as hypertension, diabetes mellitus, heart failure, myocardial infarction, hyperlipidemia, and chronic obstructive pulmonary disease, have been validated previously. 12 , 13 AF was diagnosed using the ICD‐9‐CM code (427.31) registered by the physicians responsible for the care of patients. The diagnostic accuracy of AF based on the ICD‐9‐CM code in the Taiwan NHIRD has been validated previously. 14
Predictors of AF and the Development of Scoring Scheme
To develop the risk prediction model, general principles from the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement were followed. 15 Cox proportional hazards modeling tested each variable on the time to event of the occurrence of AF during the 16‐year follow‐up. An initial saturated Cox proportional hazards model was developed that forced all candidate variables into the model. An α level of 0.1 from the saturated model was used as a threshold to enter a variable predictor into a backward elimination model. β coefficients are presented for the final Cox regression model, with significant associations reported as hazard ratios (HRs) with 95% CIs. The score weights of each significant predictors of incident AF in the multivariable Cox regression model were derived from their β coefficients using the methods described by Sullivan et al. 16 Thereafter, the score weight for each predictor variable was rounded to its closest integer to develop the score point and the scoring scheme. After the development of the risk prediction scheme, the Taiwan AF score, we reported the incidence of AF (%/year) after 1‐year, 3‐year, 5‐year, 7‐year, 10‐year, 12‐year, and 16‐year follow‐ups for each score. Patients were classified into 3 different risk categories (low‐risk, intermediate‐risk, and high‐risk groups) based on the tertile values of the Taiwan AF score of patients who developed AF after the 16‐year follow‐up.
Statistical Analysis
Data are presented as the mean±SD or median value (interquartile range) for continuous variables and proportions for categorical variables. The differences between median values were assessed using the Wilcoxon rank‐sum test. The differences between nominal variables were compared by chi‐square test. The incidence of AF was calculated from dividing the number of events by person‐time at risk. The cumulative incidence curves of AF for different scoring strata were plotted via the Kaplan‐Meier method, with statistical significance examined by the log‐rank test. The diagnostic accuracy of the Taiwan AF score in the prediction of incident AF was assessed by calculating the C statistic based on the receiver operating characteristic curve. A bootstrap method of validation using 1000 replications was applied to the final scoring scheme. The area under the receiver operating characteristic curve (AUROC) of the Taiwan AF score was compared with other reported clinical schemes, including the CHADS2, 17 , 18 CHA2DS2‐VASc, 18 and C2HEST scores, 9 using the DeLong test. The calibration, a measure of the goodness of model fit, was assessed by comparing the observed and predicted numbers of AF events in deciles of predicted risk as calculated by the Grønnesby‐Borgan chi‐square statistic. 19 The statistical significances were set at P<0.05, and all statistical analyses were carried out by SPSS 17.0 (SPSS Inc.).
The present study was approved by the institutional review board at Taipei Veterans General Hospital, Taipei, Taiwan. Informed consent was waived because of the use of anonymous data.
RESULTS
The median age of study population was 53 years, and 48.4% of them were men (Table 1). During a 16‐year follow‐up, 438 930 patients experienced incident AF with an incidence of 0.42 per 100 person‐years. The baseline clinical characteristics of patients with or without incident AF are shown in Table 1. Generally, patients who experienced AF were older and had more comorbidities.
Table 1.
Baseline Characteristics of Patients
| Variables | All Patients, n=7 220 654 | Patients With AF, n=438 930 | Patients Without AF, n=6 781 724 | P Value |
|---|---|---|---|---|
| Age, y | 53 (46–65) | 68 (58–75) | 52 (45–64) | <0.001 |
| Male sex | 3 494 582 (48.4) | 233 562 (53.2) | 3 261 020 (48.1) | <0.001 |
| Hypertension | 1 154 853 (16.0) | 150 927 (34.4) | 1 003 926 (14.8) | <0.001 |
| Diabetes mellitus | 522 767 (7.2) | 50 023 (11.4) | 472 744 (7.0) | <0.001 |
| Heart failure | 117 232 (1.6) | 29 811 (6.8) | 87 421 (1.3) | <0.001 |
| Prior stroke | 196 291 (2.7) | 25 692 (5.9) | 170 599 (2.5) | <0.001 |
| Coronary artery diseases | 423 288 (5.9) | 72 083 (16.4) | 351 205 (5.2) | <0.001 |
| Without prior MI | 391 906 (5.4) | 66 536 (15.2) | 325 370 (4.8) | <0.001 |
| With prior MI | 31 382 (0.4) | 5547 (1.3) | 25 835 (0.4) | <0.001 |
| Peripheral vascular diseases | 24 820 (0.3) | 2993 (0.7) | 21 827 (0.3) | <0.001 |
| COPD | 343 894 (4.8) | 45 725 (10.4) | 298 169 (4.4) | <0.001 |
| Autoimmune diseases | 71 341 (1.0) | 6336 (1.4) | 65 005 (1.0) | <0.001 |
| Liver cirrhosis | 52 234 (0.7) | 3127 (0.7) | 49 107 (0.7) | 0.376 |
| Cancer | 165 367 (2.3) | 11 786 (2.7) | 153 581 (2.3) | <0.001 |
| Hyperthyroidism | 14 811 (0.2) | 1218 (0.3) | 13 593 (0.2) | <0.001 |
| CKD | 142 545 (2.0) | 15 596 (3.6) | 126 949 (1.9) | <0.001 |
| Without ESRD | 100 487 (1.4) | 11 362 (2.6) | 89 125 (1.3) | <0.001 |
| With ESRD | 42 058 (0.6) | 4234 (1.0) | 37 824 (0.6) | <0.001 |
| Gout | 246 587 (3.4) | 27 634 (6.3) | 218 953 (3.2) | <0.001 |
| Alcoholism | 34 583 (0.5) | 2101 (0.5) | 32 482 (0.5) | 0.978 |
Data are provided as median (interquartile range) or number (percentage). AF indicates atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; and MI, myocardial infarction.
Predictors of Incident AF and the Calculation Rules of the Taiwan AF Score
The significant clinical predictors of incident AF from the stepwise backward selection Cox regression model are shown in Table 2. The integer risk score, herein called the Taiwan AF score, which ranged from −2 to 15, was developed, and the calculation rules are shown in Table 3. An age group between 50 and 54 years was chosen as the reference group because the median age of the study population was 53 years. Figure 1 shows the distributions of Taiwan AF score of the study population.
Table 2.
Predictors of Incident AF
| Variables | β Coefficient | Multivariate Cox Regression Analysis | |
|---|---|---|---|
| HR (95% CI) | P Value | ||
| Age, per y | 0.077 | 1.080 (1.079–1.080) | <0.001 |
| Male sex | 0.232 | 1.261 (1.253–1.268) | <0.001 |
| Hypertension | 0.343 | 1.408 (1.398–1.419) | <0.001 |
| Diabetes mellitus | 0.082 | 1.086 (1.075–1.096) | <0.001 |
| Heart failure | 0.894 | 2.444 (2.413–2.475) | <0.001 |
| Prior stroke | 0.127 | 1.136 (1.121–1.151) | <0.001 |
| Coronary artery disease | |||
| Without MI | 0.377 | 1.457 (1.444–1.471) | <0.001 |
| With MI | 0.435 | 1.545 (1.504–1.588) | <0.001 |
| Peripheral vascular diseases | −0.038 | 0.963 (0.928–0.999) | 0.042 |
| COPD | 0.151 | 1.163 (1.151–1.175) | <0.001 |
| Autoimmune diseases | 0.071 | 1.074 (1.047–1.101) | <0.001 |
| Liver cirrhosis | 0.139 | 1.149 (1.108–1.191) | <0.001 |
| Hyperthyroidism | 0.143 | 1.153 (1.090–1.216) | <0.001 |
| CKD | |||
| Without ESRD | 0.104 | 1.109 (1.080–1.139) | <0.001 |
| With ESRD | 0.375 | 1.454 (1.419–1.490) | <0.001 |
| Gout | 0.146 | 1.158 (1.143–1.172) | <0.001 |
| Alcoholism | 0.338 | 1.402 (1.342–1.464) | <0.001 |
AF indicates atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; HR, hazard ratio; and MI, myocardial infarction.
Table 3.
Calculations of Taiwan AF Score
| Variables | Score |
|---|---|
| Age, y | |
| 40–44 | −2 |
| 45–49 | −1 |
| 50–54 | 0 |
| 55–59 | 1 |
| 60–64 | 2 |
| 65–69 | 3 |
| 70–74 | 4 |
| 75–79 | 5 |
| >80 | 8 |
| Male sex | 1 |
| Hypertension | 1 |
| Heart failure | 2 |
| Coronary artery disease | 1 |
| ESRD | 1 |
| Alcoholism | 1 |
| Total score | −2 to 15 |
AF indicates atrial fibrillation; and ESRD, end‐stage renal disease.
Figure 1. The distributions of Taiwan AF score of the study population.
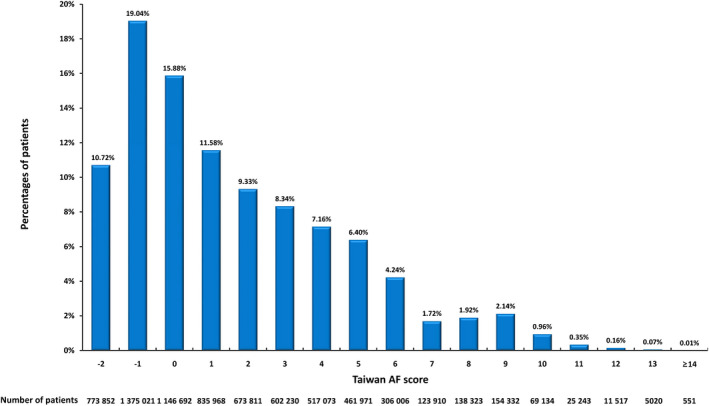
Taiwan AF score ranged from −2 to 15 with a median value of 1 (interquartile range, −1 to 5). AF indicates atrial fibrillation.
Risk of Incident AF Stratified by Taiwan AF Score
The incidences of AF (%/year) of different Taiwan AF scores with different follow‐up durations are shown in Table 4. After a 16‐year follow‐up, the risk of incident AF increased from 0.05%/year for patients with a score of −2 to 6.95%/year for those having a score ≥14. Patients were classified as low risk for score −2 to 3, intermediate risk for score 4 to 9, and high risk for score ≥10. The annual risks of AF of different scores and different risk categories based on the data of 16‐year follow‐up are shown in Figure 2. The annual risks of incident AF were 0.21%/year, 1.31%/year, and 3.37%/year for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. The cumulative incidence curves of incident AF of the low‐risk, intermediate‐risk, and high‐risk groups are shown in Figure 3. The 2‐year risks of AF were 0.08%, 2.03%, and 7.82% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. The 4‐year risks of AF were 0.31%, 4.12%, and 13.58% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. The 10‐year risks of AF were 1.26%, 11.13%, and 27.87% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. Compared with low‐risk patients, the HRs of incident AF were 5.78 (95% CI, 3.76–7.75) for the intermediate‐risk group and 8.94 (95% CI, 6.47–10.80) for the high‐risk group.
Table 4.
Incidence of AF Stratified by Taiwan AF Score After Different Follow‐Up Durations
| Follow‐Up, y | Annual Risk (%/y) of AF Stratified by Taiwan AF Score | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ≥14 | |
| 1 | 0.02 | 0.03 | 0.05 | 0.09 | 0.15 | 0.23 | 0.38 | 0.61 | 1.04 | 2.18 | 1.83 | 1.65 | 2.68 | 5.10 | 9.80 | 11.67 | 11.10 |
| 3 | 0.02 | 0.04 | 0.06 | 0.12 | 0.19 | 0.31 | 0.50 | 0.78 | 1.19 | 2.03 | 2.07 | 2.06 | 2.85 | 4.54 | 8.01 | 8.89 | 9.24 |
| 5 | 0.02 | 0.04 | 0.07 | 0.13 | 0.21 | 0.34 | 0.54 | 0.83 | 1.22 | 1.98 | 2.07 | 2.10 | 2.81 | 4.26 | 7.17 | 7.88 | 8.04 |
| 7 | 0.03 | 0.05 | 0.08 | 0.15 | 0.24 | 0.38 | 0.59 | 0.89 | 1.29 | 1.96 | 2.11 | 2.15 | 2.79 | 4.10 | 6.72 | 7.38 | 7.71 |
| 10 | 0.03 | 0.06 | 0.10 | 0.17 | 0.28 | 0.44 | 0.66 | 0.98 | 1.36 | 1.98 | 2.18 | 2.20 | 2.79 | 3.94 | 6.38 | 6.90 | 7.48 |
| 12 | 0.04 | 0.06 | 0.11 | 0.19 | 0.31 | 0.48 | 0.72 | 1.04 | 1.42 | 2.01 | 2.21 | 2.24 | 2.79 | 3.88 | 6.21 | 6.72 | 7.36 |
| 16 | 0.05 | 0.09 | 0.15 | 0.25 | 0.40 | 0.59 | 0.86 | 1.19 | 1.54 | 2.08 | 2.26 | 2.27 | 2.77 | 3.78 | 6.02 | 6.46 | 6.95 |
AF indicates atrial fibrillation.
Figure 2. The annual risks of AF of different Taiwan AF scores and different risk categories based on the data of a 16‐year follow‐up.
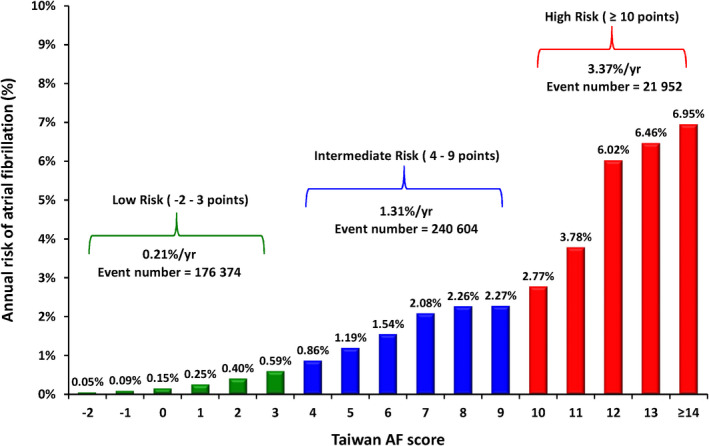
After a 16‐year follow‐up, the risk of incident AF increased from 0.05%/year for patients with a score of −2 to 6.95%/year for those having scores ≥14. Patients were classified as low risk for scores −2 to 3, intermediate risk for scores 4 to 9, and high risk for scores ≥10. The annual risks of incident AF were 0.21%/year, 1.31%/year, and 3.37%/year for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. AF indicates atrial fibrillation.
Figure 3. The cumulative incidence curves of incident AF of the low‐risk, intermediate‐risk, and high‐risk groups.
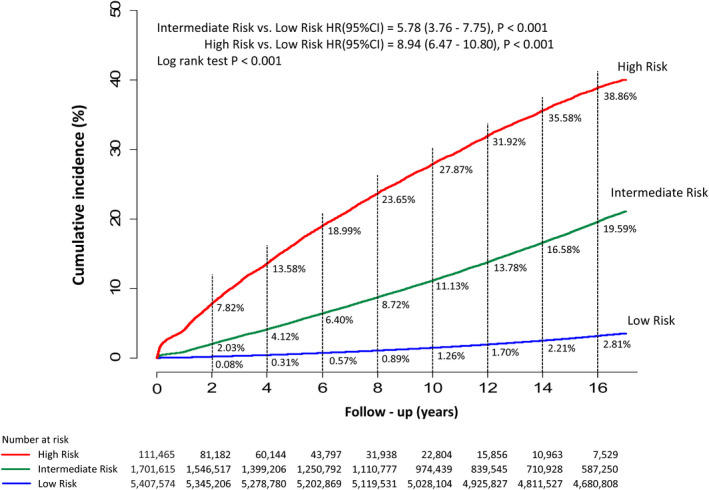
The 2‐year risks of AF were 0.08%, 2.03%, and 7.82% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. The 4‐year risks of AF were 0.31%, 4.12%, and 13.58% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. The 10‐year risks of AF were 1.26%, 11.13%, and 27.87% for the low‐risk, intermediate‐risk, and high‐risk groups, respectively. Compared with low‐risk patients, the HRs of incident AF were 5.78 (95% CI, 3.76–7.75) for the intermediate‐risk group and 8.94 (95% CI, 6.47–10.80) for the high‐risk group. AF indicates atrial fibrillation; and HR, hazard ratio.
Discrimination and Calibration of the Taiwan AF Score in the Prediction of AF
The AUROCs of the Taiwan AF score in the prediction of incident AF after different follow‐up durations are shown in Table 5. The AUROCs of the Taiwan AF scores are 0.857 (95% CI, 0.855–0.860) for the 1‐year follow‐up, 0.825 (95% CI, 0.824–0.826) for the 5‐year follow‐up, 0.797 (95% CI, 0.796–0.798) for the 10‐year follow‐up, and 0.756 (95% CI, 0.755–0.757) for the 16‐year follow‐up, which were all higher than that of other scoring schemes (all DeLong P values <0.001; Table S2). Receiver operating characteristic curves of the Taiwan AF score in the prediction of incident AF are shown in Figure S1. The model was validated internally by bootstrap with AUROCs of 0.862 (95% CI, 0.860–0.863) for the 1‐year follow‐up, 0.833 (95% CI, 0.831–0.835) for the 3‐year follow‐up, 0.830 (95% CI, 0.827–0.832) for the 5‐year follow‐up, 0.815 (95% CI, 0.814–0.816) for the 7‐year follow‐up, 0.795 (95% CI, 0.793–0.797) for the 10‐year follow‐up, and 0.755 (95% CI, 0.753–0.757) for the 16‐year follow‐up (Table 5). The AUROCs of the Taiwan AF score in the prediction of incident AF stratified by sex and age are shown in Table S3 and Table S4, respectively.
Table 5.
AUROCs of Taiwan AF Score in the Prediction of AF After Different Follow‐Up Durations
| Follow‐up Duration, y | Taiwan AF Score | Taiwan AF Score, Bootstrap | ||
|---|---|---|---|---|
| AUROC (95% CI) | P Value | AUROC (95% CI) | P Value | |
| 1 | 0.857 (0.855–0.860) | <0.001 | 0.862 (0.860–0.863) | <0.001 |
| 3 | 0.838 (0.837–0.840) | <0.001 | 0.833 (0.831–0.835) | <0.001 |
| 5 | 0.825 (0.824–0.826) | <0.001 | 0.830 (0.827–0.832) | <0.001 |
| 7 | 0.814 (0.813–0.815) | <0.001 | 0.815 (0.814–0.816) | <0.001 |
| 10 | 0.797 (0.796–0.798) | <0.001 | 0.795 (0.793–0.797) | <0.001 |
| 12 | 0.786 (0.785–0.787) | <0.001 | 0.786 (0.784–0.787) | <0.001 |
| 16 | 0.756 (0.755–0.757) | <0.001 | 0.755 (0.753–0.757) | <0.001 |
AF indicates atrial fibrillation; and AUROC, area under the receiver operating characteristic curve.
The predicted numbers of AF events in the 16‐year risk deciles were similar to the observed events (Grønnesby‐Borgan chi‐square=9.54; P=0.388). Figure S2 depicts the observed and expected risks of AF by decile of predicted risk, and the calibration was adequate (Grønnesby‐Borgan chi‐square=13.8; P=0.129).
DISCUSSION
In this nationwide cohort study, we identified clinical risk factors for new‐onset AF among 7 220 654 patients with 438 930 incident AF after a 16‐year follow‐up, and the Taiwan AF score was derived accordingly. To the best of our knowledge, it was the largest study aiming to develop a clinical risk scheme for the predictions of incident AF, especially for Asian patients.
Scoring Schemes for the Prediction of AF for Non‐Asian Patients
Several prediction schemes for AF has been proposed for non‐Asian patients, such as the FHS, ARIC, and CHARGE‐AF scores, with the AUROCs around 0.77 to 0.78. 6 , 7 , 8 The AF prediction score derived from the FHS (4764 participants with 457 incident AF), including age, body mass index, systolic blood pressure, hypertension medications, PR interval, age when cardiac murmur developed, and age of heart failure, had an AUROC of 0.78. 6 The additional incorporation of echocardiographic measurements into the FHS scheme only slightly improved the C statistic from 0.78 to 0.79 without the improvement in risk reclassification (P=0.18). 6 The CHARGE‐AF scheme was developed using individual‐level data from 3 large cohorts in the United States (ARIC study, the Cardiovascular Health Study, and the FHS), including 18 556 men and women (19% Black participants, 81% White participants) with 1186 incident AF cases in the derivation cohorts and 585 in the validation cohorts. 8 The variables included in the CHARGE‐AF model were age, race, height, weight, systolic and diastolic blood pressure, current smoking, use of antihypertensive medication, diabetes mellitus, and history of myocardial infarction and heart failure, which result in an AUROC around 0.765, and the addition of variables from the ECG did not improve the overall model discrimination. 8 The findings from the FHS and CHARG‐AF scores may suggest that a scoring scheme based on clinical factors without detailed information from the ECG and echocardiogram may be good enough for the prediction of incident AF in the clinical setting of daily practice.
Interestingly, the AUROC of the FHS score in the prediction of incident AF was only around 0.65 for Black patients in the ARIC cohort, which seems to be lower than that in the FHS cohort (AUROC=0.78). 7 It is reasonable that the FHS score performs better in the original cohort from which the scoring scheme was derived. Another possibility is that a scoring scheme for the prediction of incident AF developed for White patients may not be applied well to other races. Therefore, these preexisting scoring models may not predict incident AF for Asian patients as well as they did for non‐Asian patients.
Taiwan AF Score and the Prediction of Incident AF
In the present study, we developed an AF prediction scheme (Taiwan AF score) for Asian patients using a Taiwan nationwide database with 7 220 654 patients and 438 930 incident AF events. The AUROCs of the Taiwan AF scores are 0.857 for the 1‐year follow‐up, 0.825 for the 5‐year follow‐up, 0.797 for the 10‐year follow‐up, and 0.756 for the 16‐year follow‐up, which were higher than that of other scoring schemes, including the CHADS2, CHA2DS2‐VASc, and C2HEST scores. The Taiwan AF scheme included age, sex, and important clinical comorbidities that were significantly associated with the occurrence of incident AF. Because the Taiwan AF score is based on clinical factors with no need for ECG, echocardiogram, and laboratory evaluations, it is easy to calculate and apply in the clinical practice and allows for good identification of patients at risk for incident AF. Compared with other previously published schemes developed among the selected cohorts, 6 , 7 , 8 , 9 the present study used a nationwide Taiwanese cohort that consists of detailed healthcare data of >99% of Taiwan’s population and therefore may be less likely to have significant selection bias. A high Taiwan AF score suggesting a higher risk of incident AF may justify more aggressive and frequent evaluations and detections of AF, especially for patients with symptoms or after an ischemic stroke.
Study Limitations
There were several limitations of the present study. First, some personal information such as smoking habit, physical activity, and body mass index was not available from this nationwide registry. However, the goal of the present study was to provide a straightforward clinical scheme to estimate the risk of incident AF. Second, we were not able to compare the predictive accuracies of the Taiwan AF score to the FHS, ARIC, and CHARGE‐AF scores because some variables (eg, blood pressure and body mass index) were not recorded in our database. As we discussed in the Discussion section, scoring models derived from 1 race may not perform well among other races, and the goal of the present study was to develop a scoring scheme specific for Asian patients. However, further studies are necessary to compare the predictive accuracy of the Taiwan AF score to these published scoring schemes. Third, the diagnoses of comorbidities and alcoholism were made on the basis of ICD‐9‐CM codes registered by physicians responsible for the care of the patients without further confirmations. Although the diagnostic accuracies of important comorbidities in the Taiwan NHIRD have been validated previously, 12 , 13 the accuracy of “alcoholism” defined using ICD‐9‐CM codes may not be as accurate as that of comorbidities in the registry‐based data set, and further external validation studies using different types of databases are necessary. Lastly, the present study was performed among Chinese patients, and whether our findings could be generalized to other Asian races remains uncertain.
CONCLUSIONS
We developed a clinical prediction model, the Taiwan AF score, among 7 220 654 patients with 438 930 incident AF to assess the individual risk for Asian patients. The new score could help physicians to identify Asian patients at high risk of AF in whom more aggressive and frequent detections and screenings may be considered.
Sources of Funding
This work was supported in part by grants from the Ministry of Science and Technology (MOST 107‐2314‐B‐075‐062‐MY3), Taipei Veterans General Hospital (V108B‐015, V108B‐027, V108C‐090, V109C‐042, V109C‐186, V110C‐132, V110C‐138, V110B‐035), the Research Foundation of Cardiovascular Medicine, and the Szu‐Yuan Research Foundation of Internal Medicine, Taipei, Taiwan.
Disclosures
None.
Supporting information
Table S1–S4
Figure S1–S2
Acknowledgments
This study is based on data from the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. The interpretation and conclusions contained herein do not represent those of Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020194
For Sources of Funding and Disclosures, see page 9.
See Editorial by El Moheb and Refaat
Contributor Information
Tze‐Fan Chao, Email: eyckeyck@gmail.com.
Shih‐Ann Chen, Email: epsachen@ms41.hinet.net.
REFERENCES
- 1. Chao TF, Liu CJ, Tuan TC, Chen TJ, Hsieh MH, Lip GYH, Chen SA. Lifetime risks, projected numbers, and adverse outcomes in asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. 2018;153:453–466. DOI: 10.1016/j.chest.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2. Tse HF, Wang YJ, Ahmed Ai‐Abdullah M, Pizarro‐Borromeo AB, Chiang CE, Krittayaphong R, Singh B, Vora A, Wang CX, Zubaid M, et al. Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm. 2013;10:1082–1088. DOI: 10.1016/j.hrthm.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 3. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. DOI: 10.1038/nrcardio.2014.118. [DOI] [PubMed] [Google Scholar]
- 4. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. DOI: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, et al. 10‐year nationwide trends of the incidence, prevalence, and adverse outcomes of non‐valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. DOI: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 6. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. DOI: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:85–91. DOI: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102. DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, Wang YT, Guo YT, Lip GYH. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155:510–518. DOI: 10.1016/j.chest.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Liao JN, Chung FP, Chen TJ, et al. Age threshold for the use of non‐vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. 2019;40:1504–1514. DOI: 10.1093/eurheartj/ehy837. [DOI] [PubMed] [Google Scholar]
- 11. Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Liao JN, Chung FP, Chen TJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation. 2018;138:37–47. DOI: 10.1161/CIRCULATIONAHA.117.031658. [DOI] [PubMed] [Google Scholar]
- 12. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 13. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–242. DOI: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 14. Chang CH, Lee YC, Tsai CT, Chang SN, Chung YH, Lin MS, Lin JW, Lai MS. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. 2014;232:224–230. DOI: 10.1016/j.atherosclerosis.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 15. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. DOI: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. DOI: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 17. Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, et al. CHADS2 score and risk of new‐onset atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. 2013;168:1360–1363. DOI: 10.1016/j.ijcard.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18. Saliba W, Gronich N, Barnett‐Griness O, Rennert G. Usefulness of CHADS2 and CHA2DS2‐VASc scores in the prediction of New‐onset atrial fibrillation: a population‐based study. Am J Med. 2016;129:843–849. DOI: 10.1016/j.amjmed.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 19. Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. DOI: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Figure S1–S2


