Abstract
Background
Frailty is conceptualized as an accumulation of deficits in multiple areas and is strongly associated with the prognosis of heart failure (HF). However, the social domain of frailty is less well investigated. We prospectively evaluated the clinical characteristics and prognostic impact of social frailty (SF) in elderly patients with HF.
Methods and Results
FRAGILE‐HF (prevalence and prognostic value of physical and social frailty in geriatric patients hospitalized for heart failure) is a multicenter, prospective cohort study focusing on patients hospitalized for HF and aged ≥65 years. We defined SF by Makizako’s 5 items, which have been validated as associated with future disability. The primary end point was a composite of all‐cause death and rehospitalization because of HF. The impact of SF on all‐cause mortality alone was also evaluated. Among 1240 enrolled patients, 825 (66.5%) had SF. During the 1‐year observation period after discharge, the rates of the combined end point and all‐cause mortality were significantly higher in patients with SF than in those without SF (Log‐rank test: both P < 0.05). SF remained as significantly associated with both the combined end point (hazard ratio, 1.30; 95% CI, 1.02–1.66; P = 0.038) and all‐cause mortality (hazard ratio, 1.53; 95% CI, 1.01–2.30; P = 0.044), even after adjusting for key clinical risk factors. Furthermore, SF showed significant incremental prognostic value over known risk factors for both the combined end point (net‐reclassification improvement: 0.189, 95% CI, 0.063–0.316, P = 0.003) and all‐cause mortality (net‐reclassification improvement: 0.234, 95% CI, 0.073–0.395, P = 0.004).
Conclusions
Among hospitalized geriatric patients with HF, two thirds have SF. Evaluating SF provides additive prognostic information in elderly patients with HF.
Registration
URL: https://upload.umin.ac.jp/. Unique identifier: UMIN000023929.
Keywords: aging, heart failure, social frailty
Subject Categories: Heart Failure, Aging, Lifestyle
Nonstandard Abbreviations and Acronyms
- SF
social frailty
Clinical Perspective
What Is New?
Social frailty is prevalent among patients hospitalized for heart failure and aged ≥65 years (66.5%).
Information on social frailty yielded incremental prognostic values to known risk factors.
What Are the Clinical Implications?
Our study results suggest a prognostic role of social frailty in patients with heart failure and support the feasibility of the evaluation of social frailty in elderly patients with heart failure using this simple instrument in daily clinical practice.
Frailty is an important clinical syndrome that becomes more common with age. Frailty is recognized as a biological status associated with multiple declines in physiologic reserves and increased vulnerability to stressors, resulting in an increased risk of adverse clinical outcomes, including disability, hospitalization, and death. 1 , 2 , 3 , 4 Frailty is of particular relevance in heart failure (HF), because frailty and HF share aging as a predisposing factor, and, simultaneously, both conditions are strongly associated with systemic multisystem dysfunction. Furthermore, numerous studies have demonstrated the prognostic impact of frailty in patients with HF. 5 , 6 Although frailty is conceptualized as an accumulation of deficits in multiple areas, 7 the social domain of frailty is one of the least investigated domains. 8 , 9 As social activity frequently requires the integration of physical and mental capacities, social frailty (SF) possibly develops at a relatively early stage in the progressive trajectory of frailty. Indeed, 1 observational study, comprising community‐dwelling older people, showed that SF leads to future declines in physical and cognitive function. 10 Nonetheless, most studies on frailty in patients with HF have not focused on SF; consequently, the data on SF are limited. We recently reported that the number of expressed frailty domains (including SF) was associated with the prognosis in elderly patients with HF. However, the clinical characteristics of those with SF and the prognostic implications of SF in elderly patients with HF have not been well described. Moreover, it remains unclear whether SF provides additive prognostic impact to pre‐existing prognostic factors of HF. Therefore, we sought to detail the prevalence, clinical characteristics, and prognostic implication of SF in elderly hospitalized patients with HF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Patient Population
We performed a post hoc analysis of the FRAGILE‐HF (prevalence and prognostic value of physical and social frailty in geriatric patients hospitalized for heart failure) cohort study, which comprised 1332 hospitalized patients with decompensation of HF who were aged ≥65 years and could ambulate at discharge. The study design and main results have been published elsewhere. 11 Briefly, the main objective of the FRAGILE‐HF study was to evaluate the prevalence and prognostic impact of multifrailty domains in elderly patients with HF who require hospitalization. Exclusion criteria were as follows: previous heart transplantation or treatment with a left ventricular assist device; on either chronic peritoneal dialysis or hemodialysis; and acute myocarditis. Patients with missing brain natriuretic peptide (BNP) or N‐terminal proBNP data, and patients with a BNP level <100 pg/mL or N‐terminal proBNP level <300 pg/mL at admission were also excluded, because the admitting diagnosis could be inappropriate. We enrolled patients with reduced or preserved ejection fraction. Fifteen hospitals in Japan registered patients from September 2016 to March 2018.
All participants were notified regarding their participation in the study, and it was explained that they were free to opt out of participation at any time of the study period. The study was conducted in compliance with the Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research involving Human Subjects. Since this was an observational study without invasive procedures or interventions, written informed consent was not required under the Ethical Guidelines for Medical and Health Research Involving Human Subjects, issued by the Japanese Ministry of Health, Labor, and Welfare. The study protocol was approved by the ethics committee of each participating hospital. Study information, including the objectives, inclusion and exclusion criteria, primary outcome, and names of the participating hospitals, were published in the publicly available University hospital Medical Information Network (UMIN‐CTR, unique identifier: UMIN000023929) before the first patient was enrolled.
Evaluation and Definition of SF
Social frailty was evaluated before discharge using 5 questions that were proposed by Makizako et al, 12 as shown in Table S1. The following responses were considered positive for SF: (1) going out less frequently compared with last year; (2) not visiting friends; (3) not talking with someone every day; (4) not feeling helpful toward friends or family; and (5) living alone. This questionnaire was originally derived from community‐dwelling older adults (≥65 years old), and SF defined by this questionnaire has been shown to be associated with future disability. 12 We divided the study population into 2 groups: those with 2 or more positive criteria responses comprised the SF group, and those with none or 1 criterion response comprised the Non‐SF group, in accordance with the study by Makizako et al. 12
Outcomes
Data regarding the prognosis of registered patients within 1 year after discharge were prospectively collected up to March 2019. The predefined primary clinical outcome was a composite of death from any cause and rehospitalization because of HF, and the secondary outcome was all‐cause mortality alone. We defined readmission events as HF readmission only if the criteria for HF readmission described in the American College of Cardiology/American Heart Association Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials were fulfilled. 13 After discharge, patients were followed up in outpatient clinics at least every 3 months, as well as according to their medical needs. For those without follow‐up in clinics, prognostic data were obtained by telephone interviews with those in charge of the patient’s medical records at other medical facilities, or with the family.
Statistical Analysis
Data are expressed as the mean and standard deviation for normally distributed variables, and as the median with interquartile range for non‐normally distributed data. Categorical data are expressed as numbers and percentages. Non‐normally distributed variables were transformed into the logarithmic scale for further analyses. Group differences were evaluated using the Student t test or Mann–Whitney U test for continuous variables and the χ2 or Fisher exact test for categorical variables, as appropriate.
Event‐free survival curves were constructed using the Kaplan–Meier survival method and were compared with log‐rank statistics. For the outcome of the combined event of death from any cause and HF readmission, we selected the variables of age; sex; left ventricular ejection fraction; current smoking status; history of HF, hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, and atrial fibrillation; systolic blood pressure; estimated glomerular filtration rate; hemoglobin; serum sodium level; serum albumin; log‐transformed BNP; prescriptions of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, beta blocker, and mineralocorticoid receptor antagonist; and New York Heart Association classification III/IV at discharge as pre‐existing prognostic factors for adjustment in a multivariable model. We selected these variables according to their clinical importance and based on previous studies.
Regarding all‐cause mortality as the secondary end point, the Meta‐analysis Global Group in Chronic Heart Failure risk score was calculated for each patient as previously described. 14 The discrimination and calibration of this risk score have been well validated in Japanese patients with HF. 15 , 16 Given that adding the BNP level at discharge has been shown to be associated with discrimination improvement, with adequate calibration, 15 we used the Meta‐analysis Global Group in Chronic Heart Failure risk score and (log‐transformed) BNP as adjustment variables in a multivariable prognostic model for the outcome of all‐cause mortality.
To evaluate whether information on SF provides incremental prognostic value over that for known risk factors, we constructed 2 models: a baseline model incorporating pre‐existing risk factors, and a model incorporating the variables of the baseline model plus the presence/absence of SF. For the outcome of combined end point, the baseline model was constructed using all variables used for adjustment in the abovementioned multivariable model. For the end point of all‐cause mortality, the baseline model was built using the Meta‐analysis Global Group in Chronic Heart Failure score and log‐transformed BNP. For each outcome, we compared the area under the curve between the 2 models and calculated the continuous net‐reclassification improvement achieved by adding SF information to the baseline model. 17 A 2‐tailed P value <0.05 was considered statistically significant. Statistical analyses were performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3‐900051‐07‐0, URL: http://www.R‐project.org).
Results
Prevalence of SF and Patient Characteristics
Among 1332 patients enrolled in the FRAGILE‐HF cohort study, 1240 patients (93.1%) successfully answered all of Makizako’s questions and were analyzed. The number of patients who provided positive answers to each question and the distribution of positive responses are shown in Table S1 and Table S2, respectively. Table 1 shows the patients’ baseline profiles. Among the enrolled patients, 825 (66.5%) had SF. Patients with SF were significantly older, more likely to be living alone, and had a higher prevalence of New York Heart Association classification III/IV, higher heart rate, lower hemoglobin, lower albumin, and poorer renal function at discharge than those without SF. However, the body mass index and BNP level at discharge were comparable between the groups. Prescriptions of HF drugs also did not significantly differ between the groups.
Table 1.
Baseline Patient Profiles
| Variables | Non‐SF Group | SF Group | P Value |
|---|---|---|---|
| N = 415 | N = 825 | ||
| Age, y | 79 [73–85] | 82 [76‐87] | <0.001 |
| Male sex, % | 235 (56.6) | 478 (57.9) | 0.704 |
| Living status | |||
| Living with someone | 378 (91.1) | 561 (68.0) | <0.001 |
| Living alone | 30 (7.2) | 231 (28.0) | |
| Living in nursing home | 7 (1.7) | 33 (4.0) | |
| NYHA Class III/IV, % | 37 (8.9) | 135 (16.4) | <0.001 |
| BMI | 21.5 ± 3.6 | 21.3 ± 3.9 | 0.495 |
| Systolic blood pressure, mm Hg | 114 ± 16 | 114 ± 17 | 0.996 |
| Diastolic blood pressure, mm Hg | 62 ± 10 | 62 ± 11 | 0.556 |
| Heart rate, bpm | 70 ± 14 | 72 ± 14 | 0.03 |
| LVEF, % | 46 ± 17 | 46 ± 17 | 0.504 |
| History of heart failure, % | |||
| None | 209 (50.5) | 350 (42.4) | 0.025 |
| Less than 18 mo | 61 (14.7) | 134 (16.2) | |
| More than 18 mo | 144 (34.8) | 341 (41.3) | |
| Comorbidities, % | |||
| Atrial fibrillation | 187 (45.1) | 363 (44.0) | 0.769 |
| Coronary artery disease | 143 (34.5) | 297 (36.0) | 0.636 |
| COPD | 50 (12.0) | 83 (10.1) | 0.332 |
| Diabetes mellitus | 143 (34.5) | 295 (35.8) | 0.697 |
| Hypertension | 294 (70.8) | 584 (70.8) | >0.99 |
| Prescription of medications, % | |||
| ACE‐I/ARB | 282 (68.0) | 554 (67.2) | 0.826 |
| Beta blocker | 318 (76.6) | 592 (71.8) | 0.078 |
| MRA | 36 (8.7) | 69 (8.4) | 0.938 |
| Laboratory data at discharge | |||
| Hemoglobin, g/dL | 12.2 ± 2.0 | 11.7 ± 2.0 | <0.001 |
| Hematocrit, % | 37.2 ± 5.9 | 35.9 ± 5.8 | <0.001 |
| Albumin, g/dL | 3.5 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| Creatinine, mg/dL | 1.3 ± 0.8 | 1.4 ± 0.8 | 0.019 |
| eGFR, mL/min per 1.73m2 | 56.5 ± 22.3 | 51.3 ± 21.5 | <0.001 |
| BUN, mg/dL | 24 [19‐34] | 27 [20‐38] | 0.003 |
| Sodium, mEq/L | 139 ± 4 | 139 ± 4 | 0.060 |
| BNP, pg/mL | 251 [129‐469] | 282 [139‐499] | 0.268 |
ACE‐I indicates angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; and SF, social frailty.
Association Between SF and Prognosis
Because we could not obtain follow‐up data for 28 patients (2.3%), 1212 patients were analyzed for the impact of SF on the prognosis. Kaplan–Meier curves for the composite of death from any cause and HF readmission showed a significantly higher rate in the SF group than in the Non‐SF group during the 1‐year observation period after discharge (Log‐rank test, P < 0.001) (Figure, left panel). Likewise, all‐cause mortality was significantly higher in the SF group than in the Non‐SF group during the 1‐year observation period (P = 0.013) (Figure, right panel). Univariate Cox regression hazard modeling showed a significantly higher hazard ratio (HR) for the composite of death from any cause and HF readmission in the SF group than in the Non‐SF group (HR, 1.46; 95% CI, 1.17–1.82, P < 0.001) (Table 2). This finding persisted even after adjusting for diverse covariates on multivariable analysis (HR, 1.30; 95% CI, 1.02–1.66, P = 0.038). In terms of all‐cause mortality, the univariate analysis showed a significantly higher HR in the SF group than in the Non‐SF group (HR, 1.61; 95% CI, 1.10–2.34, P = 0.014) (Table 2). This finding persisted after the adjustment for the Meta‐analysis Global Group in Chronic Heart Failure risk score and log‐transformed BNP on multivariable analysis (HR, 1.53; 95% CI, 1.01–2.30, P = 0.044).
Figure 1. Kaplan–Meier curves for the composite end point (left panel) and all‐cause mortality (right panel).
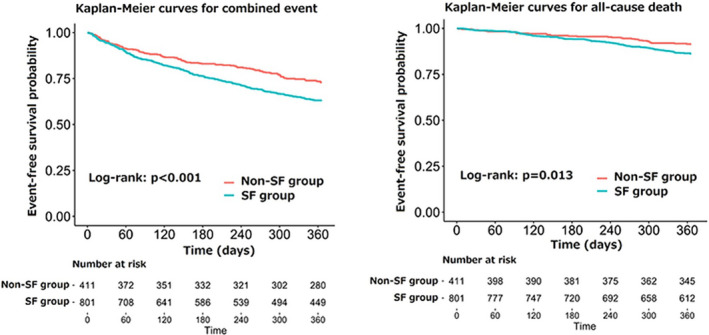
Kaplan–Meier curves for the composite end point (left panel) and all‐cause mortality (right panel) are shown for patients with SF (blue line) and those without SF (red line). SF indicates social frailty.
Table 2.
Cox Regression Models for the Combined Event and All‐Cause Mortality
| Group | Combined Event | All‐Cause Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted † | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Non‐SF group | Reference | Reference | Reference | Reference | ||||||||
| SF group | 1.46 | 1.17–1.82 | <0.001 | 1.30 | 1.02–1.66 | 0.038 | 1.61 | 1.10–2.34 | 0.014 | 1.53 | 1.01–2.30 | 0.044 |
HR indicates hazard ratio; and SF, social frailty.
Adjusted for age, estimated glomerular filtration rate, male sex, New York Heart Association III/IV, systolic blood pressure, left ventricular ejection fraction, history of atrial fibrillation, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, heart failure, and hypertension, smoking status, albumin, hemoglobin, sodium level, and log‐transformed B‐type natriuretic peptide at discharge, prescription of angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, beta blocker, and mineralocorticoid receptor antagonist at discharge.
Adjusted for Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) risk score and log‐transformed brain natriuretic peptide at discharge.
When information on SF was added to the baseline risk model (including known risk factors) for the combined end point, the area under the curve was numerically, but not significantly, increased from 0.727 (95% CI, 0.695–0.759) to 0.733 (95% CI, 0.696–0.762) (P = 0.544). However, a statistically significant incremental prognostic value of SF was shown in terms of the net‐reclassification improvement (0.189 [95% CI, 0.063–0.316], P = 0.003) (Table 3). Regarding all‐cause mortality, SF was associated with an increase in the area under the curve from 0.721(95% CI, 0.675–0.767) to 0.733 (95% CI, 0.670–0.766) (P = 0.598), and SF showed significant incremental prognostic value (net‐reclassification improvement: 0.234, 95% CI, 0.073–0.395, P = 0.004).
Table 3.
Comparisons of Predictive Ability Between the Baseline Model and the Model Incorporating the Presence/Absence of Social Frailty for the Combined End Point and All‐Cause Mortality
| Models | AUC | AUC Comparison | NRI |
|---|---|---|---|
| Combined event | |||
| Baseline model | 0.727 [95% CI, 0.695–0.759] | P = 0.544 |
0.189 [0.063–0.316], P = 0.003 |
| Baseline model + SF | 0.733 [95% CI, 0.696–0.762] | ||
| All‐cause mortality | |||
| MAGGIC + Log BNP | 0.721 [95% CI, 0.675–0.767] | P = 0.598 |
0.234 [0.073–0.395], P = 0.004 |
| MAGGIC + Log BNP+ SF | 0.733 [95% CI, 0.670–0.766] | ||
AUC indicates area under the curve; BNP, brain natriuretic peptide; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; NRI, net‐reclassification improvement; and SF, social frailty.
Discussion
In the present study, we investigated the relationships between SF and the prognosis after discharge in elderly patients with HF. We found that approximately two thirds of patients with HF aged ≥65 years had SF as assessed by 5 simple questions. Those with SF were older and more symptomatic at baseline, and had a poorer prognosis than those without SF. Furthermore, the results demonstrated that information regarding the presence of SF possesses an additive prognostic value over that of pre‐existing factors. Given its prevalence and prognostic value, a routine evaluation of SF should be implemented in daily clinical practice.
Impact of Social Issues on Clinical Outcomes
Several previous studies focused on the prevalence and prognostic impact of a weak social network in patients with HF; however, the results were not consistent. 18 , 19 , 20 One study evaluated SF in 371 patients with HF using a 4‐item questionnaire and did not find an association between SF and mortality after discharge within a short follow‐up period of 6 months. 19 Potential reasons for the nonobservation of an association include the relatively small sample size and short‐term follow‐up. Indeed, we followed up for 1 year after discharge, with a good follow‐up rate, and found consistent associations between SF and a poor prognosis, in terms of both the combined event and all‐cause mortality, independent of other known risk factors. The differences between patients with and without SF began to be evident at 2 months after discharge for the combined end point and at 6 months after discharge for all‐cause mortality. As the impact of social and environmental issues on clinical outcomes may become apparent at a later timepoint, a longer observation period may be needed to determine whether SF affects the clinical outcomes in the targeted population.
Another study investigated the prevalence and prognostic impact of social isolation in 1681 patients with HF identified by International Classification of Diseases, Ninth Revision codes. 21 Eligible patients were asked to respond to a 4‐item survey. Among enrolled patients who answered all questions, ≈25% were classified as socially isolated, and social isolation was associated with a higher risk of an emergency department visit, HF hospitalization, and death. Although these results are consistent with our findings in terms of a high vulnerability in those with social issues, the prevalence of social isolation seems to be significantly lower than that for SF in the present study. This may, of course, be attributable to differences in the questionnaires used. However, it could also be that those with social isolation are less likely to voluntarily respond to an invitation to participate in the first place, and consequently the prevalence of social isolation may have been underestimated in the previous study. SF is not a well‐explored concept; therefore, in order to better understand SF, a broad and systematic evaluation of existing insights is needed. 22 From this perspective, 1 of the strengths of our study is that most of the patients in the entire cohort (93.1%) completed the evaluations of SF.
Regarding the assessment of SF, we used a questionnaire that has already been verified to be associated with the risk of future disability in community‐dwelling elderly adults without disability. 12 In the present study, we demonstrated that SF as evaluated by Makizako’s 5 items was significantly associated with both all‐cause mortality and a composite of death from any cause and hospitalization because of HF in a prospective cohort study. This finding expands our understanding regarding the prevalence and prognostic role of SF in patients with HF and supports the feasibility of the evaluation of SF in elderly patients with HF using this simple instrument in daily clinical practice.
SF Interventions
As a future perspective, it should be noted that SF is an intervenable parameter. Indeed, 1 previous randomized study showed that a group‐based social support program could enhance social connections. 23 Facilitations provided during the index hospitalization for the transition from the hospital to the home, as seamless support, and the subsequent intentional establishment of social connections between discharged patients and their community, might be effective in decreasing mortality and preventing rehospitalization because of HF. More specifically, information technology, such as communication via smart‐phone applications, video calling, and social networking services, etc., may be promising intervention options. Additionally, a previous randomized clinical trial showed that a multicomponent exercise program, comprising a combined program of endurance, strength, coordination, balance, and flexibility exercises, improved not only physical performance, but also cognitive, emotional, and social networking parameters. 24 This might imply that frailty domains, including SF, do not occur in isolation and further prospective trials directly examining the efficacy of interventions on SF are warranted. Moreover, a previous study showed that those with SF but not physical frailty are potentially at greater risk of developing physical frailty in the near future. 25 Hence, screening for SF is important, because it potentially can identify frail older adults who are not captured otherwise. However, this hypothesis should be tested in future studies.
Study Limitations
The present study has several limitations that should be acknowledged. First, this was an observational study with a fairly large number of patients, but with a limited follow‐up period. Second, SF could be associated with different cultures or residential countries. However, previous studies have shown that ethnicity may play a limited role in frailty pathways, 26 because frailty has been associated with adverse outcomes, irrespective of race or poverty status. 27 Moreover, in 1 meta‐analysis evaluating the clinical impact of social isolation in patients with HF, including Japanese patients, the association between clinical outcomes and social isolation was shown, irrespective of race or ethnicity. 28 Nevertheless, the findings of our current study need to be validated in patients with HF who have different cultural backgrounds. Third, some (but not many) patients were excluded because of missing data on SF, which is potentially associated with a selection bias. Moreover, we also excluded those who could not ambulate by study inclusion criteria; thus, our study results may not be applicable to such populations.
Conclusions
SF is prevalent among elderly patients with HF and is also significantly associated with death from any cause and hospitalization because of HF. Evaluating and identifying those with SF provides additive prognostic information over that of pre‐existing risk factors. Future studies are warranted to determine whether SF interventions can impact other frailty domains and the prognosis of this high‐risk population.
Sources of Funding
FRAGILE‐HF was supported by Novartis Pharma Research Grants and Japan Heart Foundation Research Grant. This work was also partially supported by JSPS KAKENHI Grant Number 18K15862.
Disclosure
Dr. Yuya Matsue and Dr. Takatoshi Kasai are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi, and Dr. Yuya Matsue received an honorarium from Otsuka Pharmaceutical Co and Novartis Japan. Dr. Kagiyama reports grants from Philips, grants from Asahi KASEI Corporation, grants from Toho Holdings Co. Ltd, and grants from Inter Reha Co. Ltd outside the submitted work. Dr. Kamiya has received research funding from Eiken Chemical Co., Ltd. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
(J Am Heart Assoc. 2021;10:e019954. DOI: 10.1161/JAHA.120.019954.)
For Sources of Funding, see page 7.
See Editorial by Keshvani and Pandey
References
- 1. Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34:432–434. DOI: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. DOI: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 3. Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. DOI: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. DOI: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph SM, Rich MW. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med. 2017;19:31. DOI: 10.1007/s11936-017-0527-5. [DOI] [PubMed] [Google Scholar]
- 6. Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: implications for management. Card Fail Rev. 2018;4:104–106. DOI: 10.15420/cfr.2018.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. DOI: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 8. Hogan DB, MacKnight C, Bergman H. Steering committee CIoF and aging. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29. [PubMed] [Google Scholar]
- 9. Levers MJ, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56:282–291. DOI: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- 10. Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, Suzuki T, Shimada H. Association of social frailty with both cognitive and physical deficits among older people. J Am Med Dir Assoc. 2017;18(7):603–607. DOI: 10.1016/j.jamda.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail. 2020;22:2112–2119. DOI: 10.1002/ejhf.1926. [DOI] [PubMed] [Google Scholar]
- 12. Makizako H, Shimada H, Tsutsumimoto K, Lee S, Doi T, Nakakubo S, Hotta R, Suzuki T. Social frailty in community‐dwelling older adults as a risk factor for disability. J Am Med Dir Assoc. 2015;16(11):1003.e7–1003.e11. DOI: 10.1016/j.jamda.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 13. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: A Report of the AMERICAN college of cardiology/American heart association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation. 2015;132:302–361. DOI: 10.1161/CIR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 14. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Doughty RN and meta‐analysis global group in chronic heart F. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. DOI: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 15. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018;5:610–619. DOI: 10.1002/ehf2.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamaguchi T, Kitai T, Miyamoto T, Kagiyama N, Okumura T, Kida K, Oishi S, Akiyama E, Suzuki S, Yamamoto M, et al. Effect of optimizing guideline‐directed medical therapy before discharge on mortality and heart failure readmission in patients hospitalized with heart failure with reduced ejection fraction. Am J Cardiol. 2018;121:969–974. DOI: 10.1016/j.amjcard.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 17. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. DOI: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huynh QL, Saito M, Blizzard CL, Eskandari M, Johnson B, Adabi G, Hawson J, Negishi K, Marwick TH, Huynh Q, et al. Roles of nonclinical and clinical data in prediction of 30‐day rehospitalization or death among heart failure patients. J Card Fail. 2015;21:374–381. DOI: 10.1016/j.cardfail.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez‐Artalejo F, Guallar‐Castillon P, Herrera MC, Otero CM, Chiva MO, Ochoa CC, Banegas JR, Pascual CR. Social network as a predictor of hospital readmission and mortality among older patients with heart failure. J Card Fail. 2006;12:621–627. DOI: 10.1016/j.cardfail.2006.06.471. [DOI] [PubMed] [Google Scholar]
- 20. Park H, Jang IY, Lee HY, Jung HW, Lee E, Kim DH. Screening value of social frailty and its association with physical frailty and disability in community‐dwelling older Koreans: Aging study of PyeongChang rural area. Int J Environ Res Public Health. 2019;16:2809. DOI: 10.3390/ijerph16162809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manemann SM, Chamberlain AM, Roger VL, Griffin JM, Boyd CM, Cudjoe TKM, Jensen D, Weston SA, Fabbri M, Jiang R, et al. Perceived social isolation and outcomes in patients with heart failure. J Am Heart Assoc. 2018;7:e008069. DOI: 10.1161/JAHA.117.008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen JSM. Social frailty in older adults: A scoping review. Eur J Ageing. 2017;14:323–334. DOI: 10.1007/s10433-017-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saito T, Kai I, Takizawa A. Effects of a program to prevent social isolation on loneliness, depression, and subjective well‐being of older adults: a randomized trial among older migrants in Japan. Arch Gerontol Geriatr. 2012;55:539–547. DOI: 10.1016/j.archger.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24. Tarazona‐Santabalbina FJ, Gomez‐Cabrera MC, Perez‐Ros P, Martinez‐Arnau FM, Cabo H, Tsaparas K, Salvador‐Pascual A, Rodriguez‐Manas L, Vina J. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community‐dwelling frail elderly: A randomized clinical trial. J Am Med Dir Assoc. 2016;17:426–433. DOI: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 25. Makizako H, Shimada H, Doi T, Tsutsumimoto K, Hotta R, Nakakubo S, Makino K, Lee S. Social frailty leads to the development of physical frailty among physically non‐frail adults: a four‐year follow‐up longitudinal cohort study. Int J Environ Res Public Health. 2018;15:490. DOI: 10.3390/ijerph15030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Majid Z, Welch C, Davies J, Jackson T. Global frailty: the role of ethnicity, migration and socioeconomic factors. Maturitas. 2020;139:33–41. DOI: 10.1016/j.maturitas.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Griffin FR, Mode NA, Ejiogu N, Zonderman AB, Evans MK. Frailty in a racially and socioeconomically diverse sample of middle‐aged Americans in Baltimore. PLoS One. 2018;13:e0195637. DOI: 10.1371/journal.pone.0195637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heidari Gorji MA, Fatahian A, Farsavian A. The impact of perceived and objective social isolation on hospital readmission in patients with heart failure: a systematic review and meta‐analysis of observational studies. Gen Hosp Psychiatry. 2019;60:27–36. DOI: 10.1016/j.genhosppsych.2019.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


