Abstract
Objective
To determine the association of combined parental preconception overweight and obesity on infant birthweight.
Design
Retrospective study of fresh in vitro fertilization or intracytoplasmic sperm injection cycles (2009–2017).
Setting
Repromed, South Australia, assisted reproductive technology clinic.
Patients
Couples undergoing in vitro fertilization/intracytoplasmic sperm injection insemination with their own gametes and transfer of a fresh single blastocyst (N = 1,778).
Intervention(s)
None.
Main Outcome Measures
Parental body mass index (BMI) was recorded prior to cycle initiation. Infant birthweight was recorded at delivery. The impact of parental obesity and their interaction on first singleton term (≥37 weeks’ gestation) birthweight was assessed using linear regressions assessing nonlinearity and a pairwise linear interactions.
Results
In the base model where parental BMI is assumed linear, there was strong evidence for higher birthweight with increasing maternal BMI (11.2 g per maternal kg/m2; 95% confidence interval, 7.2, 15.1) but not paternal BMI. The inclusion of a pairwise linear interaction indicated that paternal BMI attenuates the positive association between maternal BMI and infant birthweight (interaction −0.88; 95% confidence interval, −1.49, −0.27). The inclusion of nonlinear maternal BMI terms did not change the conclusions.
Conclusions
Increases in the mean infant birthweight associated with maternal obesity are attenuated when the father is obese. While maternal BMI contributed more to the mean infant birthweight than paternal BMI, a couple-centered approach to preconception health advice is recommended, given the documented relationships between parental obesity and childhood weight beyond infancy. Further studies in both assisted reproductive technology and general population cohorts assessing the parental BMI interaction on infant birthweight are warranted.
Key Words: Maternal obesity, paternal obesity, offspring birthweight, body mass index, preconception
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00209
Obesity is a significant public health concern. There is a rising trend for increased body mass index (BMI) across all age groups, with obesity rates tripling over the past 40 years (1). A global systematic analysis found that men and women have gained weight across all age groups, with most rapid gains occurring during prime reproductive years (ages 20 and 40 years) (2). In 2014, 38.9 million pregnant women were estimated to be overweight, and 14.6 million were estimated to be obese (3). In Australia, approximately 50% of women and 70% of men who contributed to a pregnancy in 2017 were overweight or obese (4).
It is established that maternal prepregnancy BMI has a significant impact on infant birthweight, and this is irrespective of whether couples conceived naturally or through assisted reproductive technology (ART). In non-ART populations, maternal overweight or obesity increases the likelihood of an infant being born large for gestational age (odds ratio [OR], 1.45; 95% confidence interval [CI], 1.29, 1.63; and OR, 1.88; 95% CI, 1.67, 2.11, respectively) or with macrosomia (OR, 1.70; 95% CI, 1.55, 1.87; and OR, 2.92; 95% CI, 2.67, 3.20, respectively) (5). In comparison, the risk of delivering a small-for-gestational-age baby increases in mothers who are underweight (OR, 1.67; 95% CI, 1.49, 1.87) but decreases in those who are overweight (OR, 0.71; 95% CI, 0.66, 0.76) or obese mothers (OR, 0.88; 95% CI, 0.78, 0.99) (5). In women requiring ART, both maternal underweight and obesity are associated with an increased risk of low birthweight (adjusted risk ratio, 1.39; 95% CI, 1.25, 1.54; and adjusted risk ratio, 1.26; 95% CI, 1.20, 1.33, respectively) (6). The variance in results between studies is likely due to the differences seen between natural conception and ART cohorts, with several meta-analyses and large cohort studies showing that the mean birthweight in ART singletons is lower than in naturally conceived singletons (7, 8).
However, the potential impact of paternal BMI is rarely considered in these studies, despite a small body of evidence suggesting that paternal preconception overweight and obesity may also contribute to infant birthweight (9, 10). Furthermore, evidence for an interaction between the preconception obesity status of parents and infant birthweight is not possible to discern from existing studies, given that obesity tends to aggregate in family units so that obese mothers commonly have obese partners (11). One study including 2,980 parent–offspring trios reported that maternal BMI was a stronger predictor of ponderal index and birthweight than paternal BMI (12). A limitation of this study was that parental preconception body weight was estimated retrospectively by the mother at the time of child data collection at 3–18 years (12).
Therefore, while it is clearly evident that maternal preconception BMI affects infant birthweight, the influence of paternal preconception BMI remains unclear. Furthermore, it is unknown whether there is an additional effect on infant birthweight if both parents are overweight or obese. We hypothesized that the combination of both maternal and paternal preconception overweight/obesity has a greater impact on infant birthweight than either independent parental effect. This study aimed to assess the independent and combined effects of maternal and paternal preconception overweight and obesity on the mean infant birthweight utilizing an ART cohort where preconception parental BMI is routinely collected.
Materials and methods
Human Ethics
The Scientific Advisory Committee of Repromed (Adelaide, Australia) approved the retrospective study (November 14, 2019). The study was exempt from Human Research Ethics Committee review at the University of Adelaide. Patients had previously given written consent to allow their records to be accessed for low-risk retrospective investigation.
Study Population and Data Collection
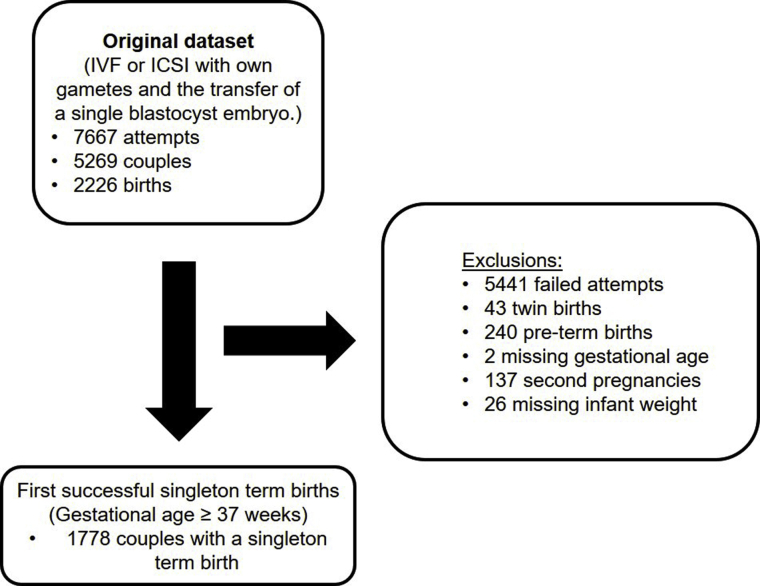
The study involved the retrospective analysis of fresh cycles from 2009 to 2017 at Repromed (Dulwich, South Australia, and Darwin, Northern Territory, clinics). Cycles including in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) with autologous sperm and eggs and the transfer of a single blastocyst embryo were assessed (Supplemental Fig. 1, available online). First singleton term births (≥37 weeks’ gestation) with birthweights recorded were included in the analysis. Preterm births (<37 weeks’ gestation), twin births, and second pregnancies from the same patient couple were excluded from the analysis (Supplemental Fig. 1). Parental, demographic, and treatment factors that are known to influence birthweight including maternal (13) and paternal age (14), socioeconomic status (15) (assessed using the Socio-Economic Indexes for Areas [SEIFA]) (16), insemination method (17), sex of baby (18), delivery method (19), and gestational age (20) were collected from case notes. A high SEIFA score indicates greater social advantage, while a low score indicates relatively greater disadvantage; the average SEIFA score is 1,000, and the middle two-thirds of the SEIFA scores generally fall between approximately 900 and 1,100 (16). Infertility diagnosis was also collected from case notes; however, it was not included in the final modeling analysis as it has been previously shown to not be associated with birthweight in term pregnancies (21, 22).
All data including infant birthweight (g), gestational age (weeks), sex (male/female), twin deliveries, and delivery method (vaginal/cesarean) were supplied by the treating obstetrician as per the ART treatment act that indicates mandatory reporting to the Australian and New Zealand Assisted Reproduction Database.
Assessment of Parental BMI
Body mass index of both parents was recorded before cycle initiation as part of routine clinical practice at Repromed. Both maternal and paternal heights were measured with a stadiometer (cm), and weight (kg) was measured with electronic scales. Body mass index was calculated using the formula weight/height2 and categorized based on the World Health Organisation criteria as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity (>30.0 kg/m2). Obesity was subclassified as obesity class I (30.0–34.9 kg/m2), obesity class II (35.0–39.9 kg/m2), and obesity class III (>40 kg/m2).
IVF Protocol
Women underwent a gonadotropin-releasing hormone antagonist protocol of treatment with vaginal progesterone gel (Crinone)/estradiol valerate luteal support or human-derived human chorionic gonadotropin luteal support (Pregnyl), as previously described (23). Over the course of the study (2009–2017), there were no substantive changes to laboratory protocols, including in culture media, consumables, gas phase, or equipment used including incubator type. During this time frame, the clinic was relatively static in its clinical protocols and policies. Extended culture was standard protocol (cleavage-stage transfer was rarely utilized), and stimulation regimes were also relatively static (antagonist protocol was standard). Eggs were fertilized by either standard IVF or ICSI in fertilization medium (G-IVF-PLUS, Vitrolife, Gothenburg, Sweden). Embryos were cultured using the sequential culture media system supplied by Vitrolife at 6% CO2, 5% O2, and 89% N2, wherein cleavage-stage embryos were grown until day 3 in G1 PLUS and then moved into G2 PLUS, which supported blastocyst development until embryo transfer on day 4 or 5. The best morphologically graded blastocyst was transferred using EmbryoGlue transfer medium (Vitrolife). Patients were in the care of their treating IVF physician until confirmation of a viable pregnancy following ultrasound at 6–8 weeks’ gestation and then referred on to primary obstetric care.
Statistical Methods
For continuous demographic, treatment, and outcome factors, means (standard deviations) and medians (ranges) are reported, and for discrete factors, frequencies (percentages) are reported. Associations between paternal and maternal BMI and infant birthweight were assessed using linear regressions, adjusting for baby sex (male or female), gestational age, delivery method (cesarean or vaginal), transfer method (IVF or ICSI), year of birth, maternal age, paternal age, and parental SEIFA score. Nonlinear associations were modeled using restricted cubic splines (knots at 5th, 35th, 65th, and 95th percentiles) for gestational age. This base model (M0) was extended in the following three stages: model M1, initially with the inclusion of the pairwise linear interaction of parental BMIs; model M2, then with the inclusion of nonlinear terms for maternal BMI; and model M3, finally with the inclusion of nonlinear terms for paternal BMI, that is, this final model included nonlinear terms for both parental BMIs and their pairwise linear interaction. The nonlinear associations were also modeled using restricted cubic splines with four knots. Multiple imputation using chained equations (100 datasets were imputed each with 100 iterations) was employed to account for the substantial missing parental BMI data (18% and 33% for maternal and paternal data, respectively). Analyses were performed in R (version 3.6.3) using the mice and rms packages. A P value of <.05 was considered statistically significant.
Results
Patient Demographics
A total of 1,778 couples were included in the analysis (Supplemental Fig. 1). The median age of mothers was 32 (range, 20.0–45.0) years, which was lower than that of fathers (35 years; range, 20.0–65.0) (Table 1). The median BMI of mothers (24.4 kg/m2; range, 16.2–55.9) was in the normal weight category, but BMI spanned from underweight (<18.5 kg/m2) to obese class III (>40 kg/m2) categories. The median BMI of fathers (27.4 kg/m2; range, 17.3–54.2) was in the overweight category and also spanned from underweight to obese class III. Moreover, 33% of paternal BMI data was missing compared with 18% of maternal BMI data. The mean SEIFA score (mean, 994; standard deviation, 72) was slightly lower than the Australian benchmark of 1,000, reflecting social disadvantage in this cohort. Male factor infertility was the most common contributing infertility diagnosis (53%) in the cohort. Intracytoplasmic sperm injection insemination was used in over 80% of cases with delivery method (vaginal vs. cesarean) and infant sex (female vs. male) split approximately 50%. Table 1 presents demographic summary statistics, with Supplemental Table 1 (available online) presenting these statistics by parental obesity categories.
Table 1.
Summary of parental demographics, treatment choices, and birth outcomes.
| Parental characteristics | N = 1,778 |
|---|---|
| Age and BMI | |
| Maternal age (years) | |
| Median (range) | 33.0 (20.0, 45.0) |
| Mean (SD) | 32.8 (4.12) |
| Paternal age (years) | |
| Median (range) | 35.0 (20.0, 65.0) |
| Mean (SD) | 36.1 (6.3) |
| Maternal BMI (kg/m2) | |
| Median (range) | 24.4 (16.2, 55.9) |
| Mean (SD) | 25.9 (6.0) |
| Missing | 315 (18%) |
| <18.5 kg/m2 | 40 (2%) |
| 18.5–24.9 kg/m2 | 754 (42%) |
| 25–29.9 kg/m2 | 371 (21%) |
| 30–34.9 kg/m2 | 155 (9%) |
| 35–39.9 kg/m2 | 91 (5%) |
| >40 kg/m2 | 52 (3%) |
| Paternal BMI (kg/m2) | |
| Median (range) | 27.4 (17.3, 54.2) |
| Mean (SD) | 28.1 (4.6) |
| Missing | 585 (33%) |
| <18.5 kg/m2 | 3 (<1%) |
| 18.5–24.9 kg/m2 | 283 (16%) |
| 25–29.9 kg/m2 | 572 (32%) |
| 30–34.9 kg/m2 | 238 (13%) |
| 35–39.9 kg/m2 | 68 (4%) |
| >40 kg/m2 | 29 (2%) |
| Couple BMI (kg/m2) | |
| Both <30 kg/m2 | 747 (42%) |
| Maternal <30 kg/m2, paternal >30 kg/m2 | 207 (12%) |
| Maternal >30 kg/m2, paternal <30 kg/m2 | 116 (7%) |
| Both >30 kg/m2 | 117 (7%) |
| Maternal <30 kg/m2 (paternal missing) | 214 (12%) |
| Maternal >30 kg/m2 (paternal missing) | 62 (3%) |
| Paternal <30 kg/m2 (maternal missing) | 2 (<1%) |
| Paternal >30 kg/m2 (maternal missing) | 4 (<1%) |
| Both missing | 309 (17%) |
| SEIFA | |
| Median (range) | 1,000 (673, 1163) |
| Mean (SD) | 994 (72) |
| Missing | 35 (2%) |
| Infertility diagnosis | |
| Tubal factor | 147 (8%) |
| Endometrial factor | 121 (7%) |
| Male factor | 949 (53%) |
| Other | 590 (33%) |
| Unexplained | 380 (21%) |
| Birth factors | |
| Insemination method | |
| IVF | 278 (16%) |
| ICSI | 1,500 (84%) |
| Delivery method | |
| Vaginal | 1,041 (59%) |
| Cesarean | 734 (41%) |
| Missing | 3 (<1%) |
| Gestational length (weeks) | |
| Median (range) | 39.1 (37.0, 42.1) |
| Mean (SD) | 39.2 (1.1) |
| Infant Sex | |
| Female | 896 (50%) |
| Male | 882 (50%) |
| Infant birthweight (g) | |
| Median (range) | 3,330 (1587, 4998) |
| Mean (SD) | 3,358 (448) |
| <2,500 g | 41 (2%) |
| 2,500–3,999 g | 1,587 (89%) |
| >4,000 g | 150 (8%) |
Note: BMI = body mass index; SEIFA = Socio-Economic Indexes for Areas; SD = standard deviation; IVF = In vitro fertilization; ICSI = intracytoplasmic sperm injection.
Parental Preconception BMI and Infant Birthweight
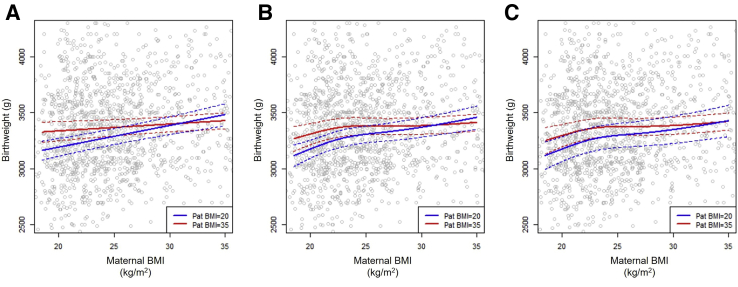
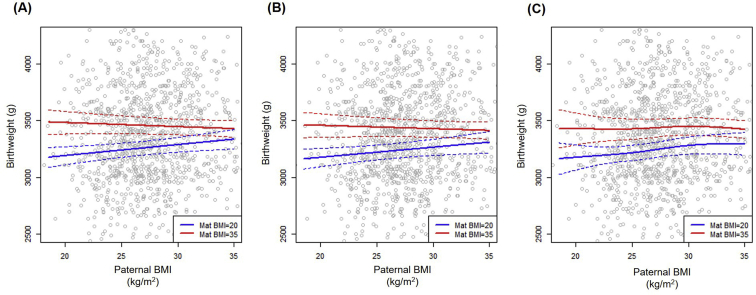
In the base model of infant birthweight (model M0) in which the influence of parental BMI was assumed linear, there was strong evidence for higher birthweight with increasing maternal BMI, with a mean increase of 11.2 g per maternal kg/m2 (95% CI, 7.2, 15.1; P<.001; Table 2). In this base model, there was no evidence of an association between paternal BMI and birthweight (P = .44, Table 2). The inclusion of a pairwise linear interaction (model M1), however, indicated that paternal BMI appears to attenuate the positive association between maternal BMI and infant birthweight (interaction ; 95% CI, −1.49, −0.27; P = .005; Table 2). As shown in Figure 1A, Supplemental Fig. 2A and Table 3, the estimated mean (95% CI) birthweights only increased from 3.33 kg (3.25, 3.42) to 3.43 kg (3.36, 3.50) in mothers who had BMIs equal to 20 kg/m2 as compared with 35 kg/m2, when the father was obese (BMI = 35 kg/m2). Comparatively, with a paternal BMI of 20 kg/m2, birthweight increased from 3.19 kg (3.11, 3.27) to 3.48 kg (3.38, 3.58) with increasing maternal BMI (20 vs. 35 kg/m2). The inclusion of nonlinear maternal BMI terms (model M2) did not qualitatively change these conclusions (paternal linear interaction P = .02, Fig. 1B, Supplemental Fig. 2B and Table 2). However, the interaction did become nonsignificant (P = .15) when both parental BMIs were modeled nonlinearly (model M3). This addition of nonlinear paternal BMI added negligible explanatory value (P = .41, Table 2), with no qualitative difference in the estimated mean (95% CI) birthweights for normal-weight versus obese parents. For example, in this full model, as presented in Figure 1C, Supplemental Fig. 2C and Table 3, the estimated birthweights increased from 3.18 kg (3.07, 3.29) to 3.43 kg (3.29, 3.57) for an increase in mothers’ BMI from 20 to 35 kg/m2, when the father had normal weight (BMI = 20 kg/m2). This is twice the change in birthweights that was observed for when fathers were obese (BMI = 35 kg/m2), for which the increase in birthweight was 3.30 kg (3.20, 3.40) to 3.42 kg (3.34, 3.50) for the same change in mothers’ BMI. The estimates for the four models, including the estimates for the adjustments (i.e., parental age, SEIFA score, baby sex, gestational age, delivery method, transfer method, and year of delivery), are shown in Supplemental Table 2 (available online).
Table 2.
The association of parental body mass index with infant birthweight, examining the influence of nonlinearity and a pairwise linear interaction.
| Parental interactions | M0 (linear BMI) |
M1 (linear + int) |
M2 (non-lin mat-BMI + int) |
M3 (non-lin BMI + int) |
||||
|---|---|---|---|---|---|---|---|---|
| Est (g) [95% CI] | P value | Est (g) [95% CI] | P value | Est (g) [95% CI] | P value | Est (g) [95% CI] | P value | |
| Mat BMI (linear) | 11.2 [7.2, 15.1] | <.001 | 12.2 [8.2, 16.2] | <.001 | 33.7 [12.1, 55.3] | <.001∗ | 33.9 [12.2, 55.6] | <.001∗ |
| Mat BMI (nonlinear 1) | −152 [−316, 12] | −156 [−320, 9] | ||||||
| Mat BMI (nonlinear 2) | 310 [−37, 657] | 318 [−31, 667] | ||||||
| Pat BMI (linear) | 2.0 [−3.1, 7.1] | .44 | 4.4 [−1.0, 9.9] | .11 | 4.2 [−1.3, 9.7] | .13 | 4.0 [−18.3, 26.2] | .10∗ |
| Pat BMI (nonlinear 1) | 26 [−75, 126] | |||||||
| Pat BMI (nonlinear 2) | −110 [−424, 204] | |||||||
| Mat BMI (linear) × Pat BMI (linear) | −0.88 [−1.49, −0.27] | .005 | −0.79 [−1.5, −0.1] | .02 | −0.56 [−1.31, 0.20] | .15 | ||
Note: BMI = body mass index; Mat = maternal; Pat = paternal; lin = linear; int = interaction. ∗A single P value is reported for all nonlinear terms. The base model M0 includes just the two parental BMIs linearly. Model M1 extends M0 with the inclusion of the pairwise linear interaction of parental BMIs. Model M2 extends M1 with the inclusion of nonlinear terms for maternal BMI. Model M3 is the full model with nonlinear terms for both parental BMIs and their pairwise linear interaction. The following adjustment factors were included in the models: gestational age, parental age, Socio-Economic Indexes for Areas score, year of delivery, and delivery method.
Figure 1.
Estimated mean infant birthweight by maternal body mass index (BMI). The interaction is illustrated by varying maternal BMI with paternal (Pat) BMI set at 20 (blue) and 35 (red) kg/m2, respectively. (A) Model M1 with both linear paternal BMIs, (B) model M2 with nonlinear maternal BMI, and (C) model M3 with both nonlinear parental BMIs. Gray circles are observed birthweights. Solid lines are the estimated means, and dashed lines are the 95% confidence intervals. Presented mean estimates are for covariates: female baby; gestational age, 3 weeks; vaginal birth in 2012; in vitro fertilization insemination; maternal age, 33 years; paternal age, 35 years; and Socio-Economic Indexes for Areas score, 1000.
Table 3.
Estimates and 95% confidence intervals for the mean infant birthweight for maternal and paternal body mass indexes.
| Maternal BMI (kg/m2) | Paternal BMI (kg/m2) | M1: birthweight (g) [95% CI] | M2: birthweight (g) [95% CI] | M3: birthweight (g) [95% CI] |
|---|---|---|---|---|
| 20 | 20 | 3191 [3112, 3270] | 3176 [3095, 3256] | 3176 [3066, 3286] |
| 35 | 20 | 3481 [3382, 3580] | 3457 [3354, 3560] | 3427 [3287, 3567] |
| 20 | 35 | 3335 [3250, 3421] | 3309 [3215, 3403] | 3296 [3196, 3395] |
| 35 | 35 | 3427 [3355, 3500] | 3412 [3336, 3487] | 3422 [3344, 3499] |
Note: BMI = body mass index. Model M1 extends M0 with the inclusion of the pairwise linear interaction of parental BMIs. Model M2 extends M1 with the inclusion of nonlinear terms for maternal BMI. Model M3 is the full model with both nonlinear terms for both parental BMIs and their pairwise linear interaction. The following adjustment factors were included in the models: gestational age, parental age, Socio-Economic Indexes for Areas score, year of delivery, and delivery method.
Discussion
In a retrospective study of 1,778 singleton term births following ART, we were unable to detect increasing birthweight when both parents were obese, as compared with just one parent alone. That is, while infants are heavier when born to overweight or obese mothers, this association appears to be substantially attenuated in the presence of paternal obesity. Importantly, the estimated increase in birthweight with maternal obesity (with normal paternal BMI) was double than that observed with paternal obesity (and normal maternal BMI), demonstrating a much larger contribution of maternal BMI than that of paternal BMI to infant birthweight.
To our knowledge, this is the first study assessing the possibility of a combined contribution of maternal and paternal preconception BMIs on infant birthweight. The strengths of our study include the use of a database in which preconception health, IVF cycle outcomes, and pregnancy rates were registered prospectively, thereby minimizing selection bias. Body mass index was calculated preconception from clinically recorded measurements of maternal and paternal weights and heights; the analysis only included first singleton term births; and the large population size from a singular ART unit limited variability in clinical protocols. The limitations of our study include the retrospective study design, which limits the ability to control and collect some key parental factors that can influence infant birthweight, including parental smoking, ethnicity, or infertility duration (24, 25, 26, 27). The use of a subfertile cohort means that our results may only be generalizable to couples undergoing ART, although infertility diagnosis has previously been shown to minimally influence infant birthweight in term pregnancies (21, 22). There was no prior power calculation performed for this analysis, although it is known that large samples sizes are required to reliably detect interactions.
Contrary to our hypothesis, we found no additional effect of having two overweight or obese parents on infant birthweight beyond the effect of maternal obesity. Evidence from our rodent model of obesity also indicates that the effect of parental obesity on infant birthweight is unlikely to be additive but is instead a combination of both maternal and paternal phenotypes (28). This is evident in the current dataset where the effect of maternal obesity on infant birthweight was substantially attenuated in the presence of paternal obesity. This is because infants born to obese fathers start out heavier (approximately 150 g). While we found no additional effect of combined parental BMI on infant birthweight, additive effects may become evident as infants grow. For instance, the Raine cohort in Western Australia demonstrated that parental obesity was the strongest predictor of offspring adult BMI (29), while the Midspan Family Study in Scotland revealed that adult offspring from two obese parents had a higher risk of cardiovascular diseases compared with offspring without obese parents (30). In contrast, a study assessing 2,980 parent–offspring trios showed that the effect of parental preconception BMI on child and adolescent BMI was minimal (12) and suggested that while parental obesity may play a role in infant birthweight, family environment likely plays a much larger role in cardio-metabolic disease risk in adult life. The clinical relevance of our results are unclear (i.e., approximately 290-g increase in birthweight is unlikely to impact clinical outcomes); however, birthweight is reported to play a significant role in the establishment of adolescent and early adulthood BMI (29, 31). Therefore, if obesity aggregates within families, then a focus on preconception planning for “healthy couples” and emphasizing the need to improve lifestyle for the family unit prior to pregnancy is recommended. This may help shape and establish later life habits to support long-term health of future generations.
There is a large body of literature demonstrating the impact of maternal BMI on infant birthweight (32), and there is some evidence that paternal BMI also impacts infant birthweight (9, 10). Unfortunately, much of the literature on paternal BMI includes self-reported paternal height and weight from the mother during pregnancy, at birth, or when the child was a toddler, rather than preconception, with adjustments for potential confounders often inadequate (33, 34). Thus, the impact of paternal BMI remains unclear. Some (35, 36) but not all (37, 38, 39) studies have demonstrated an association between paternal BMI and infant birthweight, with similar mixed reports seen on the extreme ends of infant birthweight (small for gestational age or large for gestational age) (22, 40, 41). In our study, we found that paternal BMI only minimally impacted infant birthweight, with a paternal effect only evident when paternal BMI was added to the regression models as a pairwise linear interaction (4.43 g for every 1-unit increase in paternal BMI). The lack of consensus in the reported effects of paternal overweight and obesity on infant birthweight highlights the necessity for further large cohort studies in both ART and naturally conceived populations, ensuring correct clinical measurements of preconception paternal BMI.
The mechanism on how parental obesity is altering infant birthweight is likely due to a combination of genetic and epigenetic factors (noncoding ribonucleic acid (RNAs) and deoxyribonucleic acid (DNA) and histone methylation) delivered by sperm and eggs at fertilization (42, 43, 44) and the relationship between in utero fetal programming by nutritional stimuli (45, 46). A number of genes are known to play a part in the heritability of weight (47, 48); however, these genetic loci do not fully account for the transmission, indicating that programming effects go beyond underlining genetics. Maternal obesity during gestation has been associated with an altered expression of a number of circulating microRNAs with levels directly correlating with changes in placental weight and infant birthweight (49), while a number of studies in animal models and humans directly show a link between paternal obesity at conception, sperm epigenetic changes, and altered fetal phenotypes (36, 50, 51, 52, 53, 54). These studies collectively indicate that the parental programming effect to infant birthweight goes beyond that of a shared living environment, with preconception factors able to influence the health of subsequent children.
In conclusion, utilizing close to 1,800 singleton term births from an Australian ART cohort, our results demonstrate that increases in mean infant birthweight associated with maternal obesity are attenuated when the father is obese. While maternal BMI contributed more to the mean infant birthweight than paternal BMI, a couple-centered approach to preconception health advice could be valuable, given the relationships documented between parental obesity and childhood weight beyond infancy. Further studies in both ART and general population cohorts assessing the interaction of maternal and paternal preconception BMIs on infant birthweight to support or refute our findings are warranted.
Acknowledgment
The authors thank Professor Sarah Robertson for her review and edit of the final manuscript.
Footnotes
N.O.M. has nothing to disclose. A.D.V. has nothing to disclose. D.Z.F. is a paid employee of Monash IVF Group Ltd. J.A.G. has nothing to disclose.
N.O.M. is the recipient of an NHMRC Early Career Fellowship (AA1088964). J.A.G. is the recipient of a Robinson Research Institute Career Development Fellowship.
Supplementary data
Supplemental Figure 1.
Cohort inclusion flow diagram.
Supplemental Figure 2.
Estimated mean infant birthweight by paternal body mass index (BMI). The interaction is illustrated by varying paternal BMI with maternal (Mat) BMI set at 20 (blue) and 35 (red) kg/m2, respectively. (A) Model M1 with both linear paternal BMIs, (B) model M2 with nonlinear maternal BMI, and (C) model M3 with both nonlinear parental BMIs. Gray circles are observed birthweights. Solid lines are the estimated means, and dashed lines are the 95% confidence intervals. Presented mean estimates are for covariates: female baby; gestational age, 3 weeks; vaginal birth in 2012; in vitro fertilization insemination; maternal age, 33 years; paternal age, 35 years; and Socio-Economic Indexes for Areas score, 1000.
References
- 1.National Academies Press. Global Trends in Obesity. Current Status and Response to the Global Obesity Pandemic: Proceedings of a Workshop. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Roundtable on Obesity Solutions; Callahan EA, editor. Washington (DC): National Academies Press (US); 2019 Jun 25. In. [PubMed]
- 2.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C., Xu X., Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AIHW Australian Government. Australian Institute of Health and Welfare. Australia's mothers and babies. 2017. https://www.aihw.gov.au/getmedia/2a0c22a2-ba27-4ba0-ad47-ebbe51854cd6/aihw-per-100-in-brief.pdf.aspx?inline=true Available at: In.
- 5.Liu P., Xu L., Wang Y., Zhang Y., Du Y., Sun Y., et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev. 2016;17:1091–1102. doi: 10.1111/obr.12455. [DOI] [PubMed] [Google Scholar]
- 6.Kawwass J.F., Kulkarni A.D., Hipp H.S., Crawford S., Kissin D.M., Jamieson D.J. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril. 2016;106:1742–1750. doi: 10.1016/j.fertnstert.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih W., Rushford D.D., Bourne H., Garrett C., McBain J.C., Healy D.L., et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- 8.McDonald S.D., Han Z., Mulla S., Murphy K.E., Beyene J., Ohlsson A., et al. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Campbell J.M., McPherson N.O. Influence of increased paternal BMI on pregnancy and child health outcomes independent of maternal effects: a systematic review and meta-analysis. Obes Res Clin Pract. 2019;13:511–521. doi: 10.1016/j.orcp.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Oldereid N.B., Wennerholm U.B., Pinborg A., Loft A., Laivuori H., Petzold M., et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2018;24:320–389. doi: 10.1093/humupd/dmy005. [DOI] [PubMed] [Google Scholar]
- 11.Gruber K.J., Haldeman L.A. Using the family to combat childhood and adult obesity. Prev Chronic Dis. 2009;6:A106. [PMC free article] [PubMed] [Google Scholar]
- 12.Kivimaki M., Lawlor D.A., Smith G.D., Elovainio M., Jokela M., Keltikangas-Jarvinen L., et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2007;86:1509–1514. doi: 10.1093/ajcn/86.5.1509. [DOI] [PubMed] [Google Scholar]
- 13.Kahveci B., Melekoglu R., Evruke I.C., Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. 2018;18:343. doi: 10.1186/s12884-018-1984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandwala Y.S., Baker V.L., Shaw G.M., Stevenson D.K., Lu Y., Eisenberg M.L. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ. 2018;363:k4372. doi: 10.1136/bmj.k4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astone N.M., Misra D., Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. 2007;21:310–318. doi: 10.1111/j.1365-3016.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 16.Adhikari P. Australian Bureau of Statistics; Canberra: 2006. Socio-Economic Indexes for Areas: Introduction, Use and Future Directions. 1351.0.55.015. [Google Scholar]
- 17.Pandey S., Shetty A., Hamilton M., Bhattacharya S., Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 18.Dobbins T.A., Sullivan E.A., Roberts C.L., Simpson J.M. Australian national birthweight percentiles by sex and gestational age, 1998-2007. Med J Aust. 2012;197:291–294. doi: 10.5694/mja11.11331. [DOI] [PubMed] [Google Scholar]
- 19.Weissmann-Brenner A., Simchen M.J., Zilberberg E., Kalter A., Weisz B., Achiron R., et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Scand. 2012;91:844–849. doi: 10.1111/j.1600-0412.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- 20.Hutcheon J.A., Bodnar L.M. Good practices for observational studies of maternal weight and weight gain in pregnancy. Paediatr Perinat Epidemiol. 2018;32:152–160. doi: 10.1111/ppe.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke B., Stern J.E., Kotelchuck M., Declercq E.R., Cohen B., Diop H. Birth outcomes by infertility diagnosis analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) J Reprod Med. 2015;60:480–490. [PMC free article] [PubMed] [Google Scholar]
- 22.Ma M., Zhang W., Zhang J., Liang Z., Kuang Y., Wang Y. Effect of paternal body mass index on neonatal outcomes of singletons after frozen-thawed embryo transfer cycles: analysis of 7,908 singleton newborns. Fertil Steril. 2020;113:1215–1223. doi: 10.1016/j.fertnstert.2020.02.100. [DOI] [PubMed] [Google Scholar]
- 23.Thalluri V., Tremellen K.P. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod. 2012;27:3487–3492. doi: 10.1093/humrep/des305. [DOI] [PubMed] [Google Scholar]
- 24.Abraham M., Alramadhan S., Iniguez C., Duijts L., Jaddoe V.W., Den Dekker H.T., et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andriani H., Kuo H.W. Adverse effects of parental smoking during pregnancy in urban and rural areas. BMC Pregnancy Childbirth. 2014;14:414. doi: 10.1186/s12884-014-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto V.Y., Luke B., Brown M.B., Jain T., Armstrong A., Grainger D.A., et al. Racial and ethnic disparities in assisted reproductive technology outcomes in the United States. Fertil Steril. 2010;93:382–390. doi: 10.1016/j.fertnstert.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso O., Baird D.D. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18:2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 28.McPherson N.O., Bell V.G., Zander-Fox D.L., Fullston T., Wu L.L., Robker R.L., et al. When two obese parents are worse than one! Impacts on embryo and fetal development. Am J Physiol Endocrinol Metab. 2015;309:E568–E581. doi: 10.1152/ajpendo.00230.2015. [DOI] [PubMed] [Google Scholar]
- 29.Rath S.R., Marsh J.A., Newnham J.P., Zhu K., Atkinson H.C., Mountain J., et al. Parental pre-pregnancy BMI is a dominant early-life risk factor influencing BMI of offspring in adulthood. Obes Sci Pract. 2016;2:48–57. doi: 10.1002/osp4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han T.S., Hart C.L., Haig C., Logue J., Upton M.N., Watt G.C., et al. Contributions of maternal and paternal adiposity and smoking to adult offspring adiposity and cardiovascular risk: the Midspan Family Study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham S.A., Kramer M.R., Narayan K.M. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:1660–1661. doi: 10.1056/NEJMc1402397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Z., Han S., Zhu J., Sun X., Ji C., Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Lopez L., Van Hulst A., Barnett T.A., Roy-Gagnon M.H., Tremblay A., O'Loughlin J., et al. Does parental body mass index status modify the associations among birth weight, early growth and childhood adiposity? Paediatr Child Health. 2013;18:e2–e9. doi: 10.1093/pch/18.2.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen T., Ajslev T.A., Angquist L., Morgen C.S., Ciuchi I.G., Davey Smith G. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7 y assessed in the Danish National Birth Cohort. Am J Clin Nutr. 2016;104:389–396. doi: 10.3945/ajcn.115.129171. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.P., Xiao X.M., Li J., Reichetzeder C., Wang Z.N., Hocher B. Paternal body mass index (BMI) is associated with offspring intrauterine growth in a gender dependent manner. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noor N., Cardenas A., Rifas-Shiman S.L., Pan H., Dreyfuss J.M., Oken E., et al. Association of periconception paternal body mass index with persistent changes in DNA methylation of offspring in childhood. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L'Abee C., Vrieze I., Kluck T., Erwich J.J., Stolk R.P., Sauer P.J. Parental factors affecting the weights of the placenta and the offspring. J Perinat Med. 2011;39:27–34. doi: 10.1515/jpm.2010.119. [DOI] [PubMed] [Google Scholar]
- 38.Pomeroy E., Wells J.C., Cole T.J., O'Callaghan M., Stock J.T. Relationships of maternal and paternal anthropometry with neonatal body size, proportions and adiposity in an Australian cohort. Am J Phys Anthropol. 2015;156:625–636. doi: 10.1002/ajpa.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutsaerts M.A., Groen H., Buiter-Van der Meer A., Sijtsma A., Sauer P.J., Land J.A., et al. Effects of paternal and maternal lifestyle factors on pregnancy complications and perinatal outcome. A population-based birth-cohort study: the GECKO Drenthe cohort. Hum Reprod. 2014;29:824–834. doi: 10.1093/humrep/deu006. [DOI] [PubMed] [Google Scholar]
- 40.McCowan L.M.E., North R.A., Kho E.M., Black M.A., Chan E.H., Dekker G.A., et al. Paternal contribution to small for gestational age babies: a multicenter prospective study. Obesity (Silver Spring) 2011;19:1035–1039. doi: 10.1038/oby.2010.279. [DOI] [PubMed] [Google Scholar]
- 41.Yang S., Zhou A., Xiong C., Yang R., Bassig B.A., Hu R., et al. Parental body mass index, gestational weight gain, and risk of macrosomia: a population-based case-control study in China. Paediatr Perinat Epidemiol. 2015;29:462–471. doi: 10.1111/ppe.12213. [DOI] [PubMed] [Google Scholar]
- 42.Bromfield J.J. Seminal fluid and reproduction: much more than previously thought. J Assist Reprod Genet. 2014;31:627–636. doi: 10.1007/s10815-014-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donkin I., Barres R. Sperm epigenetics and influence of environmental factors. Mol Metab. 2018;14:1–11. doi: 10.1016/j.molmet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portha B., Grandjean V., Movassat J. Mother or father: who is in the front line? Mechanisms underlying the non-genomic transmission of obesity/diabetes via the maternal or the paternal line. Nutrients. 2019;11:233. doi: 10.3390/nu11020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leddy M.A., Power M.L., Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 46.Grieger J.A., Clifton V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 2014;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrera B.M., Keildson S., Lindgren C.M. Genetics and epigenetics of obesity. Maturitas. 2011;69:41–49. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warrington N.M., Howe L.D., Wu Y.Y., Timpson N.J., Tilling K., Pennell C.E., et al. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carreras-Badosa G., Bonmati A., Ortega F.J., Mercader J.M., Guindo-Martinez M., Torrents D., et al. Altered circulating miRNA expression profile in pregestational and gestational obesity. J Clin Endocrinol Metab. 2015;100:E1446–E1456. doi: 10.1210/jc.2015-2872. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q., Yan M., Cao Z., Li X., Zhang Y., Shi J., et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 51.Lambrot R., Xu C., Saint-Phar S., Chountalos G., Cohen T., Paquet M., et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terashima M., Barbour S., Ren J., Yu W., Han Y., Muegge K. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics. 2015;10:861–871. doi: 10.1080/15592294.2015.1075691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ost A., Lempradl A., Casas E., Weigert M., Tiko T., Deniz M., et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–1364. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Soubry A., Schildkraut J.M., Murtha A., Wang F., Huang Z., Bernal A., et al. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013;11:29. doi: 10.1186/1741-7015-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.