Abstract
Objectives
To determine if bilateral salpingo-oophorectomy, compared with ovarian conservation, is associated with all cause or cause specific death in women undergoing hysterectomy for non-malignant disease, and to determine how this association varies with age at surgery.
Design
Population based cohort study.
Setting
Ontario, Canada from 1 January 1996 to 31 December 2015, and follow-up to 31 December 2017.
Participants
200 549 women (aged 30-70 years) undergoing non-malignant hysterectomy, stratified into premenopausal (<45 years), menopausal transition (45-49 years), early menopausal (50-54 years), and late menopausal (≥55 years) groups according to age at surgery; median follow-up was 12 years (interquartile range 7-17).
Exposures
Bilateral salpingo-oophorectomy versus ovarian conservation.
Main outcomes measures
The primary outcome was all cause death. Secondary outcomes were non-cancer and cancer death. Within each age group, overlap propensity score weighted survival models were used to examine the association between bilateral salpingo-oophorectomy and mortality outcomes, while adjusting for demographic characteristics, gynaecological conditions, and comorbidities. To account for comparisons in four age groups, P<0.0125 was considered statistically significant.
Results
Bilateral salpingo-oophorectomy was performed in 19%, 41%, 69%, and 81% of women aged <45, 45-49, 50-54, and ≥55 years, respectively. The procedure was associated with increased rates of all cause death in women aged <45 years (hazard ratio 1.31, 95% confidence interval 1.18 to 1.45, P<0.001; number needed to harm 71 at 20 years) and 45-49 years (1.16, 1.04 to 1.30, P=0.007; 152 at 20 years), but not in women aged 50-54 years (0.83, 0.72 to 0.97, P=0.018) or ≥55 years (0.92, 0.82 to 1.03, P=0.16). Findings in women aged <50 years were driven largely by increased non-cancer death. In secondary analyses identifying a possible change in the association between bilateral salpingo-oophorectomy and all cause death with advancing age at surgery, the hazard ratio gradually decreased during the menopausal transition and remained around 1 at all ages thereafter.
Conclusion
In this observational study, bilateral salpingo-oophorectomy at non-malignant hysterectomy appeared to be associated with increased all cause mortality in women aged <50 years, but not in those aged ≥50 years. While caution is warranted when considering bilateral salpingo-oophorectomy in premenopausal women without indication, this strategy for ovarian cancer risk reduction does not appear to be detrimental to survival in postmenopausal women.
Introduction
Bilateral salpingo-oophorectomy (the surgical removal of both ovaries and fallopian tubes) has traditionally been offered at the time of hysterectomy for non-malignant disease to prevent ovarian cancer later in life. However, this procedure is now being increasingly avoided due to recognition of potential harm from the loss of ovarian hormone production.1 2 Several observational studies have shown that bilateral salpingo-oophorectomy before age 45 or 50 years is associated with increased all cause mortality despite reduced rates of ovarian cancer.3 4 5 6 7 Therefore, current guidelines advise against bilateral salpingo-oophorectomy in premenopausal women.8 9 10 11 12 13
The risk-to-benefit ratio of bilateral salpingo-oophorectomy as women age remains unclear.2 While the ovaries produce oestrogen and androgens before menopause, they produce only androgens after menopause, and the clinical significance of this production is debated.12 13 14 Existing literature on the association between bilateral salpingo-oophorectomy and all cause mortality after the median age of natural menopause is also controversial: the Nurses’ Health Study15 16 and a decision analysis17 have suggested that bilateral salpingo-oophorectomy might be harmful even after age 50 years, but this finding has not been supported by other observational studies.3 4 7 18 In contrast to the direction provided in premenopausal women, current guidelines offer no recommendations on whether bilateral salpingo-oophorectomy should be performed or withheld in postmenopausal women.8 9 10 11 12 13
Rates of bilateral salpingo-oophorectomy vary markedly among surgeons, indicating ongoing uncertainty in the application of existing evidence.19 20 No study has identified an age threshold at which the risk-to-benefit ratio of bilateral salpingo-oophorectomy might change from supportive of ovarian conservation to removal of all ovarian tissue. Many studies enrolled selected cohorts,4 6 15 16 18 relied on patient recall to establish bilateral salpingo-oophorectomy status,4 6 15 16 18 initiated observation years to decades after the time of exposure to bilateral salpingo-oophorectomy,4 6 15 16 18 opted for referent women who did not undergo gynaecological surgery,3 4 6 7 or had few or no patients in older age groups.5 6 15 16 Hysterectomy is performed for over 400 000 women in the United States and 41 000 women in the United Kingdom annually, and additional data on the role of bilateral salpingo-oophorectomy are needed.21 22 Therefore, we examined the association between bilateral salpingo-oophorectomy and all cause and cause specific death in a population based cohort undergoing non-malignant hysterectomy, and evaluated how this association varied based on age at surgery.
Methods
Study design and population
We performed a population based cohort study using deidentified linked health administrative databases held at ICES (formerly known as the Institute for Clinical Evaluative Sciences), a non-profit research institute authorised to collect data on all residents of Ontario, Canada for the purpose of health system evaluation. Because Ontarians have universal access to hospital care and physician services, these data are comprehensive. The Research Ethics Board at the University of Toronto provided approval (No 38212).
We included adult women (30-70 years) in Ontario, Canada who were undergoing abdominal hysterectomy (open, laparoscopic, robotic assisted) for a non-malignant indication from 1 January 1996 to 31 December 2015. We used validated procedure codes to identify women who had hysterectomy from the Discharge Abstract Database, Same Day Surgery database, and Ontario Health Insurance Plan database, which hold records of inpatient surgery, outpatient surgery, and surgeon billing claims, respectively (appendix 1).20 23
We excluded non-Ontario residents ineligible for universal health coverage; patients undergoing emergency hysterectomy because of potential differences in surgical decision making in this setting; patients undergoing hysterectomy for malignant disease; patients with previous breast cancer or gynaecological cancer, or those who had undergone surgery for genetic predisposition to malignancy, because of possible confounding by indication in this population; and patients who had previously undergone bilateral salpingo-oophorectomy (appendix 2-3).
Exposure assessment
The primary exposure was bilateral salpingo-oophorectomy, defined as removal of all ovarian tissue and corresponding fallopian tubes on the date of hysterectomy (index date). This included bilateral salpingo-oophorectomy in women with both ovaries, and unilateral salpingo-oophorectomy in women with one remaining ovary because of a previous surgical procedure. We used procedure codes from the Discharge Abstract Database and Same Day Surgery databases to identify salpingo-oophorectomy with a sensitivity of 99%, positive predictive value of 98%, and κ of 99% (appendix 1).23 We compared patients undergoing bilateral salpingo-oophorectomy with patients undergoing conservation of one or both ovaries to reflect loss or retention of ovarian endocrine function, respectively.5
Outcome assessment
The primary outcome was all cause death. Secondary outcomes were non-cancer and cancer death, selected to understand the pathogenesis of any potential association of bilateral salpingo-oophorectomy with all cause death. Date of death was obtained from the Registered Persons Database. Causes of death were available to 31 December 2017 from the Ontario Cancer Registry and Ontario Registrar General-Death database. Therefore, patients were followed from the date of hysterectomy (index date) to 31 December 2017.
Covariates
Covariates were determined at the time of the index hysterectomy. Demographic characteristics included age at surgery, rural or urban residence, year of surgery (1996-2000, 2001-2005, 2006-2010, 2011-2015), residential income, ethnicity (Chinese, South Asian, other), and immigration status (long term resident, immigrant). Residential income group is a socioeconomic index derived from Canadian census data on median neighbourhood income and was assigned to patients based on their postal code of residence.24 Immigration status was assigned to patients based on their landing date in Ontario25 (long term resident: landing date absent or <1985), and was included as a covariate to account for the improved health status of immigrants relative to Canadian born residents.26 27 Ethnicity was assigned using validated surname lists that identify people of Chinese (99.7% specificity, 80.2% sensitivity, 91.9% positive predictive value) and South Asian origin (99.7% specificity, 50.4% sensitivity, 89.3% positive predictive value), Canada’s two largest visible minority groups; all other residents were classified as other.28 In this setting, sensitivity was the proportion of patients self-identified as Chinese or South Asian who were detected as such by the surname lists; specificity was the proportion of patients self-identified as not being Chinese or South Asian who were detected as such by the surname lists; and positive predictive value was the proportion of patients detected by the surname list as South Asian or Chinese who self-identified as such.
Clinical characteristics included hysterectomy type (total, subtotal), gynaecological diagnoses at the time of hysterectomy (abnormal uterine bleeding, fibroids, endometriosis, ovarian cysts, premalignant conditions such as endometrial hyperplasia and cervical dysplasia, pelvic pain or inflammation, prolapse), overall comorbidity score derived from Aggregated Diagnosis Groups of the Johns Hopkins ACG System version 10 (0-5, 6-9, ≥10),29 30 specific comorbidities (diabetes, hypertension, cardiovascular disease, chronic obstructive pulmonary disease, previous malignancy), previous abdominopelvic surgeries (0, 1, 2, ≥3), and previous ovarian surgery. Gynaecological diagnoses and surgical history were obtained from the Discharge Abstract Database and Same Day Surgery databases (appendix 4),31 32 33 34 and specific comorbidities were obtained from validated registries of affected Ontarians (appendix 5).35 36 37 38
Statistical analyses
Primary analyses were stratified by age at surgery. Because most women experience menopause between the ages of 45 and 54 years39 40 and the median age of menopause is 51 years,41 we defined the following stratums a priori: premenopause (<45 years), menopausal transition (45-49 years), early menopause (50-54 years), and late menopause (≥55 years). These stratums are also consistent with the stages of reproductive aging, as proposed by the American Society for Reproductive Medicine.42
We used overlap weighting based on the propensity score to adjust for differences in patients undergoing bilateral salpingo-oophorectomy and ovarian conservation.43 44 45 This strategy emphasises the comparison of patients at clinical equipoise who would have been eligible to receive either procedure, achieves exact balance on the mean of every covariate included in the propensity score, and is not prone to bias from extreme propensity scores (as often occurs with inverse probability weighting).43 45 46
We first generated propensity scores separately for each age stratum using logistic regression, modelling bilateral salpingo-oophorectomy as the outcome and all demographic and clinical characteristics described as covariates; exact age within each age stratum was modelled as a continuous variable using restricted cubic splines with three knots (10th, 50th, 90th percentiles).47 We then derived overlap weights for each patient, defined as the predicted probability of receiving the opposite treatment (bilateral salpingo-oophorectomy: 1−propensity score; ovarian conservation: propensity score).43 We used standardised differences to compare baseline covariates of exposed and unexposed patients before and after applying overlap weights.48
We used weighted Cox proportional hazards models to compare the rate of all cause death by bilateral salpingo-oophorectomy status, censoring at loss to follow-up (loss of eligibility for provincial health insurance) and end of follow-up (31 December 2017). We used weighted Fine-Gray subdistribution hazard models to compare the incidence of non-cancer and cancer death by bilateral salpingo-oophorectomy status,49 treating death due to the opposite cause as a competing event, and censoring at loss to follow-up and end of follow-up. We used robust variance estimators to account for weighting, and present hazard ratios with 95% confidence intervals.50
We also plotted weighted cumulative incidence curves for all cause, non-cancer, and cancer death across bilateral salpingo-oophorectomy status in each age stratum. To test the equality of curves across groups, we used P values from weighted log rank tests for all cause death,51 and from weighted Fine-Gray subdistribution hazard models for non-cancer and cancer death.49 52 We computed the risk difference in weighted cumulative incidence functions between groups at 20 years of follow-up. If the association in survival models was statistically significant, we took the inverse of the risk difference to compute the number needed to treat or harm by that time point.53 We generated 95% confidence intervals for risk difference estimates using the 2.5th and 97.5th percentiles of 1000 bootstrapped estimates.
To assess for a change in the association between bilateral salpingo-oophorectomy and mortality with advancing age at surgery, we performed a secondary analysis for all cause death in the total cohort (30-70 years). We used a multivariable Cox proportional hazards model with bilateral salpingo-oophorectomy as the primary exposure; age as a restricted cubic spline with three knots; an interaction term between bilateral salpingo-oophorectomy and age; and all demographic and clinical characteristics as covariates. We then estimated the hazard ratio for bilateral salpingo-oophorectomy at each year of age. We hypothesised that a transition in the association could occur around the population average age at menopause.
To ensure our findings in each stratum were robust, we generated traditional multivariable Cox proportional hazards models for all outcomes; and reran these models with bilateral salpingo-oophorectomy as a time varying exposure to account for patients who underwent bilateral salpingo-oophorectomy after hysterectomy; after the index date, only patients who underwent bilateral salpingo-oophorectomy for non-malignant indications (other than an ovarian mass or cancer) were able to transition from unexposed to exposed. To explore the potential impact of unmeasured confounding, we performed overlap weighted survival analyses for death due to cardiovascular disease, thought to exist on the causal pathway; and death due to upper gastrointestinal tract cancer, not thought to exist on the causal pathway but strongly associated with smoking and alcohol use, as a negative control (appendix 4).54
Datasets were linked using unique encoded identifiers and analysed at ICES. All statistical tests were two sided. No significant departures from proportionality were detected based on tests of interaction between bilateral salpingo-oophorectomy status and time, or analyses of Schoenfeld residuals. Because models were run in four stratums, we applied a Bonferroni correction such that P<0.0125 (0.05/4) was considered statistically significant, and P values from 0.0125 to 0.05 were considered marginally significant. Standardised differences ≥0.1 were considered meaningful. Complete case analyses were performed because data were rarely missing (0.04% for area of residence; 0.27% for area level income group). Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Patient and public involvement
This study was conceived through direct patient interaction and the challenges faced in providing data on the relative benefits and risks of bilateral salpingo-oophorectomy in preoperative consent discussions. Additional input was provided directly by the Toronto Health Economics and Technology Assessment Collaborative, a multidisciplinary research collaboration that aims to ensure clinical evidence will be relevant and useful to both policy makers and the public; and indirectly by the ICES Public Advisory Council, composed of members of the public from across Ontario, which regularly guides ICES on its activities, including the specific types of studies and research questions that will matter most to the public.
Results
Study population
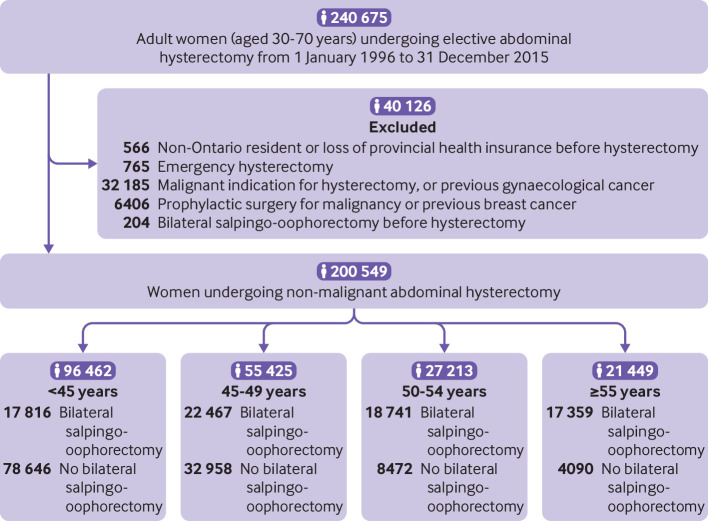
A total of 200 549 women (30-70 years) met inclusion criteria (fig 1); 76 383 (38%) underwent concurrent bilateral salpingo-oophorectomy, and only 2611 of these (3.4%) involved a second unilateral oophorectomy after previous surgery. Performance of bilateral salpingo-oophorectomy also varied with age at surgery: 18.5%, 40.5%, 68.9%, and 80.9% of women <45, 45-49, 50-54, and ≥55 years underwent bilateral salpingo-oophorectomy, respectively (fig 1, table 1).
Fig 1.
Flowchart of patients included in study
Table 1.
Baseline characteristics at time of index hysterectomy for women (aged 30-70 years) undergoing bilateral salpingo-oophorectomy versus ovarian conservation, stratified by age group at surgery (<45, 45-49, 50-54, ≥55 years) and before applying overlap propensity score weights. Data are numbers (%) unless stated otherwise
| Characteristic | <45 years | 45-49 years | 50-54 years | ≥55 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No BSO (n=78 646) | BSO (n=17 816) | Std diff | No BSO (n=32 958) | BSO (n=22 467) | Std diff | No BSO (n=8472) | BSO (n=18 741) | Std diff | No BSO (n=4090) | BSO (n=17 359) | Std diff | ||||
| Median age, years (IQR) | 40 (37-43) | 41 (38-43) | 0.19 | 47 (46-48) | 48 (46-49) | 0.35 | 52 (51-53) | 52 (51-53) | 0.23 | 60 (57-65) | 61 (57-65) | 0.00 | |||
| Year of surgery | |||||||||||||||
| 1996-2000 | 21 337 (27.1) | 6852 (38.5) | 0.24 | 5791 (17.6) | 7474 (33.3) | 0.37 | 1172 (13.8) | 5378 (28.7) | 0.37 | 581 (14.2) | 4637 (26.7) | 0.31 | |||
| 2001-2005 | 22 656 (28.8) | 4670 (26.2) | 0.06 | 8786 (26.7) | 5936 (26.4) | 0.01 | 2284 (27.0) | 4736 (25.3) | 0.04 | 1313 (32.1) | 4056 (23.4) | 0.20 | |||
| 2006-2010 | 19 796 (25.2) | 2980 (16.7) | 0.21 | 10 292 (31.2) | 4461 (19.9) | 0.26 | 2696 (31.8) | 4086 (21.8) | 0.23 | 1262 (30.9) | 3846 (22.2) | 0.20 | |||
| 2011-2015 | 14 857 (18.9) | 3314 (18.6) | 0.01 | 8089 (24.5) | 4596 (20.5) | 0.10 | 2320 (27.4) | 4541 (24.2) | 0.07 | 934 (22.8) | 4820 (27.8) | 0.11 | |||
| Area of residence | |||||||||||||||
| Urban | 65 863 (83.7) | 14 935 (83.8) | 0.00 | 28 659 (87.0) | 19 366 (86.2) | 0.02 | 7299 (86.2) | 16 287 (86.9) | 0.02 | 3430 (83.9) | 14 810 (85.3) | 0.04 | |||
| Rural | 12 758 (16.2) | 2869 (16.1) | 4285 (13.0) | 3091 (13.8) | 1171 (13.8) | 2447 (13.1) | 658 (16.1) | 2540 (14.6) | |||||||
| Area level income group (fifths)* | |||||||||||||||
| 1 (low) | 16 131 (20.5) | 3716 (20.9) | 0.01 | 5589 (17.0) | 4114 (18.3) | 0.04 | 1298 (15.3) | 2985 (15.9) | 0.02 | 658 (16.1) | 2814 (16.2) | 0.00 | |||
| 2 | 16 647 (21.2) | 3669 (20.6) | 0.01 | 6367 (19.3) | 4401 (19.6) | 0.01 | 1593 (18.8) | 3512 (18.7) | 0.00 | 797 (19.5) | 3331 (19.2) | 0.01 | |||
| 3 | 16 618 (21.1) | 3774 (21.2) | 0.00 | 6936 (21.0) | 4603 (20.5) | 0.01 | 1685 (19.9) | 3801 (20.3) | 0.01 | 861 (21.1) | 3492 (20.1) | 0.02 | |||
| 4 | 15 973 (20.3) | 3625 (20.3) | 0.00 | 7194 (21.8) | 4689 (20.9) | 0.02 | 1876 (22.1) | 4156 (22.2) | 0.00 | 898 (22.0) | 3669 (21.1) | 0.02 | |||
| 5 (high) | 13 054 (16.6) | 2973 (16.7) | 0.00 | 6771 (20.5) | 4607 (20.5) | 0.00 | 1998 (23.6) | 4250 (22.7) | 0.02 | 870 (21.3) | 4009 (23.1) | 0.04 | |||
| Immigration status | |||||||||||||||
| Long term resident | 69 830 (88.8) | 16 036 (90.0) | 0.04 | 27 878 (84.6) | 19 507 (86.8) | 0.06 | 7443 (87.9) | 16 683 (89.0) | 0.04 | 3749 (91.7) | 16 136 (93.0) | 0.05 | |||
| Immigrant | 8816 (11.2) | 1780 (10.0) | 5080 (15.4) | 2960 (13.2) | 1029 (12.1) | 2058 (11.0) | 341 (8.3) | 1223 (7.0) | |||||||
| Ethnicity | |||||||||||||||
| General population | 75 670 (96.2) | 17 108 (96.0) | 0.01 | 31 019 (94.1) | 21 213 (94.4) | 0.01 | 8013 (94.6) | 17 738 (94.6) | 0.00 | 3914 (95.7) | 16 760 (96.5) | 0.04 | |||
| South Asian | 1580 (2.0) | 326 (1.8) | 0.01 | 833 (2.5) | 537 (2.4) | 0.01 | 170 (2.0) | 387 (2.1) | 0.00 | 75 (1.8) | 291 (1.7) | 0.01 | |||
| Chinese | 1396 (1.8) | 382 (2.2) | 0.03 | 1106 (3.4) | 717 (3.2) | 0.01 | 289 (3.4) | 616 (3.3) | 0.01 | 101 (2.5) | 308 (1.8) | 0.05 | |||
| Hysterectomy type | |||||||||||||||
| Total | 68 418 (87.0) | 16 242 (91.2) | 0.13 | 27 369 (83.0) | 20 428 (90.9) | 0.24 | 7064 (83.4) | 17 095 (91.2) | 0.24 | 3396 (83.0) | 16 270 (93.7) | 0.34 | |||
| Subtotal | 10 228 (13.0) | 1574 (8.8) | 5589 (17.0) | 2039 (9.1) | 1408 (16.6) | 1646 (8.8) | 694 (17.0) | 1089 (6.3) | |||||||
| Abnormal uterine bleeding | |||||||||||||||
| Yes | 48 912 (62.2) | 7016 (39.4) | 0.47 | 18 955 (57.5) | 10 442 (46.5) | 0.22 | 4026 (47.5) | 7390 (39.4) | 0.16 | 769 (18.8) | 3480 (20.0) | 0.03 | |||
| No | 29 734 (37.8) | 10 800 (60.6) | 14 003 (42.5) | 12 025 (53.5) | 4446 (52.5) | 11 351 (60.6) | 3321 (81.2) | 13 879 (80.0) | |||||||
| Fibroids | |||||||||||||||
| Yes | 37 556 (47.8) | 6703 (37.6) | 0.21 | 23 884 (72.5) | 14 597 (65.0) | 0.16 | 6226 (73.5) | 12 729 (67.9) | 0.12 | 1648 (40.3) | 7958 (45.8) | 0.11 | |||
| No | 41 090 (52.2) | 11 113 (62.4) | 9074 (27.5) | 7870 (35.0) | 2246 (26.5) | 6012 (32.1) | 2442 (59.7) | 9401 (54.2) | |||||||
| Endometriosis | |||||||||||||||
| Yes | 20 942 (26.6) | 8831 (49.6) | 0.49 | 8176 (24.8) | 7765 (34.6) | 0.21 | 1946 (23.0) | 5105 (27.2) | 0.10 | 615 (15.0) | 3273 (18.9) | 0.10 | |||
| No | 57 704 (73.4) | 8985 (50.4) | 24 782 (75.2) | 14 702 (65.4) | 6526 (77.0) | 13 636 (72.8) | 3475 (85.0) | 14 086 (81.1) | |||||||
| Ovarian cyst | |||||||||||||||
| Yes | 8097 (10.3) | 5226 (29.3) | 0.49 | 3655 (11.1) | 6378 (28.4) | 0.45 | 1071 (12.6) | 5042 (26.9) | 0.36 | 676 (16.5) | 5219 (30.1) | 0.32 | |||
| No | 70 549 (89.7) | 12 590 (70.7) | 29 303 (88.9) | 16 089 (71.6) | 7401 (87.4) | 13 699 (73.1) | 3414 (83.5) | 12 140 (69.9) | |||||||
| Pelvic pain or inflammation | |||||||||||||||
| Yes | 22 919 (29.1) | 7430 (41.7) | 0.26 | 5985 (18.2) | 5687 (25.3) | 0.17 | 1161 (13.7) | 3319 (17.7) | 0.11 | 437 (10.7) | 2181 (12.6) | 0.06 | |||
| No | 55 727 (70.9) | 10 386 (58.3) | 26 973 (81.8) | 16 780 (74.7) | 7311 (86.3) | 15 422 (82.3) | 3653 (89.3) | 15 178 (87.4) | |||||||
| Premalignant disease | |||||||||||||||
| Yes | 4800 (6.1) | 1056 (5.9) | 0.01 | 1369 (4.2) | 1639 (7.3) | 0.14 | 480 (5.7) | 2165 (11.6) | 0.21 | 579 (14.2) | 3690 (21.3) | 0.19 | |||
| No | 73 846 (93.9) | 16 760 (94.1) | 31 589 (95.8) | 20 828 (92.7) | 7992 (94.3) | 16 576 (88.4) | 3511 (85.8) | 13 669 (78.7) | |||||||
| Prolapse | |||||||||||||||
| Yes | 3108 (4.0) | 349 (2.0) | 0.12 | 1593 (4.8) | 975 (4.3) | 0.02 | 912 (10.8) | 1541 (8.2) | 0.09 | 1722 (42.1) | 4012 (23.1) | 0.41 | |||
| No | 75 538 (96.0) | 17 467 (98.0) | 31 365 (95.2) | 21 492 (95.7) | 7560 (89.2) | 17 200 (91.8) | 2368 (57.9) | 13 347 (76.9) | |||||||
| Comorbidities (ADGs) | |||||||||||||||
| 0-5 | 14 344 (18.2) | 2073 (11.6) | 0.19 | 7279 (22.1) | 3555 (15.8) | 0.16 | 1730 (20.4) | 2989 (15.9) | 0.12 | 582 (14.2) | 2273 (13.1) | 0.03 | |||
| 6-9 | 41 436 (52.7) | 8897 (49.9) | 0.06 | 18 049 (54.8) | 11 914 (53.0) | 0.03 | 4593 (54.2) | 9966 (53.2) | 0.02 | 2145 (52.4) | 8981 (51.7) | 0.01 | |||
| ≥10 | 22 866 (29.1) | 6846 (38.4) | 0.20 | 7630 (23.2) | 6998 (31.1) | 0.18 | 2149 (25.4) | 5786 (30.9) | 0.12 | 1363 (33.3) | 6105 (35.2) | 0.04 | |||
| Hypertension | |||||||||||||||
| Yes | 8916 (11.3) | 2145 (12.0) | 0.02 | 6360 (19.3) | 4725 (21.0) | 0.04 | 2197 (25.9) | 5408 (28.9) | 0.07 | 1916 (46.8) | 8091 (46.6) | 0.00 | |||
| No | 69 730 (88.7) | 15 671 (88.0) | 26 598 (80.7) | 17 742 (79.0) | 6275 (74.1) | 13 333 (71.1) | 2174 (53.2) | 9268 (53.4) | |||||||
| Diabetes | |||||||||||||||
| Yes | 3437 (4.4) | 950 (5.3) | 0.04 | 1906 (5.8) | 1376 (6.1) | 0.01 | 510 (6.0) | 1358 (7.2) | 0.05 | 518 (12.7) | 2118 (12.2) | 0.01 | |||
| No | 75 209 (95.6) | 16 866 (94.7) | 31 052 (94.2) | 21 091 (93.9) | 7962 (94.0) | 17 383 (92.8) | 3572 (87.3) | 15 241 (87.8) | |||||||
| Chronic obstructive pulmonary disease | |||||||||||||||
| Yes | 2826 (3.6) | 874 (4.9) | 0.07 | 1925 (5.8) | 1557 (6.9) | 0.04 | 504 (5.9) | 1308 (7.0) | 0.04 | 400 (9.8) | 1838 (10.6) | 0.03 | |||
| No | 75 820 (96.4) | 16 942 (95.1) | 31 033 (94.2) | 20 910 (93.1) | 7968 (94.1) | 17 433 (93.0) | 3690 (90.2) | 15 521 (89.4) | |||||||
| Previous malignancy | |||||||||||||||
| Yes | 745 (0.9) | 206 (1.2) | 0.02 | 476 (1.4) | 373 (1.7) | 0.02 | 139 (1.6) | 348 (1.9) | 0.02 | 117 (2.9) | 528 (3.0) | 0.01 | |||
| No | 77 901 (99.1) | 17 610 (98.8) | 32 482 (98.6) | 22 094 (98.3) | 8333 (98.4) | 18 393 (98.1) | 3973 (97.1) | 16 831 (97.0) | |||||||
| Cardiovascular disease | |||||||||||||||
| Yes | 1983 (2.5) | 660 (3.7) | 0.07 | 1066 (3.2) | 1060 (4.7) | 0.08 | 338 (4.0) | 1049 (5.6) | 0.08 | 514 (12.6) | 2406 (13.9) | 0.04 | |||
| No | 76 663 (97.5) | 17 156 (96.3) | 31 892 (96.8) | 21 407 (95.3) | 8134 (96.0) | 17 692 (94.4) | 3576 (87.4) | 14 953 (86.1) | |||||||
| Previous ovarian surgery | |||||||||||||||
| Yes | 7213 (9.2) | 4293 (24.1) | 0.41 | 1875 (5.7) | 1845 (8.2) | 0.10 | 353 (4.2) | 837 (4.5) | 0.01 | 92 (2.2) | 397 (2.3) | 0.00 | |||
| No | 71 433 (90.8) | 13 523 (75.9) | 31 083 (94.3) | 20 622 (91.8) | 8119 (95.8) | 17 904 (95.5) | 3998 (97.8) | 16 962 (97.7) | |||||||
| Previous abdominopelvic surgery | |||||||||||||||
| 0 | 38 170 (48.5) | 6856 (38.5) | 0.20 | 20 567 (62.4) | 14 297 (63.6) | 0.03 | 5838 (68.9) | 13 342 (71.2) | 0.05 | 3127 (76.5) | 13 402 (77.2) | 0.02 | |||
| 1 | 24 244 (30.8) | 5640 (31.7) | 0.02 | 8564 (26.0) | 5555 (24.7) | 0.03 | 1928 (22.8) | 3992 (21.3) | 0.04 | 757 (18.5) | 3084 (17.8) | 0.02 | |||
| 2 | 10 038 (12.8) | 2926 (16.4) | 0.10 | 8564 (26.0) | 1742 (7.8) | 0.01 | 512 (6.0) | 1008 (5.4) | 0.03 | 146 (3.6) | 674 (3.9) | 0.02 | |||
| ≥3 | 6194 (7.9) | 2394 (13.4) | 0.18 | 1144 (3.5) | 873 (3.9) | 0.02 | 194 (2.3) | 399 (2.1) | 0.01 | 60 (1.5) | 199 (1.1) | 0.03 | |||
ADG=Johns Hopkins Aggregated Diagnosis Group; BSO=bilateral salpingo-oophorectomy; IQR=interquartile range; Std diff=standardised difference.
Gynaecological diagnoses were documented on admission for hysterectomy and patients could have multiple diagnoses if relevant.
Data were missing for area of residence (n=81, 0.04%) and area level income group (n=545, 0.27%).
Within each age stratum, patients undergoing bilateral salpingo-oophorectomy were older, had more comorbidities, and more often had a gynaecological indication for bilateral salpingo-oophorectomy than patients undergoing ovarian conservation; differences were less pronounced in older age stratums (table 1). After applying overlap weights, groups were balanced on baseline characteristics, with all standardised differences equal to zero (appendix 5). Median follow-up was 12 years overall (interquartile range 7-17), and there were 2268, 1516, 982, and 2267 deaths in women <45, 45-49, 50-54, and ≥55 years, respectively (appendix 6).
Primary analyses
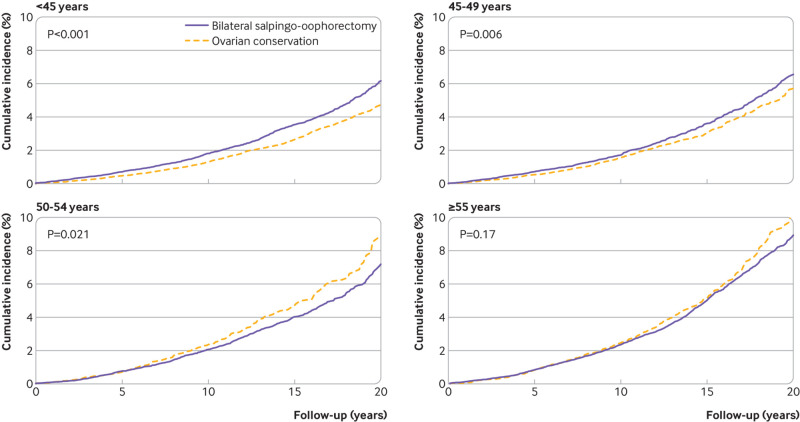
In women aged <45 years, bilateral salpingo-oophorectomy was associated with increased all cause death compared with ovarian conservation (hazard ratio 1.31, 95% confidence interval 1.18 to 1.45, P<0.001); this was driven by a significant increase in non-cancer death (1.38, 1.21 to 1.58, P<0.001) and a marginally significant increase in cancer death (1.18, 1.01 to 1.39, P=0.044; table 2, fig 2, and appendix 7-8). At 20 years, the weighted cumulative incidence of all cause death was 6.1% (95% confidence interval 5.6% to 6.6%) for bilateral salpingo-oophorectomy and 4.7% (4.4% to 5.0%) for ovarian conservation (fig 2); this corresponded to an absolute risk increase of 1.4% (0.8% to 2.1%; number needed to harm 71) at 20 years.
Table 2.
Association between bilateral salpingo-oophorectomy and all cause, non-cancer, and cancer death in women (aged 30-70 years) undergoing non-malignant hysterectomy stratified by age group at surgery (<45, 45-49, 50-54, ≥55 years)
| Outcome | <45 years | 45-49 years | 50-54 years | ≥55 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||||
| Primary analysis: overlap propensity score weighted models | |||||||||||
| All cause death | 1.31 (1.18 to 1.45) | <0.001 | 1.16 (1.04 to 1.30) | 0.007 | 0.83 (0.72 to 0.97) | 0.018 | 0.92 (0.82 to 1.03) | 0.16 | |||
| Non-cancer death | 1.38 (1.21 to 1.58) | <0.001 | 1.29 (1.10 to 1.52) | 0.002 | 0.81 (0.64 to 1.02) | 0.071 | 1.00 (0.85 to 1.17) | 0.99 | |||
| Cancer death | 1.18 (1.01 to 1.39) | 0.044 | 1.04 (0.89 to 1.21) | 0.63 | 0.87 (0.71 to 1.06) | 0.15 | 0.82 (0.69 to 0.97) | 0.023 | |||
| Sensitivity analysis: multivariable models* | |||||||||||
| All cause death | 1.30 (1.18 to 1.45) | <0.001 | 1.17 (1.05 to 1.30) | 0.006 | 0.86 (0.74 to 1.00) | 0.044 | 0.97 (0.86 to 1.09) | 0.57 | |||
| Non-cancer death | 1.38 (1.21 to 1.58) | <0.001 | 1.31 (1.11 to 1.54) | 0.001 | 0.84 (0.68 to 1.05) | 0.13 | 1.07 (0.91 to 1.25) | 0.41 | |||
| Cancer death | 1.20 (1.02 to 1.41) | 0.029 | 1.06 (0.91 to 1.24) | 0.43 | 0.88 (0.72 to 1.07) | 0.18 | 0.85 (0.72 to 1.01) | 0.072 | |||
Ovarian conservation serves as reference category. Primary analyses used overlap propensity score weighting, and sensitivity analyses used traditional multivariable Cox proportional hazards models; P<0.0125 (0.05/4) was considered statistically significant, and P values from 0.0125 to 0.05 were considered marginally significant.
Covariates were identical to those included in propensity score development: age at surgery (years), rural or urban residence, year of surgery (1996-2000, 2001-2005, 2006-2010, 2011-2015), residential income fifth, ethnicity (Chinese, South Asian, other), immigration status (long term resident, immigrant), hysterectomy type (total, subtotal), abnormal uterine bleeding (yes or no), fibroids (yes or no), endometriosis (yes or no), ovarian cysts (yes or no), premalignant conditions (yes or no), pelvic pain or inflammation (yes or no), prolapse (yes or no), Johns Hopkins Aggregated Diagnosis Groups (0-5, 6-9, ≥10), diabetes (yes or no), hypertension (yes or no), cardiovascular disease (yes or no), chronic obstructive pulmonary disease (yes or no), previous malignancy (yes or no), previous abdominopelvic surgeries (0, 1, 2, ≥3), previous ovarian surgery (yes or no).
Fig 2.
Weighted cumulative incidence of all cause death in women (aged 30-70 years) undergoing non-malignant hysterectomy with bilateral salpingo-oophorectomy or ovarian conservation stratified by age group at surgery (<45, 45-49, 50-54, ≥55 years)
In women aged 45-49 years, bilateral salpingo-oophorectomy was associated with increased all cause death (hazard ratio 1.16, 95% confidence interval 1.04 to 1.30, P=0.007) and non-cancer death (1.29, 1.10 to 1.52, P=0.002), but not cancer death (1.04, 0.89 to 1.21, P=0.63) compared with ovarian conservation (table 2, fig 2, and appendix 7-8). At 20 years, the weighted cumulative incidence of all cause death was 6.5% (95% confidence interval 6.0% to 7.0%) for bilateral salpingo-oophorectomy and 5.8% (5.3% to 6.4%) for ovarian conservation (fig 2); this corresponded to an absolute risk increase of 0.7% (−0.12% to 1.45%; number needed to harm 152) at 20 years.
In women aged 50-54 years, bilateral salpingo-oophorectomy was not associated with increased all cause death (hazard ratio 0.83, 95% confidence interval 0.72 to 0.97, P=0.018), non-cancer death (0.81, 0.64 to 1.02, P=0.071), or cancer death (0.87, 0.71 to 1.06, P=0.15) compared with ovarian conservation (table 2, fig 2, and appendix 7-8). At 20 years, the weighted cumulative incidence of all cause death was 6.9% (6.3% to 7.6%) for bilateral salpingo-oophorectomy and 8.8% (7.4% to 10.3%) for ovarian conservation (fig 2); this corresponded to an absolute risk decrease of 1.9% (−3.43% to −0.36%) at 20 years.
In women aged ≥55 years, bilateral salpingo-oophorectomy was not associated with increased all cause death (hazard ratio 0.92, 95% confidence interval 0.82 to 1.03, P=0.16), non-cancer death (1.00, 0.85 to 1.17, P=0.99), or cancer death (0.82, 0.69 to 0.97, P=0.023) compared with ovarian conservation (table 2, fig 2, and appendix 7-8). At 20 years, the weighted cumulative incidence of all cause death was 21.7% (95% confidence interval 20.4% to 22.9%) for bilateral salpingo-oophorectomy and 25.3% (22.1% to 28.5%) for ovarian conservation (fig 2); this corresponded to an absolute risk decrease of 3.6% (−7.0% to −0.24%) at 20 years.
Additional analyses
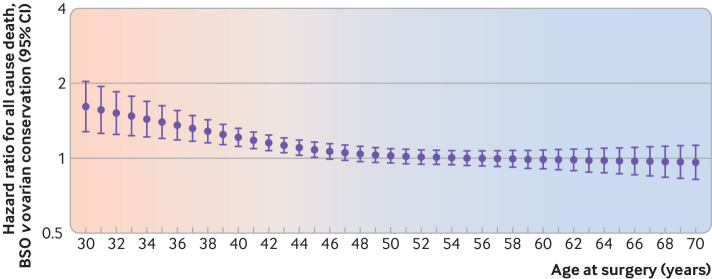
In secondary analyses exploring a potential change in the association between bilateral salpingo-oophorectomy and all cause death with advancing age at surgery, the hazard ratio was highest in the premenopausal years, gradually declined in the years representing the menopausal transition, and remained around the null in the postmenopausal years (fig 3). As age advanced, the confidence intervals for individual point estimates crossed 1, suggesting no statistical difference in the year-to-year hazard.
Fig 3.
Hazard ratios for all cause death at each year of age in women (aged 30-70 years) undergoing non-malignant hysterectomy. Bilateral salpingo-oophorectomy (BSO) is the exposure and ovarian conservation is the reference group. Associated 95% confidence intervals are represented by whiskers. Shading shows gradual change in association with advancing age
In sensitivity analyses, multivariable Cox proportional hazards models treating bilateral salpingo-oophorectomy as a static or time varying exposure yielded similar results as our overlap weighted models (table 2, appendix 9). Bilateral salpingo-oophorectomy was associated with an increase in death due to cardiovascular disease in women aged <45 years (hazard ratio 1.47, 95% confidence interval 1.07 to 2.03, P=0.019), and not significantly associated with death due to upper gastrointestinal tract cancer in any age stratums (appendix 10).
Discussion
Principal findings
In this population based cohort study of over 200 000 women undergoing non-malignant hysterectomy, the association of bilateral salpingo-oophorectomy with mortality varied based on the age at which surgery was performed. Compared with ovarian conservation, bilateral salpingo-oophorectomy appeared to be associated with significantly increased all cause mortality in women aged <50 years but not in those aged ≥50 years; in fact, marginally significant decreases were found in all cause and cancer mortality in women aged 50-54 and ≥55 years, respectively. These findings are biologically plausible: bilateral salpingo-oophorectomy before the onset of menopause will result in premature deficiency of oestrogen, whereas bilateral salpingo-oophorectomy after the onset of menopause will not. Oestrogen signalling exerts genomic and non-genomic physiological effects in multiple organ systems, and so loss of oestrogen at certain critical times might contribute to the development or progression of disease.55 56 57
Results and implications
Our study suggests that bilateral salpingo-oophorectomy might be associated with increased all cause death in women of premenopausal age. Numerous retrospective analyses of prospectively observed cohorts3 4 6 15 16 and administrative datasets3 5 7 58 59 have reported similar findings (table 3), albeit each with distinct limitations. Work by Mytton and colleagues is most comparable to ours in its overall design, methodological approach, and contemporary nature. This study included 113 679 women aged 35-45 only who were undergoing non-malignant hysterectomy in England from 2004 to 2014.5 Over a median follow-up of six years, bilateral salpingo-oophorectomy was associated with an increase in all cause death (hazard ratio 1.56, 95% confidence interval 1.37 to 1.81), cardiac death (2.00, 1.11 to 3.57), and cancer death (1.85, 1.54 to 2.22) compared with ovarian conservation. We identified similar increases in all cause and non-cancer death after adjusting for many more potential confounders and ensuring longer follow-up (median 12 years). Considering the strong methodology used in this work and by Mytton and colleagues, consistency of published literature on this association, and presence of a plausible mechanism, caution might be warranted when considering bilateral salpingo-oophorectomy in young women, namely those without a clinical indication for the procedure.
Table 3.
Cohort studies examining association between bilateral salpingo-oophorectomy and all cause death
| Study | Cohort | Follow-up | Age group | Sample size | Deaths | Hazard ratio (95% CI) | Covariates | |
|---|---|---|---|---|---|---|---|---|
| Rocca 20063 | Mayo Clinic Cohort Study; prophylactic BSO v no ovarian surgery | Median 25.0 years | <45 | 1541 | 262 | 1.67 (1.16 to 2.40) | Age | |
| –HT | 1462 | 239 | 1.93 (1.25 to 2.39) | |||||
| +HT | 1496 | 252 | 1.27 (0.67 to 2.39) | |||||
| 45-50 | 888 | 315 | 1.02 (0.78 to 1.32) | |||||
| >50 | 491 | 235 | 0.90 (0.68 to 1.19) | |||||
| Parker 200915 | Nurses’ Health Study; BSO v ovarian conservation at non-malignant hysterectomy | Maximum 24 years | Overall | 29 380 | 3197 | 1.12 (1.03 to 1.21) | Age, parity, diabetes, hypertension, hypercholesterolemia, body mass index, smoking, alcohol intake, exercise, aspirin use, tubal ligation, oral contraceptive use, HT use, family history of breast cancer, family history of myocardial infarction <60 years | |
| <45 | NR | 1627 | 1.06 (0.95 to 1.80) | |||||
| 45-54 | NR | 1300 | 1.15 (1.01 to 1.32) | |||||
| ≥55 | NR | 270 | 1.14 (0.85 to 1.52) | |||||
| Parker 201316 | Nurses’ Health Study; BSO v ovarian conservation at non-malignant hysterectomy | Maximum 28 years | Overall | 30 117 | 4599 | 1.13 (1.06 to 1.21) | Age, parity, body mass index, smoking, alcohol intake, exercise, aspirin use, tubal ligation, oral contraceptive use, HT use, family history of breast cancer, family history of myocardial infarction <60 years | |
| <50 | 21 094 | 3433 | 1.13 (1.05 to 1.22) | |||||
| –HT | NR | 292 | 1.41 (1.04 to 1.92) | |||||
| +HT | NR | 1695 | 1.05 (0.94 to 1.17) | |||||
| 50-59 | 6241 | 883 | 1.10 (0.93 to 1.31) | |||||
| ≥60 | 2782 | 283 | 1.31 (0.98 to 1.75) | |||||
| Jacoby 201118 | Women’s Health Initiative; BSO v ovarian conservation at non-malignant hysterectomy | Mean 7.6 (SD 1.6) years | <40 | 7583 | 446 | 0.90 (0.72 to 1.13) | Age, parity, ethnicity, education, insurance, health care provider, hypercholesterolemia, hypertension, diabetes, body mass index, smoking, alcohol intake, exercise, myocardial infarction, stroke, coronary revascularisation, HT use, family history of myocardial infarction or stroke | |
| 40-49 | 11 397 | 661 | 1.00 (0.84 to 1.19) | |||||
| ≥50 | 2934 | 417 | 1.07 (0.84 to 1.35) | |||||
| Gierach 20144 | Breast Cancer Detection Demonstration Project; BSO v no gynaecological surgery | Mean 22.1 years | ≤35 | 50 742 | 13 237 | 1.20 (1.08 to 1.34) | Landmark analyses at differing ages: adjusted for body mass index, alcohol intake, smoking, HT use, birth cohort | |
| ≤45 | 44 971 | 11 894 | 1.10 (1.03 to 1.17) | |||||
| ≤55 | 42 053 | 10 862 | 1.01 (0.96 to 1.06) | |||||
| Mytton 20175 | English Hospital Episode Statistics; BSO v ovarian conservation at non-malignant hysterectomy | Mean 6.2 (SD 2.8) years | 35-45 | 113 679 | 832 | 1.56 (1.37 to 1.81)* | Age, deprivation, surgery type, Charlson comorbidity score, number of admissions before hysterectomy | |
| Wilson 20196 | Australian Longitudinal Study; hysterectomy with BSO v no gynaecological surgery | Median 21.5 years | <50 | 11 069 | 734 | 1.02 (0.78 to 1.34) | Age, body mass index, smoking, alcohol intake, exercise, education, difficulty managing on income, remoteness category, number of children, diabetes, hypertension, perception of general health | |
| –HT | 8354 | 518 | 1.81 (1.01 to 3.25) | |||||
| +HT | 2708 | 216 | 0.91 (0.67 to 1.24) | |||||
| Tuesley 20207 | Western Australia electoral roll; hysterectomy with BSO v no gynaecological surgery | Median 24.2 years | Overall | 666 588 | 33 963 | 0.94 (0.88 to 1.00) | Age at entry, area of residence, area level socioeconomic status, parity (time varying), tubal ligation (time varying) | |
| <35 | 1013 | 59 | 1.44 (1.12 to 1.87) | |||||
| 35-44 | 4936 | 291 | 1.15 (1.02 to 1.29) | |||||
| 45-54 | 8599 | 414 | 0.82 (0.74 to 0.90) | |||||
| 55-64 | 2963 | 241 | 0.90 (0.79 to 1.02) | |||||
| ≥65 | 1046 | 96 | 0.90 (0.74 to 1.10) | |||||
| Cusimano 2021 | ICES Ontario Databases; BSO v ovarian conservation at non-malignant hysterectomy | Median 12.0 years | <45 | 96 462 | 2268 | 1.31 (1.18 to 1.45) | Age, year of surgery, rural or urban residence, area level income fifth, ethnicity, immigration status, hysterectomy type, abnormal uterine bleeding, fibroids, ovarian cysts, endometriosis, pelvic pain or inflammation, premalignant disease, prolapse, overall comorbidity score, chronic obstructive pulmonary disease, hypertension, diabetes, previous malignancy, cardiovascular disease, previous ovarian surgery, previous abdominopelvic surgery | |
| 45-49 | 55 425 | 1516 | 1.16 (1.04 to 1.30) | |||||
| 50-54 | 27 213 | 982 | 0.83 (0.72 to 0.97) | |||||
| ≥55 | 21 449 | 2267 | 0.92 (0.82 to 1.03) | |||||
BSO=bilateral salpingo-oophorectomy; HT=hormone therapy; NR=not reported; SD=standard deviation.
Mytton and colleagues reported BSO as the reference group (0.64, 95% confidence interval 0.55-0.73); to facilitate comparison, we present the reciprocal.
Our study also suggests that bilateral salpingo-oophorectomy might not be associated with all cause death in women of postmenopausal age. Similar findings have been reported in the Mayo Clinic Cohort Study,3 58 59 Breast Cancer Detection Demonstration Project,4 and Western Australia Data Linkage Study,7 which compared women undergoing hysterectomy with bilateral salpingo-oophorectomy with non-surgical referent women; and in the Women’s Health Initiative,18 which compared women undergoing bilateral salpingo-oophorectomy and ovarian conservation at the time of non-malignant hysterectomy (table 3). The Nurses’ Health Study is the only cohort study to suggest that the association of bilateral salpingo-oophorectomy with all cause mortality might not vary with age: the overall hazard ratio was 1.13 (95% confidence interval 1.06 to 1.21), and an interaction between bilateral salpingo-oophorectomy status and age (<50, 50-59, ≥60 years) was not significant (P=0.46).16 This study included a cohort of largely white nurses, had few women aged ≥50 years (8969 with 1166 deaths), and did not control for indications for bilateral salpingo-oophorectomy. Our study was population based, included over 48 000 women aged ≥50 years (with 3249 deaths), and controlled for gynaecological conditions that might act as confounders in older age stratums. Our study and the accumulated literature contrast with the Nurses’ Health Study, and suggest that bilateral salpingo-oophorectomy might not be associated with all cause mortality in older women.
Our study attempts to identify when the risk-to-benefit ratio of bilateral salpingo-oophorectomy might gradually shift from being supportive of ovarian conservation to removal of all ovarian tissue. Most studies have run age stratified analyses without articulating a rationale for the categories chosen, or arbitrarily changed categories in separate publications on the same cohort.15 16 We provide a clear biological basis for our stratified analyses, but also used restricted cubic splines to explicitly model how the effect of bilateral salpingo-oophorectomy changed with advancing age at surgery. These analyses showed that the hazard associated with bilateral salpingo-oophorectomy appears to gradually decrease during the 45-54 year age range and remains around the null thereafter. Since age serves as a population level surrogate for the onset of menopause, these findings provide some support to assertions that bilateral salpingo-oophorectomy could potentially be harmful in premenopausal but not postmenopausal women.4
Decisions on whether to ultimately perform opportunistic bilateral salpingo-oophorectomy at non-malignant hysterectomy must weigh the potential benefits and harms of the procedure. Bilateral salpingo-oophorectomy is known to reduce ovarian cancer incidence and ovarian cancer mortality at any age.60 If bilateral salpingo-oophorectomy is also associated with increased all cause mortality, then this alone might outweigh the benefit of ovarian cancer risk reduction. If bilateral salpingo-oophorectomy is not associated with all cause mortality, then the procedure may be a reasonable and effective strategy for ovarian cancer risk reduction. Factors such as quality of life and sexual function merit consideration as well; existing studies examining these outcomes in the context of non-malignant hysterectomy are limited, and further research is required.61 62 63 64 65 Opportunistic bilateral salpingectomy (the surgical removal of both fallopian tubes alone) is an attractive alternative to bilateral salpingo-oophorectomy that does not impact ovarian endocrine function and might still prevent high grade serous cancers that arise in the fallopian tube66; however, additional studies are required to establish the magnitude of ovarian cancer risk reduction offered by bilateral salpingectomy compared with bilateral salpingo-oophorectomy.60 67 68 69
Strengths and limitations
Our study addresses the main limitations of previous work. We included a population based cohort of all women undergoing non-malignant abdominal hysterectomy in Ontario, whose outcomes should be generalisable to patients with similar demographic and socioeconomic characteristics managed in other jurisdictions and settings. We used overlap propensity score weighting, an analytic approach that mimics pragmatic randomised trials by focusing on patients with a realistic probability of receiving either bilateral salpingo-oophorectomy or ovarian conservation. Our study includes a large population with prolonged follow-up, enabling age stratified and cause specific analyses. In contrast to most studies on this topic, which relied on longitudinal self-reported survey data,4 6 15 16 18 we observed all patients from the exact date of exposure to bilateral salpingo-oophorectomy and used validated codes to identify bilateral salpingo-oophorectomy, thereby preventing introduction of survival or misclassification bias respectively. Our data sources are of high quality and comprehensive, ensuring accurate and complete outcome ascertainment.
Several limitations require consideration. Firstly, we lacked data on preoperative menopausal status, which could confound the association observed in women aged 45-49 and 50-54 years. If women undergoing bilateral salpingo-oophorectomy are more often postmenopausal at the time of surgery, then our results in these stratums might be conservative estimates of the true effect of bilateral salpingo-oophorectomy. Secondly, our health administrative data sources lacked information on family history, intraoperative findings, genetic predisposition to malignancy, and metabolic factors such as body habitus, smoking, alcohol use, and physical activity, which might contribute to residual confounding in other age stratums as well. The importance of these factors might change as women age20; thus it is difficult to predict the direction or magnitude of possible bias in each stratum. If young women selecting bilateral salpingo-oophorectomy are also predisposed to malignancy or more likely to have an adverse metabolic profile, then the increased rate of all cause mortality observed in this population could potentially be explained by unmeasured confounding. We aimed to limit confounding by restricting our cohort on age and surgical approach to ensure all patients had an opportunity for exposure to bilateral salpingo-oophorectomy; excluding patients with previous gynaecological or breast cancer or codes indicating genetic susceptibility to malignancy; and using overlap weighting to adjust for as many relevant covariates as possible, including downstream surrogates for unmeasured confounders whenever feasible. We also performed sensitivity analyses with a plausible negative control. We did not pursue instrumental variable analysis because this approach is most suited to questions of health policy rather than clinical effectiveness, and a valid instrument was not readily apparent for the question of bilateral salpingo-oophorectomy versus ovarian conservation at non-malignant hysterectomy.70 71
A third limitation is that, despite the high quality of our databases, variables derived from administrative data remain susceptible to misclassification; for example, our covariate for ethnicity had limited sensitivity and could thereby lead to residual confounding. We aimed to limit misclassification in other critical areas by using highly accurate procedure codes to identify hysterectomy and bilateral salpingo-oophorectomy, population based vital statistics registries to determine our primary and secondary outcomes, and sensitive and specific case definition algorithms developed in Ontario for relevant comorbidities. Finally, because of data limitations, we could not explore the influence of the use of hormone therapy on our findings. Existing studies report that the association of bilateral salpingo-oophorectomy with mortality might be pronounced in never users of hormone therapy.3 6 15 16 However, such analyses are susceptible to confounding; never users might have contraindications to hormone therapy that are related to mortality72 or face sociodemographic barriers to its use.73 Because prescription and maintenance of hormone therapy will also vary among patients and providers after bilateral salpingo-oophorectomy,74 our results reflect the real world population average association of bilateral salpingo-oophorectomy with mortality, which itself is meaningful.
Conclusion
Bilateral salpingo-oophorectomy might be associated with increased all cause mortality in women of premenopausal age. Despite theoretical concerns about the loss of ongoing ovarian androgen production, we found no significant association between bilateral salpingo-oophorectomy and all cause mortality in women of postmenopausal age. Our findings apply specifically to women undergoing hysterectomy for non-malignant indications, and unmeasured confounding remains a limitation of this work and existing studies.
Caution is warranted when considering bilateral salpingo-oophorectomy in premenopausal women without an indication for the procedure. Bilateral salpingo-oophorectomy does not appear to be detrimental to survival and provides significant ovarian cancer risk reduction in postmenopausal women; additional research on other potential trade-offs in this population is required.
What is already known on this topic
Data on the potential long term health effects of bilateral salpingo-oophorectomy are inconsistent, particularly in postmenopausal women; therefore, practice guidelines on the use of bilateral salpingo-oophorectomy at the time of hysterectomy for non-malignant disease are limited
Observational studies that enrol a large representative sample of women undergoing non-malignant hysterectomy, use validated data sources, and have adequate power in older age groups are required to reliably quantify the risks of bilateral salpingo-oophorectomy
What this study adds
Advanced modelling was used to better understand how the association between bilateral salpingo-oophorectomy and mortality might change with advancing age at surgery
Estimates indicate when the risk-to-benefit ratio might potentially change from supporting ovarian conservation to removal of all ovarian tissue
In contrast to emerging hypotheses, and although unmeasured confounding remains possible, bilateral salpingo-oophorectomy might not be detrimental to survival when performed at the time of non-malignant hysterectomy in women of postmenopausal age
Acknowledgments
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information; the Ontario Ministry of Health and Long term Care; Cancer Care Ontario; Immigration, Refugees and Citizenship Canada Permanent Resident Database; the Ontario Registrar General/Ministry of Government Services, and Service Ontario. However, the conclusions, opinions, and statements expressed herein are solely those of the authors, and not those of the bodies listed. No endorsement by these bodies is intended or should be inferred.
Web extra.
Extra material supplied by authors
Web appendix: Online appendices
Contributors: MCC, NNB, and SEF contributed to study conception. All authors contributed to study design, data acquisition, and the statistical plan. MCC and SA performed statistical analyses. MCC, MC, SEF, RM, and NNB assisted in the interpretation of data. MCC wrote the first draft and created tables and figures. All authors critically revised the manuscript, approved the final version submitted, and agree to be accountable for all aspects of the work. MCC and NNB accept full responsibility for the work or the conduct of the study, had access to the data, controlled the decision to publish, and are guarantors of the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long term Care. MCC is supported by the American College of Surgeons Resident Research Scholarship and the Canadian Institutes of Health Research Vanier Canada Graduate Scholarship. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement by ICES or the Ontario Ministry of Health and Long term Care is intended or should be inferred. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: financial support from ICES, the Ontario Ministry of Health and Long term Care, the American College of Surgeons and Canadian Institutes of Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (MCC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Dissemination to participants and related patient and public communities: The findings of this study were presented at the American College of Surgeons Clinical Congress, and have been submitted to other national and international meetings. Direct engagement of physicians is planned through a series of grand rounds presentations at departments of obstetrics and gynaecology, while patients and the public will be reached through press release and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The Research Ethics Board at the University of Toronto (Toronto, Ontario, No 38212) provided ethical approval for this study.
Data availability statement
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access might be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS.
References
- 1. Mikhail E, Salemi JL, Mogos MF, Hart S, Salihu HM, Imudia AN. National trends of adnexal surgeries at the time of hysterectomy for benign indication, United States, 1998-2011. Am J Obstet Gynecol 2015;213:713. [DOI] [PubMed] [Google Scholar]
- 2. Evans EC, Matteson KA, Orejuela FJ, et al. Society of Gynecologic Surgeons Systematic Review Group . Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review. Obstet Gynecol 2016;128:476-85. 10.1097/AOG.0000000000001592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821-8. 10.1016/S1470-2045(06)70869-5 [DOI] [PubMed] [Google Scholar]
- 4. Gierach GL, Pfeiffer RM, Patel DA, et al. Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages. Menopause 2014;21:592-601. 10.1097/GME.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 2017;356:j372. 10.1136/bmj.j372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson LF, Pandeya N, Byles J, Mishra GD. Hysterectomy status and all-cause mortality in a 21-year Australian population-based cohort study. Am J Obstet Gynecol 2019;220:83. [DOI] [PubMed] [Google Scholar]
- 7. Tuesley KM, Protani MM, Webb PM, Dixon-Suen SC, Wilson LF, Stewart LM, et al. Hysterectomy with and without oophorectomy and all-cause and cause-specific mortality. Am J Obstet Gynecol 2020;223:723. [DOI] [PubMed] [Google Scholar]
- 8. RANZCOG . C-Gyn 25: prophylactic oophorectomy at the time of hysterectomy for benign gynaecological disease. O&G Magazine 2009;11:75-6. 30879487 [Google Scholar]
- 9.AUS. Five things physicians and patients should question: choosing wisely; 2015 https://www.choosingwisely.org/societies/american-urogynecologic-society/.
- 10.AAGL. Five things patients and providers should question: choosing wisely; 2019. https://www.choosingwisely.org/societies/aagl/.
- 11. Thurston J, Murji A, Scattolon S, et al. No. 377-Hysterectomy for Benign Gynaecologic Indications. J Obstet Gynaecol Can 2019;41:543-57. 10.1016/j.jogc.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 12. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847-53. 10.1210/jc.2005-0212 [DOI] [PubMed] [Google Scholar]
- 13. Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab 2007;92:3040-3. 10.1210/jc.2007-0581 [DOI] [PubMed] [Google Scholar]
- 14. Couzinet B, Meduri G, Lecce MG, et al. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab 2001;86:5060-6. 10.1210/jcem.86.10.7900 [DOI] [PubMed] [Google Scholar]
- 15. Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol 2009;113:1027-37. 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol 2013;121:709-16. 10.1097/AOG.0b013e3182864350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 2005;106:219-26. 10.1097/01.AOG.0000167394.38215.56 [DOI] [PubMed] [Google Scholar]
- 18. Jacoby VL, Grady D, Wactawski-Wende J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med 2011;171:760-8. 10.1001/archinternmed.2011.121 [DOI] [PubMed] [Google Scholar]
- 19. Mahal AS, Rhoads KF, Elliott CS, Sokol ER. Inappropriate oophorectomy at time of benign premenopausal hysterectomy. Menopause 2017;24:947-53. 10.1097/GME.0000000000000875 [DOI] [PubMed] [Google Scholar]
- 20. Cusimano MC, Moineddin R, Chiu M, Ferguson SE, Aktar S, Liu N, Baxter NN. Practice variation in bilateral oophorectomy at benign abdominal hysterectomy: a population-based study identifying opportunities for ovarian conservation. Journal of Obstetrics & Gynecology of Canada 2021;224:585. [DOI] [PubMed] [Google Scholar]
- 21. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 2013;122:233-41. 10.1097/AOG.0b013e318299a6cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NHS Digital. Hospital admissions data on hysterectomies and endometriosis 2007-2019; 2020 https://digital.nhs.uk/data-and-information/supplementary-information/2020/hospital-admissions-data-on-hysterectomies-and-endometriosis.
- 23. Juurlink DPC, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Institute for Clinical Evaluative Sciences, 2006. [Google Scholar]
- 24. CIHI . Health Indicators 2013: Definitions, Data Sources, and Rationale. Canadian Institute for Health Information, 2013. [Google Scholar]
- 25. Chiu M, Lebenbaum M, Lam K, et al. Describing the linkages of the immigration, refugees and citizenship Canada permanent resident data and vital statistics death registry to Ontario’s administrative health database. BMC Med Inform Decis Mak 2016;16:135. 10.1186/s12911-016-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tu JV, Chu A, Rezai MR, et al. The incidence of major cardiovascular events in immigrants to Ontario, Canada: The CANHEART Immigrant Study. Circulation 2015;132:1549-59. 10.1161/CIRCULATIONAHA.115.015345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheung MC, Earle CC, Fischer HD, et al. Impact of immigration status on cancer outcomes in Ontario, Canada. J Oncol Pract 2017;13:e602-12. 10.1200/JOP.2016.019497 [DOI] [PubMed] [Google Scholar]
- 28. Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol 2010;10:42. 10.1186/1471-2288-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:932-9. 10.1097/MLR.0b013e318215d5e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Johns Hopkins ACG® Case-Mix System Version 10.0 Release Notes. The Johns Hopkins University Bloomberg School of Public Health, Health Services Research & Development Center; 2011.
- 31. Hall RE, Cohen MM. Variations in hysterectomy rates in Ontario: does the indication matter? CMAJ 1994;151:1713-9. [PMC free article] [PubMed] [Google Scholar]
- 32. Bansi-Matharu L, Gurol-Urganci I, Mahmood TA, Templeton A, van der Meulen JH, Cromwell DA. Rates of subsequent surgery following endometrial ablation among English women with menorrhagia: population-based cohort study. BJOG 2013;120:1500-7. 10.1111/1471-0528.12319 [DOI] [PubMed] [Google Scholar]
- 33. Reeves GK, Balkwill A, Cairns BJ, Green J, Beral V, Million Women Study Collaborators . Hospital admissions in relation to body mass index in UK women: a prospective cohort study. BMC Med 2014;12:45. 10.1186/1741-7015-12-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MOHLTC . Quality-Based Procedures Clinical Handbook for Hysterectomy. Ministry of Health and Long-Term Care, 2016: 1-41. [Google Scholar]
- 35. Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res 2018;18:316. 10.1186/s12913-018-3148-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tu K, Chen Z, Lipscombe LL, Canadian Hypertension Education Program Outcomes Research Taskforce . Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ 2008;178:1429-35. 10.1503/cmaj.071283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009;6:388-94. 10.1080/15412550903140865 [DOI] [PubMed] [Google Scholar]
- 38. Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes 2015;8:204-12. 10.1161/CIRCOUTCOMES.114.001416 [DOI] [PubMed] [Google Scholar]
- 39. Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998;51:1271-6. 10.1016/S0895-4356(98)00119-X [DOI] [PubMed] [Google Scholar]
- 40. Li L, Wu J, Pu D, et al. Factors associated with the age of natural menopause and menopausal symptoms in Chinese women. Maturitas 2012;73:354-60. 10.1016/j.maturitas.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 41. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Am J Hum Biol 1992;4:37-46. 10.1002/ajhb.1310040107 [DOI] [PubMed] [Google Scholar]
- 42. Practice Committee of American Society for Reproductive Medicine . The menopausal transition. Fertil Steril 2008;90(Suppl):S61-5. 10.1016/j.fertnstert.2008.08.095 [DOI] [PubMed] [Google Scholar]
- 43. Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 2019;188:250-7. [DOI] [PubMed] [Google Scholar]
- 44. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 45. Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020;323:2417-8. 10.1001/jama.2020.7819 [DOI] [PubMed] [Google Scholar]
- 46. Zhou Y, Matsouaka RA, Thomas L. Propensity score weighting under limited overlap and model misspecification. Stat Methods Med Res 2020;29:3721-56. 10.1177/0962280220940334 [DOI] [PubMed] [Google Scholar]
- 47. Harrell FE. Regression modelling strategies: With applications to linear models, logistic regression, and survival analysis. Springer-Verlag, 2010. [Google Scholar]
- 48. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601-9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016;35:5642-55. 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089-110. 10.1002/sim.2174 [DOI] [PubMed] [Google Scholar]
- 52. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391-400. 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol 2010;63:46-55. 10.1016/j.jclinepi.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 54. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383-8. 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 2006;116:561-70. 10.1172/JCI27987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med 2013;19:197-209. 10.1016/j.molmed.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 2011;1379:188-98. 10.1016/j.brainres.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16:15-23. 10.1097/gme.0b013e31818888f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology 2009;33:32-40. 10.1159/000211951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cusimano MC, Ferguson SE, Moineddin R, et al. Ovarian cancer incidence and death in average-risk women undergoing bilateral salpingo-oophorectomy at benign hysterectomy. Am J Obstet Gynecol 2021;S0002-9378(21)01048-6. 10.1016/j.ajog.2021.09.020 [DOI] [PubMed] [Google Scholar]
- 61. Chen X, Guo T, Li B. Influence of prophylactic oophorectomy on mood and sexual function in women of menopausal transition or postmenopausal period. Arch Gynecol Obstet 2013;288:1101-6. 10.1007/s00404-013-2865-1 [DOI] [PubMed] [Google Scholar]
- 62. Rodríguez MC, Chedraui P, Schwager G, Hidalgo L, Pérez-López FR. Assessment of sexuality after hysterectomy using the Female Sexual Function Index. J Obstet Gynaecol 2012;32:180-4. 10.3109/01443615.2011.634035 [DOI] [PubMed] [Google Scholar]
- 63. Sözeri-Varma G, Kalkan-Oğuzhanoğlu N, Karadağ F, Ozdel O. The effect of hysterectomy and/or oophorectomy on sexual satisfaction. Climacteric 2011;14:275-81. 10.3109/13697137.2010.532251 [DOI] [PubMed] [Google Scholar]
- 64. Aziz A, Bergquist C, Nordholm L, Möller A, Silfverstolpe G. Prophylactic oophorectomy at elective hysterectomy. Effects on psychological well-being at 1-year follow-up and its correlations to sexuality. Maturitas 2005;51:349-57. 10.1016/j.maturitas.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 65. Aziz A, Bergquist C, Brännström M, Nordholm L, Silfverstolpe G. Differences in aspects of personality and sexuality between perimenopausal women making different choices regarding prophylactic oophorectomy at elective hysterectomy. Acta Obstet Gynecol Scand 2005;84:854-9. 10.1111/j.0001-6349.2005.00658.x [DOI] [PubMed] [Google Scholar]
- 66. Boerner T, Long Roche K. Salpingectomy for the risk reduction of ovarian cancer: is it time for a salpingectomy-alone approach? J Minim Invasive Gynecol 2021;28:403-8. 10.1016/j.jmig.2020.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst 2015;107:dju410. 10.1093/jnci/dju410 [DOI] [PubMed] [Google Scholar]
- 68. Madsen C, Baandrup L, Dehlendorff C, Kjaer SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand 2015;94:86-94. 10.1111/aogs.12516 [DOI] [PubMed] [Google Scholar]
- 69. Lessard-Anderson CR, Handlogten KS, Molitor RJ, et al. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol 2014;135:423-7. 10.1016/j.ygyno.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007;297:278-85. 10.1001/jama.297.3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hernán MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology 2006;17:360-72. 10.1097/01.ede.0000222409.00878.37 [DOI] [PubMed] [Google Scholar]
- 72. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:3975-4011. 10.1210/jc.2015-2236 [DOI] [PubMed] [Google Scholar]
- 73. Lawlor DA, Smith GD, Ebrahim S. Socioeconomic position and hormone replacement therapy use: explaining the discrepancy in evidence from observational and randomized controlled trials. Am J Public Health 2004;94:2149-54. 10.2105/AJPH.94.12.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Read MD, Edey KA, Hapeshi J, Foy C. Compliance with estrogen hormone replacement therapy after oophorectomy: a prospective study. Menopause Int 2010;16:60-4. 10.1258/mi.2010.010023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendices
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access might be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS.