Abstract
Background
The rise in the global prevalence of diabetes, particularly among younger people, has led to an increase in the number of pregnant women with preexisting diabetes, many of whom have diabetes-related microvascular complications. We aimed to estimate the magnitude of the risks of adverse pregnancy outcomes or disease progression in this population.
Methods and findings
We undertook a systematic review and meta-analysis on maternal and perinatal complications in women with type 1 or 2 diabetic microvascular disease and the risk factors for worsening of microvascular disease in pregnancy using a prospective protocol (PROSPERO CRD42017076647). We searched major databases (January 1990 to July 2021) for relevant cohort studies. Study quality was assessed using the Newcastle–Ottawa Scale. We summarized the findings as odds ratios (ORs) with 95% confidence intervals (CIs) using random effects meta-analysis. We included 56 cohort studies involving 12,819 pregnant women with diabetes; including 40 from Europe and 9 from North America. Pregnant women with diabetic nephropathy were at greater risk of preeclampsia (OR 10.76, CI 6.43 to 17.99, p < 0.001), early (<34 weeks) (OR 6.90, 95% CI 3.38 to 14.06, p < 0.001) and any preterm birth (OR 4.48, CI 3.40 to 5.92, p < 0.001), and cesarean section (OR 3.04, CI 1.24 to 7.47, p = 0.015); their babies were at higher risk of perinatal death (OR 2.26, CI 1.07 to 4.75, p = 0.032), congenital abnormality (OR 2.71, CI 1.58 to 4.66, p < 0.001), small for gestational age (OR 16.89, CI 7.07 to 40.37, p < 0.001), and admission to neonatal unit (OR 2.59, CI 1.72 to 3.90, p < 0.001) compared to those without nephropathy. Diabetic retinopathy was associated with any preterm birth (OR 1.67, CI 1.27 to 2.20, p < 0.001) and preeclampsia (OR 2.20, CI 1.57 to 3.10, p < 0.001) but not other complications. The risks of onset or worsening of retinopathy were increased in women who were nulliparous (OR 1.75, 95% CI 1.28 to 2.40, p < 0.001), smokers (OR 2.31, 95% CI 1.25 to 4.27, p = 0.008), with existing proliferative disease (OR 2.12, 95% CI 1.11 to 4.04, p = 0.022), and longer duration of diabetes (weighted mean difference: 4.51 years, 95% CI 2.26 to 6.76, p < 0.001) compared to those without the risk factors. The main limitations of this analysis are the heterogeneity of definition of retinopathy and nephropathy and the inclusion of women both with type 1 and type 2 diabetes.
Conclusions
In pregnant women with diabetes, presence of nephropathy and/or retinopathy appear to further increase the risks of maternal complications.
Sophie Relph and colleagues investigate adverse pregnancy outcomes in women with diabetes-related microvascular disease and risks of microvascular disease progression in pregnancy.
Author summary
Why was the study done?
The rate of diabetes in young women is increasing, meaning that more have diabetes during pregnancy.
Diabetes can cause complications in the kidneys (nephropathy), eyes (retinopathy), and nerves (nephropathy).
When planning antenatal care that enables safe pregnancy in women with diabetes and its complications, both healthcare professionals and women need robust information on the magnitude of the possible risks affecting either the mother or baby, and the factors associated with worsening of the diabetic complications during pregnancy.
What did the researchers do and find?
The researchers reviewed all the research published on this topic between January 1990 and July 2021.
Diabetic kidney disease significantly increased the risk of the woman having preeclampsia or a cesarean birth during pregnancy, the baby being born early, small or having abnormalities, the baby requiring neonatal care after birth or being stillborn, over and above the risk for diabetic women without kidney damage. Diabetic eye disease also increased the risk of early birth or preeclampsia.
Pregnant women with diabetes were more likely to get new or worsening eye damage if it was their first baby, they smoked, they already had advanced eye damage, or they had had diabetes for a long time.
What do these findings mean?
Antenatal care of pregnant women with diabetic eye or kidney damage should involve a multidisciplinary team, including maternal medicine and kidney specialists.
Women with risk factors identified in this review for worsening eye damage should be referred for closer monitoring during pregnancy, and specialist review where deterioration is noted.
Further research be carried out to study long-term outcomes beyond pregnancy for women with diabetic eye or kidney complications.
Introduction
The global prevalence of diabetes in adults doubled between 1980 and 2014 [1]. Many were diagnosed at a young age, particularly with type 2 diabetes, due to the obesity epidemic and sedentary lifestyle [2]. This trend has resulted in an increase in the numbers of reproductive aged women entering pregnancy with preexisting diabetes, with equal proportions diagnosed with type 1 and type 2 diabetes in some settings [3]. Pregnant women with long-standing diabetes are more likely to have microvascular complications manifesting as retinopathy or nephropathy [4]. Diabetic nephropathy is reported in 5% to 10% of pregnant women with type 1 diabetes [4] and about 2% to 3% with type 2 diabetes [5]. Diabetic retinopathy, the leading cause of blindness in reproductive aged women [6], affects 1 in 7 pregnant women with type 2 diabetes and almost half of pregnant women with type 1 diabetes [7]. Both nephropathy and retinopathy can worsen during pregnancy.
The recent confidential enquiries into maternal deaths across the United Kingdom highlighted that pregnant women with preexisting comorbidities are most at risk of death and major morbidity, stressing the need for accurate risk assessment and individualized management [8]. Current care of pregnant women with preexisting diabetes focuses on ascertaining the presence of microvascular complications, monitoring their progression and screening for pregnancy complications [9]. In order to plan pregnancy and optimize the antenatal management in women with preexisting diabetes and microvascular disease, both healthcare professionals and women need robust information on the magnitude of expected maternal and perinatal risks and the factors associated with deterioration of microvascular disease during pregnancy. However, existing studies are small with imprecise findings, and there are no meta-analyses to provide robust quantitative information.
We undertook a systematic review and meta-analysis to quantify the magnitude of association between the presence of diabetic nephropathy, retinopathy, and/or neuropathy on maternal and perinatal outcomes and the risk factors for microvascular disease progression during pregnancy.
Methods
Our systematic review and meta-analysis was done using a prospective protocol (PROSPERO CRD42017076647) [10] according to current recommendations. We reported our findings as per the PRISMA guidelines (S1 Appendix) [11].
Search strategy and selection criteria
We searched MEDLINE, Embase, and Cochrane databases (January 1990 to July 2021) without language restrictions for studies reporting maternal or perinatal outcomes in pregnant women with preexisting diabetes, with and without nephropathy, retinopathy, and/or neuropathy, and on risk factors associated with disease progression. We used Medical Subject Headings (MeSH) headings, free-text and expanded synonyms of “diabet*” combined with “nephropath*,” “retinopath*” or “neuropath*,” and “pregnan*.” The full search strategy is provided in S2 Appendix. We supplemented the results with a manual search of the reference lists.
Studies were selected for inclusion in 2 stages. First, we screened the titles and abstracts of all citations for potentially relevant papers. Second, we examined the full texts of these papers. Two independent reviewers (TP and SR) conducted the screening and the full-text evaluation against prespecified inclusion criteria. Any discrepancies were resolved after discussion with a third reviewer (ST). We included cohort studies if they reported on preexisting diabetes in pregnant women with and without microvascular complications (nephropathy, retinopathy, neuropathy, or any) and maternal outcomes such as early preterm birth (before 34 weeks’ gestation), any preterm birth (before 37 weeks’ gestation), preeclampsia or cesarean birth, and perinatal outcomes such as stillbirth, neonatal death, perinatal death, small or large for gestational age fetuses, congenital abnormalities, or admission to the neonatal unit (S3 Appendix). Stillbirth was defined by an intrauterine fetal death at or after 24 completed weeks. Neonatal death was defined as any death within 28 days of birth. Perinatal death included death by either definition. Small and large for gestational babies were those with birth weight less than the 10th centile for gestational age and above the 90th centile, respectively, using the centile definitions from the original studies. We accepted the study authors’ definitions or classification systems used to define diabetic microvascular diseases and all other outcomes.
We also included studies if they reported on risk factors for microvascular disease progression or onset in pregnancy such as parity, disease severity, time since diagnosis of diabetes, smoking, or ethnicity. When assessing for retinopathy progression, we included study-reported deterioration in the severity defined by any of the following grading systems: White classification of diabetes in pregnancy (class C, D, or R), English classification (background, preproliferative or proliferative retinopathy), or the Early Treatment Diabetic Retinopathy Study (ETDRS) classification (mild/moderate/severe nonproliferative or proliferative retinopathy). When assessing for progression of nephropathy, we included progression from micro- to macroalbuminuria or to end-stage renal disease, and deterioration of renal function as assessed by creatinine clearance. We did not include review articles, guidelines, editorials, case studies and case series, or animal or in vitro studies.
Study quality assessment
Two independent reviewers (SR and LD) undertook quality assessments of studies included in the meta-analysis using the Newcastle–Ottawa scale [12]. Studies with greater than 80% follow-up rates were awarded a star for outcome/exposure assessment. Studies were marked as having a low risk of bias if they scored 4 stars for selection, 2 stars for comparison, and 3 stars for exposure/outcome. Studies were marked as having a medium risk of bias if scored 2 or 3 stars for selection, 1 for comparison and 2 for outcome/exposure. Any study with a score of 1 or 0 for the selection and outcome/exposure assessments or 0 for the comparison assessment was deemed to have a high risk of bias.
Data extraction and analysis
Data were extracted independently by 2 reviewers (SR and TP) onto a predesigned spreadsheet. Where data from the same population were duplicated, the data from the larger population were included. We calculated the individual study odds ratios (ORs) of adverse pregnancy outcomes in pregnant women with preexisting diabetes with and without nephropathy, with and without retinopathy, and with either or both retinopathy and nephropathy than without any microvascular disease. The estimates were pooled using a random effects model. For continuously measured risk factors for disease progression, we computed the weighted mean difference using a random effects model. All confidence intervals (CIs) are presented at the 95% significance level. We assessed for heterogeneity between studies using I2 tests. Publication bias and the effect of small studies were assessed on outcomes with at least 10 studies using funnel plots and Egger’s tests [13]. Sensitivity analyses were conducted to see whether there was a different effect when only studying women with type 1 diabetes or by removing studies at a high risk of bias from the analysis. We assessed publication bias using Egger’s test in Stata v16. All other analyses were undertaken using Stata SE (version 12) statistical software [14].
Role of the funding source
There was no funding for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
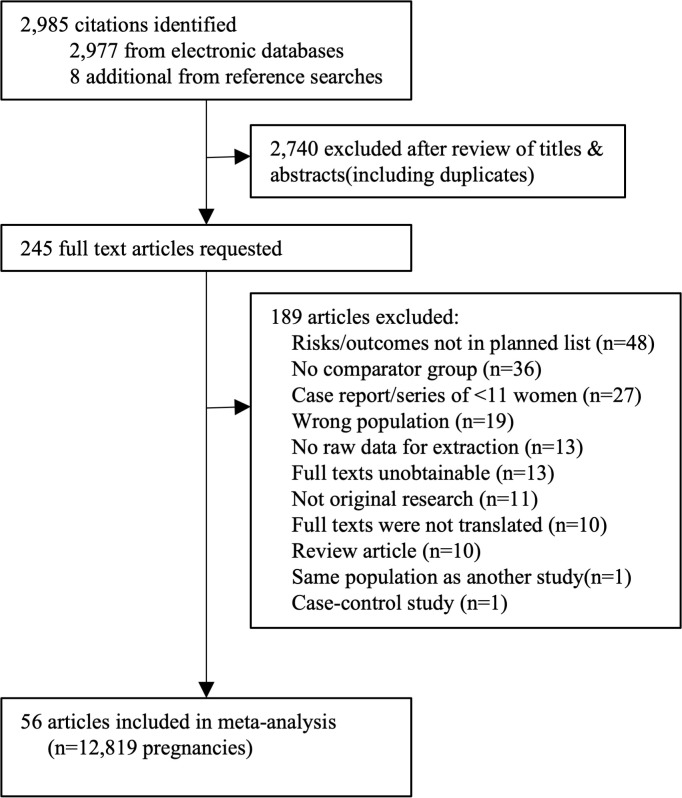
Of the 2,985 citations identified, we selected 245 titles for detailed assessment; and 56 papers were included (12,819 pregnant women) (Fig 1).
Fig 1. PRISMA flow chart of included studies in the systematic review.
Characteristics of the included studies
The majority of the studies were from Europe (40 studies), followed by North America (9 studies), Asia (4 studies), the Middle East (2 studies), and South America (1 study). Eighteen studies reported on maternal outcomes for retinopathy and 20 for nephropathy, 13 on offspring outcomes for retinopathy and 20 for nephropathy, and 21 for risk factors for disease progression. No studies reported on outcomes for women with diabetes-related neuropathy or risk factors for progressive neuropathy during pregnancy. Twelve studies included women with both type 1 and type 2 diabetes, 30 only type 1 diabetes, 6 only insulin-dependent diabetes, 1 type 2 diabetes, and 7 unspecified diabetes type. Retinopathy was either graded using modifications of White’s classification system for diabetic complications in pregnancy [15] or according to background, preproliferative, and proliferative status. Deterioration of nephropathy was described heterogeneously as either progression to nephrotic syndrome (>3 g proteinuria per day), increase in serum creatinine by >15%, creatinine clearance deterioration by >10%, elevation in creatinine greater or equal to 50% over baseline or 2-fold increase in rate of decline of glomerular filtration rate, or progression to dialysis, precluding inclusion of this outcome in meta-analysis [16–23]. The characteristics of the included studies, including inclusion and exclusion criteria, exposures, and outcomes, are provided in S4 Appendix.
Quality of the studies
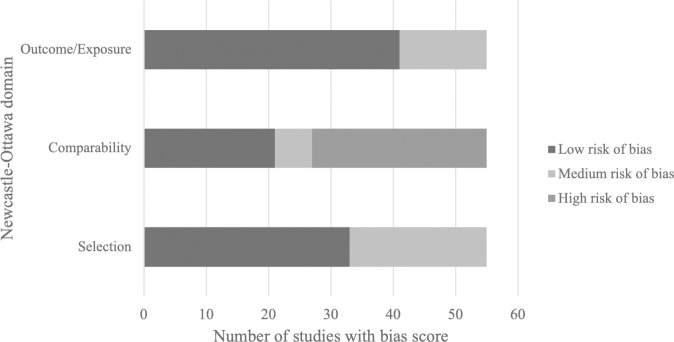
Of the 56 included studies, half (50.0%, 28/56) were considered to be at high risk of overall bias. No study had high risk of bias for adequate sample selection or for reporting outcomes. Twenty-eight studies (50.0%, 28/56) were at high risk of bias for comparability of the population. The proportion of studies deemed to have low, medium, or high risk of bias is shown in Fig 2, and details of individual study scores in S5 Appendix.
Fig 2. Quality of the studies included in the systematic review for study selection, comparability, and ascertainment of outcome.
Risk of adverse pregnancy outcomes in women with diabetic nephropathy
Maternal outcomes
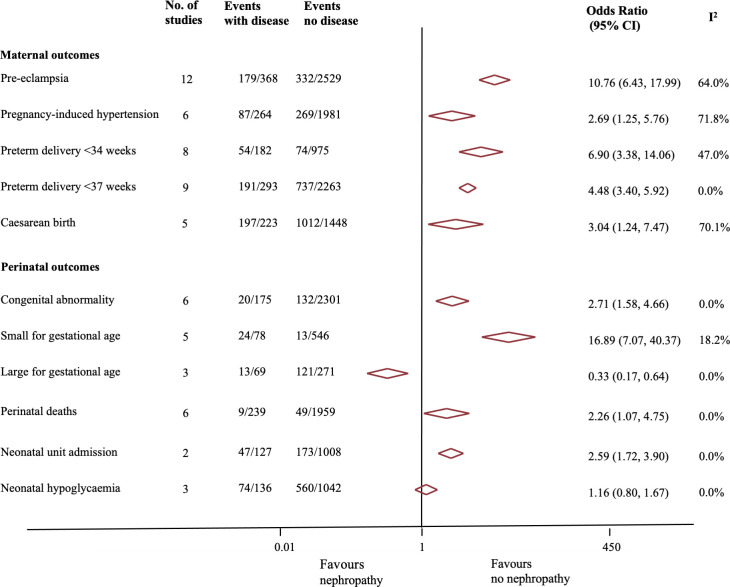
In pregnant women with preexisting diabetes, presence of nephropathy was associated with a 10-fold increase in the risk of preeclampsia (OR 10.76, 95% CI 6.43 to 17.99, p < 0.01, I2 = 64%; 12 studies) [18,21,22,24–32], 6.9-fold increased risk of preterm birth before 34 weeks’ (OR 6.90, 95% CI 3.38 to 14.06, p < 0.001, I2 = 47%; 8 studies) [5,16,18,21,22,24,27,30,33], 4.5-fold increase in preterm birth before 37 weeks’ (OR 4.48, 95% CI 3.40 to 5.92, p < 0.001, I2 = 0%; 9 studies) [5,18,22,24,25,29,30,34,35], and increased risks of pregnancy-induced hypertension (OR 2.69, 95% CI 1.26 to 5.76, p = 0.01, I2 = 72%; 6 studies) [18,24,25,30–32], and cesarean section (OR 3.04, 95% CI 1.24 to 7.47, p = 0.02, I2 = 70%; 5 studies) [16,18,21,25,36] compared to those without nephropathy (see Fig 3). Sensitivity analyses that restricted the meta-analyses to only women with type 1 diabetes or only studies with low to medium risk of bias showed similar findings (Tables A and B in S6 Appendix).
Fig 3. Association between diabetic nephropathy and adverse maternal and perinatal outcomes.
Perinatal outcomes
Presence of diabetic nephropathy was significantly associated with increased risk of adverse perinatal outcomes (Fig 3) such as congenital abnormality (OR 2.71, 95% CI 1.58 to 4.66, p < 0.001, I2 = 0%; 6 studies) [16,18,21,22,30,37], small for gestational age fetus (OR 16.89, 95% CI 7.07 to 40.37, p < 0.001, I2 = 0%; 5 studies) [5,16,22,27,30], perinatal mortality (OR 2.26, 95% CI 1.07 to 4.75, p = 0.03, I2 = 0%; 6 studies) [18,22,25,27,30,38], and neonatal unit admission (OR 2.59, 95% CI: 1.72 to 3.90, p < 0.01, I2 = 10%; 2 studies) [5,25] compared to those without nephropathy; the risk of large for gestational age fetus at birth was reduced (OR 0.33, 95% CI 0.17 to 0.64, p = 0.01, I2 = 0%; 3 studies) [5,18,27]. One study showed no difference in the risk of low Apgar score (<7 at 1 minutes) (OR 1.68, 95% CI 0.99 to 2.87, p = 0.05) or neonatal acidosis (cord arterial pH <7.05, OR 0.36, 95% CI 0.05 to 2.68, p = 0.30) [25]. Sensitivity analyses (Tables A and B in S6 Appendix) restricted to only women with type 1 diabetes, or only studies with a low to moderate risk of bias, showed similar findings, except for an even higher risk of small for gestational age fetus in the latter analysis (OR 25.75, 95% CI 10.51 to 63.08, p < 0.01. I2 = 73%, 4 studies) [16,22,27,30].
Risk of adverse pregnancy outcomes in women with diabetic retinopathy
Maternal outcomes
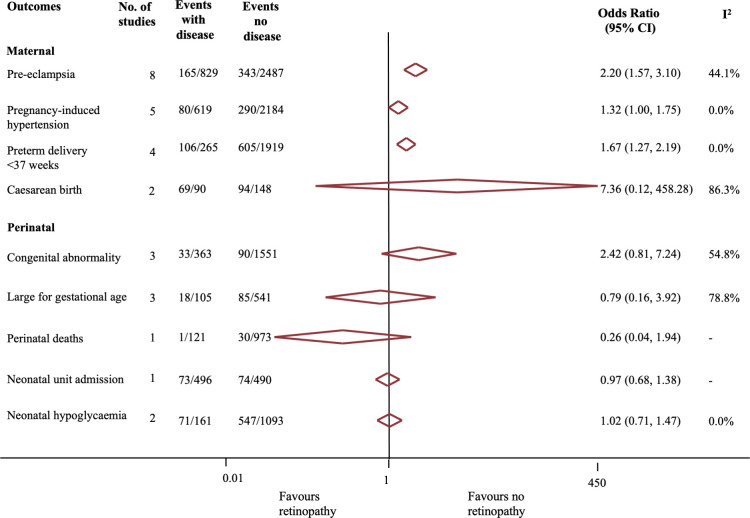
Presence of retinopathy in pregnant women with preexisting diabetes was associated with increased risk (Fig 4) of preeclampsia (OR 2.20, 95% CI:1.57 to 3.10, p < 0.001, I2 = 56%; 8 studies) [25,26,28,31,32,39–41] and preterm birth before 37 weeks’ (OR 1.67, 95% CI 1.27 to 2.20, p < 0.01, I2 = 0%; 4 studies) [25,35,42,43]. No significant differences were observed in the rates of cesarean birth (OR 7.37, 95% CI 0.12 to 458.28, p = 0.34, I2 = 86%; 2 studies) [36,44] or pregnancy-induced hypertension (OR 1.32, 95% CI 1.00 to 1.75, p = 0.05, I2 = 0%; 5 studies) [25,31,32,39,40], although the latter was of borderline statistical significance. When the analysis was restricted to only women with type 1 diabetes, or studies with a low to medium risk of bias, the findings remained similar (Tables A and B in S6 Appendix).
Fig 4. Association between diabetic retinopathy and adverse maternal and perinatal outcomes.
Perinatal outcomes
We did not observe any differences in the risk of congenital abnormality, large for gestational age fetus, perinatal death, and admission to the neonatal unit in babies born to women with versus without diabetic retinopathy (Fig 4). One study compared the pH of the umbilical arterial blood (pH <7.05, OR 1.48. 95% CI 0.50 to 4.36, p = 0.48 and Apgar score <7 at 1 minute of age (OR 0.72, 95% CI 0.38 to 1.37, p = 0.31) in women with and without retinopathy and found no difference in either outcome [25]. Findings were similar in sensitivity analyses that restricted the meta-analyses to only women with preexisting type 1 diabetes or only studies with low to medium risk of bias (Tables A and B in S6 Appendix).
Risk of adverse pregnancy outcomes in women with any diabetic microvascular complication
Maternal outcomes
Pregnant women with preexisting diabetes and any microvascular complication were at significantly increased risk in preeclampsia (OR 5.89, 95% CI 3.85 to 9.02, p < 0.01, I2 = 14%; 9 studies) [21,26,45–51], preterm birth before 34 weeks’ (OR 8.49, 95% CI 1.87 to 38.63, p = 0.01, I2 = 46%; 3 studies) [21,47,49] and 37 weeks’ (OR 2.29, 95% CI 1.85 to 2.83, p < 0.01, I2 = 0%; 7 studies) [25,26,43,47,49,50,52], and cesarean birth (OR 5.40, 95% CI 2.48 to 11.78, p < 0.01, I2 = 60%; 6 studies) [21,43,44,47,50,53] compared to those without any microvascular complication (Table 1). Findings were similar in sensitivity analyses that restricted the meta-analyses to only women with preexisting type 1 diabetes or only studies with low to medium risk of bias (Tables A and B in S6 Appendix).
Table 1. Association between diabetic nephropathy and/or retinopathy and adverse maternal and perinatal outcomes.
| Outcomes | No. of studies | Events in women with disease | Events in women with no disease | OR (95% CI) | p-value | I2 |
|---|---|---|---|---|---|---|
| Maternal outcomes | ||||||
| Preeclampsia | 9 | 92/273 | 95/1,196 | 5.89 (3.85, 9.02) | <0.01 | 13.8% |
| Pregnancy-induced hypertension | 2 | 26/172 | 15/455 | 2.33 (0.10, 51.95) | 0.59 | 86.7% |
| Preterm birth <34/40 | 3 | 19/73 | 17/371 | 8.49 (1.87, 38.63) | 0.01 | 64.9% |
| Preterm birth <37/40 | 7 | 253/502 | 575/1,882 | 2.29 (1.85, 2.83) | <0.01 | 0.0% |
| Caesarean birth | 6 | 210/248 | 341/769 | 5.40 (2.48, 11.78) | <0.01 | 60.4% |
| Perinatal outcomes | ||||||
| Congenital abnormality | 3 | 30/102 | 58/245 | 1.30 (0.75, 2.26) | 0.35 | 0.0% |
| Small for gestational age fetus | 5 | 21/349 | 11/505 | 2.49 (1.12, 5.57) | 0.03 | 0.0% |
| Large for gestational age fetus | 5 | 129/284 | 185/447 | 1.10 (0.80, 1.52) | 0.54 | 0.0% |
| Perinatal death | 5 | 18/733 | 24/1,218 | 2.85 (0.46, 17.77) | 0.26 | 75.9% |
| Neonatal unit admission | 2 | 129/643 | 149/648 | 1.21 (0.91, 1.63) | 0.19 | 0.0% |
| Neonatal hypoglycemia | 4 | 53/145 | 146/514 | 1.09 (0.56, 2.14) | 0.80 | 55.6% |
CI, confidence interval; OR, odds ratio.
Perinatal outcomes
Babies born to mothers with preexisting diabetes and any microvascular complication were at increased risk of having a small for gestational age baby (OR 2.49, 95% CI 1.12 to 5.57, p = 0.03, I2 = 0%; 5 studies) [45,47,49,50,53]. There were no differences between the groups for other perinatal outcomes (Table 1). Findings were similar with sensitivity analyses.
Risk factors for progression of diabetic microvascular disease in pregnancy
Retinopathy
In pregnant women with preexisting diabetic retinopathy, the risk of disease progression or onset was significantly increased for nulliparous women (OR 1.75, 95% CI 1.28 to 2.40, p < 0.01, I2 = 0%; 4 studies) [18,54–56] and smokers (OR 2.31, 95% CI 1.25 to 4.27, p = 0.01, I2 = 0%; 5 studies) [54,57–60] (Table 2). Pregnant women with progressive diabetic retinopathy had a mean 4.51 additional years since diabetes diagnosis (weighted mean difference 4.51 y, 95% CI 2.26 to 6.76 y; p < 0.01, I2 = 78.9%, 7 studies) than women without progressive retinopathy [55,61–66]. There were no differences in age (WMD −0.22 years, 95% CI −0.86 to 0.42, p = 0.50, I2 = 0.0%, 7 studies) [55,56,60,62,63,65,66] or BMI (WMD 0.06 kg/m2, 95% CI −1.05 to 1.16, p = 0.92, I2 = 1.2%, 4 studies) [56,60,63,66] between women with and without new or progressive retinopathy. Following exclusion of papers with high risk of bias, the risk of progressive retinopathy was no longer significant for smokers (OR 1.13, 95% CI 0.17 to 7.59, p = 0.90, I2 = 63%, 2 studies) [54,59]. The estimates from all other sensitivity analyses were similar (Table C in S6 Appendix).
Table 2. Risk factors for worsening or new onset retinopathy in pregnant women with preexisting diabetes.
| Risk factors | No. of studies | Events in women with risk factor | Events in women without risk factor | OR (95% CI) | p-value | I2 |
|---|---|---|---|---|---|---|
| Any existing retinopathy | 15 | 283/817 | 284/1,163 | 2.64 (1.47, 4.75) | <0.01 | 79.7% |
| Background/preproliferative retinopathy | 4 | 57/163 | 35/186 | 1.94 (0.69, 5.42) | 0.21 | 60.2% |
| Proliferative retinopathy | 7 | 31/73 | 89/311 | 2.12 (1.11, 4.04) | 0.02 | 12.1% |
| Macular edema | 2 | 4/14 | 29/154 | 1.54 (0.46, 5.14) | 0.49 | 0.0% |
| Previous photocoagulation | 3 | 6/48 | 42/174 | 0.85 (0.16, 4.67) | 0.85 | 65.9% |
| Nephropathy | 4 | 32/95 | 171/754 | 1.68 (1.05, 2.69) | 0.03 | 0.0% |
| White ethnicity | 2 | 101/230 | 132/160 | 1.90 (0.76, 4.73) | 0.17 | 0.0% |
| Nulliparity | 4 | 124/404 | 166/576 | 1.75 (1.28, 2.40) | <0.01 | 0.0% |
| Smoking | 5 | 24/60 | 92/396 | 2.31 (1.25, 4.27) | <0.01 | 0.0% |
CI, confidence interval; OR, odds ratio.
We found a greater risk of disease progression in pregnant women with retinopathy (OR 2.64, 95% CI 1.47 to 4.75, p <0.01, I2 = 79.7%; 15 studies) [54–56,60–71], and nephropathy (OR 1.68, 95% CI 1.05 to 2.69, p = 0.03, I2 = 0.0%, 4 studies) [54–56,71], at the time of the first antenatal consultation compared to those without the diagnoses. No studies reported progression to blindness. Presence of baseline background or preproliferative retinopathy (OR 1.94, 95% CI 0.69 to 5.42, p = 0.21, I2 = 0%; 4 studies) was not found to affect retinopathy progression) [54,62,67,72]. Pregnant women with preexisting proliferative retinopathy (OR 2.12, 95% CI 1.11 to 4.04, p = 0.02, I2 = 12.1%; 7 studies) [55,62,67,69–72], have a 2.1-fold greater risk of progression compared to women with lesser (no or background/preproliferative, respectively) changes at baseline. Proliferative retinopathy was not maintained as a risk factor after excluding studies with high risk of bias (OR 1.44, 95% CI 0.64 to 3.24, p = 0.38, I2 = 0%, 2 studies) [55,71] or when including women with type 1 diabetes only (OR 2.04, 95% CI 0.89 to 4.67, p = 0.09, I2 = 41.5%, 6 studies) [55,62,67,69–71]. The same was true for any retinopathy when studies at high risk of bias were excluded (OR 2.00, 95% CI 0.85 to 4.71, p = 0.11, I2 = 82.9%, 6 studies) [54–56,64,68,71]. Following removal of studies at high risk of bias, previous photocoagulation was found to be protective of deteriorating retinopathy (OR 0.23, 95% CI 0.06 to 0.99, p = 0.049, 1 study) [56]. Estimates from all other sensitivity analyses were similar (Table C in S6 Appendix).
Nephropathy
Two studies assessed the risk of antenatally deteriorating renal function among women with preexisting diabetic nephropathy compared to women without [21,22]. With no overall consensus on defining renal function deterioration, clinical heterogeneity between studies was too great to conduct meta-analysis. No studies assessed the risk of deterioration to end-stage renal failure or the risk contributed by any of the predefined maternal characteristics on antenatal progression of nephropathy.
Publication bias
There was evidence of small study effect (Egger’s test of asymmetry) for progression of retinopathy (p = 0.003) and preeclampsia (p = 0.001). The funnel plots are included in S7 Appendix.
Discussion
Main findings
Pregnant women with preexisting diabetes and microvascular disease (such as nephropathy and retinopathy) are at even greater risk of adverse maternal outcomes, particularly preterm birth and preeclampsia than those without microvascular complications. Mothers with diabetic nephropathy are also at high risk of offspring complications such as congenital malformations, small for gestational age fetus, and perinatal death than those without nephropathy. Nulliparity, smoking, and proliferative retinopathy at baseline are risk factors for worsening or onset of retinopathy in pregnancy.
Comparison with existing literature
Despite increasing numbers of pregnant women with preexisting diabetes presenting with microvascular complications, no meta-analysis has been published in this area. The few available systematic reviews are narrative and mainly provided noncomparative estimates of maternal–fetal pregnancy outcomes in women with preexisting diabetic nephropathy [73]. There are no systematic reviews on pregnancy outcomes in women with diabetic retinopathy. Other narrative reviews have focused mainly on progression of retinopathy rather than on its impact on pregnancy.
The recent NICE Diabetes in Pregnancy guidelines used noncomparative data from a systematic review of 681 women to report pregnancy outcomes in women with diabetic nephropathy [9]. The Australian Diabetes in Pregnancy Society guidelines also comment on the same outcomes from a narrative review with 3 small studies [74]. The American College of Obstetricians and Gynecologists guidelines only refer to the greater risk of preeclampsia among women with diabetic nephropathy [75]. Our meta-analysis provides comparative estimates with an up to 5-fold higher sample size, and reports increased risks of additional key outcomes such as perinatal death and congenital abnormality.
Current UK guidance on screening for the small for gestational age fetus in pregnant women with diabetic microvascular complications is based on a single study [26]. Our meta-analysis includes numerous studies for this outcome and provides robust and precise estimates. The Confidential Enquiry into Maternal and Child Health (CEMACH) report in the UK found no association between presence of retinopathy and poor pregnancy outcome defined as congenital anomaly or perinatal death [76]. We found diabetic retinopathy to be associated with preeclampsia and preterm birth, which are major risk factors for maternal and perinatal morbidity. Unlike the CEMACH report with 442 women, our evidence base is larger with more robust estimates. We found an increase in the rates of congenital abnormalities in women with diabetic nephropathy. It is possible that the poor glycemic control observed in women with nephropathy is more important than the disease itself in contributing to the adverse outcome.
Both the UK National Institute for Health and Care Excellence (NICE) and Australian diabetes guidelines identified severe retinopathy at conception, duration of diagnosed diabetes, poor glycemic control and hypertension as risk factors for antenatal progression of retinopathy, and disease severity at conception for worsening of nephropathy [9,74]. Many of these conclusions were based on single studies. In addition to severity of retinopathy and duration of diabetes, our meta-analysis found additional risk factors such as nulliparity and smoking for worsening of the disease. While we did not include hypertension and glycemic control in our prespecified list of risk factors (S3 Appendix), our search strategy did capture such studies. There were 6 studies identified, which assessed how glycemia control affected progression of retinopathy [62,72,77–80], one of which also assessed progression of nephropathy [79]. Glycemic control was defined heterogeneously (hypoglycemia, mean HbA1C, or change in HbA1C measured at different time points—preconception, first, second, or third trimester) and therefore would not have been amenable to meta-analysis. Five of the same studies also studied blood pressure as a risk factor for progressive microvascular disease [62,72,77–80], with the same limitation of heterogeneity (risk factors were mean diastolic blood pressure, mean systolic blood pressure–time points varied, use of antihypertensive medication, chronic hypertension, gestational hypertension).
Strengths and limitations
To our knowledge, ours is the largest and most comprehensive meta-analysis to date that quantifies the risk of adverse maternal and perinatal outcomes in pregnant women with diabetic microvascular complications and the risk factors for disease progression. We undertook the review with a prospective protocol in line with current recommendations, identified the studies with a detailed search strategy and without any language restrictions, evaluated the quality of the included studies, and performed appropriate meta-analyses with assessment of statistical heterogeneity. We reported the strength of association between diabetic microvascular complications and pregnancy outcomes separately and in combination and studied all clinically relevant outcomes. The findings were homogeneous for the risk of perinatal outcomes in women with diabetic nephropathy and for most maternal outcomes with retinopathy. Our sensitivity analyses allowed us to assess the robustness of our findings by excluding low-quality studies and limiting to studies that reported risks for women with type 1 diabetes separately.
There are limitations in our systematic review. Studies varied in their definitions of diabetic nephropathy and retinopathy and included women with both type 1 and type 2 diabetes. But our sensitivity analysis including only women with type 1 diabetes showed findings similar to the overall estimates. While many studies reported on the risks of diabetic retinopathy or nephropathy, none assessed neuropathy in detail. We were also unable to take into account other factors such as maternal age, BMI, and previous obstetric history that may have influenced the association between diabetic microvascular complications and pregnancy outcomes. Unlike retinopathy with comparable classification systems, the severity of nephropathy was reported variedly, which refrained us from identifying the risk factors for worsening disease. We only included studies published since 1990, but it is possible that some of the outcomes could be influenced by the variations in clinical practice over time and between institutions. There were very few events for some of the reported outcomes studied. This is reflected in the imprecision of the point estimates. Furthermore, the variations in the definitions of the populations, retinopathy, and nephropathy may have contributed to the high heterogeneity observed for some findings. We were only able to assess publication bias for 2 outcomes, because all other outcomes were assessed in less than 10 studies. Of the 2 outcomes where this was possible, we found evidence of small sample bias, suggesting that the results should be interpreted with caution. We limited our analysis of disease progression to only pregnancy as observational studies suggest that diabetic retinopathy deteriorates more rapidly in pregnancy [9,67,77], but the findings may not necessarily translate into worse long-term retinopathic severity when compared to women who were not pregnant [81].
Implications for clinical practice
The maternal and offspring risks are significantly increased for pregnant women with diabetic nephropathy, in particular with over 10-fold increase in the risks of preeclampsia and small for gestational age fetuses. It is essential that antenatal care of pregnant women with microvascular disease should involve a multidisciplinary team, including maternal medicine and nephrology specialists. It is possible that a higher dose (150 mg) antenatal aspirin, instead of 75 mg, may mitigate the risks of preeclampsia in women with diabetes-related microvascular disease [82]. During the Coronavirus Disease 2019 (COVID-19) pandemic, pregnant women with preexisting diabetes are also in the highest risk groups for becoming severely unwell from COVID-19, and, furthermore, there have been widespread restrictions on maternity services [83]. It is essential that the women at highest risk of adverse pregnancy outcomes are identified so that scarce resources can be appropriately targeted.
In the UK CEMACH report, pregnant women with preexisting diabetes and poor pregnancy outcomes were also less likely to have retinal assessment at the time of the first antenatal consultation than those with good outcomes [76], indicative of suboptimal diabetes care linked to suboptimal retinal monitoring. Many factors contributing to progression of retinopathy during pregnancy are modifiable such as preconception control of glucose and smoking cessation. Women with risk factors identified in this review for deteriorating eye disease should be referred for close monitoring during pregnancy [9], and specialist review where deterioration is noted.
Recommendations for research
Further research is needed to better understand the associations between diabetic microvascular diseases and pregnancy outcomes and whether this differs by type 1 or type 2 diabetes. Although the risks of preterm birth are increased in women with diabetic nephropathy and in those with retinopathy, it is unclear whether these were spontaneous or iatrogenic preterm births; this needs to be delineated. Depending on the cause for preterm birth, further research is needed to identify effective interventions to prevent spontaneous preterm birth or to reduce the risk of iatrogenic prematurity. We need consensus on criteria for deterioration of diabetic nephropathy in pregnancy, or preferred method for the assessment of renal function decline, to identify the proportion of women with worsening disease and the risk factors for disease progression. There also needs to be a standardized method of assessing both glycemic and blood pressure control throughout pregnancy, to determine what effect these have on progression of microvascular complications. Techniques worthy of further assessment for this purpose include continuous measurement of blood pressure over 12- or 24-hour periods or provision of validated monitors for home monitoring. The paucity of evidence on obstetric and disease-related outcomes for diabetic neuropathy, including autonomic neuropathies (e.g., gastroparesis) can be addressed by systematically collecting this information in the national surveillance or registry systems such as the UK Obstetric Surveillance System or Diabetes in Pregnancy audit [84]. We need data on long-term outcomes beyond pregnancy for women with diabetic microvascular complications and their babies to obtain a comprehensive overview of the risks. This information is critical to predict the risk of disease progression during pregnancy and postnatally.
Conclusions
Pregnant women with preexisting diabetes and microvascular diseases such as nephropathy and retinopathy are at greater risk of preeclampsia and preterm birth than those without the microvascular diseases. Women with diabetic nephropathy are also at higher risk of most major maternal and perinatal complications including congenital abnormalities, growth-restricted fetuses, and perinatal death. Nulliparous mothers, smokers, and women with retinopathy at baseline have an increased risk of deteriorating diabetic eye disease in pregnancy. Pregnant women with diabetic microvascular complications require management in speciality multidisciplinary teams with frequent, targeted antenatal care surveillance and interventions to improve maternal and perinatal outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Table A: Maternal and perinatal outcomes in women with type 1 diabetes and vasculopathy—A sensitivity analysis. Table B: Maternal and perinatal outcomes in women with diabetes and vasculopathy—A sensitivity analysis excluding papers with high risk of bias. Table C: Risk factors for disease progression (retinopathy)—Sensitivity analyses.
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
The authors wish to thank Dr. John Allotey for assisting them with the evaluation for publication bias.
Abbreviations
- CEMACH
Confidential Enquiry into Maternal and Child Health
- CI
confidence interval
- COVID-19
Coronavirus Disease 2019
- ETDRS
Early Treatment Diabetic Retinopathy Study
- MeSH
Medical Subject Headings
- NICE
National Institute for Health and Care Excellence
- OR
odds ratio
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organisation. Global Report on Diabetes. France; 2016.
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. Epub 2014 Jun 2. doi: 10.1016/S0140-6736(14)60460-8 ; PubMed Central PMCID: PMC4624264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHS Digital. National Pregnancy in Diabetes (NPID) Audit Report 2018. 2019 [cited 2021 Jan 11]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-pregnancy-in-diabetes-audit/national-pregnancy-in-diabetes-annual-report-2018.
- 4.Leguizamón G, Trigubo D, Pereira JI, Vera MF, Fernández JA. Vascular Complications in the Diabetic Pregnancy. Curr Diab Rep. 2015;15(4):22. doi: 10.1007/s11892-015-0586-5 [DOI] [PubMed] [Google Scholar]
- 5.Damm JA, Asbjornsdottir B, Callesen NF, Mathiesen JM, Ringholm L, Pedersen BW, et al. Diabetic nephropathy and microalbuminuria in pregnant women with type 1 and type 2 diabetes: prevalence, antihypertensive strategy, and pregnancy outcome. Diabetes Care. 2013;36(11):3489–94. Epub 2013 Sept 7. doi: 10.2337/dc13-1031 ; PubMed Central PMCID: PMC3816914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Common Eye Disorders and Diseases: Diabetic Retinopathy. 2020 [cited 2020 Jan 11]. Available from: https://www.cdc.gov/visionhealth/basics/ced/index.html#a5.
- 7.Morrison JL, Hodgson LA, Lim LL, Al-Qureshi S. Diabetic retinopathy in pregnancy: a review. Clin Exp Ophthalmol. 2016;44(4):321–34. doi: 10.1111/ceo.12760 [DOI] [PubMed] [Google Scholar]
- 8.Knight M, Bunch K, Tuffnell D, Shakespeare J, Kotnis R, Kenyon S, et al. Saving Lives, Improving Mothers’ Care. 2019. [Google Scholar]
- 9.National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. 2015. [PubMed]
- 10.Patel T, Relph S, Thangaratinam S. Maternal and offspring outcomes in pregnant women with pre-existing diabetes complicated by nephropathy and retinopathy: a systematic review and meta-analysis. 2017. [cited 2021 Oct 9]. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017076647. [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. Epub 2009 Jul 23. doi: 10.1136/bmj.b2535 ; PubMed Central PMCID: PMC2714657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2000. [cited 2017 Dec 4]. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997 Oct 6. doi: 10.1136/bmj.315.7109.629 ; PubMed Central PMCID: PMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2015.
- 15.White P. Pregnancy complicating diabetes. Am J Med. 1949;7(5):609–16. Epub 1949 Nov 1. doi: 10.1016/0002-9343(49)90382-4 . [DOI] [PubMed] [Google Scholar]
- 16.Kimmerle R, Zass R, Cupisti S, Somville T, Bender R, Pawlowski B, et al. Pregnancies in women with diabetic nephropathy: Long-term outcome for mother and child. Diabetologia. 1995;38(2):227–35. [PubMed] [Google Scholar]
- 17.Gordon M, Landon MB, Samuels P, Hissrich S, Gabbe SG. Perinatal outcome and long-term follow-up associated with modern management of diabetic nephropathy. Obstet Gynecol. 1996;87(3):401–9. Epub 1996 Mar 1. doi: 10.1016/0029-7844(95)00420-3 . [DOI] [PubMed] [Google Scholar]
- 18.Miodovnik M, Rosenn B, Khoury J, Grigsby J, Siddiqi T, Langer O, et al. Does pregnancy increase the risk for development and progression of diabetic nephropathy? Am J Obstet Gynecol. 1996;174(4):1180–91. doi: 10.1016/s0002-9378(96)70660-9 [DOI] [PubMed] [Google Scholar]
- 19.Purdy LP, Hantsch CE, Molitch ME, Metzger BE, Phelps RL, Dooley SL, et al. Effect of pregnancy on renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care. 1996;19(10):1067–74. Epub 1996 Oct 1. doi: 10.2337/diacare.19.10.1067 . [DOI] [PubMed] [Google Scholar]
- 20.Dunne FP, Chowdhury TA, Hartland A, Smith T, Brydon PA, McConkey C, et al. Pregnancy outcome in women with insulin-dependent diabetes mellitus complicated by nephropathy. QJM. 1999;92(8):451–4. Epub 2000 Jan 11. doi: 10.1093/qjmed/92.8.451 . [DOI] [PubMed] [Google Scholar]
- 21.Biesenbach G, Grafinger P, Zazgornik J, Helmut Stoger. Perinatal complications and three-year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren Fail. 2000;22(5):573–80. Epub 2000 Oct 21. doi: 10.1081/jdi-100100898 . [DOI] [PubMed] [Google Scholar]
- 22.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Molvig J, Mathiesen E. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care. 2001;24(10):1739–44. doi: 10.2337/diacare.24.10.1739 [DOI] [PubMed] [Google Scholar]
- 23.Irfan S, Arain T, Shaukat A, Shahid A. Effect of pregnancy on diabetic nephropathy and retinopathy. J Coll Physicians Surg Pak. 2004;14(2):75–8. [PubMed] [Google Scholar]
- 24.Combs CA, Rosenn B, Kitzmiller JL, Khoury JC, Wheeler BC, Miodovnik M. Early-pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol. 1993;82(5):802–7. Epub 1993 Nov 1. . [PubMed] [Google Scholar]
- 25.Klemetti MM, Laivuori H, Tikkanen M, Nuutila M, Hiilesmaa V, Teramo K. White’s classification and pregnancy outcome in women with type 1 diabetes: a population-based cohort study. Diabetologia. 2016;59(1):92–100. Epub 2015 Oct 18. doi: 10.1007/s00125-015-3787-1 . [DOI] [PubMed] [Google Scholar]
- 26.Howarth C, Gazis A, James D. Associations of Type 1 diabetes mellitus, maternal vascular disease and complications of pregnancy. Diabet Med. 2007;24(11):1229–34. Epub 2007 Aug 30. doi: 10.1111/j.1464-5491.2007.02254.x . [DOI] [PubMed] [Google Scholar]
- 27.Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care. 2009;32(1):38–44. Epub 2008 Oct 24. doi: 10.2337/dc08-1526 ; PubMed Central PMCID: PMC2606826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Boomsma F, Damm P, Mathiesen E. Higher levels of Atrial Natriuretic Peptide (ANP) are present in early pregnancy in type 1 diabetic women developing preeclampsia, September 2011, vol/is 54/. 2011:S485. [Google Scholar]
- 29.Young EC, Pires ML, Marques LP, de Oliveira JE, Zajdenverg L. Effects of pregnancy on the onset and progression of diabetic nephropathy and of diabetic nephropathy on pregnancy outcomes. Diabetes Metab Syndr. 2011;5(3):137–42. Epub 2012 Jul 21. doi: 10.1016/j.dsx.2012.02.013 . [DOI] [PubMed] [Google Scholar]
- 30.Themeli Y, Bajrami V, Zaimi K, Mustafaraj K, Lulo J, Peci E, et al. Diabetic nephropathy in pregnant women with type 1 diabetes mellitus. G Ital Ostet Ginecol. 2012;34(1):323–7. [Google Scholar]
- 31.Lauszus FF, Rasmussen OW, Lousen T, Klebe TM, Klebe JG. Ambulatory blood pressure as predictor of preeclampsia in diabetic pregnancies with respect to urinary albumin excretion rate and glycemic regulation. Acta Obstet Gynecol Scand. 2001;80(12):1096–103. Epub 2002 Feb 16. doi: 10.1034/j.1600-0412.2001.801204.x . [DOI] [PubMed] [Google Scholar]
- 32.Norgaard SK, Vestgaard MJ, Jorgensen IL, Asbjornsdottir B, Ringholm L, McIntyre HD, et al. Diastolic blood pressure is a potentially modifiable risk factor for preeclampsia in women with pre-existing diabetes. Diabetes Res Clin Pract. 2018;138:229–37. Epub 2018 Feb 24. doi: 10.1016/j.diabres.2018.02.014 . [DOI] [PubMed] [Google Scholar]
- 33.Piccoli GB, Tavassoli E, Melluzza C, Grassi G, Monzeglio C, Donvito V, et al. Severe diabetic nephropathy in type 1 diabetes and pregnancy—a case series. Rev Diabet Stud. 2013;10(1):68–78. Epub 2013 Nov 1. doi: 10.1900/RDS.2013.10.68 ; PubMed Central PMCID: PMC3932073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yogev Y, Chen R, Ben-Haroush A, Hod M, Bar J. Maternal overweight and pregnancy outcome in women with Type-1 diabetes mellitus and different degrees of nephropathy. J Matern Fetal Neonatal Med. 2010;23(9):999–1003. Epub 2010 Jan 12. doi: 10.3109/14767050903544744 . [DOI] [PubMed] [Google Scholar]
- 35.Melamed N, Chen R, Soiberman U, Ben-Haroush A, Hod M, Yogev Y. Spontaneous and indicated preterm delivery in pregestational diabetes mellitus: etiology and risk factors. Arch Gynecol Obstet. 2008;278(2):129–34. Epub 2008 Jan 15. doi: 10.1007/s00404-007-0541-z . [DOI] [PubMed] [Google Scholar]
- 36.Lepercq J, Le Meaux JP, Agman A, Timsit J. Factors associated with cesarean delivery in nulliparous women with type 1 diabetes. Obstet Gynecol. 2010;115(5):1014–20. Epub 2010 Apr 23. doi: 10.1097/AOG.0b013e3181d992ab . [DOI] [PubMed] [Google Scholar]
- 37.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012. Epub 2012 Feb 9. doi: 10.1007/s00125-012-2455-y . [DOI] [PubMed] [Google Scholar]
- 38.Herman M, Djelmis J, Ivanisevic M, Blajic J, Starcevic V. Pregnancy outcome of mothers with diabetic nephropathy. J Matern Fetal Neonatal Med. 2014;27:266–7. [Google Scholar]
- 39.Hiilesmaa V, Suhonen L, Teramo K. Glycaemic control is associated with pre-eclampsia but not with pregnancy-induced hypertension in women with type I diabetes mellitus. Diabetologia. 2000;43(12):1534–9. doi: 10.1007/s001250051565 [DOI] [PubMed] [Google Scholar]
- 40.Hanson U, Persson B. Epidemiology of pregnancy-induced hypertension and preeclampsia in type 1 (insulin-dependent) diabetic pregnancies in Sweden. Acta Obstet Gynecol Scand. 1998;77(6):620–4. Epub 1998 Aug 4. doi: 10.1034/j.1600-0412.1998.770608.x . [DOI] [PubMed] [Google Scholar]
- 41.Castiglioni MT, Valsecchi L, Cavoretto P, Pirola S, Di Piazza L, Maggio L, et al. The risk of preeclampsia beyond the first pregnancy among women with type 1 diabetes parity and preeclampsia in type 1 diabetes. Pregnancy Hypertens. 2014;4(1):34–40. Epub 2014/01/01. doi: 10.1016/j.preghy.2013.09.001 . [DOI] [PubMed] [Google Scholar]
- 42.Hoshi J, Nishida H, Takahashi N, Kabe K, Watanabe Y, Arai T, et al. Perinatal morbidity of infants of diabetic mothers. Acta Paediatr Jpn. 1991;33(2):159–65. Epub 1991 Apr 1. doi: 10.1111/j.1442-200x.1991.tb01536.x . [DOI] [PubMed] [Google Scholar]
- 43.Zhu L, Nakabayashi M, Takeda Y. Statistical analysis of perinatal outcomes in pregnancy complicated with diabetes mellitus. J Obstet Gynaecol Res. 1997;23(6):555–63. Epub 1998 Jan 20. doi: 10.1111/j.1447-0756.1997.tb00886.x . [DOI] [PubMed] [Google Scholar]
- 44.Dodesini A, Maffeis A, Corsi A, Benvenuto A, Ciriello E, Lepore G, et al. In type 1 diabetic women retinopathy and nephropathy are predictors of caesarean delivery independently of glucose control. Diabetes. 2009;58. [Google Scholar]
- 45.Patel N, Brackenridge A, Kanji A, Pasupathy D, Rajasingam D. Micro-vascular disease at booking in T1DM and associated risk of developing pre-eclampsia. Arch Dis Child Fetal Neonatal Ed. 2013;98:1359–2998. [Google Scholar]
- 46.Garner PR, D’Alton ME, Dudley DK, Huard P, Hardie M. Preeclampsia in diabetic pregnancies. Am J Obstet Gynecol. 1990;163(2):505–8. Epub 1990 Aug 1. doi: 10.1016/0002-9378(90)91184-e . [DOI] [PubMed] [Google Scholar]
- 47.Haeri S, Khoury J, Kovilam O, Miodovnik M. The association of intrauterine growth abnormalities in women with type 1 diabetes mellitus complicated by vasculopathy. Am J Obstet Gynecol. 2008;199(3):278 e1-5. Epub 2008 Sept 6. doi: 10.1016/j.ajog.2008.06.066 . [DOI] [PubMed] [Google Scholar]
- 48.Temple RC, Aldridge V, Stanley K, Murphy HR. Glycaemic control throughout pregnancy and risk of pre-eclampsia in women with type I diabetes. BJOG. 2006;113(11):1329–32. Epub 2006 Sept 29. doi: 10.1111/j.1471-0528.2006.01071.x . [DOI] [PubMed] [Google Scholar]
- 49.Samii L, Kallas-Koeman M, Donovan LE, Lodha A, Crawford S, Butalia S. The association between vascular complications during pregnancy in women with Type 1 diabetes and congenital malformations. Diabet Med. 2019;36(2):237–42. Epub 2018 Dec 1. doi: 10.1111/dme.13872 . [DOI] [PubMed] [Google Scholar]
- 50.Durackova L, Kristufkova A, Korbel M. Pregnancy and neonatal outcomes in women with type 1 diabetes mellitus. Bratisl Med J. 2017;118(1):56–60. doi: 10.4149/BLL_2017_011 [DOI] [PubMed] [Google Scholar]
- 51.Gutaj P, Zawiejska A, Mantaj U, Ożegowska E. Determinants of preeclampsia in women with type 1 diabetes. Acta Diabetol. 2017;54:1115–21. doi: 10.1007/s00592-017-1053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoetzau A, Hillebrand B. [Neonatal morbidity in infants of diabetic mothers]. Z Geburtshilfe Perinatol. 1990;194(2):58–64. German. [PubMed] [Google Scholar]
- 53.Hopp H, Vollert W, Ebert A, Weitzel H, Glockner E, Jahrig D. [Diabetic retinopathy and nephropathy—Complications during pregnancy and delivery]. Geburtshilfe Frauenheilkd. 1995;55(5):275–9. German. doi: 10.1055/s-2007-1023317 [DOI] [PubMed] [Google Scholar]
- 54.Chew EY, Mills JL, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18(5):631–7. Epub 1995 May 1. doi: 10.2337/diacare.18.5.631 . [DOI] [PubMed] [Google Scholar]
- 55.McElvy SS, Demarini S, Miodovnik M, Khoury JC, Rosenn B, Tsang RC. Fetal weight and progression of diabetic retinopathy. Obstet Gynecol. 2001;97(4):587–92. Epub 2001 Mar 29. doi: 10.1016/s0029-7844(00)01202-3 . [DOI] [PubMed] [Google Scholar]
- 56.Bourry J, Courteville H, Ramdane N, Drumez E, Duhamel A, Subtil D, et al. Progression of Diabetic Retinopathy and Predictors of Its Development and Progression During Pregnancy in Patients With Type 1 Diabetes: A Report of 499 Pregnancies. Diabetes Care. 2021;44(1):181–7. Epub 2020 Nov 13. doi: 10.2337/dc20-0904 . [DOI] [PubMed] [Google Scholar]
- 57.Biesenbach G, Grafinger P, Stoger H, Zazgornik J. How pregnancy influences renal function in nephropathic type 1 diabetic women depends on their pre-conceptional creatinine clearance. J Nephrol Nurs. 1999;12(1):41–6. [PubMed] [Google Scholar]
- 58.Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in Type 1 diabetes. Diabet Med. 2001;18(7):573–7. Epub 2001 Sept 13. doi: 10.1046/j.1464-5491.2001.00535.x . [DOI] [PubMed] [Google Scholar]
- 59.Larsen M, Colmorn LB, Bonnelycke M, Kaaja R, Immonen I, Sander B, et al. Retinal artery and vein diameters during pregnancy in diabetic women. Invest Ophthalmol Vis Sci. 2005;46(2):709–13. Epub 2005 Jan 27. doi: 10.1167/iovs.04-0604 . [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53(6):1076–83. Epub 2010 Mar 13. doi: 10.1007/s00125-010-1697-9 . [DOI] [PubMed] [Google Scholar]
- 61.Ayed S, Jeddi A, Daghfous F, El Euch M, Ben Osman N, Marrakchi S, et al. Progressive aspects of diabetic retinopathy during pregnancy. J Fr Ophtalmol. 1992;15(8–9):474–7. [PubMed] [Google Scholar]
- 62.Rosenn B, Miodovnik M, Kranias G, Khoury J, Combs CA, Mimouni F, et al. Progression of diabetic retinopathy in pregnancy: association with hypertension in pregnancy. Am J Obstet Gynecol. 1992;166(4):1214–8. Epub 1992 Apr 1. doi: 10.1016/s0002-9378(11)90608-5 . [DOI] [PubMed] [Google Scholar]
- 63.Egan AM, McVicker L, Heerey A, Carmody L, Harney F, Dunne FP. Diabetic retinopathy in pregnancy: a population-based study of women with pregestational diabetes. J Diabetes Res. 2015;2015:310239. Epub 2015 May 7. doi: 10.1155/2015/310239 ; PubMed Central PMCID: PMC4402566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Axer-Siegel R, Hod M, Fink-Cohen S, Kramer M, Weinberger D, Schindel B, et al. Diabetic retinopathy during pregnancy. Ophthalmology. 1996;103(11):1815–9. Epub 1996 Nov 1. doi: 10.1016/s0161-6420(96)30421-1 . [DOI] [PubMed] [Google Scholar]
- 65.Arun CS, Taylor R. Influence of pregnancy on long-term progression of retinopathy in patients with type 1 diabetes. Diabetologia. 2008;51(6):1041–5. Epub 2008 Apr 9. doi: 10.1007/s00125-008-0994-z . [DOI] [PubMed] [Google Scholar]
- 66.Toda J, Kato S, Sanaka M, Kitano S. The effect of pregnancy on the progression of diabetic retinopathy. Jpn J Ophthalmol. 2016;60(6):454–8. Epub 2016 Oct 23. doi: 10.1007/s10384-016-0464-y . [DOI] [PubMed] [Google Scholar]
- 67.Maayah J, Shammas A, Haddadin A. Effect of pregnancy on diabetic retinopathy. Bahrain Medical Bull. 2001;23(4):163–5. [Google Scholar]
- 68.Lovestam-Adrian M, Agardh C, Aberg A, Agardh E. Pre-eclampsia is a potent risk factor for deterioration of retinopathy during pregnancy in Type 1 diabetic patients. Diabet Med. 1997;14(12):1059–65. doi: [DOI] [PubMed] [Google Scholar]
- 69.Lauszus F, Klebe J, Bek T, Flyvbjerg A. Increased serum IGF-I during pregnancy is associated with progression of diabetic retinopathy. Diabetes. 2003;52(3):852–6. doi: 10.2337/diabetes.52.3.852 [DOI] [PubMed] [Google Scholar]
- 70.Rahman W, Rahman FZ, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35(3):231–6. Epub 2007 Apr 14. doi: 10.1111/j.1442-9071.2006.01413.x . [DOI] [PubMed] [Google Scholar]
- 71.Ringholm L, Vestgaard M, Laugesen CS, Juul A, Damm P, Mathiesen ER. Pregnancy-induced increase in circulating IGF-I is associated with progression of diabetic retinopathy in women with type 1 diabetes. Growth Hormon IGF Res. 2011;21(1):25–30. Epub 2011 Jan 8. doi: 10.1016/j.ghir.2010.12.001 . [DOI] [PubMed] [Google Scholar]
- 72.Chen HC, Newsom RS, Patel V, Cassar J, Mather H, Kohner EM. Retinal blood flow changes during pregnancy in women with diabetes. Invest Ophthalmol Vis Sci. 1994;35(8):3199–208. Epub 1994 Jul 1. . [PubMed] [Google Scholar]
- 73.Reece EA, Leguizamon G, Homko C. Pregnancy performance and outcomes associated with diabetic nephropathy. Am J Perinatol. 1998;15(7):413–21. doi: 10.1055/s-2007-993968 [DOI] [PubMed] [Google Scholar]
- 74.Rudland VL, Price SAL, Hughes R, Barrett HL, Lagstrom J, Porter C, et al. ADIPS 2020 guideline for pre-existing diabetes and pregnancy. Aust N Z J Obstet Gynaecol. 2020;60(6):E18–52. doi: 10.1111/ajo.13265 [DOI] [PubMed] [Google Scholar]
- 75.ACOG Guidance on Perinatal Management of Pregestational Diabetes [cited 2021 Mar 14]. Available from: https://www.obgproject.com/2018/12/18/updated-acog-guidance-on-perinatal-management-of-pregestational-diabetes/.
- 76.Confidential Enquiry into Maternal and Child Health. Diabetes in Pregnancy: Are we providing the best care? Findings of a National Enquiry: England, Wales and Northern Ireland. London. 2007 [cited 2021 Feb 28]. Available from: https://www.publichealth.hscni.net/sites/default/files/Diabetes%20in%20Pregnancy-%20are%20we%20providing%20the%20best%20care.pdf.
- 77.Klein B, Moss S, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13(1):34–40. Epub 1990 Jan 1. doi: 10.2337/diacare.13.1.34 . [DOI] [PubMed] [Google Scholar]
- 78.Diglas J, Bali C, Simon C, Strassegger-Böhm D, Irsigler K. [Follow-up of albumin excretion during pregnancy and post partum in type 1 diabetic patients in comparison with health pregnant probands]. Acta Med Austriaca. 1997;24(5):170–4. Epub 1997 Jan 1. . German. [PubMed] [Google Scholar]
- 79.Carr DB, Koontz GL, Gardella C, Holing EV, Brateng DA, Brown ZA, et al. Diabetic nephropathy in pregnancy: suboptimal hypertensive control associated with preterm delivery. Am J Hypertens. 2006;19(5):513–9. Epub 2006 May 2. doi: 10.1016/j.amjhyper.2005.12.010 . [DOI] [PubMed] [Google Scholar]
- 80.Stalnikiewicz L, Floriot M, Guerci B, Angioi K. Progression of diabetic retinopathy during pregnancy: a retrospective analysis of a series of 77 consecutive patients. J Fr Ophtalmol. 2010;33(7):481–6. Epub 2010 Aug 3. doi: 10.1016/j.jfo.2010.06.005 . [DOI] [PubMed] [Google Scholar]
- 81.The Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000;23(8):1084–91. doi: 10.2337/diacare.23.8.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–22. Epub 2017 Jun 29. doi: 10.1056/NEJMoa1704559 . [DOI] [PubMed] [Google Scholar]
- 83.Jardine J, Relph S, Magee L, von Dadelszen P, Morris E, Ross-Davie M, et al. Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG Int J Obstet Gynaecol. doi: 10.1111/1471-0528.16547 [DOI] [PubMed] [Google Scholar]
- 84.National Perinatal Epidemiology Unit. UK Obstetric Surveillance System. 2018 [cited 2018 Sept 6]. Available from: https://www.npeu.ox.ac.uk/ukoss.