Abstract
Background
Individuals who contract coronavirus disease 2019 (COVID-19) can suffer with persistent and debilitating symptoms long after the initial acute illness. Heart rate (HR) profiles determined during cardiopulmonary exercise testing (CPET) and delivered as part of a post-COVID recovery service may provide insight into the presence and impact of dysautonomia on functional ability.
Objective
Using an active, working-age, post–COVID-19 population, the purpose of this study was to (1) determine and characterize any association between subjective symptoms and dysautonomia; and (2) identify objective exercise capacity differences between patients classified “with” and those “without” dysautonomia.
Methods
Patients referred to a post–COVID-19 service underwent comprehensive clinical assessment, including self-reported symptoms, CPET, and secondary care investigations when indicated. Resting HR >75 bpm, HR increase with exercise <89 bpm, and HR recovery <25 bpm 1 minute after exercise were used to define dysautonomia. Anonymized data were analyzed and associations with symptoms, and CPET outcomes were determined.
Results
Fifty-one of the 205 patients (25%) reviewed as part of this service evaluation had dysautonomia. There were no associations between symptoms or perceived functional limitation and dysautonomia (P >.05). Patients with dysautonomia demonstrated objective functional limitations with significantly reduced work rate (219 ± 37 W vs 253 ± 52 W; P <.001) and peak oxygen consumption (V̇o2: 30.6 ± 5.5 mL/kg/min vs 35.8 ± 7.6 mL/kg/min; P <.001); and a steeper (less efficient) V̇e/V̇co2 slope (29.9 ± 4.9 vs 27.7 ± 4.7; P = .005).
Conclusion
Dysautonomia is associated with objective functional limitations but is not associated with subjective symptoms or limitation.
Keywords: Cardiopulmonary exercise testing, COVID-19, Dysautonomia, Exercise testing, Symptoms
Introduction
With over 275 million confirmed cases,1 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global crisis. In addition to the mortality burden, there is the important secondary challenge of ongoing morbidity. A large proportion of patients experience symptoms for many months, even after mild initial infection.2 Persistent debilitating symptoms include fatigue, breathlessness, muscle and/or joint pain, exercise intolerance, palpitations, headaches, memory loss, nausea, and dramatic mood disturbances.3 Several symptoms are impacted by the autonomic nervous system,4 with fatigue described as one of the major clinical features of dysautonomia in patients with coronavirus disease 2019 (COVID-19).5 Dysautonomia has been reported in other postviral illnesses.6 Establishing the frequency of dysautonomia and determining any association with symptoms and subjective or objective limitation in patients affected by COVID-19 is an essential step to understanding and managing this clinical entity.
Exercise provides a safe means to expose imbalances in parasympathetic and sympathetic activity that may be hidden at rest.7 Heart rate (HR) is recorded throughout a cardiopulmonary exercise test (CPET), and exercise HR profiles are prognostically important.8 Heart rate variability (HRV) (variability in the time interval between heart beats) recorded at rest is a well-validated marker of parasympathetic tone and is associated with the severity of acute COVID-19 illness.9
Most literature on the effects of COVID-19 focuses on severe acute illness, typically in vulnerable older adults with premorbid health conditions.10 The long-term effects of SARS-CoV-2 on individuals of working age in full-time employment are not known. In a military context, return to full military duty, including maximal exercise in hazardous remote environments, represents an important risk to personal health and operational effectiveness. Therefore, British Armed Forces personnel with severe acute COVID-19 illness or protracted symptoms (>12 weeks) attend a post–COVID-19 assessment clinic—the Defence Medical Services COVID-19 Recovery Service (DCRS).11 Personnel who are medically downgraded due to COVID-19 are unable to deploy on operations and are not fit to return to active duty. Consequently, there is an operational and financial imperative to understand the impact of this virus on serving personnel. The results from this service are of direct relevance to wider public health. Using an active, working-age population, this study aims to (1) determine and characterize any association between subjective symptoms and dysautonomia; and (2) identify objective exercise capacity differences between patients classified “with” and those “without” dysautonomia in a population with enduring post–COVID-19 symptoms.
Methods
The post–COVID-19 clinic received patients referred from primary care with confirmed or probable COVID-19 infection, who met specific eligibility criteria (hospitalization, life-limiting symptoms beyond 12 weeks, desaturation ≤95% on a Harvard step test, or chest pain with electrocardiographic [ECG] changes during acute illness).12 In accordance with current policy on the use of clinical data for education, evaluation, and audit purposes, permission was granted for publication by the Defence Medical Rehabilitation Centre (DMRC) Caldicott Guardian and as such did not require formal ethical approval. The patient information and clinical data routinely collected in support of DCRS were used in this service evaluation. Data include the outcomes of every consecutive patient who attended the DCRS.
Demographics
Age, sex, height, body mass, and details of acute COVID-19 illness were recorded at the start of each admission.
Protocol
CPET
CPET was performed on an electromagnetically braked cycle ergometer (Lode Carnival, Lode BV, Groningen, The Netherlands) at 16° to 18°C, with indirect calorimetry (Metalyzer 3B, Cortex Biophysik, Leipzig, Germany). The CPET protocol followed at least 5 minutes of rest. Two-minute resting recording was followed by 2-minute unloaded cycling at a cadence of 60 rpm, before introduction of a 25-W resistance followed by a progressively increasing workload to volitional fatigue. Work rate/min ramp was selected to achieve 8 to 12 minutes of loaded exercise. Continuous measurement of ventilation (V̇e), oxygen consumption (V̇o 2), expired carbon dioxide (V̇co 2), HR, ECG, peripheral oxyhemoglobin saturation (Spo 2), and workload were performed throughout the rest, exercise, and recovery phases.13 Maximal tests were defined by respiratory exchange ratio >1.1 and/or ≥30-second plateau in oxygen. Blood pressure, Spo 2, rate of perceived exertion (RPE; 6–20 on Borg scale),14 and dyspnea (0–10 on modified Borg scale)15 were recorded every 2 minutes. Following attainment of maximal exercise capacity, participants remained stationary on the ergometer for at least 3 minutes.
Outcome measures
Spirometry
Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were recorded.
Criteria for dysautonomia
Jouven’s criteria were used for the diagnosis of exercise-associated dysautonomia8: (1) resting HR >75 bpm; (2) increase in HR during exercise <89 bpm; and (3) HR recovery <25 bpm in the first 60 s after cessation of exercise. A diagnosis of dysautonomia required that all 3 criteria were met.
Symptoms
Symptoms with prevalence >25% were carried forward for further analysis.
HRV
HRV values were obtained using 12-lead ECG acquisition during rest seated upright on the cycle ergometer. HRV also was obtained during the first 3 minutes of passive recovery after exercise. Both resting and recovery values have been suggested as indicators of parasympathetic cardiac modulation.16
All individual raw data files containing R-R intervals were exported to Excel™ (Microsoft, Redmond, WA) before being read in Python. Automatic detection and removal of ectopic beats from the raw R-R intervals were performed using the method described by Acar et al,17 with the output inspected manually. Missing values were replaced using linear interpolation. Following preprocessing of the R-R intervals, the root square of the mean of squared differences of adjacent RR intervals (RMSSD), a measure of beat-to-beat variability,16 was calculated. RMSSD is the primary time-domain measure used to estimate the vagal-mediated changes reflected in HRV.18 RMSSD was measured at rest using the central 1-minute window of the 2-minute rest period. RMSSD was calculated in each 30-second window of the 3-minute recovery phase.19 , 20 All analysis was conducted using Python 3.7 (Python Software Foundation, Wilmington, DE).
Statistical analysis
The normality of all variables within the dataset was assessed using a Shapiro-Wilk test and inspection of the Q-Q plots, demonstrating normal distribution across most of variables. Given that the sample size was sufficiently large (n = 205), parametric tests were applied throughout.21 The most prevalent symptoms (prevalence >25%) were extracted, and tests of associations between the classification of dysautonomia and each symptom were performed using χ2 tests. To determine the effect of dysautonomia, independent sample t tests were conducted for CPET-related variables, identifying objective physiological differences between patients “with” and those “without” dysautonomia. For all statistical tests, an α threshold for significance of 0.05 was applied. All statistical analyses were performed using SPSS Version 27 (IBM, Armonk, NY).
Results
Mean duration from the onset of symptoms to the date of CPET assessment for the total cohort was 183 ± 77days (∼6 months). No significant differences in the duration between onset of symptoms and assessment date were observed between patients with and those without dysautonomia (180 ± 81 days vs 184 ± 76 days, respectively; P = .347).
Demographics
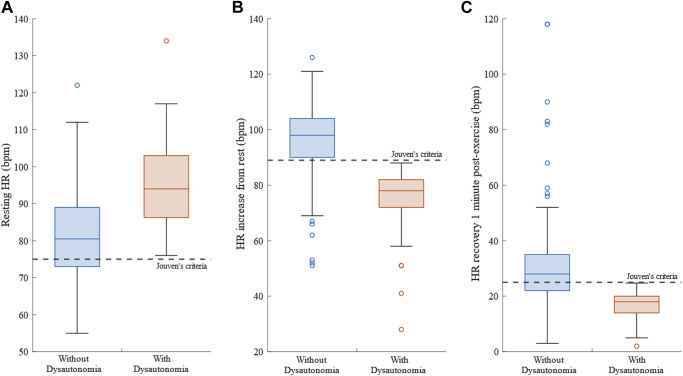
Of the 205 participants included in this study, 51 (25%) were classified with dysautonomia, according to the criteria outlined by Jouven et al8 (Figure 1 ). Intergroup analysis revealed patients with dysautonomia are older, have higher body mass index (BMI), and higher waist circumference. Body height and sex (84% men; 16% women) were comparable between groups (Table 1 ). Descriptive statistics of the HR parameters are given in Table 2 .
Figure 1.
Boxplots showing between-group differences across each of the dysautonomia criteria specified in Jouven et al.8A: Resting heart rate (HR). B: Heart rate increase from rest to peak stress. C: HR recovery 1 minute post-exercise.
Table 1.
Patient demographics
| Variable | All patients (N = 205) | Without dysautonomia (n = 154) | With dysautonomia (n = 51) | Stat | P value | Effect size |
|---|---|---|---|---|---|---|
| Sex (M/F) | 172/33 | 129/25 | 43/8 | 0.009† | .927 | 0.006 |
| Age (y) | 38 ± 10 | 37 ± 10 | 43 ± 9 | –4.053‡ | <.001∗ | 0.655 |
| Height (cm) | 177 ± 8 | 177 ± 9 | 177 ± 7 | 0.443‡ | .658 | 0.072 |
| Body mass (kg) | 90 ± 17 | 89 ± 17 | 94 ± 16 | –1.645‡ | .101 | 0.266 |
| BMI (kg/m2) | 29 ± 4 | 28 ± 4 | 30 ± 4 | –2.520‡ | .013∗ | 0.407 |
| Waist circumference (cm) | 95 ± 13 | 94 ± 13 | 100 ± 12 | –2.989‡ | .003∗ | 0.500 |
Values are given as mean ± SD.
Effect size calculated using Cohen’s d (small 0.2–0.5; medium 0.5–0.8; large >0.8). Significant P values ∗P <.05 and medium/large effect sizes are shown in bold.
BMI = body mass index.
χ2 statistic
t statistic.
Table 2.
Physiological measures during the cardiopulmonary exercise testing protocol of all patients who attended the Defence Medical Services COVID-19 Recovery Service and differences between patients classified with and those without dysautonomia
| Variable | All patients (n = 205) | Without dysautonomia (n = 154) | With dysautonomia (n = 51) | t | P value | Effect size |
|---|---|---|---|---|---|---|
| CPET | ||||||
| V̇o2 at rest (mL/kg/min) | 5.1 ± 1.04 | 5.1 ± 1.1 | 5.2 ± 0.8 | –0.454 | .650 | 0.073 |
| V̇o2 at VT1 (mL/kg/min) | 13.7 ± 3.0 | 14.1 ± 3.2 | 12.6 ± 2.1 | 3.273 | .001† | 0.529 |
| V̇o2 at peak (mL/kg/min) | 34.5 ± 7.4 | 35.8 ± 7.6 | 30.6 ± 5.5 | 4.544 | <.001‡ | 0.734 |
| Work rate at VT1 (W) | 78 ± 23 | 80 ± 24 | 71 ± 16 | 2.345 | .020∗ | 0.379 |
| Work rate at peak (W) | 245 ± 51 | 253 ± 52 | 219 ± 37 | 4.329 | <.001‡ | 0.699 |
| ΔV̇o2 (L/min)/ΔWork (W) | 10.7 ± 1.4 | 10.7 ± 1.4 | 10.7 ± 1.2 | –0.008 | .994 | 0.001 |
| Lactate at rest (mmol/L) | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | –3.254 | .001† | 0.545 |
| Lactate at peak (mmol/L) | 12.7 ± 2.6 | 12.9 ± 2.6 | 12.2 ± 2.6 | 1.582 | .115 | 0.280 |
| O2 pulse | 17.7 ± 4.4 | 17.9 ± 4.5 | 17.0 ± 3.9 | 1.264 | .208 | 0.204 |
| HR profile | ||||||
| HR at rest (bpm) | 84 ± 13 | 81 ± 12 | 95 ± 12 | –7.343 | <.001‡ | 1.186 |
| HR at VT1 (bpm) | 109 ± 16 | 107 ± 17 | 114 ± 15 | –2.408 | .017∗ | 0.389 |
| HR at peak (bpm) | 175 ± 15 | 177 ± 15 | 170 ± 13 | 2.956 | .003† | 0.478 |
| Percent of predicted max HR | 109 ± 8 | 109 ± 8 | 109 ± 8 | –0.030 | .976 | 0.005 |
| HR recovery after 1 min (bpm) | 27 ± 16 | 31 ± 17 | 17 ± 4 | 5.730 | <.001‡ | 0.926 |
| HR increase from rest to peak (bpm) | 91 ± 17 | 96 ± 13 | 75 ± 12 | 10.304 | <.001‡ | 1.665 |
| HRV | ||||||
| RMSSD at rest (m∙s-1) | 26 ± 14 | 29 ± 13 | 15 ± 9 | 6.243 | <.001‡ | 1.057 |
| RMSSD (30–60 s recovery, m∙s-1) | 8 ± 10 | 8 ± 10 | 9 ± 10 | –0.527 | .599 | 0.089 |
| RMSSD (90–120 s recovery, m∙s-1) | 9 ± 9 | 10 ± 10 | 7 ± 7 | 1.578 | .116 | 0.267 |
| RMSSD (150–180 s recovery, m∙s-1) | 9 ± 10 | 9 ± 9 | 10 ± 12 | –1.013 | .312 | 0.172 |
| BP (mm Hg) | ||||||
| Resting systolic BP (mm Hg) | 121 ± 11 | 120 ± 10 | 121 ± 12 | –0.735 | .463 | 0.119 |
| Resting diastolic BP (mm Hg) | 81 ± 9 | 81 ± 9 | 82 ± 10 | –0.682 | .496 | 0.110 |
| VT1 systolic BP (mm Hg) | 140 ± 17 | 139 ± 17 | 141 ± 16 | –0.464 | .643 | 0.076 |
| VT1 diastolic BP (mm Hg) | 83 ± 12 | 82 ± 13 | 86 ± 9 | –1.811 | .072 | 0.295 |
| Peak systolic BP (mmHg) | 169 ± 21 | 169 ± 21 | 166 ± 22 | 1.056 | .292 | 0.171 |
| Peak diastolic BP (mmHg) | 75 ± 22 | 73 ± 23 | 83 ± 16 | –2.819 | .005† | 0.455 |
| Ventilation | ||||||
| BF at rest (breaths/min) | 16 ± 5 | 16 ± 4 | 18 ± 5 | –2.756 | .006† | 0.449 |
| BF at VT1 (breaths/min) | 20 ± 6 | 19 ± 5 | 23 ± 6 | –3.609 | <.001‡ | 0.588 |
| BF at peak (breaths/min) | 47 ± 9 | 46 ± 9 | 48 ± 9 | –1.234 | .219 | 0.201 |
| V̇E/V̇co2 at rest | 30.2 ± 4.3 | 29.8 ± 4.3 | 31.3 ± 4.2 | –2.107 | .036∗ | 0.340 |
| V̇E/V̇co2 at VT1 | 26.6 ± 3.4 | 26.1 ± 3.2 | 28.0 ± 3.7 | –3.462 | .001† | 0.559 |
| V̇E/V̇co2 at peak | 33.7 ± 7.7 | 32.7 ± 4.7 | 36.4 ± 12.9 | –2.973 | .003† | 0.480 |
| V̇E/V̇co2 slope | 28.3 ± 4.8 | 27.7 ± 4.7 | 29.9 ± 4.9 | –2.839 | .005† | 0.490 |
| pco2 rest | 5.0 ± 0.6 | 5.0 ± 0.5 | 5.0 ± 0.8 | 0.037 | .970 | 0.006 |
| pco2 peak | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.2 ± 0.6 | 1.191 | .235 | 0.209 |
Values are given as mean ± SD unless otherwise indicated.
Effect size calculated using Cohen’s d (small 0.2–0.5; medium 0.5–0.8; large >0.8).
Significant P values
BF = breathing frequency; BP = blood pressure; COVID-19 = coronavirus disease 2019; CPET = cardiopulmonary exercise testing; HR = heart rate; HRV = heart rate variability; RMSSD = root mean square of successive differences of R-R intervals; V̇co2 = expired carbon dioxide; V̇e = ventilation; V̇o2 = oxygen consumption; VT1 = first ventilatory threshold.
P <.05
P <.01
P <.001 and medium/large effect sizes are shown in bold.
Associations between symptoms of COVID-19 and dysautonomia
Fourteen of 38 symptoms had prevalence >25% and underwent associative testing. There were associations between low mood (P = .007), headaches (P = .026), and poor attention (P = .047) with the classification of dysautonomia. However, the effect sizes of these associations were all small (Cohen’s d = 0.190, 0.155, and 0.139, respectively). Results of each of the symptoms associative tests are given in Table 3 .
Table 3.
Prevalence of symptoms in all patients, differences between patients with and those without dysautonomia, and associations between the presence of symptoms reported and dysautonomia
| Symptom | All patients (N = 205) | Without dysautonomia (n = 154) | With dysautonomia (n = 51) | χ2 | P value | Effect size |
|---|---|---|---|---|---|---|
| Any SOB | 61 | 58 | 69 | 1.67 | .196 | 0.09 |
| Moderate SOB | 54 | 52 | 61 | 1.205 | .272 | 0.077 |
| Fatigue | 54 | 51 | 60 | 1.386 | .239 | 0.082 |
| Any cognitive issues | 48 | 48 | 47 | 0.015 | .902 | 0.009 |
| Poor concentration | 40 | 40 | 41 | 0.039 | .843 | 0.014 |
| Mild SOB | 37 | 34 | 45 | 2.121 | .145 | 0.102 |
| Staying asleep | 32 | 25 | 31 | 0.714 | .398 | 0.059 |
| Muscle aches | 31 | 30 | 33 | 0.216 | .642 | 0.032 |
| Poor memory | 31 | 31 | 29 | 0.056 | .814 | 0.016 |
| Getting to sleep | 28 | 25 | 37 | 2.688 | .101 | 0.115 |
| Low mood | 28 | 23 | 43 | 7.374 | .007† | 0.19 |
| Anxiety | 26 | 26 | 28 | 0.043 | .836 | 0.014 |
| Headache | 25 | 17 | 31 | 4.938 | .026∗ | 0.155 |
| Poor attention | 25 | 21 | 35 | 3.941 | .047∗ | 0.139 |
Values are given as % unless otherwise indicated.
Effect size calculated using Cohen’s d (small 0.2–0.5; medium 0.5–0.8; large >0.8). Significant P values
SOB = shortness of breath.
P <.05
P <.01 are shown in bold.
Effect of dysautonomia on CPET outcomes
Notable differences in CPET findings were observed between patients who met the dysautonomia criteria8 and those who did not.
Spirometry
Although FVC (4.82 ± 0.79 L vs 5.17 ± 1.07 L; P = .031) and FEV1 (3.63 ± 0.61 L vs 3.87 ± 0.75 L; P = .036) were lower in patients with dysautonomia, the magnitudes of difference were very small (Cohen’s d <0.20).
HR profile
Mean HR values were higher in patients with dysautonomia during rest (95 ± 12 bpm vs 81 ± 12b pm; P <.001) and at the first ventilatory threshold (VT1) (114 ± 15 bpm vs 107 ± 17 bpm; P = .017) but less at peak exercise (170 ± 13 bpm vs 177 ± 15 bpm; P = .003). Increase in HR from rest to peak was lower in the dysautonomia group (75 ± 12 bpm vs 96 ± 13 bpm; P <.001). HR recovery at 1 minute following the cessation of exercise was also significantly less in patients with dysautonomia (17 ± 4b pm vs 31 ± 17 bpm; P <.001).
HRV
HRV, measured by RMSSD, was lower in patients with dysautonomia (15 ± 9 m∙s-1 vs 29 ± 13 m∙s-1; P <.001), with a large effect size (d = 1.057). No difference was identified between groups for RMSSD measured during exercise recovery (P >.05).
Work rate (watts and lactate accumulation)
Work rate at VT1 (71 ± 16 W vs 80 ± 24 W; P = .020) and work rate at peak (219 ± 37 W vs 253 ± 52 W; P <.001) were lower in patients with dysautonomia. Despite the differences in work rate between groups, lactate accumulation at peak exercise was comparable.
Ventilation
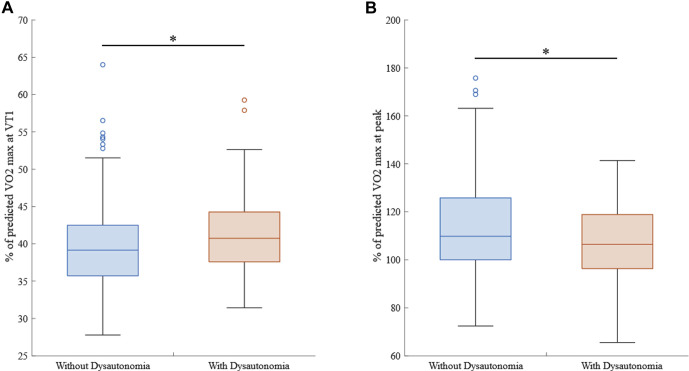
Patients with dysautonomia had lower oxygen consumption (V̇o 2) at VT1 (earlier anaerobic transition) (12.6 ± 2.1 mL/kg/min vs 14.1 ± 3.2 mL/kg/min; P = .001) and peak exercise (30.6 ± 5.5 mL/kg/min vs 35.8 ± 7.6 mL/kg/min; P <.001). This group also demonstrated lower values for V̇o 2 recorded at peak exercise (% predicted) (107% ± 18% vs 114% ± 20%; P = .028) (Figure 2B). Patients with dysautonomia demonstrated a greater percentage of their predicted peak V̇o 2 maximum at VT1 (42% ± 6% vs 40% ± 6%; P = .047), with small effect size (d = 0.322) (Figure 2A). No differences were observed in respiratory exchange ratio at peak exercise for patients with and those without dysautonomia (1.18 ± 0.05 vs 1.17 ± 0.05, respectively; P >.05).
Figure 2.
Comparison of percentages of predicted maximum oxygen consumption (Vo2max) at VT1 (A) and actual Vo2max (B) between groups. Asterisk indicates significant between-group difference.
Patients with dysautonomia demonstrated lower ventilatory efficiency (higher V̇e/V̇co 2) at rest, VT1, and peak exercise. Furthermore, the V̇e/V̇co 2 slope was steeper (less efficient) in patients with dysautonomia vs those without dysautonomia (29.9 ± 4.9 vs. 27.7 ± 4.7, respectively; P = .005). Patients with dysautonomia demonstrated a higher breathing frequency at rest and at VT1 compared to those without dysautonomia. No differences in breathing frequency at peak were demonstrated between groups (P >.05).
RPE and shortness of breath
Regarding perception of effort, RPE (rated 6–20) did not differ between groups during rest or at VT1; however, RPE at peak was significantly lower (indicating less perceived exertion) in patients with dysautonomia (16 ± 3 vs 17 ± 2; P = .021), and the effect size was small (d = 0.378). No differences in shortness of breath scores (rated 0–10) were demonstrated between patients with and those without dysautonomia during rest (0 ± 1 vs 0 ± 1; P >.05), at VT1 (1 ± 2 vs 1 ± 1; P >.05), or at peak exercise (6 ± 3 vs 7 ± 2; P >.05).
Discussion
This study identified dysautonomia in a significant proportion (25%) of active, working-age adults assessed at a median 6 months postacute COVID-19 illness. Using Jouven’s criteria to identify those with dysautonomia occurring as part of a maximal exercise test,8 no clinically significant associations were found with subjective symptoms or exercise intolerance. However, objective functional capacity was reduced in dysautonomia.
In normal physiology, maximal exercise capacity is determined by circulatory limitation (cardiac output). Patients with dysautonomia are restricted in their ability to augment HR, from rest to peak stress, which reduces their capacity to increase peak cardiac output. The mean difference in HR response to exercise was 75 ± 12 bpm in patients with dysautonomia vs 96 ± 13 bpm in those without. This is despite a somewhat elevated resting HR in all groups (84 ± 13 bpm) that the authors conclude is primarily due to anticipatory HR response prior to a maximal exercise challenge. Although peak HR was lower in the group with dysautonomia, HR at VT1 was higher. Given that this is the point of transition to mixed aerobic and anaerobic metabolism, the higher HR may be implicated in reduced exercise capacity. Work rate at VT1 and peak exercise were significantly lower in patients with dysautonomia.
Unlike previous studies of chronic fatigue syndrome and postural orthostatic tachycardia syndrome, this study did not demonstrate an association between subjective limitation (symptoms and subjective exercise intolerance) and dysautonomia. Although there was an association between dysautonomia and the symptoms of headache, low mood, and poor attention, the effect sizes were very small. There was no association with breathlessness, palpitations, or exertional intolerance, which might reasonably be anticipated to result from dysautonomia. During CPET, there were no differences in subjective symptoms of breathlessness at rest, VT1, or peak exercise, and the only differences in RPE values were demonstrated at peak RPE. The magnitude of this difference was trivial. These findings demonstrate that symptoms of post–COVID-19 syndrome are not predictive of dysautonomia. They also suggest that dysautonomia is unlikely to be a significant contributor to symptoms.
Measurement of HRV immediately following peak exercise did not reveal pathophysiological insights beyond the HR profiles used as an indirect measure of dysautonomia.8 Given the strong dependence of HRV on resting HR,22 this finding is perhaps unsurprising. The added benefit of utilizing HR recovery following maximal exercise in the indirect measure of dysautonomia8 was that HR recovery is a highly reproducible measure of an individual’s vagal tone. It is independent of other physiological measures, including resting HR.7
There was a strong association between dysautonomia and lower ventilatory efficiency. V̇e/V̇co 2 values demonstrated impaired ventilatory efficiency at rest, VT1, and peak. Two contrasting mechanisms might explain this finding: (1) lung injury sufficient to cause ventilation/perfusion mismatch; and (2) inappropriate ventilation in excess of the body’s physiological gas exchange requirements (hyperventilation). In this study, no association was identified between dysautonomia and lung pathology (computed tomographic scan and diffusing capacity) (for detailed results of clinical investigations, see the Supplemental Appendix). There also was no significant lowering of peak capillary Pco 2 in patients with vs those without dysautonomia to confirm inappropriate hyperventilation. However, patients with dysautonomia did demonstrate significantly higher breathing frequency at rest and VT1. It is possible that a central insult from this virus, with a recognized neurotropism,23 might cause both dysautonomia and central abnormalities of ventilation.
Patients with dysautonomia demonstrate a less favorable body composition compared to those without dysautonomia (higher BMI and waist circumference). It is not possible to determine whether this reflects an increased risk of complex acute COVID-19 illness, the potential of post-COVID complications in those with a high BMI, or premorbid reduced cardiovascular fitness. Another possible factor is post-COVID debility reducing exercise capacity sufficiently to contribute to weight gain. Sex has previously been shown to be a risk factor for dysautonomia.24 We identified no sex difference.
Study limitations
This was a comparatively large (n = 205) observational cohort study of a previously active, working-age population (currently underreported in the post-COVID literature) that found significant differences in autonomic function associated with reduced exercise capacity. However, there are limitations to the study. The data are cross-sectional and do not determine whether patients are improving, or regressing symptomatically, with regard to HR responses and objective physical function. To determine whether abnormalities resolve over time, follow-up testing will be required. The data presented represent every consecutive patient who met the eligibility criteria and attended the DCRS. Comparisons to an unvaccinated or healthy control group were not possible. Although not used as a primary measure to identify dysautonomia, we recognize that HRV is frequently measured with patients in a supine position with controlled breathing strategies. Body postures have been shown to affect HRV recovery, with more upright postures slowing recovery.25 Whereas resting HRV values were associated with dysautonomia, values recorded for 3 minutes of recovery were not. Only a few studies have performed test/retest reliability of HRV during postexercise recovery, and they have shown variable and inconsistent findings.16 Caution is advised when interpreting HRV measures calculated using very short epochs (eg, 30 seconds) during nonstationary conditions (particularly during the immediate postexercise recovery), as nonoscillatory changes in HR may contribute to HRV.16 Additional clinical information might be revealed by 24-hour HRV monitoring.9 Future research could tackle the question of whether blood or neuroradiology biomarkers of brain function are associated with dysautonomia in an effort to identify a mechanism for dysautonomia in this setting.
Conclusion
The prevalence of dysautonomia is high in this working-age, post–COVID-19 population. Dysautonomia is associated with objective functional limitation, but it is not associated with, or the cause of, subjective limitation or symptoms of cardiorespiratory disease.
Acknowledgments
The authors would like to thank the patients who attended the Defence Medical Services COVID-19 Recovery Service (DCRS) for their determination and positive engagement during the cardiopulmonary exercise test. We also acknowledge the tireless efforts of all the support staff at the Defence Medical Rehabilitation Centre. Data have been collected from serving UK Armed Forces personnel. As such, their data are sensitive and will not be published on an open registry. Requests for the anonymized data used for analysis can be directed to the corresponding author, who will liaise with the appropriate authority for consideration.
Footnotes
Funding Sources: The authors have no funding sources to disclose. Disclosures: The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2021.12.005.
Appendix. Supplementary data
References
- 1.Johns Hopkins Coronavirus Resource Center COVID-19 Data in Motion. https://coronavirus.jhu.edu Available from.
- 2.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goërtz Y.M., Van Herck M., Delbressine J.M., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshak N., Abdelnabi M., Ball S., et al. Dysautonomia: an overlooked neurological manifestation in a critically ill COVID-19 patient. Am J Med Sci. 2020;360:427. doi: 10.1016/j.amjms.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo Y.L. COVID -19, fatigue and dysautonomia. J Med Virol. 2021;93:1213. doi: 10.1002/jmv.26552. [DOI] [PubMed] [Google Scholar]
- 6.Loa Y., Leonga H., Hsua L., et al. Autonomic dysfunction in recovered severe acute respiratory syndrome patients. Can J Neurol Sci. 2005;32:264. [PubMed] [Google Scholar]
- 7.Gourine A.V., Ackland G.L. Cardiac vagus and exercise. Physiology. 2019;34:71–80. doi: 10.1152/physiol.00041.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jouven X., Empana J.-P., Schwartz P.J., Desnos M., Courbon D., Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 9.Pan Y., Yu Z., Yuan Y., et al. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front Physiol. 2021;12:630038. doi: 10.3389/fphys.2021.630038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIHR Living with Covid19—Second review. March 16, 2021. https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review Available from.
- 11.O'Sullivan O, Barker-Davies R, Chamley R, et al. Defence Medical Rehabilitation Centre (DMRC) COVID-19 Recovery Service. BMJ Mil Health. 2021. Published Online First: 05 February 2021. https://doi.org/10.1136/bmjmilitary-2020-001681. [DOI] [PubMed]
- 12.JSP 950 COVID Supplementary Policy Leaflet 002 v.2.0. Clinical and Occupational Assessment Prior to Return to Duty and Training post COVID-19 In: 950 JSP. Ministry of Defence; London, UK: 2021. [Google Scholar]
- 13.Radtke T., Crook S., Kaltsakas G., et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev. 2019;28:180101. doi: 10.1183/16000617.0101-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 15.Mahler D.A., Horowitz M.B. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26:1078–1081. [PubMed] [Google Scholar]
- 16.Michael S., Graham K.S., Davis G.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front Physiol. 2017;8:301. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acar B., Savelieva I., Hemingway H., Malik M. Automatic ectopic beat elimination in short-term heart rate variability measurement. Comput Meth Progr Biomed. 2000;63:123–131. doi: 10.1016/s0169-2607(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fornasiero A., Savoldelli A., Skafidas S., et al. Delayed parasympathetic reactivation and sympathetic withdrawal following maximal cardiopulmonary exercise testing (CPET) in hypoxia. Eur J Appl Physiol. 2018;118:2189–2201. doi: 10.1007/s00421-018-3945-5. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger J.J., Le F.K., Lahiri M., Kannankeril P.J., Ng J., Kadish A.H. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol. 2006;290:H2446–H2452. doi: 10.1152/ajpheart.01118.2005. [DOI] [PubMed] [Google Scholar]
- 21.Fagerland M.W. t-tests, non-parametric tests, and large studies—a paradox of statistical practice? BMC Med Res Methodol. 2012;12:1–7. doi: 10.1186/1471-2288-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monfredi O., Lyashkov A.E., Johnsen A.-B., et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavčar P., Potokar M., Kolenc M., et al. Neurotropic viruses, astrocytes, and COVID-19. Front Cell Neurosci. 2021;15:123. doi: 10.3389/fncel.2021.662578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schondorf R., Benoit J., Wein T., Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192–201. doi: 10.1016/s0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 25.Buchheit M., Al Haddad H., Laursen P., Ahmaidi S. Effect of body posture on postexercise parasympathetic reactivation in men. Exp Physiol. 2009;94:795–804. doi: 10.1113/expphysiol.2009.048041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.