Abstract
Background
Deaths attributed to Coronavirus Disease 2019 (COVID‐19) are mainly due to severe hypoxemic respiratory failure. Although the inflammatory storm has been considered the main pathogenesis of severe COVID‐19, hypersensitivity may be another important mechanism involved in severe cases, which have a perfect response to corticosteroids (CS).
Method
We detected the serum level of anti‐SARS‐CoV‐2–spike S1 protein‐specific IgE (SP‐IgE) and anti‐SARS‐CoV‐2 nucleocapsid protein‐specific IgE (NP‐IgE) in COVID‐19. Correlation of levels of specific IgE and clinical severity were analysed. Pulmonary function test and bronchial provocation test were conducted in early convalescence of COVID‐19. We also obtained histological samples via endoscopy to detect the evidence of mast cell activation.
Result
The levels of serum SP‐IgE and NP‐IgE were significantly higher in severe cases, and were correlated with the total lung severity scores (TLSS) and the PaO2/FiO2 ratio. Nucleocapsid protein could be detected in both airway and intestinal tissues, which was stained positive together with activated mast cells, binded with IgE. Airway hyperresponsiveness (AHR) exists in the early convalescence of COVID‐19. After the application of CS in severe COVID‐19, SP‐IgE and NP‐IgE decreased, but maintained at a high level.
Conclusion
Hypersensitivity may be involved in severe COVID‐19.
Keywords: Anti‐SARS‐CoV‐2 specific IgE antibodies, IgE‐mediated hypersensitivity reactions, pulse methylprednisolone therapy, severe COVID‐19
Level of IgE to SARS‐CoV‐2–spike protein and nucleocapsid protein positively correlated with clinical severity in COVID‐19 patients. Airway hyperresponsiveness (AHR) exists in early convalescence of COVID‐19. Mast cells with concurrently positive staining for IgE and CD63 were observed in lamina propria of the bronchus and duodenum in severe COVID‐19. We deduce that hypersensitivity reaction may be involved in severe COVID‐19. Note: SP‐IgE: anti‐spike protein (SP) IgE; NP‐IgE: anti‐nucleocapsid protein (NP) IgE; TLSS: Total lung severity scores; BPT: bronchial provocation test; Reff: effective airway resistance; R5‐R20: difference between the resistance at 5 Hz and 20 Hz.
Key messages.
Level of IgE to SARS‐CoV‐2–spike protein and anti‐SARS‐CoV‐2 nucleocapsid protein positively correlated with clinical severity in COVID‐19 patients.
Mast cells with concurrently positive staining for IgE and CD63 were observed in lamina propria of the bronchus and duodenum in severe COVID‐19.
Based on the pulmonary function test, airway hyperresponsiveness (AHR) exists in the early convalescence of COVID‐19.
1. INTRODUCTION
At present, Coronavirus Disease 2019 (COVID‐19) continues to spread worldwide, with more than 3,144,028 deaths reported as of 29 April 2021 and no indications that disease spread will be controlled in the near future. 1 The most common cause of death from COVID‐19 has been reported to be severe hypoxemic respiratory failure. 2 Therefore, it is extremely important to clarify the precise pathophysiology for the development of severe COVID‐19, as well as to develop appropriate treatment strategies. Increasing studies indicate that "cytokine storm" contribute to the mortality of COVID‐19. 3 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) may cause a hyperactivated innate immune response, elevating serum levels of inflammatory chemokines (interleukin‐6, interleukin‐1β, tumour necrosis factor‐α, etc.), thus resulting in the exhaustion of lymphocytes. 4 This might ultimately cause organ failure and fatal respiratory distress. On the contrary, coagulopathy in multiple vascular territories may account for severity in COVID‐19, either related to a systemic immunopathology. 5 As some data reported, referred to the clinical manifestation and lung CT performance, it is probably that the hypoxic respiratory failure caused by COVID‐19 may be related to hypersensitivity within the lungs. 6 Mast cells (MCs) are activated by SARS‐CoV‐2. 7 Although only recently recognized, MC activation syndrome (MCAS), usually due to acquired MC clonality, is a chronic multisystem disorder with inflammatory and allergic themes, and an estimated prevalence of 17%. 8 Mast cells may react to viruses in collaboration with other cells and lung autopsy findings from patients that died from the coronavirus disease that emerged in 2019 (COVID‐19) showed accumulation of mast cells in the lungs that was thought to be the cause of pulmonary oedema, inflammation and thrombosis. 9 CD63 is an important surface marker of activated human blood‐derived MCs. 10 , 11 We herein detected the anti‐SARS‐CoV‐2 specific immunoglobulin E (IgE) and CD63+ cells in COVID‐19 patients to figure out the correlations of IgE, MCs and COVID‐19.
The humoral immune system plays an important role against viral infection. Investigations in humoral immune responses provide supportive evidence to vaccine development. It is clear that most people infected with SARS‐CoV‐2 display an antibody response after infection. Seroconversion occurs approximately 10 days after the symptom onset. Specific IgM appears between 10 and 14 days after infection. 12 The IgG antibody titres rise from day 10 onwards to reach a peak whose height is likely to be influenced, on a case‐by‐case basis, by disease severity and virus load. 13 IgA serum concentrations peaked 3 weeks after symptom onset but persisted for several more weeks in saliva, and serum IgA was more potent than IgG in neutralizing SARS‐CoV‐2. 14 However, the dynamic feature of IgE after SARS‐CoV‐2 infection is still unknown. Our team successfully managed 102 COVID‐19 cases. Among these patients, 27 developed hypoxemic respiratory failure, but no deaths occurred. 15 We retrospectively reviewed our cases and conducted a study to investigate the pathophysiology of COVID‐19. We found that various COVID‐19‐related clinical features, from as severe as near‐fatal hypoxemic respiratory failure to as mild as gastrointestinal discomfort, might be due to hypersensitivity reactions. As the treatment for COVID‐19‐related hypoxemic respiratory failure, we compared specific IgE, total lung severity scores (TLSS), and the arterial oxygen tension to inspiratory oxygen concentration ratio (PaO2/FiO2) before and after administration of pulse methylprednisolone, and found the fast improved clinical manifestation and above index, which might suppress not only inflammatory storm but also the hypersensitivity reactions. There are few studies about pulmonary function in patients with COVID −19, which focus on the recovery period, and airway ventilation dysfunction is not common in these studies. 16 , 17 However, because the bronchial provocation test (BPT) is not carried out routinely, prolonged clinical symptoms of chest tightness, cough and wheezing in COVID‐19 patients were attributed to diffusion dysfunction mostly. In this study, we conducted BPT in COVID‐19 patients with prolonged clinical symptoms to find out whether airway hyperresponsiveness (AHR) exists.
2. METHODS
2.1. Study population
This was a retrospective case‐control study. COVID‐19 patients hospitalized in the Fifth Hospital of Sun Yat‐sen University from 1 January 2020‒25 April 2020 were enrolled. The diagnosis of COVID‐19 infection was made based on the criteria of the “Diagnosis and Treatment of New Coronavirus‐Infected Pneumonia” (Seventh trial version) drafted by the National Health Commission of China. We used 50 pre‐COVID‐19 sera from volunteers collected between October 2015 and November 2018 as negative controls. The samples are mainly from routine yearly health screening of staff members, who underwent chest X‐ray to rule out pulmonary diseases. According to their medical record, patients with severe systemic disease, congestive heart failure, active malignant tumour, hepatic or renal disease, were also excluded. The other exclusion criteria were: age <18 or >80 years, underlying autoimmune diseases, underlying primarily or acquired immunodeficiencies, and refusal to participate. Our study was approved by the Research Ethics Committee of the Fifth Affiliated Hospital of Sun Yat‐sen University (Ethics approval number K30‐1). Written informed consent was obtained from all the participants.
2.2. Chest CT
Chest CT was performed in all subjects using 1‐mm slice thickness CT on a UCT 760 scanner (United Imaging; Shanghai, China). CT images were read by two experienced specialists with over five years of experience, and were evaluated for the presence or absence of the following findings: ground‐glass opacity, centrilobular nodules and high‐density linear or strip shadows. The distribution of lung lobes involved was described as subpleural, central, random or diffuse. 18
2.3. COVID‐19 severity
Total lung severity scores (TLSS) are assessed as previously reported. 18 First, each of the five lung lobes was assessed for degree of involvement and classified as none (0%), minimal (1%–25%), mild (26%–50%), moderate (51%–75%) or severe (76%–100%). Then, no involvement corresponded to a lobe score of 0, minimal to a lobe score of 1, mild to a lobe score of 2, moderate to a lobe score of 3, and severe to a lobe score of 4. Subsequently, the total lung severity score was calculated by summing the five lobe scores (range of possible scores, 0‒20). In addition, the severity of COVID‐19 was assessed by PaO2/ FiO2 and respiratory dysfunction was defined as PaO2/ FiO2 less than 300 mm Hg.
2.4. Collection of biological specimens from COVID‐19 patients and control participants
All biological specimens used in this study were acquired from the specimen bank of our hospital with the approval of the participants. The sera and tissues from COVID‐19 patients were obtained during hospitalization and stored at –80°C and in liquid nitrogen, respectively. Sera of the control group were obtained for routine health screening several months prior to the COVID‐19 outbreak.
2.5. Detection of peripheral blood‐specific anti‐SARS‐CoV‐2 IgE antibody levels in patients with COVID‐19
We developed an enzyme‐linked immunosorbent assay (ELISA) system to measure the levels of anti‐SARS‐CoV‐2 IgE antibodies in the serum of all participants. The ELISA is a common method in the detection of specific IgE in clinical practice. 19 However, there lacks a specific IgE kit of SARS‐COV‐2. We developed an indirect ELISA system to detect sera anti‐SARS‐CoV‐2 spike protein antibody and anti‐SARS‐CoV‐2 N protein antibody. Checkerboard titration was used to determine the optimal antigen coating concentration, serum dilution and peroxidase‐conjugate secondary antibody working dilution. Good repeatability was showed in the experiment. We tried to determine a cut‐off value by the detection of sera from negative control individuals (detailed information in Figure S3). However, because of the small sample of the control group, it may cause some bias of the result. Therefore, instead of using a “positive” or “negative” in the paper when comparing the specific IgE between groups, we would like to compare their exact value.
The procedure of ELISA was as follows:
Coating: In this, 96‐well microtiter ELISA plates were coated with 2‐μg/ml recombinant SARS‐CoV‐2 spike S1 protein and SARS‐CoV‐2 nucleocapsid protein antigens (Sino Biological, Beijing, China), which was accepted as SARS‐CoV‐2‐specific proteins, generally, overnight at 4°C.
Blocking: Wells were blocked with 20% non‐fat dried milk overnight at 4°C and incubated at 37°C for 2 h.
Sample application: Plates were washed thrice with phosphate‐buffered saline (PBS) containing 0.04% Tween‐20 (PBST, Solarbio). For the detection of IgE antibodies, 50‐μl undiluted serum was added and incubated at 37°C for 2 h.
Antibody detection: Antigen‐specific antibodies were detected by adding 100‐μl horseradish peroxidase (HRP)‐labelled goat anti‐human IgE (1/5000 dilution, Abcam ab3901), followed by incubation at room temperature for 1 h, and after some time the plates were washed thrice.
Coloration: The plates were washed thrice with PBST, and the signal was developed by adding 100‐μl TMB (Solarbio) for 15 minutes at room temperature.
Stopping: The reaction was stopped with 2‐M sulphuric acid; a 50‐μl stop solution was added to each well.
Signal detection: Plates were read on a Spectramax Plate Reader at 450 nm using SoftMax Pro, with subtraction of the optical density (OD) values of the background.
2.6. Detection of SARS‐CoV‐2 in respiratory specimens
The presence of SARS‐CoV‐2 in nasal and pharyngeal swab specimens was detected by real‐time PCR assays approved by the China Food and Drug Administration.
2.7. Histologic and immunofluorescent staining: IgE‐mediated activated mast cells testing
Bronchial mucous and duodenal mucous tissues were obtained via endoscopy. Samples were embedded in paraffin and processed by hematoxylin and eosin (H&E) and immunofluorescent staining. For immunofluorescent staining, 3‐μm‐thick sections were de‐waxed in xylene and rehydrated in alcohol, followed by antigen retrieval by citric acid buffer microwave repairing for 20 min. After rinsing with PBST thrice, the sections were blocked with goat serum (ZSGB‐BIO, ZLI‐9022) for 1 h at room temperature and then incubated overnight at 4°C with primary antibodies anti‐CD63 (Abcam ab252919, 1:1000); anti‐IgE, (Abcam ab7382, 1:250); anti‐NP (Sino Biological, 40143‐MM08, 1:500). After rinsing with PBST, slides were incubated with secondary antibodies (Alexa Fluor 647‐conjugated goat anti‐mouse IgG; Bioss, bs‐0295G‐AF647, 1:100; Dylight 549‐conjugated goat anti‐rabbit IgG; Abbkine, A23320, 1:100) at room temperature for 1 h. Nuclei were then counterstained with 4’,6‐diamidino‐2‐phenylindole (DAPI) in the VECTASHIELD Hardset Antifade Mounting Medium after washing thrice with PBST. Slides were imaged using a laser scanning confocal microscope (LSM880; Carl Zeiss MicroImaging). At last, the immunofluorescent staining results in specimens were observed by a confocal microscope.
2.8. Pulmonary function test in early convalescence in COVID‐19 patients
Partial subjects underwent a standard pulmonary function test and bronchial provocation test 3 months after discharge (Master Screen; Jaeger). Body plethysmography, spirometry and impulse oscillation system (IOS) assessment included specific effective airway resistance (sReff), effective airway resistance (Reff), forced vital capacity (FVC), forced expiratory volume in one second (FEV1), maximal mid‐expiratory flow (MMEF), resonant frequency (Fres), airway viscosity resistance at an oscillation frequency of 5Hz(R5), and central airway resistance at an oscillation frequency of 20Hz (R20) and the difference between the resistance at 5 Hz and 20Hz (R5‐R20). In the bronchial provocation test (BPT), we used methacholine as a stimulator and observed the changes in airway resistance after medication. The measurements were expressed as a percentage of predicted normal values. To protect the lung function of the laboratory staff, lung function tests were performed in a room with a negative pressure device. Staff wore personal protective equipment, including N95 respirators, protective glasses, gloves and gowns. In addition, each patient used disposable virus and bacterial filters during the test.
2.9. Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science (SPSS) Version 13.0 (SPSS, Inc). The data of normal distribution were shown as the mean ±standard deviation (SD). Continuous variables were compared using an independent sample t‐test, whereas the rank‐sum test was used for nonparametric data. The categorical variables were expressed as count (percentage) and compared using the chi‐square test. The associations between the specific IgE and TLSS, or PaO2/FiO2 ratio were analysed by Pearson correlation coefficient. All statistical tests were two‐tailed. Statistical significance was taken as p < .05.
3. RESULTS
3.1. Characteristics of study participants
This was a retrospective case‐control study. One hundred and two patients with confirmed COVID‐19 were included in this study, 27 of whom (26.5%) were severe cases according to the criteria of PaO2/FiO2 less than 300 mm Hg. The age of the patients ranged from 15 to 80 years old (median 47.4 years old). Among the 102 patients, 1 patient (male) had a history of asthma, 20 1 had allergic rhinitis, 6 patients had a cured malignancy, 6 had a history of diabetes and 16 had a history of hypertension. The major clinical symptoms were fever, shortness of breath, cough, diarrhoea, loss of appetite, haemoptysis, fatigue, chest tightness and sputum production. The two groups (severe vs. non‐severe group) had no significant differences in coexisting disorders (all p > .05). Comparison of patients with severe vs. non‐severe disease indicated that haemoptysis (p < .001), shortness of breath (p < .001) and fever (p = .006) were more common in the severe disease group. The two groups had no significant differences in coexisting disorders (all p > .05). Among the COVID‐19 patients, a peak value of mean TLSS in severe ones was higher than that in non‐severe ones (p < .001), accompanied by a higher mean PaO2/FiO2 ratio (p < .001). At acute stage, the level of SP‐IgE and NP‐IgE were both higher than that in the control group (both p < .001), particularly in severe COVID‐19 patients (p = .002; p = .005; Table 1).
TABLE 1.
Demographic and clinical characteristics of COVID‐19 and healthy controls
| Clinical characteristics | All patients | Non‐severe group | Severe group | Control group | p‐value* | p‐value** |
|---|---|---|---|---|---|---|
| (n = 102) | (n = 75) | (n = 27) | (n = 50) | |||
| Age, median (range), years | 47.6 (15–80) | 41.0 (15–75) | 61.0 (32–80) | 49.0 (25–76) | .056 | <.001* |
| Sex | ||||||
| Male, N (%) | 45/102 (44.1) | 29/75 (38.7) | 16/27 (59.3) | 26/50 (52.0) | .121 | .083 |
| Coexisting disorders, N (%) | ||||||
| Hypertension | 16/102 (15.7) | 10/75 (13.3) | 6/27 (22.2) | 8/50 (16.0) | .571 | .454 |
| Diabetes mellitus | 6/102 (5.9) | 2/75 (2.7) | 4/27 (14.8) | 5/50 (10.0) | .069 | .072 |
| Cured malignancy | 6/102 (5.9) | 4/75 (5.3) | 2/27 (7.4) | 2/50 (4.0) | .82 | 1.000 |
| Allergic diseases | 2/102 (2.0) | 1/75 (1.3) | 1/27 (3.7) | 2/50 (4) | .598 | 0.461 |
| Chronic pulmonary disease | 6/102 (5.9) | 3/75 (4) | 3/27 (11.1) | 3/50 (6) | .730 | .188 |
| Respiratory symptoms, N (%) | ||||||
| Fever | 59/102 (57.8) | 37/75 (49.3) | 22/27 (81.5) | .006 | ||
| Hemoptysis | 7/102 (6.9) | 0/75 (0) | 7/27 (25.9) | <.001 | ||
| Shortness of breath | 34/102 (33.3) | 7/75 (9.3) | 27/27 (100.0) | <.001 | ||
| Asymptomatic on admission | 12/102 (11.8) | 12/75 (16.0) | 0/27 (0) | .062 | ||
| Gestational intestinal symptoms, N(%) | 62/102 (60.8) | 48/75 (64) | 14/27 (51.8) | .358 | ||
| Total lung severity scores (TLSS) | 5.38 ± 4.14 | 3.42 ± 2.22 | 9.37 ± 4.30 | <.001 | ||
| Worst PaO2 to FiO2 ratio (mmHg) | 295.76 ± 96.92 | 332.76 ± 61.30 | 220.38 ± 112.56 | <.001 | ||
| Specific IgE level on admission (ODvalue) | ||||||
| anti S protein | 0.47 ± 0.18 (n = 58) | 0.42 ± 0.12 (n = 45) | 0.58 ± 0.24 (n = 13) | 0.31 ± 0.01 | <.001 | .002 |
| anti N protein | 0.47 ± 0.25 (n = 58) | 0.39 ± 0.09 (n = 45) | 0.66 ± 0.38 (n = 13) | 0.30 ± 0.01 | <.001 | .005 |
p values* indicate differences between the COVID‐19 patients and control group p values** indicate differences between the non‐severe and severe groups.
3.2. Significant associations between SARS‐CoV‐2–specific IgE levels and severity of COVID‐19
Further analysis showed that the significant associations between specific SP‐IgE/NP‐IgE levels and TLSS or PaO2/ FiO2 ratio were observed (Figure 1). The level of specific IgEs were all found to have positive correlations with the TLSS (SP‐IgE, r = 0.606, p < .001; NP‐ IgE, r = 0.677, p < .001; Figure 1) and have negative correlations with the PaO2/ FiO2 ratio (SP‐IgE, r = −0.595, p < .001; NP‐ IgE, r = −0.669, p < .001). However, a negative correlation between the TLSS and PaO2/FiO2 ratio (r = −0.775, p < .001) was obtained.
FIGURE 1.
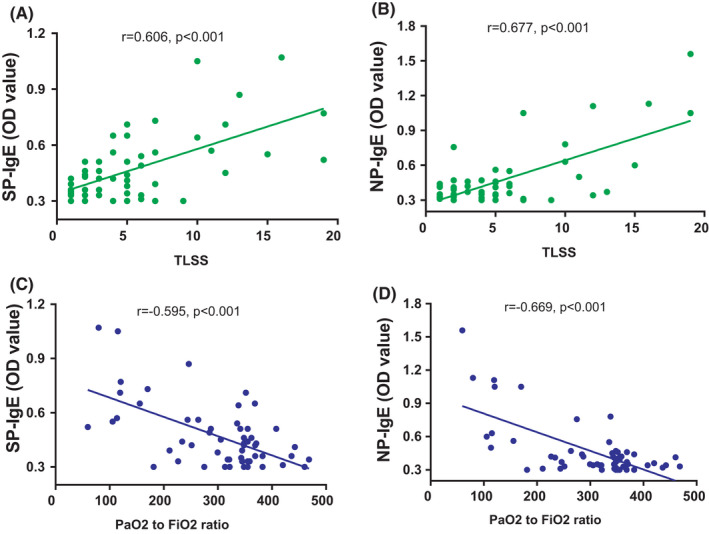
The correlations of anti‐SARS‐CoV‐2‐specific IgE with TLSS and the PaO2/ FiO2 ratio in COVID‐19 patients. SP‐ IgE: anti‐SARS‐CoV‐2‐spike S1 protein‐specific IgE; NP‐ IgE: SARS‐CoV‐2 nucleocapsid protein specific IgE; TLSS: the total lung severity scores [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Histological evidence for SARS‐CoV‐2–specific IgE‐mediated activated mast cells in COVID‐19
Bronchoscopy was performed on a patient with severe hypoxemic respiratory failure receiving mechanical ventilation, and bronchial mucosa tissues were obtained. Intestinal endoscopy was performed on selected patients with gastrointestinal symptoms, and intestinal mucosa tissues were obtained. As shown in Figure 2, activated mast cells (CD63+) with concurrently positive staining for IgE and NP were observed in lamina propria of the bronchus, and intestinal tissues, which indicated that IgE‐mediated mast cell activation is triggered by SARS‐CoV‐2 occurred in COVID‐19. In the histological aspect, hypersensitivity reactions in COVID‐19 can be partially proved.
FIGURE 2.
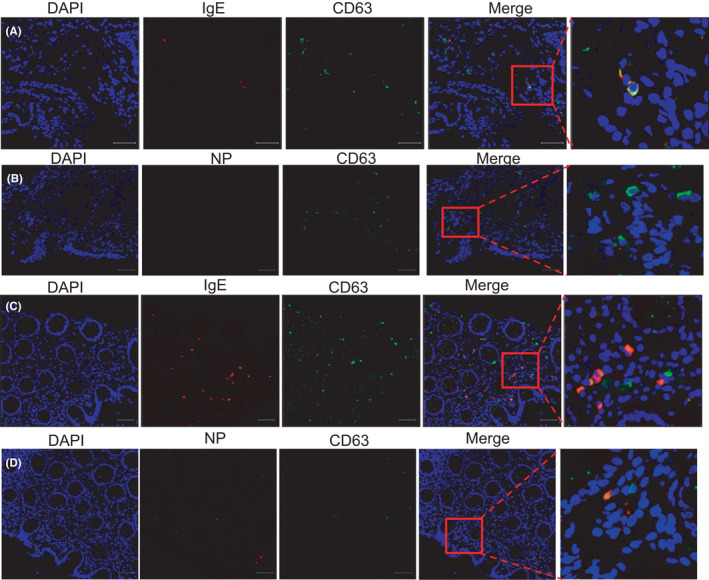
IgE‐mediated NP‐specific CD63+ mast cells in the bronchus and intestinal mucosa in COVID‐19 patients. Histologic and immunofluorescent staining had been done in specimens in bronchus (A‒B) and intestinal mucosa (C‒D) from COVID‐19 patients. DAPI: 40,6‐diamidino‐2‐phenylindole. NP: SARS‐CoV‐2 nucleocapsid protein [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Effect of corticosteroids treatment to SARS‐CoV‐2–specific‐IgE and prognosis in COVID‐19
Notably, each of the 16 critically ill patients experienced clinically significant improvements in the lung oxygenation index after each administration of systemic corticosteroids and these 16 patients all recovered without any complications. 15 Our experience is that a patient with an oxygenation index of 150 mm Hg or less should receive methylprednisolone (MP) at a dose of 4‒8 mg/kg/day to block the hypersensitivity reaction in the lungs. Among these 16 patients treated with corticosteroids, we had tested SP‐ IgE and NP‐IgE in succession. Our results indicated that the application of corticosteroids significantly decreased specific‐IgE (Figure 3), at the same time increased the PaO2/FiO2 ratio (Figure S1). We found that the level of anti‐SARS‐CoV‐2‐specific IgE antibodies decreased significantly after corticosteroids, but were still higher than healthy controls in the recovery period (Figure S2). In addition, we did not find the effect of corticosteroids application on SARS‐CoV‐2 clearance.
FIGURE 3.
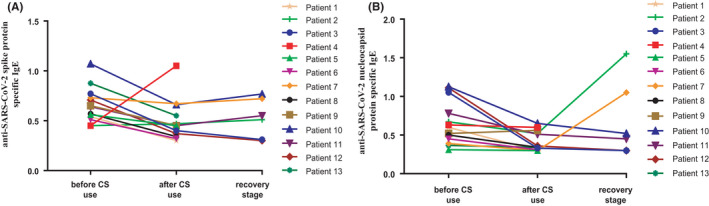
The change of SARS‐CoV‐2‐specific IgE in different stages in severe COVID‐19 patients who were treated with CS. The change of anti‐SARS‐CoV‐2 spike protein‐specific IgE (A) and anti‐SARS‐CoV‐2 nucleocapsid protein‐specific IgE (B). CS: corticosteroid [Colour figure can be viewed at wileyonlinelibrary.com]
3.5. Airway hyperresponsiveness (AHR) exists in the early convalescence of COVID‐19
During the follow up of COVID‐19 patients, we found chest tightness, cough and wheezing existed in 17 cases in early convalescence of COVID‐19. Depending on the bronchial provocation test (BPT), we considered that AHR existed in 8 patients with enhanced airway resistance (Figure 4), following the Criteria (1) FEV1 and FVC were based on the 2005 ATS/ERS criteria, ∆FEV1 ≥ 12% and 200 ml, ∆MMEF ≥ 35%, or ∆PEF ≥ 12%; (2) ∆Reff ≥ 35% (Reff, airway resistance); and (3) ∆R5 ≥ 30% or ∆Fres≥30%. 21 , 22 , 23 , 24 AHR, enhanced SP‐IgE and NP‐IgE in patients indicate that hypersensitivity might occur in the pathogenesis of COVID‐19 and go on until convalescence.
FIGURE 4.
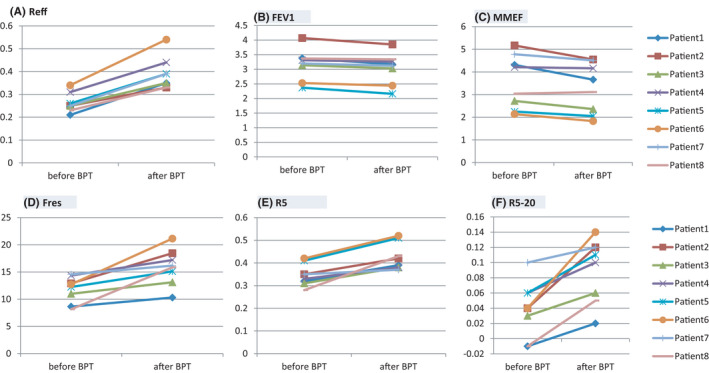
Airway hyperresponsiveness (AHR) exists in early convalescence of COVID‐19. Before and after BPT, the worsen index of lung function included Reff (A), FEV1 (B), MMEF (C), Fres (D), R5 (E) and R5‐20 (F) in early convalescence of 8 COVID‐19 patients [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The ongoing COVID‐19 outbreak has caused global disruption, with great concern for the mortality rate. There is an urgent need to determine the causal mechanisms of COVID‐19‐induced hypoxemic respiratory failure and to develop an effective treatment strategy. To date, quite a few studies presenting autopsy findings from COVID‐19 patients have been published, but there is still little evidence revealing the exact mechanism by which SARS‐CoV‐2 infection directly causes death. 25 , 26 , 27 Though vasculitis and microvascular thrombosis have been linked to COVID‐19 as a suspected pathological pattern by some authors, the evidence is still far from being conclusive. 28 , 29 , 30 Recent studies showed that SARS‐CoV‐2 may result in cytokine storm syndrome (CSS). 3 CSS is a cascade of escalated immune responses that can cause the immune system to become exhausted, which might ultimately result in organ failure and fatal respiratory distress. 31 CSS can lead to apoptosis of epithelial and endothelial cells in the lungs, vascular leakage and acute respiratory distress syndrome (ARDS). Evidence had shown that the responses of MCs to SARS‐CoV‐2 may promote cytokine storms. 9 Autopsy findings from the lungs of the patients that died from COVID‐19 showed an accumulation of MCs that was speculated to be the cause of pulmonary oedema, inflammation and thrombosis in the pathophysiology of COVID‐19. 32 , 33 The classic trigger for MCs activation is through the IgE‐mediated cross‐linking the IgE receptor, FcϵRI, thus resulting in the degranulation of MCs. Immediate hypersensitivity reactions, historically called type Ⅰ hypersensitivity reactions, are mediated by IgE antibodies produced by the immune system in response to environmental antigens. IgE antibodies secreted by plasma cells bind to the mast cells. When individuals encounter an antigen, it binds to the IgE antibody and triggers the release of histamine granules by mast cells, resulting in the dilation and increased permeability of capillaries. Although IgE is typically the least abundant isotype of immunoglobulin, it is capable of triggering the most powerful, life‐threatening inflammatory reactions. 34
The present study revealed the presence of anti‐SARS‐CoV‐2‐specific IgE antibodies in COVID‐19 patients by comparing the serum levels of these antibodies between COVID‐19 patients and healthy controls. Moreover, we discovered that the appearance of anti‐SARS‐CoV‐2‐specific IgE antibodies is an important clinical feature of COVID‐19, as the levels were significantly higher in patients who developed hypoxemic respiratory failure than in those without respiratory failure. In a recent study, it was found that the recruitment of CD45RA+ T cells in COVID‐19 is consistent with IgE‐mediated allergic response. 35 However, interestingly, the specific IgE levels are low in patients with mild COVID‐19. This means that the levels of specific IgE are associated with the progression of COVID‐19 and the severity of COVID‐19 at admission. This is explained as follows: The immune responses to viral infection are characterized by initial activation of innate immunity and production of type I and III interferons (IFN‐α/β and ‐λ, respectively), a negative link exists between IgE and IFN‐α and then antiviral responses may be suppressed in the process. 36 , 37 , 38 , 39 In addition, the serum level of a specific IgE against N protein appears to have a higher correlation coefficient with TLSS and PaO2/FiO2ratio, compared with that of specific IgE against S protein. In terms of the association between specific IgE and PaO2/ FiO2 ratio, it may be explained that during the progression of the disease, pulmonary exudation increased with increasing levels of specific IgE against SARS‐CoV‐2 antigens, and then the oxygen saturation drops gradually; however, as we all know, correlation does not always mean causality. To demonstrate causality, more rigorous empirical evidence is needed.
The risk of asthma from a severe viral infection is well documented. 40 The two major pathogens linked to asthma pathogenesis, i.e. respiratory syncytial virus and rhinovirus, are both known to potently induce the production of IgE in humans. 41 , 42 It appears that IgE may act as a signalling molecule for lung conventional dendritic cells (DCs). Once an effective adaptive immune response has been generated, virus‐specific IgE will be produced, bind to the conventional DCs, and be cross‐linked by viral antigen. 43 Anti‐IgE treatment (omalizumab) can increase virus‐induced IFN‐α responses in the presence of IgE cross‐linking and reduced DCs surface high‐affinity IgE receptor (FcεRIα) expression. 44 These results support a potential mechanism that allergic sensitization is associated with increased susceptibility to virus‐induced asthma exacerbations. We noticed some articles focus on the use of omalizumab in COVID‐19 patients who have to co‐exist asthma or urticaria. The application of omalizumab can prevent exacerbation of asthma and even the existence of pneumonia during SARS‐CoV‐2 infection, 45 and also alleviate symptoms of exacerbated urticarial rash and angioedema due to COVID‐19 in a patient with chronic spontaneous urticaria. 46 As a recombinant human anti‐IgE antibody, omalizumab is originally designed to reduce sensitivity to allergens and blocks IgE binding to FcεRI. The excellent response to omalizumab may give evidence to how IgE work on COVID‐19.
Furthermore, we detected anti‐SARS‐CoV‐2‐specific IgE on the surface of activated CD63+ cells (most likely mast cells) in the bronchial and gastrointestinal mucous membranes of patients with COVID‐19. NP‐specific activated mast cells in gastrointestinal mucous induced hypersensitivity, which may be an explanation of digestive system symptoms partially in COVID‐19. In addition, although there is no data about pulmonary function test in an acute stage, airway hyperresponsiveness proved by BPT is common in a convalescent stage in COVID‐19 patients. Considering all of this evidence together, it is reasonable to infer that SARS‐CoV‐2‐induced IgE production, which primes IgE‐mediated immediate hypersensitivity reactions, results in hypoxemic respiratory failure due to inflammatory storm. Detection of SARS‐CoV‐2 antigen‐IgE antibody on the mast cell surface represents the typical type Ⅰ hypersensitivity reaction. In conclusion, we propose that anti‐SARS‐CoV‐2–specific IgE‐mediated hypersensitivity reactions are the chief mechanism of COVID‐19‐related hypoxemic respiratory failure and other organ dysfunction.
Glucocorticoids are well‐established as the first‐line therapy for type Ⅰ hypersensitivity reactions and are also effective for inhibiting the inflammatory storm. It is reported that COVID‐19‐associated CSS could be treated with high‐dose of methylprednisolone. The use of glucocorticoids may accelerate respiratory recovery, lower hospital mortality and reduce the likelihood of invasive mechanical ventilation in COVID‐19‐associated CSS. 47 The same phenomenon was observed by our team. 15 In our study, we found that timely and appropriate application of glucocorticoid could avoid the need for invasive mechanical ventilation and improve the outcomes of critical COVID‐19 patients, compared with the outcomes in the reported studies. In the later stage in the “cytokine storm,” when the immune system becomes exhausted, continuous administration of glucocorticoid may lead to subsequent co‐infections and slows down the viral clearance. However, this did not happen in our patients. No obvious side effect has been observed during the half‐year follow‐up, probably because of the strictly controlled total dosage of methylprednisolone and the proper time of administration. The excellent response to glucocorticoids provides further evidence for the hypothesis that hypersensitivity reactions mediated by SARS‐CoV‐2‐induced specific IgE production might be the chief pathological mechanism of severe COVID‐19. Larger studies in more diverse populations are warranted to confirm this hypothesis.
The study has several limitations. First, due to the difference in the progression of COVID‐19 between patients, it is hard to determine an appropriate time to measure IgE, TLSS and PaO2/FiO2 ratio. Therefore, these patients were examined two weeks later, after admission and CS use. Second, the sample size of our study remains small. Third, some studies also found that previous allergic history (such as asthma, atopic rhinitis and allergic dermatitis) has little effect on the occurrence and severity of COVID‐19, 36 , 48 which need further research. Although our data suggested that IgE could be used to evaluate the severity of COVID‐19, more research is required to validate our findings. In addition, limited data were available and the usefulness of specific IgE in predicting mortality remains unknown. However, this research may have a good application prospect.
In COVID‐19 patients, anti‐SARS‐CoV‐2‐specific IgE, which mediated activated mast cells in the bronchial and intestinal mucosa, had been detected and significant correlations with the severity of patients were found. After the application of CS in severe COVID‐19, anti‐SARS‐CoV‐2‐specific IgE decreased but was maintained at a high level in early convalescence, which maybe is the reason for airway hyperresponsiveness (AHR) in these patients partly. Overall, our study indicated that hypersensitivity might be involved in severe COVID‐19.
CONFLICT OF INTEREST
The authors of this manuscript declare no relationships with any companies whose products or services might be related to the subject matter of the article.
AUTHOR CONTRIBUTIONS
Jin Huang and Jing Liu had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. Cuiyan Tan, Xiaobin Zheng, Fengfei Sun, Jianzhong He, Honglei Shi, Meizhu Chen, Changli Tu, Yiying Huang, Zhenguo Wang, Yingjian Liang, Jian Wu, Ye liu, Jing Liu and Jin Huang contributed substantially to the study design, data analysis and interpretation and the writing of the manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee of The Fifth Affiliated Hospital of Sun Yat‐sen University (approval series number K30‐1). The registration number of clinical trials: ChiCTR2000032895, Jing Liu.
CONSENT FOR PUBLICATION
All data published here are under the consent for publication.
Supporting information
Fig S1
Fig S2
Fig S3
Supplementary Material
ACKNOWLEDGEMENTS
Not applicable.
Tan C, Zheng X, Sun F, et al. Hypersensitivity may be involved in severe COVID‐19. Clin Exp Allergy. 2022;52:324–333. 10.1111/cea.14023
Cuiyan Tan, Xiaobin Zheng, Fengfei Sun contributed equally to this work.
Funding information
Guangdong Basic and Applied Basic Research Foundation (2020A1515011147). Zhuhai Science and Technology Project (ZH22036302200021PWC)
Contributor Information
Ye liu, Email: hjin@mail.sysu.edu.cn, Email: liujing25@sysu.edu.cn, Email: ly77219@163.com.
Jing Liu, Email: liujing25@sysu.edu.cn.
Jin Huang, Email: hjin@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the present study are available from the corresponding author on a reasonable request.
REFERENCES
- 1. World Health Organization . COVID‐19 Weekly Epidemiological Update, Data as received by WHO from national authorities, as of 25 April 2021, 10 am CET. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19. Accessed April 27, 2021.
- 2. Burki TK. Coronavirus in China. Lancet Respir Med. 2020. 8(3):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL‐6 and other mediators in the cytokine storm associated with SARS‐CoV‐2 infection. J Allergy Clin Immunol. 2020;146(3):518‐534.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID‐19). Front Immunol. 2020;1(11):827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song YG, Shin HS. COVID‐19, A Clinical Syndrome Manifesting as Hypersensitivity Pneumonitis. Infect Chemother. 2020;52(1):110‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebremeskel S, Schanin J, Coyle KM, et al. Mast cell and eosinophil activation are associated With COVID‐19 and TLR‐mediated viral inflammation: implications for an anti‐siglec‐8 antibody. Front Immunol. 2021;10(12):650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afrin LB, Weinstock LB, Molderings GJ. Covid‐19 hyperinflammation and post‐Covid‐19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hafezi B, Chan L, Knapp JP, et al. Cytokine storm syndrome in SARS‐CoV‐2 infections: a functional role of mast cells. Cells. 2021;10(7):1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol. 2018;142(2):485‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahri R, Bulfone‐Paus S. Mast Cell Activation Test (MAT). Methods Mol Biol. 2020;2163:227‐238. [DOI] [PubMed] [Google Scholar]
- 12. Long Q‐X, Liu B‐Z, Deng H‐J, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. [DOI] [PubMed] [Google Scholar]
- 13. Zhao J, Yuan Q, Wang H, et al. Antibody Responses to SARS‐CoV‐2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS‐CoV‐2. Sci Transl Med. 2021;13(577):eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Zheng X, Huang Y, Shan H, Huang J. Successful use of methylprednisolone for treating severe COVID‐19. J Allergy Clin Immunol. 2020;146(2):325‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease‐19 (COVID‐19): Relationship to Duration of Infection. Radiology. 2020;295(3):200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karsonova A, Riabova K, Villazala‐Merino S, et al. Highly sensitive ELISA‐based assay for quantification of allergen‐specific IgE antibody levels. Allergy. 2020;75(10):2668‐2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang Y, Chen M, Tan C, Tu C, Zheng X, Liu J. Successful Sequential Treatment for Severe Asthma Coexisting COVID‐19 via Budesonide/Glycopyrrolate/Formoterol Fumarate. Int J Gen Med. 2021;4(14):357‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146(3):841‐847. [DOI] [PubMed] [Google Scholar]
- 22. Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19(8):763‐768. [DOI] [PubMed] [Google Scholar]
- 23. Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4‐year‐old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112(2):317‐322. [DOI] [PubMed] [Google Scholar]
- 24. Kawai M, Kempsford R, Pullerits T, et al. Comparison of the efficacy of salmeterol/fluticasone propionate combination in Japanese and Caucasian asthmatics. Respir Med. 2007;101(12):2488‐2494. [DOI] [PubMed] [Google Scholar]
- 25. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. Covid‐19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid‐19: the first autopsy series from New Orleans. MedRxiv. 2020 Jan 1. [DOI] [PMC free article] [PubMed]
- 28. Potus F, Mai V, Lebret M, et al. Novel insights on the pulmonary vascular consequences of COVID‐19. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L277‐L288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berthelot JM, Drouet L, Lioté F. Kawasaki‐like diseases and thrombotic coagulopathy in COVID‐19: delayed over‐activation of the STING pathway? Emerg Microbes Infect. 2020;9(1):1514‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: A case series. EClinicalMedicine. 2020;25:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thiel V, Weber F. Interferon and cytokine responses to SARS‐coronavirus infection. Cytokine Growth Factor Rev. 2008;19(2):121‐132. doi: 10.1016/j.cytogfr.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motta Junior JDS, Miggiolaro AFRDS, Nagashima S, et al. Mast cells in alveolar septa of COVID‐19 patients: a pathogenic pathway that may link interstitial edema to immunothrombosis. Front Immunol. 2020;18(11):574862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Theoharides TC. Potential association of mast cells with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126(3):217‐218. doi: 10.1016/j.anai.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy K, Weaver C. Janeway's immunobiology. Garland science; 2016 Mar 1.
- 35. Bouadma L, Wiedemann A, Patrier J, et al. Immune Alterations in a Patient with SARS‐CoV‐2‐Related Acute Respiratory Distress Syndrome. J Clin Immunol. 2020;40(8):1082‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carli G, Cecchi L, Stebbing J, Parronchi P, Farsi A. Is asthma protective against COVID‐19? Allergy. 2021;76(3):866‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gonzales‐van Horn SR, Farrar JD. Interferon at the crossroads of allergy and viral infections. J Leukoc Biol. 2015;98(2):185‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated Type I Interferon and Inflammatory Monocyte‐Macrophage Responses Cause Lethal Pneumonia in SARS‐CoV‐Infected Mice. Cell Host Microbe. 2016;19(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maggi E, Parronchi P, Manetti R, et al. Immunol.Reciprocal regulatory effects of IFN‐gamma and IL‐4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148(7):2142‐2147. [PubMed] [Google Scholar]
- 40. Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elliver RC, Sun M, Rinaldo D, Ogra PL. Predictive value of respiratory syncytial virus‐specific IgE responses for recurrent wheezing following bronchiolitis. J Pediatr. 1986;109:776‐780. [DOI] [PubMed] [Google Scholar]
- 42. Kusel MM, de Klerk NH, Kebadze T, et al. Early‐life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grayson MH, Cheung D, Rohlfing MM, et al. Induction of high‐affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204(11):2759‐2769. doi: 10.1084/jem.20070360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gill MA, Liu AH, Calatroni A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735‐1743.e9. doi: 10.1016/j.jaci.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lommatzsch M, Stoll P, Virchow JC. COVID‐19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705‐2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Criado PR, Criado RFJ, Pincelli TP, Yoshimoto TA, Naufal GGA, Abdalla BMZ. Chronic spontaneous urticaria exacerbation in a patient with COVID‐19: rapid and excellent response to omalizumab. Int J Dermatol. 2020;59(10):1294‐1295. doi: 10.1111/ijd.15134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramiro S, Mostard RLM, Magro‐Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID‐19‐associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du H, Dong X, Zhang JJ, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2021;76(2):510‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Supplementary Material
Data Availability Statement
The datasets generated and analysed during the present study are available from the corresponding author on a reasonable request.


