This cohort study assesses secular changes in initial neurological severity and short-term functional outcomes of patients with acute stroke by sex using a large population.
Key Points
Question
Did the initial neurological severity and functional outcomes of patients with stroke change throughout a 20-year period?
Findings
In this hospital-based, multicenter, prospective registry involving 183 080 patients with acute stroke, initial neurological severity showed a decrease over time in all stroke types. Functional outcome at hospital discharge improved in patients with ischemic stroke but no longer showed improvement after adjustment by reperfusion therapy and others; it did not clearly improve in patients with hemorrhagic stroke.
Meaning
Twenty-year changes in functional outcomes after ischemic and hemorrhagic strokes showed different trends presumably partly owing to differences in the development of acute therapeutic strategies.
Abstract
Importance
Whether recent changes in demographic characteristics and therapeutic technologies have altered stroke outcomes remains unknown.
Objective
To determine secular changes in initial neurological severity and short-term functional outcomes of patients with acute stroke by sex using a large population.
Design, Setting, and Participants
This nationwide, hospital-based, multicenter, prospective registry cohort study used the Japan Stroke Data Bank and included patients who developed acute stroke from January 2000 through December 2019. Patients with stroke, including ischemic and hemorrhagic strokes, who registered within 7 days after symptom onset were studied. Modified Rankin Scale scores were assessed at hospital discharge for all patients.
Exposure
Time.
Main Outcomes and Measures
Initial severity was assessed by the National Institutes of Health Stroke Scale for ischemic stroke and intracerebral hemorrhage and by the World Federation of Neurological Surgeons grading for subarachnoid hemorrhage. Outcomes were judged as favorable if the modified Rankin Scale score was 0 to 2 and unfavorable if 5 to 6.
Results
Of 183 080 patients, 135 266 (53 800 women [39.8%]; median [IQR] age, 74 [66-82] years) developed ischemic stroke, 36 014 (15 365 women [42.7%]; median [IQR] age, 70 [59-79] years) developed intracerebral hemorrhage, and 11 800 (7924 women [67.2%]; median [IQR] age, 64 [53-75] years) developed subarachnoid hemorrhage. In all 3 stroke types, median ages at onset increased, and the National Institutes of Health Stroke Scale and World Federation of Neurological Surgeons scores decreased throughout the 20-year period on multivariable analysis. In ischemic stroke, the proportion of favorable outcomes showed an increase over time after age adjustment (odds ratio [OR], 1.020; 95% CI, 1.015-1.024 for women vs OR, 1.015; 95% CI, 1.011-1.018 for men) but then stagnated, or even decreased in men, on multivariate adjustment including reperfusion therapy (OR, 0.997; 95% CI, 0.991-1.003 for women vs OR, 0.990; 95% CI, 0.985-0.994 for men). Unfavorable outcomes and in-hospital deaths decreased in both sexes. In intracerebral hemorrhage, favorable outcomes decreased in both sexes, and unfavorable outcomes and deaths decreased only in women. In subarachnoid hemorrhage, the proportion of favorable outcomes was unchanged, and that of unfavorable outcomes and deaths decreased in both sexes.
Conclusions and Relevance
In this study, functional outcomes improved in patients with ischemic stroke during the past 20 years in both sexes presumably partly owing to the development of acute reperfusion therapy. The outcomes of patients with hemorrhagic stroke did not clearly improve in the same period.
Introduction
Several population-based registries have demonstrated long-term changes in age at onset, incidence, and mortality of stroke; the trends differed by changes in lifestyle and medical conditions.1,2,3,4,5,6 In Japan, both the age-adjusted incidence and mortality of stroke decreased drastically during the past half century, but these trends slowed in resent decades.2 In addition, notable technologies were introduced domestically every 5 years, with official approval for intravenous thrombolysis in 2005, official approval for the first device for mechanical thrombectomy in 2010, and far-reaching spread of stent retrievers for thrombectomy in 2015.7 However, whether the changes in demographic characteristics and therapeutic technologies altered stroke severity and functional outcomes from a long-range perspective remains unknown. Such changes could be clarified by long-term hospital-based registries.
Twenty-six countries have been reported to have nationwide standardized data sets for acute stroke.8 Some of these reported long-term changes in stroke severity; the Austrian Stroke Unit Registry, involving 53 126 patients with intracerebral hemorrhage (ICH) between 2008 and 2016,9 and the National Acute Stroke Israeli registry, involving 6693 patients with ischemic stroke (IS) and ICH between 2004 and 2013,10 showed a decrease in severity assessed by the National Institutes of Health Stroke Scale (NIHSS). Functional outcome assessed by the modified Rankin Scale (mRS) and other scales is an important indicator for most nationwide data sets,11,12 but its long-term changes were minimally discussed.
The Japan Stroke Data Bank (JSDB), previously the Japan Standard Stroke Registry Study, is a 20-year-long nationwide hospital-based registry in which the NIHSS scores on emergent hospital admission and the mRS scores at hospital discharge have been required fields from the beginning.13,14,15,16,17 Higher scores for both scales in female patients with IS than their male counterparts on multivariable analysis were reported from the registry.14 Other hospital-based registries reported similar sex-related differences.18,19,20,21,22,23,24,25,26,27 In addition to older onset age in women than men, the tendency for social isolation, poorer premorbid activity, limited access to medical resources, frequent prestroke and poststroke depression, and several more reasons could cause poor stroke conditions for women.28,29,30 Thus, we hypothesized that long-term changes in severity and outcome are also affected by sex. In the present study, secular changes in initial neurological severity and short-term outcomes of patients with stroke assessed using NIHSS and mRS scores were determined based on a large hospital-based patient population by sex.
Methods
Study Design and Setting
The JSDB study is an ongoing, hospital-based, multicenter, prospective registry of hospitalized patients with acute stroke or transient ischemic attack based on a web database from 130 academic or regional stroke centers distributed evenly throughout Japan (eTable 1 in Supplement 1). The unique aspects of this nationwide registry include standardized clinical information, detailed diagnosis, and acute management by stroke specialists. Patients’ data were prospectively recorded by the study physicians or clinical report coordinators in each institute using a standardized database form online. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The study protocol was approved by the institutional ethical board at National Cerebral and Cardiovascular Center. Individual consent for entry into the database was waived, with an opt-out consent method used instead. The data set of the JSDB study is open to the investigators who participate in this registry.
Participants
For the present analyses, patients with any stroke who were registered within 7 days after symptom onset were eligible for inclusion. Clinical information on demographics, stroke types, and IS subtypes according to the criteria of the Trial of Org 10172 in Acute Stroke Treatment,31 history of any stroke, and performance of acute reperfusion therapy (ie, intravenous thrombolysis or acute endovascular therapy mainly by mechanical thrombectomy) were collected from the database.
The outcome measures were (1) neurological severity at the emergent visit corresponding with the NIHSS score for patients with IS or ICH and the World Federation of Neurological Surgeons (WFNS) grading for those with subarachnoid hemorrhage (SAH); (2) the proportion of patients with favorable functional outcomes at hospital discharge corresponding with an mRS score of 0 to 2; (3) the proportion of patients with unfavorable functional outcomes at hospital discharge corresponding with an mRS score of 5 to 6; and (4) in-hospital mortality.
Statistical Analysis
Continuous data are reported as median and IQR and categorical data are presented as numbers and percentages. Box plots for age and NIHSS score (WFNS for SAH) were conducted for 4 categories of years (2000-2005, 2006-2010, 2011-2015, and 2016-2019) by sex. Mann-Whitney U and χ2 tests were used to test the significance of differences between 2 groups for continuous and categorical variables, respectively. To determine secular changes in the outcomes or changes in the outcomes of patients with IS by the above 4 categories, multilevel mixed-effect multivariable regression and logistic regression using the institutes as random intercepts were performed, adjusted for sex, age, NIHSS score (WFNS for SAH), history of stroke, and reperfusion therapy. The Cochran-Armitage test was also performed to check for year trends. Significance was defined as a P value less than .05. Statistical analyses were performed with STATA version 16 (StataCorp).
Results
Of 214 924 patients registered in the JSDB between January 2000 and December 2019, 15 044 had a diagnosis of transient ischemic attack, 10 412 registered 8 or more days after onset, and 6388 had unavailable data on demography or stroke types and were excluded from the study (eFigure in Supplement 1). Of the remaining 183 080 patients (77 089 women [42.1%]; median [IQR] age, 73 [64-81] years) included in the study, 135 266 developed IS (53 800 women [39.8%]; median [IQR] age, 74 [66-82] years), 36 014 developed ICH (15 365 women [42.7%]; median [IQR] age, 70 [59-79] years), and 11 800 developed SAH (7924 women [67.2%]; median [IQR] age, 64 [53-75] years).
The baseline characteristics and outcomes of patients are shown in Table 1. Of the 3 stroke types, patients with SAH were mostly female and were the youngest. Patients with ICH had higher NIHSS scores than those with IS. Discharge mRS scores were the highest in patients with ICH, and in-hospital mortality was the highest in those with SAH. Among the 3 major IS subtypes, patients with cardioembolism were mostly female, were the oldest, had the highest NIHSS and mRS scores, and had the highest mortality. History of stroke was more common in men than women with IS and SAH. Male patients with IS more often underwent acute reperfusion therapy than female patients. Initial NIHSS or WFNS scores and discharge mRS scores were higher in women than men for all stroke types. In-hospital mortality was higher in women than men with IS and lower in women with ICH. Figure 1A-C shows changes in the median age of patients by period. In all stroke types, women were older than men and ages at onset became older with time in both sexes.
Table 1. Baseline Characteristics and Outcomes of Patients.
| Variable | Total stroke | Ischemic stroke | Intracerebral hemorrhage | Subarachnoid hemorrhage | |||
|---|---|---|---|---|---|---|---|
| Total | Cardioembolism | Large-artery atherosclerosis | Small-vessel occlusion | ||||
| No. of patients | 183 082 | 135 268 | 38 896 | 42 302 | 37 541 | 36 014 | 11 800 |
| Women, No. (%) | 77 089 (42.1) | 53 800 (39.8) | 17 941 (46.1) | 14 923 (35.3) | 14 352 (38.2) | 15 365 (42.7) | 7924 (67.2) |
| Men, No. (%) | 105 993 (57.9) | 81 468 (60.2) | 20 955 (53.9) | 27 379 (64.7) | 23 189 (61.8) | 20 649 (57.3) | 3876 (32.8) |
| Age, median (IQR), y | |||||||
| Women | 77 (67-84) | 79 (70-85) | 82 (75-88) | 78 (70-85) | 76 (67-83) | 74 (63-83) | 68 (56-78) |
| Men | 70 (62-78) | 72 (64-79) | 75 (67-81) | 72 (65-79) | 69 (61-77) | 67 (57-76) | 57 (49-68) |
| P valuea | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| NIHSS score, median (IQR) | |||||||
| Women | NA | 5 (2-13) | 13 (4-22) | 4 (2-10) | 3 (1-5) | 12 (4-24) | NA |
| Men | NA | 3 (2-8) | 7 (2-18) | 4 (2-7) | 3 (1-4) | 11 (4-22) | NA |
| P valuea | NA | <.001 | <.001 | <.001 | <.001 | <.001 | NA |
| WFNS grade, median (IQR) | |||||||
| Women | NA | NA | NA | NA | NA | NA | 2 (1-5) |
| Men | NA | NA | NA | NA | NA | NA | 2 (1-4) |
| P valuea | NA | NA | NA | NA | NA | NA | <.001 |
| History of stroke, No. (%) | |||||||
| Women | 17 393 (23.9) | 13 114 (25.8) | 4554 (27.3) | 3808 (26.8) | 3490 (25.5) | 3509 (23.9) | 770 (10.5) |
| Men | 27 557 (27.5) | 22 534 (29.2) | 5639 (28.8) | 8155 (31.2) | 6545 (29.6) | 4709 (23.9) | 314 (8.8) |
| P valuea | <.001 | <.001 | .002 | <.001 | <.001 | .94 | .007 |
| Acute reperfusion therapy, No. (%) | |||||||
| Women | NA | 4629 (8.6)b | 2983 (16.3) | 758 (5.1) | 271 (1.9) | NA | NA |
| Men | NA | 7281 (8.9)c | 3528 (16.8) | 2301 (8.4) | 472 (2.0) | NA | NA |
| P valuea | NA | .03 | .58 | <.001 | .32 | NA | NA |
| Days of hospitalization, median (IQR) | |||||||
| Women | 21 (12-37) | 18 (11-32) | 22 (13-39) | 20 (13-35) | 15 (10-25) | 24 (13-43) | 31 (18-54) |
| Men | 18 (11-32) | 16 (10-29) | 20 (12-35) | 18 (12-32) | 13 (9-21) | 23 (11-41) | 29 (17-51) |
| P valuea | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Discharge mRS score, median (IQR) | |||||||
| Women | 3 (1-5) | 3 (1-4) | 4 (2-5) | 3 (1-4) | 1 (1-3) | 4 (2-5) | 3 (0-5) |
| Men | 2 (1-4) | 2 (1-4) | 3 (1-4) | 2 (1-4) | 1 (1-2) | 4 (2-5) | 2 (0-5) |
| P valuea | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| In-hospital death, No. (%) | |||||||
| Women | 7007 (9.3) | 3207 (6.1) | 2273 (12.9) | 528 (3.6) | 90 (0.6) | 2097 (13.9) | 1703 (22.1) |
| Men | 6709 (6.5) | 2919 (3.6) | 1688 (8.2) | 746 (2.8) | 135 (0.6) | 3008 (14.9) | 782 (20.9) |
| P valuea | <.001 | <.001 | <.001 | <.001 | .59 | .008 | .13 |
Abbreviations: mRS, modified Rankin Score; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; WFNS, World Federation of Neurological Surgeons.
P value for sex differences. All P values for differences among 3 ischemic stroke subtypes (cardioembolism, large-artery atherosclerosis, and small-vessel occlusion) are <.001, except for history of stroke in women (P = .002). All P values for differences among 3 stroke types (ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage) are <.001.
1.4% Between 2000 and 2005, 6.0% between 2006 and 2010, 8.8% between 2011 and 2015, and 17.3% between 2016 and 2019 (P < .05).
2.1% Between 2000 and 2005, 7.4% between 2006 and 2010, 9.3% between 2011 and 2015, and 16.6% between 2016 and 2019 (P < .05).
Figure 1. Age, National Institutes of Health Stroke Scale (NIHSS) Scores, and World Federation of Neurological Surgeons (WFNS) Grades at the Emergent Visit by Sex.
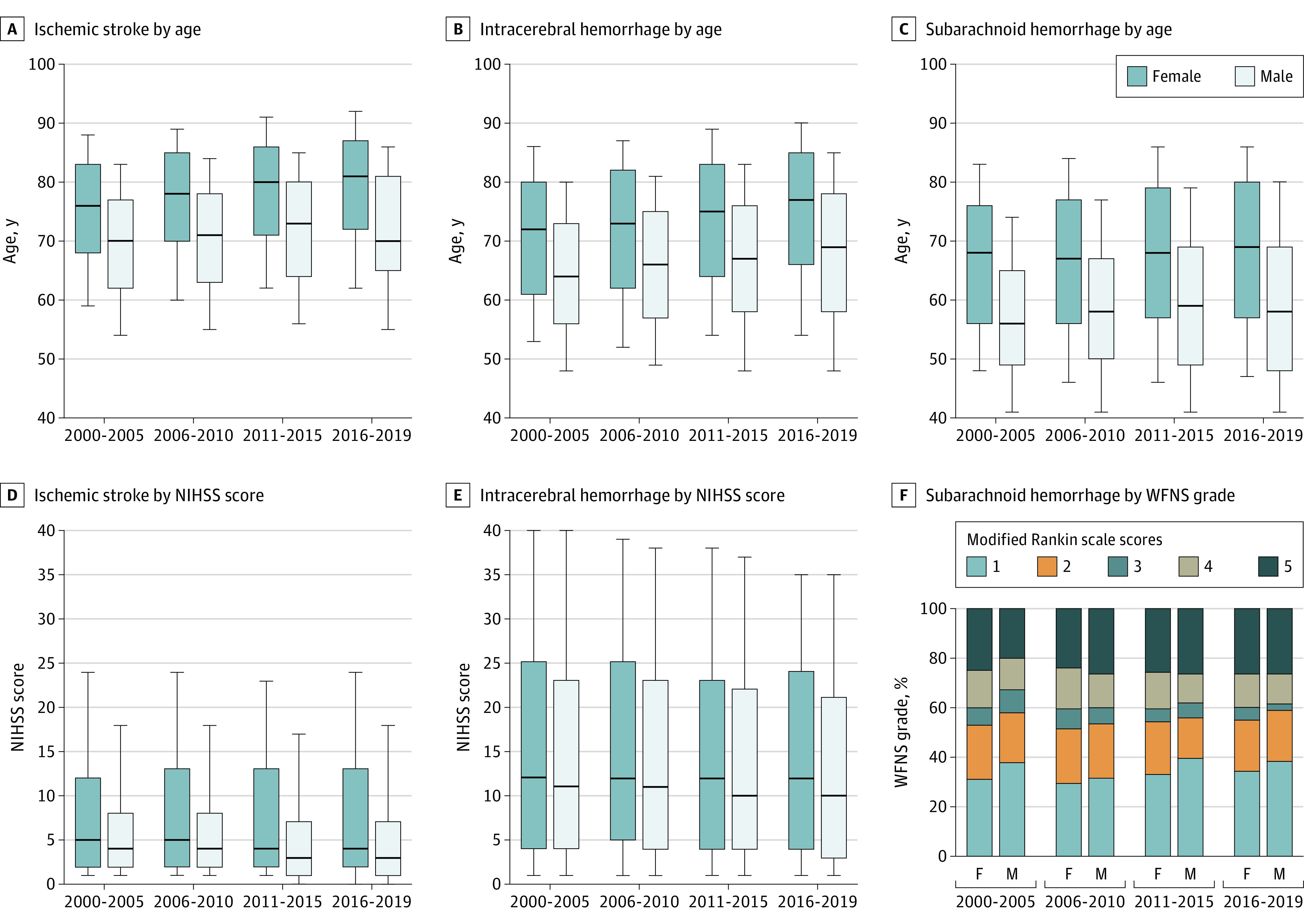
Boxes represent interquartile range, and lines across boxes indicate median values. Whiskers represent 10th percentile and 90th percentile values. All P values for sex differences in age are <.001. All P values for sex differences in NIHSS scores for ischemic stroke are <.001. P values for sex differences in NIHSS scores for intracerebral hemorrhage are .06 in 2000-2005, .006 in 2006-2010, .002 in 2011-2015, and <.001 in 2016-2019. P values for sex differences in WFNS grades are <.001 in 2000-2005, .81 in 2006-2010, 0.11 in 2011-2015, and .02 in 2016-2019. All P values for trends of age and NIHSS scores are <.001 in both sexes. P values for trends of WFNS grades are .28 for women and .64 for men.
NIHSS scores became lower throughout the 20 years regardless of sex in all stroke types and all IS subtypes, even adjusted by age and stroke history (eTable 2 in Supplement 1). Figure 1D-F shows a decrease in NIHSS scores in patients with IS and ICH over time. WFNS scores in patients with SAH did not show secular changes.
In patients with IS, the proportion of favorable outcomes increased significantly after age adjustment (model 1) but no longer increased after further adjustment by NIHSS scores and stroke history (model 2) in women and men (Table 2 and Figure 2). The odds ratios of the proportion were lower after further adjustment by achievement of reperfusion therapy in both sexes (model 3), and the proportion decreased significantly in men. Both unfavorable outcomes and death decreased over time after any adjustments in both sexes (Table 3). Because 2005, 2010, and 2015 were years for appearance of new therapeutic technologies for IS in Japan as described above,7 changes in the outcomes by 5-year categories were also determined (eTable 3 in Supplement 1). The results were similar with those of per-year changes.
Table 2. Secular Changes in Favorable Outcomes at Discharge.
| Outcome | Odds ratio (95% CI)a | |||
|---|---|---|---|---|
| Crude | Model 1b | Model 2c | Model 3d | |
| Women | ||||
| Total ischemic stroke | 0.994 (0.995-1.003) | 1.020 (1.015-1.024) | 1.003 (0.998-1.009) | 0.997 (0.991-1.003) |
| Cardioembolism | 1.009 (1.002-1.017) | 1.037 (1.029-1.045) | 1.023 (1.012-1.034) | 1.008 (0.997-1.019) |
| Large-artery atherosclerosis | 1.010 (1.003-1.018) | 1.028 (1.020-1.036) | 1.004 (0.994-1.014) | 1.002 (0.992-1.013) |
| Small-vessel occlusion | 0.997 (0.989-1.005) | 1.014 (1.005-1.022) | 0.986 (0.975-0.997) | 0.985 (0.974-0.995) |
| Intracerebral hemorrhage | 0.984 (0.976-0.992) | 0.994 (0.986-1.003) | 0.980 (0.968-0.992) | NA |
| Subarachnoid hemorrhage | 1.000 (0.990-1.010) | 1.011 (1.000-1.022) | 1.002 (0.989-1.016) | NA |
| Men | ||||
| Total ischemic stroke | 1.002 (0.999-1.005) | 1.015 (1.011-1.018) | 0.995 (0.991-1.000) | 0.990 (0.985-0.994) |
| Cardioembolism | 1.006 (1.000-1.013) | 1.023 (1.016-1.029) | 1.007 (0.998-1.016) | 0.993 (0.984-1.002) |
| Large-artery atherosclerosis | 1.009 (1.003-1.015) | 1.020 (1.014-1.026) | 1.001 (0.993-1.008) | 0.998 (0.991-1.006) |
| Small-vessel occlusion | 0.997 (0.991-1.004) | 1.009 (1.002-1.016) | 0.982 (0.973-0.991) | 0.980 (0.971-0.989) |
| Intracerebral hemorrhage | 0.983 (0.976-0.990) | 0.989 (0.982-0.996) | 0.971 (0.961-0.982) | NA |
| Subarachnoid hemorrhage | 0.996 (0.982-1.009) | 1.002 (0.988-1.017) | 0.989 (0.970-1.008) | NA |
Abbreviations: NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; WFNS, World Federation of Neurological Surgeons.
Odds ratio (95% CI) per 1 year.
Model 1 is adjusted by age.
Model 2 is adjusted by age, NIHSS score (WFNS grade for subarachnoid hemorrhage), and history of stroke.
Model 3 is adjusted by age, NIHSS score (WFNS grade for subarachnoid hemorrhage), history of stroke, and reperfusion therapy.
Figure 2. Modified Rankin Scale Scores at Discharge by Sex.
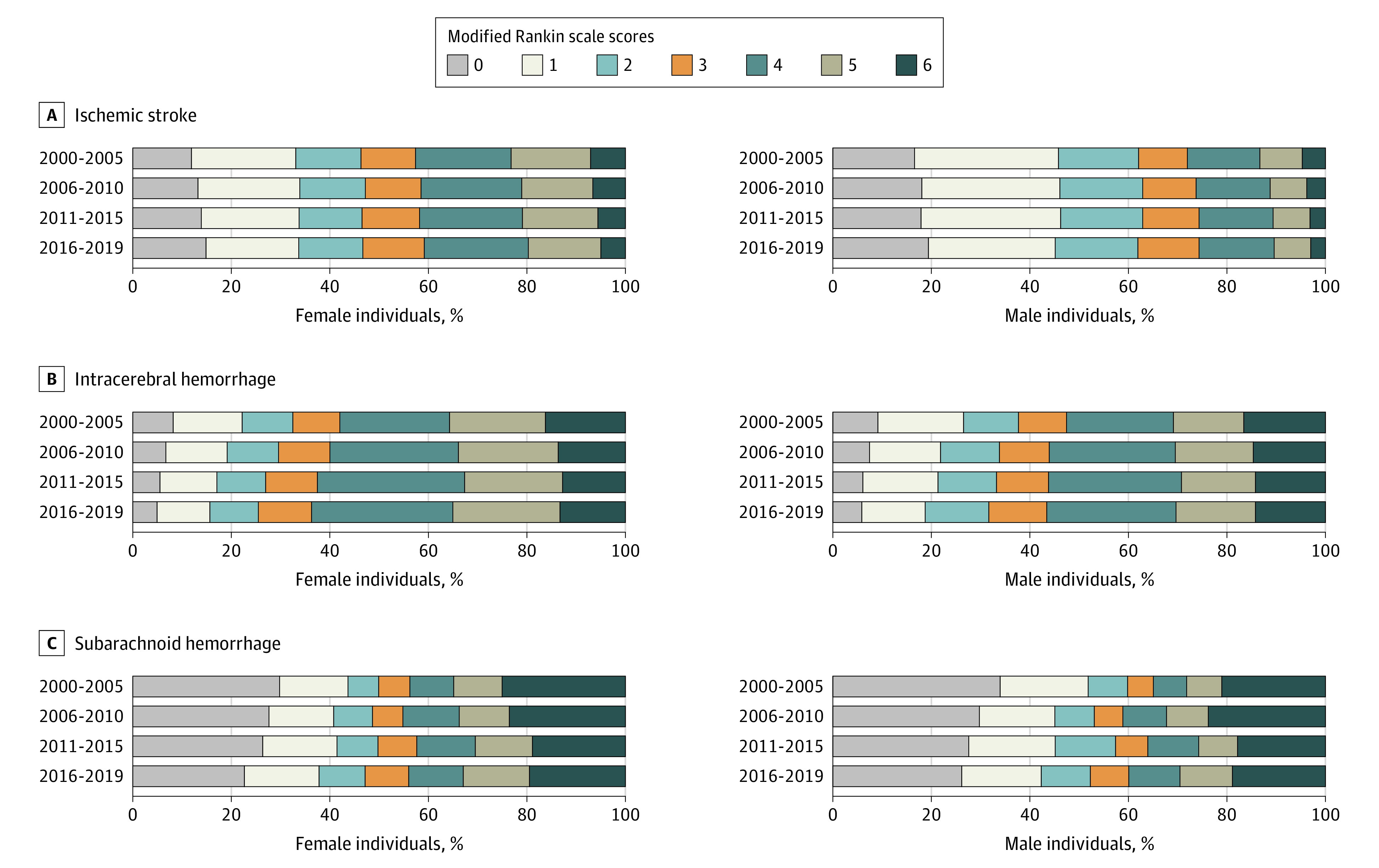
Table 3. Secular Changes in Poor Outcomes at Discharge.
| Outcome | Odds ratio (95% CI)a | ||||
|---|---|---|---|---|---|
| Crude | Model 1b | Model 2c | Model 3d | ||
| Unfavorable outcome in women | |||||
| Ischemic stroke | 0.989 (0.984-0.994) | 0.967 (0.962-0.972) | 0.974 (0.967-0.981) | 0.981 (0.973-0.988) | |
| Intracerebral hemorrhage | 0.996 (0.988-1.003) | 0.984 (0.976-0.992) | 0.987 (0.974-0.999) | NA | |
| Subarachnoid hemorrhage | 0.986 (0.975-0.996) | 0.974 (0.963-0.985) | 0.973 (0.959-0.987) | NA | |
| Unfavorable outcome in men | |||||
| Ischemic stroke | 0.982 (0.977-0.987) | 0.967 (0.962-0.972) | 0.978 (0.971-0.985) | 0.984 (0.977-0.991) | |
| Intracerebral hemorrhage | 0.999 (0.993-1.007) | 0.991 (0.985-0.998) | 1.001 (0.990-1.013) | NA | |
| Subarachnoid hemorrhage | 0.989 (0.974-1.004) | 0.981(0.966-0.997) | 0.976 (0.956-0.995) | NA | |
| In-hospital death in women | |||||
| Ischemic stroke | 0.975 (0.967-0.983) | 0.959 (0.951-0.967) | 0.969 (0.959-0.979) | 0.967 (0.957-0.978) | |
| Intracerebral hemorrhage | 0.984 (0.974-0.994) | 0.976 (0.965-0.986) | 0.978 (0.963-0.994) | NA | |
| Subarachnoid hemorrhage | 0.970 (0.958-0.982) | 0.960 (0.948-0.972) | 0.951 (0.937-0.965) | NA | |
| In-hospital death in men | |||||
| Ischemic stroke | 0.964 (0.956-0.972) | 0.950 (0.942-0.958) | 0.966 (0.956-0.976) | 0.967 (0.956-0.977) | |
| Intracerebral hemorrhage | 0.992 (0.983-1.001) | 0.985 (0.977-0.994) | 0.998 (0.985-1.012) | NA | |
| Subarachnoid hemorrhage | 0.979 (0.962-0.996) | 0.972 (0.955-0.990) | 0.965 (0.945-0.986) | NA | |
Abbreviations: NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; WFNS, World Federation of Neurological Surgeons.
Odds ratio (95% CI) per 1 year.
Model 1 is adjusted by age.
Model 2 is adjusted by age, NIHSS score (WFNS grade for subarachnoid hemorrhage), and history of stroke.
Model 3 is adjusted by age, NIHSS score (WFNS grade for subarachnoid hemorrhage), history of stroke, and reperfusion therapy.
In both patients with cardioembolism and those with large-artery atherosclerosis, the proportion of favorable outcomes increased after model 1 adjustment, remained increased only in female patients with cardioembolic IS after model 2 adjustment, and no longer increased in either sex after model 3 adjustment (Table 2). In these 2 IS subtypes, unfavorable outcomes and death became less common after any adjustments in both sexes (eTable 4 in Supplement 1).
In patients with IS with small vessel occlusion, the proportion of favorable outcomes increased after model 1 adjustment and decreased after model 2 and model 3 adjustments in both sexes (Table 2). Neither the proportions of unfavorable outcomes nor the proportions of deaths showed secular changes after any adjustments except for model 1 adjustment for unfavorable outcomes in women (eTable 4 in Supplement 1).
In patients with ICH, favorable outcomes became less common after both age adjustment (model 1) and further adjustment by NIHSS scores and stroke history (model 2) in both sexes, except for model 1 adjustment in women (Table 2 and Figure 2). Both unfavorable outcomes and deaths became less common after both model 1 and model 2 adjustment in women and did not show secular change after model 2 adjustment in men (Table 3).
In patients with SAH, favorable outcomes did not show secular change in proportions, and both unfavorable outcomes and deaths became less common after both age adjustment (model 1) and further adjustment by WFNS scores and stroke history (model 2) in both sexes.
Discussion
During the past 20 years up to 2019, the JSDB study enrolled more than 200 000 patients with acute strokes or transient ischemic attacks from stroke centers distributed evenly throughout Japan. In the present analysis, there were several major new findings. First, median ages at onset increased, and the NIHSS/WFNS scores decreased on multivariable adjustment in all stroke types. Second, functional outcomes at hospital discharge improved in patients with IS with time, but the improvement was no longer clear on multivariable adjustment including reperfusion therapy. Third, the proportion of favorable outcomes (unfavorable outcome, in-hospital death) in hemorrhagic strokes decreased or was unchanged but never increased throughout the 20 years. Sex differences in 20-year trends of each outcome were generally inconspicuous. To our knowledge, this was the first study to examine changes in functional outcomes after ischemic and hemorrhagic strokes over 2 decades using quantitative scales for a large population.
In the present cohort, NIHSS scores decreased over time in both sexes in all 3 IS subtypes and ICH, regardless of adjustment for age and stroke history. The trends were similar to the above results from the Austrian and Israeli registries.9,10 The decrease could be explained by the 2 reasons proposed previouly10: improvement in preventive therapy and changes in case mix. To give typical examples of the first reason, strict blood pressure control enabled by recent strong antihypertensives and clarification of the importance of a low-salt diet could decrease the onset of large ICH,32 and the diffusion of anticoagulation for patients with atrial fibrillation by the widespread use of direct oral anticoagulants could prevent huge infarcts due to large cardiac thrombi. In fact, the impact on the decrease of coefficients was largest in female patients with cardioembolism. The second reason, changes in case mix, means, for example, development of brain imaging modalities such as diffusion-weighted imaging that accurately differentiates minor stroke from mimics and would increase registration of such minor stroke.
Representative trends in functional outcomes after IS can be seen in patients with nonlacunar stroke in the present study. Favorable outcomes increased over time after age adjustment, and the odds ratio gradually decreased after NIHSS score adjustment and after further adjustment by reperfusion therapy, as a representative decisive therapy.33,34 In male patients with IS, the proportion of favorable outcomes finally decreased after adjustment by reperfusion therapy. Thus, reperfusion therapy seemed to be a reason for the gradual improvement of functional outcomes over 20 years. Decreased proportions of poor outcomes were also clear in patients with nonlacunar subtypes even after NIHSS score adjustment. Widespread use of dual antiplatelet therapy,35 development of early rehabilitation,36 and early initiation of anticoagulation after stroke onset using direct oral anticoagulants might also improve functional outcomes.37 In contrast, trends in favorable and poor outcomes were somewhat unclear in patients with lacunar stroke, suggesting that lacunar stroke received the least benefits from the recent development of acute therapies among the 3 subtypes, especially mechanical thrombectomy, owing to the absence of target large arteries.
In female and male patients with ICH, favorable outcomes became less common, and poor outcomes showed sex differences in trends on multivariate analysis. The tendency for improvement of functional outcome seemed unclear compared with patients with IS. This partly suggested the lack of powerful acute therapies equal to reperfusion therapy for IS, although intensive blood pressure lowering and hemostatic agents specific to each anticoagulant are regarded as promising therapies.31 Patients with SAH also showed an inconspicuous tendency for improvement of outcome. A recent increase in antithrombotics-associated ICH, also shown in the JSDB population (data not shown), might also affect the initial severity and functional outcome.38,39
There are evident differences in several stroke features between women and men.28,29,30 In the present study, women constituted 42.1% of overall stroke patients, similar to the percentage with the hospital-based registries from China (38.4%),19 Korea (42.3%),12 and Israel (43.0%).10 Age-specific stroke incidence was lower for women than men at ages 55 to 75 years and similar at the other ages in the 2016 Global Burden of Diseases, Injuries, and Risk Factors Study.40 At least in East Asia, the ratio of female inpatients with stroke to their male counterparts would be approximately 2:3. Women were around 7 or more years older than men at stroke onset in any stroke types and any IS subtypes, and ages at onset became higher with time in both sexes. Women had higher admission NIHSS/WFNS scores than men in any stroke type and any IS subtype on crude analysis. However, sex differences of 20-year trends in functional outcomes were generally unclear in any stroke types, presumably partly because Japanese women received relatively equally the benefits of medical care during acute hospitalization to Japanese men. Although limited access to medical resources has been an essential limitation for stroke in women,29 there were only modest sex-related differences in the achievement of reperfusion therapy in the present study; women underwent reperfusion therapy somewhat more frequently between 2016 and 2020 (17.3% vs 16.6%; Table 1).
Strengths and Limitations
The strengths of the present study were its duration and the population size of the database. The JSDB study belongs to the oldest group that commenced patient registration around 2000 among 28 national registries listed in a systematic review.8 Registration of approximately 10 000 patients per year enabled us to analyze a substantial number of patients by year unit. Web-based data accumulation from the beginning with thorough cleansing has enabled accurate and well-preserved data.
The limitations of the JSDB study include, first, the ongoing operation with secular changes in the participating sites, as several sites joined and a smaller number of sites became inactive midway. Second, the JSDB study registered only approximately 3% (recently, approximately 6%) of patients with stroke throughout Japan based on the estimated number of total domestic patients with stroke by regional population-based surveys. Because high-volume stroke centers tended to join the JSDB study, the present results would not be generalizable to low-volume hospitals in Japan. Third, 6388 patients (3.2%) were excluded owing to unavailable data on stroke types or demography. These patients had the lower median NIHSS scores and lower percentage of favorable outcome and in-hospital mortality than the 183 082 included patients. Unfavorable outcome was similarly common. Two-thirds of the excluded patients belonged to the third category of years (2011-2015), and the proportion of such excluded patients was 1.0% (465 of 45 849) in the latest category (2016-2019). Fourth, concepts of some of the collected data varied according to the currents of times over the 20-year period. For example, the diagnosis of IS with other determined and undetermined causes varied according to development of diagnostic technology and trends of theories, such as reevaluation of cryptogenic stroke as embolic stroke with undetermined sources.41 The present study did not focus on such inconsistent items. Fifth, functional outcomes were assessed at the time of discharge from the acute hospital around a median of 20 days after stroke onset because longer-term outcomes were not collected for all patients. Finally, data from 2020 were not included, and the effect of the COVID-19 pandemic was therefore not assessed.
Conclusions
In conclusion, stroke became milder in severity during the past 20 years regardless of sex or stroke types although age at stroke onset became older in the nationwide stroke registry in Japan. Short-term functional outcomes at hospital discharge improved gradually in patients with IS, presumably partly owing to development of acute reperfusion therapy. In contrast, outcomes of patients with hemorrhagic stroke did not clearly improve during the same period. Such differences among stroke types might reflect the existence of decisively effective acute therapeutic strategies for IS and not for ICH and SAH.
eFigure. Flow diagram
eTable 1. List of the Japan Stroke Data Bank Investigators
eTable 2. Secular changes in National Institutes of Health Stroke Scale scores at the emergent visit
eTable 3. Secular changes in ischemic stroke outcomes at discharge according to four categories of years
eTable 4. Secular changes in poor outcomes at discharge by ischemic stroke subtypes
Nonauthor Collaborators. Japan Stroke Data Bank Investigators
References
- 1.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27(4):287-295. doi: 10.1007/s10654-012-9673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hata J, Ninomiya T, Hirakawa Y, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009). Circulation. 2013;128(11):1198-1205. doi: 10.1161/CIRCULATIONAHA.113.002424 [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Krishnamurthi RV, Barker-Collo S, et al. ; ARCOS IV Group . 30-Year trends in stroke rates and outcome in Auckland, New Zealand (1981-2012): a multi-ethnic population-based series of studies. PLoS One. 2015;10(8):e0134609. doi: 10.1371/journal.pone.0134609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guéniat J, Brenière C, Graber M, et al. Increasing burden of stroke: the Dijon Stroke Registry (1987-2012). Neuroepidemiology. 2018;50(1-2):47-56. doi: 10.1159/000486397 [DOI] [PubMed] [Google Scholar]
- 5.Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in stroke incidence rates in older US adults: an update from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. JAMA Neurol. 2020;77(1):109-113. doi: 10.1001/jamaneurol.2019.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skajaa N, Adelborg K, Horváth-Puhó E, et al. Nationwide trends in incidence and mortality of stroke among younger and older adults in Denmark. Neurology. 2021;96(13):e1711-e1723. doi: 10.1212/WNL.0000000000011636 [DOI] [PubMed] [Google Scholar]
- 7.Toyoda K, Inoue M, Koga M. Small but steady steps in stroke medicine in Japan. J Am Heart Assoc. 2019;8(16):e013306. doi: 10.1161/JAHA.119.013306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadilhac DA, Kim J, Lannin NA, et al. National stroke registries for monitoring and improving the quality of hospital care: a systematic review. Int J Stroke. 2016;11(1):28-40. doi: 10.1177/1747493015607523 [DOI] [PubMed] [Google Scholar]
- 9.Teuschl Y, Brainin M, Matz K, et al. ; Austrian Stroke Unit Registry Collaborators . Time trends in patient characteristics treated on acute stroke-units: results from the Austrian Stroke Unit Registry 2003-2011. Stroke. 2013;44(4):1070-1074. doi: 10.1161/STROKEAHA.111.676114 [DOI] [PubMed] [Google Scholar]
- 10.Koton S, Geva D, Streifler JY, et al. Declining rate and severity of hospitalized stroke from 2004 to 2013: the National Acute Stroke Israeli Registry. Stroke. 2018;49(6):1348-1354. doi: 10.1161/STROKEAHA.117.019822 [DOI] [PubMed] [Google Scholar]
- 11.Quinn TJ, Singh S, Lees KR, Bath PM, Myint PK; VISTA Collaborators . Validating and comparing stroke prognosis scales. Neurology. 2017;89(10):997-1002. doi: 10.1212/WNL.0000000000004332 [DOI] [PubMed] [Google Scholar]
- 12.Kim SE, Lee H, Kim JY, et al. ; CRCS-K Investigators . Three-month modified Rankin Scale as a determinant of 5-year cumulative costs after ischemic stroke: an analysis of 11,136 patients in Korea. Neurology. 2020;94(9):e978-e991. doi: 10.1212/WNL.0000000000009034 [DOI] [PubMed] [Google Scholar]
- 13.Toyoda K, Okada Y, Kobayashi S. Early recurrence of ischemic stroke in Japanese patients: the Japan standard stroke registry study. Cerebrovasc Dis. 2007;24(2-3):289-295. doi: 10.1159/000105682 [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, Toyoda K, Minematsu K, Kobayashi S; Japan Standard Stroke Registry Study Group . Effects of sex difference on clinical features of acute ischemic stroke in Japan. J Stroke Cerebrovasc Dis. 2013;22(7):1070-1075. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Deguchi I, Hayashi T, Fukuoka T, Kobayashi S, Tanahashi N; Japan Standard Stroke Registry Study Group . Features of cardioembolic stroke with persistent and paroxysmal atrial fibrillation: a study with the Japan Stroke Registry. Eur J Neurol. 2015;22(8):1215-1219. doi: 10.1111/ene.12728 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Fukuma S, Ikenoue T, Fukuhara S, Kobayashi S. Effect of Edaravone on neurological symptoms in real-world patients with acute ischemic stroke. Stroke. 2019;50(7):1805-1811. doi: 10.1161/STROKEAHA.118.024351 [DOI] [PubMed] [Google Scholar]
- 17.Ikawa F, Ichihara N, Uno M, et al. Visualisation of the non-linear correlation between age and poor outcome in patients with aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2021;92(11):1173-1180. doi: 10.1136/jnnp-2020-325306 [DOI] [PubMed] [Google Scholar]
- 18.Koton S, Telman G, Kimiagar I, Tanne D; NASIS Investigators . Gender differences in characteristics, management and outcome at discharge and three months after stroke in a national acute stroke registry. Int J Cardiol. 2013;168(4):4081-4084. doi: 10.1016/j.ijcard.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Li J, Wang C, et al. Gender differences in 1-year clinical characteristics and outcomes after stroke: results from the China National Stroke Registry. PLoS One. 2013;8(2):e56459. doi: 10.1371/journal.pone.0056459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roquer J, Rodríguez-Campello A, Jiménez-Conde J, et al. Sex-related differences in primary intracerebral hemorrhage. Neurology. 2016;87(3):257-262. doi: 10.1212/WNL.0000000000002792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushnell CD, Reeves MJ, Zhao X, et al. Sex differences in quality of life after ischemic stroke. Neurology. 2014;82(11):922-931. doi: 10.1212/WNL.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattringer T, Ferrari J, Knoflach M, et al. Sex-related differences of acute stroke unit care: results from the Austrian stroke unit registry. Stroke. 2014;45(6):1632-1638. doi: 10.1161/STROKEAHA.114.004897 [DOI] [PubMed] [Google Scholar]
- 23.Irie F, Kamouchi M, Hata J, et al. ; FSR Investigators . Sex differences in short-term outcomes after acute ischemic stroke: the Fukuoka stroke registry. Stroke. 2015;46(2):471-476. doi: 10.1161/STROKEAHA.114.006739 [DOI] [PubMed] [Google Scholar]
- 24.Dehlendorff C, Andersen KK, Olsen TS. Sex Disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4(7):e001967. doi: 10.1161/JAHA.115.001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan HT, Blizzard CL, Reeves MJ, et al. Factors contributing to sex differences in functional outcomes and participation after stroke. Neurology. 2018;90(22):e1945-e1953. doi: 10.1212/WNL.0000000000005602 [DOI] [PubMed] [Google Scholar]
- 26.Phan HT, Reeves MJ, Blizzard CL, et al. Sex differences in severity of stroke in the INSTRUCT Study: a meta-analysis of individual participant data. J Am Heart Assoc. 2019;8(1):e010235. doi: 10.1161/JAHA.118.010235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonkhoff AK, Karch A, Weber R, Wellmann J, Berger K. Female stroke: sex differences in acute treatment and early outcomes of acute ischemic stroke. Stroke. 2021;52(2):406-415. doi: 10.1161/STROKEAHA.120.032850 [DOI] [PubMed] [Google Scholar]
- 28.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032-1037. doi: 10.1161/STROKEAHA.108.542894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordonnier C, Sprigg N, Sandset EC, et al. ; Women Initiative for Stroke in Europe (WISE) group . Stroke in women: from evidence to inequalities. Nat Rev Neurol. 2017;13(9):521-532. doi: 10.1038/nrneurol.2017.95 [DOI] [PubMed] [Google Scholar]
- 30.Dong L, Sánchez BN, Skolarus LE, Stulberg E, Morgenstern LB, Lisabeth LD. Sex difference in prevalence of depression after stroke. Neurology. 2020;94(19):e1973-e1983. doi: 10.1212/WNL.0000000000009394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 32.Broderick JP, Grotta JC, Naidech AM, et al. The story of intracerebral hemorrhage: from recalcitrant to treatable disease. Stroke. 2021;52(5):1905-1914. doi: 10.1161/STROKEAHA.121.033484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Elm JJ, Li H, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events (CHANCE) and Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trials. JAMA Neurol. 2019;76(12):1466-1473. doi: 10.1001/jamaneurol.2019.2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winstein CJ, Stein J, Arena R, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 37.Seiffge DJ, Paciaroni M, Wilson D, et al. ; CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen and Verona registry collaborators . Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85(6):823-834. doi: 10.1002/ana.25489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson D, Charidimou A, Shakeshaft C, et al. ; CROMIS-2 collaborators . Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology. 2016;86(4):360-366. doi: 10.1212/WNL.0000000000002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurogi R, Nishimura K, Nakai M, et al. ; J-ASPECT Study Collaborators . Comparing intracerebral hemorrhages associated with direct oral anticoagulants or warfarin. Neurology. 2018;90(13):e1143-e1149. doi: 10.1212/WNL.0000000000005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439-458. doi: 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429-438. doi: 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flow diagram
eTable 1. List of the Japan Stroke Data Bank Investigators
eTable 2. Secular changes in National Institutes of Health Stroke Scale scores at the emergent visit
eTable 3. Secular changes in ischemic stroke outcomes at discharge according to four categories of years
eTable 4. Secular changes in poor outcomes at discharge by ischemic stroke subtypes
Nonauthor Collaborators. Japan Stroke Data Bank Investigators


