Abstract
Corticotropin-releasing factor (CRF) signaling through CRF receptor 1 (CRFR1) regulates autonomic, endocrine, and behavioral responses to stress, as well as behavioral changes during the maternal period. Previous work in our lab reported higher levels of CRFR1 in female compared to male mice within the rostral anteroventral periventricular nucleus (AVPV/PeN), a brain region involved in maternal behaviors. In this study, we used CRFR1-GFP reporter mice to investigate whether the reproductive status (postpartum vs. nulliparous) of acutely stressed females affects levels of CRFR1 in the AVPV/PeN and other regions involved in maternal functions. Compared to nulliparous, postpartum day 14 females showed increased AVPV/PeN CRFR1-GFP immunoreactivity and an elevated number of restraint stress-activated AVPV/PeN CRFR1 cells as assessed by immunohistochemical co-localization of CRFR1-GFP and phosphorylated CREB (pCREB). The medial preoptic area (MPOA) and paraventricular hypothalamus (PVN) of postpartum mice showed modest decreases in CRFR1-GFP immunoreactivity, while increased CRFR1-GFP/pCREB co-expressing cells were found in the PVN following restraint stress relative to nulliparous mice. Tyrosine hydroxylase (TH) and CRFR1-GFP co-localization was also assessed in the AVPV/PeN and other regions and revealed a decrease in co-localized neurons in the AVPV/PeN and ventral tegmental area of postpartum mice. Corticosterone analysis of restrained mice revealed blunted peak, but elevated recovery, levels in postpartum compared to nulliparous mice. Finally, we investigated projection patterns of AVPV/PeN CRFR1 neurons using female CRFR1-Cre mice and revealed dense efferent projections to several preoptic, hypothalamic, and hindbrain regions known to control stress-associated and maternal functions. Together, these findings contribute to our understanding of the neurobiology that might underlie changes in stress-related functions during the postpartum period.
Keywords: corticotropin-releasing factor, corticotropin-releasing factor receptor type 1, rostral anteroventral periventricular nucleus, hypothalamus, preoptic area, paraventricular nucleus, postpartum, sex differences, tyrosine hydroxylase
1. Introduction
Due to the female’s ability to lactate, the mother is often established as the primary caregiver in approximately 90% of all mammals (Kleiman and Malcolm, 1981; Numan and Insel, 2003). Any disruption in normal maternal behavior, particularly maternal mood disorders, can be detrimental to the mental and physical well-being of the mother and the child. Social stress-related mood disorders, such as postpartum anxiety and depression, impact up to 20% of mothers (Steiner, 1998; Gavin et al., 2005), and previous work has shown lasting cognitive and behavioral problems in children whose mothers experienced postpartum anxiety and/or depression (Barnett et al., 1991; Brand and Brennan, 2009; Agrati et al., 2015; Choi and Sikkema, 2016; Tietz et al., 2014). Therefore, research focusing on understanding the underlying biological mechanisms of female stress response during the postpartum period are of utmost importance.
The corticotropin-releasing factor (CRF)/CRF receptor 1 (CRFR1) system, which regulates the behavioral and hormonal stress response (Vale et al., 1981; Chen et al., 1993; Smith et al., 1998), has been implicated in the pathophysiology of anxiety and depression (Zobel et al., 2000; Chrousos, 2009; Garcia-Carmona et al., 2015; da Silva et al., 2016; Holly et al., 2016; Schüssler et al., 2016; Menke, 2019). Our lab discovered CRFR1 neurons to be sexually dimorphic in the rostral anteroventral periventricular nucleus (AVPV/PeN), a brain region involved in ovulation, reproduction, and maternal behaviors (Simerly et al., 1997; Herbison, 2008; Scott et al., 2015; Wang and Moenter, 2020), where CRFR1-expressing neurons were abundant in female mice, but were nearly absent in male mice (Rosinger et al., 2017; Rosinger et al., 2019a).
The AVPV/PeN has also been shown to have female-biased sexually dimorphic expression of tyrosine hydroxylase (TH) with females having a greater number of TH-immunoreactive neurons than male mice (Simerly et al., 1997; Simerly, 2002). TH is the first rate-limiting enzyme of catecholamine synthesis and is a biomarker for dopaminergic neurons in the central nervous system (Nagatsu et al., 1964; Leshin et al., 1996; Daubner et al., 2011) and fluctuations in TH levels during the postpartum period appear relevant for maternal care (Brown et al., 2014; Scott et al., 2015; Kokay et al., 2018). Within the AVPV/PeN, TH ablation and TH overexpression have been shown to impair and promote maternal behavior, respectively (Scott et al., 2015), indicating a critical role of AVPV/PeN TH in regulating maternal behaviors. Further, previous studies have reported co-localization of CRFR1- and dopamine/TH-expressing neurons within the AVPV/PeN and other maternal-associated brain regions (Refojo et al., 2011; Rosinger et al., 2019a), suggesting a potential interaction between CRFR1 and TH neurons in regulating maternal behaviors during the postpartum period.
We used a validated CRFR1 reporter mouse line [bacterial artificial chromosome identified by green fluorescent protein (BAC CRFR1-GFP; Justice et al., 2008)] to investigate whether levels of CRFR1 and co-localization of CRFR1 with TH differed between postpartum and nulliparous (reproductively naïve) mice. Specifically, we hypothesized that CRFR1 levels would decrease in key maternal and stress-associated brain regions, including the AVPV/PeN, medial preoptic area (MPOA), paraventricular hypothalamus (PVN), and arcuate nucleus (ARN). We further predicted CRFR1/TH co-localization would decrease in the AVPV/PeN and other key TH neuron populations that regulate maternal and stress functions, including the ventral tegmental area (VTA), substantia nigra (SN), dorsal raphe nucleus (DRN), PVN, and ARN. These reductions in CRFR1 would be predicted to limit stress signaling in maternal mice to limit stress-induced perturbations in maternal behaviors but might also have consequences that relate to changes in anxiety- and depressive-like behaviors, as well as HPA axis regulation in postpartum mice.
2. Material and Methods
2.1. Experimental design.
Experiment 1: Determine whether CRFR1 levels, stress-induced activation of CRFR1 neurons, and CRFR1 co-localization with tyrosine hydroxylase differ between nulliparous and postpartum mice.
Nulliparous and postpartum day 14 CRFR1-GFP mice (N=8 per group) were subjected to acute restraint stress prior to brain and blood collection to assess CRFR1-GFP, CRFR1 co-localization with TH and pCREB, and plasma corticosterone.
Experiment 2: Determine the projection patterns of AVPV/PeN CRFR1 neurons in the female mouse.
To assess anterograde projection sites of AVPV/PeN CRFR1 neurons, CRFR1-Cre mice (N=4) were injected unilaterally with an anterograde Cre virus to visualize afferent projections of Cre-expressing neurons.
2.2. Animals and Experimental Design.
All mice were bred and born in our vivarium at University at Albany and were housed with one or two same-sex littermates after being weaned. Mice used in experiments were between 4-6 months old. Two validated mouse lines were used in this study: CRFR1-GFP (experiment 1) and CRFR1-Cre (experiment 2) (Justice et al., 2008; Jiang et al., 2018). In experiment 1, CRFR1-GFP mice were used to determine postpartum changes in CRFR1 and CRFR1 co-expression with pCREB and TH, as previously utilized in other studies (Ramot et al., 2017; Rosinger et al., 2019a, 2019b; 2020). In experiment 2, CRFR1-Cre mice were used to determine anterograde projection sites of AVPV/PeN CRFR1 neurons. All mice were housed in clear polycarbonate cages (7.25” W x 11.5” D x 5” H) with microfilter top and Aspen chip bedding, and each cage included one polycarbonate mouse igloo and a nestlet. Mice were maintained under a 12 hr light: 12 hr dark cycle (lights on at 7AM) and had access to pellets (Lab Diet 5P76 Irradiated ProLab IsoPro RMH 3000) and water ad libitum. Female CRFR1-GFP mice were co-housed with sexually experienced breeding males for 2 weeks, with the male removed prior to giving birth. Removal of the male mouse was essential to prevent impregnating the female mouse after parturition. Day of parturition was considered postpartum day 0 (PP0). Dams and pups were left undisturbed (except for cage change on PP7) until experimental day PP14. PP14 is a timepoint associated with high nursing demand as well as robust alterations in levels of kisspeptin and TH within the AVPV/PeN (Scott et al., 2015; Brown et al., 2014, 2015) and was therefore hypothesized to be a key timepoint for modifications in stress-associated genes including CRFR1. All procedures were approved by the University at Albany Institutional Animal Care and Use Committee (IACUC) and in accordance with National Institutes of Health guidelines.
2.3. Restraint stress, blood collection, and corticosterone (CORT) radioimmunoassay (RIA).
Restraint initiation and blood collection occurred between 9AM – 12PM on PP14. All experimental apparatuses and tools were cleaned and dried between mice. All CRFR1-GFP mice [N=8 nulliparous (NP), N=8 postpartum day 14 (PP14)] were transferred from the vivarium to a procedure room and were placed in ventilated plastic restraint tubes during blood collection. The restraint tubes (L: 6-4/5”, W: 3-9/10”, H: 2-3/5”) had proper ventilation and allowed mice to move their limbs freely, but not turn around. Blood samples were collected within 3 minutes by tail vein puncture (De Guzman et al., 2018) after moving the home cage to assess baseline corticosterone (CORT). After blood collection, mice remained in restraint tubes, placed into new cages, and were left undisturbed in a holding room separate from their home vivarium. Thirty minutes after restraint initiation, mice were again moved to the procedure room where blood samples were again collected via tail vein puncture to assess stress-induced CORT. Mice were taken out of the restraint tubes, placed in the same cage, and moved back to the holding room where they were left undisturbed for 1 hour. Ninety minutes following stress initiation, mice were transferred back to the procedure room and rapidly decapitated with trunk blood samples were collected to assess recovery CORT. All blood samples were kept on ice, and subsequently centrifuged at 5500 RCF for 10 minutes at 4°C. Plasma samples were stored at −80°C until radioimmunoassay for baseline, stress-induced, and recovery CORT. CORT levels were determined using a double antibody 125I radioimmunoassay kit (MP Biomedicals, Solon, USA). Samples ran in duplicate according to the manufacturer’s instructions and as previously described (Jacobskind et al., 2017; 2019). The intra-assay coefficients of variation were less than 5%.
2.4. Brain collection and sectioning.
Ninety minutes following stress initiation, brains were collected, post-fixed in 4% paraformaldehyde overnight at 4°C, and then placed into a 30% sucrose solution containing sodium azide for cryoprotection at 4°C until brain sectioning. The timing of brain collection was chosen based on a study that demonstrated a peak phosphorylated cAMP element-binding protein (pCREB) response in the mouse hypothalamus at 1-2 hours after restraint stress initiation (Kwon et al., 2006). We have also reported extensive induction of pCREB within CRFR1 cells in the AVPV/PeN, PVN, and several other regions at 1.5 to 2 hours after restraint and other stressors (Rosinger et al., 2019a, 2019b, 2020). Brains were embedded in Optimum Cutting Temperature (O.C.T.) Compound (Tissue-Tek®), coronally sectioned at 30μM on a cryostat (Microm HM505E, MICROM International GmbH, Waldorf, Germany), separated into four vials (series) containing cryopreserve, and stored at 4°C until immunohistochemistry.
2.5. CRFR1-GFP non-fluorescent immunohistochemistry.
Immunohistochemistry to visualize CRFR1-GFP-ir was performed as previously described (Rosinger et al., 2017, 2019a, 2019b). Briefly, free-floating tissue sections were rinsed with 0.1M phosphate buffered saline (PBS; pH 7.6), incubated in 0.3% Triton-X in PBS (0.3% PBS-TX) and 1% hydrogen peroxide for 10 minutes, rinsed in 0.1M PBS, blocked in 4% normal goat serum (NGS) and 0.3% PBS-TX for 1 hour, and incubated in primary GFP antisera (rabbit, Life Technologies, RRID: AB221570, 1:5000) at room temperature overnight. The following day, tissue sections were rinsed in 0.1M PBS, incubated in biotinylated antisera solution (goat anti-rabbit; Vector Laboratories; 1:500) for 1 hour, rinsed in 0.1M PBS, incubated in avidin-biotin peroxidase complex (ABC Elite kit, Vector Laboratories; 1:1000) for 1 hour, rinsed in tris buffered saline (TBS), developed in diaminobenzidine (DAB) for 10 minutes, and rinsed in 0.1M PBS. Sections were mounted onto gel-coated slides, processed with ethanol and xylene, and coverslipped using Permount. All samples for IHC were run at the same time to reduce variability in DAB labeling.
2.6. Fluorescent immunohistochemistry.
Free-floating tissue sections were rinsed with 0.1M PBS, blocked in 4% normal donkey serum (NDS) and 0.3% PBS-TX for 1 hour, and incubated in primary antisera for pCREB (rabbit, Cell Signaling Technology 87G3, RRID: AB2561044, 1:500), TH (rabbit; Millipore AB152, RRID: AB390204, 1:500), or CRFR1 (rabbit; Invitrogen, PA5-77378, RRID: AB2735685, 1:500) at room temperature overnight. The following day, tissue sections were rinsed in PBS, incubated in secondary antisera (donkey anti-rabbit alexa 594, Jackson Labs, 1:500) in 4% NDS and 0.3% PBS-TX for 2.5 hours. Tissue sections were rinsed in 0.1M PBS, incubated in 4% NDS and 0.3% PBS-TX for 60 minutes, and incubated in primary antisera for GFP (chicken; Abcam, RRID: AB300798, 1:1500) in 4% NDS and 0.3% PBS-TX at room temperature overnight. The next day, tissues were rinsed in 0.1M PBS, incubated in secondary antisera (anti-chicken alexa 488, Jackson Labs, 1:1000) in 4% NDS and 0.3% PBS-TX for 2.5 hours, and rinsed in 0.1M PBS. Sections were mounted onto gel-coated slides and coverslipped with Santa Cruz Hardset mounting media (Santa Cruz Biotechnology). GFP, TH, and pCREB antibodies have been previously validated in our laboratory and others (Rosinger et al., 2019a; 2019b; Scott et al., 2015; Kwon et al., 2006). Specificity of the CRFR1 antibody was tested by performing immunohistochemistry in the absence of primary and secondary antibodies, which yielded no labeling. Furthermore, pre-absorption with the blocking peptide corresponding to this antibody (1:50, Alomone labs, Cat # BLP-CR050) eliminated labeling.
2.7. Stereotaxic Surgery and visualization of anterograde projections of AVPV/PeN CRFR1 neurons.
This experiment was performed to determine afferent projections of AVPV/PeN CRFR1 neurons. We focused on this region as it showed the most robust alterations in CRFR1 in postpartum mice and because function and connectivity of CRFR1 in this region have not been as well described as other areas, including the PVN and MPOA (Jiang et al., 2018; 2019; Klampf et al., 2018). For these experiments, we used heterozygous CRFR1-Cre female mice on a C57BL6/J background (Jiang et al., 2018).
Female CRFR1-Cre mice were anaesthetized and maintained under an inhaled oxygen/isoflurane mixture (2.5%-3%). Using the stereotactic coordinates (Bregma; AP: +0.25, ML: +/−0.10, DV: +5.45) to target the AVPV/PeN, we unilaterally injected with 0.1 μL of rAAV5/Ef1a-DIO-EYFP (UNC Vector Core), which was previously utilized to visualize afferent projections of Cre-expressing neurons (Scott et al., 2015; Jiang et al., 2018). Using a computer-controlled stereotaxic apparatus (Neurostar Robot Stereotaxic; SD326), the AAV was delivered over a 1-minute period. Mice were returned to the colony room after restored righting reflex and were administered Carprofen (5mg/kg) subcutaneously for two consecutive days and monitored daily. Five weeks after surgery, mice were intracardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were collected, post-fixed in 4% paraformaldehyde at 4°C overnight, and cryoprotected in 30% sucrose solution containing sodium azide at 4°C until brain sectioning. Brains were embedded in O.C.T Compound, coronally sectioned at 40 μM on a cryostat, separated into three vials (series) containing cryopreserve, and stored at 4°C until immunohistochemistry. To enhance AAV signal for visualization, we used immunohistochemistry for GFP (chicken; Abcam, RRID: AB300798, 1:1000) following our standard immunohistochemistry protocol as described above.
2.7. Microscopic analyses.
We used the Allen Institute mouse brain coronal reference atlas (https://mouse.brain-map.org/static/atlas; Lein et al., 2007) to identify the following brain regions of interest (ROIs) and to quantify CRFR1-GFP neurons: AVPV/PeN (rectangle; plates 53-54), PVN (triangle; plates 62-63), MPOA (circle; plates 53-54), ARN (triangle; plates 72-73), substantia nigra (triangle; SN; plates 79-80), ventral tegmental area (VTA; ROI traced based on TH+ neurons; plates 82-83), and dorsal raphe nucleus (DRN; ROI traced based on TH+ neurons; plate 105).
Images of tissue sections from CRFR1-GFP mice were captured on a Nikon 80i Eclipse microscope at 20x magnification. ImageJ (1.51j8 Wayne Rasband, NIH, USA) was used to assess the number and density of CRFR1-GFP-ir, as well as the number of CRFR1-GFP/pCREB+, and CRFR1-GFP/TH+ cells. As previously described (Zuloaga et al., 2007), individual neurons were assigned as having a light or dark stain based on subjective criteria, with light to intermediately stained and darkly stained neurons assigned to the light and dark categories, respectively. We applied this method of quantifying dark and light GFP cells where the content of GFP within a cell, which is driven by the CRFR1 promoter, is reflective of CRFR1 gene expression (Justice et al., 2008). Therefore, darker GFP cells are interpreted as having greater CRFR1 content. CRFR1-GFP labeling density analysis was also performed in the AVPV/PeN, PVN, MPOA, and ARN. Images were captured from 8 NP and 8 PP14 mice, although select regions were excluded in 1 NP mouse due to tissue tearing or overlap.
Images of anterograde projections of AVPV/PeN CRFR1 neurons from CRFR1-Cre mice shown to have selective AAV infusion into the AVPV/PeN (N=4) were also collected on a Nikon 80i microscope equipped with a digital camera using a 10x or 20x objective. Brain region fiber density was rated based on the following scale: -, no label; −/+, minimal labeling; + moderate density; ++ dense labeling; +++ very dense labeling, similar to our previously published density rating methods (Zuloaga et al., 2014; Rosinger et al., 2017).
2.8. Statistical analyses.
Statistical analyses were performed using GraphPad (v5.01, GraphPad Software, San Diego, CA, USA). Analyses were performed using either 2-Way ANOVA (corticosterone analysis) or Student’s T-tests (all neuroanatomical analyses). Significant ANOVA effects were further analyzed using Bonferroni post hoc tests. Partial eta squared for ANOVAs and Cohen's d for pair-wise comparisons were used to calculate effect size. Parametric tests were utilized as sampled distributions for analyses met the criteria for homogeneity of variance and normal distribution, assessed using F tests and Shapiro-Wilk tests, respectively. Data are reported as mean +/− standard error of the mean (SEM) with a significance level set at p ≤ 0.05.
3. Results
3.1. Differences in CRFR1-GFP in postpartum compared to nulliparous mice.
We used non-fluorescence microscopy to assess the number of CRFR1-GFP-ir neurons within the AVPV/PeN, MPOA, PVN, and ARN. In the AVPV/PeN (Figure 1A), there were no significant differences between PP14 and NP mice in the total number of CRFR1-GFP-ir neurons. However, reproductive status impacted the number of dark (PP14 > NP; t(13) = 3.50, p = 0.0039, d = 1.94) and light (PP14 < NP; t(13) = 3.12, p = 0.0082, d = 1.73) CRFR1-GFP-ir neurons. A significant difference was also found between PP14 and NP mice in CRFR1-GFP-ir labeling density (PP14 > NP; t(13) = 3.53, p = 0.0037, d =1.96; Figure 1B). Collectively, these findings in the AVPV/PeN indicate increased CRFR1-GFP-ir in PP14 brains. Since CRFR1-GFP in the AVPV/PeN has not been as well described as other regions assessed in this study (e.g. PVN and MPOA) in terms of validation with mRNA or protein expression (Van Pett et al., 2000; Justice et al., 2008; Klampf et al., 2018; Jiang et al., 2019), we performed immunohistochemical co-labeling using GFP and an antibody directed at the CRFR1 protein. We found that 91% of AVPV/PeN CRFR1-GFP neurons (counted from 3 female mice) showed co-expression with CRFR1 protein (See Supplemental Figure 1 for a representative image).
Figure 1. CRFR1-GFP levels in the AVPV/PeN.
(A) There were significant differences in dark (postpartum > nulliparous) and light (nulliparous > postpartum CRFR1-GFP neurons, although no significant differences in total CRFR1-GFP neurons were found. (B) Density analysis revealed increased CRFR1-GFP labeling density within the AVPV/PeN of postpartum mice. Collectively, CRFR1-GFP immunoreactivity is increased in the postpartum day 14 mouse brain. (C-D) Representative images of a nulliparous (NP) and postpartum day 14 (PP14) AVPV/PeN. (E-F) High magnification images of inset boxes in C-D that indicate examples of light (yellow asterisk) and dark (red asterisk) labeled cells.
In the MPOA (Figure 2A), reproductive status significantly impacted the number of dark (PP14 < NP; t(13) = 2.20, p = 0.047, d = 1.22) neurons and there was a trend toward significance in the total number (PP14 < NP; t(13) = 1.96, p = 0.074, d = 1.09) of CRFR1-GFP-ir neurons. There was also a trend toward a decrease in CRFR1-GFP density in the MPOA of PP relative to NP mice (t(13) = 1.91, p = 0.078, d = 0.91, Figure 2D). In the PVN and ARN (Figure 2B, 2C), there were no significant differences between PP14 and NP mice in the number of light and total number of CRFR1-GFP-ir neurons, although there was a trend toward a significant decrease in the number of dark CRFR1-GFP-ir neurons in the PVN of PP14 mice (t(13) = 1.82, p = 0.092, d = 1.01). CRFR1-GFP labeling density was significantly decreased in the PVN at PP14 compared to NP mice (t(13) = 2.21, p = 0.045, d = 1.36, Figure 2E) with no differences in labeling density found in the ARN (Figure 2F).
Figure 2. CRFR1-GFP levels in the MPOA, PVN, and ARN.
(A) In the MPOA, there was a significant decrease in darkly labeled CRFR1-GFP neurons and a trend toward a decrease in total CRFR1-GFP neurons in postpartum mice compared to nulliparous mice. (B) In the PVN, there was a trend toward a decrease in darkly labeled neurons in postpartum mice. (C) There were no differences in the ARN. Density analysis revealed a trend toward decreased CRFR1-GFP labeling density in the MPOA (D), and a significant decrease in the PVN (E), of postpartum relative to nulliparous mice. No difference in CRFR1-GFP density was found in the ARN (F). NP, nulliparous; PP postpartum. Representative images of CRFR1-GFP within the MPOA (G,H) and PVN (I,J) are shown. 3v, 3rd ventricle.
3.2. Differences between postpartum and nulliparous mice in CRFR1-GFP cell activation (pCREB co-localization) following a 30-minute restraint stress.
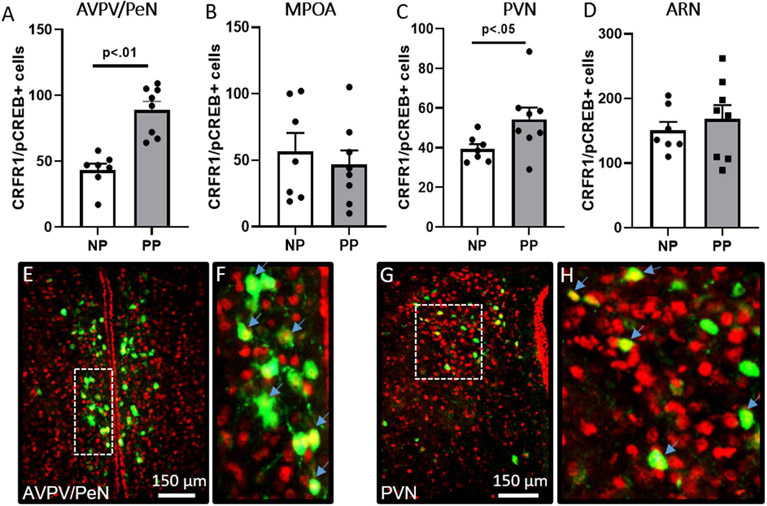
To determine whether activation of CRFR1-GFP-ir neurons differed between nulliparous and postpartum mice in response to stress, we assessed co-localization of CRFR1-GFP-ir neurons and phosphorylated CREB (pCREB; a marker for transcription/neuronal activation). In the AVPV/PeN (Figure 3A), there was a significant effect of reproductive status on the number of CRFR1/pCREB co-labeled neurons (PP14 > NP; t(13) = 4.26, p = 0.001, d = 2.36), but no difference in the number of pCREB neurons (Table 1). In the PVN, reproductive status also impacted the number of pCREB (PP14 > NP; t(13) = 2.48, p = 0.028, d = 1.38; Table 1) and CRFR1/pCREB co-labeled (PP14 > NP; t(13) = 2.16, p = 0.049, d = 1.20; Figure 3C) neurons. In the MPOA and ARN, there were no significant differences between PP14 and NP mice in the number of pCREB+ neurons (Table 1) or CRFR1/pCREB+ neurons (Figure 3B, 3D).
Figure 3. CRFR1-GFP/pCREB co-localization in postpartum and nulliparous mice after restraint stress.
Postpartum mice had greater levels of CRFR1-GFP neurons (green) expressing pCREB (red) in the (A) AVPV/PeN and (C) PVN compared to nulliparous. PP14 and nulliparous mice did not differ in the number of CRFR1 expressing pCREB in the (B) MPOA or (D) ARN. Representative images of AVPV/PeN (E,F) and PVN (G,H) are shown. High magnification images of inset boxes of E and G are shown in F and H. Arrows indicate examples of co-labeled neurons (yellow nuclear label). Data presented as mean ± SEM.
Table 1.
Total pCREB immunoreactivity in the AVPV/PeN, MPOA, PVN, and ARN of restrained mice.
| Brain Region | NP | PP14 |
|---|---|---|
| AVPV/PeN | 961 ± 166 | 1102 ± 89 |
| MPOA | 792 ± 158 | 962 ± 109 |
| PVN | 1783 ± 121 | 2141 ± 85* |
| ARN | 1594 ± 169 | 1788 ± 150 |
indicates p< 0.05 compared to NP mice. AVPV/PeN, rostral anteroventral periventricular nucleus; MPOA, medial preoptic area; PVN, paraventricular nucleus of the hypothalamus; ARN, arcuate nucleus; NP, nulliparous; PP14, postpartum day 14. Data reported as mean ± SEM of total positive cells counted bilaterally within 2 sections.
3.3. Differences in TH/CRFR1-GFP co-localization in postpartum mice compared to nulliparous mice.
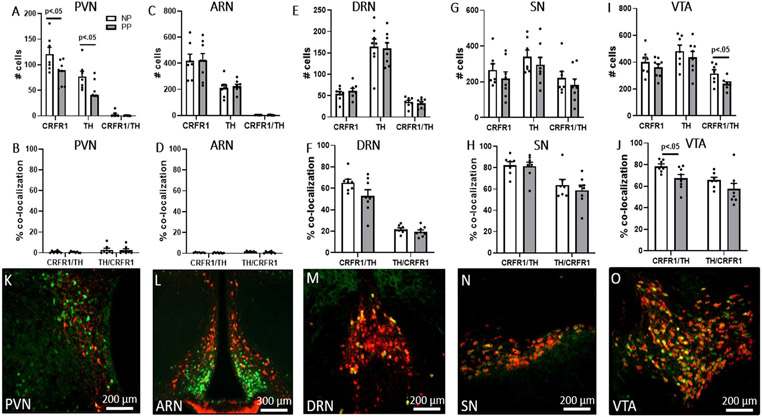
We used fluorescence microscopy to assess colocalization of TH and CRFR1-GFP within the AVPV/PeN, PVN, ARN, VTA, SN, and DRN. In the AVPV/PeN (Figure 4), reproductive status significantly impacted the number of TH (PP14 < NP; t(13) = 5.47, p < 0.001, d= 3.03; Figure 4A) and CRFR1/TH co-labeled (PP14 < NP; t(13) = 3.77, p = 0.002, d = 2.09; Figure 4A) neurons. There was a significant effect of reproductive status on the percentage of CRFR1-GFP neurons co-expressing TH (PP14 < NP; t(13) = 10.99, p < 0.001, d = 6.10; Figure 4B) and there was a trend toward significance in TH neurons co-expressing CRFR1 (PP14 <NP; t(13) = 2.12, p = 0.053, d = 1.17; Figure 4B). In the VTA, there was a significant effect of reproductive status on the number of CRFR1-GFP/TH co-expressing neurons (PP14 < NP; t(13) = 2.47, p = 0.028, d = 1.37; Figure 5I) and in the percentage of CRFR1-GFP cells co-expressing TH (PP14 < NP; t(13) = 2.43, p = 0.031, d = 1.35; Figure 5J). In the PVN (Figure 5A), PP14 and NP mice significantly differed in the number of CRFR1-GFP (PP14 < NP; t(13) = 2.45, p = 0.028, d = 1.36) and TH (PP14 < NP; t(13) = 2.31, p = 0.038, d = 1.28) neurons. There were no significant effects of reproductive status in the number of CRFR1-GFP/TH co-localized neurons and percentages of CRFR1-GFP/TH co-localizations in the PVN as co-labeled neurons were nearly absent. There were no significant differences between PP14 and NP mice in the number of TH and CRFR1-GFP/TH co-labeled neurons, in the percentages of CRFR1/TH co-localizations in the ARN, SN, or DRN (Figure 5).
Figure 4. AVPV/PeN CRFR1-GFP/TH co-localization.
Postpartum mice had lower levels of (A) TH and CRFR1-GFP/TH co-localization compared to nulliparous mice. (B) Postpartum mice also had a lower percentage of CRFR1 neurons co-localized with TH, and a trend toward lower percentage of TH neurons co-localized with CRFR1, compared to nulliparous mice. Representative images of AVPV/PeN CRFR1-GFP (green), TH (red), and CRFR1-GFP/TH+ (orange/yellow) co-localization levels in nulliparous (C-F) and PP14 (G-J) mice. High magnification of inset boxes in E and I are shown in F and J. Blue arrows indicate examples of co-labeled neurons. Data presented as mean ± SEM.
Figure 5. CRFR1-GFP/TH co-localization in the PVN, ARN, DRN, SN, and VTA.
(A) Postpartum mice had lower levels of CRFR1-GFP and TH neurons in the PVN compared to nulliparous mice. (B) Postpartum and nulliparous mice did not differ in levels and percentage of CRFR1-GFP/TH and TH/CRFR1 co-localization in the PVN. (C-H) Postpartum and nulliparous mice did not differ in CRFR1, TH, and CRFR1-GFP/TH neurons, or percentages of CRFR1 neurons co-localized with TH and TH neurons co-localized with CRFR1, in the ARN, DRN, and SN. The number of CRFR1/TH co-labeled cells (I) and percentage of CRFR1 cells expressing TH (J) were decreased in the VTA of PP14 compared to nulliparous mice. (K-O) Representative images of PVN, ARN, DRN, SN, and VTA co-labeling in a PP14 mouse. Data presented as mean ± SEM.
3.4. Differences in corticosterone levels between postpartum and nulliparous mice.
A two-way ANOVA (repeated measures) revealed a significant main effect of time (F(2,39) = 77.21) p < 0.001, N2P = 0.81) and an interaction of time and reproductive status (F(2,39) = 7.25) p < 0.01, N2P = 0.28, Figure 6). Post hoc analyses indicate no differences in baseline CORT, but postpartum mice had blunted restraint stress-induced CORT levels at 30 minutes (p = 0.027) and elevated recovery CORT levels at 90 minutes (p = 0.047) compared to nulliparous mice.
Figure 6. Baseline, restraint stress-induced, and recovery corticosterone levels.

Corticosterone levels at baseline, stress-induced (after a 30-minute restraint), and recovery corticosterone (90-min after restraint initiation and 1-hr left undisturbed) reveal attenuated corticosterone levels at 30 minutes after restraint onset and elevated corticosterone at 90 minutes in PP14 compared to nulliparous mice.
3.5. Efferent Projections of AVPV/PeN CRFR1 neurons.
See Table 2 for a list of relative density for anterograde projections within various brain regions from female CRFR1-Cre mice (N=4). Our findings indicate extensive fiber labeling within the AVPV itself as well as proximal regions including the MPOA and multiple subregions of the bed nucleus of the stria terminalis, including the dorsolateral, anteroventral, and principal nuclei. Several hypothalamic nuclei also showed dense fiber labeling, including the PVN, dorsomedial hypothalamus, ARN, and tuberal nucleus, while low to moderate labeling was found in other hypothalamic regions, including the ventromedial (ventrolateral division only), anterior, and lateral nuclei. Anterograde fiber labeling was generally sparse in the amygdala, with labeling only found at low levels within the medial division. Some hindbrain and midbrain structures also showed moderate AVPV CRFR1 anterograde fibers. These regions include the periaqueductal gray, VTA, locus coeruleus, Barrington’s nucleus, DRN, and parabrachial nucleus. Representative images of anterograde projection sites from AVPV/PeN CRFR1 neurons are shown in Figure 7.
Table 2. Anterograde projection sites of AVPV/PeN CRFR1 neurons.
Regions were assigned the following scores to indicate relative label densities: - no label; −/+ minimal labeling; + moderate density; ++ dense labeling; +++ very dense labeling. Ratings are based on labeling patterns from 4 female mice. OVLT; organum vasculosum of the lamina terminalis, BNST; bed nucleus of the stria terminalis, AVPV; anteroventral periventricular nucleus.
| Brain Region | Fiber Density |
|---|---|
| Prelimbic cortex | - |
| Infralimbic cortex | - |
| Lateral Septum (ventral) | ++ |
| Medial Septum | + |
| Medial Preoptic Area | +++ |
| AVPV | +++ |
| OVLT | +++ |
| BST (dorsolateral) | ++ |
| BST (anteroventral) | +++ |
| BST (principal nucleus) | ++ |
| Paraventricular Hypothalamus | +++ |
| Dorsomedial Hypothalamus | +++ |
| Ventromedial Hypothalamus | + |
| Anterior Hypothalamus | + |
| Lateral Hypothalamus | + |
| Supraoptic Nucleus | ++ |
| Tuberal Nucleus | ++ |
| Arcuate Nucleus | ++ |
| Paraventricular thalamus | + |
| Basolateral Amygdala | - |
| Central Amygdala | −/+ |
| Medial Amygdala | + |
| Cortical Amygdala | - |
| Periaqueductal Gray | + |
| Ventral Tegmental Area | + |
| Locus Coeruleus | + |
| Dorsal Tegmental Area | - |
| Barrington’s Nucleus | + |
| Parabrachial Nucleus | + |
| Mammillary (principal) | - |
| Dorsal Raphe | + |
| Median Raphe | - |
Figure 7. Representative images of anterograde projection sites from AVPV/PeN CRFR1 neurons.
(A) The injection site showing labeling of CRFR1-Cre neurons in the AVPV. Afferent labeling is found in numerous regions including the (B) ventral lateral septum (LSv), (C) dorsal bed nucleus of the stria terminalis (BSTd), (D) principal nucleus of the BST (BSTpr), (E) paraventricular nucleus (PVN), (F) dorsomedial hypothalamus (DMH), arcuate nucleus (ARN), and tuberal nucleus (TN), (G) lateral hypothalamus (lat hyp), (H) paraventricular thalamus (PVT), (I) medial amygdala (MeA), (J) periaqueductal gray (PAG), (K) dorsal raphe nucleus (DRN), (L) Barrington’s nucleus (BN), and (M) parabrachial nucleus (PBN). LV; lateral ventricle, 3 V; 3rd ventricle, 4v; 4th ventricle, OT; optic tract. Arrows are shown within regions with moderate to low labeling intensity to indicate specific sites that show fiber labeling.
4. Discussion
Overall, this study revealed that reproductive status impacted CRFR1 levels in a region-specific manner. The most prominent difference observed was in the AVPV/PeN, where PP14 females showed elevated CRFR1-GFP levels compared to NP females. We also report reproductive status affected the levels of CRFR1 and CRFR1 co-localization with TH in other brain regions involved in maternal- and stress- circuitries, including the MPOA and PVN. Together, these alterations in CRFR1 have potential implications for regulating changes in stress-related and maternal behavior during the postpartum period.
4.1. Differences in CRFR1 levels and co-localization with pCREB in postpartum compared to nulliparous mice.
AVPV/PeN CRFR1-GFP levels and activation of CRFR1-GFP neurons (pCREB positive) were greater in PP14 mice compared to NP mice. Interestingly, our previous study indicates that AVPV/PeN CRFR1 and CRFR1/pCREB co-localized cells are also increased in female, but not male mice, following chronic variable stress (Rosinger et al., 2020). AVPV/PeN CRFR1 neurons highly co-express glucocorticoid receptor (Rosinger et al., 2019a), thus elevations in glucocorticoids that occur either following chronic stress or during the postpartum period (Patchev and Almeida, 1996, Sárvári et al., 2017) may play a role in increasing CRFR1 levels. Fluctuations in other hormones such as estrogens, progesterone, and prolactin during pre- and post-partum periods might also contribute to alterations in AVPV/PeN CRFR1. Previous studies have demonstrated these hormones dynamically regulate various genes in the AVPV, including tyrosine hydroxylase and kisspeptin (Brown et al., 2014; Liu et al., 2014).
The functional role of AVPV CRFR1 is currently unknown but may play a role in the regulation of behavioral and neuroendocrine stress responses as well as maternal behaviors. Our previous study demonstrated a positive correlation between the number of CRFR1-GFP cells and CRFR1-GFP/pCREB co-expressing cells and anxiety-like behavior in the open field test in female mice (Rosinger et al., 2020). A similar increase in CRFR1 and CRFR1/pCREB co-expressing neurons in postpartum mice might also indicate a functional change that could drive anxiety-like behavior. Pharmacological activation of CRFR1 in the MPOA, which is in close anatomical proximity to the AVPV, increases anxiety-like responses in postpartum rats (Klampfl et al., 2018). This suggests CRFR1 stimulation in the MPOA is anxiogenic, and if similar anxiogenic effects occur with AVPV/PeN CRFR1 stimulation, higher abundance of receptor in postpartum mice would indicate a greater capacity to increase this anxiety response. Activation of CRF neurons in the PVN of postpartum mice, which project densely to rostral preoptic regions (Zhang et al., 2017), has also been demonstrated to contribute to increases in depressive-like behavior (Melon et al., 2018). Therefore, it is possible that PVN CRF signaling to AVPV/PeN CRFR1 could mediate this increase, although subsequent studies are needed to test this. Alterations in CRFR1 signaling have also been shown to affect maternal behaviors (Klampfl et al., 2018). Specifically, CRFR1 knockout mice show deficits in nursing (Gammie et al., 2007). Furthermore, pharmacological activation of CRFR1 in the rat bed nucleus of the stria terminalis and MPOA decreases nursing and arched-back nursing (Klampfl et al., 2016; 2018). CRF and CRFR1 have also been demonstrated to contribute to maternal aggression (Gammie et al., 2008; Klampfl et al., 2016). It is therefore possible that AVPV/PeN CRFR1 regulates similar maternal functions although this has not yet been tested.
Findings from our anterograde tracer study further indicate the potential for AVPV/PeN CRFR1 neurons to influence stress and reproductive/maternal functions. We demonstrate dense fibers emanating from AVPV/PeN neurons within key regions that control these functions, including the PVN, preoptic area, and BST. Lower levels of fiber innervation are also found in stress/emotion-regulating regions, including the locus coeruleus and dorsal raphe. Although we have previously shown that AVPV/PeN CRFR1 neurons are not neurosecretory (Rosinger et al., 2019a), it is possible that they can indirectly influence neuroendocrine function given the high density of projections to the PVN and ARN, both regions containing several populations of neuroendocrine neurons (Swanson and Sawchenko, 1980; Chronwall, 1985). Together, the maternal modulation of AVPV/PeN CRFR1 and dense projections of these CRFR1-expressing neurons to the aforementioned stress/maternal behavior-regulating regions suggest AVPV/PeN CRFR1 neurons may be a central hub for regulation of stress-related functions during the postpartum period.
PVN CRFR1 neurons play a critical role in behavioral and neuroendocrine stress responses (Ramot et al., 2017; Jiang et al., 2018). Previous work from our lab (Rosinger et al., 2019b) showed CRFR1 within the PVN is also sexually dimorphic, where CRFR1 levels and stress-induced activation of CRFR1 neurons were greater in adult male mice compared to adult female mice. In the current study, we demonstrated that PVN CRFR1 levels were modestly decreased at PP14, but CRFR1 activation (pCREB co-localization) and overall PVN pCREB levels were greater in PP14 mice compared to NP mice. Increased activation of CRFR1+ neurons in the PVN might contribute to the blunted stress-induced corticosterone response demonstrated here in PP14 mice, which correspond with similar findings in rats (Lightman and Young, 1989; Walker et al., 1995; Neumann et al., 1998) and humans (Altemus et al., 1995; Heinrichs et al., 2001). To our knowledge, this study is the first to show blunted stress-induced CORT and prolonged levels of recovery CORT, in postpartum female mice. In male mice, PVN CRFR1 neurons have previously been shown to provide negative feedback to CRF neurons, resulting in suppression of HPA axis responses (Jiang et al., 2018; 2019). Therefore, the increased activation of PVN CRFR1 neurons of female mice during the postpartum period might serve to suppress activation of PVN CRF neurons, thus blunting activation of the HPA axis and limiting the stress-induced rise in CORT. Furthermore, CRF-expressing neurons in the PVN have been shown to regulate depressive-like and maternal behaviors during the postpartum period (Melón et al., 2018). Therefore, it is possible the same local CRFR1 negative feedback regulation of CRF neurons might contribute to postpartum changes in these behaviors.
Total pCREB was also increased in the postpartum compared to NP PVN, suggesting increased transcription of other neuronal populations in addition to CRFR1 expressing neurons. The PVN contains several other distinct cell populations, including neurons that express CRF, oxytocin, and vasopressin, that project centrally and peripherally to mediate numerous behavioral stress responses (e.g. freezing), maternal behaviors, and neuroendocrine activity in rodents (Palkovits, 1982; Knobloch et al., 2012; Füzesi et al., 2016). It is important to note that increased transcriptional activity for the PVN and other brain regions (assessed using pCREB) is not necessarily due to the restraint stressor alone but also likely reflects neural responses to other behaviors in postpartum rodents, including those associated with pup care such as nursing (Parent et al., 2017). A similar limitation should be considered for HPA axis responses where other factors are at play to regulate CORT levels. For example, mice in this study were not returned to their pups following restraint stress, thus the prolonged elevated levels of CORT might be affected in part by another secondary stressor, separation from pups. On the contrary, the final acute restraint stress/maternal separation should have no effect on CRFR1-GFP as alterations in levels should not occur within our 90-minute time frame from stress initiation to brain extraction (Imaki et al., 1996; Rosinger et al., 2020), unlike effects on pCREB and corticosterone which occur much more rapidly.
As opposed to the adjacent AVPV/PeN, the MPOA showed decreased levels of CRFR1 in PP14 mice compared to NP mice. A wide body of research indicates that the MPOA is critical for regulation of various maternal behaviors (Numan, 2007; Numan and Stolzenberg, 2009) and recent work also suggests a critical role for MPOA CRFR1 in regulating maternal functions (Klampfl et al., 2018; Klampfl and Bosch, 2019). For example, injection of a CRFR1 agonist directly into the MPOA decreased arched-back nursing and total nursing behavior, and increased anxiety-like behavior (Klampfl et al., 2018). Thus, decreased levels of CRFR1 at PP14 could be adaptive for facilitating maternal care. By naturally decreasing CRFR1 levels, the negative effects of CRFR1 binding on maternal behaviors could also decrease. In a previous study, CRFR1 mRNA expression in PP4 rats did not differ from NP rats (Klampfl et al., 2018). The discrepancies in our results could be due to differing postpartum time points (PP4 versus PP14) and/or species differences. Regardless, changes in MPOA CRFR1 in mice could play a key role in regulating maternal care and anxiety-like behaviors during the postpartum period. Unlike other regions examined, CRFR1 in the ARN was unaltered in PP14 compared to NP mice, suggesting this population is less sensitive to modification during the postpartum period.
4.2. Differences in TH and CRFR1-GFP/TH+ co-localization levels in postpartum compared to nulliparous mice.
TH is a rate limiting enzyme in the production of both dopamine and norepinephrine, although the populations assessed in this study are largely representative of dopamine-producing neurons. TH labeling in the VTA and SN is widely known to reflect dopaminergic and not noradrenergic neurons (Swanson and Hartman, 1975). DRN TH neurons are also dopaminergic and are traditionally considered to be an extension of the VTA dopaminergic population (Lin et al., 2020;2021). Furthermore, the AVPV, PVN, and ARN TH populations are also known to be dopaminergic given the absence of dopamine β-hydroxylase (enzyme necessary to convert dopamine to norepinephrine) in these regions (Negishi et al., 2020). Dopaminergic neurons have been suggested to facilitate mother-pup interactions (Kuroda et al., 2011; Love 2014; Scott et al., 2015) and are densely distributed in brain regions involved in reward circuitry (Sombers et al., 2009). Several populations of dopaminergic cells have also been demonstrated to co-express CRFR1, including the AVPV, VTA, and SN (Refojo et al., 2011; Rosinger et al., 2019a). This co-expression with CRFR1 appears to play an important role in anxiety-like behaviors in male mice (Refojo et al., 2011), although the functional significance of CRFR1 expression in dopaminergic neurons in postpartum mice, and whether levels of co-expression change during the postpartum period, have not been examined.
In the AVPV/PeN, PP14 mice had lower levels of TH compared to NP mice. Our result is consistent with previous work, where the number of TH-immunoreactive neurons decreased in the AVPV/PeN of PP7 mice (Brown et al., 2015). One study, however, reported postpartum mice to have higher levels of TH in the AVPV/PeN compared to nulliparous mice (Scott et al., 2015). Methodological differences, including duration of co-housing with males during the postpartum period may contribute to this discrepancy. TH neurons in the AVPV have been shown to regulate maternal behaviors through a circuit involving PVN oxytocin neurons and oxytocin release (Scott et al., 2015). Therefore, postpartum changes in TH might impact behaviors, such as maternal care. However, Brown et al., (2015) demonstrated that dopamine turnover increased in conjunction with decreased TH in the AVPV/PeN, suggesting a potential compensation for the decrease in TH. Thus, it is unclear as to whether the decrease in TH actually indicates a decrease in dopamine production.
AVPV/PeN CRFR1-GFP/TH co-localization levels were also lower in PP14 mice compared to NP mice. Decreased levels of TH neurons co-localized with CRFR1 at PP14 indicate that CRF signaling could have a lesser influence on AVPV/PeN dopaminergic neurons in postpartum mice, and therefore might play a role in mediating effects of stress on maternal behaviors. PP7-PP14 constitute periods of the highest nursing demand in mice and rats suggesting that changes in TH and CRFR1-GFP/TH+ co-localization with CRFR1 might be regulated by hormones associated with nursing, such as prolactin (Sar, 1984; Ma et al., 2005). TH neurons have been shown to express receptors for prolactin at high levels at PP7, indicating the potential for prolactin to interact with these neurons (Brown et al., 2015). Stimulation of the nipples and other maternal behavioral experiences might also contribute to these alterations (Berghorn et al., 2001; Mayer et al., 2019; Stolzenberg and Mayer, 2019). Another factor that could contribute to changes in TH and TH/CRFR1 co-localization is increased drive of CRF on AVPV/PeN TH neurons. Increases in AVPV/PeN CRFR1 we report in this region might indicate a greater sensitivity to CRF signaling, which could downregulate TH. The functional significance of these changes is currently unclear, but one possibility is that lower levels of CRFR1 in TH+ neurons could decrease stress signal to CRFR1-GFP/TH+ neurons, which could otherwise interfere with maternal behaviors.
Postpartum mice also had fewer CRFR1-GFP/TH co-localized neurons in the VTA compared to nulliparous mice. VTA activity is involved in incentive valuation and goal-oriented behaviors (Koob and Volkow, 2010). Transient VTA inactivation disrupts pup retrieval (Numan et al., 2009; Seip and Morrell, 2009), suggesting that VTA is involved in responding to pup stimuli. Lower levels of CRFR1 in TH+ neurons in the VTA could decrease stress signal to CRFR1-GFP/TH+ neurons, which might otherwise interfere with incentive valuation and maternal behavioral response toward pups. However, further experiments are needed to test this hypothesis.
Co-localization of CRFR1 and TH was nearly absent in the ARN and PVN of nulliparous and postpartum mice, indicating an absence of direct CRF-CRFR1 signaling with TH neurons in these regions. On the contrary, CRFR1/TH co-localization was high in the DRN and SN, suggesting the capacity for direct CRF signaling to modify TH function and expression, although no differences were found between PP14 and NP mice. Co-expression within the SN suggests the potential for CRF to modify functions associated with SN TH neurons, including reward and anxiety (Vieira et al., 2019; Refojo et al., 2011), however whether this function changes during the postpartum period is unclear. To our knowledge, this is the first demonstration of the presence of CRFR1 in TH neurons of the DRN. Previous studies have primarily focused on CRF/CRFR1 signaling through DRN serotonergic neurons (Price et al., 1998; Valentino et al., 2001; Oshima et al., 2003; Hale and Lowry, 2011; Howerton, 2014; Hwa et al., 2016) and have demonstrated a role in various functions including the HPA axis, stress-related behaviors, and opioid conditioned place preference (Price et al., 1998; Valentino et al., 2001; Oshima et al., 2003; Hale and Lowry, 2011; Howerton, 2014; Li et al., 2021). To our knowledge, no studies have investigated CRF/CRFR1 signaling through dopaminergic (TH) neurons specifically within DRN, thus the potential function is unknown.
5. Conclusions
The primary focus of this study was to investigate potential alterations in CRFR1 within the AVPV/PeN, a region in which we previously reported higher levels of CRFR1 in females than in males. We report increases in CRFR1 and co-localization of pCREB and CRFR1 within the AVPV/PeN on PP14. PP14 mice also showed an uncoupling of AVPV/PeN TH neurons with CRFR1 as indicated by a decrease in TH/CRFR1 co-localized cells relative to NP mice. Although the function of AVPV/PeN CRFR1 is currently unknown, we demonstrate connectivity between these neurons and numerous brain regions associated with stress and maternal behaviors, suggesting a potentially key role in regulating these functions. Beyond the AVPV/PeN, other regions examined also showed alterations in CRFR1, including the MPOA which showed decreased CRFR1-GFP. Given evidence that infusion of a CRFR1 agonist into the MPOA increases anxiety and decreases maternal care (Klampfl et al., 2013, 2014, 2018), the decrease in MPOA CRFR1 may serve to buffer stress responses postpartum to facilitate maternal care. Together, these findings indicate dynamic alterations in CRFR1, which may contribute to postpartum changes in anxiety- and depressive-like behaviors, HPA axis function, and maternal care (Shoji et al., 2019; Molina-Hernandez and Téllez-Alcántara, 2001; Craft et al., 2010; Furuta et al., 2013; Lightman and Young, 1989). Beyond determining the functional significance of these alterations in CRFR1 in postpartum mice, it will also be important to determine the time course of these changes across the postpartum period. It is quite possible that increases or decreases in CRFR1 reach their peak/nadir earlier or later in the maternal phase of mice or even persist post-weaning. Determining the time course of CRFR1 changes across the postpartum period will therefore provide insight into critical periods for stress regulation of maternal behaviors.
Supplementary Material
Acknowledgements
We gratefully acknowledge Margaret Malone, Danielle Fico, Kasia Szafranska, Brianna Saglimbeni, Jaquan Vidot, Aya Caballero, Matt Sartori, Kai Shannon, Massoud Sharif, Kassandra Sturm, Marwan Abbas for their assistance in data collection and analysis. We also thank the following: Timothy Quinn, Binoy Thomas, and Kristine Klein for expert animal care; James Dias and Barbara Weaver for assistance with and use of their equipment for the radioimmunoassay. This research was supported by University at Albany Research Initiation Funds (DZ), R15-MH118692 (DZ), and R01-MH112768 (NJ).
Footnotes
Conflict of Interest
None declared.
References
- Agrati D, Browne D, Jonas W, Meaney M, Atkinson L, Steiner M, Fleming AS, 2015. Maternal anxiety from pregnancy to 2 years postpartum: transactional patterns of maternal early adversity and child temperament. Arch Womens Ment Health 18, 693–705. [DOI] [PubMed] [Google Scholar]
- Altemus M, Deuster PA, Galliven E, Carter CS, & Gold PW, 1995. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. The Journal of Clinical Endocrinology & Metabolism, 80(10), 2954–2959. [DOI] [PubMed] [Google Scholar]
- Barnett B, Schaafsma MF, Guzman AM and Parker GB, 1991. Maternal anxiety: a 5-year review of an intervention study. Journal of Child Psychology and Psychiatry, 32(3), 423–438. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Le WW, Sherman TG and Hoffman GE, 2001. Suckling stimulus suppresses messenger RNA for tyrosine hydroxylase in arcuate neurons during lactation. Journal of Comparative Neurology, 438(4), 423–432. [DOI] [PubMed] [Google Scholar]
- Brand SR and Brennan PA, 2009. Impact of antenatal and postpartum maternal mental illness: how are the children? Clinical obstetrics and gynecology, 52(3), 441–455. [DOI] [PubMed] [Google Scholar]
- Brown RSE, Herbison AE, & Grattan DR, 2014. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. Journal of neuroendocrinology, 26(12), 898–908 [DOI] [PubMed] [Google Scholar]
- Brown RSE, Herbison AE, & Grattan DR, 2015. Effects of prolactin and lactation on A15 dopamine neurones in the rostral preoptic area of female mice. Journal of neuroendocrinology, 27(9), 708–717. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH and Vale WW, 1993. Expression cloning of a human corticotropin-releasing-factor receptor. Proceedings of the National Academy of Sciences, 90(19), 8967–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Sikkema KJ, 2016. Childhood Maltreatment and Perinatal Mood and Anxiety Disorders: A Systematic Review. Trauma, Violence, & Abuse 17, 427–453. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, 1985. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides, 6, 1–11. [DOI] [PubMed] [Google Scholar]
- Chrousos GP (2009). Stress and disorders of the stress system. Nature reviews endocrinology, 5(7), 374. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kostick ML, Rogers JA, White CL and Tsutsui KT, 2010. Forced swim test behavior in postpartum rats. Pharmacology Biochemistry and Behavior, 96(4), 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva BS, Rovaris DL, Schuch JB, Mota NR, Cupertino RB, Aroche AP, Bertuzzi GP, Karam RG, Vitola ES, Tovo-Rodrigues L and Grevet EH, 2016. Effects of corticotropin-releasing hormone receptor 1 SNPs on major depressive disorder are influenced by sex and smoking status. Journal of affective disorders, 205, 282–288. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T and Wang S, 2011. Tyrosine hydroxylase and regulation of dopamine synthesis. Archives of biochemistry and biophysics, 508(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guzman RM, Saulsbery AI, & Workman JL, 2018. High nursing demand reduces depression-like behavior despite increasing glucocorticoid concentrations and reducing hippocampal neurogenesis in late postpartum rats. Behavioural brain research, 353, 143–153. [DOI] [PubMed] [Google Scholar]
- Furuta M, Numakawa T, Chiba S, Ninomiya M, Kajiyama Y, Adachi N, Akema T, Kunugi H Estrogen, predominantly via estrogen receptor a, attenuates postpartum-induced anxiety- and depression-like behaviors in female rats. Endocrinology. 2013; 154(10):3807–16. [DOI] [PubMed] [Google Scholar]
- Füzesi T, Daviu N, Cusulin JIW, Bonin RP and Bains JS, 2016. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature communications, 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Seasholtz AF, Stevenson SA, 2008. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience. 157(3), 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Bethea ED, & Stevenson SA, 2007. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC neuroscience, 8(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carmona JA, Baroja-Mazo A, Milanés MV and Laorden ML, 2015. Sex differences between CRF1 receptor deficient mice following naloxone-precipitated morphine withdrawal in a conditioned place aversion paradigm: implication of HPA axis. PLoS One, 10(4), e0121125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G and Swinson T, 2005. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics & Gynecology, 106(5 Part 1), 1071–1083. [DOI] [PubMed] [Google Scholar]
- Hale MW and Lowry CA, 2011. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology, 213(2), 243–264. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, & Hellhammer DH, 2001. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. The journal of clinical endocrinology & metabolism, 86(10), 4798–4804. [DOI] [PubMed] [Google Scholar]
- Herbison AE, 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Research Reviews, 57(2), 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF and Miczek KA, 2016. Episodic social stress-escalated cocaine self-administration: Role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. Journal of Neuroscience, 36(14), 4093–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG and Bale TL, 2014. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biological psychiatry, 75(11), 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, Miczek KA, 2016. Dissociation of μ-opioid receptor and CRF-R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addiction Biology, 21(1), 111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Naruse M, Harada S, Chikada N, Imaki J, Onodera H, Demura H and Vale W (1996. Corticotropin-releasing factor up-regulates its own receptor mRNA in the paraventricular nucleus of the hypothalamus. Molecular brain research, 38(1), 166–170. [DOI] [PubMed] [Google Scholar]
- Jacobskind JS, Rosinger ZJ, & Zuloaga DG, 2017. Hypothalamic-pituitary-adrenal axis responsiveness to methamphetamine is modulated by gonadectomy in males. Brain research, 1677, 74–85. [DOI] [PubMed] [Google Scholar]
- Jacobskind JS, Rosinger ZJ, Brooks ML and Zuloaga DG, 2019. Stress-induced neural activation is altered during early withdrawal from chronic methamphetamine. Behavioural brain research, 366, 67–76. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rajamanickam S, & Justice NJ, 2018. Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. Journal of Neuroscience, 38(8), 1874–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Rajamanickam S, Justice NJ. 2019. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiology of Stress, 11, 100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, & Vale W, 2008. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. Journal of Comparative Neurology, 511(4), 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Schramm MM, Gaßner BM, Hübner K, Seasholtz AF, Brunton PJ, & Bosch OJ, 2018. Maternal stress and the MPOA: activation of CRF receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology, 133, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM and Bosch OJ, 2019. Mom doesn’t care: When increased brain CRF system activity leads to maternal neglect in rodents. Frontiers in neuroendocrinology, 53, p. 100735. [DOI] [PubMed] [Google Scholar]
- Klampfl SM, Brunton PJ, Bayerl DS, Bosch OJ. CRF-R1 activation in the anterior-dorsal BNST induces maternal neglect in lactating rats via an HPA axis-independent central mechanism. Psychoneuroendocrinology. 64, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman Devra G., and Malcolm James R., 1981. "The evolution of male parental investment in mammals." Parental care in mammals. Springer, Boston, MA. 347–387. [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, & Grinevich V, 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73(3), 553–566. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Wyatt A, Phillipps HR, Aoki M, Ectors F, Boehm U, & Grattan DR, 2018. Analysis of prolactin receptor expression in the murine brain using a novel prolactin receptor reporter mouse. Journal of neuroendocrinology, 30(9), e12634. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda KO, Tachikawa K, Yoshida S, Tsuneoka Y, & Numan M, 2011. Neuromolecular basis of parental behavior in laboratory mice and rats: with special emphasis on technical issues of using mouse genetics. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(5), 1205–1231. [DOI] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, & Suh HW, 2006. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience, 142(4), 1281–1292. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ and Chen L, 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature, 445(7124), 168–176. [DOI] [PubMed] [Google Scholar]
- Leshin LS, Kraeling RR, Kineman RD, Barb CR, Rampacek GB, 1996. Immunocytochemical distribution of catecholamine-synthesizing neurons in the hypothalamus and pituitary gland of pigs: tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol, 364:151–68. [DOI] [PubMed] [Google Scholar]
- Li C, McCloskey N, Phillips J, Simmons SJ and Kirby LG, 2021. CRF-5-HT interactions in the dorsal raphe nucleus and motivation for stress-induced opioid reinstatement. Psychopharmacology, 238(1), 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, & Young WS III, 1989. Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotropin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology, 124(5), 2358–2364. [DOI] [PubMed] [Google Scholar]
- Lin R, Liang J, Luo M, 2021. The Raphe Dopamine System: Roles in Salience Encoding, Memory Expression, and Addiction. Trends in Neuroscience. 44(5), 366–377. [DOI] [PubMed] [Google Scholar]
- Lin R, Liang J, Wang R, Yan T, Zhou Y, Liu Y, Feng Q, Sun F, Li Y, Li A, Gong H, Luo M, 2020. The Raphe Dopamine System Controls the Expression of Incentive Memory. Neuron. 106(3), 498–514. [DOI] [PubMed] [Google Scholar]
- Liu X, Brown RS, Herbison AE, Grattan DR, 2014. Lactational anovulation in mice results from a selective loss of kisspeptin input to GnRH neurons. Endocrinology, 155, 193–203. [DOI] [PubMed] [Google Scholar]
- Mayer HS, Helton J, Torres LY, Cortina I, Brown WM, Stolzenberg DS 2019. Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Hormones and Behav, 108, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Payant MA, Schumacker KS, Wittmann G, Butler RM, Lechan RM, Steinbusch HWM, Khan AM, Chee MJ, 2020. Distributions of hypothalamic neuron populations coexpressing tyrosine hydroxylase and the vesicular GABA transporter in the mouse. Journal of Comparative Neurology. 528(11), 1833–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM, 2014. Oxytocin, motivation and the role of dopamine. Pharmacology Biochemistry and Behavior, 119, pp.49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer HS, Helton J, Torres LY, Cortina I, Brown WM, Stolzenberg DS, 2019. Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Hormones and Behavior, 108, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Hooper A, Yang X, Moss SJ and Maguire J, 2018. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology, 90, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, 2019. Is the HPA axis as target for depression outdated, or is there a new hope? Frontiers in psychiatry, 10, p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Hernández M and Téllez-Alcántara NP, 2001. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology, 26(5), 479–491. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M and Udenfriend S, 1964. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. Journal of Biological Chemistry, 239(9), 2910–2917. [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Liebsch G, Holsboer F and Landgraf R, 1998. Increased basal activity of the hypothalamo–pituitary–adrenal axis during pregnancy in rats bred for high anxiety-related behaviour. Psychoneuroendocrinology, 23(5), 449–463. [DOI] [PubMed] [Google Scholar]
- Numan M and Insel TR, 2003. Paternal behavior. The Neurobiology of Parental Behavior, 246–267. [Google Scholar]
- Numan M, 2007. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 49(1), 12–21. [DOI] [PubMed] [Google Scholar]
- Numan M and Stolzenberg DS, 2009. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in neuroendocrinology, 30(1), pp.46–64. [DOI] [PubMed] [Google Scholar]
- Oshima A, Flachskamm C, Reul JM, Holsboer F and Linthorst AC, 2003. Altered serotonergic neurotransmission but normal hypothalamic–pituitary–adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology, 28(12), 2148–2159. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Eiden LE, Beinfeld MC, Russell J, Arimura A and Szabo S, 1982. Selective depletion of somatostatin in rat brain by cysteamine. Brain Research, 240(1), 178–180. [DOI] [PubMed] [Google Scholar]
- Parent C, Wen X, Dhir SK, Ryan R, Diorio J and Zhang TY, 2017. Maternal care associates with differences in morphological complexity in the medial preoptic area. Behavioural brain research, 326, 22–32. [DOI] [PubMed] [Google Scholar]
- Patchev VK and Almeida OF, 1996. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. Journal of Neuroscience, 16(21), 7077–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML and Lucki I, 2001. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. Journal of Neuroscience, 21(8), 2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, & Chen A, 2017. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nature neuroscience, 20(3), 385. [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, Von Wolff G, Avrabos C and Touma C, 2011. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science, 333(6051), 1903–1907. [DOI] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, & Zuloaga DG, 2017. Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: a novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience, 361, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, Bulanchuk N, Malone M, Fico D, Justice NJ, & Zuloaga DG, 2019a. Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group. Journal of Comparative Neurology, 527(6), 1056–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, De Guzman RM, Justice NJ, Zuloaga DG. 2019b. A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience, 409, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, De Guzman RM, Jacobskind JS, Saglimbeni B, Malone M, Fico D, & Zuloaga DG, 2020. Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations. Physiology & Behavior, 219, 112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, 1984. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science, 223(4639), 938–940. [DOI] [PubMed] [Google Scholar]
- Sárvári M, Kalló I, Hrabovszky E, Solymosi N and Liposits Z, 2017. Ovariectomy alters gene expression of the hippocampal formation in middle-aged rats. Endocrinology, 158(1), 69–83. [DOI] [PubMed] [Google Scholar]
- Schüssler P, Kluge M, Gamringer W, Wetter TC, Yassouridis A, Uhr M, Rupprecht R and Steiger A, 2016. Corticotropin-releasing hormone induces depression-like changes of sleep electroencephalogram in healthy women. Psychoneuroendocrinology, 74, 302–307. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, & Kimchi T, 2015. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature, 525(7570), 519. [DOI] [PubMed] [Google Scholar]
- Seip KM, & Morrell JI, 2009. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup-but not cocaine-paired contexts. Behavioral neuroscience, 123(6), 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H and Miyakawa T, 2019. Increased depression-related behavior during the postpartum period in inbred BALB/c and C57BL/6 strains. Molecular brain, 12(1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB and Korach KS, 1997. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proceedings of the National Academy of Sciences, 94(25), 14077–14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual review of neuroscience, 25(1), 507–536. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA and Sawchenko PE, 1998. Corticotropin releasing factor receptor 1–deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron, 20(6), 1093–1102. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, & Wightman RM, 2009. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. Journal of Neuroscience, 29(6), 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, 1998. Perinatal mood disorders: position paper. Psychopharmacology Bulletin 34(3):301–6. [PubMed] [Google Scholar]
- Stolzenberg DS and Mayer HS, 2019. Experience-dependent mechanisms in the regulation of parental care. Frontiers in neuroendocrinology, 54,100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK, 1975. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. Journal of Comparative Neurology. 163(4), 467–505. [DOI] [PubMed] [Google Scholar]
- Swanson LW and Sawchenko PE, 1980. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology, 31(6), 410–417. [DOI] [PubMed] [Google Scholar]
- Tietz A, Zietlow A-L, Reck C, 2014. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health 17, 433–442. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, & Rivier J, 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L and Van Bockstaele EJ, 2001. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. Journal of Comparative Neurology, 435(4), 450–463. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W and Sawchenko PE, 2000. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. Journal of Comparative Neurology, 428(2), 191–212. [DOI] [PubMed] [Google Scholar]
- Vieira JCF, Bassani TB, Santiago RM, de O Guaita G, Zanoveli JM, da Cunha C, Vital MABF, 2019. Anxiety-like behavior induced by 6-OHDA animal model of Parkinson's disease may be related to a dysregulation of neurotransmitter systems in brain areas related to anxiety. Behavioral Brain Research, 2019, 371, 111981. [DOI] [PubMed] [Google Scholar]
- Walker CD, Trottier G, Rochford J, & Lavallée D, 1995. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. Journal of neuroendocrinology, 7(8), 615–622. [DOI] [PubMed] [Google Scholar]
- Wang L, Moenter SM, 2020. Differential Roles of Hypothalamic AVPV and Arcuate Kisspeptin Neurons in Estradiol Feedback Regulation of Female Reproduction. Neuroendocrinology, 110(3-4), 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Asai M, Mahoney CE, Joachim M, Shen Y, Gunner G, Majzoub JA, 2017. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Molecular Psychiatry, 22(5), 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Künzel HE, Ackl N, Sonntag A, Ising M, & Holsboer F, 2000. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. Journal of psychiatric research, 34(3), 171–181. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Monks DA, Breedlove SM and Jordan CL, 2007. Androgen-sensitivity of somata and dendrites of spinal nucleus of the bulbocavernosus (SNB) motoneurons in male C57BL6J mice. Hormones and behavior, 51(2), 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, & Handa RJ, 2014. Estrogen receptor β expression in the mouse forebrain: age and sex differences. Journal of Comparative Neurology, 522(2), 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.