Abstract
BACKGROUND:
Ketamine provides rapid antidepressant response in those struggling with major depressive disorder (MDD). This study measured acute changes in brain activity over 24 hours after a single infusion of ketamine using arterial spin labeled (ASL) functional magnetic resonance imaging (fMRI) in patients with MDD. ASL is a novel technique that provides quantitative values to measure cerebral blood flow (CBF).
METHODS:
A single sub-anesthetic dose (0.5 mg/kg) of ketamine was delivered intravenously. Treatment-refractory patients (n=11) were assessed at: Baseline (pre-infusion), and approximately 1hr, 6hrs, and 24hrs post-infusion. Linear mixed-effects models detected changes in CBF with respect to treatment outcome, and results were corrected for false discovery rate (FDR).
RESULTS:
After ketamine infusion, increased CBF was observed in the thalamus, while decreased CBF was observed in lateral occipital cortex in all patients. Time-by-response interactions were noted in ventral basal ganglia and medial prefrontal cortex, where CBF change differed according to antidepressant response.
LIMITATIONS:
Modest sample size is a limitation of this pilot study; strict statistical correction and visualization of single-subject data attempted to ameliorate this issue.
CONCLUSION:
In this pilot study, a sub-anesthetic dose of ketamine was associated with acute neurofunctional changes that may be consistent with altered attention, specifically increased thalamus activity coupled with decreased cortical activity. By contrast, antidepressant response to ketamine was associated with changes in reward-system regions, specifically ventral basal ganglia and medial prefrontal cortex. Further work is needed to determine whether these results generalize to larger samples and/or serial ketamine infusions associated with longer-lasting clinical effects.
Keywords: ketamine, antidepressant, treatment resistant depression, arterial spin labeling, cerebral blood flow, functional magnetic resonance imaging
INTRODUCTION
Depression is a pervasive health issue, with an estimated 16.2 million U.S. adults having experienced at least one depressive episode (Ahrnsbrak et al., 2017). However, an estimated 12%–20% of those adults were unsuccessful in achieving remission after two or more pharmacotherapies, rendering them treatment resistant (McIntyre et al., 2014; Souery et al., 1999). Seeing as this is a major issue, rapid-acting treatment alternatives like ketamine are a focus of ongoing clinical research.
At high doses, ketamine is used clinically as an anesthetic, first synthesized by chemists Calvin Stevens and Parke Davis consultant in 1962 (Li and Vlisides, 2016; Schwartz et al., 2016). At subanesthetic doses, ketamine has been shown to provide analgesic and psychotomimetic effects (Gorlin et al., 2016; Krystal et al., 1994), as well as rapid antidepressant effects at lower doses (Newport et al., 2015; Zarate et al., 2006).Indeed, multiple clinical trials have shown the efficacy of ketamine’s rapid antidepressant response in those with severe depression (Lapidus et al., 2014; McGirr et al., 2015; Wan et al., 2015; Zarate et al., 2006)and acute suicidality (Lee et al., 2015; Price et al., 2009). However, it remains unclear how ketamine affects the brain to induce these antidepressant effects.
Ketamine is an antagonist of the N-methyl-D-aspartate (NMDA) receptor, inhibiting the binding of glutamate, the major excitatory neurotransmitter of the CNS (Li and Vlisides, 2016). Literature suggests glutamate levels in the brain may be affected in mood disorders (Mathews et al., 2012), also demonstrated in animal models of depression (Koike et al., 2011). Low levels of glutamate-related metabolites have been detected in depressed patients by a large number of neuroimaging studies in the medial prefrontal cortex, anterior cingulate cortex, left dorsal lateral prefrontal cortex, amygdala, and hippocampus (Abdallah et al., 2018; Auer et al., 2000; Block et al., 2009; Hasler et al., 2007; Luykx et al., 2012; Nikolaus Michael et al., 2003; N. Michael et al., 2003; Pfleiderer et al., 2003; Stone et al., 2012; Taylor et al., 2012; Yüksel and Öngür, 2010). This corresponds well with studies implicating the prefrontal cortex, limbic, basal ganglia, and brainstem regions in depression using other neuroimaging modalities (Pandya et al., 2012; Price and Drevets, 2010), including changes in resting brain function and connectivity in these regions (Evans et al., 2018; Leaver et al., 2016). We hypothesize that, while ketamine may have nonspecific effects on resting brain function in all patients, the drug may preferentially influence these brain regions previously associated with depression in patients who respond to ketamine. Indeed, recent neuroimaging studies suggest that ketamine may influence activity in these brain regions in people with depression (Abdallah et al., 2017b; Sahib et al., 2020), and a compelling study demonstrated decreased activity in medial prefrontal and subgenual anterior cingulate after ketamine in nondepressed controls (Deakin et al., 2008). However, evidence remains sparse.
In this open-label mechanistic pilot study, we investigated the effects of ketamine on resting brain activity via a functional neuroimaging technique, arterial spin-labelled (ASL) MRI, which yields a quantitative measure of cerebral blood flow as a proxy for brain activity. Based on previous clinical trials of ketamine for severe depression, a subanesthetic dose of 0.5 mg/kg was administered intravenously, which optimizes bioavailability in comparison to intranasal or intramuscular methods (Chilukuri et al., 2014; Li and Vlisides, 2016). Our objective was to observe how ketamine treatment affects resting brain activity acutely in the hours after treatment, measured with MRI before, and 1, 6, and 24 hours after infusion. Critically, statistical analyses measured both monotonic and nonmonotonic trajectories of CBF change, while also attempting to dissociate nonspecific effects of ketamine from changes associated with antidepressant response (i.e., CBF change occurring in all patients while controlling for outcome vs. CBF changes correlated with symptom improvement). We anticipate that the results of this exploratory pilot study will inform approaches to larger scale studies of longitudinal effects of ketamine therapy on the depressed brain.
MATERIALS AND METHODS
Subjects
Depressed patients (n=11) provided informed written consent and acknowledgment from their treating physician prior to participating in this pilot study. Volunteers were between the ages of 27 and 62 years old, and met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV TR) (American Psychiatric Association, 2000) criteria for non-psychotic major depression. Patients (1) were in a current depressive episode for at least 6 months, (2) had not responded to at least 2 adequate antidepressant trials, (3) had not changed antidepressant usage for the past 1-month, and (4) had no contraindications to a joint trial of ketamine infusion.
Ketamine Therapy
Participants were administered a single sub-anaesthetic dose (0.5 mg/kg) of ketamine diluted in 60 cc normal saline administered intravenously over 40 minutes (Ibrahim et al., 2012; Niciu et al., 2014; Zarate et al., 2006). Vital signs were monitored every 3 minutes for any detections of blood pressure increase, respiratory rate decrease, or any unusual behavioral or psychological effects. No complications were experienced during ketamine infusion for any of the patients in this study.
Clinical Assessments
The following assessment scores were documented for each of the pre- and post-infusion time points: Hamilton Depression Rating Scale, 17 item (HAMD-17) (Hamilton, 1980), Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR) (Rush et al., 2003).
Image Acquisition
MR images were obtained using a 3T Siemens Allegra MRI scanner across 4 timepoints: at baseline prior to ketamine infusion and approximately 1hr, 6hrs and 24hrs after the end of infusion. Timepoints were chosen to capture well-established robust antidepressant response occurring 24 hours after infusion, as well as more modest response demonstrated to occur within hours of infusion in some studies (Ibrahim et al., 2011; Zarate et al., 2006). T1-weighted anatomical (MPRAGE) and fMRI continuous ASL (fMRI-ASL) scans were acquired at each scanning time point. The following continuous ASL sequence was used: 60 volumes (30 label, 30 control), 4×4×7.5 mm3 resolution, 18 axial slices, repetition time 4000 ms, echo time 16 ms, label time 2100 ms, post label delay 1000 ms, and 95% duty cycle. Structural imagines were T1-weighted echo-planar navigated multiecho MRPAGE: echo/repetition times 1.74, 3.6, 5.46, 7.32/2530ms, inversion time 1260ms, flip angle 7 °, field of view 256 × 256 mm2, 192 sagittal slices, 1.3×1×1 mm3 resolution. Subjects were asked to close their eyes and rest while ASL data was acquired. Due to technical issues during image acquisition, ASL-fMRI data were not collected for one baseline scan and four follow-up scans (across 3 subjects); adjusted sample sizes are displayed in Table 1. Data for remaining timepoints were retained for analysis, and mixed effects statistical models were applied to compensate for missing datapoints (below).
Table 1.
Patient Characteristics
| Demographics | Depressed | |||
|---|---|---|---|---|
|
| ||||
| Sample Size | n = 11 | |||
| Age, Mean (SD) | 47.7 (11.90) | |||
| Sex, Females : Males | 3:8 | |||
| Baseline | Post-1hr | Post-6hr | Post-24hr | |
|
| ||||
| HDRS-17, Mean (SD) | 22.44 (3.68) | 13.33 (9.39) | 9.80 (8.48) | 9.00 (7.67) |
| MADRS, Mean (SD) | 34.89 (3.44) | 21.11 (13.52) | 15.30 (12.54) | 16.00 (13.58) |
| QIDS-SR, Mean (SD) | 17.78 (7.60) | 13.44 (7.60) | 10.80 (8.50) | 10.40 (6.69) |
| Corrected Sample Size | n = 10 | n = 9 | n = 10 | n = 10 |
Abbreviations: HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptoms.
Image Preprocessing
Image preprocessing was completed in ASLtoolbox using MATLAB (The Mathworks, Inc.), and FSL (FMRIB, Functional MRI of the Brain). ASL offers a quantitative measure of CBF directly measured with MRI in units mL/100g/min, which can be interpreted as reflecting metabolic needs (i.e., activity) of neurons and related cells. The quantitative nature of ASL offers an advantage over the more typical blood-oxygenation-level-dependent (BOLD) signal, where inferences regarding neuronal activity are made through relative contrast of T2* images reflecting blood oxygenation in BOLD rather than direct measurements of CBF in ASL (Detre and Wang, 2002). ASL images were corrected for motion in FSL prior to CBF quantification using ASLtoolbox (simple subtraction) to create CBF and pseudo-BOLD images (Wang et al., 2008). After voxelwise CBF was quantified for each series of ASL images with ASLtoolbox, an additional quality control step identified and removed volumes within each ASL series with non-neurobiological global CBF (gCBF). For each 30-volume ASL series, gCBF was calculated for each volume by averaging CBF values across all voxels within a mask (pseudo-BOLD > 100) (Wang et al., 2008). Per convention (Wang et al., 2008), gCBF calculations include gray- and white-matter tissue. Volumes with gCBF < 20 or >90 mL/100g/min were removed from the ASL series. Remaining volumes for each series were averaged using FSL to yield a single mean CBF image per study session and subject for subsequent statistical analyses (i.e., 1 averaged CBF image for each patient’s baseline scan, 1 CBF image for each patient’s ~1hr post-infusion scan, etc.). During this quality control process, one volume was removed for 8 ASL series, and two volumes were removed for one ASL series prior to averaging. For all other series, no volumes were removed prior to averaging.
Pseudo-BOLD images derived from fMRI-ASL data with the ASLtoolbox were used for anatomical registration and creation of masks. Intrasession alignment was performed between pseudo-BOLD (and CBF) images to their respective MPRAGE images, followed by intersession alignment to each patient’s baseline MPRAGE image. Non-linear MNI transformation was then applied to each image. Each normalized CBF image (i.e. baseline, 1hr, 6hr, and 24hr) was smoothed (6 mm3 FWHM) prior to statistical analyses. A group mask was created using SPM’s grey matter (GM) template and averaged pseudo-BOLD images for statistical analyses and calculation of global CBF. Global CBF was calculated by averaging voxelwise CBF within this mask for each of the CBF images. All fMRI-ASL images were inspected for the presence of susceptibility or other artifacts, or low global CBF values (< 20 mL/100g/min). No CBF images were removed during these quality control checks.
Statistical Analysis
All statistical analyses were performed in R (https://www.r-project.org) using lmer (Bates, Douglas et al., 2015) and oro.nifti (Whitcher et al., 2011) packages. First, linear mixed effects models were used to analyze CBF (dependent variable) focusing on the main effect of time (baseline, ~1hr, ~6hrs, ~24hrs). In one model, time was a numerical variable to examine monotonic increases (or decreases) in CBF over time. In a second model, time was a categorical factor to examine nonmonotonic changes in CBF over time (e.g., if CBF peaked after 1 hour but returned to baseline levels at 24 hours). In both models, nuisance regressors were modeled as such: subjects were the random factor while the independent variables age, gCBF, and ketamine response (% change in mean depression score 24 hours post-infusion) were fixed factors to control any variability in the magnitude of change in CBF over time related to these factors. Age has known correlations with CBF (Moeller et al., 1996; Zhang et al., 2018), and was therefore included as a nuisance variable. However, biological sex is not currently known to influence CBF or ketamine response, and was therefore not included in our statistical models for this pilot study. Future studies better powered to address biological sex are warranted. For multiple voxelwise comparisons, a false discovery rate (FDR) correction q < 0.05 was applied across all voxels (using R software).
Time by response interactions were also analyzed using linear mixed effects models, where time was modeled as a numerical or categorical variable as above, and response was defined as % change in mean depression score at 24 hours post-infusion (a numerical variable). Thus, all available data were used to estimate effects of response (i.e., 10 patients and 39 MRI total scans, Table 1). Again, subject was a random factor and time, age, gCBF, and ketamine response fixed. Similarly, multiple voxelwise comparisons were corrected at FDR q < 0.05.
In clusters exhibiting significant time-by-response interactions, additional pairwise analyses were applied post hoc to assess the direction of CBF change across the range of antidepressant response. Time by response interactions were calculated for 3 timepoint pairs: baseline vs. 1hr, baseline vs. 6hrs, and baseline vs. 24hrs post infusion. Linear mixed models included age, gCBF, and subject as nuisance factors and multiple comparisons (Bonferroni) correction was applied (α = 0.05/3 = 0.017).
RESULTS
Demographic information and clinical assessments
With a sample size of 11, the average age of the participants was 47.7 with a spread of 11.9 (Table 1). For this study, depression scores decreased significantly 24 hours after infusion, and improved by at least 50% for 5 of the 10 patients to qualify as responders (Table 1).
Global CBF
There was no significant effect of time on monotonic or nonmonotonic change in global CBF (i.e., numerical time x2 = 1.44 p = 0.69; categorical time x2 = 1.15 p = 0.28). Change in global CBF also did not differ with respect to antidepressant response; no significant time by response interactions were noted (numerical time x2 = 2.90 p = 0.09; categorical time x2 = 3.75 p = 0.29).
Regional CBF changes after ketamine infusion
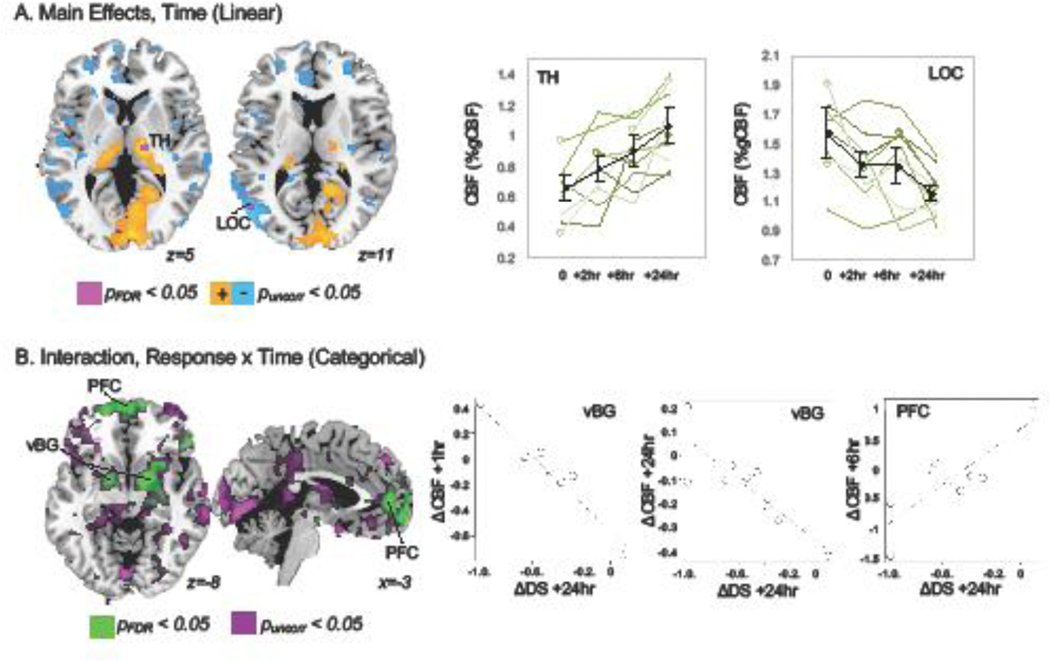
Monotonic increases or decreases in CBF were detected across all subjects (i.e., time modeled as numerical factor controlling for antidepressant response). These changes are shown in Figure 1, panel A. Increased CBF was observed in the thalamus while decreases were shown in the lateral occipital cortex, pFDR< 0.05. Significant nonmonotonic changes in CBF (i.e., time as categorical factor) were not noted, pFDR> 0.05.
Figure 1.
A. Monotonic changes in CBF were noted after ketamine infusion in patients with depression. A cluster in the thalamus (TH) exhibited significant increased CBF over time, while a cluster in lateral occipital cortex (LOC) showed significant decreases, pFDR < 0.05 (green). At more permissive thresholds (puncorr < 0.05, orange/blue), decreased CBF was also noted in anterior cingulate and dorsomedial prefrontal cortex, and increased CBF was noted in medial visual cortex. Mean CBF (% global CBF) is plotted for TH and LOC clusters in the top-right panels for all patients in black and for each individual subject in shades of green to demonstrate consistency of these changes across subjects. Subjects with missing data are plotted with open circles. B. Non-monotonic changes in CBF differed according to antidepressant response in time-by-response interactions. Bilateral ventral basal ganglia (vBG, including pallidum and ventral striatum) and medial prefrontal regions (PFC) exhibited significant interactions, pFDR <0.05 (green). Scatter plots display relationships between change in (delta, Δ) in CBF and change in depression symptoms (averaged across three inventories) meeting statistical criterion in post hoc analyses (pBonf < 0.05).
Time by response interactions
Significant time by antidepressant response interactions were detected in several depression relevant brain regions when considering nonmonotonic changes (i.e., time as categorical), including ventral basal ganglia overlapping the pallidum and ventral striatum, and in medial prefrontal cortex (Figure 1B). Post hoc region of interest (ROI) tests assessed the direction of CBF change across antidepressant response in these regions. In ventral basal ganglia, significant negative relationships (pBonf < 0.05) were noted between change in depression score (baseline vs. 24h post infusion) and change in CBF from baseline to 1h (beta(SE) = −35.86(8.05), t = −4.46, p = 0.0008) and from baseline to 24h post infusion (beta(SE) = −20.86(4.56), t=−4.57, p=0.002). Here, nonresponders tended to exhibit decreased CBF after ketamine infusion. Positive associations were also noted in medial prefrontal cortex for CBF change from baseline to 6h post infusion (pBonf < 0.05), where responders tended to have decreased CBF after 6 hours (beta(SE) = 67.70(13.49), t=5.02, p=0.0002). Significant interactions were not apparent for monotonic CBF changes in voxelwise analyses, pFDR > 0.05.
DISCUSSION
In this pilot study, we demonstrate that ketamine influences brain function measured with ASL-fMRI. In the hours after a single administration of ketamine, resting brain function increased in thalamus and decreased in lateral occipital cortex in all patients, regardless of antidepressant response to ketamine. CBF changes in ventral basal ganglia and medial prefrontal cortex were correlated with antidepressant response, suggesting that ketamine’s effects on fronto-striatal regions may associate with clinical outcome, corroborating recent functional connectivity studies (Abdallah et al., 2017b, 2017a). Our study targets CBF change in a small sample of depressed patients at three 3 timepoints in the 24 hours after a single dose of ketamine, offering novel insight into the rapid effects of ketamine on brain function. Future studies with larger sample sizes and using multiple administrations of this drug are needed to assess the mechanisms of the rapid and long-term antidepressant actions of ketamine.
Ketamine and altered attention
Ketamine is likely to affect brain function even in those patients who did not experience relief from depressive symptoms. Indeed, a number of studies have reported changes in brain function in participants without depression (Deakin et al., 2008; Scheidegger et al., 2012; Stone et al., 2012). Therefore, dissociating the “nonspecific” effects of ketamine on brain activity from those associated with antidepressant response may aid researchers attempting to understand the antidepressant mechanisms of this promising treatment. In our study, all patients exhibited increased CBF in the thalamus and medial occipital cortex, and decreased CBF in lateral occipital regions. This supports previous studies, which also demonstrated changes after ketamine in thalamus and visual cortex function in both depressed and nondepressed volunteers (Abdallah et al., 2017b; Deakin et al., 2008; Evans et al., 2018; Sahib et al., 2020). These effects could be interpreted in several different ways. In high doses, ketamine is used as an anesthetic to render a patient unconscious; therefore, lower doses of ketamine may influence perceptual awareness and attention (Sousa, 2013) and brain regions associated with awareness and attention (Scheidegger et al., 2012). Subanesthetic ketamine can also be associated with visual disturbances not related to psychotomimetic effects (e.g., double vision; (Wan et al., 2015)); visual cortex CBF changes may reflect general effects of ketamine on basic visual perception. Note that volunteers were instructed to keep their eyes closed during this study, which may explain discrepancies between the direction of brainactivity change in this study vs. others where volunteers had eyes open (Abdallah et al., 2017b; Evans et al., 2018; Sahib et al., 2020). It is also possible that these so-called nonspecific effects of ketamine play a role in antidepressant or placebo response, though they appear to occur in responders, nonresponders, and controls without depression. Future studies designed to target the interactions between these “nonspecific” effects in attentional/perceptual systems and antidepressant effects in limbic/default-mode systems may be illuminating.
Rapid antidepressant response to ketamine correlates with fronto-striatal function
The purpose of this study was to get a better understanding of the antidepressant mechanisms of ketamine through observation of its effects on resting cerebral blood flow with ASL-fMRI. Previous functional neuroimaging studies have noted changes in basal ganglia, dorsal anterior cingulate cortex, and default mode networks in depressed patients undergoing ketamine infusion (Abdallah et al., 2018, 2017b, 2017a; Sahib et al., 2020). In our study, CBF changes in the pallidum, ventral striatum, and medial prefrontal cortex were correlated with changes in depressive symptoms, suggesting that fronto-striatal networks associated with the reward and default-mode systems may be involved in acute antidepressant response to single infusions of ketamine. Though our study was open-label, our results are consistent with studies that included a placebo control (Reed et al., 2019) and offer additional novel insight into acute changes in brain function occurring in the first hours after infusion. Confirmation of these ASL-fMRI results with larger sample size, placebo- or active-comparator, and/or long-term follow-up is needed to determine the relationship between ketamine’s effects on fronto-striatal function and sustained antidepressant response.
Limitations
Limitations of this pilot study are as follows: sample size, variation in the sample such as age and gender, limited number of ketamine infusions, and dosage. Lack of placebo or active control must also be considered. However, CBF changes occurring in all patients (i.e., in thalamus and visual cortex) are less likely to be influenced by the placebo effect because they occurred even in those volunteers whose symptoms did not improve with treatment. Furthermore, given the clear superiority of ketamine over placebo in reducing depressive symptoms (Singh et al., 2016; Zarate et al., 2006), frontostriatal changes correlated with antidepressant response may be more related to true antidepressant response, rather than the placebo effect. Volunteers were also treatment refractory, and current/past medication use may have influenced response to ketamine, though we are not aware of studies reporting such effects. For future studies, a larger sample size with placebo or active comparator, comprehensive mediation histories, and more variability in age and gender may be informative. Scanning time points taken more or less frequently may provide additional insight into the effect of ketamine in the brain, particularly with respect to response maintenance. Results obtained after multiple ketamine infusions or increased/decreased dosage may improve the efficacy of ketamine and its antidepressant affects, which may also impact ketamine’s effects on brain function.
Conclusions
Despite the limitations of this pilot study, these results nevertheless constitute a novel contribution to the ketamine MRI literature by measuring quantitative CBF at four timepoints in the 24 hours after a single infusion of ketamine to treat depression. Consistent with previous studies in depression and nondepressed controls (Abdallah et al., 2017b; Evans et al., 2018; Sahib et al., 2020), ketamine associated with acute CBF change in the thalamus and visual cortex in all patients regardless of antidepressant outcome in our study. Despite our relatively small sample size, these effects survived strict statistical correction and appeared to be robust across all subjects when single-subject data were visualized (i.e., Figure 1A right panels). CBF change in medial frontal cortex and basal ganglia associated with antidepressant response 24 hours after ketamine infusion. These results were also consistent with previous studies (Abdallah et al., 2018, 2017b, 2017a) and survived strict statistical corrections; however, these results in particular should be interpreted with caution given our sample size and lack of an active or placebo comparator. Future studies are needed with larger sample sizes targeting multiple infusions of ketamine, and perhaps using PET-fMRI to simultaneously measure the molecular and brain-network consequences of this promising treatment for depression.
Table 2.
MNI Coordinates
| Peak Coordinate | MNI | ||||
|---|---|---|---|---|---|
| Statistical Test | Brain Region | Volume | X | Y | Z |
|
| |||||
| Time, numerical | Right Thalamus | 232 | 16 | −18 | 4 |
| Left Lateral Occipital Cortex | 168 | −52 | −66 | 12 | |
| Time, categorical | n/a | ||||
| Response*Time, numerical | n/a | ||||
| Response*Time, categorical | Medial Prefrontal & Anterior Cingulate Cortex | 8578 | 44 | 79 | 17 |
| Right Ventral Striatum | 9176 | 42 | 57 | 27 | |
Highlights.
Ketamine’s effects on cerebral blood flow were measured over 24 hours
Blood flow in thalamus and visual cortex changed after ketamine
Ketamine’s antidepressant response associated with fronto-striatal change
These promising pilot results require further study
ACKNOWLEDGEMENTS
This study was supported by the NIH, including U01 MH110008 to Drs. Narr and Espinoza, K24 MH102743 to Dr. Narr, the Muriel Harris Chair in Geriatric Psychiatry to Dr. Espinoza, as well as the Brain and Behavior Research Foundation, a NARSAD Young Investigator award to Dr. Leaver.
Financial Disclosure
The authors report no financial conflicts of interest.
This study was supported by the NIH, including U01 MH110008 to Drs. Narr and Espinoza, K24 MH102743 to Dr. Narr, the Muriel Harris Chair in Geriatric Psychiatry to Dr. Espinoza, as well as the Brain and Behavior Research Foundation, a NARSAD Young Investigator award to Dr. Leaver.
Role of the Funding Source: The sponsors had no role in the study design, data collection, analysis, or interpretation of the data, the writing of this manuscript, or in the decision to submit this manuscript for publication.
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
Ethical Statement
This research was conducted with approval and oversight by the UCLA Institutional Review Board.
Supplemental information: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, Mathew SJ, Mathalon DH, 2017a. Prefrontal Connectivity and Glutamate Transmission: Relevance to Depression Pathophysiology and Ketamine Treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 566–574. 10.1016/j.bpsc.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW, 2017b. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology 42, 1210–1219. 10.1038/npp.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Feyter HMD, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, Graaf R.A. de, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G, Mason GF, 2018. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43, 2154–2160. 10.1038/s41386-018-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrnsbrak R, Bose J, Hedden S, Lipari R, Park-Lee E, 2017. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. SAMHSA. [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR®. American Psychiatric Association. [Google Scholar]
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F, 2000. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry 47, 305–313. 10.1016/S0006-3223(99)00159-6 [DOI] [PubMed] [Google Scholar]
- Bates Douglas, Mächler Matin, Bolker Ben, Walker Steve, 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Block W, Träber F, von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F, 2009. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol 12, 415–422. 10.1017/S1461145708009516 [DOI] [PubMed] [Google Scholar]
- Chilukuri H, Reddy NP, Pathapati RM, Manu AN, Jollu S, Shaik AB, 2014. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian J Psychol Med 36, 71–76. 10.4103/0253-7176.127258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM, 2008. Glutamate and the Neural Basis of the Subjective Effects of Ketamine: A Pharmaco–Magnetic Resonance Imaging Study. Arch Gen Psychiatry 65, 154–164. 10.1001/archgenpsychiatry.2007.37 [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, 2002. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol 113, 621–634. 10.1016/s1388-2457(02)00038-x [DOI] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA, 2018. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biological Psychiatry, Cannabinoids, Ketamine, Connectivity, and Depression 84, 582–590. 10.1016/j.biopsych.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin AW, Rosenfeld DM, Ramakrishna H, 2016. Intravenous sub-anesthetic ketamine for perioperative analgesia. J Anaesthesiol Clin Pharmacol 32, 160–167. 10.4103/0970-9185.182085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1980. Rating depressive patients. J Clin Psychiatry 41, 21–24. [PubMed] [Google Scholar]
- Hasler G, Veen JW van der, Tumonis T, Meyers N, Shen J, Drevets WC, 2007. Reduced Prefrontal Glutamate/Glutamine and γ-Aminobutyric Acid Levels in Major Depression Determined Using Proton Magnetic Resonance Spectroscopy. Arch Gen Psychiatry 64, 193–200. 10.1001/archpsyc.64.2.193 [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA, 2012. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37, 1526–1533. 10.1038/npp.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA, 2011. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1155–1159. 10.1016/j.pnpbp.2011.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S, 2011. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav. Brain Res 224, 107–111. 10.1016/j.bbr.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS, 1994. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch Gen Psychiatry 51, 199–214. 10.1001/archpsyc.1994.03950030035004 [DOI] [PubMed] [Google Scholar]
- Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW, 2014. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry 76, 970–976. 10.1016/j.biopsych.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Espinoza R, Joshi SH, Vasavada M, Njau S, Woods RP, Narr KL, 2016. Desynchronization and Plasticity of Striato-frontal Connectivity in Major Depressive Disorder. Cereb. Cortex 26, 4337–4346. 10.1093/cercor/bhv207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Narang P, Enja M, Lippmann S, 2015. Use of ketamine in acute cases of suicidality. Innov Clin Neurosci 12, 29–31. [PMC free article] [PubMed] [Google Scholar]
- Li L, Vlisides PE, 2016. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci 10, 612. 10.3389/fnhum.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykx JJ, Laban KG, van den Heuvel MP, Boks MPM, Mandl RCW, Kahn RS, Bakker SC, 2012. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of 1H-MRS findings. Neuroscience & Biobehavioral Reviews 36, 198–205. 10.1016/j.neubiorev.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Mathews DC, Henter ID, Zarate CA, 2012. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 72, 1313–1333. 10.2165/11633130-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW, 2015. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45, 693–704. 10.1017/S0033291714001603 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau M-J, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M, 2014. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 156, 1–7. 10.1016/j.jad.2013.10.043 [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B, 2003. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychological Medicine 33, 1277–1284. 10.1017/S0033291703007931 [DOI] [PubMed] [Google Scholar]
- Michael Nikolaus, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B, 2003. Neurotrophic Effects of Electroconvulsive Therapy: A Proton Magnetic Resonance Study of the Left Amygdalar Region in Patients with Treatment-Resistant Depression. Neuropsychopharmacology 28, 720–725. 10.1038/sj.npp.1300085 [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D, 1996. The metabolic topography of normal aging. J. Cereb. Blood Flow Metab 16, 385–398. 10.1097/00004647-199605000-00005 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, 2015. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. AJP 172, 950–966. 10.1176/appi.ajp.2015.15040465 [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Charney DS, 2014. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu. Rev. Pharmacol. Toxicol 54, 119–139. 10.1146/annurev-pharmtox-011613-135950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA, Anand A, 2012. Where in the brain is depression? Curr Psychiatry Rep 14, 634–642. 10.1007/s11920-012-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W, 2003. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Research: Neuroimaging 122, 185–192. 10.1016/S0925-4927(03)00003-9 [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC, 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ, 2009. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66, 522–526. 10.1016/j.biopsych.2009.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA, 2019. Effects of Ketamine on Brain Activity During Emotional Processing: Differential Findings in Depressed Versus Healthy Control Participants. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 610–618. 10.1016/j.bpsc.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Sahib AK, Loureiro JRA, Vasavada MM, Kubicki A, Joshi SH, Wang K, Woods RP, Congdon E, Wang DJJ, Boucher ML, Espinoza R, Narr KL, 2020. Single and repeated ketamine treatment induces perfusion changes in sensory and limbic networks in major depressive disorder. Eur Neuropsychopharmacol. 10.1016/j.euroneuro.2020.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E, 2012. Ketamine Decreases Resting State Functional Network Connectivity in Healthy Subjects: Implications for Antidepressant Drug Action. PLOS ONE 7, e44799. 10.1371/journal.pone.0044799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Murrough JW, Iosifescu DV, 2016. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health 19, 35–38. 10.1136/eb-2016-102355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L, 2016. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry 173, 816–826. 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, Racagni G, Zohar J, Mendlewicz J, 1999. Treatment resistant depression: methodological overview and operational criteria. European Neuropsychopharmacology 9, 83–91. 10.1016/S0924-977X(98)00004-2 [DOI] [PubMed] [Google Scholar]
- Sousa AD, 2013. Towards An Integrative Theory Of Consciousness: Part 1 (Neurobiological And Cognitive Models). Mens Sana Monographs 11, 100–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ, 2012. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Molecular Psychiatry 17, 664–665. 10.1038/mp.2011.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ, 2012. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol 26, 733–737. 10.1177/0269881111405359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L-B, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW, 2015. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76, 247–252. 10.4088/JCP.13m08852 [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA, 2008. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging 26, 261–269. 10.1016/j.mri.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcher B, Schmid VJ, Thornton A, 2011. Working with the DICOM and NIfTI Data Standards in R. Journal of Statistical Software 44. 10.18637/jss.v044.i06 [DOI] [Google Scholar]
- Yüksel C, Öngür D, 2010. Magnetic Resonance Spectroscopy Studies of Glutamate-Related Abnormalities in Mood Disorders. Biological Psychiatry, Glutamate in Mood Disorders 68, 785–794. 10.1016/j.biopsych.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, Ma Y, Chi B, Gomar JJ, Peng S, Kingsley PB, Eidelberg D, Goldberg TE, 2018. The Age-Related Perfusion Pattern Measured With Arterial Spin Labeling MRI in Healthy Subjects. Front. Aging Neurosci 10. 10.3389/fnagi.2018.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]