Abstract
Background
Echocardiography is considered the cornerstone of the diagnostic workup of heart failure with preserved ejection fraction. Thus far, validation of the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) echo‐algorithm for evaluation of diastolic (dys)function in a patient suspected of heart failure with preserved ejection fraction has been limited.
Methods and Results
The diagnostic performance of the 2016 ASE/EACVI algorithm was assessed in 204 patients evaluated for unexplained dyspnea or pulmonary hypertension with echocardiogram and right heart catheterization. Invasively measured pulmonary capillary wedge pressure (PCWP) was used as the gold standard. In addition, the diagnostic performance of H2FPEF score and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were evaluated. There was a poor correlation between indexed left atrial volume, E/e′ (septal and average) or early mitral inflow (E), and PCWP (r=0.25–0.30, P values all <0.01). No correlation was found in our cohort between e′ (septal or lateral) or tricuspid valve regurgitation and PCWP. The correlation between diastolic function grades of the ASE/EACVI algorithm and PCWP was poor (r=0.17, P<0.05). The ASE/EACVI algorithm had a sensitivity and specificity of 35% and 87%, respectively; an accuracy of 67% and an area under the curve of 0.56. Moreover, in 30% of cases the algorithm was not applicable or indeterminate. H2FPEF score had a modest correlation with PCWP (r=0.44, P<0.0001), and accuracy was 73%; NT‐proBNP correlated weakly with PCWP (r=0.24, P<0.001), and accuracy was 57%.
Conclusions
The 2016 ASE/EACVI algorithm for the assessment of diastolic function has a limited diagnostic accuracy in patients evaluated for unexplained dyspnea and/or pulmonary hypertension, and especially sensitivity to detect diastolic dysfunction was low.
Keywords: diagnostics, echocardiography, heart failure with preserved ejection fraction, right heart catheterization
Subject Categories: Diagnostic Testing, Echocardiography, Heart Failure
Nonstandard Abbreviations and Acronyms
- ASE
American Society of Echocardiography
- EACVI
European Association of Cardiovascular Imaging
- HFpEF
heart failure with preserved ejection fraction
- LAVi
maximal left atrial volume indexed for body surface area
- LAVi‐min
minimal left atrial volume indexed for body surface area
- PCWP
pulmonary capillary wedge pressure
- PH
pulmonary hypertension
- RHC
right heart catheterization
- TRV
tricuspid regurgitation peak velocity
Clinical Perspective
What Is New?
The American Society of Echocardiography/European Association of Cardiovascular Imaging algorithm has a low sensitivity (35%) to detect elevated filling pressures in patients with unexplained dyspnea and a preserved left ventricular ejection fraction.
Individual echo‐parameters of the algorithm (E/e′, e′, indexed left atrial volume, and tricuspid valve regurgitation) have no significant correlations or only modest correlations with invasively measured filling pressures.
What Are the Clinical Implications?
Normal or grade I diastolic dysfunction on echocardiogram does not rule out relevant diastolic dysfunction in a symptomatic patient.
In case of unexplained dyspnea, other diagnostic tools (eg, H2FPEF score, exercise echocardiography, or right heart catheterization) should be considered to diagnose or rule out heart failure with preserved ejection fraction.
In our aging population, the incidence of heart failure with preserved ejection fraction (HFpEF) is rising and it is soon expected to be the predominant phenotype in heart failure. 1 Guidelines position echocardiography at the center of the diagnostic workup in patients suspected for HFpEF. 2 , 3 Echocardiography is a powerful tool to detect systolic dysfunction or valvular abnormalities. However, left ventricular (LV) diastolic dysfunction, the hallmark feature of HFpEF, is more difficult to assess with echocardiography, especially early in the disease.
It is important to determine whether echocardiography is sensitive and specific enough to diagnose diastolic dysfunction in patient with a preserved ejection fraction. Knowing the strengths and pitfalls of our diagnostic instruments will allow for a critical appraisal of our diagnostic workup and a cordate use of advanced diagnostic tools, such as exercise echocardiogram and right heart catheterization (RHC) in the setting of unexplained dyspnea and/or pulmonary hypertension (PH). 4
Over 20 parameters have been suggested to noninvasively assess LV compliance and LV filling pressures. 2 In 2016, the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) proposed a simple algorithm to assess LV diastology. 2 The algorithm has demonstrated a moderate to high diagnostic accuracy in assessing LV filling pressures in patients evaluated for coronary artery disease and known myocardial disease, when validated against invasively measured LV filling pressures as the gold standard. 5 , 6 , 7 , 8 However, thus far validation of the echo‐parameters and the echo‐algorithm specifically for patients suspected for HFpEF has been limited. 9 , 10 , 11
The present paper examines the diagnostic performance of the 2016 ASE/EACVI algorithm in a large cohort of patients that are referred to a tertiary center for unexplained dyspnea and/or PH, using invasively measured pulmonary capillary wedge pressure (PCWP) as reference. In addition, this paper examines the diagnostic performance of other commonly used diagnostic tools, including NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and the H2FPEF score, a recently developed clinical score to predict the likelihood of HFpEF. 12
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We retrospectively analyzed all patients evaluated for unexplained dyspnea and/or PH between 2015 and 2017. Patients were included if both RHC and trans‐thoracic echocardiography (with complete evaluation of diastolic function) were performed in our center during the diagnostic workup (typically the same day or the day before/after). Because of a low incidence of elevated filling pressures in the initial cohort, the cohort was complemented with additional cases with PCWP ≥15 mm Hg at rest between 2011 and 2014 and 2018 and 2020.
Patients with other cardiac disease (eg, LV ejection fraction (LVEF) <50%, relevant left‐sided valvular pathology, congenital heart disease, obstructive coronary artery disease, and hypertrophic cardiomyopathy) as potential causes for elevated LV filling pressures and symptoms were excluded (Figure S1). 3 In addition, atrial fibrillation, left bundle branch block, and ventricular paced rhythm at time of echocardiography were excluded, as the 2016 ASE/EACVI algorithm cannot be used in these cases. 2 In case of doubles, the first case was used for analysis. Before referral, significant other cardiac and extracardiac explanations of symptoms were already explored (this does not preclude the presence of comorbidity), such as coronary artery disease, chronic obstructive lung disease, obstructive sleep apnea syndrome, and interstitial lung disease. In accordance with the European Society of Cardiology guideline, PCWP ≥15 mm Hg at rest, in the absence of other causes for elevated LV filling pressures, was diagnostic for (manifest) HFpEF. 3
These investigations were performed for clinical reasons and all data was collected for quality control. Therefore, our medical ethical committee waived the requirement of informed consent.
Echocardiography
Echocardiography was performed by experienced sonographers using commercially available ultrasound systems (Philips iE33/EPIQ7 system equipped with a X5‐1 xMATRIX array transducer). Patients underwent a comprehensive echocardiographic examination. 13 Images were stored and the following measurement were analyzed off line (Philips Xcelera R4.1): LV end‐systolic and end‐diastolic volume and left atrial volume by Simpson's biplane, maximal and minimal left atrial volume were indexed for body surface area (LAVi, LAVi‐min, respectively); pulsed‐wave Doppler mitral inflow E(‐max) and A(‐max) velocity, tissue Doppler septal and lateral e′ velocity, continuous wave tricuspid regurgitation peak velocity (TRV); LVEF, left atrial ejection fraction, mitral valve E/A ratio, and averaged E/e′ were derived.
Based on the 2016 ASE/EACVI recommendations, in patients with a preserved LVEF (≥50%) and no structural heart disease, LV diastolic function was graded as normal, diastolic dysfunction, or indetermined (if equal number of parameters for LV diastology are either positive or negative), using parameters E/e′, e′ velocity (septal and/or lateral), TRV, and LAVi. 2 Patients with a structural heart disease (eg, LV hypertrophy, right ventricular dysfunction, or ischemic heart disease) and/or diastolic dysfunction were further graded according to transmitral valve E/A ratio, E velocity and E/e′, TRV, and LAVi; with diastolic dysfunction grade I, LV filling pressure is considered normal, or with diastolic dysfunction grade II or III, LV filling pressures are considered elevated. Diastolic dysfunction grade II or higher, in the absence of other causes for elevated LV filling pressures, was diagnostic for HFpEF. In the recalibrated ASE/EACVI algorithm, echo‐parameter cutoffs were replaced by the cutoffs optimal for our cohort. In additional analyses of the algorithm, LAVi was replaced by left atrial ejection fraction or LAVi‐min. Echocardiographic analyses were performed by 2 investigators (V.E. and A.A.B), who were blinded for the hemodynamic assessments.
Right Heart Catheterization
RHC was performed in supine position, while patients were on their chronic medication. Procedures were performed on patients in a nonfasting state, to prevent confounding of dehydration. RHC was performed by an experienced team, 14 through a 9F sheath via the right internal jugular vein, using a fluid‐filled 7F Swan‐Ganz catheter (131HF7, Baxter Healthcare). Transducers were zeroed at midthoracic level in each patient. 15 , 16 Pressure tracings were digitized and analyzed by 2 investigators experienced in hemodynamic assessment (F.T.P.O and M.L.H.), who were blinded for the echocardiographic assessments. Pressures were measured at end‐expiration. Mean right atrium pressure and PCWP were taken at mid‐A wave. Cardiac output was assessed by thermodilution, using the average values of ≥3 measurements with maximally 10% variance.
H2FPEF Score
The H2FPEF score was calculated based on the following parameters: body mass index >30, 2 points; hypertension, 1 point; atrial fibrillation, 3 points; PH (as evaluated by echocardiography), 1 point; age >60 years, 1 point; early mitral inflow velocity/septal mitral annular early diastolic velocity (E/e′ septal) >9, 1 point. 12 Conforming with Reddy et al, a H2FPEF score of ≥6 was used to diagnose and ≤1 was used to rule out HFpEF. 12 Indeterminate H2FPEF scores (2–5) were used in the correlation analysis but excluded in further analyses of the diagnostic performance. In addition, the diagnostic properties of the discrete and continuous variant of the H2FPEF score were compared.
Statistical Analysis
Normal distribution of the data was checked and data were log‐transformed if applicable. Results are reported as mean±SD in case of a normal distribution or median [interquartile range] in case of a nonnormal distribution. Pearson's correlation coefficient was used to evaluate the correlation between PCWP at rest and the individual echocardiographic parameters; in case of nonnormal distribution Spearman's rank correlation was used. Missing data were not imputed. A P<0.05 was considered significant. NT‐proBNP ≥125 ng/L was considered elevated in accordance with the European Society of Cardiology guideline. 3 Receiver operating characteristics analysis was performed in order to assess the discriminative properties for elevated PCWP of the individual echo‐parameters, the original and recalibrated 2016 ASE/EACVI algorithm, the H2FPEF score, and NT‐proBNP. DeLong's method was used to compare areas under the curve (AUC). The optimal cutoff of individual echo‐parameters for our cohort was determined using Youden's index, with exception of E and E/A's lower limits where the optimal cutoff was defined having a sensitivity ≥90%, and E/A's upper limit optimal cutoff was defined by a specificity of ≥90%. In a subanalysis the ASE/EACVI algorithm was also compared using PCWP >12 mm Hg as cutoff, as this was used in the original validation study. 5 Potential (confounding) effect of conduction disorders, revealed by the ECG, on the diagnostic performance of the echo‐algorithm was assessed using receiver operating characteristics analyses. To adjust for the relatively high prevalence of precapillary PH (PCWP <15 mm Hg and mean pulmonary arterial pressure ≥25 mm Hg), subanalyses were performed without precapillary PH. Time in days between RHC and echocardiogram was calculated and added to the linear regressions in order to assess the possible confounding effect of time. R Statistics (version 3.6.1) was used for analyses.
Results
Patient Characteristics
This study evaluated 476 patients, of which 204 unique patients met the inclusion and exclusion criteria (Figure S1. Flowchart of patient selection). The patient characteristics and echocardiographic and hemodynamics data are shown in Tables 1 and 2. All patients with PCWP at rest ≥15 mm Hg (n=87) were diagnosed with HFpEF. The diagnoses of patients with PCWP <15 mm Hg (n=117) consisted mainly of chronic tromboembolic PH, other types of PH and no pulmonary or cardiac disease (Table S1). Patients with elevated filling pressures were more likely to be on diuretics compared with patients with low filling pressures (72% versus 40%, respectively, P<0.001).
Table 1.
Patient Characteristics
| PCWP <15 mm Hg (n=117) | PCWP ≥15 mm Hg (n=87) | |
|---|---|---|
| Sex (female) | 67 (57%) | 57 (66%) |
| Age, y | 62 (14) | 66 (10) |
| Body mass index, kg/m2 | 27.5 (5.2) | 32.0 (7.0) |
| Body surface area, m2 | 1.97 (0.27) | 2.09 (0.27) |
| Mean blood pressure, mm Hg | 100 (13) | 100 (14) |
| Heart rate, beats per min | 78 (16) | 75 (15) |
| New York Heart Association class | ||
| 1 | 10 (9%) | 4 (5%) |
| 2 | 48 (41%) | 23 (26%) |
| 3 | 55 (47%) | 50 (58%) |
| 4 | 3 (3%) | 10 (12%) |
| N‐terminal pro‐B‐type natriuretic peptide, ng/L* | 168 [87–932] | 484 [131–1200] |
| Physical examination † | ||
| Jugular venous pressure elevated | 2 (4%) | 13 (30%) |
| Pulmonary congestion | 8 (7%) | 21 (25%) |
| Edema | 24 (22%) | 49 (61%) |
| Medication | ||
| Calcium‐antagonist | 20 (17%) | 22 (25%) |
| Beta blocker | 20 (17%) | 40 (46%) |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 38 (33%) | 45 (52%) |
| Diuretics | 47 (40%) | 63 (72%) |
| Hydrochlorothiazide | 15 (13%) | 13 (15%) |
| Loop diuretic | 31 (27%) | 49 (56%) |
| Mineralocorticoid receptor antagonist | 14 (12%) | 26 (30%) |
| Comorbidities | ||
| Pulmonary hypertension ‡ | 79 (68%) | 79 (91%) |
| Hypertension | 42 (36%) | 51 (59%) |
| Renal dysfunction § | 49 (42%) | 37 (43%) |
| Obesity | 41 (35%) | 43 (49%) |
| Hypercholesterolemia | 34 (29%) | 21 (24%) |
| Obstructive sleep apnea syndrome | 14 (12%) | 22 (25%) |
| Rheumatoid disorders | 23 (20%) | 9 (10%) |
| Diabetes mellitus | 14 (12%) | 17 (20%) |
| Coronary artery disease | 13 (11%) | 16 (18%) |
| Chronic obstructive pulmonary disease | 12 (10%) | 17 (20%) |
| Atrial fibrillation | 8 (7%) | 17 (20%) |
| H2FPEF score | ||
| H2FPEF score | 3 (2) | 4 (2) |
PCWP indicates pulmonary capillary wedge pressure.
Available in 97% of cases.
Jugular venous pressure, pulmonary congestion, and edema assessment were inconclusive or unavailable in 113, 11, and 13 of the cases, respectively.
Based on invasive measurements (mean pulmonary arterial pressure ≥25 mm Hg).
Defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2.
Table 2.
Echocardiography and Hemodynamics
|
PCWP <15 mm Hg (n=117) |
Measurable (%) |
PCWP ≥15 mm Hg (n=87) |
Measurable (%) | |
|---|---|---|---|---|
| Echocardiogram | ||||
| Left ventricular ejection fraction (%) | 55 (53–60) | 89% (100% teichert) | 55 (53–60) | 49% (100% teichert) |
| E, cm/s | 65.2 (19.1) | 99% | 75.5 (23.9) | 100% |
| Septal e′, cm/s | 6.7 (2.0) | 93% | 6.9 (2.3) | 91% |
| Lateral e′, cm/s | 9.5 (2.8) | 96% | 9.7 (3.2) | 98% |
| E/e′ septal, cm/s | 9.7 [7.9–11.8] | 93% | 11.5 [9.4–14.3] | 91% |
| E/e′ average, cm/s | 8.1 [6.4–9.7] | 89% | 9.4 [7.3–11.1] | 89% |
| E/A | 0.82 [0.66–1.0] | 97% | 0.94 [0.77–1.4] | 93% |
| Tricuspid regurgitation velocity, m/s | 3.4 (0.8) | 72% | 3.3 [2.8–3.9] | 70% |
| Maximal left atrial volume indexed for body surface area, mL/m2 | 26.0 [21–32.2] | 87% | 32.6 [27.3–43.0] | 75% |
| Minimal left atrial volume indexed for body surface area, mL/m2 | 11.6 [8.6–15.8] | 78% | 17.4 [11.7–29.0] | 62% |
| Left atrial ejection fraction (%) | 55 [46–62] | 78% | 41 [29–58] | 62% |
| Hemodynamics | ||||
| Arterial saturation (%) | 95 [92–97] | 95 [92–97] | ||
| Venous saturation (%) | 67 (10) | 65 (10) | ||
| Mean right atrial pressure, mm Hg | 6 [4–9] | 10 [8–13] | ||
| Diastolic RVP, mm Hg | 4 [2–7] | 6 [4–10] | ||
| Systolic RVP, mm Hg | 54 [35–77] | 61 [46–81] | ||
| Diastolic PAP, mm Hg | 20 [14–28] | 25 [19–31] | ||
| Systolic PAP, mm Hg | 55 [35–76] | 58 [45–80] | ||
| Mean PAP, mm Hg | 34 [22–46] | 39 [29–49] | ||
| PCWP, mm Hg | 11 [8–12] | 17 [16–20] | ||
| PVR5 | 292 [143–566] | 222 [123–489] | ||
| SVR, dyn×s/cm5 | 1410 (507) | 1230 (490) | ||
| Cardiac index, L/min per m2 | 3.0 (1.0) | 3.2 (1.3) | ||
E indicates early diastolic transmitral flow velocity; e', early diastolic mitral annular tissue velocity; E/A, early to late diastolic transmitral flow velocity; PAP indicates pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RVP, right ventricular pressure; and SVR, systemic vascular resistance.
Weak Correlation Between PCWP and Individual Echo‐Parameters for LV Diastology
Correlation between the individual echo‐parameters and PCWP are shown in Table 3 (regression plots are shown in Figure S2). Echo‐parameters TRV, and e′ septal and lateral did not correlate statistically significant with PCWP. All other echo‐parameters for diastolic function significantly correlated with PCWP, although the relation was only fair at best (r=0.15–0.30). AUC by receiver operating characteristics analysis of the ASE/EACVI algorithm parameters ranged from 0.47 to 0.69. Using the 2016 ASE/EACVI algorithm cutoff values, sensitivity and specificity of parameters TRV, LAVi, E/e′, and e′ ranged from 16% to 74% and 21% to 96%, respectively, with an accuracy ranging from 43% to 67%.
Table 3.
Relation Between Individual Echo‐Parameters and PCWP
| r | P Value | AUC | ASE/EACVI Cutoff | Sensitivity | Specificity | Accuracy | Optimized Cutoff | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| e′ septal, cm/s | 0.01 | 0.87 | 0.49 | <7 | 52% | 49% | 50% | <7.8 | 73% | 29% | 48% |
| e′ lateral, cm/s | −0.04 | 0.61 | 0.47 | <10 | 51% | 43% | 46% | <5.0 | 11% | 96% | 59% |
| E, cm/s | 0.28 | <0.0001 | 0.63 | ≥50 | 89% | 19% | 49% | ≥50 | 89% | 19% | 49% |
| E/e′ average | 0.30* | <0.0001 | 0.62 | >14 | 16% | 96% | 62% | >9.0 | 57% | 67% | 63% |
| E/e′ septal | 0.26* | <0.001 | 0.62 | >15 | 20% | 90% | 61% | >11.5 | 49% | 72% | 62% |
| E/A | 0.15 | 0.04 | 0.61 |
≤0.8 ≥2.0 |
68% 11% |
43% 100% |
53% 63% |
>0.56 ≥1.4 |
90% 28% |
12% 93% |
45% 66% |
| TRV, m/s | 0.04 | 0.67 | 0.47 | >2.8 | 74% | 21% | 43% | >2.7 | 78% | 15% | 42% |
| TRVexcluding precapillary pulmonary hypertension, m/s | 0.34 | <0.001 | 0.77 | >2.8 | 74% | 62% | 71% | >3.2 | 54% | 95% | 65% |
| Maximal left atrial volume indexed for body surface area, mL/m2 | 0.25* | 0.001 | 0.69 | >34 | 42% | 83% | 67% | >32 | 54% | 74% | 66% |
| Minimal left atrial volume indexed for body surface area, mL/m2 | 0.35* | <0.0001 | 0.72 | … | … | … | … | >15.8 | 61% | 75% | 70% |
| Left atrial ejection fraction, % | −0.35 † | <0.0001 | 0.68 | … | … | … | … | <40% | 50% | 86% | 73% |
ASE/EACVI indicates American Society of Echocardiography/European Association of Cardiovascular Imaging; AUC, area under the curve; E, early diastolic transmitral flow velocity; e', early diastolic mitral annular tissue velocity; E/A, early to late diastolic transmitral flow velocity;PCWP, pulmonary capillary wedge pressure; r, correlation; and TRV, tricuspid regurgitation velocity.
Pearson's correlation coefficient after log transformation.
Pearson's correlation coefficient after exponential transformation.
Sensitivity and specificity only marginally improved using cutoff values optimized for our study cohort. After exclusion of precapillary PH, TRV correlated statistically significant with PCWP. As a marker of quality control, TRV2 assessed by echocardiogram and invasive measured systolic pulmonary arterial pressure had a very strong correlation (r=0.82, P<0.001). There was no confounding effect of time in days (median [interquartile range]: 0 [−6, 45]) between RHC and echocardiogram, and the relationship between the individual echo‐parameters or NT‐proBNP with PCWP. In addition to the ASE/EACVI parameters two alternative left atrial parameters were evaluated. LAVi‐min and left atrial ejection fraction showed a stronger correlation with PCWP (r=−0.35 and r=0.35) and a better diagnostic performance (combined sensitivity and specificity, and accuracy) compared with LAVi; however, AUC was not statistically significant between the 3 parameters (P=0.10–0.76).
Limited Diagnostic Accuracy of the 2016 ASE/EACVI Algorithm
Conform the ASE/EACVI algorithm, all patients with myocardial disease (eg, left or right ventricular hypertrophy, right ventricular dysfunction, or history of coronary artery disease) started in step B of the algorithm (n=185). According to the algorithm, LV filling pressures were normal in 128 of the patients, of which 9 patients had a normal diastolic function and 119 patients had a diastolic dysfunction grade I (Figure 1). Noninvasively assessed LV filling pressures were elevated in 35 of the patients, of which 27 patients and 8 patients had diastolic dysfunction grade II or grade III, respectively.
Figure 1. Diastolic function according to the 2016 ASE/EACVI algorithm.
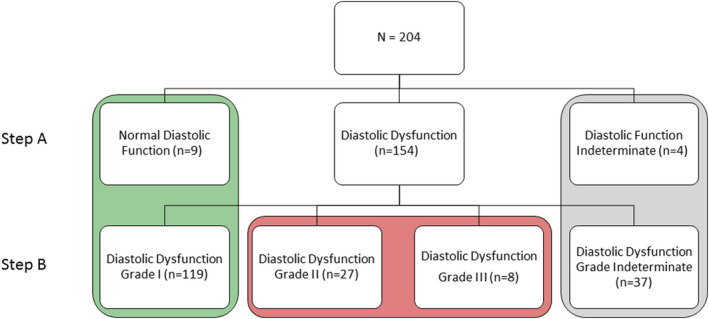
ASE/EACVI indicates American Society of Echocardiography/European Association of Cardiovascular Imaging.
In 30% of the patients, the echo‐algorithm could not be used to determine diastolic function. In 12% of the patients evaluated for diastolic dysfunction, the ASE/EACVI algorithm could not be used because of limitations of the algorithm (of which 8% were due to atrial fibrillation and 4% to other causes (Figure S1). In addition, in 41 patients (20%) of the final cohort diastolic function could not be determined (step 1 of the echo‐algorithm) or diastolic dysfunction grade could not be determined (step 2) by the echo‐algorithm.
The correlation between individual diastolic function grades and PCWP was poor (r=0.17, P<0.01). With diastolic dysfunction grade II as cutoff for predicted elevated filling pressures, the ASE/EACVI algorithm had an AUC of 0.56 (P<0.001); sensitivity and specificity were 35% and 87%, respectively, with an overall accuracy of 67% (Figures 2 and 3, Table 4).
Figure 2. Relationship between the ASE/EACVI algorithm and PCWP.
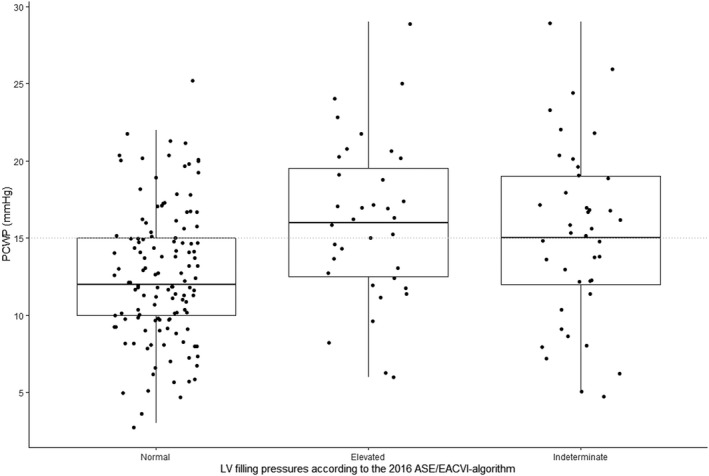
Normal LV filling pressures: normal diastolic function and diastolic dysfunction grade I. Elevated LV filling pressures: diastolic dysfunction grade II and III. Dotted line indicates the border between normal and elevated filling pressures. ASE/EACVI indicates American Society of Echocardiography/European Association of Cardiovascular Imaging; LV, left ventricular; and PCWP, pulmonary capillary wedge pressure.
Figure 3. Receiver operating characteristics analysis.
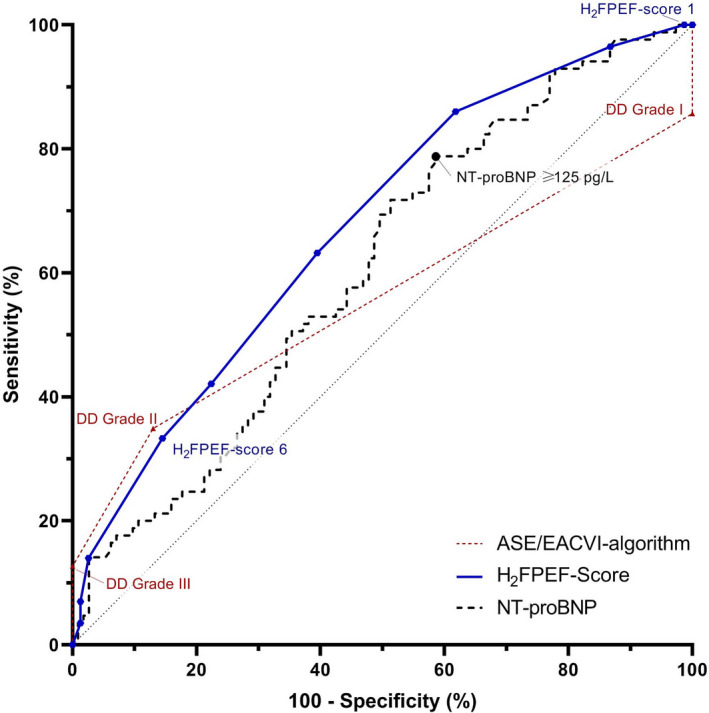
ASE/EACVI indicates American Society of Echocardiography/European Association of Cardiovascular Imaging; DD, diastolic dysfunction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 4.
Diagnostic Accuracy of the Tested Algorithms and Diagnostic Tools
| Algorithms | r | P Value | AUC | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| ASE/EACVI | 0.17 | 0.03 | 0.56 | 35% | 87% | 67% | 63% | 68% |
| ASE/EACVI—excluding precap PH | 0.20 | 0.05 | 0.56 | 35% | 88% | 53% | 85% | 41% |
| ASE/EACVI—optimized cutoffs | 0.30 | <0.0001 | 0.68 | 71% | 62% | 66% | 56% | 76% |
| ASE/EACVI—minimal left atrial volume indexed for body surface area | 0.24 | <0.01 | 0.58 | 43% | 85% | 70% | 62% | 73% |
| ASE/EACVI—left atrial ejection fraction | −0.02 | 0.80 | 0.53 | 43% | 37% | 40% | 37% | 43% |
| ASE/EACVI vs PCWP >12 mm Hg | 0.17 | 0.03 | 0.55 | 30% | 88% | 56% | 74% | 48% |
| Other diagnostic tools | ||||||||
| NT‐proBNP | 0.24* | <0.001 | 0.61 | 78% | 42% | 57% | 50% | 71% |
| NT‐proBNP—excl. precap PH | 0.44* | <0.0001 | 0.78 | 78% | 65% | 74% | 85% | 54% |
| H2FPEF score | 0.44 | <0.0001 | 0.67 | 88% | 59% | 73% | 67% | 84% |
ASE/EACVI indicates American Society of Echocardiography/European Association of Cardiovascular Imaging; AUC, area under the curve; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCWP, pulmonary capillary wedge pressure; PPV, positive predictive value, NPV, negative predictive value; precap PH., precapillary pulmonary hypertension; and r, correlation.
Pearson's correlation coefficient after log‐transformation.
Exclusion of patients with precapillary PH did not improve the diagnostic performance of echocardiography (Table 4). Recalibrating the ASE/EACVI algorithm using cutoffs optimized for our cohort (Table 3 for cutoff values and specifications) improved the sensitivity, but this was at the expense of specificity. Overall diagnostic accuracy, therefore, remained unchanged.
Replacing LAVi by LAVi‐min in the ASE/EACVI algorithm resulted in a minimal improvement in diagnostic performance. Replacing LAVi by left atrial ejection fraction worsened the diagnostic accuracy of the echo‐algorithm. Assessing the diagnostic performance of the ASE/EACVI algorithm using PCWP >12 mm Hg as reference did not result in a higher diagnostic accuracy of the echo‐algorithm either (Table 4). We found no effect of (incomplete) right bundle branch block (n=57) or intraventricular conduction disorders in general (n=89) on diagnostic performance of the ASE/EACVI algorithm (P=0.63 and P=0.33, respectively).
Diagnostic Performance of NT‐proBNP and the H2FPEF Score
As shown in Figure 4A, the correlation between NT‐proBNP and PCWP was poor (r=0.24, P<0.001). Sensitivity of NT‐proBNP to detect elevated LV filling pressures in patients with a preserved ejection fraction was moderate, but specificity and AUC were low (Figure 3 and Table 4). Subanalyses showed an increase in diagnostic performance after exclusion of precapillary PH. Of note, 22% of the patients with elevated PCWP had a NT‐proBNP <125 pg/L and 58% of the patients with normal PCWP had an elevated NT‐proBNP.
Figure 4. Relationship of PCWP with NT‐proBNP and the H2FPEF score.
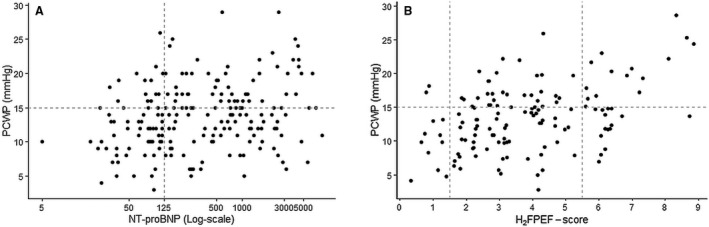
Regression plot between PCWP and (A) NT‐proBNP and (B) H2FPEF score. NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; and PCWP, pulmonary capillary wedge pressure.
As shown in Figure 4B, the H2FPEF score and PCWP had a fair correlation (r=0.44, AUC=0.67, P<0.0001). Using a H2FPEF score of ≥6 to diagnose and a H2FPEF score of ≤1 to rule out manifest‐HFpEF (n=52); sensitivity and specificity were 88% and 59%, respectively, with an overall diagnostic accuracy of 73% (Figure 3 and Table 4). The diagnostic accuracy of the continuous variant of the H2FPEF score (r=0.38, AUC=0.65) was comparable to the discrete variant of the H2FPEF score. Receiver operating characteristics curves and diagnostic properties per cutoff values of both H2FPEF scores are shown in Table S2 and Figure S3. Of note, 9 patients with low PCWP at rest were later diagnosed with masked‐HFpEF, these patients had a H2FPEF score of 4 [4–6]; of whom 7 patients were stratified as intermediate likelihood and 2 were stratified as high likelihood of HFpEF.
Discussion
This study evaluated the diagnostic accuracy of the 2016 ASE/EACVI echo‐algorithm in a large cohort of patients with unexplained dyspnea or PH with LVEF ≥50%, that is, patients with potential HFpEF. The current study demonstrated that the echo‐parameters used to assess diastolic function in the ASE/EACVI algorithm have a poor correlation with filling pressures in patients with a preserved LVEF. Consequently, the ASE/EACVI algorithm has only a modest diagnostic performance in these patients, primarily driven by a low sensitivity. In other words, with echocardiography relevant diastolic dysfunction is easily missed. Interestingly, we found that the ASE/EACVI algorithm did not perform better than some of its individual components (E/e′ and LAVi). Moreover, in almost 1 out of 3 patients, the echo‐algorithm could not be used to determine whether elevated filling pressures were present.
Previous validation studies have reported conflicting results regarding the diagnostic performance of 2016 ASE/EACVI echo‐algorithm. In contrast to our results, initial validation studies of the echo‐algorithm have reported a moderate to good overall diagnostic performance. 5 , 6 , 7 , 8 This large difference in diagnostic performance may be explained as these initial studies included only patients with known cardiac disease or (suspected) coronary artery disease, 5 , 6 , 7 , 8 while excluding patients with pulmonary disease (the main differential diagnosis for HFpEF). 5 Also, Andersen et al and Lancellotti et al reported that diagnostic performance was lower in patients with LVEF ≥50%, compared with patients with LVEF <50%. 5 , 6 Although Lancellotti et al and Balanay et al reported a modest accuracy in patients with a LVEF ≥50% (67% and 76%, respectively), this was because of a high specificity in contrast to a low sensitivity (sensitivity 11% and 57%, respectively). 6 , 7
More recently, 3 papers evaluated the diagnostic performance of the 2016 ASE/EACVI algorithm in patients with LVEF ≥50%. 10 , 11 , 17 Ran et al reported high sensitivity and specificity (84% and 80%, respectively) in patients with PH of different etiologies. 17 This was in a relatively small study (N=63) and other cardiac causes for elevated filling pressure were not excluded, which may have overestimated diagnostic performance. In accordance with our study, the 2 remaining studies reported low diagnostic accuracy of the echo‐algorithm in patients suspected for HFpEF. 10 , 11 Furthermore, a recently published systematic review underlined the limited evidence available for the individual echo‐parameters in patients with HFpEF and found only a modest correlation between E/e′ and LV filling pressures in patients with HFpEF (r=0.62). 9
When developing the 2016 ASE/EACVI algorithm, specificity was preferred over sensitivity, and the observed low(er) sensitivity may therefore not come as a surprise. 2 Therefore, we explored whether recalibrating of the algorithm using the Youden's index improved diagnostic accuracy, which however was not the case.
Our study did not show a statistically significant difference in diagnostic performance between LAVi‐min and LAVi, despite previous literature reporting a superiority of LAVi‐min. 18 , 19 Further research is needed to evaluate whether this parameter should be implemented in a more accurate diagnostic tool for the evaluation of HFpEF. In this respect, other novel echo‐parameters, such as global longitudinal strain and left atrial strain by speckle tracking, 20 , 21 , 22 are also of interest.
Alternatives for the 2016 ASE/EACVI Algorithm for Diastolic Dysfunction
In our study, the H2FPEF score had the best diagnostic performance, and NT‐proBNP had the worst. However, subanalysis showed that the low discriminative power of NT‐proBNP in this study was because of the high prevalence of precapillary PH, which is also a known causes for elevated NT‐proBNP (because of increased right ventricular filling pressures). It is important to note that almost a quarter of the patients with elevated LV filling pressures had normal NT‐proBNP levels. This phenomenon has been frequently reported and is commonly linked to the relative low LV wall tension in HFpEF compared with HFrEF. 23 , 24 , 25 In a recent systematic review, the low sensitivity of NT‐proBNP was again confirmed. 26 Nevertheless, some clinicians still use the low NT‐proBNP as an absolute rule‐out method.
On the other hand, the H2FPEF score showed a good diagnostic accuracy in diagnosing HFpEF in our cohort, and these results are in line with other validation studies. 12 , 27 Of note, the H2FPEF score was developed to diagnose manifest and masked HFpEF combined. In our study the H2FPEF score was compared with PCWP at rest rather than to PCWP at exercise. As a result the specificity found in our study will likely be an underestimation. Combined with the simplicity of the score, and its applicability in all patients (despite, for example, the presence of atrial fibrillation), the H2FPEF score deserves to have an important position in the workup in the evaluation of HFpEF.
Strengths and Limitations
The strength of our study is the comparison of the echocardiogram to the gold standard (PCWP), and to our knowledge, the largest validation study of the 2016 ASE/EACVI algorithm for diastolic dysfunction in patients evaluated for unexplained dyspnea or PH. RHCs and echocardiograms were performed and evaluated by experienced staff in a tertiary center specialized in HFpEF and PH, blinded for echocardiography. In addition, other causes for elevated filling pressures (eg, ischemia and severe valve disease) were thoroughly investigated and ruled out. Moreover, integrating the H2FPEF score and NT‐proBNP in this study enabled easy comparison between different diagnostic tools.
A limitation is that echocardiography and RHC were not always performed on the same day, so day‐to‐day fluctuations in filling pressure could have influenced the correlations. However, statistical analysis showed no signs of confounding by time. Moreover, HFpEF is a relatively stable chronic disease, which implies that days between the echocardiogram and the RHC (in the same diagnostic workup) should not influence the diagnosis of HFpEF.
Another important limitation of our study is a potential patient bias: this study was performed in a tertiary center. Therefore, our cohort mainly contained referred patients, in whom there was doubt about the diagnosis, which may have resulted in a lower sensitivity for our particular patient cohort. However, our study evaluated the diagnostic performance of echocardiography and compared it with invasive measurements in patients at rest. It is now established that a large number of patients with HFpEF have normal filling pressures at rest but a significant and pathophysiological rise in LV filling pressures during exercise, a patient group that has been named “masked” HFpEF or “early” HFpEF. 4 , 28 For this reason, guidelines nowadays recommend to perform exercise echocardiography or exercise ‐RHC in patients suspected for HFpEF, despite “normal” echocardiography at rest in symptomatic patients. 2 , 3 If the 2016 ASE/EACVI algorithm would have been compared with PCWP at exercise instead of PCWP at rest, sensitivity of the echo‐algorithm would be even lower than was found now. 4
Clinical Implications
In patients with dyspnea and/or PH, echocardiography remains the first screening tool to evaluate for possible cardiac involvement. Systolic dysfunction and/or valvular disease are easily detected, but because of low sensitivity, a “normal” echo does not safely rule out relevant diastolic dysfunction in a symptomatic patient. Therefore, when dyspnea remains unexplained, even when NT‐proBNP is low, we propose to calculate the H2FPEF score to quantify the pretest likelihood for HFpEF. In case of an intermediate score (H2FPEF score 2–5), more advanced diagnostics is advised (exercise echocardiography: E/e′ at exercise; exercise RHC) to definitely rule out or to rule in. Furthermore, the low diagnostic accuracy of NT‐proBNP and echocardiogram in diagnosing HFpEF has important consequences for clinical trials using mainly NT‐proBNP and echocardiogram as inclusion criteria. Therefore, more robust inclusion criteria (eg, invasive or exercise tests) are warranted for clinical trials. In addition, multiple studies over the years have compared or validated other methods to evaluate/predict diastolic dysfunction (eg, ECG, cardiac magnetic resonance imaging, and biomarkers) to echocardiogram. 29 , 30 These results should be interpreted with the knowledge that correlation between echocardiogram and filling pressures is modest at best, repeating these studies with invasive measurements as comparator is to be considered.
Conclusions
The 2016 ASE/EACVI algorithm for the assessment of diastolic function has a limited diagnostic accuracy for diagnosing HFpEF. This paper underlines the importance of advanced diagnostics, such as exercise echocardiogram or exercise‐RHC, in symptomatic patients with an intermediate a priori likelihood for HFpEF.
Sources of Funding
None.
Disclosures
Handoko received an educational/speaker/consultancy fees from Novartis, Boehringer Ingelheim, Daiichi Sankyo, Vifor Pharma, AstraZeneca, Bayer, MSD, and Quin. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S3
Arno A. van de Bovenkamp and Vidya Enait are co‐authors.
For Sources of Funding and Disclosures, see page 11.
See Editorial by MacNamara and Sarma
Shared first authorship for Arno A. van de Bovenkamp and Vidya Enait.
References
- 1. Gladden JD, Linke WA, Redfield MM. Heart failure with preserved ejection fraction. Pflugers Arch. 2014;466:1037–1053. DOI: 10.1007/s00424-014-1480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. DOI: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. DOI: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. DOI: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69:1937–1948. DOI: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 6. Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, Magne J, Laginha S, Hagendorff A, Haland TF, et al. Echo‐Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro‐Filling study. Eur Heart J Cardiovasc Imaging. 2017;18:961–968. DOI: 10.1093/ehjci/jex067. [DOI] [PubMed] [Google Scholar]
- 7. Balaney B, Medvedofsky D, Mediratta A, Singh A, Ciszek B, Kruse E, Shah AP, Addetia K, Lang RM, Mor‐Avi V. Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head‐to‐head comparison with the 2009 guidelines. J Am Soc Echocardiogr. 2018;31:79–88. DOI: 10.1016/j.echo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato K, Grant ADM, Negishi K, Cremer PC, Negishi T, Kumar A, Collier P, Kapadia SR, Grimm RA, Desai MY, et al. Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Am Heart J. 2017;189:28–39. DOI: 10.1016/j.ahj.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 9. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20:1303–1311. DOI: 10.1002/ejhf.1220. [DOI] [PubMed] [Google Scholar]
- 10. Berthelot E, Jourdain P, Bailly MT, Bouchachi A, Gellen B, Rouquette A, Damy T, Hervé P, Chemla D, Assayag P. Echocardiographic evaluation of left ventricular filling pressure in patients with heart failure with preserved ejection fraction: usefulness of inferior vena cava measurements and 2016 EACVI/ASE recommendations. J Card Fail. 2020;26:507–514. DOI: 10.1016/j.cardfail.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 11. Hummel YM, Liu LCY, Lam CSP, Fonseca‐Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, et al. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;19:1651–1660. DOI: 10.1002/ejhf.957. [DOI] [PubMed] [Google Scholar]
- 12. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. DOI: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. DOI: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 14. Huis In't Veld AE, Oosterveer FPT, De Man FS, Marcus JT, Nossent EJ, Boonstra A, Van Rossum ACB, Vonk Noordegraaf A, Bogaard HJ, Handoko ML. Hemodynamic effects of pulmonary arterial hypertension‐specific therapy in patients with heart failure with preserved ejection fraction and with combined post‐ and precapillay pulmonary hypertension. J Card Fail. 2020;26:26–34. DOI: 10.1016/j.cardfail.2019.07.547. [DOI] [PubMed] [Google Scholar]
- 15. Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42:1586–1594. DOI: 10.1183/09031936.00050713. [DOI] [PubMed] [Google Scholar]
- 16. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. DOI: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 17. Ran H, Schneider M, Pistritto AM, Gerges C, Heidari H, Binder T, Lang I, Goliasch G. Echocardiographic evaluation of left ventricular filling pressures in patients with pulmonary hypertension. Int J Cardiovasc Imaging. 2019;35:861–868. DOI: 10.1007/s10554-019-01528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu V‐C, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, Haruki N, Yoshitani H, Tamura M, Abe H, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6:1025–1035. DOI: 10.1016/j.jcmg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. DOI: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frydas A, Morris DA, Belyavskiy E, Radhakrishnan A‐K, Kropf M, Tadic M, Roessig L, Lam CSP, Shah SJ, Solomon SD, et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail. 2020;7:1956–1965. DOI: 10.1002/ehf2.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takagi T. Impaired global longitudinal strain in elderly patients with preserved ejection fraction is associated with raised post‐exercise left ventricular filling pressure. J Echocardiogr. 2021;19:37–44. DOI: 10.1007/s12574-020-00481-x. [DOI] [PubMed] [Google Scholar]
- 22. Ito H, Ishida M, Makino W, Goto Y, Ichikawa Y, Kitagawa K, Omori T, Dohi K, Ito M, Sakuma H. Cardiovascular magnetic resonance feature tracking for characterization of patients with heart failure with preserved ejection fraction: correlation of global longitudinal strain with invasive diastolic functional indices. J Cardiovasc Magn Reson. 2020;22:42. DOI: 10.1186/s12968-020-00636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meijers WC, Hoekstra T, Jaarsma T, van Veldhuisen DJ, de Boer RA. Patients with heart failure with preserved ejection fraction and low levels of natriuretic peptides. Neth Heart J. 2016;24:287–295. DOI: 10.1007/s12471-016-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, et al. Neprilysin, obesity and the metabolic syndrome. Int J Obes. 2011;35:1031–1040. DOI: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. DOI: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remmenzwaal S, van Ballegooijen AJ, Schoonmade LJ, Dal Canto E, Handoko ML, Henkens MTHM, van Empel V, Heymans SRB, Beulens JWJ. Natriuretic peptides for the detection of diastolic dysfunction and heart failure with preserved ejection fraction—a systematic review and meta‐analysis. BMC Med. 2020;18:290. DOI: 10.1186/s12916-020-01764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lundberg A, Johnson J, Hage C, Bäck M, Merkely B, Venkateshvaran A, Lund LH, Nagy AI, Manouras A. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol. 2019;108:703–715. DOI: 10.1007/s00392-018-1399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huis in ’t Veld AE, de Man FS, van Rossum AC, Handoko ML. How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J. 2016;24:244–251. DOI: 10.1007/s12471-016-0811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kagiyama N, Piccirilli M, Yanamala N, Shrestha S, Farjo PD, Casaclang‐Verzosa G, Tarhuni WM, Nezarat N, Budoff MJ, Narula J, et al. Machine learning assessment of left ventricular diastolic function based on electrocardiographic features. J Am Coll Cardiol. 2020;76:930–941. DOI: 10.1016/j.jacc.2020.06.061. [DOI] [PubMed] [Google Scholar]
- 30. Kawaji K, Codella NCF, Prince MR, Chu CW, Shakoor A, LaBounty TM, Min JK, Swaminathan RV, Devereux RB, Wang YI, et al. Automated segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging. 2009;2:476–484. DOI: 10.1161/CIRCIMAGING.109.879304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


