Abstract
Background
Ovarian function is closely related to the degree of vascular network development surrounding the ovary. Maternal aging‐related construction defects in this vascular network can cause ovarian hypoxia, which impedes oocyte nutrient supply, leading to physiological changes in the ovaries and oocytes. The anti‐aging gene Sirtuin 1 (SIRT1) senses and adapts to ambient stress and is associated with hypoxic environments and mitochondrial biogenesis.
Methods
The present study is a literature review focusing on investigations involving the changes in SIRT1 and mitochondrial expression during hypoxia and the cytoprotective effects of the SIRT1 activator, resveratrol.
Main findings
Hypoxia suppresses SIRT1 and mitochondrial expression. Resveratrol can reverse the hypoxia‐induced decrease in mitochondrial and SIRT1 activity. Resveratrol suppresses the production of hypoxia‐inducible factor‐1α and vascular endothelial growth factor proteins.
Conclusion
Resveratrol exhibits protective activity against hypoxic stress and may prevent hypoxia‐ or aging‐related mitochondrial dysfunction. Resveratrol treatment may be a potential option for infertility therapy.
Keywords: aging, hypoxia, mitochondria, resveratrol, sirtuin 1
Resveratrol may prevent mitochondrial dysfunction due to hypoxia or aging and that resveratrol treatment may be a potential therapy for treating infertility.
1. INTRODUCTION
Angiogenesis is a critical process during folliculogenesis, ovulation, and corpus luteum formation in the human ovary. 1 , 2 , 3 , 4 , 5 Ovarian follicular microvasculature development is regulated by angiogenic factors, including the hepatocyte growth factor, angiopoietin, and members of the vascular endothelial growth factor (VEGF) family and the CXC chemokine family. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Ovarian function is closely related to the degree of ovarian tissue vascular network development. Maternal age‐related defects in the construction of the vascular network surrounding the ovary can lead to ovarian hypoxia, which is assumed to affect ovarian follicle growth and development. Hypoxia and the aneuploid oocyte increase associated with advanced reproductive age can also result from age‐related dominant follicle microvasculature deficiencies. 10 , 15
Mitochondrial quantity is highly positively correlated with oocyte maturation and fertilization and the subsequent embryo development. 16 , 17 , 18 , 19 Cellular hypoxia substantially suppresses mitochondrial gene expression, and the mitochondria count reduction caused by aging and hypoxia may be the primary source of infertility in advanced age animals. 20 , 21 , 22 , 23
Sirtuin‐1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)‐dependent protein deacetylase, detects and adapts to ambient stress. 24 , 25 , 26 SIRT1 is activated by caloric restriction and natural polyphenolic compounds such as resveratrol 27 , 28 , 29 , 30 , 31 and is involved in the regulation of various cellular physiological and pathological processes, including gene silencing, stress resistance, apoptosis, and inflammation, all of which are associated with aging‐related diseases. 32 , 33 , 34 , 35 SIRT1 activity is primarily nuclear but significantly impacts mitochondrial biogenesis and turnover. 23 , 36 , 37 , 38 , 39 , 40 , 41 Recent studies have demonstrated that SIRT1 promotes mitochondrial biogenesis via deacetylation of target proteins such as peroxisome proliferator‐activated receptor‐gamma coactivator (PGC)‐1α and hypoxia‐inducible factor (HIF)‐1α, indicating potential therapeutic benefits of SIRT1 activation in metabolic and other aging‐related diseases. 36 , 42 , 43
To further elucidate the molecular and cellular mechanisms involved in the role of SIRT1 in hypoxia, we focused this review on the changes in both SIRT1 and mitochondrial expression during hypoxia and the protective effects of resveratrol. Furthermore, we present possible remediation measures for ovarian hypoxia.
2. SIRT1 AND OVARIAN FUNCTION
2.1. SIRT1
Sirtuins are protein members of the class III NAD+‐dependent histone deacetylase family, or the silent information regulator 2 family. Because changes in the NAD+/NADH ratio regulate sirtuin activity, all members of this family may play crucial roles in the detection of cellular energy status. 27 , 44 To date, seven members of the sirtuin family, SIRT1–7, have been identified in mammals, with each member exhibiting unique subcellular localization, function, and substrate specificity. 45 , 46 SIRT1 and SIRT2 have been found in both the nucleus and cytosol, SIRT3, SIRT4, and SIRT5, exclusively in mitochondria, and SIRT6 and SIRT7, exclusively in the nuclear compartment. 47 , 48 , 49 The presence of all sirtuins has been confirmed in granulosa cells (GCs) and human ovarian granulosa‐like tumor (KGN) cells. 50
Among the sirtuins, SIRT1 is the most phylogenetically similar to yeast Sir2, the most prominent, and the most extensively studied. 51 Chiefly located in the nucleus, with some environmental signal‐triggered shuttling to the cytosol, SIRT1 has the capability to extend lifespan, delay aging, and prevent aging‐related diseases, primarily via catalysis of histone deacetylation and regulation of transcription factors or coactivators, such as p53, forkhead box O (FOXO), nuclear factor‐κB (NF‐κB), PGC‐1α, and Ku70. 52 , 53 , 54 It is involved in the regulation of biological and pathological processes, such as apoptosis via inhibition of p53‐dependent transcription, inflammation via reduction of NF‐κB activity, and energy metabolism via regulation of metabolic enzymes like PPAR‐γ. 32 , 55 , 56 , 57 , 58 , 59 The SIRT1 preventative mechanisms in aging and aging‐related diseases are likely diverse and dependent on the regulated proteins. In addition, SIRT1 endogenous levels are related to aging‐related disease development. 60 Besides affecting the protein levels of SIRT1, aging also impacts its activity. 61 Age‐dependent decrease in the level of SIRT1 is observed in the brain, liver, muscle, and arteries. 62 , 63 , 64 , 65 , 66 SIRT1 deficiency promotes the expression of genes characteristic of aging. 67
SIRT1 is expressed not only in GCs and cumulus cells but also in oocytes and theca cells. 65 , 68 , 69 , 70 In the reproductive system, SIRT1 plays a role in GC apoptosis during follicular atresia, has been linked to follicular reserve preservation and ovarian lifespan extension, and is an essential factor in the activation of the steroidogenesis associated with luteinization and terminal differentiation. 68 , 71 , 72
2.2. Resveratrol
Numerous studies have explored controlling sirtuin‐dependent downstream pathways via pharmacological‐ and nonpharmacological‐based sirtuin activation. Resveratrol (3,5,4′‐hydroxystilbene), a natural polyphenolic compound commonly found in grapes, berries, red wine, and several botanical extracts, was one of the first compounds recognized as a SIRT activator. 30 , 73 Resveratrol has a chemical structure similar to that of some estrogens, such as diethylstilbestrol, and is considered a natural phytoestrogen. 74 , 75 As the most potent natural SIRT1 ligand, resveratrol has received a great deal of attention due to its beneficial anti‐oxidant, anti‐inflammatory, anti‐aging, anti‐carcinogenic, and anti‐angiogenic qualities. 76 , 77 , 78 , 79 , 80 , 81 Stressful events induce SIRT1 activation and binding to various molecular targets, including NF‐κB, tumor protein p53, FOXO, PGC‐1α, liver X receptor, and HIF‐2α. 25 , 49 By activating these molecules via SIRT1, resveratrol plays a pivotal role in energy homeostasis regulation, gene silencing, genomic stability, and cell survival. Resveratrol has been found to extend the lifespan of Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster, and increase energy metabolism and mitochondrial oxidative capacity. 82
2.3. Effect of resveratrol on GCs and oocyte via SIRT1
Sirtuin expression has been detected in mammalian ovaries, GCs, oocytes, and embryos. 65 , 68 , 69 , 70 , 83 Resveratrol may provide protection against ovarian aging through SIRT1‐related cellular mechanisms, via an anti‐oxidative effect, protecting oocytes from age‐dependent defects. 44
Various studies have reported the effects of resveratrol, such as increased ATP production and the promotion of mitochondrial biogenesis, on mammalian—including human—GCs. 84 In rat GCs, resveratrol treatment induces transcription‐level upregulation of SIRT1, the luteinizing hormone receptor, the steroidogenic acute regulatory protein, and P450 aromatase, but does not affect the regulation of the follicle‐stimulating hormone receptor, suggesting that resveratrol and SIRT1 can modulate ovarian functions via folliculogenesis‐related molecule and gonadotropin receptor activation. 68
In swine GCs, resveratrol increases SIRT1 mRNA and protein levels in a dose‐dependent manner, accelerating cell apoptosis, and follicular atresia. 71 Resveratrol supplementation of cultured porcine ovarian GCs increases SIRT1 protein levels, induces apoptosis, promotes testosterone and estrogen release, and inhibits cell proliferation. 85
Interestingly, resveratrol treatment also promotes mitochondrial synthesis, ATP production, and autophagy in GCs from advanced age cows, improving mitochondrial function and in vitro oocyte development. 86 Improved regulation of GC function is an expected result of stimulation of mitochondrial biogenesis by resveratrol. Resveratrol supplementation of maturation medium enhances SIRT1 protein expression and increases the ATP content in bovine oocytes, resulting in improved fertilization outcomes. 87 In human oocytes, resveratrol increases the emission rate of first polar body and reduces the percentage of spindle with abnormal morphology. 88 These reports on the impact of resveratrol on GCs correlate with current evidence for the overall effect exerted by resveratrol on ovarian physiology and with the results of a recent study suggesting that mitochondrial function in cumulus cells and GCs can directly influence pregnancy outcomes. 89 , 90 , 91
These findings led us to investigate the direct impact of hypoxia and resveratrol on the SIRT1/PGC‐1α pathway and on the quantity of mitochondria in KGN cells (Figure 1A–F). 92 Resveratrol significantly and dose‐dependently upregulates, whereas hypoxic stress induced by cobalt chloride (CoCl2, a hypoxia‐mimicking agent) significantly downregulates the expression of SIRT1, PGC‐1α, and mitochondrial DNA (mtDNA) in comparison to the controls.
FIGURE 1.
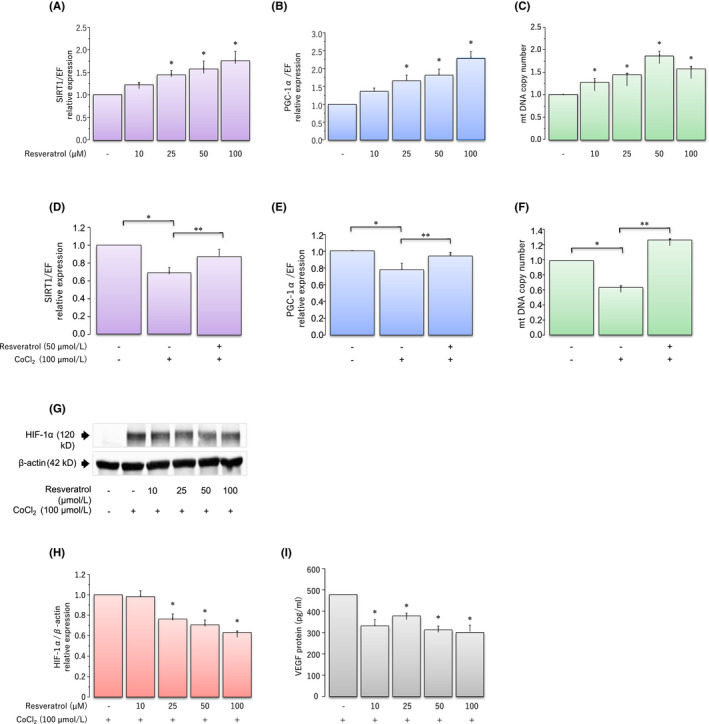
Protective effects of resveratrol during CoCl2‐induced hypoxic stress. Effects of various concentrations of the resveratrol on KGN cells. (A) Sirtuin 1 (SIRT1) mRNA and (B) peroxisome proliferator‐activated receptor‐gamma coactivator (PGC)‐1α mRNA levels were assessed via real‐time PCR and calculated after normalization to elongation factor 1α mRNA levels. (C) Mitochondrial DNA copy number was determined via real‐time PCR. Effects of CoCl2‐induced hypoxic stress on mRNA expression and protein secretion. (D) SIRT1 mRNA and (E) PGC‐1α mRNA levels were assessed via real‐time PCR and calculated after normalization to elongation factor 1α mRNA levels. (F) Mitochondrial DNA copy number was determined via real‐time PCR. (G) The expression of hypoxia‐inducible factor (HIF)‐1α was quantified using western blotting. The levels of HIF‐1α were normalized to β‐actin levels. (H) The protein levels were quantified using ImageJ. (I) Levels of vascular endothelial growth factor protein were analyzed via enzyme‐linked immunosorbent assay. Fold differences are shown in comparison with the control, for which the value was defined as 1.0. Data are presented as mean ±SEM, n = 3. Statistically significant differences are indicated in brackets: * p < 0.05 versus the control group; ** p < 0.05 versus the 100 μmol/L CoCl2 treatment group. These figures have been modified from Nishigaki et al. 92 Reprod Med Biol. 2020
To further examine the protective effect of resveratrol from CoCl2‐induced hypoxic stress, KGN cells were cultured in medium containing 100 μmol/l CoCl2, with or without 50 μM resveratrol. CoCl2‐induced hypoxic stress resulted in the downregulation of the expression of SIRT1 and PGC‐1α, and reduced mtDNA copy number, and resveratrol reversed the CoCl2‐induced inhibitory effects of hypoxia.
The cumulative results indicate that upregulation of SIRT1 by resveratrol partially improves the condition of hypoxiated GCs by increasing mitochondria quantity. Thus, resveratrol may have a potentially beneficial effect in ameliorating reproductive function via SIRT1 regulation.
2.4. SIRT1 activators other than resveratrol
Several SIRT1 activators other than resveratrol have been reported to improve reproductive function. They have also been reported to improve reproductive function. Melatonin protects premature ovarian insufficiency by reducing oxidative stress and apoptotic damage via activation of SIRT1 signaling in a receptor‐dependent manner in rats. 93 N‐acetyl‐L‐cysteine treatment has been demonstrated to increase the quality of the oocytes from old mice in association with the higher expression level of Sirt1 and Sirt2, and increased telomerase activity and length. 94 On the contrary, it was demonstrated that an inhibitor of SIRT1 increased the ratio of abnormal fertilization. 95 Thus, SIRT1 is clearly involved in the protection of ovarian function.
2.5. Clinical studies with resveratrol
Surprisingly, resveratrol has been reported to have a negative effect on pregnancy outcomes. Ochiai et al. demonstrated that women with regular resveratrol supplementation (200 mg/day) during IVF–embryo transfer cycles had lower pregnancy rates and higher miscarriage rates. Resveratrol has potential therapeutic effects in improving ovarian function; however, it also has anti‐deciduogenic activity. Therefore, it is recommended to avoid the consumption of the compound during the luteal and gestational phases. 96 Initial clinical applications of resveratrol have taken into account its various positive and negative effects.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age and is primarily characterized by hyperandrogenism and ovulatory dysfunction. 97 The effect of resveratrol on the endocrine and metabolic functions of PCOS patients was evaluated during a 3‐month placebo‐controlled randomized clinical trial. This study revealed that resveratrol (1500 mg/day p.o.) reduces the levels of serum testosterone, serum dehydroepiandrosterone sulfate, and insulin, while increasing the insulin sensitivity index. Improvement in hyperandrogenemia observed in response to resveratrol was comparable to, or greater than, that observed in response to metformin, an effect possibly related to improvements in insulin sensitivity and level. 98
The impact of resveratrol on the management of endometriosis‐related pain was investigated in a clinical trial involving 12 patients of reproductive age (range 22–37 years), with a laparoscopic diagnosis of endometriosis, who had failed to obtain pain relief using an oral contraceptive containing drospirenone +ethinylestradiol alone for 6 months. The addition of 30 mg of resveratrol to the contraceptive regimen resulted in a significant reduction in pain scores, with 82% of patients reporting complete resolution of dysmenorrhea and pelvic pain after 2 months of use. These results demonstrate that resveratrol potentiates the efficacy of oral contraceptives in the management of endometriosis‐associated dysmenorrhea. 99
Although most studies that have revealed the excellent anti‐cancer properties of resveratrol have been performed in cell culture and pre‐clinical models, a small number of clinical trials involving cancer patients have been reported. 100 In addition to the effects in subjects with cancer, the effect of resveratrol in subjects with a higher cancer risk has also been demonstrated. A pilot study was conducted in postmenopausal women with a high body mass index (BMI ≥25 kg/m2) to determine the clinical effect of resveratrol on systemic steroid hormones. A 12‐week 1 g/day resveratrol supplementation has been shown to increase the concentration of sex steroid hormone‐binding globulin, a protein that has been linked to reduction in breast cancer risk, and resulted in an average of 73% increase in urinary 2‐hydroxyestrone (2‐OHE1) levels leading to a favorable change in urinary 2‐OHE1/16α‐OHE1 ratio. 101 The results indicate that resveratrol supplementation has a favorable influence on estrogen metabolism and lowered breast cancer risk factors in obese and overweight postmenopausal women. 102 Therefore, resveratrol has provided some benefit in cancer prevention and treatment, and the efficacy and safety of resveratrol in human trials must be further investigated to better understand and develop its therapeutic potential.
At present, clinical trials of resveratrol have been limited due to unresolved issues, such as its extensive metabolism leading to poor bioavailability and its adverse side effects, including diarrhea, nausea, and abdominal pain. 103 , 104
3. HYPOXIA AND OVARIAN FUNCTION
3.1. HIF‐1α expression during hypoxia
Hypoxia potentially contributes to aging‐related functional decline, and reducing hypoxic damage and senescence induction requires improved understanding of the molecular and cellular responses to hypoxia. 21 An appropriate response to changes in oxygen availability, particularly coping with oxygen deficiency in hypoxic environments, is essential for survival.
Cellular level hypoxic responses are largely mediated by HIFs, which act as transcriptional regulators of the genes involved in survival during periods of low oxygen and are regulated primarily by oxygen‐dependent proteasomal degradation. HIF‐1α is stably expressed during hypoxia. 105 , 106 , 107
One such contribution involves VEGF, a 46‐kDa disulfide‐linked homodimeric glycoprotein that stimulates vascular endothelial cell proliferation, migration, tubule organization, and permeability. 108 Repeated evidence has demonstrated that VEGF production is regulated by HIF‐1α, particularly under hypoxic conditions. 109 During hypoxia‐stimulated angiogenesis, HIF‐1 transcriptionally regulates VEGF expression by directly binding to the hypoxia‐response elements in the VEGF promoter. 110 , 111 Recent studies have demonstrated that VEGF is an essential regulator of ovarian angiogenesis, a critical process in follicular development and corpus lutea formation. 108 , 112 In addition, both hypoxia and CoCl2 have been reported to induce VEGF production in ovarian GCs. 10 VEGF acts as a key angiogenic factor in ovarian vascularization regulation and may play a modulatory role in GC functional activity and follicular growth.
As a follicle grows, follicular fluid oxygen concentration decreases, causing the accumulation of HIF‐1α, which is speculated to promote VEGF transcription and angiogenesis around the follicle. HIF‐1α, which is involved in follicle development and luteinization in the ovaries of mammals, including those of humans, is expressed in human corpus luteum and luteinized GCs. Hypoxia increases VEGF directly, and nuclear HIF‐1α is most active in the early luteal phase at the time of maximal angiogenesis. 113
Aging contributes to ovarian hypoxic stress, which affects ovarian follicle growth and development. Aging‐related hormonal imbalances cause non‐optimal microvasculature to develop around maturing and mature follicles. The resulting reduction in perifollicular capillary bed size and blood flow through the area leads to an oxygen deficit and the concomitant accumulation of carbon dioxide and anaerobic products, such as lactic acid, inside the follicle. The consequent decrease in oocyte intracellular pH diminishes the spindle size, causing chromosome displacement and nondisjunction. 15
Higher follicular fluid VEGF concentrations have been reported to correlate positively with female age. 10 Thus, aging‐associated deficient microvasculature around the dominant follicle results in hypoxia, and the predisposition toward increased aneuploid oocyte incidence is associated with advanced reproductive age.
Mitochondria are multifunctional organelles essential to energy production, apoptosis, and calcium homeostasis, 114 , 115 and their quantity is closely related to oocyte maturation, fertilization, and subsequent development. 16 , 17 , 18 , 19 Cellular hypoxia significantly suppresses mitochondrial gene expression, 21 and the reduction in mitochondrial quantity due to aging and hypoxia may be the primary cause of infertility in advanced age animals. 16 , 23 , 116 Therefore, HIF‐1α regulation mechanisms have been implicated in the prevention of premature cellular senescence and the pathogenesis of numerous aging‐related chronic diseases.
Recent reports have suggested an important connection between HIF‐1α and SIRT1. 26 , 117 , 118 Lim et al. observed that SIRT1 interacts with HIF‐1α in multiple cell lines and mouse tissues, and modulates cellular hypoxia responses via HIF‐1α deacetylation. 26
3.2. SIRT1, a key regulator of hypoxic stress
During hypoxia, expression of SIRT1 is suppressed, whereas that of HIF‐1α is activated. 21 , 118 At least two distinct mechanisms have been suggested of the former effect: reduced transcription of SIRT1 mRNA and a decrease in the NAD+/NADH ratio. 119 , 120 The ratio of NAD+, a SIRT‐mediated deacetylation substrate, to NADH is an important physiological regulator of SIRT activity, and intracellular levels of both are modulated by nutrient deprivation, energy consumption, or hypoxia. 49
During hypoxia, decreased NAD+ levels downregulate SIRT1 activity, which leads to increased HIF‐1α acetylation and enhanced induction of hypoxic response genes, implying that crosstalk between hypoxia and metabolism detection pathways ensures cellular adaptation to hypoxia. 26 In addition, HIF‐1α is inactivated by SIRT1 activators, such as resveratrol, and is activated by SIRT1 inhibitors, such as nicotinamide and splitomicin. 117 SIRT1 knockdown reverses the inhibition of HIF‐1α acetylation and activation by resveratrol, suggesting that resveratrol inhibits HIF‐1α through SIRT1 activation.
3.3. Effect of resveratrol on hypoxia
Resveratrol inhibits hypoxia‐induced HIF‐1α and VEGF expression in human cancer cells via multiple mechanisms, including interference with protein translational regulation and promotion of HIF‐1α protein degradation. 121 , 122 , 123 In one such mechanism, inhibition of protein kinase B and mitogen‐activated protein kinase phosphorylation by resveratrol plays a partial role in the downregulation of HIF‐1α expression. An additional translation‐level mechanism involves the inhibition of protein translational regulators, including Mr 70,000 ribosomal protein S6 kinase 1, S6 ribosomal protein, eukaryotic initiation factor 4E‐binding protein 1, and eukaryotic initiation factor 4E. Finally, resveratrol also induces substantial HIF‐1α protein degradation via the proteasome pathway. 123
Based on these findings, we examined the effects of resveratrol on HIF‐1α and VEGF expression in KGN cells under CoCl2‐induced hypoxic conditions (Figure 1G–I). 92 HIF‐1α protein levels significantly increase in response to hypoxia, an induction which is significantly suppressed by treatment with resveratrol in a dose‐dependent manner. CoCl2 rapidly induces HIF‐1α protein accumulation due to a marked decrease in HIF‐1α protein degradation, indicating that resveratrol may substantially induce HIF‐1α protein degradation under hypoxic conditions. Resveratrol also attenuates CoCl2‐induced production of VEGF.
4. MITOCHONDRIAL BIOGENESIS AND ITS RELATION TO HYPOXIA
4.1. Mitochondrial biogenesis
Mitochondria are the most dynamically responsive detection systems in eukaryotic cells, satisfying metabolic energy demands, supplying biosynthetic precursors, and consequently regulating diverse processes including proliferation, immune response, apoptosis, and cell viability. 124 , 125 , 126 , 127 Cells can degrade damaged mitochondria via the process of mitophagy and, under appropriate conditions, stimulate functional mitochondria to proliferate through mitochondrial biogenesis, a complex process consisting of both the growth and the division of preexisting mitochondria. 128
4.2. Deacetylation of PGC‐1 and mitochondrial biogenesis by SIRT1
Recent studies have demonstrated that SIRT1 promotes mitochondrial biogenesis by deacetylation of target proteins, such as PGC‐1α, which suggests the potential therapeutic benefits of SIRT1 activation in metabolic and aging‐related diseases. 129 , 130 , 131 Both SIRT1 and the nuclear transcription factor PGC‐1α have been found in the mitochondria of human cell lines and platelets, as well as in various mouse organs.
Within the mitochondria, deacetylase and its substrate are associated with mtDNA nucleoids and mitochondrial transcription factor A (TFAM), a key mitochondrial gene transcription factor and mtDNA copy number regulator. 132 , 133 These findings suggest that SIRT1 and PGC‐1α may also directly affect mitochondrial transcription (Figure 2).
FIGURE 2.
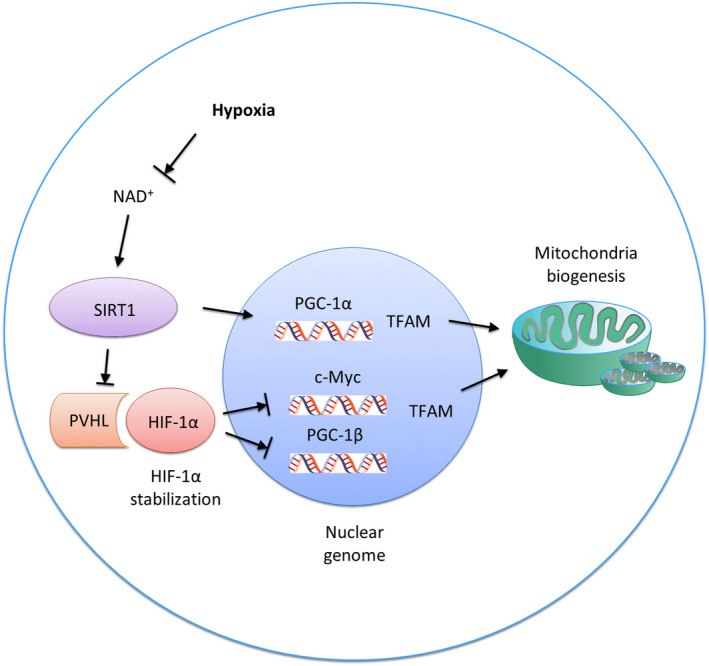
Regulation of mitochondria biogenesis by SIRT1 and HIF‐1α during hypoxia. During hypoxia, the activity of SIRT1 in the nucleus is reduced, which decreases Von Hippel Lindau tumor suppressor levels and subsequently stabilizes HIF‐1α. Activated HIF‐1α reduces c‐Myc activity and subsequently reduces transcription of mitochondrial transcription factor A. PGC‐1β activity is also inhibited by HIF‐1α, resulting in the downregulation of mitochondrial genes. SIRT1 reduction suppresses PGC‐1α activity and prevents mitochondrial synthesis
TFAM and mitochondrial transcription factors B1 and B2 are critical in the regulation of replication, transcription, and maintenance during mitochondrial biogenesis. 130 SIRT1 is primarily located in the nucleus, but its activities greatly impact mitochondrial biogenesis and turnover. 36 Mitochondrial biogenesis involves the transcription of both nuclear and mtDNA‐encoded genes and is orchestrated by the PGC‐1 family of transcriptional coactivators. 42 , 134 The PGC‐1 family consists of three members: PGC‐1α, PGC‐1β, and the PGC‐related coactivator. 135 PGC‐1α, often cited as a master regulator of mitochondrial biogenesis, co‐activates the transcription of nuclear respiratory factor 1 and 2, which, in turn, regulate TFAM transcription. 130 , 136 , 137 TFAM translocates to the mitochondrial matrix, where it stimulates mtDNA replication and mitochondrial gene expression. PGC‐1α undergoes several modes of post‐translational modifications, including acetylation and phosphorylation.
Acetylation alters the localization of PGC‐1α in the nucleus and inhibits its transcriptional activity. Conversely, several studies have demonstrated that PGC‐1α deacetylation is dependent on SIRT1 activity, which increases PGC‐1α transcriptional activity. 138 , 139 , 140 Given the reliance of PGC‐1α activity on its acetylation status, investigations on metabolic regulation and mitochondrial biogenesis have focused on the connection between SIRT1 and PGC‐1α. Nemoto's group provided clear biochemical evidence that SIRT1 physically and functionally interacts with PGC‐1α. 139
4.3. Hypoxia and mitochondrial biogenesis
During the aging process, HIF‐1α suppresses mitochondrial biogenesis, impairing energy‐dependent cellular processes, including cell and tissue repair. 32 HIF‐1α involvement in mitochondrial biogenesis regulation and nuclear‐mitochondrial communication modulation during aging is independent of PGC‐1α. 43 The upregulation of HIF‐1α activates the Mxi1 gene, which encodes a c‐Myc transcriptional repressor, resulting in an interruption of the c‐Myc and TFAM binding, thus further suppressing TFAM promoter activity and mitochondrial biogenesis. 43
PGC‐1β, an additional master regulator of mitochondrial biogenesis, oxidative metabolism, and antioxidant defense, is preferentially expressed in high oxidative capacity tissue, where it participates in the metabolic response to high energy demand by regulating mitochondrial biogenesis. 141 HIF‐1α also negatively regulates PGC‐1β activity. In the cardiac ventricles of hypoxic mice, increased HIF‐1α expression results in decreased PGC‐1β mRNA and protein levels due to HIF‐1α binding. Conversely, degradation of HIF‐1α leads to PGC‐1β release, which subsequently promotes mitochondrial biogenesis. 142
During the hypoxia‐ or aging‐induced decline in nuclear energy state or NAD+ levels, the nuclear SIRT1 activity is reduced, downregulating the Von Hippel‐Lindau protein and stabilizing the expression of HIF‐1α. The latter subsequently reduces c‐Myc activity and prevents TFAM transcription, which is required for replication, transcription, and maintenance of mitochondrial biogenesis. PGC‐1β activity is also inhibited by its interaction with HIF‐1α, resulting in the downregulation of mitochondrial genes 21 , 26 (Figure 2).
5. CONCLUSION AND FUTURE PERSPECTIVE
Due to defects in the construction of vascular network surrounding the ovaries, maternal aging can cause ovarian hypoxia, which is assumed to affect the growth and development of ovarian follicles. Resveratrol enhances SIRT1 expression and mitochondrial function under hypoxic conditions, suggesting that it exerts a protective effect against hypoxia (Figure 3). Resveratrol may prevent mitochondrial dysfunction due to hypoxia or aging, and resveratrol treatment may be a potential therapy for treating infertility.
FIGURE 3.
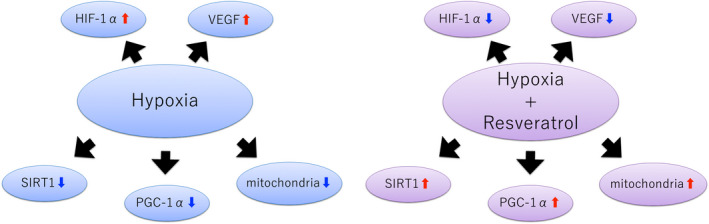
Effects of resveratrol on hypoxia. Resveratrol exerts protective effects against hypoxic stress
CONFLICT OF INTEREST
All authors declare having no conflicts of interest.
HUMAN RIGHTS STATEMENT AND INFORMED CONCENT
This article does not contain any study with human participants that have been performed by any of the authors.
ANIMAL STUDIES
This article does not contain any study with animal participants that have been performed by any of the authors.
ACKNOWLEDGMENTS
We thank Yumi Kono for her editorial assistance. This work was supported by a grant from the Smoking Research Foundation. This work was also supported by the Japan Society for the Promotion of Science KAKENHI, Grant Number # JP21K09480. We thank Editage (www.editage.com) for English language editing.
Nishigaki A, Tsubokura H, Tsuzuki‐Nakao T, Okada H. Hypoxia: Role of SIRT1 and the protective effect of resveratrol in ovarian function. Reprod Med Biol. 2021;21:e12428. doi: 10.1002/rmb2.12428
REFERENCES
- 1. Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson RS, Woad KJ, Hammond AJ, et al. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869‐881. [DOI] [PubMed] [Google Scholar]
- 3. Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(6):883‐900. [DOI] [PubMed] [Google Scholar]
- 4. Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol. 2003;1:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fraser HM, Duncan WC. Vascular morphogenesis in the primate ovary. Angiogenesis. 2005;8(2):101‐116. [DOI] [PubMed] [Google Scholar]
- 6. Osuga Y, Tsutsumi O, Momoeda M, et al. Evidence for the presence of hepatocyte growth factor expression in human ovarian follicles. Mol Hum Reprod. 1999;5(8):703‐707. [DOI] [PubMed] [Google Scholar]
- 7. Nishigaki A, Okada H, Tsuzuki T, et al. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011;96(6):1378‐1383. [DOI] [PubMed] [Google Scholar]
- 8. Kawano Y, Zeineh Hasan K, Fukuda J, et al. Production of vascular endothelial growth factor and angiogenic factor in human follicular fluid. Mol Cell Endocrinol. 2003;202(1–2):19‐23. [DOI] [PubMed] [Google Scholar]
- 9. Anasti JN, Kalantaridou SN, Kimzey LM, et al. Human follicle fluid vascular endothelial growth factor concentrations are correlated with luteinization in spontaneously developing follicles. Hum Reprod. 1998;13(5):1144‐1147. [DOI] [PubMed] [Google Scholar]
- 10. Friedman CI, Danforth DR, Herbosa‐Encarnacion C, et al. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68(4):607‐612. [DOI] [PubMed] [Google Scholar]
- 11. Kamat BR, Brown LF, Manseau EJ, et al. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995;146(1):157‐165. [PMC free article] [PubMed] [Google Scholar]
- 12. Kryczek I, Frydman N, Gaudin F, et al. The chemokine SDF‐1/CXCL12 contributes to T lymphocyte recruitment in human pre‐ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol. 2005;54(5):270‐283. [DOI] [PubMed] [Google Scholar]
- 13. Nishigaki A, Okada H, Okamoto R, et al. Concentrations of stromal cell‐derived factor‐1 and vascular endothelial growth factor in relation to the diameter of human follicles. Fertil Steril. 2011;95(2):742‐746. [DOI] [PubMed] [Google Scholar]
- 14. Nishigaki A, Okada H, Okamoto R, et al. The concentration of human follicular fluid stromal cell‐derived factor‐1 is correlated with luteinization in follicles. Gynecol Endocrinol. 2013;29(3):230‐234. [DOI] [PubMed] [Google Scholar]
- 15. Gaulden ME. Maternal age effect: the enigma of Down syndrome and other trisomic conditions. Mutat Res. 1992;296(1–2):69‐88. [DOI] [PubMed] [Google Scholar]
- 16. Reynier P, May‐Panloup P, Chrétien MF, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425‐429. [DOI] [PubMed] [Google Scholar]
- 17. Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584‐591. [DOI] [PubMed] [Google Scholar]
- 18. Smith LC, Thundathil J, Filion F. Role of the mitochondrial genome in preimplantation development and assisted reproductive technologies. Reprod Fertil Dev. 2005;17(1–2):15‐22. [DOI] [PubMed] [Google Scholar]
- 19. Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76(2):327‐335. [DOI] [PubMed] [Google Scholar]
- 20. Shiratsuki S, Hara T, Munakata Y, et al. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol. 2016;437:75‐85. [DOI] [PubMed] [Google Scholar]
- 21. Yeo EJ. Hypoxia and aging. Exp Mol Med. 2019;51(6):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan CC, Liu VW, Lau EY, et al. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11(12):843‐846. [DOI] [PubMed] [Google Scholar]
- 23. Iwata H, Goto H, Tanaka H, et al. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23(3):424‐432. [DOI] [PubMed] [Google Scholar]
- 24. Tatone C, Di Emidio G, Barbonetti A, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267‐289. [DOI] [PubMed] [Google Scholar]
- 25. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim JH, Lee YM, Chun YS, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia‐inducible factor 1alpha. Mol Cell. 2010;38(6):864‐878. [DOI] [PubMed] [Google Scholar]
- 27. Lee SH, Lee JH, Lee HY, et al. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52(1):24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390‐392. [DOI] [PubMed] [Google Scholar]
- 29. Chen D, Bruno J, Easlon E, et al. Tissue‐specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22(13):1753‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187‐17195. [DOI] [PubMed] [Google Scholar]
- 31. Gertz M, Nguyen GT, Fischer F, et al. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One. 2012;7(11):e49761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasaki T, Maier B, Bartke A, et al. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5(5):413‐422. [DOI] [PubMed] [Google Scholar]
- 33. Chung S, Yao H, Caito S, et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501(1):79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaRocca TJ, Hearon CM, Henson GD, et al. Mitochondrial quality control and age‐associated arterial stiffening. Exp Gerontol. 2014;58:78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Revollo JR, Li X. The ways and means that fine tune Sirt1 activity. Trends Biochem Sci. 2013;38(3):160‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang BL. Sirt1 and the Mitochondria. Mol Cells. 2016;39(2):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan Y, Cruzat VF, Newsholme P, et al. Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis. Mech Ageing Dev. 2016;155:10‐21. [DOI] [PubMed] [Google Scholar]
- 38. Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104(2):150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang HT, Hwang ES. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell. 2009;8(4):426‐438. [DOI] [PubMed] [Google Scholar]
- 40. Abe K, Tani K, Fujiyoshi Y. Conformational rearrangement of gastric H(+), K(+)‐ATPase induced by an acid suppressant. Nat Commun. 2011;2:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cantó C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high‐fat diet‐induced obesity. Cell Metab. 2012;15(6):838‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wenz T. Regulation of mitochondrial biogenesis and PGC‐1α under cellular stress. Mitochondrion. 2013;13(2):134‐142. [DOI] [PubMed] [Google Scholar]
- 43. Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear‐mitochondrial communication during aging. Cell. 2013;155(7):1624‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tatone C, Di Emidio G, Vitti M, et al. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015:659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pucci B, Villanova L, Sansone L, et al. Sirtuins: the molecular basis of beneficial effects of physical activity. Intern Emerg Med. 2013;8(Suppl 1):S23‐25. [DOI] [PubMed] [Google Scholar]
- 46. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2‐like proteins. Biochem Biophys Res Commun. 2000;273(2):793‐798. [DOI] [PubMed] [Google Scholar]
- 47. Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med. 2013;56:133‐171. [DOI] [PubMed] [Google Scholar]
- 48. Imai S, Guarente L. Ten years of NAD‐dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31(5):212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. González‐Fernández R, Martín‐Ramírez R, Rotoli D, et al. Granulosa‐lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. Int J Mol Sci. 2019;21(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wątroba M, Szukiewicz D. The role of sirtuins in aging and age‐related diseases. Adv Med Sci. 2016;61(1):52‐62. [DOI] [PubMed] [Google Scholar]
- 52. Tanno M, Sakamoto J, Miura T, et al. Nucleocytoplasmic shuttling of the NAD+‐dependent histone deacetylase SIRT1. J Biol Chem. 2007;282(9):6823‐6832. [DOI] [PubMed] [Google Scholar]
- 53. Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3‐mediated reduction of premature senescence in mice. J Clin Invest. 2012;122(6):2032‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van der Veer E, Ho C, O'Neil C, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282(15):10841‐10845. [DOI] [PubMed] [Google Scholar]
- 55. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF‐kappaB‐dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369‐2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Z, Lowry SF, Guarente L, et al. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J Biol Chem. 2010;285(53):41391‐41401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tran D, Bergholz J, Zhang H, et al. Insulin‐like growth factor‐1 regulates the SIRT1‐p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD‐dependent p53 deacetylase. Cell. 2001;107(2):149‐159. [DOI] [PubMed] [Google Scholar]
- 59. Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR‐gamma. Nature. 2004;429(6993):771‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arunachalam G, Samuel SM, Marei I, et al. Metformin modulates hyperglycaemia‐induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gong H, Pang J, Han Y, et al. Age‐dependent tissue expression patterns of Sirt1 in senescence‐accelerated mice. Mol Med Rep. 2014;10(6):3296‐3302. [DOI] [PubMed] [Google Scholar]
- 62. Cho SH, Chen JA, Sayed F, et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL‐1β. J Neurosci. 2015;35(2):807‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marton O, Koltai E, Nyakas C, et al. Aging and exercise affect the level of protein acetylation and SIRT1 activity in cerebellum of male rats. Biogerontology. 2010;11(6):679‐686. [DOI] [PubMed] [Google Scholar]
- 64. Braidy N, Guillemin GJ, Mansour H, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6(4):e19194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem. 2013;288(42):30515‐30526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bai B, Vanhoutte PM, Wang Y. Loss‐of‐SIRT1 function during vascular ageing: hyperphosphorylation mediated by cyclin‐dependent kinase 5. Trends Cardiovasc Med. 2014;24(2):81‐84. [DOI] [PubMed] [Google Scholar]
- 67. Hwang JW, Yao H, Caito S, et al. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morita Y, Wada‐Hiraike O, Yano T, et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: an implicative role of SIRT1 in the ovary. Reprod Biol Endocrinol. 2012;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Di Emidio G, Falone S, Vitti M, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29(9):2006‐2017. [DOI] [PubMed] [Google Scholar]
- 70. Wang F, Tian X, Zhang L, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril. 2014;101(2):577‐586. [DOI] [PubMed] [Google Scholar]
- 71. Zhao F, Zhao W, Ren S, et al. Roles of SIRT1 in granulosa cell apoptosis during the process of follicular atresia in porcine ovary. Anim Reprod Sci. 2014;151(1–2):34‐41. [DOI] [PubMed] [Google Scholar]
- 72. Zhang XM, Li L, Xu JJ, et al. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523(1):82‐87. [DOI] [PubMed] [Google Scholar]
- 73. Hasan MM, Bashir T, Ghosh R, et al. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules. 2017;22(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bowers JL, Tyulmenkov VV, Jernigan SC, et al. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657‐3667. [DOI] [PubMed] [Google Scholar]
- 75. Gehm BD, McAndrews JM, Chien PY, et al. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94(25):14138‐14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22(8):1111‐1117. [DOI] [PubMed] [Google Scholar]
- 77. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF‐induced activation of nuclear transcription factors NF‐kappa B, activator protein‐1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509‐6519. [DOI] [PubMed] [Google Scholar]
- 78. Joe AK, Liu H, Suzui M, et al. Resveratrol induces growth inhibition, S‐phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8(3):893‐903. [PubMed] [Google Scholar]
- 79. Juhasz B, Varga B, Gesztelyi R, et al. Resveratrol: a multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010;11(8):810‐818. [DOI] [PubMed] [Google Scholar]
- 80. Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218‐220. [DOI] [PubMed] [Google Scholar]
- 81. Labinskyy N, Csiszar A, Veress G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti‐inflammatory phytoestrogen. Curr Med Chem. 2006;13(9):989‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191‐196. [DOI] [PubMed] [Google Scholar]
- 83. Bódis J, Sulyok E, Kőszegi T, et al. Serum and follicular fluid levels of serotonin, kisspeptin, and brain‐derived neurotrophic factor in patients undergoing. J Int Med Res. 2020;48(4):300060519879330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ragonese F, Monarca L, De Luca A, et al. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertil Steril. 2021;115(4):1063‐1073. [DOI] [PubMed] [Google Scholar]
- 85. Sirotkin AV. The role and application of sirtuins and mTOR signaling in the control of ovarian functions. Cells. 2016;5(4):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sugiyama M, Kawahara‐Miki R, Kawana H, et al. Resveratrol‐induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev. 2015;61(4):251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takeo S, Sato D, Kimura K, et al. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev. 2014;60(2):92‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu MJ, Sun AG, Zhao SG, et al. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril. 2018;109(5):900‐907. [DOI] [PubMed] [Google Scholar]
- 89. Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229(3):353‐361. [DOI] [PubMed] [Google Scholar]
- 90. Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715‐725. [DOI] [PubMed] [Google Scholar]
- 91. Tsai HD, Hsieh YY, Hsieh JN, et al. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J Reprod Med. 2010;55(11–12):491‐497. [PubMed] [Google Scholar]
- 92. Nishigaki A, Kido T, Kida N, et al. Resveratrol protects mitochondrial quantity by activating SIRT1/PGC‐1α expression during ovarian hypoxia. Reprod Med Biol. 2020;19(2):189‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ma M, Chen XY, Li B, et al. Melatonin protects premature ovarian insufficiency induced by tripterygium glycosides: role of SIRT1. Am J Transl Res. 2017;9(4):1580‐1602. [PMC free article] [PubMed] [Google Scholar]
- 94. Liu J, Liu M, Ye X, et al. Delay in oocyte aging in mice by the antioxidant N‐acetyl‐L‐cysteine (NAC). Hum Reprod. 2012;27(5):1411‐1420. [DOI] [PubMed] [Google Scholar]
- 95. Takeo S, Kawahara‐Miki R, Goto H, et al. Age‐associated changes in gene expression and developmental competence of bovine oocytes, and a possible countermeasure against age‐associated events. Mol Reprod Dev. 2013;80(7):508‐521. [DOI] [PubMed] [Google Scholar]
- 96. Ochiai A, Kuroda K. Preconception resveratrol intake against infertility: friend or foe? Reprod Med Biol. 2020;19(2):107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544‐551. [DOI] [PubMed] [Google Scholar]
- 98. Banaszewska B, Wrotyńska‐Barczyńska J, Spaczynski RZ, et al. Effects of resveratrol on polycystic ovary syndrome: a double‐blind, randomized, placebo‐controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322‐4328. [DOI] [PubMed] [Google Scholar]
- 99. Maia H, Haddad C, Pinheiro N, et al. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis‐related pain. Int J Womens Health. 2012;4:543‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ko JH, Sethi G, Um JY, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12):2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606‐616. [DOI] [PubMed] [Google Scholar]
- 102. Chow HH, Garland LL, Heckman‐Stoddard BM, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Patel KR, Scott E, Brown VA, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161‐169. [DOI] [PubMed] [Google Scholar]
- 104. Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377‐1382. [DOI] [PubMed] [Google Scholar]
- 105. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia‐inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551‐578. [DOI] [PubMed] [Google Scholar]
- 106. Semenza GL. HIF‐1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474‐1480. [DOI] [PubMed] [Google Scholar]
- 107. Semenza GL. Regulation of tissue perfusion in mammals by hypoxia‐inducible factor 1. Exp Physiol. 2007;92(6):988‐991. [DOI] [PubMed] [Google Scholar]
- 108. Ferrara N, Chen H, Davis‐Smyth T, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4(3):336‐340. [DOI] [PubMed] [Google Scholar]
- 109. Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia‐inducible factor 1. Mol Cell Biol. 1996;16(9):4604‐4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Semenza GL. Hypoxia, clonal selection, and the role of HIF‐1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35(2):71‐103. [DOI] [PubMed] [Google Scholar]
- 111. Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ortega I, Duleba AJ. Ovarian actions of resveratrol. Ann N Y Acad Sci. 2015;1348(1):86‐96. [DOI] [PubMed] [Google Scholar]
- 113. van den Driesche S, Myers M, Gay E, et al. HCG up‐regulates hypoxia inducible factor‐1 alpha in luteinized granulosa cells: implications for the hormonal regulation of vascular endothelial growth factor A in the human corpus luteum. Mol Hum Reprod. 2008;14(8):455‐464. [DOI] [PubMed] [Google Scholar]
- 114. Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128(3):269‐280. [DOI] [PubMed] [Google Scholar]
- 115. Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797‐813. [DOI] [PubMed] [Google Scholar]
- 116. Hamatani T, Falco G, Carter MG, et al. Age‐associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13(19):2263‐2278. [DOI] [PubMed] [Google Scholar]
- 117. Laemmle A, Lechleiter A, Roh V, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF‐1α protein under hypoxic conditions. PLoS One. 2012;7(3):e33433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Leiser SF, Kaeberlein M. A role for SIRT1 in the hypoxic response. Mol Cell. 2010;38(6):779‐780. [DOI] [PubMed] [Google Scholar]
- 119. Zhang Q, Wang SY, Fleuriel C, et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci USA. 2007;104(3):829‐833. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120. Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+‐dependent deacetylase SIRT1 modulates CLOCK‐mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mitani T, Harada N, Tanimori S, et al. Resveratrol inhibits hypoxia‐inducible factor‐1α‐mediated androgen receptor signaling and represses tumor progression in castration‐resistant prostate cancer. J Nutr Sci Vitaminol (Tokyo). 2014;60(4):276‐282. [PubMed] [Google Scholar]
- 122. Zhang Q, Tang X, Lu QY, et al. Resveratrol inhibits hypoxia‐induced accumulation of hypoxia‐inducible factor‐1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005;4(10):1465‐1474. [DOI] [PubMed] [Google Scholar]
- 123. Cao Z, Fang J, Xia C, et al. trans‐3,4,5'‐Trihydroxystibene inhibits hypoxia‐inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10(15):5253‐5263. [DOI] [PubMed] [Google Scholar]
- 124. Kim S, Kim MJ, Park DY, et al. Mitochondrial reactive oxygen species modulate innate immune response to influenza A virus in human nasal epithelium. Antiviral Res. 2015;119:78‐83. [DOI] [PubMed] [Google Scholar]
- 125. Kuo CY, Chiu YC, Lee AY, et al. Mitochondrial Lon protease controls ROS‐dependent apoptosis in cardiomyocyte under hypoxia. Mitochondrion. 2015;23:7‐16. [DOI] [PubMed] [Google Scholar]
- 126. Li D, Li X, Guan Y, Guo X. Mitofusin‐2‐mediated tethering of mitochondria and endoplasmic reticulum promotes cell cycle arrest of vascular smooth muscle cells in G0/G1 phase. Acta Biochim Biophys Sin (Shanghai). 2015;47(6):441‐450. [DOI] [PubMed] [Google Scholar]
- 127. Radogna F, Albertini MC, De Nicola M, et al. Melatonin promotes Bax sequestration to mitochondria reducing cell susceptibility to apoptosis via the lipoxygenase metabolite 5‐hydroxyeicosatetraenoic acid. Mitochondrion. 2015;21:113‐121. [DOI] [PubMed] [Google Scholar]
- 128. Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang T, Chi Y, Kang Y, et al. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC‐1α mediated attenuation of mitochondrial oxidative stress. J Cell Physiol. 2019;234(4):5033‐5043. [DOI] [PubMed] [Google Scholar]
- 130. Brenmoehl J, Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. 2013;13(6):755‐761. [DOI] [PubMed] [Google Scholar]
- 131. Anderson RM, Barger JL, Edwards MG, et al. Dynamic regulation of PGC‐1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7(1):101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bogenhagen DF. Mitochondrial DNA nucleoid structure. Biochim Biophys Acta. 2012;1819(9–10):914‐920. [DOI] [PubMed] [Google Scholar]
- 133. Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819(9–10):921‐929. [DOI] [PubMed] [Google Scholar]
- 134. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC‐1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Handschin C, Spiegelman BM. Peroxisome proliferator‐activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728‐735. [DOI] [PubMed] [Google Scholar]
- 136. Springer M, Moco S. Resveratrol and its human metabolites‐effects on metabolic health and obesity. Nutrients. 2019;11(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dominy JE, Lee Y, Gerhart‐Hines Z, et al. Nutrient‐dependent regulation of PGC‐1alpha's acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim Biophys Acta. 2010;1804(8):1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC‐1α. J Biol Chem. 2005;280(16):16456‐16460. [DOI] [PubMed] [Google Scholar]
- 140. Amat R, Planavila A, Chen SL, et al. SIRT1 controls the transcription of the peroxisome proliferator‐activated receptor‐gamma Co‐activator‐1alpha (PGC‐1alpha) gene in skeletal muscle through the PGC‐1alpha autoregulatory loop and interaction with MyoD. J Biol Chem. 2009;284(33):21872‐21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dillon LM, Rebelo AP, Moraes CT. The role of PGC‐1 coactivators in aging skeletal muscle and heart. IUBMB Life. 2012;64(3):231‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Carabelli J, Burgueño AL, Rosselli MS, et al. High fat diet‐induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med. 2011;15(6):1329‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


