ABSTRACT
Chronic wasting disease (CWD) is a transmissible prion disease first observed in the 1960s in North America. This invariably fatal disease affects multiple cervid species in the wild and in captivity. In addition to the several known transmission pathways involving cervid host species, prions have been detected in the feces of crows and coyotes after consumption of experimentally spiked tissues. This raises questions about the role of cervid consumers in the perpetuation of CWD. Mountain lions have been shown to preferentially select CWD-infected prey and are also apparently resistant to infection. In this study, two captive mountain lions were fed ground mule deer muscle tissue spiked with brain-derived CWD prions, and lion feces were collected for 1 week afterward. The input brain and resulting fecal materials were analyzed using the highly sensitive real-time quaking-induced conversion (RT-QuIC) assay to quantify prion seeding activity. We recovered only 2.8 to 3.9% of input CWD prions after passage through the mountain lions’ gastrointestinal tracts. Interestingly, CWD prions were shed only in the first defecation following consumption. Our data support the possibility that mountain lions feeding upon infected carcasses could excrete CWD prions in their feces over a short period of time but also suggest that most of the ingested prions are eliminated or sequestered by this large predator.
IMPORTANCE CWD prions appear to spread naturally among susceptible cervid species in captivity and in the wild. A better understanding of all the ways these prions move, persist, and subsequently infect target species through the environment is critical to developing comprehensive disease control strategies. In our study, we show limited, transient pass-through of CWD prions in an apex predator, the mountain lion, using the highly sensitive RT-QuIC assay on feces collected after lions were fed prion-spiked muscle tissue. Prions were detected in feces only in the first defecation after exposure. Moreover, the amount of CWD prions recovered in feces was reduced by >96% after passing through the lion digestive system. This indicates that mountain lions may have some potential to distribute CWD prions within their home ranges but that they also effectively eliminate most of the CWD prions they consume.
KEYWORDS: chronic wasting disease, prion, PrP, mountain lion, Puma concolor, RT-QuIC
INTRODUCTION
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) found in captive and free ranging cervids. CWD was first observed as early as the 1960s in Colorado, USA (1), but is now known to be widespread in North America and focally in Scandinavia and South Korea (2, 3). Multiple cervid species, including mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and wapiti (Cervus canadensis) have been shown to be naturally susceptible to CWD (4). The disease is transmissible to cervid hosts via contact with infected individuals and/or environmental exposure to CWD prions (5). Near the end stages of disease, infected cervids lose considerable amounts of weight and become neurologically compromised (1). Clinical disease can kill infected individuals outright, but in the wild, it also increases their vulnerability to other hazards, including hunting and predation (6–8).
Consumption of CWD-infected cervids by predators and/or scavengers presents two potential causes for concern. Prion diseases have been shown to cross some species barriers in the past (9, 10). During the bovine spongiform encephalopathy (BSE) epidemic, in addition to the humans that developed variant Creutzfeldt-Jakob disease (vCJD) (9), some domestic and nondomestic felids that apparently were fed tissues from cattle infected with BSE prions developed fatal prion disease (10). The potential for CWD transmission to felids also has been suggested (11), although a study by Wolfe et al. reported no evidence for CWD transmission to mountain lions following over a decade of dietary prion exposure (12). Beyond the potential for cross-species transmission of CWD lies the concern that scavengers and/or predators that consume CWD prions may be capable of shedding CWD prions in feces and thus increasing the environmental contamination of CWD prions. Infectious prions have been detected in the feces of both crows (Corvus brachyrhynchos) (13) and coyotes (Canis latrans) (14) for a brief time after consuming rodent-adapted scrapie and CWD prions, respectively. Balancing these understandable concerns is the possibility that predators also may play a useful role in the fight against CWD. Predation as a means of natural control has a theoretical basis (15). Mountain lions (Puma concolor) in particular preferentially prey upon CWD-infected mule deer (6–8). It follows that removal of infected animals by mountain lions and other predators could complement broader disease management efforts. Removal of prion-infected cervids by predators would be even more beneficial if consuming infected carcasses also reduced the abundance of prions left in the environment.
In this study, we quantified the amount of CWD prions able to withstand digestion and passage through the mountain lion gastrointestinal tract to be shed in feces. Captive mountain lions were fed a meal of CWD prion-spiked ground mule deer muscle tissue, and feces from each lion were collected daily for the following week. Ingested CWD prions and CWD prions recovered from fecal samples were quantified using the real-time quaking-induced conversion (RT-QuIC) assay, and the percentage of prion seeding activity recovered was determined. In our experiments, the RT-QuIC assay was used as a substitute for infectivity detection by mouse bioassay. Typically, prion seeding activity levels measured by RT-QuIC are about 10 times higher than corresponding prion infectivity levels measured in laboratory studies (16). Our results indicate that CWD prions can be readily detected in feces following consumption by mountain lions, but the majority of the ingested prions were not passed in feces.
RESULTS
Mountain lion intestinal transit time.
Initial experiments were performed to estimate intestinal transit time in the subject mountain lions to better understand the time frame that feces should be collected following a specific feeding event. Food-grade dye was added to food items, and feces were collected daily (as available) thereafter until staining was no longer detectable. Blue staining was visibly detectable in mountain lion feces for 1 to 4 days after consumption of a bolus of dyed meat, with intensity diminishing over time (Fig. 1). These results were comparable to values reported for other felid species (17). Based on these observations, we regarded a 7-day sampling window as sufficient for assessing the passage of dietary prions. We also regarded a 7-day washout period as minimally sufficient to isolate the prion exposure associated with our experiment from other potential dietary sources of long-term exposure in the two subject animals (12).
FIG 1.
Use of food-grade dye (FD&C Blue No. 1 and 2; ≤1% of food mass consumed) facilitated intestinal transit time estimation in captive mountain lions (Puma concolor). Blue staining was visible in feces for 1 to 4 days after consumption of a bolus of dyed meat (top row), with intensity diminishing over time. Unstained feces collected at same time points are shown for comparison (bottom row).
Detection of CWD prion seeding activity in feces following ingestion.
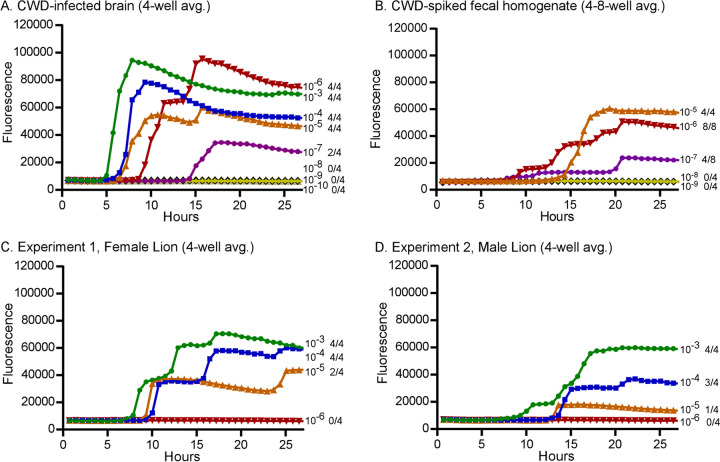
Prior to testing any experimental samples, we first confirmed that the RT-QuIC assay used to detect prion seeding activity would not be inhibited by components in fecal homogenates. CWD-positive brain homogenates were either diluted into RT-QuIC buffer (routine method) or used to spike normal mountain lion feces prior to dilution in RT-QuIC buffer. Endpoint titration was used to compare prion seeding activities between the CWD-positive brain homogenates diluted in RT-QuIC buffer (Fig. 2A) and the those used to spike 10% mountain lion fecal homogenate prior to dilution (Fig. 2B). Both samples gave 100% positive wells at the 10−5 and 10−6 dilutions of CWD brain homogenate and 50% positive wells at the 10−7 dilution, before becoming negative at the 10−8 dilution (Fig. 2A and B), suggesting that CWD prions were not sequestered by fecal homogenates or were unavailable to react in the RT-QuIC assay. We attempted to increase our sensitivity by testing fecal homogenates more concentrated than 10−3; unfortunately, we found complete inhibition of the RT-QuIC assay when testing 10−1 and 10−2 fecal homogenates (data not shown). This finding is not unique to our study, and inhibition of the RT-QuIC assay has been reported previously when feces, brain, and other sample types have been tested (18–21). In the experiments described below, all lion fecal samples were initially tested using 10−3 fecal homogenates.
FIG 2.
End-point titrations to determine the prion 50% seeding dose (SD50) present in the CWD-infected brain stock used for the feeding experiments and in the resulting prion-positive fecal samples. (A and B) CWD-infected brain was diluted directly in RT-QuIC buffer (A) or used to spike a 10% mountain lion fecal homogenate prior to dilution in RT-QuIC buffer (B). Positive fecal samples from experiments 1 and 2 were also diluted to determine prion SD50 (C and D). Each curve shown is the average fluorescence of 4 wells tested per dilution. As samples become more dilute, the time to reach a positive reaction increases. The dilutions are labeled to the right of each curve and are shown with different symbols and colors, followed by a fraction indicating the number of positive wells of the total tested.
To quantify the amount of CWD prions able to pass through the mountain lion gastrointestinal tract, we hand-fed CWD prion-spiked ground mule deer muscle tissue to two lions (one male and one female), searched for and collected feces twice daily for the following week, and quantified prions present in the feces using RT-QuIC. Two trials were performed; in the first trial, CWD prion-spiked ground venison was fed to the female and normal uninfected ground venison was fed to the male. In the second trial, the male received the CWD prion-spiked meal and the female received the uninfected meal. In trial 1, only the first feces passed by the female 3 days after ingesting the CWD prions contained detectable prion seeding activity (Table 1). In trial 2 (35 days later), the first feces collected from the male following ingestion of the CWD prions (3 days postingestion) was also positive (Table 1). Neither mountain lion had detectable prion seeding in feces in the respective trials when they received only test-negative muscle tissue. In both trials, samples with >50% positive RT-QuIC wells at the 10−3 dilution were further diluted and assayed by RT-QuIC to determine the endpoint prion 50% seeding dose (SD50) (Fig. 2C and D and Table 2). A prion SD50 is equivalent to the level of seeding activity that would produce positive RT-QuIC reactions in 50% of the wells tested. An aliquot of the CWD-positive brain material used for feeding the lions was also analyzed by RT-QuIC assay to determine the starting value of prion seeding activity present (Fig. 2A and B and Table 2.) Final SD50 recovery calculations were made by comparing the input (fed) brain SD50 values to the recovered (fecal) SD50s. Fecal and brain SD50 calculations were adjusted according the mass of prion-positive materials fed or recovered. In trials 1 and 2, 3.9% and 2.8% of the input prion seeding activity were recovered, respectively (Table 2). Our experiment with the two mountain lions revealed remarkably consistent patterns in prion passage after ingestion.
TABLE 1.
Summary of prion seeding activity in mountain lion feces following oral exposure to CWD prions
| Fecal sample | CWD prion-spiked meal on day 0 | Days post-feeding | RT-QuIC resultsb |
|---|---|---|---|
| Expt 1 | |||
| Female | Yes | 3 | 10/12 |
| 5 | 0/8 | ||
| 6 | 0/12 | ||
| 7 | 0/8 | ||
| Male | No | 2 | 0/8 |
| 4 | 0/8 | ||
| 6 | 0/8 | ||
| 7 | 0/8 | ||
| Expt 2 | |||
| Female | No | 3 | 0/8 |
| 5a | 0/8 | ||
| 5b | 0/8 | ||
| 6 | 0/8 | ||
| Male | Yes | 3 | 12/12 |
| 5 | 0/8 | ||
| 6 | 2/12 | ||
| Natural feeding 1 | NAa | 2 | 7/8 |
| Natural feeding 2 | NA | 2 | 4/8 |
| Control lions (n = 5) | No | NA | 1/48 |
NA, not applicable; the lions in these experiments were allowed to feed freely on a CWD-infected carcass.
Number of positive RT-QuIC reactions over the total number of wells tested.
TABLE 2.
RT-QuIC analysis of endpoint dilutions of input brain and output fecal samples to determine prion recovery
| Sample | RT-QuIC results at dilutiona |
SD50/gb | Recovered |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feces (g) | SD50 | % SD50 | ||||||||
| 10−3 | 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |||||
| CWD brain | 4/4 | 4/4 | 4/4 | 4/4 | 2/4 | 0/4 | 5 × 109 (2.5 × 1011) | |||
| Female, day 3 | 4/4 | 4/4 | 2/4 | 0/4 | NT | NT | 5 × 107 | 195.9 | 9.8 × 109 | 3.9 |
| Male, day 3 | 4/4 | 3/4 | 1/4 | 0/4 | NT | NT | 1.6 × 107 | 450.9 | 7.1 × 109 | 2.8 |
Number of positive RT-QuIC reactions over the total number of wells tested. NT, not tested.
The calculated prion seeding activity that would produce positive responses in 50% of replicate RT-QuIC reactions. The value in parentheses is the total SD50 fed to each lion.
The above-described experiment used a known but somewhat artificial challenge dose of CWD prions to provide evidence that prion seeding activity could be detected in feces following oral exposure. Although mountain lions will consume brain and spinal cord from some carcasses and ingest high levels of infectious prions, most of their intake would consist of nonneuronal tissues, such as muscle, fat, and connective tissues, which harbor much lower overall prion infectivity levels (22, 23). To test for CWD prions in feces following more natural consumption of a carcass, we opportunistically sampled both lions after they fed on a headless portion of a CWD-positive mule deer carcass in the course of another study (12). The lions were cohoused during this opportunistic sampling session. Two fecal samples collected 2 days following carcass feeding were both positive for prion seeding activity by RT-QuIC (Table 1). Endpoint dilution of the most positive sample (natural feeding 1) examined produced an SD50 of 1.6 × 107/g of feces, a value equivalent to the SD50 measured in the male’s feces after the experimental exposure.
DISCUSSION
We used an ultrasensitive prion detection assay (RT-QuIC) to quantify the amount of CWD prion seeding activity that persisted following transit through the mountain lion gastrointestinal tract. In separate trials using CWD prion-spiked meals, we found that 3.9% and 2.8% of the input prion seeding activity was recovered. We also tested feces collected from the same mountain lions after they fed on a portion of a CWD-infected carcass. Both fecal samples collected 2 days after carcass feeding harbored detectable levels of prion seeding activity, and one of the samples had prion levels equivalent to those measured in the feces from lions after the high experimental challenge dose. These data demonstrate that consumption of CWD-infected cervids in a more natural presentation could also result in transient prion shedding in mountain lion feces. This is not altogether surprising considering that ample prion accumulation and infectivity occur in a variety of peripheral tissues, including lymph nodes and nerves, in later stages of clinical CWD (22–25).
In both our spiked and natural exposure trials, we were limited by a small sample size of just two aged mountain lions. Despite the consistency in our limited data, it is unclear if prion shedding would be similar in other mountain lions, or how much prion shedding would vary depending on the stage of CWD infection in the carcass consumed. It is also unclear how intestinal transit time may alter prion shedding. Perhaps rapid passage of digesta may lead to increased shedding. We encourage further study to understand such nuances in mountain lions and other predatory species.
Two previous studies, one using rodent-adapted scrapie exposure of crows and another using CWD exposure of coyotes, also looked for prion pass-through in feces (13, 14). Both studies used mouse bioassay as a detection method to show prion infectivity persisting through a digestive tract, but neither study attempted to quantify total recovery. In the coyote study, 3 of the 4 subjects shed infective CWD prions in their feces for up to 3 days following exposure (14). This period of reported shedding was somewhat longer than we observed, albeit still relatively brief. The liquefied inoculum and artificial (“dry dog food”) diet provided to study coyotes (14) may have slowed intestinal transit compared to the more natural diet consumed by mountain lions in our study. Nonetheless, available data suggest that any prion shedding by canids or felids feeding upon infected carcasses would most likely be transient. Given the extensive exposure history of the mountain lions used in our study (12), the lack of seeding activity in their feces, absent known dietary input, provides some assurance that observed shedding was passive and that this species does not naturally propagate CWD prions.
It remains unclear how CWD prions spread by predators and/or scavengers may contribute to natural CWD (or other animal prion disease) transmission. Recently, it has been demonstrated that deer can become infected with CWD from consumption of as little as 300 nanograms of CWD-infected brain (26). Because prion-infected cervids shed infective prions in saliva, feces, and urine for much of the disease course, social contact between infected and uninfected cervids remains the most likely means of transmission (4, 5). However, environmental contamination has been demonstrated as a viable means of prion disease transmission (5, 27, 28), and prions in feces from cervids (29), or from animals consuming CWD-infected cervids (13, 14), could add to the environmental burden. Although long-distance movements of predators could pose some risk of accelerating the spread of CWD, lions typically remain near a recent cervid kill for several days, until they have consumed most of the carcass. Human transportation of infected cervids (dead or alive) (30, 31) or more continuous shedding by infected cervids during seasonal migrations (32) seems more likely to have driven the extended geographic distribution of CWD.
We estimated that only approximately 3% of the prion seeding activity ingested by mountain lions was excreted in their feces. This outcome was comparable to the ∼95% reduction of ingested scrapie prions subsequently excreted by Syrian hamsters after oral dosing (33). The fate of the unrecovered prion activity remains uncertain. Typically, proteins are digested by alimentary fluids and absorbed by the gastrointestinal tract. In mountain lions, overall digestibility of crude protein has been measured at 89.7% (34), leaving approximately 10% undigested to pass in feces. Despite resistance to some proteases, prion proteins are not exempt from all digestive processes and are transported from the lumen of the gut across the absorptive epithelium (35). In susceptible species, this process is likely the first step in infection; however, in unsusceptible species such as the mountain lion, the digestive process appears to be an efficient mechanism to remove prions from the ingesta and reduce potential prion contamination of the environment.
CWD prion infectivity following digestion by coyotes also appeared to be substantially reduced, based on the longer incubation periods in the mice used in the bioassay (14). The parallels to our study suggest the possibility that mammalian predation and scavenging could lower the prion burden associated with carcasses of infected cervids, at least to some degree. Even though some passive shedding may occur, repellency of predator excrements may limit direct interactions in deer (36, 37) and perhaps other cervids that could lead to prion exposure. Additionally, selective predation upon CWD-infected deer by predators such as the mountain lion (6–8) may further reduce both direct CWD transmission and environmental prion contamination by removing actively shedding animals from the population. Further detailed ecological studies would be required to analyze the effects of predator densities to reduce prion contamination at the expense of increased overall predation on all cervids.
MATERIALS AND METHODS
Mountain lion husbandry and experimental dietary prion exposure.
The experiment reported herein was subsidiary to a long-term study of dietary CWD prion exposure involving the same subject animals (12; Colorado Division of Parks and Wildlife animal care and use committee file 04–2002). Consequently, animal acquisition, housing, caretaking, and disposition have been described in detail elsewhere (12).
Intestinal transit time.
To estimate intestinal transit time, we offered one of the two subject animals an ∼250-g bolus of ground wapiti muscle tissue mixed with ∼1 g food-grade dye (FD&C Blue No. 1 and No. 2 food dye; ≤1% of food mass consumed) to food items. After the dyed meat had been voluntarily consumed, we then searched for and collected (as available) feces from the enclosure twice daily thereafter until staining was no longer detectable in any of the feces collected. After dye had cleared from the first animal, we repeated the process using the second subject animal.
CWD feeding experiment.
We performed the experiment with two available mountain lions (one male and one female) included in each trial. The lions were housed together and fed CWD-negative carcasses and supplemental meat chunks for 7 days prior to the start of each trial. At day 0, the lions were separated; one lion was hand-fed CWD-negative meat mixed with brain homogenate from a CWD-infected deer, and the other lion was hand-fed only ground CWD-negative meat (500 g). Because both mountain lions were tame and accustomed to receiving food rewards (12), we directly observed intake to ensure consumption. Lions readily consumed the entire meal offering in <5 min. For dietary prion input, we spiked 450 g of ground muscle tissue from an uninfected mule deer with 50 g of homogenized, CWD-infected mule deer brain tissue and offered the total 500-g mixture to an individual mountain lion. Following ingestion of the spiked material (or control meat), the two animals remained separately housed for 7 days and received meat portions derived from a CWD-negative mule deer. During that time, we searched enclosures and collected lion feces twice daily. Feces were labeled, weighed, and stored frozen (−70°C). Feces were not always present, and lions often did not defecate each day. We switched treatment assignments and repeated the trial 35 days later using the same prion inoculum source and subject animals.
Control mountain lion feces.
Control feces were collected from five free-ranging mountain lions. Four of these with unknown prion exposure history had been injured or otherwise required euthanasia because of human conflicts in portions of Colorado, USA, where CWD is endemic (WHL20 253, WHL20 311, WHL20 549, and WHL20 569); one had been harvested by a hunter in Montana, USA, hunting district 270, currently considered CWD free. Initial RT-QuIC optimization and CWD spiking experiments were performed using the Montana mountain lion.
CWD-positive brain homogenate and carcass.
The CWD-infected brain homogenate was made from whole brain (∼167 g) collected from the carcass of a female mule deer that died in end-stage CWD (sample number 18008196; CWD enzyme-linked immunosorbent assay [ELISA] optical density [OD] = 3.405). The homogenate was prepared in a dedicated blender. The resulting slurry was divided into ∼50-g aliquots, stored frozen, and thawed overnight before use.
The infected carcass portion fed upon as part of the umbrella exposure study prior to opportunistic sampling was part of an adult female mule deer carcass submitted by field personnel as a “CWD suspect” (sample number 19017412; ELISA OD = 0.421). The head had been removed from the carcass for laboratory testing, and a portion (∼1/3) of the carcass was provided intact.
Fecal homogenate preparation.
Frozen fecal samples were thawed and homogenized to prepare them for prion seeding activity testing using the RT-QuIC assay. All fecal samples were similar in fluid content but varied in the amount of hair present. Fecal samples were homogenized to 10% (wt/vol) in phosphate-buffered saline (PBS) by placing 7 mL of PBS inside a 15-mL conical tube (Corning) followed by 0.7 g of feces and subsequently vortexed until suspended. Homogenates were then centrifuged for 1 min at 2,000 rpm, and 1-mL aliquots of supernatant were removed and frozen for later analysis. This initial centrifugation step did not produce a large, dense pellet and was used to facilitate removal of supernatant that was free from hair present in the feces.
RT-QuIC assay.
RT-QuIC reactions were performed as previously described using recombinant hamster prion protein 90–231 (Ha rPrP) prepared at Rocky Mountain Laboratory as the substrate (38). Prior to testing the experimental sample, CWD-positive brain homogenate was used to spike normal mountain lion feces to test for potential inhibitors of the RT-QuIC assay. No inhibition was observed when fecal homogenates were used as the sample solution. For the experimental samples, 1,000-μL to 200-μL frozen homogenate supernatants were thawed, vortexed and then centrifuged for 1 min at 2,000 × g. Supernatants were then further diluted in 0.1% SDS (sodium dodecyl sulfate; Sigma)–PBS–N-2 supplement (Gibco) to produce 10−3 sample concentrations. For endpoint titrations, additional serial 10-fold dilutions were made. Two-microliter sample volumes were added to reaction wells of a black, 96-well, clear-bottom plate (Nunc) containing 98 μL of RT-QuIC reaction mix. Plates were sealed with a plate sealer film (Nunc) and incubated at 50°C in a BMG FLUOstar Omega plate reader with a repeating protocol of 1 min shaking (700 rpm; double orbital) and 1 min rest throughout the indicated incubation time. Thioflavin T (ThT) fluorescence measurements (450 ± 10 nm excitation and 480 ± 10 nm emission; plate bottom read) were taken every 45 min. The plate reader gain was set at 1,600 for each run. The maximum fluorescence readout on our plate reader is 260,000 units. Each 10−3-diluted fecal sample screened was tested using a minimum of 8 total wells by performing quadruplicate reactions on two separate plates. Samples giving >50% positive wells at the 10−3 dilution were considered positive, and additional RT-QuIC was performed in quadruplicate on serial 10-fold dilutions to determine an SD50 using the Spearman-Karber formula for titer calculation (39).
ACKNOWLEDGMENTS
We thank Clayton Winkler, Katie Williams, and Bruce Chesebro for critical review of the manuscript.
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases, the Colorado Division of Parks and Wildlife, and U.S. Fish and Wildlife Service Federal Aid to Wildlife Restoration funding. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
L.L.W. and M.W.M. conceptualized the study and experiments; L.L.W., M.W.M., K.C.S., and K.A.G. planned the animal studies; K.A.G. acquired and prepared challenge material; L.L.W., K.C.S., and M.W.M. performed the animal experiments; B.R., C.B., and A.G.H. developed laboratory methods, provided necessary materials, and conducted analyses; B.R., C.B., and K.C.S. generated the figures and tables; B.R., C.B., L.L.W., and M.W.M. drafted the manuscript; all authors contributed to the revised the manuscript.
We declare no conflict of interest.
Contributor Information
Michael W. Miller, Email: mike.miller@state.co.us.
Brent Race, Email: raceb@niaid.nih.gov.
Michael J. Imperiale, University of Michigan—Ann Arbor
REFERENCES
- 1.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 2.EFSA Panel on Biological Hazards, Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Norrung B, Robertson L, Sanaa M, Skandamis P, Snary E, Speybroeck NT, Kuile B, Threlfall J, Wahlstrom H, Benestad S, Gavier-Widen D, Miller MW, Ru G, Telling GC, Tryland M, Ortiz Pelaez A, Simmons M. 2017. Chronic wasting disease (CWD) in cervids. EFSA J 15:e04667. doi: 10.2903/j.efsa.2017.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mysterud A, Edmunds DR. 2019. A review of chronic wasting disease in North America with implications for Europe. European J Wildlife Res 65:26. doi: 10.1007/s10344-019-1260-z. [DOI] [Google Scholar]
- 4.Miller MW, Williams ES. 2004. Chronic wasting disease of cervids. Curr Top Microbiol Immunol 284:193–214. doi: 10.1007/978-3-662-08441-0_8. [DOI] [PubMed] [Google Scholar]
- 5.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH, Lukacs PM. 2008. Lions and prions and deer demise. PLoS One 3:e4019. doi: 10.1371/journal.pone.0004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krumm CE, Conner MM, Hobbs NT, Hunter DO, Miller MW. 2010. Mountain lions prey selectively on prion-infected mule deer. Biol Lett 6:209–211. doi: 10.1098/rsbl.2009.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeVivo MT, Edmunds DR, Kauffman MJ, Schumaker BA, Binfet J, Kreeger TJ, Richards BJ, Schatzl HM, Cornish TE. 2017. Endemic chronic wasting disease causes mule deer population decline in Wyoming. PLoS One 12:e0186512. doi: 10.1371/journal.pone.0186512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature 389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood JK, Cunningham AA. 2007. Portrait of prion diseases in zoo animals, p 250–256. In Hörnlimann B, Riesner D, Kretzschmar HA (ed), Prions in humans and animals. De Gruyter, Berlin, Germany. [Google Scholar]
- 11.Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA. 2013. Susceptibility of domestic cats to chronic wasting disease. J Virol 87:1947–1956. doi: 10.1128/JVI.02592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe LL, Fox KA, Griffin KA, Miller MW. 2021. Mountain lions (Puma concolor) resist long-term dietary exposure to chronic wasting disease. J Wildl Dis doi: 10.7589/JWD-D-21-00029. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.VerCauteren KC, Pilon JL, Nash PB, Phillips GE, Fischer JW. 2012. Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos). PLoS One 7:45774. doi: 10.1371/journal.pone.0045774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols TA, Fischer JW, Spraker TR, Kong Q, VerCauteren KC. 2015. CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion 9:367–375. doi: 10.1080/19336896.2015.1086061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild MA, Hobbs NT, Graham MS, Miller MW. 2011. The role of predation in disease control: a comparison of selective and nonselective removal on prion disease dynamics in deer. J Wildl Dis 47:78–93. doi: 10.7589/0090-3558-47.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, Meade-White KD, Taubner LM, Timmes A, Caughey B. 2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathogens 6:1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller G, Margulis SW, Santymire R. 2011. The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol 30:379–398. doi: 10.1002/zoo.20339. [DOI] [PubMed] [Google Scholar]
- 18.Tennant JM, Li M, Henderson DM, Tyer ML, Denkers ND, Haley NJ, Mathiason CK, Hoover EA. 2020. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS One 15:e0227094. doi: 10.1371/journal.pone.0227094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davenport KA, Mosher BA, Brost BM, Henderson DM, Denkers ND, Nalls AV, McNulty E, Mathiason CK, Hoover EA. 2018. Assessment of chronic wasting disease prion shedding in deer saliva with occupancy modeling. J Clin Microbiol 56:e01243-17. doi: 10.1128/JCM.01243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoover CE, Davenport KA, Henderson DM, Zabel MD, Hoover EA. 2017. Endogenous brain lipids inhibit prion amyloid formation in vitro. J Virol 91:e02162-16. doi: 10.1128/JVI.02162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosko T, Galuskova S, Matej R, Bruzova M, Holada K. 2021. Detection of prions in brain homogenates and CSF samples using a second-generation RT-QuIC assay: a useful tool for retrospective analysis of archived samples. Pathogens 10:750. doi: 10.3390/pathogens10060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Race B, Meade-White K, Race R, Chesebro B. 2009. Prion infectivity in fat of deer with chronic wasting disease. J Virol 83:9608–9610. doi: 10.1128/JVI.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117–1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 24.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. 2001. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 25.Fox KA, Jewell JE, Williams ES, Miller MW. 2006. Patterns of Prp(CWD) accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 26.Denkers ND, Hoover CE, Davenport KA, Henderson DM, McNulty EE, Nalls AV, Mathiason CK, Hoover EA. 2020. Very low oral exposure to prions of brain or saliva origin can transmit chronic wasting disease. PLoS One 15:e0237410. doi: 10.1371/journal.pone.0237410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gough KC, Maddison BC. 2010. Prion transmission: prion excretion and occurrence in the environment. Prion 4:275–282. doi: 10.4161/pri.4.4.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins SAC, Simmons HA, Gough KC, Maddison BC. 2015. Persistence of ovine scrapie infectivity in a farm environment following cleaning and decontamination. Vet Rec 176:99. doi: 10.1136/vr.102743. [DOI] [PubMed] [Google Scholar]
- 29.Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–U590. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams ES, Miller MW. 2003. Transmissible spongiform encephalopathies in non-domestic animals: origin, transmission and risk factors. Rev Sci Tech 22:145–156. doi: 10.20506/rst.22.1.1385. [DOI] [PubMed] [Google Scholar]
- 31.Kahn S, Dube C, Bates L, Balachandran A. 2004. Chronic wasting disease in Canada: part 1. Can Vet J 45:397–404. [PMC free article] [PubMed] [Google Scholar]
- 32.Conner MM, Miller MW. 2004. Movement patterns and spatial epidemiology of a prion disease in mule deer population units. Ecol Appl 14:1870–1881. doi: 10.1890/03-5309. [DOI] [Google Scholar]
- 33.Kruger D, Thomzig A, Lenz G, Kampf K, McBride P, Beekes M. 2009. Faecal shedding, alimentary clearance and intestinal spread of prions in hamsters fed with scrapie. Vet Res 40:4. doi: 10.1051/vetres:2008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbiers RB, Vosburgh LM, Ku PK, Ullrey DE. 1982. Digestive efficiencies and maintenance energy requirements of captive wild felidae: cougar (Felis concolor); leopard (Panthera pardus); lion (Panthera leo); and tiger (Panthera tigris). J Zoo Anim Med 13:32–37. doi: 10.2307/20094560. [DOI] [Google Scholar]
- 35.Jeffrey M, Gonzalez L, Espenes A, Press CM, Martin S, Chaplin M, Davis L, Landsverk T, MacAldowie C, Eaton S, McGovern G. 2006. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol 209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 36.Mullerschwarze D. 1972. Responses of young black-tailed deer to predator odors. J Mammal 53:393. doi: 10.2307/1379188. [DOI] [Google Scholar]
- 37.Sullivan TP, Nordstrom LO, Sullivan DS. 1985. Use of predator odors as repellents to reduce feeding damage by herbivores.1. Snowshoe hares (Lepus americanus). J Chem Ecol 11:903–919. doi: 10.1007/BF01012077. [DOI] [PubMed] [Google Scholar]
- 38.Williams K, Hughson AG, Chesebro B, Race B. 2019. Inactivation of chronic wasting disease prions using sodium hypochlorite. PLoS One 14:e0223659. doi: 10.1371/journal.pone.0223659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dougherty RM. 1964. Animal virus titration techniques. Academic Press, Inc., New York, NY. [Google Scholar]