Abstract
Background
Dopamine neurotransmission plays a critical role in reward in drug abuse and drug addiction. However, the role of dopamine in the recognition of drug-associated environmental stimuli, retrieval of drug-associated memory, and drug-seeking behaviors is not fully understood.
Methods
Roles of dopamine neurotransmission in the prefrontal cortex (PFC) and nucleus accumbens (NAc) in the cocaine-conditioned place preference (CPP) paradigm were evaluated using in vivo microdialysis.
Results
In mice that had acquired cocaine CPP, dopamine levels in the PFC, but not in the NAc, increased in response to cocaine-associated cues when mice were placed in the cocaine chamber of an apparatus with 2 separated chambers. The induction of the dopamine response and the development of cocaine CPP were mediated through activation of glutamate NMDA (N-methyl-D-aspartate)/AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor signaling in the PFC during conditioning. Activation of dopamine D1 or D2 receptor signaling in the PFC was required for cocaine-induced locomotion, but not for the induction of the dopamine response or the development of cocaine CPP. Interestingly, dopamine levels in the NAc increased in response to cocaine-associated cues when mice were placed at the center of an apparatus with 2 connected chambers, which requires motivated exploration associated with cocaine reward.
Conclusions
Dopamine neurotransmission in the PFC is activated by the exposure to the cocaine-associated cues, whereas dopamine neurotransmission in the NAc is activated in a process of motivated exploration of cues associated with cocaine reward. Furthermore, the glutamate signaling cascade in the PFC is suggested to be a potential therapeutic target to prevent the progression of drug addiction.
Keywords: Conditioned place preference, D1 receptor, D2 receptor, glutamate, microdialysis
Significance Statement.
Dopamine neurotransmission is critically involved in cocaine reward-associated behaviors. The present study demonstrates the distinct roles of dopamine in the PFC and NAc during recognition and exploration of cocaine-associated cues. In the PFC, activation of ionotropic glutamate receptor signaling during conditioning leads to the induction of the dopamine response to cocaine-associated cues and the development of cocaine-conditioned place preference (CPP). In contrast, dopamine neurotransmission in the NAc is not activated by the exposure to cocaine-associated cues but during the motivated exploratory process of cues associated with cocaine reward. Thus, the present findings indicate the difference in the nature of dopamine neurotransmission in the PFC and NAc in response to psychological reward cues.
Introduction
Mesocorticolimbic dopamine pathways play a critical role in the rewarding effects of drugs of abuse (Shinohara et al., 2017; Thibeault et al., 2019; Zhou et al., 2019; Porter, 2020). Environmental cues are thought to trigger drug craving and relapse of addiction via mechanisms involving activation of dopamine neurons in the ventral tegmental area (VTA), leading to the enhancement of dopamine neurotransmission in the nucleus accumbens (NAc) (Volkow and Morales, 2015; Li et al., 2016) and prefrontal cortex (PFC) (Bassareo and Di Chiara, 1997; Milella et al., 2016; Ellwood et al., 2017). In the cocaine self-administration paradigm, cocaine-associated cues have been shown to induce dopamine release in the NAc core of rats using in vivo microdialysis (Ito et al., 2000) and fast-scan cyclic voltammetry (Gratton and Wise, 1994; Phillips, 2003). The dopamine response to cocaine-associated cues in the NAc core has also been reported in the cocaine-conditioned place preference (CPP) paradigm in rats (Duvauchelle et al., 2000). However, failures of the dopamine response to conditioned cocaine-associated cues have also been reported in the NAc core of rats (Brown and Fibiger, 1992; Neisewander et al., 1996) and the mesolimbic striatum of nonhuman primates (Bradberry et al., 2000), suggesting the involvement of dopaminergic circuits other than the mesolimbic pathway, such as the mesocortical pathway.
Alterations in glutamatergic transmission underlie the mechanisms of cocaine-induced synaptic and structural plasticity of D1 and D2 receptor-expressing medium spiny neurons (MSNs) in the NAc (Ceglia et al., 2017; Barrientos et al., 2018) and pyramidal neurons in the medial PFC (Hearing et al., 2013). It has been shown that modifications of glutamate receptors (e.g., phosphorylation, palmitoylation, ubiquitination and sumoylation) are sensitive to addictive drugs, and altered modifications are directly linked to persistent drug-related synaptic plasticity and addictive behaviors (Mao et al., 2011). In fact, glutamate AMPA receptors have been shown to mediate the cocaine reward-context association (Shukla et al., 2017) and the expression (Cervo and Samanin, 1995) of cocaine CPP. Activation of NMDA receptors within the NAc shell has been shown to regulate cocaine memory reactivation (Li et al., 2016). Thus, glutamate NMDA (N-methyl-D-aspartate)/AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor signaling is implicated in the development and expression of cocaine CPP.
The CPP paradigm is one of the most popular behavioral animal models used to assess the rewarding effects of drugs (Prus et al., 2009). In cocaine CPP, the cocaine-associated cue induces the retrieval of memories associated with the cocaine reward and the expectation of cocaine administration. These processes are mediated through activation of mesocorticolimbic dopamine pathways by dynamically interacting with glutamate pathways. A better understanding of the neural mechanisms of the development and expression of cocaine CPP is required to develop novel therapeutic strategies for cocaine addiction.
Using the cocaine CPP paradigm, we investigated the response of dopamine to cocaine-associated cues in the PFC as well as the NAc because dopamine neurotransmission in the PFC has been implicated in the expression of cocaine CPP (Milella et al., 2016; Shinohara et al., 2017), but there is no direct evidence of increased dopamine levels in the PFC. Interestingly, we found that dopamine levels increased in response to cocaine-associated cues (2-separated chamber apparatus) in the PFC, but not in the NAc, using in vivo microdialysis. Based on these findings, we investigated the mechanism of the induction of the dopamine response in the PFC and the development of cocaine CPP, especially the roles of glutamate and dopamine receptor signaling. We further analyzed the effects of cocaine-associated cues on the dopamine response in the NAc and PFC using a 2-connected chamber apparatus that allows exploration of cues associated with cocaine reward.
METHODS
Animals
Male C57BL/6NCr mice (Japan SLC, Shizuoka, Japan) at 9 to 13 weeks of age were used. Mice were housed 2 to 5 per cage and maintained in a 12-hour-light/-dark cycle with access to standard mouse chow and water ad libitum. All mice used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health, and the specific protocols were approved by the Institutional Animal Care and Use Committee of Kurume University. All efforts were made to minimize the number of animals used. For chemogenetic modulation experiments, BAC transgenic [Drd1]-Cre mice (EY262) (12 mice) were used (GENSAT, Rockefeller University) (Gong et al., 2007).
Drugs
Cocaine (Takeda, Osaka, Japan) and clozapine N-oxide (CNO; Cayman Chemical, Ann Arbor, MI) were dissolved in saline for systemic injection. 6-Cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX; MilliporeSigma, St. Louis, MO), (R,S)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (3-2-Cpp; MilliporeSigma), R(+)-SCH-23390 hydrochloride (MilliporeSigma), and S(–)-raclopride (+)-tartrate salt (MilliporeSigma) were dissolved in Ringer’s solution for local infusion into the PFC.
EXPERIMENTAL DESIGN
Cocaine CPP Paradigms and Dopamine Recording
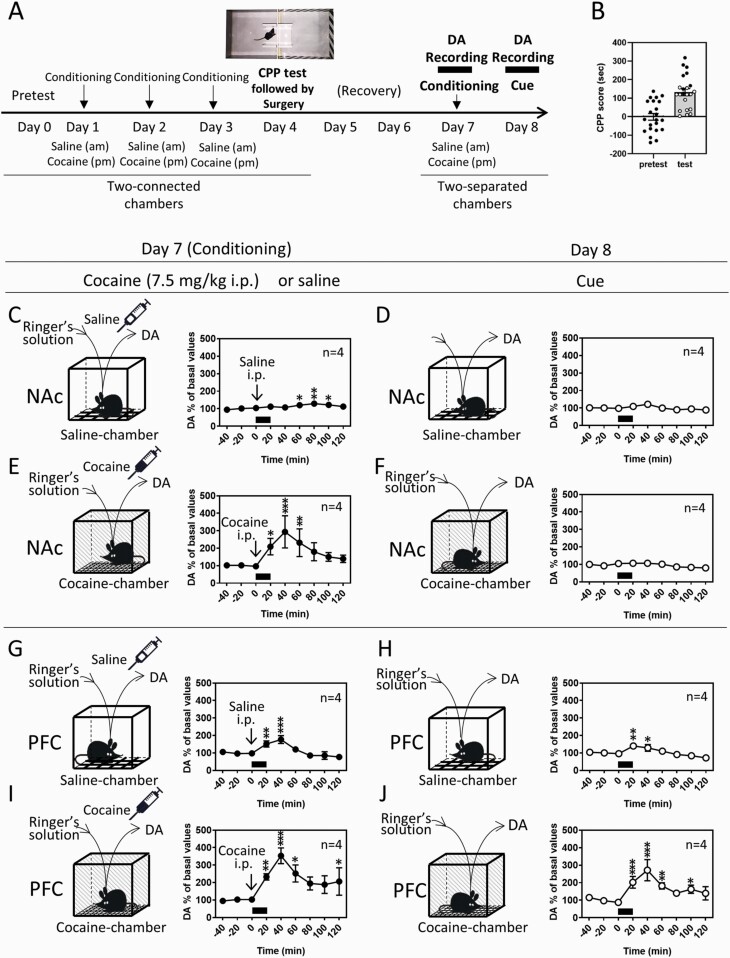
Cocaine conditioning consisted of 2 sessions of training with saline and cocaine for development (day 1–3) and maintenance (day 7) of CPP (Figure 1A). Mice were injected with saline (10 mL/kg) between 10:00 am and 11:00 am, confined to 1 chamber for 30 minutes, and placed back into their home cage. Between 4:00 pm and 5:00 pm, the mice were injected with cocaine (7.5 or 15 mg/kg) and confined to the other chamber for 30 minutes. On 3 consecutive days (day 1–3), a total of 3 rounds of association training was conducted. CPP was tested on day 4 between 12:00 pm and 2:00 pm. After the CPP test, surgery for implantation of microdialysis probes in the NAc or PFC was conducted (day 4). The dopamine levels were recorded on day 7 and day 8 with in vivo microdialysis. The CPP apparatus was changed to a 2-separated chamber apparatus consisting of saline and cocaine chambers (32 × 22 × 12 cm), which have the same contexts with the 2-connected chamber apparatus used for the CPP test. On day 7, additional cocaine conditioning was conducted to maintain the memory association 2 days after recovery from the surgery (day 5 and 6). Extracellular dopamine recording was initiated in the home cage from 2 to 3 hours before the saline session (am) and continued until 2 hours after the cocaine session (pm). After obtaining 3 stable consecutive samples of dopamine differing by <10%, mice received injections of saline (am) and cocaine (pm) and were immediately exposed to the saline and cocaine chambers for 20 minutes, respectively. On day 8, the dopamine levels were recorded in the same mice, while the mice were exposed to the saline and cocaine chambers for 20 minutes, which were considered saline- and cocaine-associated cues, respectively. Microdialysis experiments require several steps of procedures and can be performed maximally in 2 mice per week in our setup of dopamine recording. Therefore, the 2 mice, which exhibited high CPP scores, were chosen from the 3 to 5 mice tested for CPP every week and used for in vivo microdialysis (Figure 1A, closed circles) on day 7 and 8. The mice withdrawn for brain dialysis are shown in Figure 1B with open circles. Because the 2 mice were selected from the mouse set in each week, the mice used for microdialysis analysis occasionally showed a lower CPP score than the withdrawn mice.
Figure 1.
Dopamine (DA) responses to saline and cocaine injections in additional place conditioning (day 7) and to saline- and cocaine-associated cues (day 8) in the nucleus accumbens (NAc) and prefrontal cortex (PFC). (A) Protocols for the conditioned place preference (CPP) to cocaine and subsequent measurements of DA. Following the pretest of chamber preference (day 0), saline and cocaine (7.5 mg/kg i.p.) were injected daily, and mice were placed in saline- and cocaine-paired chambers, respectively, for cocaine conditioning for 3 days (day 1–3) using the 2-connected chamber apparatus with a shielding plate between chambers. CPP was tested on day 4 using the 2-connected chamber apparatus without a shielding plate between chambers, and only the mice showing a preference for the cocaine-associated chamber were used for the following analyses. Additional cocaine conditioning for the developed CPP to cocaine was conducted on day 7 using the 2-separated chamber apparatus consisting of saline and cocaine chambers. The extracellular DA level was recorded from 2 to 3 hours before saline conditioning until 2 hours after the cocaine conditioning. Mice were kept in their home cage throughout the dopamine recording except for when they were placed in the saline or cocaine chamber for 20 minutes, where mice received injections. The saline or cocaine chamber has the same contexts as each paired chamber used for conditioning. On day 8, extracellular DA was subsequently recorded when mice were exposed to the saline or cocaine chambers without injections for 20 minutes, which was considered a cue. (B) CPP scores recorded on day 0 and day 4. CPP scores on day 4 of the mice used and unused for microdialysis experiments were shown as closed and open circles, respectively. (C–J) Extracellular levels of DA were measured in the NAc (C–F) and PFC (G–J) using in vivo microdialysis. Effects of the injections of saline (C, G) and cocaine (7.5 mg/kg, i.p.) (E, I) for additional cocaine conditioning (day 7) and the saline (D, H)- and cocaine (F, J)-associated cues (day 8) on DA levels were determined. Exposure of mice to the cues is indicated with black bars. Data represent mean ± SEM. The number of mice is indicated in each graph. *P < .05, **P < .01, ***P < .001 vs the basal levels of DA; mixed linear models.
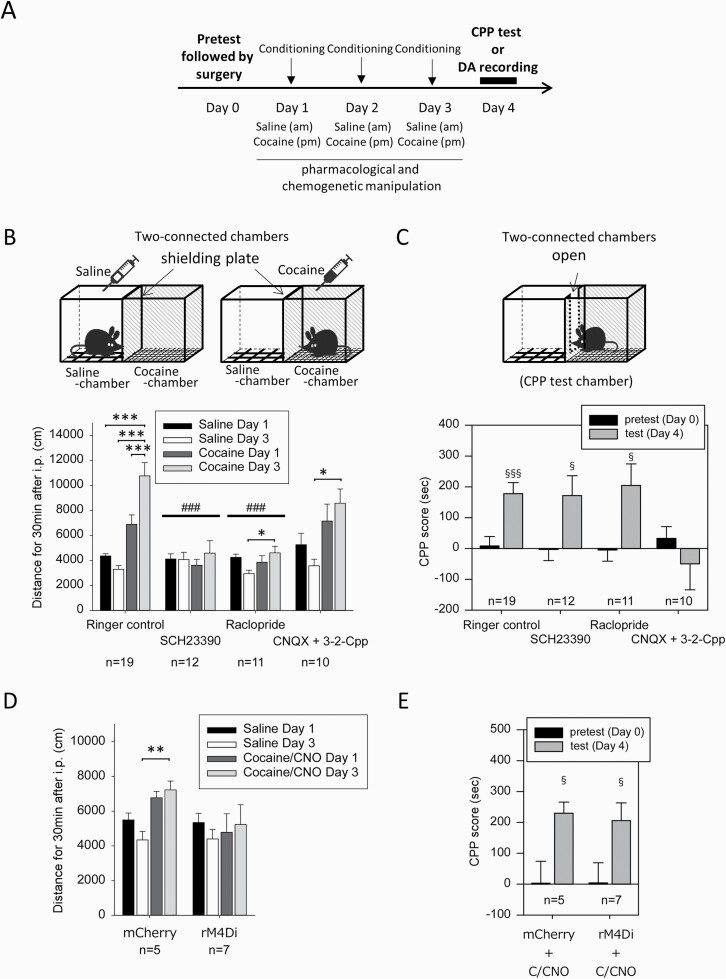
In the other experimental paradigm of cocaine CPP, mice received infusions of the receptor ligands (SCH23390, raclopride, or CNQX plus 3-2-Cpp) in the PFC via a microdialysis probe from 2 hours before the initiation of saline conditioning in the morning until the end of cocaine conditioning in the afternoon (day 1–3) (Figure 2A). Effective doses of SCH23390, raclopride (Kobayashi and Inoue, 1993; West and Grace, 2002; Ginovart, 2005; Lupinsky et al., 2017; Shuto et al., 2020), and CNQX plus 3-2-Cpp (Kawahara et al., 2000, 2002; Catarzi et al., 2010; Osorio-Gómez et al., 2017) were chosen based on previous receptor binding and microdialysis studies. Ringer’s solution was infused for 30 minutes before and after the infusion of receptor ligands. In [Drd1]-Cre mice that received Gi-coupled-designer receptors exclusively activated by designer drugs (Gi-DREADD) or control (mCherry) viral injection, CNO (1 mg/kg, i.p.) was administered 30 minutes before initiation of cocaine conditioning for 3 consecutive days. Biological effects of CNO in DREADD-expressing experimental mice have been shown to last long term (6–10 hours) despite its short half-life in plasma (<2 hours) (Alexander et al., 2009; Guettier et al., 2009). Therefore, before initiation of saline conditioning, saline was administered instead of CNO to avoid the accumulative effects of CNO or clozapine during cocaine conditioning.
Figure 2.
Effects of antagonist infusion into the prefrontal cortex (PFC) during cocaine conditioning on the locomotor response and conditioned place preference (CPP) to cocaine. (A) Daily injections of saline and cocaine (7.5 mg/kg) for conditioning were conducted as described in Figure 1A. Surgery for microdialysis probe implantation was conducted on day 0 following the pretest of chamber preference. Pharmacological and chemogenetic manipulation was performed during conditioning (day 1–3), and the CPP test was performed on day 4. (B–C) Pharmacological ligands (a D1 receptor antagonist, SCH23390 [10 µM]; a D2 receptor antagonist, raclopride [10 µM]; ionotropic glutamate antagonists, CNQX [10 µM] plus 3-2-Cpp [30 µM]) were infused into the PFC during conditioning (day 1–3). The locomotor response to saline or cocaine injection was evaluated in paired chambers using the 2-connected chamber apparatus with a shielding plate on day 1 and day 3 (B), and cocaine CPP was evaluated using the 2-connected chamber apparatus without a shielding plate on day 4 (C). (D, E) For chemogenetic inhibition of D1 receptor-expressing neurons in the PFC, saline and CNO (1 mg/kg, i.p.) were administered 30 minutes before the saline-paired place conditioning and cocaine-paired place conditioning (day 1–3), respectively, in [Drd1]-Cre mice injected with Gi-DREADD virus (rAAV-hsyn-DIO-rM4D(Gi)-mCherry) or control virus (rAAV2-Ef1a-DIO-mCherry) into the PFC. The locomotor response to saline injection or cocaine injection after CNO administration (C/CNO) was evaluated on day 1 and day 3 (D), and cocaine CPP was evaluated on day 4 (E). Data represent mean ± SEM. The number of mice is indicated under each experimental group. *P < .05, **P < .01, ***P < .001 as indicated; 1-way ANOVA and Bonferroni post hoc test. ###P < .001 vs Ringer’s control; mixed effect models. §P < .05, §§§P < .001 vs CPP score in pretest, Welch’s t test.
In the microdialysis experiments presented in Figures 3 and 4, dopamine levels were determined on day 4 in mice subjected to saline and/or cocaine conditioning for 3 days (day 1–3) without performing the CPP test (Figure 2A), because the cocaine conditioning procedure was shown to induce the cocaine CPP (Figure 2C).
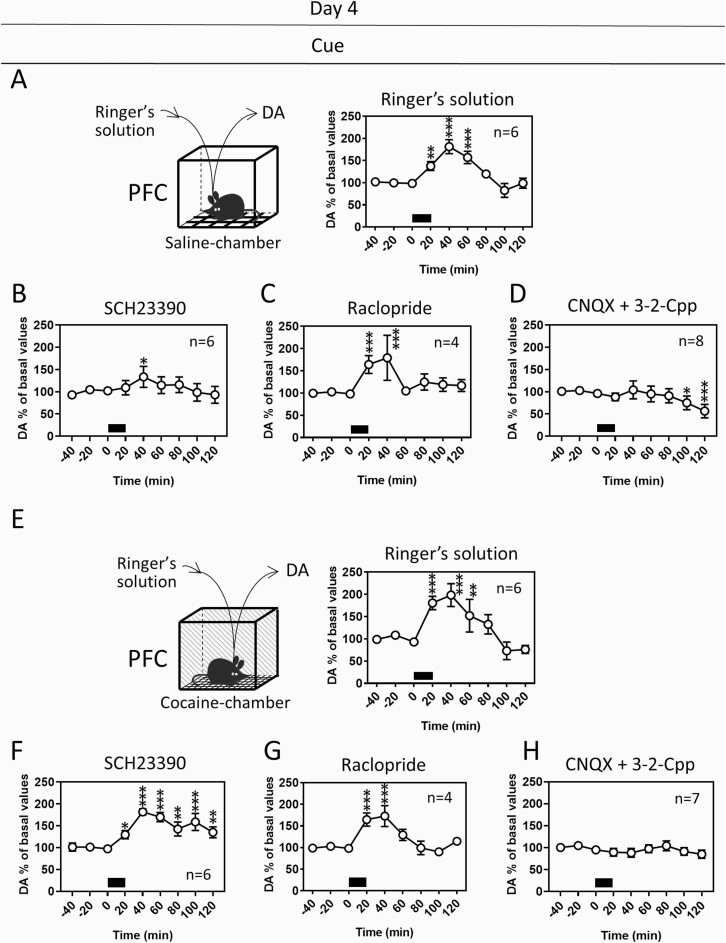
Figure 3.
Dopamine (DA) responses to the saline- and cocaine-associated cues in the PFC on day 4 after cocaine place conditioning with pharmacological manipulation. In another group of animals, DA levels were determined on day 4 by in vivo microdialysis following cocaine conditioning as shown in Figure 2A. DA responses to the saline (A–D)- and cocaine (E–H)-associated cues in mice that received infusion of Ringer’s solution (A, E), the D1 receptor antagonist SCH23390 (10 µM) (B, F), the D2 receptor antagonist raclopride (10 µM) (C, G), or the ionotropic glutamate receptor antagonists CNQX (10 µM) plus 3-2-Cpp (30 µM) (D, H) into the PFC during conditioning (day 1–3). Exposure of mice to the cues is indicated with black bars. Data represent mean ± SEM. The number of mice is indicated in each graph. *P < .05, **P < .01, ***P < .001 vs the basal levels of DA; mixed linear models.
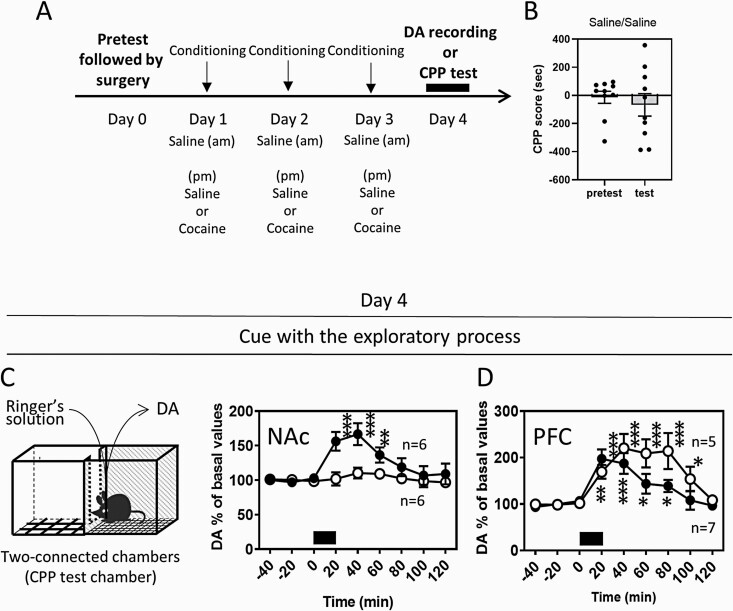
Figure 4.
The dopamine (DA) response to the cocaine-associated cues in the NAc and PFC using the 2-connected chamber apparatus. (A) Daily injections of saline (am) and saline or cocaine (7.5 mg/kg) (pm) for conditioning were conducted. Surgery for microdialysis probe implantation was conducted on day 0 following the pretest of chamber preference. DA levels were determined on day 4 by in vivo microdialysis. Mice were conditioned with saline in 1 chamber and cocaine in the other chamber (the cocaine CPP group: black circles) or with saline in both chambers (the control group: open circles) using the 2-connected chamber apparatus with a shielding plate between chambers for 3 days (day 1–3). (B) In different experimental groups subjected to saline (am) and saline (pm) for conditioning, CPP was tested on day 4. The cocaine conditioning procedure was shown to induce the cocaine CPP (Figure 1B). The DA levels in the NAc (C) or PFC (D) were determined on day 4, when mice were placed at the center of the 2-connected chamber apparatus without a shielding plate and allowed to explore both the saline- and cocaine-associated cues. Exposure of mice to the cues is indicated with a black bar. Data represent mean ± SEM. The number of mice is indicated in the graph. *P < .05, **P < .01, ***P < .001 vs the basal levels of DA; mixed linear models.
CPP Test and Locomotor Responses to Cocaine
The side preference for cocaine was calculated as the time spent in the cocaine-paired chamber minus the time spent in the saline-paired chamber, which is defined as the CPP score in the pretest and the CPP test (posttest). For the pretest, mice were placed on the center point of the 2-connected chamber apparatus (64 × 22 × 12 cm), which consists of 2 contextually distinct chambers on either side. Pretest bias was declared in a 20-minute pretest session. There were no group differences for the bias for either chamber. The environments were adjusted by floor and wall design so that animals could differentiate one environment from the other but did not exhibit an innate preference for either environment. Based on the pretest, the CPP score in each experimental group was balanced and minimized.
Locomotor activity was recorded during the conditioning sessions. Saline (10 mL/kg, i.p.) or cocaine (7.5 mg/kg, i.p.) was injected into the mice while they were confined to their paired chamber in the 2-connected chamber apparatus (Figure 2A–B). The side preference for cocaine and locomotor activity were analyzed using the video-tracking software SMART (Panlab, Barcelona, Spain). For the evaluation of the CPP test and locomotor activity, 64 mice, including BAC transgenic [Drd1]-Cre mice, were used.
Surgery and Brain Dialysis
Microdialysis probes (I-shaped cannulas: customized home-made probes using dialysis membrane [Hospal Industrie, Meyzieu, France]) were implanted in the unilateral NAc (exposed length = 1.5 mm) or PFC (exposed length = 2.8 mm) of mice under pentobarbital (50 mg/kg i.p.) and xylazine (8 mg/kg i.p.) anesthesia and local application of 0.1% lidocaine. The coordinates of the implantation were A/P +1.4 mm, L/M 0.6 mm from the bregma, and V/D −4.5 mm from the dura and A/P +1.9 mm, L/M 0.3 mm from the bregma, and V/D −2.8 mm from the dura to target the NAc and PFC, respectively (supplementary Figure 1) (Paxinos and Franklin, 2004). After surgery, the mice were housed individually in plastic cages (30 × 30 × 40 cm) for recovery.
Microdialysis experiments were performed 3 or 4 days after implantation of the probe as previously described (Hanada et al., 2018). An online approach for real-time quantification of dopamine was used. The flow rate of Ringer’s solution was 2.0 µL/min. The 20-minute sample fractions collected through the dialysis probes were directly injected into high-performance liquid chromatography using a reverse-phase column (150 × 4.6 mm, Supelcosil LC18; Merck, Darmstadt, Germany) with electrochemical detection. An LC-20AD pump (Shimadzu Corporation, Kyoto, Japan) was used in conjunction with an electrochemical detector (potential of the first cell, +180 mV; potential of the second cell, −180 mV) (ESA, Chelmsford, MA). The mobile phase was a mixture of 4.1 g/L sodium acetate adjusted to pH 4.1, 100 mg/L Na2EDTA, 120 mg/L octanesulfonic acid, and 10% methanol. The flow rate was 0.4 mL/min. The detection limit of the assay was approximately 0.9 fmol per sample (on-column). The composition of Ringer’s solution was (in mM) NaCl 140.0, KCl 4.0, CaCl2 1.2, and MgCl2 1.0. At the end of the experiments, the mice were given an overdose of sevoflurane, and the brains were fixed with 4% paraformaldehyde via intracardiac infusion. Coronal sections (50 µm) were cut, and dialysis probe placement was localized using the atlas of Paxinos and Franklin (Paxinos and Franklin, 2004) (supplementary Figure 1). Misplacement of the microdialysis probes was not observed in any experimental animals used for the study. The representative traces of the microdialysis probe locations for the NAc and PFC are shown in supplementary Figure 1.
D1 Cell-Specific Expression of rM4D (Gi-DREADD) Using AAV Vectors
—For chemogenetic inhibition of PFC neurons expressing dopamine D1 receptors, rAAV-hsyn-DIO-rM4D(Gi)-mCherry (3.7 × 1012 virus molecules/mL), which was purchased from the University of North Carolina Vector Core (Chapel Hill, NC), and the control vector rAAV2-Ef1a-DIO-mCherry (3.2 × 1012 virus molecules/mL), which was purchased from the University of Pennsylvania Viral Core (Philadelphia, PA), were used. Viruses were infused bilaterally into the PFC of [Drd1]-Cre mice at 8 weeks of age under pentobarbital (50 mg/kg i.p.) and xylazine (8 mg/kg i.p.) anesthesia and local application of 10% lidocaine. The coordinates of the infusions into the PFC were A/P +1.9 mm, L/M ±0.3 mm from bregma, and V/D −2.25 mm from the dura (supplementary Figure 1). All infusions were performed using a 5-μL Hamilton syringe with a 33-G needle attached at a rate of 0.1 μL/min. To prevent reflux after infusion, the injection needle was left in place for 15 minutes and then withdrawn by a short distance (0.3 mm) every 3 minutes until the needle was completely removed. Four weeks later, cocaine conditioning and the CPP test were performed.
Statistical Analysis
The data are displayed as the mean ± SEM. For analyses of the microdialysis data, all values were expressed as a percentage of the basal values (100%) for each group, which was obtained as the average of 3 stable baseline samples for dopamine. The values obtained after injection of saline or cocaine and cue exposure were compared with the basal values using mixed models with time as a covariate, and Bonferroni’s correction was applied for multiple comparisons using the SAS MIMED procedure (version 9.4, SAS Institute, Cary, NC). Repeated-measures 2-way ANOVA was used to compare the experimental groups (JMP Pro, SAS Institute). The basal values of dopamine were analyzed by 1-way ANOVA and Welch’s t test (GraphPad Prism 8.4.3, GraphPad Software, San Diego, CA), as shown in supplementary Table 1. For analyses of behavioral data, the mixed model was used for the comparison of the changes in locomotor activity between groups and within the group, and 1-way ANOVA was used for the comparison of changes in locomotor activity within the group. Welch’s t test was used for the comparison of CPP scores between groups (SigmaPlot 14.0, Systat Software, San Jose, CA). P <.05 was considered to be significant.
RESULTS
Dopamine Responses to Cocaine-Associated Place Conditioning and Cocaine-Associated Cues in the NAc and PFC
After 3 days of cocaine conditioning (day 1–3) using the 2-connected chamber apparatus with a shielding plate (Figure 1A), CPP for cocaine was developed on day 4 (Welch’s t test, t(42) = −5.062, P < .0001) (Figure 1B). Dopamine levels in response to additional conditioning (day 7) and exposure to saline- and cocaine-associated cues (day 8) were determined using the 2-separated chamber apparatus consisting of saline and cocaine chambers with in vivo microdialysis (Figure 1A). In agreement with previous studies (Airavaara et al., 2004; Zhang et al., 2013), cocaine injection (7.5 mg/kg i.p.) on day 7 significantly increased dopamine levels in both the NAc (Figure 1E) and PFC (Figure 1I). The saline injections for conditioning also increased dopamine levels in both the NAc and PFC, although the effects on dopamine levels were relatively small (Figure 1C, G). The effects of saline injection were larger in the PFC (175% of basal levels) than in the NAc (120% of basal levels) (2-way ANOVA, region F [1, 54] = 0.0203, P = .8871; time F [8, 54] = 4.2452, P = .0005; region time interaction F [8, 54] = 5.3743, P < .0001).
Exposure of mice to cocaine-associated contextual cues (day 8) significantly increased dopamine levels in the PFC (Figure 1J) but did not affect dopamine levels in the NAc (Figure 1F). Exposure to saline-associated contextual cues slightly increased dopamine levels in the PFC (Figure 1H), but not in the NAc (Figure 1D).
We primarily speculated that cocaine-associated cues could increase dopamine levels in the NAc, because dopamine in the NAc is highly responsive to rewarding stimuli (Volkow and Morales, 2015), including cocaine-associated cues (Ito et al., 2000). To exclude the influence of the cocaine dose chosen for the conditioning, cocaine at a higher dose (15 mg/kg) (dela Cruz et al., 2009) was also used for conditioning (supplementary Figure 2). However, cocaine conditioning at the high dose also failed to induce the dopamine response to the cocaine-associated cue in the NAc (supplementary Figure 2D), indicating that dopamine in the NAc does not respond to the cocaine-associated cue (2-separated chamber apparatus) under the present experimental conditions. Based on these results, we focused on the PFC for further analyses of the role of dopaminergic neurotransmission in the cocaine-induced locomotor response and cocaine CPP.
Role of Dopamine and Ionotropic Glutamate Receptors in the PFC During Cocaine Conditioning in the Locomotor Response and Development of CPP
To analyze the role of dopamine and glutamate receptor signaling in the PFC in the locomotor response and development of CPP to cocaine, the activities of dopamine D1 and D2 receptors and ionotropic glutamate receptors were pharmacologically inhibited during conditioning for 3 consecutive days (day 1–3) (Figure 2A). In the control with infusion of Ringer’s solution into the PFC via a microdialysis probe, cocaine injections (7.5 mg/kg i.p.) induced an increase in locomotor activity on day 1 and day 3 compared with saline injection (Figure 2B) (1-way ANOVA, F [3, 72] = 24.320, P < .001). Infusion of a dopamine D1 receptor antagonist, SCH23390 (10 µM), or a D2 receptor antagonist, raclopride (10 µM), into the PFC suppressed cocaine-induced locomotion (Figure 2B) (SCH23390: 1-way ANOVA, F [3, 44] = 0.360, P = .782; raclopride: 1-way ANOVA, F [3, 40] = 2.955, P = .044) but failed to suppress the development of cocaine CPP (Figure 2C) (SCH23390: Welch’s t test, t(22) = −2.255, P = .0380; raclopride: Welch’s t test, t(20) = −2.655, P = .0180).
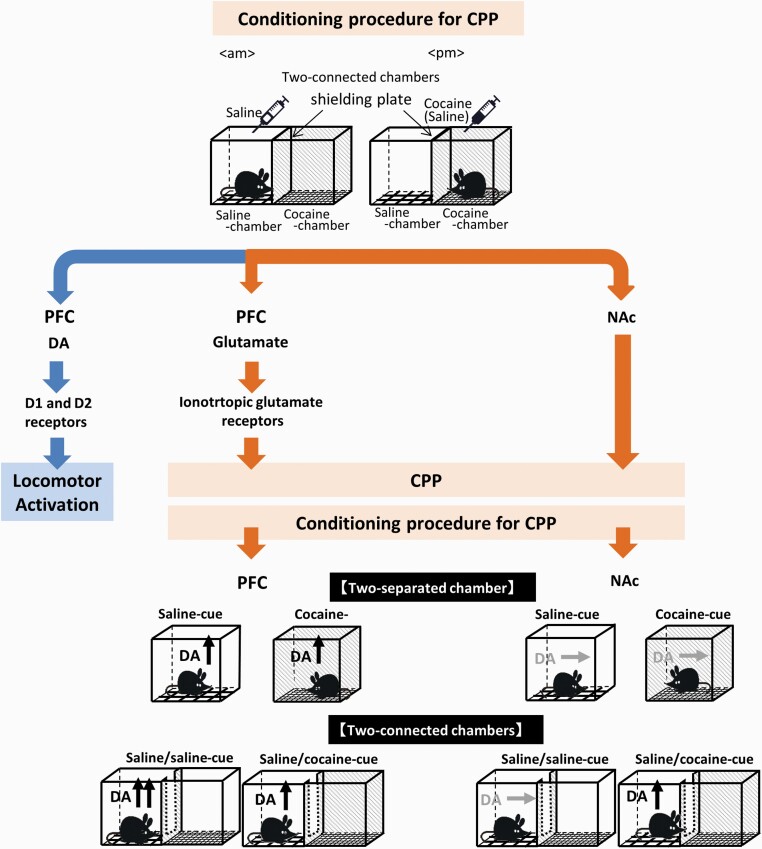
Infusion of the ionotropic glutamate receptor antagonists CNQX (10 µM) plus 3-2-Cpp (30 µM) into the PFC did not affect the locomotor response to cocaine during conditioning (Figure 2B) (1-way ANOVA, F [3, 36] = 4.511, P = .009) but suppressed cocaine CPP (Figure 2C) (Welch’s t test, t(18) = 0.634, P = .538). The results suggest that D1 and D2 receptors mediate the locomotor response to cocaine, whereas ionotropic NMDA/AMPA receptors mediate the development of CPP to cocaine and dopamine responses to cues (Figure 5).
Figure 5.
Schematic representation of the distinct role of the dopamine pathways in the prefrontal cortex (PFC) and nucleus accumbens (NAc) in the cocaine conditioned place preference (CPP). This study demonstrates that the dopamine (DA) response in the PFC plays an important role in the recognition of cocaine-associated cues in the cocaine CPP, whereas the DA response in the NAc is related to motivated exploration of cocaine reward. Ionotropic glutamate receptor signaling in the PFC is activated during the cocaine conditioning, leading to the development of cocaine CPP and the induction of the DA response to the cocaine-associated cues in the PFC. The DA responses in the PFC did not differ between 2-separated and 2-connected chambers. In contrast, dopamine in the NAc is responsive to the cocaine-associated cues when 2-connected chambers, but not 2-separated chambers, are used, suggesting that DA in the NAc plays important roles in the exploratory process of the cocaine-associated cues. DA in the PFC also responded to the saline-associated cues via mechanisms involving ionotropic glutamate receptor and DA D1 receptor signaling. In addition, the locomotor response to cocaine is mediated through activation of DA D1 and D2 receptor signaling in the PFC.
We also investigated the effects of chemogenetic inhibition of D1 receptor-expressing neurons in the PFC. Gi-DREADD or control (mCherry) virus was injected into the PFC of [Drd1]-Cre mice to induce expression of Gi-DREADD in D1 receptor-positive neurons (supplementary Figure 3). CNO was administered before cocaine conditioning, but not before saline conditioning, to avoid an overdose of CNO. In [Drd1]-Cre mice expressing mCherry, cocaine with CNO injections induced an increase in locomotor activity during conditioning compared with locomotor activity during saline conditioning without CNO (Figure 2D) (1-way ANOVA, F [3, 16] = 8.542, P = .001). CNO injections in [Drd1]-Cre mice expressing Gi-DREADD suppressed cocaine-induced locomotion on day 1 and day 3 (Figure 2D) (1-way ANOVA, F [3, 20] = 0.247, P = .863) but failed to suppress cocaine CPP (Figure 2E) (Welch’s t test, t(10) = 2.316, P = .0434). These results suggest that activation of D1 receptor-expressing neurons is required for the locomotor response to cocaine, but not for the development of cocaine CPP.
Effects of Dopamine Receptor or Ionotropic Glutamate Receptor Antagonists on the Dopamine Response to Saline- or Cocaine-Associated Cues in the PFC
Because dopamine receptors and ionotropic glutamate receptors in the PFC contributed to the locomotor response and CPP to cocaine, respectively, we next examined whether dopamine and/or glutamate receptor signaling is involved in the dopamine response to cocaine-associated cues in the PFC. For this purpose, dopamine or glutamate receptor antagonists were infused into the PFC during conditioning (day 1–3), as shown in Figure 2A. Following conditioning, the dopamine responses to the cocaine-associated cue in the PFC were determined on day 4 without performing the CPP test (Figure 3). The basal levels of dopamine in the PFC were similar among groups that received the infusion of various antagonists during conditioning (1-way ANOVA, F [3, 20] = 0.4711, P = .7058). The dopamine response to the cocaine-associated cue was suppressed in the group that received infusion of CNQX plus 3-2-Cpp (Figure 3H) compared with that in the control group infused with Ringer’s infusion (Figure 3E) (2-way ANOVA, group F [1, 99] = 30.4289, P < .0001; time F [8, 99] = 5.4126, P < .0001; group time interaction F [8, 99] = 5.8059, P < .0001). However, the dopamine responses to the cocaine-associated cue were not affected by the infusion of SCH23390 (Figure 3F) (2-way ANOVA, group F [1, 90] = 0.0009, P = .9757; time F [8, 90] = 8.9483, P < .0001; group time interaction F [8, 90] = 2.9035, P = .0063) or raclopride (Figure 3G) (2-way ANOVA, group F [1, 72] = 2.7678, P = .1005; time F [8, 72] = 7.3933, P < .0001; group time interaction F [8, 72] = 1.0255, P = .4272). Because blockade of NMDA/AMPA receptors in the PFC also suppressed cocaine CPP (Figure 2C), the dopamine response to the cocaine-associated cue could be related to the reward effect of cocaine.
Interestingly, the dopamine response to the saline-associated cue was suppressed by CNQX plus 3-2-Cpp (Figure 3D) (2-way ANOVA, group F [1, 104] = 19.6877, P < .0001; time F [8, 104] = 7.1483, P < .0001; group time interaction F [8, 104] = 2.6771, P = .0102) or SCH23390 (Figure 3B) (2-way ANOVA, group F [1, 91] = 6.1804, P = .0147; time F [8, 91] = 5.8942, P < .0001; group time interaction F [8, 91] = 2.1441, P = .0393), but not by raclopride (Figure 3C) (2-way ANOVA, group F [1, 73] = 0.7891, P = .3773; time F [8, 73] = 6.3099, P < .0001; group time interaction F [8, 73] = 1.1956, P = .3137). These results suggest that the dopamine response to saline-associated cues is mediated through ionotropic glutamate and D1 receptor signaling, whereas the dopamine response to reward-associated cues is largely mediated through ionotropic glutamate receptor signaling.
Dopamine Responses in the PFC and NAc to Exploration of Cocaine- or Saline-Associated Cues
The dopamine response to the cocaine-associated cue was absent in the NAc, as shown in Figure 1F. In the experimental paradigm, mice were exposed to the saline and cocaine chambers separately since the 2-separated chamber apparatus was used during dopamine measurements. However, in many previous studies, a 2- or 3-connected chamber apparatus, where mice were allowed to freely explore the entire apparatus, was used for the assessment of CPP expression (Prus et al., 2009; Alvarez, 2016). The difference in the CPP apparatus between the 2-separated and 2-connected chambers could affect the dopamine response in the NAc. Therefore, mice were exposed to the 2-connected chambers, where mice were allowed to explore both the saline and cocaine chambers (Figure 4C). When mice subjected to cocaine conditioning (saline/cocaine) explored the entire apparatus, an increase in dopamine levels in the NAc was observed (Figure 4C). However, dopamine response in the NAc was not observed in mice subjected to saline conditioning (saline/saline) (Figure 4C), in which no place preference was detected (Figure 4B) (Welch’s t test, t(14) = −0.5906, P = .5643) in contrast to mice subjected to cocaine conditioning (Figure 1B). In the PFC, when mice were exposed to the 2-connected chambers, dopamine levels increased in both mice subjected to cocaine and saline conditioning (Figure 4D). The dopamine response in mice subjected to saline conditioning was larger than that in mice subjected to cocaine conditioning (2-way ANOVA, group F [1, 84] = 6.7190, P = .0112; time F [8, 84] = 11.0094, P < .0001; group time interaction F [8, 84] = 1.7732, P = .0938). These results suggest that the motivated exploration of cocaine-associated cues and the expectation of cocaine injection likely trigger the dopamine response in the NAc, whereas the dopamine response in the PFC is triggered both by the exploration of saline- and cocaine-associated cues.
Because the increases in dopamine in the PFC were induced by the exposure to the saline-associated chamber(s) of 2-separated and 2-connected chamber apparatus (Figures 1H, 3A, and 4D), we asked the question of which procedure of saline conditioning is responsible for the dopamine response as stressful stimuli: repeated injections of saline, temporal holding for dopamine recording, or transfer to chamber(s) from home cage. For this purpose, dopamine levels were determined on day 4 in mice subjected to the conditioning procedures without saline injections (supplementary Figure 4). Returning to home cage (acrylic box for microdialysis) after temporal holding or exposure to the 2-separated chamber or 2-connected chambers after handling did not affect dopamine levels in the PFC. These findings suggest that aversive stimuli of the repeated injections of saline are associated with contexts of saline chamber(s), leading to the induction of dopamine response to the saline-associated cues in the PFC.
Discussion
This in vivo microdialysis study demonstrated that the extracellular levels of dopamine increased in the PFC, but not in the NAc, in response to cocaine-associated cues (2-separated chamber apparatus) in our cocaine CPP and dopamine measurement paradigms. The dopamine response to cocaine-associated cues and cocaine CPP was abolished by blockade of ionotropic glutamate receptors, but not by blockade of D1 or D2 receptors, in the PFC during cocaine conditioning. These results indicate that ionotropic glutamate receptor signaling in the PFC is required for the induction of the dopamine response to cocaine-associated cues and the development of cocaine CPP. Analyses using a 2-connected chamber apparatus revealed that the cocaine-associated cue that allows exploration of the 2-connected chambers induces the dopamine response in the NAc. Taken together, the results suggest that the dopamine response in the PFC plays an important role in the recognition of cocaine reward-associated cues, whereas the dopamine response in the NAc is related to the motivated exploration associated with cocaine reward or the decision-making that involves weighing 2 choices (Figure 5).
Role of the Dopamine Response to Cocaine-Associated Cues in the PFC in the Expression of Cocaine CPP
The role of dopaminergic neurotransmission in the PFC has been implicated in the expression of cocaine CPP (Milella et al., 2016; Shinohara et al., 2017). In the CPP paradigm, Shinohara et al. (2017) demonstrated that the increase in dopaminergic neurotransmission from the VTA to PFC and subsequent activation of dopamine D1 receptors in the PFC play a critical role in the expression of cocaine CPP in rats. In our cocaine CPP paradigms, we were able to demonstrate that dopamine levels in the PFC increase in response to cocaine-associated cues in C57BL/6N mice. The dopamine response in the PFC is likely associated with the recognition of the cocaine chamber context, which is required for the expression of cocaine CPP.
Induction of the Dopamine Response to Cocaine-Associated Cues and Development of Cocaine CPP Are Regulated by Ionotropic Glutamate Receptors in the PFC
Activation of glutamatergic pyramidal neurons in the medial PFC (mPFC) is clearly shown to mediate both the development and expression of cocaine CPP using chemogenetic techniques (Zhang et al., 2020). In addition, optogenetic stimulation of VTA-PFC dopaminergic circuits has been shown to activate mPFC pyramidal neurons (Buchta et al., 2017) via mechanisms involving D1 receptors (Shinohara et al., 2017). In this study, pharmacological blockade of ionotropic glutamate NMDA/AMPA receptors in the PFC during conditioning abolished the induction of the dopamine response to cocaine-associated cues and the development of cocaine CPP. The present results are in agreement with previous reports that showed that repeated cocaine administration induces the alteration of the synaptic plasticity of excitatory synapses in the layer V mPFC (Huang et al., 2007). Ionotropic NMDA/AMPA receptor signaling may be required for the enhanced activation of a subset of pyramidal neurons in the PFC during cocaine conditioning. We hypothesize that cocaine conditioning induces the activation of glutamatergic neurotransmission in the PFC via mechanisms involving VTA-PFC dopaminergic circuits. After conditioning, the cocaine-associated cues invoke retrieval of the cocaine-context association by activating the subset of pyramidal neurons in the PFC, which sends output projections to the VTA (Carr and Sesack, 2000), activates VTA-PFC dopamine neurons, and induces the dopamine response in the PFC (Enrico et al., 1998; Butts and Phillips, 2013).
Pharmacological blockade of dopamine D1 or D2 receptors or chemogenetic inhibition of D1 receptor-expressing neurons in the PFC during conditioning had no effect on the induction of the dopamine response to cocaine-associated cues and the development of cocaine CPP. It has been reported that D1 receptor signaling in the PFC is required for the expression of cocaine CPP (Shinohara et al., 2017). It is likely that D1 receptor signaling in the PFC plays a critical role in the expression of cocaine CPP but a limited role in the development of cocaine CPP. However, D1 receptors, but not D2 receptors, are reported to play an essential role in the development of cocaine CPP (Cervo and Samanin, 1995; Gu et al., 2020). In fact, cocaine CPP does not develop in D1 receptor knockout mice (Chen and Xu, 2010). In the mesocorticolimbic reward pathway, D1 receptors are highly expressed in D1-type MSNs in the NAc. The CPP acquisition test induces activation of ERK in the PFC and NAc via D1 receptor-mediated mechanisms (Chen and Xu, 2010). D1-type MSNs in the NAc core are recruited in the hippocampus (ventral CA1)-NAc core engram cell circuit, which stores cocaine reward-associated contextual information (Zhou et al., 2019). This information suggests that D1 receptors in the NAc and PFC may be involved in the induction of the dopamine response and the development of cocaine CPP, but D1 receptors in the NAc play a dominant role compared with those in the PFC. Therefore, cocaine CPP could be developed under blockade of D1 receptors in the PFC during conditioning in our CPP paradigms.
Cocaine-Induced Locomotion Is Regulated by Dopamine Receptors in the PFC
Pharmacological blockade of D1 receptors and chemogenetic inhibition of D1 receptor-expressing neurons in the PFC during conditioning suppressed the locomotor response to cocaine, suggesting the requirement of D1 receptor signaling in the PFC for cocaine-induced locomotion. Several studies indicate that region-specific D1 receptor signaling in the PFC contributes to the locomotor response to cocaine in sensitized animals (Sorg et al., 2001; Cosme et al., 2018). mPFC neurons expressing D1 receptors project strongly to the motor control loop, especially to the components of the motor cortex, striatum, and thalamic nuclei (Han et al., 2017). PFC neurons expressing D1 receptors could be activated by dopamine following cocaine injections and mediate the motor effect of cocaine through activation of the motor control loop. Indeed, cocaine injection induces activation of ERK in D1-type MSNs in the striatum via mechanisms involving dopamine and glutamate (Valjent et al., 2005).
Pharmacological blockade of D2 receptors in the PFC during conditioning also suppressed cocaine-induced locomotion. However, there are several reports showing that activation of D2 receptors in the PFC suppresses the excitability of pyramidal neurons (Seamans and Yang, 2004), blocks the acute locomotor response to cocaine (Beyer and Steketee, 2000), and suppresses the development and expression of behavioral sensitization to cocaine (Beyer and Steketee, 2002). The reason for the discrepancy is not currently understood, and further studies are required.
Dopamine Responses to Saline-Associated Cues Are Regulated by Ionotropic Glutamate and D1 Receptors in the PFC
Dopamine levels in the PFC increased in response to saline injection and saline-associated cues, suggesting that repeated saline injections were recognized as aversive stimuli. This interpretation is supported by our findings that the mice subjected to handling and/or transferring to chambers without repeated injections of saline/cocaine did not show the increase in dopamine levels in the PFC. Activation of ionotropic glutamate and D1 receptor signaling during conditioning is required for the induction of the dopamine response to saline-associated cues. Glutamate transmission evokes dopamine release when stressful stimuli are applied (Kawahara et al., 2002). Stressful stimuli may induce plastic changes in the subset of pyramidal neurons in the PFC due to activated ionotropic glutamate and dopamine D1 receptor signaling. Saline-associated cues can be recognized as aversive cues when the subset of aversive stimuli-associated pyramidal neurons and VTA-PFC dopamine circuits is activated.
Dopamine increased in the PFC in response to both cocaine- and injection stress–associated cues, but these conditioned dopamine responses likely involved different mechanisms: the involvement of D1 receptor signaling in addition to ionotropic glutamate receptor signaling in saline conditioning but not in cocaine conditioning. It was surprising that, in the 2-connected chamber apparatus, the dopamine response in mice subjected to saline conditioning (saline/saline) was larger than that in mice subjected to cocaine conditioning (saline/cocaine). This raises the possibility that the dopamine response to aversive cues in the PFC is quite large, but it can be attenuated when the repeated injections of cocaine, which induce rewarding effects, are combined.
Dopamine in the NAc Responds to the Exploratory Process of Cocaine-Associated Cues
The dopamine response to the cocaine-associated cue was absent in the NAc when mice were directly placed in the cocaine-associated chamber (2-separated chamber apparatus), although there was a report showing a dopamine response in the NAc core in rats using a 1-chamber apparatus (Duvauchelle et al., 2000). The absence of a dopamine response was unexpected, because dopamine neurotransmission in the NAc has been linked to reward-associated cues (Volkow and Morales, 2015). The absence of dopamine response in this study is not likely due to the localization of dialysis probes at the small anatomical structure of mouse NAc, which are placed between shell and core and can collect dopamine from the NAc core. Various differences of experimental conditions involving strain (mouse vs rat), route of cocaine administration (i.p. injection vs i.v. administration) (Wenzel et al., 2015; Estave et al., 2020), and conditioning (forced administration vs self-administration) (Ito et al., 2000) could account for the inconsistency between this and the previous studies (Duvauchelle et al., 2000), respectively.
However, the dopamine response in the NAc was observed when mice were placed in 2-connected chambers requiring a motivated exploratory process. Interestingly, in line with our findings, D1-type MSNs in the NAc encode contextual information on cocaine reward, and the increased activity of D1-type MSNs precedes entry into the cocaine-associated context (Calipari et al., 2016). Together with the present results, the difference in the CPP apparatus between 1-chamber and 2-connected chambers critically affects the dopamine response in the NAc in mice, and dopamine in the NAc responds to motivated exploration associated with cocaine reward or alternatively to decision-making that involves weighing 2 choices.
Daily cocaine administration has been shown to induce cocaine expectation associated with the increased basal dopamine levels in the rat NAc core at the time of chronic cocaine injection (Puig et al., 2012). Because dopamine in the NAc was measured at the time of cocaine conditioning (midafternoon), a possibility that cocaine expectation affects the dopamine response in the NAc cannot be ruled out. However, dopamine responded to exposure to 2-connected chambers, but not to 2-separated chambers, despite the measurement at the similar time, suggesting that the differences of the dopamine response are attributable to apparatus configuration rather than cocaine expectation at the specific time. It is not known whether cocaine expectation at the time of chronic cocaine injection affects the dopamine levels in the PFC. The contribution of cocaine expectation to the dopamine response in the PFC needs to be clarified in future studies.
In conclusion, the present study provided evidence for the distinct roles of the mesocortical and mesolimbic dopamine pathways in the recognition of cocaine-associated context and drug-seeking behaviors. Full elucidation of the mechanisms of the activation of the mesocortical and mesolimbic dopamine pathways in response to drug-associated contextual cues may facilitate the development of novel therapeutic strategies for drug addiction.
Supplementary Material
Acknowledgments
We acknowledge Prof. Tatsuyuki Kakuma for his assistance with the statistical analysis. This manuscript was edited for English language by American Journal Experts (AJE).
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP26460328 and JP19K08332 to Y.K.; JP15K01837 and JP18K07401 to Y.N.O.; JP16K16648 to Y.H.O.; JP18K09756 to H.K.; JP16H05135 and JP19H03410 to A.N.) and a grant from the Ishibashi Foundation for the Promotion of Science to Y.K.
Interest Statement
None.
References
- Airavaara M, Planken A, Gäddnäs H, Piepponen TP, Saarma M, Ahtee L (2004) Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci 20:2336–2344. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA (2016) Clues on the coding of reward cues by the nucleus accumbens. Proc Natl Acad Sci U S A 113:2560–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos C, Knowland D, Wu MMJ, Lilascharoen V, Huang KW, Malenka RC, Lim BK (2018) Cocaine-induced structural plasticity in input regions to distinct cell types in nucleus accumbens. Biol Psychiatry 84:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G (1997) Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci 17:851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD (2000) Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology 151:211–218. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD (2002) Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex 12:526–535. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR (2000) Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci 20:3874–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC (1992) Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience 48:621–629. [DOI] [PubMed] [Google Scholar]
- Buchta WC, Mahler SV, Harlan B, Aston-Jones GS, Riegel AC (2017) Dopamine terminals from the ventral tegmental area gate intrinsic inhibition in the prefrontal cortex. Physiol Rep 5:e13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Phillips AG (2013) Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol 16:1799–1807. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ (2016) In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A 113:2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR (2000) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20:3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarzi D, Lenzi O, Colotta V, Varano F, Poli D, Filacchioni G, Lingenhöhl K, Ofner S (2010) Pharmacological characterization of some selected 4,5-dihydro-4-oxo-1,2,4-triazolo[1,5-a]quinoxaline-2-carboxylates and 3-hydroxyquinazoline-2,4-diones as (S)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)-propionic acid receptor antagonists. Chem Pharm Bull 58:908–911. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Lee KW, Cahill ME, Graves SM, Dietz D, Surmeier DJ, Nestler EJ, Nairn AC, Greengard P, Kim Y (2017) WAVE1 in neurons expressing the D1 dopamine receptor regulates cellular and behavioral actions of cocaine. Proc Natl Acad Sci U S A 114:1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R (1995) Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res 673:242–250. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu M (2010) Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem 114:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, Worth WR, LaLumiere RT (2018) D1, but not D2, receptor blockade within the infralimbic and medial orbitofrontal cortex impairs cocaine seeking in a region-specific manner. Addict Biol 23:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Cruz AM, Herin DV, Grady JJ, Cunningham KA (2009) Novel approach to data analysis in cocaine-conditioned place preference. Behav Pharmacol 20:720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castaneda E (2000) Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav Neurosci 114:1156–1166. [DOI] [PubMed] [Google Scholar]
- Ellwood IT, Patel T, Wadia V, Lee AT, Liptak AT, Bender KJ, Sohal VS (2017) Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. J Neurosci 37:8315–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrico P, Bouma M, de Vries JB, Westerink BH (1998) The role of afferents to the ventral tegmental area in the handling stress-induced increase in the release of dopamine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Brain Res 779:205–213. [DOI] [PubMed] [Google Scholar]
- Estave PM, Spodnick MB, Karkhanis AN (2020) KOR control over addiction processing: an exploration of the mesolimbic dopamine pathway. Handb Exp Pharmacol 10.1007/164_2020_421. doi: 10.1007/164_2020_421. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N (2005) Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol 7:45–52. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27:9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Wise RA (1994) Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci 14:4130–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SM, Cha HJ, Seo SW, Hong JT, Yun J (2020) Dopamine D1 receptor antagonist reduces stimulant-induced conditioned place preferences and dopamine receptor supersensitivity. Naunyn Schmiedebergs Arch Pharmacol 393:131–138. [DOI] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J (2009) A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A 106:19197–19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Kim YC, Narayanan NS (2017) Projection targets of medial frontal D1DR-expressing neurons. Neurosci Lett 655:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada Y, Kawahara Y, Ohnishi YN, Shuto T, Kuroiwa M, Sotogaku N, Greengard P, Sagi Y, Nishi A (2018) p11 in cholinergic interneurons of the nucleus accumbens is essential for dopamine responses to rewarding stimuli. eNeuro 5:ENEURO. 0332-18.2018. Doi:10.1523/ENEURO.0332-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K (2013) Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lin HJ, Hsu KS (2007) Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cereb Cortex 17:1877–1888. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000) Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20:7489–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH (2000) The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol 387:279–286. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH (2002) Hypotension-induced dopamine release in prefrontal cortex is mediated by local glutamatergic projections at the level of nerve terminals. J Neurochem 81:285–291. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inoue O (1993) An increase in the in vivo binding of [3H]SCH 23390 induced by MK-801 in the mouse striatum. Neuropharmacology 32:341–348. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, Wu H, Wang X, Wang X (2016) NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience 315:45–69. [DOI] [PubMed] [Google Scholar]
- Lupinsky D, Moquin L, Gratton A (2017) Interhemispheric regulation of the rat medial prefrontal cortical glutamate stress response: role of local GABA- and dopamine-sensitive mechanisms. Psychopharmacology 234:353–363. [DOI] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ (2011) Post-translational modification biology of glutamate receptors and drug addiction. Front Neuroanat 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella MS, Fotros A, Gravel P, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Reader AJ, Dagher A, Benkelfat C, Leyton M (2016) Cocaine cue-induced dopamine release in the human prefrontal cortex. J Psychiatry Neurosci 41:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castañeda E, Fuchs RA (1996) Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15:506–514. [DOI] [PubMed] [Google Scholar]
- Osorio-Gómez D, Guzmán-Ramos K, Bermúdez-Rattoni F (2017) Memory trace reactivation and behavioral response during retrieval are differentially modulated by amygdalar glutamate receptors activity: interaction between amygdala and insular cortex. Learn Mem 24:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB (2004) The mouse brain in stereotaxic coordinates. Compact. 2nd ed. San Diego, CA: Academic Press. [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618. [DOI] [PubMed] [Google Scholar]
- Porter JT, Sepulveda-Orengo MT (2020) Learning-induced intrinsic and synaptic plasticity in the rodent medial prefrontal cortex. Neurobiol Learn Mem 169:107117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA (2009) Conditioned place preference. In: Methods of behavior analysis in neuroscience. 2nd ed (Buccafusco JJ, ed) pp81–90. Boca Raton, FL: CRC Press/Taylor & Francis. [Google Scholar]
- Puig S, Noble F, Benturquia N (2012) Short- and long-lasting behavioral and neurochemical adaptations: relationship with patterns of cocaine administration and expectation of drug effects in rats. Transl Psychiatry 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Shinohara F, Kamii H, Minami M, Kaneda K (2017) The role of dopaminergic signaling in the medial prefrontal cortex for the expression of cocaine-induced conditioned place preference in rats. Biol Pharm Bull 40:1983–1989. [DOI] [PubMed] [Google Scholar]
- Shukla A, Beroun A, Panopoulou M, Neumann PA, Grant SG, Olive MF, Dong Y, Schlüter OM (2017) Calcium-permeable AMPA receptors and silent synapses in cocaine-conditioned place preference. Embo J 36:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Kuroiwa M, Sotogaku N, Kawahara Y, Oh YS, Jang JH, Shin CH, Ohnishi YN, Hanada Y, Miyakawa T, Kim Y, Greengard P, Nishi A (2020) Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol Psychiatry 25:1229–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Li N, Wu WR (2001) Dopamine D1 receptor activation in the medial prefrontal cortex prevents the expression of cocaine sensitization. J Pharmacol Exp Ther 297:501–508. [PubMed] [Google Scholar]
- Thibeault KC, Kutlu MG, Sanders C, Calipari ES (2019) Cell-type and projection-specific dopaminergic encoding of aversive stimuli in addiction. Brain Res 1713:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Hervé D, Girault JA (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A 102:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Rauscher NA, Cheer JF, Oleson EB (2015) A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem Neurosci 6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA (2002) Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Yanagida J, Kamii H, Wada S, Domoto M, Sasase H, Deyama S, Takarada T, Hinoi E, Sakimura K, Yamanaka A, Maejima T, Mieda M, Sakurai T, Nishitani N, Nagayasu K, Kaneko S, Minami M, Kaneda K (2020) Glutamatergic neurons in the medial prefrontal cortex mediate the formation and retrieval of cocaine-associated memories in mice. Addict Biol 25:e12723. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ (2013) Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology 67:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, Tian Z, Huang B, Yan E, Liu X, Ma L (2019) A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci 22:1986–1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.