Abstract
The angiotensin-converting enzyme (ACE)/Angiotensin II (Ang II) and angiotensin-converting enzyme 2 (ACE2)/angiotensin-(1-7) (Ang-(1-7)) pathways are coexpressed in most tissues. The balance between these pathways determines, at least in part, whether tissue damage will occur in response to pathological stimuli. The present study tested the hypothesis that male sex and high blood pressure are associated with ACE/ACE2 imbalance in the lungs. Experiments were conducted in male and female Wistar rats and spontaneously hypertensive rats (SHRs). Lung ACE and ACE2 gene expression was also evaluated in normotensive and hypertensive humans using the Genotype-Tissue Expression (GTEx) project. Compared with Wistar rats and female SHRs, male SHRs displayed reduced lung ACE2 mRNA, ACE2 protein abundance and ACE2 activity, and increased Ang II concentration. Lung ACE mRNA levels were higher in male SHRs than in Wistar rats, whereas lung ACE protein abundance and activity were similar among the four groups of rats. Lung Ang-(1-7) concentration was higher in female than in male SHRs (89 ± 17 vs. 43 ± 2 pg/g, P<0.05). Lung ACE to ACE2 mRNA expression in hypertensive patients was significantly higher than that in normotensive subjects. Taken together, these results demonstrate that male hypertensive rats display imbalance between the ACE/Ang II and ACE2/Ang-(1-7) pathways in the lungs mainly attributable to ACE2 down-regulation. Further studies should be conducted to investigate whether this imbalance between ACE/ACE2 may promote and accelerate lung injury in respiratory infections, including coronavirus disease 2019 (COVID-19).
Keywords: angiotensin II, angiotensin-(1-7), Genotype-Tissue Expression project, hypertension, sexual dimorphism
Introduction
Biological sex is recognized as a crucial determinant of susceptibility and clinical outcomes in non-communicable and infectious diseases. Sex differences have been well investigated in cardiology since several epidemiological studies demonstrated that premenopausal women are less prone to developing cardiovascular disease (CVDs) than age-matched men [1–3]. In accordance, high blood pressure, a major risk factor for CVDs, prevails in men before the age of 50 [4–6]. Similarly, male bias has emerged in the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Sex differences have been consistently implicated in the severity of COVID-19 [7], including mortality and the need for admission to intensive care [8]. One potential mechanism responsible for sex dimorphism in hypertension and COVID-19 is the effect of sex steroid hormones on the renin–angiotensin system (RAS) [9,10].
Abnormal activation of the classical RAS components, ultimately leading to the up-regulation of angiotensin II (Ang II) and activation of the Ang II type 1 receptor (AT1R), contributes to the development and progression of hypertension [11–15]. Conversely, angiotensin-converting enzyme 2 (ACE2) functions as a counterregulatory enzyme that cleaves Ang II to form angiotensin-(1-7) (Ang-(1-7)), which in turn activates the Mas receptor (MasR). The ACE2/Ang-(1-7) pathway exerts a protective role against hypertension by reducing blood pressure, promoting vasodilation, increasing the renal excretion of sodium and water, and exerting anti-inflammatory and antioxidant effects [16]. Interestingly, renal ACE2 activity and expression were reduced in ovariectomized hypertensive rats, whereas administration of 17-β estradiol restored ACE2 levels and conferred renoprotection to these animals [17]. These findings raise the possibility that estradiol-replete women may be protected from hypertensive renal disease at least partly by estradiol-mediated up-regulation of ACE2.
ACE2 is also the functional receptor for SARS-CoV-2 host cell entry [18], thus providing a biological link among hypertension, biological sex, and COVID-19. Loss of the ACE2 protective arm in the lungs may create an imbalance that favors the classic RAS, unleashing a cascade of harmful effects that leads to severe acute respiratory failure in COVID-19 [19–23]. Indeed, a causal relationship between imbalance of the angiotensin-converting enzyme (ACE)/ACE2 axis and acute respiratory distress syndrome has been established through the use of genetically modified animals, demonstrating that the ACE/Ang II/AT1R axis drives severe acute lung failure, whereas ACE2 protects against it [24]. Sex modulation of the lung ACE to ACE2 ratio in systemic arterial hypertension, however, remains elusive. The present study was undertaken to test the hypothesis that male sex and high blood pressure are associated with ACE/ACE2 imbalance in the lungs. To this end, we investigated the sex-specific modulation of ACE and ACE2 at the transcriptional, translational, and post-translational levels in the lungs of normotensive and hypertensive rats. Lung ACE to ACE2 gene expression was further evaluated in hypertensive and normotensive humans using the Genotype-Tissue Expression (GTEx) project.
Methods
Animals
All experiments were carried out following the ethical principles of animal research of the Brazilian College of Animal Experimentation and had been approved by the Institutional Animal Care and Use Committee (protocol number #1550/2020). All animal experiments took place in the Heart Institute (InCor), University of São Paulo Medical School, São Paulo, SP, Brazil. Blood pressure in conscious, restrained rats was measured noninvasively by tail-cuff plethysmography (BP-2000 Blood Pressure Analysis System, Visitech Systems, Apex, NC) 1 day before euthanasia as previously described [25]. Experiments were performed using 12-week-old male (n=10, BP = 129 ± 1 mmHg) and female (n=10, BP = 118 ± 1 mmHg) Wistar rats and age-matched male (n=9, BP = 185 ± 3 mmHg) and female (n=8, BP = 161 ± 2 mmHg) spontaneously hypertensive rats (SHRs) (Supplementary Figure S1). Rats were housed at the Heart Institute (InCor) animal facility under a 12-h dark–light cycle. Access to food and water was provided ad libitum. The rats were anesthetized intraperitoneally with pentobarbital (50 mg/kg body weight), terminal arterial blood samples were collected from the abdominal aorta to obtain serum, and the lungs were rapidly removed. Anesthetized rats were killed by decapitation.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated from lung tissue using TRIzol reagent (Thermo Fisher Scientific, Carlsbad, CA). The Super-Script IV Reverse Transcriptase (Thermo Fisher Scientific) was used to synthesize lung first-strand cDNA. Gene expression was determined by quantitative reverse transcription-polymerase chain reaction (RT-PCR). Real-time RT-PCRs were performed using SYBR Green PCR Master Mix-PE (Applied Biosystems, Foster City, CA) in the ABI Prism 7700 Sequence Detection System (Applied Biosystems). The following oligonucleotide primers were used: ACE, CCAGAGGGAATTGACCTAGAGAC (forward) and ACACTTCCTTGTTTTTCTGAAGCA (reverse); ACE2, GAGCCCATATGCCGACCAAA (forward) and CTCACCCATACGTCTGCCTC (reverse); and cyclophilin, AATGCTGGACCAAACACAAA (forward) and CCTTCTTTCACCTTCCCAAA (reverse). All samples were assayed in triplicate. The relative expression was normalized to that of cyclophilin.
Lung homogenate and membrane protein isolation
The lungs were homogenized in ice-cold PBS buffer (150 mM sodium chloride, 2.8 mM monobasic sodium phosphate, 7.2 mM dibasic sodium phosphate, pH 7.4) containing protease (1 μM pepstatin A, 1 μM leupeptin, 230 μM phenylmethylsulfonyl fluoride (PMSF)) (Sigma–Aldrich) and phosphatase inhibitors (15 mM sodium fluoride and 50 mM sodium pyrophosphate) (Sigma–Aldrich) using a Potter–Elvehjem-style tissue grinder (POLYMIX PX-SR50E, Kinematica, Luzern, Switzerland). The homogenate was centrifuged at 2000×g for 10 min at 4°C. Part of the supernatant was stored at −80°C, and the other part was centrifuged at 100000×g for 1 hour at 4°C. The pellet was then resuspended in fresh PBS with protease and phosphatase inhibitors and stored at −80°C. The determination of protein concentration in the samples was by the Lowry method [26].
SDS/PAGE and immunoblotting
Equal protein amounts of lung membrane proteins were run on an SDS/PAGE polyacrylamide gel (7.5%) and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Merck Millipore, Darmstadt, Germany) at 350 mA for 8–10 h at 4°C. Then, the PVDF membranes were incubated with blocking solution (5% nonfat dry milk or 5% bovine serum albumin and 0.1% Tween 20 in PBS, pH 7.4) for 1 h and overnight (4°C) with specific primary antibodies: a rabbit monoclonal antibody against ACE (1:1000; ab254222, Abcam, Cambridge, MA), a rabbit polyclonal antibody against ACE2 (1:1000; ab15348, Abcam) or a mouse monoclonal antibody against β-actin (1:5000; ab6276, Abcam). Proteins were detected using horseradish peroxidase-conjugated secondary antibodies (1:2000; Jackson ImmunoResearch, West Grove, PA). The bound antibodies were detected using an enhanced chemiluminescence system (GE Healthcare, United Kingdom) according to the manufacturer’s protocols. The visualized bands were digitized using an Image Scanner (GE HealthCare) and quantified using the Scion Image Software package (Scion Corporation, Frederick, MD).
Lung ACE and ACE2 activity
ACE and ACE2 activity from lung membrane proteins (2 µg for ACE and 20 µg for ACE2) were determined using the ACE activity assay kit (fluorometric) from Sigma–Aldrich (CS0002, St. Louis, MO) and the ACE2 activity assay kit (fluorimetric) from Abcam (ab273373), according to the manufacturer’s instructions. Both assays are based on the cleavage of synthetic fluorogenic peptides and were conducted in the presence or absence of selective inhibitors of ACE (10 µM captopril) and ACE2 (20 µM). Fluorescence was measured at 320 nm excitation and 420 nm emission wavelength in kinetic mode for 1 h at 37°C (Molecular Devices, San José, CA, U.S.A.). All samples and standards were assayed in duplicate. Enzyme activity was expressed as milliunits (mU) per mg, i.e., the amount of enzyme that releases 1 pmol of fluorescent product from the substrate, in 1 min, under the conditions of the assay method.
Lung ADAM17 activity
A disintegrin and metalloprotease 17 (ADAM17) activity was measured using a fluorescence-quenched ADAMs substrate (Mca–Pro–Leu–Ala–Gln–Ala–Val–Dpa–Arg–Ser–Ser–Ser–Arg–NH2 (R&D Systems). Briefly, 5 µg of lung membrane proteins were incubated with reaction buffer (50 mM acetic acid, pH 4.5, 100 mM sodium chloride) in the presence or absence of the ADAM17 inhibitor TAPI 0 (10 µM; Tocris Biosciences, Minneapolis, MN, U.S.A.). Samples were incubated with 10 µM of the fluorescent substrate in reaction buffer (final volume of 200 µl) at 37°C for 5 h. Enzymatic activity was continuously monitored with SpectraMax M5 (Molecular Devices, San José, CA) by measuring fluorescence at 320 nm excitation and 420 nm emission wavelength. Experiments were carried out in duplicate. Results were expressed as relative fluorescence units (RFUs/µg/h).
Lung Ang II and Ang-(1-7) concentration
Enzyme-linked immunosorbent assay (ELISA) was employed to measure the concentration of Ang II (EKC38669, Biomatik, Cambridge, ON) and Ang-(1-7) (EKC38664, Biomatik) in lung homogenates containing protease inhibitors, according to the manufacturer’s instructions.
Serum ACE and ACE2 concentration
The arterial blood was collected into vacuum tubes with a gel separator (Vacutainer SST Advance, Franklin Lakes, NJ) and centrifuged at 1200×g for 10 min at 4°C to obtain serum. The serum concentrations of ACE and ACE2 were determined by ELISA (Biomatik). Experiments were conducted according to the manufacturers’ instructions.
Human gene expression data
Lung ACE and ACE2 gene expression analysis in male (n=94) and female (n=18) hypertensive and male (n=216) and female (n=56) normotensive subjects was performed using the GTEx database (https://www.gtexportal.org/home/). Detailed information regarding tissue and patient collection, as well as gene expression experiments, are described elsewhere [27]. Briefly, paired-end RNA-seq was generated using Illumina TruSeq Protocol and aligned to the human genome (GRCh38/hg38) using STAR v2.5.3a, based on the GENCODE v26 annotation (https://www.gencodegenes.org/human/release_26.html). Gene-level quantification was estimated as transcripts per million using RNA-SeQC v1.1.9 [28]. Patient information was obtained upon request for controlled-access data (dbGaP accession phs000424.v8.p2 #90490-2) [29]. Briefly, patients’ information was extracted from medical reports obtained from deceased donors’ next-of-kin. Thus, information about medical history and the use of medications is not specific. We considered as hypertensive those individuals who displayed the following terms in their medical history: hypertension, essential hypertension, high blood pressure, or medication for hypertension, including nicardipine, enalapril, hydrochlorothiazide, bisoprolol, and clevidipine. Patients’ systolic and diastolic blood pressure were not available since samples were collected postmortem.
Statistical analyses
The experimental animal results are reported as the mean ± standard error of the mean (SEM). The normality of the distribution of all variables was evaluated using the Kolmogorov–Smirnov test. If the numerical data were normally distributed, comparisons among the means were carried out using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Otherwise, the nonparametric Kruskal–Wallis test was used. The Pearson correlation test was used to measure the strength of associations between two parametric variables, and the Spearman correlation test was employed to assess the relationships between nonparametric variables. Differences for which P<0.05 were considered significant. Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software, La Jolla, CA). Differential gene expression in the human lung was assessed using the DESeq2 R package from Bioconductor, which employs a negative binomial distribution to estimate dispersion and model differential expression (http://bioconductor.org/packages/release/bioc/html/DESeq2.html). Differences in the ACE/ACE2 mRNA ratio between normotensive and hypertensive individuals were assessed using the generalized linear model with gamma distribution using transcripts per million normalized data using the glm function in R after outlier removal (GTEx_Analysis_2017-06-05_v8_RNASeQCv1.1.9_gene_tpm.gct).
Results
Lung ACE and ACE2 mRNA expression, protein abundance, and enzymatic activity in male and female normotensive and hypertensive rats
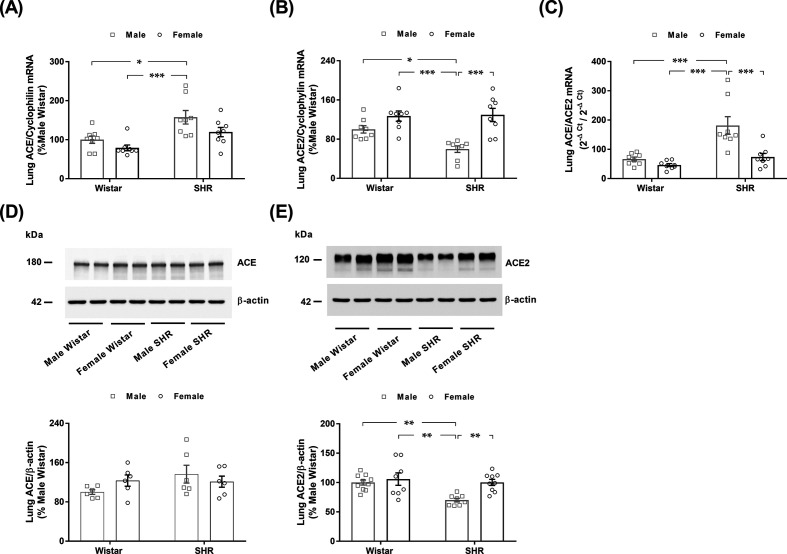
Lung ACE mRNA expression was similar in males and females of both strains (Figure 1A). Conversely, male SHRs displayed higher lung ACE mRNA expression than male and female Wistar rats. No difference in the expression of ACE2 mRNA was found between male and female Wistar rats (Figure 1B). However, male SHRs exhibited lower lung ACE2 mRNA expression than female SHRs and male and female Wistar rats. Figure 1C shows that the ACE to ACE2 mRNA levels were remarkably higher in the lungs of male SHRs than in the lungs of the three other groups of animals (Figure 1C).
Figure 1. Lung ACE and ACE2 mRNA and protein expression in male and female normotensive (Wistar) and hypertensive (SHR) rats.
The gene expression of (A) ACE and (B) ACE2 was quantitatively measured by RT-PCR and normalized to cyclophilin and is expressed as the % of male Wistar rats. (C) Lung ACE/ACE2 gene expression ratio was expressed as 2−ΔCt/2−ΔCt. The protein expression of (D) ACE and (E) ACE2 was determined by immunoblotting of lung membrane proteins and normalized to β-actin and is expressed as the % of male Wistar rats (Supplementary Figures S3 and S4). The values represent individual measurements and the means ± SEMs. *P<0.05; **P<0.01; ***P<0.001.
As seen in Figure 1D, the protein abundance of ACE did not vary among the four groups of rats. According to the mRNA data, male SHRs displayed an ∼40% decrease in the lung membrane protein abundance of ACE2 (Figure 1E). No difference in the lung membrane protein abundance of ACE2 was observed between male and female Wistar rats (Figure 1E). We could not determine the protein ratio of ACE to ACE2 in the lung, given the semi-quantitative nature of immunoblotting analysis and the different antibody characteristics for each protein.
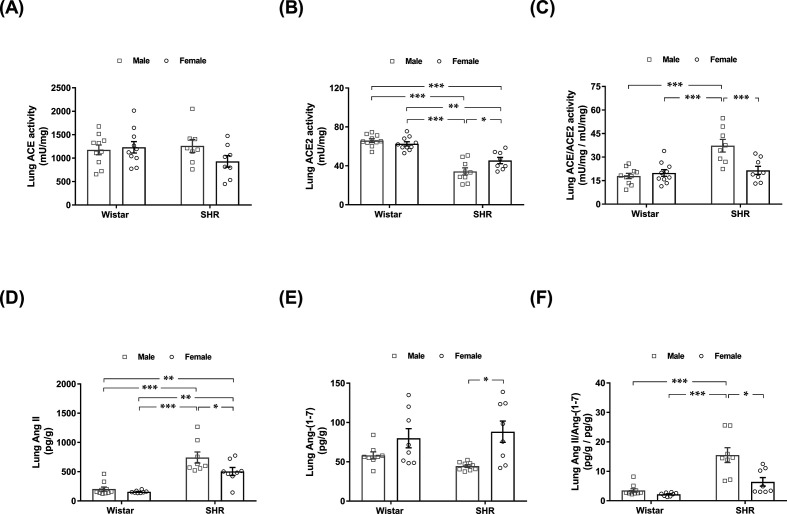
Figure 2A shows that ACE activity in the lungs was similar among the four groups of rats. Additionally, no difference in lung ACE2 activity was found between male and female Wistar rats (Figure 2B). In contrast, lung ACE2 activity was reduced in SHRs compared with Wistar rats, regardless of biological sex. In addition, lung ACE2 activity was decreased by 25% in male SHRs compared with female SHRs (P<0.05) (Figure 2B). As seen in Figure 2C, lung ACE to ACE2 activity was approximately two-fold higher in male SHRs than in Wistar rats and female SHRs.
Figure 2. Lung activity of ACE and ACE2 and lung concentration of their respective products, Ang II and Ang-(1-7), in male and female normotensive (Wistar) and hypertensive (SHR) rats.
The activities of (A) ACE and (B) ACE2 were assayed by a fluorogenic method. (C) Lung ACE/ACE2 activity ratio. Lung (D) Ang II and (E) Ang-(1-7) concentrations were determined by ELISA. (F) Lung Ang II/Ang-(1-7) peptide concentration ratio. The values represent individual measurements and the means ± SEMs. *P<0.05; **P<0.01; ***P<0.001.
Ang II and Ang-(1-7) concentrations in the lungs of male and female normotensive and hypertensive rats
As shown in Figure 2D, the lung concentrations of Ang II in male and female normotensive rats were similar (203 ± 34 vs. 156 ± 8 pg/g). Both male and female hypertensive rats displayed increased Ang II levels than those in Wistar rats; however, this increase was most prominent in male SHRs (∼3-fold higher in male hypertensive rats vs. male normotensive animals and ∼4.5-fold higher than female Wistar rats). A significant difference in lung Ang II concentration was also found between male and female SHRs (743 ± 95 vs. 501 ± 68 pg/g, P<0.05). Biological sex did not affect the concentration of Ang-(1-7) in the lungs of Wistar rats (Figure 2E). Conversely, the lung content of Ang-(1-7) was lower in hypertensive males than in hypertensive females (43 ± 2 vs. 89 ± 17 pg/g, P<0.05). The lung ratios of Ang II to Ang-(1-7) in male and female Wistar rats were similar. In accordance with the enzymatic activity data (Figure 2C), the lung ratio of Ang II to Ang-(1-7) was remarkably increased in male SHRs compared with the other three groups of rats (Figure 2F).
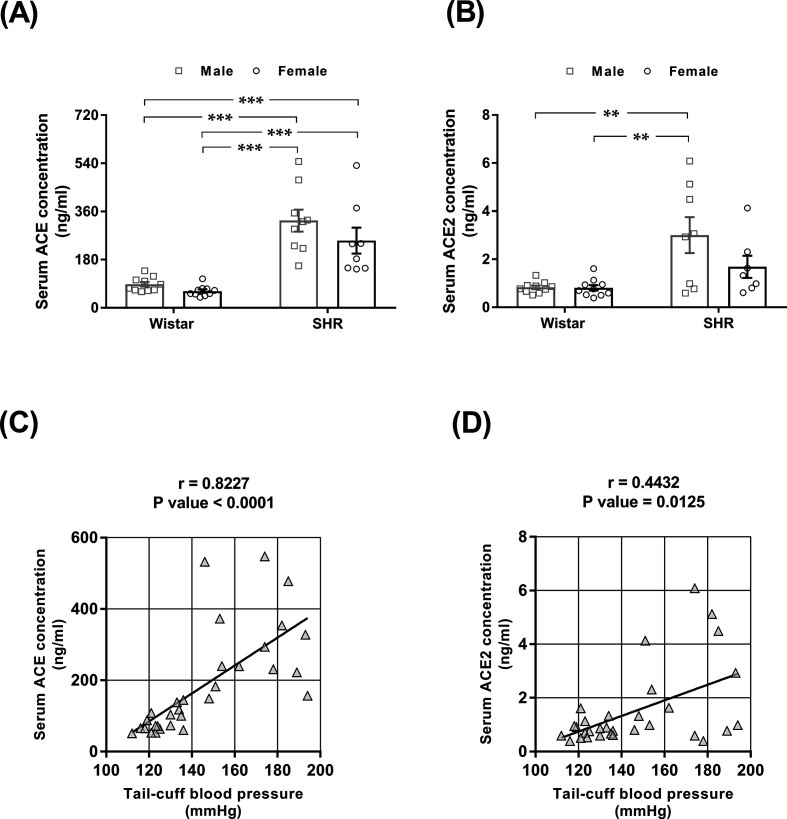
Serum concentrations of ACE and ACE2 in male and female normotensive rats
No difference in serum ACE concentration was noted between males and females of either strain (Figure 3A). SHRs exhibited a higher serum ACE concentration than normotensive rats, regardless of sex. The serum ACE2 concentration was increased in male SHRs compared with the three other groups of animals (Figure 3B). The relationships between blood pressure and ACE and ACE2 serum concentrations are shown in Figure 3C,D. As seen in Figure 3C,D, the higher the blood pressure was, the higher the serum concentrations of ACE (Figure 3C) and ACE2 were (Figure 3D).
Figure 3. Serum ACE and ACE2 concentrations and their correlation with blood pressure in male and female normotensive (Wistar) and hypertensive (SHR) rats.
Serum (A) ACE and (B) ACE2 concentrations were determined by ELISA. The values represent individual measurements and the means ± SEMs. **P<0.01 and ***P<0.001. Correlations between the serum (C) ACE and (D) ACE2 concentrations and tail-cuff blood pressure. The correlation coefficients (r) and P-values were obtained by Pearson’s correlation test, and lines were obtained by linear regression plotting.
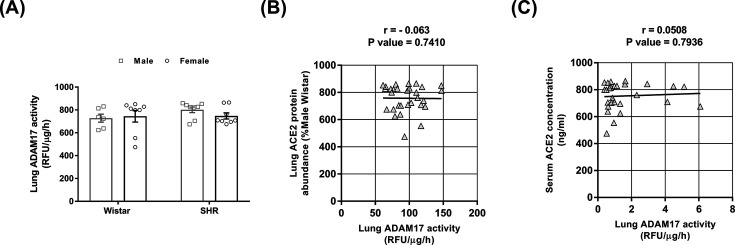
Lung ADAM17 activity in male and female normotensive and hypertensive rats
To evaluate whether down-regulation of lung ACE2 in male SHRs could be at least partially attributed to ectodomain shedding, we measured the activity of ADAM17, a disintegrin, and metalloprotease that cleaves and releases the catalytically active ectodomain of ACE2 to the circulation [30]. The lung activity of ADAM17 was similar among the four groups of rats (Figure 4A). Accordingly, no significant correlation was found between ADAM17 activity and lung ACE2 protein abundance (Figure 4B) or serum ACE2 abundance (Figure 4C).
Figure 4. Lung ADAM17 activity and its correlation with lung ACE2 protein and serum abundance in male and female normotensive (Wistar) and hypertensive (SHR) rats.
(A) Lung ADAM17 activity was determined by a fluorescent enzymatic assay in the presence or absence of TAPI 0. The TAPI 0 sensitive enzymatic activity was expressed as RFUs/µg/h. The values represent individual measurements and the means ± SEMs. The absence of a correlation between lung ADAM17 activity and (B) lung ACE2 protein or (C) serum ACE2 concentration. The correlation coefficients (r) and P-values were obtained by Pearson’s correlation test, and lines were obtained by linear regression plotting.
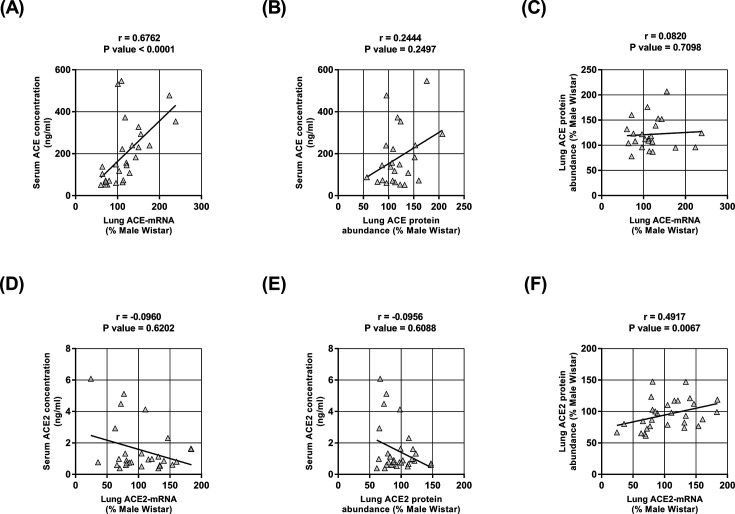
Relationship between serum and the lung expression of ACE and ACE2
The serum abundance of ACE significantly correlated with lung ACE mRNA level (Figure 5A) but not with ACE protein abundance (Figure 5B). Accordingly, no association between ACE mRNA and ACE protein abundance was found (Figure 5C). Serum ACE2 abundance did not correlate with either lung ACE2 mRNA level (Figure 5D) or ACE2 protein abundance (Figure 5E); however, lung ACE2 mRNA levels and ACE2 protein abundance were positively correlated (Figure 5F).
Figure 5. The relationship between the lung expression and serum concentrations of ACE and ACE2.
(A) Correlation between serum ACE concentration and lung ACE mRNA level. (B) The absence of a correlation between serum ACE concentration and lung ACE protein abundance. (C) The absence of a correlation between lung ACE protein abundance and lung ACE mRNA level. (D) The absence of a correlation between serum ACE2 concentration and lung ACE2 mRNA level. (E) The absence of a correlation between serum ACE2 abundance and lung ACE2 protein abundance. (F) Correlation between lung ACE2 protein abundance and lung ACE2 mRNA level. The correlation coefficients (r) and P-values were obtained by Pearson’s correlation test, and lines were obtained by linear regression plotting.
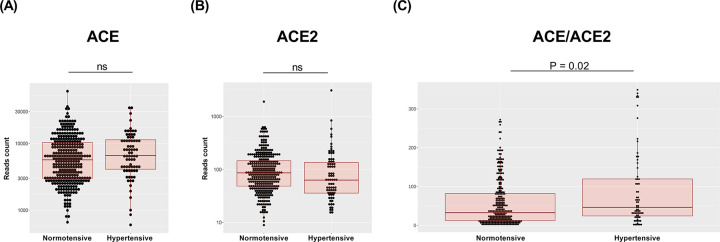
Lung ACE and ACE2 mRNA expression in normotensive and hypertensive humans
Figure 6 presents data on lung ACE and ACE2 mRNA expression in hypertensive and normotensive subjects. No difference in lung ACE gene expression level was found between normotensive and hypertensive patients (log2 fold change = 0.169; P=0.22) (Figure 6A). A trend towards decreased lung ACE2 mRNA expression was observed in hypertensive patients (Figure 6B), but the difference did not reach statistical significance (log2 fold change = −0.168; P=0.08). However, as seen in Figure 6C, lung ACE to ACE2 mRNA expression in hypertensive patients was significantly higher than that in normotensive subjects.
Figure 6. Lung ACE and ACE2 mRNA expression in normotensive and hypertensive patients.
Normalized (A) ACE mRNA and (B) ACE2 mRNA expression data and the (C) ACE to ACE2 mRNA ratio from normotensive and hypertensive patients whose lung samples were available in the GTEx database. Boxplots show the minimum, first quartile, median, third quartile, and maximum values. Each dot represents an individual data point.
Discussion
The main findings of our study can be summarized as follows: (i) male hypertensive rats displayed imbalance between the ACE/Ang II and ACE2/Ang-(1-7) pathways in the lungs; (ii) this RAS imbalance resulted in a higher lung Ang II concentration that is mainly attributable to decreased lung ACE2 activity since neither high blood pressure nor biological sex was associated with changes in lung enzymatic ACE function; (iii) down-regulation of lung ACE2 activity in male hypertensive rats was accompanied by decreased ACE2 protein abundance and ACE2 mRNA expression but did not seem to be dependent on increased ADAM17-mediated shedding; (iv) the lung ACE/ACE2 mRNA ratio was increased in hypertensive patients, thereby suggesting that the RAS balance may be shifted towards the classical RAS in human subjects with high blood pressure.
The present study mainly focused on the relationships between biological sex, high blood pressure, and the balance between the RAS pathways in the lungs. However, it also provides interesting insights regarding sex dimorphism-mediated modulation of lung ACE2 in hypertension. Based on the results shown here, we suggest that down-regulation of ACE2 activity in the lungs leads to the decreased consumption of Ang II and that lower Ang-(1-7) production may be primarily due to decreased ACE2 mRNA transcription and translation. Indeed, a high degree of correlation was found between lung ACE2 mRNA and ACE2 protein abundance. The fact that the activity of the ACE2 sheddase ADAM17 [30] was similar among the four groups of rats demonstrates that neither biological sex nor blood pressure affects its enzymatic activity in the lungs. This finding also suggests that pulmonary plasma membrane ACE2 expression is not primarily regulated by ADAM17-induced post-translational modifications in SHRs. Accordingly, no correlation was found between lung ADAM17 activity and lung ACE2 protein abundance or serum concentration. In addition, no relationship was found between lung ACE2 expression and serum ACE2 concentration, thereby suggesting that the lungs are not the primary source of serum ACE2. It is worth mentioning that several cellular processes, such as protein degradation and post-translational modification mechanisms other than ADAM17-mediated shedding, might impact ACE2 activity in the lungs, but these processes were not evaluated in the present study.
High-serum ACE and ACE2 are known risk factors for CVD [31–35] and, as revealed more recently, COVID-19 severity [21,36]. Consistent with these studies, we found that rats with high blood pressure, regardless of biological sex, displayed higher serum ACE and ACE2 concentrations. In addition, a strong positive correlation between blood pressure and serum ACE levels was noted, and a moderate correlation between blood pressure and serum ACE2 content was noted. Because the circulating pool of ACE and ACE2 represents the sum of ectodomain shedding from the cell surface among a wide variety of tissues minus plasma clearance [37,38], the relative contributions from different tissues should be weighed based on the organ-specific gene expression of ACE/ACE2 and local shedding [39,40]. As mentioned above, our results suggest that in the setting of chronic hypertension, the contribution of lung ACE2 shedding to serum ACE2 is probably minor. However, chronic hypertension seems to influence lung ACE2 expression since a moderate correlation between blood pressure and lung ACE2 protein abundance has been found (Supplementary Figure S2). In contrast, high blood pressure is associated with both augmented lung ACE mRNA and ACE shedding from the cell surface, leading to increased serum ACE concentrations but not ACE cell surface lung protein abundance. These data also suggest that under not only physiological conditions [41] but also chronic hypertension, the lung is one of the primary sources of serum ACE.
The finding that ACE/ACE2 mRNA expression is elevated in the lungs of hypertensive patients compared with those of age-matched normotensive individuals provides biological plausibility that our experimental results in rats can be translated into humans. In addition, since our samples were from both male and presumably postmenopausal women in the majority (average age higher than 55 years, Supplementary Table S1), we can speculate that the RAS imbalance observed in hypertensive males may be primarily due to the influence of sex hormones. Nevertheless, we have to acknowledge as a limitation of our study that samples from females for gene expression analysis were underrepresented. Therefore, we could not reliably analyze the influence of biological sex, and no conclusions about the sex-biased lung ACE/ACE2 mRNA-expression ratio in hypertensive patients could be made. Future studies assessing the impact of biological sex and menopausal status on human lung tissues will add important information about the mechanisms regulating the ACE/ACE2 expression ratio and might reveal the exact role of sex hormones and sex chromosomes.
Over the last year, several reviews and opinion articles have defended the view that a disequilibrium between the classic RAS and its opposing protective arm in the lungs plays a crucial role in the pathophysiology of COVID-19 [19,22,23,42]. This study found that high blood pressure and male sex shift the RAS balance in the lungs towards the vasoconstrictive, profibrotic, and proinflammatory ACE/Ang II axis. Notably, during the early stages of the pandemic, concerns that increased ACE2 cell surface expression could be a causal factor for COVID-19 severity due to augmented viral cell invasion and replication were raised. Some authors even postulated that hypertensive patients treated with angiotensin II type 1 receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEis), which up-regulate ACE2, could be at higher risk for increased COVID-19 severity, and replacement with alternative pharmacological treatments was advocated [43–45]. However, data from observational studies and randomized clinical trials have consistently contradicted this hypothesis by demonstrating a lack of an association between the chronic use of either ACEis or ARBs and increased disease severity [46–48] or susceptibility to COVID-19 [49]. Indeed, a large observational study found a lower COVID-19 risk in hypertensive patients treated over a long period with ACEis or ARBs than with other antihypertensive drugs [50], suggesting that RAS inhibitors may, in fact, exert a protective effect. The data showed herein strengthen the notion that future research should consider the impact of sex on RAS dysregulation in COVID-19 patients, as sex-biased differences due to hypertension or a class of antihypertensive medications might occur.
To the best of our knowledge, this is the first study to demonstrate sexual dimorphism in lung ACE2 regulation at the transcriptional and translational levels in hypertensive rats. The combination of down-regulated lung ACE2 and unchanged ACE protein abundance and activity shifts the lung RAS balance towards the proinflammatory ACE/Ang II axis. Additionally, gene expression analysis in human lung samples showed higher ACE/ACE2 in hypertensive patients, suggesting that our findings in experimental animals can potentially be extrapolated to humans. Further research is needed to address whether the use of RAS inhibitors, including AT1R receptor blockers and ACEis, which up-regulate the ACE2/Ang-(1-7)/MasR axis, may help to mitigate the effects of COVID-19 in male and possibly postmenopausal female hypertensive patients.
Supplementary Material
Abbreviations
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- ACEi
angiotensin-converting enzyme inhibitor
- ADAM17
a disintegrin and metalloprotease 17
- Ang II
angiotensin II
- Ang-(1-7)
angiotensin-(1-7)
- ARB
angiotensin II type 1 receptor blocker
- AT1R
angiotensin II type 1 receptor
- COVID-19
coronavirus disease 2019
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- GTEx
Genotype-Tissue Expression
- MasR
Mas receptor
- PVDF
polyvinylidene difluoride
- RAS
renin–angiotensin system
- RT-PCR
reverse transcription-polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SHR
spontaneously hypertensive rat
Data Availability
The raw data supporting the conclusions of this article will be made available by authors without undue reservation.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) [grant numbers 2016/22140-7, 2020/05338-3].
CRediT Author Contribution
Flavia L. Martins: Data curation, Formal analysis, Investigation, Visualization, Methodology. Caio A.M. Tavares: Validation, Visualization, Writing—original draft, Writing—review & editing. Pamella A. Malagrino: Data curation, Formal analysis, Investigation, Methodology. Thiago Rentz: Data curation, Formal analysis, Investigation, Methodology. Acaris Benetti: Data curation, Visualization, Methodology. Thiago M.S. Rios: Methodology. Gabriel M.D. Pereira: Methodology. Bruno Caramelli: Writing—review & editing. Samantha K. Teixeira: Formal analysis, Validation, Investigation, Visualization, Methodology. José E. Krieger: Resources, Supervision, Validation. Adriana C.C. Girardi: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing.
Ethics Approval
The ethics committee of the University of São Paulo School of Medicine approved the protocol for animal studies (Approval Number 1550/2020 as of 26 August 2020).
References
- 1.Pérez-López F.R., Larrad-Mur L., Kallen A., Chedraui P. and Taylor H.S. (2010) Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod. Sci. 17, 511–531 10.1177/1933719110367829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shufelt C.L., Pacheco C., Tweet M.S. and Miller V.M. (2018) Sex-specific physiology and cardiovascular disease. Adv. Exp. Med. Biol. 1065, 433–454 10.1007/978-3-319-77932-4_27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V. and Kararigas G. (2017) Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 97, 1–37 10.1152/physrev.00021.2015 [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff J.F. (2018) Gender differences in hypertension. Curr. Opin. Nephrol. Hypertens. 27, 176–181 10.1097/MNH.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y. and Moran A.E. (2017) Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension 70, 736–742 10.1161/HYPERTENSIONAHA.117.09801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J., Lu Y., Wang X., Li X., Linderman G.C., Wu C.et al. (2017) Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet 390, 2549–2558 10.1016/S0140-6736(17)32478-9 [DOI] [PubMed] [Google Scholar]
- 7.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C. and Klein S.L. (2020) Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 20, 442–447 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R.et al. (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 11, 6317 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maric-Bilkan C. and Manigrasso M.B. (2012) Sex differences in hypertension: contribution of the renin-angiotensin system. Gend. Med. 9, 287–291 10.1016/j.genm.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Viveiros A., Rasmuson J., Vu J., Mulvagh S.L., Yip C.Y.Y., Norris C.M.et al. (2021) Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 320, H296–H304 10.1152/ajpheart.00755.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley S.D., Gurley S.B., Oliverio M.I., Pazmino A.K., Griffiths R., Flannery P.J.et al. (2005) Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J. Clin. Invest. 115, 1092–1099 10.1172/JCI23378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurley S.B., Riquier-Brison A.D.M., Schnermann J., Sparks M.A., Allen A.M., Haase V.H.et al. (2011) AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 13, 469–475 10.1016/j.cmet.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley S.D., Gurley S.B., Herrera M.J., Ruiz P., Griffiths R., Kumar A.P.et al. (2006) Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. U.S.A. 103, 17985–17990 10.1073/pnas.0605545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlöf B., Devereux R.B., Kjeldsen S.E., Julius S., Beevers G., de Faire U.et al. (2002) Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359, 995–1003 10.1016/S0140-6736(02)08089-3 [DOI] [PubMed] [Google Scholar]
- 15.Carson P., Giles T., Higginbotham M., Hollenberg N., Kannel W. and Siragy H.M. (2001) Angiotensin receptor blockers: evidence for preserving target organs. Clin. Cardiol. 24, 183–190 10.1002/clc.4960240303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M.et al. (2018) The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol. Rev. 98, 505–553 10.1152/physrev.00023.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji H., Menini S., Zheng W., Pesce C., Wu X. and Sandberg K. (2008) Role of angiotensin-converting enzyme 2 and angiotensin (1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp. Physiol. 93, 648–657 10.1113/expphysiol.2007.041392 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S.et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavares C.A.M., Bailey M.A. and Girardi A.C.C. (2020) Biological context linking hypertension and higher risk for COVID-19 severity. Front. Physiol. 11, 599729 10.3389/fphys.2020.599729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavares C.A.M., Avelino-Silva T.J., Benard G., Cardozo F.A.M., Fernandes J.R., Girardi A.C.C.et al. (2020) ACE2 expression and risk factors for COVID-19 severity in patients with advanced age. Arq. Bras. Cardiol. 115, 701–707 10.36660/abc.20200487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S.K., Juno J.A., Lee W.S., Wragg K.M., Hogarth P.M., Kent S.J.et al. (2021) Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J 57, 2003730 10.1183/13993003.03730-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarzani R., Giulietti F., Di Pentima C., Giordano P. and Spannella F. (2020) Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L325–L336 10.1152/ajplung.00189.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sriram K. and Insel P.A. (2020) A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 177, 4825–4844 10.1111/bph.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B.et al. (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436, 112–116 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins F.L., Bailey M.A. and Girardi A.C.C. (2020) Endogenous activation of glucagon-like peptide-1 receptor contributes to blood pressure control: role of proximal tubule Na. Hypertension 76, 839–848 10.1161/HYPERTENSIONAHA.120.14868 [DOI] [PubMed] [Google Scholar]
- 26.Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 10.1016/S0021-9258(19)52451-6 [DOI] [PubMed] [Google Scholar]
- 27.eGTEx Project (2017) Enhancing GTEx by bridging the gaps between genotype, gene expression, and disease. Nat. Genet. 49, 1664–1670 10.1038/ng.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLuca D.S., Levin J.Z., Sivachenko A., Fennell T., Nazaire M.D., Williams C.et al. (2012) RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 10.1093/bioinformatics/bts196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. GTEx Consortium (2020) The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 10.1126/science.aaz1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R.et al. (2014) Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell Cardiol. 66, 167–176 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 31.Ramchand J., Patel S.K., Kearney L.G., Matalanis G., Farouque O., Srivastava P.M.et al. (2020) Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovasc. Imaging 13, 655–664 10.1016/j.jcmg.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 32.Epelman S., Shrestha K., Troughton R.W., Francis G.S., Sen S., Klein A.L.et al. (2009) Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J. Card. Fail. 15, 565–571 10.1016/j.cardfail.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narula S., Yusuf S., Chong M., Ramasundarahettige C., Rangarajan S., Bangdiwala S.I.et al. (2020) Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet 396, 968–976 10.1016/S0140-6736(20)31964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cambien F., Costerousse O., Tiret L., Poirier O., Lecerf L., Gonzales M.F.et al. (1994) Plasma level and gene polymorphism of angiotensin-converting enzyme in relation to myocardial infarction. Circulation 90, 669–676 10.1161/01.CIR.90.2.669 [DOI] [PubMed] [Google Scholar]
- 35.Ribichini F., Steffenino G., Dellavalle A., Matullo G., Colajanni E., Camilla T.et al. (1998) Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme: a major risk factor and a marker of risk for coronary stent restenosis. Circulation 97, 147–154 10.1161/01.CIR.97.2.147 [DOI] [PubMed] [Google Scholar]
- 36.Reindl-Schwaighofer R., Hödlmoser S., Eskandary F., Poglitsch M., Bonderman D., Strassl R.et al. (2021) ACE2 elevation in severe COVID-19. Am. J. Respir. Crit. Care Med. 203, 1191–1196 10.1164/rccm.202101-0142LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danilov S.M., Lunsdorf H., Akinbi H.T., Nesterovitch A.B., Epshtein Y., Letsiou E.et al. (2016) Lysozyme and bilirubin bind to ACE and regulate its conformation and shedding. Sci. Rep. 6, 34913 10.1038/srep34913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert D.W., Clarke N.E., Hooper N.M. and Turner A.J. (2008) Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 582, 385–390 10.1016/j.febslet.2007.11.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G. and van Goor H. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P.et al. (2020) Ang II (Angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (Prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 75, 173–182 10.1161/HYPERTENSIONAHA.119.14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdos E.G. and Skidgel R.A. (1987) The angiotensin I-converting enzyme. Lab. Invest. 56, 345–348 [PubMed] [Google Scholar]
- 42.Pagliaro P. and Penna C. (2020) ACE/ACE2 ratio: a key also in 2019 coronavirus disease (Covid-19)? Front. Med. (Lausanne) 7, 335 10.3389/fmed.2020.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang L., Karakiulakis G. and Roth M. (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 8, e21 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B.et al. (2020) Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 382, 2441–2448 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A.et al. (2020) Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 395, 1705–1714 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C.et al. (2021) Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir. Med. 9, 275–284 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes R.D., Macedo A.V.S., de Barros E.S.P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L.et al. (2021) Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA 325, 254–264 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer A., Schreinlechner M., Sappler N., Dolejsi T., Tilg H., Aulinger B.A.et al. (2021) Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir. Med. 9, 863–872 10.1016/S2213-2600(21)00214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopes R.D., Macedo A.V.S., de Barros E., Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M.et al. (2021) Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA 325, 254–264 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenzato L., Botton J., Drouin J., Baricault B., Vabre C., Cuenot F.et al. (2021) Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension 77, 833–842 10.1161/HYPERTENSIONAHA.120.16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by authors without undue reservation.