Abstract
The COVID-19 pandemic is one of the greatest threats to human health in the 21st century with more than 257 million cases and over 5.17 million deaths reported worldwide (as of November 23, 2021. Various agents were initially proclaimed to be effective against SARS-CoV-2, the etiological agent of COVID-19. Hydroxychloroquine, lopinavir/ritonavir, and ribavirin are all examples of therapeutic agents, whose efficacy against COVID-19 was later disproved. Meanwhile, concentrated efforts of researchers and clinicians worldwide have led to the identification of novel therapeutic options to control the disease including PAXLOVID™ (PF-07321332). Although COVID-19 cases are currently treated using a comprehensive approach of anticoagulants, oxygen, and antibiotics, the novel Pfizer agent PAXLOVID™ (PF-07321332), an investigational COVID-19 oral antiviral candidate, significantly reduced hospitalization time and death rates, based on an interim analysis of the phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness. The scheduled interim analysis demonstrated an 89 % reduction in risk of COVID-19-related hospitalization or death from any cause compared to placebo in patients treated within three days of symptom onset (primary endpoint). However, there still exists a great need for the development of additional treatments, as the recommended therapeutic options are insufficient in many cases. Thus far, mRNA and vector vaccines appear to be the most effective modalities to control the pandemic. In the current review, we provide an update on the progress that has been made since April 2020 in clinical trials concerning the effectiveness of therapies available to combat COVID-19. We focus on currently recommended therapeutic agents, including steroids, various monoclonal antibodies, remdesivir, baricitinib, anticoagulants and PAXLOVID™ summarizing the latest original studies and meta-analyses. Moreover, we aim to discuss other currently and previously studied agents targeting COVID-19 that either show no or only limited therapeutic activity. The results of recent studies report that hydroxychloroquine and convalescent plasma demonstrate no efficacy against SARS-CoV-2 infection. Lastly, we summarize the studies on various drugs with incoherent or insufficient data concerning their effectiveness, such as amantadine, ivermectin, or niclosamide.
Keywords: SARS-CoV-2, COVID-19, Baricitinib, Casirivimab, Dexamethasone, Imdevimab, Remdesivir, Sotrovimab, Tocilizumab, Paxlovid, Omicron
1. Introduction
Coronaviruses (CoVs) are enveloped, spherical viruses, whose genome contains a positive-sense, single-strained RNA (Cui et al., 2019; Pollard et al., 2020). They are responsible for respiratory and interstitial infections, whose severity varies from cold-like symptoms to severe respiratory failure (Fehr and Perlman, 2015; Giovanetti et al., 2021). The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causes the Coronavirus Disease 2019 (COVID-19), whose symptoms can vary from mild, self-limiting respiratory distress to severe pneumonia leading to multiple organ failure and death (Huang et al., 2020). To date, the World Health Organization (WHO) has reported nearly 257 million COVID-19 cases and more than 5.17 million deaths worldwide (World Health Organization, 2021) (as of November 23, 2021).
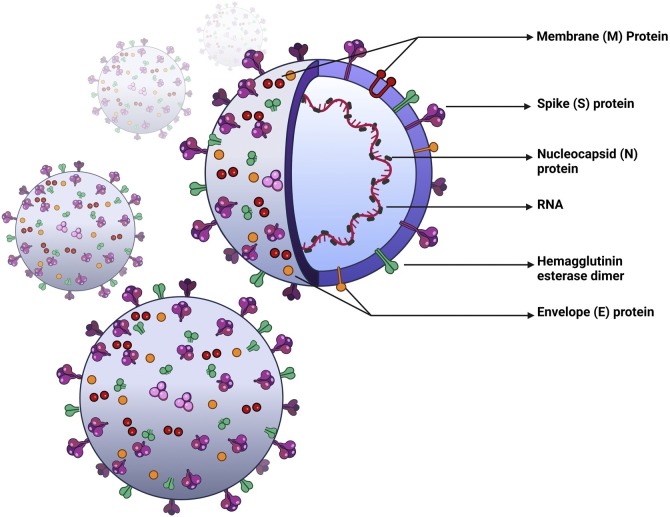
The genome of the SARS-CoV-2 encodes multiple structural, as well as 16 non-structural proteins necessary for transcription and replication (Fehr and Perlman, 2015; Perlman and Netland, 2009), such as the membrane protein (M), spike protein (S), envelope protein (E), and nucleocapsid protein (N) (Fig. 1 ) (Kirtipal et al., 2020). Similar to other RNA viruses, the genome of SARS-CoV-2 is prone to random mutations that affect both structural and non-structural genes (Giovanetti et al., 2021; Aleem et al., 2021). As a result of this genetic diversity, SARS-CoV-2 variants of concern (VOC) have emerged around the world, posing a possible threat to public health. The genetic alterations change the viral phenotype and affect its transmissibility, virulence, and severity of clinical manifestation (World Health Organization, 2021; Aleem et al., 2021). Since the beginning of the pandemic, the WHO has named five variants as VOCs, namely the Alpha, Beta, Gamma, Delta, and Omicron variants, which have spread worldwide (World Health Organization, 2021). With the emergence of novel variants, the rapid evaluation of possible resistance to anti-viral therapies and vaccines is highly required. However, data on the efficacy of available therapeutic agents and vaccines against VOC is clearly insufficient. For example, the Beta and Gamma variants demonstrated decreased susceptibility in vitro to treatment with bamlanivimab and etesevimab, a combination of anti-SARS-CoV-2 monoclonal antibodies (mAb) (COVID-19 Treatment Guidelines Panel, 2021; Food and Drug Administration, 2021a). However, this combination shows no reduced susceptibility (<5-fold reduction) towards the Alpha, Delta and Lambda variants. The clinical implication of these findings has yet to be established. Nevertheless, sotrovimab and a combination of casirivimab and imdevimab showed sufficient activity against all VOCs (COVID-19 Treatment Guidelines Panel, 2021; Food and Drug Administration, 2020, 2021b). The emergence of highly transmissible variants, combined with the easing of travel restrictions and low vaccination rates in some countries may lead to a further rise in reported cases, hospitalization rates, and deaths (World Health Organization, 2021).
Fig. 1.
Schematic depiction of SARS-Cov-2. SARS-Cov-2 is an enveloped, spherical virus belonging to the coronaviridae family. RNA – genomic, positive-sense, single-stranded RNA, M – membrane protein, S – spike protein, N – nucleocapsid protein, E – envelope protein.
Since the beginning of the pandemic, multiple antivirals, antibiotics, antimalarials, and immunomodulatory drugs were predicted to be effective against SARS-CoV-2 (Fig. 2 ). However, further studies reported limited or no clinical usefulness for most proposed drugs. However, identification of agents that are ineffective is of paramount importance, so that both proper and effective treatment is applied, and possible undesired side-effects of treatment are avoided. In the current review, we aim to provide an update on the advancements in clinical trials assessing the clinical efficacy of those treatment modalities that has been made since April 2020 and provide insight into future perspectives (Table 1, Table 2 ). The current recommendations for COVID-19 treatment are summarized in Table 3 .
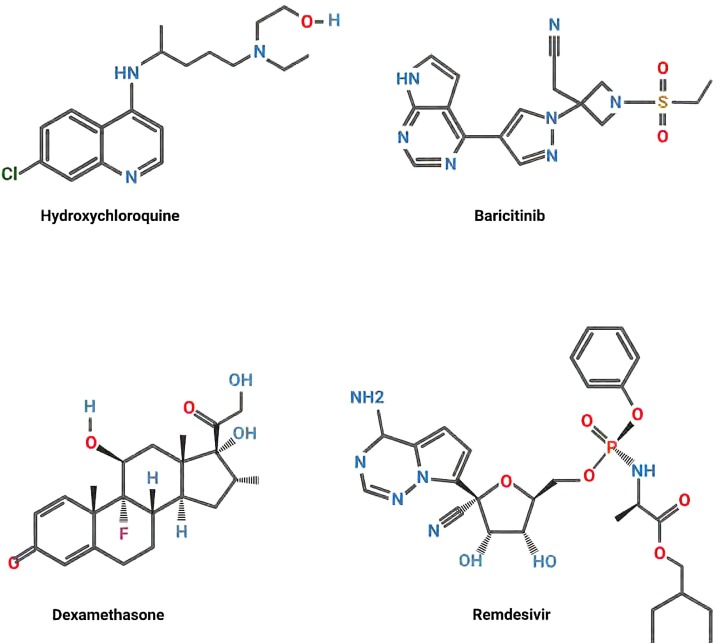
Fig. 2.
Examples of drugs proposed for the treatment of SARS-CoV-2. Structural renderings of Hydroxychloroquine (antimalarial drug, potential blocker of viral maturation), Baricitinib (anti-inflammatory: blocker of JAK-1, JAK-2 kinases), Dexamethasone (steroid anti-inflammatory drug), and Remdesivir (blocks viral replication) are shown.
Table 1.
Summary of currently conducted studies on COVID-19 drugs according to: drugvirus.info (Andersen et al., 2020; Drugvirus.info, 2021), clinicaltrials.gov (US National Library of Medicine, 2020) (updated on – 27th of July 2021).
| Therapeutic agent | Number of phase III-IV clinical trials |
|---|---|
| Amantadine | 3 |
| ASA | 10 |
| Azithromycin | 41 |
| Bamlanivimab - etesevimab | 3 |
| Baricitinib | 13 |
| Camostat mesylate | 6 |
| Casirivimab/ imdevimab | 3 |
| Chloroquine | 13 |
| Dexamethasone | 29 |
| Favipiravir | 21 |
| HCQ | 117 |
| Imatinib | 2 |
| IFN-β-1a | 11 |
| Isotretinoin | 3 |
| Ivermectin | 37 |
| Lopinavir/ritonavir | 20 |
| Mefloquine | 2 |
| Nafamostat mesylate | 5 |
| Niclosamide | 4 |
| Nitazoxanide | 18 |
| Oseltamivir | 7 |
| Remdesivir | 46 |
| Ribavirin | 3 |
| Sofosbuvir | 8 |
| Sotrovimab | 2 |
| Tocilizumab | 23 |
| Umifenovir | 4 |
Legend: ASA – acetylsalicylic acid, aspirin; HCQ – hydroxychloroquine; IFN-interferon.
Table 2.
An update on the clinical trials on COVID (as of the 29th of July 2021) (US National Library of Medicine, 2020).
| Therapeutic agent | Clinical trial ID | Number of participants | status | Additional information |
|---|---|---|---|---|
| Abidol | NCT04255017 | 400 | recruiting | compared to oseltamivir, lopinavir/ritonavir, standard of care |
| Adalimumab | NCT04705844 | 1444 | not yet recruiting | compared to placebo |
| Adalimumab | ChiCTR2000030089 | 60 | active, not recruiting | compared to standard treatment |
| Adamumab + Tozumab | ChiCTR2000030580 | 60 | recruiting | compared to standard treatment |
| Amantadine | NCT04952519 | 500 | recruiting | compared to placebo |
| Amantadine | NCT04894617 | 226 | not yet recruiting | compared to placebo |
| Amantadine | NCT04854759 | 200 | recruiting | compared to placebo |
| Amiodarone | NCT04351763 | 804 | recruiting | compared to verapamil, standard of care |
| Anakinra | NCT04680949 | 606 | active | compared to placebo |
| Anakinra | NCT04424056 | 216 | not yet recruiting | combined with ruxolitinib; compared to tocilizumab, tocilizumab + ruxolitinib, standard of care |
| Anakinra | NCT04362111 | 30 | recruiting | compared to placebo |
| Anakinra | NCT04443881 | 179 | completed | compared to standard of care |
| Anakinra | NCT04643678 | 80 | recruiting | compared to standard of care |
| Anakinra | NCT04341584 | 240 | completed | – |
| Anakinra | NCT04339712 | 20 | completed | compared to tocilizumab |
| Anakinra | NCT04324021 | 54 | terminated | compared to emapalumab and standard treatment |
| Angiotensin 1−7 | NCT04332666 | 60 | not yet recruiting | – |
| ACE-I | NCT04345406 | 60 | not yet recruiting | compared to standard of care |
| ACE-Is & ARBs | NCT04353596 | 216 | completed | stopping of ACEI/ARB treatment compared to further ACEI/ARB treatment |
| ACE-Is & ARBs | NCT04591210 | 1155 | recruiting | compared to no treatment |
| ACE-Is & ARBs | NCT04493359 | 240 | recruiting | compared to standard of care |
| ARBs | NCT04394117 | 1500 | recruiting | compared to placebo |
| Anti-SARS-CoV-2 equine hyperimmune serum | NCT04838821 | 156 | active | compared to placebo |
| Apremilast | NCT04590586 | 516 | active | compared to landelumab, zilucoplan, placebo |
| Arbidol | NCT04260594 | 304 | completed, has results | compared to standard of care |
| ASC09 | NCT04261270 | 60 | recruiting | combined with oseltamivir; compared to ritonavir + oseltamivir, oseltamivir |
| ASC09 | NCT04261270 | 60 | recruiting | compared to ritonavir; combined with oseltamivir |
| ASC09 | NCT04261907 | 160 | not yet recruiting | compared to lopinavir/ritonavir; combined with ritonavir |
| ASA | NCT04365309 | 128 | recruiting | compared to standard of care |
| Atazanavir | NCT04468087 | 1005 | recruiting | compared to daclatasvir, sofosbuvir + daclatasvir, placebo |
| Atovaquone | NCT04339426 | 25 | recruiting | combined with azithromycin |
| Aviptadil | NCT04311697 | 196 | completed | compared to placebo |
| AZD7442 | NCT04723394 | 1700 | recruiting | compared to placebo |
| Azithromycin | NCT04359316 | 40 | not yet recruiting | combined with HCQ |
| Azithromycin | NCT04381962 | 298 | completed | compared to standard of care |
| Azithromycin | NCT04363060 | 104 | not yet recruiting | combined with amoxicillin/clavulanate; compared to amoxycillin/clavulanate |
| Azithromycin | NCT04341727 | 500 | suspended | compared to chloroquine and hydroxychloroquine |
| Azithromycin | NCT04324463 | 1500 | recruiting | compared to chloroquine |
| Azithromycin | NCT04339816 | 240 | terminated | combined with hydroxychloroquine |
| Azithromycin | NCT04336332 | 160 | active, not recruiting | compared to hydroxychloroquine; combined with hydroxychloroquine |
| Azithromycin | NCT04332107 | 2271 | active, not recruiting | – |
| Azithromycin + Hydroxychloroquine | NCT04322123 | 630 | active, not recruiting | compared to HCQ |
| Azithromycin + Hydroxychloroquine | NCT04321278 | 440 | completed | compared to HCQ |
| Azoximer Bromide | NCT04381377 | 394 | active | compared to placebo |
| Azvudine | NCT04668235 | 342 | recruiting | compared to placebo |
| Azvudine | ChiCTR2000029853 | 20 | recruiting | compared to standard treatment |
| Azvudine | ChiCTR2000030041 | 40 | not yet recruiting | – |
| Azvudine | ChiCTR2000030424 | 30 | not yet recruiting | – |
| Azvudine | ChiCTR2000030487 | 10 | recruiting | – |
| Bactek-R | NCT04363814 | 100 | recruiting | compared to standard of care |
| Baloxavir marboxil | ChiCTR2000029544 | 30 | not yet recruiting | compared to favipiravir and standard treatment |
| Baloxavir marboxil | ChiCTR2000029548 | 30 | not yet recruiting | compared to favipiravir and lopinavir/ritonavir |
| Bamlanivimab | NCT04656691 | 4000 | completed | single group assignment |
| Bamlanivimab | NCT04796402 | 576 | recruiting | compared to standard of care |
| Bamlanivimab | NCT04748588 | 648 | recruiting | compared to standard of care |
| Bamlanivimab | NCT04518410 | 2000 | recruiting | compared to BRII-196/BRII-198, AZD7442, SGN001, Camostat, C135-LS + C144-LS, SAB-185, placebo |
| Baricitinib | NCT04401579 | 1033 | completed | combined with remdesivir; compared to remdesivir + placebo |
| Baricitinib | NCT04640168 | 1010 | active | combined with remdesivir; compared to dexamethasone and remdesivir |
| Baricitinib | NCT04970719 | 382 | recruiting | combined with remdesivir; compared to dexamethasone plus remdesivir |
| Baricitinib | NCT04421027 | 1585 | completed | compared to placebo |
| Baricitinib | NCT04358614 | 12 | completed | crossover assignment |
| Baricitinib | NCT04320277 | 60 | not yet recruiting | – |
| Baricitinib | NCT04340232 | 80 | withdrawn | – |
| Baricitinib | NCT04321993 | 1000 | recruiting | compared to HCQ, lopinavir/ritonavir and sarilumab |
| BDB-001 | NCT04449588 | 368 | recruiting | compared to standard of care |
| BLD-2660 | NCT04334460 | 120 | active, not recruiting | – |
| BNO 1030 | NCT04797936 | 133 | completed | compared to standard of care |
| Brazilian Green Propolis Extract | NCT04480593 | 120 | completed | compared to placebo |
| Brensocatib | NCT04817332 | 400 | completed | compared to placebo |
| Bromhexidine | NCT04355026 | 90 | recruiting | combined with HCQ; compared to HCQ |
| Bucillamine | NCT04504734 | 1000 | recruiting | compared to placebo |
| Budesonid | NCT04361474 | 120 | completed | compared to placebo |
| Budesonid | NCT04355637 | 300 | recruiting | compared to standard of care |
| C21 | NCT04880642 | 600 | not yet recruiting | compared to placebo |
| Camostat Mesylate | NCT04608266 | 596 | recruiting | compared to placebo |
| Camostat Mesylate | NCT04657497 | 155 | completed | compared to placebo |
| Camostat Mesylate | NCT04321096 | 180 | recruiting | – |
| Canakinumab | NCT04362813 | 451 | completed | compared to placebo |
| Canakinumab | NCT04510493 | 116 | recruiting | compared to placebo |
| Cannabidiol | NCT04467918 | 100 | active | compared to placebo |
| Cannabidiol | NCT04615949 | 422 | recruiting | compared to placebo |
| Carrimycin | NCT04672564 | 300 | recruiting | compared to placebo |
| CD24Fc | NCT04317040 | 243 | completed | compared to placebo |
| CD24Fc | NCT04317040 | 230 | completed | – |
| Cefditoren pivoxil | NCT04709172 | 30 | recruiting | single group assignment |
| Cetirizine + Famotidine | NCT04836806 | 160 | recruiting | compared to placebo |
| Chloroquine | ChiCTR2000029542 | 20 | recruiting | compared to standard treatment |
| Chloroquine | ChiCTR2000029609 | 200 | not yet recruiting | compared to lopinavir/ritonavir |
| Chloroquine | ChiCTR2000029741 | 112 | recruiting | compared to lopinavir/ritonavir |
| Chloroquine | ChiCTR2000029826 | 45 | not yet recruiting | – |
| Chloroquine | ChiCTR2000029837 | 120 | not yet recruiting | – |
| Chloroquine | ChiCTR2000029935 | 100 | recruiting | – |
| Chloroquine | ChiCTR2000029939 | 100 | recruiting | compared to standard treatment |
| Chloroquine | ChiCTR2000029975 | 10 | not yet recruiting | – |
| Chloroquine | ChiCTR2000029988 | 80 | recruiting | compared to standard treatment |
| Chloroquine | ChiCTR2000029992 | 100 | not yet recruiting | compared to standard treatment; combined with HCQ |
| Chloroquine | ChiCTR2000030031 | 120 | suspended | – |
| Chloroquine | ChiCTR2000030417 | 30 | suspended | – |
| Chloroquine | ChiCTR2000030718 | 80 | recruiting | compared to standard treatment |
| Chloroquine | ChiCTR2000029898 | 100 | recruiting | compared to hydroxychloroquine |
| Chloroquine | ChiCTR2000029899 | 100 | recruiting | compared to HCQ |
| Chloroquine | NCT04341727 | 500 | suspended | compared to azithromycin and CQ |
| Chloroquine | NCT04324463 | 1500 | recruiting | compared to azithromycin |
| Chloroquine | NCT04323527 | 440 | completed | – |
| Chloroquine | NCT04333628 | 210 | terminated | compared to standard treatment |
| Chloroquine | NCT04331600 | 400 | completed | – |
| Chloroquine | NCT04328493 | 250 | completed | compared to standard treatment |
| Chlorpromazine | NCT04366739 | 40 | not yet recruiting | compared to standard of care |
| Ciclesonide | NCT04377711 | 400 | completed | compared to placebo |
| Ciclesonide | NCT04330586 | 141 | completed | compared to standard treatment; combined with HCQ |
| CimertrA | NCT04802382 | 252 | recruiting | compared to placebo |
| Colchicine | NCT04667780 | 102 | completed | compared to standard of care |
| Colchicine | NCT04350320 | 102 | completed | compared to standard of care |
| Colchicine | NCT04818489 | 250 | recruiting | compared to standard of care |
| Colchicine | NCT04472611 | 466 | recruiting | combined with rosuvastatin; compared to standard of care |
| Colchicine | NCT04328480 | 1279 | completed | compared to standard of care |
| Colchicine | NCT04492358 | 144 | recruiting | combined with prednisone; compared to standard of care |
| Colchicine | NCT04416334 | 954 | recruiting | compared to standard of care |
| Colchicine | NCT04328480 | 2500 | completed | – |
| Colchicine | NCT04322682 | 6000 | completed | – |
| Colchicine | NCT04322565 | 100 | recruiting | – |
| Comega-3 Oil | NCT04836052 | 372 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04425915 | 400 | completed | compared to standard of care |
| Convalescent Plasma Therapy | NCT04355767 | 511 | completed | compared to placebo |
| Convalescent Plasma Therapy | NCT04547660 | 160 | completed | compared to standard of care |
| Convalescent Plasma Therapy | NCT04589949 | 690 | recruiting | compared to Fresh Frozen Plasma |
| Convalescent Plasma Therapy | NCT04535063 | 200 | recruiting | single group assignment |
| Convalescent Plasma Therapy | NCT04381858 | 196 | completed | compared to human immunoglobulin |
| Convalescent Plasma Therapy | NCT04361253 | 220 | recruiting | compared to standard plasma |
| Convalescent Plasma Therapy | NCT04539275 | 702 | active | compared to placebo |
| Convalescent Plasma Therapy | NCT04516811 | 600 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04836260 | 100 | recruiting | single group assignment |
| Convalescent Plasma Therapy | NCT04567173 | 136 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04345289 | 1100 | recruiting | compared to infusion placebo |
| Convalescent Plasma Therapy | NCT04747158 | 350 | completed | single group assignment |
| Convalescent Plasma Therapy | NCT04385043 | 400 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04388410 | 410 | recruiting | compared to placebo |
| Convalescent Plasma Therapy | NCT04873414 | 364 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04342182 | 426 | active | compared to standard of care |
| Convalescent Plasma Therapy | NCT04502472 | 200 | recruiting | single group assignment |
| Convalescent Plasma Therapy | NCT04374526 | 29 | completed | compared to standard of care |
| Convalescent Plasma Therapy | NCT04380935 | 60 | recruiting | compared to standard of care |
| Convalescent Plasma Therapy | NCT04384588 | 100 | recruiting | parallel assignment - cancer patients and non-cancer patients |
| Convalescent Plasma Therapy | NCT04816942 | 102 | completed | single group assignment |
| Convalescent Plasma Therapy | NCT04332835 | 92 | completed | compared to standard of care |
| Convalescent Plasma Therapy | NCT04376034 | 240 | recruiting | compared to standard of care |
| Cretan IAMA | NCT04705753 | 20 | completed | single group assignment |
| CSA0001 | ChiCTR2000030939 | 10 | recruiting | – |
| CT-P59 | NCT04602000 | 1020 | recruiting | compared to placebo |
| Cyclosporine | NCT04392531 | 120 | recruiting | compared to standard of care |
| Dalargin | NCT04346693 | 320 | completed | compared to standard of care |
| Danoprevir | NCT04345276 | 10 | completed | combined with ritonavir |
| Danoprevir/Ritonavir | ChiCTR2000030000 | 50 | recruiting | compared to IFN-α, peginterferon α-2a and standard treatment |
| Danoprevir/Ritonavir | ChiCTR2000030259 | 60 | recruiting | compared to standard treatment |
| Danoprevir/Ritonavir | ChiCTR2000030472 | 20 | recruiting | compared to standard treatment |
| Dapagliflozin | NCT04350593 | 1250 | active | compared to placebo |
| Dapsone | NCT04935476 | 3000 | not yet recruiting | compared to placebo |
| Darunavir/Cobicistat | NCT04252274 | 30 | recruiting | compared to standard treatment |
| Darunavir/Cobicistat | NCT04304053 | 3040 | completed | – |
| Darunavir/Ritonavir | NCT04291729 | 50 | completed | compared to IFN-α, lopinavir/ritonavir and peginterferon α-2a; combined with IFN-α |
| DAS181 | NCT04324489 | 4 | completed | – |
| Deferoxamine | NCT04333550 | 50 | recruiting | compared to standard treatment |
| Defibrotide | NCT04335201 | 50 | recruiting | – |
| Desferal | NCT04389801 | 200 | not yet recruiting | compared to placebo |
| Dexamethasone | NCT04726098 | 198 | recruiting | high dose compared to low dose |
| Dexamethasone | NCT04663555 | 300 | recruiting | high dose compared to low dose |
| Dexamethasone | NCT04509973 | 1000 | active | high dose compared to low dose |
| Dexamethasone | NCT04509973 | 1000 | active | high dose compared to low dose |
| Dexamethasone | NCT04499313 | 60 | recruiting | compared to methylprednisolone |
| Dexamethasone | NCT04347980 | 122 | recruiting | combined with HCQ; compared to HCQ |
| Dexamethasone | NCT04834375 | 142 | recruiting | weight-based dexamethasone use compared to standard dexamethasone dose |
| Dexamethasone | NCT04765371 | 220 | recruiting | compared to prednisolone |
| Dexamethasone | NCT04780581 | 290 | recruiting | compared to methylprednisolone |
| Dexamethasone | NCT04327401 | 290 | terminated | – |
| Dihydroartemisinin/Piperaquine | ChiCTR2000030082 | 40 | suspended | compared to IFN-α + umifenovir; combined with antiviral treatment |
| Dipyridamole | NCT04410328 | 132 | recruiting | combined with ASA; compared to standard of care |
| Dornase alfa | NCT04355364 | 100 | recruiting | compared to standard of care |
| Dornase alfa | NCT04402970 | 30 | completed | compared to standard of care |
| Doxycycline | NCT04715295 | 200 | recruiting | combined with rivaroxaban; compared to standard of care |
| Doxycycline | NCT04584567 | 1100 | recruiting | monotherapy or combined with Zinc; compared to placebo |
| Doxycycline | NCT04371952 | 330 | not yet recruiting | compared to placebo |
| Dutasteride | NCT04729491 | 138 | completed | combined with azithromycin + nitazoxanide; compared to azithromycin + nitazoxanide + placebo |
| DWJ1248 | NCT04713176 | 1022 | recruiting | combined with remdesivir; compared to placebo |
| Ebastine | ChiCTR2000030535 | 100 | recruiting | combined with IFN-α and lopinavir |
| EDP1815 | NCT04393246 | 1407 | recruiting | compared to dapagliflozin + ambrisentan, standard of care |
| Emapalumab | NCT04324021 | 54 | terminated | compared to anakinra and standard treatment |
| Emtricitabine/Tenofovir | NCT04890626 | 2193 | recruiting | compared to baricitinib + dexamethasone, dexamethasone, standard of care |
| Emtricitabine/Tenofovir | NCT04359095 | 1200 | recruiting | compared to colchicine + rosuvastatin, emtricitabine/tenofovir + colchicine + rosuvastatin, standard of care |
| Emtricitabine/Tenofovir + Lopinavir/Ritonavir | ChiCTR2000029468 | 120 | not yet recruiting | – |
| Enisamium Iodide | NCT04682873 | 700 | recruiting | compared to placebo |
| Ensovibep | NCT04828161 | 2100 | recruiting | compared to placebo |
| Evolocumab | NCT04941105 | 60 | recruiting | compared to placebo |
| Famotidine | NCT04370262 | 233 | completed | compared to placebo |
| Favipiravir | NCT04529499 | 780 | active | compared to placebo |
| Favipiravir | NCT04542694 | 200 | completed | compared to standard of care |
| Favipiravir | NCT04359615 | 40 | not yet recruiting | combined with HCQ; compared to HCQ |
| Favipiravir | NCT04558463 | 100 | recruiting | compared to oseltamivir |
| Favipiravir | NCT04501783 | 168 | active | compared to standard of care |
| Favipiravir | NCT04600895 | 826 | recruiting | compared to placebo |
| Favipiravir | NCT04818320 | 500 | active | compared to standard of care |
| Favipiravir | NCT04694612 | 676 | recruiting | compared to remdesivir, placebo |
| Favipiravir | NCT04425460 | 256 | not yet recruiting | compared to placebo |
| Favipiravir | NCT04411433 | 1008 | active | monotherapy or combined with HCQ or azithromycin; compared to HCQ, HCQ + azithromycin |
| Favipiravir | NCT04600999 | 150 | recruiting | compared to standard of care |
| Favipiravir | NCT04434248 | 330 | active | compared to standard of care |
| Favipiravir | NCT04464408 | 576 | recruiting | compared to placebo |
| Favipiravir | NCT04351295 | 90 | recruiting | compared to placebo |
| Favipiravir | NCT04402203 | 50 | recruiting | compared to standard of care |
| Favipiravir | NCT04373733 | 502 | active | compared to standard of care |
| Favipiravir | NCT04319900 | 150 | recruiting | monotherapy or combined with favipiravir; compared to placebo |
| Favipiravir | ChiCTR2000029544 | 30 | not yet recruiting | compared to baloxavir marboxil and standard treatment |
| Favipiravir | ChiCTR2000029548 | 30 | not yet recruiting | compared to baloxavir marboxil and lopinavir/ritonavir |
| Favipiravir | ChiCTR2000029600 | 90 | recruiting | compared to lopinavir/ritonavir; combined with IFN-α |
| Favipiravir | ChiCTR2000029996 | 60 | recruiting | – |
| Favipiravir | ChiCTR2000030113 | 20 | recruiting | compared to ritonavir |
| Favipiravir | ChiCTR2000030254 | 240 | completed | compared to umifenovir |
| Favipiravir | ChiCTR2000030987 | 150 | recruiting | combined with chloroquine |
| Favipiravir | JPRN jRCTs041190120 | 86 | completed | – |
| Favipiravir | NCT04273763 | 60 | active, not recruiting | combined with bromohexine, IFN α-2b and umifenovir |
| Favipiravir | NCT04310228 | 150 | recruiting | compared to tocilizumab; combined with tocilizumab |
| Favipiravir | NCT04336904 | 100 | active, not recruiting | – |
| Fenofibrate | NCT04661930 | 50 | recruiting | compared to placebo |
| Fingolimod | NCT04280588 | 30 | withdrawn | compared to standard treatment |
| Fluoxetine | NCT04377308 | 2000 | recruiting | compared to standard of care |
| Fluvoxamine (Lenze et al., 2020) | NCT04342663 | 152 | completed, has results | – |
| Fluvoxamine | NCT04727424 | 3645 | recruiting | compared to doxazosin, ivermectin, peginterferon λ-1a, peginterferon β-1A, placebo |
| Fluvoxamine | NCT04668950 | 1100 | active | compared to placebo |
| Fostamatinib | NCT04629703 | 308 | recruiting | compared to placebo |
| FP-025 | NCT04750278 | 403 | recruiting | compared to placebo |
| Furosemide | NCT04588792 | 640 | recruiting | compared to placebo |
| Hydrocortisone | NCT04348305 | 1000 | active | compared to placebo |
| HCQ | NCT04359953 | 1600 | recruiting | compared to azithromycin, telmisartan, standard of care |
| HCQ | NCT04466540 | 1300 | recruiting | compared to placebo |
| HCQ | NCT04358081 | 20 | completed | monotherapy or combined with azithromycin; compared to placebo |
| HCQ | NCT04344444 | 600 | active | monotherapy or combined with azithromycin; compared to placebo |
| HCQ | NCT04429867 | 700 | active | compared to placebo |
| HCQ | NCT04370782 | 18 | completed | combined with Zinc + either azithromycin or doxycycline |
| HCQ | NCT04405921 | 200 | not yet recruiting | combined with azithromycin; compared to HCQ |
| HCQ | NCT04355052 | 250 | recruiting | combined with azithromycin or camostat mesylate; compared to no treatment |
| HCQ | NCT04491994 | 540 | completed | compared to standard of care |
| HCQ | NCT04420247 | 142 | completed | compared to standard of care |
| HCQ | NCT04354428 | 300 | active | monotherapy or combined with folic acid or azithromycin; compared to lopinavir / ritonavir, placebo |
| HCQ | NCT04351724 | 500 | recruiting | compared to lopinavir / ritonavir, remdesivir, asunercept, standard of care |
| HCQ | NCT04964583 | 105 | recruiting | combined with azithromycin; compared to HCQ, placebo |
| HCQ | NCT04573153 | 400 | recruiting | combined with cofactor supplementation; compared to HCQ + sorbitol |
| HCQ | NCT04353336 | 194 | completed | compared to standard of care |
| HCQ | NCT04652648 | 54 | completed | compared to control |
| HCQ | NCT04322123 | 630 | active | monotherapy or combined with azithromycin; compared to control |
| HCQ | NCT04788355 | 176 | completed | monotherapy or combined with apixaban; compared to apixaban or placebo |
| HCQ | 2020−000890-25 (EU-CTR) | 25 | ongoing | – |
| HCQ | ChiCTR2000029559 | 300 | recruiting | – |
| HCQ | ChiCTR2000029740 | 78 | recruiting | compared to standard treatment |
| HCQ (Tang et al., 2020) | ChiCTR2000029868 | 200 | completed, has results | – |
| HCQ | ChiCTR2000029898 | 100 | recruiting | compared to chloroquine |
| HCQ | ChiCTR2000029899 | 100 | recruiting | compared to chloroquine |
| HCQ | ChiCTR2000030054 | 100 | not yet recruting | compared to standard treatment |
| HCQ | NCT04261517 | 30 | completed | compared to standard treatment |
| HCQ | NCT04315896 | 500 | active, not recruiting | – |
| HCQ | NCT04315948 | 3100 | active, not recruiting | compared to IFNβ-1a, lopinavir/ritonavir and remdesivir |
| HCQ | NCT04316377 | 202 | active, not recruiting | compared to standard treatment |
| HCQ | NCT04342221 | 220 | terminated | – |
| HCQ | NCT04340544 | 2700 | terminated | – |
| HCQ | NCT04338698 | 500 | recruiting | compared to azithromycin and oseltamivir |
| HCQ | NCT04335552 | 500 | terminated, has results - poor recruitment, strong evidence from larger trials of no therapeutic benefit | compared with azithromycin, HCQ and standard treatment; combined with azithromycin |
| HCQ | NCT04334512 | 600 | recruiting | combined with azithromycin |
| HCQ | NCT04334382 | 1550 | recruiting | combined with azithromycin |
| HCQ | NCT04329832 | 300 | active, not recruiting | combined with azithromycin |
| HCQ | NCT04329572 | 400 | suspended | combined with azithromycin |
| HCQ | NCT04328272 | 75 | not yet recruiting | combined with azithromycin |
| HCQ | NCT04323631 | 1116 | withdrawn | compared to standard treatment |
| HCQ | NCT04321993 | 1000 | recruiting | compared to baricitinib, lopinavir/ritonavir and sarilumab |
| HCQ | NCT04342169 | 400 | recruiting | – |
| HCQ | NCT04341727 | 500 | suspended | compared to azithromycin and chloroquine |
| HCQ | NCT04341493 | 86 | terminated | compared to nitazoxanide |
| HCQ | NCT04334967 | 1250 | suspended | compared to standard treatment |
| HCQ | NCT04333654 | 210 | terminated | compared to standard treatment |
| HCQ (Self et al., 2020) | NCT04332991 | 510 | completed, has results | – |
| HCQ | NCT04321616 | 700 | recruiting | compared to remdesivir and standard treatment |
| HCQ + IFN β-1b + Lopinavir/Ritonavir | IRCT20100228 003449N27 | 30 | completed | – |
| HCQ + IFN β-1b + Lopinavir/Ritonavir | IRCT20100228 003449N28 | 30 | completed, has results (Effat et al., 2021) | doi: 10.1128/AAC.01061−20 |
| HCQ + Lopinavir/Ritonavir | JPRN jRCTs031190227 | 50 | completed | – |
| HCQ + Lopinavir/Ritonavir + Sofosbuvir/Ledipasvir | IRCT20100228 003449N29 | 50 | completed | – |
| HCQ + Camostat Mesylate | NCT04338906 | 334 | withdrawn | – |
| Hyperimmune Anti SARS-CoV-2 serum | NCT04913779 | 200 | recruiting | compared to placebo |
| Ibuprofen | NCT04334629 | 230 | recruiting | compared to standard of care |
| Ifenprodil (NP-120) | NCT04382924 | 168 | completed | compared to standard of care |
| IFN α | ChiCTR2000029496 | 90 | recruiting | compared to lopinavir/ritonavir; combined with lopinavir/ritonavir |
| IFN α | ChiCTR2000029600 | 90 | recruiting | compared to lopinavir/ritonavir and favipiravir |
| IFN α | ChiCTR2000029638 | 100 | recruiting | compared to rSIFN-co |
| IFN α | NCT04291729 | 11 | completed | compared to darunavir/ritonavir, lopinavir/ritonavir and peginterferon α-2a |
| IFN α-1b | ChiCTR2000029989 | 300 | not yet recruiting | – |
| IFN α-1b | NCT04293887 | 328 | not yet recruiting | compared to standard treatment |
| IFN α-1b + Lopinavir/Ritonavir + Ribavirin | ChiCTR2000029387 | 108 | recruiting | – |
| IFN α-2b | NCT04273763 | 60 | active, not recruiting | combined with bromohexine, favipiravir and umifenovir |
| IFNα-2b + Lopinavir/Ritonavir | ChiCTR2000030166 | 20 | not yet recruiting | – |
| IFN β-1a | NCT04492475 | 969 | completed | combined with remdesivir; compared to placebo |
| IFN β-1a | NCT04350671 | 40 | recruiting | combined with lopinavir/ritonavir + HCQ, compared with lopinavir/ritonavir + HCQ |
| IFN β-1a | 2020−001023-14 (EU-CTR) | 400 | completed, has results (Monk et al., 2021) | – |
| IFN β-1a | NCT04343768 | 60 | completed | compared to HCQ + lopinavir / ritonavir and IFN β-1b; combined with HCQ + lopinavir / ritonavir |
| IFN β-1b | NCT04343768 | 60 | completed | compared to HCQ + lopinavir / ritonavir and IFN β-1a; combined with HCQ + lopinavir / ritonavir |
| IFN β-1b + Ribavirin | NCT04276688 | 70 | completed | combined with lopinavir/ritonavir |
| IFN α and Lopinavir/Ritonavir | NCT04251871 | 150 | recruiting | – |
| IFN α and Lopinavir/Ritonavir | NCT04275388 | 348 | not yet recruiting | – |
| IFX-1 | NCT04333420 | 130 | recruiting | compared to standard treatment |
| Imatinib | NCT04394416 | 204 | recruiting | compared to placebo |
| Imatinib | NCT04422678 | 30 | not yet recruiting | compared to standard of care |
| Imatinib | NCT04422678 | 30 | not yet recruiting | compared to standard of care |
| IMU-838 | NCT04379271 | 223 | completed | compared to placebo |
| INB03 | NCT04370236 | 366 | recruiting | compared to placebo |
| Infliximab | NCT04593940 | 2160 | recruiting | combined with remdesivir and standard of care; compared to abatacept, ceniciviroc, standard of care |
| INM005 | NCT04494984 | 242 | completed | compared to placebo |
| Interleukin-2 | ChiCTR2000030167 | 80 | not yet recruiting | compared to standard treatment |
| Isavuconazole | NCT04707703 | 162 | recruiting | compared to placebo |
| Isotretinoin | NCT04361422 | 300 | not yet recruiting | compared to standard of care |
| Isotretinoin | NCT04353180 | 10,000 | not yet recruiting | compared to standard of care |
| Ivermectin | NCT04523831 | 400 | completed | combined with doxycycline; compared to standard of care |
| Ivermectin | NCT04920942 | 500 | recruiting | compared to standard of care |
| Ivermectin | NCT04646109 | 66 | completed | compared to standard of care |
| Ivermectin | NCT04729140 | 150 | recruiting | combined with doxycycline; compared to placebo |
| Ivermectin | NCT04681053 | 80 | recruiting | compared to standard of care |
| Ivermectin | NCT04739410 | 50 | completed | compared to standard of care |
| Ivermectin | NCT04937569 | 1644 | not yet recruiting | compared to standard of care |
| Ivermectin | NCT04885530 | 15,000 | recruiting | compared to fluvoxamine, fluticasone, placebo |
| Ivermectin | NCT04746365 | 300 | completed | compared to HCQ, placebo |
| Ivermectin | NCT04944082 | 60 | not yet recruiting | combined with remdesivir; compared to remdesivir |
| Ivermectin | NCT04391127 | 108 | completed | monotherapy or combined with HCQ; compared to placebo |
| Ivermectin | NCT04703608 | 1200 | recruiting | compared to ASA, placebo |
| Ivermectin | NCT04834115 | 400 | recruiting | compared to placebo |
| Ivermectin | NCT04435587 | 80 | recruiting | compared to darunavir/ritonavir + HCQ |
| Ivermectin | NCT04445311 | 100 | recruiting | compared to standard of care |
| Ivermectin | NCT04403555 | 160 | recruiting | compared to standard of care |
| Ivermectin | NCT04351347 | 300 | recruiting | compared to standard of care |
| Ivermectin | NCT04529525 | 501 | completed | compared to placebo |
| Ivermectin | NCT04405843 | 476 | completed | compared to placebo |
| Ivermectin | NCT04959786 | 100 | recruiting | combined with ribavirin, nitazoxanide, Zinc; compared to standard of care |
| Ivermectin | NCT04716569 | 150 | recruiting | compared to standard of care |
| Ivermectin | NCT04951362 | 117 | recruiting | compared to placebo |
| Ivermectine | NCT04343092 | 50 | completed, has results | combined with HCQ; compared to placebo |
| IVIG | NCT04500067 | 76 | completed | compared to standard of care |
| IVIG | NCT04350580 | 146 | completed | compared to placebo |
| IVIG | NCT04546581 | 593 | active | combined with remdesivir; compared to placebo + remdesivir |
| IVIG | NCT04842435 | 376 | recruiting | compared to placebo |
| IVIG | NCT04891172 | 310 | recruiting | compared to standard of care |
| Ixekizumab | NCT04724629 | 60 | recruiting | compared to adesleukin, colchicine, standard of care |
| Ixekizumab | ChiCTR2000030703 | 40 | recruiting | compared to antiviral therapy; combined with antiviral therapy |
| Leflunomide (Wang et al., 2020b) | ChiCTR2000030058 | 200 | completed, has results | compared to standard treatment |
| Lenalidomide | NCT04361643 | 120 | not yet recruiting | compared to placebo |
| Lenlizumab | NCT04351152 | 520 | active | compared to standard of care |
| Leronlimab | NCT04901689 | 306 | not yet recruiting | compared to placebo |
| Leronlimab | NCT04343651 | 70 | active, not recruiting | – |
| Levamisole | NCT04331470 | 30 | recruiting | compared to standard treatment; combined with budesonide, formoterol and hydroxychloroquine + lopinavir/ritonavir |
| Levilimab | NCT04397562 | 206 | completed | compared to placebo |
| Lianhua Qingwen | NCT04433013 | 300 | not yet recruiting | compared to placebo |
| Lidocaine | NCT04609865 | 100 | recruiting | compared to placebo |
| Lilly Bamlanivimab | NCT04790786 | 5000 | recruiting | compared to regeneron casirivimab + imdevimab, Lilly Bamlanivimab + etesevimab, sotrovimab |
| Lipid Emulsion Infusion | NCT04957940 | 90 | recruiting | compared to placebo |
| Liposomal Lactoferrin | NCT04475120 | 92 | completed | compared to standard of care |
| Lopinavir / Ritonavir | NCT04738045 | 90 | recruiting | combined with remdesivir; compared to remdesivir |
| Lopinavir / Ritonavir | NCT04466241 | 294 | recruiting | monotherapy or combined with telmisartan, atorvastatin |
| Lopinavir / Ritonavir | NCT04403100 | 1968 | recruiting | monotherapy or combined with HCQ; compared to HCQ, placebo |
| Lopinavir / Ritonavir | NCT04381936 | 45,000 | recruiting | compared to corticosteroid, HCQ, azithromycin, convalescent plasma, tocilizumab, immunoglobulin, neutralizing antibodies, ASA, colchicine, baricitinib, anakinra, dimethyl fumarate, empagliflozin |
| Lopinavir/Ritonavir | 2020−000936-23 (EU-CTR) | 3000 | ongoing | compared to IFN β-1a and remdesivir |
| Lopinavir/Ritonavir (Cao et al., 2020) | ChiCTR2000029308 | 160 | completed, has results | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR2000029400 | 60 | recruiting | – |
| Lopinavir/Ritonavir (Zheng et al., 2020) | ChiCTR2000029496 | 90 | completed, has results | compared to IFN α; combined with IFN α |
| Lopinavir/Ritonavir | ChiCTR2000029539 | 328 | recruiting | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR2000029548 | 30 | not yet recruiting | compared to baloxavir marboxil and favipiravir |
| Lopinavir/Ritonavir | ChiCTR2000029573 | 480 | recruiting | combined with IFN-α and umifenovir |
| Lopinavir/Ritonavir | ChiCTR2000029600 | 90 | recruiting | compared to favipiravir; combined with IFN α |
| Lopinavir/Ritonavir | ChiCTR2000029609 | 200 | not yet recruiting | compared to chloroquine |
| Lopinavir/Ritonavir | ChiCTR2000030187 | 60 | recruiting | compared to standard treatment |
| Lopinavir/Ritonavir | ChiCTR2000030218 | 80 | recruiting | – |
| Lopinavir/Ritonavir | NCT04252885 | 125 | completed | compared to standard treatment and umifenovir |
| Lopinavir/Ritonavir | NCT04255017 | 400 | recruiting | compared to oseltamivir and umifenovir |
| Lopinavir/Ritonavir | NCT04261907 | 160 | not yet recruiting | compared to ASC09 |
| Lopinavir/Ritonavir | NCT04291729 | 11 | completed | compared to darunavir/ritonavir, IFN α and peginterferon α-2a |
| Lopinavir/Ritonavir | NCT04315948; 2020−000936-23 (EU-CTR) | 3100 | active, not recruiting | compared to hydroxychloroquine and remdesivir; combined with IFN β-1a |
| Lopinavir/Ritonavir | NCT04330690 | 440 | recruiting | compared to standard care |
| Lopinavir/Ritonavir | NCT04321993 | 1000 | recruiting | compared to baricitinib, hydroxychloroquine and sarilumab |
| Losartan | NCT04606563 | 1372 | recruiting | compared to standard of care |
| Losartan | NCT04328012 | 100 | recruiting | compared to placebo |
| Losartan (Geriak et al., 2021) | NCT04340557 | 200 | completed, has results | – |
| Losmapimod | NCT04511819 | 410 | active | compared to placebo |
| LY3127804 | NCT04342897 | 200 | terminated | – |
| LY3819253 | NCT04501978 | 10,000 | recruiting | compared to remdesivir, VIR-7831, BRII-196/BRII-198, AZD7442, MP0420, placebo |
| LY3819253 | NCT04427501 | 577 | recruiting | monotherapy or combined with LY3832479; compared to placebo |
| MAD0004J08 | NCT04952805 | 800 | recruiting | compared to placebo |
| Mavrilimumab | NCT04447469 | 588 | recruiting | compared to placebo |
| Mefloquine | NCT04347031 | 320 | completed | monotherapy or combined with azithromycin +/- tocilizumab; compared to HCQ; HCQ + azithromycin +/- tocilizumab |
| Meplazumab | NCT04275245 | 28 | completed | – |
| Mesenchymal Stem Cells | NCT04366063 | 60 | recruiting | compared to standard of care |
| Mesenchymal Stromal Cells | NCT04371393 | 223 | active | compared to placebo |
| Metenkefalin | NCT04374032 | 120 | completed | combined with tridecactide; compared to standard of care |
| Metformin | NCT04510194 | 1160 | recruiting | combined and compared with ivermectin, fluvoxamine, placebo |
| Methylprednisolone | NCT04673162 | 260 | not yet recruiting | compared to standard of care |
| Methylprednisolone | NCT04438980 | 72 | completed | compared to placebo |
| Methylprednisolone | NCT04636671 | 680 | recruiting | compared to dexamethasone |
| Methylprednisolone | NCT04244591 | 80 | completed | compared to standard of care |
| Methylprednisolone | NCT04263402 | 100 | recruiting | – |
| Methylprednisolone | ChiCTR2000029386 | 48 | recruiting | compared to standard treatment |
| Methylprednisolone | ChiCTR2000029656 | 100 | not yet recruiting | compared to standard treatment |
| Methylprednisolone | NCT04244591 | 80 | completed | compared to standard treatment |
| Methylprednisolone | NCT04273321 | 400 | completed | compared to standard treatment |
| Methylprednisolone | NCT04323592 | 104 | completed, has results | compared to standard treatment |
| Molixan | NCT04780672 | 330 | recruiting | compared to placebo |
| Molnupiravir | NCT04575584 | 304 | active | compared to placebo |
| Molnupiravir | NCT04575597 | 1850 | recruiting | compared to placebo |
| Montelukast | NCT04389411 | 600 | not yet recruiting | compared to placebo |
| MultiStem | NCT04367077 | 400 | recruiting | compared to placebo |
| NA-831 | NCT04452565 | 525 | recruiting | monotherapy or combined with atazanavir or dexamethasone; compared to atazanavir + dexamethasone |
| N-acetylcysteine | NCT04792021 | 60 | recruiting | compared to standard of care |
| Nafamostat Mesilate | NCT04390594 | 186 | recruiting | compared to standard of care |
| Nafamostat Mesilate | NCT04483960 | 2400 | recruiting | compared to standard of care |
| Nafamostat Mesilate | NCT04352400 | 256 | recruiting | compared to placebo |
| Nafamostat Mesilate | NCT04473053 | 60 | recruiting | compared to TD139, standard of care |
| Nangibotide | NCT04429334 | 730 | recruiting | compared to placebo |
| Naproxen | NCT04325633 | 584 | terminated | compared to standard treatment |
| Neurokinin-1 Receptor | NCT04468646 | 100 | recruiting | compared to placebo |
| Niagen | NCT04809974 | 100 | recruiting | compared to placebo |
| Niclosamide | NCT04558021 | 200 | recruiting | compared to placebo |
| Niclosamide | NCT04603924 | 436 | recruiting | compared to placebo |
| Nintedanib | NCT04541680 | 250 | recruiting | compared to placebo |
| Nintedanib | NCT04619680 | 120 | recruiting | compared to placebo |
| Nitazoxanide | NCT04486313 | 1092 | completed | compared to placebo |
| Nitazoxanide | NCT04423861 | 380 | not yet recruiting | compared to placebo |
| Nitazoxanide | NCT04392427 | 100 | not yet recruiting | combined with ribavirin and ivermectin; compared to standard of care |
| Nitazoxanide | NCT04382846 | 160 | recruiting | compared to standard of care |
| Nitazoxanide | NCT04523090 | 440 | recruiting | compared to placebo |
| Nitazoxanide | NCT04463264 | 135 | recruiting | compared to placebo |
| Nitazoxanide | NCT04920838 | 600 | recruiting | combined with ciclesonide; compared to paracetamol, telmisartan |
| Nitazoxanide | NCT04341493 | 86 | terminated | compared to hydroxychloroquine |
| Nivolumab | NCT04343144 | 92 | not yet recruiting | compared to standard treatment |
| Novaferon | NCT04669015 | 914 | recruiting | compared to placebo |
| Octagam | NCT04400058 | 208 | completed | compared to placebo |
| Octagam | NCT04411667 | 34 | completed | compared to standard of care |
| Omega 3 | NCT04553705 | 200 | recruiting | combined with sativa oil, Indian Costus, quinine pills, anise seed capsules |
| Opaganib | NCT04467840 | 475 | completed | compared to placebo |
| Oseltamivir | NCT04255017 | 400 | recruiting | compared to lopinavir/ritonavir and umifenovir |
| Oseltamivir | NCT04261270 | 60 | recruiting | compared to ASC09 and ritonavir |
| Oseltamivir | NCT04303299 | 80 | recruiting | compared to favipiravir, lopinavir/ritonavir and standard treatment; combined with chloroquine, darunavir/ritonavir and lopinavir/ritonavir |
| Ozone therapy | NCT04359303 | 50 | not yet recruiting | compared to standard of care |
| Ozone therapy | NCT04370223 | 208 | not yet recruiting | compared to standard of care |
| P2Et | NCT04410510 | 100 | recruiting | compared to placebo |
| Pacritinib | NCT04404361 | 200 | active | compared to placebo |
| Palmitoylethanolamide | NCT04568876 | 40 | recruiting | compared to standard of care |
| PD-1 monoclonal antibody | ChiCTR2000030028 | 40 | not yet recruiting | compared to standard treatment |
| PD-1 monoclonal antibody | NCT04268537 | 120 | not yet recruiting | compared to standard treatment and thymosin |
| Peginterferon Lambda-1a | NCT04331899 | 120 | completed, has results | doi: 10.1038/s41467−021-22177−1 |
| Peginterferon α-2a | NCT04291729 | 11 | completed | compared to darunavir/ritonavir, IFN α and lopinavir/ritonavir |
| Piclidenoson | NCT04333472 | 40 | recruiting | compared to standard treatment |
| Pioglitazone | NCT04535700 | 76 | recruiting | compared to standard of care in DM2 patients |
| Pirfenidone | NCT04282902 | 294 | recruiting | compared to standard of care |
| Plitidepsin | NCT04784559 | 609 | recruiting | combined with dexamethasone; compared to remdesivir + dexamethasone |
| Polyinosinic polycytidylic acid | ChiCTR2000029776 | 40 | compared to standard treatment | |
| Propolis extract | NCT04800224 | 200 | recruiting | compared to placebo |
| Proxalutamide | NCT04869228 | 724 | not yet recruiting | compared to placebo |
| Proxalutamide | NCT04853134 | 200 | active | compared to standard of care |
| Proxalutamide | NCT04728802 | 645 | completed | compared to placebo |
| Proxalutamide | NCT04870606 | 668 | recruiting | compared to placebo |
| Psidii guava | NCT04810728 | 90 | completed | compared to standard of care |
| PTC299 | NCT04439071 | 380 | recruiting | compared to placebo |
| PTC299 | NCT04439071 | 380 | recruiting | compared to standard of care |
| PUL-042 | NCT04312997 | 100 | completed | – |
| PVP-I | NCT04872686 | 798 | recruiting | compared to placebo |
| Pyridostigmine Bromide | NCT04343963 | 436 | recruiting | compared to placebo |
| Pyronaridine-artesunate | NCT04701606 | 402 | recruiting | compared to placebo |
| Quercetin | NCT04468139 | 60 | recruiting | combined with Zinc, Vitamin C, bromelain; single group assessment |
| Quercetin phytosome | NCT04578158 | 152 | completed | compared to standard of care |
| Radiation Therapy | NCT04433949 | 52 | recruiting | compared to standard of care |
| Ramdicivir | NCT04693026 | 150 | recruiting | combined with baricitinib; compared to remdesivir + tocilizumab |
| Ravulizumab | NCT04390464 | 1167 | recruiting | compared to baricitinib, standard of care |
| Ravulizumab | NCT04369469 | 270 | active | compared to standard of care |
| REGN10933+REGN10987 | NCT04425629 | 6420 | recruiting | compared to placebo |
| REGN10933+REGN10987 | NCT04452318 | 3750 | active | compared to placebo |
| Remdesivir | NCT04843761 | 640 | recruiting | compared to aviptadil, steroids, placebo |
| Remdesivir | NCT04853901 | 77 | completed | compared to standard of care |
| Remdesivir | NCT04647669 | 100 | not yet recruiting | compared to acalabrutinib, IFN β-1a, standard of care |
| Remdesivir | NCT04779047 | 150 | recruiting | compared to HCQ, tocilizumab, lopinavir / ritonavir, ivermectin |
| Remdesivir | NCT04745351 | 1116 | recruiting | compared to standard of care |
| Remdesivir | NCT04610541 | 2000 | active | single group assignment |
| Remdesivir | NCT04431453 | 52 | recruiting | single group assignment |
| Remdesivir | NCT04575064 | 400 | active | compared to standard of care |
| Remdesivir | NCT04345419 | 200 | completed | compared to standard of care |
| Remdesivir | NCT04315948 | 2416 | active | compared to lopinavir/ritonavir, lopinavir / ritonavir + IFN β-1a, HCQ, AZD7442, standard of care |
| Remdesivir | 2020−000936-23 (EU-CTR) | 3000 | ongoing | compared to IFN β-1a and lopinavir/ritonavir |
| Remdesivir | NCT04252664 | 308 | suspended | – |
| Remdesivir | NCT04257656 | 453 | terminated | – |
| Remdesivir (Beigel et al., 2020) | NCT04280705 | 394 | completed, has results | – |
| Remdesivir (Spinner et al., 2020) | NCT04292730; 2020−000842-32 (EU-CTR) | 600 | completed, has results | compared to standard treatment |
| Remdesivir (Goldman et al., 2020) | NCT04292899; 2020−000841-15 (EU-CTR) | 400 | completed, has results | compared to standard treatment |
| Remdesivir | NCT04315948 | 3100 | active, not recruiting | compared to hydroxychloroquine, IFN β-1a and lopinavir/ritonavir |
| Remdesivir | NCT04321616 | 700 | recruiting | compared to hydroxychloroquine and standard treatment |
| Remdesivir + Baricitinib | NCT04832880 | 4000 | not yet recruiting | combined with dexamethasone; compared to remdesivir + dexamethasone, baricitinib + dexamethasone, dexamethasone |
| Remdesivir + Tocilizumab | NCT04678739 | 205 | completed | compared to standard of care |
| Reparixin | NCT04878055 | 312 | recruiting | compared to placebo |
| Reparixin | NCT04878055 | 312 | recruiting | compared to placebo |
| RESP301 | NCT04460183 | 300 | recruiting | compared to standard of care |
| RhACE2 APN01 | NCT04335136 | 200 | completed | – |
| rhG-CSF (Cheng et al., 2021) | ChiCTR2000030007 | 200 | completed, has results | compared to standard treatment |
| Ribavirin | ChiCTR2000030922 | 30 | recruiting | combined with IFN α-2a and umifenovir |
| Ritonavir | ChiCTR2000030113 | 20 | recruiting | compared to favipiravir |
| RO7496998 | NCT04889040 | 1386 | recruiting | compared to placebo |
| RPH-104 | NCT04380519 | 372 | completed | compared to olokizumab, placebo |
| rSIFN-co | ChiCTR2000029638 | 100 | recruiting | compared to IFN α |
| Ruconest | NCT04705831 | 40 | recruiting | compared to placebo |
| Ruxolitinib | NCT04362137 | 432 | completed | compared to placebo |
| Ruxolitinib | NCT04338958 | 200 | recruiting | – |
| Ruxolitinib | NCT04331665 | 64 | completed | – |
| Sargramostim | NCT04326920 | 80 | completed | compared to standard of care |
| Sargramostim | NCT04642950 | 60 | recruiting | compared to placebo |
| Sarilumab (Lescure et al., 2021a) | NCT04327388 | 300 | completed, has results | doi: 10.1016/S2213−2600(21)00099−0 |
| Sarilumab | NCT04322773 | 200 | terminated | compared to standard treatment and tocilizumab |
| Sarilumab | NCT04341870 | 60 | suspended | combined with azithromycin and HCQ; compared with sarilumab |
| Sarilumab | NCT04315298 | 400 | completed | – |
| Sarilumab | NCT04321993 | 1000 | recruiting | compared to baricitinib, HCQ, and lopinavir/ritonavir |
| SARS-CoV-2 Convalescent Plasma | NCT04372979 | 80 | recruiting | compared to standard plasma |
| SARS-CoV-2 Convalescent Plasma | NCT04432103 | 36 | not yet recruiting | parallel assignment - two groups depending on the stage of the disease |
| SCTA01 | NCT04644185 | 795 | recruiting | compared to placebo |
| Sildenafil | NCT04304313 | 10 | recruiting | single group assignment |
| Sildenafil | NCT04304313 | 10 | recruiting | – |
| Siltuximab | NCT04329650 | 100 | recruiting | compared to methylprednisolone |
| Silymarin | NCT04816682 | 30 | recruiting | compared to standard of care |
| Silymarin | NCT04394208 | 50 | recruiting | compared to placebo |
| Sirolimus | NCT04948203 | 60 | recruiting | parallel assignment - varying doses of sirolimus |
| Sirolimus | NCT04341675 | 30 | recruiting | – |
| SNG001 | NCT04732949 | 610 | recruiting | compared to placebo |
| Sodium Pyruvate | NCT04824365 | 60 | recruiting | compared to placebo |
| Sofosbuvir | NCT04535869 | 50 | recruiting | combined with daclatasvir |
| Sofosbuvir | NCT04460443 | 60 | recruiting | combined with ledipsavir; compared to sofosbuvir + daclatasvir, standard of care |
| Sofosbuvir | NCT04497649 | 100 | recruiting | combined with daclatasvir; compared to standard of care |
| Sofosbuvir + Daclatasvir | NCT04773756 | 54 | completed | single group assignment |
| Sofosbuvir + Ledipasvir | NCT04530422 | 250 | completed | compared to oseltamivir + HCQ + azithromycin |
| Sofosbuvir + Ledipasvir | NCT04498936 | 240 | completed | compared to nitazoxanide, standard of care |
| Sofosbuvir + Ledipasvir | NCT04460443 | 60 | recruiting | compared to sofosbuvir + daclatasvir, standard of care |
| Sofosbuvir/Daclatasvir (Simmons et al., 2021) | IRCT20200128 046294N2 | 70 | completed; has results | compared to standard treatment |
| Sotrovimab | NCT04913675 | 1020 | recruiting | i.v. administration versus i.m. administration |
| Spironolactone | NCT04424134 | 80 | recruiting | combined with bromhexine; compared to standard of care |
| Spironolactone | NCT04826822 | 440 | recruiting | combined with dexamethasone; compared to standard of care |
| Suleoxide | NCT04483830 | 243 | completed | compared to placebo |
| Tacrolimus | NCT04341038 | 84 | recruiting | compared to standard treatment; combined with methylprednisolone |
| Telmisartan | NCT04355936 | 400 | completed | compared to standard of care |
| Telmisartan | NCT04356495 | 820 | recruiting | compared to ciclesonide, IFN β-1b, vitamins |
| Tenofovir | NCT04685512 | 60 | completed | combined with emtricitabine; compared to standard of care |
| Tetrandrine | NCT04308317 | 60 | recruiting | compared to standard of care |
| Therapeutic Plasma Exchange | NCT04973488 | 38 | completed | compared to standard of care |
| Thymosin | ChiCTR2000029541 | 100 | not yet recruiting | combined with darunavir/cobicistat or lopinavir/ritonavir |
| Thymosin | ChiCTR2000029806 | 120 | recruiting | compared to camrelizumab and conventional treatment |
| Tigerase | NCT04459325 | 100 | completed | compared to standard of care |
| TJ003234 | NCT04341116 | 144 | recruiting | – |
| Tocilizumab | NCT04577534 | 88 | completed | compared to standard of care |
| Tocilizumab | NCT04730323 | 93 | completed | compared to methylprednisolone + standard of care |
| Tocilizumab | NCT04600141 | 308 | recruiting | combined with heparin |
| Tocilizumab | NCT04377750 | 500 | recruiting | compared to placebo |
| Tocilizumab | NCT04412772 | 300 | recruiting | compared to placebo |
| Tocilizumab | NCT04372186 | 388 | active | compared to placebo |
| Tocilizumab | NCT04409262 | 649 | completed | combined with remdesivir; compared to remdesivir + placebo |
| Tocilizumab | NCT04356937 | 243 | completed | compared to placebo |
| Tocilizumab | ChiCTR2000029765 | 188 | recruiting | compared to standard treatment |
| Tocilizumab | ChiCTR2000030196 | 60 | not yet recruiting | – |
| Tocilizumab | ChiCTR2000030442 | 100 | not yet recruiting | – |
| Tocilizumab | NCT04310228 | 150 | recruiting | compared to favipiravir; combined with favipiravir |
| Tocilizumab | NCT04315480 | 30 | active, not recruiting | – |
| Tocilizumab | NCT04317092 | 400 | active, not recruiting | – |
| Tocilizumab | NCT04339712 | 20 | completed | compared to anakinra |
| Tocilizumab | NCT04331808 | 240 | active, not recruiting | – |
| Tocilizumab | NCT04322773 | 200 | terminated | compared to sarilumab and standard treatment |
| Tocilizumab | NCT04335305 | 24 | recruiting | compared to standard treatment; combined with pembrolizumab |
| Tocilizumab | NCT04335071 | 100 | terminated | – |
| Tocilizumab | NCT04332913 | 30 | recruiting | – |
| Tocilizumab | NCT04332094 | 276 | recruiting | compared with azithromycin + hydroxychloroquine; combined with azithromycin + HCQ |
| Tocilizumab | NCT04331795 | 50 | recruiting | – |
| Tocilizumab | NCT04330638 | 342 | active, not recruiting | compared with anakinra and siltuximab; combined with anakinra and siltuximab |
| Tocilizumab (Rosas et al., 2021) | NCT04320615 | 330 | completed, has results | – |
| Tofacitinib | NCT04332042 | 50 | not yet recruiting | – |
| Tradipitant | NCT04326426 | 300 | enrolling by invitation | – |
| Traditional Chinese Medicine | NCT04323332 | 50 | not yet recruiting | compared to standard of care |
| Tranexamic acid | NCT04338126 | 60 | withdrawn | – |
| Tranexamic acid | NCT04338074 | 100 | terminated (lack of recruitment) | – |
| Tranilast | ChiCTR2000030002 | 60 | recruiting | compared to standard treatment |
| Triazavirin | ChiCTR2000030001 | 240 | recruiting | compared to standard treatment |
| Triazavirin (Riamilovir) | NCT04581915 | 420 | recruiting | compared to placebo |
| TY027 | NCT04649515 | 1305 | recruiting | compared to placebo |
| Ulinastatin | ChiCTR2000030779 | 100 | recruiting | compared to standard treatment |
| Umifenovir | NCT04350684 | 40 | recruiting | combined with IFN β-1a + lopinavir / ritonavir + HCQ + standard of care; compared to IFN β-1a + lopinavir / ritonavir + HCQ + standard of care |
| Umifenovir | ChiCTR2000029573 | 480 | recruiting | combined with IFN α and lopinavir/ritonavir |
| Umifenovir | ChiCTR2000029621 | 380 | recruiting | compared to standard treatment |
| Umifenovir | ChiCTR2000029993 | 40 | recruiting | – |
| Umifenovir (Chen et al., 2020) | ChiCTR2000030254 | 240 | completed, has results | compared to favipiravir |
| Umifenovir | NCT04252885 | 125 | completed | compared standard treatment and tolopinavir/ritonavir |
| Umifenovir | NCT04254874 | 100 | recruiting | combined with peginterferon α-2a |
| Umifenovir | NCT04255017 | 400 | recruiting | compared to lopinavir/ritonavir and oseltamivir |
| Umifenovir | NCT04273763 | 60 | active, not recruiting | combined with bromohexine, favipiravir and IFN α-2b |
| Upamostat | NCT04723537 | 310 | recruiting | compared to placebo |
| Valsartan | NCT04335786 | 651 | recruiting | compared to placebo |
| Valsartan | NCT04335786 | 651 | recruiting | – |
| VIR-7831 | NCT04545060 | 1360 | active | compared to placebo |
| Vitamin C | NCT04401150 | 800 | recruiting | compared to placebo |
| Vitamin D | NCT04411446 | 1264 | recruiting | compared to placebo |
| Vitamin D | NCT04536298 | 2700 | recruiting | compared to placebo |
| Vitamin D | NCT04641195 | 700 | recruiting | monotherapy or combined with Zinc; compared to Zinc, placebo |
| Vitamin D | NCT04385940 | 64 | recruiting | high dose vitamin D compared to low dose vitamin D |
| Vitamin D | NCT04636086 | 100 | recruiting | compared to placebo |
| Vitamin D | NCT04552951 | 80 | recruiting | compared to standard of care |
| Vitamin D | NCT04780061 | 200 | recruiting | compared to vitamin C + Zinc, vitamin K2 + D, triglyceride oil, microcrystalline cellulose |
| Vitamin D | NCT04579640 | 6200 | active | compared to standard of care |
| Vitamin D | NCT04482673 | 140 | recruiting | compared to standard of care |
| Vitamin D | NCT04502667 | 40 | recruiting | compared to standard of care |
| Vitamin D | NCT04386850 | 1500 | recruiting | compared to placebo |
| Vitamin D | NCT04344041 | 260 | completed | high dose vitamin D compared to low dose vitamin D |
| Vitamin D | NCT04621058 | 108 | recruiting | compared to placebo |
| XAV-19 | NCT04928430 | 722 | recruiting | compared to placebo |
| XC221 | NCT04940182 | 274 | recruiting | compared to placebo |
| XC221 | NCT04487574 | 118 | completed | compared to placebo |
| Zafirlukast | NCT04871828 | 66 | recruiting | compared to placebo |
| Zavegepant (BHV-3500) | NCT04346615 | 120 | recruiting | compared to placebo |
| Zinc | NCT04447534 | 200 | recruiting | combined with Chloroquine; compared to Chloroquine |
| Zinc | NCT04621461 | 3 | completed | compared to placebo |
Legend: ACE-I – Angiotensin Converting Enzyme Inhibitors, ARB – Angiotensin Receptor Blockers; ASA – acetylsalicylic acid, aspirin; HCQ – hydroxychloroquine; IFN – interferon.
Table 3.
COVID-19 – summary of World Health Organization (WHO), National Institute of Health, and Infectious Diseases Society of America guidelines (COVID-19 Treatment Guidelines Panel, 2021; Organization, 2021; Bhimraj et al., 2021).
| Drug | WHO | Dose | Patient condition |
|---|---|---|---|
| Baricitinib | N/A | 4 mg daily for 14 days or until hospital discharge (whichever is first) | Patients with SpO2 ≤ 94 % on room air and CRP ≥ 75 mg/L, and no invasive mechanical ventilation Patients with contraindications to receive dexamethasone or other corticosteroids |
| Dexamethasone | Recommended | 6 mg iv or per os daily for 10 days or until hospital discharge (whichever is first) | Patients with SpO2 ≤ 94 % on room air |
| Neutralizing antibodies (casirivimab/ imdevimab, or sotrovimab) | N/A | – | COVID-19 at high risk for progression |
| Remdesivir | Not recommended | 200 mg iv – 1st day one 100 mg iv daily - days 2−5 |
Patients with SpO2 ≤ 94 % on room air |
| Tocilizumab | Recommended | 4 – 8 mg/kg iv (single dose) | Patients with SpO2 ≤ 94 % on room air and CRP ≥ 75 mg/L |
| HCQ | Not recommended | N/A | N/A |
2. Vaccines
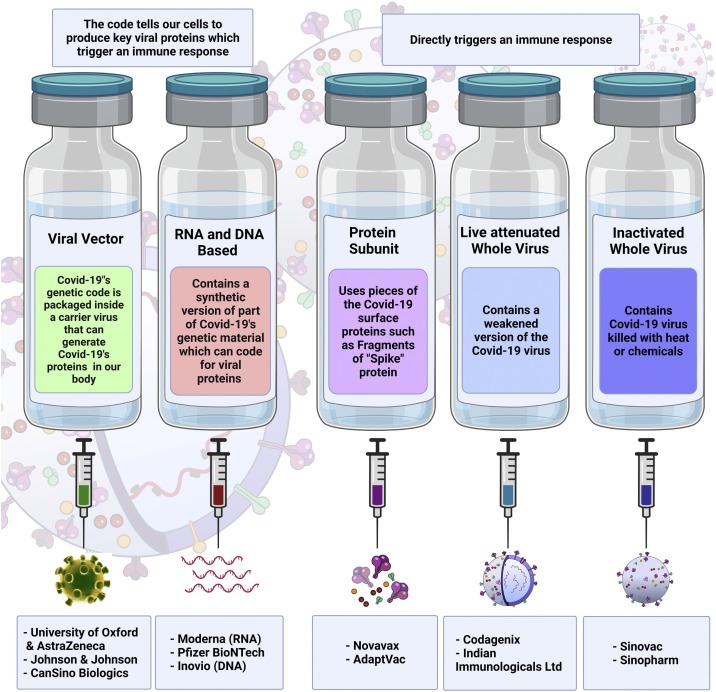
The introduction of COVID-19 vaccines in late 2020 has provided an opportunity to restrict the transmission of the SARS-CoV-2 virus and reduce the number of hospitalizations and deaths (Fig. 3 ). The US Food and Drugs Administration (FDA) has approved the Pfizer-BioNTech COVID-19 vaccine, Moderna COVID-19 Vaccine, and Janssen COVID-19 Vaccine for emergency use in the USA, while the European Medicines Agency (EMA) also authorized the vaccine developed by AstraZeneca. Furthermore, other vaccines are being used around the world and many more are still being developed. The efficacy and safety of the most frequently used vaccines are summarized in Table 4 . According to the WHO, almost 7.7 billion doses of vaccines have been administered and approximately 53.2 % of the world’s population have received at least the first vaccine dose. However, most vaccines were distributed in a small number of highly developed countries, leaving most of the developing world susceptible to SARS-CoV-2 infection. Furthermore, the data evaluating the efficacy of vaccines against VOC is limited and inconsistent, yet full vaccination appears to protect against a severe course of illness and death from all occurring VOCs (World Health Organization, 2021; Fontanet et al., 2021; Lopez Bernal et al., 2021). Moreover, multiple studies have shown waning immunity acquired after vaccination, especially in immunocompromised patients, for example those undergoing hemodialysis or cytotoxic cancer drug treatment. This contributes to an increasing number of breakthrough infections (Shroff et al., 2021; Juno and Wheatley, 2021; Goldberg et al., 2021; Fowlkes et al., 2021; Davidovic et al., 2021; Campo et al., 2021). Currently, several countries have developed various strategies to tackle this problem, among which, additional doses of COVID-19 vaccines have shown to be safe and efficient in boosting immune response (Yue et al., 2021; Falsey et al., 2021; Dekervel et al., 2021; Choi et al., 2021; Barros-Martins et al., 2021). Nonetheless, the low vaccination rate, coupled with the risk of emergence of vaccine-resistant SARS-CoV-2 variants and waning immunity, emphasizes the burning need to develop novel drugs and therapeutic modalities for COVID-19 (Artese et al., 2020; Twomey et al., 2020; Drożdżal et al., 2020).
Fig. 3.
Schematic representation of available anti-SARS-CoV-2 vaccines. The principle, main components and mechanism of action of each vaccine type has been explained in detail in the text.
Table 4.
Efficacy of FDA Approved Vaccines Against Selected Sars-Cov2 Variants (Gubbay et al., 2021).
|
Virus variant | |||
|---|---|---|---|
| Name of the vaccine | Alpha Variant (B.1.1.7) | Beta Variant (B.1.351) | Delta Variant (B.1.617.2) |
| Comirnaty (Pfizer BioNTech) | Vaccine effectiveness Vs symptomatic infection | Vaccine effectiveness Vs symptomatic infection | Vaccine effectiveness Vs symptomatic infection |
| Dose 1 | 95 % CI 64–68 % | 95 % CI 52–67 % | ∼56 % |
| Dose 2 | 95 % CI 86–91 % | 95 % CI 69–92 % | 95 % CI 64–95 % |
| Spikevax (Moderna) | Vaccine effectiveness Vs Hospitalization rate | Vaccine effectiveness Vs Hospitalization rate | Vaccine effectiveness Vs Hospitalization rate |
| Dose 1 | 95 % CI 80–86 % | 95 % CI 69–92 % | ∼78 % |
| Dose 2 | 95 % CI 86–96 % | No information | No information |
| Janssen COVID-19 Vaccine (Johnson & Johnson) | Vaccine effectiveness Vs symptomatic infection rate | Vaccine effectiveness Vs symptomatic infection rate | Vaccine effectiveness Vs symptomatic infection rate |
| Dose 1 | effective according to the manufacturer | effective according to the manufacturer | effective according to the manufacturer |
Legend: 95 % CI – 95 % confidence interval.
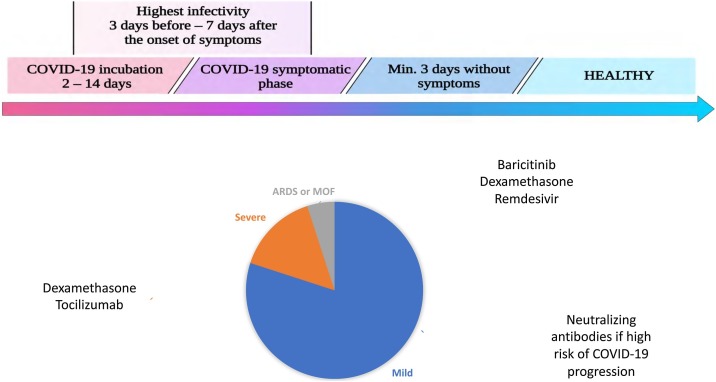
3. Recommended therapeutic agents/potential treatment
3.1. Monoclonal antibodies
Bamlanivimab (LY-CoV555) is a potent neutralizing IgG1 mAb against the SARS-CoV-2 spike protein. It is designed to block viral attachment and entry into human cells, thus neutralizing the virus and potentially preventing and treating COVID-19 (Anon, 2006; Jones et al., 2021).
Etesevimab (also known as JS016 or LY-CoV016) is a fully humanized recombinant neutralizing mAb that specifically binds to the SARS-CoV-2 surface protein receptor-binding domain (RBD) with high affinity and can effectively block virus binding to the host angiotensin converting enzyme 2 (ACE-2) receptor on the cell surface (Anon, 2006).
In a phase 3 study, Dougan et al., randomized a 1:1 cohort of outpatients with mild to moderate COVID-19, who were at high risk of progressing to severe disease, have received a single intravenous infusion of mAbs. This therapy was administered to patients at doses of 2800 mg (bamlanivimab) and 2800 mg (etesevimab) or a placebo within 3 days following laboratory diagnosis of SARS-CoV-2 infection. The primary endpoint was the overall clinical status of the patients, defined as hospitalization for COVID-19 or all-cause death by day 29. A total of 1035 patients participated in the study, with a mean age (± SD) of 53.8 ± 16.8 years. By day 29, a total of 11 out of 518 patients (2.1 %) in the bamlanivimab-etesevimab group were hospitalized or died from COVID-19, compared with 36 of 517 patients (7.0 %) in the placebo group [absolute risk difference = -4.8 percentage points (95 % CI: -7.4 – -2.3); relative risk difference = 70 %; p < 0.001]. There were no deaths in the bamlanivimab-etesevimab group, although there were 10 deaths in the placebo group, 9 of which were assessed by the investigators as related to COVID-19. At Day 7, there was a greater log reduction from baseline in viral load for patients who received bamlanivimab with etesevimab than for patients who received a placebo (p < 0.001). The authors of the study have concluded that in high-risk outpatients, the use of mAbs led to fewer hospitalizations and deaths associated with COVID-19 than with a placebo. Moreover, such therapy accelerated the decline in SARS-CoV-2 viral load (Dougan et al., 2021).
Gottlieb et al., in their randomized phase 2/3 BLAZE-1 trial, evaluated the effect of bamlanivimab monotherapy and combined therapy with etesevimab on SARS-CoV-2 virus load in mild to moderate COVID-19. The first group of patients received a single infusion of bamlanivimab, the second received both mAbs, and the third group received placebo. Compared to the placebo, the difference in log viral load-change at day 11 was statistically significant [-0, 57 (95 % CI: -1.00 – -0.14; p = 0.01)] only for combined therapy, and there were no deaths recorded during study treatment. The authors of the study concluded that in non-hospitalized patients with mild to moderate COVID-19 disease, treatment with bamlanivimab and etesevimab compared to a placebo was associated with a statistically significant reduction in SARS-CoV-2 viral load on day 11 (Gottlieb et al., 2021).
Sotrovimab (Xevudy, GlaxoSmithKline and Vir Biotechnology, Inc.) is a recombinant engineered human IgG1 mAb that binds to a highly conserved epitope on the S protein RBD of SARS-CoV-2 with high affinity, but it does not compete with human ACE-2 receptor binding (Anon, 2021). The efficacy of sotrovimab was evaluated in an interim analysis of the ongoing COMET-ICE study. Patients were treated with a single 500 mg infusion of sotrovimab (N = 291) or a placebo (N = 292) over 1 h. The median age of the overall randomized population was 53 years (range: 18–96). The clinical progression of COVID-19 at Day 29 in recipients of sotrovimab was reduced by 85 % compared with the placebo group (p = 0.002) (Anon, 2021).
Casirivimab (IgG1-κ) and imdevimab (IgG1-λ) are recombinant human mAbs, which are unmodified in the Fc regions. The mAbs bind to non-overlapping epitopes of the spike protein RBD of SARS-CoV-2, and thereby block binding to the human ACE-2 receptor (Anon, 2020). An ongoing phase 1–3 trial in non-hospitalized COVID-19 patients investigated the effect of the mix of these antibodies (REGN−COV2) to reduce the risk of developing a refractory mutant virus. Patients were randomly assigned (1:1:1) to receive a placebo, 2.4 g of REGN−COV2, or 8.0 g of REGN−COV2 and were prospectively characterized at baseline for the endogenous immune response against SARS−COV-2 (serum antibody-positive or serum antibody-negative). Key endpoints included the time-weighted average change in viral load from baseline (day 1) through day 7 and the percentage of patients with at least one COVID-19-related co-morbidity who attended a clinic visit through day 29. Data from 275 patients are reported; the least-squares mean difference (the combined REGN−COV2 dose groups vs. the placebo group) in the time-weighted average change in viral load from day 1 through day 7 was -0.56 log10 copies per milliliter (95 % CI: -1.02 – -0.11) among patients who were serum antibody-negative at baseline and -0.41 log10 copies per milliliter (95 % CI: -0.71 – -0.10) in the overall trial population. In this interim analysis, REGN−COV2 reduced viral load, and to a greater extent in patients whose immune response had not yet been initiated or who had a high viral load at baseline (Weinreich et al., 2021).
Tocilizumab (RoActemra, Roche Pharma AG) is a recombinant humanized IgG1 mAb that binds specifically to both soluble and membrane-bound receptors for IL-6 (sIL-6R and mIL-6R), thereby inhibiting this signaling pathway, and reducing the pro-inflammatory effect of IL-6 (Sebba, 2008). In their dissertation, Malgie et al., reviewed and performed a meta-analysis of observational studies evaluating the effect of tocilizumab on COVID-19 patient mortality. The authors included 10 studies related to the use of tocilizumab, totaling 1358 patients, with nine out of ten studies found to be of high quality. The meta-analysis showed that the mortality in the tocilizumab group was lower than in the control group [RR = 0.27 (95 % CI: 0.12 – 0.59); the risk difference = 12 % (95 % CI: 4.6%–20%)]. With only a few studies available, no difference in side effects has been observed. Mortality was 12 % lower in the group of patients who received tocilizumab compared to those who did not, although these results require confirmation in randomized controlled trials (RCTs) (Malgie et al., 2021).
In another review by Arthur et al., researchers analyzed 10 RCTs evaluating the effect of tocilizumab in COVID-19 in which they allocated patients to two groups. The control group received the standard care, while the treatment group was comprised of patients who received tocilizumab in addition to standard care; the primary outcome was 28 to 30-day mortality. Secondary endpoints included progression to severe disease, defined as the need for mechanical ventilation, intensive care unit (ICU) admission, or complex disease. Out of 6493 patients, 3358 (52.2 %) were allocated to tocilizumab. The results demonstrated that tocilizumab use was associated with decreased mortality [24.4 % vs. 29.0 %; odds ratio (OR) = 0.87 (95 % CI: 0.74–1.01); p = 0.07]. Tocilizumab did reduce the need for mechanical ventilation and was associated with an advantage in the composite secondary endpoint, but did not reduce the number of ICU admissions (Arthur et al., 2021).
However, the results of a phase 3 trial were contradictory. The NCT04320615 study described by Rosas et al., did not present a difference between tocilizumab and placebo groups [mortality at day 28 was 19.7 % – the tocilizumab group and 19.4 % – the placebo group (95 % CI = -7.6–8.2; p = 0.94)] (Rosas et al., 2021). A Study authors suggests considering the use of tocilizumab in hospitalized COVID-19 patients with hypoxia and laboratory signs of significant inflammation.
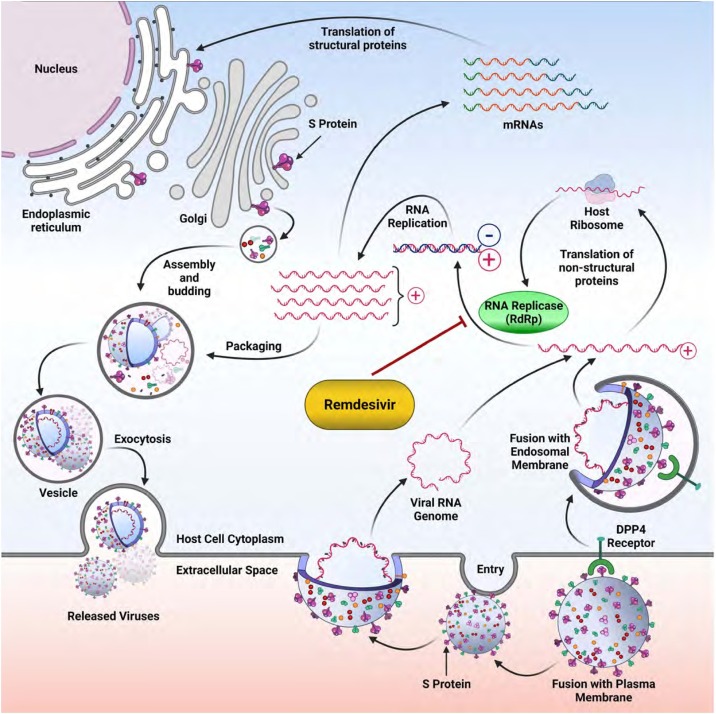
3.2. Remdesivir
Remdesivir is an adenosine analogue that is metabolized to its active metabolite, remdesivir triphosphate. Remdesivir triphosphate is a structural analogue of adenosine triphosphate (ATP) and competes with the natural substrate for the incorporation by RNA polymerase into nascent viral RNA, which results in delayed chain termination during replication and consequently inhibition of viral replication (Fig. 4 ) (Singh et al., 2020).
Fig. 4.
The viral cycle of SARS-CoV-2 and the Remdesivir target. Remdesivir is an inhibitor of the RNA-replicase (RdRp), therefore inhibition of this enzyme impairs the replication of the viral genome and hence, blocks the life cycle of the whole virus, or renders it defective.
One of the most recent and largest studies that describes the effectiveness of remdesivir in SARS-CoV-2 infection reports that despite its conditional recommendation, remdesivir may still be effective in achieving early clinical improvement. It reduces early-stage mortality and the need for high flow oxygen supplementation and invasive mechanical ventilation among hospitalized COVID-19 patients. Treatment with remdesivir was associated with an increase in clinical recovery rate by 21 % [risk ratio (RR) = 1.21 (95 % CI: 1.08–1.35)] on day 7 and 29 % [RR = 1.29 (95 % CI: 1.22–1.37)] on day 14. The likelihoods of requiring high-flow supplemental oxygen and invasive mechanical ventilation in the remdesivir group were lower than in the placebo group by 27 % [RR = 0.73 (95 % CI: 0.54 – 0.99)] and 47 % [RR = 0.53 (95 % CI: 0.39 – 0.72)], respectively. Remdesivir-treated patients showed a 39 % [(RR = 0.61 (95 % CI: 0.46 – 0.79)] reduction in the risk of mortality on day 14 compared to the control group; however, there was no significant difference on day 28 (Angamo et al., 2021). A Study authors suggests considering the use of remdesivir in patients with confirmed SARS-CoV-2 infection during the period of viral replication (i.e., not later than 5–7 days from the onset of the first symptoms of the disease) in patients with documented pneumonia and peripheral blood oxygen saturation (SpO2) ≤ 94 % (when breathing atmospheric air).
3.3. Baricitinib
Baricitinib is a selective inhibitor of janus activated kinase 1 (JAK1) and janus activated kinase 2 (JAK2), the two of which mediate signaling for cytokines and growth factors involved in hematopoiesis, inflammation, and the immune response. It modulates intracellular signaling by partially inhibiting JAK1 and JAK2 enzymatic activity, thereby reducing phosphorylation and activation of STAT proteins. Baricitinib inhibits the induction of IL-6 in a dose dependent manner while also reducing the serum concentration of C-reactive protein (CRP) (Stebbing et al., 2020).
In a multi-center study, the beneficial impact of baricitinib was tested in COVID-19 patients with moderate pneumonia (Cantini et al., 2020). At baseline, 113 patients were included in the baricitinib-arm, and 78 in the control-arm. The results indicate that the 2-week case fatality rate was significantly lower in the baricitinib-arm compared with controls [0% (0/113) vs. 6.4 % (5/78) (p = 0.010; 95 % CI: 0.0000 – 0.4569)]. ICU admission was necessary in 0.88 % (1/113) patients in the baricitinib-arm compared to the 17.9 % (14/78) in the control-arm in week 1 (p = 0.019; 95 % CI: 0.0092 – 0.6818), and week 2 (p < 0.0001; 95 % CI: 0.0038 – 0.2624). Discharge rate was significantly higher in the baricitinib-arm at week 1 [9.7 % (11/113) vs. 1.3 % (1/78); p = 0.039; 95 % CI: 1.41–90.71], and at week 2 [77.8 % (88/113) vs. 12.8 % (10/78); p < 0.0001; 95 % CI: 10.79–51.74] (Cantini et al., 2020). In a randomized trial, Marconi et al., demonstrated that baricitinib may be an important drug that can be used in patients hospitalized for COVID-19 (Marconi et al., 2021). The 60-day all-cause mortality was 10 % (= 79) for baricitinib and 15 % (n = 116) for placebo (HR 0.62 [95 % CI 0.47–0.83]; p = 0.0050). The use of this drug did not significantly increase the side effects (Marconi et al., 2021). The authors of this study recommend the use baricitinib in hospitalized patients diagnosed with COVID-19 with moderate and severe disease.
3.4. Tofacitinib
Tofacitinib is a potent and selective inhibitor of the JAK family of kinases. Tofacitinib has been shown to inhibit the activity of JAK1, JAK2, and JAK3, and to a lesser extent tyrosine-protein 2 kinases (TyK2). In human cells, tofacitinib inhibits the signaling of heterodimeric cytokine receptors which bind JAK3 and/or JAK1, and that possess greater functional selectivity than that of cytokine receptors that signal through JAK2 kinase pairs. Inhibition of JAK1 and JAK3 kinases by tofacitinib attenuates interleukin signaling (IL-2, IL-4, IL-6, IL-7, IL-9, IL-15, and IL-21), as well as interferon type I and type II signaling, resulting in modulation of the immune response (Maeshima et al., 2012).
Guimarães et al., assessed the efficacy and safety of tofacitinib in patients hospitalized for coronavirus pneumonia. Two groups of adult patients (n = 289 in total) with COVID-19 pneumonia were randomized in a 1:1 ratio, receiving either 10 mg of tofacitinib or a placebo twice daily for up to 14 days or until hospital discharge. Efficacy was assessed after 28 days and examined the death or respiratory failure rate. Furthermore, 89.3 % of patients were receiving glucocorticoids during their hospitalization. The cumulative incidence of death or respiratory failure up to day 28 was 18.1 % in the tofacitinib group and 29.0 % in the placebo group (hazard ratio (HR) = 0.63; 95 % CI: 0.41 – 0.97; p = 0.04). By day 28, death from any cause had occurred in 2.8 % of patients in the tofacitinib group and in 5.5 % of patients in the placebo group (HR = 0.49; 95 % CI: 0.15–1.63). The authors summarized the study by stating that among patients hospitalized with COVID-19 pneumonia, tofacitinib led to a decrease in the risk of death or respiratory failure by day 28 in comparison with a placebo (Gunay et al., 2021). According to the authors, the use of tofacitinib in hospitalized patients diagnosed with COVID-19 may be considered.
3.5. Application of autophagy and UPR in targeting SARS-CoV-2 infection
The endoplasmic reticulum (ER) is the site of both protein translation and protein folding (Sureda et al., 2020). However, if the protein load that is shuffled into the ER exceeds its folding capacity, there is an accumulation of unfolded proteins which triggers the ER stress response, and activates a pathway known as the unfolded protein response (UPR) (Almanza et al., 2019). UPR aims to improve ER folding capacity by reducing global protein synthesis and inducing molecular chaperone expression (Hombach-Klonisch et al., 2018). However, if ER stress is not resolved, UPR directs the cell towards programmed cell death (Mehrbod et al., 2019).
Multiple studies have shown that CoV replication in the cytoplasm directly induces ER stress, leading to the activation of UPR in infected cells. As an intricate interplay between UPR and the inflammatory response, apoptosis, autophagy, and innate immunity exists, ER stress can significantly affect the patient’s antiviral response (Fung and Liu, 2019; Shi et al., 2019). Recent evidence suggests that upon coronavirus infection, ER stress and UPR are induced by excessive synthesis, modification, and folding of viral proteins that results in ER membrane restructuring and its subsequent exhaustion due to continued formation of new virions (Fung et al., 2014; Fung and Liu, 2014). Moreover, some members of the coronaviridae family are capable of utilizing certain aspects of UPR to overcome protein translation shutdown and ensure the production of their own proteins (Fung et al., 2016). Moreover, in severe COVID-19 cases, hypoxemia may trigger a response from both mitochondria and ER, which is directed towards restoring oxygen level and promoting cell survival (Bartoszewska and Collawn, 2020). However, if this state persists, the role of UPR would then be altered from pro-survival to induction of apoptosis, which is possibly one of the molecular causes of organ damage in COVID-19 (Sureda et al., 2020).
Unsurprisingly, multiple therapeutic drug candidates for COVID-19 infection are autophagy modulators. It is therefore possible that the beneficial effect of these drugs is perhaps due to the over-accumulation of autophagosomes that can induce apoptotic cell death of virally infected cells (Shojaei et al., 2020). Further research exploring CoV-induced UPR could help identify novel therapeutic targets that are based directly on the pathogenesis of the disease.
Studies exploring UPR reveal that the inositol-requiring enzyme 1 (IRE1) axis is involved in the regulation of the secretome of cells via production of spliced XBP (Logue et al., 2018). Moreover, SARS-CoV activates NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasomes in macrophages as well as induces UPR through its Open Reading Frame-8b (ORF-8b) (Shi et al., 2019). The latter is involved in autophagy flux activation and cytokine processing. Hence, targeting the RNase activity of IRE1 could potentially modulate COVID-19 infection via modulation of the macrophage secretome.
In another study, SARS-CoV activated the protein kinase R-like reticulum kinase (PERK) arm of UPR, thereby increasing the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α). As PERK activation suppresses type 1 interferon signaling, it could be a potential mechanism through which innate immunity is suppressed in CoV infected cells (Minakshi et al., 2009). Therefore, PERK inhibitors could potentially aid in halting SARS-CoV-2 infection.
3.5.1. Paxlovid
Paxlovid is a therapeutic combination consisting of two compounds: PF-07,321,332, an oral covalent 3CL protease inhibitor of SARS-CoV-2 and ritonavir, an inhibitor of HIV-1 and HIV-2 protease. Ritonavir is also an inhibitor of cytochrome P450 3A and CYP2D6, thus inhibiting the metabolism of PF-07,321,332 and allowing the administration of a lower dose of the substance. In contrast, P-07,321,332 binds to the catalytic cysteine residue of CyS145 in all coronavirus proteases infecting humans (Mahase, 2021a).
In a recent study, the participants were randomized 1:1; half of which received paxlovid and the other half received placebo administered orally every 12 h for five consecutive days (Mahase, 2021b). The study revealed that among patients who were treated with paxlovid within three days of symptom onset, 3 out of 339 (0.8 %) participants were admitted to hospital by day 28 after randomization and no deaths were reported. In comparison, 7% (27/385) of patients who received placebo were admitted to the hospital, with seven deaths reported. The statistical significance of these results was assessed as high (p < 0.0001). In subjects treated within five days of symptom onset, 1% (6/607) of those treated with paxlovid were admitted to hospital by day 28 compared to 6.7 % (41/612) of patients in the placebo group. Up to day 28, no deaths were reported in the paxlovid group as compared to 10 deaths (1.6 %) in the placebo group (Mahase, 2021b).
3.5.2. Molnupiravir
The mechanism of action of molnupiravir (Lagevrio) is based on a novel approach to fighting viruses. The compound is converted in the patient's body into a synthetic cytidine nucleoside. It then introduces errors into the genetic material of the viruses RNA as it replicates. The mutations lead to defective viral elements, hence neutralizing the pathogen, ultimately exerting an antiviral effect (Painter et al., 2021). Among 202 participants of a recent study, significantly lower number of participants receiving 800 mg dose of molnupiravir (1.9 %) were carried virus that could be isolated, as compared to placebo (16.7 %) at day 3 (p = 0.02). At day 5, virus could not be isolated from any participants receiving 400 or 800 mg molnupiravir, versus 11.1 % of those receiving placebo (p = 0.03). Molnupiravir was generally well tolerated, with similar adverse events across all groups (Fischer et al., 2021).
3.5.3. Regdanvimab
Regdanvimab (Regkirona) is a recombinant human IgG1 monoclonal antibody. The mechanism of action for regdanvimab in treating patients with SARS-CoV-2 infection is binding of regdanvimab to the receptor binding domain (RBD) of the spike(s) protein of SARS-CoV-2 with dissociation constant KD = 0.065 nM, thus, inhibiting the interaction between the SARS-CoV-2 RBD and the cellular receptor, namely the angiotensin-converting enzyme 2 (ACE2), and consequently blocking cellular entry and SARS-CoV-2 infection. Regkirona is recommended for treating COVID-19 in adults who do not require supplemental oxygen and who are at increased risk of their disease becoming severe (European Medicines Agency, 2021). The main study in patients with COVID-19 showed that Regkirona treatment led to fewer patients requiring hospitalizations or oxygen therapy or dying when compared with placebo. Among the patients at increased risk of their illness becoming severe, 3.1 % of patients treated with Regkirona (14 out 446) were hospitalized, required supplemental oxygen or died within 28 days of treatment compared with 11.1 % of patients on placebo (48 out of 434) (Kreuzberger et al., 2021).
3.5.4. Anakinra
Anakinra (Kineret) inhibits the biological activity of interleukin 1. It counteracts the production of NO, PGE2 and collagenase in the synovium, fibroblasts and chondrocytes. A systematic review and patient-level meta-analysis performed by Kyriazopoulou et al. examined pooled data for 1185 patients from nine studies, as well as individual patient data for 895 patients from six of the analyzed studies (Kyriazopoulou et al., 2021). Eight trials were observational studies, and one was a randomized controlled trial. The data taken into account were age, comorbidities, baseline partial pressure of oxygen in arterial blood, the ratio of arterial partial pressure of oxygen divided by inspired fraction of oxygen (PaO2/FiO2), C-reactive protein and lymphopenia. The mortality was significantly lower in patients treated with anakinra (38 [11 %] out of 342 patients) as compared with subjects receiving standard care with or without placebo (137 [25 %] out of 553; adjusted odds ratio [OR] 0.32 [95 % CI 0.20−0.51]). The mortality benefit was comparable between all subgroups, regardless of existing comorbidities, levels of ferritin l, or baseline PaO2/FiO2. Anakinra was more effective in reducing mortality in patients with a C-reactive protein concentration exceeding 100 mg/l (OR 0.28 [95 % CI 0.17−0.47]). Anakinra showed significant improvement in survival when administered without dexamethasone (OR 0.23 [95 % CI 0.12−0.43]), but not with additional dexamethasone (0.72 [95 % CI 0.37–1.41]). The use of anakinra, as compared to standard of care was not associated with a significantly increased risk of secondary infections (OR 1.35 [95 % CI 0.59–3.10]) (Kyriazopoulou et al., 2021).
3.5.5. Sotrovimab
Sotrovimab (Xevudy, also known as VIR-7831 and GSK4182136) is a monoclonal antibody with an activity against COVID-19. Sotrovimab was designed to attach to S protein of SARS-CoV-2. When it binds to S protein, the ability of the virus to enter the cells of the body are reduced. This is expected to reduce both the severity of the disease and need for hospitalization in COVID-19 (Sotrovimab, 2021). One article reported that the drug was administered at a dose of 500 mg or placebo. The primary efficacy outcome was hospitalization exceeding 24 h for any cause or death within 29 days of randomization. In this pre-specified interim analysis, which included an intention-to-treat population of 583 patients (291 in the sotrovimab group and 292 in the placebo group), 3 patients (1%) in the sotrovimab group, as compared with 21 patients (7%) in the placebo group, experienced disease progression leading to hospitalization or death (relative risk reduction, 85 %; 97.24 % confidence interval, 44–96; p = 0.002). In the placebo group, 5 patients were admitted to the ICU, including 1 who died by day 29. The safety assessment was performed in 868 patients (430 in the sotrovimab group and 438 in the placebo group). The adverse events were reported in 17 % of subjects in the sotrovimab group and 19 % of those in the placebo group; serious adverse events were less common with sotrovimab than with placebo (in 2% and 6% of the patients, respectively) (Gupta et al., 2021).
3.5.6. Tixagevimab and cilgavimab
Tixagevimab and cilgavimab (Evusheld), two monoclonal antibodies have been designed to attach to the spike protein of SARS-CoV-2 at two different sites. By attaching to the spike protein, the medicine is expected to stop the virus from entering the body’s cells and causing infection. Because the antibodies attach to different parts of the protein, using them in a combination may be more effective than using either of them alone. The results of a recent trial funded by Astra Zeneca met the primary endpoint, with a dose of 600 mg of AZD7442 given by intramuscular (IM) injection reducing the risk of developing severe COVID-19 or death (from any cause) by 50 % compared to placebo in outpatients who had been symptomatic for seven days or less. The trial recorded 18 events in the AZD7442 arm (18/407) and 37 in the placebo arm (37/415). The LAAB was generally well tolerated in the trial. In a pre-specified analysis of participants who received treatment within five days of symptom onset, AZD7442 reduced the risk of developing severe COVID-19 or death (from any cause) by 67 % compared to placebo, with nine events in the AZD7442 arm (9/253) and 27 in the placebo arm (27/251) (AstraZeneca, 2021).
4. Other agents tested for potential efficacy in treating COVID-19 infection
4.1. Hydroxychloroquine
During the early days of the COVID-19 pandemic, many scientists and physicians placed hope in hydroxychloroquine (HCQ) and other antimalarial drugs. Moreover, non-randomized studies describing the positive effects of this drug are cited more often than any subsequent randomized trials about its lack of clinical benefit or even harmful side-effects (Bellos, 2021). With time, the severity of adverse effects and long-term consequences of HCQ treatment were elucidated (Drożdżal et al., 2020; Diaz-arocutipa and Hernandez, 2021). HCQ used both in monotherapy and in combination with azithromycin has been shown to increase the prevalence of a prolonged QTc as a side effect. An association with higher incidence of arrhythmias has not been demonstrated, although this is possibly due to underestimated reporting frequency72].
According to studies with a high level of certainty surrounding their evidence, HCQ does not reduce mortality in patients with COVID-19 (Self et al., 2020; Kashour et al., 2021). Moreover, a meta-analysis performed by Axfors et al., showed that patients had an all-cause combined mortality OR of 1.11 for hydroxychloroquine (95 % CI: 1.02–1.20) (Axfors et al., 2021). The effect of pharmacological prophylaxis in COVID-19 has also been disputed. Bartoszko et al., showed that taking HCQ has practically no effect on hospital admission or mortality, but it significantly increased the incidence of side effects. A meta-analysis of the available RCTs demonstrated no positive effects of the drug, but instead the incidence of side effects increased [RR = 1.81 (95 % CI: 1.36–2.42); p < 0.05] (Bartoszko et al., 2021). The study authors, do not recommend the use of chloroquine and hydroxychloroquine for either post-exposure prophylaxis or the treatment of COVID-19.
4.2. Colchicine
Colchicine may play a role in reducing the symptoms of COVID-19, as it binds to b-tubulin hence blocking microtubule polymerization. This in turn affects the spindle, and therefore reduces the movement and degranulation of intracellular lysosomes and the release of lysozymes, chemoattractants, and lactic acid. It inhibits the phagocytosis of sodium urate crystals by leukocytes, and reduces the breakdown of leukocyte cell membranes through their mobilization, migration, and the ability to adhere (Leung et al., 2015). It is characterized by anti-inflammatory effects achieved through a reduction of leukocyte migration, inhibition of endothelial adhesion, reduction in interleukin production, and cytokine storm prevention (Vitiello and Ferrara, 2021). Colchicine is a powerful anti-inflammatory agent routinely used to treat gout, viral pericarditis, coronary artery disease, and familial Mediterranean fever. Golpour et al., in a meta-analysis analyzed the effect of colchicine on the treatment of COVID-19. Colchicine was shown to be responsible for reducing mortality and length of hospitalization, and may therefore be an effective therapeutic option to improve COVID-19 treatment (Golpour et al., 2021).
4.3. Convalescent plasma
The concept of using convalescent plasma in the treatment of COVID-19 was enthusiastically received by clinicians, internationally. The premise was based on the theory that antibodies produced by convalescent patients would help the recipients’ body combat the infection and improve their prognosis. The initial results were very promising, but the intervention group not only included COVID-19 patients, but also those with SARS, MERS, and influenza (Aviani et al., 2021). In a meta-analysis of COVID-19 patients, Bansal et al., showed that adding convalescent plasma to the standard of care reduced mortality among patients (Bansal et al., 2021a). A second meta-analysis by Janiaud et al., did not demonstrate the beneficial effect of administering convalescent plasma to patients (Janiaud et al., 2021). Furthermore, Prasad et al., considered the most recent data in both randomized clinical trials and cohort studies, suggesting a possible weak association, although underlined the need for further randomized trials (Prasad et al., 2021). Finally, Korley et al., published the results of a recent trial investigating the effect of convalescent plasma on the progression of COVID-19 in high-risk patients (n = 511). This study showed no effect on disease progression and length of hospitalization (Korley et al., 2021). The study authors do not recommend the routine use of convalescent plasma in patients hospitalized with COVID-19.
4.4. Amantadine
Amantadine hydrochloride, a synthetic tricyclic amine, is an antiviral drug known since the 1960s for the treatment of influenza A. It works by blocking M2 ion channels, inhibiting viral entry into cells, and inhibiting viral replication (Raupp-Barcaro et al., 2018a).
A model was proposed by Abreu et al., in which amantadine blocks viroportin E of the SARS-CoV-2 virus, preventing the release of genetic material into the host nucleus (Aranda-Abreu et al., 2020). It was also shown to inhibit the replication of the virus in vitro, however, this occurred only at a concentration higher than that achievable with oral supplementation (Fink et al., 2021).
When discussing amantadine, it is worth mentioning the neurological complications of COVID-19, i.e. agitation, myoclonus, abulia, alogy (Baller et al., 2020), brain fog, and chronic fatigue (Graham et al., 2021). Studies are emerging to assess the effects of amantadine on alleviating theses neurological symptoms. It has been suggested that amantadine can potentially help in the treatment of catatonia, especially in patients with contraindications to benzodiazepines due to respiratory failure (Raupp-Barcaro et al., 2018b). Additionally, amantadine may support the treatment of depressive disorders (Zaidi and Dehgani-Mobaraki, 2021). The study authors did not recommend the routine use of amantadine in COVID-19 patients limiting its use to a clinical trial.
4.5. Ivermectin
Ivermectin is one of the most commonly used drugs to treat parasitic infections in humans as well as in animals in veterinary medicine. Its mechanism is based on the selective, positive allosteric modulation of glutamate chloride channels found in nematodes and insects. It acts by binding to these channels, leading to an influx of chloride ions, causing cell hyperpolarization and thus dysfunction. Moreover, at higher concentrations, ivermectin can also bind to GABA receptors (Zaidi and Dehgani-Mobaraki, 2021). Ivermectin is rapidly absorbed orally and has high liposome solubility. Moreover, it is metabolized in the liver (by the cytochrome P450 system) and almost exclusively excreted in feces (González Canga et al., 2008). One of the main potential mechanisms of ivermectin action is based on binding to the importin α (IMPα)/β1 heterodimer complex. IMPα/β1 participates in binding to the CoV load protein in the cytoplasm and transports it through the nuclear pore complex (NPC) into the nucleus, where it breaks down and the viral load assists in reducing the host cell's antiviral response, thereby increasing the infection. Ivermectin binds to the IMPα/β1 and destabilizes it, thus preventing it from binding the viral protein and entering the nucleus. This likely results in decreased inhibition of the immune response, leading to a normal, more effective antiviral reaction (Wagstaff et al., 2012).
Ivermectin has been examined in several studies, including that by Zein et al., who performed a review of the meta-analyses and meta-regression of randomized controlled trials. Among the available trials, they searched for the effectiveness of ivermectin in SARS-CoV-2 virus infections as compared to control patients with standard of care or a placebo. The primary endpoint that was evaluated was mortality. In total, 9 RCTs involving 1788 patients were analyzed in this meta-analysis, revealing that ivermectin was associated with a reduction in mortality [RR = 0.39 (95 % CI: 0.20 – 0.74); p = 0.004]. However, the benefit of ivermectin and this reduced mortality were impeded by hypertension [RR = 1.08 (95 % CI: 1.03–1.13); p = 0.001]. A sensitivity analysis using the fixed effects model showed that ivermectin reduced all-cause mortality [RR = 0.43 (95 % CI: 0.29 – 0.62); p < 0.001] and the severe COVID-19 subgroup [RR = 0.48 (95 % CI: 0.32–0.72); p < 0.001] (AFMZ et al., 2021).
However, other studies did not report statistically significant differences in mortality (Ravikirti and Pattadar, 2021), length of hospitalization (Abdulamir et al., 2021a) and clinical endpoints, disease progression, recovery, the occurrence of symptoms (Okumuş et al., 2021). The study authors did not recommend the routine use of ivermectin in COVID-19 patients, limiting its use to a clinical trial.
4.6. Niclosamide
Niclosamide (NIC) is an oral chlorinated salicylanilide. In clinical practice, it is a drug used to treat tapeworm infections. Its mechanism of action is centered around decoupling the electron transport chain from ATP synthase, thereby abolishing ATP synthesis. When administered orally, NIC specifically induced the degradation of the androgen receptor variant V7 (AR-V7) via a proteasome-mediated pathway. This action decreased the expression of the AR variant, inhibiting its transcriptional activity and reducing the recruitment of AR-V7 into the prostate-specific antigen (PSA) gene promoter. NIC also prevented AR-V7-mediated phosphorylation and activation of STAT3 (Kadri et al., 2018). In addition, there are reports of the antiviral activity of NIC against the influenza virus and HRV (Jurgeit et al., 2012). Various drug repurposing screens identified NIC as a potential drug candidate against COVID-19. Prevention of viral entry by altering endosomal pH and prevention of viral replication by inhibition of autophagy are the plausible mechanisms of action of NIC against COVID-19. Therefore, the clinical efficacy of NIC against COVID-19 therefore needs to be further evaluated (Pindiprolu and Pindiprolu, 2020).
One study in an animal model assessed the efficacy of NIC-Lysozyme (NIC-hLYS) particles against the SARS-CoV-2 infection. A once-daily administration in the form of nasal NIC-hLYS particles suspended in 0.45 % NaCl resulted in a 30 % survival rate in fatal SARS-CoV-2 infection. Moreover, it caused a statistically significant decrease in viral load in the lung after 10 days of treatment. By day 6 of treatment with 240 μg/kg NIC, interstitial pneumonia was significantly reduced and further resolved by day 14 (Brunaugh et al., 2020).
A randomized trial by Abdulamir et al., investigated the efficacy and safety of NIC as an adjunct to the standard of care in COVID-19 infection. This study was a randomized, controlled, open-label clinical study including 75 COVID-19 patients treated with standard of care plus NIC and 75 COVID-19 patients treated only with standard care therapy. Each group consisted of 25 mild, 25 moderate, and 25 severe COVID-19 patients. The main endpoints of the analysis were survival rate, time to recovery, and adverse reactions. NIC did not increase the survival rate as three severe COVID-19 patients in the NIC and control groups died (p > 0.05). However, when compared to the control group, NIC reduced recovery time in patients with moderate and severe COVID-19 by 5 and 3 days, respectively, but not in mild patients (p ≤ 0.05). Interestingly, NIC reduced recovery time to five days in patients with comorbidities (P ≤ 0.05), while shortening it by only one day in patients without comorbidities (p > 0.05). The authors concluded that NIC speeds up recovery by approximately 3–5 days in patients with moderate to severe COVID-19, especially those with underlying medical conditions. Hence NIC achieved clinical benefits by freeing up hospital beds for more patients in a pandemic crisis (Abdulamir et al., 2021b). The authors did not recommend the routine use of NIC in COVID-19 patients, limiting its use to a clinical trial.
4.7. Sarilumab
Sarilumab (Kevzara) is a human monoclonal antibody that acts to inhibit the binding of IL-6 to its α receptor. This drug is approved for the treatment of adults with moderately to severely active rheumatoid arthritis. Due to sarilumab ability to inhibit both soluble and membrane-bound IL-6 receptor, it has the potential to exert a therapeutic effect in patients with SARS-CoV-2 infection (KEVZARA (Sarilumab), 2017).
A study by Lescure et al., describes the effects of sarilumab in patients admitted to the hospital with severe or critical COVID-19. This was a phase 3 randomized, double-blind, placebo-controlled study on 416 patients allocated to 3 groups. Group one received a placebo, the second group received sarilumab at a dose of 200 mg and the third group received the drug at a dose of 400 mg. The authors concluded that the use of sarilumab was not effective in patients admitted to the hospital with COVID-19 and receiving oxygen supplementation. In patients with critical illness due to COVID-19, appropriately enhanced trials of targeted immunomodulatory therapies assessing survival as a primary endpoint, are suggested (Lescure et al., 2021a).
4.8. Chinese herbal medicine
In many environments, folk medicine plays an important role in the treatment of various diseases, especially those that people fear, or when conventional medicine is powerless or unable to propose effective treatment. This can be seen during the course of some cancers, and the beginning of the COVID-19 pandemic. Patients' questions often relate to Chinese herbal medicine (CHM) as a popular representative of alternative medicine. Currently, protocols of systematic reviews and meta-analyses for 7 preparations have been announced: Shufeng Jiedu (Wang et al., 2020a), Xuanfei Baidu (Zhao et al., 2021), Maxingshigan Decoction (Shao et al., 2020), Reyanning mixture (Li et al., 2021), Xiaoqinglong decoction (Ren et al., 2020), Lianhua Qingwen (Liu et al., 2020a), and Xiyanping (Zhou et al., 2020). As the authors suggest, these drugs have been used to treat COVID-19 in China, so scientific evidence is needed to evaluate their effectiveness. The study authors did not recommend the use of CHM in COVID-19 patients.
4.9. Dietary supplements
Vitamin C has been used as a remedy for cold-like symptoms for years. Studies on animal models show that vitamin C reduces vascular permeability, improves blood circulation, and due to its antioxidant effect, reduces the amount of free radicals (Armour et al., 2001; Chakrabarty et al., 1992). Furthermore, there have been reports of vitamin C used in combination with hydrocortisone and thiamine to treat sepsis and acute respiratory distress syndrome, significantly reducing mortality (Marik et al., 2017).
Gao et al., conducted a study in which vitamin C was administered at high doses to patients with COVID-19 (n = 46) and compared them with standard treatment (n = 30). The study showed a significant reduction in mortality and a lower need for respiratory support. Given the availability of vitamin C, there is a lack of large adequately powered studies confirming or contradicting the effectiveness of this supplement in treating COVID-19 (Gao et al., 2021). Huang et al., have published a protocol for a systematic review and meta-analysis of high-dose intravenous vitamin C administration, but have not released the results as of November 2021 (Huang et al., 2021).
Vitamin D supplementation during viral infections is also very popular. Vitamin D possess an immunomodulatory effect by altering the expression and secretion of proinflammatory cytokines (e.g. Il-6, TNF), interferon, and chemokines (Greiller and Martineau, 2015). A meta-analysis published by Rawat et al., examining the use of vitamin D in patients with COVID-19 demonstrated no significant reduction in mortality, ICU admission, or the need for invasive ventilation in patients receiving vitamin D supplementation (Rawat et al., 2021).
It is also worth mentioning that zinc, one of the micronutrients, was postulated to be effective in the combat against COVID-19. It was shown that supplementation with zinc reduced mortality in pneumonia without increasing the risk of therapy failure (Wang and Song, 2018). Its role is to reduce oxidative stress and inflammation (Prasad, 2014), thereby potentially alleviating the symptoms of COVID-19. Szarpak et al., performed a meta-analysis of the effect of zinc supplementation in COVID-19, although no statistically significant difference was found on mortality between patients using supplementation and those that were not (Szarpak et al., 2021). An overview on the COVID-19 drug effectiveness is presented in Table 5, Table 6 .
Table 5.
A summary of COVID-19 drug effectiveness meta-analyses.
| Drug | No. patients | Outcome | Effect |
|---|---|---|---|
| Vitamin D (Rawat et al., 2021) | 467 | Mortality reduction | No effect; R = 0.55 (95 % CI 0.22–1.39), p = 0.21 |
| HCQ (Amani et al., 2021) | 6059 | Mortality reduction | No effect, RR = 0.7 (95 % CI: 0.24–1.99) |
| HCQ (Bartoszko et al., 2021) | 8161 | Side effects | RR = 1.81 (95 % CI: 1.36–2.42), p < 0.05 |
| HCQ (Axfors et al., 2021) | 10,012 | Increase of mortality | OR = 1.11 (95 % CI: 1.02–1.20) |
| Convalescent plasma (Bansal et al., 2021b) | 27,706 | Mortality reduction | OR 0.76 (95 % CI: 0.53–1.08), p = 0.13 |
| Sarilumab (Lescure et al., 2021b) | 416 | Positive effect | HR = 1.03 (95 % CI 0.75–1.40]; p = 0.96 |
Legend: HCQ – hydroxychloroquine; HR – hazard ratio; OR – odds ratio; RR – risk ratio; 95 % Cl – 95 % confidence interval.
Table 6.
Effectiveness of therapeutic agents in COVID-19.
| Drug | No. patients | Dose | Outcome | Effect |
|---|---|---|---|---|
| Ivermectin (AFMZ et al., 2021) | 1788 | 140 - 400 μg/kg | Mortality reduction | RR = 0.39 (95 % CI: 0.20−0.74); p = 0.004 |
| Colchicine (Golpour et al., 2021) | 5901 | NA | Mortality reduction | RR = 0.644 (95 % CI: 0.555 – 0.748) |
| Niclosamide (Abdulamir et al., 2021a) | 150 | 3 g per day | Reduced recovery time | p ≤ 0.05 |
| Tofacitinib (Gunay et al., 2021) | 289 | 10 mg twice a day | Mortality reduction | HR = 0.49 (95 % CI: 0.15–1.63) |
| Bamlanivimab - Etesevimab (Dougan et al., 2021) | 1035 | 2.8 g + 2.8 g | Hospitalizations or death | absolute risk difference = −4.8%; (95% CI − 7.4 - −2.3); RR = 0.3; p < 0.001 |
| Bamlanivimab - Etesevimab (Gottlieb et al., 2021) | 577 | 2.8 g + 2.8 g | Viral load | Viral load change = - 0.57 (95 % CI: −1.00 to −0.14); p = 0.01 |
| Anticoagulants (Parisi et al., 2021) | 25,719 | therapeutic and prophylactic dose | Mortality reduction | RR = 0.50 (95 % CI: 0.40−0.62) |
| ASA (RECOVERY Collaborative Group, 2021) | 14,892 | 150 mg | Mortality reduction | RR = 0.96 (95 % CI 0.89–1.04); p = 0.35 |
| Dexamethasone (Lim et al., 2021) | 6425 | 6 mg | Mortality reduction | RR = 0.83; (95 % CI: 0.75−0.93); p < 0.001 |
| Budesonide (Ramakrishnan et al., 2021) | 139 | 400 μg | Emergency visit/hospitalization | RR = 0.131 (95 % CI: 0.043−0.218); p = 0.004 |
Legend: ASA – acetylsalicylic acid, aspirin, HR – hazard ratio; RR – risk ratio; 95 % Cl – 95 % confidence interval.
5. Adjuvants/supportive treatment
5.1. Steroids
5.1.1. Dexamethasone
Dexamethasone is a synthetic glucocorticoid, a fluorinated derivative of prednisone that possesses a strong and long-lasting anti-inflammatory and immunosuppressive effect. The mechanism of action is based on the reduction of accumulated leukocytes and their adhesion to the endothelium. Moreover, dexamethasone inhibits phagocytosis and lysosomal breakdown, reduces the number of lymphocytes, eosinophils, monocytes, and blocks IgE-dependent secretion of histamine and leukotrienes. Finally, it inhibits the synthesis and release of cytokines, including interferon γ, TNF-α, GM-CSF, and interleukins IL-1, IL-2, IL-3, and IL-6. By inhibiting the activity of phospholipase A2 through lipocortin, it prevents the release of arachidonic acid, therefore reducing mediators of inflammation such as leukotrienes and prostaglandins (Ahmed and Hassan, 2020; Sinner, 2019).
In one of the most comprehensive trials, patients were randomized to receive 6 mg oral or intravenous dexamethasone once daily for up to 10 days or to a control group that received the standard of care. The primary endpoint was mortality at 28 days. A total of 2104 patients were assigned to receive dexamethasone and 4321 received standard of care. Overall, 482 patients (22.9 %) in the dexamethasone group and 1110 patients (25.7 %) in the standard of care group died within 28 days after randomization [age-adjusted rate ratio = 0.83 (95 % confidence interval [CI]: 0.75–0.93); p < 0.001]. In the dexamethasone group, the death rate was lower than in the standard care group receiving invasive mechanical ventilation [29.3 % vs. 41.4 %; rate ratio = 0.64 (95 % CI: 0.51–0.81)] and receiving oxygen without invasive mechanical ventilation [23.3 % vs. 26.2 %; rate ratio = 0.82 (95 % CI: 0.72–0.94)], but not among those who did not receive respiratory support at the time of randomization [17.8 % vs. 14.0 %; rate ratio = 1.19 (95 % CI: 0.92–1.55)]. This study showed that dexamethasone treatment resulted in a lower 28-day mortality in patients hospitalized for COVID-19 who were undergoing mechanical ventilation or oxygen therapy, but not for those patients who did not receive respiratory support (Lim et al., 2021).
The results of the most recent trial pertaining the use of dexamethasone, the COVID STEROID 2 Trial provided by Munch et al. in October 2021 have shown that in COVID-19 patients with severe hypoxemia, the use of 12 mg/d of dexamethasone as compared with 6 mg/d of dexamethasone did not reduce 28-day survival without life support (Munch et al., 2021). In the 12 mg dexamethasone group the mortality at 28 days was lower (27.1 %) and in the 6 mg dexamethasone group was higher (32.3 %) (adjusted relative risk, 0.86 [99 % CI, 0.68–1.08]). Similarly, the death rate at 90 days was lower (32.0 %) in the 12 mg dexamethasone group as compared to mortality in the 6 mg dexamethasone group (37.7 %), with adjusted relative risk of 0.87 [99 % CI, 0.70–1.07]). Although the results of the by Munch et al. are supportive, but not definitive of improved outcomes when using 12 mg/d of dexamethasone, the study was underpowered. Therefore, the results of COVID STEROID 2 Trial do not satisfy the usual criteria to support change in practice, but further trials are needed to define the optimal dose of dexamethasone with definite survival benefit. The results of three on-going trials (NCT04381936, NCT04726098, NCT04663555) are highly awaited. Hence, the study authors recommended the use of dexamethasone in the routine care of patients with COVID-19, especially during hospitalization, but the optimal dose is yet to be established.
5.1.2. Budesonide
Another member of the glucocorticoid family which has recently been used to treat SARS-CoV-2 infections is budesonide. A randomized, phase 2 trial of inhaled budesonide versus standard of care (Steroids in COVID-19; STOIC study) was conducted in adults within 7 days of onset of mild COVID-19 symptoms. The dry powder of budesonide was administered via a turbine inhaler at a dose of 400 μg. Participants were asked to perform two inhalations twice a day. The primary endpoint was a COVID-19 related emergency department visit. Secondary endpoints were patient-reported symptom relief, body temperature, blood oxygen saturation, and SARS-CoV-2 virus load. For the pre-protocol population (n = 139), the primary endpoint was met in 10 (14 %) of 70 participants receiving the standard of care and 1 (1%) of 69 participants receiving budesonide [difference = 0.131 (95 % CI: 0.043 – 0.218); p = 0.004]. In the intention-to-treat population, the primary endpoint occurred in 11 (15 %) participants in the usual care group and two (3%) participants in the budesonide group [difference = 0.123 (95 % CI: 0.033 – 0.213); p = 0.009]. The number needed to treat with inhaled budesonide to reduce the worsening of COVID-19 was 8. Budesonide was also found to be safe, and only five (7%) participants reported self-limiting adverse events (Ramakrishnan et al., 2021). The study authors recommend the inhalation of steroids in the routine use in patients with COVID-19 in the early stages of the disease.
5.2. Anticoagulants
Heparin possesses potent anticoagulant activity, induced by catalyzing the thrombin-antithrombin reaction. In addition, heparin exerts an anti-inflammatory effect that may improve endothelial function, which may be beneficial for patients with COVID-19. To date, there are two studies comparing the low-molecular-weight (LMW) to unfractionated heparin, and both demonstrated a reduced risk of death with LMW compared with unfractionated heparin (Kirkup et al., 2021; Pawlowski et al., 2021). In one study, mortality for the primary population was 270/1939 vs. 390/1012 with an OR = 0.258 (95 % CI: 0.215–0.309); in-hospital mortality for the matched populations was 154/711 (22 %) vs. 268/733 (37 %) with an OR = 0.480 (95 % CI: 0.380–0.606) and 28-day mortality for matched populations 12/528 (2.3 %) vs. 44/463 (9.5 %) with an OR = 0.221 (95 % CI: 0.115 – 0.425). In addition, the addition of LMW heparin reduced hospitalization (10.99 days vs. 13.33 days; p = 0.005), and ICU admission (10.7 vs. 12.16; p = 0.00008), and finally reduced the number of patients transferred to the ICU [primary populations: 988/1936 vs. 717/1009, OR = 0.424 (95 % CI: 0.361 – 0.499); comparison of matched populations: 399/714 (56 %) vs. 481/732 (66 %), OR = 0.661 (95 % CI: 0.534 – 0.817)] (Kirkup et al., 2021).
In the second study, Pawlowski et al., showed that all-cause mortality for primary populations was reduced [11 (2.5 %) vs. 28 (17 %), RR = 6.76 (95 % CI: 3.39–12.7)], with the 28-day mortality for the primary populations of 9/244 (3.7) vs. 20/118 (17) (RR = 4.60, 95 % CI: 2.13–9.29)]. Additionally, end-points in favor of LMW heparin were reached in terms of patients transferred to the ICU primary population (88 (20 %) vs. 50 (30 %) RR = 1.51 (95 % CI: 1.12–2.03) (Pawlowski et al., 2021). The study authors recommended the routine use of anticoagulants in patients with COVID-19, especially during hospitalization.
5.3. Acetylsalicylic acid
Acetylsalicylic acid (ASA, aspirin) belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs) that possess anti-inflammatory, antipyretic, and analgesic properties. Its mechanism of action is based mainly upon inhibiting cyclooxygenases (COX) in two distinct ways. Constitutive COX (COX-1) is responsible for the synthesis of prostaglandins that fulfill physiological functions. On the other hand, inducible COX (COX-2) is responsible for the synthesis of pro-inflammatory prostaglandins at the site of inflammation. ASA mainly inhibits COX-1, and to a lesser extent, COX-2. By irreversibly inhibiting platelet COX-1 and crippling thrombogenesis, it exerts an anti-aggregating effect. At higher doses, it acts as an antithrombotic agent by antagonizing vitamin K (Tanasescu et al., 2000). Moreover, the pleiotropic effects of ASA include the modulation of endothelial function (Sayed Ahmed et al., 2021), and therefore it may have a role in preventing COVID-19 complications (Dzeshka et al., 2016). Moreover, ASA has been shown to carry antiviral activity against RNA viruses in the respiratory tract, such as influenza A virus and human rhinoviruses, but its mode of action is still unknown and requires further research (Glatthaar-Saalmüller et al., 2017).
In the RECOVERY study, Horby et al., described the effectiveness of ASA in COVID-19 infection. In this randomized, controlled, open-label platform study, several possible treatments were compared with standard of care in patients hospitalized for COVID-19. Eligible and consenting adults were randomly assigned in a 1:1 ratio to either standard care (7541 patients) or standard care plus 150 mg of ASA (7351 patients) once a day until discharge from the hospital. The primary endpoint was mortality at 28 days. This study demonstrated that 1222 (17 %) patients assigned to ASA and 1299 (17 %) patients assigned to ordinary care died within 28 days (RR = 0.96; 95 % CI: 0.89–1.04; p = 0.35). Among subjects who did not require invasive mechanical ventilation at baseline, there was no significant difference in the proportion meeting the composite endpoint of invasive mechanical ventilation or death (21 % vs. 22 %; HR = 0.96; 95 % CI: 0.90–1.03; p = 0.23). The use of ASA was associated with an absolute reduction in the number of thrombotic events by 0.6 % and an absolute increase in the number of major bleeding events by 0.6 % (RECOVERY Collaborative Group, 2021). Furthermore, the study by Chow et al., reported promising effects of ASA in SARS-CoV-2 infection. Among the 412 patients included in the study, 314 did not receive ASA (76.3 %) while 98 patients (23.7 %) did. The significant differences were reported between the two groups in the ICU admission rate (51 % non-ASA vs. 38.8 % ASA; p < 0.05) and the rate of mechanical ventilation (48.4 % non-ASA vs. 35.7 % ASA; p < 0.05). After the adjustment of confounding variables, the ASA use was reported to decrease the risk of mechanical ventilation (HR = 0.56; 95 % CI: 0.37 – 0.85; p = 0.007), admission to intensive care unit (HR = 0.57; 95 % CI: 0.38–0.85; p = 0.005) and in-hospital death adjusted (HR = 0.53; 95 % CI: 0.31–0.90; p = 0.02) (Chow et al., 2021). Accordingly, the study authors suggested potential use of acetylsalicylic acid in patients with COVID-19, especially in clinical trials.
5.4. Statins
Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors, are lipid-lowering drugs that display pleiotropic effects. As acute respiratory distress syndrome (ARDS), the main cause of death from COVID-19, is caused by exaggerated inflammatory response, the immunomodulatory properties of statins have become of interest in the context of COVID-19 research, and have previously shown a beneficial effect in the treatment of autoimmune, inflammatory, and infectious diseases (Lima Martínez et al., 2020). These agents could potentially limit the cytokine storm by blocking NF-κB and NLRP3 inflammasomes (Rodrigues-Diez et al., 2020). Moreover, statins also affect the cell cycle, even leading to its arrest, induce autophagy and apoptosis, which is likely to further limit viral replication (Ahmadi et al., 2020). However, the significance of the mechanism in which statins possibly increase SARS-CoV-2 virus entry by inducing ACE-2 expression is still not fully known (Rodrigues-Diez et al., 2020; Zhang et al., 2020).
The wide-spread use of statins has enabled the researchers to conduct large-scale retrospective studies among COVID-19 patients. Members of our team performed such a study of statin-treated vs non-treated people, who were infected with SARS-CoV-2. However, data from a group of 150 patients, 75 of which received statins, failed to reach statistical significance. However, these data have encouraged us to conduct larger retrospective analyses or even prospective studies (Peymani et al., 2021). A large retrospective study on 13,981 patients from China found an association between the statin use and lower risk of mortality (Zhang et al., 2020).
A meta-analysis of 4 studies showed that the use of statins is associated with a significantly reduced hazard for fatal or severe disease (pooled HR = 0.70; 95 % CI: 0.53–0.94), although these results based on 8990 patients strongly highlight a need for prospective studies (Kow and Hasan, 2020).
The currently available data seems encouraging and suggests that in no case should the use of statins be abandoned during COVID-19 infection. However, it is too soon to include statins in the routine therapeutic plan for COVID-19 treatment (Subir et al., 2020). Moreover, people, who start therapy with statins due to cardiovascular diseases during the pandemic should be aware that some of the potential side effects might mimic COVID-19. Muscle-related symptoms especially, are similar when comparing the side-effects of statins or viral infection (Karalis DG, 2020).
6. Treatment of COVID-19 complications
COVID-19 symptoms can, in some cases, persist for months. The virus can damage the lungs, heart and brain, which significantly increases the risk of long-term health issues. This group of conditions has been called post−COVID-19 syndrome or long COVID-19 (Datta et al., 2020). In general, they are considered to be the effects of COVID-19 that persist for more than four weeks after diagnosis (Silva Andrade et al., 2021). SARS-CoV-2 can cause severe inflammation that is triggered by the immune system, which responds by increasing the rate of coagulation, which is triggered largely due to other systems in the body being affected by blood clots, such as the lungs, kidneys, liver, or heart. Moreover, COVID-19 can also weaken blood vessels and cause them to leak, which further contributes to the potential long-term complications affecting the kidneys and liver (Jin et al., 2020). The SARS-CoV-2 infection requires the cooperation of several essential systems to maintain homeostasis. The direct effect of SARS-CoV-2 hyperinflammation induces the production of endogenous compounds that promote the alteration of vascular hemostasis (Liu et al., 2020b). Furthermore, the release of pro-inflammatory and pro-thrombotic cytokines has a direct effect on blood coagulation. These factors result in disseminated intravascular coagulation and the formation of thromboembolic conditions that can affect various tissues, especially those which are more sensitive to ischemic processes, such as pulmonary, cardiovascular, and cerebrovascular tissues (Jin et al., 2020; Giustino et al., 2020). The cardiopulmonary system especially is severely affected (Cobos-Siles et al., 2020). The lungs suffer from gradual functional failure, which is reflected by hypoxia and pathological findings (Silva Andrade et al., 2021; Al-Khawaga and Abdelalim, 2020). Among the most common pathologies of the lung, respiratory failure, pulmonary thromboembolism, pulmonary embolism, pneumonia, pulmonary vascular damage, and post-viral pulmonary fibrosis should be highlighted (Sakr et al., 2020; George et al., 2020; Lechowicz et al., 2020). So far, there is no single, proper guideline for treating pulmonary complications after COVID-19. It has been suggested that physical exercise and appropriate rehabilitation, including breathing exercises, may help to resolve pulmonary symptoms (Crook et al., 2021). In more severe cases, the use of opioids may reduce respiratory effort (Jennings, 2002). However, lung fibrosis may be a long-term complication. Due to the relatively short follow-up period from the first infection, the available data on this phenomenon is limited. Therefore, it is suggested that the treatment recommendations regarding idiopathic pulmonary fibrosis be followed.152] There have been reports in the literature that the use of spironolactone during COVID-19 infection can prevent fibrosis(Kotfis et al., 2021).
The most experienced cardiac complications include angina, acute coronary syndromes, and arrhythmias. The NICE recommendations point to the use of beta blockers in these cases (National Institute for Health and Care Excellence, 2021, 2020; National Institute for Health and Care Excellence, 2016). Furthermore, remission of one complication, myocarditis, might depend on immunomodulatory effect (Sinagra et al., 2016). Complications related to the nervous system following COVID-19 infection include loss of taste, smell and hearing, headaches, spasms, convulsions, confusion, visual disturbances, neuralgia, dizziness, disturbance of consciousness or delirium, nausea and vomiting, hemiplegia, ataxia, stroke, as well as cerebral hemorrhage (Favas et al., 2020; Samaranayake et al., 2020; Almufarrij et al., 2020; Kennedy et al., 2020; Kotfis et al., 2020; Pun et al., 2021). According to Crook et al., chronic fatigue syndrome can be compared to the myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) so treatment may include cognitive behavioral therapy (CBT) and graded exercise therapy (GET) (Crook et al., 2021). In the case of cognitive impairment, the so-called brain fog, apart from psychological support, methylphenidate, donepezil, modafinil, and memantine may also be helpful (Crook et al., 2021; Chemo brain, 2021; Theoharides et al., 2021).
COVID-19 infections can cause macro- and micro-thromboembolic renal dysfunction as well as trigger microvascular obstruction and infarction. Idilman et al., found that a large number of patients with mild to moderate COVID-19 had perfusion deficits (PD) in their lungs and kidneys, which may be suggestive of the presence of systemic microangiopathy with microthrombosis (Acharya et al., 2020; Idilman et al., 2021). In addition to kidney damage, the other system affected by complications from COVID-19 infection is the digestive system and liver. A meta-analysis of thirty-one studies examining the incidence of gastrointestinal symptoms in 4682 patients found that diarrhea and anorexia were among the most significant gastrointestinal symptoms associated with COVID-19. In addition, it was observed that patients admitted to ICU or with high intensity were more likely to develop abdominal pain and increased hepatic inflammatory markers such as aspartate aminotransferase or alanine aminotransferase (Dong et al., 2021).
One of the other potential long-term complications of COVID-19, due to long-term persistence of viral particles in organs, is interaction with autophagy machinery (Habibzadeh et al., 2021). This interaction induces inhibition of autophagy flux, which potentially is involved in potentiation of cancer progression and metastasis and immune escape in COVID-19 survivors (Habibzadeh et al., 2021).
7. Summary
Prophylaxis with SARS-CoV-2 vaccines is the most effective modality to prevent and eliminate COVID-19. COVID-19 symptomatology varies between patients and treatment needs to be tailored towards specific symptoms, as there are many critical points of disease progression that can be targeted. The development and progression of COVID-19 can be viewed as a multi-stage process (Fig. 5 ) that begins with the exposure to the virus, followed by the SARS-CoV-2 infection phase, and then the initiation of COVID-19 disease processes such as early infection, pulmonary phase and inflammatory storm phase. Pharmacological interventions at any of these stages are required in order to minimize the effects. Moreover, the timing of the intervention is critical. Currently, behavioral modifications are necessary to prevent exposure to SARS-CoV-2, and public health guidelines for social distancing, masking, and hygiene are recommended. Rigorously tested pharmacological strategies to reduce and block SARS-CoV-2 virus infection and COVID-19 development are the subject of thousands of trials around the world to reduce and contain the global epidemic. In the latter respect, Pfizer Inc., recently announced that its investigational novel COVID-19 oral antiviral candidate, PAXLOVID™ (PF-07321332), significantly reduced hospitalization and death, based on an interim analysis of the phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness. The scheduled interim analysis demonstrated an 89 % reduction in risk of COVID-19-related hospitalization or death from any cause compared to placebo in patients treated within three days of symptom onset (primary endpoint); 0.8 % of patients who received PAXLOVID™ were hospitalized through Day 28 following randomization (3/389 hospitalized with no deaths), compared to 7.0 % of patients who received placebo and were hospitalized or died (27/385 hospitalized with 7 subsequent deaths). The statistical significance of these results was high (p < 0.0001). Similar reductions in COVID-19-related hospitalization or death were observed in patients treated within five days of symptom onset; 1.0 % of patients who received PAXLOVID™ were hospitalized through Day 28 following randomization (6/607 hospitalized, with no deaths), compared to 6.7 % of patients who received a placebo (41/612 hospitalized with 10 subsequent deaths), with high statistical significance (p < 0.0001). In the overall study population through Day 28, no deaths were reported in patients who received PAXLOVID™ as compared to 10 deaths (1.6 %) in patients who received placebo.
Fig. 5.
Graphical representation of currently recommended therapeutic agents depending on the clinical condition.Top shows the natural course of COVID-19 infection. The symptomatic phase occurs after incubation at >20 % infection. Out of patients in critical condition even around 50 % die. Bottom shows suggested therapeutic interventions depending on the course of the disease 80 % – mild course of the disease; neutralizing antibodies recommended if high risk of disease progression. 15 % - severe course of the disease; dexamethasone and remdesivir recommended for patients with SpO2 ≤94 % on room air; if rapidly increasing oxygen need and systemic inflammation – consider baricitinib or tocilizumab. 5% - acute respiratory distress syndrome (ARDS) or multi-organ failure develops; use dexamethasone and consider tocilizumab.
Funding
MJŁ acknowledge partial support from grant # 32/007/RGJ21/0034 from Silesian University of Technology.
References
- Abdulamir A.S., Gorial F.I., Saadi S.J., Maulood M.F., Hashim H.A., Abdulrrazaq M.K. Effectiveness and safety of Niclosamaide as add-on therapy to the standard of care measures in COVID-19 management: randomized controlled clinical trial. medRxiv. 2021;(January) doi: 10.1101/2021.06.10.21258709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulamir A.S., Gorial F.I., Saadi S.J., Fauzi M., Hashim H.A., Manal K. Measures in COVID-19 Management : randomized controlled clinical trial. medrxiv.org. 2021 doi: 10.1101/2021.06.10.21258709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya S., Anwar S., Siddiqui F.S., Shabih S., Manchandani U., Dalezman S. Renal artery thrombosis in COVID-19. IDCases. 2020;22 doi: 10.1016/j.idcr.2020.e00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AFMZ Zein, Sulistiyana C.S., Raffaelo W.M., Pranata R. Ivermectin and mortality in patients with COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Diabetes Metab. Syndr. 2021;15(4):102186. doi: 10.1016/j.dsx.2021.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M., Amiri S., Pecic S., et al. Pleiotropic effects of statins: a focus on cancer. Biochim. Biophys. Acta – Mol. Basis Dis. 2020;1866(12):165968. doi: 10.1016/j.bbadis.2020.165968. [DOI] [PubMed] [Google Scholar]
- Ahmed M.H., Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr. Clin. Med. 2020;2(12):2637–2646. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem A., Akbar Samad A.B., Slenker A.K. Treasure Island (FL); 2021. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19) [PubMed] [Google Scholar]
- Al-Khawaga S., Abdelalim E.M. Potential application of mesenchymal stem cells and their exosomes in lung injury: an emerging therapeutic option for COVID-19 patients. Stem Cell Res. Ther. 2020;11(1):437. doi: 10.1186/s13287-020-01963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanza A., Carlesso A., Chintha C., et al. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J. 2019;286(2):241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almufarrij I., Uus K., Munro K.J. Does coronavirus affect the audio-vestibular system? A rapid systematic review. Int. J. Audiol. 2020;59(7):487–491. doi: 10.1080/14992027.2020.1776406. [DOI] [PubMed] [Google Scholar]
- Amani B., Khanijahani A., Amani B. Hydroxychloroquine plus standard of care compared with standard of care alone in COVID-19: a meta-analysis of randomized controlled trials. Sci. Rep. 2021;11(1):11974. doi: 10.1038/s41598-021-91089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P.I., Ianevski A., Lysvand H., et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angamo M.T., Mohammed M.A., Peterson G.M. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 2021;(July):1–15. doi: 10.1007/s15010-021-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . 2006. Etesevimab and Bamlanivimab. Bethesda (MD)http://www.ncbi.nlm.nih.gov/pubmed/33630482 [Google Scholar]
- Anon An EUA for casirivimab and imdevimab for COVID-19. Med. Lett. Drugs Ther. 2020;62(1614):201–202. [PubMed] [Google Scholar]
- Anon An EUA for sotrovimab for treatment of COVID-19. Med. Lett. Drugs Ther. 2021;63(1627):97–98. [PubMed] [Google Scholar]
- Aranda-Abreu G.E., Aranda-Martínez J.D., Araújo R., Hernández-Aguilar M.E., Herrera-Covarrubias D., Rojas-Durán F. Observational study of people infected with SARS-Cov-2, treated with amantadine. Pharmacol. Rep. 2020;72(6):1538–1541. doi: 10.1007/s43440-020-00168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour J., Tyml K., Lidington D., Wilson J.X. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J. Appl. Physiol. 2001;90(3):795–803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- Artese A., Svicher V., Costa G., et al. Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2020;53 doi: 10.1016/j.drup.2020.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur T., Snow C., Saleem N., Ambler G., Nastouli E., Singer M. Tocilizumab in COVID ‑ 19 : a meta ‑ analysis, trial sequential analysis, and meta ‑ regression of randomized ‑ controlled trials. Intensive Care Med. 2021 doi: 10.1007/s00134-021-06416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca . 2021. AZD7442 Reduced Risk of Developing Severe COVID-19 or Death in TACKLE Phase III Outpatient Treatment Trial.www.astrazeneca.com/ [Google Scholar]
- Aviani J.K., Halim D., Soeroto A.Y., Achmad T.H., Djuwantono T. Current views on the potentials of convalescent plasma therapy (CPT) as Coronavirus disease 2019 (COVID‐19) treatment: a systematic review and meta‐analysis based on recent studies and previous respiratory pandemics. Rev. Med. Virol. 2021;(October 2020) doi: 10.1002/rmv.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axfors C., Schmitt A.M., Janiaud P., et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021;12(1):2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baller E.B., Hogan C.S., Fusunyan M.A., et al. Neurocovid: pharmacological recommendations for delirium associated with COVID-19. Psychosomatics. 2020;61(6):585–596. doi: 10.1016/j.psym.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V., Mahapure K.S., Mehra I., Bhurwal A., Tekin A. Mortality Benefit of Convalescent Plasma in COVID-19 : A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2021;8(April) doi: 10.3389/fmed.2021.624924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V., Mahapure K.S., Mehra I., et al. Mortality benefit of convalescent plasma in COVID-19: a systematic review and meta-analysis. Front. Med. 2021;8(April) doi: 10.3389/fmed.2021.624924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Martins J., Hammerschmidt S.I., Cossmann A., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewska S., Collawn J.F. Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell. Mol. Biol. Lett. 2020;25(1):1–20. doi: 10.1186/s11658-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszko Jessica J., Siemieniuk Reed A.C., Kum Elena, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021:373. doi: 10.1136/BMJ.N949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellos I. A metaresearch study revealed susceptibility of Covid-19 treatment research to white hat bias: first, do no harm. J. Clin. Epidemiol. 2021;136(January):55–63. doi: 10.1016/j.jclinepi.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C., Edwards K.M., Gandhi R., Gallagher J., Muller W.J., O’Horo J.C., Shoham S., Murad M.H., Mustafa R.A., F-YY Sultan S. Infectious Diseases Society of America; 2021. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients With COVID-19.https://www.Idsociety.Org/Practice-Guideline/Covid-19-Guideline-Treatment Version 4.4.1. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunaugh A.A.D., Seo H., Warnken Z., Ding L., Seo S.H. Broad-Spectrum, Patient-Adaptable Inhaled Niclosamide-Lysozyme Particles are Efficacious Against Coronaviruses in Lethal Murine Infection Models. bioRxiv. 2020;2(8) doi: 10.1101/2020.09.24.310490. [DOI] [Google Scholar]
- Campo F., Venuti A., Pimpinelli F., et al. Antibody persistence 6 months post-vaccination with BNT162b2 among health care workers. Vaccines. 2021;9(10) doi: 10.3390/vaccines9101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L., Nannini C., et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S., Nandi A., Mukhopadhyay C.K., Chatterjee I.B. Protective role of ascorbic acid against lipid peroxidation and myocardial injury. Mol. Cell. Biochem. 1992;111(1–2):41–47. doi: 10.1007/BF00229572. [DOI] [PubMed] [Google Scholar]
- Chemo brain . Mayo Clinic; 2021. Diagnosis and Treatment.https://www.mayoclinic.org/diseases-conditions/chemo-brain/diagnosis-treatment/drc-20351065 [Google Scholar]
- Chen C., Zhang Y., Huang J., et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;(January) doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- Cheng L., Guan W., Duan C., et al. Effect of recombinant human granulocyte colony–stimulating factor for patients with coronavirus disease 2019 (COVID-19) and lymphopenia: a randomized clinical trial. JAMA Intern. Med. 2021;181(1):71–78. doi: 10.1001/jamainternmed.2020.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A., Koch M., Wu K., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J.H., Khanna A.K., Kethireddy S., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 2021;132(4) doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- Cobos-Siles M., Cubero-Morais P., Arroyo-Jiménez I., et al. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern. Emerg. Med. 2020;15(8):1533–1544. doi: 10.1007/s11739-020-02485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at https://www.Covid19treatmentguidelines.Nih.Gov/. Accessed July 8, 2021. [PubMed] [Google Scholar]
- Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021:374. doi: 10.1136/BMJ.N1648. [DOI] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.D., Talwar A., Lee J.T. A proposed framework and timeline of the Spectrum of disease due to SARS-CoV-2 infection. JAMA. 2020;324(22):2251. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- Davidovic T., Schimpf J., Abbassi-Nik A., et al. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021;100(6):1334–1335. doi: 10.1016/j.kint.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekervel M., Henry N., Torreggiani M., et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021;14(11):2349–2355. doi: 10.1093/ckj/sfab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-arocutipa C., Hernandez A.V. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir / ritonavir : a systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2021;(November 2020):694–706. doi: 10.1002/pds.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z.-Y., Xiang B.-J., Jiang M., Sun M.-J., Dai C. The prevalence of gastrointestinal symptoms, abnormal liver function, digestive system disease and liver disease in COVID-19 infection: a systematic review and meta-analysis. J. Clin. Gastroenterol. 2021;55(1):67–76. doi: 10.1097/MCG.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M., Nirula A., Azizad M., et al. 2021. Bamlanivimab Plus Etesevimab in Mild or Moderate Covid-19; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drożdżal S., Rosik J., Lechowicz K., et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist. Updat. 2020;53 doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugvirus.info . 2021. Drugvirus. August. [Google Scholar]
- Dzeshka M.S., Shantsila A., Lip G.Y.H. Effects of aspirin on endothelial function and hypertension. Curr. Hypertens. Rep. 2016;18(11):83. doi: 10.1007/s11906-016-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effat D.-M., Hamid R., Hossein K., et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2021;64(9) doi: 10.1128/AAC.01061-20. e01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . 2021. COVID-19: EMA Recommends Authorisation of Two Monoclonal Antibody Medicines.https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-two-monoclonal-antibody-medicines [Google Scholar]
- Falsey A.R., Frenck R.W.J., Walsh E.E., et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021;385(17):1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favas T.T., Dev P., Chaurasia R.N., et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020;41(12):3437–3470. doi: 10.1007/s10072-020-04801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Vol 1282. 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. (Methods in Molecular Biology (Clifton, N.J.)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K., Nitsche A., Neumann M., Grossegesse M., Eisele K.H., Danysz W. Amantadine inhibits sars-cov-2 in vitro. Viruses. 2021;13(4):1–10. doi: 10.3390/v13040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Eron J.J., Holman W., et al. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv Prepr. Serv. Heal. Sci. 2021;(June) doi: 10.1101/2021.06.17.21258639. [DOI] [Google Scholar]
- Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397(10278):952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration . 2020. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of REGEN-COV (Casirivimab and Imdevimab) [Google Scholar]
- Food and Drug Administration . 2021. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. [Google Scholar]
- Food and Drug Administration . 2021. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Sotrovimab. [Google Scholar]
- Fowlkes A., Gaglani M., Groover K., Thiese M.S., Tyner H., Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance - eight U.S. locations, December 2020-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(34):1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology. 2019;533:34–44. doi: 10.1016/j.virol.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Huang M., Liu D.X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus–host interactions. Virus Res. 2014;194:110–123. doi: 10.1016/j.virusres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liao Y., Liu D.X. Regulation of stress responses and translational control by coronavirus. Viruses. 2016;8(7):184. doi: 10.3390/v8070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Xu M., Wang G., et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging (Albany NY) 2021;13(5):7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P.M., Barratt S.L., Condliffe R., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- Geriak M., Haddad F., Kullar R., et al. Randomized prospective open label study shows No impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia. Infect. Dis. Ther. 2021;10(3):1323–1330. doi: 10.1007/s40121-021-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Benedetti F., Campisi G., et al. Evolution patterns of SARS-CoV-2: snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021;538:88–91. doi: 10.1016/j.bbrc.2020.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino G., Pinney S.P., Lala A., et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J. Am. Coll. Cardiol. 2020;76(17):2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatthaar-Saalmüller B., Mair K.H., Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Resp. Viruses. 2017;11(1):85–92. doi: 10.1111/irv.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., et al. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;(October) doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpour M., Mousavi T., Alimohammadi M., et al. The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: meta-analysis. Int. J. Immunopathol. Pharmacol. 2021;35 doi: 10.1177/20587384211031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb R.L., Nirula A., Chen P., et al. Effect of Bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19. JAMA. 2021;325(7):632. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., et al. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized Covid‐19 “long haulers.”. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J.B., Buchan S.A., Wilson S.E., Austin P.C., Schwartz K.L., Pharmd M.T. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv. 2021:1–27. [Google Scholar]
- Gunay L.M., Deuring J.J., Ph D., Rizzo L.V., Ph D. 2021. Tofacitinib in Patients Hospitalized With Covid-19 Pneumonia; pp. 1–11. [DOI] [Google Scholar]
- Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- Habibzadeh P., Dastsooz H., Eshraghi M., Łos * M.J., Klionsky D.J., Ghavami S. Autophagy: The Potential Link between SARS-CoV-2 and Cancer. Cancers. 2021;13:5721. doi: 10.3390/cancers13225721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Klonisch S., Mehrpour M., Shojaei S., et al. Glioblastoma and chemoresistance to alkylating agents: involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Ther. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Wang L., Tan J., Liu H., Ni Y. High-dose vitamin C intravenous infusion in the treatment of patients with COVID-19. Medicine (Baltimore) 2021;100(19):e25876. doi: 10.1097/MD.0000000000025876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idilman I.S., Telli Dizman G., Ardali Duzgun S., et al. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur. Radiol. 2021;31(2):1090–1099. doi: 10.1007/s00330-020-07155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiaud P., Axfors C., Schmitt A.M., et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19. JAMA. 2021;325(12):1185. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings A.-L. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57(11):939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020;5(1):293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.E., Brown-Augsburger P.L., Corbett K.S., et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021;13(593):eabf1906. doi: 10.1126/scitranslmed.abf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno J.A., Wheatley A.K. Boosting immunity to COVID-19 vaccines. Nat. Med. 2021;27(11):1874–1875. doi: 10.1038/s41591-021-01560-x. [DOI] [PubMed] [Google Scholar]
- Jurgeit A., McDowell R., Moese S., Meldrum E., Schwendener R., Greber U.F. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. Lee B, ed. PLoS Pathog. 2012;8(10):e1002976. doi: 10.1371/journal.ppat.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri H., Lambourne O.A., Mehellou Y. Niclosamide, a Drug with Many (Re)purposes. ChemMedChem. 2018;13(11):1088–1091. doi: 10.1002/cmdc.201800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis DG Are statins safe in patients with COVID-19? J. Clin. Lipidol. 2020;14(4):396–397. doi: 10.1016/j.jacl.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashour Z., Kashour T., Gerberi D., Tleyjeh I.M. Mortality, viral clearance, and other clinical outcomes of hydroxychloroquine in COVID‐19 patients: a systematic review and meta‐analysis of randomized controlled trials. Clin. Transl. Sci. 2021;14(3):1101–1112. doi: 10.1111/cts.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Helfand B.K.I., Gou R.Y., et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.29540. e2029540-e2029540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEVZARA (Sarilumab) 2017. Summary of Product Characteristics. [Google Scholar]
- Kirkup C., Pawlowski C., Puranik A., et al. Healthcare disparities among anticoagulation therapies for severe COVID-19 patients in the multi-site VIRUS registry. J. Med. Virol. 2021;93(7):4303–4318. doi: 10.1002/jmv.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtipal N., Bharadwaj S., Kang S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korley F.K., Durkalski-Mauldin V., Yeatts S.D., et al. Early convalescent plasma for high-risk outpatients with Covid-19. N. Engl. J. Med. 2021:1–11. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Lechowicz K., Drożdżal S., et al. COVID-19—the potential beneficial therapeutic effects of spironolactone during SARS-CoV-2 infection. Pharmaceuticals. 2021;14(1):71. doi: 10.3390/ph14010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am. J. Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberger N., Hirsch C., Chai K.L., et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst. Rev. 2021;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazopoulou E., Huet T., Cavalli G., et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3(10):e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechowicz K., Drożdżal S., Machaj F., et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J. Clin. Med. 2020;9(6):1–21. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E.J., Mattar C., Zorumski C.F., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F., Honda H., Fowler R.A., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021;9(5):522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F., Honda H., Fowler R.A., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19 : a randomised, double-blind, placebo- controlled phase 3 trial. Lancet Respir. Med. 2021;9(May) doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine—update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang J., Li S., et al. Efficacy and safety of Reyanning mixture combined with conventional Western medicine for treating COVID-19. Medicine (Baltimore) 2021;100(3):e24169. doi: 10.1097/MD.0000000000024169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.S., Emberson J.R., Mafham M., et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Martínez M.M., Contreras M.A., Marín W., D’Marco L. Estatinas en COVID-19: ¿existe algún fundamento? Clínica e Investig en Arterioscler. 2020;32(6):278–281. doi: 10.1016/j.arteri.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhang T., Ma L., et al. Efficacy and safety of Lianhua Qingwen in the treatment of patients with moderate COVID-19 infection. Medicine (Baltimore) 2020;99(33):e21614. doi: 10.1097/MD.0000000000021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Blet A., Smyth D. Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Logue S.E., McGrath E.P., Cleary P., et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018;9(1):1–14. doi: 10.1038/s41467-018-05763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;(July) doi: 10.1056/NEJMoa2108891. NEJMoa2108891. [DOI] [PubMed] [Google Scholar]
- Maeshima K., Yamaoka K., Kubo S., et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–1798. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375:n2697. doi: 10.1136/bmj.n2697. [DOI] [PubMed] [Google Scholar]
- Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- Malgie J., Schoones J.W., Pijls B.G. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin. Infect. Dis. 2021;72(11):e742–e749. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi V.C., Ramanan A.V., de Bono S., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;(September) doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017;151(6):1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Mehrbod P., Ande S.R., Alizadeh J., et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10(1):376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS One. 2009;4(12):e8342. doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P.D., Marsden R.J., Tear V.J., et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021;9(2):196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326(18):1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . 2016. Stable Angina: Management Clinical Guideline. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . 2020. Acute Coronary Syndromes NICE Guideline.https://www.nice.org.uk/guidance/ng185 Accessed September 3, 2021. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . 2021. Atrial Fibrillation: Diagnosis and Management NICE Guideline. [PubMed] [Google Scholar]
- Okumuş N., Demirtürk N., Çetinkaya R.A., et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect. Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. 2021. Therapeutics and COVID-19: Living Guideline. August. [PubMed] [Google Scholar]
- Painter W.P., Holman W., Bush J.A., et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65(5) doi: 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi R., Costanzo S., Di Castelnuovo A., de Gaetano G., Donati M.B. Iacoviello L. Different Anticoagulant Regimens, Mortality, and Bleeding in Hospitalized Patients with COVID-19: A Systematic Review and an Updated Meta-Analysis. Semin. Thromb. Hemost. 2021;47(04):372–391. doi: 10.1055/s-0041-1726034. [DOI] [PubMed] [Google Scholar]
- Pawlowski C., Venkatakrishnan A., Kirkup C., et al. Enoxaparin is associated with lower rates of mortality than unfractionated Heparin in hospitalized COVID-19 patients. EClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peymani P., Dehesh T., Aligolighasemabadi F., et al. Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Transl. Med. Commun. 2021;6(1):3. doi: 10.1186/s41231-021-00082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindiprolu S.K.S.S., Pindiprolu S.H. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard C.A., Morran M.P., Nestor-Kalinoski A.L. The COVID-19 pandemic: a global health crisis. Physiol. Genomics. 2020;52(11):549–557. doi: 10.1152/physiolgenomics.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A.S. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front. Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M., Seth T., Elavarasi A. Efficacy and safety of convalescent plasma for COVID-19: a systematic review and meta-analysis. Indian J. Hematol. Blood Transfus. 2021;37(3):347–365. doi: 10.1007/s12288-021-01417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun B.T., Badenes R., Heras La Calle G., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir. Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S., Nicolau D.V., Langford B., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021;9(7):763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupp-Barcaro I.F., Vital M.A., Galduróz J.C., Andreatini R. Potential antidepressant effect of amantadine: a review of preclinical studies and clinical trials. Rev. Bras. Psiquiatr. 2018;40(4):449–458. doi: 10.1590/1516-4446-2017-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupp-Barcaro I.F., Vital M.A., Galduróz J.C., Andreatini R. Potential antidepressant effect of amantadine: a review of preclinical studies and clinical trials. Rev. Bras. Psiquiatr. 2018;40(4):449–458. doi: 10.1590/1516-4446-2017-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikirti Roy R., Pattadar C., et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India. J. Pharm. Pharm. Sci. 2021;24:343–350. doi: 10.18433/jpps32105. [DOI] [PubMed] [Google Scholar]
- Rawat D., Roy A., Maitra S., Shankar V., Khanna P., Baidya D.K. Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102189. doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group . 2021. Aspirin in Patients Admitted to Hospital With COVID-19 (RECOVERY): a Randomised, Controlled, Open-label, Platform Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Shi Y., Li G. Traditional Chinese medicine formula Xiaoqinglong decoction for cough caused by COVID-19. Medicine (Baltimore) 2020;99(48):e23261. doi: 10.1097/MD.0000000000023261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Diez R.R., Tejera-Muñoz A., Marquez-Exposito L., et al. Statins: Could an old friend help in the fight against COVID-19? Br. J. Pharmacol. 2020;177(21):4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr Y., Giovini M., Leone M., et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann. Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L.P., Fakhruddin K.S., Panduwawala C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontol. Scand. 2020;78(6):467–473. doi: 10.1080/00016357.2020.1787505. [DOI] [PubMed] [Google Scholar]
- Sayed Ahmed H.A., Merrell E., Ismail M., et al. Rationales and uncertainties for aspirin use in COVID-19: a narrative review. Fam. Med. Commun. Heal. 2021;9(2) doi: 10.1136/fmch-2020-000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebba A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health. Syst. Pharm. 2008;65(15):1413–1418. doi: 10.2146/ajhp070449. [DOI] [PubMed] [Google Scholar]
- Self W.H., Semler M.W., Leither L.M., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G., Huang S., Cui Y., Yang D. Maxingshigan decoction for treating COVID-19. Medicine (Baltimore) 2020;99(48):e23224. doi: 10.1097/MD.0000000000023224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5(1):1–12. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei S., Suresh M., Klionsky D.J., Labouta H.I., Ghavami S. 2020. Autophagy and SARS-CoV-2 Infection: a Possible Smart Targeting of the Autophagy Pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R.T., Chalasani P., Wei R., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021;27(11):2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Andrade B., Siqueira S., de Assis Soares W.R., et al. Long-COVID and Post-COVID health complications: an up-to-Date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4) doi: 10.3390/v13040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons B., Wentzel H., Mobarak S., et al. Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis. J. Antimicrob. Chemother. 2021;76(2):286–291. doi: 10.1093/jac/dkaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinagra Gianfranco, Anzini Marco, Pereira Naveen L., et al. Myocarditis in clinical practice. Mayo Clin. Proc. 2016;91(9):1256–1266. doi: 10.1016/J.MAYOCP.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Singh R., Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diab. Metab. Syndr. Clin. Res. Rev. 2020;14(4):641–648. doi: 10.1016/j.dsx.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner B. [Perioperative dexamethasone] Anaesthesist. 2019;68(10):676–682. doi: 10.1007/s00101-019-00672-x. [DOI] [PubMed] [Google Scholar]
- Sotrovimab . 2021. Drugs and Lactation Database (LactMed). Bethesda (MD)http://www.ncbi.nlm.nih.gov/pubmed/34165945 [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Krishnan V., de Bono S., et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020;12(8):e12697. doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subir R., Jagat J.M., Kalyan K.G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19) Diab. Metab. Syndr. Clin. Res. Rev. 2020;14(5):1225–1229. doi: 10.1016/j.dsx.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureda A., Alizadeh J., Nabavi S.F., et al. Endoplasmic reticulum as a potential therapeutic target for covid-19 infection management? Eur. J. Pharmacol. 2020;882 doi: 10.1016/j.ejphar.2020.173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarpak L., Pruc M., Gasecka A., et al. Should we supplement zinc in COVID-19 patients? Evidence from meta-analysis. Polish. Arch. Intern. Med. 2021;(June) doi: 10.20452/pamw.16048. [DOI] [PubMed] [Google Scholar]
- Tanasescu S., Lévesque H., Thuillez C. Pharmacologie de l’aspirine. La Rev Médecine Interne. 2000;21:S18–S26. doi: 10.1016/S0248-8663(00)88721-4. [DOI] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides Theoharis C., Cholevas Christos, Polyzoidis Konstantinos, Politis Antonios. Long-COVID syndrome-associated brain fog and chemofog: luteolin to the rescue. Biofactors. 2021;47(2):232–241. doi: 10.1002/BIOF.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J.D., Luo S., Dean A.Q., Bozza W.P., Nalli A., Zhang B. COVID-19 update: the race to therapeutic development. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2020;53 doi: 10.1016/j.drup.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US National Library of Medicine . 2020. ClinicalTrials.gov. [Google Scholar]
- Vitiello A., Ferrara F. Colchicine and SARS-CoV-2: Management of the hyperinflammatory state. Respir. Med. 2021;178 doi: 10.1016/j.rmed.2021.106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: A meta-analysis of randomized, double-blind and placebo-controlled trials. Clin. Respir. J. 2018;12(3):857–864. doi: 10.1111/crj.12646. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhao Y., Hu W., et al. Treatment of coronavirus disease 2019 patients with prolonged postsymptomatic viral shedding with leflunomide: a single-center randomized controlled clinical trial. Clin. Infect. Dis. 2020;(September) doi: 10.1093/cid/ciaa1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., et al. Outpatients With Covid-19. 2021. REGN-COV2, a neutralizing antibody cocktail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . vol edition 67. Geneva PP; Geneva: 2021. Weekly Epidemilogical Update on COVID-19-23 November. [Google Scholar]
- Yue L., Zhou J., Zhou Y., et al. Antibody response elicited by a third boost dose of inactivated SARS-CoV-2 vaccine can neutralize SARS-CoV-2 variants of concern. Emerg. Microbes Infect. 2021;10(1):2125–2127. doi: 10.1080/22221751.2021.1996210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A.K., Dehgani-Mobaraki P. The mechanisms of action of Ivermectin against SARS-CoV-2: an evidence-based clinical review article. J Antibiot (Tokyo) 2021;(June) doi: 10.1038/s41429-021-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-J., Qin J.-J., Cheng X., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;(June) doi: 10.1016/j.cmet.2020.06.015. S1550-4131(20)30316-30318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Guo D., Fan M., Liu Y. Efficacy and safety of Xuanfei Baidu granules for treating COVID-19. Medicine (Baltimore) 2021;100(20):e25653. doi: 10.1097/MD.0000000000025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Zhou Y., Zhou Z., et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int. J. Infect. Dis. 2020;99:84–91. doi: 10.1016/j.ijid.2020.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Chen D., Zhang Y., et al. Efficacy and safety of xiyanping for COVID-2019. Medicine (Baltimore) 2020;99(46):e22962. doi: 10.1097/MD.0000000000022962. [DOI] [PMC free article] [PubMed] [Google Scholar]