Abstract
Obesity is marked by the build-up of fat in adipose tissue that results in increase in body weight and many associated health problems including diabetes and cardiovascular risk. Treatment options for obesity are limited and available medications have many side effects therefore, emphasizing the need to investigate for alternative medicine for treating obesity. This study explores the anti-adipogenic potential of n-butanol fraction of Cissus quadrangularis (CQ-B) on 3T3-L1 mouse pre-adipocyte cell line. The expression of various lipogenic marker genes such as adiponectin, peroxisome proliferator-activated receptor gamma, leptin, fatty acid binding protein, sterol regulatory element-binding protein, fetal alcohol syndrome, steroyl-CoA desaturase-1, lipoprotein, acetyl CoA carboxylase alpha and acetyl CoA carboxylase beta were variously significantly down regulated. After establishing the anti-adipogenic potential of CQ-B, it was fractionated to isolate anti-adipogenic compounds. We observed significant reduction in neutral lipid content of differentiated cells treated with various fractions of CQ-B. GCMS analysis revealed presence of thirteen compounds with reported anti-adipogenic activities. Further studies to purify these compounds can offer efficacious and viable treatment options for obesity and related complications.
Keywords: Cissus quadrangularis, Adipogenic differentiation, Obesity, Oil red O staining, Triglycerides, Lipogenesis, Fatty acid synthase gene expression, Peroxisome proliferator activated receptor-γ gene expression, Lipogenic marker genes, Steroyl-CoA desaturase-1, Lipoprotein, Acetyl CoA carboxylase alpha and Acetyl CoA carboxylase beta, Leptin, Fatty acid binding protein, Sterol regulatory element-binding protein
I. INTRODUCTION
Despite the splendid development in use of modern medicine, the treatment of diseases with traditional medicine is still practiced. A large percentage of population in developing countries depends on the use of traditional herbal medicines for treating various ailments and to meet basic health needs.1 It is true that modern medicines are used along with the traditional herbal medicines in many parts of the world but a tremendous popularity is won by the traditional medicines due to cultural and historical reasons.2 One such traditional herbal medicine of immense importance is Cissus quadrangularis (CQ).
CQ, a perdurable medicinal plant of Vitaceae or grape family, commonly known as hadjod is indigenous to Africa, some regions of Asia and Arabia.3 Its historical use is mainly based on its two features (i) treating feminine disorders including menstrual irregularity and (ii) curing bone fractures which accord it the historical name of bone setter. It has been employed for healing injured tendons and ligaments and broken bones.4 In addition to its osteogenic potential, it has been used for treating a variety of risk factors associated with cardiac disease, rheumatoid arthritis, gout, hemorrhoids, peptic ulcer, asthma, malaria, seizures, and pain.3 Although sufficient information on analgesic, anti-inflammatory and antioxidant characteristics of CQ are known5, but its mechanism of action has still not been worked out. In addition to all these, CQ has been reported to cause weight reduction in overweight people.6
Obesity is the prevalent and underrated condition of public health and clinical importance. It is globally epidemic in developing and developed countries and in every age group. In 2016, more than 1.9 billion adults, 18 years and older, were overweight. Of these 650 million were obese. In 2019, about 38 million children under the age of 5 were obese. Obesity is a multifactorial disease. Its main causative agent is imbalance of energy i.e. the consumed calories exceed the expended ones.7 Enhanced energy imbalance result in excess fat being stored in fat cells or adipocytes that exhibit hyperplasia and hypertrophy. These processes are linked to intracellular aberrations in adipocyte functions especially mitochondrial and endoplasmic reticulum stress.8 Other harmful effects of obesity include augmented blood pressure levels, atherosclerosis, osteoarthritis and various respiratory problems like sleep apnea,9,10 some cancers such as breast cancer and prostate cancer.7
Obesity should be minimized to have a better lifestyle. For this purpose, drug therapy needs to be employed with increased physical activity, balanced diet and behavioral therapy.11 This study explores the anti-adipogenic potential of Cissus quadrangularis for which pre-adipocyte cell line 3T3-L1 derived from 3T3 cells of mouse has been used for experimental purpose. This cell line has a played a vital role in advancement of understanding the fundamental cellular mechanisms linked to obesity, diabetes and accompanying complex disorders.12 These cells possess fibroblast like morphology and under suitable conditions can differentiate into mature adipocytes.13 Differentiation of 3T3-L1 into adipocytes is accompanied by adiponectin and leptin production, accumulation of yellow colored lipid droplets and expression of adipocyte markers.14
This study aims at isolating specific active chemical of C. quadrangularis which has anti-adipogenic property. It is expected that this study will pave a way for development of a fruitful remedial drug for obesity, which is emerging as a serious medical condition.
II. MATERIALS AND METHODS
A. Chemicals
The reagents for cell culturing used in this study were purchased from ThermoFisher Scientific (California, US), chemicals were purchased from Sigma Aldrich (now Merck, KGaA, Darmstadt, Germany), and plasticware were purchased from Nunc™ (Nunc Cell Culture, ThermoFisher Scientific, California, US) unless stated otherwise
B. CQ Fractionation and Sub-Fractionation
The air dried CQ herb was grounded and passed through 40-mesh sieve to collect fine powder. To prepare crude ethanolic extract, finely ground powder was soaked in absolute ethanol (cat# 1070174000) at 1:4 in a conical flask and kept in shaking incubator (Orbital incubator model 1–4000, IRMECO GmbH, Germany) at 110 rpm, 30°C for 48h. The ethanolic extract was then filtered through 0.22μm filtration assembly (Nalgene™ Rapid-Flow™ sterile disposable filtration system - 2923320). The procedure was repeated thrice with the residue for maximum extraction. The filtrate was collected after repeated extractions and subjected to rotary evaporation (Heidolph Labrota 4000-efficient) at 60°C, 20 rpm. A sticky brown mass was obtained at the end.
Fractionation was carried out by solvent-solvent extraction (1:1) of crude ethanolic extract using n-hexane (cat# 4371), dichloromethane (cat# 644501), ethyl acetate (cat# K1500364) and n-butanol (cat# K15461288). The four fractions i.e. n-hexane (CQ-H), dichloromethane (CQ-D), ethyl acetate (CQ-EA) and n-butanol (CQ-B) fractions were concentrated by rotary evaporation (60°C, 20 rpm), followed by concentration by cooling trap rotating evaporator (Christ-RVC 2–18 CD Plus) and eppendorf concentrator (Hamburg Eppendorf concentrator-AG 22331) to obtain completely dried sticky fractions. Fractions were stored at −20°C until further use. This study explores anti-adipogenic potential of CQ-B fraction.
Sub-fractionation of CQ-B was carried out by HPLC (Sykam System with peak simple software for analysis) using semi-preparatory C-18 column (cat# AG880975–202 Agilent Technologies, California, US). CQ-B sample 10mg/ml was prepared in absolute ethanol, containing 0.2% Trifluoroacetic acid (cat#302031) and filtered through 0.22μm syringe filter. Running conditions included: injection volume 5ml, mobile phase: distilled water (3 min hold) and 0 – 100% gradient of acetonitrile (cat# 34998) with 1% ramp), flow rate 2ml/min, and detection at 238nm. HPLC was run multiple times, flow through (5ml) of each run was collected in labeled conical tubes, concentrated and stored at −20°C until use.
C. Maintenance of 3T3-L1 Cell Line
Murine pre-adipocyte cell line 3T3-L1 MBX (ATCC® CRL-3242™) competent to differentiate into adipocytes upon induction, was acquired from ATCC. The cells were maintained in Dulbecco modified Eagle medium (DMEM) (SLM-120-B, Millipore, Burlington, Massachusetts, USA), containing 10% FBS, 1% penicillin/streptomycin under standard culture conditions. Cells were passaged at 60 – 70% confluence. Cells of passage number 5 – 20 were used for the study. All experiments were carried out in technical and biological triplicates. For all experiments, cells were seeded at 3000 cells/cm2 density.
D. Cell Viability, Proliferation, and Growth Analysis
To find the optimum concentration of CQ-B for 3T3-L1 cells, the cells were passaged and seeded in 24-well cell culture plate (cat# 142475) in independent experiments. After 24h, complete growth medium was replaced with fresh complete growth medium supplemented with different concentrations of CQ-B such as 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 and 200μg/ml. Treatment lasted for 72h with a single change of medium after 48h.
Cell viability of 3T3-L1 cells was assessed by neutral red assay according to Repetto et al. (2008). Metabolic activity and proliferation of cells were determined by MTT (3–4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide) assay and BrdU (5-bromo-2-deoxyuridine) incorporation assay. Cells after 72h treatment were labeled with MTT (cat# M6494) for 4h, destained with acidified isopropanol (0.1N HCL in isopropanol) and absorbance was taken at 570nm using BioTek Elx-808 absorbance reader. For cell proliferation, BrdU cell proliferation kit (Roche Pakistan Limited, Lahore, Pakistan – cat # 11647229001) was used as per manufacturer’s instructions
For generating growth curve, 3T3-L1 cells were seeded in 24-well culture plate. Cells were harvested and counted by hemocytometer (Counting chamber, Improved Neubauer Type without snap on clips, John Barron, Raegecon, Germany; Raegecon Diagnostics Ltd., Clare, Ireland) on day 1, 3, 5 and 7 of treatment with change of medium after 48h. Growth curve indicating lag, log (exponential), stationary and decline phases of cells was plotted by taking logarithmic number of cells on y-axis and duration of treatment on x-axis. Doubling time and specific growth rate were determined as described by Butler.15 All experiments were conducted in technical and biological triplicates.
E. Adipogenic Differentiation
3T3-L1 pre-adipocyte cell line was seeded in 6-well cell culture plates (cat # 140675) in normal complete growth medium. Adipogenic induction medium (complete growth medium supplemented with 0.5mM 3-Isobutyl-1-methylxanthine (IBMX) (cat# I708), 100nM insulin and 0.25μM dexamethasone) supplemented with mitogenic doses of CQ-B (0.0001, 0.01, 1, and 10μg/ml) and 0.05% DMSO (positive control cultures) was added to 48h post-confluence cultures. Control cultures were treated with complete growth medium supplemented with 0.05% DMSO. Medium was replaced with fresh adipogenic induction medium (complete growth medium supplemented with 100nM insulin only) after 48h. Cells were allowed to differentiate into adipocytes for 9 days after induction. Trizol was added to the plates at day 0, 1, 3, 6 and 9 of adipogenic induction. Samples were also fixed with 10% NBF on day 9 for staining.
For analysis of CQ-B subfractions (1 – 27), cells were induced to differentiate as mentioned above and treated with 0.1 and 1μg/ml of each sub-fraction with appropriate controls. ORO staining and semi-quantification of neutral lipids was carried out to access the anti-adipogenic sub-fractions. The sub-fraction with ≥40% reduction in neutral lipids was considered effective anti-adipogenic sub-fraction. Experiments were conducted independently in biological triplicates.
F. Oil Red O Staining and Semi-Quantification of Neutral Lipids
Oil red O stains neutral lipids. After 9th day of adipogenic induction, cells were washed with 1x PBS and fixed with 10% NBF for 15 min at 4°C. The fixed cells were washed with distilled water, followed by rinsing with 60% isopropanol. Oil Red O stain (O0625 Sigma Aldrich) was prepared by dissolving 5mg of stain in 60% isopropanol. Stain was filtered and added to the wells for 20 min. After The stained cells were washed with distilled water to remove excessive stain. Images were taken from Nikon Eclipse TS-100 inverted microscope at 10x magnification.
For semi-quantification of lipid droplets, isopropanol was added to the stained cells for 1 – 2 min to destain the cells. Absorbance was taken at 570nm using BioTek Elx-808 ELISA absorbance reader. Dye up-taken by cells was neutralized by subtracting the absorbance of control cells (negative control) from positive control and CQ- solvent fractions treated cells. Percentage of dye up-take was calculated taking value for positive control cells as 100%.
G. Triglycerides Estimation
After 9 days of adipogenic induction, the cells were incubated with 1:1 mixture of hexane and chloroform for 1h to remove all the lipids from the cells. Hexane and chloroform were removed by using eppendorf vacuum centrifuge at 30°C. Triglycerides (TG) reagent (Analyticon – 5052) was used to estimate TGs in differentiated adipocytes. Triglycerides mixture (I7811 AMP – Sigma Aldrich) was used to prepare TG standards (0, 10, 20, 30, 40 and 50μg/ml). Concentrated lipids were incubated with TG reagent as per manufacturer’s instructions. Absorbance of samples and standards was taken at 570nm using BioTek Elx-808 ELISA reader. Standard curve was plotted and TGs were estimated for the samples using Microsoft Excel spreadsheet 2016.
H. Relative Quantification of Adipocyte Marker Genes
Total RNA was isolated from the trizol samples as per manufacturer’s instructions. Isolated RNA was treated with DNase I (Ambion, ThermoFisher Scientific – Cat# AM2222). Purification was carried out using PureLink™ RNA mini kit (Cat # 12183018A). Complementary DNA was synthesized by using first strand cDNA synthesis kit (Cat # K1622). Real Time PCR was performed for relative quantification of osteoblast marker genes, using Maxima SYBR Green/Rox qPCR master mix – 2x – (Cat # K0222), cDNA (2.5ng/μl) and 500nM forward and reverse primers (Table I). Two-step protocol with initial denaturation at 95°C for 3min and 40 cycles of denaturation at 95°C for 30s, annealing/ extension at 60°C for 30s was used to amplify cDNA on PikoReal Real-Time PCR system (ThermoFisher Scientific). For melt curve analysis, data were generated at 60°C – 90°C with 0.2°C increment. Expression of housekeeping gene, hydroxymethylbilane synthase (HMBS), was used to normalize the expression of adipocyte and lipogenic marker genes such as peroxisome proliferation-activated receptor-gamma (PPAR-γ), adiponectin (ADIN), fatty acid binding protein-4 or adipocyte protein-2 (FAB4 or AP2), leptin (LPN), fatty acid synthase (FASN), sterol regulatory element binding protein-1 (SREBP-1), steroyl-CoA desaturase-1 (SCD-1), lipoprotein lipase (LPL), acetyl-CoA carboxylase-alpha (ACACα), and acetyl-CoA carboxylase-beta (ACACβ). The sequences of primers for all marker genes are given in Table I. Relative expression (fold) was calculated by taking ratio of expression of osteoblast marker genes under experimental conditions to the expression of marker genes under control conditions.
Table I:
Details of primers used for quantitative determination of relative expression of adipocyte and lipogenic marker genes by real time PCR
| Primer ID | Gene | Sequence 5’ – 3’ |
|---|---|---|
| PPARγF PPARγR |
Peroxisome Proliferator Activator Receptor γ | TCGCTGATGCACTGCCTATG GAGAGGTCCACAGAGCTGATT |
| ADINF ADINR |
Adiponectin | TGTTCCTCTTAATCCTGCCCA CCAACCTGCACAAGTTCCCTT |
| LEPF LEPR |
Leptin | GAGACCCCTGTGTCGGTTC CTGCGTGTGTGAAATGTCATTG |
| FAB4F FAB4R |
Fatty Acid Binding Protein −4 | AAGGTGAAGAGCATCATAACCCT TCACGCCTTTCATAACACATTCC |
| SREBP1cF SREBP1cR |
Sterol Regulatory Binding Element 1c | CCGTCACTTCCAGCTAGACC GGTGCCTACAGAGCAAGAGG |
| FASNF FASNR |
Fatty Acid Synthase | TGGGTTCTAGCCAGCAGAGT ACCACCAGAGACCGTTATGC |
| SCD1F SCD1R |
Steroyl CoA Desaturase | CGAGGGTTGGTTGTTGATCT GCCCATGTCTCTGGTGTTTT |
| LPLF LPLR |
Lipoprotein Lipase | CATAGAGGGTCCTGCTCTGC CTTTCCACTGCGACTCTTCC |
| ACACAF ACAFAR |
Acetyl CoA Carboxylase Alpha | TCCACGAAAAGAGCTGACCT ACTAAGGATGCTCCCCACCT |
| ACACBF ACACBR |
Acetyl CoA Carboxylase Beta | TGGAGTCCATCTTCCTGTCC CGTAGGCGATGTAGCCTCTC |
I. Gas Chromatography/ Mass Spectrometry (GC-MS)
To identify the chemical constituents in effective sub-fractions of CQ-B, GC-MS analysis was performed. For sample preparation, 10mg/ml solution of effective sub-fractions was prepared in n-butanol and was filtered through 0.22μm syringe filter (MiniSart-16534-K).
GC-MS analysis was performed using GC (Clarus 500 Perkin Elmer Gas Chromatogram system) consisting of AOC-20i auto-sampler and MS (Clarus 500 Perkin Emer Mass Spectometer system) interfaced with silica capillary column (30mm x 0.25mm I.D. x 1μm df, composed of 100% dimethylpolysiloxane). GC-MS was run with inlet temperature of 250°C, oven temperature set at 110°C to 270°C with 4°C ramp, and ion source temperature set at 280°C. Sample (0.5μl) with 10:1 split ratio was injected using micro-syringe with 99.999% helium as carrier gas with flow rate of 1ml/min. Mass spectra were collected using electron impact mode at 70eV, scan interval of 0.5 seconds and fragment size ranging from 45 to 450Da. The profile run was of 41 min.
The identification of the compound was carried out based on the retention time and the mass spectrum of unknown compound(s) with a library of known compounds (NIST library). The spectrum of each unknown compound was matched with that of known compound in the library and compounds predicted based on the mass spectra that matched the best with the unknown compound.
J. Statistical Analyses
Data were represented as mean ± SD of technical and independent biological replicates. Statistical analyses (when required) were conducted by Student’s t test, one-way analysis of variance (ANOVA) followed by multiple comparison by Dunnett’s test, with statistical significance when p ≤ 0.05 (95% confidence interval) using Graphpad Prism 7.03 software.
III. RESULTS
A. Effect on Cell Viability, Proliferation and Growth Parameters
Figure 1 shows the effect of CQ-B fraction on the viability, metabolic activity, proliferation, and growth parameters of 3T3-L1 cell line. For viability analysis, the cells were treated with 0.0001, 0.001, 0.01, 0.1, 1, 10, 100, and 200μg/ml of CQ-B. We observed that compared to control cells, lower doses of CQ-B enhanced (P≤0.05) viability of the cells. However, significant reduction in viability was observed at higher doses of CQ-B (Fig. 1A). EC50 was calculated to be 207.9μg/ml. The doses 0.0001, 0.01, 1 and 10μg/ml were selected for further experiments.
Fig. 1.
Effect of different concentrations of CQ-B on Cell viability (A), Metabolic activity (B), Cell proliferation (C), and Growth curve (D) of 3T3-L1 cells in independent experiments. Cell viability was estimated by neutral red assay. Concentration range of 0.0001μg/ml - 200μg/ml was tested and the concentrations with ≥95% cell viability were selected for further analyses. Cell metabolic activity and cell proliferation were assessed by MTT and BrdU assay respectively. For growth curve, the cells were harvested and counted on day 1, 3, 5 and 7 of treatment. In all experiments, the control was treated with 0.05% DMSO. Viability (%), Metabolic activity (%), Proliferation (%) was determined taking control as 100%. Data has been presented as mean ± SD of technical and biological triplicates. Data were analyzed by one-way ANOVA and multiple comparison was carried out by Dunnett test with 95% confidence interval using Prism 7 software (p≤0.05) (ns ≥ 0.05, * P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001).
Compared to control cells, we observed significant increase in metabolic activity of cells when treated with aforementioned selected doses of CQ-B. Cells treated with 0.01, 1 and 10μg/ml CQ-B showed 18.5%, 25.6% and 23.4% enhanced metabolic activity respectively. Whereas, metabolic activity of cells treated with 0.0001μg/ml CQ-B was non-significantly enhanced compared to control cells (Fig. 1B).
The cell proliferation in terms of BrdU incorporation in the DNA was found to be reduced compared to control cells, when treated with the same doses of CQ-B. The proliferation was comparable to control in 0.0001 CQ-B treated cells. Treatment with10μg/ml CQ-B, cells showed >90% cell proliferation. The cells treated with 0.01 and 1μg/ml had reduced proliferation (P≤0.05), however as the proliferation in both cases was ~85% (15% reduction compared to control cells) both concentrations were considered as non-detrimental for the cells (Fig. 1C).
Figure 1D shows the growth curve after treatment with 0.0001, 0.01, 1, and 10μg/ml of CQ-B. For all these doses, the growth curves are comparable to those of control cells. Doubling time was 24.1h for control cells and 23.4, 23.6, 23.5 and 23.5h for 0.0001, 0.01, 1, and 10μg/ml CQ-B treated cells, respectively. Specific growth rate was found to be 0.013 for control cells and 0.014 for treated cells.
B. Effect on Adipogenic Differentiation and Lipogenesis
To analyze the anti-adipogenic effect of CQ-B, 3T3-L1 cells were differentiated into adipocytes by feeding them adipocyte induction medium supplemented with the selected concentrations (0.0001, 0.01, 1 and 10μg/ml) of CQ-B). The control cultures had normal complete growth medium supplemented with 0.05% DMSO. Positive control cells were fed with adipocyte induction medium supplemented with 0.05% DMSO. The cells were grown to confluence and induction was carried out in 48h post-confluence cultures.
After 24h of induction, changes in cell morphology were observed. The cells excreted extracellular matrix and acquired elongated shape up to 48h. Some of the cells started attaining oval morphology after 72h of induction. In day 4 post induction cultures, small oil droplets started appearing in oval cells. The formation of oil droplets continued afterwards. By 9th day of induction, the cells were fully differentiated into adipocytes.
Oil red O (ORO) staining was carried out at the end of adipogenic differentiation to confirm formation of neutral lipid droplets in the cultures, thus indicating formation of mature adipocytes. Semi-quantification of neutral lipids was carried out by destaining and quantifying ORO stain. Furthermore, to establish anti-adipogenic effect of CQ-B, estimation of triglycerides was carried out in the mature adipocytes at the end of differentiation. Expression of adipogenic and lipogenic marker genes was carried out to study the anti-adipogenic effect of CQ-B at molecular level.
The cells treated with non-cytotoxic concentrations of CQ-B during differentiation exhibited a smaller number of adipocytes compared to the positive control (Fig. 2). We observed formation of ECM in day 3 and in day 6 cultures; formation of lipid droplets was observed. In positive control cultures, mature adipocytes with oil droplets were observed in day 9 cultures (Fig. 3). The cultures treated with non-cytotoxic concentrations of CQ-B had lesser number of adipocytes compared to positive control cells in day 9 cultures that exhibit potential anti-adipogenic potential of CQ-B solvent fraction.
Fig. 2.

Effect of mitogenic concentrations of CQ-B (0.0001, 0.01, 1 and 10μg/ml) on adipocyte differentiation of 3T3-L1 cell line. The control cells were treated with 0.05% DMSO supplemented medium. Positive control cells were treated with 0.05% DMSO supplemented adipocyte induction medium. The CQ-B treated medium had respective concentrations of CQ-B in adipocyte induction medium. Cells were differentiated after 9 days of adipocyte induction.
Fig. 3.

Differentiated 3T3-L1 pre-adipocytes in response to treatment with different concentrations of CQ-B 9 days after induction. Control (undifferentiated) cells were fed with 0.05% DMSO supplemented normal complete medium and do not have any adipocytes. Positive control cells were induced to differentiate into adipocytes. Adipogenic medium was supplemented with 0.05% DMSO. CQ-B treated cells had induction medium supplemented with different concentrations of CQ-B. The cultures treated with CQ-B show lesser number of adipocytes compared to positive control cells. Moreover, CQ-B treated cultures do not show properly differentiated adipocytes or lipid droplets as seen in the positive control culture. The arrows show differentiated adipocytes.
Neutral lipids formed in differentiated adipocytes were stained with ORO stain (Fig. 4). It can be observed that positive control culture has more stained adipocytes indicating differentiation of cells into adipocytes and formation of neutral lipids. In CQ-B treated cells, the number of stained cells is less compared to positive control culture. To semi-quantify adipogenesis in positive control and CQ-B treated cultures, ORO was destained and absorbance was measured. Comparison of values of CQ-B treated cultures taking positive control cultures as 100% indicated anti-adipogenic potential of CQ-B. The cells treated with CQ-B concentrations showed 36.5% and 30.1% reduction in lipid content of the differentiated cells treated with 0.0001 and 0.01μg/ml CQ-B respectively. The reduction was found to be significant (P≤0.05) in comparison to the positive control. The cells treated with 1 and 10μg/ml showed 14.6% and 28.4% decrease in lipid content of the cells that was not significant compared to positive control cells (Fig. 5A).
Fig. 4.

ORO stained differentiated 3T3-L1 pre-adipocytes in response to treatment with different concentrations of CQ-B 9 days after induction. Control (undifferentiated) cells were fed with 0.05% DMSO supplemented normal complete medium and do not have any adipocytes. Positive control cells were induced to differentiate into adipocytes. Adipogenic medium was supplemented with 0.05% DMSO. CQ-B treated cells had induction medium supplemented with different concentrations of CQ-B. The cultures treated with CQ-B show a smaller number of adipocytes compared to positive control cells.
Fig. 5.
(A) Semi-quantification of lipid content in the differentiated adipocytes treated with mitogenic concentrations of CQ-B (0.0001, 0.01, 1 and 10 μg/ml) determined by ORO staining method. The ORO content was quantified using ELISA reader. The ORO content is proportional to lipid content of the cells; therefore, comparison with the positive control (100%) gives relative lipid content of the cells. (B). Estimation of triglycerides in differentiated adipocytes treated with mitogenic concentrations of CQ-B (0.0001, 0.01, 1 and 10 μg/ml). The data are presented as Mean ± SD of technical and biological triplicates. (P≤0.05; ns = not significant, * P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001).
To estimate the extent of anti-adipogenic effect of CQ-B on the differentiation of 3T3-L1 into adipocytes, total lipids were isolated from the cells and triglycerides were estimated using standard curve. The triglyceride content was normalized with the undifferentiated cells (Control). Treatment with 0.0001 μg/ml CQ-B significantly reduced (P≤0.05, 51.3%) the triglycerides content of the cells compared to positive control cells. However, in the case of treatment with 0.01, 1 and 10μg/ml, the reduction was not significant (Fig. 5B).
C. Relative Quantification of Adipogenic and Lipogenic Genes
Differentiation process is accompanied by changes in gene expression with time. In adipocytes, the differentiation is accompanied by formation of lipid droplets in the cells that indicate formation of mature adipocytes. To get an insights of molecular processes involved in adipogenesis and lipogenesis, and to study the effect of CQ solvent fractions on the process, real time expression of adipocyte and lipogenic marker genes was studied. The marker genes selected for the study were PPAR-γ, ADIN, FAB4/AP2, LPN, FASN, SREBP-1, SCD-1, LPL, ACACα, and ACACβ.
Total RNA was isolated from differentiating 3T3-L1 cells during different stages of the process on day0, day1, day3, day6 and day9 of adipogenic induction. c-DNA was synthesized and real time PCR each of adipogenic and lipogenic marker gene was performed.
PPAR-γ is a transcription factor and a key regulator of adipogenic differentiation. It controls the expression of most of adipocyte marker genes, therefore it is expressed during early phases of adipocyte differentiation. It was observed that the expression of PPAR-γ in the untreated cells increased 24h after induction and peaked 72h after induction. Compared to the positive control cells, PPAR-γ expression was downregulated 77.5% in differentiated cells treated with 0.0001μg/ml CQ-B, 71% in cells treated with 0.01 μg/ml CQ-B, 69% in cells treated with 1 μg/ml CQ-B and 80% in cells treated with 10 μg/ml CQ-B 72h after adipogenic induction (Fig. 6). The downregulation of PPAR-γ expression indicates the inhibition of adipogenic differentiation of 3T3-L1 cells indicating anti-adipogenic potential of CQ-EA, CQ-B, CQ-H and CQ-D. In all cases, the un-induced, untreated cells (Control) had no expression of PPAR-γ indicating the undifferentiated state of the cells.
SREBP-1 gene encodes protein that acts as transcription factor and regulates expression of genes that play role in biosynthesis of sterol. SREBP-1 is also one of early phase genes during adipocyte differentiation. In untreated induced cells (positive control) maximum upregulation in SREBP-1 expression was observed after 72h of adipogenic induction. In the cells treated with CQ-B, the expression of SREBP-1 was delayed compared to the positive control. The maximum upregulation was observed 6 days after adipogenic induction in the treated cells. Compared to expression of SREBP-1 in day 3 positive control cells, treatment with 1 and 10μg/ml CQ-B downregulated expression by 36% and 28%, respectively. Whereas, in comparison to the expression of SREBP-1 in positive control (day 3), the downregulation was 79% both in 0.0001 and 10μg/ml CQ-B treated cells (Fig. 6).
SCD-1 gene encodes a key enzyme that plays a role in the formation of monounsaturated fatty acids specifically palmitioleate and oleate from palmitoyl-CoA and steroyl-CoA, respectively. As the formation of lipid droplets starts after 72 – 96h of adipocyte induction, the expression is observed in the early phase of adipocyte differentiation. Peak expression of SCD-1 gene was observed after 72h of adipogenic induction in positive control cells. Expression of SCD-1 was downregulated 74% in cells treated with 1 μg/ml CQ-B. The expression of SCD-1 was delayed and was downregulated 69%, 73% and 88% after treatment with 0.0001, 0.01, and 10μg/ml CQ-B, respectively when compared to SCD-1 expression in day-3 positive control cells (Fig. 6).
FASN is an enzyme that is involved in the synthesis of triglycerides. The expression is observed in adipocytes when the cells start forming oil droplets. It is a late phase gene during adipocyte differentiation. Maximum expression of FASN was observed 6 days after induction in the positive control cells. Compared to positive control cells, CQ-B treatment did not affect FASN expression except for treatment with 0.0001μg/ml CQ-B dose that showed 94% downregulation on day 6 and 67% downregulation on day 9 (Fig. 6) indicating delayed lipid synthesis in 0.0001 μg/ml CQ-B treated cells.
FAB4/AP2 is a carrier protein that is involved in transport, uptake and metabolism of fatty acids. It is mainly expressed by adipocytes and hence is an adipocyte marker gene. In the positive control cells, the upregulated expression of AP2/FAB4 was observed during day 3–6 of adipogenic induction. In the cells treated with CQ-B, compared to positive control (day-6), 53% and 51% downregulation was observed in 0.01 and 1μg/ml CQ-B treated cells, respectively, whereas 92.5% and 80% downregulation was observed in FAB4/AP2 expression in 0.0001 and 10μg/ml CQ-B treated cells, respectively (Fig. 6).
LPL is an enzyme that is involved in the breakdown of fats into triglycerides and their uptake to be transported to adipose tissue for storage as fats. It is produced by mature adipocytes and is a late phase gene to be expressed during adipogenesis. In untreated induced cells, the expression of LPL starts after 72h of induction and maximizes after 6 days of induction. In CQ-B treated cells, in comparison to positive control, the expression of LPL was 77%, 29%, 53% and 70.5% downregulated in 0.0001μg/ml, 0.01μg/ml, 1μg/ml and 10μg/ml CQ-B treated cells, respectively (Fig. 6).
ACACα enzyme catalyzes conversion of acetyl-CoA to malonyl-CoA, which is a rate-limiting step of biosynthesis of fatty acids. In positive control cells, maximum expression of ACACα was observed after 6 days of induction. The expression of ACACα was found to be 83%, 99.5%, 76.5%, and 72% downregulated in 0.0001μg/ml, 0.01μg/ml, 1μg/ml and 10μg/ml CQ-B treated cells, respectively compared to positive control cells (Fig. 6).
ACACβ plays role in the inhibition of glucose and fatty acid oxidation and increasing of fat storage. It is speculated to play a role in mitochondrial oxidation of fatty acids via inhibition of carnitine palmitoyltransferase 1 by malonyl-CoA. In the positive control cells, the maximum expression of ACACβ was observed after 6 days of induction. The expression of ACACβ was 75%, 37%, 31% and 79.5% downregulated compared to positive control (day 6) in 0.0001μg/ml, 0.01μg/ml, 1μg/ml and 10μg/ml CQ-B treated cells, respectively (Fig. 6).
ADIN is an adipocytokine or hormone that are secreted by mature adipocytes. Expression of ADIN is specific to the adipocytes; therefore, hence considered as marker of differentiated adipocytes. ADIN n plays a role in the breakdown of fatty acids and glucose metabolism. It is expressed during the late phase of adipocyte differentiation. Maximum expression of ADIN was observed 6 days after induction in the positive control cells. Compared to the positive control (day 6), 82%, 87%, 48.5% and 74% downregulation was observed in the expression of ADIN (Fig. 6) in 0.0001μg/ml, 0.01μg/ml, 1μg/ml and 10μg/ml CQ-B treated cultures, respectively.
LPN is another adipocytokine (hormone) that is secreted by mature adipocytes and is involved in energy regulation, utilization of fat and inhibition of hunger, thus inhibiting the storage of fats in adipocytes. LPN gene expression was upregulated 6 days after adipocyte induction in untreated induced cells. Compared to the positive control (day 6), 82%, 87%, 48.5% and 74% downregulation was observed in the expression of ADIN (Fig. 6) in 0.0001μg/ml, 0.01μg/ml, 1μg/ml and 10μg/ml CQ-B treated cultures, respectively.
The expression profiles of adipogenic and lipogenic marker genes correlated with the results of triglycerides estimation and semi-quantification of neural lipids by ORO staining and indicated anti-adipogenic potential of CQ-B.
Fig. 6.
Expression profile of adipocyte and lipogenesis marker genes (PPAR-γ, SREBP-1, SCD-1, FASN, FAB4/aP2, LPL, ACACA, ACACB, ADIN and LPN) during the process of differentiation of 3T3-L1 cells into adipocytes treated with non-detrimental concentrations of CQ-B (0.0001, 0.01, 1 and 10μg/ml). The control culture was treated with 0.05% DMSO in normal complete medium. Positive control cultures were treated with adipogenic induction medium supplemented with 0.05%DMSO only. CQ-B treated cells had adipogenic induction medium supplemented with non-detrimental concentrations of CQ-B. The cells were allowed to differentiate for 9 days with medium change after 48h. Total RNA was isolated and cDNA was synthesized. Real-time PCR was used to analyze the relative expression profile of adipocyte and lipogenic marker genes with time during the process of adipogenesis. The target genes were normalized using Ct values of HMBS as housekeeping gene. Relative expression (n-fold) of target genes was calculated as ratio of relative expression of target genes (normalized to HMBS) under experimental conditions to the relative expression of target genes (normalized to HMBS) under control conditions.
D. Bioactivity Analysis of CQ-B Subfractions
CQ-B fraction was sub-fractionated by HPLC to isolate and identify the bioactive constituents. Twenty-seven sub-fractions were collected and labeled as B-1 – B-27. Based on the cell viability analysis of CQ-B, 0.1 and 1μg/ml doses of each sub-fraction were checked for their effect on cell viability of 3T3-L1 cells by neutral red uptake assay. We observed that all sub-fractions had no detrimental effect on viability of the cells. Compared to control cells (0.05% DMSO treated cells) taken as 100%, cells treated with CQ-B subfractions had increased cell viability (Fig. 7).
Fig. 7.
Cell viability of 3T3-L1 cells when treated with 0.1μg/ml and 1μg/ml of CQ-B sub-fractions. Both doses of twenty-seven sub-fractions (B-1 – B-27) were tested in independent experiments. Both doses were found to be non-cytotoxic for the cells. Compared to control (0.05% DMSO treated cells) taken as 100%, increased viability of cells was observed in some cultures, however, the increase was non-significant (P≥0.05). Data are presented as Mean ± SD of technical and biological replicates. (P≤0.05; ns = not significant, * P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001).
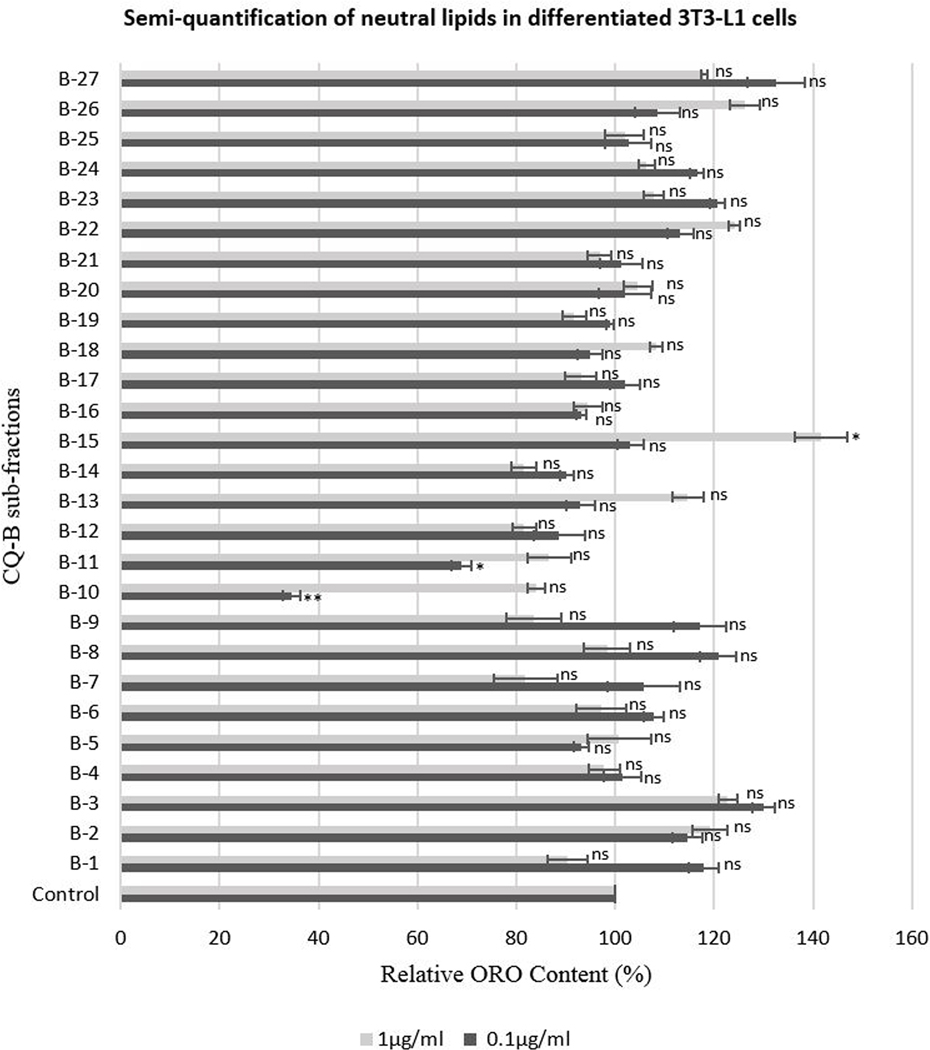
3T3-L1 cells were allowed to differentiate into adipocytes and treated with 0.1 and 1μg/ml concentrations of CQ-B subfractions (B-1 – B-27), with appropriate controls, following aforementioned procedure. Neutral lipids in differentiated cells were stained with ORO stain and semi-quantification of neutral lipids was performed as described previously. We observed significant reduction (P≤0.05) in relative ORO content (%) of differentiated cells treated with 0.1μg/ml B-10 and B-11 subfractions (Fig. 8). Relative ORO content was reduced by 65.5% and 31.1% in cells treated with 0.1μg/ml B-10 and B-11 subfractions, respectively. In the cells treated with other sub-fractions, relative ORO content was comparable to positive control. The results indicated presence of anti-adipogenic compounds in B-10 and B-11 subfractions.
Fig. 8.
Semi-quantification of lipid content in the differentiated adipocytes treated with mitogenic concentrations of CQ-B (0.1 and 1μg/ml) determined by ORO staining method. The ORO content was quantified using ELISA reader. The ORO content is proportional to lipid content of the cells; therefore, comparison with the positive control (100%) gives relative lipid content of the cells. The data are presented as Mean ± SD of technical and biological triplicates. (P≤0.05; ns = not significant, * P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001).
To identify the chemical constituents in B-10 and B-11, these subfractions were subjected to GCMS. Seventeen compounds (1) Phenol, 2,4-bis(1,1-dimethylethyl), (2) 2-propyn-1-ol, propionate, (3) Oxazole, 4,5-dihydro-2,5-dimethyl, (4) 2-nonadecanone 2,4-dinitrophenylhydrazine, (5) 3-hexanol, 2-methyl, (6) Pentane, 2-methoxy-2,4,4-trimethyl, (7) Oxalic acid, ethyl neopentyl ester, (8) 1,3-dioxolane, 2-pentadecyl-, (9) 3-hexanol, 2-methyl-, (10) 2-hydroxy-2-methylbutyric acid and 1-hexen-4-ol, 1-chloro- 3,5-dimethyl, (11) Cis-inositol, (12) butanedioic acid, 2-[(tert-butyldimethylsilyl) oxy]-, bis (tert- butyldime, (13) 1,2,5,6-di-o isopropylidene-3-o-methanesulfonyl glucofuranose, (14) 1-hexen-4-ol, 1-chloro-3,5-dimethyl-, (15) 9-octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl) methyl ester, (16) Glucitol, 6-o-octyl-, (17) Benzene propanoic acid, alpha -[[(trimethylsilyl)oxy] imino]-, trimethyl-, were identified in B-10 subfraction. Figure 9A shows the gas chromatogram of B-10 subfraction. The predicted compounds along with their reported biological activities are listed in Table II. Five of these compounds have reported anti-adipogenic activity that account for the anti-adipogenic potential of B-10 subfraction.
Fig. 9.
(A) GCMS chromatogram of B-10 subfraction, (B) GCMS chromatogram of B-11 subfraction.2
Table 2.
List of predicted compounds in B-10 sub-fraction with their reported bioactivities
| Sr. No. | Retention time (min) | Predicted compounds | Biological Activity |
|---|---|---|---|
| 1 | 16.16 | Phenol, 2,4-bis(1,1-dimethylethyl) | Antioxidant, Antimicrobial, analgesic, anti-inflammatory and anti-adipogenic activity (Alghamdi et al.44). |
| 2 | 25.57 | 2-propyn-1-ol, propionate | Not identified |
| 3 | 26.62 | Oxazole, 4,5-dihydro-2,5-dimethyl | Anti-inflammatory activity (Zhang et al. 45). |
| 4 | 30.38 | 2-nonadecanone 2,4-dinitrophenylhydrazine | Not identified |
| 5 | 32.29 | 3-hexanol, 2-methyl- | Not identified |
| 6 | 33.91 | Pentane, 2-methoxy-2,4,4-trimethyl | Not identified |
| 7 | 34.06 | Oxalic acid, ethyl neopentyl ester | Used as anti-obesity drug (Kalayoglu and Singer46) |
| 8 | 34.20 | 1,3-dioxolane, 2-pentadecyl- | Not identified |
| 9 | 34.28 | 3-hexanol, 2-methyl- | Precursor of HIV-1 protease inhibitor (Ziółkowska et al.47) |
| 10 | 34.40 | 2-hydroxy-2-methylbutyric acid and 1-hexen-4-ol, 1-chloro- 3,5-dimethyl- | Anti-diabetic effect (Gao et al. 48). |
| 11 | 34.42 | Cis-inositol | Anti-adipogenic effect (Rapiejko et al. 49) |
| 12 | 34.436 | Butanedioic acid, 2-[(tert-butyldimethylsilyl) oxy]-, bis (tert- butyldime | Not identified |
| 13 | 34.54 | 1,2,5,6-di-o isopropylidene-3-o-methanesulfonyl glucofuranose | Suppress Appetite https://www.google.com/patents/CN100378118C?cl=en |
| 14 | 34.73 | 1-hexen-4-ol, 1-chloro-3,5-dimethyl- | Not identified |
| 15 | 37.65 | 9-octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl) methyl ester | Anti-bacterial activity (Rodríguez et al. 50) |
| 16 | 38.32 | Glucitol, 6-o-octyl- | Anti-adipogenic effect (Conforti and Pan 51). |
| 17 | 38.62 | Benzene propanoic acid, alpha -[[(trimethylsilyl)oxy] imino]-, trimethyl- | Not identified |
Twenty-nine compounds (1) 1,2:5,6 Dianhydrogalactitol, (2) 1,3-propanediol, 2-(hydroxymethyl)-2-nitro-, (3) 2,4,4-trimethyl-1-pentanol, (4) sydnone, 3,3’-tetramethylenedi-, (5) 1-propanol, 2-methyl-2-[(2-methyl-2-propenyl)oxy]-, (6) 1,3-propanediol, 2-(hydroxymethyl)-2-nitro-, (7) nonadecane, 1-bromo-, (8) 4-undecene, 2-methyl-, (E)-, (9) piperazine-1,4-dicarboxylic acid di-tert-butyl ester, (10) 2-propenoic acid, butyl ester, (11) oleic acid, (12) 3,4-hexanediol, 2,5-dimethyl-, (13) trifluoromethyl T-butyl disulfide, (14) 3-pentanol, 2-chloro-4-methyl-, (R*,S*)-(.+/−.)-, (15) 3-hexanol, 2,2-dimethyl-, (16) 4-tetradecanol, (17) 3-chloro-2,2-dimethyl-1-propanol, (18) 2-pteridinamine, 6-methyl-N,N-bis(trimethylsilyl)-4-[(trimethylsilyl)oxy, (19) dodecane, 1-fluoro-, (20) 2-T-butylperoxy-2-ethylbutan-1-ol, propionate ester, (21) oxalic acid, ethyl neopentyl ester, (22) benzoic acid 1-methoxy-1h-tetrazol-5-ylmethyl ester, (23) 3-hexanol, 2,2-dimethyl-, (24) 1-hexen-4-Ol, 1-chloro-3,5-dimethyl-, (25) 1,2-benzenedicarboxylic acid, dinonyl ester, (26) 2-propenoic acid, butyl ester (27) 2-pteridinamine, 6-methyl-N,N-bis(trimethylsilyl)-4-[(trimethylsilyl)oxy, (28) butanedioic acid, 2-[(tert-butyldimethylsilyl)oxy]-, bis(tert-butyldime, (29) 1,3-dioxolane, 2-(1-phenylethyl)-, were identified in B-11 subfraction. . Figure 9B shows the gas chromatogram of B-11 subfraction. The predicted compounds along with their reported biological activities are listed in Table 3. Eight of these compounds have reported anti-adipogenic activity that account for the anti-adipogenic potential of B-11 subfraction.
Table 3:
List of predicted compounds in B-11 subfraction with their reported biological activities
| Sr. No. | Retention Time | Predicted Compound | Biological Activity |
|---|---|---|---|
| 1 | 8.967 | 1,2:5,6 Dianhydrogalactitol | Treatment of glioblastoma (Peng et al. 52) |
| 2 | 15.063 | 1,3-Propanediol, 2-(Hydroxymethyl)-2-Nitro- | Not identified |
| 3 | 15.302 | 2,4,4-Trimethyl-1-pentanol | Metabolite (https://pubchem.ncbi.nlm.nih.gov/compound/2_4_4-Trimethyl-1-pentanol). |
| 4 | 17.749 | Sydnone, 3,3’-Tetramethylenedi- | Antibacterial activity (Bhosale et al. 53) |
| 5 | 18.42 | 1-Propanol, 2-Methyl-2-[(2-Methyl-2-Propenyl)Oxy]- | Antibacterial activity (https://pubchem.ncbi.nlm.nih.gov/compound/12517151) |
| 6 | 19.392 | 1,3-Propanediol, 2-(Hydroxymethyl)-2-Nitro- | Antimicrobial activity (https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6810). |
| 7 | 21.177 | Nonadecane, 1-Bromo- | Antimicrobial/ Antioxidant activity (Thakur et al. 54). |
| 8 | 22.343 | 4-Undecene, 2-Methyl-, (E)- | Anti-obesity activity (Chatzigeorgiou et al. 55) |
| 9 | 24.384 | Piperazine-1,4-Dicarboxylic Acid Di-Tert-Butyl Ester | Anti-obesity activity (https://pubchem.ncbi.nlm.nih.gov/compound/143540–05-0). |
| 10 | 25.78 | 2-Propenoic Acid, Butyl Ester | Anti-obesity activity (https://www.science.gov/topicpages/a/acid+tert-butyl+ester |
| 11 | 26.54 | Oleic acid | Anti-obesity activity (Kojima et al. 56) |
| 12 | 27.459 | 3,4-Hexanediol, 2,5-Dimethyl- | Anti-diabetic activity (Khan et al. 57) |
| 13 | 27.494 | Trifluoromethyl T-Butyl Disulfide | Anti-obesity activity (Sahib et al. 58) |
| 14 | 28.431 | 3-Pentanol, 2-Chloro-4-Methyl-, (R*,S*)-(.+/−.)- | Not identified |
| 15 | 29.526 | 3-Hexanol, 2,2-Dimethyl- | Not identified |
| 16 | 30.339 | 4-Tetradecanol | Antifungal activity (Obłąk et al. 59) |
| 17 | 30.896 | 3-Chloro-2,2-Dimethyl-1-Propanol | Anticancer/ Anti-inflammatory activity (https://pubchem.ncbi.nlm.nih.gov/compound/3-Chloro-2_2-dimethyl-1-propanol). |
| 18 | 32.239 | 2-Pteridinamine, 6-Methyl-N,N-Bis(Trimethylsilyl)-4-[(Trimethylsilyl)Oxy | Not identified |
| 19 | 32.707 | Dodecane, 1-Fluoro- | Antidiabetic activity (https://pubchem.ncbi.nlm.nih.gov/compound/1-fluorododecane). |
| 20 | 33.82 | 2-T-Butylperoxy-2-Ethylbutan-1-Ol, Propionate Ester | Antimalarial activity (Avery et al.60) |
| 21 | 34.024 | Oxalic Acid, Ethyl Neopentyl Ester | Anti-obesity activity (https://worldwidescience.org/topicpages/p/phthalic+acid+esters.html). |
| 22 | 35.428 | Benzoic Acid 1-Methoxy-1h-Tetrazol-5-Ylmethyl Ester | Antidiabetic/ Anti-obesity activity (Rakse et al. 61). |
| 23 | 36.126 | 3-Hexanol, 2,2-Dimethyl- | Not identified |
| 24 | 36.391 | 1-Hexen-4-Ol, 1-Chloro-3,5-Dimethyl- | Antidiabetic activity (www.chemspider.com/Chemical-Structure.4518247.html). |
| 25 | 36.6 | 1,2-Benzenedicarboxylic Acid, Dinonyl Ester | Ameliorative effect (Jung Choi et al. 62) |
| 26 | 37.107 | 2-Propenoic Acid, Butyl Ester | Anti-obesity activity (https://www.science.gov/topicpages/a/acid+tert-butyl+ester). |
| 27 | 37.31 | 2-Pteridinamine, 6-Methyl-N,N-Bis(Trimethylsilyl)-4-[(Trimethylsilyl)Oxy | Not identified |
| 28 | 38.477 | Butanedioic Acid, 2-[(Tert-Butyldimethylsilyl)Oxy]-, Bis(Tert-Butyldime | Not identified |
| 29 | 39.413 | 1,3-Dioxolane, 2-(1-Phenylethyl)- | Metabolite (https://pubchem.ncbi.nlm.nih.gov/compound/2-_1-Phenylethyl_-1_3-dioxolane). |
IV. DISCUSSION
Obesity is characterized as a condition in which body weight exceeds healthy or normal body weight. It is a serious disorder that is associated with myriad of medical conditions including hypertension, diabetes type II, fatty liver disease, cardiovascular disease, dyslipidemia, cancer, stroke, and physical disability.16–18 Obese people are generally recommended to be physically more active and reduce calorie intake, however modifying routine behavior is difficult for people. Other options to control and treat obesity include surgical removal of fat or taking anti-obesity medicines. However, the medicines available currently have limitations that emphasizes the need to look for new approaches to control and treat obesity. Herbal medicines or plant-based medicines are one of the alternative approaches to treat and control obesity.18
Cissus quadrangularis is one of such medicinal herbs that is reported for its role in weight management in ancient and modern literature. CQ is reported to consist of phytochemical constituents including alkaloids, terpenoids, steroids, flavonoids, resins, tannins, oxalates and others like resveratrol, quercetin, kaempferol, palllidol, stilbenes, ascorbic acid, beta-carotene, ascorbic acid, piceatannol and their derivatives.19 The phytochemicals such as saponins, alkaloids, phenol, flavonoids, cardiac glycosides and terpenoids have been reported for their beneficial effects for treatment of obesity and related diseases.20
With the main goal to isolate and identify the main anti-obesity constituents in CQ-B, this study elucidated the anti-adipogenic effect of CQ-B solvent fraction and its subsequent sub-fractions on 3T3-L1 mouse pre-adipocyte cell line. We observed dose-dependent effect of CQ-B on viability of 3T3-L1 cells with lower concentrations (0.0001, 0.01, 0.1 and 10 μg/ml) being non-cytotoxic for the cells whereas, higher concentrations 100 and 200 μg/ml significantly reduced cell viability. EC50 was determined to be 207.9 μg/ml. The dose-dependent effect is in correlation with previous studies on crude CQ extracts and CQ-solvent fractions that have reported similar findings with lower non-cytotoxic doses.21–27 To validate the observation of cell viability analysis, effect of non-cytotoxic concentrations of CQ-B was observed on metabolic activity, proliferation and growth parameters of 3T3-L1 cells and selected concentrations (0.0001, 0.01, 0.1 and 10 μg/ml) were found to be non-detrimental for the cells (Fig. 1). Doubling time of the cells was found to be 24.1h with specific growth rate of 0.013. We observed non-significant alteration in doubling time and specific growth rate of the cells in response to CQ-B treatment.
Several growth factors and hormones regulate the process of adipogenesis through a cascade of intracellular signaling pathways that involve different proteins and transcription factors. The differentiating 3T3-L1 cells undergo one round of mitosis 24h after induction at confluence followed by growth seizure. This round of mitosis is essential for differentiation as it allows the access of transcription factors to the response elements of the genes 28 that are required for the formation of adipogenic and lipogenic proteins. 29,30 The induction medium for adipogenesis consists of insulin and glucocorticoids. Insulin leads to cross-activation of IGF-1 (insulin like growth factor) receptor that activates downstream signaling pathways ultimately leading to the differentiation of cells.31–32 Glucocorticoid treatment results in differentiation of pre-adipocytes into mature adipose tissues. For instance, the adipose induction medium consists of dexamethasone. A synthetic glucocorticoid that belongs to the family of PPAR-γ and is essential for optimal induction of adipogenesis. Another glucocorticoid that is added in induction medium is iso-butyl methyl xanthine (IBMX) that positively regulates adipogenesis, by inhibiting phosphodiesterases and elevating the intracellular cAMP levels. This induces expression of C/EBP-β through a transcriptional activator - cAMP response element binding protein. Consequently, the expression of PPAR-γ and C/EBP-α, the essential transcription factors for adipogenesis, is upregulated and differentiation of pre-adipocytes into mature adipocytes is initiated. 33–35
The cells were induced to differentiate in response to treatment with non-cytotoxic, mitogenic concentrations of CQ-B. Fewer adipocytes with impaired lipogenesis were observed in differentiating cells treated with CQ-B (Figs. 2, 3). ORO staining and semi-quantification of neutral lipids was performed to analyze anti-adipogenic potential of CQ-B. We observed 36.5% and 30.1% reduction in neutral lipids in cells treated with 0.0001 and 0.01 μg/ml CQ-B. As triglycerides are the major form in which fat is deposited in adipose tissue, therefore triglyceride content of cells was estimated in cells treated with CQ-B. In cells treated with 0.0001μg/ml of CQ-B, 51.3% reduction in TG content was observed (Fig. 5). In correlation to the findings of this study, Lee et al. 25 have reported 44% reduction in adipocyte differentiation in cells treated with different concentrations of CQR-300. Previous animal and clinical studies on the role of CQ on body weight and lipid profile have reported that CQ treatment have resulted in reduction of body weight and fat content in obese subjects. 36–43
To elucidate anti-adipogenic potential of CQ-B at molecular level, expression profiles of adipogenic and lipogenic marker genes including PPAR-γ, SREBP-1, SCD-1, FASN, FAB-4, ACACA, ACACB, LPL, LPN, and ADIN were also studied. PPAR-γ, SREBP-1, and SCD-1 are the adipogenic and lipogenic transcription factors that regulate the expression of downstream genes including FASN, FAB-4, ACACA, ACACB, LPL, LPN, and ADIN. FASN, FAB-4, ACACA, ACACB, and LPL are the genes that play role in formation of fatty acids and triglycerides.25 We observed that CQ-B treatment downregulated the expression of adipogenic and lipogenic marker genes (Fig.6). Adiponectin and leptin are the adipokines secreted by adipose tissue that play role in energy metabolism and inhibition of hunger. It was speculated that downregulation in expression of these adipokines is related to inhibition of differentiation rather than due to effect of CQ solvent fractions. Previously, Lee et al.25 have reported downregulation in expression of PPAR-γ, C/EBP-α, SREBP-1, SCD-1, FASN, FAB-4, ACC and LPL accompanied by activation of AMPK after treatment of cells with CQR-300.
To further purify active compounds in CQ-B, the solvent fraction was sub-fractionated by reverse phase HPLC using C-18 column. Fractions were collected and analyzed for their bioactivity. The concentrations 0.1 and 1μg/ml were tested for all CQ-B subfractions (B-1 – B-27) for their effect on cell viability. We observed that both concentrations were non-cytotoxic for the cells (Fig. 7). To identify the sub-fraction with anti-adipogenic potential, 3T3-L1 cells were exposed to non-cytotoxic concentrations of CQ-B subfractions during differentiation. The sub-fractions B-10 and B-11 were identified as anti-adipogenic subfractions (Fig. 8). We observed 0.1μg/ml as the efficacious concentration for both sub-fractions and enhanced anti-adipogenic activity in B-10 subfraction compared to B-11. To identify the chemical constituents that are responsible for the bioactivity of these sub-fractions, GCMS was performed.
We identified seventeen compounds in B-10 fraction, of which five compounds (1) Phenol, 2,4-bis(1,1-dimethylethyl), (2) Oxalic acid, ethyl neopentyl ester, (3) Cis-inositol, (4) 1,2,5,6-di-o isopropylidene-3-o-methanesulfonyl glucofuranose, (5) Glucitol, 6-o-octyl have reported anti-adipogenic properties (Table II).
Of the twenty-nine compounds identified in B-11 subfraction, eight (1) 4-undecene, 2-methyl-, (E)-, (2) piperazine-1,4-dicarboxylic acid di-tert-butyl ester, (3) 2-propenoic acid, butyl ester, (4) oleic acid, (5) trifluoromethyl T-butyl disulfide, (6) oxalic acid, ethyl neopentyl ester, (7) benzoic acid 1-methoxy-1h-tetrazol-5-ylmethyl ester, (8) 2-propenoic acid, butyl ester, have reported anti-adipogenic activity (Table III).
The results of this study highlight the presence of active anti-adipogenic compounds in CQ-B fraction. The bioactive compounds identified in the sub-fractions can be further purified by analytical techniques to get pure anti-adipogenic compounds that can be used to develop potential drugs to treat obesity and related complications.
ACKNOWLEDGEMENT
The project is financially supported by the research grant of Pakistan Academy of Sciences Ref. # 5-9/PAS/22 dated 27.7.2016.
Footnotes
Statement of conflict of interest
The authors have declared no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Evans S. Changing the knowledge base in western herbal medicine. Soc Sci Med. 2008; 67(12), 2098–2106. doi: 10.1016/j.socscimed.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Giordano J, Engebretson J, Garcia MK. Challenges to complementary and alternative medical research: Focal issues influencing integration into a cancer care model. Integr Cancer Therap. 2005; 4(3), 210–218. DOI: 10.1177/1534735405279179 [DOI] [PubMed] [Google Scholar]
- 3.Mishra G, Srivastava S, Nagori BP. Pharmacological and therapeutic activity of Cissus quadrangularis: an overview. Int J Pharm Tech Res. 2010; 2(2): 1298–1310. [Google Scholar]
- 4.Baby J, Raj SJ. Pharmacognostic and traditional properties of Cissus quadrangularis Linn – An overview. Int J Pharma BioSci., 2011, 2:131–139. [Google Scholar]
- 5.Bloomer RJ, Farney TM, McCarthy CG, Lee SR. Cissus quadrangularis reduces joint pain in exercise-trained men: a pilot study. Physic Sportsmed. 2013; 41(3): 29–35. doi: 10.3810/psm.2013.09.2021 [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Shah N, & Thakar AB (2012). Effect of Majja Basti (therapeutic enema) and Asthi Shrinkhala (Cissus quadrangularis) in the management of osteoporosis (Asthi-Majjakshaya). Ayu, 33(1), 110–113. doi: 10.4103/0974-8520.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008; 54(6): 945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 8.Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, Rheaume C. Tchernof A. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes. 2008; 32(2): 283–291. doi: 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- 9.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J Clin. Res. 2000; 320(7244): 1240–1243. doi: 10.1136/bmj.320.7244.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djalalinia S, Qorbani M, Peykari N, Kelishadi R. Health impacts of obesity. Pakistan J Med Sci. 2014; 31(1):239–242. doi: 10.12669/pjms.311.7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα. Mol Cell Biol. 2007; 27(13): 4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012; 425(1): 88–90. doi: 10.1016/j.ab.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Stolic M, Russell A, Hutley L, Fielding G, Hay J, MacDonald G, Whitehead J, Prins J. Glucose uptake and insulin action in human adipose tissue—influence of BMI, anatomical depot and body fat distribution. Int J Obes Relat Metab Disord. 2002; 26(1): 17–23. 10.1038/sj.ijo.0801850 [DOI] [PubMed] [Google Scholar]
- 15.Butler M. Animal cell culture and technology. Taylor & Francis, 2004. ISBN: 9781135325381. [Google Scholar]
- 16.Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009; 15(25): 3073. doi: 10.3748/wjg.15.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopelman PG. Obesity as a medical problem. Nature 2000; 404(6778): 635–643. 10.1038/35007508 [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Chang B, Chen XY, Zhou SP, Zhen Z, Zhang LL, Xu Y. Chinese herbal medicine for obesity: a randomized, double-blinded, multicenter, prospective trial. Am J Chin. Med. 2014; 42(06): 1345–1356. 10.1142/S0192415X14500840 [DOI] [PubMed] [Google Scholar]
- 19.Stohs SJ, Ray SD. A review and evaluation of the efficacy and safety of Cissus quadrangularis extracts. Phytother Res. 2013; 27(8): 1107–1114. DOI: 10.1002/ptr.4846 [DOI] [PubMed] [Google Scholar]
- 20.Chinonye I, Lynda O, Fidelis O, Maureen C. Characterization and antimicrobial properties of volatile component of the ethanol leaf extract of Momordica charantia. Int Res J Nat Sci. 2019; 7:14–27. [Google Scholar]
- 21.Parisuthiman D, Singhatanadgit W, Dechatiwongse T, Koontongkaew S. Cissus quadrangularis extract enhances biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts. In Vitro Cell Develop Biol - Anim. 2009; 45(3–4), 194–200. doi: 10.1007/s11626-008-9158-1. [DOI] [PubMed] [Google Scholar]
- 22.Potu BK, Bha KM, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR, Muttigi MS. Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics 2009; 64(10): 993–998. doi: 10.1590/S1807-59322009001000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthusami S, Senthilkumar K, Vignesh C, Ilangovan R, Stanley J, Selvamurugan N, Srinivasan N. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J Cell Biochem. 2011; 112(4): 1035–1045. 10.1002/jcb.23016 [DOI] [PubMed] [Google Scholar]
- 24.Tasadduq R, Gordon J, Al-Ghanim KA, Lian JB, Van Wijnen AJ, Stein JL, Shakoori AR. Ethanol extract of Cissus quadrangularis enhances osteoblast differentiation and mineralization of murine pre-osteoblastic MC3T3-E1 cells. J Cell Physiol. 2017; 232(3): 540–547. doi: 10.1002/jcp.25449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Le B, Lee DR, Choi BK, Yang SH. Cissus quadrangularis extract (CQR-300) inhibits lipid accumulation by downregulating adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol Rep. 2018; 5: 608–614. DOI: 10.1016/j.toxrep.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toor RH, Tasadduq R, Adhikari A, Chaudhary MI, Lian JB, Stein JL, Stein GS, Shakoori AR. Ethyl acetate and n-butanol fraction of Cissus quadrangularis promotes the mineralization potential of murine pre-osteoblast cell line MC3T3-E1 (sub-clone 4). J Cell Physiol. 2019a; 234: 10300–10314. DOI: 10.1002/jcp.27707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toor RH, Malik S, Qamar H, Batool F, Tariq M, Nasir Z, Tassaduq R, Lian JB, Stein JL, Stein GS, Shakoori AR. Osteogenic potential of hexane and dichloromethane fraction of Cissus quadrangularis on murine preosteoblast cell line MC3T3-E1 (sub-clone 4). J Cell Physiol. 2019b; 234 (12): 23082–23096. doi: 10.1002/jcp.28869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutri. 1994; 14: 99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 29.Erickson RL, Longo KA, Ross SE, Hemati N, MacDougald OA. Structure and function of C/EBPa. In: Ntambi JM, editor. Adipocyte biology and hormone signaling. Burke, VA: IOS Press Inc; 2000. [Google Scholar]
- 30.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999; 266(3): 677–683. 10.1006/bbrc.1999.1885 [DOI] [PubMed] [Google Scholar]
- 31.Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes, 2004; 53(suppl 1): S43–S50. 10.2337/diabetes.53.2007.S43 [DOI] [PubMed] [Google Scholar]
- 32.Tomlinson JJ, Boudreau A, Wu D, Salem HA, Carrigan A, Gagnon A, Hache RJ. Insulin sensitization of human preadipocytes through glucocorticoid hormone induction of forkhead transcription factors. Mol Endocrinol. 2010; 24(1): 104–113. 10.1210/me.2009-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehrke M, Lazar MA. The many faces of PPARγ. Cell, 2005; 123(6): 993–999. 10.1016/j.cell.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 34.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutri. 2000; 130(12): 3122S–3126S. 10.1093/jn/130.12.3122S [DOI] [PubMed] [Google Scholar]
- 35.Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. Topics Tissue Engin. 2008; 4(1),:26. [Google Scholar]
- 36.Oben J, Kuate D, Agbor G, Momo C, Talla X. The use of a Cissus quadrangularis formulation in the management of weight loss and metabolic syndrome. Lipids Hlth Dis. 2006; 5(1): 24. 10.1186/1476-511X-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oben JE, Enyegue DM, Fomekong GI, Soukontoua YB, Agbor GA. The effect of Cissus quadrangularis (CQR-300) and a Cissus formulation (CORE) on obesity and obesity-induced oxidative stress. Lipids Hlth Dis. 2007; 6(1): 4. doi: 10.1186/1476-511X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oben JE, Ngondi JL, Momo CN, Agbor GA, Sobgui CSM. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Hlth Dis. 2008; 7(1): 12. doi: 10.1186/1476-511X-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiji MJ, Visalakshi S, Meenakshi P, Rathi MA, Thirumoorthi LD, Chinna Guravaiah D, Gopalakrishnan VK. Antilipidemic activity of Cissus quadrangularis and Tribulus terrestris on obesity in high fat fed rats. Pharmacologyonline, 2009; 2: 1250–1258. [Google Scholar]
- 40.Kuate D, Nash RJ, Bartholomew B, Penkova Y. The use of Cissus quadrangularis (CQR-300) in the management of components of metabolic syndrome in overweight and obese participants. Nat Prod Commun. 2015; 10(7): 817–822. 10.1177//1934578X1501000737 [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Dong-Ryung CB. Cissus quadrangularis extracts decreases body fat through regulation of fatty acid synthesis in high-fat diet-induced obese mice. J Appl Biol Chem. 2016; 59(1): 49–56. 10.3839/jabc.2016.010 [DOI] [Google Scholar]
- 42.Talreja T, Kumar M, Sirohi P, Sharma T. Preparation and use of Cissus quadrangularis and Achyranthes aspera formulation in the management of weight loss. Pharma Innov J. 2017; 6(3, Part C): 143–151. [Google Scholar]
- 43.Nash R, Azantsa B, Kuate D, Singh H, Oben J. The use of a stem and leaf aqueous extract of Cissus quadrangularis (CQR-300) to reduce body fat and other components of metabolic syndrome in overweight participants. J Altern Complement Med. 2019; 25(1): 98–106. doi: 10.1089/acm.2018.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alghamdi S, Migdadi H, Khan M, El-Harty EH, Ammar M, Farooq M, Afzal M. Phytochemical Profiling of Soybean (Glycine max (L.) Merr.) Genotypes Using GC-MS Analysis. Phytochemicals-Source of Antioxidants and Role in Disease Prevention. Oshiki Asao and Md Asaduzzaman, IntechOpen, DOI: 10.5772/intechopen.78035. Available from: https://www.intechopen.com/books/phytochemicals-source-of-antioxidants-and-role-in-disease-prevention/phytochemical-profiling-of-soybean-glycine-max-l-merr-genotypes-using-gc-ms-analysis; 2018. [DOI] [Google Scholar]
- 45.Zhang HZ, LV Damu G, Cai GX, Zhou CH. Current developments in the syntheses of 1, 2, 4-triazole compounds. Curr Org Chem. 2014; 18(3): 359–406. DOI : 10.2174/13852728113179990025 [DOI] [Google Scholar]
- 46.Kalayoglu M, Singer M. U.S. Patent No. US20130178525A1. Washington, DC: U.S. Patent and Trademark Office, 2013.
- 47.Ziołkowska NE, Bujacz A, Randad RS, Erickson JW, Skálová T, Hašek J, Bujacz G. New active HIV-1 protease inhibitors derived from 3-hexanol: Conformation study of the Ffree inhibitors in crystalline state and in complex with the enzyme. Chem Biol Drug Design 2012; 79(5): 798–809. 10.1111/j.1747-0285.2012.01328.x [DOI] [PubMed] [Google Scholar]
- 48.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes, 2009; 58(7): 1509–1517. doi: 10.2337/db08-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapiejko PJ, Northup JK, Evans T, Brown JE, Malbon CC. G-proteins of fat-cells Role in horm- A, Mahomoodally F, Abdul Hamid, A. Plants’ metabolites as potential antiobesity agents. The Scient World J. 2012; 23(4): 1–8. 10.1100/2012/436039 [DOI] [Google Scholar]
- 50.de Rodríguez DJ, García-Hernández LC, Rocha-Guzmán NE, Moreno-Jiménez MR, Rodríguez-García R, Díaz-Jiménez MLV, Carrillo-Lomelí DA. Psacalium paucicapitatum has in vitro antibacterial activity. Indust Crops Prod. 2017; 107: 489–498. DOI: 10.1016/j.indcrop.2017.05.025 [DOI] [Google Scholar]
- 51.Conforti F, Pan MH. Natural products in anti-obesity therapy. Molecules 2016; 21(12): 1750–65. doi: 10.3390/molecules21121750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng C, Qi XM, Miao LL, Ren J. 1, 2: 5, 6-dianhydrogalactitol inhibits human glioma cell growth in vivo and in vitro by arresting the cell cycle at G 2/M phase. Acta Pharmacol Sin. 2017; 38(4): 561–570. doi: 10.1038/aps.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhosale SK, Deshpande SR, Wagh RD. Ultrasound assisted one pot synthesis, spectral, antimicrobial and antioxidant studies of novel 4-[1-Oxo-3-(substituted phenyl)-2-propenyl]-3-substituted phenyl sydnones. Asian J Chem. 2015; 27(8): 3063–3068. 10.14233/ajchem.2015.18864 [DOI] [Google Scholar]
- 54.Thakur K, Navdeep A, Jaswal S, Bhatt AK. Evaluation of antimicrobial potential of root extract of Asparagus racemosus Willd. and bark extract of Juglans regia L. against pathogenic bacterial isolates. Annls Phytomed. 2018; 7(2): 64–69. [Google Scholar]
- 55.Chatzigeorgiou A, Kandaraki E, Papavassiliou AG, Koutsilieris M. Peripheral targets in obesity treatment: a comprehensive update. Obesity Rev. 2014; 15(6): 487–503. doi: 10.1111/obr.12163 [DOI] [PubMed] [Google Scholar]
- 56.Kojima M, Tachibana N, Yamahira T, Seino S, Izumisawa A, Sagi N, Arishima T, Kohno M, Takamatsu K, Hirotsuka M, Ikeda I. Structured triacylglycerol containing behenic and oleic acids suppresses triacylglycerol absorption and prevents obesity in rats. Lipids Hlth Dis. 2010; 9(1): 77. 10.1186/1476-511X-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan M, Yusufzai SK, Kaun LP, Shah MD, Idris R. Chemical composition and antioxidant activity of essential oil of leaves and flowers of Alternanthera sessilis red from Sabah. J Appl Pharmaceut Sci. 2016; 6(12): 157–161. DOI: 10.7324/JAPS.2016.601222 [DOI] [Google Scholar]
- 58.Sahib NG, Saari N, Ismail A, Kghatib A, Mahomoodally F, Hsamid AA. Plants’ metabolites as potential antiobesity agents. Scient World J 2012; Article ID 436039, pp. 1–8. DOI 109.1100/2012/436039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obłąk E, Piecuch A, Krasowska A, Łuczyński J. Antifungal activity of gemini quaternary ammonium salts. Microbiol Res. 2013; 168(10): 630–638. 10.1016/j.micres.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 60.Avery MA, Mehrotra S, Johnson TL, Bonk JD, Vroman JA, Miller, R. Structure− activity relationships of the antimalarial agent artemisinin. 5. Analogs of 10-deoxoartemisinin substituted at C-3 and C-9. J med Chem. 1996; 39(21): 4149–4155. 10.1021/jm960200e [DOI] [PubMed] [Google Scholar]
- 61.Rakse M, Karthikeyan C, Deora GS, Moorthy NSHN, Rathore V, Rawat AK, Trivedi P. Design, synthesis and molecular modelling studies of novel 3-acetamido-4-methyl benzoic acid derivatives as inhibitors of protein tyrosine phosphatase 1B. European J Med Chem. 2013; 70: 469–476. 10.1016/j.ejmech.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 62.Jung Choi S, Kim MJ, Jin Heo H, Kim JK, Jin Jun W, Kim HK, Jun Kim Y. Ameliorative effect of 1, 2-benzenedicarboxylic acid dinonyl ester against amyloid beta peptide-induced neurotoxicity. Amyloid, 2009; 16(1): 15–24. doi: 10.1080/13506120802676997. [DOI] [PubMed] [Google Scholar]