ABSTRACT
The formation of dormant spores is essential for the anaerobic pathogen Clostridioides difficile to survive outside the host gastrointestinal tract. The regulatory pathways and environmental signals that initiate C. difficile spore formation within the host are not well understood. One second-messenger signaling molecule, cyclic diguanylate (c-di-GMP), modulates several physiological processes important for C. difficile pathogenesis and colonization, but the impact of c-di-GMP on sporulation is unknown. In this study, we investigated the contribution of c-di-GMP to C. difficile sporulation. The overexpression of a gene encoding a diguanylate cyclase, dccA, decreased the sporulation frequency and early sporulation gene transcription in both the epidemic R20291 and historical 630Δerm strains. The expression of a dccA allele encoding a catalytically inactive DccA that is unable to synthesize c-di-GMP no longer inhibited sporulation, indicating that the accumulation of intracellular c-di-GMP reduces C. difficile sporulation. A null mutation in dccA slightly increased sporulation in R20291 and slightly decreased sporulation in 630Δerm, suggesting that DccA contributes to the intracellular pool of c-di-GMP in a strain-dependent manner. However, these data were highly variable, underscoring the complex regulation involved in modulating intracellular c-di-GMP concentrations. Finally, the overexpression of dccA in known sporulation mutants revealed that c-di-GMP is likely signaling through an unidentified regulatory pathway to control early sporulation events in C. difficile. c-di-GMP-dependent regulation of C. difficile sporulation may represent an unexplored avenue of potential environmental and intracellular signaling that contributes to the complex regulation of sporulation initiation.
IMPORTANCE Many bacterial organisms utilize the small signaling molecule cyclic diguanylate (c-di-GMP) to regulate important physiological processes, including motility, toxin production, biofilm formation, and colonization. c-di-GMP inhibits motility and toxin production and promotes biofilm formation and colonization in the anaerobic, gastrointestinal pathogen Clostridioides difficile. However, the impact of c-di-GMP on C. difficile spore formation, a critical step in this pathogen’s life cycle, is unknown. Here, we demonstrate that c-di-GMP negatively impacts sporulation in two clinically relevant C. difficile strains, the epidemic strain R20291 and the historical strain 630Δerm. The pathway through which c-di-GMP controls sporulation was investigated, and our results suggest that c-di-GMP is likely signaling through an unidentified regulatory pathway to control C. difficile sporulation. This work implicates c-di-GMP metabolism as a mechanism to integrate environmental and intracellular cues through c-di-GMP levels to influence C. difficile sporulation.
KEYWORDS: Clostridioides difficile, Clostridium difficile, sporulation, spore, cyclic diguanylate, c-di-GMP, anaerobe, cyclic diguanylate synthase
INTRODUCTION
Nucleotide second messengers, such as the nearly ubiquitous cyclic diguanylate (c-di-GMP), serve as central intracellular signaling molecules in bacteria. c-di-GMP promotes the switch between a planktonic, motile stage and a sessile, surface-associated lifestyle and controls virulence factor production in numerous pathogenic and nonpathogenic bacteria. c-di-GMP is synthesized from GTP by diguanylate cyclases (DGCs) (synthases), which contain the conserved catalytic GGDEF motif (1). Phosphodiesterases (PDEs) (hydrolases) containing either the EAL or HD-GYP domain degrade c-di-GMP to pGpG or GMP, respectively (2–5). Often, DGC and PDE proteins contain additional sensory or regulatory domains that potentially control enzymatic activity, suggesting that environmental and bacterial cues influence the intracellular concentration of c-di-GMP (6). Most Gram-negative bacteria encode numerous DGC and PDE proteins, resulting in complex c-di-GMP metabolic pathways, while Gram-positive bacteria often contain more modest numbers of DGC and PDE proteins (7–9). However, the gastrointestinal pathogen Clostridioides difficile encodes many c-di-GMP metabolic proteins; 37 genes encoding GGDEF and/or EAL domains were identified in the historical 630 strain (10, 11). Many of the C. difficile c-di-GMP metabolic proteins have been demonstrated to be enzymatically active (10, 12), suggesting that the regulation of c-di-GMP metabolism in C. difficile is physiologically important and tightly controlled. High intracellular concentrations of c-di-GMP have been demonstrated to inhibit C. difficile motility and toxin production while promoting cell aggregation, biofilm formation, and colonization (13–20).
C. difficile is an obligate anaerobe and relies on the formation of a dormant spore for long-term persistence outside the host and transmission to new hosts. Spore formation in all endospore-forming bacteria, including C. difficile, is initiated by the highly conserved transcriptional regulator Spo0A (21, 22). Spo0A activity is tightly controlled by phosphorylation through a large regulatory network of kinases, phosphatases, and additional regulators (23, 24). Once phosphorylated, Spo0A∼P activates the expression of early sporulation genes, triggering sporulation (25; M. A. DiCandia and S. M. McBride, unpublished data). However, many of the regulatory proteins and pathways that control early sporulation events in the model organism Bacillus subtilis are not conserved in C. difficile (26, 27). Although recent progress has uncovered several important sporulation regulatory factors in C. difficile (reviewed in reference 28), the environmental cues and regulatory pathways that control Spo0A activation are largely unknown.
Environmental conditions and nutrient availability likely trigger C. difficile spore formation within the host gastrointestinal tract. Two global transcriptional regulators, CodY and CcpA, control sporulation initiation in C. difficile in response to nutrient availability. CodY represses target gene expression when GTP and branched-chain amino acids are abundant, and CcpA activates or represses target gene transcription based on carbohydrate availability (29, 30). Mutations in either CodY or CcpA result in increased sporulation frequencies (30, 31). Some sporulation genes serve as direct targets for CodY and CcpA regulation, but the molecular mechanisms are not delineated (29–31). Additionally, the uptake of peptides, a critical nutrient source for C. difficile (32), by the Opp and App oligopeptide permeases affects the timing of sporulation, as opp and app inactivation significantly increases sporulation frequencies (33). It is reasonable to consider that other global signaling systems in C. difficile link nutrient availability and/or other environmental conditions to the decision to initiate sporulation.
In this study, we examined the impact that c-di-GMP has on C. difficile sporulation. We show that the overexpression of dccA, a gene encoding a DGC, resulted in decreased sporulation in two important C. difficile strains. The conserved catalytic motif GGDEF was required for DccA-dependent inhibition of C. difficile spore formation, indicating that c-di-GMP metabolic activity is responsible for this phenotype. Finally, we provide evidence that c-di-GMP does not depend on signaling through several known sporulation factors, suggesting that c-di-GMP influences sporulation through an unidentified pathway.
RESULTS
Overexpression of a diguanylate cyclase reduces C. difficile sporulation frequency.
As c-di-GMP affects many physiological processes in C. difficile, we hypothesized that C. difficile sporulation is also influenced by c-di-GMP. To test this hypothesis, dccA, which encodes a DGC in C. difficile (10, 13), was overexpressed on a multicopy plasmid using the nisin-inducible cpr promoter (13, 33, 34) in two different C. difficile backgrounds. We included R20291, which is a clinically prevalent epidemic strain, and 630Δerm, which is a spontaneous erythromycin-sensitive derivative of 630, a clinical isolate that has served as a long-term laboratory model strain (35, 36). Of note, the amino acid sequences of DccA from R20291 and 630Δerm are 100% identical. To assess sporulation frequency, we performed ethanol-resistant sporulation assays in these strains after 24 h of growth (H24) on 70:30 sporulation agar.
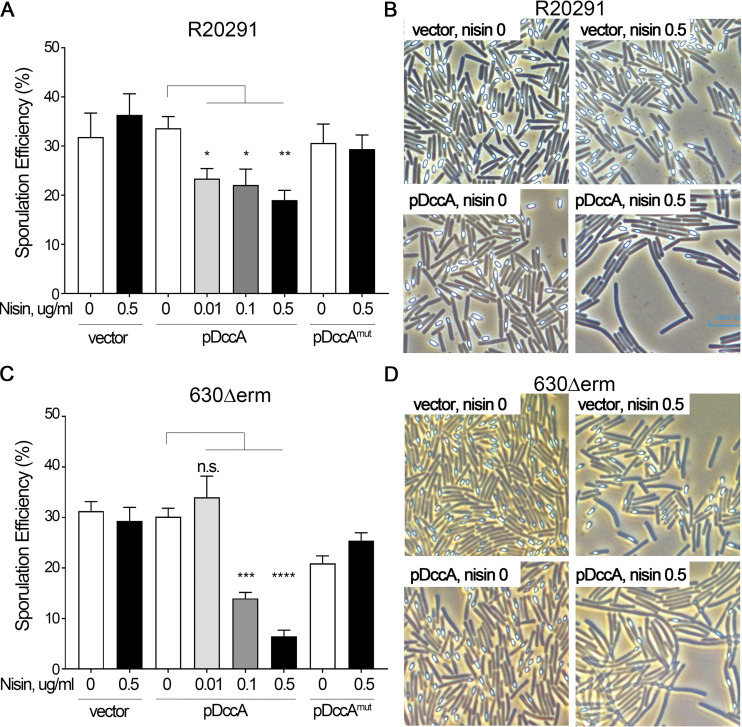
When a plasmid copy of dccA (pDccA) was overexpressed from the nisin-inducible promoter in the R20291 background, the sporulation frequency significantly decreased from 33.7% in the absence of nisin to 19% in the presence of 0.5 μg/ml nisin (Fig. 1A), suggesting that the overexpression of dccA and, presumably, high intracellular levels of c-di-GMP inhibit C. difficile sporulation. To assess whether the decrease in the sporulation frequency was due to the production of c-di-GMP by DccA, we overexpressed a dccA allele encoding a mutated GGDEF domain (AADEF) that is unable to synthesize c-di-GMP (pDccAmut) (13). Here, even at the highest expression level of dccA (0.5 μg/ml nisin), the sporulation efficiency remained unaffected, indicating that the DccA-dependent reduction in the sporulation frequency is due to DccA’s diguanylate cyclase activity. We also visualized sporulation in these strains using phase-contrast microscopy, in which spores appear phase bright and vegetative cells are phase-dark rods. When dccA was overexpressed in R20291, not only were fewer spores visible, but this strain also formed long chains (Fig. 1B; discussed below). The reduced sporulation frequency and cell morphology phenotypes are dependent on increased c-di-GMP concentrations as these effects were not seen in the R20291 strain overexpressing pDccAmut (data not shown).
FIG 1.
Overexpression of dccA inhibits sporulation in R20291 and 630Δerm and is dependent upon a functional cyclic diguanylate (GGDEF) domain. (A and B) Ethanol-resistant sporulation assays (A) and representative phase-contrast micrographs (B) of R20291 pMC211 (RT526), R20291 pDccA (RT527), and R20291 pDccAmut (RT539) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0 to 0.5 μg/ml nisin, as indicated, at H24. (C and D) Ethanol-resistant sporulation assays (C) and representative phase-contrast micrographs (D) of 630Δerm pMC211 (RT762), 630Δerm pDccA (RT763), and 630Δerm pDccAmut (RT764) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0 to 0.5 μg/ml nisin, as indicated, at H24. The means and standard errors of the means from at least three biological replicates are shown. n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (by one-way analysis of variance [ANOVA] with Dunnett’s posttest comparing values to that of uninduced WT pDccA).
When dccA was overexpressed in the 630Δerm background, we observed an ∼4-fold decrease in the sporulation efficiency (29.7% in the absence of nisin to 8.5% with 0.5 μg/ml nisin) (Fig. 1C). This reduction in sporulation frequency in the 630Δerm pDccA strain was dose dependent, with the lowest sporulation frequency occurring at the highest expression level of dccA (0.5 μg/ml nisin). Again, the overexpression of the dccAmut allele resulted in wild-type (WT) levels of sporulation, indicating that the ability of DccA to repress sporulation relies on a functional GGDEF domain and its diguanylate cyclase activity. Similar to R20291, the overexpression of dccA in the 630Δerm background resulted in fewer spores and a change in cell morphology when observed by phase-contrast microscopy, and these phenotypes were also dependent on a functional DccA GGDEF domain (Fig. 1D and data not shown).
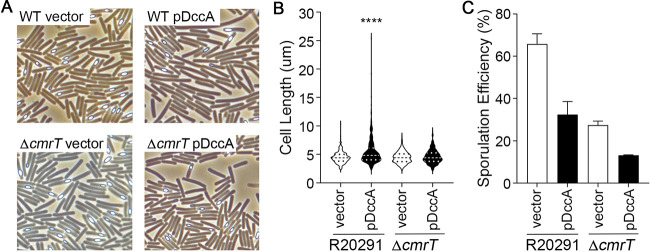
As noted above, the overexpression of dccA resulted in dramatic cell morphology changes in R20291 and 630Δerm that are dependent on increased intracellular concentrations of c-di-GMP (Fig. 1B and D). This change in cell morphology with dccA overexpression was expected and is likely due to the c-di-GMP-dependent increase in the expression of cmrRST, which encodes an atypical signal transduction system that regulates C. difficile colony morphology and motility (37, 38). CmrT promotes bundled cell chaining. To determine whether CmrT is responsible for the formation of long chains at high c-di-GMP concentrations, we overexpressed dccA in the R20291 cmrT background. As predicted, the elongated cells observed at high c-di-GMP concentrations are no longer present in the cmrT mutant (Fig. 2A and B), indicating that c-di-GMP promotes cell chaining through the activation of cmrT expression. CmrT is not involved in the c-di-GMP-dependent inhibition of sporulation, as the sporulation frequency of the cmrT mutant is decreased ∼2-fold, similar to the R20291 parent strain, when dccA is overexpressed (Fig. 2C).
FIG 2.
c-di-GMP-dependent cell chaining is dependent on CmrT. (A and B) Representative phase-contrast micrographs (A) and quantification of cell length using ImageJ (B) of R20291 pMC211 (RT526), R20291 pDccA (RT527), R20291 cmrT pMC211 (RT2283), and R20291 cmrT pDccA (RT2284) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0.5 μg/m nisin at H24. For panel B, cell lengths of 100 bacterial cells from 3 biological replicates were measured (n = 300). The violin plot shows distributions, medians, and quartiles. ****, P < 0.0001 (by one-way ANOVA and Tukey’s posttest compared to all other strains). (C) Ethanol-resistant sporulation assays of R20291 pMC211 (RT526), R20291 pDccA (RT527), R20291 cmrT pMC211 (RT2283), and R20291 cmrT pDccA (RT2284) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0.5 μg/m nisin at H24. The means and standard errors of the means from at least three biological replicates are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (by one-way ANOVA and Tukey’s posttest).
Overexpression of a cyclic diguanylate decreases sporulation-specific gene expression.
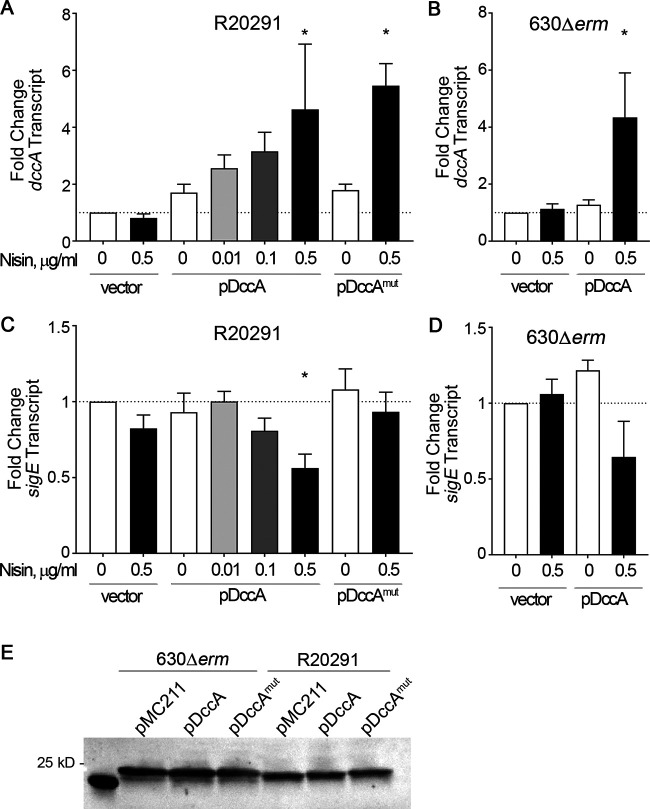
To ensure that dccA and dccAmut transcription are activated in a dose-dependent manner with increasing concentrations of nisin, we measured dccA transcript levels using quantitative reverse transcription-PCR (qRT-PCR). Cells were harvested after 12 h of growth on 70:30 sporulation agar, which marks early stationary phase and the onset of sporulation (39). As expected, dccA and dccAmut transcript levels were increased ∼4- to 6-fold in both R20291 and 630Δerm in the presence of 0.5 μg/ml nisin (Fig. 3A and B), showing that dccA and dccAmut expression levels are consistent in both backgrounds. To better understand the effect of c-di-GMP on early sporulation events in C. difficile, we measured the steady-state transcript levels of an early sporulation-specific gene, sigE (spoIIG), using qRT-PCR. The transcription of sigE is dependent on active, phosphorylated Spo0A (40). The relative expression levels of sigE in both R20291 pDccA and 630Δerm pDccA grown on 0.5 μg/ml nisin were decreased ∼2-fold compared to the levels in their respective parent strains (Fig. 3C and D). The decreased sigE transcript levels in R20291 pDccA were dependent upon a functional GGDEF domain as sigE transcript levels were unchanged when dccAmut was overexpressed in R20291 (Fig. 3C).
FIG 3.
Overexpression of dccA decreases Spo0A-dependent gene expression. (A and C) qRT-PCR analyses of dccA (A) and sigE (C) transcript levels in R20291 pMC211 (RT526), R20291 pDccA (RT527), and R20291 pDccAmut (RT539) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0 to 0.5 μg/ml nisin, as indicated, at H12. (B and D) qRT-PCR analyses of dccA (B) and sigE (D) transcript levels in 630Δerm pMC211 (RT762) and 630Δerm pDccA (RT763) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol in the absence or presence of 0.5 μg/ml nisin at H12. The means and standard errors of the means from at least three biological replicates are shown. *, P < 0.05 (by one-way ANOVA with Dunnett’s posttest comparing values to that of the uninduced WT vector). (E) Anti-Spo0A Western blot analysis of 630Δerm pMC211 (RT762), 630Δerm pDccA (RT763), 630Δerm pDccAmut (RT764), R20291 pMC211 (RT526), R20291 pDccA (RT527), and R20291 pDccAmut (RT539) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0.5 μg/ml nisin at H12.
To further assess how c-di-GMP impacts early sporulation events, we measured the transcript levels of additional early-sporulation-specific genes, including spo0A, murG (a Spo0A-dependent gene), sigF (encodes the early sporulation-specific sigma factor that is expressed in the forespore compartment), gpr (a SigF-dependent gene), and spoIID (a SigE-dependent gene). The transcript levels of early sporulation genes were minimally affected in the R20291 background; however, with the exception of spo0A, there was a significant decrease in early sporulation gene expression when dccA was overexpressed in the 630Δerm background (Table 1). These results may reflect the stronger impact on sporulation that dccA overexpression has on 630Δerm than on R20291. Mirroring spo0A transcript levels in R20291 and 630Δerm, Spo0A protein levels were unchanged regardless of dccA overexpression (Fig. 3E). The lack of changes in spo0A transcript and protein levels is expected given that Spo0A activity is controlled by posttranslational phosphorylation. Furthermore, Spo0A autoregulation has not been observed in other C. difficile sporulation mutants, including the hypersporulating opp app and oligosporogenous rstA mutants (33, 39), despite affecting Spo0A activity and Spo0A-dependent gene expression. These data indicate that the overexpression of dccA reduces early sporulation gene expression and confirm that the increased production of c-di-GMP is responsible for reduced sporulation in C. difficile.
TABLE 1.
Effect of dccA overexpression on sporulation-specific gene expression in R20291 and 630Δerm
| Transcripta | Strainb | Mean fold change ± SDc |
||
|---|---|---|---|---|
| pMC211 | pDccA | pDccAmut | ||
| spo0A | R20291 | 0.91 ± 0.12 | 0.96 ± 0.27 | 0.77 ± 0.11 |
| 630Δerm | 0.79 ± 0.07 | 0.96 ± 0.16 | ND | |
|
murG (Spo0A dependent) |
R20291 | 0.85 ± 0.08 | 0.72 ± 0.08 | 0.79 ± 0.06 |
| 630Δerm | 1.19 ± 0.15 | 0.60 ± 0.05 | ND | |
| sigF | R20291 | 0.97 ± 0.20 | 1.15 ± 0.38 | 0.95 ± 0.23 |
| 630Δerm | 0.74 ± 0.10 | 0.47 ± 0.11 | ND | |
|
gpr (SigF dependent) |
R20291 | 0.82 ± 0.10 | 0.6 ± 0.17 | 0.9 ± 0.10 |
| 630Δerm | 1.16 ± 0.19 | 0.44 ± 0.15 | ND | |
|
spoIID (SigE dependent) |
R20291 | 0.73 ± 0.14 | 0.48 ± 0.08 | 0.67 ± 0.13 |
| 630Δerm | 1.32 ± 0.13 | 0.72 ± 0.06 | ND | |
All mean fold change values are relative to the respective parent strain containing pMC211 grown in the presence of no nisin.
Strains were harvested at H12 from 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0.5 μg/ml nisin.
Overexpression of dccA increases c-di-GMP-dependent gene expression in a dose-dependent manner on 70:30 sporulation agar.
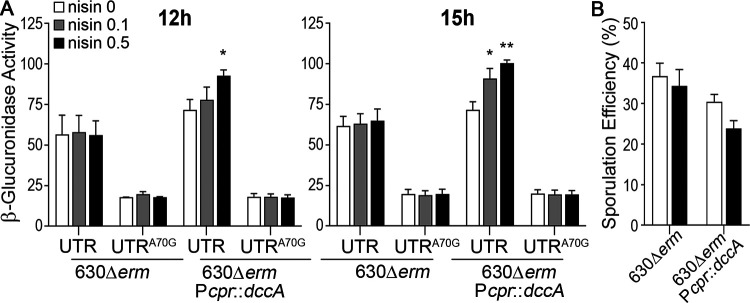
We previously utilized high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) to measure the intracellular concentration of c-di-GMP when dccA is overexpressed from the nisin-inducible promoter (13). To assess the relative increase in intracellular c-di-GMP with dccA induction during growth on 70:30 sporulation agar, we employed the previously described reporter controlled by the regulatory region of the pilA1 locus: the pilA1 promoter (PpilA) and the 5′ untranslated region (UTR) containing a c-di-GMP riboswitch (41). c-di-GMP directly and positively regulates pilA transcription via the c-di-GMP-responsive riboswitch located in the pilA 5′ UTR (16). This PpilA1-UTR-gusA reporter was introduced into 630Δerm with chromosomal nisin-inducible dccA (41). As a control, we also utilized a PpilA1-UTRA70G-gusA reporter where a single nucleotide substitution renders the riboswitch unresponsive to c-di-GMP (16, 41). We measured PpilA1-UTR-gusA reporter activity after growth on 70:30 sporulation agar in the presence of no nisin or 0.1 μg/ml or 0.5 μg/ml nisin at H12 and H15. These time points represent early stationary phase, at the point where the sporulation regulatory cascade is initiated and sporulation-specific gene expression is active. Without induction, basal expression from the chromosomally encoded Pcpr-dccA construct resulted in a slight increase (∼1.2-fold) in β-glucuronidase activity compared to that of the parent strain at both time points (Fig. 4A). We observed ∼1.4-fold and ∼1.6-fold increases in β-glucuronidase activity in the presence of 0.1 μg/ml and 0.5 μg/ml nisin, respectively, compared to the parental control. As expected, the c-di-GMP-blind PpilA1-UTRA70G-gusA reporter exhibited significantly reduced and constitutive activity under all tested conditions (Fig. 4A).
FIG 4.
Overexpression of dccA results in a dose-dependent increase in c-di-GMP-dependent gene expression. (A) β-Glucuronidase reporter activity in 630Δerm pPpilA1-UTR-gusA (RT1050), 630Δerm pPpilA1-UTRA70G-gusA (RT1051), 630Δerm::PcprA-dccA pPpilA1-UTR-gusA (RT1054), and 630Δerm::PcprA-dccA pPpilA1-UTRA70G-gusA (RT1055) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol in the absence or presence of 0.1 μg/ml or 0.5 μg/ml nisin at H12 and H15. (B) Ethanol-resistant sporulation assays of 630Δerm and 630Δerm::PcprA-dccA (RT993) grown on 70:30 sporulation agar in the presence or absence of 0.5 μg/ml nisin at H24. The means and standard errors of the means from three biological replicates are shown. *, P < 0.05; **, P < 0.01 (by one-way ANOVA and Dunnett’s posttest comparing values to that of the WT vector, with 0.5 μg/ml nisin).
Because dccA is overexpressed from the chromosome in these reporter assays and not from a plasmid as we did in our previous experiments, we assessed the impact on sporulation frequency when dccA expression was induced from the chromosome. The sporulation frequency of 630Δerm::PcprA-dccA grown on 70:30 sporulation agar plates supplemented with 0.5 μg/ml nisin was ∼1.44-fold decreased compared to the 630Δerm parent (Fig. 4B). As anticipated, the decrease in the sporulation efficiency was muted compared to dccA overexpression on the plasmid, likely due to differences in copy numbers. However, increasing the intracellular c-di-GMP concentration through dccA overexpression impacts both c-di-GMP-specific gene regulation and sporulation frequency. Altogether, these data confirm that c-di-GMP levels are modestly induced to physiologically relevant concentrations that affect c-di-GMP-dependent physiological processes under these conditions.
Deletion of dccA results in variable sporulation frequencies in R20291 and 630Δerm.
Sporulation may be impacted by one or a subset of c-di-GMP metabolic enzymes. C. difficile 630 encodes 37 proteins containing GGDEF and/or EAL domains, and R20291 encodes 31 (10, 11, 35). The overexpression of dccA bypasses the endogenous control of c-di-GMP. To better understand the c-di-GMP regulatory mechanism, we next asked whether a null mutation in dccA alone affects the C. difficile sporulation frequency. To test this hypothesis, we employed the pseudo-suicide vectors pMSR and pMSR0 (tailored for the 630 and R20291 backgrounds, respectively), which take advantage of allelic exchange and an inducible C. difficile toxin-antitoxin system to create a markerless gene deletion (42).
The R20291 ΔdccA mutant produced slightly more spores than R20291 (42.3% in R20291 ΔdccA versus 31.7% in the isogenic parent) (Fig. 5A). However, the sporulation frequencies were highly variable, and the differences between strains were not statistically significant. The R20291 ΔdccA mutant was complemented by integrating the dccA gene into the chromosome using the conjugative transposon Tn916. dccA is the second gene in a two-gene operon in R20291 and 630Δerm, and the promoter of the upstream gene, CD1421, was used to drive dccA transcription in the complementation construct. The sporulation frequency of R20291 ΔdccA Tn916::PCD1421-dccA was reduced (34.7%) compared to that of the dccA mutant, but again, the data were variable and not statistically significant.
FIG 5.
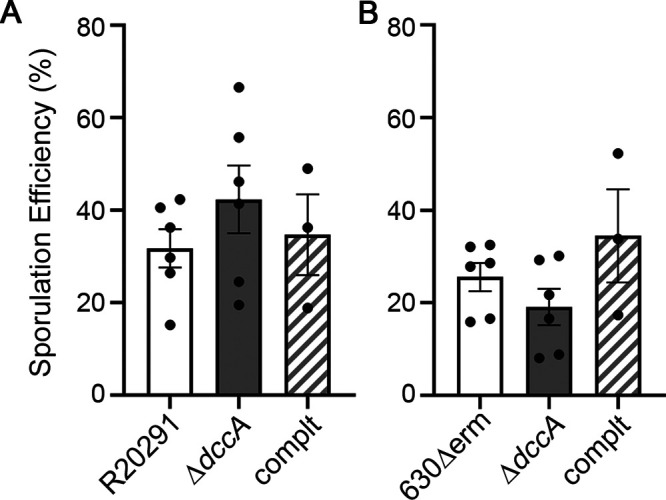
A null dccA mutation variably affects R20291 and 630Δerm sporulation. Shown are data from ethanol-resistant sporulation assays of R20291, R20291 ΔdccA (RT2656), and R20291 ΔdccA Tn916::PCD1421-dccA (MC1961) (A) and 630Δerm, 630Δerm ΔdccA (RT2703), and 630Δerm ΔdccA Tn916::PCD1421-dccA (MC1960) (B) grown on 70:30 sporulation agar at H24. The means and standard errors of the means from at least three biological replicates are shown. No significant differences were determined by one-way ANOVA. complt, complemented strain.
The 630Δerm ΔdccA mutant exhibited a slightly reduced sporulation frequency compared to the 630Δerm parent (19.1% in the dccA mutant versus 25.6% in the parent strain) (Fig. 5B), an opposite trend compared to that of the R20291 ΔdccA mutant. The complemented strain 630Δerm ΔdccA Tn916::PCD1421-dccA showed an increased sporulation frequency (34.4%). But, as in the R20291 background, the sporulation frequencies were variable and did not achieve statistical significance. Altogether, these data suggest that c-di-GMP synthesis by DccA does not greatly and/or consistently contribute to sporulation initiation under the conditions tested or that other c-di-GMP metabolic enzymes are redundant with or compensate for the loss of DccA.
c-di-GMP likely does not signal through known C. difficile sporulation factors.
To attempt to identify the regulatory pathway(s) through which c-di-GMP influences sporulation in C. difficile, we overexpressed dccA in several well-studied 630Δerm sporulation mutants and performed ethanol-resistant sporulation assays after 24 h of growth on 70:30 sporulation agar. We included the parent 630Δerm strain overexpressing dccA in the absence and presence of 0.5 μg/ml nisin as a reference in these experiments (Fig. 6A).
FIG 6.
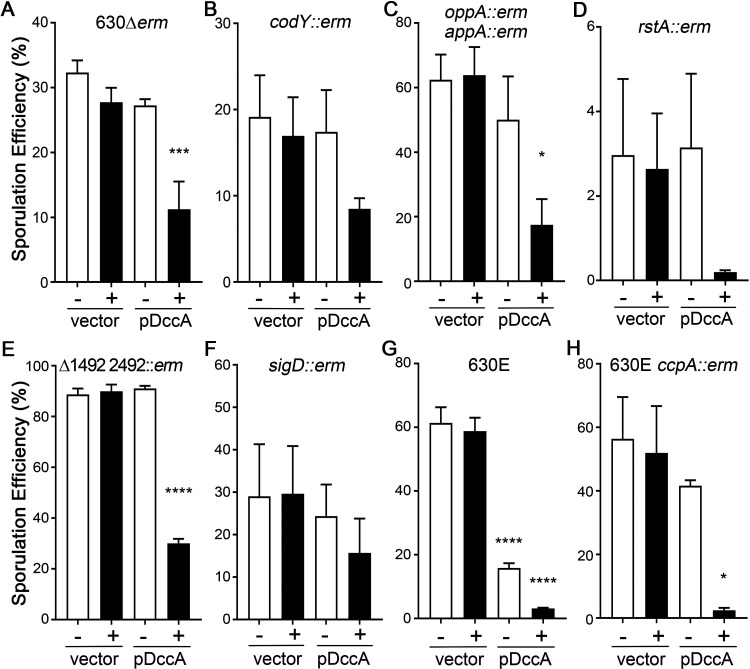
c-di-GMP does not inhibit sporulation through known sporulation factors. Shown are data from ethanol-resistant sporulation assays of 630Δerm pMC211 (RT762) and 630Δerm pDccA (RT763) (A), 630Δerm codY::erm pMC211 (MC947) and 630Δerm codY::erm pDccA (MC948) (B), 630Δerm oppB::erm appA::erm pMC211 (MC924) and 630Δerm oppB::erm appA::erm pDccA (MC925) (C), 630Δerm rstA::erm pMC211 (MC926) and 630Δerm rstA::erm pDccA (MC927) (D), 630Δerm ΔCD1492 CD2492::erm pMC211 (MC928) and 630Δerm ΔCD1492 CD2492::erm pDccA (MC929) (E), 630Δerm sigD::erm pMC211 (MC864) and 630Δerm sigD::erm pDccA (MC865) (F), 630E pMC211 (MC943) and 630E pDccA (MC944) (G), and 630E ccpA::erm pMC211 (MC945) and 630E ccpA::erm pDccA (MC946) (H) grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol in the absence or presence of 0.5 μg/ml nisin, as indicated, at H24. The means and standard errors of the means from at least three biological replicates are shown. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001 (by one-way ANOVA with Dunnett’s posttest comparing values to those of the respective parent strains with an uninduced vector). Note that the y axes for each panel vary depending on the sporulation frequency of the strain tested.
First, we assessed the effect of dccA overexpression on 630Δerm codY and 630Δerm opp app mutants. CodY is a global transcriptional regulator that binds to target DNA at high intracellular concentrations of the effectors GTP and branched-chain amino acids (BCAAs) (43). The loss of GTP and BCAA binding changes the conformation of CodY, the differential expression of metabolic pathways, and other physiological processes, including toxin production and sporulation (29, 31, 44, 45). The opp and app operons each encode oligopeptide permeases that import small, heterogeneous peptides into the cell (46, 47). The inactivation of these permeases in C. difficile results in significantly increased sporulation, suggesting that limited nutrient uptake triggers sporulation (33). The regulatory pathways and molecular mechanisms by which CodY, Opp, and App affect sporulation are not fully understood, although null mutations in these loci affect sporulation timing and result in increased sporulation frequencies (31, 33). The sporulation frequencies of the codY mutant and the opp app double mutant decreased when dccA was overexpressed using 0.5 μg/ml nisin (2-fold and 3.7-fold, respectively, compared to each strain’s vector controls grown in 0.5 μg/ml nisin) (Fig. 6B and C). These results indicate that c-di-GMP does not require CodY or the Opp and App oligopeptide permeases to inhibit C. difficile sporulation.
Next, we ascertained whether RstA or the phosphotransfer proteins CD1492 and CD2492 are part of the regulatory pathway that c-di-GMP employs to control sporulation. RstA is a multifunctional protein that serves as a transcriptional regulator and, through a separate domain, positively influences C. difficile sporulation initiation via an unknown mechanism (39). The overexpression of dccA in the rstA background resulted in an ∼13-fold-decreased sporulation frequency compared to that of the rstA mutant containing the vector control (Fig. 6D), indicating that RstA is not necessary for c-di-GMP-dependent inhibition of sporulation. CD1492 and CD2492 are predicted histidine kinases that function as phosphotransfer proteins to repress sporulation and are hypothesized to directly impact Spo0A phosphorylation (28, 48). A CD1492 CD2492 double mutant exhibited significantly increased sporulation compared to the 630Δerm parent (Fig. 6A and E), and the overexpression of dccA in the CD1492 CD2492 background reduced the sporulation frequency ∼3-fold (Fig. 5E), demonstrating that CD1492 and CD2492 are not a required part of the c-di-GMP signaling pathway.
We also asked whether c-di-GMP signals through SigD to control C. difficile sporulation. SigD is the flagellar alternative sigma factor that is required for both motility and efficient toxin production in C. difficile (14, 39, 49). Although a null sigD mutation does not affect sporulation under these conditions (comparing the 630Δerm and 630Δerm sigD vector control strains) (Fig. 6A and F) (39, 49), we chose to investigate the sigD mutant because c-di-GMP directly represses sigD transcription, inhibiting C. difficile motility and toxin production (13, 15, 50). The sporulation frequency was reduced only 1.9-fold when dccA was overexpressed in the sigD background, and the difference was not statistically significant (Fig. 6F). Furthermore, in the 630E background, where the phase-variable switch that controls the transcription of the flagellar operon is locked off, resulting in low sigD expression levels (51–53), the overexpression of dccA also significantly decreased sporulation by ∼20-fold (Fig. 6G). The DccA-dependent effect was not as dramatic in the 630Δerm sigD background as in the other mutant backgrounds tested; however, these data altogether suggest that c-di-GMP does not primarily influence sporulation through SigD.
Finally, we tested whether c-di-GMP affects sporulation frequency through the catabolite control protein CcpA. As a transcriptional regulator, CcpA responds to glycolytic carbohydrate availability to regulate carbon and nitrogen metabolism as well as other physiological processes, including sporulation and toxin production (30, 54). The sporulation frequency was again significantly decreased by ∼20-fold when dccA was overexpressed in the 630E ccpA mutant (Fig. 6H), indicating that CcpA is not involved in c-di-GMP signaling to control sporulation initiation. Altogether, these data indicate that c-di-GMP does not significantly impact C. difficile sporulation through these known regulatory factors under the conditions tested.
DISCUSSION
In this work, we set out to determine the impact of the bacterial second messenger c-di-GMP on C. difficile sporulation. We found that the overexpression of dccA, encoding a DGC that synthesizes high intracellular levels of c-di-GMP when overexpressed (13), resulted in significant decreases in early sporulation gene expression and spore formation in the epidemic R20291 and historical 630Δerm strains. The conserved catalytic GGDEF motif was required for DccA-dependent inhibition of sporulation, indicating that the diguanylate cyclase synthase activity of DccA is responsible for decreased sporulation. Consistent with this result, sporulation was inhibited in a dose-dependent manner, as higher transcript levels of dccA coincided with fewer detected transcripts of sigE, which encodes an early sporulation-specific sigma factor.
Because DccA overexpression drastically altered the sporulation frequency in R20291 and 630Δerm, we had anticipated a stronger sporulation phenotype in the corresponding dccA mutants. However, there are many additional DGCs encoded in the C. difficile genome as well as many PDEs, and these likely contribute to the intracellular concentration of c-di-GMP also, as most are catalytically active (10). Given the sheer number of encoded DGCs and that their functions may inherently exhibit redundancy, it is, in retrospect, unsurprising that the deletion of a single GGDEF domain protein does not significantly impact sporulation. Any contribution of DccA to the intracellular c-di-GMP pool may be masked by the redundant functions of similar proteins. As such, we previously found that the overexpression of DccA resulted in modest increases in the transcript levels of several encoded PDEs, suggesting that C. difficile is able to somewhat compensate for high levels of intracellular c-di-GMP (55). It is also possible that changes in c-di-GMP levels upon the loss of DccA are compensated for by the upregulation or activation of other DGCs, the downregulation or inhibition of PDEs, or both. Importantly, previous studies have shown that distinct DGCs control different c-di-GMP-regulated phenotypes, suggesting that localized pools of c-di-GMP influence only a subset of phenotypes (56, 57). It is plausible that the deletion of one or more of the other encoded DGCs in C. difficile could result in a stronger impact on the sporulation frequency. A recent report describes that the overexpression of a phosphodiesterase containing an EAL domain increases sporulation and that the deletion of that PDE decreases sporulation in C. difficile UK1, an epidemic strain that is nearly identical to R20291, corroborating our findings (58).
Interestingly, two early C. difficile sporulation regulators that are orthologous to the B. subtilis SinR transcriptional repressor regulate C. difficile intracellular c-di-GMP levels. Null mutations in the two C. difficile SinR orthologs, known as SinRR′ in R20291 and CD2214-CD2215 in 630Δerm, result in increased dccA transcription and c-di-GMP levels and an asporogenous phenotype (20, 59). Furthermore, the deletion of CD2214-CD2215 resulted in the differential expression of additional DGC and PDE genes in C. difficile 630Δerm (20). It will be intriguing to determine if the effect of SinRR′ on C. difficile sporulation occurs through alteration of c-di-GMP levels by regulating DGC/PDE gene expression or if SinRR′ affects sporulation initiation through multiple regulatory pathways.
Identification of the c-di-GMP effector(s) that mediates c-di-GMP-dependent sporulation regulation in C. difficile is of great interest. A variety of c-di-GMP receptors that directly bind to c-di-GMP have been characterized in bacteria. These encompass a number of protein-based receptors, including proteins containing degenerate GGDEF and/or EAL domains, and two distinct types of riboswitches that alter downstream gene expression in response to c-di-GMP binding (8). C. difficile encodes a single PilZ domain protein (6), a domain that often directly binds c-di-GMP (60, 61), and a type IV pilus PilB ATPase similar to orthologs that have been shown to bind c-di-GMP (62), but there are no other known or predicted c-di-GMP protein receptors reported in C. difficile (8). C. difficile encodes at least 11 functional riboswitches that alter gene expression in response to c-di-GMP and contains 5 additional predicted riboswitches (15, 55). None of these riboswitches appear to affect the expression of sporulation-related genes; however, the conditions used in this study do not support efficient sporulation initiation in C. difficile (55). Performing transcriptomics under conditions that favor sporulation may provide insights into which regulatory pathway(s) or factor(s) is required to mediate this c-di-GMP-dependent response.
The regulatory pathway that c-di-GMP utilizes to influence sporulation remains unknown. The decrease in spore formation mediated by dccA overexpression does not appear to signal through CodY, CcpA, the Opp or App oligopeptide permeases, RstA, or the CD1492 and CD2492 phosphotransfer proteins. Although the effect of DccA-mediated inhibition of sporulation was slightly decreased in the sigD mutant, c-di-GMP is unlikely to signal solely through SigD under these conditions. Given that we know that c-di-GMP directly affects sigD transcription in C. difficile through the Cdi-1-3 riboswitch (13, 15, 50), it is attractive to hypothesize that SigD might have a role in sporulation. It may be possible that decreased levels of SigD are necessary for c-di-GMP to affect sporulation; in this case, testing the c-di-GMP-dependent effects on sporulation in a sigD mutant or in JIR8094, a phase-off, nonmotile strain with low SigD levels, may not directly answer this question. Thus far, there is no published evidence of a regulatory role for SigD in sporulation (39, 49, 55).
The impact of c-di-GMP on sporulation has been investigated in only a few other spore-forming bacteria. Interestingly, using an mCherry reporter fused to a c-di-GMP-regulated riboswitch, high c-di-GMP levels were observed in sporulating B. subtilis cells, suggesting a correlation (63), but the impact of c-di-GMP on B. subtilis sporulation has remained relatively unexplored. Studies in Bacillus thuringiensis and Bacillus anthracis found no effect on the sporulation efficiency when any of the catalytically active GGDEF/EAL/HD-GYP-encoding genes were individually deleted (64, 65). These studies in Bacillus sp. further underscore the differences between C. difficile and other endospore-forming bacteria in their early sporulation regulatory networks. Finally, direct c-di-GMP regulation of sporulation has been identified only in Streptomyces sp., where c-di-GMP inhibits spore formation directly by antagonizing the sporulation-specific sigma factor WhiG and binding directly to the transcriptional regulator BldD (66, 67). The contribution of c-di-GMP to sporulation remains an understudied field.
Utilizing c-di-GMP to inhibit sporulation may be advantageous to C. difficile, as c-di-GMP can be rapidly degraded when environmental and intracellular conditions favor sporulation. The c-di-GMP metabolic activity of a protein is often controlled by the corresponding domains encoded within the same protein. Identifying the DGCs and PDEs that affect C. difficile sporulation and investigating the function of their associated domains may reveal the environmental and intracellular signals that promote or delay sporulation. Finally, the finding that c-di-GMP is a regulator of early sporulation events in C. difficile creates new research opportunities for discovering the potentially novel regulatory pathways, c-di-GMP effectors, and molecular mechanisms that control spore formation in this significant pathogen.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used for this study are listed in Table 2. Clostridioides difficile was routinely cultured in brain heart infusion-supplemented (BHIS) medium in a 37°C vinyl anaerobic chamber (Coy) with an atmosphere of 5% CO2, 10% H2, and 85% N2 (68). C. difficile cultures were supplemented with 2 to 10 μg/ml thiamphenicol as necessary for plasmid maintenance. Escherichia coli strains were grown aerobically at 37°C in LB with 100 μg/ml ampicillin and/or 10 to 20 μg/ml chloramphenicol, and counterselection against E. coli after conjugation with C. difficile was performed using 100 μg/ml kanamycin (13).
TABLE 2.
Bacterial strains and plasmids
| Strain (lab annotation) or plasmid | Relevant genotype or feature(s) | Source and/or reference(s) |
|---|---|---|
| Strains | ||
| E. coli | ||
| HB101 | F− mcrB mrr hsdS20(rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 pRK24 | B. Dupuy |
| B. subtilis | ||
| BS49 | CU2189::Tn916 | P. Mullany |
| MC1959 | BS49 Tn916::PCD1421-dccA | This study |
| C. difficile | ||
| 630Δerm | Erms derivative of strain 630 | N. Minton; 36 |
| R20291 | 35 | |
| JIR8094 | Erms derivative of strain 630 (630E) | B. Dupuy; 75 |
| JIR8094 ccpA::erm | 54 | |
| RT526 | R20291 pMC211 | 13 |
| RT527 | R20291 pDccA | 13 |
| RT539 | R20291 pDccAmut | This study |
| RT762 | 630Δerm pMC211 | This study |
| RT763 | 630Δerm pDccA | This study |
| RT764 | 630Δerm pDccAmut | This study |
| RT993 | 630Δerm::PcprA-dccA | 41 |
| RT1050 | 630Δerm pPpilA1-UTR-gusA | 41 |
| RT1051 | 630Δerm pPpilA1-UTRA70G-gusA | 41 |
| RT1054 | 630Δerm::PcprA-dccA pPpilA1-UTR-gusA | 41 |
| RT1055 | 630Δerm::PcprA-dccA pPpilA1-UTRA70G-gusA | 41 |
| RT1075 | 630Δerm sigD::erm | 39 |
| RT2257 | R20291 ΔcmrT | 37 |
| RT2283 | R20291 ΔcmrT pMC211 | This study |
| RT2284 | R20291 ΔcmrT pDccA | This study |
| RT2656 | R20291 ΔdccA | This study |
| RT2703 | 630Δerm ΔdccA | This study |
| MC307 | 630Δerm oppB::erm appA::erm | 33 |
| MC364 | 630Δerm codY::erm | 31 |
| MC391 | 630Δerm rstA::erm | 39 |
| MC802 | 630Δerm ΔCD1492 CD2492::erm | This study |
| MC864 | 630Δerm sigD::erm pMC211 | This study |
| MC865 | 630Δerm sigD::erm pDccA | This study |
| MC924 | 630Δerm oppB::erm appA::erm pMC211 | This study |
| MC925 | 630Δerm oppB::erm appA::erm pDccA | This study |
| MC926 | 630Δerm rstA::erm pMC211 | This study |
| MC927 | 630Δerm rstA::erm pDccA | This study |
| MC928 | 630Δerm ΔCD1492 CD2492::erm pMC211 | This study |
| MC929 | 630Δerm ΔCD1492 CD2492::erm pDccA | This study |
| MC943 | JIR8094 pMC211 | This study |
| MC944 | JIR8094 pDccA | This study |
| MC945 | JIR8094 ccpA::erm pMC211 | This study |
| MC946 | JIR8094 ccpA::erm pDccA | This study |
| MC947 | 630Δerm codY::erm pMC211 | This study |
| MC948 | 630Δerm codY::erm pDccA | This study |
| MC1960 | 630Δerm ΔdccA Tn916::PCD1421-dccA | This study |
| MC1961 | R20291 ΔdccA Tn916::PCD1421-dccA | This study |
| Plasmids | ||
| pRK24 | Tra+ Mob+; bla tet | 76 |
| pSMB47 | Tn916 integrational vector; catP erm | 77 |
| pCR2.1 | bla kan | Invitrogen |
| pMSR | Allelic exchange in C. difficile 630 | 42 |
| pMSR0 | Allelic exchange in C. difficile R20291 | 42 |
| pMSR::ΔdccA | dccA deletion construct in pMSR | This study |
| pMSR0::ΔdccA | dccA deletion construct in pMSR0 | This study |
| pCE240 | C. difficile Targetron construct based on pJIR750ai (group II intron; ermB::RAM ltrA); catP | C. Ellermeier |
| pMC330 | pCR2.1 with group II intron targeted to CD2492 | This study |
| pMC333 | pCE240 with CD2492-targeted intron | This study |
| pMC336 | pMC123 with CD2492-targeted intron | This study |
| pMC211 | E. coli-C. difficile shuttle vector; bla catP | 13, 33 |
| pDccA | CD1420 from 630 in pMC211 | 13 |
| pDccAmut | CD1420 (AADEF) in pMC211 | 13 |
| pMC1094 | PCD1421-dccA in pSMB47 | This study |
| pPpilA1-UTR-gusA | 41 | |
| pPpilA1-UTRA70G-gusA | 41 | |
Strain and plasmid construction.
C. difficile strains 630 (GenBank accession no. NC_009089.1) and R20291 (GenBank accession no. FN545816.1) were used as the basis for primer design and PCR amplification (oligonucleotides used in this study are listed in Table 3). The dccA mutants were constructed using the pseudo-suicide vectors pMSR and pMSR0 (42). Upstream and downstream homology regions were amplified from the 630Δerm genome with primer pairs R2928/R2929 and R2930/R2931, respectively. The fragments were Gibson assembled (New England BioLabs [NEB]) into SalI/XhoI-digested pMSR to create pMSR::ΔdccA. Similar fragments were amplified from the R20291 chromosome using the same primers and assembled into pMSR0 to create pMSR0::ΔdccA. Chloramphenicol-resistant colonies were confirmed by PCR with plasmid-specific primer pair R838/R1832 (pMSR) or R2743/R2744 (pMSR0).
TABLE 3.
Oligonucleotides
| Primer | Sequence (5′→3′) | Reference, source, or use (reference) |
|---|---|---|
| CD1420qF | 5′-AAGAAACTCCCTGATAATATTGCTAA | 13 |
| CD1420qR | 5′-ACATTCCAATAGCTTGTAGTATCTTT | 13 |
| EBSu | 5′-CGAAATTAGAAACTTGCGTTCAGTAAAC | Sigma-Aldrich |
| oMC44 | 5′-CTAGCTGCTCCTATGTCTCACATC | Forward primer for rpoC (34) |
| oMC45 | 5′-CCAGTCTCTCCTGGATCAACTA | Reverse primer for rpoC (34) |
| oMC309 | 5′-GGAGAATACAGAGATTTGATTGATTC | Forward primer for CD2492::erm verification |
| oMC317 | 5′-AAAAGCTTTTGCAACCCACGTCGATCGTGAAGTGATCTTAATCGTGCGCCCAGATAGGGTG | IBS for CD2492-targeted intron |
| oMC318 | 5′-CAGATTGTACAAATGTGGTGATAACAGATAAGTCTTAATCTCTAACTTACCTTTCTTTGT | EBS1 for CD2492-targeted intron |
| oMC319 | 5′-CGCAAGTTTCTAATTTCGATTATCACTCGATAGAGGAAAGTGTCT | EBS2 for CD2492-targeted intron |
| oMC338 | 5′-TCCCATTTGCCTTTATTTGAACTTGA | Reverse primer for CD2492::erm verification (39) |
| oMC339 | 5′-GGGCAAATATACTTCCTCCTCCAT | Forward primer for sigE (CD2643) (33) |
| oMC340 | 5′-TGACTTTACACTTTCATCTGTTTCTAGC | Reverse primer for sigE (CD2643) (33) |
| oMC2910 | 5′-GACCACACCCGTCCTGTGGATCCCCATCTCTATGTAATATTTTTCATATTAAAACTGATTTC | Forward primer for PCD1421 |
| oMC2911 | 5′-CTTTAAACATATTAATTTCTCCAAAATAAAATACTTTGTACTGGTATTCCTCCATAAGATACTTTAAATTTTG | Reverse primer for PCD1421 |
| oMC2912 | 5′-CAAAATTTAAAGTATCTTATGGAGGAATACCAGTACAAAGTATTTTATTTTGGAGAAATTAATATGTTTAAAG | Forward primer for dccA (CD1420) |
| oMC2913 | 5′-CCGCCGCAAGGAATGGTGCATGCTTAATAATCATTTTTATCAAATTTTTTCTTGTTTTTCTCC | Reverse primer for dccA (CD1420) |
| R838 | 5′-GTAAAACGACGGCCAGT | Reverse screening primer for pMSR |
| R1832 | 5′-TATTTCGATGCCCTGGACT | Forward screening primer for pMSR |
| R2743 | 5′-GTGTTATCAATTGCACTACTCATGG | Forward screening primer for pMSR0 |
| R2744 | 5′-GTTGAACCATTAGCTAAGGATTCAG | Reverse screening primer for pMSR0 |
| R2926 | 5′-CTGATATAGGAAAATCTTTAATAGAGAAG | Forward screening primer for dccA chromosomal deletion |
| R2927 | 5′-TCCATGAACTCATCATATGTGTATCC | Reverse screening primer for dccA chromosomal deletion |
| R2928 | 5′-TTCGGATCCTCTAGAGTCGACTGCAAGATATGAAAAAACTAAGAGC | Forward primer for upstream fragment of dccA deletion construct |
| R2929 | 5′-CAAATTTTTTCTTGTTTTTCTCCATATTAATTTCTCCAAAATAAAATACTTTGTACTAG | Reverse primer for upstream fragment of dccA deletion construct |
| R2930 | 5′-GTATTTTATTTTGGAGAAATTAATATGGAGAAAAACAAGAAAAAATTTGATAAAAATG | Forward primer for downstream fragment of dccA deletion construct |
| R2931 | 5′-ATGTCTGCAGGCCTCGAGGAGTATGTACTATCATCATTGCTACC | Reverse primer for downstream fragment of dccA deletion construct |
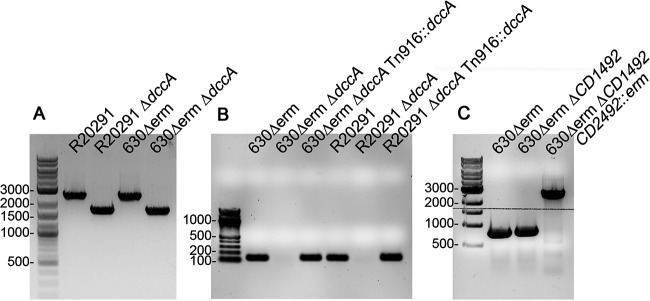
To create the dccA mutants, the pMSR::ΔdccA and pMSR0::ΔdccA plasmids were transformed into E. coli HB101 pRK24 for conjugation with C. difficile 630Δerm and R20291, respectively. Subsequent steps were done essentially as previously described (42). Briefly, large thiamphenicol- and kanamycin-resistant colonies, which presumably had integrated the plasmid into the chromosome to allow for more rapid growth, were streaked again on BHIS medium with 10 μg/ml thiamphenicol and 100 μg/ml kanamycin to ensure purity. Next, large colonies were streaked onto BHIS medium with 100 ng/ml anhydrotetracycline (ATc) to induce the expression of the toxin gene and eliminate bacteria that still contained the vector. Colonies were screened for the 0.8-kb deletion of dccA using primer pair R2926/R2927 (Fig. 7A). Genomic DNA was isolated from potential mutants, and the dccA region was PCR amplified using primer pair R2926/R2927 and sequenced to confirm the integrity of the sequence.
FIG 7.
PCR verification of the constructed strains. (A) PCR confirmation of ΔdccA in the R20291 and 630Δerm backgrounds using primer pair R2926/R2927. Shown are strains R20291, R20291 ΔdccA (RT2656), 630Δerm, and 630Δerm ΔdccA (RT2703). The expected PCR products’ sizes are 2.5 kb for the wild-type allele and 1.7 kb for the deletion mutants. (B) PCR verification of Tn916::PCD1421-dccA chromosomal integration in 630Δerm and R20291 using the internal qRT-PCR dccA primer pair CD1420qF/CD1420qR. Shown are strains 630Δerm ΔdccA (RT2703), 630Δerm ΔdccA Tn916::PCD1421-dccA (MC1960), R20291, R20291 ΔdccA (RT2656), and R20291 ΔdccA Tn916::PCD1421-dccA (MC1961). The expected PCR product size is 140 bp. (C) PCR confirmation of the CD2492-targeted intron in the 630Δerm ΔCD1492 background (MC674). Shown are strains 630Δerm, 630Δerm ΔCD1492 (MC674), and 630Δerm ΔCD1492 CD2492::erm (MC802) using primer pair oMC309/oMC338. The expected PCR products’ sizes are 811 bp for the wild-type CD2492 allele and ∼2,811 bp for CD2492::erm.
The 630Δerm and R20291 dccA mutants were complemented using a Bacillus subtilis donor strain, BS49, carrying the conjugative transposon Tn916 to transfer the dccA gene driven by its native promoter, which is encoded upstream of CD1421 (MC1959). To create the plasmid carrying the Tn916::PCD1421-dccA construct (pMC1094), PCD1421 and dccA were spliced by overlapping PCR using primer pairs oMC2910/2911 and oMC2912/2913 and Gibson assembled into BamHI/SphI-digested pSMB47. Erythromycin-resistant colonies were confirmed by PCR with primer pair CD1420qF/CD1420qR (Fig. 7B).
To create the 630Δerm ΔCD1492 CD2492::erm double mutant, the Targetron-based group II intron from pCE240 was retargeted using the targeting site reported previously by Underwood et al. to create pMC336 (69). Briefly, the CD2492-targeted intron was amplified using primers oMC317, oMC318, oMC319, and EBSu and TA cloned into pCR2.1 to create pMC330. A BsrGI/HindIII-digested fragment containing the CD2492-targeted intron was subcloned into pCE240 to create pMC333. Finally, an SphI/SfoI-digested fragment containing the CD2492-targeted intron was subcloned into pMC123 to create pMC336. The resulting construct, pMC336, was conjugated into the 630Δerm CD1492 strain (MC674), and erythromycin-resistant mutants were screened for the 2-kb Targetron insertion within CD2492 using primer pair oMC309/338 (Fig. 7C). Notably, the targeting site was not located in the 254a site within the CD2492 coding region noted by Underwood et al. (69) but rather was located at 318s (data not shown).
Sporulation assays.
C. difficile strains were grown overnight in BHIS medium supplemented with 0.1% taurocholate and 0.2% fructose to aid in spore germination and prevent spore formation, respectively (70, 71). Thiamphenicol (5 μg/ml) was included for plasmid maintenance when necessary. When strains reached mid-exponential phase (optical density at 600 nm [OD600] of ∼0.5), 250-μl aliquots were applied to the surface of 70:30 agar containing 2 μg/ml thiamphenicol and 0 to 0.5 μg/ml nisin (71). After 24 h of growth, ethanol-resistant sporulation assays were performed as previously described (48, 72). Briefly, cells were scraped from the plate surface and suspended in BHIS medium to an OD600 of ∼1. To enumerate vegetative cells, the cell suspensions were serially diluted in BHIS medium and plated onto BHIS plates. Simultaneously, 0.5-ml aliquots of the cell suspensions were mixed thoroughly with 0.3 ml 95% ethanol and 0.2 ml distilled water (dH2O) (final concentration, 28.5% ethanol) and incubated for 15 min to eliminate all vegetative cells. Ethanol-treated cells were then serially diluted in 1× phosphate-buffered saline (PBS) with 0.1% taurocholate and plated onto BHIS medium supplemented with 0.1% taurocholate. Total CFU were enumerated after at least 24 h of growth, and the sporulation frequency was calculated as the number of ethanol-resistant spores divided by the total number of vegetative and ethanol-resistant spores combined. A spo0A mutant (MC310) was used as a negative control in all assays.
Phase-contrast microscopy.
Phase-contrast microscopy was performed at H24 as described previously (33). Briefly, cells were concentrated by pelleting 0.5 ml of the cell suspension, and the concentrated cell suspension was applied to a 0.7% agarose pad on a slide. Cells were imaged with a 100× Ph3 oil immersion objective on a Nikon Eclipse Ci-L microscope. At least two fields of view were captured with a DS-Fi2 camera from at least three independent experiments for each strain tested.
Quantitative reverse transcription-PCR analysis.
RNA was isolated from C. difficile strains grown on 70:30 sporulation agar at H12 and DNase I treated as previously described (13, 29, 33, 34). cDNA was synthesized using random hexamers, and quantitative real-time-PCRs were performed in triplicate (Bioline) and monitored using a Roche LightCycler 96 system. The rpoC transcript (primer pair oMC44/oMC45) was used as the reference gene (34). Controls with no reverse transcriptase were included for all templates and primer sets. The data were analyzed by the 2−ΔΔCT method (73), with normalization to rpoC and the indicated reference condition or strain. The results represent the means and standard errors of the means from at least three independent experiments.
Western blotting.
The indicated strains were grown on 70:30 sporulation agar supplemented with 2 μg/ml thiamphenicol and 0.5 μg/ml nisin and harvested at H12. Total protein from the cell lysates was quantitated using the Pierce Micro bicinchoninic acid (BCA) protein assay kit (Thermo Scientific), 2.5 μg of total protein was separated by electrophoresis on a precast 4 to 20% TGX stain-free gradient gel (Bio-Rad), and total protein was imaged using a ChemiDoc system (Bio-Rad). Protein was transferred to a 0.45-μm nitrocellulose membrane, and Western blot analysis was performed with mouse anti-Spo0A (71) as the primary antibody and goat anti-mouse conjugated with Alexa Fluor 488 (Invitrogen) as the secondary antibody. Imaging and densitometry were performed with a ChemiDoc system and Image Lab software (Bio-Rad), respectively, for three independent experiments.
β-Glucuronidase reporter assays.
β-Glucuronidase assays were performed as previously detailed (41, 74). Briefly, strains were grown on 70:30 sporulation agar as indicated above and harvested at H12 and H15 by scraping the plates and suspending the cells in BHIS medium to an OD600 of ∼0.5 to 0.7. Two 1-ml aliquots were pelleted and stored at −20°C overnight. The pellets were suspended in 0.8 ml Z buffer and 0.05 ml 0.01% SDS. The samples were vortexed, incubated at 37°C for 5 min, and then chilled on ice for 5 min. After a 1-min incubation at 37°C to warm the samples up to room temperature, the enzymatic reaction was started with the addition of 100 μl of 40 μg/ml p-nitrophenol-β-d-glucuronide and stopped with 0.4 ml 1 M Na2CO3. Cell debris was pelleted, and the A420 and A550 were measured using a BioTek Synergy H1 plate reader. Specific activity was normalized by the OD600. Three independent biological replicates were used to calculate the means and standard errors of the means.
ACKNOWLEDGMENTS
We are grateful to the members of the Tamayo and McBride labs for helpful suggestions and discussions during the course of this work.
This research was supported by the U.S. National Institutes of Health through research grants AI107029 and AI143638 to R.T. and AI116933 and AI156052 to S.M.M. C.L.W. is supported by an Institutional Research and Academic Career Development Award (IRACDA) fellowship under K12-GM000678. The content of the manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Contributor Information
Adrianne N. Edwards, Email: anehrli@emory.edu.
Craig D. Ellermeier, University of Iowa
REFERENCES
- 1.Hecht GB, Newton A. 1995. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J Bacteriol 177:6223–6229. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt AJ, Ryjenkov DA, Gomelsky M. 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamayo R, Tischler AD, Camilli A. 2005. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J Biol Chem 280:33324–33330. doi: 10.1074/jbc.M506500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan RP, Lucey J, O’Donovan K, McCarthy Y, Yang L, Tolker-Nielsen T, Dow JM. 2009. HD-GYP domain proteins regulate biofilm formation and virulence in Pseudomonas aeruginosa. Environ Microbiol 11:1126–1136. doi: 10.1111/j.1462-2920.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- 5.Hammer BK, Bassler BL. 2009. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. J Bacteriol 191:169–177. doi: 10.1128/JB.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou SH, Galperin MY. 2016. Diversity of cyclic di-GMP-binding proteins and mechanisms. J Bacteriol 198:32–46. doi: 10.1128/JB.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seshasayee AS, Fraser GM, Luscombe NM. 2010. Comparative genomics of cyclic-di-GMP signalling in bacteria: post-translational regulation and catalytic activity. Nucleic Acids Res 38:5970–5981. doi: 10.1093/nar/gkq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell EB, Tamayo R. 2016. Cyclic diguanylate signaling in Gram-positive bacteria. FEMS Microbiol Rev 40:753–773. doi: 10.1093/femsre/fuw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galperin MY, Higdon R, Kolker E. 2010. Interplay of heritage and habitat in the distribution of bacterial signal transduction systems. Mol Biosyst 6:721–728. doi: 10.1039/b908047c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordeleau E, Fortier LC, Malouin F, Burrus V. 2011. c-di-GMP turn-over in Clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLoS Genet 7:e1002039. doi: 10.1371/journal.pgen.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, Thomson NR, Roberts AP, Cerdeno-Tarraga AM, Wang H, Holden MT, Wright A, Churcher C, Quail MA, Baker S, Bason N, Brooks K, Chillingworth T, Cronin A, Davis P, Dowd L, Fraser A, Feltwell T, Hance Z, Holroyd S, Jagels K, Moule S, Mungall K, Price C, Rabbinowitsch E, Sharp S, Simmonds M, Stevens K, Unwin L, Whithead S, Dupuy B, Dougan G, Barrell B, Parkhill J. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet 38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Dong X, Subramanian S, Matthews PM, Cooper CA, Kearns DB, Dann CE, III.. 2014. Engineering of Bacillus subtilis strains to allow rapid characterization of heterologous diguanylate cyclases and phosphodiesterases. Appl Environ Microbiol 80:6167–6174. doi: 10.1128/AEM.01638-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J Bacteriol 194:3307–3316. doi: 10.1128/JB.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee RW, Mangalea MR, Purcell EB, Borchardt EK, Tamayo R. 2013. The second messenger cyclic Di-GMP regulates Clostridium difficile toxin production by controlling expression of sigD. J Bacteriol 195:5174–5185. doi: 10.1128/JB.00501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soutourina OA, Monot M, Boudry P, Saujet L, Pichon C, Sismeiro O, Semenova E, Severinov K, Le Bouguenec C, Coppee JY, Dupuy B, Martin-Verstraete I. 2013. Genome-wide identification of regulatory RNAs in the human pathogen Clostridium difficile. PLoS Genet 9:e1003493. doi: 10.1371/journal.pgen.1003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordeleau E, Purcell EB, Lafontaine DA, Fortier LC, Tamayo R, Burrus V. 2015. Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J Bacteriol 197:819–832. doi: 10.1128/JB.02340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purcell EB, McKee RW, Courson DS, Garrett EM, McBride SM, Cheney RE, Tamayo R. 2017. A nutrient-regulated cyclic diguanylate phosphodiesterase controls Clostridium difficile biofilm and toxin production during stationary phase. Infect Immun 85:e00347-17. doi: 10.1128/IAI.00347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee RW, Aleksanyan N, Garrett EM, Tamayo R. 2018. Type IV pili promote Clostridium difficile adherence and persistence in a mouse model of infection. Infect Immun 86:e00943-17. doi: 10.1128/IAI.00943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson LF, Peltier J, Hall CL, Harrison MA, Derakhshan M, Shaw HA, Fairweather NF, Wren BW. 2021. Extracellular DNA, cell surface proteins and c-di-GMP promote biofilm formation in Clostridioides difficile. Sci Rep 11:3244. doi: 10.1038/s41598-020-78437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poquet I, Saujet L, Canette A, Monot M, Mihajlovic J, Ghigo JM, Soutourina O, Briandet R, Martin-Verstraete I, Dupuy B. 2018. Clostridium difficile biofilm: remodeling metabolism and cell surface to build a sparse and heterogeneously aggregated architecture. Front Microbiol 9:2084. doi: 10.3389/fmicb.2018.02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD. 2012. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun 80:2704–2711. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbusch KE, Bakker D, Kuijper EJ, Smits WK. 2012. C. difficile 630Δerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA. PLoS One 7:e48608. doi: 10.1371/journal.pone.0048608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ireton K, Rudner DZ, Siranosian KJ, Grossman AD. 1993. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev 7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 24.Sonenshein AL. 2000. Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol 3:561–566. doi: 10.1016/s1369-5274(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 25.Fimlaid KA, Bond JP, Schutz KC, Putnam EE, Leung JM, Lawley TD, Shen A. 2013. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet 9:e1003660. doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paredes CJ, Alsaker KV, Papoutsakis ET. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol 3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 27.Edwards AN, McBride SM. 2014. Initiation of sporulation in Clostridium difficile: a twist on the classic model. FEMS Microbiol Lett 358:110–118. doi: 10.1111/1574-6968.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen A, Edwards AN, Sarker MR, Paredes-Sabja D. 2019. Sporulation and germination in clostridial pathogens. Microbiol Spectr 7:GPP3-0017-2018. doi: 10.1128/microbiolspec.GPP3-0017-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dineen SS, McBride SM, Sonenshein AL. 2010. Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol 192:5350–5362. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. 2012. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res 40:10701–10718. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nawrocki KL, Edwards AN, Daou N, Bouillaut L, McBride SM. 2016. CodY-dependent regulation of sporulation in Clostridium difficile. J Bacteriol 198:2113–2130. doi: 10.1128/JB.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith EA, Macfarlane GT. 1998. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol 25:355–368. doi: 10.1111/j.1574-6941.1998.tb00487.x. [DOI] [Google Scholar]
- 33.Edwards AN, Nawrocki KL, McBride SM. 2014. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect Immun 82:4276–4291. doi: 10.1128/IAI.02323-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride SM, Sonenshein AL. 2011. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect Immun 79:167–176. doi: 10.1128/IAI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol 10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Δerm) and demonstration that the conjugative transposon Tn916ΔE enters the genome of this strain at multiple sites. J Med Microbiol 54:137–141. doi: 10.1099/jmm.0.45790-0. [DOI] [PubMed] [Google Scholar]
- 37.Garrett EM, Sekulovic O, Wetzel D, Jones JB, Edwards AN, Vargas-Cuebas G, McBride SM, Tamayo R. 2019. Phase variation of a signal transduction system controls Clostridioides difficile colony morphology, motility, and virulence. PLoS Biol 17:e3000379. doi: 10.1371/journal.pbio.3000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrett E, Mehra A, Sekulovic O, Tamayo R. 2021. Multiple regulatory mechanisms control the production of CmrRST, an atypical signal transduction system in Clostridioides difficile. bioRxiv 10.1101/2021.10.06.463453. [DOI] [PMC free article] [PubMed]
- 39.Edwards AN, Tamayo R, McBride SM. 2016. A novel regulator controls Clostridium difficile sporulation, motility and toxin production. Mol Microbiol 100:954–971. doi: 10.1111/mmi.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettit LJ, Browne HP, Yu L, Smits WK, Fagan RP, Barquist L, Martin MJ, Goulding D, Duncan SH, Flint HJ, Dougan G, Choudhary JS, Lawley TD. 2014. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genomics 15:160. doi: 10.1186/1471-2164-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purcell EB, McKee RW, Bordeleau E, Burrus V, Tamayo R. 2016. Regulation of type IV pili contributes to surface behaviors of historical and epidemic strains of Clostridium difficile. J Bacteriol 198:565–577. doi: 10.1128/JB.00816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peltier J, Hamiot A, Garneau JR, Boudry P, Maikova A, Hajnsdorf E, Fortier L-C, Dupuy B, Soutourina O. 2020. Type I toxin-antitoxin systems contribute to the maintenance of mobile genetic elements in Clostridioides difficile. Commun Biol 3:718. doi: 10.1038/s42003-020-01448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonenshein AL. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8:203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol 66:206–219. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 45.Daou N, Wang Y, Levdikov VM, Nandakumar M, Livny J, Bouillaut L, Blagova E, Zhang K, Belitsky BR, Rhee K, Wilkinson AJ, Sun X, Sonenshein AL. 2019. Impact of CodY protein on metabolism, sporulation and virulence in Clostridioides difficile ribotype 027. PLoS One 14:e0206896. doi: 10.1371/journal.pone.0206896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiles ID, Gallagher MP, Jamieson DJ, Higgins CF. 1987. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol 195:125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- 47.Koide A, Hoch JA. 1994. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol Microbiol 13:417–426. doi: 10.1111/j.1365-2958.1994.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 48.Childress KO, Edwards AN, Nawrocki KL, Woods EC, Anderson SE, McBride SM. 2016. The phosphotransfer protein CD1492 represses sporulation initiation in Clostridium difficile. Infect Immun 84:3434–3444. doi: 10.1128/IAI.00735-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Meouche I, Peltier J, Monot M, Soutourina O, Pestel-Caron M, Dupuy B, Pons JL. 2013. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS One 8:e83748. doi: 10.1371/journal.pone.0083748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anjuwon-Foster BR, Maldonado-Vazquez N, Tamayo R. 2018. Characterization of flagellum and toxin phase variation in Clostridioides difficile ribotype 012 isolates. J Bacteriol 200:e00056-18. doi: 10.1128/JB.00056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anjuwon-Foster BR, Tamayo R. 2017. A genetic switch controls the production of flagella and toxins in Clostridium difficile. PLoS Genet 13:e1006701. doi: 10.1371/journal.pgen.1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anjuwon-Foster BR, Tamayo R. 2018. Phase variation of Clostridium difficile virulence factors. Gut Microbes 9:76–83. doi: 10.1080/19490976.2017.1362526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antunes A, Martin-Verstraete I, Dupuy B. 2011. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol Microbiol 79:882–899. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 55.McKee RW, Harvest CK, Tamayo R. 2018. Cyclic diguanylate regulates virulence factor genes via multiple riboswitches in Clostridium difficile. mSphere 3:e00423-18. doi: 10.1128/mSphere.00423-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI. 2010. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science 328:1295–1297. doi: 10.1126/science.1188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abel S, Bucher T, Nicollier M, Hug I, Kaever V, Abel Zur Wiesch P, Jenal U. 2013. Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the Caulobacter cell cycle. PLoS Genet 9:e1003744. doi: 10.1371/journal.pgen.1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhungel BA, Govind R. 2021. Phase-variable expression of pdcB, a phosphodiesterase, influences sporulation in Clostridioides difficile. Mol Microbiol 116:1347–1360. doi: 10.1111/mmi.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girinathan BP, Ou J, Dupuy B, Govind R. 2018. Pleiotropic roles of Clostridium difficile sin locus. PLoS Pathog 14:e1006940. doi: 10.1371/journal.ppat.1006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Chai Y, Guo JH, Losick R. 2012. Evidence for cyclic di-GMP-mediated signaling in Bacillus subtilis. J Bacteriol 194:5080–5090. doi: 10.1128/JB.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao X, Mukherjee S, Matthews PM, Hammad LA, Kearns DB, Dann CE, III.. 2013. Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. J Bacteriol 195:4782–4792. doi: 10.1128/JB.00373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang YC, Chin KH, Tu ZL, He J, Jones CJ, Sanchez DZ, Yildiz FH, Galperin MY, Chou SH. 2016. Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain. Nat Commun 7:12481. doi: 10.1038/ncomms12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss CA, Hoberg JA, Liu K, Tu BP, Winkler WC. 2019. Single-cell microscopy reveals that levels of cyclic di-GMP vary among Bacillus subtilis subpopulations. J Bacteriol 201:e00247-19. doi: 10.1128/JB.00247-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fagerlund A, Smith V, Rohr AK, Lindback T, Parmer MP, Andersson KK, Reubsaet L, Okstad OA. 2016. Cyclic diguanylate regulation of Bacillus cereus group biofilm formation. Mol Microbiol 101:471–494. doi: 10.1111/mmi.13405. [DOI] [PubMed] [Google Scholar]
- 65.Hermanas TM, Subramanian S, Dann CE, III, Stewart GC. 2021. Spore-associated proteins involved in c-di-GMP synthesis and degradation of Bacillus anthracis. J Bacteriol 203:e00135-21. doi: 10.1128/JB.00135-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher KA, Schumacher MA, Bush MJ, Bibb MJ, Chandra G, Holmes NA, Zeng W, Henderson M, Zhang H, Findlay KC, Brennan RG, Buttner MJ. 2020. c-di-GMP arms an anti-sigma to control progression of multicellular differentiation in Streptomyces. Mol Cell 77:586–599.e6. doi: 10.1016/j.molcel.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tschowri N, Schumacher MA, Schlimpert S, Chinnam NB, Findlay KC, Brennan RG, Buttner MJ. 2014. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 158:1136–1147. doi: 10.1016/j.cell.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards AN, Suarez JM, McBride SM. 2013. Culturing and maintaining Clostridium difficile in an anaerobic environment. J Vis Exp 2013:e50787. doi: 10.3791/50787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, Wilcox MH, Stephenson K. 2009. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J Bacteriol 191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr Protoc Microbiol Chapter 9:Unit9A.1. doi: 10.1002/9780471729259.mc09a01s12. [DOI] [PubMed] [Google Scholar]
- 71.Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195:1214–1225. doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards AN, McBride SM. 2017. Determination of the in vitro sporulation frequency of Clostridium difficile. Bio Protoc 7:e2125. doi: 10.21769/BioProtoc.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 74.Dupuy B, Sonenshein AL. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol 27:107–120. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 75.O’Connor JR, Lyras D, Farrow KA, Adams V, Powell DR, Hinds J, Cheung JK, Rood JI. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol Microbiol 61:1335–1351. doi: 10.1111/j.1365-2958.2006.05315.x. [DOI] [PubMed] [Google Scholar]
- 76.Thomas CM, Smith CA. 1987. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu Rev Microbiol 41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- 77.Manganelli R, Provvedi R, Berneri C, Oggioni MR, Pozzi G. 1998. Insertion vectors for construction of recombinant conjugative transposons in Bacillus subtilis and Enterococcus faecalis. FEMS Microbiol Lett 168:259–268. doi: 10.1111/j.1574-6968.1998.tb13282.x. [DOI] [PubMed] [Google Scholar]