Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic is an unprecedented global health emergency causing more than 4.2 million fatalities as of 30 July 2021. Only three antiviral therapies have been approved or granted emergency use authorization by the FDA. The SARS‐CoV‐2 3CL protease (3CLpro) is deemed an attractive drug target as it plays an essential role in viral polyprotein processing and pathogenesis, although no inhibitors have been approved. This patent review discusses SARS coronavirus 3CLpro inhibitors that have been filed up to 30 July 2021, giving an overview on the types of inhibitors that have generated commercial interest, especially amongst drug companies. Insights into the common structural motifs required for active site binding is also discussed.
Keywords: 3CLpro, Mpro, SARS-CoV-2, cysteine protease inhibitor, COVID-19
Necessity is the mother of invention: The current SARS‐CoV‐2 pandemic is causing an unprecedented global health crisis. The coronavirus main protease 3CLpro is deemed an attractive drug target due to its critical role in viral polyprotein processing. In this patent review, 24 coronavirus 3CLpro inhibitor patents filed up to 30 July 2021 are described. Important structural features required for protease binding are discussed to facilitate the design of more potent inhibitors.
1. Introduction
In late 2019, a number of severe pneumonia clusters were reported in Wuhan, Hubei province, China.[ 1 , 2 ] Genome sequencing revealed the responsible pathogen was a coronavirus with 80 % nucleotide sequence identity to the severe acute respiratory syndrome coronavirus (SARS‐CoV), responsible for the 2002–2004 outbreak originating from Foshan, Guangdong province, China.[ 3 , 4 , 5 , 6 ] This new coronavirus was named ‘severe acute respiratory syndrome coronavirus 2’ (SARS‐CoV‐2) by the International Committee on Taxonomy of Viruses [7] and the disease was called ‘coronavirus disease 2019’ (COVID‐19) by the World Health Organization (WHO). The virus has since spread globally, infecting more than 196 million people and causing more than 4.2 million deaths as of 30 July 2021. [8] Although a number of vaccines have been granted authorization, only three antiviral therapies (Remdesivir, Casirivimab+Imdevimab and Sotrovimab)[ 9 , 10 , 11 ] have so far been approved or granted emergency use authorization by the United States Food and Drug Administration (FDA), signifying an urgent need for more antiviral drugs.
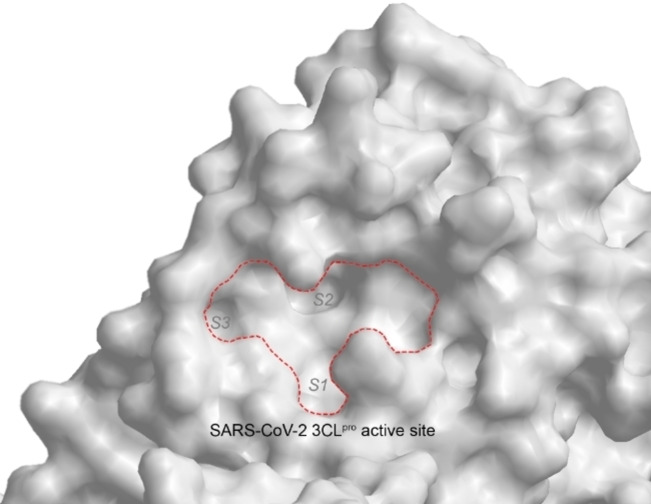
The coronavirus 3‐chymotrypsin‐like protease (3CLpro; also known as ‘main protease’ or ‘Mpro’) is deemed an attractive drug target due to its essential role in coronavirus polyprotein processing. It cleaves the polyprotein at more than 11 sites to yield essential proteins required for virus replication and pathogenesis.[ 12 , 13 , 14 , 15 , 16 ] As 3CLpro has no reported human homologues, a 3CLpro inhibitor should not inhibit any human proteases and hence, reduce the risk of side‐effects.[ 15 , 16 ] 3CLpro is a cysteine protease with a unique substrate preference for a P1 glutamine, hydrolysing the peptide bond at the C‐terminus of glutamine.[ 12 , 13 , 14 , 15 , 16 ] Particularly noteworthy is that the 3CLpro of SARS‐CoV and SARS‐CoV‐2 share 96 % amino acid sequence identity so inhibitors designed for the former should work equally well on the latter.[ 12 , 17 ] Indeed, the first coronavirus 3CLpro inhibitor to enter clinical trials is Pfizer's PF‐07304814 (ClinicalTrials.gov identifier NCT04535167) in September 2020. First described in a patent filed in 2005 as a SARS‐CoV 3CLpro inhibitor (WO 2005/113580), its synthesis, structure‐activity relationship study and preclinical development details were published only much later in 2020. [18] .
2. Patent Review
Although many SARS‐CoV and SARS‐CoV‐2 3CLpro inhibitors have been reported, collated and reviewed in academic journals since the 2002–2004 SARS‐CoV pandemic, none have so far been approved as antiviral drugs.[ 12 , 13 , 14 ] A plausible explanation could be that commercial interest in coronavirus drug development waned rapidly after the SARS‐CoV pandemic ended. However, since the start of the SARS‐CoV‐2 pandemic in late 2019, academic and commercial interest in coronavirus 3CLpro inhibitors returned with a vengeance. This review collates SARS‐CoV and SARS‐CoV‐2 3CLpro inhibitors reported in patent applications filed since the 2002 SARS‐CoV pandemic up to 30 July 2021. SciFinder® software (CAS) [19] was employed and search terms: ‘coronavirus 3CLpro inhibitor’, ‘coronavirus 3CL protease inhibitor’, ‘coronavirus Mpro inhibitor’, ‘coronavirus main protease inhibitor’ and ‘coronavirus protease inhibitor’ were used. The patents are summarized in Table 1 in chronological order with detailed analysis included vide infra.
Table 1.
Summary of SARS coronavirus 3CLpro inhibitor patents in chronological order up to 30 July 2021.
|
Patent number |
Applicant/Assignee |
Filing date (dd.mm.yyyy) |
Inhibitor type |
Fig. |
Ref. |
|---|---|---|---|---|---|
|
WO 2004/093860 |
Pfizer |
13.04.2004 |
peptidomimetic |
1 |
[20] |
|
WO 2004/101742 |
Cytovia |
06.05.2004 |
peptidomimetic |
2 |
[21] |
|
US 2006/0014821 |
Agouron Pharmaceuticals |
13.08.2004 |
peptidomimetic |
3 |
[22] |
|
WO 2005/041904 |
Fulcrum Pharmaceuticals |
01.11.2004 |
small molecule |
4 |
[23] |
|
WO 2005/066123 |
Taigen Biotechnology |
28.12.2004 |
peptidomimetic |
5 |
[24] |
|
WO 2005/113580 |
Pfizer |
09.05.2005 |
peptidomimetic |
6 |
[25] |
|
US 2006/0019967 |
National Health Research Institutes, Taiwan |
20.07.2005 |
small molecule |
7 |
[26] |
|
WO 2006/042478 |
Tsinghua University, Shanghai Institute of Organic Chemistry |
24.10.2005 |
peptidomimetic |
8 |
[27] |
|
CN 1965833 A |
Peking University |
18.11.2005 |
small molecule |
9 |
[28] |
|
WO 2006/061714 |
Pfizer |
06.12.2005 |
peptidomimetic |
10 |
[29] |
|
WO 2006/095624 |
Tokyo Medical and Dental University, RIKEN |
01.03.2006 |
small molecule |
11 |
[30] |
|
WO 2007/075145 |
Singapore Polytechnic, Shanghai Institute of Materia Medica |
15.12.2006 |
small molecule |
12 |
[31] |
|
CN 103159665B |
Tianjin International Joint Academy of Biotechnology and Medicine |
09.12.2011 |
small molecule |
13 |
[32] |
|
WO 2013/049382 |
Kansas State University, Ohio State University, Wichita State University |
27.09.2012 |
peptidomimetic |
14 |
[33] |
|
KR 1020130002975 |
Chonnam National University |
20.12.2012 |
small molecule |
15 |
[34] |
|
WO 2013/166319 |
Kansas State University, Wichita State University |
02.05.2013 |
peptidomimetic |
16 |
[35] |
|
CN 106176728 A |
Institute of Microbiology, Chinese Academy of Sciences |
07.07.2016 |
small molecule |
17 |
[36] |
|
WO 2017/114509 |
Shanghai Institute of Materia Medica, University of Lübeck |
30.12.2016 |
peptidomimetic |
18 |
[37] |
|
US 2017/0313685 |
Purdue Research Foundation |
28.04.2017 |
small molecule |
19 |
[38] |
|
WO 2017/222935 |
Kansas State University, Wichita State University |
16.07.2017 |
peptidomimetic |
20 |
[39] |
|
CN 108785293 A |
Tianjin International Joint Academy of Biomedicine |
28.04.2017 |
small molecule |
21 |
[40] |
|
WO 2018/042343 |
GSK |
30.08.2017 |
peptidomimetic |
22 |
[41] |
|
WO 2020/030143 |
Shanghai Institute of Materia Medica, Fudan University |
09.08.2019 |
peptidomimetic |
23 |
[42] |
|
WO 2021/176369 |
Pfizer |
03.03.2021 |
peptidomimetic |
24 |
[43] |
SciFinder® revealed 18 patents on CoV 3CLpro inhibitors and together with a 2020 patent review on coronavirus therapeutic agents, [44] a total 24 patents are summarised in Table 1. Unsurprisingly, majority of the patents (23 out of 24) described inhibitors designed to inhibit SARS‐CoV 3CLpro as the SARS‐CoV‐2 pandemic started only in late 2019. However, since both viral proteases share 96 % sequence identity,[ 12 , 17 ] inhibitors designed for the former should also inhibit the latter protease. Indeed, Pfizer's PF‐00835231 (6 b), first described in WO 2005/113580, was originally designed to inhibit SARS‐CoV 3CLpro (IC50 4 nM) [18] and was later shown to be a potent inhibitor of SARS‐CoV‐2 3CLpro (IC50 8 nM). [45]
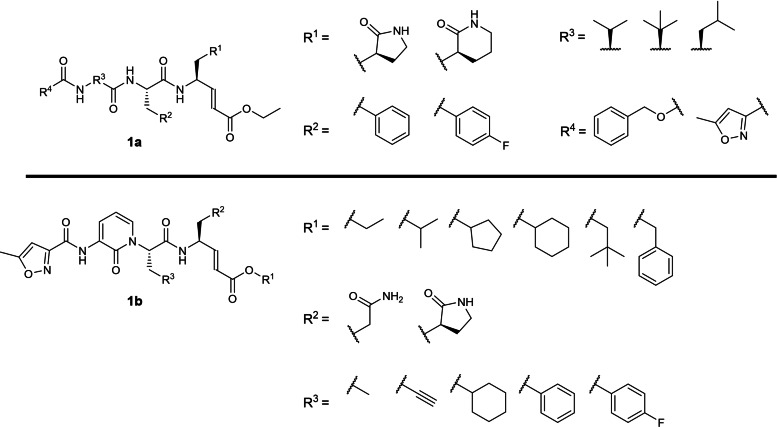
To our best knowledge, the earliest patent application describing SARS‐CoV 3CLpro inhibitors was filed by Pfizer in 2004 (WO 2004/093860; Table 1) [20] involving 3‐residue peptidomimetics bearing a P1 glutamine or lactam glutamine mimic with a C‐terminal electrophilic α,β‐unsaturated ester warhead (Figure 1). Some inhibitors had their P3 amino acid residue substituted by a 2‐pyridone moiety (1 b), presumably to improve their pharmacokinetic properties. All the described compounds were originally designed to inhibit the rhinovirus 3C protease which shares some structural similarity to coronavirus 3CLpro. [20] Unfortunately, no coronavirus 3CLpro inhibition data were reported in the patent. We believe this is the first patent describing peptidomimetics bearing a P1 lactam glutamine mimic (1 a) used for inhibiting SARS‐CoV 3CLpro and were originally designed as rhinovirus 3C protease inhibitors in 1999 by Agouron Pharmaceuticals (WO 99/57135). [46] We are currently unable to recommend these peptidomimetics for further drug development until their SARS‐CoV‐2 3CLpro inhibitory potencies are revealed.
Figure 1.
General scaffolds from WO 2004/093860.
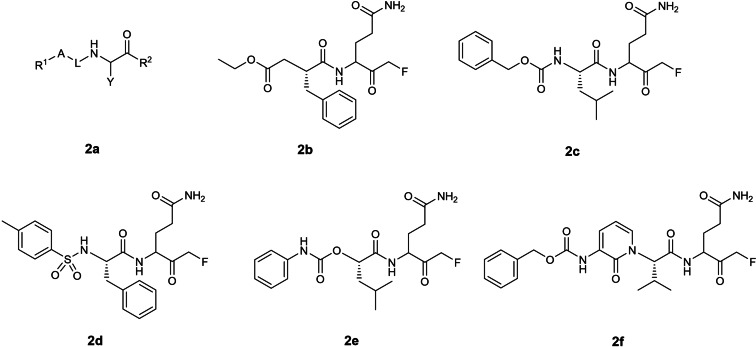
The second earliest patent application on SARS‐CoV 3CLpro inhibitors was filed by Cytovia in 2004 (WO 2004/101742; Table 1) [21] describing 2‐residue peptidomimetics containing a P1 glutamine residue with a C‐terminal electrophilic monofluoromethylketone warhead (Figure 2). P1 alanine, glycine and N‐dimethylated glutamine mimics were also exemplified although their inhibitory activities were not disclosed. Compound 2 b was revealed to be the most potent inhibitor with an EC50 of 0.02 μg/mL in a SARS‐CoV Vero cell assay. No biochemical 3CLpro inhibition (IC50) data were reported in the patent. A publication was found for 2 e containing a P1 N‐dimethylated glutamine with an EC50 of 2.5 μM in a SARS‐CoV Vero cell assay. [47] The authors revealed that the P1 glutamine residue was incompatible with the C‐terminal electrophilic warhead due to intramolecular cyclisation with the P1 glutamine side‐chain primary amide, resulting in bioactivity loss. [47] To solve this problem, Pfizer filed a patent in 2004 describing peptidomimetic inhibitors where the P1 glutamine was modified to either 5‐ or 6‐membered lactams (WO 2004/093860; Figure 1a). [20] Notably, this P1 modification strategy was adopted in subsequent patents involving coronavirus 3CLpro peptidomimetic inhibitors after 2004. In addition, the idea of incorporating a 2‐pyridone moiety at the P3 position, exemplified in compound 2 f, was reported in an earlier Pfizer patent (WO 2004/093860; Figure 1b). [20] Although there are currently no follow‐up publications for 2 f, this idea was later adopted for peptidomimetic ketoamide SARS‐CoV‐2 3CLpro inhibitors in a 2020 Science paper. [48]
Figure 2.
Structures from WO 2004/101742. (a) general scaffold; (b–f) exemplified structures.
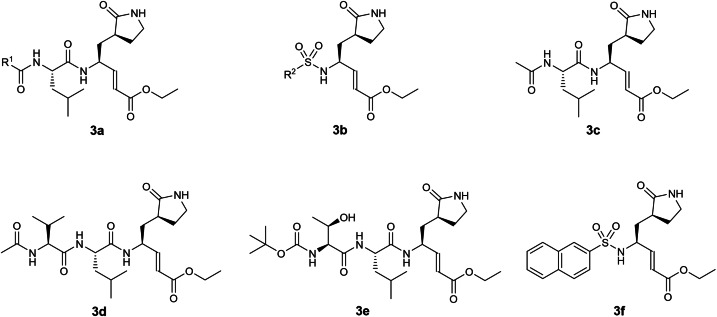
The third earliest patent on coronavirus 3CLpro inhibitors was filed in 2004 by Agouron Pharmaceuticals involving 1‐, 2‐ and 3‐residue peptidomimetics bearing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic α,β‐unsaturated ester warhead (US 2006/0014821; Table 1). [22] 8 structures were exemplified (Figure 3) but no biochemical 3CLpro inhibition (IC50) data were reported in the patent. A structure search on all 8 compounds, including compounds 3 c to 3 f, revealed no follow‐up publications. Notably, peptidomimetics with α,β‐unsaturated ester warheads inhibiting SARS‐CoV 3CLpro were described in an earlier patent (WO 2004/093860; Figure 1). [20] We are currently unable to gauge their potential for further antiviral drug development due to the lack of inhibitory potency data. Interestingly, another patent involving peptidomimetic inhibitors with electrophilic α,β‐unsaturated ester warheads was filed in 2004 by Taigen Biotechnology (WO 2005/066123; Figure 5), [24] discussed vide infra.
Figure 3.
Structures from US 2006/0014821. (a, b) general scaffolds; (c–f) exemplified structures.
Figure 5.
Structures from WO 2005/066123. (a) general scaffold; (b–f) exemplified structures.
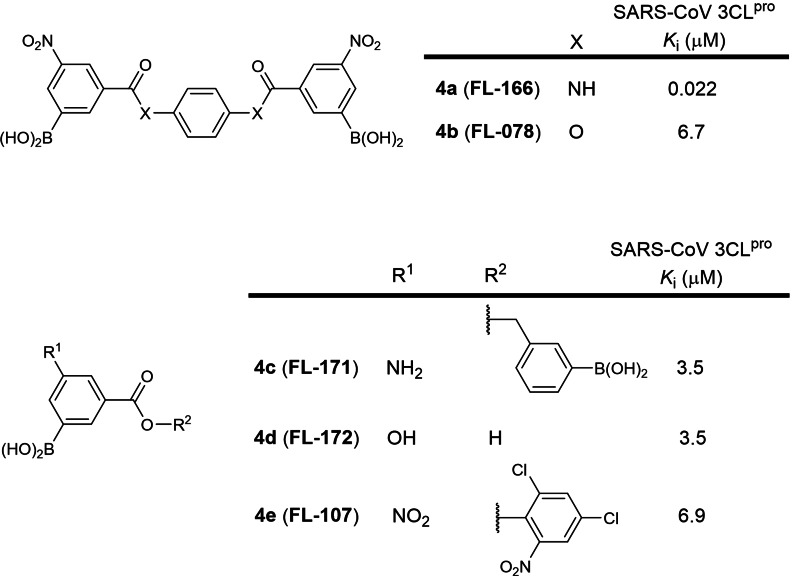
The fourth earliest patent was filed in 2004 by Fulcrum Pharmaceuticals involving small molecules containing one or two boronic acid moieties (WO 2005/041904; Table 1). [23] 403 structures were described. The strongest binder was identified to be compound 4 b, also named FL‐166, with a SARS‐CoV 3CLpro K i of 22 nM, followed by 4 c to 4 f with K is ranging between 3.5 and 6.9 μM (Figure 4). Compounds 4 b, 4 c and 4 d were reported in a research article to possess K is of 0.04, 4.5 and 16 μM against SARS‐CoV 3CLpro respectively. [49] No SARS‐CoV‐2 3CLpro inhibition data have been reported for these compounds using SciFinder® structure searches. In our view, cell‐based EC50 values should first be obtained for 4 a to gauge its potential for further development as an antiviral drug.
Figure 4.
Structures from WO 2005/041904. (a–e) exemplified structures with the most potent K is against SARS‐CoV 3CLpro.
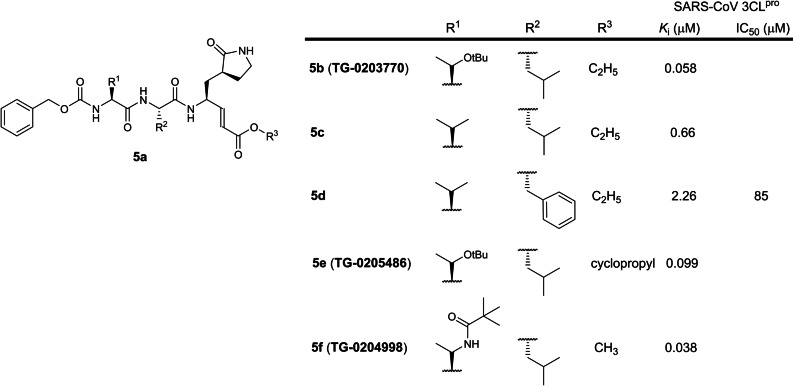
The fifth patent was filed in 2004 by Taigen Biotechnology involving 3‐residue peptidomimetics containing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic α,β‐unsaturated ester warhead (WO 2005/066123; Table 1). [24] 145 compounds were reported and 77 were disclosed to be sub‐micromolar SARS‐CoV 3CLpro inhibitors. Unfortunately, no K i or IC50 values were assigned to any of the structures. However, a literature search revealed compound 5 b, also named TG‐0203770, to be the strongest binder with a SARS‐CoV 3CLpro K i of 58 nM, followed by 5 c and 5 d (K is 0.66 and 2.26 μM respectively; Figure 5). [50] Compound 5 c was later reported to inhibit SARS‐CoV‐2 3CLpro with an IC50 of 286 nM. [45] In contrast, 5 d was reported to be a weak SARS‐CoV 3CLpro inhibitor (IC50 85 μM), [51] suggesting that a P2 phenylalanine should be avoided in the design of new SARS‐CoV 3CLpro inhibitors. Compounds 5 e and 5 f, known as TG‐0205486 and TG‐0204998 respectively, were reported with K is of 99 and 38 nM respectively. [52] Notably, two earlier patents involving electrophilic α,β‐unsaturated ester warhead peptidomimetics were filed by Pfizer and Agouron Pharmaceuticals (WO 2004/093860 and US 2006/0014821), discussed vide supra. We opine compounds 5 b, 5 e and 5 f warrant further investigation as SARS‐CoV‐2 drug candidates.
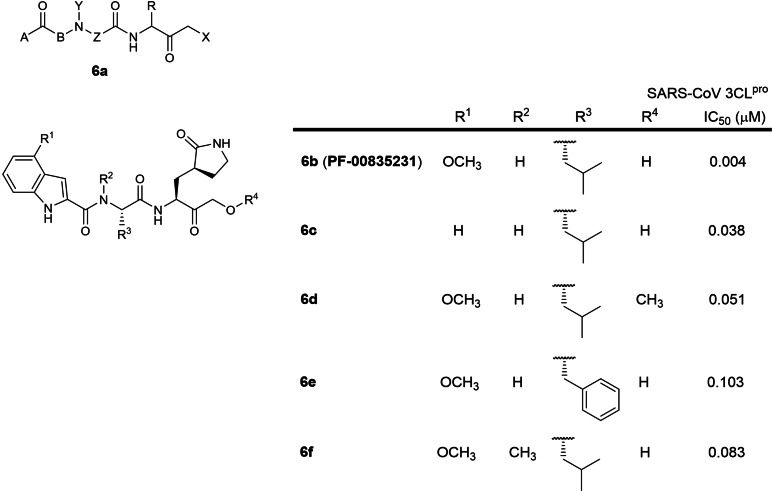
The sixth earliest patent was filed in 2005 by Pfizer involving 2‐residue peptidomimetics containing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic hydroxymethylketone warhead (WO 2005/113580; Table 1). [25] 61 compounds were reported with 35 exhibiting sub‐micromolar IC50s against SARS‐CoV 3CLpro. A literature search revealed PF‐00835231 (6 b; Figure 6) as the lead candidate with a very potent IC50 of 4 nM against SARS‐CoV 3CLpro and a SARS‐CoV EC50 of 4.8 μM in a SARS‐CoV infection cellular assay while a recent paper reported an IC50 of 8 nM against SARS‐CoV‐2 3CLpro.[ 18 , 45 ] This strongly suggests that inhibitors designed to inhibit SARS‐CoV 3CLpro can be repurposed for SARS‐CoV‐2 3CLpro as both proteases share 96 % sequence identity.[ 12 , 17 ] The phosphate prodrug of PF‐0835231 entered phase 1 clinical trials in September 2020 to evaluate safety and pharmacokinetics in hospitalised COVID‐19 patients (ClinicalTrials.gov identifier: NCT04535167). Notably, substituting the P2 leucine with phenylalanine resulted in a 25‐fold activity loss (IC50 103 vs. 4 nM; 6 e and 6 b respectively), suggesting that the SARS‐CoV 3CLpro S2 binding pocket preferred smaller sized residues. [18] N‐methylating the P2 leucine resulted in a 20‐fold activity loss (IC50 83 vs. 4 nM; 6 f and 6 b respectively), suggesting that the P2 NH plays an important role in 3CLpro binding. [18] 6‐membered lactam glutamine mimics were also described in the patent but none were exemplified.
Figure 6.
Structures from WO 2005/113580. (a) general scaffold; (b–f) exemplified structures.
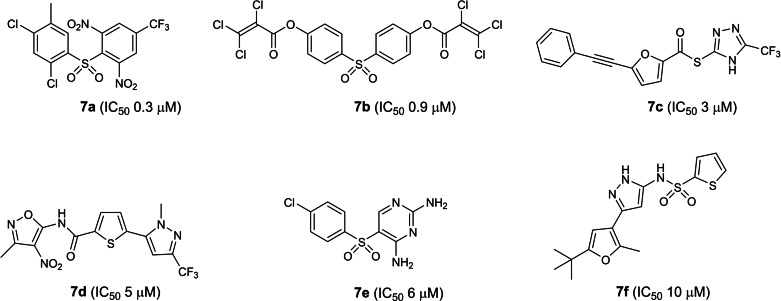
The seventh earliest patent was filed in 2005 by the National Health Research Institutes, Taiwan, involving small molecules from a commercially available small molecule library screen (US 2006/0019967; Table 1). [26] 21 compounds were reported to possess inhibitory activities with 4 exhibiting ‘very low’ micromolar IC50s against SARS‐CoV 3CLpro (compounds 7 a to 7 d; Figure 7). Unfortunately, IC50 values were not disclosed in the patent. A literature search revealed compound 7 a to be the most potent with an IC50 of 300 nM against SARS‐CoV 3CLpro. [53] The next most potent is 7 b with an IC50 of 900 nM while 7 c to 7 f exhibited IC50s between 3 to 10 μM. [53] Structure searches revealed there are currently no reports on their SARS‐CoV‐2 3CLpro inhibitory activities. Interestingly, compound 7 c, also known as PNU‐136592, was earlier reported by Pharmacia scientists to inhibit MurB, a bacteria enzyme involved in cell wall peptidoglycan biosynthesis. [54] In our view, cell‐based EC50 values should first be obtained to gauge their potential for further antiviral drug development.
Figure 7.
Six most potent SARS‐CoV 3CLpro inhibitors reported in US 2006/0019967.
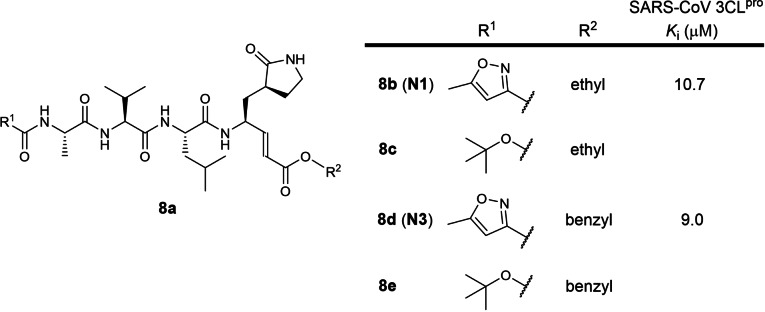
The eighth earliest patent was filed in 2005 by Tsinghua University and the Shanghai Institute of Organic Chemistry involving 3‐ and 4‐residue peptidomimetics bearing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic α,β‐unsaturated ester warhead (WO 2006/042478; Table 1). [27] 10 compounds were exemplified and no IC50 data was reported (Figure 8). The patent is written in Chinese and an English version could not be found online. A research article published in the same month of patent filing reported inhibitors 8 b and 8 d (named N1 and N3 respectively) with K is of 10.7 and 9.0 μM against SARS‐CoV 3CLpro respectively. [55] A 2020 Nature paper reported 8 d/N3 to be a very potent SARS‐CoV‐2 3CLpro inhibitor and that its K i could not be feasibly measured. [15] No SARS‐CoV‐2 3CLpro inhibition data have been reported so far for compounds 8 c and 8 e using SciFinder® structure searches. In our opinion, 8 d/N3 warrants further investigation as an antiviral drug.
Figure 8.
Structures from WO 2006/061714. (a) general scaffold; (b–e) exemplified structures.
The ninth earliest patent was filed in 2005 by Peking University involving the discovery of the oral H1 histamine receptor antagonist oxatomide possessing SARS‐CoV 3CLpro inhibitory activity (CN 1965833 A; Figure 9). [28] No IC50 or K i data was reported. The patent is written in Chinese and an English version could not be found online. A SciFinder® structure search did not reveal any academic journals reporting its SARS‐CoV 3CLpro inhibitory activity. Due to the lack of inhibitory data, we are currently unable to gauge its potential for further development as an antiviral drug.
Figure 9.
Exemplified structure from CN 1965833 A.
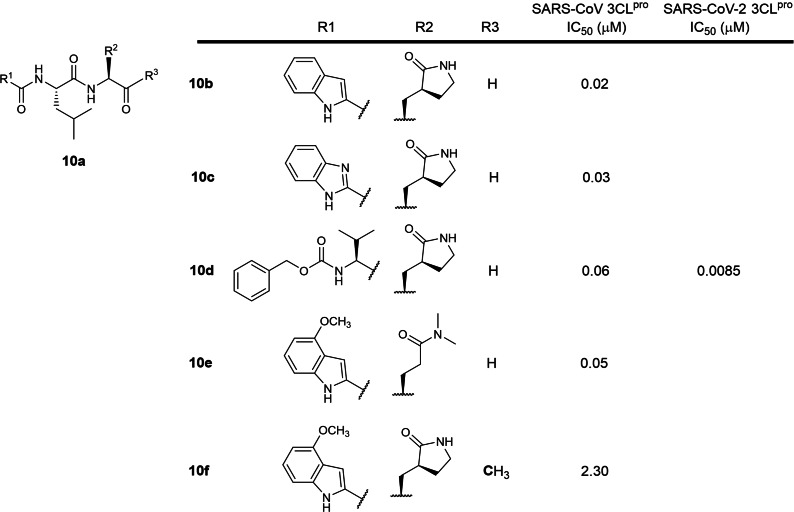
The tenth earliest patent was filed in 2005 by Pfizer involving 2‐ and 3‐residue peptidomimetics containing P1 5‐membered lactam and tertiary amide glutamine mimics with C‐terminal electrophilic aldehyde or ketone warheads (WO 2006/061714; Table 1). [29] 15 compounds were exemplified with 4 (10 b to 10 e; Figure 10) exhibiting sub‐micromolar IC50s against SARS‐CoV 3CLpro. Ketone peptidomimetic 10 f exhibited a moderate IC50 of 2.3 μM, suggesting that aldehyde warheads are much more reactive than ketones. Compound 10 d has been resynthesized and reported to inhibit SARS‐CoV‐2 3CLpro with an IC50 of 8.5 nM. [56] Aldehydes are known to be highly reactive towards endogenous biological nucleophiles, potentially rendering compounds 10 b to 10 e cytotoxic. Aldehydes are also metabolically unstable due to their susceptibility to oxidation and reduction by liver enzymes.[ 57 , 58 ] Hence, we believe aldehyde peptidomimetics lack potential for further drug development due to their metabolic liabilities.
Figure 10.
Structures from WO 2006/061714. (a) general scaffold; (b–f) exemplified structures.
The eleventh earliest patent was filed in 2006 by Tokyo Medical and Dental University and RIKEN involving small molecules with a 3‐cyanopyridine scaffold (WO 2006/095624; Table 1; Figure 11). [30] 102 compounds were described. The patent is published in Japanese and no English version can be found online. To our best knowledge, 3CLpro IC50 inhibition data was not reported in the patent. Structure searches using SciFinder® did not reveal any follow‐up journal publications. Due to the lack of inhibitory data, we are unable to gauge their potential for further drug development.
Figure 11.
Structures from WO 2006/095624. (a) general scaffold; (b–f) exemplified structures.
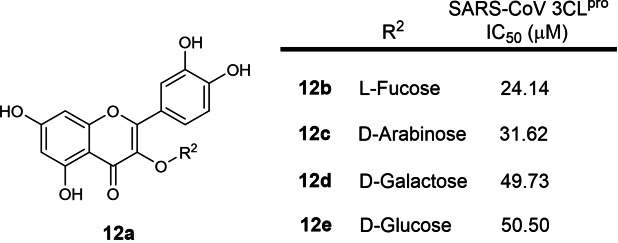
The twelfth earliest patent was filed in 2006 by Singapore Polytechnic and Shanghai Institute of Materia Medica involving small molecules with a chromone (1,4‐benzopyrone) scaffold (WO 2007/075145; Table 1). [31] 17 structures were exemplified and 4 compounds, 12 b to 12 e (Figure 12), were reported with SARS‐CoV 3CLpro IC50s ranging from 24 to 49 μM. These compounds were published in a research article in the same year of patent filing. [59] Due to their weak inhibitory activities, we opine that these compounds are not potent enough for further drug development.
Figure 12.
Structures from WO 2007/075145. (a) general scaffold; (b–e) exemplified structures.
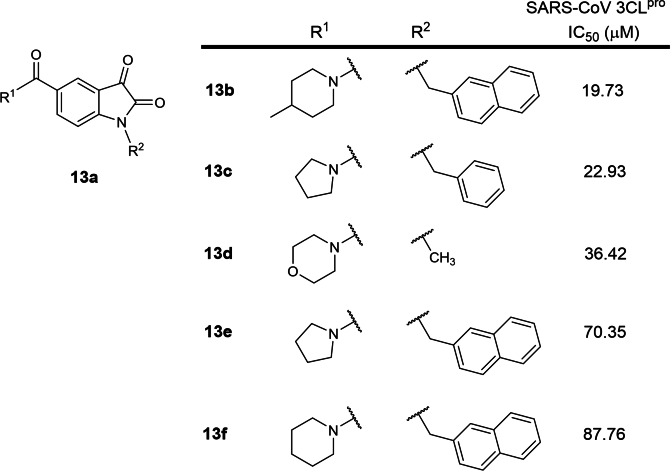
The thirteenth earliest patent was filed in 2011 by Tianjin International Joint Academy of Biotechnology and Medicine involving 2,3‐dioxindole SARS‐CoV 3CLpro inhibitors (CN 103159665B; Table 1). [32] The patent is written in Chinese and an English version could not be found online. 5 structures (Figure 13) were exemplified with SARS‐CoV 3CLpro IC50s between 20 to 88 μM. Structure searches on the compounds using SciFinder® did not reveal any follow‐up journal publications. In our opinion, these compounds are not potent enough to be developed as antiviral drugs.
Figure 13.
Structures from CN 103159665B. (a) general scaffold; (b–f) exemplified structures.
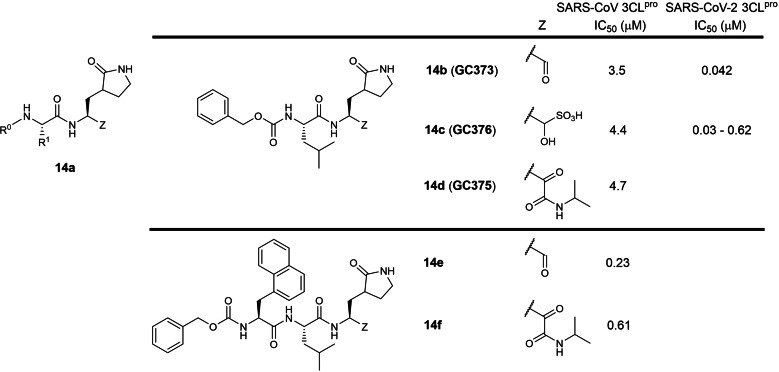
The fourteenth patent was filed in 2012 by Kansas State, Ohio State and Wichita State Universities involving 2‐ and 3‐residue peptidomimetics containing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic aldehyde or ketoamide warhead (WO 2013/049382; Table 1). [33] A hydroxymethyl sulfonic acid prodrug masking the reactive aldehyde warhead was also described (14 c; Figure 14). Compounds 14 b, 14 c and 14 d exhibited IC50s ranging between 3.5 to 4.7 μM in a SARS‐CoV 3CLpro biochemical assay. No SARS‐CoV 3CLpro IC50s were reported for tripeptide aldehyde 14 e and ketoamide 14 f in the patent but were later reported in a research article (0.23 and 0.61 μM respectively). [60] Compounds 14 b and 14 c, also named GC373 and GC376 respectively, were reported to inhibit SARS‐CoV 3CLpro with 3.5 and 4.4 μM IC50s in a research paper. [61] Interestingly, 14 b/GC373 was shown to be much more active against SARS‐CoV‐2 3CLpro, exhibiting an IC50 of 0.042 μM in a recent paper. [45] Similarly, 14 c/GC376 was also shown to be much more active against SARS‐CoV‐2 3CLpro, exhibiting IC50s ranging between 0.03 to 0.62 μM.[ 45 , 62 , 63 ] We opine ketoamide peptidomimetics 14 d and 14 f can potentially be further developed as SARS‐CoV‐2 3CLpro antivirals because ketoamide peptidomimetic protease inhibitors such as Boceprevir and Telaprevir have been approved for treating hepatitis C virus infections. [64]
Figure 14.
Structures from WO 2013/049382. (a) general scaffold; (b–f) exemplified structures.
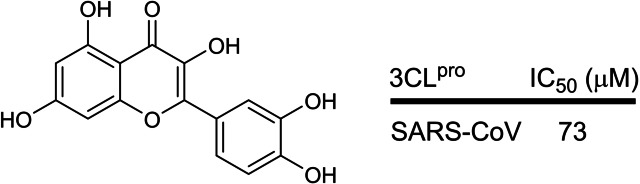
The fifteenth earliest patent was filed in 2012 by Chonnam National University, South Korea revealing that the plant flavonoid, quercetin (KR 1020130002975; Table 1; Figure 15), was able to inhibit SARS‐CoV 3CLpro with an IC50 of 73 μM and suggested that it could potentially be used to treat SARS‐CoV infections. The patent is written in Korean and no English version could be found online. In our opinion, we believe that its inhibitory activity is too weak to be considered for development as an antiviral drug.
Figure 15.
Structure of quercetin from KR 1020130002975.
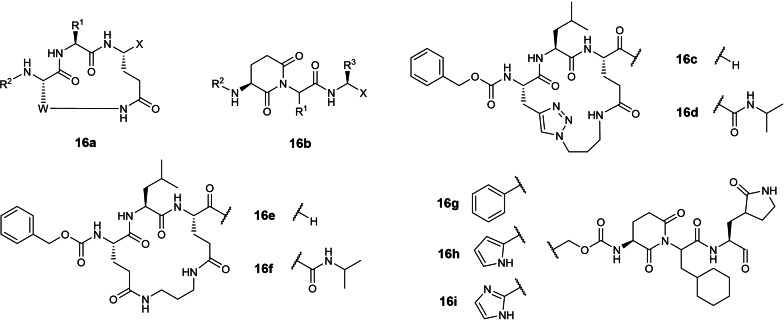
The sixteenth earliest patent was filed in 2013 by Kansas State and Wichita State Universities involving 3‐residue cyclic peptidomimetics and linear piperidine‐2,6‐dione peptidomimetics with C‐terminal aldehyde or ketoamide warheads (WO 2013/166319; Table 1). [35] Although SARS‐CoV 3CLpro IC50s were not disclosed in the patent, a research article by the inventors reported compound 16 c (Figure 16) inhibited SARS‐CoV 3CLpro with an IC50 of 15.5 μM. [65] No SARS‐CoV‐2 3CLpro inhibitory activities have so far been reported for compounds 16 c to 16 i. In our view, 16 c lacks potency for further drug development.
Figure 16.
Structures from WO 2013/166319. (a, b) general scaffolds; (c–i) exemplified structures.
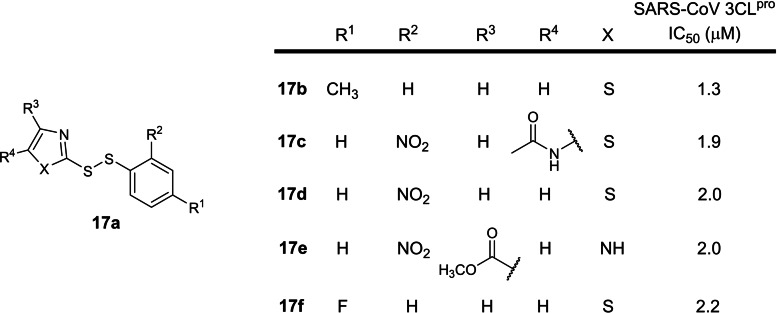
The seventeenth earliest patent was filed in 2016 by The Institute of Microbiology, Chinese Academy of Sciences involving small molecule disulphide SARS‐CoV 3CLpro inhibitors (CN 106176728 A; Table 1). [36] The patent is written in Chinese and an English version could not be found online. 12 structures were exemplified and the 5 most potent compounds possess IC50s between 1.3 to 2.2 μM (17 b to 17 f; Figure 17). A SciFinder® structure search revealed one follow‐up academic paper reporting their SARS‐CoV 3CLpro inhibitory activities. [66] There are currently no reports on SARS‐CoV‐2 3CLpro inhibitory activities. We opine that these compounds lack drug development potential due to their disulphide moiety, reported to be unstable in physiological conditions due to the presence of biological reducing agents, free thiols and isomerases. [67] Hence, pharmacokinetic studies should be conducted first before these compounds can be evaluated further.
Figure 17.
Structures from CN 106176728 A. (a) general scaffold; (b–f) top five most potent inhibitors.
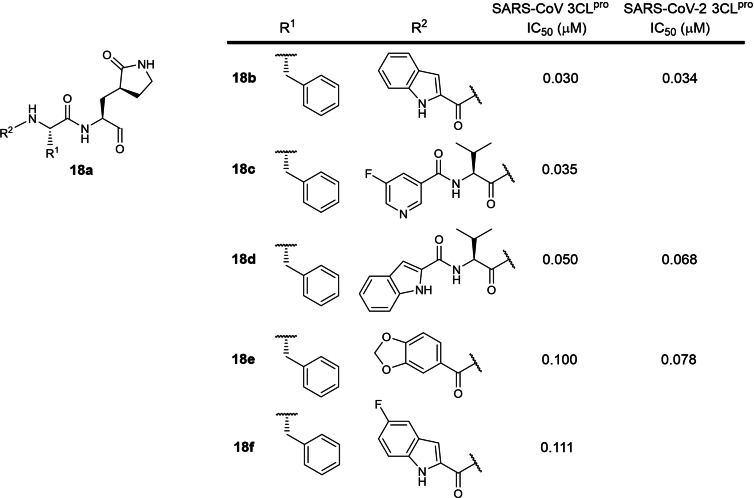
The eighteenth patent was filed in 2016 by Shanghai Institute of Materia Medica and the University of Lübeck involving 2‐ and 3‐residue peptidomimetics bearing a P1 5‐membered lactam glutamine mimic and a C‐terminal electrophilic aldehyde warhead (WO 2017/114509; Table 1). [37] 74 structures were described with SARS‐CoV 3CLpro IC50s ranging from 30 nM to 7.4 μM. The four most potent structures are shown in Figure 18 (compounds 18 b to 18 e) with SARS‐CoV 3CLpro IC50s ranging from 30 to 100 nM. The most potent inhibitor 18 b is structurally similar to Pfizer's 10 b (Figure 10), differing at the P2 residue. 18b’s SARS‐CoV 3CLpro inhibitory activity is 1.5‐fold weaker than Pfizer's 10 b (IC50s 30 and 20 nM respectively), suggesting that SARS‐CoV 3CLpro prefers a P2 leucine over phenylalanine. This distinction is an important consideration when designing new coronavirus 3CLpro inhibitors and may be the reason why a P2 leucine was selected for Pfizer's lead compound PF‐00835231 (Figure 6b). [18] 18 b was also reported to be the most potent SARS‐CoV‐2 3CLpro inhibitor (IC50 34 nM) amongst 13 peptide aldehydes in a recent publication, [68] suggesting that inhibitors designed for SARS‐CoV 3CLpro can be effectively used to inhibit SARS‐CoV‐2 3CLpro. Interestingly, the addition of a P3 valine to 18 b to yield tripeptide aldehyde 18 d resulted in an approximate 2‐fold activity reduction for SARS‐CoV and SARS‐CoV‐2 3CLpro (IC50s 30 to 50 nM and 34 to 68 nM respectively). [68] Notably, all the inhibitors described in the patent possess highly electrophilic aldehyde warheads which are known to be highly reactive towards endogenous biological nucleophiles, potentially rendering them cytotoxic. Aldehydes are also metabolically unstable due to their susceptibility to oxidation and reduction by liver enzymes.[ 57 , 58 ] Hence, we believe these aldehyde peptidomimetics lack potential for further drug development.
Figure 18.
Structures from WO 2017/114509. (a) general scaffold; (b–f) most potent exemplified inhibitors against SARS‐CoV 3CLpro.
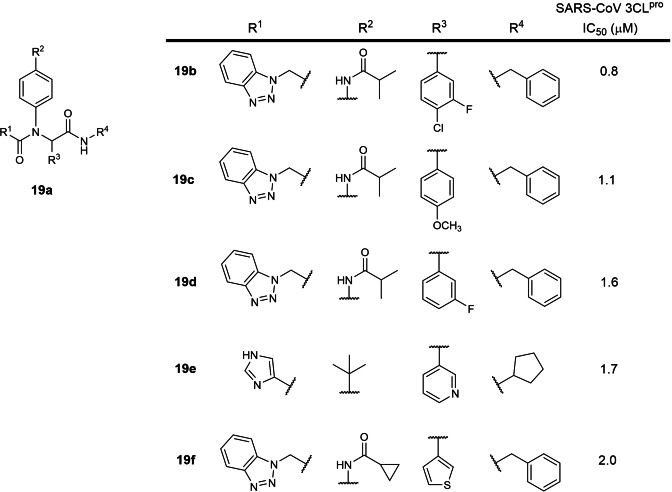
The nineteenth earliest patent was filed in 2017 by Purdue Research Foundation involving small molecules with amidophenyl scaffolds (US 2017/0313685; Table 1). [38] 77 structures were reported with SARS‐CoV 3CLpro IC50s between 0.8 to 27.3 μM. The five most potent inhibitors were identified to be compounds 19 b to 19 f (Figure 19) with 0.8 to 2.0 μM IC50s. Interestingly, the structure of compound 19 f was reported in an earlier 2015 research paper with an IC50 of 1.9 μM against HKU4‐CoV 3CLpro. [69] There are currently no reports on their SARS‐CoV‐2 3CLpro inhibitory activities. We opine that the most potent inhibitor, 19 b, should be further evaluated for drug development.
Figure 19.
Structures from US 2017/0313685. (a) general scaffold; (b–f) most potent exemplified structures.
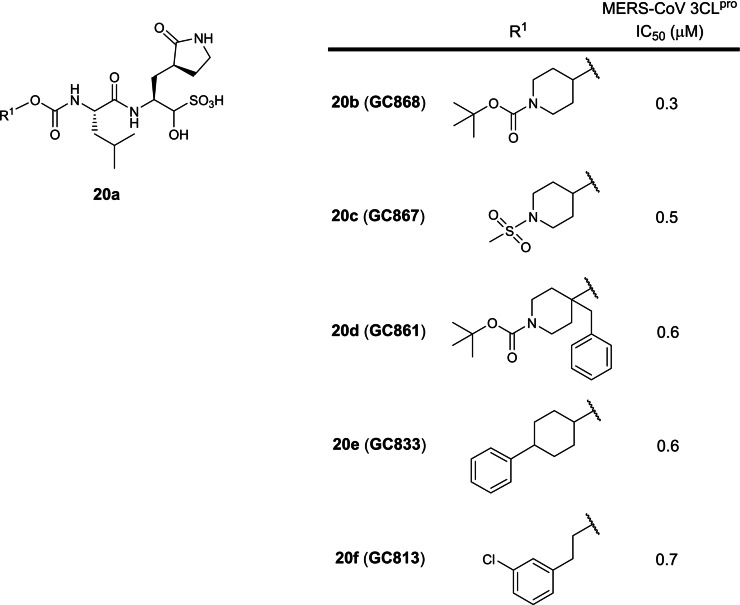
The twentieth earliest patent was filed in 2017 by Kansas State and Wichita State Universities involving dipeptide peptidomimetics with a P1 5‐membered lactam glutamine analog and a C‐terminal hydroxymethyl sulfonic acid prodrug warhead against the Middle East Respiratory Syndrome (MERS) coronavirus 3CLpro (WO 2017/222935; Table 1). [39] The hydroxymethyl sulfonic acid moiety changes to a reactive electrophilic aldehyde warhead at physiological pH, first described in WO 2013/049382. [33] Although no SARS‐CoV 3CLpro inhibitory activities were disclosed, the MERS‐CoV and SARS‐CoV‐2 3CLpro share approximately 50 % sequence identity [16] so inhibitors designed to inhibit MERS‐CoV 3CLpro will likely be able to inhibit SARS‐CoV‐2 3CLpro. 8 compounds were described with IC50s ranging from 0.3 to 3.1 μM with compounds 20 b to 20 f being the most potent against MERS‐CoV 3CLpro (Figure 20). There are currently no reports on their SARS‐CoV‐2 3CLpro inhibitory activities. As the hydroxymethyl sulfonic acid moiety transforms into a reactive aldehyde warhead in physiological conditions, we opine these compounds lack drug development potential as explained vide supra.
Figure 20.
Structures from WO 2017/222935. (a) general scaffold; (b–f) exemplified structures.
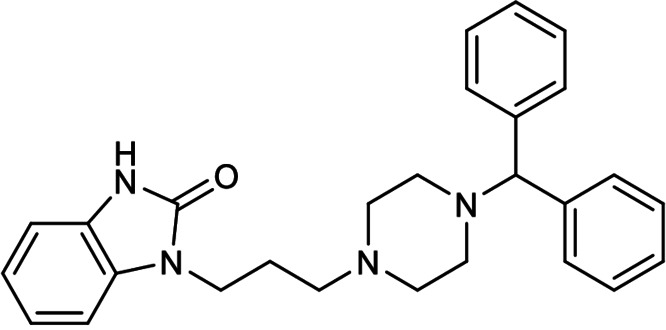
The twenty‐first earliest patent was filed in 2017 by Tianjin International Joint Academy of Biomedicine revealing that the N‐methyl‐D‐aspartate (NMDA) receptor antagonist and gamma aminobutyric acid (GABA) receptor modulator, acamprosate calcium (Figure 21), was able to inhibit SARS‐CoV 3CLpro (CN 108785293 A; Table 1). [40] The patent is written in Chinese and no English version could be found online. Unfortunately, no IC50 or K i value was reported and there are no follow‐up reports in academic journals. Hence, we are currently unable to comment on its drug development potential for treating COVID‐19.
Figure 21.
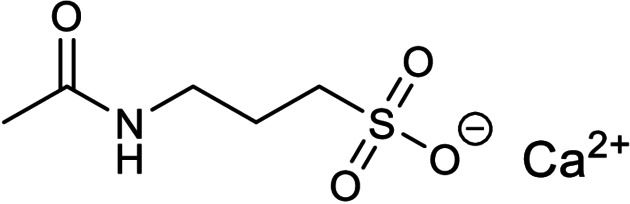
Structure of acamprosate calcium from CN 108785293 A.
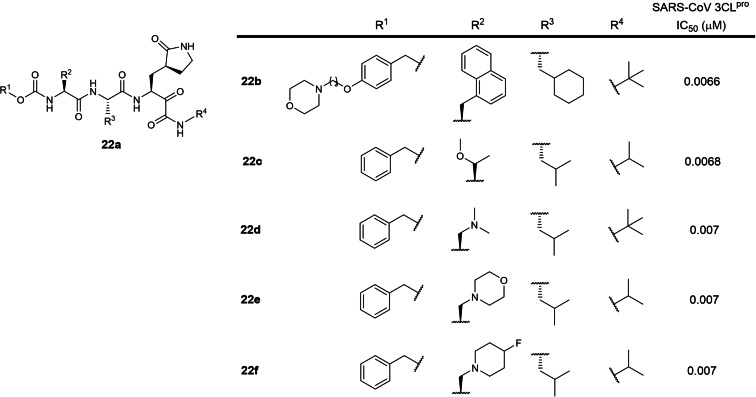
The twenty‐second patent was filed by GSK in 2017 involving 3‐residue peptidomimetics containing a P1 5‐membered lactam glutamine mimic with a C‐terminal electrophilic ketoamide warhead (WO 2018/042343; Table 1). [41] 48 structures were described with SARS‐CoV 3CLpro IC50s of approximately 7 nM. The five most potent inhibitors against SARS‐CoV 3CLpro are shown in Figure 22. There are currently no reports on their SARS‐CoV‐2 3CLpro inhibitory activities based on a SciFinder® search. In our opinion, these compounds can potentially be developed as SARS‐CoV‐2 3CLpro inhibitors to treat COVID‐19 as oral ketoamide peptidomimetic protease inhibitors such as Boceprevir and Telaprevir have been approved for treating hepatitis C virus infections. [64]
Figure 22.
Structures from WO 2018/042343. (a) general scaffold; (b–e) exemplified structures.
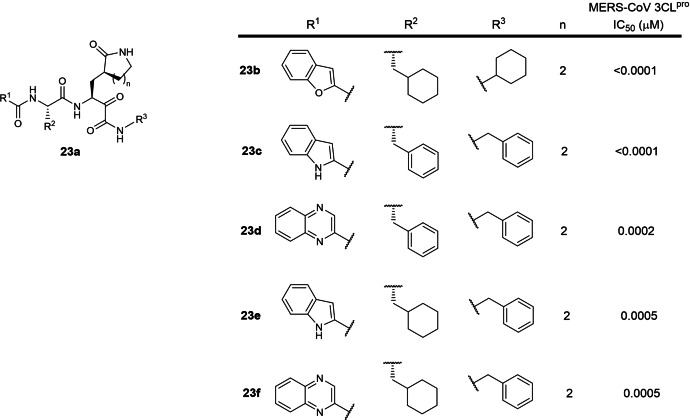
A special note is also made for WO 2020/030143 (Table 1), filed by Shanghai Institute of Materia Medica and Fudan University in 2019. [42] It involves 2‐residue peptidomimetics bearing a P1 5‐ or 6‐membered lactam glutamine mimic with a C‐terminal electrophilic ketoamide warhead designed to inhibit MERS‐CoV 3CLpro. The patent is written in Chinese and the English version could not be found online. No SARS‐CoV or SARS‐CoV‐2 3CLpro IC50 data were reported in the patent. However, this patent is included in this review as the MERS‐CoV and SARS‐CoV‐2 3CLpro share approximately 50 % sequence identity [16] so inhibitors designed to inhibit MERS‐CoV 3CLpro will likely be able to inhibit SARS‐CoV‐2 3CLpro. 253 compounds were described with IC50s ranging from sub‐nanomolar to 786 nM using a MERS pseudovirus neutralization assay. The five most potent MERS‐CoV 3CLpro inhibitors, 23 b to 23 f, possess sub‐nanomolar to 5 nM IC50s (Figure 23). There are currently no reports on their SARS‐CoV‐2 3CLpro inhibitory activities based on a SciFinder® search. In our opinion, these compounds can potentially be developed as SARS‐CoV‐2 3CLpro inhibitors to treat COVID‐19 as oral ketoamide peptidomimetic protease inhibitors such as Boceprevir and Telaprevir have been approved for treating hepatitis C virus infections. [64]
Figure 23.
Structures from WO 2020/030143. (a) general scaffold; (b–f) most potent exemplified MERS‐CoV 3CLpro inhibitors.
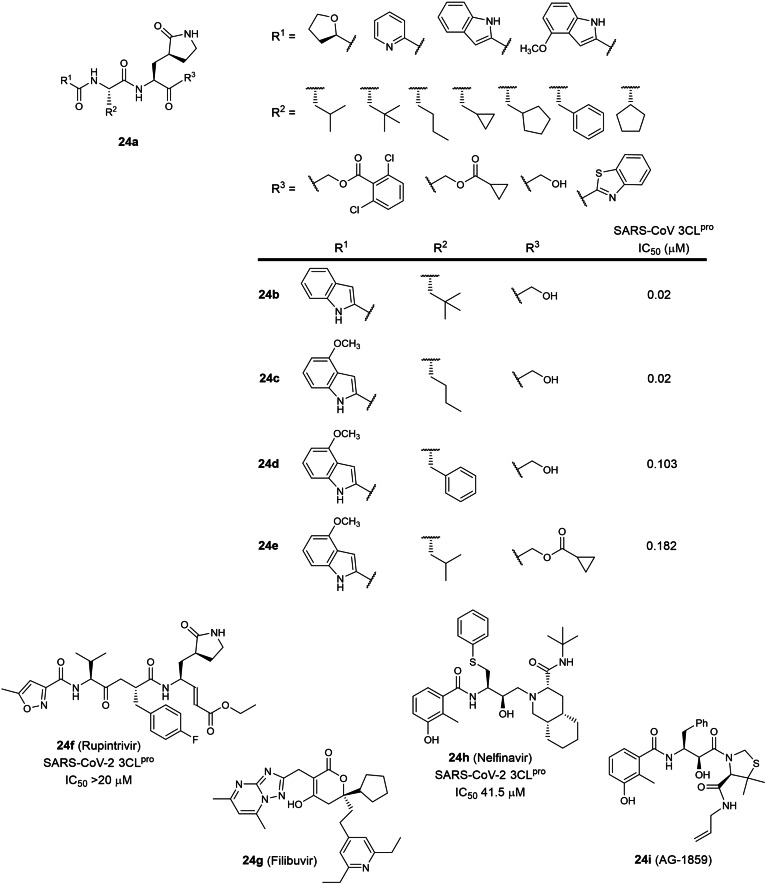
The final patent in this review is an application patent involving 13 peptidomimetic protease inhibitors (WO 2021/176369; Table 1). [43] Interestingly, 6 peptidomimetics with various C‐terminal electrophilic warheads have been described in an earlier patent (WO 2005/113580), [25] all bearing a P1 5‐membered lactam glutamine mimic. No 3CLpro inhibitory data were disclosed in the patent but SARS‐CoV 3CLpro IC50s for compounds 24 b to 24 e (Figure 24) were reported in a recent research paper. [18] Surprisingly, the patent also described the peptidomimetic rhinovirus 3C protease inhibitor, Rupintrivir (24 f), as a potential COVID‐19 therapeutic despite its lack of SARS‐CoV‐2 3CLpro inhibitory activity. [62] Three hepatitis C and human immunodeficiency virus protease inhibitors were also described; Filibuvir (24 g), Nelfinavir (24 h) and AG‐1859 (24 i). No IC50s were reported in the patent but a literature search revealed Nelfinavir to possess weak SARS‐CoV‐2 3CLpro inhibitory activity (IC50 41.5 μM). [70] In our view, the peptidomimetic hydroxymethylketones 24 b and 24 c possess the highest potential for further drug development if converted to a phosphate prodrug as observed for clinical candidate PF‐00835231 (6 b). [18] In contrast, we opine that Rupintrivir (24 f) and Nelfinavir (24 h) are not potent enough for further development. We are unable to comment on the drug development potential for Filibuvir (24 g) and AG‐1859 (24 i) due to the lack of SARS‐CoV‐2 3CLpro inhibitory data.
Figure 24.
Structures from WO 2021/176369. (a) peptidomimetic general scaffold; (b–i) exemplified inhibitors.
Lastly, a special mention is made for Pfizer's oral SARS‐CoV‐2 3CLpro peptidomimetic inhibitor PF‐07321332 (Figure 25) which entered phase 3 clinical trials in July 2021 for treating non‐hospitalised SARS‐CoV‐2 infected adults (ClinicalTrials.gov identifier NCT04960202). [71] Like previous Pfizer 3CLpro inhibitor patents, it bears a P1 5‐membered lactam glutamine mimic and interestingly, a nitrile warhead not described in any patents found in this review. This is particularly intriguing as nitrile warhead peptidomimetics have been reported to be relatively weak SARS‐CoV 3CLpro inhibitors with IC50s ranging between 4.6 to 49 μM. [72] To our best knowledge, there are currently no published reports on its SARS‐CoV‐2 3CLpro IC50 and a SciFinder® structure search did not reveal any patents for PF‐07321332.
Figure 25.
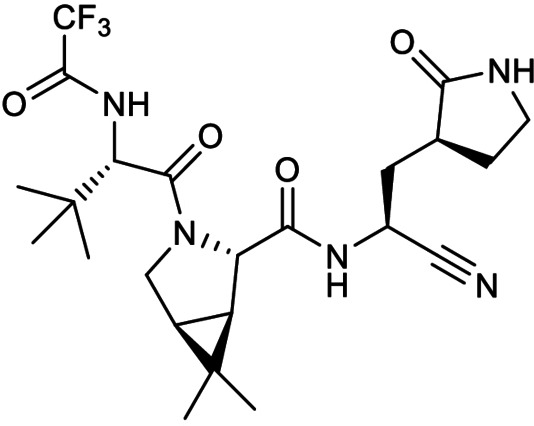
Structure of PF‐07321332.
3. Summary and Outlook
24 patents describing coronavirus 3CLpro inhibitors have so far been filed up to 30 July 2021 (Table 1). 9 of the patents were filed by pharmaceutical companies while the remaining 15 from academia. 10 of the 24 patents described inhibitors that have not been reported in academic journals. 14 of the 24 patents involved peptidomimetics with electrophilic warheads, signifying that this modality has generated more commercial interest compared to small molecules. A plausible reason could be that warhead peptidomimetics generally show more potent inhibitory activities, typically in the nanomolar range, compared to small molecules. In addition, warhead peptidomimetics have been successfully developed into antiviral drugs, exemplified by the hepatitis C virus protease inhibitors Boceprevir and Telaprevir. [64]
The peptidomimetic inhibitors covered in this review bear a P1 5‐ of 6‐membered lactam glutamine‐mimicking residue first described by Agouron Pharmaceuticals in 1999 (WO 99/57135). [46] Reported P2 residues ranged from leucine to cyclohexylalanine and phenylalanine, suggesting that the SARS‐CoV S2 subsite could accommodate bulky residues with 6‐membered side‐chains. However, a 2020 research paper by Pfizer revealed that a P2 leucine exhibited 11‐ and 25‐fold IC50 improvement over cyclohexylalanine and phenylalanine respectively, suggesting that leucine was highly preferred at the P2 position. [18] Indeed, Pfizer utilised a P2 leucine in their phosphate prodrug of PF‐00835231 (6 b) which entered phase 1 clinical trials in September 2020 (ClinicalTrials.gov identifier: NCT04535167). Other plausible P2 residue substitutions include norvaline and t‐butylalanine (Figure 24). The P3 position is more accommodating to different moieties including indoles (Figures 6, 10 and 18), amino acid residues like threonine (Figure 3), valine (Figures 5 and 8) and naphthylalanine (Figures 14 and 22). The longest peptidomimetic inhibitor in this review constituted 4 residues (Figure 8) but unfortunately, no IC50s were reported as it would be interesting to study the correlation of peptidomimetic length to inhibition potency.
Small molecule inhibitors constituted 10 of the 24 patents (Table 1). In contrast to the peptidomimetic inhibitors, their reported IC50s or K is were generally inferior (close to or above 1 μM), with the exception of two patents filed by Fulcrum Pharmaceuticals and the National Health Research Institutes, Taiwan (Table 1; Figures 4 and 7 respectively). The former described a small molecule containing two boronic acid moieties (K i 22 nM) and the latter, a biphenyl sulphone (IC50 300 nM). In our view, both warrant further investigations into their drug development potential.
We believe this review serves to bolster the knowledge of existing coronavirus 3CLpro inhibitors reported beyond academic journals and the general structural motifs required for binding and inhibiting SARS‐CoV‐2 3CLpro. We are hopeful that some of these inhibitors will be successfully developed and approved as drugs for treating COVID‐19.
Abbreviations
- 3CLpro
3‐chymotrypsin‐like protease
- CoV
coronavirus
- COVID‐19
coronavirus disease 2019
- EC50
half‐maximal effective concentration
- FDA
Food and Drug Administration
- GABA
gamma aminobutyric acid
- IC50
half‐maximal inhibitory concentration
- MERS
Middle East Respiratory Syndrome
- Mpro
main protease
- NMDA
N‐methyl‐D‐aspartate
- SARS
severe acute respiratory syndrome
- WHO
World Health Organization
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Brian Chia obtained his B.Sc. with first‐class honours and a Ph.D. from the University of Adelaide followed by postdoctoral training at Cambridge University, UK. In 2005, he joined the Institute of Molecular and Cell Biology before becoming a group leader at the Experimental Drug Development Centre, Agency for Science, Technology and Research (A*STAR). He has co‐authored more than 50 research papers and is an inventor/co‐inventor of 8 granted or pending patents involving small molecules, peptides and peptidomimetics with antibacterial, anticancer, antifungal and antiviral properties.

Biographical Information
Weijun Xu obtained his B.Sc. with first‐class honours in Biochemistry and a Ph.D. from the University of Queensland followed by postdoctoral training at the University of California, San Diego. He lectured at the Singapore Polytechnic School of Chemical and Life Sciences for 8 years before his current position at the Experimental Drug Development Centre, Agency for Science, Technology and Research (A*STAR) as a senior research fellow. His research interests include computational biology, modelling of protein−ligand and protein−protein interactions including GPCRs, proteases and kinases inhibitors.

Biographical Information
Pearly S. Ng is a medicinal chemist who has been involved in the development of various novel small molecule therapeutics for infectious diseases in the past decade. Prior to being a medicinal chemist, she spent 6 years as a research chemist in various companies working on diversity‐oriented synthesis and parallel synthesis technologies. She obtained her Ph.D. in organic chemistry from Nanyang Technological University and has co‐authored several research publications in medicinal and organic chemistry. She is also a co‐inventor of 3 patent applications.

Acknowledgements
This review was supported by the Agency for Science, Technology and Research (A*STAR).
C. S. B. Chia, W. Xu, P. Shuyi Ng, ChemMedChem 2022, 17, e202100576.
References
- 1. Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W., N. Engl. J. Med. 2020, 382(13), 727. DOI: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K. S. M., Lau E. H. Y., Wong J. Y., Xing X., Xiang N., N. Engl. J. Med. 2020, 382(13), 1199. DOI: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ksiazek T. G., Erdman D., Goldsmith C. S., Zaki S. R., Peret T., Emery S., Tong S., Urbani C., Comer J. A., Lim W., Rollin P. E., Dowell S. F., Ling A., Humphrey C. D., Shieh W., Guarner J., Paddock C. D., Rota P., Fields B., DeRisi J., Yang J., Cox N., Hughes J. M., LeDuc J. W., Bellini W. J., Anderson L. J., N. Engl. J. Med. 2003, 348(20), 1953. DOI: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4. Drosten C., Günther S., Preiser W., van der Werf S., Brodt H., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R. A. M., Berger A., Burguière A., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H., Osterhaus A. D., Schmitz H., Doerr H. W., N. Engl. J. Med. 2003, 348(20), 1967. DOI: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 5. Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W. J., Wang D., Xu W., Holmes E. C., Gao G. F., Wu G., Chen W., Shi W., Tan W., Lancet. 2020, 395, 565. DOI: 10.1016/S0140-6736(20)30251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W., Si H., Zhu Y., Li B., Huang C., Chen H., Chen J., Luo Y., Guo H., Jiang R., Liu M., Chen Y., Shen X., Wang X., Zheng X., Zhao K., Chen Q., Deng F., Liu L., Yan B., Zhan F., Wang Y., Xiao G., Shi Z., Nature. 2020, 579, 270. DOI: 10.1038/s41586-020-2012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, Nat. Microbiol. 2020, 5, 536. DOI: 10.1038/s41564-020-0695-z.
- 8.“World Health Organization Coronavirus (COVID-19) Dashboard” can be found under https://covid19.who.int.
- 9. Rubin D., Chan-Tack K., Farley J., Sherwat A., N. Engl. J. Med. 2020, 383, 2598. DOI: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 10. Taylor P. C., Adams A. C., Hufford M. M., de la Torre I., Winthrop K., Gottlieb R. L., Nat. Rev. Immunol. 2021, 21, 382. DOI: 10.1038/s41577-021-00542- x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolgin E., Nat. Biotechnol. 2021, 39, 783. DOI: 10.1038/s41587-021-00980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banerjee R., Perera L., Tillekeratne L. M. V., Drug Discovery Today 2021, 26(3), 804. DOI: 10.1016/j.drudis.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong W., Su H., Zhao W., Xie H., Shao Q., Xu Y., Med. Res. Rev. 2021, 41, 1965. DOI: 10.1002/med.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang H., Yang J., RSC Med. Chem. 2021, 12, 1026. DOI: 10.1039/d1md00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Guddat L. W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H., Nature. 2020, 582, 289. DOI: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 16. Ullrich S., Nitsche C., Bioorg. Med. Chem. Lett. 2020, 30, 127377. DOI: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosh A. K., Brindisi M., Shahabi D., Chapman M. E., Mesecar A. D., ChemMedChem. 2020, 15, 907. DOI: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffman R. L., Kania R. S., Brothers M. A., Davies J. F., Ferre R. A., Gajiwala K. S., He M., Hogan R. J., Kozminski K., Li L. Y., Lockner J. W., Lou J., Marra M. T., Mitchell L. J. Jr., Murray B. W., Nieman J. A., Noell S., Planken S. P., Rowe T., Ryan K., Smith G. J., Solowiej J. E., Steppan C. M., Taggart B., J. Med. Chem. 2020, 63(21), 12725. DOI: 10.1021/acs.jmedchem.0c01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabrielson S. W., J. Med. Libr. Assoc. 2018, 106(4), 588. DOI: 10.5195/jmla.2018.515. [Google Scholar]
- 20.S. A. Fuhrman, D. A. Matthews, A. K. Patick, P. A. Rejto (Pfizer Inc.), WO 2004/093860, 2004.
- 21.S. X. Cai, W. E. Kemnitzer, H. Zhang, H. Zhang (Cytovia Inc.), WO 2004/101742, 2004.
- 22.M. He, R. S. Kania, J. Lou, S. Planken (Agouron Pharmaceuticals Inc.), US 2006/0014821, 2004.
- 23.E. Freire, R. Ottenbrite, Y. Xiao, A. Velazquez-Campoy, S. Leavitt, U. Bacha, J. Barrila (Fulcrum Pharmaceuticals Inc.), WO 2005/041904, 2004.
- 24.S. Yang, J. Wu, Y. Xiao, F. Su, S. Leavitt, C. Kuo, W. Chen, Y. Xiang, C. Wang, S. Liao (Taigen Biotechnology), WO 2005/066123, 2004.
- 25.R. L. Hoffman, R. S. Kania, J. A. Nieman, S. P. Planken, G. J. Smith (Pfizer Inc.), WO 2005/113580, 2005.
- 26.S. Wu, H. Hsieh, T. Hsu, I. Lu (National Health Research Institutes, Taiwan), WO 2005/066123, 2004.
- 27.H. Yang, X. Xue, M. Bartlam, K. Yang, D. Ma, W. Xie (Tsinghua University, Shanghai Institute of Organic Chemistry), WO 2006/042478, 2005.
- 28.L. Lai, Y. Liu, Z. Liu, K. Fan, C. Huang, P. Wei, J. Pei, S. Liu, H. Chen (Peking University), CN 1965833 A, 2005.
- 29.R. S. Kania, L. J. Mitchell, J. A. Nieman (Pfizer Inc.), WO 2006/061714, 2005.
- 30.N. Yamamoto, N. Yamamoto, S. Yokoyama, H. Hirota, H. Hiroshi, O. Hiroyuki, S. Kazuhito, T. Akiko, M. Takehisa, U. Hideaki, S. Mayuko, M. Shigeru, S. Masayuki, K. Ichiro (Tokyo Medical and Dental University, RIKEN) WO 2006/095624, 2006.
- 31.C. M. Puah, W. Zhu, J. Li, L. Chen, C. Luo, G. Chen, Z. Zuo, X. Luo, X. Shen, K. Chen, H. Jiang (Singapore Polytechnic, Shanghai Institute of Materia Medica), WO 2007/075145, 2006.
- 32.Z. Rao, C. Yang, Z. Lou, Y. Sun, H. Liu, W. Liu, B. Sun, H. Zhu, W. Chen (Tianjin International Joint Academy of Biotechnology and Medicine), CN 103159665B, 2011.
- 33.K. Chang, Y. Kim, W. C. Groutas, D. Hua, L. J. Saif (Kansas State University, Ohio State University, Wichita State University), WO 2013/049382, 2012.
- 34.D. Kim, H. Ryu, T. Hanh, S. Hwang (Chonnam National University), KR 1020130002975, 2012.
- 35.K. Chang, Y. Kim, W. C. Groutas (Kansas State University, Wichita State University), WO 2013/166319, 2013.
- 36.L. Zhang, F. Song, H. Dai, Q. Wang (Institute of Microbiology, Chinese Academy of Sciences), CN 106176728 A, 2016.
- 37.H. Liu, Y. Nian, J. Li, R. Hilgenfeld, D. Lin, H. Liu, Y. Zhou, H. Jiang, K. Chen (Shanghai Institute of Materia Medica, University of Lübeck), WO 2017/114509, 2016.
- 38.S. E. St. John, A. D. Mesecar (Purdue Research Foundation), WO 2017/0313685, 2017.
- 39.K. Chang, Y. Kim, W. Groutas (Kansas State University, Wichita State University), WO 2017/222935, 2017.
- 40.H. Yang, K. Zhou, C. Hu, Z. Wang, C. Chen, Y. Cai, S. Li, S. Fu (Tianjin International Joint Academy of Biomedicine), CN 108785293 A, 2017.
- 41.J. Botyanszki, J. G. Catalano, P. Y. Chong, H. Dickson, Q, Jin, A, Leivers, A. Maynard, X. Liao, J. Miller, J. B. Shotwell, V .W. Tai, R. Thalji (Glaxosmithkline Intellectual Property Limited), WO 2004/101742, 2004.
- 42.H. Liu, S. Jiang, W. Dai, L. Lu, J. Peng, S. Xia, J. Wang, J. Li, H. Jiang, K. Chen (Shanghai Institute of Materia Medica, University of Lübeck), WO 2020/030143, 2019.
- 43.P. Bezawada, B. J. Burke, E. L. Hawking, R. L. Hoffman, R. S. Kania, J. R. Lillis, M. N. O'Brien Laramy, K. D. Pencheva, B. P. Sullivan, A. J. Thiel, M. D. Ticehurst (Pfizer Inc.), WO 2021/176369, 2021.
- 44. Carvalho Nascimento Junior J. A., Santos A. M., Quintans L. J., Walker C. I. B., Borges L. P., Serafini M. R., Expert Opin. Ther. Pat. 2020, 106(4), 588. DOI: 10.1080/13543776.2020.1772231. [DOI] [PubMed] [Google Scholar]
- 45. Vankadara S., Wong Y. X., Liu B., See Y. Y., Tan L. H., Tan Q. W., Wang G., Karuna R., Guo X., Tan S. T., Fong J. Y., Joy J., Chia C. S. B., Bioorg. Med. Chem. Lett. 2021, 48, 128263. DOI: 10.1016/j.bmcl.2021.128263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.P. S. Dragovich, J. T. Marakovits, T. J. Prins, J. G. Tikhe, S. E. Webber, R. Zhou, T. O. Johnson (Agouron Pharmaceuticals Inc.), WO 99/57135, 1999.
- 47. Zhang H., Zhang H., Kemnitzer W., Tseng B., Cinatl J. Jr, Michaelis M., Doerr H. W., Cai S. X., J. Med. Chem. 2006, 49(3), 1198. DOI: 10.1021/jm0507678. [DOI] [PubMed] [Google Scholar]
- 48. Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R., Science. 2020, 368(6489), 409. DOI: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bacha U., Barrila J., Velazquez-Campoy A., Leavitt S. A., Freire E., Biochemistry. 2004, 43(17), 4906. DOI: 10.1021/bi0361766. [DOI] [PubMed] [Google Scholar]
- 50. Yang S., Chen S., Hsu M., Wu J., Tseng C. K., Liu Y., Chen H., Kuo C., Wu C., Chang L., Chen W., Liao S., Chang T., Hung H., Shr H., Li C., Huang Y., Chang L., Hsu J., Peters C. J., Wang A. H., Hsu M., J. Med. Chem. 2006, 49(16), 4971. DOI: 10.1021/jm0603926. [DOI] [PubMed] [Google Scholar]
- 51. Shie J., Fang J., Kuo T., Kuo C., Liang P., Huang H., Wu Y., Jan J., Cheng Y. E., Wong C., Bioorg. Med. Chem. 2005, 13, 5240. DOI: 10.1016/j.bmc.2005.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee C., Kuo C., Ko T., Hsu M., Tsui Y., Chang S., Yang S., Chen S., Chen H., Hsu M., Shih S., Liang P., Wang A. H., J. Biol. Chem. 2009, 284(12), 7646. DOI: 10.1074/jbc.M807947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu I., Mahindroo N., Liang P., Peng Y., Kuo C., Tsai K., Hsieh H., Chao Y., Wu S., J. Med. Chem. 2006, 49(17), 5154. DOI: 10.1021/jm060207o. [DOI] [PubMed] [Google Scholar]
- 54. Sarver R. W., Rogers J. M., Epps D. E., J. Biomol. Screening 2002, 7(1), 21. DOI: 10.1177/108705710200700104. [DOI] [PubMed] [Google Scholar]
- 55. Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K. Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z., PLoS Biol. 2005, 3(10), e324. DOI: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang K. S., Ma X. R., Ma Y., Alugubelli Y. R., Scott D. A., Vatansever E. C., Drelich A. K., Sankaran B., Geng Z. Z., Blankenship L. R., Ward H. E., Sheng Y. J., Hsu J. C., Kratch K. C., Zhao B., Hayatshahi H. S., Liu J., Li P., Fierke C. A., Tseng C. K., Xu S., Liu W. R., ChemMedChem. 2021, 16, 942. DOI: 10.1002/cmdc.202000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Manevski N., King L., Pitt W. R., Lecomte F., Toselli F., J. Med. Chem. 2019, 62(24), 10955. DOI: 10.1021/acs.jmedchem.9b00875. [DOI] [PubMed] [Google Scholar]
- 58. Gampe C., Verma V. A., J. Med. Chem. 2020, 63(23), 14357. DOI: 10.1021/acs.jmedchem.0c01177. [DOI] [PubMed] [Google Scholar]
- 59. Chen L., Li J., Luo C., Liu H., Xu W., Chen G., Liew O. W., Zhu W., Puah C. M., Shen X., Jiang H., Bioorg. Med. Chem. 2006, 14(24), 8295. DOI: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prior A. M., Kim Y., Weerasekara S., Moroze M., Alliston K. R., Uy R. A. Z., Groutas W. C., Chang K., Hua D. H., Bioorg. Med. Chem. Lett. 2013, 23, 6317. DOI: 10.1016/j.bmcl.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim Y., Lovell S., Tiew K., Mandadapu S. R., Alliston K. R., Battaile K. P., Groutas W. C., Chang K., J. Virol. 2012, 86(21), 11754. DOI: 10.1128/JVI.01348–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma C., Sacco M. D., Hurst B., Townsend J. A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M. T., Chen Y., Wang J., Cell Res. 2020, 30, 678. DOI: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rathnayake A. D., Zheng J., Kim Y., Perera K. D., Mackin S., Meyerholz D. K., Kashipathy M. M., Battaile K. P., Lovell S., Perlman S., Groutas W. C., Chang K., Sci. Transl. Med. 2020, 12(557), eabc5332. DOI: 10.1126/scitranslmed.abc5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manns M. P., von Hahn T., Nat. Rev. Drug Discovery 2013, 12, 595. DOI: 10.1038/nrd4050. [DOI] [PubMed] [Google Scholar]
- 65. Mandadapu S. R., Weerawarna P. M., Prior A. M., Uy R. A. Z., Aravapalli S., Alliston K. R., Lushington G. H., Kim Y., Hua D. H., Chang K., Groutas W. C., Bioorg. Med. Chem. Lett. 2013, 23, 3709. DOI: 10.1016/j.bmcl.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang L., Bao B., Song G., Chen C., Zhang X., Lu W., Wang Z., Cai Y., Li S., Fu S., Song F., Yang H., Wang J., Eur. J. Med. Chem. 2017, 137, 450. DOI: 10.1016/j.ejmech.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gori A., Gagni P., Rinaldi S., Chem. Eur. J. 2017, 23(60), 14987. DOI: 10.1002/chem.201703199. [DOI] [PubMed] [Google Scholar]
- 68. Dai W., Jochmans D., Xie H., Yang H., Li J., Su H., Chang D., Wang J., Peng J., Zhu L., Nian Y., Hilgenfeld R., Jiang H., Chen K., Zhang L., Xu Y., Neyts J., Liu H., J. Med. Chem. 2021. DOI: 10.1021/acs.jmedchem.0c02258. [DOI] [PubMed] [Google Scholar]
- 69. St. John S. E., Tomar S., Stauffer S. R., Mesecar A. D., Bioorg. Med. Chem. 2015, 23, 6036. DOI: 10.1016/j.bmc.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Y., Zhang J., Duan Z., Wang N., Sun X., Zhang Y., Fu L., Liu K., Yang Y., Pan S., Shi Y., Zeng H., Guo G., Lai R., Zou Q., Signal Transduct. Target Ther. 2021, 6, 356. DOI: 10.1038/s41392-021-00763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vandyck K., Deval J., Curr. Opin. Virol. 2021, 49, 36. DOI: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chuck C., Chen C., Ke Z., Wan D. C., Chow H., Wong K., Eur. J. Med. Chem. 2013, 59, 1. DOI: 10.1016/j.ejmech.2012.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]