Abstract
Objectives
There is a scarcity of longitudinal cohort studies in sub-Saharan Africa to understand the epidemiology of cardiovascular disease as a basis for intervention. We estimated incident hypertension and associated sociodemographic, health and behavioural risk factors in a population aged 40 years and older over a 5-year period.
Design
We assessed the association between incident hypertension and sociodemographic, health and behavioural factors using Poisson regression. We adjusted for non-response in 2015 using inverse probability sampling weights from a logistic regression including sex and age at baseline.
Setting
Rural South Africa.
Participants
We used a population-based cohort of normotensive adults in 2010 who were aged 40 years and older at retest in 2015.
Results
Of 676 individuals completing baseline and 5-year follow-up, there were 193 incident cases of hypertension. The overall hypertension incidence rate was 8.374/100 person-years. In multivariable analyses, those who became hypertensive were more likely to be older, have a high waist circumference (incidence rate ratio (IRR): 1.557, 95% CI: 1.074 to 2.259) and be employed (IRR: 1.579, 95% CI: 1.071 to 2.329) at baseline. Being HIV positive and not on antiretroviral therapy at baseline was associated with lower risk of incident hypertension.
Conclusions
Over a 5-year period, 29% of respondents developed hypertension. Given the high burden of hypertension in South Africa, continued longitudinal follow-up is needed to understand the complex interplay of non-communicable and infectious diseases and their underlying and modifiable risk factors to inform public health prevention strategies and programmes.
Keywords: hypertension, incidence, South Africa, rural population, cohort
Strengths and limitations of this study.
We provide longitudinal evidence on hypertension incidence from a population-based cohort in rural South Africa including both HIV-positive and HIV-negative individuals.
Associations between HIV status and incident hypertension may be sensitive to survivorship bias if those who died due to HIV/AIDS over the 5-year period were also more likely to develop hypertension.
A longer period of follow-up is needed to assess the effects of HIV and antiretroviral therapy on hypertension and related cardiometabolic conditions.
Introduction
Hypertension is one of the most important non-communicable disease (NCD) risk factors and the largest contributor to the global burden of disease, with high blood pressure (BP) accounting for 7% of global disability-adjusted life years.1 The burden of hypertension is greatest in low/middle-income countries (LMICs),2 and has increased rapidly in sub-Saharan Africa.3–7 A study of people aged 50 years and over from six countries found markedly high prevalence in South Africa (77.9%).8
Rapid demographic and epidemiological changes in LMICs, such as population ageing, are expected to dramatically increase hypertension prevalence. Results from a modelling study found that without any changes in the age-specific prevalence of hypertension, the population with hypertension in South Africa is expected to grow by 105% by 2050.9 These dramatic changes on the epidemiology of hypertension are further complicated by a lack of awareness by those with a hypertensive condition, with serious consequences of a low proportion of individuals with hypertension being on treatment.10–12 In South Africa, an estimated 38%–64% of hypertensives were aware of their status and 7.8%–22.8% effectively controlled.8 13
Longitudinal data from sub-Saharan Africa are needed to examine changes in population-specific hypertension risk factors over time,14 particularly given differences in sociocultural environments and related health factors (eg, diet, concurrent infectious diseases), and differentials in rural versus urban risk factor levels.15 This is particularly important as wide-scale availability of antiretroviral therapy (ART) has reduced HIV/AIDS-related mortality16 17 thereby increasing the population of those ageing with HIV.18 The ageing population will be at higher risk of developing hypertension, and the effect of HIV and ART may also increase the incidence of hypertension.19–22 In South Africa, an emerging dual burden of disease, along with urban–rural differences due to the legacy of the apartheid era, also highlight the importance of understanding location-specific hypertension risk factors over time.23 However, there are currently a limited number of longitudinal studies examining risk factors for incident hypertension in the region, with most of these restricted to HIV-positive individuals only.24–27
Methods
We use a population-based cohort of adults in rural South Africa who were normotensive in 2010–2011 and were 40 years or older in 2014–2015 to estimate hypertension incidence and identify sociodemographic, health and behavioural risk factors over a 5-year period.
We use data from two survey studies conducted in 2010–2011 and 2014–2015 in the Agincourt Health and socio-Demographic Surveillance System (HDSS) study area in rural northeast South Africa.28 The area is a low rainfall setting with limited subsistence farming. Since 1992, the Medical Research Council/Wits Rural Public Health and Health Transitions Unit has been conducting an annual census update of the population living in the study site, including information on vital events (births, deaths, migrations) and household and individual sociodemographic information. In 2010–2011, the baseline study (Ha Nakekela) included a sex/age-stratified random sample of 7662 men and women aged 15 years and older who were permanent residents from the 2009 HDSS census.29 A follow-up study from November 2014 to November 2015 (the Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) included a random sample of 6281 men and women aged 40 years and older who were permanent residents from the 2013 HDSS census,30 including those in the baseline study who fulfilled the inclusion criteria. Both studies included information on sociodemographic factors and self-reported health and conditions, anthropometric and BP measurements, and point-of-care blood tests for glucose and lipids, and dried blood spots (DBS) for HIV status.
Outcome measure
BP and hypertension
BP was measured three times using a Boso BP instrument 2 min apart in 2010 and an Omron M6W automated cuff 2 min apart in 2015. Validation studies of similar BP monitoring devices indicate that they can provide accurate measurements.31–33 Consistent with national surveillance guidance, we used the average of the second and third measurements.34 Hypertension was defined as a systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or if the respondent self-reported taking antihypertensive medication.
2010 risk factors
Anthropometry and high waist circumference
Height, weight and waist circumference were measured using a flexible stadiometer (Seca). High waist circumference was defined as >102 cm for men and >88 cm for women.35 Obesity was classified as a body mass index (BMI; kg/m2) ≥30.35
Random blood glucose and diabetes
Point-of-care instruments were used to measure glucose (Caresens POP blood glucose metre). Diabetes was defined as a random blood glucose level of ≥11.1 mmol/L or if the respondent self-reported medication use for diabetes.36
High triglycerides
A Cardiocheck instrument was used to measure lipid levels. High triglycerides were defined as ≥1.7 mmol/L.37
HIV status
HIV DBS were tested using screening assay Vironostika Uniform 11 (Biomeriuex, France); with positive results retested using the SD Bioline HIV ELISA test (SD; Standard Diagnostics, Korea). If the two tests were inconsistent, we conducted a third assay (Elecys, Roche, USA) that determined the final result.
Sociodemographics and behaviours
Respondents were asked about smoking (never, prior, current) and alcohol history (not in past 30 days, less than weekly, weekly), physical activity (using the International Physical Activity Questionnaire) and if they were using ART. Information on years of completed education, employment (currently working for pay), union (informal or formal) and socioeconomic status (based on tertiles of an asset index38) was extracted from the most recent surveillance census.
Cause of death
For those who died between the baseline and follow-up study and for whom a death was identified from census updates, a verbal autopsy (VA) was conducted using a standardised VA instrument. For each identified death, a specially trained team conducted a VA interview with the closest living caretaker to record signs and symptoms experienced before the death. We categorised cause of death using InterVA-439—assigning a single cause for the largest likelihood for each death.
Analysis
We calculated hypertension incidence (over 5 years) for those aged 40 years and older at the time of the second survey overall, and by sex, age, and other sociodemographic factors. We calculated age-adjusted incidence using the Agincourt 2009 census population. We used Poisson regression with robust SEs to examine the association of hypertension status with sociodemographic, health and behavioural risk factors from the baseline study. To adjust for non-response in the follow-up study, we developed inverse probability sampling weights (IPSW) based on a logistic regression including sex and age in August 2010. We multiplied the IPSW for non-response by the inverse probability weights from the 2010 sample selection to derive our final weights for analysis. For our fully adjusted multivariable models, we fit separate models with and without HIV/ART status given a reduced sample of 2010 respondents with measured HIV status (particularly for the eligible sample estimates, see below).
We used two approaches to estimating exposure time for our incidence estimates. For the first approach, we included only those individuals who participated in both surveys. For incident cases, we defined exposure time as the midpoint between the dates of the first and second survey assessments. For the second approach, we included all eligible individuals from the first survey who were able to be tracked from census data. For those who out-migrated or died before the start of the second study, we allowed them to contribute exposure time between their BP measurement in the first study and time at death or out-migration. For those who were not found or refused to participate in the second survey, we allowed exposure time between the first study’s measurement and the start of the second study. As the second approach includes additional exposure time but no new incident cases, it provides a lower bound for our estimate of hypertension incidence. Individuals who aged to 40 years during the follow-up time only contributed to exposure when they had reached 40 years or older. We used Stata V.15 for all statistical analyses.40
We also tested the sensitivity of our results. We tested models using either BMI or waist-to-hip ratio instead of waist circumference. We also tested a model of hypertension based on only BP thresholds to assess if there were differences in the associations between predictors and incident BP only. Finally, we tested a competing risk model for those eligible individuals who either died, migrated or completed the follow-up study to test for bias in our risk factor associations. We modelled incident hypertension as the main event and death due to any cause as a competing event (censoring those who out-migrated) using the Fine-Grey model.41
Patient and public involvement
Neither study participants nor the public were involved in study design or conduct of the study. The HDSS Learning, Information dissemination and Networking with Community office manages community liaison activities with the HDSS study communities and their leaders. Annual feedback of findings from the HDSS census and research projects conducted in the site are provided through open village meetings, with frequent participation from local service providers.
Results
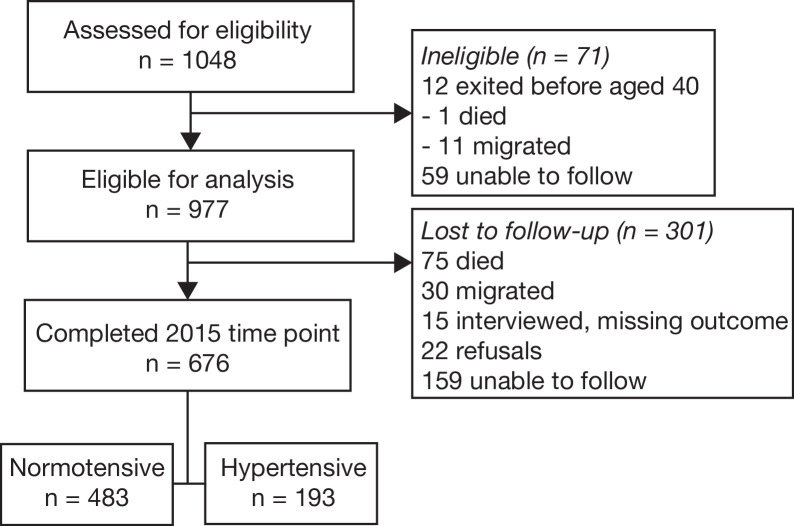
Figure 1 shows the participant flow chart. A total of 977 individuals were eligible for analysis from the first study and 676 (69%) also completed the second study. Table 1 presents sample characteristics from the baseline study comparing those who completed the second study and those who did not. Women, those with a high waist circumference, those in older ages and in a union with lower completed education were more likely to participate in the second study.
Figure 1.
Participant flow chart.
Table 1.
Sample characteristics at baseline (2010), by study participation in 2015 for eligible individuals (n=977)
| Lost to follow-up (n=301) | Completed 2015 (n=676) | P value | Total (n=977) | ||||
| n | % | n | % | n | % | ||
| Gender | |||||||
| Male | 140 | 46.5 | 239 | 35.4 | 0.001 | 379 | 38.8 |
| Female | 161 | 53.5 | 437 | 64.6 | 598 | 61.2 | |
| Age groups | |||||||
| 35–44 | 130 | 43.2 | 227 | 33.6 | 0.003 | 357 | 36.5 |
| 45–54 | 71 | 23.6 | 180 | 26.6 | 251 | 25.7 | |
| 55–64 | 34 | 11.3 | 126 | 18.6 | 160 | 16.4 | |
| 65–74 | 29 | 9.6 | 81 | 12.0 | 110 | 11.3 | |
| 75+ | 37 | 12.3 | 62 | 9.2 | 99 | 10.1 | |
| Education | |||||||
| None | 40 | 34.2 | 277 | 41.0 | 0.018 | 317 | 40.0 |
| Less than secondary | 54 | 46.2 | 327 | 48.4 | 381 | 48.0 | |
| Secondary or more | 23 | 19.7 | 72 | 10.7 | 95 | 12.0 | |
| Union status | |||||||
| Not in union | 166 | 55.1 | 302 | 44.7 | 0.002 | 468 | 47.9 |
| Formal/informal union | 135 | 44.9 | 374 | 55.3 | 509 | 52.1 | |
| SES* | |||||||
| Low | 130 | 43.2 | 253 | 37.7 | 0.258 | 383 | 39.4 |
| Middle | 86 | 28.6 | 216 | 32.2 | 302 | 31.1 | |
| High | 85 | 28.2 | 202 | 30.1 | 287 | 29.5 | |
| Employment status | |||||||
| Not employed | 80 | 68.4 | 498 | 74.1 | 0.196 | 578 | 73.3 |
| Employed | 37 | 31.6 | 174 | 25.9 | 211 | 26.7 | |
| Smoking history | |||||||
| Never | 236 | 78.4 | 548 | 81.1 | 0.206 | 784 | 80.2 |
| Prior | 18 | 6.0 | 49 | 7.2 | 67 | 6.9 | |
| Current | 47 | 15.6 | 79 | 11.7 | 126 | 12.9 | |
| Alcohol use | |||||||
| Not in past 30 days | 235 | 78.1 | 544 | 80.5 | 0.686 | 779 | 79.7 |
| Less than weekly | 24 | 8.0 | 47 | 7.0 | 71 | 7.3 | |
| Weekly | 42 | 14.0 | 85 | 12.6 | 127 | 13.0 | |
| Physical activity† | |||||||
| Low | 27 | 9.4 | 40 | 6.0 | 0.128 | 67 | 7.0 |
| Moderate | 80 | 27.9 | 209 | 31.3 | 289 | 30.3 | |
| High | 180 | 62.7 | 419 | 62.7 | 599 | 62.7 | |
| High waist circumference‡ | |||||||
| No | 205 | 72.7 | 430 | 65.8 | 0.04 | 635 | 67.9 |
| Yes | 77 | 27.3 | 223 | 34.2 | 300 | 32.1 | |
| Diabetes§ | |||||||
| No | 293 | 98.0 | 655 | 97.2 | 0.46 | 948 | 97.4 |
| Yes | 6 | 2.0 | 19 | 2.8 | 25 | 2.6 | |
| High triglycerides¶ | |||||||
| No | 213 | 72.7 | 479 | 73.5 | 0.805 | 692 | 73.2 |
| Yes | 80 | 27.3 | 173 | 26.5 | 253 | 26.8 | |
HIV/ART is not included given missing values (n=297) for the vast majority of those lost to follow-up.
*Based on a household asset index score.
†Based on the International Physical Activity Questionnaire.
‡Greater than 102 cm for men and 88 cm for women.
§Blood glucose greater than or equal to 11.1.
¶Greater than or equal to 1.7 mmol/L.
ART, antiretroviral therapy; SES, socioeconomic status.
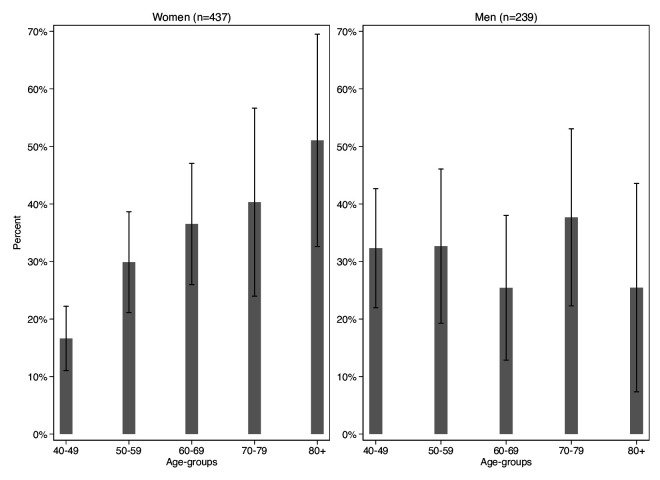
There were 193 incident cases of hypertension since baseline. The overall hypertension incidence rate was 8.374 per 100 person-years (95% CI: 7.242 to 9.721) for those completing both studies (men 9.097 (95% CI: 7.266 to 11.496); women 8.159 (95% CI: 6.832 to 9.804); table 2). The overall age-adjusted hypertension incidence rate for those completing both studies was 8.372 per 100 person-years (men 8.955; women 8.50). Rates were lower when including the full eligible sample (online supplemental table 1). Men in their 40s and 50s had higher incidence compared with same-aged women; from ages 60+ years, women showed higher rates than men (figure 2).
Table 2.
Hypertension incidence rates (IRs) and incidence rate ratios (IRRs) per 100 person-years (PYRS) over 5 years of follow-up (2010–2015), by sociodemographic, health and behavioural factors among those completing both time points
| Value | Events | PYRS | IR | 95% CI | IRR | 95% CI | P value | ||
| Lower | Upper | Lower | Upper | ||||||
| Overall | 193 | 2311 | 8.374 | 7.242 | 9.721 | ||||
| Gender | |||||||||
| Male | 74 | 815 | 9.097 | 7.266 | 11.496 | 1 | |||
| Female | 119 | 1496 | 8.159 | 6.832 | 9.804 | 0.897 | 0.67 | 1.2 | 0.463 |
| Age groups | |||||||||
| 40–49 | 56 | 975 | 5.04 | 3.837 | 6.73 | 1 | |||
| 50–59 | 47 | 556 | 8.897 | 6.667 | 12.077 | 1.765 | 1.177 | 2.647 | 0.006 |
| 60–69 | 42 | 399 | 11.104 | 8.282 | 15.14 | 2.203 | 1.464 | 3.315 | <0.001 |
| 70–79 | 28 | 239 | 11.875 | 8.285 | 17.436 | 2.356 | 1.486 | 3.735 | <0.001 |
| 80+ | 20 | 141 | 16.197 | 10.647 | 25.379 | 3.213 | 1.931 | 5.348 | <0.001 |
| Education | |||||||||
| None | 85 | 986 | 9.256 | 7.491 | 11.536 | 1 | |||
| Less than secondary | 92 | 1090 | 8.21 | 6.645 | 10.229 | 0.887 | 0.655 | 1.202 | 0.439 |
| Secondary or more | 16 | 235 | 5.318 | 3.112 | 9.652 | 0.575 | 0.319 | 1.034 | 0.065 |
| Union status | |||||||||
| Not in union | 91 | 1034 | 8.613 | 6.983 | 10.709 | 1 | |||
| Formal/informal union | 102 | 1277 | 8.165 | 6.685 | 10.051 | 0.948 | 0.706 | 1.273 | 0.722 |
| SES* | |||||||||
| Low | 68 | 867 | 8.064 | 6.328 | 10.397 | 1 | |||
| Middle | 64 | 736 | 8.715 | 6.798 | 11.305 | 1.081 | 0.759 | 1.539 | 0.667 |
| High | 60 | 689 | 8.389 | 6.449 | 11.056 | 1.04 | 0.723 | 1.497 | 0.831 |
| Employment status | |||||||||
| Not employed | 144 | 1701 | 8.336 | 7.048 | 9.911 | 1 | |||
| Employed | 48 | 595 | 8.511 | 6.375 | 11.557 | 1.021 | 0.726 | 1.435 | 0.905 |
| Smoking history | |||||||||
| Never | 163 | 1861 | 8.644 | 7.392 | 10.155 | 1 | |||
| Prior | 11 | 171 | 6.356 | 3.501 | 12.447 | 0.735 | 0.391 | 1.381 | 0.339 |
| Current | 19 | 279 | 6.715 | 4.293 | 10.956 | 0.777 | 0.478 | 1.262 | 0.308 |
| Alcohol use | |||||||||
| Not in past 30 days | 153 | 1852 | 8.416 | 7.167 | 9.931 | 1 | |||
| Less than weekly | 10 | 167 | 5.459 | 2.879 | 11.284 | 0.649 | 0.331 | 1.272 | 0.208 |
| Weekly | 30 | 292 | 9.789 | 6.708 | 14.649 | 1.163 | 0.766 | 1.765 | 0.478 |
| Physical activity† | |||||||||
| Low | 20 | 122 | 15.468 | 9.85 | 24.91 | 1 | |||
| Moderate | 71 | 687 | 10.641 | 8.402 | 13.61 | 0.688 | 0.412 | 1.149 | 0.153 |
| High | 100 | 1471 | 6.98 | 5.718 | 8.59 | 0.451 | 0.275 | 0.742 | 0.002 |
| High waist circumference‡ | |||||||||
| No | 104 | 1512 | 6.519 | 5.326 | 8.046 | 1 | |||
| Yes | 78 | 735 | 10.571 | 8.483 | 13.283 | 1.621 | 1.197 | 2.196 | 0.002 |
| Diabetes§ | |||||||||
| No | 186 | 2240 | 8.298 | 7.156 | 9.662 | 1 | |||
| Yes | 6 | 64 | 10.06 | 4.451 | 26.006 | 1.212 | 0.529 | 2.778 | 0.649 |
| High triglycerides¶ | |||||||||
| No | 122 | 1660 | 7.262 | 6.049 | 8.775 | 1 | |||
| Yes | 59 | 575 | 10.59 | 8.177 | 13.88 | 1.458 | 1.057 | 2.012 | 0.022 |
| HIV and ART status | |||||||||
| Negative | 155 | 1514 | 10.452 | 8.909 | 12.318 | 1 | |||
| Positive, not on ART | 25 | 486 | 4.749 | 3.15 | 7.439 | 0.454 | 0.289 | 0.713 | 0.001 |
| Positive, on ART | 6 | 178 | 3.553 | 1.52 | 10.122 | 0.34 | 0.142 | 0.811 | 0.015 |
*Based on a household asset index score.
†Based on the International Physical Activity Questionnaire.
‡Greater than 102 cm for men and 88 cm for women.
§Blood glucose greater than or equal to 11.1.
¶Greater than or equal to 1.7 mmol/L.
ART, antiretroviral therapy; SES, socioeconomic status.
Figure 2.
Proportion of participants with incident hypertension, by age and gender, 2015.
bmjopen-2021-049621supp001.pdf (136.1KB, pdf)
Table 2 shows incidence rates and ratios (unadjusted) for those completing both studies by baseline sociodemographic, health and behavioural risk factors. Older individuals had higher incident hypertension risk compared with those aged 40–49 years. Those with high waist circumference and elevated triglycerides had a higher risk of incident hypertension. Respondents engaging in high physical activity levels had a lower risk of incident hypertension compared with those with low physical activity levels. Compared with those HIV negative at baseline, those HIV positive and not on ART had a 55% lower risk of developing hypertension over the 5 years of follow-up, while those on ART also had lower hypertensive risk. Results for the full eligible sample are presented in online supplemental table 1.
Table 3 shows the multivariable-adjusted results from the full Poisson regression excluding HIV status for those completing both studies (see online supplementary table 2 for the full eligible sample results). Older ages (eg, ages 60–69 years adjusted incidence rate ratio (aIRR): 2.4, 95% CI: 1.463 to 3.938), being employed (aIRR: 1.579, 95% CI: 1.071 to 2.329) and having a high waist circumference (aIRR: 1.557, 95% CI: 1.074 to 2.259) were associated with higher risk of incident hypertension in 2015. Those engaging in high levels of physical activity had an approximately 43% lower risk of incident hypertension, although the 95% CI overlapped with the null value of 1 (95% CI: 0.319 to 1.018).
Table 3.
Multivariable Poisson regression of incident hypertension on sociodemographic, health and behavioural risk factors among those completing both time points (n=616)
| aIRR | 95% CI | P value | ||
| Lower | Upper | |||
| Gender | ||||
| Male | 1 | |||
| Female | 0.818 | 0.512 | 1.305 | 0.399 |
| Age groups | ||||
| 40–49 | 1 | |||
| 50–59 | 1.831 | 1.193 | 2.811 | 0.006 |
| 60–69 | 2.4 | 1.463 | 3.938 | <0.001 |
| 70–79 | 2.607 | 1.451 | 4.684 | <0.001 |
| 80+ | 2.561 | 1.196 | 5.488 | <0.001 |
| Education | ||||
| None | 1 | |||
| Less than secondary | 1.061 | 0.732 | 1.537 | 0.755 |
| Secondary or more | 0.741 | 0.372 | 1.478 | 0.395 |
| Union status | ||||
| Not in union | 1 | |||
| Formal/informal union | 1.023 | 0.724 | 1.445 | 0.899 |
| SES* | ||||
| Low | 1 | |||
| Middle | 1.068 | 0.715 | 1.593 | 0.749 |
| High | 0.915 | 0.59 | 1.42 | 0.693 |
| Employment status | ||||
| Not employed | 1 | |||
| Employed | 1.579 | 1.071 | 2.329 | 0.021 |
| Smoking history | ||||
| Never | 1 | |||
| Prior | 0.758 | 0.37 | 1.55 | 0.447 |
| Current | 0.709 | 0.373 | 1.349 | 0.295 |
| Alcohol use | ||||
| Not in past 30 days | 1 | |||
| Less than weekly | 0.717 | 0.345 | 1.492 | 0.373 |
| Weekly | 1.07 | 0.652 | 1.755 | 0.789 |
| Physical activity† | ||||
| Low | 1 | |||
| Moderate | 0.781 | 0.447 | 1.364 | 0.385 |
| High | 0.57 | 0.319 | 1.018 | 0.057 |
| High waist circumference‡ | ||||
| No | 1 | |||
| Yes | 1.557 | 1.074 | 2.259 | 0.02 |
| Diabetes§ | ||||
| No | 1 | |||
| Yes | 0.932 | 0.399 | 2.178 | 0.87 |
| High triglycerides¶ | ||||
| No | 1 | |||
| Yes | 1.297 | 0.932 | 1.805 | 0.123 |
*Based on a household asset index score.
†Based on the International Physical Activity Questionnaire.
‡Greater than 102 cm for men and 88 cm for women.
§Blood glucose greater than or equal to 11.1.
¶Greater than or equal to 1.7 mmol/L.
aIRR, adjusted incidence rate ratio; SES, socioeconomic status.
Table 4 shows the same multivariable-adjusted Poisson model as table 3 including HIV status, with similar results to those risk factors from the model without HIV status. The results for high waist circumference were in the same direction but the 95% CI overlapped with the null value of 1. Those who were HIV positive and not on ART had an approximately 52% lower risk of incident hypertension compared with those HIV negative at baseline (95% CI 0.301 to 0.778), while those HIV positive and on ART showed similar associations to those not on ART.
Table 4.
Multivariable Poisson regression of incident hypertension on sociodemographic, health and behavioural risk factors, and HIV and ART status among those completing both time points (n=581)
| aIRR | 95% CI | P value | ||
| Lower | Upper | |||
| Gender | ||||
| Male | 1 | |||
| Female | 0.854 | 0.533 | 1.369 | 0.512 |
| Age groups | ||||
| 40–49 | 1 | |||
| 50–59 | 1.846 | 1.183 | 2.879 | 0.007 |
| 60–69 | 2.128 | 1.281 | 3.535 | 0.004 |
| 70–79 | 2.339 | 1.256 | 4.356 | 0.007 |
| 80+ | 2.139 | 0.978 | 4.676 | 0.057 |
| Education | ||||
| None | 1 | |||
| Less than secondary | 1.124 | 0.765 | 1.652 | 0.552 |
| Secondary or more | 0.754 | 0.368 | 1.542 | 0.439 |
| Union status | ||||
| Not in union | 1 | |||
| In union | 0.939 | 0.662 | 1.332 | 0.724 |
| SES* | ||||
| Low | 1 | |||
| Middle | 0.97 | 0.647 | 1.454 | 0.883 |
| High | 0.812 | 0.519 | 1.269 | 0.36 |
| Employment status | ||||
| Not employed | 1 | |||
| Employed | 1.604 | 1.064 | 2.419 | 0.024 |
| Smoking history | ||||
| Never | 1 | |||
| Prior | 0.727 | 0.34 | 1.554 | 0.411 |
| Current | 0.661 | 0.345 | 1.267 | 0.213 |
| Alcohol use | ||||
| Not in past 30 days | 1 | |||
| Less than weekly | 0.751 | 0.358 | 1.574 | 0.448 |
| Weekly | 1.111 | 0.668 | 1.846 | 0.685 |
| Physical activity† | ||||
| Low | 1 | |||
| Moderate | 0.77 | 0.434 | 1.365 | 0.371 |
| High | 0.56 | 0.309 | 1.015 | 0.056 |
| High waist circumference‡ | ||||
| No | 1 | |||
| Yes | 1.448 | 0.975 | 2.149 | 0.066 |
| Diabetes§ | ||||
| No | 1 | |||
| Yes | 0.907 | 0.392 | 2.102 | 0.82 |
| High triglycerides¶ | ||||
| No | 1 | |||
| Yes | 1.34 | 0.956 | 1.877 | 0.089 |
| HIV and ART status | ||||
| Negative | 1 | |||
| Positive, not on ART | 0.484 | 0.301 | 0.778 | 0.003 |
| Positive, on ART | 0.462 | 0.197 | 1.082 | 0.075 |
*Based on a household asset index score.
†Based on the International Physical Activity Questionnaire.
‡Greater than 102 cm for men and 88 cm for women.
§Blood glucose greater than or equal to 11.1.
¶Greater than or equal to 1.7 mmol/L.
aIRR, adjusted incidence rate ratio; ART, antiretroviral therapy; SES, socioeconomic status.
Results of the sensitivity analyses of alternate anthropometry measures showed similar associations for BMI as for waist circumference (online supplementary table 3). There were not enough cases of high waist-to-hip ratio to include in the models. A model examining an outcome based only on BP thresholds also showed similar associations to the original models (online supplemental table 4). For the competing risk model, high rates of missing data on HIV/ART status precluded including that indicator. Results omitting HIV/ART status at baseline are presented in online supplemental table 5, showing similar results to the full eligible sample (online supplemental table 2). Cause of death information according to broad cause groups is presented in online supplemental table 6.
Discussion
In 1998, South Africa had approximately 6.3 million adults with hypertension.42 Now it is estimated to be close to 12 million, nearly doubling despite population growth of about 34% over the same time period, with prevalence increasing from 24% to over 40% in some populations.43 Based on our finding of 8.37 per 100 person-years, we estimate that roughly 1.4 million adults over the age of 40 years will develop hypertension over the next 5 years. Given an increase of nearly 50% in the risk of ischaemic heart disease and stroke death for each 10 mm Hg increase,44 the results suggest both a significant increase in the number of people in need of additional treatment and premature mortality if not adequately controlled.
We found that 29% of middle-aged and older adults in our study developed hypertension over a 5-year period. Our results were similar to another study from South Africa following individuals aged 30+ over 5 years (2005–2010) who started with optimal BP. They found a relatively similar incidence of 24%27 given the slightly younger age range.
We showed that men have higher hypertension incidence rates in midlife, while women had higher rates at older ages. This is likely due at least in part to the smaller sample size of men in our study. A potentially similar pattern was shown in a study in South Africa (2004–2016) of patients initiating ART at 10 public sector clinics (9 urban, 1 rural) which included a wider age range (ages 18–50+).24 They found that men had higher hypertension incidence rates at ages 18–39 years, while women had higher rates at ages 40–49 and 50+ years. Our finding may also be due to greater employment for middle-aged men45 and higher survival17 46 or obesity47 48 among older women.
In multivariable-adjusted models, we found that being employed and having a high waist circumference at baseline were risk factors for incident hypertension. Another study in South Africa also found that high waist circumference was a key risk factor, along with alcohol intake.27 While we showed no association with alcohol use, our sample also had low self-reported use of alcohol, with 80% reporting not drinking in the past month, which may be due to response bias.49 Given the limited employment opportunities in our setting,28 a higher risk of hypertension among employed individuals may represent those more likely to be exposed to workplace-related stress and other behavioural factors such as diet50 51 that may differ from those not employed.
We found that being HIV positive at baseline was associated with a lower risk of incident hypertension. This also aligns with an earlier study in South Africa that showed that being HIV positive was inversely related to increased BP.27 However, our results may be sensitive to survivorship bias if those who died due to HIV/AIDS over the 5-year period were also more likely to develop hypertension. Of the 71 individuals for whom mortality information is available, about 28% died due to HIV/AIDS or tuberculosis. If a substantial portion of those individuals developed hypertension, this may affect our estimates of the association between HIV/ART status and risk of incident hypertension. Further, as we lacked information on HIV/ART status for many individuals in the eligible sample who did not complete the follow-up study, this may affect our estimates if those individuals were more likely to be HIV positive.52 A longer period of follow-up is needed to assess the effects of HIV and ART on hypertension and related cardiometabolic conditions. Longitudinal studies restricted to HIV-positive individuals have shown high hypertension incidence rates over relatively short periods of follow-up and similar risk factors to the HIV-negative population.25 26
Our longitudinal findings are particularly important given the complex health transition occurring in South Africa, with a concomitant burden of infections and NCDs.17 23 29 47 A study from the same community as our study demonstrated a high and increasing burden of stroke morbidity and mortality.53 While our findings are consistent with hypertension-related risk factors found in other regions, population-specific studies such as ours are important to contextualise the epidemiological findings from elsewhere and inform local prevention and treatment strategies.14 They also provide an opportunity to understand the interaction between cardiometabolic and infectious diseases such as HIV. A longer period of follow-up, which will be possible as future waves of the study are completed, will permit a greater understanding of the interplay between hypertension, HIV, and treatment of both and related conditions.
We acknowledge our study limitations. While our study is one of the few population-based, longitudinal cohorts on hypertension incidence in Africa, the study comes from a defined region in rural northeast South Africa. Additional studies are needed in other settings, particularly given differences in exposures and differential risk factors in rural and urban contexts. We include a wide range of potential risk factors based on existing studies. Other factors, however, such as migration history, would be important to consider given the high levels of circular labour migration in this setting and potential links of rural to urban migration to increased BP.54–57 Other important factors to consider include nutritional factors such as consumption of fruits and vegetables and salt intake. Further, food insecurity is highly prevalent in this setting58 and may lead to differential hypertension risk due to dietary differences. Given the high level of missing data on HIV/ART status among the eligible population who did not complete the follow-up study, we were unable to assess the effect of HIV/ART in a competing risk framework. Our measure of ART status is also based on self-report and may be subject to response bias, as well as factors related to HIV awareness such as engagement with health services.59 Our self-reported measures may also be subject to social desirability and recall bias.
Over a period of 5 years, 29% of individuals developed hypertension in a population-based cohort of individuals aged 40 years and older given an incidence rate of 8.374 per 100 person-years. Abdominal obesity was one of the most consistent risk factors. Being employed was also a predictor of incident hypertension. As South Africa continues to undergo a complex health and epidemiological transition, continued longitudinal follow-up is needed to understand the complex interplay of non-communicable and infectious diseases, along with their underlying and modifiable risk factors. In response to the call for longitudinal studies from sub-Saharan Africa on hypertension risk, this study contributes to the evidence base that can help inform and target public health strategies to reduce preventable morbidity and mortality.
Supplementary Material
Acknowledgments
The authors thank the study participants and all those involved in the successful field operations in Ha Nakekela, HAALSI and the Agincourt HDSS.
Footnotes
Contributors: BH wrote the first draft and designed and completed the statistical analyses. FXG-O, BH, TAG and ST conceptualised the work. FXG-O, NA, CK and ST designed and implemented the baseline study. TAG, FXG, CK and ST designed and implemented the follow-up study. TAG, NA, SAM, CK, ST and FXG-O revised the manuscript for important intellectual content and contributed to interpretation of the data. All authors read and approved the final manuscript. BH is the guarantor of the study.
Funding: The Ha Nakekela Study was supported by the National Institutes of Health (R24 AG032112-05) and the William and Flora Hewlett Foundation 2009-4060 African Population Research and Training Program. Data analysis for this study, part of the HIV after 40 in rural South Africa project, was funded by the US National Institute on Aging (R01 AG049634) and the University of Colorado, Innovative Seed Grant (not applicable). HAALSI was supported by the US National Institute on Aging (P01AG041710; 1R01AG051144-01; 3U54HG006938-03S1). The MRC/Wits Rural Public Health and Health Transitions Research Unit and Agincourt Health and socio-Demographic Surveillance System, a node of the South African Population Research Infrastructure Network (SAPRIN), is supported by the Department of Science and Innovation (not applicable), the University of the Witwatersrand (not applicable), and the Medical Research Council, South Africa (not applicable), and previously the Wellcome Trust, UK (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The datasets generated and/or analysed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) programme website: wwwhaalsiorg. The data supporting the findings of this study are available from the corresponding author on reasonable request. The datasets generated and/or analysed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) programme website: www.haalsi.org. The data supporting the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical clearance for both surveys and the HDSS were obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) (M10458 and M141159) and the Mpumalanga Provincial Research and Ethics Committee. The baseline study also received ethical approval from the Institutional Review Board of the University of Colorado–Boulder (11-0549) and the follow-up study from the Harvard TH Chan School of Public Health, Office of Human Research Administration (C13-1608-02). Written consent to participate was obtained for all participants in the baseline study. Each respondent in the follow-up study also provided written informed consent (or by a proxy, when needed).
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. The Lancet 2013;380:2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim MM, Damasceno A. Hypertension in developing countries. The Lancet 2012;380:611–9. [DOI] [PubMed] [Google Scholar]
- 3.Bosu WK, Reilly ST, Aheto JMK, et al. Hypertension in older adults in Africa: a systematic review and meta-analysis. PLoS One 2019;14:e0214934. 10.1371/journal.pone.0214934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health 2015;15:1211. 10.1186/s12889-015-2546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaze AD, Schutte AE, Erqou S, et al. Prevalence of hypertension in older people in Africa. Journal of Hypertension 2017;35:1345–52. [DOI] [PubMed] [Google Scholar]
- 6.Sarki AM, Nduka CU, Stranges S, et al. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine 2015;94:e1959. 10.1097/MD.0000000000001959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twagirumukiza M, De Bacquer D, Kips JG, et al. Current and projected prevalence of arterial hypertension in sub-Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens 2011;29:1243–52. 10.1097/HJH.0b013e328346995d [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Sherlock P, Beard J, Minicuci N, et al. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol 2014;43:116–28. 10.1093/ije/dyt215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudharsanan N, Geldsetzer P. Impact of coming demographic changes on the number of adults in need of care for hypertension in Brazil, China, India, Indonesia, Mexico, and South Africa. Hypertension 2019;73:770–6. 10.1161/HYPERTENSIONAHA.118.12337 [DOI] [PubMed] [Google Scholar]
- 10.Addo J, Smeeth L, Leon DA. Hypertension in sub-Saharan Africa: a systematic review. Hypertension 2007;50:1012–8. 10.1161/HYPERTENSIONAHA.107.093336 [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Olivé FX, Ali SA, Made F, et al. Regional and sex differences in the prevalence and awareness of hypertension: an H3Africa AWI-Gen study across 6 sites in sub-Saharan Africa. Glob Heart 2017;12:81-90. 10.1016/j.gheart.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataklte F, Erqou S, Kaptoge S, et al. Burden of undiagnosed hypertension in sub-Saharan Africa: a systematic review and meta-analysis. Hypertension 2015;65:291–8. 10.1161/HYPERTENSIONAHA.114.04394 [DOI] [PubMed] [Google Scholar]
- 13.Jardim TV, Reiger S, Abrahams-Gessel S, et al. Hypertension management in a population of older adults in rural South Africa. J Hypertens 2017;35:1283–9. 10.1097/HJH.0000000000001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kengne AP, Ntyintyane LM, Mayosi BM. A systematic overview of prospective cohort studies of cardiovascular disease in sub-Saharan Africa. Cardiovasc J Afr 2012;23:103–12. 10.5830/CVJA-2011-042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes MD, Dalal S, Volmink J, et al. Non-Communicable diseases in sub-Saharan Africa: the case for cohort studies. PLoS Med 2010;7:e1000244. 10.1371/journal.pmed.1000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst AJ, Cooke GS, Bärnighausen T, et al. Adult mortality and antiretroviral treatment roll-out in rural KwaZulu-Natal, South Africa. Bull World Health Organ 2009;87:754–62. 10.2471/blt.08.058982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabudula CW, Houle B, Collinson MA, et al. Progression of the epidemiological transition in a rural South African setting: findings from population surveillance in Agincourt, 1993-2013. BMC Public Health 2017;17:424. 10.1186/s12889-017-4312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmer S, Harttgen K, Alfven T, et al. The HIV epidemic in sub-Saharan Africa is aging: evidence from the demographic and health surveys in sub-Saharan Africa. AIDS Behav 2016;21:101–13. [DOI] [PubMed] [Google Scholar]
- 19.Anand AR, Rachel G, Parthasarathy D. Hiv proteins and endothelial dysfunction: implications in cardiovascular disease. Front Cardiovasc Med 2018;5:185. 10.3389/fcvm.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. New England Journal of Medicine 2005;352:48–62. [DOI] [PubMed] [Google Scholar]
- 21.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and art with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–71. 10.1093/ije/dyt198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigaud AS, Forette B. Hypertension in older adults. J Gerontol A Biol Sci Med Sci 2001;56:M217–25. 10.1093/gerona/56.4.m217 [DOI] [PubMed] [Google Scholar]
- 23.Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet 2009;374:934–47. 10.1016/S0140-6736(09)61087-4 [DOI] [PubMed] [Google Scholar]
- 24.Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS One 2018;13:e0204020. 10.1371/journal.pone.0204020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens 2015;33:2039–45. 10.1097/HJH.0000000000000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania - A prospective cohort study. PLoS One 2017;12:e0172089. 10.1371/journal.pone.0172089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutte AE, Schutte R, Huisman HW, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in black South Africans? A 5-year prospective study. Int J Epidemiol 2012;41:1114–23. 10.1093/ije/dys106 [DOI] [PubMed] [Google Scholar]
- 28.Kahn K, Collinson MA, Gómez-Olivé FX, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol 2012;41:988–1001. 10.1093/ije/dys115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Olivé FX, Angotti N, Houle B, et al. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care 2013;25:1122–8. 10.1080/09540121.2012.750710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Olivé FX, Montana L, Wagner RG, et al. Cohort profile: health and ageing in Africa: a longitudinal study of an indepth community in South Africa (HAALSI). Int J Epidemiol 2018;47:689–90. 10.1093/ije/dyx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altunkan S, Oztaş K, Altunkan E. Validation of the Omron 637IT wrist blood pressure measuring device with a position sensor according to the International protocol in adults and obese adults. Blood Press Monit 2006;11:79–85. 10.1097/01.mbp.0000200483.49540.dc [DOI] [PubMed] [Google Scholar]
- 32.Saladini F, Benetti E, Palatini P. Accuracy of the visomat handy wrist blood pressure measuring device according to the International protocol. Blood Press Monit 2010;15:281–4. 10.1097/MBP.0b013e32833e50f2 [DOI] [PubMed] [Google Scholar]
- 33.Omboni S, Riva I, Giglio A, et al. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International protocol of the European Society of hypertension. Blood Press Monit 2007;12:233–42. 10.1097/MBP.0b013e32813fa386 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) . National health and nutrition examination survey. 1999-2000 Data Documentation, Codebook, and Frequencies 2002. [Google Scholar]
- 35.US Department of Health and Human Services . The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. National Heart, Lung, and Blood Institute 2004. NIH Publication No. 04-5230. [PubMed] [Google Scholar]
- 36.World Health Organization . Prevention of cardiovascular disease. pocket guidelines for assessment and management of cardiovascular risk: (WHO/ISH cardiovascular risk prediction charts for the African region). World Health Organization, Geneva 2007. [Google Scholar]
- 37.US Department of Health and Human Services . The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. National Heart, Lung, and Blood Institute 2004. [PubMed] [Google Scholar]
- 38.Kabudula CW, Houle B, Collinson MA, et al. Assessing changes in household socioeconomic status in rural South Africa, 2001-2013: a distributional analysis using household asset indicators. Soc Indic Res 2016;133:1047–73. 10.1007/s11205-016-1397-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byass P, Chandramohan D, Clark SJ, et al. Strengthening standardised interpretation of verbal autopsy data: the new InterVA-4 tool. Glob Health Action 2012;5:1–8. 10.3402/gha.v5i0.19281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. StataCorp. Stata statistical software: release 15. StataCorp, LP, College Station, TX 2017. [Google Scholar]
- 41.Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 42.Steyn K, Gaziano TA, Bradshaw D, et al. Hypertension in South African adults: results from the demographic and health survey, 1998. J Hypertens 2001;19:1717–25. 10.1097/00004872-200110000-00004 [DOI] [PubMed] [Google Scholar]
- 43.Ntuli ST, Maimela E, Alberts M, et al. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. Afr J Prim Health Care Fam Med 2015;7:847. 10.4102/phcfm.v7i1.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–71. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 45.Blalock CL. Labor migration and employment in post-apartheid rural South Africa. Department of SociologyDoctor of Philosophy 2014. [Google Scholar]
- 46.Houle B, Clark SJ, Gómez-Olivé FX, et al. The unfolding counter-transition in rural South Africa: mortality and cause of death, 1994-2009. PLoS One 2014;9:e100420. 10.1371/journal.pone.0100420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark SJ, Gómez-Olivé FX, Houle B, et al. Cardiometabolic disease risk and HIV status in rural South Africa: establishing a baseline. BMC Public Health 2015;15:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: the HAALSI (health and aging in Africa: longitudinal studies of indepth communities) study. BMC Public Health 2017;17:206. 10.1186/s12889-017-4117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houle B, Angotti N, Gómez-Olivé FX, et al. Fieldworker effects on substance use reporting in a rural South African setting. Int J Alcohol Drug Res 2018;7:29–39. 10.7895/ijadr.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maredza M, Hofman KJ, Tollman T. A hidden menace: cardiovascular disease in South Africa and the costs of an inadequate policy response: health policy and cardiovascular disease. SA Heart 2011;8:48–57. [Google Scholar]
- 51.Feeley ABB, Kahn K, Twine R, et al. Exploratory survey of informal vendor-sold fast food in rural South Africa. South African Journal of Clinical Nutrition 2011;24:199–201. [Google Scholar]
- 52.Clark SJ, Houle B. Validation, replication, and sensitivity testing of Heckman-type selection models to adjust estimates of HIV prevalence. PLoS One 2014;9:e112563. 10.1371/journal.pone.0112563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurol 2015;15:54. 10.1186/s12883-015-0311-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collinson MA, White MJ, Bocquier P, et al. Migration and the epidemiological transition: insights from the Agincourt sub-district of northeast South Africa. Glob Health Action 2014;7:23514. 10.3402/gha.v7.23514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pheiffer CF, McGarvey ST, Ginsburg C, et al. Dimensions of internal migration and their relationship to blood pressure in South Africa. J Biosoc Sci 2019;51:1–16. 10.1017/S0021932019000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulter NR, Khaw KT, Hopwood BE, et al. The Kenyan Luo migration study: observations on the initiation of a rise in blood pressure. BMJ 1990;300:967–72. 10.1136/bmj.300.6730.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steyn K, Bradshaw D, Norman R, et al. Determinants and treatment of hypertension in South Africans: the first demographic and health survey. S Afr Med J 2008;98:376–80. [PubMed] [Google Scholar]
- 58.Hunter LM, Twine W, Patterson L. "Locusts are now our beef": adult mortality and household dietary use of local environmental resources in rural South Africa. Scand J Public Health Suppl 2007;69:165–74. 10.1080/14034950701356385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manne-Goehler J, Montana L, Gómez-Olivé FX, et al. The art advantage: health care utilization for diabetes and hypertension in rural South Africa. J Acquir Immune Defic Syndr 2017;75:561–7. 10.1097/QAI.0000000000001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049621supp001.pdf (136.1KB, pdf)
Data Availability Statement
The datasets generated and/or analysed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) programme website: wwwhaalsiorg. The data supporting the findings of this study are available from the corresponding author on reasonable request. The datasets generated and/or analysed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) programme website: www.haalsi.org. The data supporting the findings of this study are available from the corresponding author on reasonable request.